-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSetting Research Priorities to Reduce Mortality and Morbidity of Childhood Diarrhoeal Disease in the Next 15 Years
article has not abstract
Published in the journal: . PLoS Med 10(5): e32767. doi:10.1371/journal.pmed.1001446
Category: Guidelines and Guidance
doi: https://doi.org/10.1371/journal.pmed.1001446Summary
article has not abstract
Summary Points
-
This paper aims to identify research priorities, using the Child Health and Nutrition Research Initiative's (CHNRI's) method, for global childhood diarrhoeal disease over the next 15 years.
-
Ten teams were established, and over 150 experts participated on one or more teams, generating and scoring 466 research questions.
-
Research questions involving improving implementation, especially through behaviour change and other delivery strategies ranked highly; oral rehydration and zinc were also seen as priorities, as research questions asking to identify driving factors of caregiver demand for oral rehydration solution (ORS) and zinc and development of an ORS formulation that reduces stool output were ranked highly.
-
Despite a range of discovery-related research topics, implementation research questions related to known interventions for childhood diarrhoeal diseases were ranked highly by most experts.
-
In tandem with the Global Action Plan for Pneumonia and Diarrhoea, concerted efforts by a range of stakeholders in implementation research will be needed to equitably scale up already proven, effective interventions.
Introduction
While considerable progress has been made towards the Millennium Development Goals (MDGs) and childhood diarrheal diseases have reduced from 4.6 million to 0.8 million over the last three decades, the number of diarrhoeal deaths remains unacceptably high [1]–[8]. These deaths remain concentrated in a relatively small number of countries and in poor and difficult-to-reach populations. For diarrhoeal disease in particular, coverage indicators for key preventive and curative interventions remain suboptimal, suggesting that efforts to reduce diarrhoea-related child deaths by two-thirds have stalled [4],[5],[9]–[14]. Moreover, although deaths have declined globally, the proportion of decline has been greater in high-income countries, suggesting that significant inequities between the developed and developing countries have persisted [6].
As major growth and brain development occur in the first two years of life, the impact of diarrhoeal morbidity on disability-adjusted life years (DALYs) is likely to remain substantial even as diarrhoeal mortality diminishes following current trends [15]–[17]. Nutritional deficits caused by diarrhoea can affect a child's growth, fitness, cognition, and performance at school [15],[17]. It is estimated that each diarrhoeal episode experienced by a child in the months preceding the child's second birthday increases the risk of being stunted by 5% [18]. Moreover, diarrhoeal illness in early childhood is associated with long-term adverse cognitive effects and decreased work productivity later in life [15].
Trends in coverage of many life-saving interventions have varied [9],[10]. Santosham et al. [12] reported that from 1982 to 1988 the proportion of children under age 5 receiving oral rehydration solution (ORS) grew from 5% to 60% as a result of substantial investment in diarrhoeal control programs. During this time, Brazil and Egypt made enormous progress in reducing childhood mortality due to diarrhoea [12],[19], reporting a 67% and 74% reduction, respectively. Regrettably, the median coverage of ORS has dropped since the late 1980s. Currently, the median percentage of access to ORS is 30.9% [13],[14]. The previous Countdown Report, published in 2008, combined data for ORS and ORS with continued feeding, reporting a median coverage of 38% with a range of 7%–76% [9]. Thus, coverage indicators for diarrhoea treatment have not progressed over the past four years.
Mismatch of Burden and Investments in Programs and Research
Investment into diarrhoeal disease control has been disproportionately low in comparison to other diseases, and coverage has stalled [4],[12],[20]. Only about 2 cents for every 10 US dollars in health research annually is allocated to pneumonia and diarrhoea [21]. While diarrhoeal disease control programs receive US $10 per DALY globally, diabetes and cardiovascular disease programmes receive US $102.07 per DALY and US $63.45 per DALY, respectively [4],[20]. A recognized limitation of research investments in the area relate to lack of consensus on priorities.
The 2009 Global Forum Report on financial flows in health research highlights the importance of researchers and policy makers ensuring economic and social returns on research. It emphasizes transparency in investments and attention to health inequities [22]. The Child Health and Nutrition Research Initiative (CHNRI) developed a method to systematically and transparently identify research gaps and resource priorities [4],[7],[23]–[27]. Two previous exercises have employed the CHNRI method to identify research priorities to reduce mortality from childhood diarrhoea by 2015, but these previous CHNRI exercises had several limitations [4],[23]. Firstly, the main focus was on mortality and disease burden within the time frame of the MDGs, i.e. 2015, which influenced the choice of interventions and research options. The exercises were also limited to 10 [23] and 13 [4] participants, and 46 and 154 scored research priorities, respectively. Given the need to focus on achieving major reduction in diarrheal deaths and morbidity over a longer time frame to make it consistent with the Global Action Plan for Pneumonia and Diarrhoea (GAPPD) [28], we undertook a fresh exercise building and expanding the previous two exercises in terms of the timeframe for the research options, number of research options generated, and number of participants. We employed the CHNRI method to identify research gaps and resource priorities to reduce morbidity and mortality caused by childhood diarrhoeal disease over the next 15 years.
Methods
The CHNRI methodology was created to assist those who develop research policy and/or invest in health research by identifying research gaps and resource priorities systematically and transparently in a specified context [26],[27],[29]. The aim is to help policy makers understand the potential risks and benefits of a range of research options. This methodology has been used previously to identify research gaps and resource priorities in areas such as birth asphyxia and childhood pneumonia [24],[25].
The CHNRI method has four stages: (i) the context of the problem and the criteria for priority setting are defined; (ii) technical experts generate and rank research questions; (iii) stakeholders give input regarding the weighting of the CHNRI criteria; and, (iv) research scores for the research questions are calculated and agreement between experts is analysed [25]. Detailed information on the CHNRI methodology has been provided in previous publications [24],[25]. We supplemented the CHNRI method by hosting an international workshop on the identified research priorities, which is reported elsewhere [30].
(i) Context of the Problem Is Defined
In consideration of the substantial mortality rates of diarrhoea and its effect on morbidity as described above, we used the CHNRI method to identify research gaps and address resource priorities to questions related to both mortality and morbidity caused by childhood diarrhoeal disease. Our exercise specifically targeted a time trajectory over the next 15 years to be broadly consistent with the emerging targets for eliminating diarrhoea and pneumonia deaths by 2035 [31].
(ii) Technical Experts Provide Input
Ten areas of focus, defined by the results of the previous CHNRI exercises [4],[23], were identified and experts in each area were invited to be team leaders (Figure 1). Researchers identified team leaders by their scientific and subject expertise, contributions, and willingness to lead respective work groups. Each team leader was instructed to assemble a diverse virtual team of approximately 20 global experts representing different genders, age groups, and geographical locations. We also aimed to have representation of high-, middle - and low-income countries. Global experts were not permitted to participate in more than two teams. Information on the composition of each of the ten teams, including team leaders, global experts, countries represented, and institutional affiliations, is available in Table S16 of Supporting Information S1.
Fig. 1. Ten “teams” of research focus and distribution of research questions into Description, Discovery, Development, and Delivery areas. 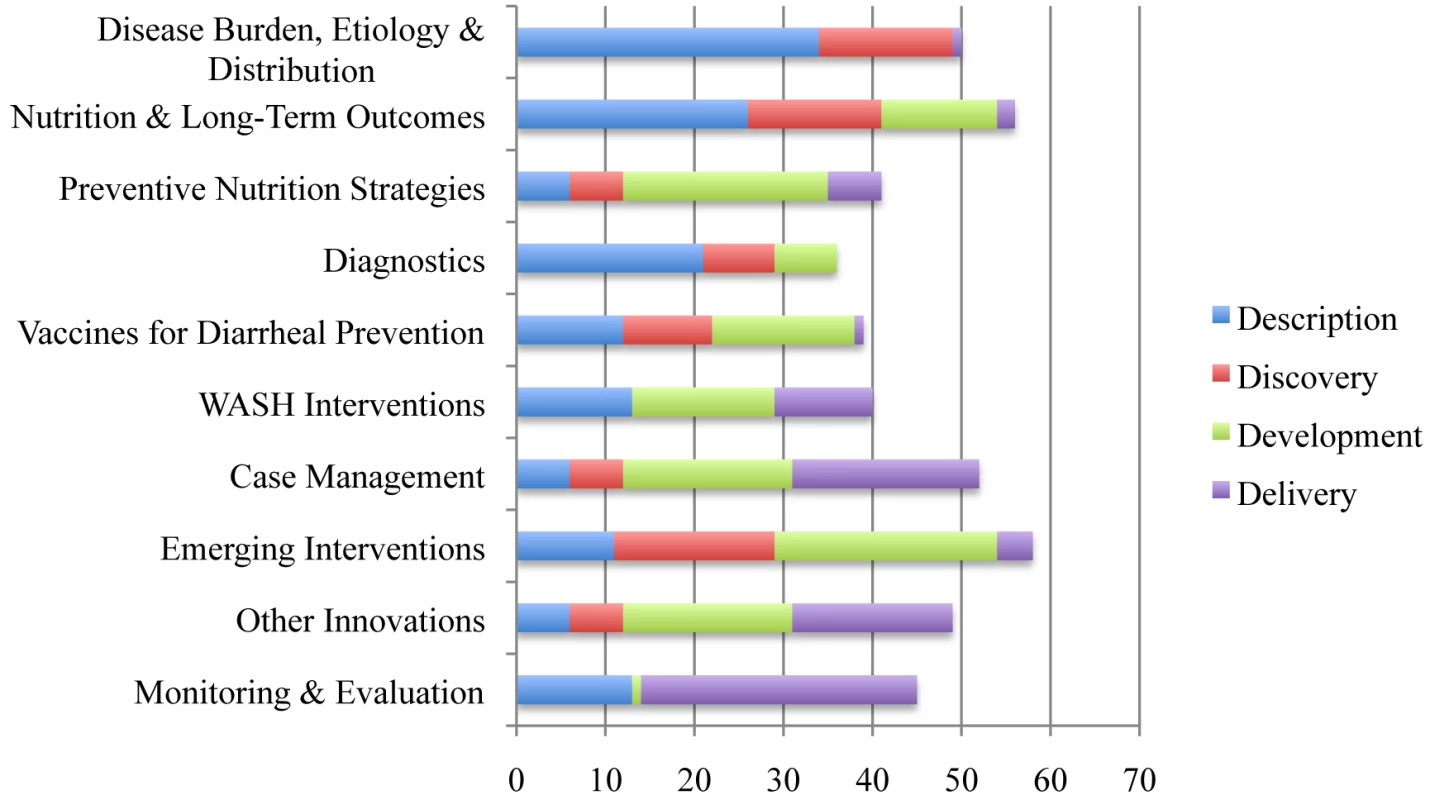
The global experts were asked to generate distinct, answerable research questions to be priorities over the next 15 years in their corresponding teams, covering the broad research domains of:
-
Description (epidemiology)
-
Discovery (new interventions)
-
Development (improving existing interventions)
-
Delivery (health policy systems, including cost-effectiveness)
These four domains (also termed D4), intended to be universally applicable to all health research, were proposed by the Council on Health Research and Development and modified by Rudan et al. [29]. Sample “seed questions” were provided to each team; these questions were taken from a previous CHNRI exercise to identify research priorities in childhood diarrhoea mortality [4].
Team leaders collected research questions submitted by their experts, eliminated redundancies, and chose the top questions to be scored. Research questions were scored using five criteria: (a) answerability; (b) likelihood of effectiveness; (c) likelihood of deliverability; (d) disease burden reduction; and, (e) effect on equity. A detailed description of the criteria can be found in Table S17 of Supporting Information S1. Experts were asked to score the questions, giving a 1 for yes, 0 for no, and 0.5 if they were undecided. Experts were asked to leave cells blank if they did not feel knowledgeable enough to answer the question. Blank cells did not affect the questions' scores. The Monitoring and Evaluation team recognized at an early stage that the traditional CHNRI criteria were not applicable to the research priorities their team generated; thus, this team revised some of the criteria to be more applicable to their research priorities (Table S18 of Supporting Information S1).
(iii) External Stakeholders Agree on Weighting
Because we needed to identify a balanced portfolio of research and we lacked an empiric basis to weigh various aspects objectively, we chose to weigh all CHNRI criteria equally. This decision differs from previous CHNRI exercises where such an approach was not used [24],[25]. It was anticipated, however, that the final ranking and hierarchy of questions would reflect the priority listing of questions irrespective of individual ranking by the groups themselves.
(iv) Research Scores Calculated for Each Team
The research priority score (RPS) and average expert agreement (AEA) were used to generate a ranking of research priorities. The RPS was computed as a mean of the scores given, by all global experts and across all criteria scored, to a particular question. The AEA score was calculated as a proportion of experts who gave the most common answer for a particular research question.
We have used an AEA to display agreement rather than a Kappa statistic, as the large number of scorers and few possible scoring options make it impossible to rule out chance, even with complete agreement among experts.
The AEA was computed using the following formula:
where q is a question that experts are being asked to evaluate competing research investment options, ranging from 1 to 15.Results
RPSs from the 466 questions (Table S15 of Supporting Information S1) ranged from 95.63 to 36.95 with a median of 68.50. The AEA scores ranged from 0.94 to 0.40, with a median of 0.56. The top ten questions from each team are shown in Tables S1–S10 of Supporting Information S1. The distribution of research questions into the D4 categories can be found in Figure 1. The top 20 research questions in the four categories are located in Tables S11–S14 of Supporting Information S1. The top 20 research questions overall are displayed in Table 1.
Tab. 1. Top 20 research questions overall. 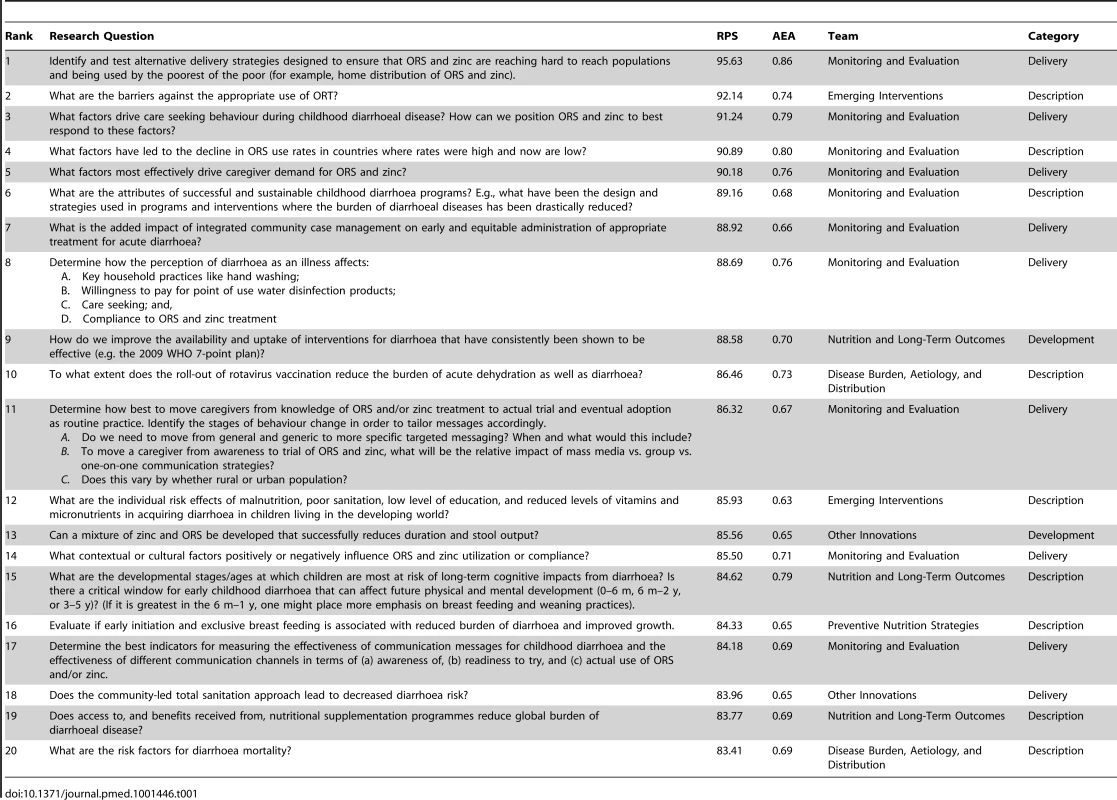
ORS/ORT and Zinc
Across the ten teams, many research questions addressed various aspects of ORS or oral rehydration therapy (ORT), many of which were also connected to zinc. The top five research questions overall were related to ORS or ORT, and had RPSs over 90.00. Of the top 50 research questions overall, 20 concerned some aspect of ORS or ORT, many of which addressed ORS/ORT and zinc concurrently. Of these 20, 13 related to some feature of delivery of ORS and ORT, such as barriers to use, driving care-seeking behaviour, and characteristics of mothers that are associated with high use of ORS/ORT. Three of the top 50 research questions concerned improving the formulation of ORS and two of the top 50 research questions concerned monitoring ORS use.
Delivery Strategies
Questions relating to delivery strategies were found in three of four D4 categories. Research questions regarding descriptions of barriers to appropriate ORS use and attributes of successful programs ranked highly in the Description category. Questions regarding how to improve the uptake of the UNICEF/WHO's seven-point plan and the effect of low-cost, sustainable education packages were ranked highly in the Development category. Twenty-six of the top 50 research questions overall were either in the Delivery category, related to further developing a delivery strategy, or were of a descriptive nature that would inform a delivery strategy.
Behaviour Change
There were numerous highly ranked questions regarding behaviour changes in mothers and other caregivers. Many research questions focused on driving care-seeking behaviour and moving caregivers from awareness to action in general and for specific interventions, including zinc, ORS, and hand washing with soap. Other behaviour change–related research priorities focused on interventions to support mothers, to encourage responsive care/parenting, or on the effect of women's peer groups and counselling on childhood diarrhoeal outcomes. Of the top 50 research questions overall, 23 involved understanding how to change the behaviour of caregivers.
Observations on Specific Teams and D4 Categories
The Disease Burden, Aetiology and Distribution team prioritized developing a clear understanding of the prevalence and distribution and risk factors of diarrhoea globally. Understanding long-term child development outcomes was a priority in the Nutrition and Long-Term Outcomes team, which emphasized a need for education for diarrhoea prevention and in promoting child development.
The Preventive Nutrition Strategies team highlighted the importance of community involvement and education regarding the relationship between ideal nutrition strategies and prevention of diarrhoea. The Emerging Interventions team also prioritised research questions regarding the importance of nutritional factors in diarrhoeal disease.
The Diagnostics team prioritised research questions involving availability of technology both in diagnostic labs and in the field for accurate diagnosis of the causative agents in diarrhoea. The Vaccines for Diarrhoea Prevention team prioritized understanding barriers to effectiveness of oral vaccines in developing countries so as to develop oral vaccines with improved efficacy, and development and implementation of vaccines.
Both the Case Management team and the Monitoring and Evaluation team emphasized research priorities around ORS and zinc use, including determinants of use, factors that drive care-seeking behaviour, delivery strategies, and social marketing. The Monitoring and Evaluation team also prioritized finding factors that have led to decline in ORS use as well as defining attributes of successful and sustainable childhood diarrhoea programs.
In addition to placing an emphasis on ORS and zinc, the Other Innovations team's highly ranked questions included research questions involving feeding practices during diarrhoea and research questions regarding hand washing and sanitation. The WASH Interventions team also prioritized hand washing, highlighting the importance of better understanding the relative contribution of different transmission routes to disease burden. The team identified a need to study the effectiveness of programs to improve sanitation, water supply and hygiene behaviour in the home and in schools, and to better understand the transmission routes of diarrhoea pathogens through the environment.
Research questions in the category of Discovery tended to rank lower than those in Description, Development, and Delivery. The highest-ranked research question in the area of Discovery was twenty-third overall; the second highest Discovery question was sixty-fifth overall. Furthermore, research questions in the Diagnostics and the Vaccines for Diarrhoea Prevention areas ranked much lower relative to the research questions generated by other teams.
Discussion
This is the largest exercise to date using the CHNRI methods and a range of subject experts building on previous exercises to develop priorities for diarrhoeal disease research. The latter utilized a more limited set of experts and specifically focused on diarrhoea mortality and morbidity in the relatively short time frame of the MDGs [4],[23]. The results of this multidisciplinary exercise emphasize strengthening the use of ORS and ORT, through new formulations, better delivery systems, and an improved understanding of the barriers to appropriate use. Research questions regarding various aspects of delivery strategies, in general, ranked highly, as did research questions regarding behaviour change. Research questions concerning new interventions ranked relatively low, which mirrors the results of the exercises conducted by Fontaine et al. and Kosek et al. [4],[23]. Fontaine and colleagues propose that the relatively low ranking of discovery-related questions was likely due to the short time frame that participants were instructed to consider [4]. However, since the time frame considered in the current exercise is one-third greater than that considered in the 2009 exercise, an alternative explanation for the low ranking of Discovery questions may be the certainty of results. As Discovery questions focused on developing entirely new interventions, research questions in Description, Development, and Delivery may have more predictable results than the Discovery research questions.
The current CHNRI exercise expanded the time lines of the previous exercise to set research priorities in childhood diarrhoeal disease over the next 15 years [4]. Fontaine et al. [4] argue that lack of implementation and coverage of cost-effective interventions, particularly in low-income settings, is a central reason that progress in preventing childhood diarrhoeal morbidity and mortality has stalled. The plentiful amount of high-priority research questions focusing on delivery of interventions supports this contention.
This CHNRI exercise differed from previous exercises by creating ten separate teams, representing different areas within the topic of diarrhoeal disease, that generated and ranked their own sets of research questions. This approach ensured a wide variety of research questions within each discipline, representation from each discipline, and experts specific to each discipline generating and ranking research questions. However, there were also disadvantages to this approach. As investments in a specific intervention can relate to multiple disciplines, there were many duplicate questions across the ten teams. Furthermore, the approach to creating research questions differed across teams; for example, research questions from the Vaccines team were much more detailed than research questions from other teams. The different styles of composing research questions could affect the scores given to those questions. Moreover, the use of multiple coordinators, some of whom had more familiarity with the CHNRI method than others, made standardization between teams challenging. Although each team was given the same format to record scores, research questions from some teams seemed to score lower as a whole than research questions from others. The research questions from the Monitoring and Evaluation team, for which custom CHNRI criteria were created (as described above), scored notably higher than research questions from other teams and 12 of the top 20 Delivery questions came from this team. Research questions from the Vaccines, Diagnostics, WASH Interventions, and Preventive Nutrition Strategies had relatively lower scores. This difference could be attributable to the research questions themselves being less important and impactful than research questions from other teams, or it could be a result of the experts being more critical scorers.
Although we strove to achieve global representation through our “global experts,” we were unable to achieve equal representation from all regions across all areas of D4. This could bias the types of research questions generated. In addition, questions more relevant regions that were more heavily represented through expert participation could have received higher scores as a result of this potential bias.
Conclusions
While there is an unprecedented amount of investment in health research [10],[32], vast inequities exist in the conditions being funded. MDG 4 seeks to reduce global childhood mortality by two-thirds by 2015. Despite proven cost-effective interventions, diarrhoeal disease remains the second most important cause of death in children under five [6].
This exercise represents an important effort to assist policy-makers in identifying research gaps and resource priorities in childhood global diarrhoeal disease. Results of this CHNRI exercise emphasize a need for research to improve delivery and implementation of existing interventions.
The research priorities of this CHNRI exercise and the previous CHNRI exercise addressing childhood pneumonia [25] will be incorporated into the integrated GAPPD and will be shared with funders, donors, and science bodies over the next 24 months. The GAPPD will include contributions from key stakeholders, such as WHO, UNICEF, and USAID. Relevant stakeholders and UN agencies in six pilot countries in South Asia and East Africa have agreed to implement the GAPPD. Further development and implementation of GAPPD is planned for South Asia and East Africa.
Reducing the diarrheal morbidity and mortality in low - and middle-income countries to the levels of that in high-income countries is within our grasp. Combined efforts from all stakeholders, including donors and bilateral agencies, as well as country-level commitments and strong political will, are necessary to achieve this goal through effective implementation of currently available interventions.
Supporting Information
Zdroje
1. BlackRE, CousensS, JohnsonHL, LawnJE, RudanI, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375 : 1969–1987.
2. BryceJ, Boschi-PintoC, ShibuyaK, BlackRE (2005) Group WHOCHER (2005) WHO estimates of the causes of death in children. Lancet 365 : 1147–1152.
3. BryceJ, TerreriN, VictoraCG, MasonE, DaelmansB, et al. (2006) Countdown to 2015: tracking intervention coverage for child survival. Lancet 368 : 1067–1076.
4. FontaineO, KosekM, BhatnagarS, Boschi-PintoC, ChanKY, et al. (2009) Setting research priorities to reduce global mortality from childhood diarrhoea by 2015. PLoS Med 6: e1000041 doi:10.1371/journal.pmed.1000041.
5. JonesG, SteketeeRW, BlackRE, BhuttaZA, MorrisSS, et al. (2003) How many child deaths can we prevent this year? Lancet 362 : 65–71.
6. LiuL, JohnsonHL, CousensS, PerinJ, ScottS, et al. (2012) Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379 : 2151–2161.
7. RudanI, LawnJ, CousensS, RoweAK, Boschi-PintoC, et al. (2005) Gaps in policy-relevant information on burden of disease in children: a systematic review. Lancet 365 : 2031–2040.
8. SnyderJD, MersonMH (1982) The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Org 60 : 605–613.
9. Bryce J, Requejo JH, editors. (2008) The Countdown to 2015: maternal, newborn and child survival. Tracking progress in maternal, newborn and child health: the 2008 report. New York: UNICEF.
10. Requejo J, Bryce J, Victora C, editors. (2012) The Countdown to 2015: Maternal, Newborn and Child Survival. Building a Future for Women and Children: the 2012 Report. Washington, DC: WHO and UNICEF.
11. BryceJ, el ArifeenS, PariyoG, LanataC, GwatkinD, et al. (2003) Reducing child mortality: can public health deliver? Lancet 362 : 159–164.
12. SantoshamM, ChandranA, FitzwaterS, Fischer-WalkerC, BaquiAH, et al. (2010) Progress and barriers for the control of diarrhoeal disease. Lancet 376 : 63–67.
13. BhuttaZA, DasJK, WalkerN, RizviA, CampbellH, et al. (2013) Interventions to address deaths from childhood diarrhoea and pneumonia equitably: what works and at what costs? Lancet doi: 10.1016/S0140-6736(13)60648-0.
14. WalkerC, RudanI, LiuL, NairH, TheodoratouE, et al. (2013) Global Burden of Childhood Diarrhoea and Pneumonia. Lancet doi: 10.1016/S0140-6736(13)60222-6.
15. GuerrantRL, KosekM, LimaAAM, LorntzB, GuyattHL (2002) Updating the DALYs for diarrhoeal disease. Trends Parasitol 18 : 191–193.
16. KosekM, BernC, GuerrantRL (2003) The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Org 81 : 197–204.
17. PetriWAJr, MillerM, BinderHJ, LevineMM, DillinghamR, et al. (2008) Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 118 : 1277–1290.
18. BhuttaZA, NelsonEA, LeeWS, TarrPI, ZablahR, et al. (2008) Recent advances and evidence gaps in persistent diarrhea. J Pediatr Gastroenterol Nutr 47 : 260–265.
19. MillerP, HirschhornN (1995) The effect of a national control of diarrheal diseases program on mortality: the case of Egypt. Soc Sci Med 40: S1–S30.
20. Monitoring Financial Flows for Health Research 2006: the changing landscpare of health research for development. Global Forum for Health Research
21. Child Health Research: A Foundation for Improving Child Health.
22. Monitoring Financial Flows for Health Research 2009: Behind the Global Numbers. Global Forum for Health Research
23. KosekM, LanataCF, BlackRE, WalkerDG, SnyderJD, et al. (2009) Directing diarrhoeal disease research towards disease-burden reduction. J Health Popul Nutr 27 : 319–331.
24. LawnJE, BahlR, BergstromS, BhuttaZA, DarmstadtGL, et al. (2011) Setting research priorities to reduce almost one million deaths from birth asphyxia by 2015. PLoS Med 8: e1000389 doi:10.1371/journal.pmed.1000389.
25. RudanI, El ArifeenS, BhuttaZA, BlackRE, BrooksA, et al. (2011) Setting research priorities to reduce global mortality from childhood pneumonia by 2015. PLoS Med 8: e1001099 doi:10.1371/journal.pmed.1001099.
26. RudanI, GibsonJ, KapiririL, LansangMA, HyderAA, et al. (2007) Setting priorities in global child health research investments: assessment of principles and practice. Croat Med J 48 : 595–604.
27. RudanI, GibsonJL, AmeratungaS, El ArifeenS, BhuttaZA, et al. (2008) Setting priorities in global child health research investments: guidelines for implementation of CHNRI method. Croat Med J 49 : 720–733.
28. Pneumonia and diarrhoea: Tackling the deadliest diseases for the world's poorest children. United Nations Children's Fund (UNICEF)
29. RudanI, ChopraM, KapiririL, GibsonJ, Ann LansangM, et al. (2008) Setting priorities in global child health research investments: universal challenges and conceptual framework. Croat Med J 49 : 307–317.
30. ZipurskyA, WaznyK, BlackR, KeenanW, DugganC (2013) Global Action Plan for Childhood Diarrhea, Developing Research Priorities: Report from a Workshop of the Programme for Global Paediatric Research. Journal of Global Health In press.
31. NutritionECo, AgostoniC, BraeggerC, DecsiT, KolacekS, et al. (2009) Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 49 : 112–125.
32. GrecoG, Powell-JacksonT, BorghiJ, MillsA (2008) Countdown to 2015: assessment of donor assistance to maternal, newborn, and child health between 2003 and 2006. Lancet 371 : 1268–1275.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 5- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Measuring Coverage in MNCH: Challenges and Opportunities in the Selection of Coverage Indicators for Global Monitoring
- Measuring Coverage in MNCH: Tracking Progress in Health for Women and Children Using DHS and MICS Household Surveys
- Measuring Coverage in MNCH: Population HIV-Free Survival among Children under Two Years of Age in Four African Countries
- Tobacco Company Efforts to Influence the Food and Drug Administration-Commissioned Institute of Medicine Report An Analysis of Documents Released through Litigation
- Irreconcilable Conflict: The Tobacco Industry and the Public Health Challenge of Tobacco Use
- Providing Impetus, Tools, and Guidance to Strengthen National Capacity for Antimicrobial Stewardship in Viet Nam
- Measuring Coverage in MNCH: New Findings, New Strategies, and Recommendations for Action
- Grand Challenges: Integrating Maternal Mental Health into Maternal and Child Health Programmes
- Measuring Coverage in MNCH: Challenges in Monitoring the Proportion of Young Children with Pneumonia Who Receive Antibiotic Treatment
- Measuring Coverage in MNCH: Current Indicators for Measuring Coverage of Diarrhea Treatment Interventions and Opportunities for Improvement
- Measuring Coverage in MNCH: Evaluation of Community-Based Treatment of Childhood Illnesses through Household Surveys
- Measuring Coverage in MNCH: Accuracy of Measuring Diagnosis and Treatment of Childhood Malaria from Household Surveys in Zambia
- Grand Challenges: Integrating Mental Health Care into the Non-Communicable Disease Agenda
- Integrating Global and National Knowledge to Select Medicines for Children: The Ghana National Drugs Programme
- Grand Challenges: Integrating Mental Health Services into Priority Health Care Platforms
- Disability Transitions and Health Expectancies among Adults 45 Years and Older in Malawi: A Cohort-Based Model
- Comparative Efficacy of Seven Psychotherapeutic Interventions for Patients with Depression: A Network Meta-Analysis
- Measuring Coverage in MNCH: Design, Implementation, and Interpretation Challenges Associated with Tracking Vaccination Coverage Using Household Surveys
- Measuring Coverage in MNCH: A Prospective Validation Study in Pakistan and Bangladesh on Measuring Correct Treatment of Childhood Pneumonia
- Contribution of and Smoking Trends to US Incidence of Intestinal-Type Noncardia Gastric Adenocarcinoma: A Microsimulation Model
- Carriage of in the Upper Respiratory Tract of Symptomatic and Asymptomatic Children: An Observational Study
- Setting Research Priorities to Reduce Mortality and Morbidity of Childhood Diarrhoeal Disease in the Next 15 Years
- Grand Challenges: Improving HIV Treatment Outcomes by Integrating Interventions for Co-Morbid Mental Illness
- Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value
- Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis
- Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies
- Effect of Facilitation of Local Maternal-and-Newborn Stakeholder Groups on Neonatal Mortality: Cluster-Randomized Controlled Trial
- Intimate Partner Violence and Population Mental Health: Why Poverty and Gender Inequities Matter
- Assessing Population Aging and Disability in Sub-Saharan Africa: Lessons from Malawi?
- The Paradox of Mental Health: Over-Treatment and Under-Recognition
- Measuring Coverage in MNCH: Determining and Interpreting Inequalities in Coverage of Maternal, Newborn, and Child Health Interventions
- Measuring Coverage in MNCH: Total Survey Error and the Interpretation of Intervention Coverage Estimates from Household Surveys
- Measuring Coverage in MNCH: Indicators for Global Tracking of Newborn Care
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value
- Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis
- Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies
- Measuring Coverage in MNCH: Challenges in Monitoring the Proportion of Young Children with Pneumonia Who Receive Antibiotic Treatment
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




