-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEffect of Supplementation with Zinc and Other Micronutrients on Malaria in Tanzanian Children: A Randomised Trial
Background:
It is uncertain to what extent oral supplementation with zinc can reduce episodes of malaria in endemic areas. Protection may depend on other nutrients. We measured the effect of supplementation with zinc and other nutrients on malaria rates.Methods and Findings:
In a 2×2 factorial trial, 612 rural Tanzanian children aged 6–60 months in an area with intense malaria transmission and with height-for-age z-score≤−1.5 SD were randomized to receive daily oral supplementation with either zinc alone (10 mg), multi-nutrients without zinc, multi-nutrients with zinc, or placebo. Intervention group was indicated by colour code, but neither participants, researchers, nor field staff knew who received what intervention. Those with Plasmodium infection at baseline were treated with artemether-lumefantrine. The primary outcome, an episode of malaria, was assessed among children reported sick at a primary care clinic, and pre-defined as current Plasmodium infection with an inflammatory response, shown by axillary temperature ≥37.5°C or whole blood C-reactive protein concentration ≥8 mg/L. Nutritional indicators were assessed at baseline and at 251 days (median; 95% reference range: 191–296 days). In the primary intention-to-treat analysis, we adjusted for pre-specified baseline factors, using Cox regression models that accounted for multiple episodes per child. 592 children completed the study. The primary analysis included 1,572 malaria episodes during 526 child-years of observation (median follow-up: 331 days). Malaria incidence in groups receiving zinc, multi-nutrients without zinc, multi-nutrients with zinc and placebo was 2.89/child-year, 2.95/child-year, 3.26/child-year, and 2.87/child-year, respectively. There was no evidence that multi-nutrients influenced the effect of zinc (or vice versa). Neither zinc nor multi-nutrients influenced malaria rates (marginal analysis; adjusted HR, 95% CI: 1.04, 0.93–1.18 and 1.10, 0.97–1.24 respectively). The prevalence of zinc deficiency (plasma zinc concentration <9.9 µmol/L) was high at baseline (67% overall; 60% in those without inflammation) and strongly reduced by zinc supplementation.Conclusions:
We found no evidence from this trial that zinc supplementation protected against malaria.
Trial Registration: ClinicalTrials.gov NCT00623857
: Please see later in the article for the Editors' Summary.
Published in the journal: . PLoS Med 8(11): e32767. doi:10.1371/journal.pmed.1001125
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001125Summary
Background:
It is uncertain to what extent oral supplementation with zinc can reduce episodes of malaria in endemic areas. Protection may depend on other nutrients. We measured the effect of supplementation with zinc and other nutrients on malaria rates.Methods and Findings:
In a 2×2 factorial trial, 612 rural Tanzanian children aged 6–60 months in an area with intense malaria transmission and with height-for-age z-score≤−1.5 SD were randomized to receive daily oral supplementation with either zinc alone (10 mg), multi-nutrients without zinc, multi-nutrients with zinc, or placebo. Intervention group was indicated by colour code, but neither participants, researchers, nor field staff knew who received what intervention. Those with Plasmodium infection at baseline were treated with artemether-lumefantrine. The primary outcome, an episode of malaria, was assessed among children reported sick at a primary care clinic, and pre-defined as current Plasmodium infection with an inflammatory response, shown by axillary temperature ≥37.5°C or whole blood C-reactive protein concentration ≥8 mg/L. Nutritional indicators were assessed at baseline and at 251 days (median; 95% reference range: 191–296 days). In the primary intention-to-treat analysis, we adjusted for pre-specified baseline factors, using Cox regression models that accounted for multiple episodes per child. 592 children completed the study. The primary analysis included 1,572 malaria episodes during 526 child-years of observation (median follow-up: 331 days). Malaria incidence in groups receiving zinc, multi-nutrients without zinc, multi-nutrients with zinc and placebo was 2.89/child-year, 2.95/child-year, 3.26/child-year, and 2.87/child-year, respectively. There was no evidence that multi-nutrients influenced the effect of zinc (or vice versa). Neither zinc nor multi-nutrients influenced malaria rates (marginal analysis; adjusted HR, 95% CI: 1.04, 0.93–1.18 and 1.10, 0.97–1.24 respectively). The prevalence of zinc deficiency (plasma zinc concentration <9.9 µmol/L) was high at baseline (67% overall; 60% in those without inflammation) and strongly reduced by zinc supplementation.Conclusions:
We found no evidence from this trial that zinc supplementation protected against malaria.
Trial Registration: ClinicalTrials.gov NCT00623857
: Please see later in the article for the Editors' Summary.Introduction
Preventive zinc supplementation among children in low-income countries can reduce the burden of diarrhoea and respiratory tract infections [1]. If effective against malaria, it would be an important advance in public health, particularly in Africa, where 90% of malarial deaths occur. Anti-malarial efficacy of zinc supplementation has thus far been investigated in four trials of which we are aware, with discordant results. In one trial in Gambian children, twice-weekly zinc seemed to reduce the mean number of clinic visits for malaria by 32% [2], but this trial was not designed to evaluate protection from malaria, and the statistical evidence was weak. In another trial in Papua New Guinean children, daily zinc reduced rates of malaria due to Plasmodium falciparum by 38% [3]. By contrast, in trials in Burkina Faso and Peru [4],[5], there was no evidence of protection against malaria in the children enrolled, despite zinc deficiency being highly prevalent at baseline and reversed by supplementation.
In these trials, malaria was detected among children self-reporting at clinics in Papua New Guinea and The Gambia, whereas cases were detected through regular home visits in Burkina Faso and Peru. This could indicate that zinc supplementation is more efficacious in preventing severe malaria than mild episodes, for which mothers do not immediately seek treatment. Alternatively, the response to zinc may be suppressed when children are also deficient in other nutrients [6],[7]. Simultaneous supplementation with other nutrients may be required to overcome a lack of effect of zinc supplements when given alone.
There are concerns, however, about the safety of supplementation with micronutrients in malaria-endemic areas. In a large trial among children aged 1–35 months in Pemba [8], supplementation with iron and folic acid increased the incidence of serious adverse events by 12%, which was assumed to be due to malaria. Results from a sub-study within this trial suggested that the overall effect tended to be beneficial in settings with improved basic care facilities. Also, the increase in adverse events appeared restricted to those who were iron-replete, whereas supplementation seemed beneficial in those who were iron-deficient [8],[9]. Based on these findings, the World Health Organization now advocates a policy of restricting iron supplementation to children with iron deficiency [10]. To strengthen the basis of this recommendation, the interaction with iron status and the safety of iron supplements in iron deficient children should be confirmed in other studies.
Although randomised trials in malaria-endemic areas have consistently shown that preventive iron supplementation (with or without other micronutrients) reduces the prevalence of anaemia [11]–[15], we have not found reports on its effect on haemoglobin concentrations during febrile episodes of malaria. By increasing parasite propagation during such episodes, supplementation may potentially exacerbate haemolysis. Thus, gains in haemoglobin concentrations due to supplementation before febrile malaria attacks may be more than compensated for by greater haemoglobin losses during such episodes. In a study in Papua New Guinean infants (aged 6–12 months), the decrease in haemoglobin concentration associated with asymptomatic Plasmodium infection at two surveys seemed more pronounced among those receiving iron [16]. By contrast, while supplementation with iron and folic acid increased rates of cerebral malaria in Pemba, it seemed to reduce rates of severe anaemia, although the statistical evidence for this reduction was not strong [8].
The primary aim of our study was to assess the effect of supplementation with zinc, alone or in combination with other nutrients, on rates of uncomplicated malaria. Secondary aims were to compare intervention effects in subgroups pre-defined by age, presence of parasitaemia, and zinc and iron status at baseline. We also assessed the effect of multi-nutrient supplementation on haemoglobin concentrations during febrile malaria episodes.
Methods
Study Population
We conducted the study between February 2008 and March 2009, in a rural area in Handeni District, Tanzania [17],[18] where malaria transmission is intense [19]. The trial protocol (Text S1) and the CONSORT checklist (Text S2) are available online as supporting information. The study (ClinicalTrials.gov: NCT00623857) was approved by the Ethical Review Committee of Wageningen University, The Netherlands and the National Health Research Ethics Review sub-Committee, Dar es Salaam, Tanzania. We sought written individual informed consent; parents or primary caretakers were invited to sign (or thumbprint if illiterate) the informed consent form witnessed by a member of the community (who countersigned the form). There were no important changes to methods during the trial.
Study Design
In this trial with a parallel design, children were randomized to receive daily supplements with: (a) zinc; (b) both zinc and multi-nutrients; (c) multi-nutrients without zinc; or (d) placebo. Such a 2×2 factorial design allows the investigation of interaction between interventions; in the absence of interaction (i.e., the interventions act independently), data can be analysed ‘at the margins’ [20], and the design can ‘achieve two trials for the price of one’: every participant contributes information to each of the randomized factors simultaneously [21]. The multi-nutrient supplement included iron (18 mg as ferrous fumarate), folic acid (93.75 µg), vitamin A (450 µg, as retinyl palmitate), vitamin B12 (1.17 µg), vitamin B1 (0.625 mg as thiamine nitrate), vitamin B2 (0.55 mg riboflavin), copper (340 µg), and vitamin E (6.6 mg as RRR-α-tocopherol), all of which may have haematinic effects. For practical reasons, children participating in the study daily received one standard dose of supplements, regardless of age class. The amount of iron was set to supply on average 2 mg/kg body weight/day, as recommended to prevent iron deficiency anaemia [22]. For the youngest children (6–12 months), this amount was very close to the RNI under conditions of low bioavailability (18.6 mg). The average intakes of iron actually achieved were 2.3 mg/kg, 1.7 mg/kg, and 1.3 mg/kg bodyweight, for age classes 6–17, 18–35, and 36–59 months, respectively. Table S1 provides further details of the composition of the supplements.
Sample Size Calculations
Our aim was to measure a malaria rate reduction by 30% unambiguously [2], and to exclude the null value from the 95%CI with a minimum probability of 80%. With an anticipated malaria incidence in the reference group of 1.05 episodes per child-year, this required an effective sample size of 142 child-years per group. It should be noted, however, that the relevance of sample size calculations to the interpretation of study findings is controversial [23],[24]; specifically, in our study, the incidence actually found in the placebo group was much higher than originally foreseen. No interim analyses were foreseen or done.
Recruitment
In four villages, resident children aged 6–60 months were listed, screened, and enrolled in daily batches until attaining the target number (n = 600) in August 2008 (Figure S1). Anthropometric indices were computed as the average of two measurements taken on consecutive days. Following a physical examination, venous blood was collected in EDTA-tubes suitable for trace element analyses (Becton-Dickinson, Franklin Lakes, NJ, USA). One aliquot was centrifuged immediately after collection and plasma stored in liquid nitrogen; a second aliquot was examined by haematology analyser (Sysmex KX21, Kobe, Japan).
Malaria dipstick tests (CareStart, G0121, Access Bio, Monmouth Jct, NJ) were used to detect lactate dehydrogenase produced by live P. falciparum or other human Plasmodium species. This test has a sensitivity of 96% for samples with >50 P. falciparum parasites/mL, and detects only current parasitaemia [25]. Blood films were prepared for all children. All children with current Plasmodium infection, as indicated by a positive result for a dipstick test, received a therapeutic course of artemether-lumefantrine upon enrolment so that they were at risk of malaria after a post-treatment prophylactic period [26] in which lumefantrine levels exceeded the minimum concentration inhibiting parasite multiplication.
Children with height-for-age z-scores>−1.5 SD (who are at lower risk of zinc deficiency) [27], weight-for-height z-score<−3 SD, haemoglobin concentration <70 g/L, those unlikely to comply with interventions, whose parents/guardians refused consent, or with signs of severe or chronic disease, were excluded from participation.
Randomisation and Masking
We used stratified block randomisation to allocate interventions. A colleague not otherwise involved in the trial used tables with random numbers to generate the allocation sequence consisting of randomly permuted blocks with random size (4 or 8) within each of six strata defined by Plasmodium infection (yes/no infected) and age class (6–17 months, 18–35 months, and 36–60 months). Interventions were indicated by colour code on paper slips in opaque, consecutively numbered envelopes that were prepared in advance, in excess of the expected number required. A simple colour code (one colour to distinguish each of the four supplement types) was used to minimize the possibility of children receiving the wrong supplements. This code was not revealed to researchers, field staff, or participants, who therefore did not know who received what intervention. At the end of each screening day, the names of eligible children were listed by screening number and each name was randomly allocated to an intervention by drawing the next envelope from a box that corresponded to the age - and malaria-specific stratum for that child.
Supplements, as powder in colour-coded capsules, were contained in blister packs, and administered orally after suspending capsule contents in clean water or breast milk. All types of powder had similar appearance, smell, and taste. At the end of each screening day, when eligibility had been fully established, children were individually allocated in order of their screening number to intervention groups by drawing successive envelopes from a box corresponding to the infection - and age-specific stratum for that child. The number of the envelope was then recorded on a list before the envelope was opened. The randomisation code was not revealed to researchers, field workers, or participants until data collection was completed and the database had been finalised and sent to the Trial Oversight Committee. The colour of the supplements received by each child was known to participants and field workers but not by the clinical outcome assessors.
Follow-up and Case Detection
Community volunteers administered supplements 7 days per week close to the homes of participating children and reported daily to field staff, who followed up the same day in cases of non-compliance. Field staff made regular, unannounced spot checks to ensure adherence to procedures. Supplementation and follow-up continued for all children until 12 March 2009, when the trial was stopped (Figure 1). Because we could not start the study on the date originally foreseen, we had to stop the trial when running out of resources, before the planned number of person-years were accrued, but after the desired number of events had been accrued.
Fig. 1. Flow chart of study recruitment and follow-up. 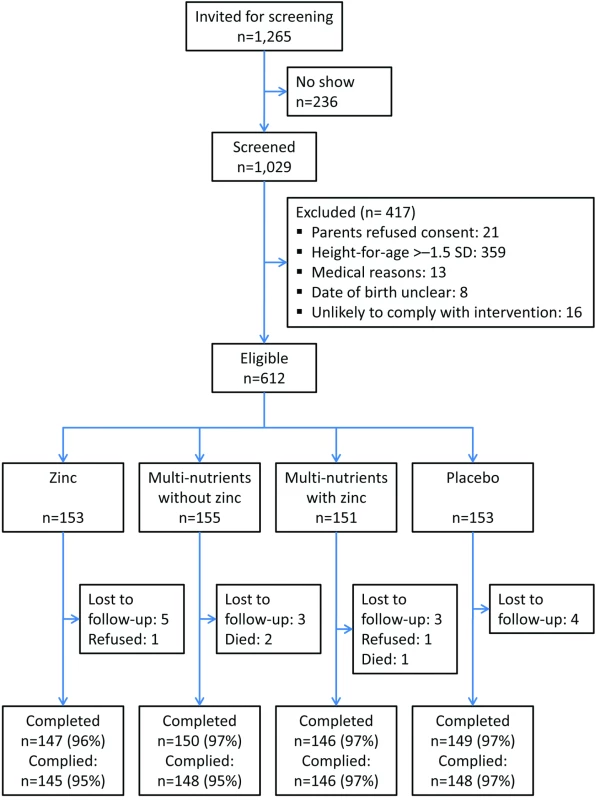
Compliance was measured as the proportion of children who consumed >95% of scheduled supplements. Parents were requested to bring study children to the clinic if their child developed a fever or became unwell. A clinical officer was on 24-hour duty and collected medical information on standardised forms. Axillary temperature was measured using an electronic thermometer and dipstick tests administered for children with guardian-reported fever; for those with positive test results, we prepared two blood films and measured whole-blood C-reactive protein concentrations using a point-of-care test (QuikRead, Orion Diagnostica, Espoo, Finland).
In accordance with national guidelines, we treated uncomplicated malaria with artemether-lumefantrine (Novartis Pharma, Basel, Switzerland). This drug combination is highly efficacious [28],[29], and was available free of charge at government health-facilities but not in local shops. Participating children received free medical care for common illnesses. Because of the strategic location of the research clinic, and based on interviews with local informants, we believe that very few sick participants were brought to other health facilities or were treated at home.
A second survey, at 251 days (median; 95% reference range: 191–296 days) after enrolment, followed similar procedures.
Laboratory Analyses
Peripheral blood parasite density was determined by microscopy; slides with results that were inconsistent with those from the dipstick test were read twice. Asexual Plasmodium parasites were counted against at least 200 leukocytes, and density, expressed per µL of blood, was estimated using an assumed leukocyte density of 8,000/µL. For children with very high densities, parasites were counted per 2,000 erythrocytes, in which case we used the estimated erythrocyte count at the time of the episode to determine the number of parasites per µL. The erythrocyte density was estimated based on haemoglobin concentration measured by HemoCue meter, using a linear model describing the relationship between haemoglobin concentrations and erythrocyte counts as assessed during surveys. Plasma concentrations of C-reactive protein and ferritin were measured (Meander Medical Centre, Amersfoort, The Netherlands) on a Beckman Coulter Unicel DxC880i system according to the manufacturer's instructions. Plasma zinc concentrations were determined by inductively-coupled plasma-mass spectrometry (Varian 820-MS; CV: 9% at 26.8 µM; 13% at 21.25 µM and 13% at 15 µM; n = 32, V = 10 µL).
Statistical Analysis
Data were analysed following a pre-specified plan, by intention-to-treat, using SPSS (v15·0 for Windows, SPSS, Chicago, IL, USA), CIA (v2.1.2) [30] and STATA (v11; College Station, TX, USA). Compliance was measured as the proportion of children who consumed >95% of scheduled supplements. Nutritional status was defined by the presence of iron deficiency (plasma ferritin concentration <12 µg/L) [22], zinc deficiency (plasma zinc concentration <9.9 µmol/mL) [31] or being stunted (height-for-age z-score<−2 SD).
The primary outcome, an episode of malaria, was pre-defined as a positive result for the malaria dipstick test in children with guardian-reported fever in the previous 24 hours and either: (a) confirmed fever (axillary temperature ≥37.5°C), or (b) unconfirmed fever with inflammation (whole blood C-reactive protein concentrations ≥8 mg/L), separated by at least 14 days from a previous malaria episode. It has been recommended that only measured fever should be used to identify malaria cases, and to exclude cases of unconfirmed fever from the analysis [32]. We considered this approach would miss many malaria episodes because temperature can fluctuate strongly over the day and many fever cases would remain undetected during the relatively short visit to the health facility. Thus we included inflammation as additional criterion in the case definition for cases of unconfirmed fever. In the primary analysis we did not use a parasite density threshold in the malaria case definition [33],[34], because this can lead to biased estimates of intervention effects when the interventions affect parasite density [35],[36]; in addition, density estimates can vary greatly within short time spans, and ideally require leukocyte counts to be determined simultaneously [35],[37],[38]. To increase the specificity of malaria case definitions, Plasmodium-infected participants were treated at baseline to clear parasitaemia before the start of surveillance. Episodes with pre-defined parasitaemia thresholds (1,000, 3,000, and 5,000 asexual parasites/µL) were considered as secondary outcomes. We also assessed the effect of the intervention on relatively severe episodes (with parasite densities exceeding 10,000 or 100,000 parasites/µL).
Because we considered a priori a reduction in overall malaria disease burden of primary public health importance [39],[40], our primary analysis included all malaria events. We used Cox models with robust estimates of the standard error to account for correlation between episodes within children and interpreted the hazard ratio as a proxy for the incidence ratio. We calculated the percentage reduction (or increase) due to the intervention as 100×(1−hazard ratio). Following the analysis plan, we adjusted for prognostic factors at baseline (age class [6–18 months, 18–35 months, and 36–59 months], Plasmodium infection, mosquito net use, distance between homestead and clinic, height-for-age z-score). We evaluated possible interaction between zinc and multi-nutrients by including an interaction term in the Cox regression model. We also conducted a pre-specified secondary analysis to assess the influence on effect estimates of excluding observations in a 14-day post-treatment prophylactic period. To assess changes in intervention effect over time, we explored effects on all malaria episodes within the first 100 days of supplementation versus the subsequent period. We arbitrarily defined a cut-point of 100 days because this period covered almost half of all episodes, and adjusted for baseline factors as described above. We similarly explored intervention effects within the first 50 days.
In a secondary analysis we assessed intervention effects on time-to-first malaria episode using Kaplan-Meier analysis, and compared hazard rates of first episodes using Cox regression.
After we concluded that there was no evidence for interaction between zinc and multi-nutrients on malaria rates, we conducted pre-specified subgroup analyses (all events; unadjusted) to explore to what extent the magnitude of marginal intervention effects on malaria frequency depended on age class, presence of parasitaemia, and zinc and iron status at baseline, by including (for each factor in turn) interaction terms in the Cox regression models. Lastly, we explored whether differences in intervention effects between subgroups were consistent when using higher parasite density cut-offs.
Results
Of 1,029 screened children, 662 had height-for-age z-scores≤−1.5 SD; of these, 612 were eligible and randomised. Twenty children (3%) did not complete the trial: three died, two were withdrawn by parents, and 15 emigrated from the area (Figure 1). Another two children discontinued the intervention but were available for follow-up. Compliance was high (96%) and similar in all four groups.
Groups were similar in baseline characteristics except that there were slightly more boys and zinc-deficient children in the multi-nutrient group (Table 1). The prevalence of zinc deficiency was 67% overall, and 60% in those without inflammation; the prevalence of zinc deficiency was dramatically reduced by zinc supplementation, whether given alone or with other micronutrients (Table 2).
Tab. 1. Baseline characteristics of study participants, by intervention group. 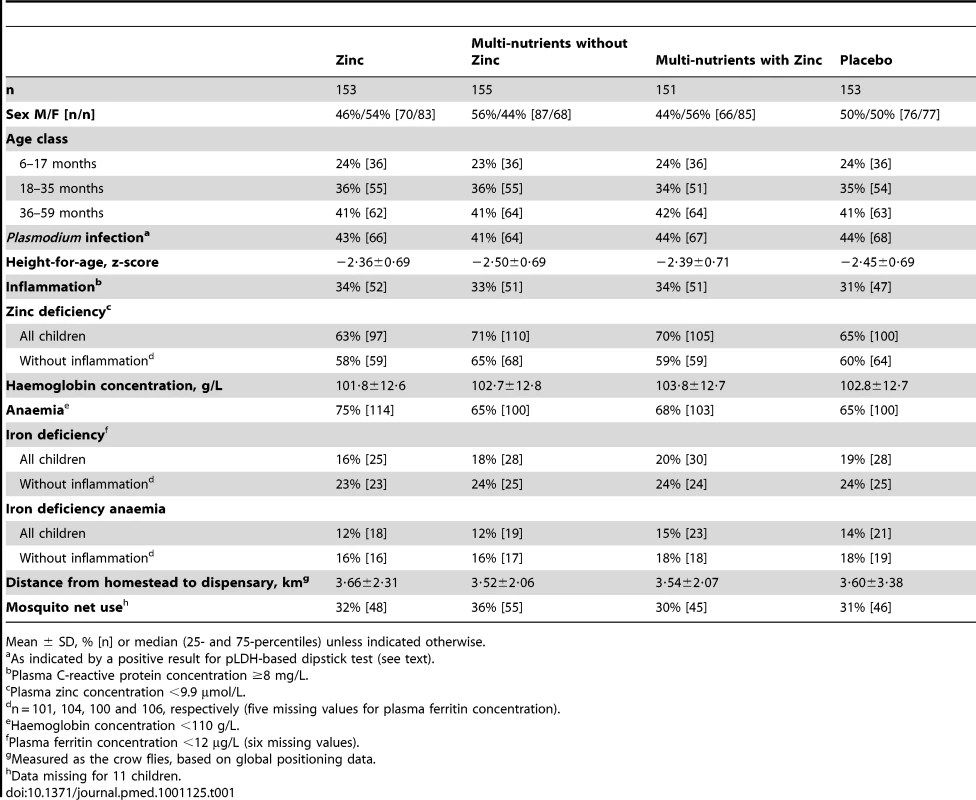
Mean ± SD, % [n] or median (25- and 75-percentiles) unless indicated otherwise. Tab. 2. Intervention effects on indicators of nutritional status, inflammation and malaria at the second survey. 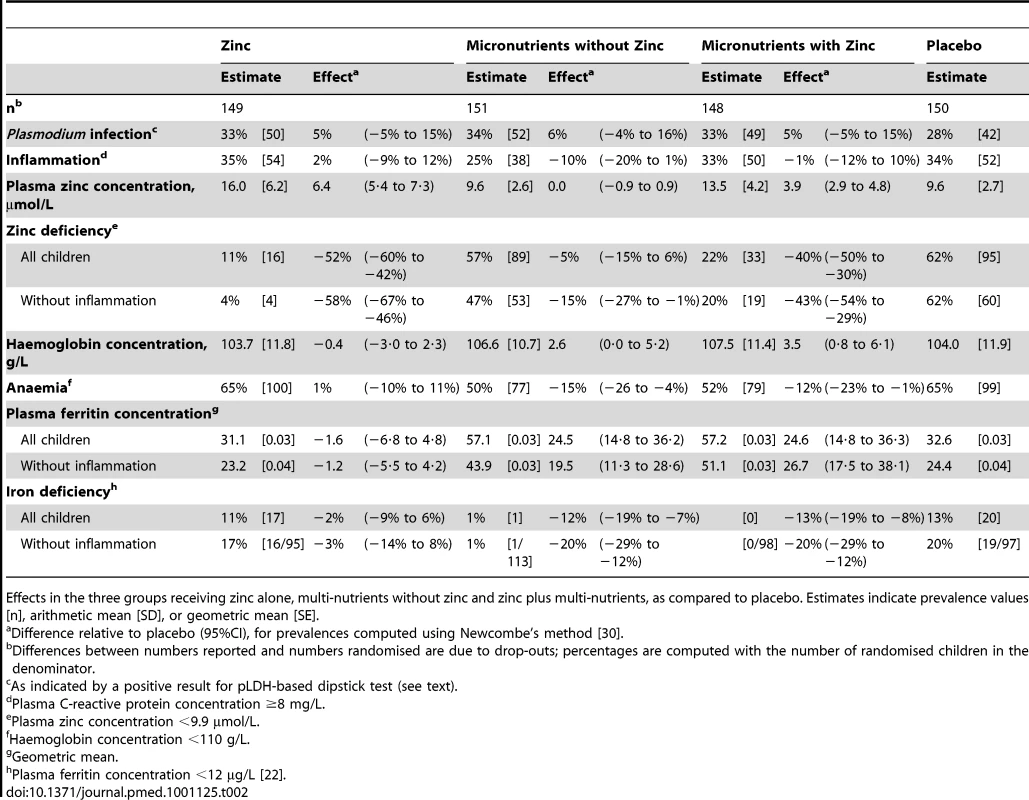
Effects in the three groups receiving zinc alone, multi-nutrients without zinc and zinc plus multi-nutrients, as compared to placebo. Estimates indicate prevalence values [n], arithmetic mean [SD], or geometric mean [SE]. There were 3,268 clinic visits during the study period, of which 2,462 (75%) were accompanied by guardian-reported fever. Of 2,462 episodes of reported fever, 1,618 (66%) were accompanied by Plasmodium infection as detected by dipstick test; for 46 children parasitaemia was not accompanied by confirmed fever or inflammation. Hence, 1,572 episodes classified as malaria: 1,499 (95%) due to P. falciparum and 73 (5%) due to other Plasmodium species. The incidence of malaria was 3.0 events/child-year. There were 1,408, 1,314, 1,248, 1,119, and 263 episodes with densities exceeding 1,000, 3,000, 5,000, 10,000, and 100,000 parasites/µL, respectively. Of all hospital admissions (68), almost half (30 cases occurring in 27 children) were due to malaria: five were admitted for life-threatening disease (respiratory distress or prostration; none had neurological manifestations); 25 lived far from the dispensary, and the clinician referred them as a precaution (18 for haemoglobin concentrations <60 g/L without respiratory distress or dehydration and seven for other reasons). There were no episodes of cerebral malaria.
Primary Analysis: Effects on All Episodes of Malaria
Compared to placebo, there was no evidence of a meaningful effect of either zinc or multi-nutrients alone on the incidence of malaria. The hazard ratio for all episodes of malaria by zinc was 0.99; 95% CI 0.82–1.18 (adjusted model, as compared to placebo). Thus for zinc, the lower limit of the confidence interval is compatible with a reduction in incidence of all episodes of malaria of 18% only. The hazard ratio for all episodes of malaria by multi-nutrients (as compared to placebo) was 1.04, 95% CI 0.87–1.23 (adjusted model; see Table 3). Incidence of all episodes of malaria was slightly higher in the group that received both zinc and multi-nutrients, as compared to placebo (HR 1.14, 95% CI 0.96–1.35), but there was no evidence of an interaction between zinc and multi-nutrients in their effects on malaria incidence (interaction ratio 1.10, 95% CI 0.84–1.43). In the remainder of this paper, we will assume that the interventions acted independently, and report marginal analyses accordingly [20]. Thus analysed, there was no evidence that zinc supplementation affected malaria rates (adjusted HR: 1.04, 95% CI 0.93–1.18), whereas supplementation with multi-nutrients seemed to slightly increase malaria rates (adjusted HR 1.10, 95% CI 0.97–1.24). Excluding observations for 14 days post-treatment or restricting the analysis to P. falciparum cases did not change our conclusions (unpublished data).
Tab. 3. Incidence rates by intervention group and intervention effects on malaria rates, relative to placebo. 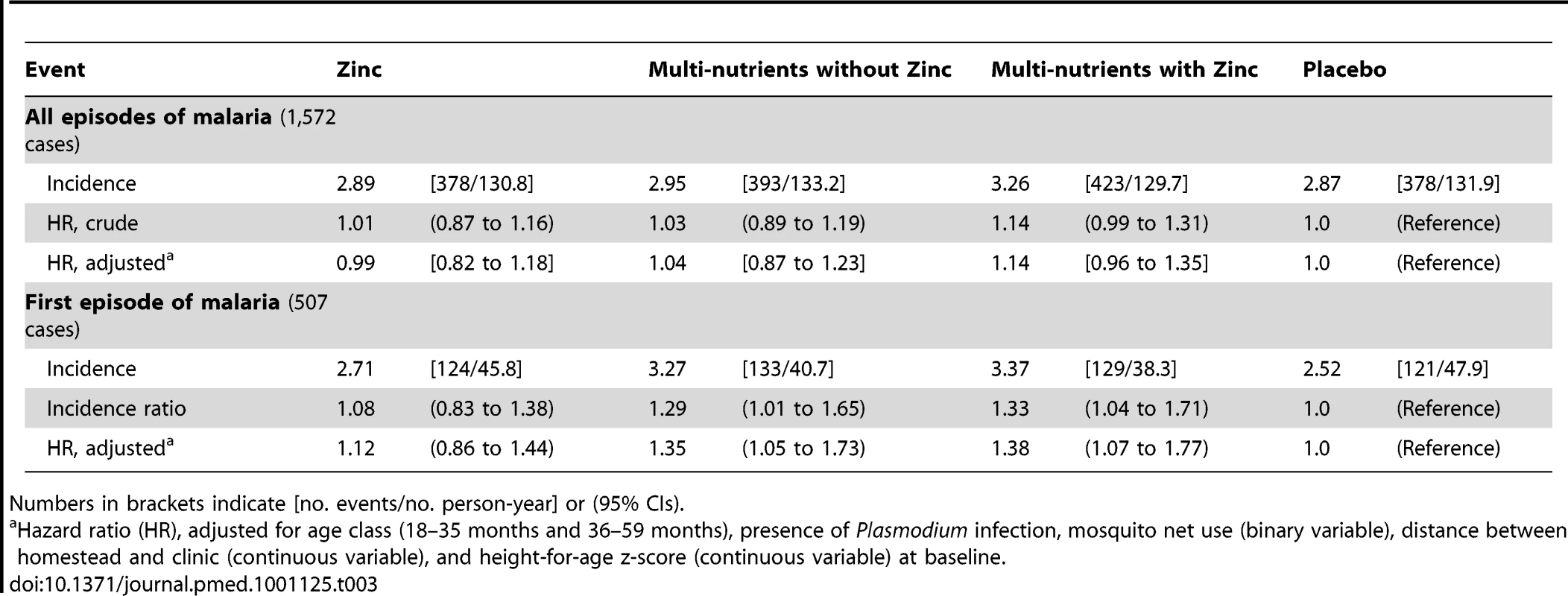
Numbers in brackets indicate [no. events/no. person-year] or (95% CIs). Secondary Analyses: Effects of Zinc on Malaria Rates
Likewise, when using different case definitions, we found no evidence that zinc protected against malaria when episodes were defined using density thresholds of 1,000 parasites/µL (HR 1.02, 95% CI 0.90–1.16), 3,000 parasites/µL (HR 1.01, 95% CI 0.88–1.17) or 5,000 parasites/µL (HR 1.00, 95% CI 0.87–1.16), or that zinc influenced the incidence or time-to-first episode of malaria (Table 3; Figure 2). Nor was there evidence that rates of episodes with parasite densities exceeding 10,000 parasites/µL and 100,000 parasites/µL were reduced by zinc (HR 1.00, 95% CI 0.87–1.16 and HR 1.19, 95% CI 0.89–1.58, respectively).
Fig. 2. Effect of zinc (panel A) or multi-nutrients (panel B) on time to first malaria episode. 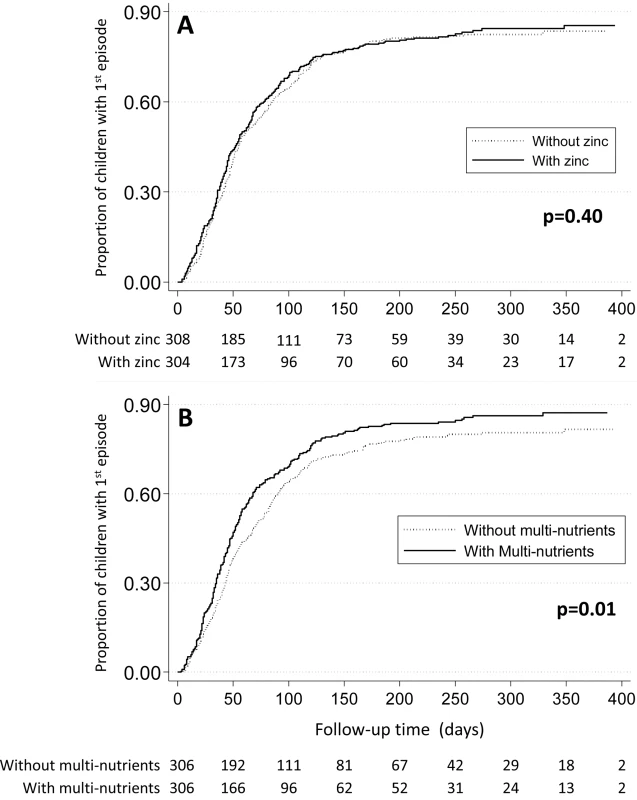
Marginal group comparisons (Kaplan-Meier analysis), with p-values obtained by Tarone-Ware test. Values below each panel indicates the number of children at risk. Lastly, we found no evidence that zinc protected against malaria after 100 days of supplementation (HR 1.01, 95% CI 0.86–1.18), or that the intervention effect depended on the duration of supplementation (100 days or less versus more than 100 days, p = 0.29), or by any of the pre-specified baseline factors (age class, Plasmodium infection, height-for-age z-score or zinc status) or by sex (unpublished data).
Secondary Analyses: Effects of Multi-nutrients on Malaria Rates
There was some indication that the effect of multi-nutrients on malaria rates decreased over time: when analysis was limited to the first 100 days of supplementation, the hazard ratio was 1.17 (95% CI 1.02–1.34), and in the first 50 days 1.23 (95% CI 1.02–1.50), but the statistical evidence for a change in the effect of multi-nutrients over time was weak (p-value for the change in effect 0.10 and 0.17 with time entered as continuous or categorical [< or ≥100 days] variable). When analysis was limited to the first or only episode, malaria rates were greater in those who received multi-nutrients (HR 1.29, 95% CI 1.08–1.54), and the median time to the first episode of malaria was 54 days for children receiving multi-nutrients, compared to 72 days for those without (p = 0.01; Tarone-Ware test, Figure 2).
When analysing the data by iron status at baseline, multi-nutrient supplementation appeared to increase the overall number of malaria episodes by 41% (95% CI 9%–82%) in children with iron deficiency, whereas there was no evident effect in their iron-replete peers (Figure 3; p-value for difference in effect 0.01). For episodes with densities >10,000 and >100,000 parasites/µL, similar effect modification was observed, whereby the increase in rates remained consistently and, with similar magnitude, restricted to children with iron deficiency (Figure S2).
Fig. 3. Effect of multi-nutrient supplementation on malaria rates, by iron status and age class. 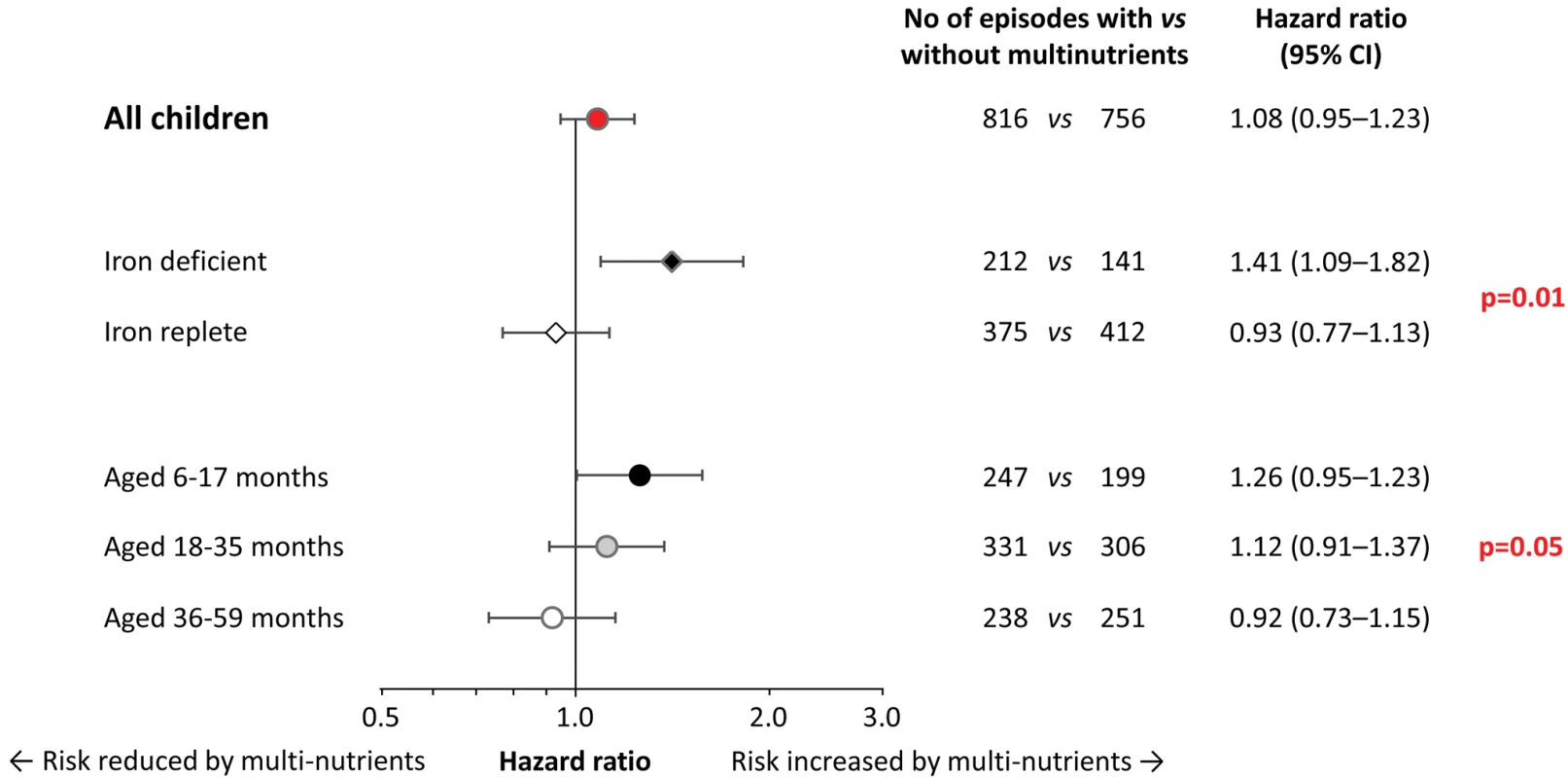
Malaria with case definition as pre-defined in the analysis plan. Values on the right indicate crude hazard ratios (95% CIs); p-values for differences in intervention effects between subgroups (with age class entered on an ordinal scale). Initial iron status was defined as iron-deficient (black diamond; plasma ferritin concentration <12 µg/L, n = 111) or iron replete (white diamond; plasma ferritin concentration ≥12 µg/L, without inflammation; n = 312). In the analysis, we excluded children in whom iron status was uncertain (plasma ferritin concentration ≥12 µg/L, with inflammation); hence analyses are restricted to 423 subjects. Adjustment for distance between homestead and dispensary, height-for-age z-scores, mosquito net use, and Plasmodium infection at baseline led to similar estimates (unpublished data). The prevalence of iron deficiency at baseline decreased rapidly with age (38%, 20% and 5% in children aged 6–17 months, 18–35 months, and 36–60 months, respectively; p<0.001; corresponding values in those without inflammation: 45%, 28%, and 7%; p<0.001). As with initial iron status, age strongly modified the effect of multi-nutrients on malaria incidence (Figure 3). Contrasts between age-specific effects on malaria rates increased when defining episodes with higher parasite density cut-offs (Figure S3). For episodes with hyper-parasitaemia (>100,000 parasites/µL), multi-nutrients increased rates by 54% (95% CI 1%–137%) in the youngest children, but reduced rates by 58% (95% CI −3% to −82%) in the eldest (p-value for interaction: 0.006), with an overall neutral effect.
Secondary Analyses: Effect of Multi-nutrients on Haemoglobin Concentrations
Analysis of haemoglobin concentrations at the second survey (when virtually all children were symptom-free) showed that the youngest children and those with iron deficiency at baseline responded most to multi-nutrient supplementation (8.4 g/L, 95% CI 4.2 −12.5 g/L and 8.7 g/L, 95% CI 5.3–12.2 g/L, respectively; Figure 4). Similar beneficial effects were found at time of the first malaria episode: multi-nutrients increased haemoglobin concentrations by 6.5 g/L (95% CI 1.2–11.9 g/L) in children with iron deficiency, while no marked effect was apparent in those who were iron replete (−2.1 g/L, 95% CI −4.7 to 0.5 g/L; difference in effect: 8.6 g/L, 95% CI 2.7–14.6 g/L; p-value 0.004); (Figure 5). Similar patterns were observed for the intervention effects on haemoglobin concentrations during the second malaria episode (unpublished data).
Fig. 4. Effect of multi-nutrients on haemoglobin concentration at the second survey, by iron status and age class. 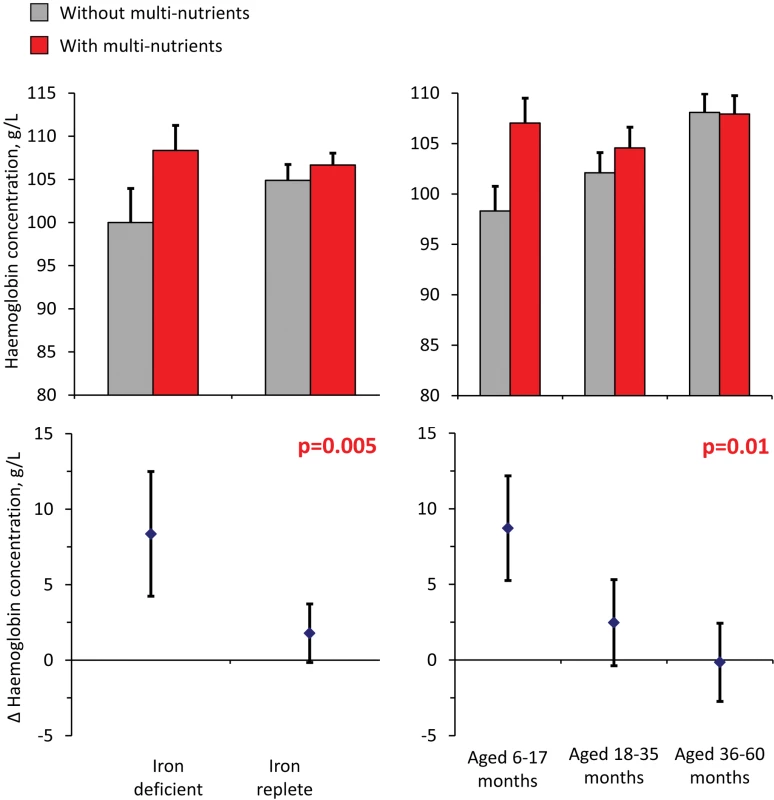
Top: haemoglobin concentrations, by supplementation group. Bottom: effects of multi-nutrients on haemoglobin concentrations. Left: By initial iron status. Right: By age class. Line bars indicate 95% CIs (only upper half of the interval indicated in top panel). Analysis based on 598 children. All estimates are adjusted for standardized haemoglobin concentrations at baseline. p-Values indicate interaction between age class or iron status and intervention effects on haemoglobin concentration. Fig. 5. Effect of multi-nutrients on haemoglobin concentration during the first malaria episode, by iron status and age class. 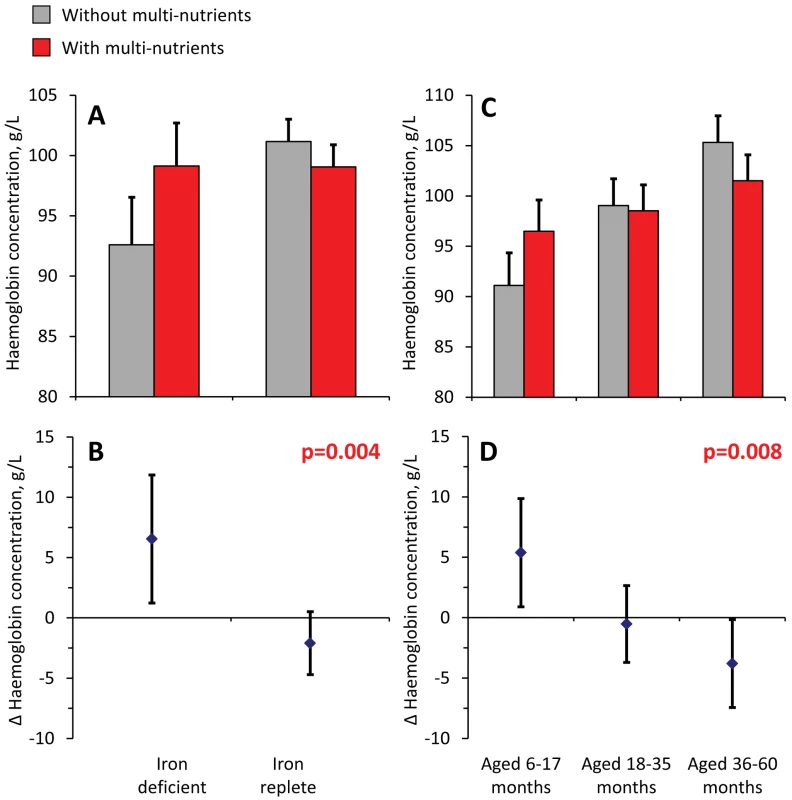
Top: haemoglobin concentrations, by supplementation group. Bottom: effects of multi-nutrients on haemoglobin concentrations. (A and B) By initial iron status. C and D) By age class. Line bars indicate 95% CIs (only upper half of the interval indicated in top panel). Analysis is based on 507 episodes. All estimates are adjusted for standardized haemoglobin concentrations at baseline. Further adjustment for time between start of intervention and episode led to virtually identical estimates. p-Values indicate interaction between age class or iron status and intervention effects on haemoglobin concentration. Discussion
Despite a high prevalence of zinc deficiency, excellent compliance, and few drop-outs, we found no evidence from this trial that preventive zinc supplementation, alone or with multi-nutrients, reduced rates of febrile attacks of malaria. In the primary analysis, there was no evidence that multi-nutrients influenced malaria rates, but results from a secondary analysis suggest that multi-nutrient supplementation may have increased the incidence of first malaria episodes by approximately 30%. The effect of supplementation on overall malaria rates seemed to vary with iron status at baseline: among children with iron deficiency, multi-nutrients increased rates of malaria episodes by 41%, while they had no effect in those who were iron replete. This difference in effect was unlikely to have been observed by chance (p = 0.01).
One limitation of our study is that supplements were colour-coded and not in packs labelled with individual identity numbers. During the field work, however, we received no indication that participants or field staff discovered who received what intervention, or that participants favoured a particular type of supplement. It should be noted also that the clinical outcome assessors were blinded to what intervention had been assigned to individual children. HIV infection was not assessed in our study but is unlikely to have confounded its results: the prevalence of HIV infection in this paediatric population is probably below 5% and, due to the randomisation process, marked imbalances in HIV infection at baseline are unlikely.
By excluding children with height-for-age z-score>−1.5 SD, the children enrolled in our trial probably were more zinc deficient than the general population from which they were sampled. Thus the effect on malaria rates that could potentially be attained in the general population, without this exclusion criterion, is probably smaller than that reported here. Two previous trials indicated that zinc can protect against malaria. The Gambian trial [2] was not designed to assess this association, and episodes were recorded through routine clinical procedures. In Papua New Guinea, zinc reduced the incidence of febrile episodes with parasitaemia ≥9,200/µL and ≥100,000/µL by 38% (95% CI 3%–60%) and 69% (95% CI 36%–87%), respectively [3]. Both studies were smaller than our study (91 cases of P. falciparum malaria in Papua New Guinea, as compared to 1,499 in our study). It seems unlikely that discrepancies with our findings are explained by differences in access to health care and a differential effect on rates of severe malaria: even with episodes with parasitaemia ≥100,000/µL, our confidence intervals (Table 3) are incompatible with the magnitude of the protective effect reported from Papua New Guinea. In addition, the overall and malaria-specific mortality reduction found in Pemba (7% and 10%, respectively) [41], seems inconsistent with the 69% reduction in episodes with parasitaemia ≥100,000/µL that was reported from Papua New Guinea. The intensity of malaria transmission in Papua New Guinea was much lower than in Burkina Faso, Pemba, or our study, raising the question of whether zinc affords protection only in populations with low acquired immunity. The absence of an age-dependent decrease in efficacy of zinc supplementation in our study makes this unlikely. Thus the divergent results from Papua New Guinea may be due to unknown differences in host, parasite, or environmental factors that predict the response to zinc.
Because malaria parasites are transmitted through mosquito bites, they bypass epithelial barriers that form the first line of defence against pathogens that cause diarrhoea and respiratory infections. Zinc is known to play a critical role in maintaining epithelial barrier function [42]–[46]. In vitro studies using Caco-2 cells suggest that tight junctions between intestinal epithelial cells have impaired function in zinc deficiency [46], whilst morphological studies and findings from a zinc supplementation trial in Bangladeshi children with diarrhoea suggest that zinc deficiency adversely affects intestinal permeability [47],[48]. The protective effect of zinc against diarrhoea and respiratory infections, as compared to the absence of such an effect against malaria, suggest that this nutrient primarily acts by strengthening barrier function rather than through immunity.
Our results also suggest that an increase in overall malaria rates may have occurred in children with iron deficiency. Subgroup analyses should generally be interpreted with caution [49]. On one hand, we did not specify interactions between multi-nutrient supplementation and iron status a priori, and the direction of the subgroup effect is in contrast with the findings previously obtained in the Pemba sub-study with iron and folic acid [8]. On the other hand, the difference in effects of multi-nutrients between children with and without iron deficiency was large, the statistical evidence for effect modification was strong and consistent for other malaria-related outcomes (haemoglobin concentration and malaria episodes with higher parasite densities).
Because the effect of multi-nutrient supplementation on malaria in our trial depended on initial iron status, it would seem most likely that this was caused by iron, and not by other nutrients in the supplement. Diverse lines of evidence suggest that, of all the micronutrients, iron is the most critical mediator of host–pathogen interactions [50], and our findings build on existing evidence suggesting that supplementation with iron can increase malaria risk [8],[51]–[54].
In their report of the findings from the Pemba sub-study [8], the authors were cautious in their interpretation of the subgroup effect, stating that their results suggested that supplementation with iron and folic acid is beneficial in children with iron deficiency, but unsafe in those who are iron-replete. Based on these findings, an expert group convened by the World Health Organization recommended that iron supplements should be administered routinely to iron-deficient infants in settings with adequate access to anti-malarial treatment [10]. Unfortunately, our findings contradict those from the Pemba study, and thereby cast doubt on the assumption that iron is safe in iron-deficient children. In the Pemba study, iron deficiency (found in 75% of children) was defined by elevated molar ratios of zinc protoporphyrin and haem. Although to their credit, ZPP∶H ratios react more slowly to inflammation than plasma ferritin concentrations, elevated ratios may occur due to (longstanding) inflammation, (asymptomatic) Plasmodium infection, and thalassaemia (Veenemans, unpublished data), and hence falsely indicate iron deficiency. Plasma ferritin concentration below 12 µg/L as indicator of iron deficiency may fail to detect deficient children in the presence of inflammation, but it is a highly specific marker of iron deficiency, probably more so than the ZPP∶H ratio.
We speculate that iron may have enhanced parasite proliferation specifically in children with iron deficiency, because iron absorption in this subgroup is more efficient and thus may lead to transient production of non-transferrin bound iron [55]–[57], which may act as a nutritional source and favour the proliferation of Plasmodium parasites [10]. Evidence in support of this hypothesised mechanism is, however, lacking. In addition, children with a low plasma ferritin concentration are probably free of inflammation and likely to absorb iron rapidly. Because children with iron deficiency were substantially younger, the difference in effect between subgroups of iron-replete and -deficient children may also (partly) have been mediated by the lack of protective immunity in these younger children, and the higher dose of supplemental iron per kilogram of bodyweight. It seems plausible that the magnitude of potential adverse effects of iron is determined by a combination of the host's immune status, dose of iron per kg bodyweight, and the amount of iron absorbed. The qualitative interaction with age and iron status as observed in our study emphasizes the need for trials to take these into account in the analysis, as overall estimates may tend towards a neutral effect [58].
We found no support for our initial hypothesis that, at the time of malaria episodes, haemoglobin concentrations would be lower in children receiving multi-nutrients. In fact, young children with iron deficiency (who were most vulnerable to declines in haemoglobin) were better able to maintain their haemoglobin concentration when they received multi-nutrients. The critical issue remains whether the benefits of higher haemoglobin concentrations during acute malaria and thus the expected reduced risk of severe malarial anaemia, on one hand, outweigh the potential risk of severe disease manifestations that can be associated with an increased frequency of malaria, on the other hand. Access to primary care facilities may be a critical factor determining this balance, and in the absence of early treatment, the youngest children with iron deficiency may be a vulnerable subgroup.
The estimated amount of iron absorbed from our supplement is close to that absorbed from multi-nutrient mixes used for home fortification [59]–[61]. Our data question the safety of supplying these mixes to vulnerable populations, particularly in settings with poor malaria prevention, but even in conditions where access to diagnosis and therapy for malaria is excellent, as in our study.
In conclusion, when results from all trials are considered together, there is no evidence that zinc interventions can reduce the burden of malaria in African children. We have presented evidence that multi-nutrient supplementation may increase the risk of malaria in children with iron deficiency, strengthening earlier concerns about the safety of multi-nutrient supplementation in malaria-endemic areas, even in settings with good access to health care and appropriate treatment. Similar risks may apply to home fortification in such settings. Although safety may be improved by modifying the formulation of supplements (in particular by reducing the iron dose) implementing multi-nutrient supplementation or home fortification as a public health measure should be carefully monitored until this has been demonstrated. The World Health Organization recommendation that iron should be supplemented to iron-deficient children needs reconsideration, not because we claim that our study provides conclusive evidence that targeting this group is unsafe, but because the current guideline is based on weak evidence from a single study that is contradicted by our findings using highly specific markers of iron deficiency.
Supporting Information
Zdroje
1. IZiNCG 2009 Systematic reviews of zinc intervention strategies. International Zinc Nutrition Consultative Group Technical Document #2. BrownKHHessSY Food Nutr Bull 30 Suppl. S1 S184
2. BatesCJEvansPHDardenneM 1993 A trial of zinc supplementation in young rural Gambian children. Br J Nutr 69 243 255
3. ShankarAHGentonBBaisorM 2000 The influence of zinc supplementation on morbidity due to Plasmodium malaria: a randomized trial in preschool children in Papua New Guinea. Am J Trop Med Hyg 62 663 669
4. MüllerOBecherHvan ZweedenABYeYDialloDA 2001 Effect of zinc supplementation on malaria and other causes of morbidity in West African children: randomised double blind controlled trial. Brit Med J 322 1567 1570
5. RichardSAZavaletaNCaulfieldLEBlackREWitzigRS 2006 Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg 75 126 132
6. RonaghyHAReinholdJGMahloudjiMGhavamiPFoxMR 1974 Zinc supplementation of malnourished schoolboys in Iran: increased growth and other effects. Am J Clin Nutr 27 112 121
7. RonaghyHSpivey FoxMRGarnSM 1969 Controlled zinc supplementation for malnourished school boys: a pilot experiment. Am J Clin Nutr 22 1279 1289
8. SazawalSBlackRERamsanM 2006 Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367 133 143
9. StolzfusRJHeidkampRKenkelDHabichtJP 2007 Iron supplementation of young children: Learning from the new evidence. Food Nutr Bull 28 S572 S584
10. WHO 2007 Conclusions and recommendations of the WHO consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull 28 S621 S627
11. EkvallHPremjiZBjorkmanA 2000 Micronutrient and iron supplementation and effective antimalarial treatment synergistically improve childhood anaemia. Trop Med Intern Health 5 696 705
12. OuédraogoHZDramaix-WilmetMZebaANHennartPDonnenP 2008 Effect of iron or multiple micronutrient supplements on the prevalence of anaemia among anaemic young children of a malaria-endemic area: a randomized double-blind trial. Trop Med Int Health 13 1257 1266
13. FriisHMwanikiDOmondiB 2003 Effects on haemoglobin of multi-micronutrient supplementation and multi-helminth chemotherapy: a randomized, controlled trial in Kenyan school children. Eur J Clin Nutr 57 573 579
14. OjukwuJUOkebeJUYahavDPaulM 2009 Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas. Cochrane Database Sys Rev 3 CD006589
15. VerhoefHWestCENzyukoSM 2002 Intermittent administration of iron and sulfadoxine-pyrimethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet 360 908 914
16. OppenheimerSJGibsonFDMacfarlaneSB 1986 Iron supplementation increases prevalence and effects of malaria: report on clinical studies in Papua New Guinea. Trans R Soc Trop Med Hyg 80 603 612
17. VeenemansJAndang'oPEAMbugiEV 2008 α+-Thalassemia protects against anemia associated with asymptomatic malaria: evidence from community-based surveys in Kenya and Tanzania. J Infect Dis 198 401 408
18. VeenemansJMankTOttenhofM 2011 Protection against diarrhea associated with Giardia intestinalis is lost with multi-nutrient supplementation: a study in Tanzanian children. PLoS Negl Trop Dis 5 6 e1158 doi:10.1371/journal.pntd.0001158
19. EllmanRMaxwellCFinchRShayoD 1998 Malaria and anaemia at different altitudes in the Muheza district of Tanzania: childhood morbidity in relation to level of exposure to infection. Ann Trop Med Parasitol 92 741 753
20. McAllisterFAStraussSESackettDLAltmanDG 2003 Analysis and reporting of factorial trials: a systematic review. JAMA 289 2545 2553
21. LubsenJPocockSJ 1994 Factorial trials in cardiology: pros and cons. Eur Heart J 15 585 588
22. UNICEF/UNU/WHO 2001 Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. Document reference no. WHO/NHD/01.3 Geneva, Switzerland World Health Organization Available: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. Accessed 2 November 2011
23. GoodmanSNBerlinJA 1994 The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med 121 200 206
24. VerhoefH 2007 Malnutrition, zinc deficiency and malaria in Africa. Lancet 369 2156
25. PiperRLebrasJWentworthL 1999 Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). Am J Trop Med Hyg 60 109 118
26. WhiteNJ 2008 How antimalarial drug resistance affects post-treatment prophylaxis. Malaria J 7 9
27. BrownKHPeersonJMRiveraJAllenLH 2002 Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr 75 1062 1071
28. OgbonnaAUnekeCJ 2008 Artemisinin-based combination therapy for uncomplicated malaria in sub-Saharan Africa: the efficacy, safety, resistance and policy implementation since Abuja 2000. Trans R Soc Trop Med Hyg 102 621 627
29. WhiteNJ 2008 Qinghaosu (artemisinin): the price of success. Science 320 330 334
30. AltmanDGMachinDBryantTNGardnerMJ 2000 Statistics with confidence: confidence intervals and statistical guidelines London, UK BMJ Books
31. IZiNCG 2004 Assessment of the risk of zinc deficiency in populations and options for its control (Hotz C, Brown KH, eds.). International Zinc Nutrition Consultative Group Technical Document #1. Food Nutr Bull 25 Suppl. 2 S91 S204
32. MoorthyVReedZSmithPG 2007 Meeting report. Vaccine 25 5115 5123
33. SchellenbergJRMSmithTAlonsoPLHayesRJ 1994 What is clinical malaria? Finding case definitions for field research in highly endemic areas. Parasitol Today 10 439 442
34. SmithTSchellenbergJAHayesR 1994 Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med 13 2345 2358
35. O'MearaWPHallBFMcKenzieFE 2007 Malaria vaccine efficacy: the difficulty of detecting and diagnosing malaria. Malar J 6 36
36. SmithTA 2007 Measures of clinical malaria in field trials of interventions against Plasmodium falciparum. Malar J 6 53
37. KoramKAMolyneuxME 2007 When is “malaria” malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. Am J Trop Med Hyg 77 Suppl 6 1 5
38. DelleyVBouvierPBreslowN 2000 What does a single determination of malaria parasite density mean? A longitudinal survey in Mali. Trop Med Int Health 5 404 412
39. CheungYBXuYTanSHCuttsFMilliganP 2010 Estimation of intervention effects using first or multiple episodes in clinical trials: the Andersen-Gill model re-examined. Statist Med 29 328 336
40. O'MearaWPLangT 2009 Malaria vaccine trial endpoints – bridging the gaps between trial design, public health and the next generation of vaccines. Parasite Immunol 31 574 581
41. SazawalSBlackRERamsanM 2007 Effect of zinc supplementation on mortality in children aged 1–48 months: a community-based randomised placebo-controlled trial. Lancet 369 927 934
42. PrenticeAMGhattasHCoxSE 2007 Host-pathogen interactions: can micronutrients tip the balance? J Nutr 137 1334 1337
43. KeenCLGershwinME 1990 Zinc deficiency and immune function. Ann Rev Nutr 10 415 431
44. AlamANSarkerSAWahedMAKhatunMRahamanMM 1994 Enteric protein loss and intestinal permeability changes in children during acute shigellosis and after recovery: effect of zinc supplementation. Gut 35 1707 1711
45. Truong-TranAQCarterJRuffinRZalewskiPD 2001 New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol 79 170 177
46. ZalewskiPDTruong-TranAQGrosserD 2005 Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets. A review. Pharmacol Ther 105 127 149
47. FinamoreAMassimiMConti DevirgiliisLMengheriE 2008 Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr 138 1664 1670
48. MoranJRLewisJC 1985 The effects of zinc deficiency on intestinal permeability: an ultrastructural study. Pediatr Res 19 968 973
49. SunXBrielMWalterSDGuyattGH 2010 Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 340 c117
50. RoySKBehrenRHHaiderRAkramuzzamanSMMahalanabisD 1992 Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J Peadiatr Gastroenterol Nutr 15 289 296
51. MurrayMJMurrayNJMurrayABMurrayMB 1970 Refeeding malaria and hyperferraemia. Lancet 1 653 654
52. OppenheimerSJ 2001 Iron and its relation to immunity and infectious disease. J Nutr 131 616S 635S
53. GeraTSachdevHPS 2002 Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ 325 1142
54. ShankarAH 2000 Nutritional modulation of malaria morbidity and mortality. J Infect Dis 182 Suppl 1 S37 S53
55. HutchinsonCAl-AshgarWLiuDY 2004 Oral ferrous sulphate leads to a marked increase in pro-oxidant nontransferrin-bound iron. Eur J Clin Invest 34 782 784
56. BaronJBen-DavidGHallakM 2008 Changes in non-transferrin-bound iron (NTBI) in pregnant women on iron supplements. Eur J Obstet Gynecol Reprod Biol 140 281 282
57. DresowBPetersenDFischerRNielsenP 2008 Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals 21 273 276
58. PrenticeAM 2008 Iron metabolism, malaria and other infections: What is all the fuss about? J Nutr 138 2537 2541
59. WHO 2011 Guideline: use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age Geneva, Switzerland World Health Organization, 2011 Available: http://whqlibdoc.who.int/publications/2011/9789241502047_eng.pdf. Accessed 9 September 2011
60. TroeschBEgliIZederCHurrellRFde PeeS 2009 Optimization of a phytase-containing micronutrient powder with low amounts of highly bioavailable iron for in-home fortification of complementary foods. Am J Clin Nutr 89 539 544
61. VerhoefHVeenemansJ 2009 Safety of iron-fortified foods in malaria-endemic areas. Am J Clin Nutr 89 1949 1950
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Voluntary Medical Male Circumcision: Strategies for Meeting the Human Resource Needs of Scale-Up in Southern and Eastern Africa
- Voluntary Medical Male Circumcision: Translating Research into the Rapid Expansion of Services in Kenya, 2008–2011
- Quality of Maternal Health Care: A Call for Papers for a Maternal Health Task Force–PLoS Collection
- Voluntary Medical Male Circumcision: Matching Demand and Supply with Quality and Efficiency in a High-Volume Campaign in Iringa Region, Tanzania
- Priorities for Research on Equity and Health: Towards an Equity-Focused Health Research Agenda
- What Research Is Needed to Stop TB? Introducing the
- Responsible Governance for Mental Health Research in Low Resource Countries
- Voluntary Medical Male Circumcision: Modeling the Impact and Cost of Expanding Male Circumcision for HIV Prevention in Eastern and Southern Africa
- Effect of Supplementation with Zinc and Other Micronutrients on Malaria in Tanzanian Children: A Randomised Trial
- HIV, Gender, Race, Sexual Orientation, and Sex Work: A Qualitative Study of Intersectional Stigma Experienced by HIV-Positive Women in Ontario, Canada
- Voluntary Medical Male Circumcision: A Framework Analysis of Policy and Program Implementation in Eastern and Southern Africa
- Voluntary Medical Male Circumcision: Logistics, Commodities, and Waste Management Requirements for Scale-Up of Services
- Optimal Uses of Antiretrovirals for Prevention in HIV-1 Serodiscordant Heterosexual Couples in South Africa: A Modelling Study
- Managing the Demand for Global Health Education
- Post-neonatal Mortality, Morbidity, and Developmental Outcome after Ultrasound-Dated Preterm Birth in Rural Malawi: A Community-Based Cohort Study
- Voluntary Medical Male Circumcision: An Introduction to the Cost, Impact, and Challenges of Accelerated Scaling Up
- On the Futility of Screening for Genes That Make You Fat
- Rapid Diagnosis of Tuberculosis with the Xpert MTB/RIF Assay in High Burden Countries: A Cost-Effectiveness Analysis
- Physical Activity Attenuates the Influence of Variants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children
- A Head-to-Head Comparison of Four Artemisinin-Based Combinations for Treating Uncomplicated Malaria in African Children: A Randomized Trial
- Evidence-Based Guidelines for Mental, Neurological, and Substance Use Disorders in Low- and Middle-Income Countries: Summary of WHO Recommendations
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evidence-Based Guidelines for Mental, Neurological, and Substance Use Disorders in Low- and Middle-Income Countries: Summary of WHO Recommendations
- Voluntary Medical Male Circumcision: Strategies for Meeting the Human Resource Needs of Scale-Up in Southern and Eastern Africa
- Rapid Diagnosis of Tuberculosis with the Xpert MTB/RIF Assay in High Burden Countries: A Cost-Effectiveness Analysis
- Physical Activity Attenuates the Influence of Variants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



