-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIncident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis
Background:
Women may have persistent risk of HIV acquisition during pregnancy and postpartum. Estimating risk of HIV during these periods is important to inform optimal prevention approaches. We performed a systematic review and meta-analysis to estimate maternal HIV incidence during pregnancy/postpartum and to compare mother-to-child HIV transmission (MTCT) risk among women with incident versus chronic infection.Methods and Findings:
We searched PubMed, Embase, and AIDS-related conference abstracts between January 1, 1980, and October 31, 2013, for articles and abstracts describing HIV acquisition during pregnancy/postpartum. The inclusion criterion was studies with data on recent HIV during pregnancy/postpartum. Random effects models were constructed to pool HIV incidence rates, cumulative HIV incidence, hazard ratios (HRs), or odds ratios (ORs) summarizing the association between pregnancy/postpartum status and HIV incidence, and MTCT risk and rates. Overall, 1,176 studies met the search criteria, of which 78 met the inclusion criterion, and 47 contributed data. Using data from 19 cohorts representing 22,803 total person-years, the pooled HIV incidence rate during pregnancy/postpartum was 3.8/100 person-years (95% CI 3.0–4.6): 4.7/100 person-years during pregnancy and 2.9/100 person-years postpartum (p = 0.18). Pooled cumulative HIV incidence was significantly higher in African than non-African countries (3.6% versus 0.3%, respectively; p<0.001). Risk of HIV was not significantly higher among pregnant (HR 1.3, 95% CI 0.5–2.1) or postpartum women (HR 1.1, 95% CI 0.6–1.6) than among non-pregnant/non-postpartum women in five studies with available data. In African cohorts, MTCT risk was significantly higher among women with incident versus chronic HIV infection in the postpartum period (OR 2.9, 95% CI 2.2–3.9) or in pregnancy/postpartum periods combined (OR 2.3, 95% CI 1.2–4.4). However, the small number of studies limited power to detect associations and sources of heterogeneity.Conclusions:
Pregnancy and the postpartum period are times of persistent HIV risk, at rates similar to “high risk” cohorts. MTCT risk was elevated among women with incident infections. Detection and prevention of incident HIV in pregnancy/postpartum should be prioritized, and is critical to decrease MTCT.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(2): e32767. doi:10.1371/journal.pmed.1001608
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001608Summary
Background:
Women may have persistent risk of HIV acquisition during pregnancy and postpartum. Estimating risk of HIV during these periods is important to inform optimal prevention approaches. We performed a systematic review and meta-analysis to estimate maternal HIV incidence during pregnancy/postpartum and to compare mother-to-child HIV transmission (MTCT) risk among women with incident versus chronic infection.Methods and Findings:
We searched PubMed, Embase, and AIDS-related conference abstracts between January 1, 1980, and October 31, 2013, for articles and abstracts describing HIV acquisition during pregnancy/postpartum. The inclusion criterion was studies with data on recent HIV during pregnancy/postpartum. Random effects models were constructed to pool HIV incidence rates, cumulative HIV incidence, hazard ratios (HRs), or odds ratios (ORs) summarizing the association between pregnancy/postpartum status and HIV incidence, and MTCT risk and rates. Overall, 1,176 studies met the search criteria, of which 78 met the inclusion criterion, and 47 contributed data. Using data from 19 cohorts representing 22,803 total person-years, the pooled HIV incidence rate during pregnancy/postpartum was 3.8/100 person-years (95% CI 3.0–4.6): 4.7/100 person-years during pregnancy and 2.9/100 person-years postpartum (p = 0.18). Pooled cumulative HIV incidence was significantly higher in African than non-African countries (3.6% versus 0.3%, respectively; p<0.001). Risk of HIV was not significantly higher among pregnant (HR 1.3, 95% CI 0.5–2.1) or postpartum women (HR 1.1, 95% CI 0.6–1.6) than among non-pregnant/non-postpartum women in five studies with available data. In African cohorts, MTCT risk was significantly higher among women with incident versus chronic HIV infection in the postpartum period (OR 2.9, 95% CI 2.2–3.9) or in pregnancy/postpartum periods combined (OR 2.3, 95% CI 1.2–4.4). However, the small number of studies limited power to detect associations and sources of heterogeneity.Conclusions:
Pregnancy and the postpartum period are times of persistent HIV risk, at rates similar to “high risk” cohorts. MTCT risk was elevated among women with incident infections. Detection and prevention of incident HIV in pregnancy/postpartum should be prioritized, and is critical to decrease MTCT.
Please see later in the article for the Editors' SummaryIntroduction
Antenatal HIV testing is essential to identify HIV-infected women who need to start antiretrovirals (ARVs) both to decrease risk of mother-to-child HIV transmission (MTCT) and improve maternal health. As part of this process, women who test HIV negative during antenatal screening may feel reassured that neither they nor their infants are at risk for HIV. However, HIV may be acquired during pregnancy and postpartum and would not be detected unless repeat HIV testing is conducted. Despite guidelines recommending repeat HIV testing during the third trimester or at delivery in settings where the HIV epidemic is generalized [1],[2], repeat testing is rarely implemented or documented [3],[4]. The lack of retesting during pregnancy and postpartum represents a missed opportunity to identify women who have recently acquired HIV infection and have an increased risk of MTCT because of their high HIV viral loads during incident infection, and to initiate ARVs for prevention of mother-to-child HIV transmission (PMTCT) among HIV-infected women who did not access antenatal care and were not tested during pregnancy [5].
Several individual studies suggest that the incidence of HIV infection during pregnancy and the postpartum period is high, with some suggesting increased incidence among pregnant/postpartum women compared to non-pregnant women [3],[5],[6]. However, differences in antenatal and postpartum follow-up, study designs, assays used to detect infection, and underlying HIV prevalence have made it difficult to compare findings between studies. Potential mechanisms for increased susceptibility to HIV during pregnancy and postpartum include both biological and behavioral characteristics unique to this period [6]. If the risk of HIV acquisition is increased during the pregnancy and/or postpartum periods, this higher risk may translate to a substantial cumulative period of risk for women in areas where fertility rates and HIV prevalence are high.
While a systematic review conducted in 1992 by Dunn et al. quantified risk of MTCT through breastfeeding among women infected postnatally, risk of in utero or intrapartum transmission among women who acquired HIV during pregnancy was not included [7]. In addition, the review was conducted prior to the implementation of ARV prophylaxis for PMTCT and was unable to characterize risk of MTCT among mothers with incident HIV infection in the era of PMTCT ARVs. Thus, the relative contribution of maternal HIV acquisition during pregnancy or postpartum and use of maternal PMTCT ARVs is unknown.
In 2012, the World Health Organization announced a global plan to eliminate MTCT by 2015 that includes reducing HIV incidence among women and MTCT rates, and increasing uptake of maternal and infant ARVs for PMTCT [8]. However, achievement of these objectives will be hindered without a greater understanding of the risk of HIV infection in pregnant and postpartum populations, and the subsequent risk of MTCT both in the presence and absence of PMTCT ARVs. We performed a systematic review and meta-analysis to synthesize and compare the risk of HIV acquisition during pregnancy and the postpartum period, and risk of MTCT among women with incident HIV infection.
Methods
Systematic Review and Article/Abstract Selection
We conducted a systematic review for all peer-reviewed published articles and conference abstracts on recent HIV infection during pregnancy or through 12 months postpartum (Figure 1). PubMed and Embase databases were searched from January 1, 1980, through October 31, 2013, to identify articles to review using combinations of the following terms: HIV infections, HIV seropositivity, pregnancy, human, incidence, acquisition, and acute. For PubMed (Medline) our search strategy was the following: (HIV infections[mh] or hiv-seropositivity[mh]) AND pregnancy[mh] AND human[mh] AND (incidence[mh] OR incidence[ti] OR acquisition[ti] OR acute[ti]). Our search strategy for Embase (excluding Medline results) was: ‘Human immunodeficiency virus infection’/exp AND ‘pregnancy’/exp AND (‘incidence’/exp OR ‘incidence’:ti OR ‘acquisition’:ti OR ‘acute’:ti) AND [embase]/lim NOT [medline]/lim. Titles of abstracts from the 19th and 20th Conference on Retroviruses and Opportunistic Infections (2011 and 2012), 6th and 7th International AIDS Society Conference (2011 and 2013), and 19th International AIDS Conference (2012) were also searched using the terms pregnancy, postpartum, and lactating. Titles of articles without abstracts were reviewed for consideration of full-text review; however, duplicate abstracts were not considered for full-text review. Articles were included in full-text review if the abstract or title mentioned HIV incidence, HIV seroconversion, or recent HIV infection during pregnancy, the postpartum period, or lactation. Review articles and articles on unrelated topics were excluded from full-text review. Titles of references cited in studies selected for full-text review were also evaluated for relevance and full-text review. Full-text articles and conference abstracts were excluded from analyses if (1) HIV incidence rates; cumulative HIV incidence; risk of HIV acquisition during pregnancy, postpartum, and/or during lactation; risk of MTCT during incident infection; or MTCT rates among women with incident infections were not reported and could not be calculated; (2) HIV infection preceded pregnancy; (3) HIV incidence estimates were based on mathematical modeling; (4) postpartum follow-up was >12 mo postpartum and HIV incidence during pregnancy was not reported; or (5) HIV prevalence and incidence could not be distinguished from one another. Additionally, conference abstracts that were subsequently published as articles were excluded, studies reporting results from the same cohort were compared and only the article with the most detailed data on incidence and follow-up was included, and articles in English were included if some or all of the data were also published in a different journal and in a language other than English. All other non-English articles/abstracts were considered for full-text review, but either were found to not be relevant based on review of English abstracts and titles, or did not have available abstracts to review. The combined search of articles and conference abstracts resulted in 1,176 studies for review: 626 articles and 559 conference abstracts. An additional nine articles were included for review from references cited in these articles, for a total of 1,185 studies identified to review. Among these, 78 studies (70 articles and eight conference abstracts) were identified for full-text review.
Fig. 1. Flowchart of studies reviewed and included in meta-analyses. 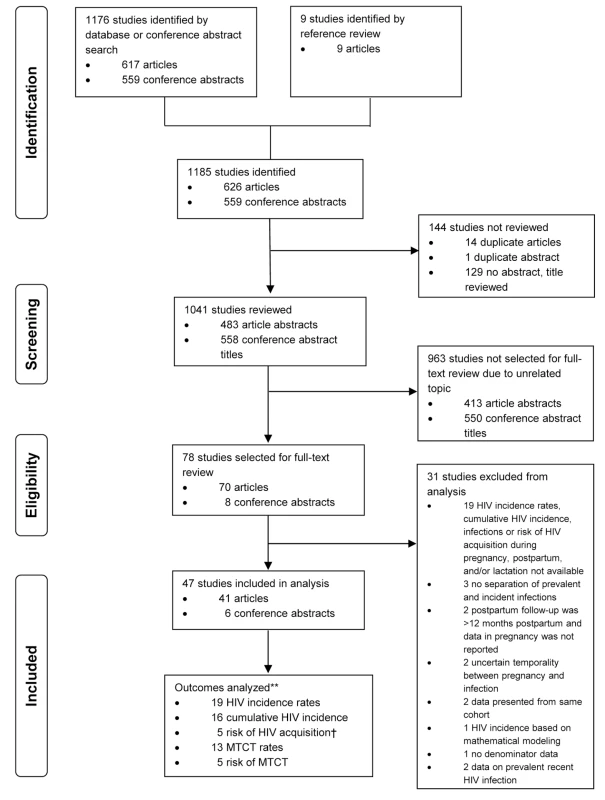
**Not mutually exclusive. †One study excluded that reported relative risk rather than hazard ratio. Data Extraction and Statistical Analysis
Two independent reviewers (A. L. D. and A. W.) reviewed full-text articles for inclusion and exclusion and abstracted data on the following outcomes occurring during pregnancy and/or postpartum periods: (1) HIV incidence rates; (2) cumulative incidence of HIV; (3) risk of HIV acquisition (hazard ratio [HR]); (4) risk of MTCT (odds ratio [OR]); and (5) MTCT rate. Variables extracted for analysis included sample size, number of incident HIV infections, number of women at risk of incident infection, person-time of follow-up, HIV incidence rates, cumulative HIV incidence, number of infant infections, assay used to detect incident infections, retesting and/or follow-up intervals, postpartum and lactation status, and study location. All postpartum follow-up time was restricted to 12 months postpartum unless women were reported to be lactating; there was no restriction on follow-up time for lactating women. Articles that did not report maximum postpartum follow-up time but presented a mean or median postpartum follow-up ≤12 months were also included in the analysis. Lactating women were combined with postpartum women for the meta-analysis; however, a separate subgroup analysis of postpartum women who were defined as lactating versus postpartum was also conducted.
Incidence rates and cumulative incidence
Incidence rates are reported as the number of new infections per 100 person-years; rates were calculated if data were available but not presented as an incidence rate. Cumulative incidence was calculated as the number of new infections per number at risk for studies with repeat testing. Cumulative incidence based on cross-sectional testing of HIV-positive samples using assay algorithms designed to detect incident infections was included as presented in the article or abstract. Assays included in the cumulative incidence meta-analysis included serological testing algorithm for recent HIV seroconversion (STARHS) using BED capture enzyme immunoassay or bioMerieux Vironosticka less sensitive enzyme immunoassay, avidity index, nucleic acid amplification tests, and p24 antigen tests. Studies presenting annual cumulative incidence were summarized for the meta-analysis by calculating a weighted incidence based on sample size. We calculated 95% CIs if they were not presented in the study. The Poisson distribution was used to calculate 95% CIs for incidence rates using person-time denominators, and the binomial distribution was used to calculate 95% CIs for cumulative incidence using individuals as the denominator. 95% CIs were calculated using the weighted estimate if annual cumulative incidence was calculated by weighting. Authors were contacted if additional information was required to calculate incidence rates or cumulative incidence.
Pooled summary statistics and plots
Meta-analyses were conducted to summarize cumulative HIV incidence and incidence rates of HIV during pregnancy and postpartum, MTCT rates among women with incident HIV infections, the association between pregnancy/postpartum periods and HIV acquisition, and the association between incident maternal HIV infection during pregnancy/postpartum periods and MTCT. Pooled HRs were calculated to summarize the association between pregnancy and postpartum periods and HIV acquisition if HRs were presented, and pooled ORs were used to summarize the association between incident infection during pregnancy/postpartum periods and MTCT. Fixed effects models were constructed to pool HIV incidence rates, cumulative incidence, MTCT rates, HRs, and ORs; models were tested for heterogeneity using the I2 statistic. Models pooling HIV incidence rates, MTCT rates, and HRs for HIV acquisition were a priori stratified by pregnancy, postpartum period, or pregnancy and postpartum periods combined. Significant heterogeneity was detected in all models, and the models were reconstructed with random effects using estimates of heterogeneity from the Mantel-Haenszel model to calculate pooled measures. We conducted both a priori and post hoc analyses to identify sources of heterogeneity. Pooled incidence rates during pregnancy and the postpartum period for studies conducted in Africa were a priori stratified by region (south, southeast, east, central, and west; in descending order of HIV prevalence) as a strategy to adjust for differences in HIV prevalence between these regions. We compared cumulative incidence detected by assay algorithms versus repeat testing and MTCT risk by use of maternal or infant ARV prophylaxis through additional stratified analysis. We also conducted post hoc stratified analysis of MTCT rates, MTCT risk, and cumulative incidence by study location (Africa or outside of Africa), and multivariate meta-regression for cumulative incidence by study location and testing algorithm. Forest plots of HIV incidence rates, cumulative HIV incidence, HRs, ORs, and MTCT rates were generated for individual articles, pooled subgroup estimates (when indicated), and pooled overall estimates. Meta-regression was used to compare log-transformed incidence, log-transformed cumulative incidence, MTCT rates, ORs, and HRs between subgroups and in multivariate models. Statistical analysis was performed using Stata version 13.1 (StataCorp).
The Newcastle-Ottawa Scale was used to assess the quality of the studies included in the meta-analyses that compared MTCT risk or risk of maternal HIV acquisition between groups; this scale ranges from 0 to 9 and assesses study quality based on study characteristics for selection of the cohort, comparability of exposed and unexposed groups, and outcome ascertainment. The quality of studies for studies with comparison groups was not compared statistically since quality scoring adds subjective bias to the results unless individual score components are used in the meta-regression, and adding several covariates for individual score components would result in over-stratification [9],[10]. Two independent reviewers (A. L. D. and A. W.) scored each study, and the average score for all studies, stratified by outcome, was calculated [11].
Results
Study Selection for Review
Among the 1,176 studies identified, 78 were selected for further review of the full-text article or conference abstract, and 47 were included in the analysis (41 articles and six conference abstracts) (Figure 1). Characteristics of studies included in the meta-analysis and data summaries are presented in Table 1. Studies were identified from six continents: 35 from Africa; four each from Asia, North America, and South America; and one each from Australia and Europe. Only ten studies comparing MTCT risk (n = 5) or risk of maternal HIV acquisition (n = 5) between groups were eligible for evaluation by the Newcastle-Ottawa Scale. Studies evaluated by the Newcastle-Ottawa Scale received a mean score of 7.4 for studies comparing risk of MTCT and a mean score of 8.2 for studies comparing risk of maternal HIV acquisition, indicating that the quality of included studies was high; individual Newcastle-Ottawa Scores for MTCT and risk of maternal HIV acquisition are shown in Table 1.
Tab. 1. Study characteristics and data included in meta-analyses and data summaries. 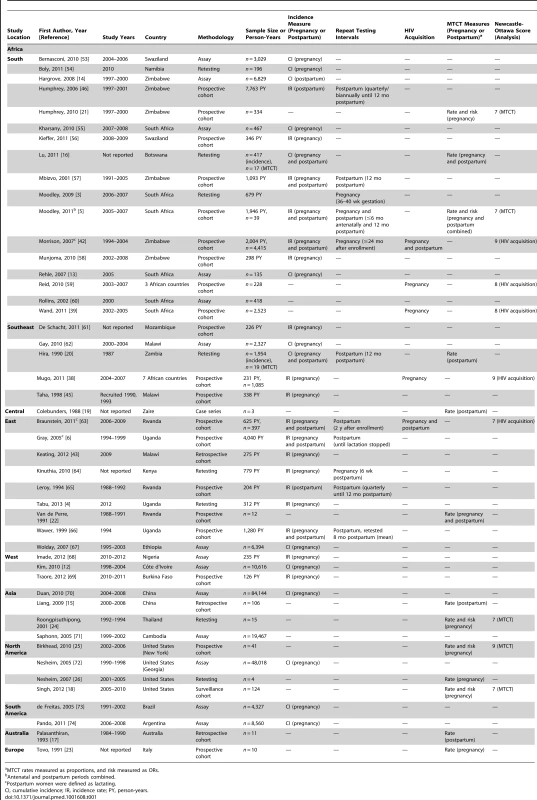
MTCT rates measured as proportions, and risk measured as ORs. Incidence Rates during Pregnancy and Postpartum
Nineteen studies reported HIV incidence rates during pregnancy and/or the postpartum period: 16 during pregnancy, seven during the postpartum period, and one during pregnancy and postpartum periods combined (Figure 2), representing 19 cohorts and 22,803 person-years in total. All incidence rates were from publications reported from sub-Saharan Africa. Incidence rates during pregnancy ranged from 0 to 16.8 per 100 person-years, with a pooled incidence rate of 4.7 (95% CI 3.3–6.1). During the postpartum period, the pooled incidence rate was 2.9 (95% CI 1.8–4.0) and not significantly different from the pooled incidence rate during pregnancy (4.7, 95% CI 3.3–6.1; p = 0.18). After combining results from all studies, the pooled incidence during pregnancy and/or the postpartum period was 3.8 (95% CI 3.0–4.6). In subgroup analyses separating postpartum women who were defined as lactating versus postpartum, stratified pooled incidence rates among women defined as lactating (2.0, 95% CI 0.2–3.8) or postpartum (3.2, 95% CI 2.2–4.2) were not significantly different from that of the overall postpartum population combining these groups. Incidence rates during pregnancy and/or postpartum were also stratified by African region (Figure 3). Pooled incidence was highest in southeast Africa (6.2, 95% CI 4.2–8.1), followed by south Africa (4.8, 95% CI 3.5–6.2), east Africa (2.7, 95% CI 1.8–3.6), and west Africa (0.7, 95% CI 0–2.3); the pooled incidence rate was significantly lower in east Africa than in southeast Africa (p = 0.04), but there were no other significant differences by region.
Fig. 2. Forest plot of HIV incidence rates, by pregnancy and postpartum status. 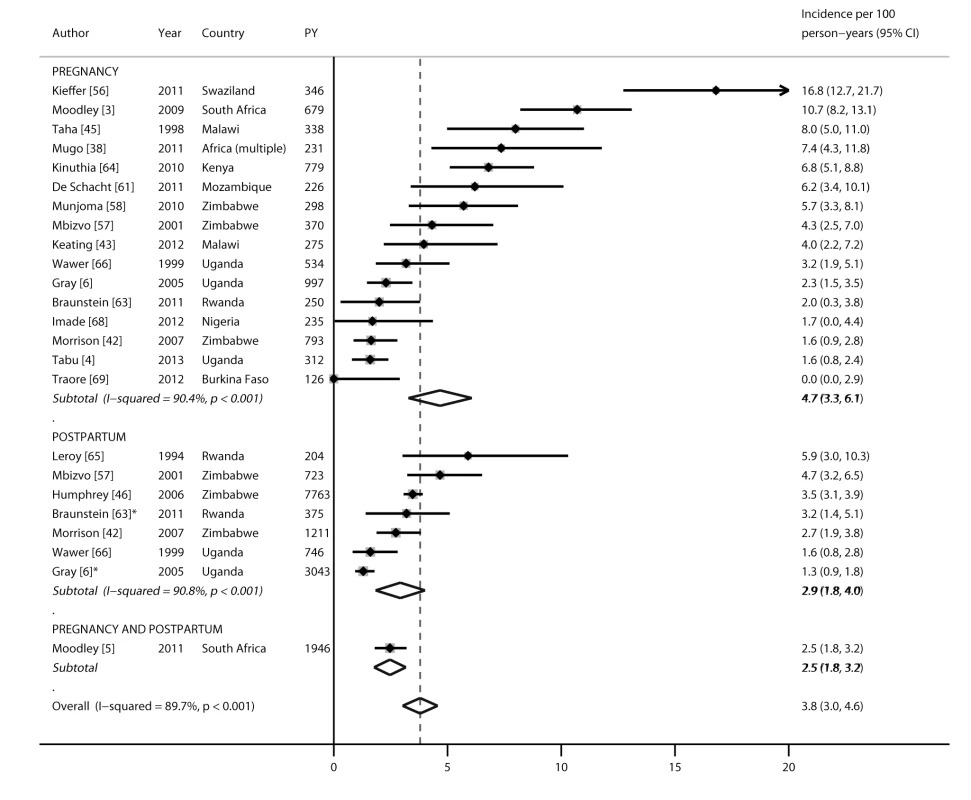
PY, person-years. *Defined as lactating. Fig. 3. Forest plot of HIV incidence rates during pregnancy and postpartum, by African region. 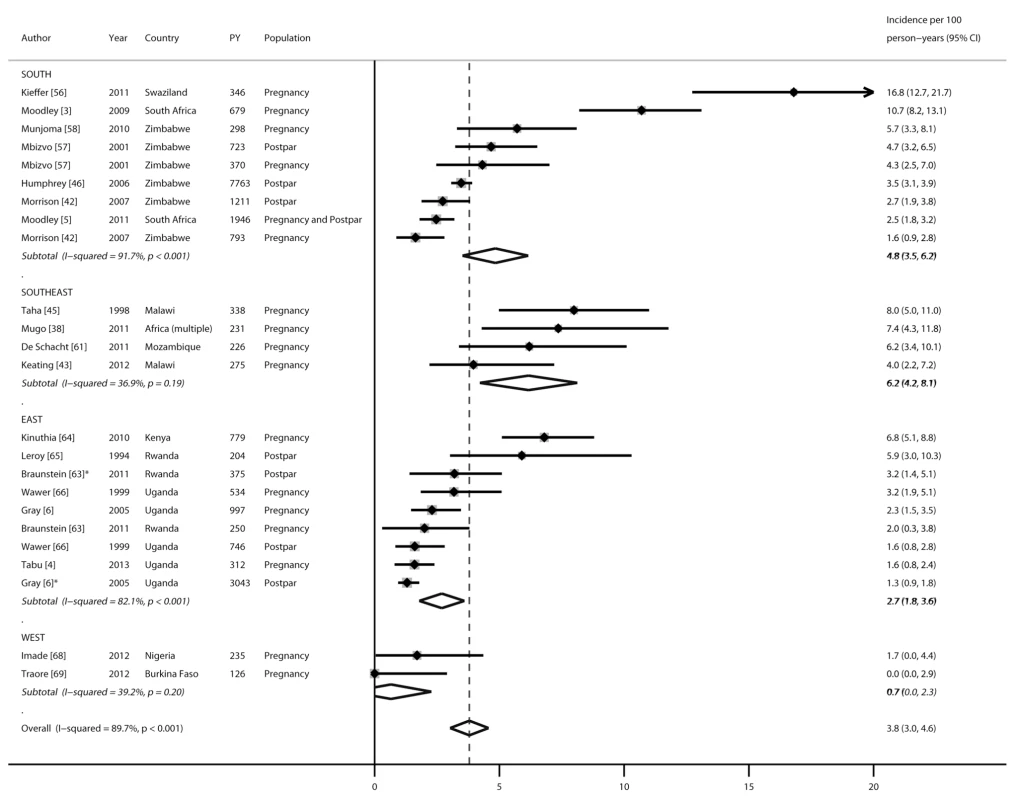
PY, person-years. *Defined as lactating. Cumulative HIV Incidence during Pregnancy and Postpartum
Cumulative HIV incidence during pregnancy was reported in 16 studies, during the postpartum period in three studies, and during the combined pregnancy/postpartum period in one study (Figure 4). Thirteen studies reported cumulative incidence based on assay-specific algorithms from cross-sectional testing, two studies were based on repeat testing, and one study reported both assay and repeat testing results during pregnancy, delivery, or postpartum. Incident infections from assays were detected using an avidity index, STARHS, p24 antigen detection, or nucleic acid amplification tests. The estimate of cumulative incidence using BED capture enzyme immunoassay as the assay for STARHS was adjusted to account for assay sensitivity and specificity in the studies by Kim et al. [12], Rehle et al. [13], and Hargrove et al. [14], as reported in the articles. Pooled cumulative incidence was 1.4% (95% CI 1.1%–1.7%) when infections were detected by assay algorithms from cross-sectional testing and 2.7% (95% CI 1.0%–4.4%) among populations retested (p = 0.4). Cumulative incidence was significantly higher in African countries (3.6%, 95% CI 1.9%–5.3%) than in non-African countries (0.3%, 95% CI 0.1%–0.4%; p<0.001) and remained significant (p<0.001) in a meta-regression model including study location (Africa or outside of Africa).
Fig. 4. Forest plot of cumulative incidence of incident HIV infection during pregnancy and postpartum, by testing algorithm. 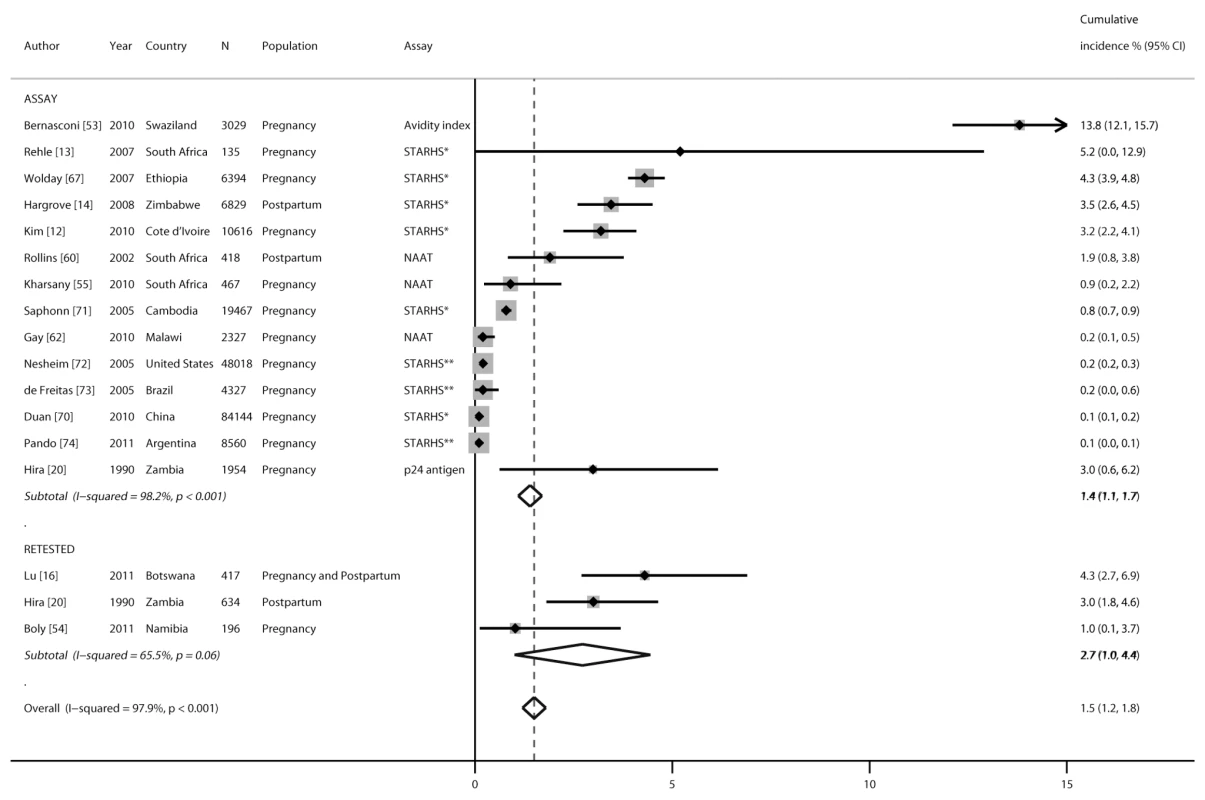
The retested cumulative incidence category is calculated as the number of new infections per number at risk for studies, and the assay cumulative incidence category is calculated based on cross-sectional testing of HIV-positive samples using assay algorithms designed to detect incident infections; cumulative incidence expressed as percent for both retested and assay categories. NAAT, nucleic acid amplification test; STARHS*, STARHS using BED capture enzyme immunoassay; STARHS**, STARHS using bioMerieux Vironosticka less sensitive enzyme immunoassay. Association between Pregnancy and/or Postpartum Period and Risk of HIV Acquisition
Two studies compared the HIV incidence rate during pregnancy and/or postpartum, separately, to the incidence rate of non-pregnant/non-lactating women. Three studies reported HIV incidence only for pregnant women compared to non-pregnant women. Risk of HIV acquisition among pregnant (pooled HR 1.3, 95% CI 0.5–2.1) and postpartum women (pooled HR 1.1, 95% CI 0.6–1.6) was not significantly different from non-pregnant/non-lactating women (p = 0.92). A non-significantly higher risk of HIV acquisition was also observed when data from the pregnancy and postpartum periods were combined and compared to non-pregnant/non-lactating women (pooled HR 1.2, 95% CI 0.7–1.8) (Figure 5).
Fig. 5. Forest plot of risk of HIV acquisition, by pregnancy and postpartum status. 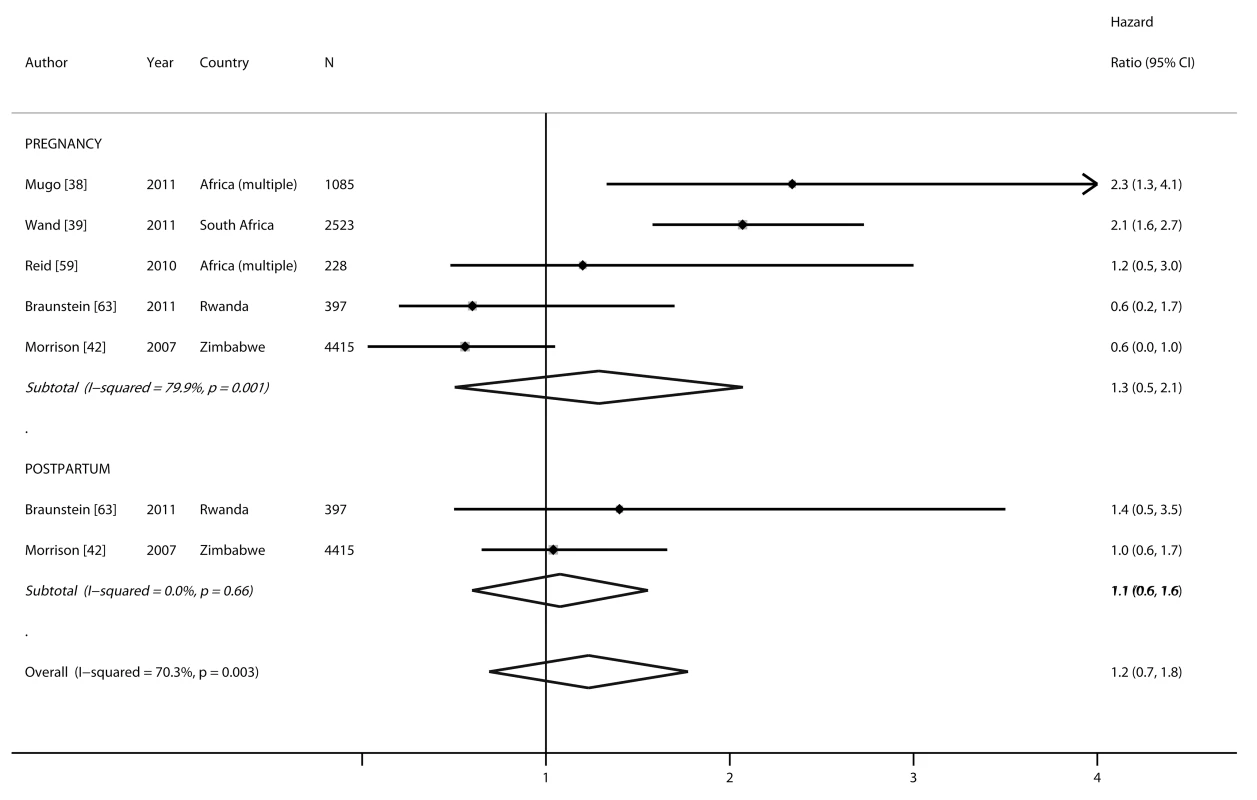
Mother-to-Child Transmission among Women with Incident HIV during Pregnancy or Postpartum
MTCT rates among women with incident infections detected during pregnancy or postpartum were presented in 13 studies, representing 11 countries and four continents (Figure 6). Five of these studies identified women who became infected during the postpartum period (three studies documented infections following postnatal blood transfusions), three reported MTCT rates among women infected during pregnancy or postpartum, and five reported MTCT rates among women infected during pregnancy. Ten studies provided no PMTCT: four studies provided no PMTCT because of cross-sectional, simultaneous ascertainment of maternal and infant HIV infections or testing after infant exposure to HIV had stopped [15]–[18], and six studies were conducted prior to PMTCT implementation at the study sites [19]–[24]. Three studies documented provision of PMTCT ARVs [5],[25],[26]: one used single-dose nevirapine for women with CD4 count >200 cells/mm3 and antiretroviral therapy (ART) for women with CD4 count ≤200 cells/mm3, one used zidovudine and nevirapine regimens for women and infants, and the last did not specify ARV regimen. MTCT rates across all studies ranged from 12.9% in the United States to 58.0% in Rwanda, with a pooled rate of 22.7% (95% CI 17.5%–27.8%). A similar MTCT rate (23.5%) resulted from the pooled analysis of the subset of these studies that did not use ARVs (p = 0.70); MTCT rates also did not differ by African versus non-African countries (23.6% versus 22.0%, respectively; p = 0.49). The pooled MTCT rate among women with incident infection during pregnancy was 17.8% (95% CI 12.2%–23.4%), compared to 26.9% (95% CI 19.3%–34.5%) among women with incident infection postpartum (p = 0.10).
Fig. 6. Forest plot of mother-to-child HIV transmission rates among women with incident infection during pregnancy/postpartum. 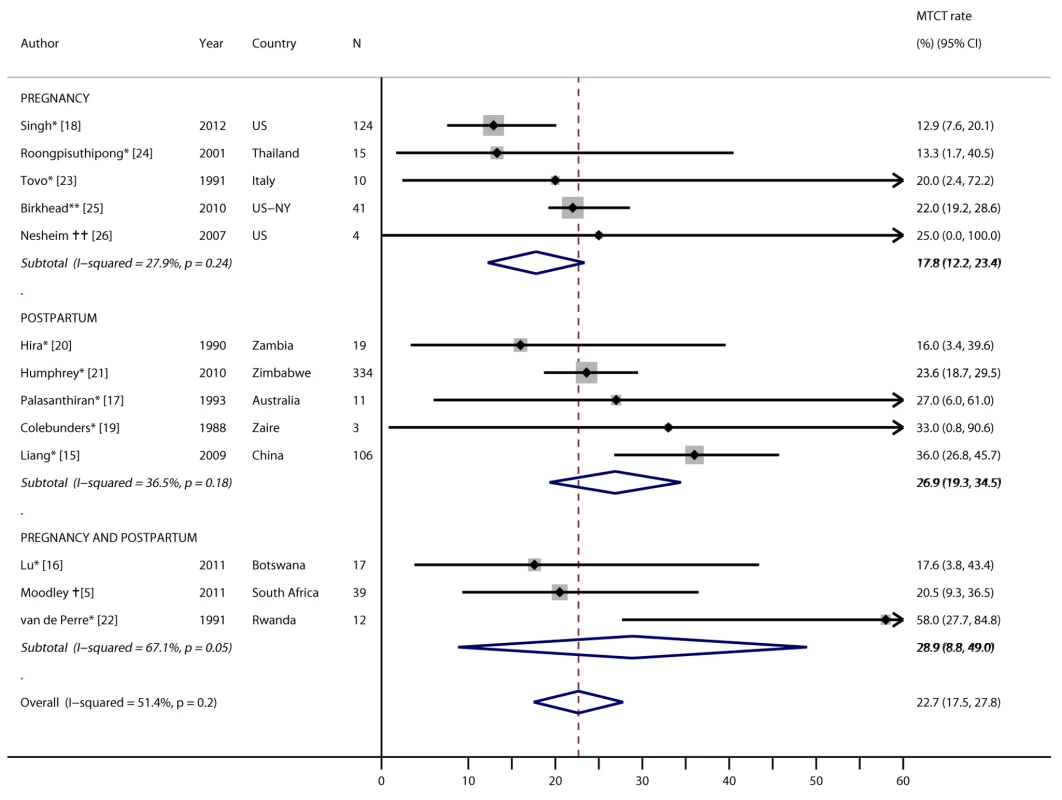
*No PMTCT ARVs. **Unspecified ARV regimen. †Single-dose nevirapine if maternal CD4 count >200 cells/mm3 and ART for women with CD4 count ≤200 cells/mm3. ††Zidovudine and nevirapine regimens for women and infants. Five studies compared risk of MTCT among women with incident versus chronic HIV infection (Figures 7 and 8). MTCT risk was 9 - to 15-fold higher among US women who seroconverted during pregnancy than among women with chronic HIV infection, because of effective early ART treatment of chronically HIV-infected mothers. In African cohorts, compared to women with chronic HIV, MTCT risk was 2 - to 3-fold higher among women with incident HIV infection in the postpartum period (pooled OR 2.9, 95% CI 2.2–3.9) or in the pregnancy and postpartum periods combined (pooled OR 2.3, 95% CI 1.2–4.4). The pooled OR for MTCT with incident HIV versus chronic HIV among mothers was 2.8 (95% CI 0.9–4.7) (Figure 8); the pooled OR after adjusting for maternal or infant ARV prophylaxis was 5.6 (95% CI 0.1–311.5).
Fig. 7. Effect of incident maternal HIV infection and antiretroviral prophylaxis on mother-to-child HIV transmission. 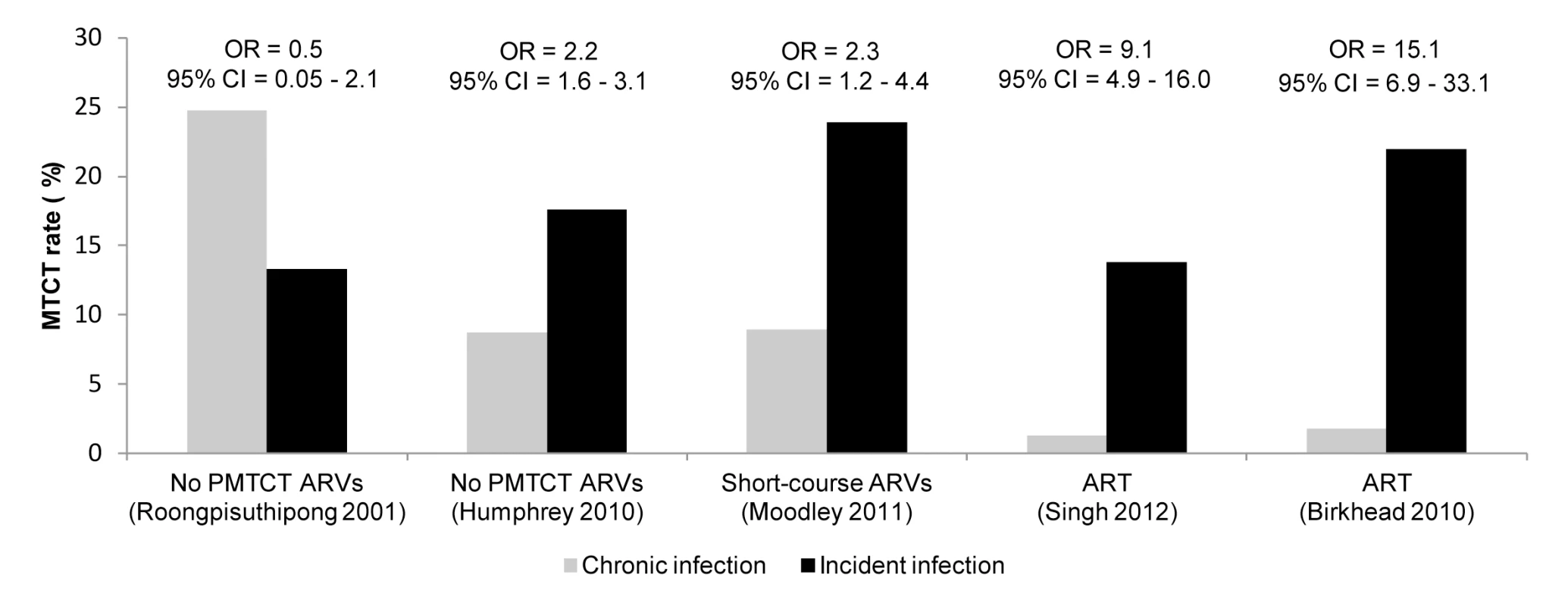
Incident infection defined as maternal HIV acquisition during pregnancy or postpartum; chronic infection defined as established HIV infection during pregnancy or postpartum. MTCT rates and ORs are derived from the studies indicated in parentheses; PMTCT ARVs represent the regimens available during the studies. For Moodley [5], women with CD4 count >200 cells/mm3 were eligible to receive ART; all other women received single-dose nevirapine regimens. For Singh [18], women were assumed to receive ART as per national guidelines in place during the study. Fig. 8. Forest plot of risk of mother-to-child HIV transmission and maternal HIV infection status. 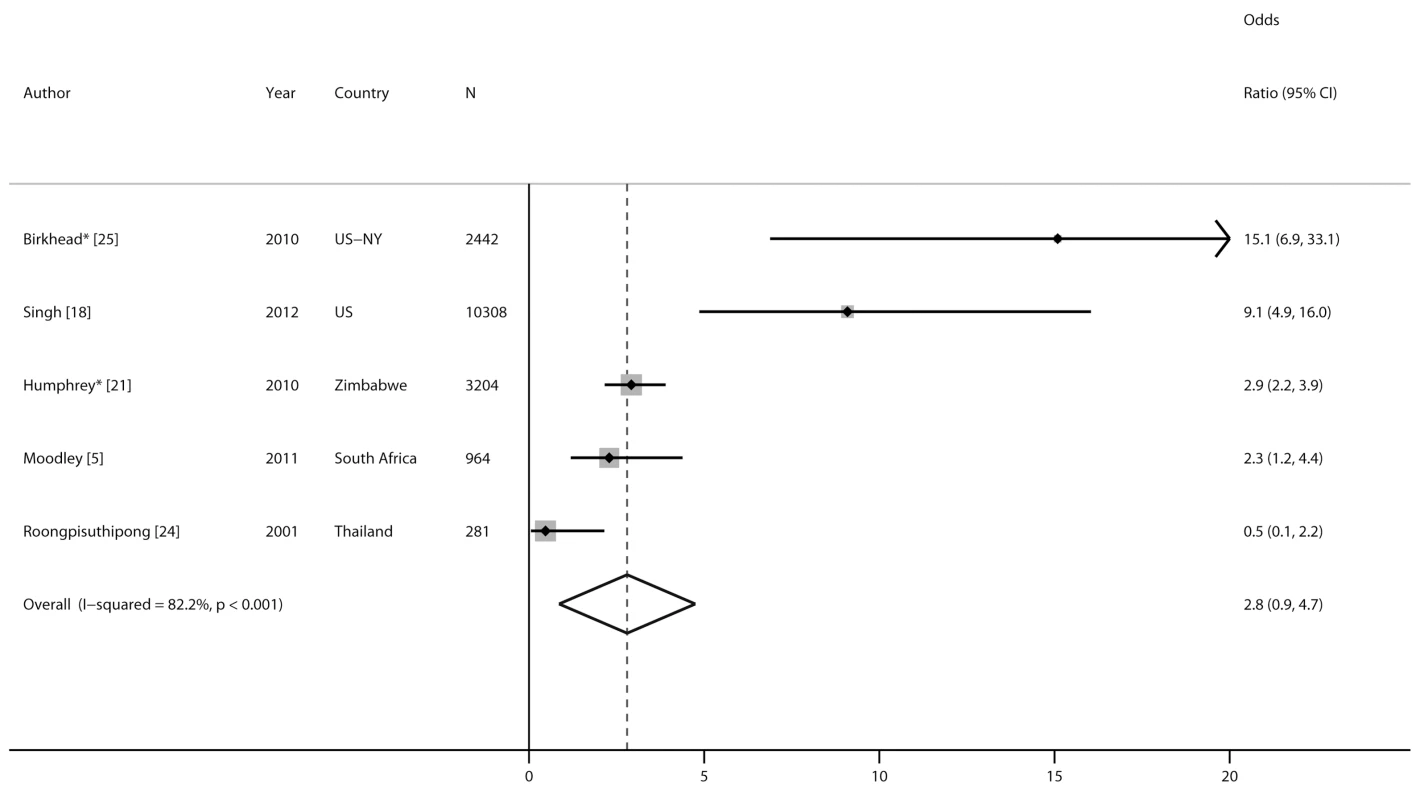
Discussion
In this meta-analysis we found that HIV incidence rates were high among pregnant and postpartum women, with pooled incidence rates of 4.7 per 100 person-years among pregnant women and 2.9 per 100 person-years among postpartum women. In our pooled analysis, we did not find a significantly higher risk of HIV infection among pregnant or postpartum women compared to non-pregnant women. The pooled MTCT rate was high, 22.7%, among pregnant and postpartum women with incident HIV infection, and risk of MTCT was 2.8-fold higher among these women compared to women with chronic HIV infection. Pooled MTCT rate was non-significantly higher among women with postpartum versus antenatal incident infections (26.9% versus 17.8%, respectively).
The highest HIV incidence estimates during pregnancy/postpartum were >10 per 100 person-years and were from South Africa and Swaziland, countries with high HIV prevalence (>15%) [27] and in which high incidence has been noted in non-pregnant adults [28],[29]. The pooled incidence rates we observed are comparable to, or higher than, those of non-pregnant “high risk” individuals, including female sex workers (2.7 per 100 person-years), HIV-discordant couples (2.0 to 3.6 per 100 person-years) from south and east Africa, and men who have sex with men (6.1 per 100 person-years) from North and South America [30]–[35]. Studies of discordant couples involve a known HIV-infected partner, which would be expected to result in higher HIV incidence than in pregnant women with unknown partner status, the majority of whom presumably have HIV-uninfected partners. However, discordant couples in cohort studies, by virtue of knowing their status, have reinforced messages to use condoms, access ART treatment, and decrease risk, leading to lower HIV incidence. Similarly, studies in female sex workers have noted dramatic reductions in HIV incidence following initial enrollment in cohort studies [36],[37], because of either increased HIV preventive measures or selective inclusion of relatively resistant individuals in the cohort.
While single studies have noted increased risk of HIV acquisition during pregnancy/postpartum compared to non-pregnant women [38],[39], the pooled estimate did not suggest that pregnancy or the postpartum period significantly elevated risk. However, it is difficult to design a study that would definitively determine the incremental risk of the pregnancy/postpartum periods compared to non-pregnant time periods because of a variety of confounding factors. One notable potential confounder is use of contraceptives in the non-pregnant/postpartum comparison group, some of which (injectable and oral contraceptives) have also been linked to higher risk of HIV acquisition [40],[41]. One study in this meta-analysis included hormonal contraception within the multivariate model and found that the effect of pregnancy on HIV acquisition risk was attenuated when potential confounders were included in the model [38], whereas another study did not observe any difference in risk when non-pregnant/non-lactating women using versus not using hormonal contraception were used as comparison groups [42]. In addition, differential risk during early versus late pregnancy may modify the association between pregnancy and HIV risk. Studies included in this meta-analysis included both early and late stages of pregnancy in their risk intervals, but did not capture associations with specific periods in pregnancy, for example, whether risk increases as hormone levels rise. Mugo et al. [38] did not observe any difference in associations, based on stage of pregnancy, but the sample size was small, limiting the power to detect effect modification by pregnancy stage. Finally, while HIV incidence was non-significantly higher during pregnancy versus non-pregnant time periods, the lower coital frequency among pregnant and postpartum African women previously reported [6],[43],[44] may indicate that pregnancy is associated with increased per-coital-act susceptibility to HIV, as has been observed in a study conducted in Uganda [6]. Thus, an alternative approach to determining the impact of the pregnancy/postpartum periods would be to pool estimates of risk per coital act, as was done in the Ugandan study, which estimated 1.4-fold higher HIV risk per coital act during pregnancy/postpartum compared to non-pregnant/non-lactating periods [6]. However, detailed data on frequency of intercourse were not provided in the studies reviewed, limiting ability to estimate risk per coital act.
Both biological and behavioral changes have been hypothesized to explain the potentially higher risk of HIV acquisition observed during pregnancy and the postpartum period. Behavioral factors were not associated with HIV acquisition in two prior studies of pregnant and postpartum women [3],[6]; however, behavior changes in male partners during the pregnancy/postpartum period have not been well characterized and could play a role in maternal HIV risk, particularly if partners increase sexual activity outside of the relationship. Pregnancy-induced physiological changes have been hypothesized to increase HIV susceptibility, through changes in systemic and mucosal immunity, disturbances in vaginal flora, and alterations of the genital mucosa. However, prospective studies to examine biological risk factors for HIV acquisition during pregnancy and postpartum have been limited. Taha et al. showed that sexually transmitted infections (gonorrhea and trichomoniasis) and other genital tract infections (bacterial vaginosis) were associated with increased risk of HIV acquisition during pregnancy and postpartum: risk was 2-fold higher for trichomonas infection during pregnancy or postpartum, and 4-fold higher for gonorrhea during pregnancy [45]. Other potential biological risk factors for HIV acquisition include vitamin A deficiency, severe anemia, and younger age [46].
Incident maternal infections during pregnancy/postpartum may increase MTCT because of high levels of maternal viral load during incident infection, low levels of passively transferred maternal antibody, and absence of PMTCT ARVs because maternal infection is initially undetected. While half of the studies included in our MTCT rate analysis were conducted prior to implementation of PMTCT ARV prophylaxis, transmission rates were similar among women receiving and not receiving ARVs. In addition, although current World Health Organization guidelines for PMTCT are shifting from short-course ARV regimens to lifelong maternal ART, short-course regimens continue to be used in many resource-limited settings [47]. Thus, our pooled MTCT rates remain relevant to pregnant and postpartum women with both undiagnosed and treated incident infections, and highlight the need to improve detection of infections and early initiation of PMTCT ARVs.
In early MTCT studies, prior to PMTCT interventions, the increased relative risk for incident versus chronic maternal infection was due to high maternal viral load and lower maternal HIV-specific immune responses in incident infection. With PMTCT ARVs, rates of transmission among women with chronic HIV decreased from ∼20%–35% to 1%–5%. Since incident maternal HIV is typically detected weeks after infection, there is a relative delay in the initiation and effect of ARVs, amplifying the relative risk in incident versus chronic MTCT rates. Thus, earlier studies comparing MTCT in incident versus chronic maternal infection noted 2 - to 3-fold increased MTCT, while later studies note 6 - to >15-fold increased MTCT because of differential PMTCT. A recent modeling study projected that the absolute number of MTCT events could be reduced by 28% in South Africa if HIV screening was repeated during late pregnancy or at 6-week infant immunization visits, demonstrating a greater need for identification and early treatment for women acquiring HIV during pregnancy [48].
Our systematic review and meta-analysis had several strengths. We used a broad search strategy that included peer-reviewed articles in addition to conference abstracts presented at recent HIV conferences. We also contacted authors to acquire additional data for some summary measures. Two independent reviewers evaluated full-text articles for relevance and abstraction of data. HIV incidence rates, cumulative HIV incidence, and risk of HIV acquisition were pooled separately by pregnancy and postpartum status, as well as together, to better understand risks specific to pregnancy versus the postpartum period. To complement the meta-analysis of HIV incidence, we also pooled MTCT rates among mothers with incident infection, and compared the risk of MTCT for women with incident versus chronic infection.
Our analysis is subject to limitations resulting from pooling data from studies with heterogeneous research methodologies. Incident HIV infections reported in the studies were estimated using tests that varied in sensitivity and with different intervals for follow-up testing. Variability in test performance has previously been noted to result in overestimation of incidence when lower sensitivity tests are used initially and higher sensitivity tests for subsequent tests [49]. Timing of seroconversion is also related to timing of testing; women seeking antenatal care and testing earlier in their pregnancy have more person-time and opportunity to be detected as an incident rather than chronic infection. Conversely, seroconversions early in pregnancy are not captured as incident infections if antenatal care is sought later. Another limitation of this analysis is that cumulative incidence based on repeat testing was estimated from cohorts with differing duration of follow-up and cross-sectional testing using sensitive assays. While the performance of assays for detecting recent infections has been shown to vary across HIV clades and subpopulations, a recent study suggests pregnancy does not influence performance of BED or avidity assays [50],[51]. However, there is considerable evidence that early iterations of testing algorithms for incident infection misclassified a proportion of individuals with chronic infections as incident infections (false recent rate) and prompted the World Health Organization to issue guidance on conducting assays, with specific criteria for appropriate sampling designs, sample size, and statistical analysis considerations [51]. Thus, inclusion of different assays and testing algorithms likely overestimates our pooled cumulative incidence. These differences partially explain why our estimates of pooled cumulative incidence and pooled incidence rate differ. While we were unable to adjust for national HIV prevalence at the time of the study—since HIV prevalence estimates are not available for all countries and years included in this meta-analysis—we did consider African region as a marker of HIV prevalence; however, this approach may result in residual confounding. In addition, none of the included studies were primarily designed to estimate HIV incidence in pregnancy and the postpartum period; most studies excluded pregnant women from initial study participation, and report HIV incidence during pregnancy and postpartum as a secondary research objective, limiting generalizability. Finally, the number of studies included in our meta-regression models was small; therefore, the models may lack power to detect associations and are unable to ascertain multiple potential sources of confounding.
In conclusion, HIV incidence among pregnant and postpartum populations was high in this meta-analysis and may substantially increase risk of MTCT. Our results have several implications for antenatal care/PMTCT programs. First, women in high prevalence settings should be offered repeat HIV testing to detect incident infections and to diagnose women in the postpartum period who did not receive antenatal care. This approach is beneficial because it detects maternal HIV infection while women are accessing the health care system and prompts referral to appropriate HIV care and treatment. While specific recommendations regarding ARV regimens for women with incident infection do not currently exist, maternal ART during pregnancy is likely the best option given high maternal viral loads during incident infection. Second, there is a need for wider distribution of more sensitive HIV tests, such as the fourth generation rapid tests, to enhance early detection of incident HIV. These more sensitive assays, which detect both HIV antibodies and HIV p24 antigen, can reduce the number of women who have early HIV infection and are incorrectly classified as HIV negative. Third, since pregnant and postpartum women are a vulnerable population at risk of HIV and sexually transmitted infections, they should receive continued counseling on the need for condoms to prevent transmission during this time. Pregnant and postpartum populations should also be considered, and included early in the process, of developing and evaluating female-controlled prevention methods, such as microbicides, for safety and efficacy [52].
Supporting Information
Zdroje
1. Perinatal HIV prevention aims for elimination. AIDS Read 12 : 146, 150.
2. BransonBM, HandsfieldHH, LampeMA, JanssenRS, TaylorAW, et al. (2006) Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 55 : 1–17.
3. MoodleyD, EsterhuizenTM, PatherT, ChettyV, NgalekaL (2009) High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS 23 : 1255–1259.
4. Tabu F, Ngonzi J, Mugyenyi G, Bajunirwe F, Mayanja R, et al. (2013) Prevalence of HIV infection among parturients with a negative primary test during the antenatal period at Mbarara Regional Referral Hospital, Uganda [abstract]. SpROGs 2013 Conference; 5–6 Dec 2013; Nottingham, United Kingdom.
5. MoodleyD, EsterhuizenT, ReddyL, MoodleyP, SinghB, et al. (2011) Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis 203 : 1231–1234.
6. GrayRH, LiX, KigoziG, SerwaddaD, BrahmbhattH, et al. (2005) Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 366 : 1182–1188.
7. DunnDT, NewellML, AdesAE, PeckhamCS (1992) Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet 340 : 585–588.
8. World Health Organization (2012) Global monitoring framework and strategy for the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva: World Health Organization.
9. GreenlandS (1994) Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol 140 : 290–296.
10. ThompsonSG, HigginsJP (2002) How should meta-regression analyses be undertaken and interpreted? Stat Med 21 : 1559–1573.
11. Wells G, Shea B, O'Connell D, Peterson J, Welch V, et al. (1999) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Ottawa: Ottawa Hospital Research Institute.
12. KimAA, McDougalJS, HargroveJ, RehleT, Pillay-Van WykV, et al. (2010) Evaluating the BED capture enzyme immunoassay to estimate HIV incidence among adults in three countries in sub-Saharan Africa. AIDS Res Hum Retroviruses 26 : 1051–1061.
13. RehleT, ShisanaO, PillayV, ZumaK, PurenA, et al. (2007) National HIV incidence measures—new insights into the South African epidemic. S Afr Med J 97 : 194–199.
14. HargroveJW, HumphreyJH, MutasaK, ParekhBS, McDougalJS, et al. (2008) Improved HIV-1 incidence estimates using the BED capture enzyme immunoassay. AIDS 22 : 511–518.
15. LiangK, GuiX, ZhangYZ, ZhuangK, MeyersK, et al. (2009) A case series of 104 women infected with HIV-1 via blood transfusion postnatally: high rate of HIV-1 transmission to infants through breast-feeding. J Infect Dis 200 : 682–686.
16. Lu L, Motswere-Chirwa C, Kegwaila K, Matambo S, Kolobe T, et al. (2011) HIV incidence in women during the first postpartum year and implications for prevention of mother-to-child transmission (PMTCT) programs—Francistown, Botswana, 2010 [abstract]. 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 Jul 2011; Rome, Italy.
17. PalasanthiranP, ZieglerJB, StewartGJ, StuckeyM, ArmstrongJA, et al. (1993) Breast-feeding during primary maternal human immunodeficiency virus infection and risk of transmission from mother to infant. J Infect Dis 167 : 441–444.
18. Singh A, Lampe MA, Surendera Babu A, Rao S, Borkowf CB, et al. (2012) HIV seroconversion during prgnancy and mother-to-child HIV transmission: data from the Enhanced Perinatal Surveillance Project, United States, 2005–2010 [abstract]. 19th International AIDS Conference; 22–27 Jul 2012; Washington, District of Columbia, US.
19. ColebundersR, KapitaB, NekweiW, BahweY, LebugheI, et al. (1988) Breastfeeding and transmission of HIV. Lancet 2 : 1487.
20. HiraSK, MangrolaUG, MwaleC, ChintuC, TemboG, et al. (1990) Apparent vertical transmission of human immunodeficiency virus type 1 by breast-feeding in Zambia. J Pediatr 117 : 421–424.
21. HumphreyJH, MarindaE, MutasaK, MoultonLH, IliffPJ, et al. (2010) Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ 341: c6580.
22. Van de PerreP, SimononA, MsellatiP, HitimanaDG, VairaD, et al. (1991) Postnatal transmission of human immunodeficiency virus type 1 from mother to infant. A prospective cohort study in Kigali, Rwanda. N Engl J Med 325 : 593–598.
23. TovoPA, PalombaE, GabianoC, GalliL, de MartinoM (1991) Human immunodeficiency virus type 1 (HIV-1) seroconversion during pregnancy does not increase the risk of perinatal transmission. Br J Obstet Gynaecol 98 : 940–942.
24. RoongpisuthipongA, SiriwasinW, SimondsRJ, SangtaweesinV, VanpraparN, et al. (2001) HIV seroconversion during pregnancy and risk for mother-to-infant transmission. J Acquir Immune Defic Syndr 26 : 348–351.
25. BirkheadGS, PulverWP, WarrenBL, HackelS, RodriguezD, et al. (2010) Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 115 : 1247–1255.
26. NesheimS, JamiesonDJ, DannerSP, MaupinR, O'SullivanMJ, et al. (2007) Primary human immunodeficiency virus infection during pregnancy detected by repeat testing. Am J Obstet Gynecol 197 : 149.e141–5.
27. Joint United Nations Programme on HIV/AIDS (2012) UNAIDS World AIDS Day Report 2012. Geneva: Joint United Nations Programme on HIV/AIDS.
28. Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, et al. (2013) Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003) [abstract]. 20th Conference on Retroviruses and Opportunistic Infections 3–6 Mar 2013; Atlanta, Georgia, US.
29. Abdool KarimQ, Abdool KarimSS, FrohlichJA, GroblerAC, BaxterC, et al. (2010) Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329 : 1168–1174.
30. PriceMA, RidaW, MwangomeM, MutuaG, MiddelkoopK, et al. (2012) Identifying at-risk populations in Kenya and South Africa: HIV incidence in cohorts of men who report sex with men, sex workers, and youth. J Acquir Immune Defic Syndr 59 : 185–193.
31. KaulR, KimaniJ, NagelkerkeNJ, FonckK, NgugiEN, et al. (2004) Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA 291 : 2555–2562.
32. CelumC, WaldA, HughesJ, SanchezJ, ReidS, et al. (2008) Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 371 : 2109–2119.
33. CelumC, WaldA, LingappaJR, MagaretAS, WangRS, et al. (2010) Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 362 : 427–439.
34. Watson-JonesD, WaldA, CelumC, LingappaJ, WeissHA, et al. (2010) Use of acyclovir for suppression of human immunodeficiency virus infection is not associated with genotypic evidence of herpes simplex virus type 2 resistance to acyclovir: analysis of specimens from three phase III trials. J Clin Microbiol 48 : 3496–3503.
35. BaetenJM, DonnellD, NdaseP, MugoNR, CampbellJD, et al. (2012) Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367 : 399–410.
36. BaetenJM, RichardsonBA, MartinHLJr, NyangePM, LavreysL, et al. (2000) Trends in HIV-1 incidence in a cohort of prostitutes in Kenya: implications for HIV-1 vaccine efficacy trials. J Acquir Immune Defic Syndr 24 : 458–464.
37. FowkeKR, NagelkerkeNJ, KimaniJ, SimonsenJN, AnzalaAO, et al. (1996) Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet 348 : 1347–1351.
38. MugoNR, HeffronR, DonnellD, WaldA, WereEO, et al. (2011) Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS 25 : 1887–1895.
39. WandH, RamjeeG (2011) Combined impact of sexual risk behaviors for HIV seroconversion among women in Durban, South Africa: implications for prevention policy and planning. AIDS Behav 15 : 479–486.
40. HeffronR, DonnellD, ReesH, CelumC, MugoN, et al. (2012) Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis 12 : 19–26.
41. MorrisonCS, ChenPL, KwokC, RichardsonBA, ChipatoT, et al. (2010) Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS 24 : 1778–1781.
42. MorrisonCS, WangJ, Van Der PolB, PadianN, SalataRA, et al. (2007) Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS 21 : 1027–1034.
43. KeatingMA, HamelaG, MillerWC, MosesA, HoffmanIF, et al. (2012) High HIV incidence and sexual behavior change among pregnant women in Lilongwe, Malawi: implications for the risk of HIV acquisition. PLoS ONE 7: e39109.
44. ClelandJG, AliMM, Capo-ChichiV (1999) Post-partum sexual abstinence in West Africa: implications for AIDS-control and family planning programmes. AIDS 13 : 125–131.
45. TahaTE, HooverDR, DallabettaGA, KumwendaNI, MtimavalyeLA, et al. (1998) Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12 : 1699–1706.
46. HumphreyJH, HargroveJW, MalabaLC, IliffPJ, MoultonLH, et al. (2006) HIV incidence among post-partum women in Zimbabwe: risk factors and the effect of vitamin A supplementation. AIDS 20 : 1437–1446.
47. World Health Organization (2010) Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants: recommendations for a public health approach. Geneva: World Health Organization.
48. JohnsonLF, StinsonK, NewellML, BlandRM, MoultrieH, et al. (2012) The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr 59 : 417–425.
49. BlackV, OsihR, ReesHV, ChersichMF (2009) High HIV incidence or poor test performance? AIDS 23 : 2234–2235.
50. LaeyendeckerO, ChurchJD, OliverAE, MwathaA, OwenSM, et al. (2010) Pregnancy does not affect HIV incidence test results obtained using the BED capture enzyme immunoassay or an antibody avidity assay. PLoS ONE 5: e13259.
51. World Health Organization (2011) When and how to use assays for recent infection to estimate HIV incidence at a population level. Geneva: World Health Organization.
52. DimitrovD, BoilyMC, MarrazzoJ, BeigiR, BrownER (2013) Population-level benefits from providing effective HIV prevention means to pregnant women in high prevalence settings. PLoS ONE 8: e73770.
53. BernasconiD, TavoschiL, RegineV, RaimondoM, GamaD, et al. (2010) Identification of recent HIV infections and of factors associated with virus acquisition among pregnant women in 2004 and 2006 in Swaziland. J Clin Virol 48 : 180–183.
54. Boly L, Ndile J, Zwartz F, Sabwa H, Musyoka K, et al. (2011) Screening for HIV seroconversion among pregnant women in rural Namibia [abstract]. 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 Jul 2011; Rome, Italy.
55. KharsanyAB, HancockN, FrohlichJA, HumphriesHR, Abdool KarimSS, et al. (2010) Screening for ‘window-period’ acute HIV infection among pregnant women in rural South Africa. HIV Med 11 : 661–665.
56. KiefferMP, NhlabatsiB, MahdiM, HoffmanHJ, KudiaborK, et al. (2011) Improved detection of incident HIV infection and uptake of PMTCT services in labor and delivery in a high HIV prevalence setting. J Acquir Immune Defic Syndr 57: e85–e91.
57. MbizvoMT, KasuleJ, MahomedK, NathooK (2001) HIV-1 seroconversion incidence following pregnancy and delivery among women seronegative at recruitment in Harare, Zimbabwe. Cent Afr J Med 47 : 115–118.
58. MunjomaMW, MhlangaFG, MapingureMP, KurewaEN, MashavaveGV, et al. (2010) The incidence of HIV among women recruited during late pregnancy and followed up for six years after childbirth in Zimbabwe. BMC Public Health 10 : 668.
59. ReidSE, DaiJY, WangJ, SichalweBN, AkpomiemieG, et al. (2010) Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr 53 : 606–613.
60. RollinsNC, DedicoatM, DanaviahS, PageT, BishopK, et al. (2002) Prevalence, incidence, and mother-to-child transmission of HIV-1 in rural South Africa. Lancet 360 : 389.
61. De Schacht C, Ismael N, Santos I, Calu N, Vubil A, et al. (2011) HIV incidence during pregnancy and post-partum period in Southern Mozambique: impact on vertical HIV transmission [abstract]. 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 Jul 2011; Rome, Italy.
62. GayCL, MwapasaV, MurdochDM, KwiekJJ, FiscusSA, et al. (2010) Acute HIV infection among pregnant women in Malawi. Diagn Microbiol Infect Dis 66 : 356–360.
63. BraunsteinSL, IngabireCM, KestelynE, UwizeraAU, MwamarangweL, et al. (2011) High human immunodeficiency virus incidence in a cohort of Rwandan female sex workers. Sex Transm Dis 38 : 385–394.
64. KinuthiaJ, KiarieJN, FarquharC, RichardsonB, NduatiR, et al. (2010) Cofactors for HIV-1 incidence during pregnancy and postpartum period. Curr HIV Res 8 : 510–514.
65. LeroyV, Van de PerreP, LepageP, SabaJ, NsengumuremyiF, et al. (1994) Seroincidence of HIV-1 infection in African women of reproductive age: a prospective cohort study in Kigali, Rwanda, 1988–1992. AIDS 8 : 983–986.
66. WawerMJ, SewankamboNK, SerwaddaD, QuinnTC, PaxtonLA, et al. (1999) Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet 353 : 525–535.
67. WoldayD, MelesH, HailuE, MesseleT, MengistuY, et al. (2007) Temporal trends in the incidence of HIV infection in antenatal clinic attendees in Addis Ababa, Ethiopia, 1995–2003. J Intern Med 261 : 132–137.
68. Imade G, Sagay S, Musa J, Ocheke A, Adeniyl D, et al. (2012) Declining rates of maternal HIV infection detected at delivery settings in north central Nigeria [abstract]. 19th International AIDS Conference; 22–27 Jul 2012; Washington, District of Columbia, US.
69. Traore CAH, Dielbeogo R, Bagre I, Konate D, Meda N, et al. (2012) Evaluation of HIV incidence during pregnancy in Ouagadougou, Burkina Faso [abstract]. 19th International AIDS Conference; 22–27 Jul 2012; Washington, District of Columbia, US.
70. DuanS, ShenS, BulterysM, JiaY, YangY, et al. (2010) Estimation of HIV-1 incidence among five focal populations in Dehong, Yunnan: a hard hit area along a major drug trafficking route. BMC Public Health 10 : 180.
71. SaphonnV, ParekhBS, DobbsT, MeanC, BunLH, et al. (2005) Trends of HIV-1 seroincidence among HIV-1 sentinel surveillance groups in Cambodia, 1999–2002. J Acquir Immune Defic Syndr 39 : 587–592.
72. NesheimS, ParekhB, SullivanK, BulterysM, DobbsT, et al. (2005) Temporal trends in HIV Type 1 incidence among inner-city childbearing women in Atlanta: use of the IgG-capture BED-enzyme immunoassay. AIDS Res Hum Retroviruses 21 : 537–544.
73. de Freitas OliveiraCA, UedaM, YamashiroR, RodriguesR, SheppardHW, et al. (2005) Rate and incidence estimates of recent human immunodeficiency virus type 1 infections among pregnant women in Sao Paulo, Brazil, from 1991 to 2002. J Clin Microbiol 43 : 1439–1442.
74. PandoMA, Gomez-CarrilloM, VignolesM, RubioAE, dos Ramos FariasMS, et al. (2011) Incidence of HIV type 1 infection, antiretroviral drug resistance, and molecular characterization in newly diagnosed individuals in Argentina: a Global Fund Project. AIDS Res Hum Retroviruses 27 : 17–23.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 2- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Data Access for the Open Access Literature: PLOS's Data Policy
- Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap
- Effect of Water, Sanitation, and Hygiene on the Prevention of Trachoma: A Systematic Review and Meta-Analysis
- Biomarker Profiling by Nuclear Magnetic Resonance Spectroscopy for the Prediction of All-Cause Mortality: An Observational Study of 17,345 Persons
- Physicians as Fundraisers: Medical Philanthropy and the Doctor-Patient Relationship
- Molecular Diagnostics for Gonorrhoea: Implications for Antimicrobial Resistance and the Threat of Untreatable Gonorrhoea
- Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis
- Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study
- Quantitative Measurement of Melanoma Spread in Sentinel Lymph Nodes and Survival
- Effect of an Educational Toolkit on Quality of Care: A Pragmatic Cluster Randomized Trial
- Patterns of Obesity Development before the Diagnosis of Type 2 Diabetes: The Whitehall II Cohort Study
- Long-Term Survival and Dialysis Dependency Following Acute Kidney Injury in Intensive Care: Extended Follow-up of a Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study
- Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap
- Quantitative Measurement of Melanoma Spread in Sentinel Lymph Nodes and Survival
- Physicians as Fundraisers: Medical Philanthropy and the Doctor-Patient Relationship
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



