-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPoint-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries
article has not abstract
Published in the journal: . PLoS Med 9(9): e32767. doi:10.1371/journal.pmed.1001306
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001306Summary
article has not abstract
Summary Points
-
Enthusiasm and hope are increasing around point-of-care (POC) diagnostics for diseases of global health importance.
-
The mere availability of rapid or simple tests does not automatically ensure their adoption or scale-up. A range of barriers prevent the successful use of POC testing—economic, regulatory, and policy-related, as well as user/provider perceptions and cultural barriers.
-
Technology as such does not define a POC test. Rather, it is the successful use at the POC that defines a diagnostic process as POC testing. Thus, the focus must be on POC testing programs, rather than POC technologies.
-
We discuss a framework that envisions POC testing as a spectrum of technologies (simplest to more sophisticated), users (lay persons to highly trained), and settings (homes, communities, clinics, peripheral laboratories, and hospitals).
-
A deeper appreciation of this diversity in target product profiles, and likely barriers in each setting, might help test developers and public health managers to identify the most impactful product and delivery model.
The Promise of Point-of-Care Testing
Point-of-care (POC) tests have the potential to improve the management of infectious diseases, especially in resource-limited settings where health care infrastructure is weak, and access to quality and timely medical care is a challenge [1],[2]. These tests offer rapid results, allowing for timely initiation of appropriate therapy, and/or facilitation of linkages to care and referral. Most importantly, POC tests can be simple enough to be used at the primary care level and in remote settings with no laboratory infrastructure. POC tests can potentially empower patients to self-test in the privacy of their homes, especially for stigmatized diseases such as HIV [3]. In fact, home-based, over-the-counter HIV testing was approved in July 2012 by the Food and Drug Administration in the United States [4].
Several agencies, notably, the Bill & Melinda Gates Foundation and Grand Challenges Canada, have recently announced grants for the development of new POC diagnostics for global health. Several million dollars are being invested in this area, and substantial enthusiasm and hope are increasing around POC diagnostics. Efforts are also underway to engage diagnostic and biotech companies in emerging economies such as India and China in developing new and affordable diagnostics for TB and HIV [5]. Furthermore, donors such as UNITAID clearly value the importance of good diagnostics and are actively supporting projects on TB, HIV, and malaria diagnostics [6].
In this context, various stakeholders need deeper insights into the challenges for use and scale-up of POC testing, and a framework for thinking about the diversity of product profiles involved in POC testing, including where and how POC testing can be implemented successfully, what barriers need to be overcome, and what characteristics are necessary for impact.
Diversity of Definitions and Target Product Profiles within POC Testing
According to one textbook, point-of-care testing (POCT) can be defined as the “provision of a test when the result will be used to make a decision and to take appropriate action, which will lead to an improved health outcome” [7]. Another definition is: “patient specimens assayed at or near the patient with the assumption that test results will be available instantly or in a very short timeframe to assist caregivers with immediate diagnosis and/or clinical intervention” [8]. However, there are dozens of definitions of POCT and no accepted universal definition [9]. Regardless of the exact definition, we believe that the most critical elements of POCT are rapid turn-around and communication of results to guide clinical decisions and completion of testing and follow-up action in the same clinical encounter [10]–[12].
Rapid turn-around of results is critical for the test results to impact clinical management (e.g., triage, referral, treatment decisions, decision to discharge, etc.). Indeed, without a clear link to a treatment or counseling plan, test results, even if rapid, are unlikely to have an impact [13]. “Rapid” can range from within seconds, to minutes, to a few hours (“while the patient waits”). At the least, results “on the same day” can still help disposition of clients with a clear plan (e.g., initiation of anti-tuberculosis or anti-retroviral therapy). Convenience to patients and care providers mainly derives from the fact that the POC diagnostic process is completed “in the same clinical encounter,” as compared to conventional testing where clients/patients may not come back for testing or go far away (or wait long) for testing. One of the biggest concerns about conventional laboratory-based testing is the long turn-around times and delays, and the resultant loss of patients from the testing and treatment pathway. This is, for example, a well-recognized concern with conventional sputum smear microscopy for tuberculosis (TB) [14], and laboratory-based CD4 and viral load testing for HIV [15].
We suggest that the technology as such does not define a POC test nor determine its use at the POC. Rather, it is the successful use at the POC that defines a diagnostic process as POC testing. So, it may be best to think of POC testing programs, rather than POC tests. It is how the tests are deployed or implemented in a health system that defines a POC testing program. For example, one could implement a rapid diagnostic test (RDT) or dipstick in a reference laboratory, and that will not be a POCT program. Indeed, laboratories in resource-limited countries often use RDTs, but results are often delivered after days. On the other hand, one could implement a molecular test in an out-patient clinic and successfully allow POC usage. The Xpert MTB/RIF test based on GeneXpert technology (Cepheid Inc) is one such technology that can potentially be implemented in TB clinic settings and peripheral laboratories [12].
Thus, systems for rapid reporting of test results to care providers, and a mechanism to link test results to appropriate counseling and treatment are as important as the technology itself. If systems for reporting the results and follow-up care are not in place, then POC testing is unlikely to have an impact on clinical or public health outcomes [13]. Also, POCT programs require viable business models to ensure that they are sustainable in the real world and will actually get used. This means POC testing must fit within real-world workflow patterns and economic/incentive structures.
It is widely believed that POC tests should be equipment free, simple RDTs (that is, those that meet the “ASSURED” criteria: affordable, sensitive, specific, user friendly, rapid and robust, equipment-free, and delivered [2]) and always be done outside of laboratories and hospitals by non-laboratorians. Such criteria were probably necessary when RDTs were introduced, but in today's context, they impose artificial restrictions on the concept of POCT. POC testing done at the point of clinical contact is preferable but not required, so long as a system is in place for rapid reporting of results that can inform clinical decisions. For example, testing at a peripheral laboratory attached to (or near) a clinic or hospital can still allow for POCT. Such POC testing can be done by hospital staff in emergency rooms, operating rooms, intensive care units, and labour wards, without waiting for results to come from laboratories, and this means POCT can also occur in hospital settings [16]. Indeed, there are successful examples of POC testing by non-laboratorians in hospital-based settings such as emergency rooms (e.g., rapid influenza testing [17]) and labour wards (e.g., rapid HIV testing to reduce mother-to-child transmission [18]).
Also, restricting POCT to really cheap and equipment-free tests (e.g., RDTs—also called “first-generation POC tests”) imposes barriers for use of newer technologies such as cartridge-based POC nucleic acid amplification tests (NAATs; “second-generation POC tests”) and hand-held devices such as mobile phone-based technologies (“third-generation POCTs”) [19]. These newer POCTs may not be as cheap and equipment free as RDTs and dipsticks, but may prove to be very impactful and cost-effective in the right context [20].
Thus, we propose that it is easier to think of POC testing as a spectrum of technologies (simplest to more sophisticated), users (lay persons to highly trained), and settings (homes to hospitals). This diversity of target product profiles (TPPs) within POCT is illustrated in Figure 1. This framework shows that POCT can be done in at least five distinct settings: homes (TPP1), communities (TPP2), clinics (TPP3), peripheral laboratories (TPP4), and hospitals (TPP5). Unique barriers may operate at each level, and prevent the adoption and use of POCTs. As shown in the schematic, there are several examples of POC tests in each of these settings. The relative importance of these settings may vary by country, and also within a country, where there may be important differences in public versus private sectors, and rural versus urban areas.
Fig. 1. Diversity of target product profiles, users, and settings within the spectrum of POC testing. 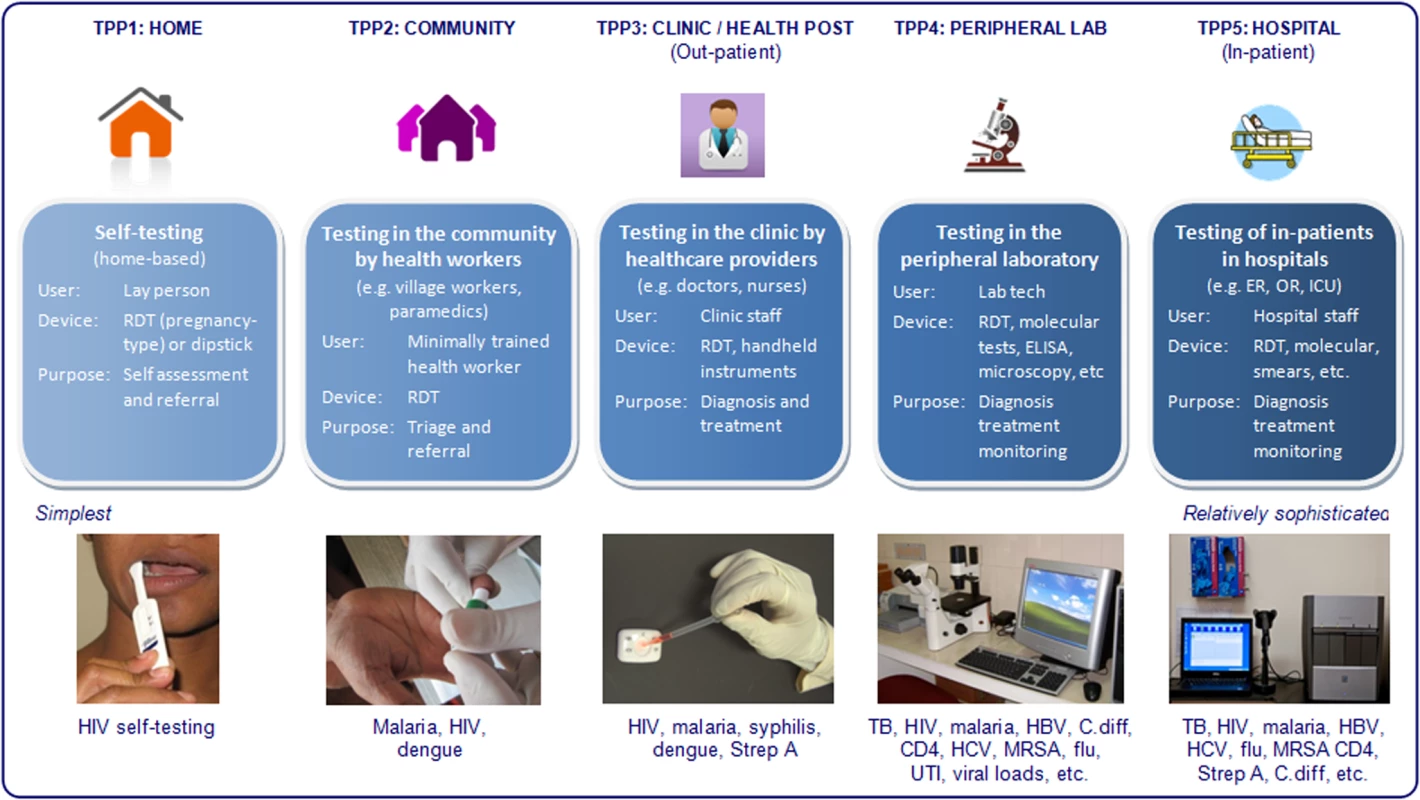
HBV, hepatitis B virus; HCV, hepatitis C virus; UTI, urinary tract infection; MRSA, methicillin-resistant staphylococcus aureus; C. diff, clostridium difficile; RDT, rapid diagnostic test; Strep A, group A streptococcus. In the framework that we propose (Figure 1), the type of device does not define a POC test. As mentioned, POC tests can range from the simplest dipsticks to sophisticated automated molecular tests, portable analysers, and imaging systems. The same lateral flow assay, for example, could be used across all TPPs. Hence, the device does not automatically define the TPP, although some types of devices will immediately rule out some TPPs or users. For example, conventional ELISA cannot be performed by lower level health workers or even doctors. Microscopy is another technology that requires a trained user and quality assurance mechanism—this restricts the technology to laboratories and hospitals. In general, even the simplest molecular tests require basic infrastructure such as power supply and temperature control, and are therefore unlikely to be used in TPP1–TPP3 in resource-limited countries.
Also, the end-user of the test does not automatically define a POC test. The same device (e.g., lateral flow assay), can be performed by several users across the TPPs—from untrained (lay) people, to community health workers, to nurses, to doctors, and laboratory technicians. Rapid oral-fluid–based HIV tests are a good example of such a test [9], because it now spans the entire spectrum—from in-home testing to hospital-based testing. So, the actual user does not immediately identify the TPP, although targeting the end-user helps narrow down the type of TPP needed (e.g., lay person or lower level health worker necessarily means the simplest type of device and the most robust design). For example, in-home testing for HIV demands the simplest type of device, along the lines of a pregnancy test, and may also require telephonic counseling and support services [4],[21],[22].
Depending on the end-user and the actual setting, the purpose of POC testing may vary—from triage and referral, to diagnosis, treatment, and monitoring. Some POC test users may not be empowered to prescribe medicines, while others can use POCT results for treatment. This has implications for test developers. A test that is intended for triage and referral can have different accuracy (e.g., lower specificity), compared to a test that is used to make treatment decisions [23].
The Need to Understand Barriers for POC Testing
The best POCT will not have any impact unless it is widely used and followed-up with appropriate treatment interventions [24]. The mere availability of rapid or simple tests does not automatically ensure their adoption or scale-up [24]. This observation is evident from the global experience with rapid HIV tests and malaria RDTs [5],[25]–[28]. In India, for example, simple RDTs are available for a variety of diseases (e.g., HIV, malaria, dengue, syphilis, hepatitis), are quite inexpensive (US$1 per test or less), and some meet all the ASSURED criteria. However, these RDTs are often not used in community or clinic settings to make clinical decisions (with the possible exception of pregnancy tests and possibly HIV and malaria RDTs). It appears that very little POCT occurs in homes, communities, or clinics (TPP1–TPP3). Testing predominantly takes place in laboratories and hospitals (TPP4 and TPP5). In fact, small, stand-alone laboratories are the biggest consumers of RDTs.
What are the most important barriers to widespread use of POCT at lower levels of the health care delivery system, where we hope POC testing will reduce diagnostic delays and interrupt transmission? On the basis of our observations and the published literature [2],[11],[26]–[30], we believe there are a variety of barriers to successful use of POCT—from economic, regulatory, and policy-related barriers to user/provider perceptions and cultural barriers. Table 1 provides illustrative examples of these barriers. Some barriers are generic, while others are restricted to a specific TPP or setting. Barriers for POC testing may depend on the country, and may also differ across public versus private, and urban versus rural settings. In addition, some barriers may be disease-specific, while others will apply to all types of tests. For example, stigma and confidentiality may be important barriers for HIV testing [26],[28],[31], while they may be less relevant with malaria or dengue testing.
Tab. 1. Barriers to adoption and scale-up of POC technologies. 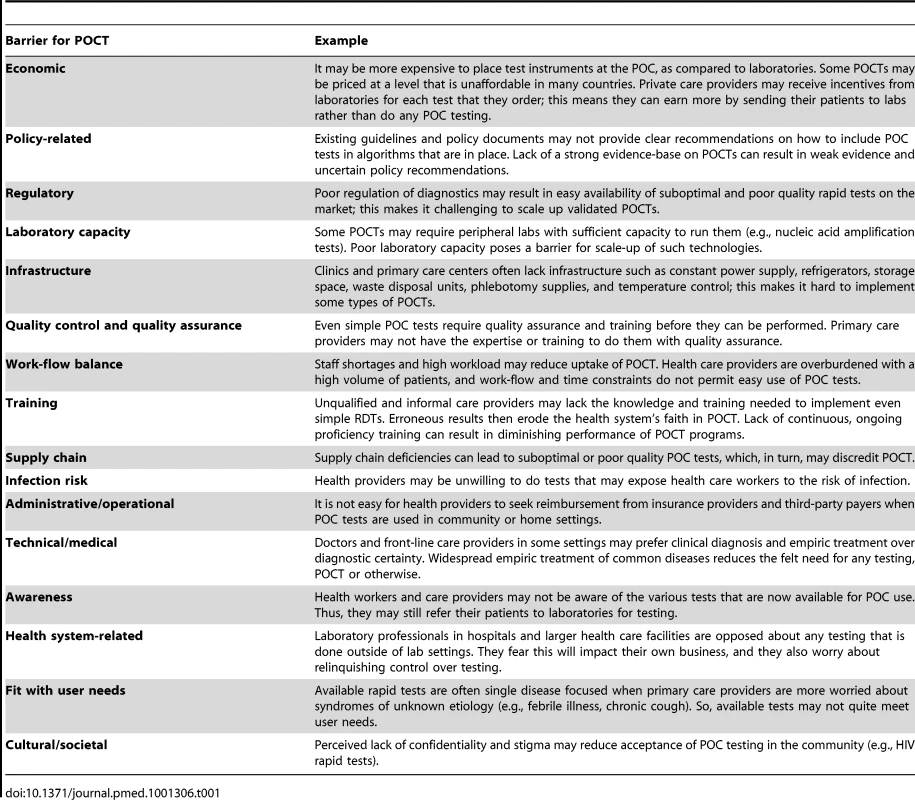
Why Do We Need to Understand the Diagnostic Ecosystem in Countries?
It is particularly important to look beyond the technology, and understand current diagnostic practices and the health systems within which POC testing has to get scaled up. At the country level, POC diagnostics ultimately need to be integrated within health systems, supported by financing (who will pay and how much?), incentives (do various stakeholders benefit from the economics?), training and information and communications technology (ICT). These other factors (“the business model”) may be as important as the POCT itself and need to be taken into account when developing tests [32].
Indeed, the best POCT without a good business model is unlikely to get scaled up, while, paradoxically, inaccurate tests can become popular because of economic reasons, as illustrated by the apparent market success of inaccurate TB serological tests in many developing countries [33],[34]. In India, we have shown that although serological TB tests are inaccurate, various players along the value chain profit from their use, and this sustains a market for these tests [34].
ICT, when combined with POC, can help expand care to lower tiers of the health care delivery system, all the way to home-based, self-testing [30]. Thus, the rapid expansion of mobile telephony makes telephonic counseling and rapid reporting of results (to patients as well as to public health programs) feasible in many settings. Diagnostic devices linked with mobile phones can also allow for automatic data capture, external quality assurance, and proficiency testing. Smart phones can also provide decision support to health workers on what follow-up action is necessary after testing. Indeed, new TPPs and business models are now feasible, thanks to the rapid expansion of ICT. Interestingly, the mobile phone itself is becoming a POC testing device, and this is a vibrant area for incentive prizes [35].
In India, the diagnostic ecosystem is worrisome with systematic market failures throughout the value chain for diagnostics—private doctors receiving payments or incentives for tests ordered, over-reliance on useless tests, and under-use of good diagnostics [33],[34],[36]–[38]. There is little quality assurance for laboratories in India and private labs offer tests of doubtful value. Laboratories in the public health sector suffer from poor infrastructure and limited capacity, while dealing with massive volumes. The regulatory framework for in vitro diagnostics in India is weak and most diagnostics do not undergo rigorous validation before approval [34]. As a result a large number of inaccurate and ineffective products can be found on the market; many of these are imported, but not approved by the US Food and Drug Administration (FDA) or other such credible regulatory bodies outside of India [34]. In fact, the Government of India has recently banned TB serological antibody tests, which are widely used in the private sector [34].
It is within this context that we need to understand the barriers for use of POC diagnostics in India. Based on our work in India on TB and HIV diagnostics [3],[18],[29],[33],[34],[36],[37],[39]–[43], we have identified several potential barriers to implementation of POC tests by primary care providers (Table 2). Additional barriers may operate at the community level in India. Much of the community-level health care in India is done by village health nurses, auxiliary nurse midwives, and Accredited Social Health Activist workers, under the National Rural Health Mission. These workers are generally not well trained or empowered to adequately use POC tests or prescribe drugs on the basis of test results (with some exceptions). In fact, the medical lobby in India vigorously prevents any move to empower health workers to prescribe drugs. Furthermore, these community health workers are heavily burdened with paperwork associated with maternal and child health-related programs and often do not have the time to conduct any testing. Furthermore, the incidence of many diseases is quite low at the community level, and this might reduce the motivation and resources for community-based testing.
Tab. 2. Barriers for use of point-of-care tests in India. 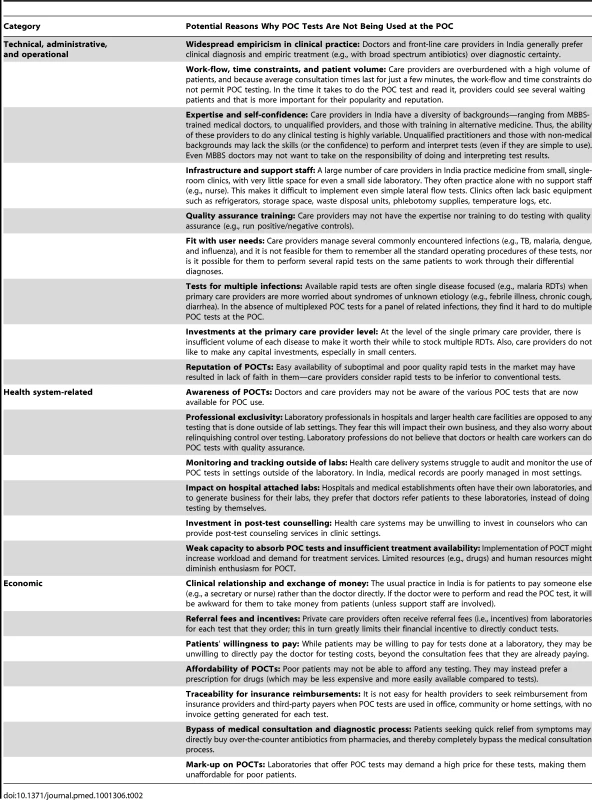
In South Africa, discussions with TB/HIV experts suggest that much of the POC testing currently occurs in laboratories and hospitals (TPP4 and TPP5). For example, the scale-up of Xpert MTB/RIF in South Africa is happening via the National Health Laboratory Service (NHLS) network of laboratories. At the clinic level (TPP3), tests for infections like HIV, syphilis, and malaria are widely used. At the community level (TPP2), HIV rapid tests are the most widely used POC tests. HIV self-testing is known to happen at home in South Africa (TPP1), especially among health care workers who avoid conventional voluntary counseling and testing (VCT) to protect their confidentiality.
South African experts identified several potential barriers for POCT in their setting. For example, laboratory professions are concerned about widespread use of POC tests for many reasons: (1) NHLS cannot control or provide oversight to any testing that is done outside of the NHLS lab network; (2) it is not clear which agency will accept ownership of a POC testing program in South Africa—who will conduct training, quality assurance, maintenance; (3) it is unclear who will provide overall management of a decentralized POC testing program at the level of communities and clinics. Cost can also be a major barrier for POCT—a recent study from South Africa suggests that placing the Xpert MTB/RIF test at the POC will be substantially more expensive than placing the instruments in the NHLS laboratories [44].
Overcoming Barriers to POCT Programs
POC testing holds a lot of promise, but it is important to understand the complexity and diversity of POCT, and identify the biggest barriers to successful implementation of POCT programs. This step is critical for development and scale-up of POCTs, because it will allow test developers and public health programs to target the TPP that is most likely to succeed and has the most impact.
The framework we have proposed may have utility in shaping many of the ongoing efforts to develop and deploy POC tests for global health. Firstly, test developers and manufacturers need to understand the real-world context (e.g., conditions, settings, users, resources) within which tests need to get scaled up. Only then can TPPs by test developers match the TPPs required by public health programs. Indeed, technologies may need to be designed in resource-limited settings (“frugal or reverse innovation”), from the ground up, to ensure that they are robust, field-tested in a variety of conditions, have built-in capacity for reporting/notification, and appropriately priced. Secondly, donors and funding agencies must consider the downstream implications of the health technologies that they are funding, and ensure that product development initiatives are simultaneously coordinated with pricing and delivery mechanisms, supported by innovative business models for scale-up. Lastly, health care managers must invest in POCT programs, rather than merely purchase rapid tests, and ensure the mechanisms are put in place for quality assurance, reporting of results, notification of cases, and initiation of action on the results of the tests. Only then will the true benefits of POC testing be realized.
Zdroje
1. YagerP, DomingoGJ, GerdesJ (2008) Point-of-care diagnostics for global health. Annu Rev Biomed Eng 10 : 107–144.
2. PeelingRW, MabeyD (2010) Point-of-care tests for diagnosing infections in the developing world. Clin Microbiol Infect 16 : 1062–1069.
3. PaiNP, KleinMB (2008) Are we ready for home-based, self-testing for HIV? Future HIV Therapy 2 : 515–520.
4. US Food and Drug Administration (2012) FDA approves first over-the-counter home-use rapid HIV test. FDA news release, 3 July 2012. Available: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm310542.htm. Accessed 5 August 2012.
5. PalamountainKM, BakerJ, CowanEP, EssajeeS, MazzolaLT, et al. (2012) Perspectives on introduction and implementation of new point-of-care diagnostic tests. J Infect Dis 205 Suppl 2: S181–S190.
6. UNITAID (2011) UNITAID Annual Report 2011. UNITAID, Geneva. Available: http://www.unitaid.eu/images/Annual_Report_2011/UNITAID_AR2011_EN.pdf. Accessed 5 August 2012.
7. Price CP, St. John A, Hicks JM (2004) Point-of-care testing. 2nd edition. Washington (D.C.): American Association for Clinical Chemistry.
8. EhrmeyerSS, LaessigRH (2007) Point-of-care testing, medical error, and patient safety: a 2007 assessment. Clin Chem Lab Med 45 : 766–773.
9. PaiNP, PaiM (2012) Point-of-care diagnostics for HIV and tuberculosis: landscape, pipeline, and unmet needs. Discov Med 13 : 35–45.
10. PriceCP (2001) Point of care testing. BMJ 322 : 1285–1288.
11. BissonnetteL, BergeronMG (2010) Diagnosing infections–current and anticipated technologies for point-of-care diagnostics and home-based testing. Clin Microbiol Infect 16 : 1044–1053.
12. BoehmeCC, NicolMP, NabetaP, MichaelJS, GotuzzoE, et al. (2011) Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377 : 1495–1505.
13. The PLoS Medicine Editors (2011) Speed and convenience aren't everything with diagnostics. PLoS Med 8: e1001113 doi:10.1371/journal.pmed.1001113.
14. SquireSB, BelayeAK, KashotiA, SalaniponiFM, MundyCJ, et al. (2005) ‘Lost’ smear-positive pulmonary tuberculosis cases: where are they and why did we lose them? Int J Tuberc Lung Dis 9 : 25–31.
15. BassettIV, WangB, ChettyS, MazibukoM, BearnotB, et al. (2009) Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr 51 : 135–139.
16. Cohen-BacrieS, NinoveL, NougairedeA, CharrelR, RichetH, et al. (2011) Revolutionizing clinical microbiology laboratory organization in hospitals with in situ point-of-care. PLoS One 6: e22403 doi:10.1371/journal.pone.0022403.
17. ChartrandC, LeeflangMM, MinionJ, BrewerT, PaiM (2012) Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med 156 : 500–511.
18. PaiNP, BarickR, TulskyJP, ShivkumarPV, CohanD, et al. (2008) Impact of round-the-clock, rapid oral fluid HIV testing of women in labor in rural India. PLoS Med 5: e92 doi:10.1371/journal.pmed.0050092.
19. NiemzA, FergusonTM, BoyleDS (2011) Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol 29 : 240–250.
20. VassallA, van KampenS, SohnH, MichaelJS, JohnKR, et al. (2011) Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med 8: e1001120 doi:10.1371/journal.pmed.1001120.
21. PaiM, MinionJ, SteingartK, RamsayA (2010) New and improved tuberculosis diagnostics: evidence, policy, practice, and impact. Curr Opin Pulm Med 16 : 271–284.
22. ChokoAT, DesmondN, WebbEL, ChavulaK, Napierala-MavedzengeS, et al. (2011) The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med 8: e1001102 doi:10.1371/journal.pmed.1001102.
23. GiftTL, PateMS, HookEW3rd, KasslerWJ (1999) The rapid test paradox: when fewer cases detected lead to more cases treated: a decision analysis of tests for Chlamydia trachomatis. Sex Transm Dis 26 : 232–240.
24. CobelensF, van den HofS, PaiM, SquireSB, RamsayA, et al. (2012) Which new diagnostics for tuberculosis, and when? J Infect Dis 205 (Suppl 2) S191–S198.
25. SchitoML, PeterTF, CavanaughS, PiatekAS, YoungGJ, et al. (2012) Opportunities and challenges for cost-efficient implementation of new point-of-care diagnostics for HIV and tuberculosis. J Infect Dis 205 Suppl 2: S169–S180.
26. DeblondeJ, De KokerP, HamersFF, FontaineJ, LuchtersS, et al. (2010) Barriers to HIV testing in Europe: a systematic review. Eur J Public Health 20 : 422–432.
27. AsiimweC, KyabayinzeDJ, KyalisiimaZ, NabakoozaJ, BajabaiteM, et al. (2012) Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators. Implement Sci 7 : 5.
28. HeunisJC, WoutersE, NortonWE, EngelbrechtMC, KigoziNG, et al. (2011) Patient - and delivery-level factors related to acceptance of HIV counseling and testing services among tuberculosis patients in South Africa: a qualitative study with community health workers and program managers. Implement Sci 6 : 27.
29. PaiNP, JoshiR, MoodieEE, TaksandeB, KalantriSP, et al. (2009) Profile of adults seeking voluntary HIV testing and counseling in rural Central India: results from a hospital-based study. AIDS Care 21 : 294–300.
30. PriceCP (2011) Point-of-care testing. Care closer to home. Point of Care 10 : 88–92.
31. MacPhersonP, WebbEL, ChokoAT, DesmondN, ChavulaK, et al. (2011) Stigmatising attitudes among people offered home-based HIV testing and counselling in Blantyre, Malawi: construction and analysis of a stigma scale. PLoS One 6: e26814 doi:10.1371/journal.pone.0026814. doi:10.1371/journal.pone.0026814.
32. BalochNA, PaiM (2012) Tuberculosis control: business models for the private sector. Lancet Infect Dis 12 : 579–580.
33. GrenierJ, PintoL, NairD, SteingartK, DowdyD, et al. (2012) Widespread use of serological tests for tuberculosis: data from 22 high-burden countries. Eur Respir J 39 : 502–505.
34. JarosawlskiS, PaiM (2012) Why are inaccurate tuberculosis serological tests widely used in the Indian private healthcare sector? A root-cause analysis. J Epidemiol Global Health 2 : 39–50.
35. X Prize Foundation (2012) Qualcomm Tricorder X PRIZE. Available: http://www.qualcommtricorderxprize.org/.
36. PaiM (2011) Improving TB diagnosis: difference between knowing the path and walking the path. Expert Rev Mol Diagn 11 : 241–244.
37. DowdyDW, SteingartKR, PaiM (2011) Serological testing versus other strategies for diagnosis of active tuberculosis in India: a cost-effectiveness analysis. PLoS Med 8: e1001074 doi:10.1371/journal.pmed.1001074.
38. BhargavaA, PintoLM, PaiM (2011) Mismanagement of tuberculosis in India: causes, consequences, and the way forward. Hypothesis 9 : 1–13.
39. PaiNP, KleinMB (2009) Rapid testing at labor and delivery to prevent mother-to-child HIV transmission in developing settings: issues and challenges. Womens Health 5 : 55–62.
40. EngelN, KennethJ, PaiM (2012) TB diagnostics in India: creating an ecosystem for innovation. Expert Rev Mol Diagn 12 : 21–24.
41. DowdyDW, CattamanchiA, SteingartKR, PaiM (2011) Is scale-up worth it? Challenges in economic analysis of diagnostic tests for tuberculosis. PLoS Med 8: e1001063 doi:10.1371/journal.pmed.1001063.
42. Pant PaiN, JoshiR, DograS, TaksandeB, KalantriSP, et al. (2007) Evaluation of diagnostic accuracy, feasibility and client preference for rapid oral fluid-based diagnosis of HIV infection in rural India. PLoS One 2: e367 doi:10.1371/journal.pone.0000367. doi:10.1371/journal.pone.0000367.
43. PaiNP, BalramB, ShivkumarS, Martinez-CajasJL, ClaessensC, et al. (2012) Head-to-head comparison of accuracy of a rapid point-of-care HIV test with oral versus whole-blood specimens: a systematic review and meta-analysis. Lancet Infect Dis 12 : 373–380.
44. SchnippelK, Meyer-RathG, LongL, MacleodW, SanneI, et al. (2012) Scaling up Xpert MTB/RIF technology: the costs of laboratory - vs. clinic-based roll-out in South Africa. Trop Med Int Health Epub ahead of print. doi:10.1111/j.1365-3156.2012.03028.x.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 9- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Misrepresentation of Randomized Controlled Trials in Press Releases and News Coverage: A Cohort Study
- Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
- The Challenges and Possibilities of Reducing Deaths from Cryptococcal Meningitis in Sub-Saharan Africa
- The World Health Report 2012 That Wasn't
- External Financial Aid to Blood Transfusion Services in Sub-Saharan Africa: A Need for Reflection
- Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries
- A Systematic Review and Meta-Analysis of Utility-Based Quality of Life in Chronic Kidney Disease Treatments
- Statins and Venous Thrombosis: A Story Too Good to Be True?
- Who Sets the Global Health Research Agenda? The Challenge of Multi-Bi Financing
- Reduced Risk of Malaria in Papua New Guinean Children with Southeast Asian Ovalocytosis in Two Cohorts and a Case-Control Study
- Gender Differences in Survival among Adult Patients Starting Antiretroviral Therapy in South Africa: A Multicentre Cohort Study
- Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition?
- The Effect of Adding Ready-to-Use Supplementary Food to a General Food Distribution on Child Nutritional Status and Morbidity: A Cluster-Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition?
- Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
- The Effect of Adding Ready-to-Use Supplementary Food to a General Food Distribution on Child Nutritional Status and Morbidity: A Cluster-Randomized Controlled Trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



