-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaErectile Dysfunction Severity as a Risk Marker for Cardiovascular Disease Hospitalisation and All-Cause Mortality: A Prospective Cohort Study
Background:
Erectile dysfunction is an emerging risk marker for future cardiovascular disease (CVD) events; however, evidence on dose response and specific CVD outcomes is limited. This study investigates the relationship between severity of erectile dysfunction and specific CVD outcomes.Methods and Findings:
We conducted a prospective population-based Australian study (the 45 and Up Study) linking questionnaire data from 2006–2009 with hospitalisation and death data to 30 June and 31 Dec 2010 respectively for 95,038 men aged ≥45 y. Cox proportional hazards models were used to examine the relationship of reported severity of erectile dysfunction to all-cause mortality and first CVD-related hospitalisation since baseline in men with and without previous CVD, adjusting for age, smoking, alcohol consumption, marital status, income, education, physical activity, body mass index, diabetes, and hypertension and/or hypercholesterolaemia treatment. There were 7,855 incident admissions for CVD and 2,304 deaths during follow-up (mean time from recruitment, 2.2 y for CVD admission and 2.8 y for mortality). Risks of CVD and death increased steadily with severity of erectile dysfunction. Among men without previous CVD, those with severe versus no erectile dysfunction had significantly increased risks of ischaemic heart disease (adjusted relative risk [RR] = 1.60, 95% CI 1.31–1.95), heart failure (8.00, 2.64–24.2), peripheral vascular disease (1.92, 1.12–3.29), “other” CVD (1.26, 1.05–1.51), all CVD combined (1.35, 1.19–1.53), and all-cause mortality (1.93, 1.52–2.44). For men with previous CVD, corresponding RRs (95% CI) were 1.70 (1.46–1.98), 4.40 (2.64–7.33), 2.46 (1.63–3.70), 1.40 (1.21–1.63), 1.64 (1.48–1.81), and 2.37 (1.87–3.01), respectively. Among men without previous CVD, RRs of more specific CVDs increased significantly with severe versus no erectile dysfunction, including acute myocardial infarction (1.66, 1.22–2.26), atrioventricular and left bundle branch block (6.62, 1.86–23.56), and (peripheral) atherosclerosis (2.47, 1.18–5.15), with no significant difference in risk for conditions such as primary hypertension (0.61, 0.16–2.35) and intracerebral haemorrhage (0.78, 0.20–2.97).Conclusions:
These findings give support for CVD risk assessment in men with erectile dysfunction who have not already undergone assessment. The utility of erectile dysfunction as a clinical risk prediction tool requires specific testing.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(1): e32767. doi:10.1371/journal.pmed.1001372
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001372Summary
Background:
Erectile dysfunction is an emerging risk marker for future cardiovascular disease (CVD) events; however, evidence on dose response and specific CVD outcomes is limited. This study investigates the relationship between severity of erectile dysfunction and specific CVD outcomes.Methods and Findings:
We conducted a prospective population-based Australian study (the 45 and Up Study) linking questionnaire data from 2006–2009 with hospitalisation and death data to 30 June and 31 Dec 2010 respectively for 95,038 men aged ≥45 y. Cox proportional hazards models were used to examine the relationship of reported severity of erectile dysfunction to all-cause mortality and first CVD-related hospitalisation since baseline in men with and without previous CVD, adjusting for age, smoking, alcohol consumption, marital status, income, education, physical activity, body mass index, diabetes, and hypertension and/or hypercholesterolaemia treatment. There were 7,855 incident admissions for CVD and 2,304 deaths during follow-up (mean time from recruitment, 2.2 y for CVD admission and 2.8 y for mortality). Risks of CVD and death increased steadily with severity of erectile dysfunction. Among men without previous CVD, those with severe versus no erectile dysfunction had significantly increased risks of ischaemic heart disease (adjusted relative risk [RR] = 1.60, 95% CI 1.31–1.95), heart failure (8.00, 2.64–24.2), peripheral vascular disease (1.92, 1.12–3.29), “other” CVD (1.26, 1.05–1.51), all CVD combined (1.35, 1.19–1.53), and all-cause mortality (1.93, 1.52–2.44). For men with previous CVD, corresponding RRs (95% CI) were 1.70 (1.46–1.98), 4.40 (2.64–7.33), 2.46 (1.63–3.70), 1.40 (1.21–1.63), 1.64 (1.48–1.81), and 2.37 (1.87–3.01), respectively. Among men without previous CVD, RRs of more specific CVDs increased significantly with severe versus no erectile dysfunction, including acute myocardial infarction (1.66, 1.22–2.26), atrioventricular and left bundle branch block (6.62, 1.86–23.56), and (peripheral) atherosclerosis (2.47, 1.18–5.15), with no significant difference in risk for conditions such as primary hypertension (0.61, 0.16–2.35) and intracerebral haemorrhage (0.78, 0.20–2.97).Conclusions:
These findings give support for CVD risk assessment in men with erectile dysfunction who have not already undergone assessment. The utility of erectile dysfunction as a clinical risk prediction tool requires specific testing.
Please see later in the article for the Editors' SummaryIntroduction
Erectile dysfunction is a common health problem that increases rapidly in prevalence with age. While erectile dysfunction can be a distressing symptom in itself, there is increasing recognition of its importance as a risk marker for potentially life-threatening cardiovascular disease (CVD) events and premature death [1],[2].
CVD is the leading cause of morbidity and mortality globally [3]. Many effective interventions for the primary and secondary prevention of CVD rely on identifying individuals at increased risk. Despite the robustness of current risk prediction models, it is well recognised that a significant proportion of cases of CVD will occur in individuals without classic risk factors [4]. In men without known CVD, erectile dysfunction has potential as a sentinel symptom indicating the presence or increased risk of systemic CVD [1]. Although the possibility has been less widely investigated, erectile dysfunction could also serve as a marker of increased risk of repeated events in men known to have existing CVD.
Currently, evidence on the nature of the relationship of erectile dysfunction to the risk of developing specific subtypes of CVD is limited. Previous prospective studies indicate that, among men without known CVD, those with erectile dysfunction have a significantly increased risk of grouped CVD outcomes, such as coronary heart disease, stroke, and peripheral vascular disease, and all-cause mortality, compared to men without erectile dysfunction [1],[5]–[8]. However, such evidence is limited by the following: the predominant use of dichotomised data on erectile dysfunction status, which precludes evaluation of a dose–response relationship [1],[7]–[9]; the lack of large-scale evidence on how erectile dysfunction relates to different subtypes of CVD; the lack of evidence on how erectile dysfunction relates to CVD in men with existing CVD, who constitute an important high risk group; and the relatively small size and selective nature of most of the studies to date [1],[7],[8],[10].
The aim of this large prospective population-based study is to investigate the relationship of severity of erectile dysfunction, as a marker of risk, to a range of CVD outcomes and all-cause mortality, among men with and without CVD at baseline.
Methods
Data Collection and Definitions
The 45 and Up Study is a large-scale Australian cohort study of 123,775 men and 143,378 women aged 45 y and over, randomly sampled from the general population of New South Wales (NSW), Australia. Its methods are described elsewhere [11]. Only men are included in the study presented here. In brief, from 1 February 2006 to 30 April 2009, individuals from the general population of NSW joined the study by completing a postal questionnaire and giving informed consent for follow-up through repeated contact and population health databases.
Data from study participants have been linked probabilistically to the NSW Admitted Patient Data Collection, which is a complete census of all public and private hospital admissions in NSW. It contains details of admissions in participants from 1 July 2000 to 30 June 2010, including dates of admission and discharge, the primary reason for admission using the World Health Organization International Classification of Diseases, 10th revision—Australian Modification (ICD-10-AM) [12], up to 55 additional clinical diagnoses, and up to 50 operations or procedures, coded using Australian Classification of Health Interventions procedure codes [13]. The validity of administrative coding for specific CVD outcomes varies, ranging from fair to very good, with kappa scores for agreement between chart review and recorded diagnoses of 0.6–0.8 [14] and positive predictive values of 66%–99% [15]–[18] for acute myocardial infarction, cerebral infarction, and heart failure. Where misclassification is present, it is unlikely to be biased by prior knowledge of erectile dysfunction status and would therefore tend to result in more conservative estimates of relative risk (RR). Vital status and date of death were ascertained from the date of recruitment up to 31 December 2010 using linkage to the NSW Registry of Births, Deaths and Marriages. Death registrations capture all deaths in NSW. Cause of death information was not available at the time of analysis.
Over the relatively short follow-up period, a small but unknown number of participants are likely to have moved out of the area. Although hospitalisations occurring in neighbouring states would not be captured, these are estimated to make up fewer than 2% of admissions in NSW residents. Hence, follow-up for hospitalisations is considered to be ∼98% complete among those continuing to reside in NSW, and loss to follow-up is considered to be minimal. Quality assurance data show false positive and negative rates for data linkage of <0.5% and <0.1%, respectively.
Baseline questionnaire data included information on socio-demographic factors, lifestyle, height and weight, medical and surgical history, functional capacity, physical activity, and sexual health. The questionnaire for men included a single self-assessment question on erectile functioning: “How often are you able to get and keep an erection that is firm enough for satisfactory sexual activity?”, with the response categories being “always”, “usually”, “sometimes”, “never”, and “I would rather not answer this question”. The first four categories were used to define erectile dysfunction as none, mild, moderate, and severe, respectively. This questionnaire item was based on that used in the Massachusetts Male Aging Study, which has shown good agreement with the International Index of Erectile Function (correlation coefficient = 0.71, kappa = 0.6) [19].
Statistical Methods
At the time of writing, data from 123,750 male 45 and Up Study participants (99.98%) had been linked to data on hospital admissions and deaths. Among these, men who were missing data (n = 4,264; 3.4%), who chose not to answer the question on erectile dysfunction (n = 11,034; 8.9%), or whose records had linkage errors (n = 25; 0.02%) were excluded. Because cancer and its treatment may lead to erectile dysfunction and affect future survival and CVD risk, those with a self-reported history of prostatectomy and/or cancer other than melanoma and skin cancer (n = 13,389; 10.8%) were also excluded. We did not have specific data on other procedures that can result in erectile dysfunction, such as pelvic irradiation, abdomino-pelvic resection, and low anterior resection; however, as these are largely used as treatments for cancer, individuals with these interventions would have been mostly excluded from the analyses. Following exclusions, data were available on 95,038 men for the main analyses.
The main CVD outcome was defined as the first hospitalisation following recruitment into the 45 and Up Study with a primary diagnosis of CVD at discharge, based on ICD-10-AM three-character codes I00–I99, G45, and G46. Diagnoses were grouped consistent with the NSW 2010 health report [20] (see Figure 1), and were also examined individually for diagnoses for which at least 50 events were reported in men with no previous CVD. In the analyses of incident CVD hospitalisation since baseline, eligible men contributed person-years from the date of recruitment until the CVD admission date, date of death, or end of follow-up (30 June 2010), whichever was the earliest. In the analysis of all-cause mortality, eligible men contributed person-years from recruitment until death or end of follow-up (31 December 2010), whichever was the earliest.
Fig. 1. Age-standardised rates (per 1,000 person-years) of grouped CVD hospitalisations since baseline and all-cause mortality by degree of erectile dysfunction and previous CVD history, directly age-standardised to the 2006 New South Wales population. 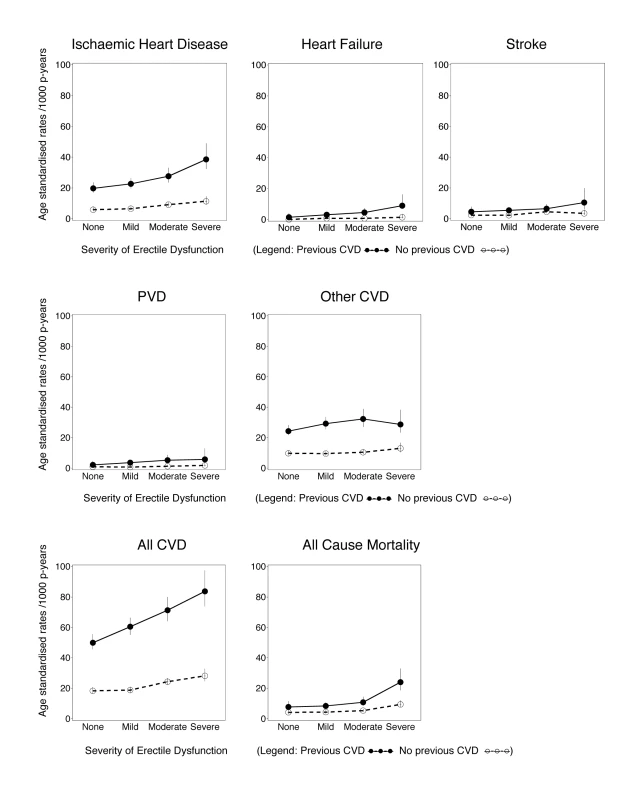
All CVD includes ICD-10-AM diagnosis codes I00–I99, G45, and G46; ischaemic heart disease includes I20–I25; peripheral vascular disease (PVD) includes I70–I74; stroke includes I60–I69, G45, and G46; heart failure is I50; and other CVD is all CVDs except those listed above. Vertical lines indicate 95% CIs. Estimates of incident CVD hospitalisation rates since baseline and 95% confidence intervals (95% CIs) were calculated for the different levels of erectile dysfunction at baseline, age-standardised to the 2006 NSW population, in 5-y age groups, using the direct method [21]. Hazard ratios (RRs) for specific CVD outcomes, all CVD, and all-cause mortality according to erectile dysfunction at baseline were estimated using Cox regression modelling, using age as the underlying time variable to finely adjust for age. Each outcome of interest was considered as an end point in the model, except where used for censoring. RRs and 95% CIs take account of age (the underlying time variable), adjusting where appropriate for tobacco smoking (current, past, never), alcohol consumption (0, 1–14, ≥15 drinks/week), marital status (living alone [single/widowed/divorced], living with a partner [married/living with a partner]), annual household pre-tax income (in Australian dollars: <$20,000, $20,000–$39,999, $40,000–$69,999, ≥$70,000), education (secondary school incomplete, secondary school graduation, certificate or diploma, university graduation), tertiles of physical activity (based on number of weekly sessions, weighted for intensity [22]), body mass index (BMI, based on self-reported height and weight, which has been shown to correlate well with that based on measured values in this population [r = 0.95], with a relatively small mean difference in values [23]) (15.0–18.49, 18.5–24.99, 25.0–29.99, ≥30.0 kg/m2), self-reported doctor-diagnosed diabetes (yes, no), current treatment for hypertension (yes, no), and current treatment for hypercholesterolaemia (yes, no). Missing values for covariates were included in the models as separate categories. No material differences in the main exposure–outcome relationships (ischaemic heart disease and all CVD) were observed when BMI, alcohol consumption, and physical activity were modelled as continuous variables (Table S1). Sensitivity analyses modelling the effect of assuming that men who stated that they would rather not answer the question on erectile dysfunction had severe erectile dysfunction demonstrated that the main findings did not vary materially according to this assumption (Table S2).
Analyses of CVD outcomes were stratified throughout by previous CVD status. Previous CVD was defined as self-reported heart disease, stroke, or blood clot (including peripheral blood clots) on the baseline questionnaire, or a hospital admission in the 6 y prior to study entry with a CVD diagnosis code in any diagnostic field or a CVD-related procedure code in any procedure code field (Table 1).
Tab. 1. Characteristics of study population according to CVD history and degree of erectile dysfunction at baseline. 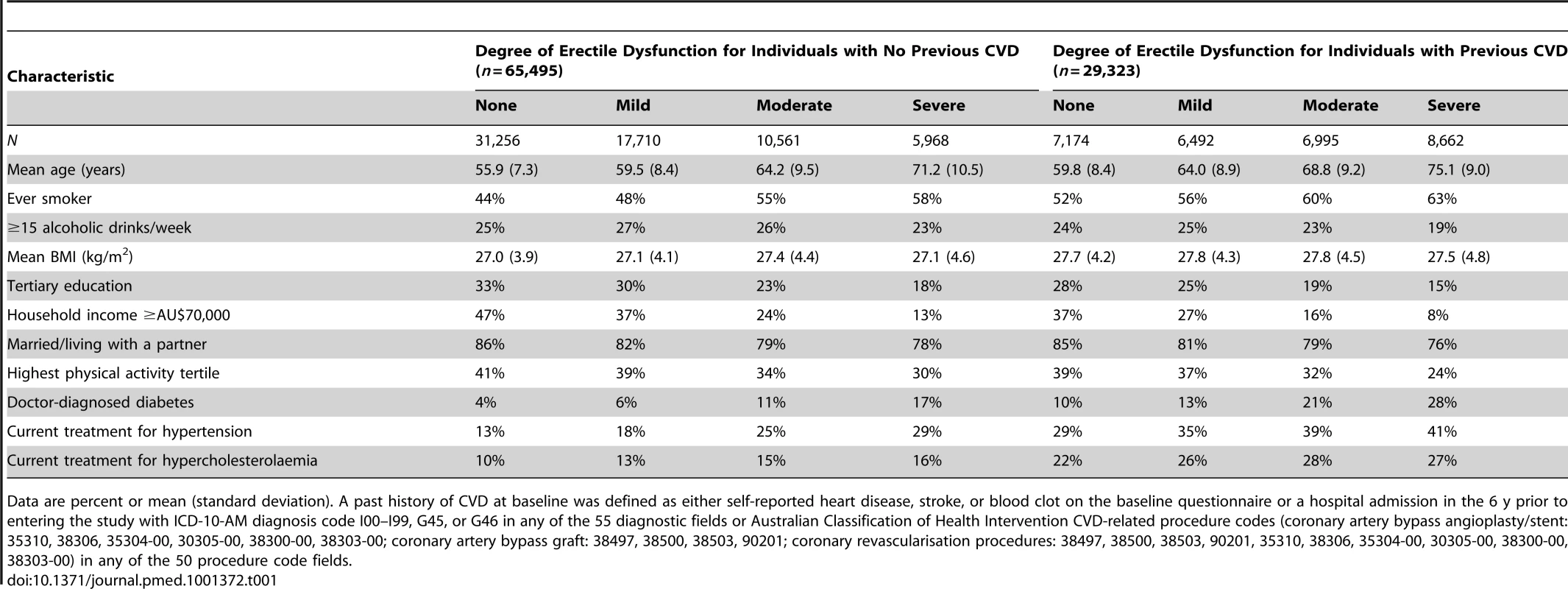
Data are percent or mean (standard deviation). A past history of CVD at baseline was defined as either self-reported heart disease, stroke, or blood clot on the baseline questionnaire or a hospital admission in the 6 y prior to entering the study with ICD-10-AM diagnosis code I00–I99, G45, or G46 in any of the 55 diagnostic fields or Australian Classification of Health Intervention CVD-related procedure codes (coronary artery bypass angioplasty/stent: 35310, 38306, 35304-00, 30305-00, 38300-00, 38303-00; coronary artery bypass graft: 38497, 38500, 38503, 90201; coronary revascularisation procedures: 38497, 38500, 38503, 90201, 35310, 38306, 35304-00, 30305-00, 38300-00, 38303-00) in any of the 50 procedure code fields. The proportionality assumption was verified by plotting the Schoenfeld residuals against the time variable in each model, with a stratified form used where covariates displayed non-proportionality of hazards. Tests for trend were performed by modelling erectile dysfunction as an ordinal variable. To test for interaction between erectile dysfunction categories and previous CVD, the likelihood-ratio test was used, stratified by age because of strong age–previous CVD relationships, to compare the model with and without the interaction term. The weighted least-squares method was used to test for heterogeneity in the RR for dichotomised erectile dysfunction (severe/moderate versus mild/none) in different study subgroups. All analyses were carried out using SAS version 9.3 [24]. All statistical tests were two-sided, using a significance level of 5%.
Ethical approval for the study was provided by the NSW Population and Health Services Research Ethics Committee (2010/05/234).
Results
The study population ranged in age from 45 to 106 y, with a mean of 62.0 y (standard deviation 10.5 y). Erectile dysfunction increased steeply with age, with a prevalence of severe erectile dysfunction of 2.2% at age 45–54 y, 6.8% at age 55–64 y, 20.2% at age 65–74 y, 50.0% at age 75–84 y, and 75.4% at age ≥85 y. Overall, the prevalence of severe erectile dysfunction was higher in smokers and in men with lower incomes, lower consumption of alcohol, lower education, and/or single/widower marital status, compared to other men (Table 1). Severe erectile dysfunction was also more common in men who had been diagnosed with diabetes and/or CVD in the past and in those who were receiving treatment for hypertension and/or hypercholesterolaemia, compared to men without these risk factors.
Median (mean) follow-up time for the cohort was 1.9 (2.2) y for CVD hospitalisation and 2.2 (2.8) y for mortality; over this time, 7,855 incident hospital admissions with a primary diagnosis of CVD and 2,304 deaths occurred. The age-standardised incidences of all-cause mortality and of hospitalisation for ischaemic heart disease, heart failure, peripheral vascular disease, “other” CVD, and all CVD combined generally increased with increasing severity of erectile dysfunction and generally were higher for men with prior CVD at baseline, compared to men without prior CVD (Figure 1).
The adjusted RRs of ischaemic heart disease, heart failure, stroke, peripheral vascular disease, “other” CVD, and all CVD combined increased significantly with increasing severity of baseline erectile dysfunction, in men with and without a past history of CVD (Figure 2; p[trend]<0.001 for all comparisons, except stroke and other CVD in men without previous CVD, where p[trend] = 0.008 and 0.04, respectively). These RRs varied according to the type of CVD outcome examined, with greater RRs for heart failure than for other grouped CVD outcomes (Figure 2). The relationship of severity of erectile dysfunction to CVD outcomes did not vary significantly according to history of CVD prior to baseline (p[interaction] for all strata >0.05, except for stroke, where p[interaction] = 0.02). Men with severe erectile dysfunction had approximately a doubling in the risk of death during follow-up compared to men without erectile dysfunction (Figure 2), regardless of past CVD history. The relation of erectile dysfunction to subsequent CVD events did not vary materially according to duration of follow-up (i.e., <2 y versus ≥2 y; Table S3).
Fig. 2. Relative risk of grouped CVD admissions and all-cause mortality since baseline, according to severity of erectile dysfunction at baseline. 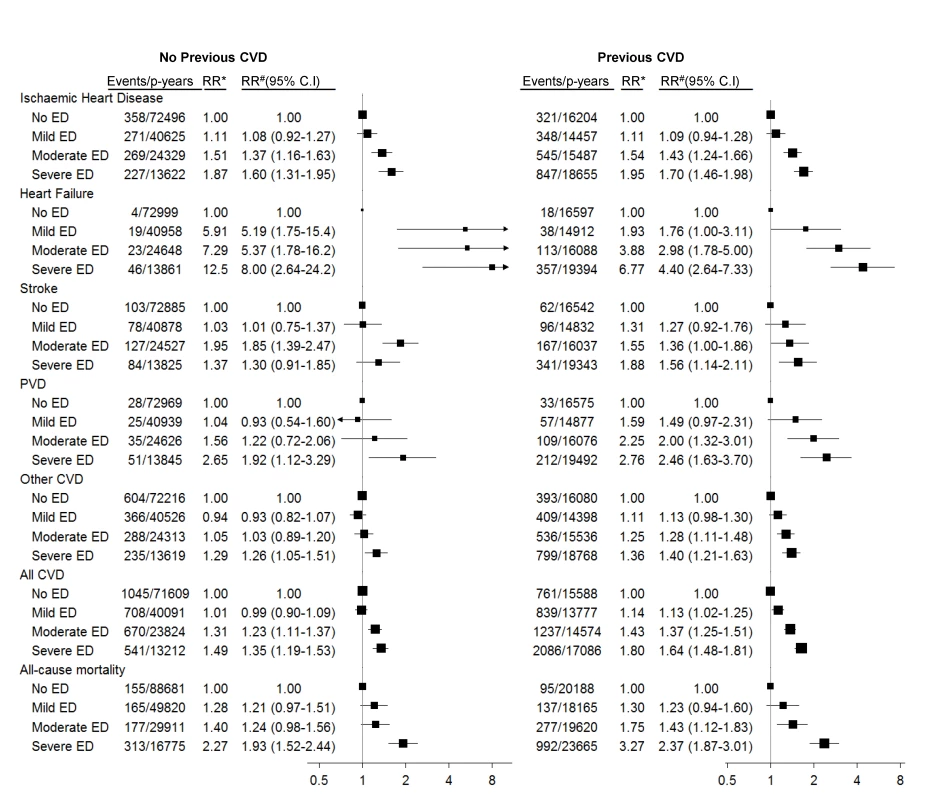
*RR adjusted for age only. #RR adjusted for age, tobacco smoking, alcohol consumption, marital status, income, education, physical activity, BMI, diabetes, and current treatment for hypertension and hypercholesterolaemia. RRs are plotted on a log scale and are represented with squares with areas inversely proportional to the variance of the logarithm of the RR, providing an indication of the amount of statistical information available; 95% CIs are indicated by horizontal lines. ED, erectile dysfunction, PVD, peripheral vascular disease. Among men with no previous history of CVD, significant trends of increasing hospitalisation risk for specific ICD-10-AM primary diagnostic codes with increasing erectile dysfunction were seen for angina pectoris, acute myocardial infarction, chronic ischaemic heart disease, atrioventricular and left bundle branch block, heart failure, and (peripheral) atherosclerosis (Table 2). No clear patterns of increasing risk were observed for conditions such as pulmonary embolism, dysrhythmias, intracerebral haemorrhage, aortic aneurysm, phlebitis and thrombophlebitis, and haemorrhoids. Findings were broadly similar for men with previous CVD (Table 3). There were fewer than 50 admissions for cardiomyopathy (I42) among men without previous CVD, so the findings are not included in Table 2; however, there were more than 50 events for men with previous CVD, with noteworthy findings: RRs (95% CIs) for this outcome were 2.38 (0.95–5.97), 3.61 (1.47–8.84), and 3.21 (1.23–8.35) for mild, moderate, and severe erectile dysfunction versus no dysfunction (p[trend] = 0.02).
Tab. 2. Relative risk (95% CI) of incident CVD-related hospital admission since baseline among men with no previous history of CVD by ICD-10-AM diagnosis code, according to degree of erectile dysfunction (ED). 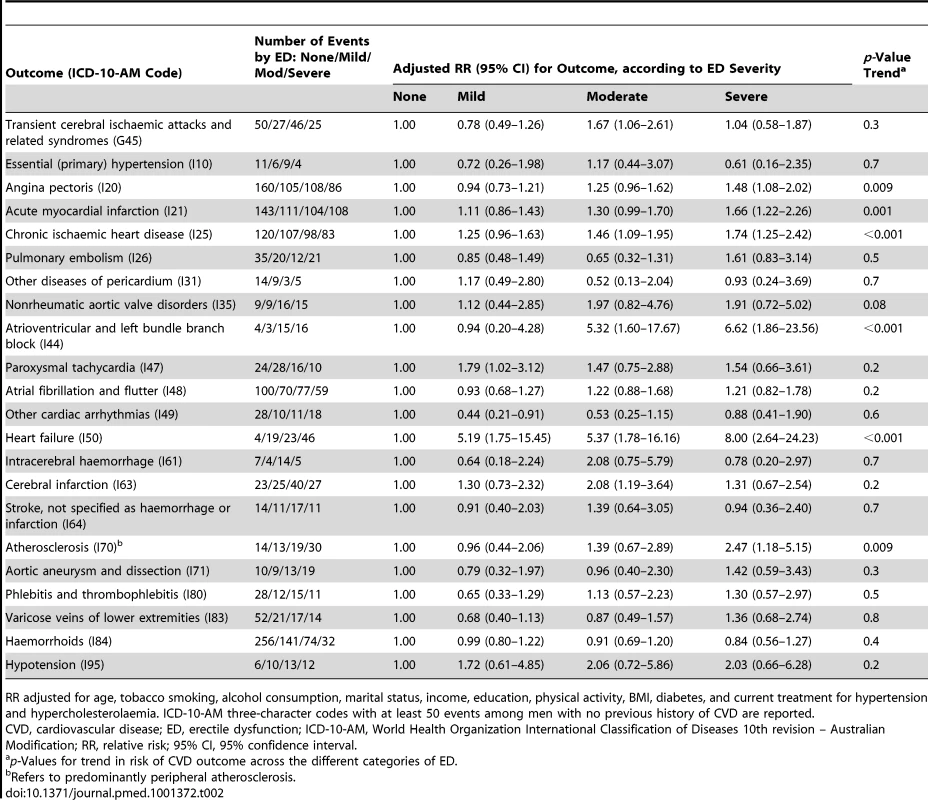
RR adjusted for age, tobacco smoking, alcohol consumption, marital status, income, education, physical activity, BMI, diabetes, and current treatment for hypertension and hypercholesterolaemia. ICD-10-AM three-character codes with at least 50 events among men with no previous history of CVD are reported. Tab. 3. Relative risk of incident CVD-related hospital admission since baseline among men with previous history of CVD by ICD-10-AM diagnosis code, according to degree of erectile dysfunction. 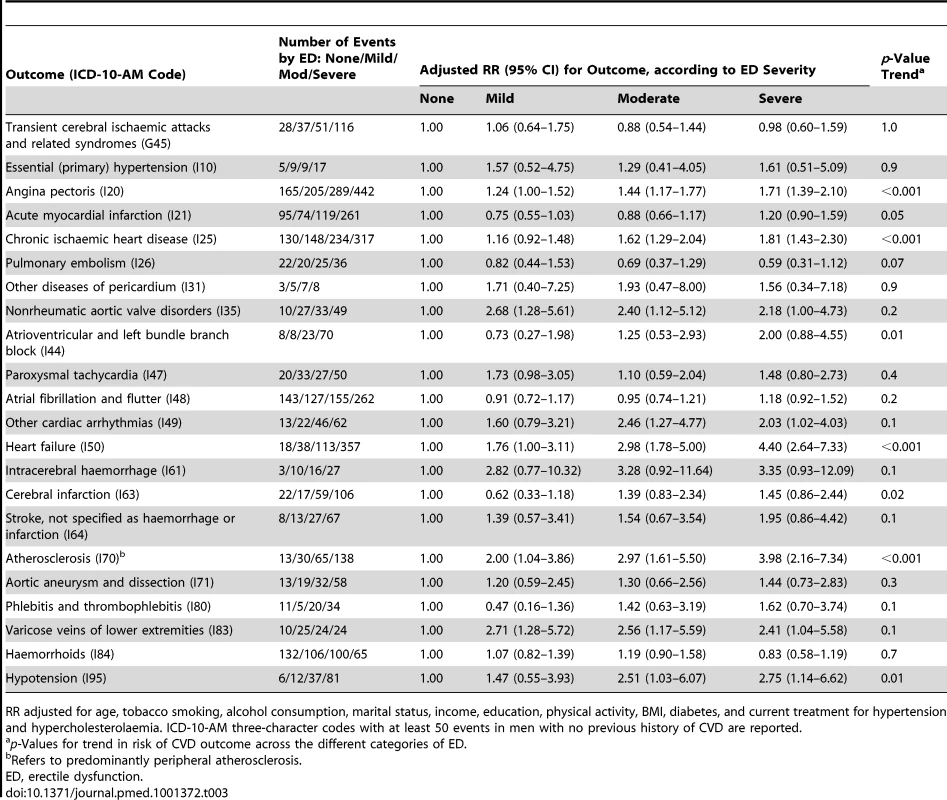
RR adjusted for age, tobacco smoking, alcohol consumption, marital status, income, education, physical activity, BMI, diabetes, and current treatment for hypertension and hypercholesterolaemia. ICD-10-AM three-character codes with at least 50 events in men with no previous history of CVD are reported. When categorised as a dichotomous variable (severe/moderate versus no/mild erectile dysfunction), the relationship of erectile dysfunction to all CVD combined was significantly attenuated in those reporting greater alcohol consumption and in those without diabetes, but did not vary significantly (i.e., p [heterogeneity] was not significant) by age, smoking, education, income, treatment for hypertension, and a range of other personal characteristics (Figure 3). When adjusted only for age, the relationship of erectile dysfunction to all-cause mortality appeared stronger in younger men (Figure 4). However, this difference was not apparent following more comprehensive adjustment for potential confounding factors (p[heterogeneity by age] = 0.2; Figure 4). The association between erectile dysfunction and all-cause mortality did not vary significantly according to any of the factors examined, apart from BMI, where the relationship showed non-systematic variation in the RRs, of borderline statistical significance (p[heterogeneity by BMI] = 0.04; Figure 4).
Fig. 3. Relative risk of any CVD admission since baseline among men with severe/moderate erectile dysfunction (ED) compared to those with mild/no erectile dysfunction (no ED), in a range of population subgroups, among men with no previous CVD. 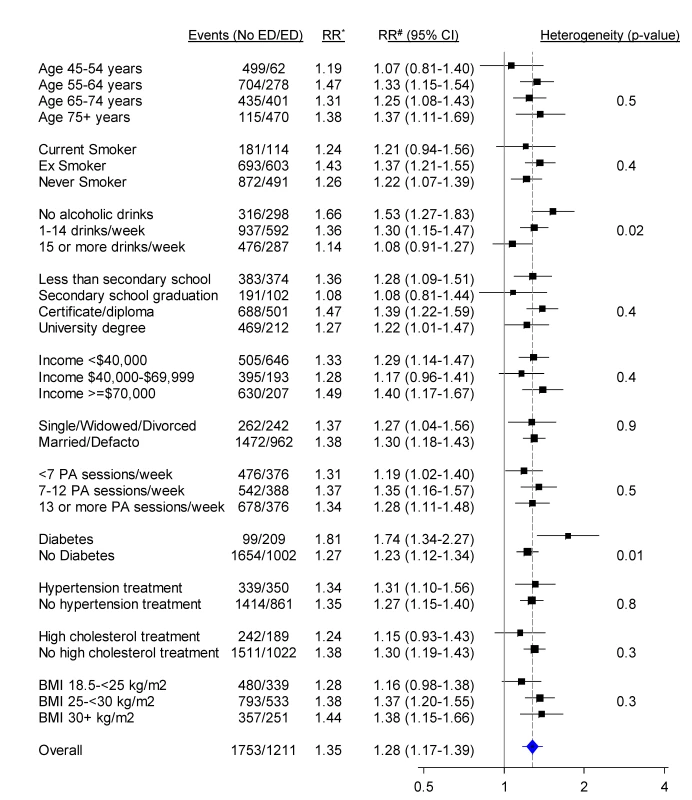
*RR adjusted for age. #RR adjusted for age, tobacco smoking, alcohol consumption, marital status, income, education, physical activity (PA), BMI, diabetes, and current treatment for hypertension and hypercholesterolaemia where appropriate. RRs are represented with squares with areas inversely proportional to the variance of the logarithm of the RR, providing an indication of the amount of statistical information available. 95% CIs are indicated by horizontal lines. The vertical dotted line represents the overall RR of CVD events in men with moderate/severe erectile dysfunction versus no/mild erectile dysfunction. Defacto, living with a partner. Fig. 4. Relative risk of all-cause mortality among men with severe/moderate erectile dysfunction (ED) compared to those with mild/no erectile dysfunction (no ED), in a range of population subgroups, among men with no previous CVD. 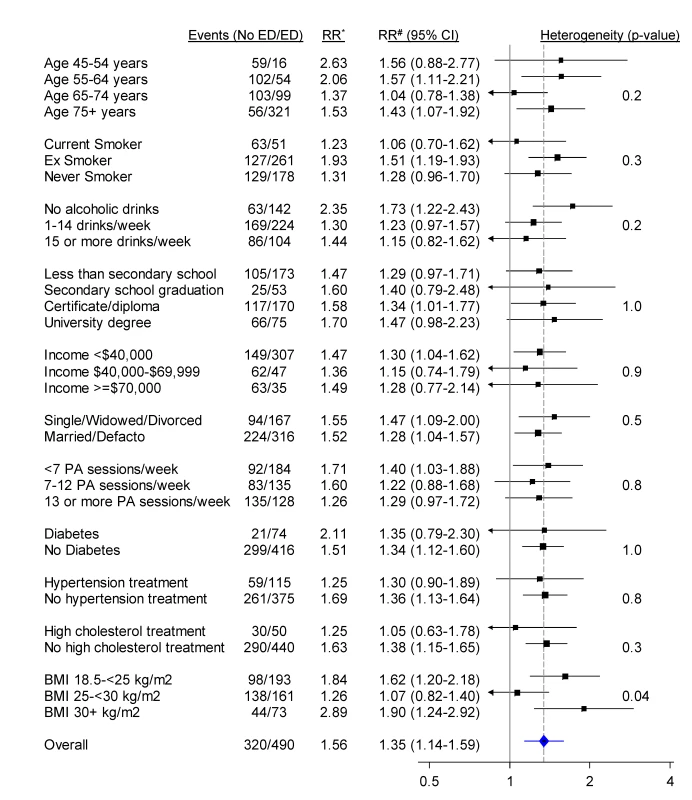
*RR adjusted for age. #RR adjusted for age, tobacco smoking, alcohol consumption, marital status, income, education, physical activity (PA), BMI, diabetes, and current treatment for hypertension and hypercholesterolaemia where appropriate. RRs are represented with squares with areas inversely proportional to the variance of the logarithm of the RR, providing an indication of the amount of statistical information available. 95% CIs are indicated by horizontal lines. The vertical dotted line represents the overall RR of all-cause mortality in men with moderate/severe erectile dysfunction versus no/mild erectile dysfunction. Defacto, living with a partner. Discussion
In this large population-based prospective cohort study, the risk of hospitalisation for CVD increased progressively with the self-reported severity of erectile dysfunction in men without and with known CVD at baseline, and this pattern was present after adjusting for established risk factors for CVD. Compared to men without erectile dysfunction, men with severe erectile dysfunction had RRs of approximately 1.5 to 2.0 for ischaemic heart disease, peripheral vascular disease, combined CVD events, and all-cause mortality, and even greater point estimates for risk (albeit with wide confidence intervals) for conditions such as heart failure and atrioventricular and left bundle branch block.
The current study is an order of magnitude larger than any previous prospective study of erectile dysfunction and CVD known to us, and provides the strongest evidence to date of a relationship of increasing CVD risk with increasing self-reported severity of erectile dysfunction [7],[8],[25]. Our results lend strong support to previous studies, which have found that among men without known CVD at baseline, those with moderate or severe erectile dysfunction have increased risks of a subsequent CVD event [1],[7],[8],[10],[25], including ischaemic heart disease [1],[5],[7],[8],[10], stroke [1],[7],[8],[10], and peripheral vascular disease [6],[10], as well as all-cause mortality [7],[26], compared to men with mild or no erectile dysfunction, based on data from over 30,000 participants in relevant prospective studies (∼15,000 in prospective population-based studies) [7],[8]. Although heart failure is often a consequence of ischaemic heart disease, our finding on the relationship between severity of erectile dysfunction and future admissions for heart failure is, to our knowledge, novel and warrants further investigation, given that this condition is common and results in a considerable burden of disease. Similarly, we were unable to locate any previous prospective studies demonstrating the increased risks of chronic ischaemic heart disease and atrioventricular and left bundle branch block with increasing levels of erectile dysfunction.
Erectile dysfunction per se is unlikely to be a major independent cause of CVD, and is best considered as a risk marker rather than a risk factor for CVD. That is, it is likely to serve as an indicator, or “biomarker”, of the severity of underlying pathological processes such as atherosclerosis and endothelial dysfunction. Hence, the study presented here does not aim to investigate erectile dysfunction as an independent cause of CVD. It quantifies the gradient in CVD risk with increasing degrees of erectile dysfunction because this relationship is likely to inform the potential usefulness of erectile dysfunction as a risk marker in predicting events and in discriminating at what level clinical concerns should be raised. Additional CVD risk factors are adjusted for, where possible, with the aim of demonstrating how well the marker predicts risk above and beyond these additional factors.
Although the pathophysiology of erectile dysfunction is multifactorial and includes arterial, neurogenic, hormonal, cavernosal, iatrogenic, and psychogenic causes [27], it is now widely accepted that erectile dysfunction is predominantly due to underlying vascular causes, particularly atherosclerosis [28]. Endothelial dysfunction is considered a key pathophysiological cause of erectile dysfunction and CVD [28]. The normal function of the corpus cavernosum is highly dependent on intact endothelium-dependent relaxation, which potentially explains why erectile dysfunction may precede other clinical manifestations of systemic atherosclerosis. It has also been hypothesized that progressive occlusive/atherosclerotic disease might manifest itself earlier in smaller blood vessels, such as those in the penis, than in larger blood vessels [29]. The observed relationship of erectile dysfunction to subsequent hospitalisation for atherosclerotic conditions such as angina [1], acute myocardial infarction [1], ischaemic stroke, and peripheral vascular disease, over and above known CVD risk factors, is therefore biologically plausible. A large proportion of deaths in older men are attributable to CVD, so the relationship of erectile dysfunction to CVD is likely to be an important contributor to the relationship of erectile dysfunction to all-cause mortality.
The mechanisms underlying the relationship of erectile dysfunction to hospitalisation for the other CVD diagnoses observed in this study require further exploration. Erectile dysfunction is extremely common in men with heart failure [30], and since a large proportion of heart failure is undiagnosed, particularly at its early stages [31], erectile dysfunction may serve as an early marker of occult heart failure that subsequently manifests itself and necessitates treatment in hospital. Conditions categorised as atrioventricular and left bundle branch block are likely to be multifactorial and include ischaemic heart disease as well as fibrotic degeneration of the cardiac conducting system related to primary causes or secondary to ischaemic heart disease and/or cardiomyopathies. The large sample size permitted a large number of comparisons to be performed, which raises type 1 error as a possible explanation for some relationships identified, but the lack of a clear relationship between erectile dysfunction and certain other CVD diagnoses (e.g., pulmonary embolism) suggests a degree of specificity in its potential to predict disease.
The relationship of severity of erectile dysfunction to the different types of CVD was similar for those with and without a prior history of CVD, indicating that erectile dysfunction remains a risk marker even in those with known CVD. Men with existing CVD should, theoretically, already be receiving maximal secondary preventative therapy, so the clinical utility of markers of increased risk of recurrent events is open to question. Since in reality there are consistent and often large gaps between recommended treatment and actual practice for those with existing CVD [32], markers of increased risk of recurrent events could potentially be useful for improving secondary prevention of CVD. However, the findings reported here for individuals with a prior history of CVD should be interpreted with caution, since a number of treatments for CVD increase the risk of erectile dysfunction.
Using data from the cohort prior to exclusions, 16% of men aged 50–59 y, 34% of men aged 60–69 y, and 60% of men aged ≥70 y reported moderate or severe erectile dysfunction; this compares with 11%, 31%, and 68% affected in the same age groups in a representative population-based telephone survey of Australian men [33]. Hence, although representativeness is not necessary for valid and reliable estimates of RR from within-cohort comparisons [34], the degree of erectile dysfunction within the cohort is broadly similar to that in the general population.
Our study included men aged 45 y and over and, following adjustment for a wide range of potential confounding factors, found that the relationship of erectile dysfunction to CVD and to all-cause mortality did not differ significantly for men of different ages. Two previous studies found stronger erectile dysfunction–CVD relationships in younger than in older men [5],[35], particularly in men under 40 y [35], while another found consistent relationships across age groups [36]. In our study, the increased risk of CVD hospitalisation in men with moderate/severe versus mild/no erectile dysfunction was more pronounced in men with diabetes. Other evidence on this is heterogeneous, with previous studies in men with diabetes suggesting slightly lesser and greater erectile-dysfunction-associated risk of CVD compared to studies including the broader population [7],[10]. The attenuation in the erectile dysfunction–CVD relationship with increasing alcohol consumption could potentially be explained by the fact that heavy drinkers may have erectile dysfunction for reasons other than underlying CVD.
In studies of the relationship between risk factors and disease, short follow-up time is often seen as a limitation, particularly if there are issues with reverse causality, where the presence of disease can influence the risk factor itself. In contrast, where a risk marker is being considered, with the aim of measuring something that relates to the presence and severity of underlying disease, short follow-up time is often desirable, as is the case here. Provided there are sufficient numbers of health events, a short follow-up time allows quantification of the relationship of the risk marker to the immediate risk of disease. In particular, because erectile dysfunction status is likely to change increasingly with time since baseline, longer follow-up might lead to reduced accuracy of prediction.
Several study limitations may potentially influence the interpretation of results in our study. (i) While erectile dysfunction at baseline was measured using a single validated question, the duration of dysfunction was unknown. (ii) Data on potential confounding factors were mostly based on self-report. (iii) Measured data on CVD risk factors such as blood pressure and blood lipids were not available. (iv) Information on use of medications, including CVD medications and those used to treat erectile dysfunction, such as phosphodiesterase type 5 inhibitors, was not available for this study. Use of phosphodiesterase type 5 inhibitors is likely to be relatively uncommon in Australia, with the most relevant survey showing that 5% of men aged 40 y and over reported ever having received medical treatment for erectile dysfunction [33]. Given that users may have been misclassified as having no erectile dysfunction, and that use of phosphodiesterase type 5 inhibitors does not appear to influence the risk of myocardial infarction or cardiovascular death [37], this bias, if it were present, would tend to lead to conservative estimates of RR. (v) Administrative data were used for CVD hospitalisation end points—these are independent and virtually complete for cohort members, with fair to very good validity, but were not confirmed individually by chart review or other methods. (vi) Power was limited for some subtypes of CVD. (vii) Finally, we cannot exclude the possibility that the findings presented here are affected by unmeasured confounding. The fact that a range of disease processes, apart from those related to erectile dysfunction, may lead to a CVD event should also be considered.
The findings of this study highlight the need to consider erectile dysfunction in relation to the risk of a wide range of CVDs that extends beyond ischaemic heart disease and stroke and includes conditions such as heart failure and conduction disorders. They also provide evidence that CVD risk is elevated across a spectrum of severity of erectile dysfunction and that men with mild or moderate erectile dysfunction should be considered at increased risk, in addition to those with severe disease. Nevertheless, this does not translate automatically into utility as part of a clinical risk score, such as using erectile dysfunction in addition to the Framingham score [36]. Rather, the findings provide general support for the Princeton consensus [29] that men with erectile dysfunction require assessment for CVD risk, while the quantitative ability of erectile dysfunction to predict risk in the clinical setting, over and above clinically measured risk factors, requires specific testing.
Supporting Information
Zdroje
1. ThompsonIM, TangenCM, GoodmanPJ, ProbstfieldJL, MoinpourCM, et al. (2005) Erectile dysfunction and subsequent cardiovascular disease. JAMA 294 : 2996–3002.
2. KingA (2010) Erectile dysfunction and CVD. Nat Rev Cardiol 7 : 241.
3. World Health Organization (2011) Global status report on noncommunicable diseases 2010. Geneva: World Health Organization.
4. HelfandM, BuckleyD, FreemanM, FuR, RogersK, et al. (2009) Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Int Med 151 : 496–507.
5. InmanBA, SauverJLS, JacobsonDJ, McGreeME, NehraA, et al. (2009) A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clinic Proc 84 : 108–113.
6. BlumentalsWA, Gomez-CamineroA, JooS, VannappagariV (2003) Is erectile dysfunction predictive of peripheral vascular disease? Aging Male 6 : 217–221.
7. DongJY, ZhangYH, QinLQ (2011) Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol 58 : 1378–1385.
8. GuoW, LiaoC, ZouY, LiF, LiT, et al. (2010) Erectile dysfunction and risk of clinical cardiovascular events: a meta-analysis of seven cohort studies. J Sex Med 7 : 2805–2816.
9. HotalingJM, WalshTJ, MacleodLC, HeckbertSR, PocobelliG, et al. (2012) Erectile dysfunction is not independently associated with cardiovascular death: data from the Vitamins and Lifestyle (VITAL) Study. J Sex Med 9 : 2104–2110.
10. BattyG, LiQ, CzernichowS, NealB, ZoungasS, et al. (2010) Erectile dysfunction and later cardiovascular disease in men with type 2 diabetes: prospective cohort study based on the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation) trial. J Am Coll Cardiol 56 : 1908–1913.
11. BanksE, RedmanS, JormL, ArmstrongB, BaumanA, et al. (2008) Cohort profile: the 45 and up study. Int J Epidemiol 37 : 941–947.
12. National Centre for Classification in Health (2006) International statistical classification of diseases and related health problems, 10th revision, Australian modification (ICD-10-AM)—fifth edition. Sydney: National Centre for Classification in Health.
13. National Centre for Classification in Health (2007) The Australian Classification of Health Interventions (ACHI), 6th edition. Tabular list of interventions and alphabetic index of interventions. Sydney: National Centre for Classification in Health.
14. QuanH, ParsonsGA, GhaliWA (2004) Assessing accuracy of diagnosis-type indicators for flagging complications in administrative data. J Clin Epidemiol 57 : 366–372.
15. BoyleCA, DobsonAJ (1995) The accuracy of hospital records and death certificates for acute myocardial infarction. Aust N Z J Med 25 : 316–323.
16. TirschwellDL, LongstrethWTJr (2002) Validating administrative data in stroke research. Stroke 33 : 2465–2470.
17. TengTH, FinnJ, HungJ, GeelhoedE, HobbsM (2008) A validation study: how effective is the Hospital Morbidity Data as a surveillance tool for heart failure in Western Australia? Aust N Z J Public Health 32 : 405–407.
18. RuigomezA, Martin-MerinoE, RodriguezLA (2010) Validation of ischemic cerebrovascular diagnoses in the health improvement network (THIN). Pharmacoepidemiol Drug Saf 19 : 579–585.
19. DerbyCA, AraujoAB, JohannesCB, FeldmanHA, McKinlayJB (2000) Measurement of erectile dysfunction in population-based studies: the use of a single question self-assessment in the Massachusetts Male Aging Study. Int J Impot Res 12 : 197–204.
20. Population Health Division (2010) The health of the people of New South Wales: summary report—report of the Chief Health Officer. Sydney: New South Wales Department of Health. Available: http://www0.health.nsw.gov.au/pubs/2010/pdf/chorep_summary_2010.pdfwww.health.nsw.gov.au/publichealth/chorep/. Accessed (17 Dec 2012).
21. FayMP, FeuerEJ (1997) Confidence intervals for directly adjusted rates: a method based on the gamma distribution. Stat Med 16 : 791–801.
22. Australian Institute of Health and Welfare (2003) The Active Australia Survey: a guide and manual for implementation, analysis and reporting. Canberra: Australian Institute of Health and Welfare.
23. NgSP, KordaR, ClementsM, LatzI, BaumanA, et al. (2011) Validity of self-reported height and weight and derived body mass index in middle-aged and elderly individuals in Australia. Aust N Z J Public Health 35 : 557–563.
24. SAS Institute (2011) SAS version 9.3 [computer program]. Cary (North Carolina): SAS Institute.
25. SchoutenBWV, BohnenAM, BoschJLHR, BernsenRMD, DeckersJW, et al. (2008) Erectile dysfunction prospectively associated with cardiovascular disease in the Dutch general population: results from the Krimpen Study. Int J Impot Res 20 : 92–99.
26. AraujoAB, TravisonTG, GanzP, ChiuGR, KupelianV, et al. (2009) Erectile dysfunction and mortality. J Sex Med 6 : 2445–2454.
27. LueTF (2000) Erectile dysfunction. N Engl J Med 342 : 1802–1813.
28. SolomonH, ManJ, JacksonG (2003) Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart 89 : 251–253.
29. JacksonG, RosenRC, KlonerRA, KostisJB (2006) The second Princeton consensus on sexual dysfunction and cardiac risk: new guidelines for sexual medicine. J Sex Med 3 : 28–36.
30. HerbertK, LopezB, CastellanosJ, PalacioA, TamarizL, et al. (2008) The prevalence of erectile dysfunction in heart failure patients by race and ethnicity. Int J Impot Res 20 : 507–511.
31. AbhayaratnaW, SmithW, BeckerN, MarwickT, JeffreyI, et al. (2006) Prevalence of heart failure and systolic ventricular dysfunction in older Australians: the Canberra Heart Study. Med J Aust 184 : 151–154.
32. HuangN, DaddoM, CluneE (2009) Heart health—CHD management gaps in general practice. Aust Fam Physician 38 : 241–245.
33. HoldenC, McLachlanR, PittsM, CummingRG, WittertG, et al. (2005) Men in Australia Telephone Survey (MATeS): a national survey of the reproductive health and concerns of middle-aged and older Australian men. Lancet 366 : 218–224.
34. MealingN, BanksE, JormL, SteelD, ClementsM, et al. (2010) Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol 10 : 26.
35. ChewKK, FinnJ, StuckeyB, GibsonN, SanfilippoF, et al. (2010) Erectile dysfunction as a predictor for subsequent atherosclerotic cardiovascular events: findings from a linked-data study. J Sex Med 7 : 192–202.
36. AraujoAB, HallSA, GanzP, ChiuGR, RosenRC, et al. (2010) Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham risk score? J Am Coll Cardiol 55 : 350–356.
37. MittlemanMA, GlasserDB, OrazemJ (2003) Clinical trials of sildenafil citrate (Viagra) demonstrate no increase in risk of myocardial infarction and cardiovascular death compared with placebo. Int J Clin Pract 57 : 597–600.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- The Effectiveness of Mobile-Health Technologies to Improve Health Care Service Delivery Processes: A Systematic Review and Meta-Analysis
- The Effectiveness of Mobile-Health Technology-Based Health Behaviour Change or Disease Management Interventions for Health Care Consumers: A Systematic Review
- More Data and Appropriate Statistical Methods Needed to Fully Measure the Displacement Effects of Development Assistance for Health
- Preventing Antiretroviral Treatment Interruptions among HIV/AIDS Patients in Africa
- Translating Coverage Gains into Health Gains for All Women and Children: The Quality Care Opportunity
- Implementation of a Mental Health Care Package for Children in Areas of Armed Conflict: A Case Study from Burundi, Indonesia, Nepal, Sri Lanka, and Sudan
- Toward an Understanding of Disengagement from HIV Treatment and Care in Sub-Saharan Africa: A Qualitative Study
- Tackling Non-Communicable Diseases In Low- and Middle-Income Countries: Is the Evidence from High-Income Countries All We Need?
- Change in the Body Mass Index Distribution for Women: Analysis of Surveys from 37 Low- and Middle-Income Countries
- Getting More Generous with the Truth: Clinical Trial Reporting in 2013 and Beyond
- Reducing Vulnerability to Climate Change in Sub-Saharan Africa: The Need for Better Evidence
- Erectile Dysfunction Severity as a Risk Marker for Cardiovascular Disease Hospitalisation and All-Cause Mortality: A Prospective Cohort Study
- Differences in Reporting of Analyses in Internal Company Documents Versus Published Trial Reports: Comparisons in Industry-Sponsored Trials in Off-Label Uses of Gabapentin
- Why Do Women Not Use Antenatal Services in Low- and Middle-Income Countries? A Meta-Synthesis of Qualitative Studies
- Considerations in Assessing the Evidence and Implications of Aid Displacement from the Health Sector
- Prioritizing Congenital Syphilis Control in South China: A Decision Analytic Model to Inform Policy Implementation
- Balancing Community and Hospital Care: A Case Study of Reforming Mental Health Services in Georgia
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Differences in Reporting of Analyses in Internal Company Documents Versus Published Trial Reports: Comparisons in Industry-Sponsored Trials in Off-Label Uses of Gabapentin
- The Effectiveness of Mobile-Health Technologies to Improve Health Care Service Delivery Processes: A Systematic Review and Meta-Analysis
- The Effectiveness of Mobile-Health Technology-Based Health Behaviour Change or Disease Management Interventions for Health Care Consumers: A Systematic Review
- Why Do Women Not Use Antenatal Services in Low- and Middle-Income Countries? A Meta-Synthesis of Qualitative Studies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



