-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLong-Term Exposure to Silica Dust and Risk of Total and Cause-Specific Mortality in Chinese Workers: A Cohort Study
Background:
Human exposure to silica dust is very common in both working and living environments. However, the potential long-term health effects have not been well established across different exposure situations.Methods and Findings:
We studied 74,040 workers who worked at 29 metal mines and pottery factories in China for 1 y or more between January 1, 1960, and December 31, 1974, with follow-up until December 31, 2003 (median follow-up of 33 y). We estimated the cumulative silica dust exposure (CDE) for each worker by linking work history to a job–exposure matrix. We calculated standardized mortality ratios for underlying causes of death based on Chinese national mortality rates. Hazard ratios (HRs) for selected causes of death associated with CDE were estimated using the Cox proportional hazards model. The population attributable risks were estimated based on the prevalence of workers with silica dust exposure and HRs. The number of deaths attributable to silica dust exposure among Chinese workers was then calculated using the population attributable risk and the national mortality rate. We observed 19,516 deaths during 2,306,428 person-years of follow-up. Mortality from all causes was higher among workers exposed to silica dust than among non-exposed workers (993 versus 551 per 100,000 person-years). We observed significant positive exposure–response relationships between CDE (measured in milligrams/cubic meter–years, i.e., the sum of silica dust concentrations multiplied by the years of silica exposure) and mortality from all causes (HR 1.026, 95% confidence interval 1.023–1.029), respiratory diseases (1.069, 1.064–1.074), respiratory tuberculosis (1.065, 1.059–1.071), and cardiovascular disease (1.031, 1.025–1.036). Significantly elevated standardized mortality ratios were observed for all causes (1.06, 95% confidence interval 1.01–1.11), ischemic heart disease (1.65, 1.35–1.99), and pneumoconiosis (11.01, 7.67–14.95) among workers exposed to respirable silica concentrations equal to or lower than 0.1 mg/m3. After adjustment for potential confounders, including smoking, silica dust exposure accounted for 15.2% of all deaths in this study. We estimated that 4.2% of deaths (231,104 cases) among Chinese workers were attributable to silica dust exposure. The limitations of this study included a lack of data on dietary patterns and leisure time physical activity, possible underestimation of silica dust exposure for individuals who worked at the mines/factories before 1950, and a small number of deaths (4.3%) where the cause of death was based on oral reports from relatives.Conclusions:
Long-term silica dust exposure was associated with substantially increased mortality among Chinese workers. The increased risk was observed not only for deaths due to respiratory diseases and lung cancer, but also for deaths due to cardiovascular disease.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(4): e32767. doi:10.1371/journal.pmed.1001206
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001206Summary
Background:
Human exposure to silica dust is very common in both working and living environments. However, the potential long-term health effects have not been well established across different exposure situations.Methods and Findings:
We studied 74,040 workers who worked at 29 metal mines and pottery factories in China for 1 y or more between January 1, 1960, and December 31, 1974, with follow-up until December 31, 2003 (median follow-up of 33 y). We estimated the cumulative silica dust exposure (CDE) for each worker by linking work history to a job–exposure matrix. We calculated standardized mortality ratios for underlying causes of death based on Chinese national mortality rates. Hazard ratios (HRs) for selected causes of death associated with CDE were estimated using the Cox proportional hazards model. The population attributable risks were estimated based on the prevalence of workers with silica dust exposure and HRs. The number of deaths attributable to silica dust exposure among Chinese workers was then calculated using the population attributable risk and the national mortality rate. We observed 19,516 deaths during 2,306,428 person-years of follow-up. Mortality from all causes was higher among workers exposed to silica dust than among non-exposed workers (993 versus 551 per 100,000 person-years). We observed significant positive exposure–response relationships between CDE (measured in milligrams/cubic meter–years, i.e., the sum of silica dust concentrations multiplied by the years of silica exposure) and mortality from all causes (HR 1.026, 95% confidence interval 1.023–1.029), respiratory diseases (1.069, 1.064–1.074), respiratory tuberculosis (1.065, 1.059–1.071), and cardiovascular disease (1.031, 1.025–1.036). Significantly elevated standardized mortality ratios were observed for all causes (1.06, 95% confidence interval 1.01–1.11), ischemic heart disease (1.65, 1.35–1.99), and pneumoconiosis (11.01, 7.67–14.95) among workers exposed to respirable silica concentrations equal to or lower than 0.1 mg/m3. After adjustment for potential confounders, including smoking, silica dust exposure accounted for 15.2% of all deaths in this study. We estimated that 4.2% of deaths (231,104 cases) among Chinese workers were attributable to silica dust exposure. The limitations of this study included a lack of data on dietary patterns and leisure time physical activity, possible underestimation of silica dust exposure for individuals who worked at the mines/factories before 1950, and a small number of deaths (4.3%) where the cause of death was based on oral reports from relatives.Conclusions:
Long-term silica dust exposure was associated with substantially increased mortality among Chinese workers. The increased risk was observed not only for deaths due to respiratory diseases and lung cancer, but also for deaths due to cardiovascular disease.
: Please see later in the article for the Editors' SummaryIntroduction
Crystalline silica is one of the most ubiquitous minerals on earth, with widespread exposure in working and living environments. Multiple serious diseases and increased mortality have been associated with exposure to crystalline silica, making it a high-priority public health concern. Occupational silica exposure and its related health effects rank among the most important public health concerns in developing and developed nations. Recent reports indicate that more than 23 million workers are exposed to crystalline silica in China [1] and over 10 million in India alone [2]. In the United States and Europe, the respective figures are 1.7 million [3] and over 3 million [4]. Silica dust is generated at industrial sources and transported to environments, and it is also generated by such natural phenomena as volcanic explosions and sandstorms.
Adverse health effects from exposure to silica dust are of increasing public health concern worldwide, and have been studied for many years [5]. Silicosis is a well known consequence of silica dust exposure, and exposure has also been associated with the risk of lung cancer, pulmonary tuberculosis, and other airway diseases [6]–[9]. However, silica-related health effects are not limited to those diseases [10]. The potential health effects of particulate exposure on cardiovascular diseases (CVDs) have drawn recent attention, but have yet to be well studied in workers exposed to silica dust. Several studies suggest that ambient particulates (mainly combustion-sourced) are associated with an elevated risk of death [11]–[13] and CVD [14],[15]. Silica is a non-combustion-sourced particle, but its role in the pathogenesis of CVD also needs to be addressed. In addition, adverse health effects from low levels of silica exposure (below legally set exposure limits) need further evaluation.
Therefore, we present results from a retro-prospective cohort study of 74,040 Chinese workers followed from January 1, 1960, to December 31, 2003. Cumulative silica dust exposure (CDE) was calculated for each worker using a job–exposure matrix (JEM) based on a large number of measurements broken down by job title and collected since 1950. Our objectives were to quantify the health effects of silica exposure on cause-specific mortality and to determine population attributable risks (PARs) of mortality associated with the exposure in Chinese workers.
Methods
Study Population and Health Data
We identified 74,040 workers from 20 metal mines and nine pottery factories in central and southern China. All individuals were unrelated ethnic Han Chinese. We selected workplaces with systematically collected data on silica dust exposure and workers' health condition. The study included ten tungsten mines in Jiangxi and Hunan provinces, six iron and copper mines in Hubei province, four tin mines in Guangxi province, and nine pottery factories in Jiangxi, Hunan, and Henan provinces (Figure S1). The cohort included all 74,040 workers who were registered in company employment records—which included personnel files, individual medical records, occupational records, and wage rosters—for at least 1 y between January 1, 1960, and December 31, 1974. We collected retrospective data on vital status, work history, and newly diagnosed pneumoconiosis (silicosis) until 1986, with mortality follow-up until the end of 2003.
Trained investigators used a questionnaire to collect data on demographic information, cigarette use, and drinking habits since 1986. In 2004, occupational history and other updates were collected from survivors or those still employed. We defined positive silica dust exposure status as employment in a silica-dust-exposed job for 6 mo or more. Work histories for silica-dust-exposed workers were taken from company occupational records. Data included job titles, work start and end dates, and reasons for leaving (e.g., retirement or workplace change).
All individuals were tracked for their vital status by local hygienists (or occupational health doctors) from January 1, 1960, through December 31, 2003. We classified cause of death evidence by levels of confidence in the data: Level 1—medical record from a hospital or a personal doctor at a local hospital (60.5%); Level 2—cause of death recorded in an employment register, accident record, or death certificate (35.2%); and Level 3—oral reports from relatives (4.3%). Results did not change materially after excluding Level 3 deaths. We used the 10th International Classification of Diseases (ICD-10) to code causes of death.
All workers exposed to silica dust received chest radiographs every 2 to 4 y, even after cessation of dust exposure. National diagnostic criteria for pneumoconiosis were standardized as stage I, II, or III. These categories have been previously described [16]. The study was approved by the Tongji Medical College Institutional Review Board and the US National Institute for Occupational Safety and Health Institutional Review Board.
Occupational Exposure Data
We conducted a detailed quantitative occupational exposure evaluation using data from historical industrial health records. Industrial health record-keeping for occupational hazards in each mine or factory started in the early 1950s, when the Chinese government enforced systematic dust sampling regulations that required monthly measurement of dust concentrations in workplaces. The dust monitoring scheme involved measuring total airborne dust concentration by a gravimetric method for each dust-exposed job title, and using a microscopic sizing method to determine particle size distribution and crystalline silica content (quartz by X-ray diffraction method) in bulk samples of settled dust [17].
For the purpose of this study, more than 4,200,000 environmental measurements of total dust concentrations from 29 mines and factories from 1950 to 2003 were used to create a JEM. In this matrix, the total dust concentrations associated with each job title were averaged by year, then listed, along with specific facility and job titles, for each calendar year [18]. For missing data for years or jobs (less than 20%), total dust concentrations were estimated using monitoring data for similar jobs or for the same job at different times. In the matrix, there were 1,090 facility–job title combinations for 54 calendar-year periods from 1950 to 2003.
We used the JEM of total dust concentrations to estimate silica dust exposure for each worker. In this matrix, total silica dust concentrations were listed along with specific facility and job titles for each calendar year. We used all available total dust concentrations for each job to create this JEM. The results indicated good agreement for measured total dust concentrations (r2 = 0.84) between Chinese and US methods [19]. To convert the total dust JEM into a respirable silica JEM, each respirable silica concentration was estimated by total dust concentration multiplied by a conversion factor. The conversion factors from Chinese total dust to US respirable silica concentrations (quartz by X-ray diffraction method) were developed based on paired side-by-side dust measurements. The conversion factors of respirable silica concentration to total dust concentration were estimated to be 0.0143 for iron/copper mines, 0.0355 for pottery factories, 0.0429 for tin mines, and 0.0861 for tungsten mines [17]. These conversion factors were updated in a recent analysis with additional data from the same side-by-side measurements conducted from September 1, 2003, to June 30, 2009. The new analysis confirmed that the mean crystalline silica percentage of total dust measurements did not change substantially over time.
Complete work histories for each study individual were taken from personal employment records in mine/factory files. Work histories include job titles and calendar years for each worker's full duration of employment. They were used with the JEM to estimate CDE for individual workers as follows:where CDE is cumulative respirable silica dust exposure in milligrams/cubic millimeter–years; n is the total number of job titles held by the individual during his or her work history; Cj is 8-h time-weighted mean concentrations of dust in milligrams/cubic millimeter for the jth job title within a facility and employment period; and Tj is duration of employment in years in the jth job. We calculated CDE from the starting date of dust-exposed work until employees were either lost to follow-up, ended employment, or died.
We used data from a standardized monitoring program in all industrial facilities to track potential environmental hazards, including radon, polycyclic aromatic hydrocarbons, asbestos, talc, and metal elements. Findings indicated very low exposure to asbestos, nickel, talc, and cadmium in the studied workplaces.
Statistical Analysis
We used Cox proportional hazards regressions to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for selected causes of death by different levels of CDE compared with no exposure. CDE was categorized into low, medium, and high levels based on equally spaced percentiles from the exposure distribution in the entire cohort. Further, tests of linear trend were conducted by including the median value for each level of CDE as a continuous variable in the models. We also estimated the HRs associated with a 1 mg/m3-y increase in CDE by entering CDE into the models as a continuous variable. In addition, nonlinear association was assessed by adding a quadratic term (CDE and square of CDE, continuous) to the model. Other covariates included in the model were gender, year of hire (five categories, 1955 or earlier, 1956–1960, 1961–1965, 1966–1970, and 1970 or later), age at hire (continuous), and type of mine/factory (four categories, tungsten, iron/copper, tin, and pottery) as potential confounders. For mortality with possible nonlinear associations, we further examined the detailed exposure–response relationship of mortality risk across the range of CDEs using a penalized spline regression model [20]. The sample size (2.3 million person-years) was too large for fitting a Cox proportional hazards model with penalized splines; therefore, we created a nested case–control sample for each specific cause of death by randomly selecting 20 controls (matched for type of mine/factory and gender) for each case who were alive at the time of the case's death. The penalized spline regression model was fitted with and without adjustment for smoking (never smoked/ever smoked) to detect the potential confounding effect of smoking. The sensitivity of the model was tested by selecting different degrees of freedom and excluding influential outliers.
The PAR was calculated with the following equation:where P is the prevalence of silica-exposed workers among all industrial workers (16.3%) [1], and RR is the multivariate-adjusted relative risk. The HRs from Cox proportional hazards models of this cohort were used as estimates of RR.
Standardized mortality ratios (SMRs) were defined as the ratio of observed to expected deaths [21]. National death rate data were not available before 1970; therefore, individuals who died before that time were not included in the SMR analysis. In total, SMRs were calculated using 17,783 deaths. We calculated the expected number of cause-specific deaths by multiplying the gender-, age-, period-, and cause-specific person-years at risk (5-y intervals for age and period) by the corresponding mortality rates in the Chinese national population [22]. We obtained the 95% CIs for SMRs by setting limits for the numerator and the observed number of cases, and by assuming the denominator to be a constant [21]. A p-value≤0.05 was considered statistically significant. The penalized spline regression analyses were conducted using S-Plus version 8.0 (Insightful Corporation); all other analyses were performed using SAS version 9.1 (SAS Institute).
Results
The cohort included 74,040 individuals (63,529 males, 85.8%). The average age was 27.2 y for individuals entering into the cohort. And 16.2% were still working at the end of follow-up (Table S1). The baseline characteristics of the cohort and follow-up information are summarized in Table 1. A total of 49,309 (66.6%) of workers were exposed to silica dust. The largest number of exposed workers (78.9%) worked in tungsten mines; the lowest (48.5%), in iron and copper mines. During a median follow-up period of 33.1 y (2,306,428 person-years), 19,516 deaths were reported. Mortality was 846.2 per 100,000 person-years, with 992.6 per 100,000 person-years among dust-exposed workers and 550.7 per 100,000 person-years among non-dust-exposed workers.
Tab. 1. Description of the cohort (<i>n</i> = 74,040) based on different types of mine/factory, 1960–2003. 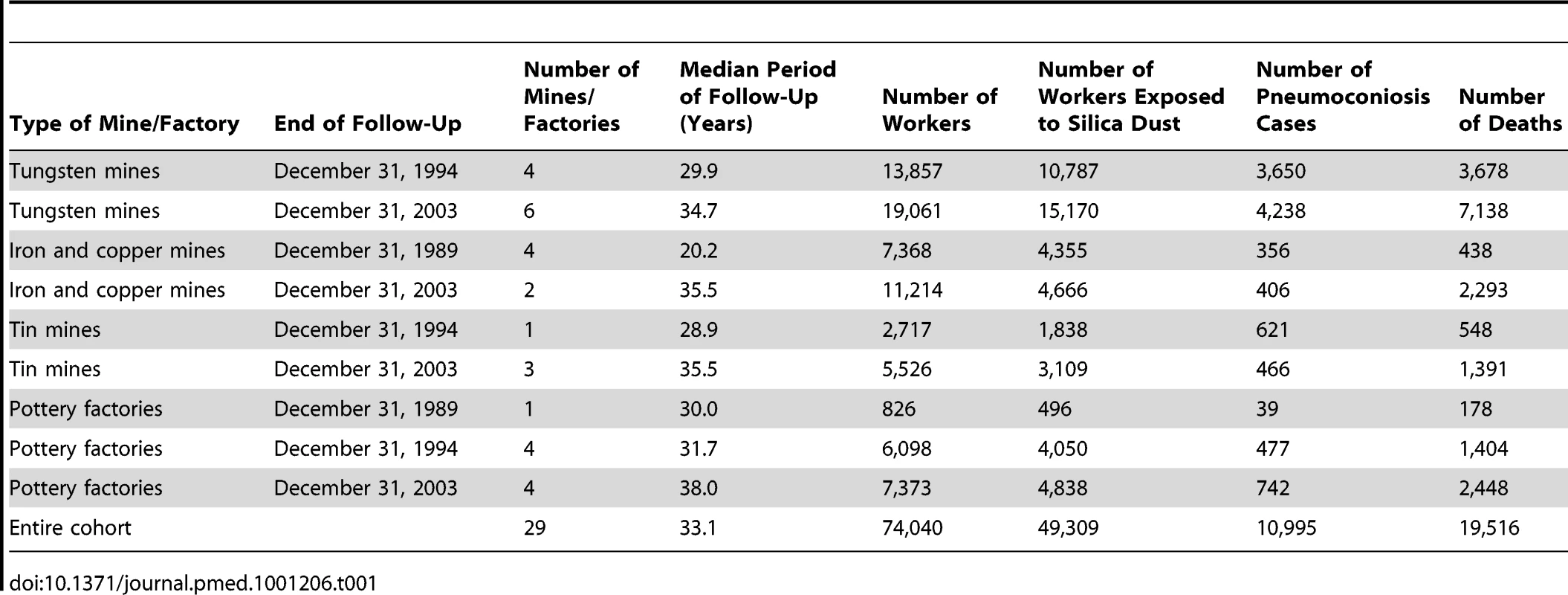
Respirable silica dust levels in the four types of mines/factories from 1960 to the end of 2003 are shown in Figure 1. The mean respirable silica dust concentrations ranged from approximately 0.08 mg/m3 in iron mines to 0.52 mg/m3 in tungsten mines. Starting in 1960, safer working practices and increased protection measures led to decreased dust concentration and exposure. Mean dust concentration in mines fell to less than 0.1 mg/m3 after 1970. Mean dust concentrations in pottery factories were approximately 0.15 to 0.30 mg/m3 from the 1960s to the 1980s, and 0.12 to 0.15 mg/m3 after 1990.
Fig. 1. Annual silica dust concentrations, average of all job titles in different mines/factories in China, 1950–2003. 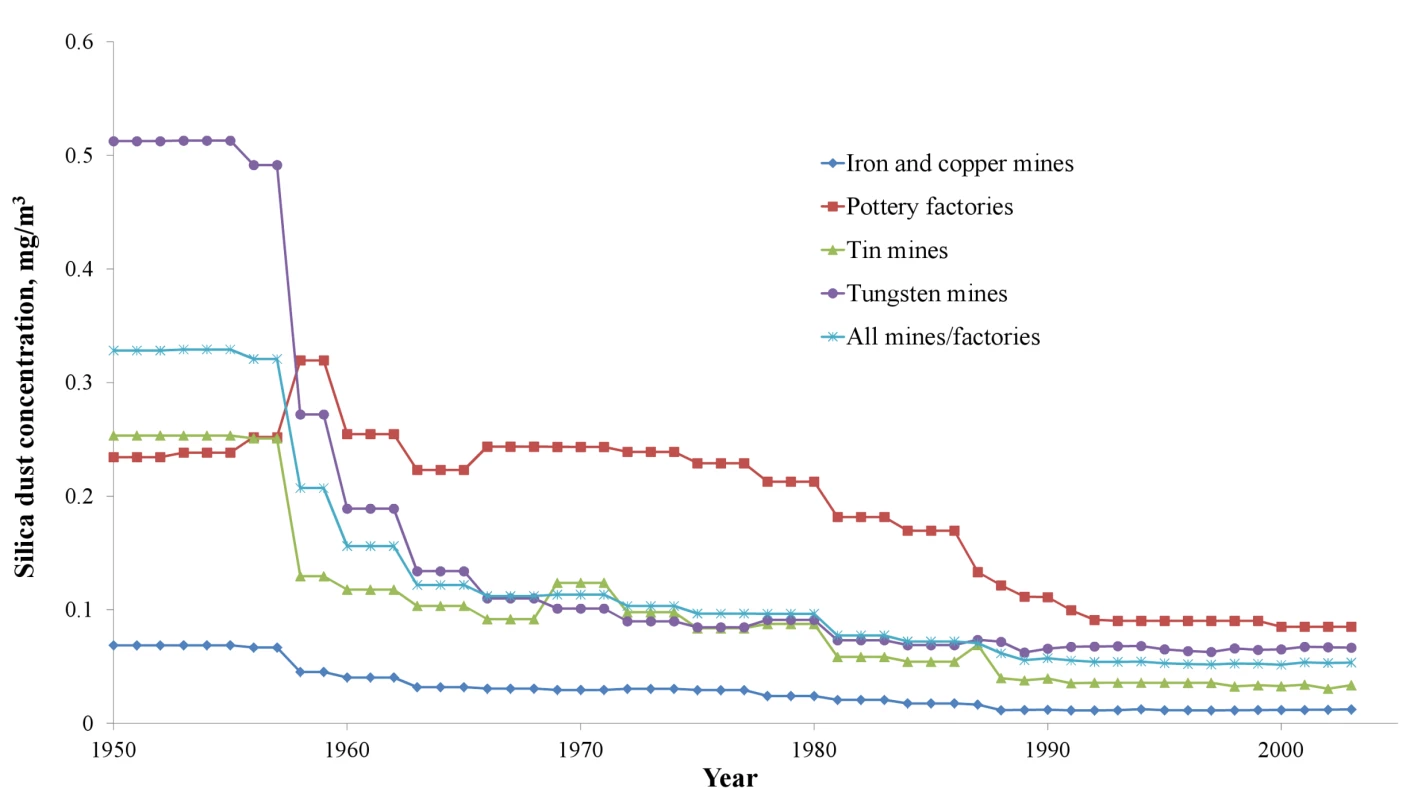
Table 2 shows the distribution of individuals according to different silica dust exposure levels, the year of birth, age at first hire, CDE, and prevalence of pneumoconiosis. Males accounted for 92.6% of those exposed to silica dust. The prevalence of smokers (current and former) among the entire cohort and the dust-exposed workers was 61.7% (98.7% for males) and 69.4% (99.1% for males), respectively.
Tab. 2. Characteristics of the cohort (n = 74,040) based on CDE, 1960–2003. 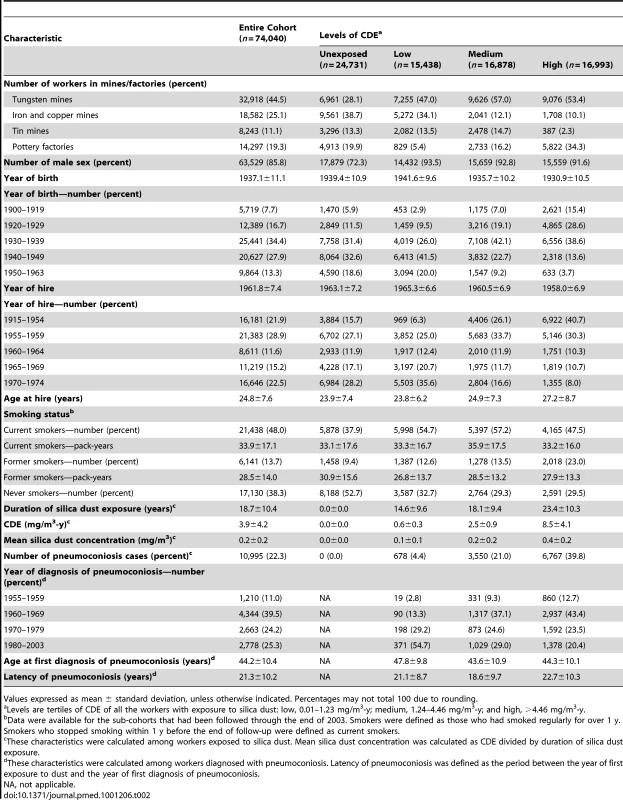
Values expressed as mean ± standard deviation, unless otherwise indicated. Percentages may not total 100 due to rounding. The numbers of deaths and the HRs for the main mortality causes are shown in Table 3. CVD was the leading cause of death in this cohort. Non-malignant respiratory diseases, malignant neoplasms, infectious diseases, and cerebrovascular disease were the second to fifth causes of death for all cohort members. Mortality from all causes was significantly higher in the group exposed to silica dust compared with the non-exposed group (HR 1.38, 95% CI 1.33–1.43). For both categorical and continuous CDE variables, a positive exposure–response relationship was observed for mortality from all causes, from CVDs (including pulmonary heart disease), from respiratory diseases (including pneumoconiosis), and from infectious diseases (including respiratory tuberculosis). Each 1 mg/m3-y increase in CDE was associated with a 2.6%, 6.9%, and 3.1% increase in the mortality risk for all causes, respiratory diseases, and CVDs, respectively. For lung cancer, a positive exposure–response association was observed for categorical CDE (HRs 1.45, 1.53, and 1.46 for low, medium, and high levels of CDE, respectively; p-value for linear trend = 0.01); however, the HR for each 1 mg/m3-y increase in CDE did not reach statistical significance (HR 1.005, 95% CI 0.987–1.023). The HRs for the association between total and cause-specific mortality and the continuous CDE variable, estimated using penalized spline regressions, are shown in Figure 2. The risk of mortality from all causes increased monotonically with increased CDE; risk of mortality from lung cancer, CVDs (including pulmonary heart disease), and diseases of the respiratory system (including pneumoconiosis) increased monotonically when CDE was lower than about 10 mg/m3-y, but became attenuated or even decreased (lung cancer) with higher CDE. Adjustment for smoking slightly attenuated the estimates for lung cancer and pneumoconiosis, but did not change the results for other causes of death (Figure 2).
Fig. 2. Estimated HRs for total and cause-specific mortality associated with a continuous CDE variable in nested case–control samples from workers with detailed data on historical silica exposure and smoking, 1960–2003. 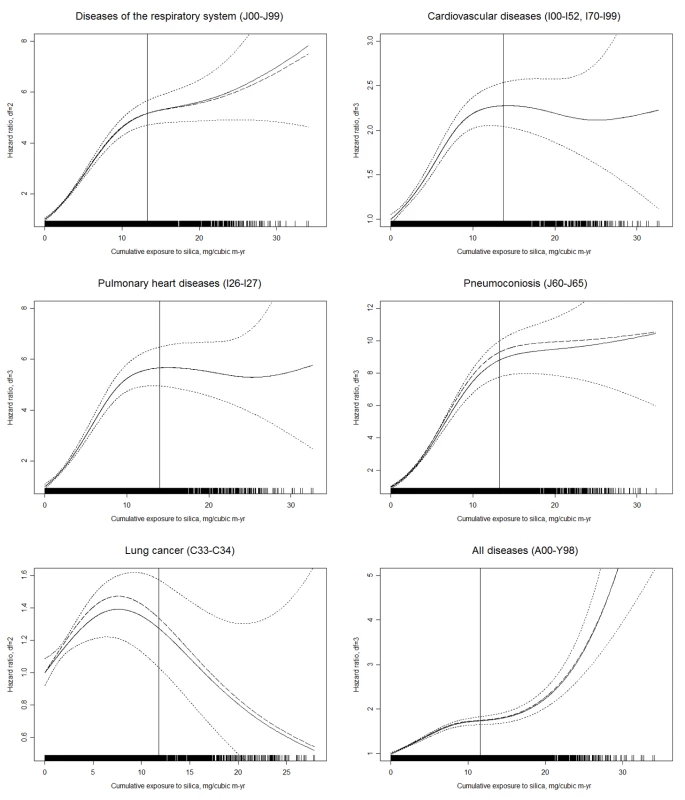
HRs and 95% CIs were derived from penalized spline regression models to examine the nonlinear relation of CDE to mortality. The vertical solid line in each panel represents the 95th percentile of CDE. Dashed lines represent the point estimate of the HR adjusted for duration of follow-up (time-dependent, continuous) and calendar time (time-dependent, continuous); solid lines represent HR further adjusted for smoking (never smoked/ever smoked), with dotted lines indicating the 95% CI; the rug plots along the horizontal axes give the distribution of CDE values. For simplicity of presentation, the reference value of CDE was set to 0 mg/m3-y (0.01 mg/m3-y for pneumoconiosis). Tab. 3. Estimated HRs for total and cause-specific mortality associated with CDE in the cohort (n = 74,040), 1960–2003. 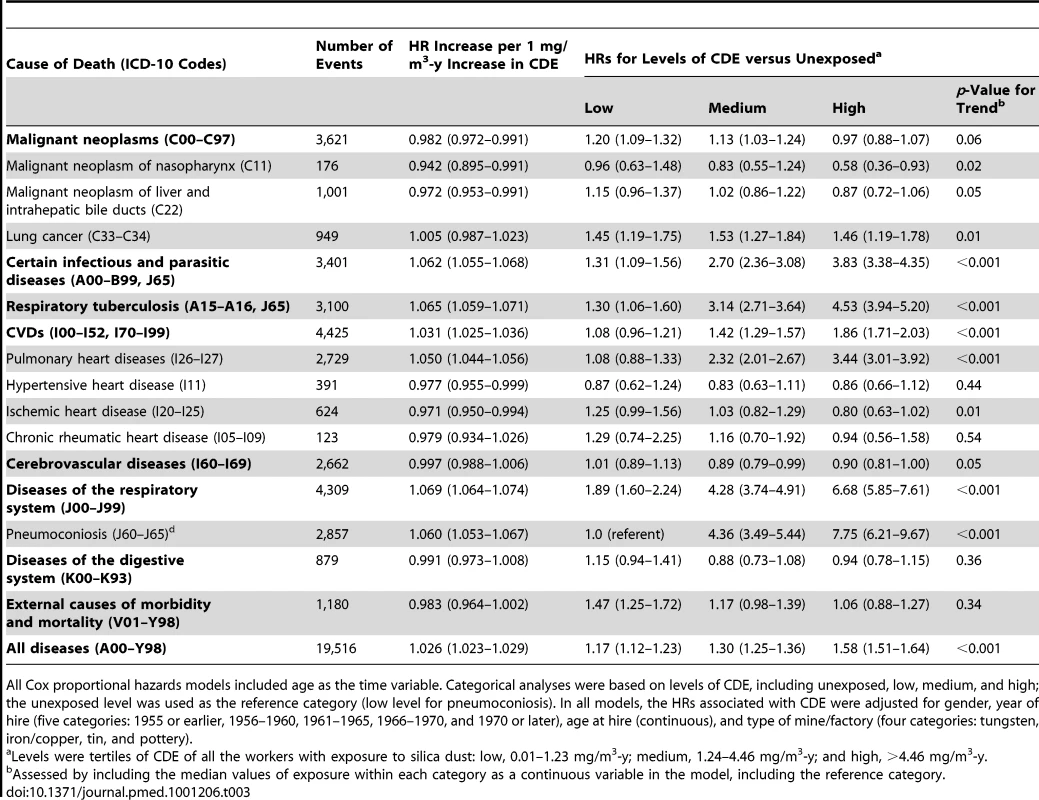
All Cox proportional hazards models included age as the time variable. Categorical analyses were based on levels of CDE, including unexposed, low, medium, and high; the unexposed level was used as the reference category (low level for pneumoconiosis). In all models, the HRs associated with CDE were adjusted for gender, year of hire (five categories: 1955 or earlier, 1956–1960, 1961–1965, 1966–1970, and 1970 or later), age at hire (continuous), and type of mine/factory (four categories: tungsten, iron/copper, tin, and pottery). For a subset of workers with exposures under the respirable silica concentration limit of 0.1 mg/m3 during their lifetime work histories (mean and median CDE were 0.64 and 0.56 mg/m3-y, respectively), each 0.1 mg/m3-y increase in CDE was associated with a 2.1% (95% CI 1.4%–2.7%), 7.2% (5.2%–9.4%), and 2.4% (0.7%–4.1%) increase in the mortality risk for all diseases, respiratory diseases, and CVDs, respectively. After adjusting for gender, year of hire, age at hire, type of mine/factory, and smoking, the respective mortality risk were 0.8% (0.1%–1.5%), 6.3% (4.1%–8.6%), and 2.2% (0.4%–4.1%) for all diseases, respiratory diseases, and CVDs, respectively; for CVDs, the mortality risk of pulmonary heart disease and ischemic heart disease increased by 6.0% (2.8%-9.3%) and 4.2% (1.4%-7.2%), respectively.
After adjustment for potential confounders including smoking, we estimated the PAR for silica dust exposure. Silica exposure accounted for 15.2% of mortality from all deaths, 63.9% of mortality from respiratory diseases, and 21.0% of mortality from CVDs among the silica-exposed workers. According to an annual health statistical report in China, the prevalence of silica-dust-exposed workers was 16.3% among Chinese industrial workers in 2008 [1]. We estimate that 4.2% of the deaths (231,104) among industrial workers in 2008 were attributable to silica dust exposure based on the relative risks derived from this study.
Table 4 shows the SMRs for deaths from all causes and from the main exposure-related diseases among dust-exposed workers from January 1, 1970, to December 31, 2003. Compared with national mortality in China, workers exposed to silica had significantly elevated mortality from all causes of death (SMR 1.21), and elevated mortality for nasopharynx cancer (1.76), liver cancer (1.16), infectious diseases (6.83), respiratory tuberculosis (4.88), CVDs (1.91), and respiratory diseases (2.32). Among CVDs, mortality for pulmonary heart diseases (4.03) and hypertensive heart disease (2.45) were significantly elevated. For those who worked at annual respirable silica dust concentrations at or below 0.1 mg/m3, mortality was significantly elevated for all causes (SMR 1.06, 95% CI 1.01–1.11), pneumoconiosis (11.01, 7.67–14.95), infectious diseases (1.88, 1.55–2.25), and malignant neoplasms (1.10, 1.01–1.19), including nasopharynx cancer (1.63, 1.01–2.40) and liver cancer (1.55, 1.33–1.78). Elevated mortality from CVDs (1.09, 0.97–1.23) included ischemic heart disease (1.65, 1.35–1.99) and hypertensive heart disease (2.53, 1.76–3.44).
Tab. 4. Estimated SMRs for underlying cause of death of silica-dust-exposed workers in the cohort (n = 72,248), 1970–2003. 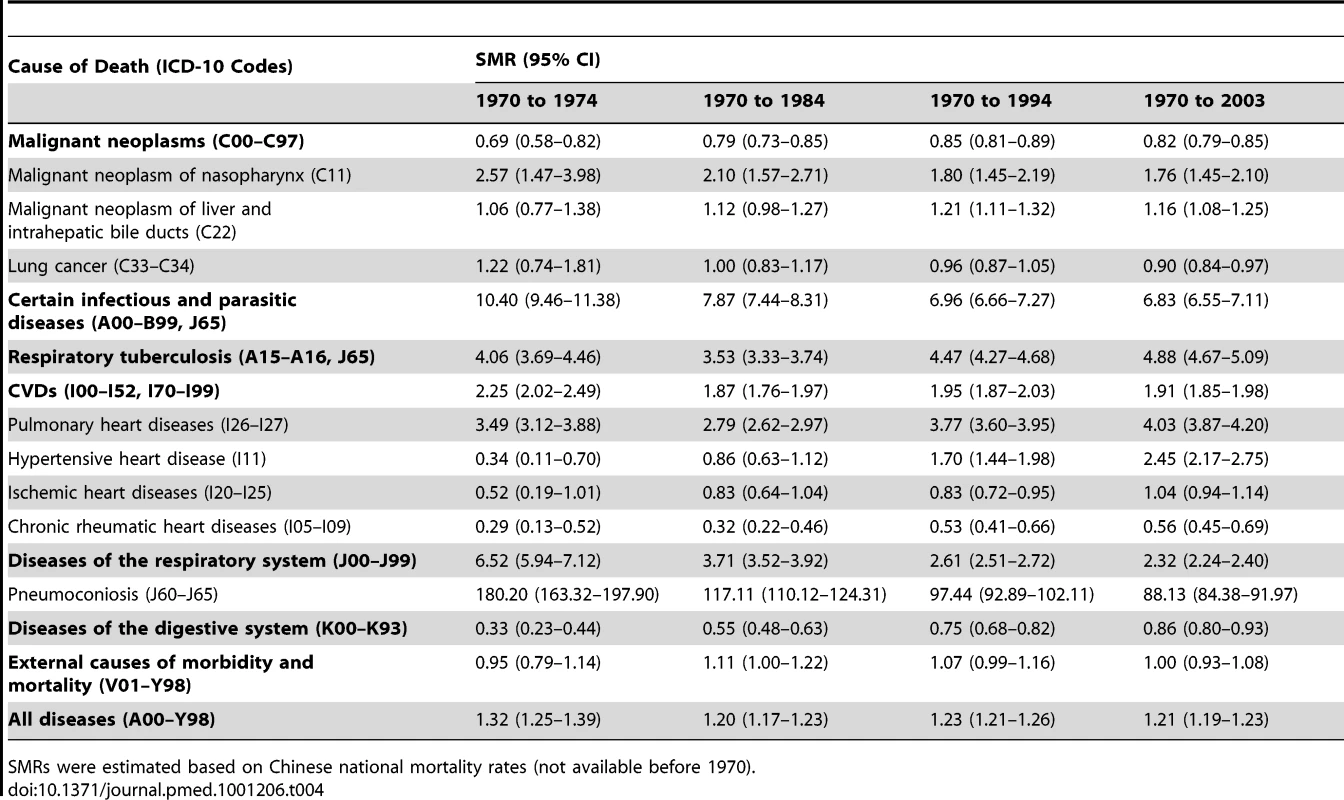
SMRs were estimated based on Chinese national mortality rates (not available before 1970). The SMR from all causes was 0.83 (95% CI 0.80–0.85) among non-dust-exposed workers. In this group, we observed elevated SMRs for nasopharynx cancer (SMR 1.91, 95% CI 1.41–2.48), liver cancer (1.17, 1.04–1.31), hypertensive heart disease (2.24, 1.84–2.68), pulmonary heart disease (1.17, 1.04–1.32), and infectious diseases (1.98, 1.76–2.22), including respiratory tuberculosis (1.24, 1.08–1.41).
Discussion
Our findings provide strong evidence that long-term silica dust exposure is associated with substantially increased mortality among Chinese workers. We not only confirmed significant relationships between increased silica dust exposure and heightened risk of death from respiratory diseases and lung cancer, but also found a significant exposure–response relationship between silica dust exposure and mortality from CVD, even at lower exposure levels.
These findings have important public health implications. Silica dust exposure is very common and is associated with increased morbidity and mortality from pneumoconiosis. Our study showed that the cumulative incidence of pneumoconiosis was 20.3% and the death rate from this disease in those with the disease was very high (61.7%). A report from the Chinese Ministry of Health indicated that the death rate from all reported pneumoconiosis was 23.1% between 1949 and 2008 [1]. Data from this study and prior ones provide strong evidence to support an association between silica dust exposure and increased mortality from cardiopulmonary diseases. We estimated that 4.2% of deaths (231,104) among industrial workers were attributable to dust exposure in China in 2008. It is well known that silica dust exposure is a preventable health hazard. These data underscore an urgent need to tighten regulations on dust control at worksites.
Dust exposure has been linked to risk of death in previous environmental and occupational health studies. The World Health Organization has estimated that 1.4% of all deaths result from exposure to various dust particles [23]. It is interesting that two cohort studies conducted in Germany among 17,644 porcelain production workers [24] and 19,943 construction workers [25] showed no significant increase in SMR from any cause. However, the mortality rates in both studies were very low, and the follow-up was relatively short. In addition, neither study determined levels of dust exposure. Our results are consistent with those from a study conducted among 3,010 tin miners in the UK (SMR 1.27) [26], although excess mortality in the UK cohort resulted from malignant neoplasms and accidents, not from respiratory diseases and CVDs. In our study, after 44 y of follow-up, we confirmed the adverse health effects of silica dust through exposure–response analysis of personal silica exposure and total mortality among 74,040 workers. After adjustment for potential confounders including cigarette smoking, silica dust exposure accounted for approximately 15.2% of all deaths in our cohort.
Our data suggest that silica dust substantially raises the risk of death from respiratory diseases as well as CVDs. Traditionally, non-malignant respiratory diseases were thought to be the main causes of death among dust-exposed workers [27]–[29]. However, this study showed that the proportion of deaths from respiratory diseases to all deaths decreased from 36.6% to 23.1%, while the proportion of deaths from diseases of the circulatory system increased from 29.4% to 37.9% from 1974 to 2003. Increased mortality from CVD was mainly due to higher rates of pulmonary heart disease from 1970 to 1974. Pulmonary heart disease was caused directly by high dust concentrations leading to a high prevalence of pneumoconiosis. From the second half of the 1960s onwards, there was a gradual decline in silica dust concentrations because of safer working practices and increased protective measures. From 1970 to 2003, the subtype of CVD changed: the proportion of deaths from pulmonary heart diseases decreased from 90.7% to 37.8%, while the proportion of deaths from hypertensive, ischemic, and chronic rheumatic heart disease increased from 5.4% to 41.3% during the same period. The SMRs of hypertensive, ischemic, and chronic rheumatic heart diseases gradually increased with ongoing follow-up of the cohort. Among workers exposed to respirable silica concentrations lower than 0.1 mg/m3 in their lifetime work histories, we observed elevated mortality from hypertensive heart disease, and ischemic heart disease. Among these workers, each 0.1 mg/m3-y increase in CDE was associated with a 2.2%, 6.0%, and 4.2% increase in the death rate from CVDs, pulmonary heart disease, and ischemic heart disease after adjusting for smoking and other confounder factors, respectively. These results indicate that low dust exposure is likely to contribute to CVDs without the presence of respiratory disease. Increased cardiovascular mortality in this study may be an independent and novel complication of silica exposure.
Several prior reports on the relationship between ambient particulate matter and cardiovascular mortality have focused on combustion-sourced particulate matter in cities [30],[31]. However, silica particles are not combustion-sourced. Rather, they are made up of a continuous framework of silicon–oxygen tetrahedral crystals, an essential constituent of granite and other felsitic igneous rocks. Increased risk of ischemic heart diseases from silica dust exposure was observed in South African gold miners and in the Swedish national census [32],[33], although these studies did not examine a dose–response relationship. Our study showed that non-combustion-sourced particles of crystalline silica were associated with elevated cardiovascular mortality, and this finding needs to be confirmed in further studies. The mechanisms by which non-combustion-sourced silica particulates increase the risk of CVD are largely unknown. Possibilities may involve the direct effects of fine particulates that cross the pulmonary epithelium into the cardiovascular system and lung receptors, or an indirect effect mediated through pulmonary oxidative stress and inflammatory responses [34]–[36].
In addition, we found elevated mortality from all causes, pneumoconiosis, infectious diseases, malignant neoplasms including nasopharynx cancer and liver cancer, and CVDs including ischemic heart disease and hypertensive heart disease among individuals who worked in an environment with respirable silica dust concentrations equal to or lower than 0.1 mg/m3. The 0.1-mg/m3 level is the exposure limit for respirable silica in the workplace specified by the US Occupational Safety and Health Administration. In China, the limit for respirable silica (0.07–0.35 mg/m3, depending on the percentage of silica dust) is similar to the US standard. However, even keeping silica exposure lower than 0.1 mg/m3 may not fully protect workers.
The association of silica dust exposure and lung cancer risk has been controversial for decades. In the present study, silica dust exposure was associated with lung cancer; risk ratios based on exposure levels ranged from 1.45 to 1.53. The penalized spline curve suggested a positive exposure–response association between silica exposure and lung cancer risk, although the HR decreased at higher levels of CDE. Possible explanations for the decrease in lung cancer risk at higher CDE include (1) a depletion of the number of susceptible workers in the cohort at high exposure levels and (2) bias introduced by the healthy worker survivor effect. This phenomenon was also observed in studies of other occupational populations [37]. Although adjustment for smoking did not change the overall shape of the exposure–response curve, it decreased the lung cancer mortality risk across the range of CDE levels, indicating a confounder effect of smoking in the association of silica exposure and lung cancer risk.
The strengths of this study include a large sample size, a long duration of follow-up, and a low rate of loss to follow-up (4.6%). We collected detailed information on silica dust exposure and cause-specific mortality; the diversity of mine types provided a wide range of exposures.
There were several limitations to this study. First, we did not collect data on dietary patterns and leisure time physical activity, and, therefore, we were unable to evaluate the confounding influence of these factors, especially on CVDs. However, diet and physical activity patterns were likely to be relatively homogenous in this cohort. Second, long-term exposure to silica dust was estimated carefully, but measurement errors were inevitable. Silica dust concentrations before 1950 were estimated using the concentrations in 1950, which may have led to underestimation of exposure for those who worked before 1950 (6,164 workers). Third, although the majority of deaths were ascertained by reviewing medical or accident records or death certificates, 4.3% of deaths were reported orally by relatives, yielding cause of death data that might not be reliable. However, results did not change after excluding these deaths. Finally, silica dust levels vary across different industries and companies, and thus the use of HRs estimated from this cohort may lead to inaccurate estimation of the PAR due to silica dust exposure for the entire population of Chinese industrial workers.
In summary, in this large cohort study, we found a significant exposure–response relationship between silica dust exposure and mortality from all causes, pneumoconiosis, and respiratory disease. Importantly, we also demonstrated a significant exposure–response relationship between silica dust exposure and CVDs. Findings from this study have important public health implications for improving occupational safety among those exposed to silica dust in China and around the world.
Supporting Information
Zdroje
1. Ministry of Health of the People's Republic of China 2009 [Chinese annual health statistical report in 2009.] Beijing Ministry of Health of the People's Republic of China
2. World Health Organization Global Occupational Health Network 2007 Elimination of silicosis. GOHNET Newsletter 12 Geneva World Health Organization Global Occupational Health Network
3. US National Institute for Occupational Safety and Health 2002 Health effects of occupational exposure to respirable crystalline silica Washington District of Columbia) US Department of Health and Human Services
4. KauppinenTToikkanenJPedersenDYoungRKogevinasM 1998 Occupational exposure to carcinogens in the European Union in 1990–93 Carex International Information System on Occupational Exposure to Carcinogens. Helsinki: Finnish Institute of Occupational Health
5. SchenkerMBPinkertonKEMitchellDVallyathanVElvine-KreisB 2009 Pneumoconiosis from agricultural dust exposure among young California farmworkers. Environ Health Perspect 117 988 994
6. MerchantJA 1986 Occupational respiratory diseases Washington (District of Columbia) US Department of Health and Human Services
7. OxmanADMuirDCShannonHSStockSRHnizdoE 1993 Occupational dust exposure and chronic obstructive pulmonary disease. A systematic overview of the evidence. Am Rev Respir Dis 148 38 48
8. CalvertGMRiceFLBoianoJMSheehyJWSandersonWT 2003 Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med 60 122 129
9. BrownTPRushtonL 2005 Mortality in the UK industrial silica sand industry: 1. assessment of exposure to respirable crystalline silica. Occup Environ Med 62 442 445
10. SteenlandK 2005 One agent, many diseases: exposure-response data and comparative risks of different outcomes following silica exposure. Am J Ind Med 48 16 23
11. PopeCAIIIEzzatiMDockeryDW 2009 Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 360 376 386
12. SamoliEPengRRamsayTPipikouMTouloumiG 2008 Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA study. Environ Health Perspect 116 1480 1486
13. LadenFSchwartzJSpeizerFEDockeryDW 2006 Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med 173 667 672
14. BrookRDFranklinBCascioWHongYHowardG 2004 Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109 2655 2671
15. DominiciFPengRDBellMLPhamLMcDermottA 2006 Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295 1127 1134
16. ChenWZhuangZAttfieldMDChenBTGaoP 2001 Exposure to silica and silicosis among tin miners in China: exposure-response analyses and risk assessment. Occup Environ Med 58 31 37
17. ZhuangZHearlFJOdencrantzJChenWChenBT 2001 Estimating historical respirable crystalline silica exposures for Chinese pottery workers and iron/copper, tin, and tungsten miners. Ann Occup Hyg 45 631 642
18. DosemeciMChenJQHearlFChenRGMcCawleyM 1993 Estimating historical exposure to silica among mine and pottery workers in the People's Republic of China. Am J Ind Med 24 55 66
19. WuZHearlFPengKMcCawleyMChenA 1992 Current occupational exposures in Chinese iron and copper mines. Appl Occup Environ Hyg 7 735 743
20. ThurstonSWEisenEASchwartzJ 2002 Smoothing in survival models: an application to workers exposed to metalworking fluids. Epidemiology 13 685 692
21. RothmanKJBoiceJDAustinH 1982 Epidemiologic analysis with a programmable calculator, 2nd edition Boston Epidemiology Resources
22. ChenWYangJChenJBruchJ 2006 Exposures to silica mixed dust and cohort mortality study in tin mines: exposure-response analysis and risk assessment of lung cancer. Am J Ind Med 49 67 76
23. World Health Organization 2002 The world health report 2002—reducing risks, promoting healthy life Geneva World Health Organization
24. BirkTMundtKAGuldnerKParsonsWLuippoldRS 2009 Mortality in the German porcelain industry 1985–2005: first results of an epidemiological cohort study. J Occup Environ Med 51 373 385
25. ArndtVRothenbacherDDanielUZschenderleinBSchuberthS 2004 All-cause and cause specific mortality in a cohort of 20 000 construction workers; results from a 10 year follow up. Occup Environ Med 61 419 425
26. HodgsonJTJonesRD 1990 Mortality of a cohort of tin miners 1941–86. Br J Ind Med 47 665 676
27. McDonaldADMcDonaldJCRandoRJHughesJMWeillH 2001 Cohort mortality study of North American industrial sand workers. I. Mortality from lung cancer, silicosis and other causes. Ann Occup Hyg 45 193 199
28. MeijersJMSwaenGMSlangenJJ 1996 Mortality and lung cancer in ceramic workers in The Netherlands: preliminary results. Am J Ind Med 30 26 30
29. CheckowayHHeyerNJSeixasNSWelpEADemersPA 1997 Dose-response associations of silica with nonmalignant respiratory disease and lung cancer mortality in the diatomaceous earth industry. Am J Epidemiol 145 680 688
30. BellMLEbisuKPengRDWalkerJSametJM 2008 Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am J Epidemiol 168 1301 1310
31. ItoKMathesRRossZNadasAThurstonG 2011 Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect 119 467 473
32. WyndhamCHBezuidenhoutBNGreenacreMJSluis-CremerGK 1986 Mortality of middle aged white South African gold miners. Br J Ind Med 43 677 684
33. WeinerJBarlowLSjogrenB 2007 Ischemic heart disease mortality among miners and other potentially silica-exposed workers. Am J Ind Med 50 403 408
34. NemmarAHoetPHVanquickenborneBDinsdaleDThomeerM 2002 Passage of inhaled particles into the blood circulation in humans. Circulation 105 411 414
35. VinzentsPSMollerPSorensenMKnudsenLEHertelO 2005 Personal exposure to ultrafine particles and oxidative DNA damage. Environ Health Perspect 113 1485 1490
36. KellyFJ 2003 Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 60 612 616
37. StaynerLSteenlandKDosemeciMHertz-PicciottoI 2003 Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health 29 317 324
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Medical Evidence of Human Rights Violations against Non-Arabic-Speaking Civilians in Darfur: A Cross-Sectional Study
- New Methodology for Estimating the Burden of Infectious Diseases in Europe
- Reappraisal of Metformin Efficacy in the Treatment of Type 2 Diabetes: A Meta-Analysis of Randomised Controlled Trials
- Does Conflict of Interest Disclosure Worsen Bias?
- Open Clinical Trial Data for All? A View from Regulators
- The Imperative to Share Clinical Study Reports: Recommendations from the Tamiflu Experience
- Where There Is No Health Research: What Can Be Done to Fill the Global Gaps in Health Research?
- The Role of Public Health Institutions in Global Health System Strengthening Efforts: The US CDC's Perspective
- Long-Term Exposure to Silica Dust and Risk of Total and Cause-Specific Mortality in Chinese Workers: A Cohort Study
- Ovarian Cancer and Body Size: Individual Participant Meta-Analysis Including 25,157 Women with Ovarian Cancer from 47 Epidemiological Studies
- Is Food Insecurity Associated with HIV Risk? Cross-Sectional Evidence from Sexually Active Women in Brazil
- Induction of Labor versus Expectant Management in Women with Preterm Prelabor Rupture of Membranes between 34 and 37 Weeks: A Randomized Controlled Trial
- Prioritizing CD4 Count Monitoring in Response to ART in Resource-Constrained Settings: A Retrospective Application of Prediction-Based Classification
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Induction of Labor versus Expectant Management in Women with Preterm Prelabor Rupture of Membranes between 34 and 37 Weeks: A Randomized Controlled Trial
- The Imperative to Share Clinical Study Reports: Recommendations from the Tamiflu Experience
- Long-Term Exposure to Silica Dust and Risk of Total and Cause-Specific Mortality in Chinese Workers: A Cohort Study
- Prioritizing CD4 Count Monitoring in Response to ART in Resource-Constrained Settings: A Retrospective Application of Prediction-Based Classification
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



