-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaReappraisal of Metformin Efficacy in the Treatment of Type 2 Diabetes: A Meta-Analysis of Randomised Controlled Trials
Background:
The UK Prospective Diabetes Study showed that metformin decreases mortality compared to diet alone in overweight patients with type 2 diabetes mellitus. Since then, it has been the first-line treatment in overweight patients with type 2 diabetes. However, metformin-sulphonylurea bitherapy may increase mortality.Methods and Findings:
This meta-analysis of randomised controlled trials evaluated metformin efficacy (in studies of metformin versus diet alone, versus placebo, and versus no treatment; metformin as an add-on therapy; and metformin withdrawal) against cardiovascular morbidity or mortality in patients with type 2 diabetes. We searched Medline, Embase, and the Cochrane database. Primary end points were all-cause mortality and cardiovascular death. Secondary end points included all myocardial infarctions, all strokes, congestive heart failure, peripheral vascular disease, leg amputations, and microvascular complications. Thirteen randomised controlled trials (13,110 patients) were retrieved; 9,560 patients were given metformin, and 3,550 patients were given conventional treatment or placebo. Metformin did not significantly affect the primary outcomes all-cause mortality, risk ratio (RR) = 0.99 (95% CI: 0.75 to 1.31), and cardiovascular mortality, RR = 1.05 (95% CI: 0.67 to 1.64). The secondary outcomes were also unaffected by metformin treatment: all myocardial infarctions, RR = 0.90 (95% CI: 0.74 to 1.09); all strokes, RR = 0.76 (95% CI: 0.51 to 1.14); heart failure, RR = 1.03 (95% CI: 0.67 to 1.59); peripheral vascular disease, RR = 0.90 (95% CI: 0.46 to 1.78); leg amputations, RR = 1.04 (95% CI: 0.44 to 2.44); and microvascular complications, RR = 0.83 (95% CI: 0.59 to 1.17). For all-cause mortality and cardiovascular mortality, there was significant heterogeneity when including the UK Prospective Diabetes Study subgroups (I2 = 41% and 59%). There was significant interaction with sulphonylurea as a concomitant treatment for myocardial infarction (p = 0.10 and 0.02, respectively).Conclusions:
Although metformin is considered the gold standard, its benefit/risk ratio remains uncertain. We cannot exclude a 25% reduction or a 31% increase in all-cause mortality. We cannot exclude a 33% reduction or a 64% increase in cardiovascular mortality. Further studies are needed to clarify this situation.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(4): e32767. doi:10.1371/journal.pmed.1001204
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001204Summary
Background:
The UK Prospective Diabetes Study showed that metformin decreases mortality compared to diet alone in overweight patients with type 2 diabetes mellitus. Since then, it has been the first-line treatment in overweight patients with type 2 diabetes. However, metformin-sulphonylurea bitherapy may increase mortality.Methods and Findings:
This meta-analysis of randomised controlled trials evaluated metformin efficacy (in studies of metformin versus diet alone, versus placebo, and versus no treatment; metformin as an add-on therapy; and metformin withdrawal) against cardiovascular morbidity or mortality in patients with type 2 diabetes. We searched Medline, Embase, and the Cochrane database. Primary end points were all-cause mortality and cardiovascular death. Secondary end points included all myocardial infarctions, all strokes, congestive heart failure, peripheral vascular disease, leg amputations, and microvascular complications. Thirteen randomised controlled trials (13,110 patients) were retrieved; 9,560 patients were given metformin, and 3,550 patients were given conventional treatment or placebo. Metformin did not significantly affect the primary outcomes all-cause mortality, risk ratio (RR) = 0.99 (95% CI: 0.75 to 1.31), and cardiovascular mortality, RR = 1.05 (95% CI: 0.67 to 1.64). The secondary outcomes were also unaffected by metformin treatment: all myocardial infarctions, RR = 0.90 (95% CI: 0.74 to 1.09); all strokes, RR = 0.76 (95% CI: 0.51 to 1.14); heart failure, RR = 1.03 (95% CI: 0.67 to 1.59); peripheral vascular disease, RR = 0.90 (95% CI: 0.46 to 1.78); leg amputations, RR = 1.04 (95% CI: 0.44 to 2.44); and microvascular complications, RR = 0.83 (95% CI: 0.59 to 1.17). For all-cause mortality and cardiovascular mortality, there was significant heterogeneity when including the UK Prospective Diabetes Study subgroups (I2 = 41% and 59%). There was significant interaction with sulphonylurea as a concomitant treatment for myocardial infarction (p = 0.10 and 0.02, respectively).Conclusions:
Although metformin is considered the gold standard, its benefit/risk ratio remains uncertain. We cannot exclude a 25% reduction or a 31% increase in all-cause mortality. We cannot exclude a 33% reduction or a 64% increase in cardiovascular mortality. Further studies are needed to clarify this situation.
: Please see later in the article for the Editors' SummaryIntroduction
Type 2 diabetes mellitus (T2DM), is a major health problem because of its cardiovascular complications and economic costs [1]. Epidemiological evidence indicates that T2DM is an independent risk factor for cardiovascular diseases (CVDs). The rate of CVDs is approximately two times higher in diabetic patients than non-diabetic patients [2]. Since publication of the results of the UK Prospective Diabetes Study (UKPDS 34) in 1998 [3], metformin, a biguanid glucose-lowering agent, has been recommended as the first-line treatment by international guidelines [4],[5]. When compared with diet alone, metformin showed a reduction of all-cause mortality in overweight patients (risk ratio [RR] = 0.64; 95% CI: 0.45 to 0.91 [3]). In the same study, non-overweight patients were randomised to receive various glucose-lowering treatments, and some took either metformin and sulphonylurea or sulphonylurea alone. An increase of overall mortality (RR = 1.60; 95% CI: 1.02 to 2.52) was observed in the metformin add-on sulphonylurea group when compared with sulphonylureas alone. The authors attributed this disturbing result to chance. The authors of recently published Cochrane systematic reviews on metformin efficacy did not include this result in their analyses [6]. Their conclusion, based on the results of the overweight patient group, is that metformin reduces overall and cardiovascular mortality. Selvin et al. [7] and Bennett et al. [8] also did not include the results of non-overweight group, even though they mentioned this subgroup. They concluded that “treatment with metformin hydrochloride was associated with a decreased risk of cardiovascular mortality (pooled OR, 0.74; 95% CI, 0.62–0.89) compared with any other oral diabetes agent or placebo” [7]. Lamanna et al. [9] integrated both subgroups, but included non-diabetic patients as well as patients with HIV or polycystic ovary syndrome. They also did not include safety studies as Rachmani et al. [10] and COSMIC [11] did. They concluded that “it is likely that metformin monotherapy is associated with improved survival (MH-OR: 0.801[0.625–1.024], p = 0.076). However, concomitant use with sulphonylurea was associated with reduced survival (MH-OR: 1.432[1.068–1.918], p = 0.016)” [9].
Phenformin, a drug belonging to the same biguanid family as metformin, was withdrawn from the market after an increased cardiovascular mortality rate was observed in the University Group Diabetes Program study [12].
Our aim was to review all available evidence to evaluate the risk-to-benefit balance of metformin in T2DM patients based on cardiovascular morbidity and mortality using a systematic review and meta-analysis of controlled trials.
Methods
Data Sources
Studies were identified by searching Medline, Embase, and the Cochrane database of systematic reviews (1 January 1950 through 31 July 2010) with the following key words: type 2 diabetes, diabetes mellitus; macrovascular; cardiovascular or coronary diseases, stroke, peripheral vascular disease; microvascular; retinopathy; neuropathy; nephropathy; and metformin. No language restrictions were applied. Reference lists of published meta-analyses were reviewed.
Study Selection
Included studies were randomised controlled trials that evaluated metformin effects in T2DM patients on cardiovascular morbidity or mortality as primary outcomes, secondary outcomes, or adverse events. We included studies comparing metformin to diet alone, placebo, or no treatment, as well as studies of metformin as an add-on therapy, i.e., a comparison of metformin versus no treatment combined with another treatment, and studies of metformin withdrawal. We did not include active-control metformin monotherapy studies.
Two investigators (R. B. and I. S.) independently reviewed the identified abstracts or manuscripts to determine which studies were eligible for inclusion in the meta-analysis.
Quality Assessment
The quality of selected articles was assessed by two independent investigators (R. B. and I. S.) using the Jadad score [13].
End Points
Two reviewers (R. B. and I. S.), independently and in duplicate, extracted numerical data for all the outcomes of interest from the included trials.
Primary end points were all-cause mortality and cardiovascular death. Secondary end points included: all myocardial infarctions (fatal and non-fatal), all strokes (fatal and non-fatal), congestive heart failure, peripheral vascular disease, leg amputations, and microvascular complications. End-point definitions referred to what was reported in the originally published papers. End points were not available for all studies included in this meta-analysis. Therefore, our evaluation was not always based on the overall studied population.
Statistical Analysis
For each trial, RRs and 95% CIs were calculated from the number of events in each group using a fixed-effects model. Summary data for each end point were obtained by pooling the RRs across studies. Statistical heterogeneity across trials was assessed with the χ2 statistic (p<0.1) and the I2 statistic [14]. The I2 statistic measures the proportion of overall variation that is attributable to between-study heterogeneity. The heterogeneity test was considered statistically significant if the p-value was under 0.1. Heterogeneity was considered high if the I2 was above 50%. Tau2 was calculated in order to determine the size and clinical relevance of heterogeneity when detected by the previous calculations [15]. A random-effects model was used when the heterogeneity test was statistically significant. Sensitivity analyses and an interaction test were performed based on (a) the Jadad score (≤3 versus >3) and (b) sulphonylurea as an add-on treatment (absent versus present).
Statistical analyses were performed according to the intention-to-treat principle. All p-values were two-sided (p<0.05). Analyses were performed using Revman software, version 5 (http://ims.cochrane.org/revman).
Results
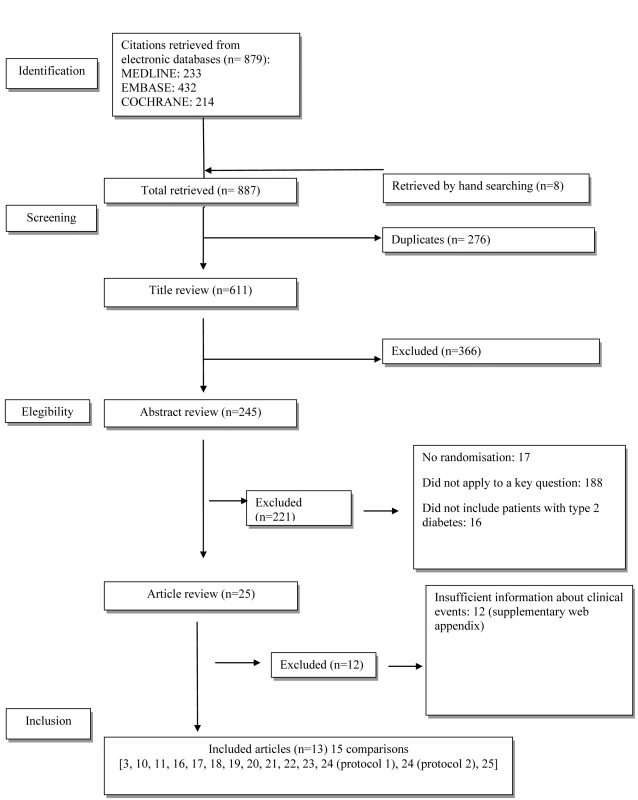
The flow diagram of study selection is shown in Figure 1. Overall, 25 trials met the inclusion criteria. Twelve trials were excluded because they did not report sufficient information about clinical events (see Text S2). Only four had a clinical event as the primary outcome: one double-blind controlled trial, Hyperinsulinemia: The Outcome of its Metabolic Effects (HOME) [16], and three open trials, UKPDS 34 [3], Rachmani et al. [10], and COSMIC [11]. Nine trials had clinical events as adverse events [17]–[25]. In five studies, metformin was given as an add-on to sulphonylurea [3],[18],[22]–[24], and in two studies, as an add-on to insulin [16],[20]; two studies were versus diet [3],[17], and two were versus usual care [10],[11].
The baseline characteristics of the selected studies are summarised in Table 1.
Tab. 1. Characteristics of Studies or Subgroups Included in the Meta-Analysis. 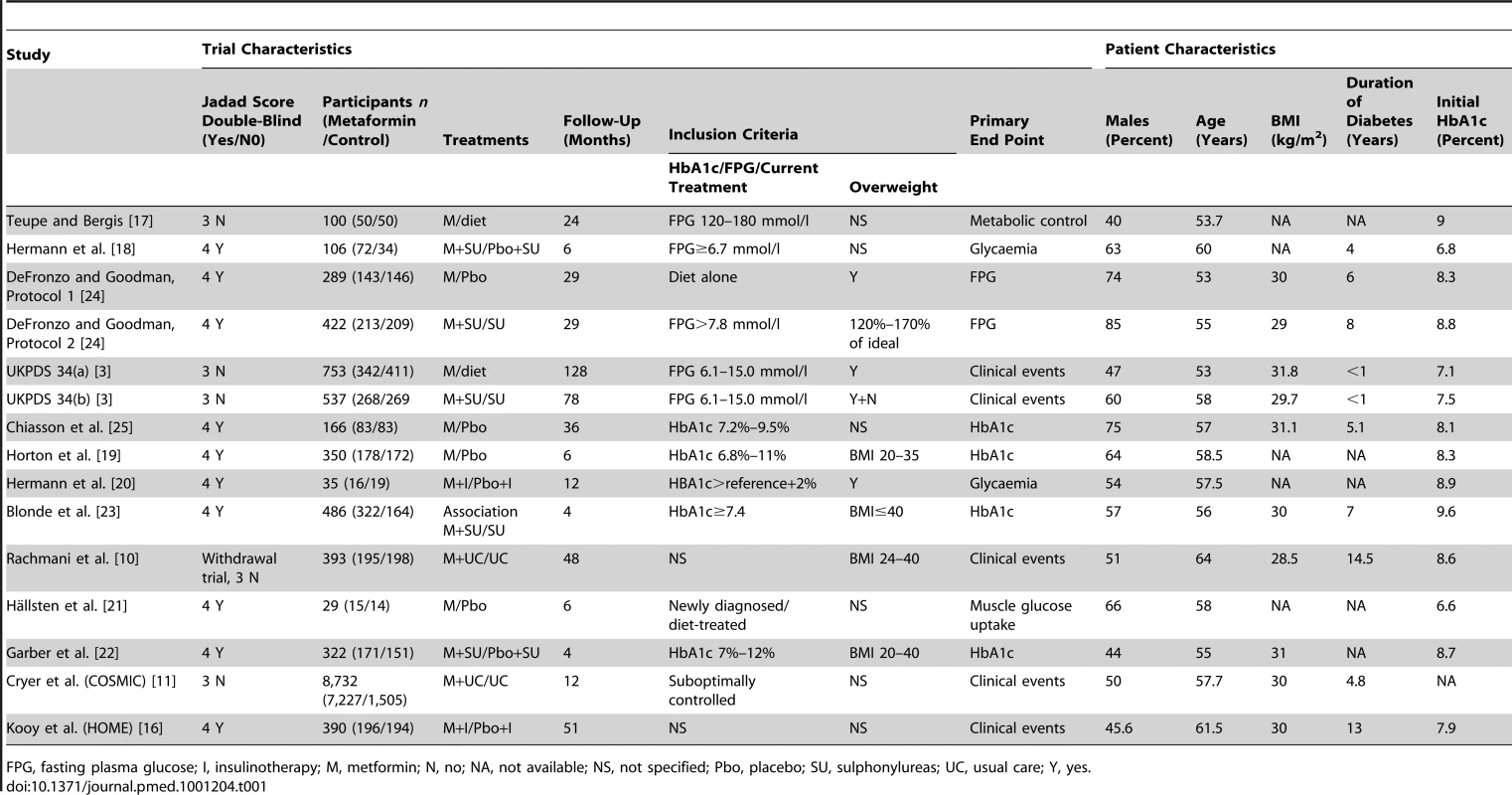
FPG, fasting plasma glucose; I, insulinotherapy; M, metformin; N, no; NA, not available; NS, not specified; Pbo, placebo; SU, sulphonylureas; UC, usual care; Y, yes. UKPDS 34 was divided into two parts. UKPDS 34(a) evaluated metformin plus diet versus diet alone, and UKPDS 34(b) evaluated metformin plus sulphonylurea versus sulphonylurea alone.
The present meta-analysis included 13,110 patients (Table 1). Among them, 50% were men; their mean age (range) was 57.7 (53–64) y; baseline mean body mass index (BMI) (range) was 30 (28.5–31.8) kg/cm2. The mean (range) duration of diabetes was 4.8 (0–14.5) y. In total, 9,560 patients were randomised to receive metformin, and 3,550 to receive the conventional or placebo treatment.
The effect of metformin on mortality and macrovascular complications is summarised in Figure 2.
Fig. 2. Forest plot for primary end points. 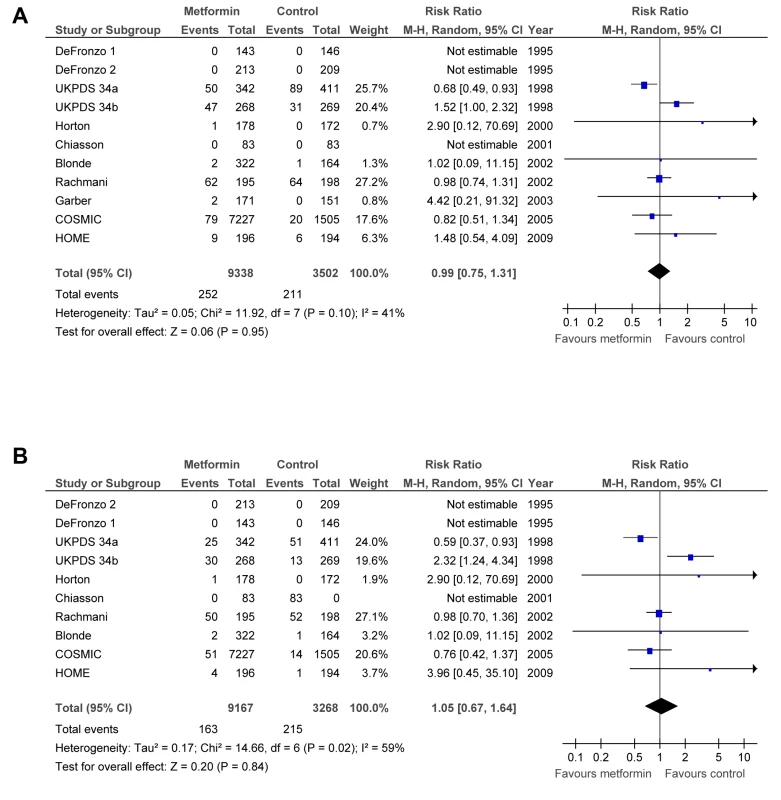
(A) All-cause mortality. (B) Cardiovascular mortality. df, degrees of freedom; M-H, Mantel–Haenszel odds ratio method. Primary End Points
Metformin did not significantly affect the primary end points: all-cause mortality (RR = 0.99; 95% CI: 0.75 to 1.31) or cardiovascular deaths (RR = 1.05; 95% CI: 0.67 to 1.64) (Figure 2). There was significant heterogeneity between trials for all-cause mortality (p = 0.10, Tau2 = 0.05, I2 = 41%) and cardiovascular deaths (p = 0.02, Tau2 = 0.17, I2 = 59%). The results did not change after restricting the analysis to trials with a Jadad score >3 or trials with clinical events as outcomes. The analysis of trials where metformin plus sulphonylurea was compared to sulphonylurea alone (see Text S1) shows a significant increase in all-cause mortality, RR = 1.53 (95% CI: 1.02 to 2.31), and in cardiovascular deaths, RR = 2.20 (95% CI: 1.20 to 4.03). UKPDS 34(a) represents most of the weight of this analysis.
After excluding UKPDS 34, the estimated RR for all-cause mortality (RR = 0.98; 95% CI: 0.77 to 1.24) and cardiovascular deaths (RR = 0.95; 95% CI: 0.72 to 1.26) did not change (plot not shown), but no heterogeneity was detected for all-cause mortality (p = 0.77, Tau2 = 0.00, I2 = 0%) or cardiovascular deaths (p = 0.61; Tau2 = 0.00, I2 = 0%). After excluding UKPDS 34(a), UKPDS 34(b), or both, the results remained not significant (plot not shown), and heterogeneity disappeared.
Secondary End Points
The rates of all myocardial infarctions (RR = 0.90; 95% CI: 0.74 to 1.09), all strokes (RR = 0.76; 95% CI: 0.51 to 1.14), heart failure (RR = 1.03; 95% CI: 0.67 to 1.59), peripheral vascular disease (RR = 0.90; 95% CI: 0.46 to 1.78), leg amputations (RR = 1.04; 95% CI: 0.44 to 2.44), and microvascular complications (RR = 0.83; 95% CI: 0.59 to 1.17) did not significantly differ between groups (Figure 3). There was no heterogeneity between trials for these end points. The results did not change after sensitivity analyses were performed (see Text S1).
Fig. 3. Forest plot for secondary end points. 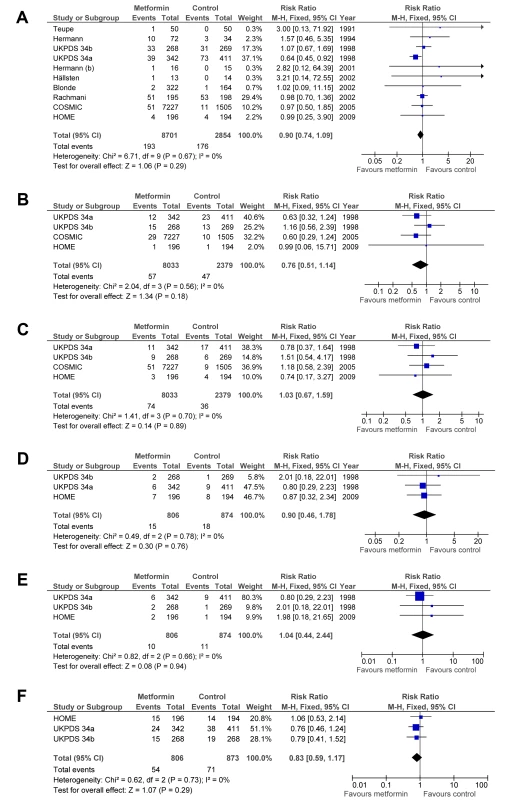
(A) All myocardial infarctions. (B) All strokes. (C) Heart failure. (D) Peripheral vascular events. (E) Amputation. (F) Microvascular complications. df, degrees of freedom; M-H, Mantel–Haenszel odds ratio method. Discussion
Major Results
The aim of this meta-analysis was to evaluate the clinical efficacy of metformin in the treatment of T2DM. Surprisingly, this meta-analysis shows no evidence for benefits of metformin in terms of all-cause or cardiovascular mortality and all diabetes macrovascular complications.
Considering the low number of randomised controlled trials included in this meta-analysis and the limited number of events, these results must be interpreted with caution. The consensus recommendations of diabetes experts are that the positive effects of metformin against mortality and CVD observed in UKPDS 34 need confirmation [4],[5]. According to our results, we cannot exclude beyond a reasonable doubt a 25% reduction or a 31% increase in all-cause mortality. We cannot exclude a 33% reduction or a 64% increase cardiovascular mortality.
We used the Mantel–Haenszel odds ratio method with a 0.5 zero-cell correction. This might have somehow biased the results. However, trials with very few or zero events have a very low weight in the meta-analysis. Even though 25 trials met the inclusion criteria, 12 trials could not be included in the meta-analysis because they did not report sufficient information about outcomes of interest.
The observed heterogeneity between studies on the end points mortality and cardiovascular mortality is not totally explained. Trial designs are heterogeneous: follow-up duration (e.g., 4 mo for Garber et al. [22] and Blonde et al. [23], up to 10 y for UKPDS 34 [3]), associated treatments, prior diabetes duration at inclusion, etc. Heterogeneity remained in the subgroup of studies where metformin was not associated with sulphonylurea for the outcome cardiovascular death. Therefore, concomitant treatment with sulphonylurea does not totally explain heterogeneity.
The inclusion of the UKPDS 34(b) subgroup (metformin plus sulphonylurea versus sulphonylurea alone) is what makes our meta-analysis unique. It may partially explain why our results are contradictory with those of previous systematic reviews [6],[7]. The authors of Cochrane systematic reviews excluded this subgroup because their aim was to analyse metformin only as a monotherapy. It is also noteworthy that the international community has emphasised and often cited the favourable results—i.e., showing a benefit from metformin—of UKPDS 34(a), but often not cited the unfavorable results of UKPDS 34(b). However, both groups are randomised and present the same level of evidence. The fact that UKPDS 34(a) is often cited but UKPDS 34(b) is not may be an example of biased knowledge created by excessively citing of a positive result [26]. Lamanna and al. [9] included the UKPDS 34(b) group in their meta-analysis, which had non-diabetic patients and those with type 1 and 2 diabetes, and obtained the same results as we did. Although they put forward the lack of proof for the overall benefit of metformin on cardiovascular events, they concluded that compared to placebo or no treatment, metformin has a benefit. Unlike Lamanna et al., we included different types of control groups (i.e., diet alone, placebo, no treatment) and included add-on therapy and metformin withdrawal studies, and we considered only T2DM. Our conclusion is that the clinical benefit of metformin is far from being demonstrated.
The deleterious effect of the combination of metformin plus sulphonylurea remains unexplained. Five studies in this meta-analysis compared metformin as an add-on therapy in patients receiving sulphonylurea [3],[18],[22]–[24]. There were more deaths, RR = 1.55 (95% CI: 1.03 to 2.33), but this result was mainly related to UKPDS 34 (35.1% of weight). In the ADVANCE study, the combination of sulphonylurea plus metformin was more frequent in the intensive treatment group. No increased risk of mortality was shown [27]. The RECORD study found the combination of metformin plus sulphonylurea “equivalent” to rosiglitazone on both outcomes: all-cause deaths and cardiovascular deaths [28]. However, rosiglitazone was removed from the European market because of safety concerns. Observational studies of metformin combination with sulphonylurea show contradictory results. Two recent studies did not find an increased risk [29],[30], whereas another study [31] and a meta-analysis of observational studies [32] suggest an increased risk of composite end points of CVD, hospital stays, or mortality (fatal and non-fatal events): RR = 1.43 (95% CI: 1.10 to 1.85).
The results of UKPDS 34(a) and (b) may be due to chance alone. Even though it was a randomised study, UKPDS 34 presents methodological weaknesses: the primary end point and study length were modified during the study, after notification of unfavourable results [33]–[36]. The absence of a placebo group and double-blinding could overestimate the benefits of metformin [37],[38]. There may be a bias in the follow-up and assessment of patients or an imbalance of concomitant treatments (such as statins or antihypertensive agents). Details on concomitant treatments received by the study participants in UKPDS 34 have not been published. The authors of the UKPDS 34 10-y follow-up did not provide explanations for and did not discuss the possible toxicity of the combination of metformin and sulphonylurea [39].
Moreover, metformin has no proven efficacy against the occurrence of microvascular complications. The fact that metformin might be ineffective is a possibility that should not be excluded. Metformin belongs to the biguanid class. The first molecule of this class, phenformin, induced increased cardiovascular risk in the University Group Diabetes Program study, which was a double-blind randomised controlled trial versus placebo [12].Pharmacologically speaking, there are few differences between metformin and phenformin [40]. Phenformin is monosubstituted by a longer side chain than metformin, thus conferring lipophilic characteristics and a greater affinity for mitochondrial membranes and an inhibitory effect on the functioning of the mitochondrial respiratory chain. These small molecular differences may explain a decreased risk of lactic acidosis with metformin [40], but are they enough to explain the only favourable results observed in the UKPDS 34(a) subgroup?
Our dataset did not allow a valid evaluation of the benefit of metformin on intermediate end points. Hirst et al. [41] performed a meta-analysis to address this question. Their work supports a clinically important lowering of glycated haemoglobin c (HbA1c) when metformin is used as a monotherapy and in combination with other therapeutic agents.
We were surprised by the small number of studies with enough evidence to evaluate the efficacy of metformin. This is consistent with the findings of Shaughnessy and Slawson [42] and Gandhi et al. [43]. They show that in a sample of registered, ongoing randomised controlled trials on diabetes, only 18% included patient-relevant outcomes as primary outcomes. The vast majority of clinical trials evaluating the efficacy of glucose-lowering drugs in diabetic patients use HbA1c levels as the primary outcome. This is often considered sufficient for licensing. However, because there is a lack of clinical evidence supported by a double-blind randomised controlled trial versus placebo on the clinical efficacy of antidiabetic drugs, it is not possible to prove the ability of HbA1c to predict and capture the effect of treatments [44]. HbA1c cannot be considered as a valid surrogate end point to establish the clinical efficacy of antidiabetic drugs according to the current state of scientific knowledge. In the UKPDS 34(b) subgroup [3], the combination of sulphonylurea and metformin lowered HbA1c levels more than in the group that took only sulphonylurea. The median rate at 4 y was 7.7% versus 8.2%, respectively. However, an excess of mortality was found in the group receiving the combined therapy.
Policy Implications
Metformin is universally recommended as the first-line treatment for T2DM, even though available evidence of its clinical efficacy is scarce. What should we think about the efficacy of other antidiabetic treatments? Lamanna et al. [9] compared metformin with other hypoglycaemic drugs, and found no difference for cardiovascular end points (OR = 1.03; 95% CI: 0.72 to 1.77, p = 0.89). This may be because all treatments have a real clinical benefit that was not demonstrated, or that none of them is beneficial. A large number of patients have taken these treatments over many years, even though there is the possibility of an overall unfavourable benefit/risk ratio. Of note, metformin can induce severe adverse effects such as lactic acidosis in the case of acute renal failure [45] or vitamin B12 deficiency [46].
If doctors doubt the efficacy of metformin because of our results, they may be tempted to prescribe other antidiabetic drugs whose benefits are even less well known. It is not certain whether this is beneficial for patients. In their meta-analysis of retrospective cohort studies, Tzoulaki et al. [47] compared metformin monotherapy with first - or second-generation sulphonylureas on the risk of mortality and congestive heart failure. Their results showed a significant increase (24%–61%) in all-cause mortality associated with first-generation sulphonylureas, while second-generation sulphonylureas were associated with an 18% to 30% increase in congestive heart failure. Insulin therapy is potentially associated with an increase in all-cause mortality [48], especially in patients with heart failure [49]. Sulphonylurea and insulin therapy may be associated with an increase in cancer mortality [50]. In a recent cohort study including more than 62,000 patients, Currie et al. [51] provided evidence that sulphonylurea and insulin treatments in monotherapy are associated with an increased risk of solid cancers (HR = 1.36 and 1.42, respectively) compared to metformin [51]. After a marketing period of more than 10 y, the European Medicines Agency decided to withdraw rosiglitazone from the European market because of its unfavourable benefit/risk ratio, while the US Food and Drug Administration restricted its use. The adverse effects, such as myocardial infarction or death from cardiovascular causes, are well documented [52]. The increased risk of congestive heart failure and weight gain makes the benefit/risk ratio of pioglitazone unclear [53].
Compared with other antidiabetic treatments, metformin may be the one with the least disadvantages. It does not induce hypoglycaemia, weight gain, and heart failure. It is also associated with a reduced rate of mortality among patients with atherothrombosis [54].
Conclusion
The specific efficacy of metformin to prevent death or cardiovascular events has not been proven by current studies. The number and quality of available studies are insufficient. We cannot exclude beyond any reasonable doubt that metformin use increases or decreases the risk of all-cause mortality or cardiovascular mortality. Further studies are needed to clarify this problematic situation. Metformin may not be the best comparator for evaluating new hypoglycaemic drugs. However, it is not clear which comparator has the most favourable risk/benefit ratio.
Supporting Information
Zdroje
1. WildSGreenASicreeRKingH 2004 Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27 1047 1053
2. The Emerging Risk Factors Collaboration 2010 Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375 2215 2222
3. UK Prospective Diabetes Study (UKPDS) Group 1998 Effect of Intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352 854 865
4. NathanDMBuseJBDavidsonMBFerranniniEHolmanRR 2009 Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 32 193 203
5. National Institute for Health and Clinical Excellence 2008 May Type 2 diabetes: the management of type 2 diabetes. Available: http://www.nice.org.uk/CG66. Accessed 18 May 2011
6. SaenzAFernandez-EstebanIMataixAAusejo SeguraMRoqué i FigulsM 2005 Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005 CD002966
7. SelvinEBolenSYehHCWileyCWilsonLM 2008 Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med 168 2070 2080
8. BennettWLMaruthurNMSinghSSegalJBWilsonLM 2011 Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med 154 602 613
9. LamannaCMonamiMMarchionniNMannucciE 2011 Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomised clinical trials. Diabetes Obes Metab 13 221 228
10. RachmaniRSlavachevskiILeviZZadokBKedarY 2002 Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med 13 428 433
11. CryerDMillsDNicholasSPStadelBVHenryDH 2005 Comparative outcomes study of metformin intervention versus conventional approach. Diabetes Care 28 539 543
12. The University Group Diabetes Program 1975 A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of phenformin therapy. Diabetes 24 Suppl 1 65 184
13. JadadARMooreRACarrollDJenkinsonCReynoldsDJ 1996 Assessing the quality of reports on randomised clinical trials: Is blinding necessary? Control Clin Trials 17 1 12
14. HigginsJPThompsonSG 2002 Quantifying heterogeneity in a meta-analysis. Stat Med 21 1539 1558
15. RückerGSchwarzerGCarpenterJRSchumacherM 2008 Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 27 79 Available: http://www.biomedcentral.com/1471-2288/8/79. Accessed 5 March 2012
16. KooyADe JagerJLehertPBetsDWulffeléMG 2009 Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 169 616 625
17. TeupeBBergisK 1991 Prospective randomised two-years clinical study comparing additional metformin treatment with reducing diet in type 2 diabetes. Diabete Metab 17 213 217
18. HermannLSSchersténBBitzénPOKjellströmTLindgärdeF 1994 Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care 17 1100 1109
19. HortonEFoleyJClinkingbeardCMallowsSGatlinM 2000 Nateglinide alone and in combination with Metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care 23 1660 1665
20. HermannLSKalénJKatzmanPLagerINilssonA 2001 Long-term glycaemic improvement after addition of metformin to insulin in insulin-treated obese type 2 diabetes patients. Diabetes Obes Metab 3 428 434
21. HällstenKVirtanenKALönnqvistFSipiläHOksanenA 2002 Rosiglitazone but not metformin enhances insulin - and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes 51 3479 3485
22. GarberAJDonovanDSDandonaPBruceSParkJS 2003 Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J Clin Endocrinol Metab 88 3598 3604
23. BlondeLRosenstockJMooradianADPiperBAHenryD 2002 Glyburide/metformin combination product is safe and efficacious in patients with type 2 diabetes failing sulphonylureas therapy. Diabetes Obes Metab 4 368 375
24. DeFronzoRAGoodmanAM 1995 Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 333 541 549
25. ChiassonJLNadtichL for the Miglitol Canadian University Investigator Group 2001 The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care 24 989 994
26. GreenbergSA 2009 How citation distortions create unfounded authority: analysis of a citation network. BMJ 339 b2680 doi:10.1136/bmj.b2680
27. ADVANCE Collaborative Group 2008 Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358 2560 2572
28. HomePDPocockSJBeck-NielsenHCurtisPSGomisR 2009 Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373 2125 2135
29. AzoulayLSchneider-LindnerVDell'anielloSSchiffrinASuissaS 2010 Combination therapy with sulphonylureas and metformin and the prevention of death in type 2 diabetes: a nested case-control study. Pharmacoepidemiol Drug Saf 19 335 342
30. AnderssonCOlesenJBHansenPRWeekePNorgaardML 2010 Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia 53 2546 2553
31. SillarsBDavisWAHirschIBDavisTM 2010 Sulphonylureas-metformin combination therapy, cardiovascular disease and all-cause mortality: the Fremantle Diabetes Study. Diabetes Obes Metab 9 757 765
32. RaoADKuhadiyaNReynoldsKFonsecaVA 2008 Is the combination of sufonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality. Diabetes Care 31 1672 1678
33. NathanDM 1998 Some answers, more controversy, from UKPDS. Lancet 352 832 833
34. EwartRM 2001 The case against agressive treatment of type 2 diabetes: critique of the UK prospective diabetes study. BMJ 323 854 858
35. McCormackJGreenhalghT 2000 Seeing what you want to see in randomised controlled trials: versions and perversions of UKPDS data. BMJ 320 1720 1723
36. UK Prospective Diabetes Study (UKPDS) Group 1998 Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352 837 853
37. SchulzKFChalmersIHayesRJAltmanDG 1995 Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273 408 412
38. MoherDPhamBJonesACookDJJadadAR 1998 Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352 609 613
39. HolmanRRMatthewsDRNeilHA 2009 Follow-up of intensive glucose control in type 2 diabetes. The authors reply. N Engl J Med 360 416 418
40. BaileyC 1996 Metformin. N Engl J Med 334 574 579
41. HirstJAFarmerAJAliRRobertsNWStevensRJ 2012 Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care 35 446 454
42. ShaughnessyAFSlawsonDC 2003 What happened to the valid POEMs? A survey of review articles on the treatment of type 2 diabetes. BMJ 327 266 273
43. GandhiGYMuradMHFujiyoshiAMullanRJFlynnDN 2008 Patient-important outcomes in registered diabetes trials. JAMA 299 2543 2549
44. PrenticeRL 1989 Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 8 431 440
45. FitzgeraldEMathieuSBallA 2009 Metformin associated lactic acidosis. BMJ 339 b3660 doi:10.1136/bmj.b3660
46. De JagerJKooyALehertPWulffeléMGvan der KolkJ 2010 Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 340 c2181 doi:10.1136/bmj.c2181
47. TzoulakiIMolokhiaMCurcinVLittleMPMillettCJ 2009 Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 339 b4731 doi:10.1136/bmj.b4731
48. GambleJMSimpsonSHEurichDTMajumdarSRJohnsonJA 2010 Insulin use and increased risk of mortality in type 2 diabetes: a cohort study. Diabetes Obes Metab 12 47 53
49. EurichDTMcAlisterFABlackburnDFMajumdarSRTsuyukiRT 2007 Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ 335 497
50. BowkerSLMajumdarSRVeugelersPJohnsonJA 2006 Increased cancer-related mortality for patients with type 2 diabetes who use sulphonylureas or insulin. Diabetes Care 29 254 258
51. CurrieCJPooleCDGaleEA 2009 The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52 1766 1777
52. NissenSEWolskiK 2007 Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Eng J Med 356 2457 2471
53. RichterBBandeira-EchtlerEBergerhoffKClarCEbrahimSH 2006 Pioglitazone for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006 CD006060
54. RousselRTravertFPasquetBWilsonPWFSmithSCJr 2010 Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 170 1892 1899
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Medical Evidence of Human Rights Violations against Non-Arabic-Speaking Civilians in Darfur: A Cross-Sectional Study
- New Methodology for Estimating the Burden of Infectious Diseases in Europe
- Reappraisal of Metformin Efficacy in the Treatment of Type 2 Diabetes: A Meta-Analysis of Randomised Controlled Trials
- Does Conflict of Interest Disclosure Worsen Bias?
- Open Clinical Trial Data for All? A View from Regulators
- The Imperative to Share Clinical Study Reports: Recommendations from the Tamiflu Experience
- Where There Is No Health Research: What Can Be Done to Fill the Global Gaps in Health Research?
- The Role of Public Health Institutions in Global Health System Strengthening Efforts: The US CDC's Perspective
- Long-Term Exposure to Silica Dust and Risk of Total and Cause-Specific Mortality in Chinese Workers: A Cohort Study
- Ovarian Cancer and Body Size: Individual Participant Meta-Analysis Including 25,157 Women with Ovarian Cancer from 47 Epidemiological Studies
- Is Food Insecurity Associated with HIV Risk? Cross-Sectional Evidence from Sexually Active Women in Brazil
- Induction of Labor versus Expectant Management in Women with Preterm Prelabor Rupture of Membranes between 34 and 37 Weeks: A Randomized Controlled Trial
- Prioritizing CD4 Count Monitoring in Response to ART in Resource-Constrained Settings: A Retrospective Application of Prediction-Based Classification
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Induction of Labor versus Expectant Management in Women with Preterm Prelabor Rupture of Membranes between 34 and 37 Weeks: A Randomized Controlled Trial
- The Imperative to Share Clinical Study Reports: Recommendations from the Tamiflu Experience
- Long-Term Exposure to Silica Dust and Risk of Total and Cause-Specific Mortality in Chinese Workers: A Cohort Study
- Prioritizing CD4 Count Monitoring in Response to ART in Resource-Constrained Settings: A Retrospective Application of Prediction-Based Classification
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání