-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGender Differences in Survival among Adult Patients Starting Antiretroviral Therapy in South Africa: A Multicentre Cohort Study
Background:
Increased mortality among men on antiretroviral therapy (ART) has been documented but remains poorly understood. We examined the magnitude of and risk factors for gender differences in mortality on ART.Methods and Findings:
Analyses included 46,201 ART-naïve adults starting ART between January 2002 and December 2009 in eight ART programmes across South Africa (SA). Patients were followed from initiation of ART to outcome or analysis closure. The primary outcome was mortality; secondary outcomes were loss to follow-up (LTF), virologic suppression, and CD4+ cell count responses. Survival analyses were used to examine the hazard of death on ART by gender. Sensitivity analyses were limited to patients who were virologically suppressed and patients whose CD4+ cell count reached >200 cells/µl. We compared gender differences in mortality among HIV+ patients on ART with mortality in an age-standardised HIV-negative population.
Among 46,201 adults (65% female, median age 35 years), during 77,578 person-years of follow-up, men had lower median CD4+ cell counts than women (85 versus 110 cells/µl, p<0.001), were more likely to be classified WHO stage III/IV (86 versus 77%, p<0.001), and had higher mortality in crude (8.5 versus 5.7 deaths/100 person-years, p<0.001) and adjusted analyses (adjusted hazard ratio [AHR] 1.31, 95% CI 1.22–1.41). After 36 months on ART, men were more likely than women to be truly LTF (AHR 1.20, 95% CI 1.12–1.28) but not to die after LTF (AHR 1.04, 95% CI 0.86–1.25). Findings were consistent across all eight programmes. Virologic suppression was similar by gender; women had slightly better immunologic responses than men. Notably, the observed gender differences in mortality on ART were smaller than gender differences in age-standardised death rates in the HIV-negative South African population. Over time, non-HIV mortality appeared to account for an increasing proportion of observed mortality. The analysis was limited by missing data on baseline HIV disease characteristics, and we did not observe directly mortality in HIV-negative populations where the participating cohorts were located.Conclusions:
HIV-infected men have higher mortality on ART than women in South African programmes, but these differences are only partly explained by more advanced HIV disease at the time of ART initiation, differential LTF and subsequent mortality, and differences in responses to treatment. The observed differences in mortality on ART may be best explained by background differences in mortality between men and women in the South African population unrelated to the HIV/AIDS epidemic.
Please see later in the article for the Editors' Summary.
Published in the journal: . PLoS Med 9(9): e32767. doi:10.1371/journal.pmed.1001304
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001304Summary
Background:
Increased mortality among men on antiretroviral therapy (ART) has been documented but remains poorly understood. We examined the magnitude of and risk factors for gender differences in mortality on ART.Methods and Findings:
Analyses included 46,201 ART-naïve adults starting ART between January 2002 and December 2009 in eight ART programmes across South Africa (SA). Patients were followed from initiation of ART to outcome or analysis closure. The primary outcome was mortality; secondary outcomes were loss to follow-up (LTF), virologic suppression, and CD4+ cell count responses. Survival analyses were used to examine the hazard of death on ART by gender. Sensitivity analyses were limited to patients who were virologically suppressed and patients whose CD4+ cell count reached >200 cells/µl. We compared gender differences in mortality among HIV+ patients on ART with mortality in an age-standardised HIV-negative population.
Among 46,201 adults (65% female, median age 35 years), during 77,578 person-years of follow-up, men had lower median CD4+ cell counts than women (85 versus 110 cells/µl, p<0.001), were more likely to be classified WHO stage III/IV (86 versus 77%, p<0.001), and had higher mortality in crude (8.5 versus 5.7 deaths/100 person-years, p<0.001) and adjusted analyses (adjusted hazard ratio [AHR] 1.31, 95% CI 1.22–1.41). After 36 months on ART, men were more likely than women to be truly LTF (AHR 1.20, 95% CI 1.12–1.28) but not to die after LTF (AHR 1.04, 95% CI 0.86–1.25). Findings were consistent across all eight programmes. Virologic suppression was similar by gender; women had slightly better immunologic responses than men. Notably, the observed gender differences in mortality on ART were smaller than gender differences in age-standardised death rates in the HIV-negative South African population. Over time, non-HIV mortality appeared to account for an increasing proportion of observed mortality. The analysis was limited by missing data on baseline HIV disease characteristics, and we did not observe directly mortality in HIV-negative populations where the participating cohorts were located.Conclusions:
HIV-infected men have higher mortality on ART than women in South African programmes, but these differences are only partly explained by more advanced HIV disease at the time of ART initiation, differential LTF and subsequent mortality, and differences in responses to treatment. The observed differences in mortality on ART may be best explained by background differences in mortality between men and women in the South African population unrelated to the HIV/AIDS epidemic.
Please see later in the article for the Editors' Summary.Introduction
South Africa has the largest antiretroviral therapy (ART) programme worldwide. The programme has undergone rapid expansion with nearly 1.64 million adults initiating ART since 2004 [1]. Given the unprecedented scale of this initiative there is an urgent need to evaluate the outcomes of the programme in order to improve delivery of services. There is particular interest in gender differences in ART programme access and survival. Disproportionately more women than men have accessed ART in sub-Saharan Africa [2],[3]. Studies from Europe and North America suggest a higher risk of death on ART for women than men [4]; in contrast, across sub-Saharan Africa men appear to experience greater mortality than women on treatment [5]–[8].
A range of possible explanations for gender differences in mortality on ART have been suggested but there has been no comprehensive evaluation of the putative mechanisms. Baseline characteristics strongly predict mortality on ART [9]–[11], and men initiating ART in many African programmes have more advanced HIV disease than women [2],[6],[12]. In addition, loss to follow-up (LTF) is associated with mortality [13], and men are more likely to become LTF than women in many settings [14],[15]. Evidence regarding gender differences in immunologic and virologic responses is mixed [4]. It is vital to understand such differentials in order to improve health outcomes in this large and rapidly expanding health service.
We examined the magnitude of, and risk factors for, gender differences in mortality. We included data from eligible adults starting ART in the South African sites of the International Epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA-SA) collaboration between 2002 and 2009, representing about 10% of all patients enrolled nationally during this period [1]. We hypothesized that increased mortality in men on ART, if present, would be explained by differences in: (1) baseline characteristics; (2) differential risk of LTF and subsequent mortality; and/or (3) gender differences in virologic and immunologic responses.
Methods
Study Design, Population, and Eligibility Criteria
The South African cohorts of IeDEA-SA have been described in detail elsewhere [16],[17]. Briefly, the collaboration includes eight adult cohorts providing ART services in three of the most populous provinces (Gauteng, KwaZulu-Natal, and Western Cape). Cohorts range in size and are predominantly government-funded and follow national HIV treatment guidelines. The multicentre cohort is broadly representative of patients accessing public sector ART in rural and urban centres. This retrospective cohort analysis included all ART-naïve HIV-positive adults (≥16 and ≤80 y) who initiated ART between 2002 and 2009.
Variables and Definitions
Baseline characteristics measured immediately before ART initiation included demographics (age, gender), available measures of HIV disease severity (CD4+ cell count, WHO stage, HIV viral load), clinical and laboratory characteristics (haemoglobin, weight), and calendar year of ART initiation. CD4+ cell count and viral load measures were taken after 12, 24, and 36 mo on ART. When measurements were not available at these time points, we included the closest laboratory measurement within a 3-mo period on either side of the date as available. All laboratory tests were performed by the South African National Health Laboratory Services.
We treated the following variables as categorical: age (16–24, 25–34, 35–44, 45+ y), CD4+ cell count (0–24, 25–49, 50–99, 100–199, 200+ cells/µl), WHO stage (I and II, III, IV), and haemoglobin; we treated weight and log viral load as continuous variables. Because of gender variability in haemoglobin levels, we generated a categorical variable (anaemia) from the haemoglobin level (measured in g/dl), which was defined as: none: females >11.9, males >13.1; mild: females 10–11.9, males 11–13.1; moderate: females 8.1 to <10, males 8.1 to <11; severe: <8.1 [18]. We defined virologic failure as a viral load measurement >400 copies/ml.
The primary outcome was mortality. Secondary outcomes were LTF, virologic suppression, and CD4+ cell count responses. Deaths were identified by the sites or by linkage to the National Population Register (NPR) of the Department of Home Affairs. Transfers were recorded by programmes and observation time was right-censored at the date of transfer. Patients were defined as LTF if there was no patient contact between analysis closure and database closure. Analysis closure preceded database closure by 6 mo to allow patients to meet the LTF definition. LTF date was defined as the last patient contact date. In order to differentiate between patients who were truly LTF and patients who had died within 3 mo of being LTF (misclassified deaths), we used linkage information to trace patients LTF with South African civil identification (ID) numbers (Figure S1). Patients who had a date of death within 3 mo after LTF were defined as misclassified deaths. Those with ID numbers who were not found in the population register in this period were defined “true” LTF [19]. For patients who started ART but had no further contact, we added 1 d of follow-up to allow their inclusion in survival analyses.
Missing Data
On the basis of the assumption that data were likely missing at random, we used multiple imputation [20] by chained equation methods [21] to impute missing baseline data. We multiply imputed (20 times) baseline CD4+ cell count, WHO stage, viral load, weight, and haemoglobin. The multiple imputation models included all measured variables.
Given that a high proportion of patients LTF are likely to have died [13], we used inverse probability weighting [22] to correct mortality and LTF for missing deaths among those defined as LTF. Briefly, LTF patients with ID numbers (approximately 50%) were linked to the South African National Population Register to determine their true vital status (and date of death if deceased) and weighted to represent all patients LTF, enabling more accurate estimates of vital status.
Analysis
Data were analysed using STATA 11.0 (STATA Corporation). Baseline characteristics were described with summary statistics (median, interquartile range [IQR] and proportions) by gender. Differences between proportions and medians were tested with Pearson's chi-squared test for proportions or the two-sample Wilcoxon rank-sum test. Two-sided statistical tests were used at alpha = 0.05. Time to death and time to “true” LTF were analysed from date of ART initiation using Kaplan-Meier curves.
Cox's proportional hazards regression models were used to assess crude and adjusted associations between patient characteristics and outcomes. All available plausible demographic and clinical variables were considered potential confounders and were included in multivariable models if they altered the association between gender and mortality or were significantly associated with the outcome under study. Results are presented as hazard ratios (HRs) with a 95% CI by duration on ART. The proportional hazards assumption was confirmed by testing gender/time and gender/log time interaction terms. We undertook sensitivity analyses limited to patients who were virologically suppressed and patients whose CD4+ cell count reached >200 cells/µl. We explored heterogeneity in analyses stratified by cohort. The gender mortality ratio was defined as the male divided by the female mortality rate.
To compare gender differences in our cohort with expected gender differences in the HIV-negative population, we calculated HIV-negative mortality in the South African male population and female population, age-standardised to our patients. Age-specific HIV-negative mortality rates in males and females were obtained from the Actuarial Society of South Africa (ASSA) estimates of non-HIV mortality in the year 2005 [23], which are derived from vital registration statistics as well as census and survey data (further explanation of the derivation of these rates is provided in Text S1).
All IeDEA-SA sites obtained ethical approval from relevant local institutions before contributing anonymised patient data to this collaborative analysis. In addition, the collaboration has approval from the University of Cape Town Human Research Ethics Committee to receive and analyse these collaborative data.
Results
Among 58,124 patients assessed for eligibility, 11,923 were ineligible for the following reasons: age <16 or >80 y (n = 4,344), missing or invalid dates (birth, ART initiation, last visit, outcomes) (n = 4,770), non-ART naïve (n = 2,806), unknown gender (n = 3) (Figure 1). This analysis included 46,201 adults who started ART between 1 January 2002 and 31 December 2009 (median age 35 y; 65% female) (Table 1), contributing a total of 77,578 person-years of follow-up. Men had a shorter median time to death (483 versus 532 person-days) and “true” LTF (434 versus 495 person-days) than women. By the end of the study period, 29,901 patients were still on ART, 67% (n = 20,151) of these female.
Fig. 1. Patient flowchart. Description of a combined cohort of adult patients initiating public sector ART in South Africa, 2002–2009. 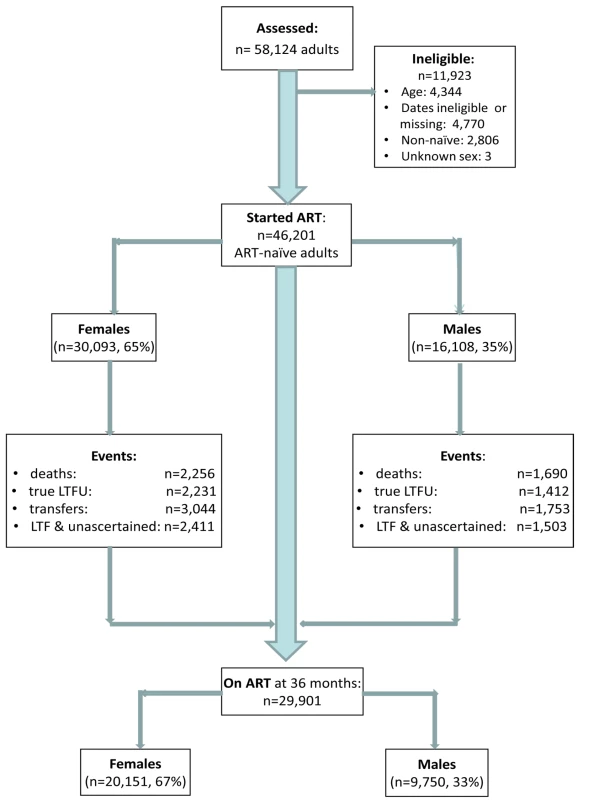
Tab. 1. Patient characteristics among 46,201 adults initiating public-sector ART in South Africa, 2002–2009. 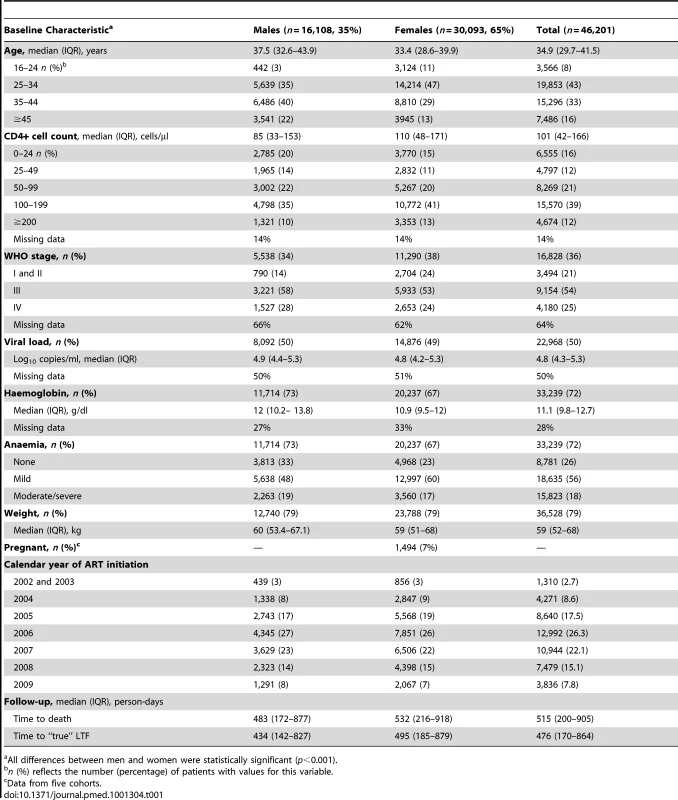
All differences between men and women were statistically significant (p<0.001). At initiation of ART, men were older than women (38 versus 33 y) and had lower median CD4+ cell counts (85 versus 110 cells/µl) (Table 1). Men were more likely than women to have a CD4+ cell count <50 cells/µl (34% versus 26%) and to be classified WHO stage III/IV (86% versus 77%). The median haemoglobin level was similar for men and women (12 versus 11 g/dl). Among females initiating ART, 7% were pregnant. Gender differences in baseline characteristics were consistent across all the eight cohorts (Figure 2).
Fig. 2. Male versus female mortality. Baseline characteristics and adjusted hazard ratios for male versus female mortality, by cohort. 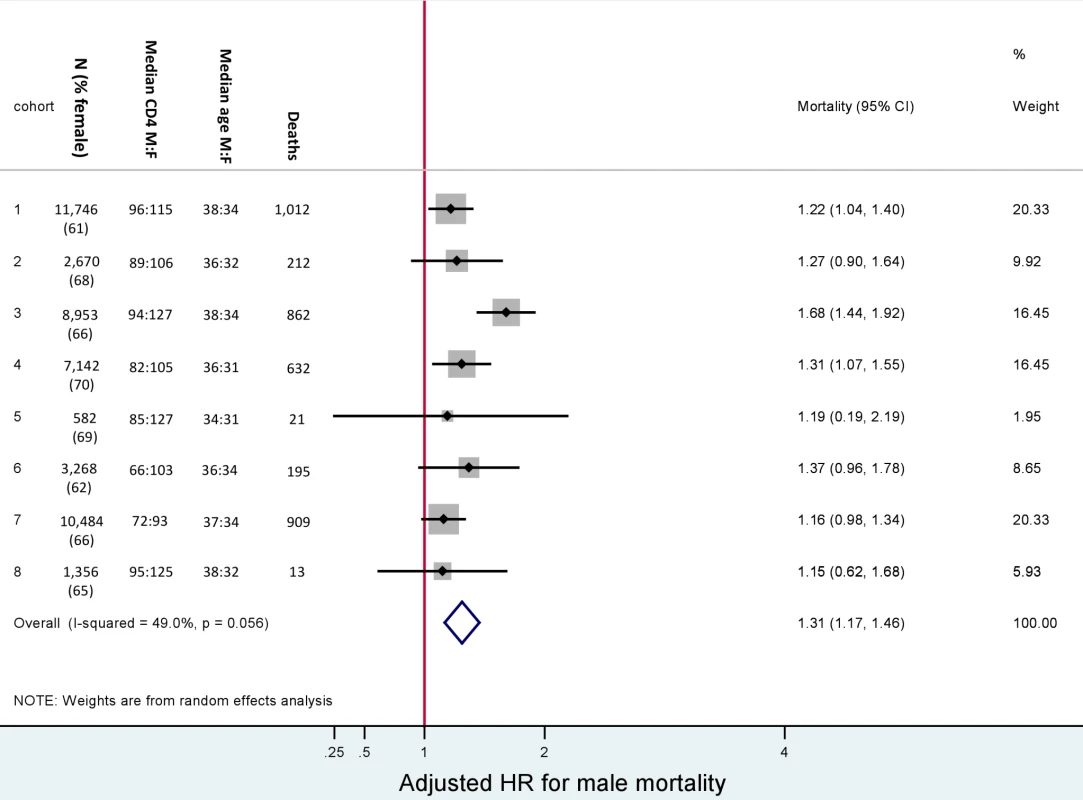
Adjusted for baseline age, CD4+ cell count, WHO stage, anaemia, weight, and viral load. Male versus female is reported as M∶F. Gender and Mortality
In total, after correction via linkage to the National Population Register, there were 3,946 deaths, 57% among women. Men had a higher risk of mortality (Figure 3). The crude mortality on ART was higher for men than women: 8.5 versus 5.7/100 person-years, unadjusted HR 1.46 (1.37–1.56), p<0.001. In multivariable analysis, after adjusting for baseline age, cohort, CD4+ cell count, WHO stage, log viral load, anaemia, and weight, men had a 31% higher risk of death than women (adjusted HR [AHR] 1.31, 95% CI 1.22–1.41) (Table 2). Other baseline factors associated with mortality were age >35 y, CD4+ cell count, WHO stage, anaemia, weight, and viral load (Table S1). The association between gender and death persisted with increasing duration on ART. In a stratified analysis, the elevated risk of death for men compared with women was consistent across cohorts (Figure 2). Excluding WHO stage from the adjusted model did not change our main finding (AHR 1.34, 95% CI 1.25–1.44). There was no evidence of an interaction between gender and age.
Fig. 3. Mortality by gender and ART duration. 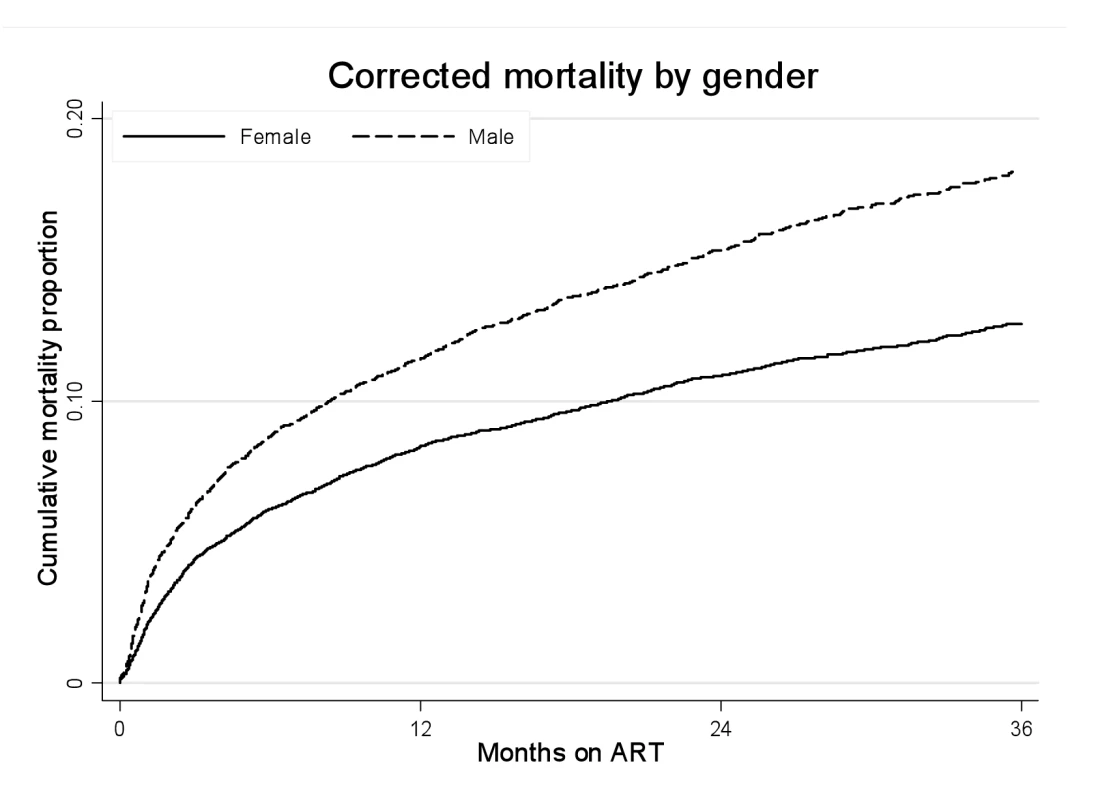
Kaplan-Meier estimates of mortality by gender and duration on ART, corrected via linkage to the National Population Register. Tab. 2. Crude and adjusted associations between male gender and mortality by duration on ART. 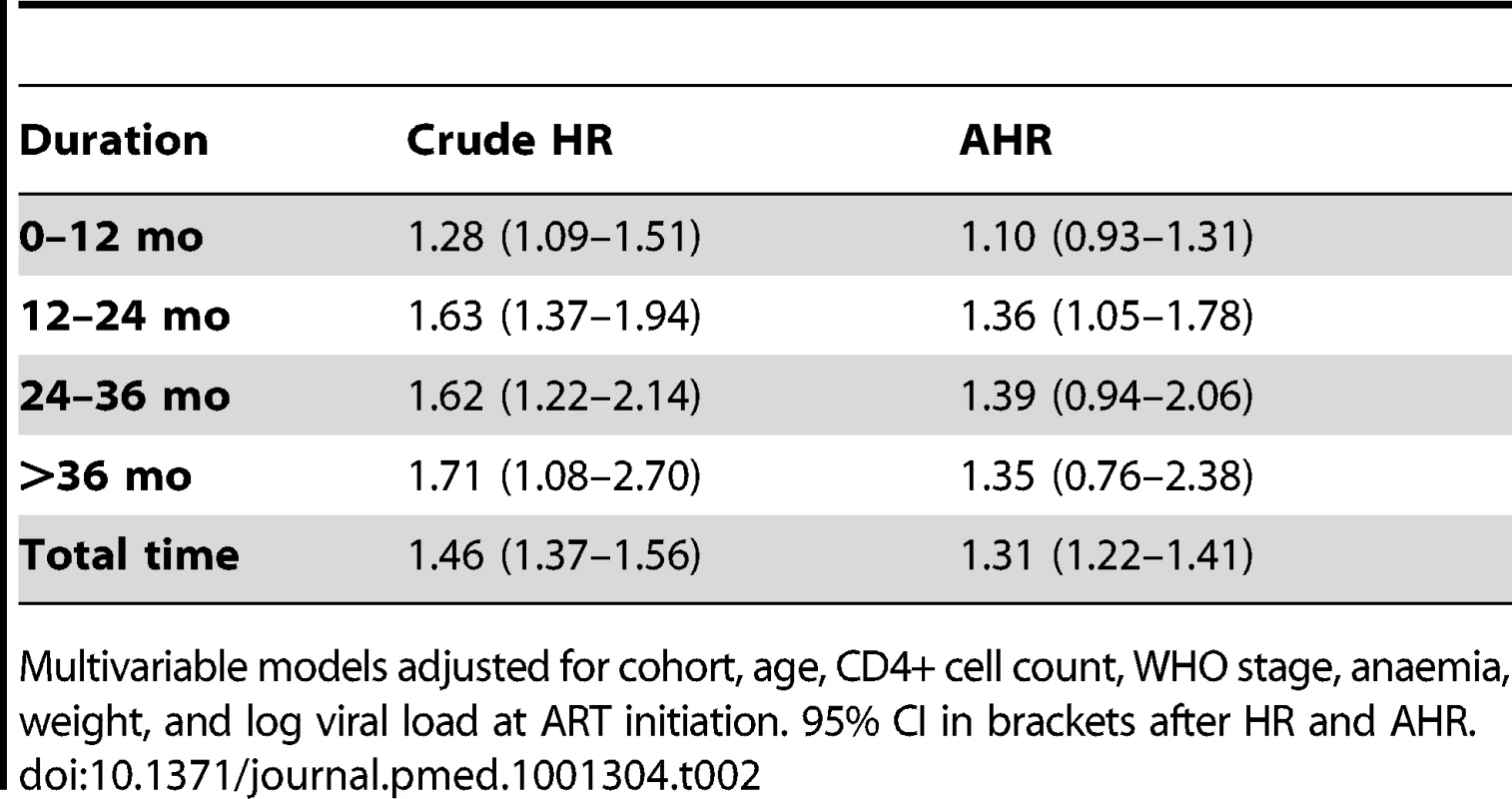
Multivariable models adjusted for cohort, age, CD4+ cell count, WHO stage, anaemia, weight, and log viral load at ART initiation. 95% CI in brackets after HR and AHR. Gender and Loss to Follow-up
Using our initial definition, 8,303 adults were suspected LTF, of whom 61% were female (Figure S1). We were able to ascertain the vital status of 4,389 patients who had ID numbers (61% female). Among those patients who were LTF without ID numbers and whose outcomes could not be ascertained (n = 3,914), 62% were female. After linkage to the NPR, we identified 746 patients who had died within 3 mo of being suspected LTF. We regarded these as misclassified deaths (57% female). After correction for these deaths, a total of 3,643 patients were defined as “true” LTF, 61% female.
Men were more likely than women to experience “true” LTF (Figure 4). The crude “true” LTF rate was 10.03/100 person-years, lower among women than men (9.18 versus 11.77/100 person-years, respectively). In multivariable analysis, after adjusting for baseline age, cohort, CD4+ cell count, WHO stage, anaemia, weight, viral load, and calendar year of initiation, men were more likely to be truly LTF than women (AHR 1.20, 95% CI 1.12–1.28). Within each cohort, the trend towards increased risk of “true” LTF was consistent but estimates were less precise. Using linkage to the NPR, we then explored mortality among those who were truly LTF with ID numbers (i.e., still alive 3 mo after being suspected LTF). We found no gender difference in the hazard of death among those patients who were truly LTF (AHR 1.04, 95% CI 0.86–1.25).
Fig. 4. “True” loss to follow-up by gender and ART duration. 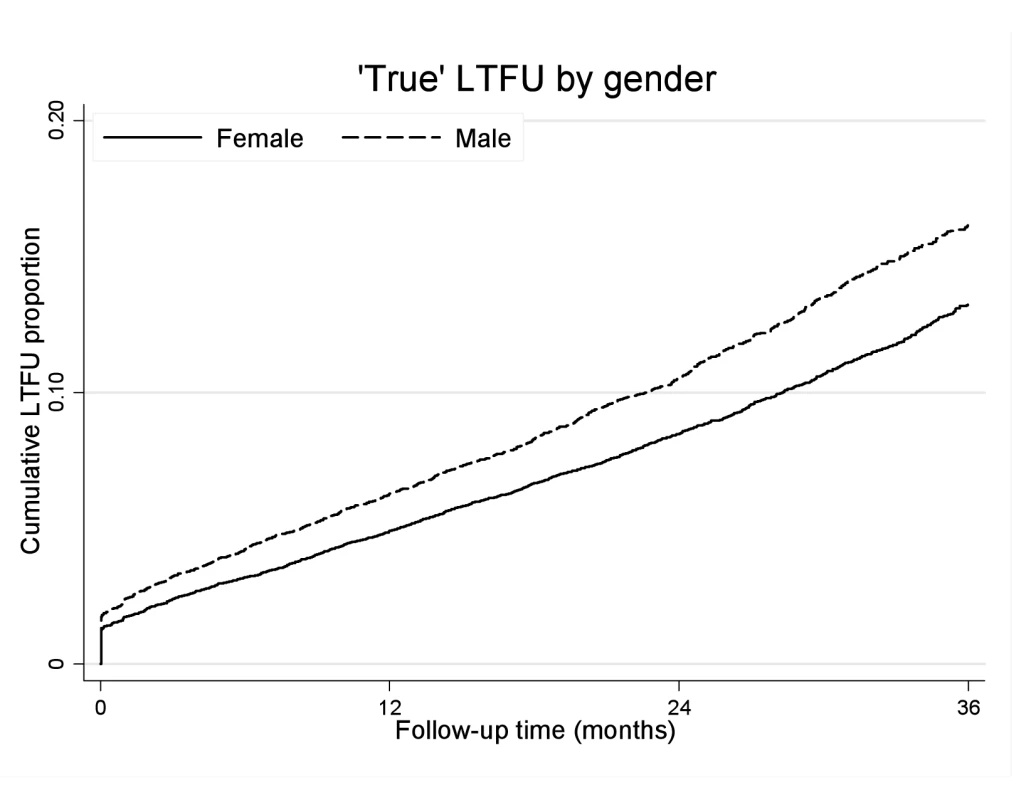
Kaplan-Meier estimates of “true” LTF by gender and duration on ART. Gender and Virologic and Immunologic Responses to ART
There was a high proportion of virologically suppressed patients at 12, 24, and 36 mo on ART, with no evident gender difference in proportions (Table 3). When analysis of mortality was restricted to individuals who were virologically suppressed at 12 mo, men still had a higher risk of death than women (AHR 1.38, 95% CI 1.07–1.79). Women initiating ART had a higher baseline CD4+ cell count and better CD4+ cell count responses than men over 36 mo (Figure S2). The median incremental CD4+ cell gains for women and men at 12, 24, and 36 mo were 179 versus 145, 89 versus 70, and 63 versus 39 cells/µl, respectively (Table 3, p<0.001 for all comparisons). In a sensitivity analysis limited to patients who had reached a CD4+ cell count ≥200 after a year on ART, the gender difference in mortality persisted (AHR 1.37, 95% CI 1.03–1.83) (unpublished data).
Tab. 3. Virologic and immunologic responses by gender and duration on ART. 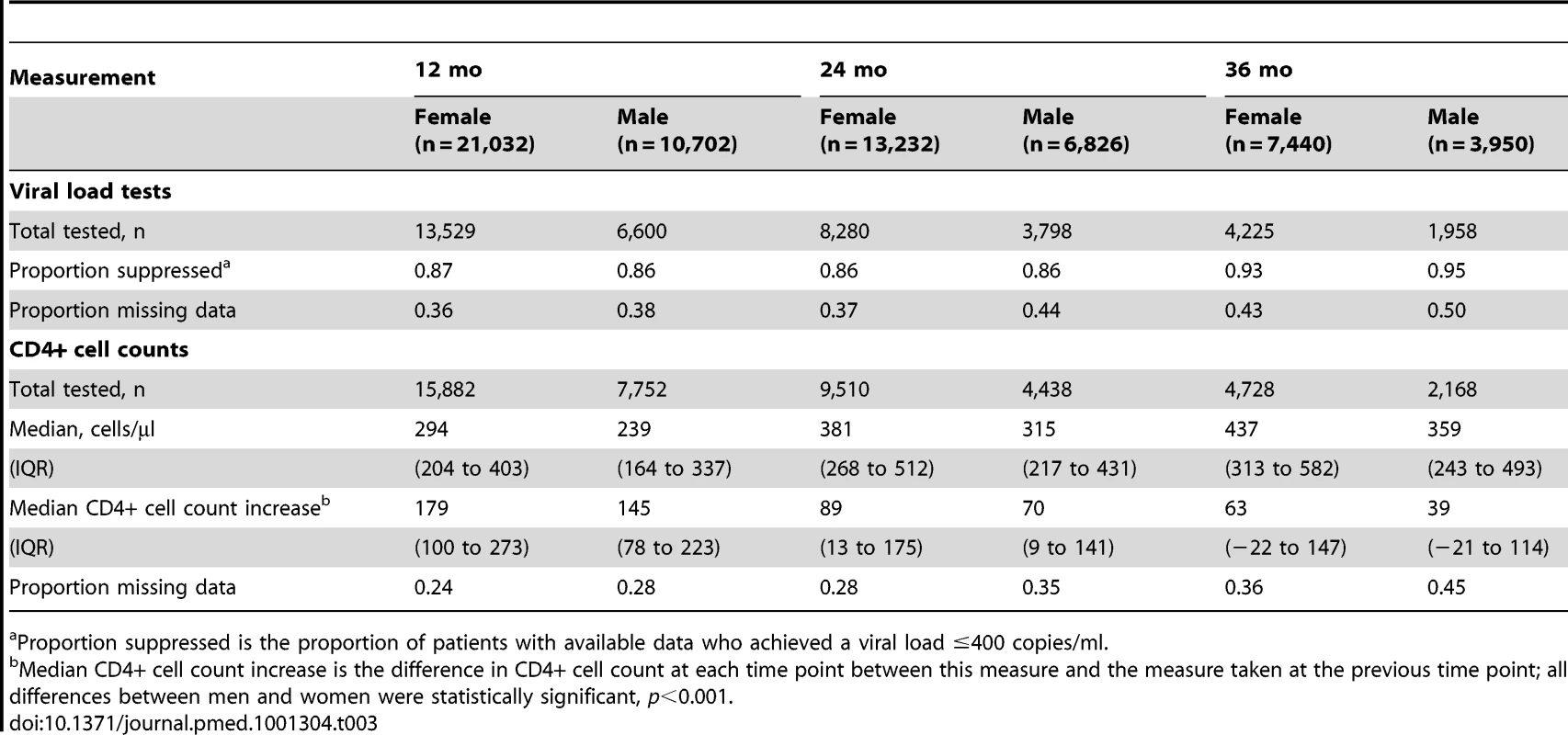
Proportion suppressed is the proportion of patients with available data who achieved a viral load ≤400 copies/ml. Gender and Non-HIV Mortality
Given the persistence of the gender differential in mortality among patients on ART, we compared this to the background gender differential in mortality in the South African population. Figure 5 shows that the gender mortality ratio among patients on ART appears to be smaller than the age-standardised HIV-negative mortality ratio for men versus women in South Africa. HIV-negative men with the same age distribution as that in the ART cohort would be expected to die at twice the rate in HIV-negative women of the same age, compared with our AHR of 1.31 on ART. With increasing duration of ART, the contribution of expected non-HIV mortality to observed mortality increased from an estimated 5% in the first 6 mo to 36% after 36 mo among men and from 3% to 25% among women during the same periods (Table 4). In further analyses stratified by age (16–34 and 35+ y), expected non-HIV mortality accounted for a larger proportion of deaths in the older men than in the younger men (Table S2). One exception was the proportion of observed male mortality attributable to non-HIV mortality at >36 mo, likely to be random error due to the small number of deaths at the longest duration. In both age groups, non-HIV mortality contributed more substantially to male mortality than to female mortality. In addition, over all periods on ART, although the expected ratio of male to female non-HIV deaths differed at younger and older ages, all ratios were greater than the male to female ratio of observed mortality on ART.
Fig. 5. Male versus female mortality ratios over time. 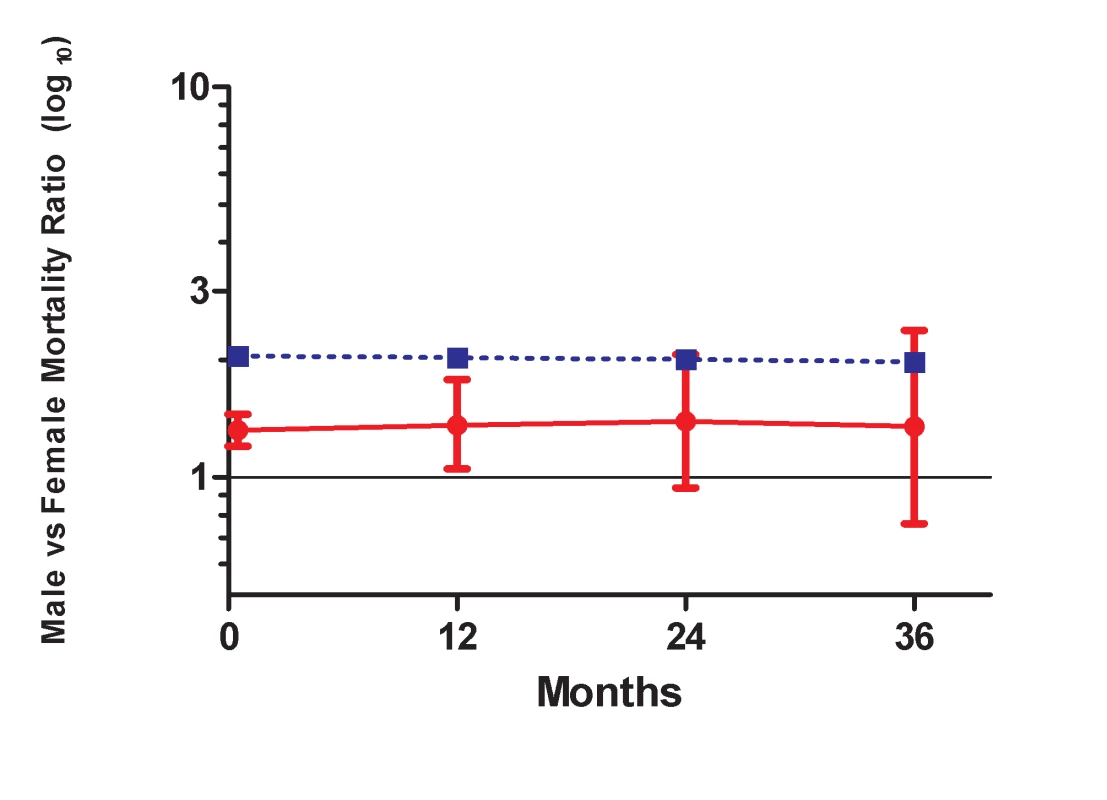
Solid line, observed mortality ratio for men versus women on ART. Dotted line, age-standardised HIV-negative mortality ratio for men versus women in South Africa. Tab. 4. Observed crude mortality<sup>a</sup>, age-standardised HIV-negative mortality<sup>a</sup>, and expected non-HIV mortality as a percentage of observed mortality among men and women, by duration on ART. 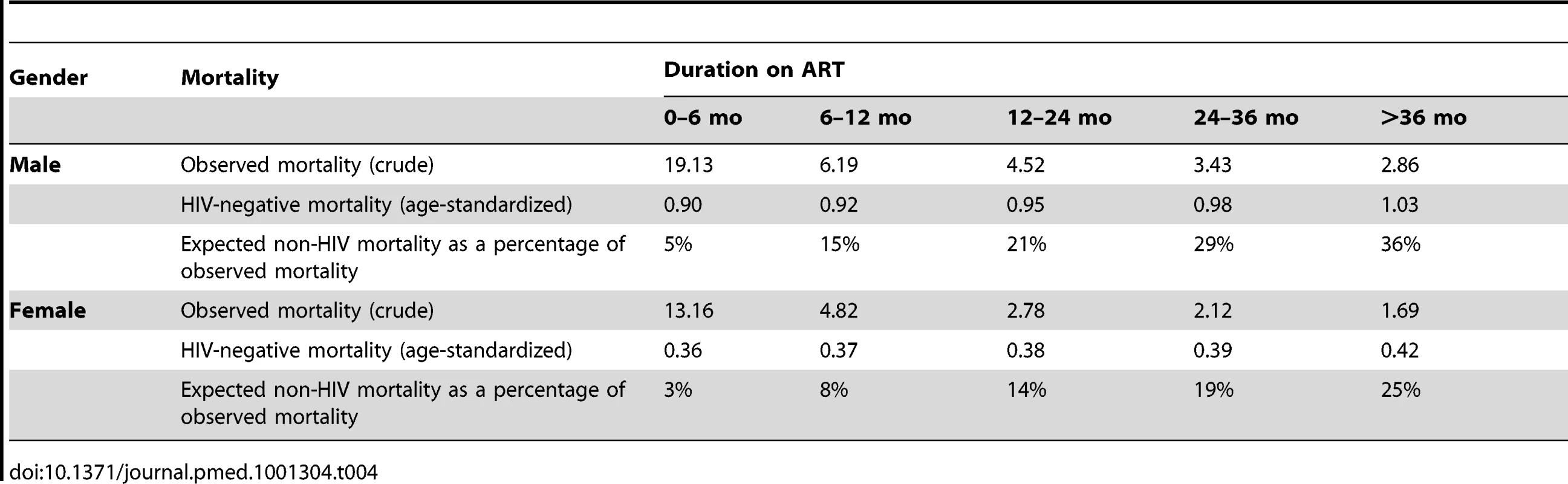
Discussion
These analyses demonstrate that among patients initiating ART between 2002 and 2009 at sites across South Africa, men had higher mortality than women. We hypothesized that gender differences in mortality would be explained by differences in baseline characteristics, LTF and subsequent mortality, and/or virologic and immunologic responses. However, we found that the increased mortality risk among men persisted throughout each analysis, including after adjustment for measures of HIV disease at the time of ART initiation, in the subset of patients who achieved virologic suppression, and among patients with good immune responses to treatment.
There is a large and growing body of work on gender and ART in developing countries, much of which documents the same association repeatedly [24]: disproportionately fewer men than women access ART [2],[3],[25], and there is higher mortality among men than women on ART [5],[8],[15],[16],[26],[27]. Such studies typically speculate as to the possible mechanisms underlying the observed associations. Putative mechanisms include: poor health-seeking behaviours among men leading to more advanced disease at the time of ART initiation, differential rates of LTF leading to higher mortality, behavioural factors such as poor adherence, and/or biologic factors such as gender differences in immunologic responses to ART. However, there have been few systematic attempts to evaluate each of these possible mechanisms.
In this study, women comprised the majority of adults starting ART across the country. In the early years of ART programmes there were understandable concerns that due to gender imbalances, women may have reduced access to ART services [28]. Contrary to this, however, there is mounting evidence that men appear disadvantaged in their access to ART programmes. Numerous papers and a systematic review have suggested gender inequalities in ART access, particularly in sub-Saharan Africa [2],[3],[8], and ART initiation in South Africa, relative to ART need, is substantially higher in women than in men [1].
Among patients who do start ART, late presentation has been cited as one of the main reasons for increased male mortality in ART programmes [4]–[6],[8]. In sub-Saharan Africa, men appear to initiate ART at older ages and with more advanced HIV disease than women [2],[3],[6],[16], and markers of advanced HIV disease at the time of ART initiation strongly predict early mortality on ART [9],[12],[29]–[31]. However, in our multivariable models we found that adjustment for baseline characteristics accounted for only part of the gender difference in mortality, and that the gender differentials in mortality continued to appear for several years after ART initiation. The reasons for men's later entry into ART programmes are poorly understood but frequently are attributed to gender differences in health-seeking behaviour or in routes of referral [32]. An alternate and more compelling explanation may be that while prioritising maternal and child health services in many public health systems, men's primary health care needs may have been neglected [32]–[35], a possibility that warrants further research attention.
We then assessed the role of LTF in explaining mortality differentials. Increased mortality among patients LTF, especially within the first 3 mo after LTF, has been well documented [19],[36]–[40]. In turn, it is plausible that if males are more likely to be LTF, this could explain their elevated mortality risk. Through linkage to the South African Population Register we were able to identify a group of patients who were truly LTF, and to trace their vital status after being LTF. Although men were more likely to be LTF compared with women, we found no gender difference in mortality after LTF, and deaths after LTF contributed only a small proportion of all deaths. Thus in these data, LTF alone did not appear to explain men's increased mortality.
Behavioural factors such as treatment adherence could also help to explain the observed increased male mortality on ART. Poor adherence to ART, measured by virologic non-suppression, significantly increases the risk of mortality on treatment. We found no apparent gender difference in virologic suppression, although previous studies have suggested that adherence may vary by gender [41]. Of note, this finding was consistent across all cohorts, and in a sensitivity analysis restricted to patients who were virologically suppressed at 12 mo, men still had a higher risk of subsequent death than women. As a result, gender differences in adherence to treatment do not appear to explain differences in mortality.
Biologic differences between men and women have been suggested as shaping immunologic responses to ART and mortality risk. We found that women had higher CD4+ cell counts at ART initiation than men, and slightly better absolute CD4+ cell increases on treatment. These data are in line with results from two collaborative studies in sub-Saharan Africa that documented greater immune recovery in women than in men, with gender-based differences increasing with time on ART [43],[44]. However, even among patients with similar immunologic responses by 1 y on treatment, the gender difference in mortality persisted.
In these data, the finding of persistently increased male mortality for up to 3 y on ART does not appear to be explained entirely by baseline differences in HIV disease status, variation in LTF, differences in virologic suppression, or sex-linked differences in immune responses to treatment. Having thus refuted our a priori hypotheses, we explored alternate explanations for the observed association. We examined evidence for gender differences in non-HIV adult mortality in South Africa, and found pronounced differences that appear independent of the HIV/AIDS epidemic. Specifically, evidence from actuarial modelling suggests that HIV-negative South African men in the same age groups as our study population have twice the mortality risk of women, although the male-female differences tend to be more pronounced in young adults than in older adults. This pattern is not unique to South Africa: similar trends are seen elsewhere in Africa and worldwide [44]–[48]. This evidence places these data, and previous studies showing increased mortality among men in ART services across Africa, into perspective: among patients on ART, men have an increased risk of death on ART compared to women, but this same phenomenon operates in HIV-negative individuals. In fact, in South Africa the mortality ratio for men versus women on ART appears to be somewhat smaller than the age-standardised ratio for men versus women estimated for the HIV-negative population. Gender differentials in mortality of HIV-negative individuals are well-documented in South Africa and include an increased burden of mortality among younger men due to traumatic causes and non-HIV tuberculosis [49]. It is possible that through accessing ART services, men and women may access other preventive and curative services that reduce non-HIV mortality. While these findings are intriguing, it is important to recognize that our original hypotheses did not include gender differences in non-HIV mortality, and this phenomenon clearly requires further investigation.
Observational studies of patients on ART face major constraints in terms of mortality ascertainment, LTF, and missing measurements. Most cohort analyses from sub-Saharan Africa—and especially large collaborative analyses—have limited ability to ascertain true outcomes as they have little capacity to follow patients actively [6]. In addition, few African countries have high quality vital statistics [47],[50],[51]. In turn, most large ART programmes have difficulty confirming patients' true vital status [52], and in the absence of reliable death registration, researchers have undertaken targeted tracing studies [40]. In South Africa, the National Population Register captures nearly 90% of adult deaths [53]. Through our linkage with this register, we are able to distinguish between “true” LTF and unrecognised mortality in many patients. Moreover, consistency of findings across different cohorts strengthens the generalizability of our results.
Interpretation of these results is subject to several important limitations. These data come from multiple service delivery programmes from across South Africa, and there is a substantial amount of missing data in this analysis. For example, South African national identity numbers were only available for 53% of those who were suspected to be LTF, and it is possible that there are systematic differences between patients with and without ID numbers for which we have not accounted. There was also a high proportion of missing values for WHO stage and viral load; we tried to address this with multiple imputation and sensitivity analyses confirming our main findings. Missing data on specific risk factors for mortality, particularly prevalent tuberculosis at ART initiation [54], further limited this analysis. Finally, we did not observe mortality in HIV-negative populations where the participating cohorts are located, and instead used a national-level actuarial model to estimate mortality in men and women who are HIV-negative. These findings are likely to be generalizable to the South African national ART programme, and potentially to other parts of the region, but further investigation in other settings is warranted.
In summary, there have been concerns raised about the presence of gender differences in mortality on ART in South Africa and many other parts of the continent. In this study we systematically explored this phenomenon and found that none of the explanations posited for this association adequately explains the increased mortality observed among men on ART. Instead, the observed differences in mortality on ART may be best explained by background differences in death rates between men and women in the South African population, unrelated to the HIV/AIDS epidemic.
Supporting Information
Zdroje
1. JohnsonL (2012) Access to antiretroviral treatment in South Africa, 2004–2011. S Afr J HIV Med 13 : 22–27.
2. BraitsteinP, BoulleA, NashD, BrinkhofM, DabisF, et al. (2008) Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health (Larchmt) 17 : 47–55.
3. MuulaA, NgulubeT, SiziyaS, MakupeC, UmarE, et al. (2007) Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health 7 : 63.
4. StenehjemE, ShlayJC (2008) Sex-specific differences in treatment outcomes for patients with HIV and AIDS. Expert Rev Pharmacoecon Outcomes Res 8 : 51–63.
5. HawkinsC, ChalamillaG, OkumaJ, SpiegelmanD, HertzmarkE, et al. (2011) Gender differences in antiretroviral treatment outcomes among HIV-infected adults in Dar es Salaam, Tanzania. AIDS 25 : 1189–1197.
6. StringerJSA, ZuluI, LevyJ, StringerEM, MwangoA, et al. (2006) Rapid scale-up of antiretroviral therapy at primary care sites in Zambia. JAMA 296 : 782–793.
7. KlausnerJ, SerenataC, O'BraH, MattsonC, BrownJ, et al. (2011) Scale-up and continuation of antiretroviral therapy in South African treatment programs, 2005–2009. J Acquir Immune Defic Syndr 56 : 292–295.
8. Taylor-SmithK, TweyaH, HarriesA, SchouteneE, JahnA (2010) Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J 22 : 49–56.
9. BrinkhofM, DabisF, MyerL, BangsbergDR, BoulleA, et al. (2008) Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ 86 : 559–567.
10. HoggRS, YipB, ChanKJ, WoodE, CraibKJ, et al. (2001) Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 286 : 2568–2577.
11. LawnSD, HarriesAD, AnglaretX, MyerL, WoodR (2008) Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 22 : 1897–1908.
12. CornellM, GrimsrudA, FairallL, FoxM, van CutsemG, et al. (2010) Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS 24 : 2263–2270.
13. BrinkhofMWG, Pujades-RodriguezM, EggerM (2009) Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One 4: e5790 doi:10.1371/journal.pone.0005790.
14. Ochieng-OokoV, OchiengD, SidleJ, HoldsworthM, Wools-KaloustianK, et al. (2010) Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ 88 : 681–688.
15. NglaziM, LawnS, KaplanR, KranzerK, OrrellC, et al. (2011) Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr 56 : 1e1–e8.
16. CornellM, TechnauK, FairallL, WoodR, MoultrieH, et al. (2009) Monitoring the South African National Antiretroviral Treatment Programme, 2003–2007: the IeDEA Southern Africa collaboration. S Afr Med J 99 : 653–660.
17. EggerM, EkoueviD, WilliamsC, LyamuyaR, MukumbiH, et al. (2011) Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 1–9.
18. MayM, BoulleA, PhiriS, MessouE, MyerL, et al. (2010) Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet 376 : 449–457.
19. Van CutsemG, FordN, HildebrandK, GoemaereE, MatheeS, et al. (2011) Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS One 6: e14684 doi:10.1371/journal.pone.0014684.
20. RubinD (1996) Multiple imputation after 18+ years. J Am Stat Assoc 91 : 473–489.
21. van BuurenS, BoshuizenHC, KnookDL (1999) Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 18 : 681–694.
22. BoulleA, Van CutsemG, HilderbrandK, CraggC, AbrahamsM, et al. (2010) Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 24 : 563–572.
23. Actuarial Society of South Africa (2011) ASSA 2008 AIDS and Demographic Model (lite version 110207). Available: http://aids.actuarialsociety.org.za/ASSA2008-Model-3480.htm
24. KullerLH (1999) Invited commentary: circular epidemiology. Am J Epidemio 150 : 897–903.
25. JohannessenA (2011) Are men the losers of the antiretroviral treatment scale-up? AIDS 25 : 1225–1226.
26. MillsE, BakandaC, BirungiJ, ChanK, HoggR, et al. (2011) Male gender predicts mortality in a large cohort of patients receiving antiretroviral therapy in Uganda. J Int AIDS Soc 14 : 52.
27. Wools-KaloustianK, KimaiyoS, DieroL, SiikaA, SidleJ, et al. (2006) Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS 20 : 41–48.
28. MontanerJSG, HoggR, WoodE, KerrT, TyndallM, et al. (2006) The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 368 : 531–536.
29. OjikutuB, ZhengH, WalenskyR, LuZ, LosinaE, et al. (2008) Predictors of mortality in patients initiating antiretroviral therapy in Durban, South Africa. S Afr Med J 98 : 204–208.
30. RussellE, CharalambousS, PembaL, ChurchyardGJ, GrantA, et al. (2010) Low haemoglobin predicts early mortality among adults starting antiretroviral therapy in an HIV care programme in South Africa: a cohort study. BMC Public Health 10 : 433.
31. ZachariahR, HarriesK, MosesM, ManziM, LineA, et al. (2009) Very early mortality in patients starting antiretroviral treatment at primary health centres in rural Malawi. Trop Med Int Health 14 : 713–721.
32. CornellM, McIntyreJ, MyerL (2011) Men and antiretroviral therapy in Africa: our blind spot. Trop Med Int Health 828–829.
33. HawkesS, HartG (2000) Men's sexual health matters: promoting reproductive health in an international context. Trop Med Int Health 5: A37–44.
34. KalmussD, TatumC (2007) Patterns of men's use of sexual and reproductive health services. Perspect Sex Reprod Health 39 : 74–81.
35. SternbergP, HubleyJ (2004) Evaluating men's involvement as a strategy in sexual and reproductive health promotion. Health Promot Int 19 : 389–396.
36. BrinkhofMWG, BoulleA, WeigelR, MessouE, MathersC, et al. (2009) Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med 6: e1000066 doi:10.1371/journal.pmed.1000066.
37. McGuireM, MunyenyembeT, SzumilinE, HeinzelmannA, Le PaihM, et al. (2010) Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health 15 : 55–62.
38. TweyaH, GaretaD, ChagweraF, Ben-SmithA, MwenyemasiJ, et al. (2010) Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the ‘Back-to-Care’ project in Lilongwe, Malawi. Trop Med Int Health 15 : 82–89.
39. WeigelR, HochgesangM, BrinkhofMW, HosseinipourM, BoxshallM, et al. (2011) Outcomes and associated risk factors of patients traced after being lost to follow-up from antiretroviral treatment in Lilongwe, Malawi. BMC Infect Dis 11 : 31.
40. YuJ, ChenS-C, WangK-Y, ChangC-S, MakombeS, et al. (2007) True outcomes for patients on antiretroviral therapy who are ‘lost to follow-up’ in Malawi. Bull World Health Organ 85 : 550–554.
41. NachegaJB, HislopM, DowdyDW, LoM, OmerSB, et al. (2006) Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African Adults. J Acquir Immune Defic Syndr 43 : 78–84.
42. MamanD, Pujades-RodriguezM, SubtilF, PinogesL, McGuireM, et al. (2012) Gender differences in immune reconstitution: a multicentric cohort analysis in sub-Saharan Africa. PLoS One 7: e31078 doi:10.1371/journal.pone.0031078.
43. NashD, KatyalM, BrinkhofMWG, KeiserO, MayM, et al. (2008) Long-term immunologic response to antiretroviral therapy in low-income countries: collaborative analysis of prospective studies. AIDS 22 : 2291–2302.
44. BlackerJ (2004) The impact of AIDS on adult mortality: evidence from national and regional statistics. AIDS 18: S19–S26.
45. CaseA, PaxsonC (2005) Sex differences in morbidity and mortality. Demography 42 : 189–214.
46. OwensIPF (2002) Sex differences in mortality rate. Science 297 : 2008–2009.
47. RajaratnamJK, MarcusJR, Levin-RectorA, ChalupkaAN, WangH, et al. (2010) Worldwide mortality in men and women aged 15–59 years from 1970 to 2010: a systematic analysis. Lancet 375 : 1704–1720.
48. RigbyJE, DorlingD (2007) Mortality in relation to sex in the affluent world. J Epidemiol Commun Health 61 : 159–164.
49. NormanR, MatzopoulosR, GroenewaldP, BradshawD (2007) The high burden of injuries in South Africa. Bull World Health Organ 85 : 695–702.
50. HillK, LopezAD, ShibuyaK, JhaP (2007) Interim measures for meeting needs for health sector data: births, deaths, and causes of death. Lancet 370 : 1726–1735.
51. Jamison D, Feachem R, Makgoba M, Bos E, Baingana F, et al. (2006) Disease and mortality in sub-Saharan Africa, 2nd edition. Washington (D.C.): World Bank.
52. Bradshaw D, Timaeus I (2006) Levels and trends of adult mortality. Jamison D, Feachem R, Makgoba Mea, editors. Disease and mortality in sub-Saharan Africa. Washington (D.C.): World Bank.
53. Dorrington R, Bourne D, Bradshaw D, Laubscher R, Timaeus I (2001) The impact of HIV/AIDS on adult mortality in South Africa. Cape Town: Burden of Disease Research Unit, Medical Research Council.
54. BassettI, ChettyS, WangB, MazibukoM, GiddyJ, et al. (2012) Loss to follow-up and mortality among HIV-infected people co-infected with TB at ART initiation in Durban, South Africa. J Acquir Immune Defic Syndr 59 : 25–30.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 9- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Misrepresentation of Randomized Controlled Trials in Press Releases and News Coverage: A Cohort Study
- Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
- The Challenges and Possibilities of Reducing Deaths from Cryptococcal Meningitis in Sub-Saharan Africa
- The World Health Report 2012 That Wasn't
- External Financial Aid to Blood Transfusion Services in Sub-Saharan Africa: A Need for Reflection
- Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries
- A Systematic Review and Meta-Analysis of Utility-Based Quality of Life in Chronic Kidney Disease Treatments
- Statins and Venous Thrombosis: A Story Too Good to Be True?
- Who Sets the Global Health Research Agenda? The Challenge of Multi-Bi Financing
- Reduced Risk of Malaria in Papua New Guinean Children with Southeast Asian Ovalocytosis in Two Cohorts and a Case-Control Study
- Gender Differences in Survival among Adult Patients Starting Antiretroviral Therapy in South Africa: A Multicentre Cohort Study
- Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition?
- The Effect of Adding Ready-to-Use Supplementary Food to a General Food Distribution on Child Nutritional Status and Morbidity: A Cluster-Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition?
- Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
- The Effect of Adding Ready-to-Use Supplementary Food to a General Food Distribution on Child Nutritional Status and Morbidity: A Cluster-Randomized Controlled Trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



