-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPersistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
Background:
The beneficial effects of statins in rheumatoid arthritis (RA) have been suggested previously, but it is unclear whether statins may prevent its development. The aim of this retrospective cohort study was to explore whether persistent use of statins is associated with onset of RA.Methods and Findings:
The computerized medical databases of a large health organization in Israel were used to identify diagnosed RA cases among adults who began statin therapy between 1998 and 2007. Persistence with statins was assessed by calculating the mean proportion of follow-up days covered (PDC) with statins for every study participant. To assess the possible effects of healthy user bias, we also examined the risk of osteoarthritis (OA), a common degenerative joint disease that is unlikely to be affected by use of statins.
A total of 211,627 and 193,770 individuals were eligible for the RA and OA cohort analyses, respectively. During the study follow-up period, there were 2,578 incident RA cases (3.07 per 1,000 person-years) and 17,878 incident OA cases (24.34 per 1,000 person-years). The crude incidence density rate of RA among nonpersistent patients (PDC level of <20%) was 51% higher (3.89 per 1,000 person-years) compared to highly persistent patients who were covered with statins for at least 80% of the follow-up period. After adjustment for potential confounders, highly persistent patients had a hazard ratio of 0.58 (95% confidence interval 0.52–0.65) for RA compared with nonpersistent patients. Larger differences were observed in younger patients and in patients initiating treatment with high efficacy statins. In the OA cohort analysis, high persistence with statins was associated only with a modest decrement in risk ratio (hazard ratio = 0.85; 0.81–0.88) compared to nonadherent patients.Conclusions:
The present study demonstrates an association between persistence with statin therapy and reduced risk of developing RA. The relationship between continuation of statin use and OA onset was weak and limited to patients with short-term follow-up.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(9): e32767. doi:10.1371/journal.pmed.1000336
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000336Summary
Background:
The beneficial effects of statins in rheumatoid arthritis (RA) have been suggested previously, but it is unclear whether statins may prevent its development. The aim of this retrospective cohort study was to explore whether persistent use of statins is associated with onset of RA.Methods and Findings:
The computerized medical databases of a large health organization in Israel were used to identify diagnosed RA cases among adults who began statin therapy between 1998 and 2007. Persistence with statins was assessed by calculating the mean proportion of follow-up days covered (PDC) with statins for every study participant. To assess the possible effects of healthy user bias, we also examined the risk of osteoarthritis (OA), a common degenerative joint disease that is unlikely to be affected by use of statins.
A total of 211,627 and 193,770 individuals were eligible for the RA and OA cohort analyses, respectively. During the study follow-up period, there were 2,578 incident RA cases (3.07 per 1,000 person-years) and 17,878 incident OA cases (24.34 per 1,000 person-years). The crude incidence density rate of RA among nonpersistent patients (PDC level of <20%) was 51% higher (3.89 per 1,000 person-years) compared to highly persistent patients who were covered with statins for at least 80% of the follow-up period. After adjustment for potential confounders, highly persistent patients had a hazard ratio of 0.58 (95% confidence interval 0.52–0.65) for RA compared with nonpersistent patients. Larger differences were observed in younger patients and in patients initiating treatment with high efficacy statins. In the OA cohort analysis, high persistence with statins was associated only with a modest decrement in risk ratio (hazard ratio = 0.85; 0.81–0.88) compared to nonadherent patients.Conclusions:
The present study demonstrates an association between persistence with statin therapy and reduced risk of developing RA. The relationship between continuation of statin use and OA onset was weak and limited to patients with short-term follow-up.
: Please see later in the article for the Editors' SummaryIntroduction
Rheumatoid arthritis (RA) is a leading cause of disability that often reduces patients' quality of life and impairs their ability to work [1]. Prevalence estimates of RA worldwide indicate that the prevalence of RA range between 2.0 to 10.7 per 1,000 adults, based on the 1987 revised American College of Rheumatology (ACR) criteria [2].
RA is a chronic systemic inflammatory condition characterized by leukocyte recruitment into synovial tissue. There is growing evidence that statins have anti-inflammatory and immunumodulatory properties, demonstrated by reducing the level of C-reactive protein (CRP) that may play an important role in RA, independent of their cholesterol lowering effects [3],[4]. A modest beneficial effect of statins in RA has been demonstrated by several small randomized clinical trials and observational studies that reported on decreased disease activity [5],[6], decreased CRP levels, reduced number of swollen joints [6],[7], and improved vascular function [8],[9]. In contrast, a large US medical and pharmacy claims study [10] showed no beneficial effect for statins among 31,451 RA patients as measured by initiation and cessation of oral steroids. Consequently, there is a need for large investigations with long follow-up periods to explore whether statins can be shown not only to improve the clinical manifestation of RA, but perhaps also to relate to lower RA occurrence. In our previous studies we examined the effect of persistent use of statins on all-cause mortality [11], incident cataract [12], and age-related macular degeneration. In the present study, we evaluated the association between persistence with statins and onset of RA among a large, unselected population of statin users who were at least 18 y of age and did not have RA or a related disease, including symptomatic osteoarthritis (OA) or rheumatic fever, at study entry.
Methods
Study Population
We conducted a retrospective cohort study among the members of Maccabi Healthcare Services (MHS), a 1.8-million enrollee health maintenance organization (HMO) operating in Israel. According to the Israeli National Health Insurance Act, MHS may not bar any citizen who wishes to join it, and therefore every section in the Israeli population is represented in MHS. According to the most recent report of the Israel National Insurance [13], the mean age and proportion of women among MHS members (31.0 y, 48.6%) is similar to the general population (32.4 y, 48.9%). All data were obtained from MHS automated databases that have previously been described [11] and were used to elicit information on all dispensed community prescriptions, hospital discharge data, biochemistry results, using a unique nine-digit national identification number. Research ethics approval was obtained from Assuta Medical Center institutional review board.
Study Outcome
Incident cases of RA were defined by the date of first diagnostic codes associated with RA (Rheumatoid arthritis; International Classification of Diseases, 9th revision [ICD-9] codes 714.x) during the study follow-up period. The tendency of healthier patients to be more likely to persist with preventive treatments leads to a bias that has been termed “healthy user bias” [14]. To assess the potential effects of this possibly important bias, we also examined the association between persistence with statins and OA (Osteoarthritis; ICD-9 codes 715.x), a common degenerative joint disease that is unlikely to be affected by statin use.
Cohort Definition
The cohort covered the period 1998–2007 and included members who were continuously enrolled in the HMO from 1995 to 1998. New users of statins were identified among all MHS enrollees aged 18 y or older, who from January 1, 1998 to July 1, 2007 had at least one dispensed prescription of 3-hydroxy-3-methylgluraryl coenzyme A (HMG-CoA) reductase inhibitors (e.g., lovastatin, pravastatin, simvastatin, atorvastatin). The date of first purchased statin was defined as the index date. We only included patients who were enrolled in MHS and did not have a statin prescription at least 3 y prior to the index date. Also excluded were patients who had been diagnosed with RA, OA, or rheumatic fever, and patients who had any dispensed relevant medications (methotrexate, sulphasalzine, prednisone, leflunomide, azathioprine, infliximab, etanercept, adalimumab, hydroxychloroquine, auranofin, rituximab [abatecept is currently not available in Israel]) prior to the index date or within 1 y after the index date.
Proportion of Days Covered
Following previous studies [15]–[17], we calculated the mean proportion of days covered (PDC) by dividing the quantity of statins dispensed by the total time interval from index date to first diagnosis of RA or OA, death, leaving MHS, or December 31, 2007, whichever occurred first.
Other Study Variables
Demographic variables at index date included baseline values of age, gender, marital status, place of residency, years of stay in Israel (for new immigrants). Socioeconomic level was categorized into quintiles according to the poverty index of the member's enumeration area, as defined by 1995 national census. The poverty index is based on several parameters including, household income, educational qualifications, crowding, material conditions, and car ownership [18]. Study subjects' electronic medical records were reviewed for a diagnosis of chronic conditions defined by ICD-9 codes. Diabetes mellitus patients were identified by using the MHS computerized diabetes mellitus patient registry [19]. Information on cancer history was provided by the Israel National Cancer Registry (INCR), which has collected information of diagnosed cancer cases from all medical institutions in Israel since 1960.
Information on health services utilization, such as number of hospitalizations in general hospitals, visits to outpatient clinics, and filled prescriptions of antihypertensive drugs and diuretics, was based on data collected for the year prior to the index date. Laboratory test results included liver function and the median of all low-density lipoprotein (LDL)-cholesterol tests during the year prior to the index date, as well as presence of rheumatoid factor (RF) in patients diagnosed with RA.
Lipid-Lowering Pharmacotherapy
On the basis of previous clinical trials [20]–[24], statin therapy was categorized into three relative efficacy levels that were created from expected amounts of LDL-cholesterol reduction from baseline: (a) low efficacy (≤30% LDL reduction): daily dose of fluvastatin ≤40 mg, pravastatin ≤40 mg, simvastatin ≤10 mg, cerivastatin 0.2 mg, or lovastatin ≤40 mg or 10 mg twice daily; (b) moderate efficacy (31%–40% LDL reduction): daily dose of fluvastatin 80 mg, cerivastatin 0.3 mg or 0.4 mg, rosuvastatin <10 mg, simvastatin 20 mg or 40 mg, atorvastatin 10 mg; or (c) high efficacy (≥41% LDL reduction): simvastatin 80 mg, atorvastatin ≥20 mg, rosuvastatin ≥10 mg, pravastatin 80 mg, or lovastatin 80 mg).
Statistical Analysis
A chi-square test for categorical variables and a Kruskal-Wallis test for continuous variables were performed to determine significant differences in baseline characteristics and levels of PDC with statins that were categorized into <20% (reference category), 20%–39%, 40%–59%, 60%–79%, and 80% or above. In the primary analysis, a Cox proportional hazards model with years of follow-up as the time scale was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) [25] and to identify variables significantly associated with increased risk of RA. In the secondary analysis, similar regression models were computed with OA as a dependent variable. In both analyses, each participant was followed from the first purchase of statin to first diagnosis of RA (or OA), leaving MHS, or December 31, 2007, whichever occurred first. The maximum follow-up was approximately 10 y. To examine a potential prevalence incidence bias, we excluded patients with less than 1 y of follow-up. In addition, we conducted sensitivity analyses limited to patients with at least 5 y of follow-up.
The full multivariate model included the following baseline values: age at baseline (in 1-y intervals), gender, marital status, nationality, socioeconomic level, presence of chronic comorbidity, utilization of health services, LDL cholesterol level, and efficacy of the initial statin therapy. Tests for trend of ordinal variables were based on the category median values. Analyses were stratified by age categories, sex, baseline LDL levels, and efficacy of initial statin therapy. A chi-square test was performed to assess heterogeneity.
Results
The median number of health plan enrollees during the study period was more than 1.6 million, with persons aged 18 y or above accounting for 66% of the population. After applying the inclusion and exclusion criteria, a total of 211,627 individuals for the RA analysis and 193,770 individuals for the OA analysis were identified as being newly treated with statin agents during the study period. During the study follow-up period 11,692 individuals died and 3,343 left MHS.
Baseline characteristics of the study population eligible for the RA analysis cohort and for the OA analysis are given in Tables 1 and 2, respectively. Patients with high PDC were more likely to be older, belong to a lower socioeconomic level, have higher prevalence of chronic conditions such as cancer, diabetes, and cardiovascular diseases, and have more frequent hospitalizations and visits to primary physicians. During 4.97 and 4.79 y of mean follow-up in the RA and OA analyses, there were 2,578 (3.07 per 1,000 person-years) RA cases and 17,878 OA cases (24.34 per 1,000 person-years), respectively. The age - and sex-specific incidence rates of RA and OA in the study population during the 10 y of study period are depicted in Figure 1. In most age groups, the incidence rate of RA and OA was 2 - to 3-fold higher in women compared to men, and increased with age to a maximum in women aged 65–74 y (4.78 for RA and 50.45 per 1,000 for OA). RF tests were available for 76.6% of the diagnosed RA cases, of whom 1,478 (76.0%) were positive for RF at ≥10 IU/ml and 714 (36.7%) were positive for RF at ≥40 IU/ml.
Fig. 1. Incidence density rate (per 1,000 person-years) of RA and OA in study cohort, by age and sex, 1998–2006. 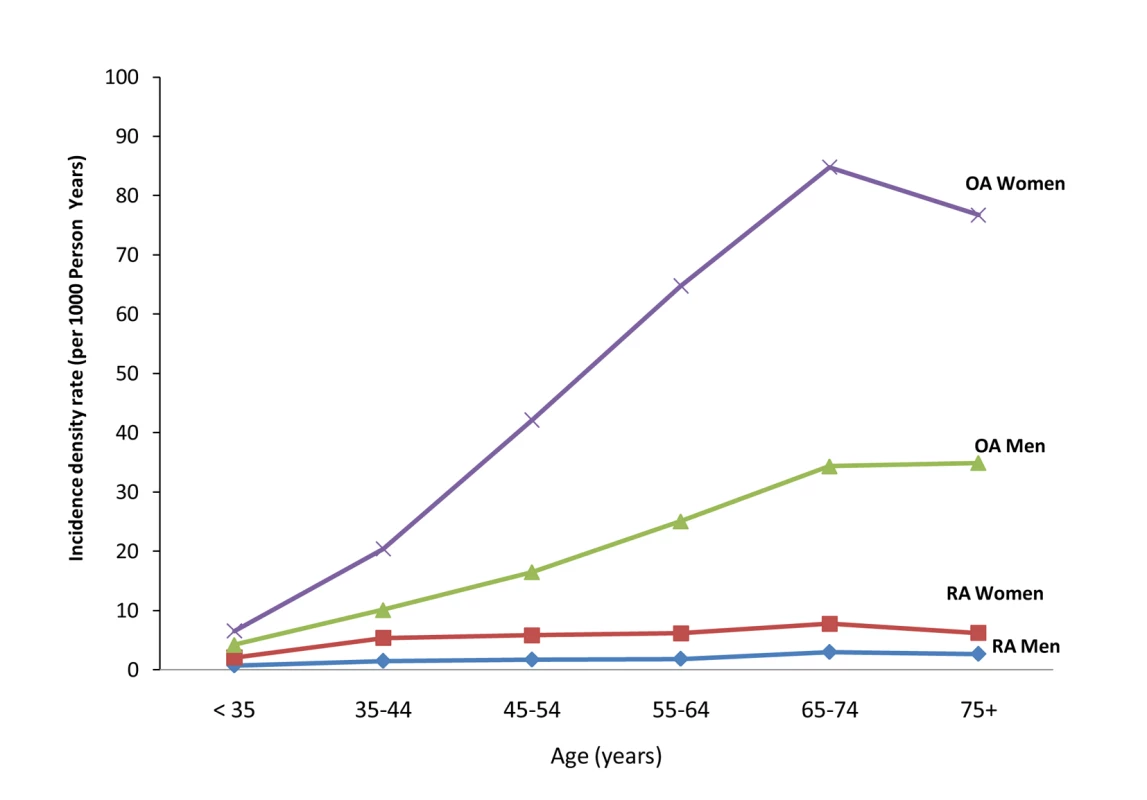
Tab. 1. Study population characteristics, according to PDC with statins, patients eligible for the RA analysis (n = 211,627). 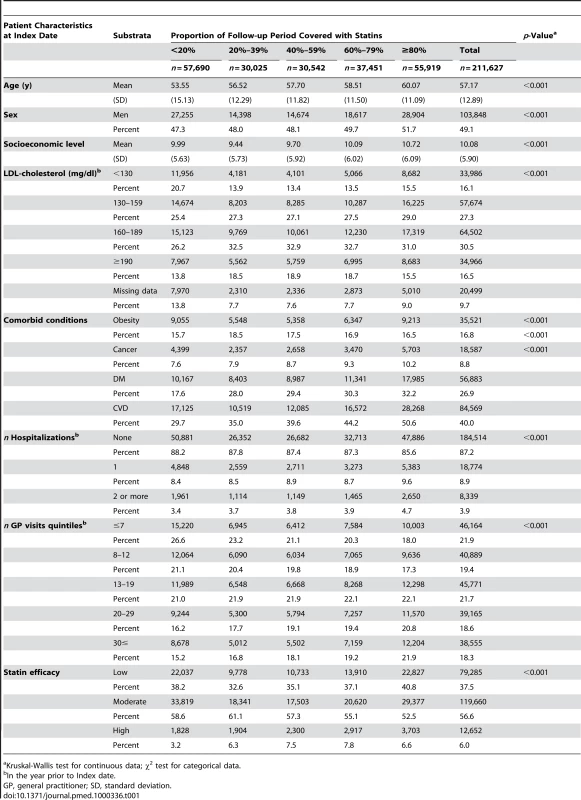
Kruskal-Wallis test for continuous data; χ2 test for categorical data. Tab. 2. Study population characteristics, according to PDC with statins, patients eligible for the OA (n = 193,770). 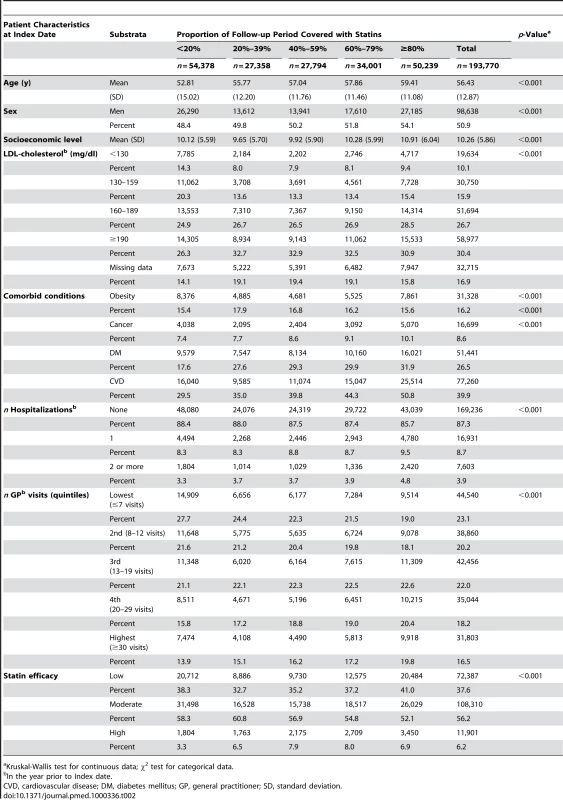
Kruskal-Wallis test for continuous data; χ2 test for categorical data. The crude incidence density rate of RA among patients with a PDC level of less than 20% (3.89 per 1,000 [95% CI 3.62–4.17]) was higher by 51% compared to patients with a PDC level of 80% (2.57 per 1,000 [2.37–2.78]). No similar pattern was observed in the OA analysis where the incidence density rate of OA in the lowest PDC level category (23.61 per 1,000 : 95% CI 22.92–24.31) was comparable to the incidence in the highest PDC level (24.12 per 1,000) (Table 3).
Tab. 3. Incidence density rates (IDR) of RA and OA according to the PDC with statins, MHS 1998–2007. 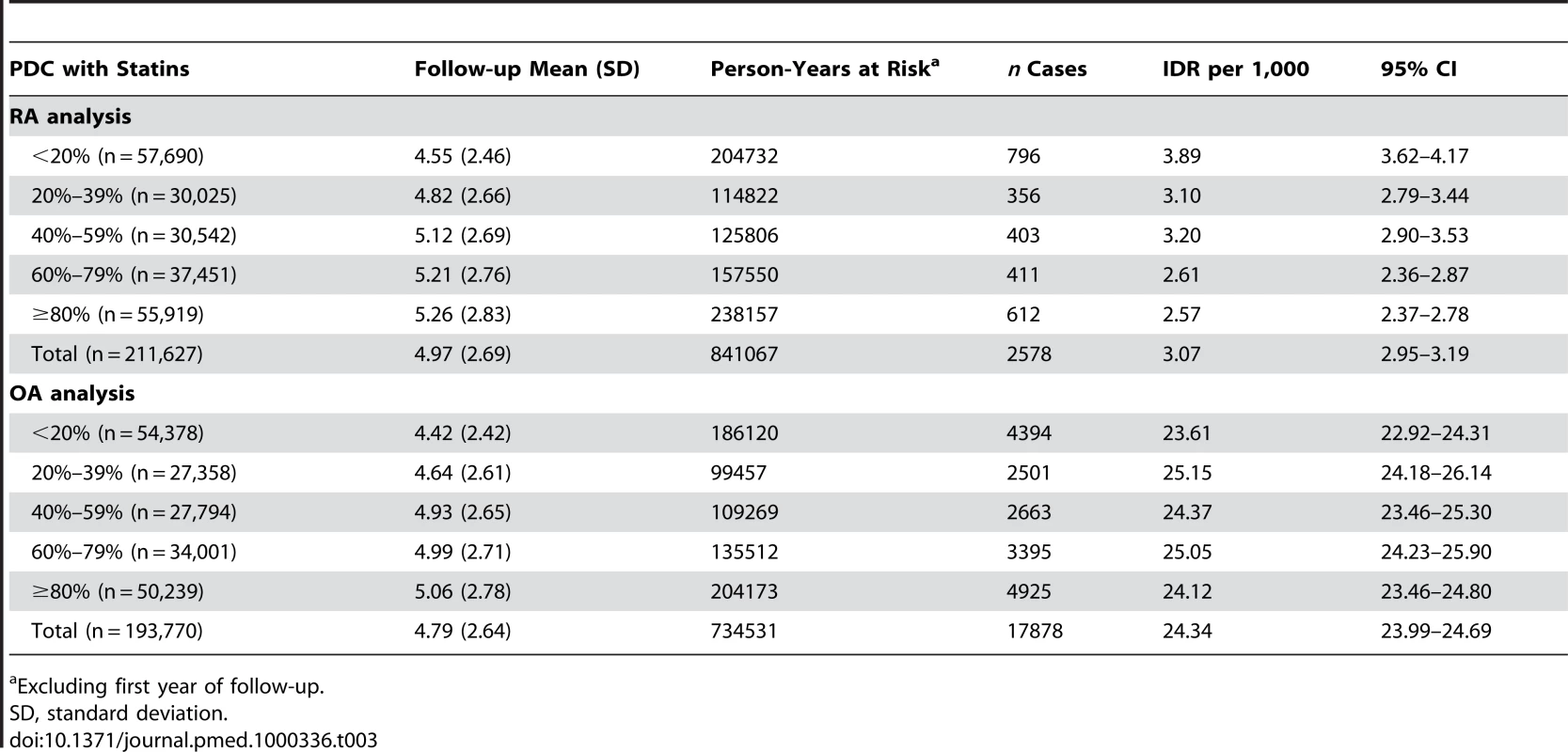
Excluding first year of follow-up. Baseline characteristics associated with increased risk for RA and OA included age, female gender, and frequent visits to primary physicians (Tables 4 and 5). Morbid obesity was related to a substantially higher risk of OA (HR = 1.72; 95% CI 1.65–1.78), but not to RA. Similar results were obtained when analyses were limited to patients with at least 5 y of follow-up.
Tab. 4. Mutually adjusted HRs and 95% CIs for RA according to PDC with statins and baseline characteristics, MHS 1998–2007. 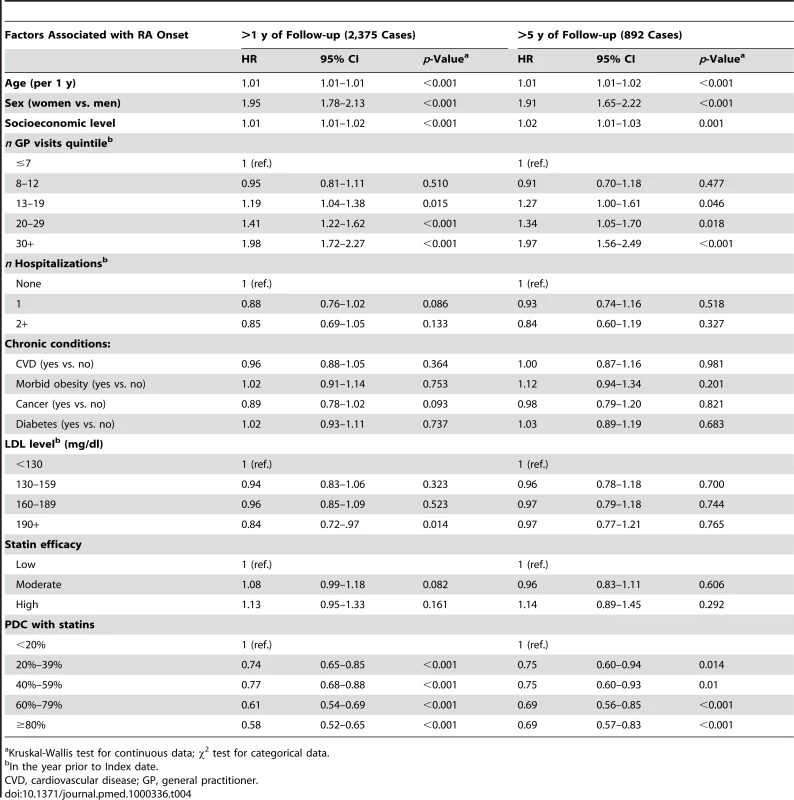
Kruskal-Wallis test for continuous data; χ2 test for categorical data. Tab. 5. Mutually adjusted HRs and 95% CIs for OA according to PDC with statins and baseline characteristics, MHS 1998–2007. 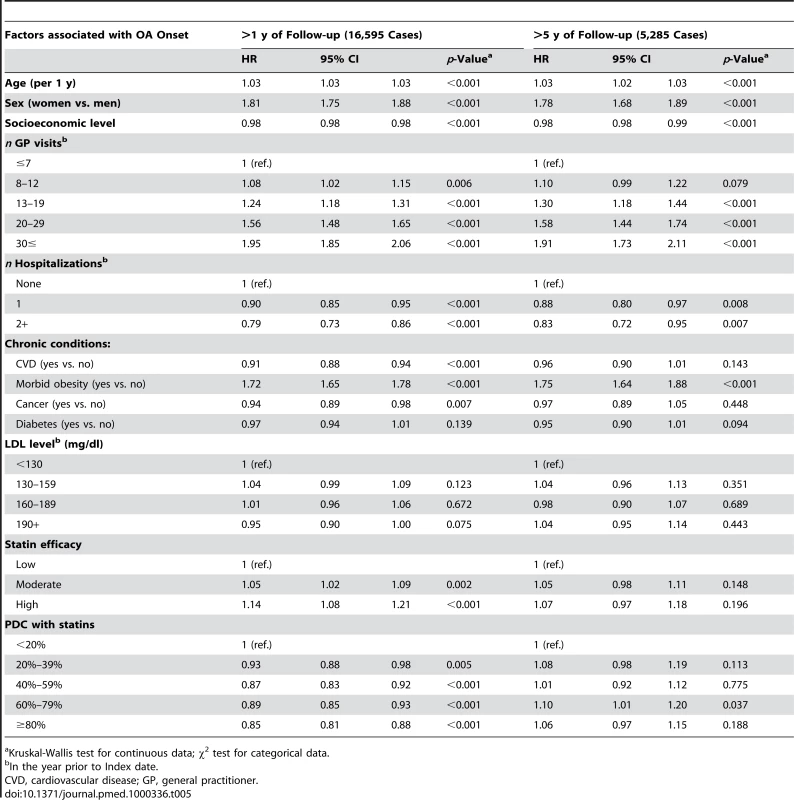
Kruskal-Wallis test for continuous data; χ2 test for categorical data. After adjustment for the variables in Table 4, patients with a PDC with statins of 80% or above, had a HR of 0.58 (95% CI 0.52–0.65) for RA compared with patients with PDC levels of less than 20% (Table 4). Similar results were obtained when analyses were restricted to patients with ≥5 y of follow-up. In the OA cohort analysis (Table 5), high persistence with statins (PDC level ≥80%) was associated only with a modest decrement in risk ratio (HR = 0.85; 0.81–0.88) compared to nonadherent patients (PDC level <20%). In contrast with the RA analysis, the negative association between OA risk and PDC with statins was not observed when analyses were limited to patients with at least 5 y of follow-up (Figure 2).
Fig. 2. Adjusted HR and 95% CI for RA and OA, according to PDC with statins in patients with at least 5 y of follow-up. 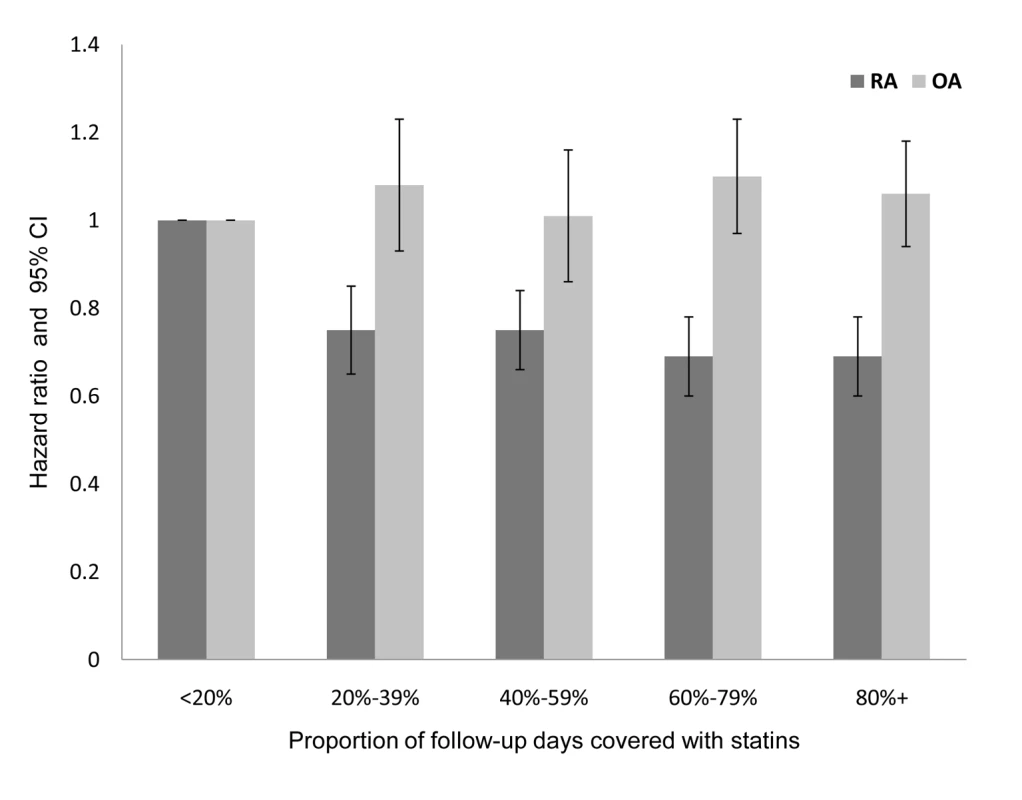
Adjusted for baseline values of age, sex, socioeconomic level, utilization of healthcare services in the year prior to index date, chronic comorbidity (cardiovascular diseases, diabetes mellitus, cancer, morbid obesity), LDL level, and statin efficacy. When PDC with statins was analyzed as a continuous variable, an increase of 10% in PDC level was associated with an adjusted HR of 0.95 (95% CI 0.94–0.96) or a 5.3% lower risk of RA. Similar results were obtained when analyses were limited to patients with ≥5 y of follow-up. In the OA analysis, a 10% increase in PDC was related with an adjusted HR of 0.99 (95% CI 0.99–1.00) for OA. No association (p = 0.23) was calculated when analyses were limited to patients with ≥5 y of follow-up. In stratified analyses, younger age at index date was associated with larger differences in RA risk. In patients aged 35–44 y, an increase of 10% in days covered with statins was associated with an adjusted HR of 0.90 (95% CI 0.87–0.97), whereas in patients aged 75 y or above, there was no significant association between persistence with statins and reduced RA risk. However, a heterogeneity test between all age strata did not reach statistical significance. Similarly, substantially lower risk of RA was calculated for patients persistently treated with high efficacy statins with a statistically significant heterogeneity (Figure 3).
Fig. 3. Proportional effects of persistence with statins on reduction of risk for RA per 10% of follow-up days covered with statins. 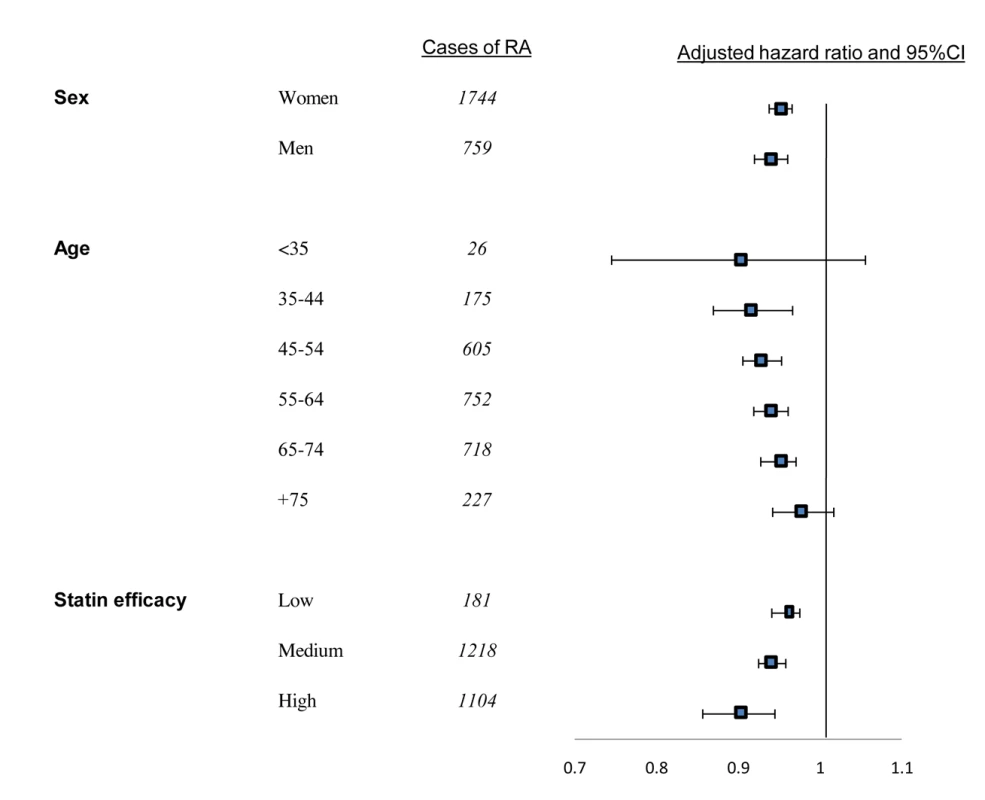
Squares indicate adjusted HRs, horizontal lines, 95% CIs. Mutually adjusted for all covariates listed in Table 4. Discussion
The present study demonstrates a significant negative association between persistence with statin therapy and RA onset, particularly in adult patients who began treatment at a relatively young age and with high efficacy statins. Our results agree with a previous nested case-control study [26] in hyperlipidemia patients, which compared 313 RA patients and 1,252 matched controls. In that analysis, the adjusted OR for development of RA in subjects taking statins compared to the reference group was 0.59 (95% CI 0.37–0.96). Similar to the present study, it was also found that patients receiving high efficacy statins (atorvastatin) had a lower odds ratio for contracting RA, although the difference did not reach statistical significance.
Several of the cholesterol-independent effects of statins are exerted by the modulation of the synthesis of isoprenoids. Post-translational modification by isoprenylation is inhibited by statins; statins decrease isoprenylation of the GTP-binding proteins Ras and Rho, which consequently leads to the modulation of signaling pathways involving endothelial nitric oxide synthase [27], tissue plasminogen activator [28], endothelin 1 [29], plasminogen activator inhibitor 1 [30], and CRP [31]. Statins were also shown to effectively suppress the induction of class II major histocompatibility complex (MHC) protein and gene expression by interferon-γ (IFNγ), resulting in suppressed class II MHC-mediated T cell activation [32]. Interestingly, statins also selectively block β2 integrin and lymphocyte function-associated antigen 1 (LFA-1). In addition LFA-1 binding to intercellular adhesion molecule 1 (ICAM-1) was also abrogated by statins [33].
In a murine model of autoimmune encephalomyelitis, atorvastatin altered cytokine secretion favoring Th2 cytokine (interleukin [IL]-4, IL-5, IL-10, and transforming growth factor β) secretion while inhibiting the expression of Th1 cytokines (IL-2, IL-12, IFNγ, and tumor necrosis factor α [TNFα]). Interestingly, CD40 signaling, which is implicated in the pathogenesis of many autoimmune conditions, was reduced by statins in atheroma-associated cells in vitro and in atherosclerotic lesions; this change hinders proinflammatory cytokines, chemokines, matrix metalloproteinases, and tissue factor secretion and activity [34].
Additional features of statins are bone-specific anabolic and antiresorptive effects that may prevent osteoporosis, which often occurs in patients with active RA. These mechanisms may elucidate the improved, yet modest overall, RA disease activity, swollen joint scores, and reduced CRP in the TARA (Trial of Atorvastatin in Rheumatoid Arthritis) trial, a 6-mo, placebo-controlled, randomized clinical trial with Atorvastatin [6].
The JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial was designed to investigate the preventive effects of Rosuvastatin against vascular events among individuals with LDL cholesterol <130 mg/dl and enhanced innate immune response, as determined by a high-sensitivity CRP (hsCRP) level. The results of the JUPITER trial indicated that statin therapy may also reduce all cause mortality in patients with low LDL cholesterol, probably by decreased inflammation [35]. Although RA patients were not included in the JUPITER trial, the questionable validity of its conclusions [36], and the uncertainty of whether or not CRP itself is a marker of risk or the target for therapy, our findings may support the potential relationship between statin use and RA risk [37].
The strengths of the current study include prospective data collection, the use of administrative databases to avoid the problem of differential recall bias, the systematic and comprehensive collection of personal sociodemographic data, medical history, and utilization of health services prior to the index date, which reduces the possibility for bias due to study outcomes. The present retrospective cohort is one of the largest undertaken to date on the relationship between statin therapy and RA, with respect to the length of follow-up and the size of the study population. Furthermore, survivor-treatment selection bias and competing medical issues, two potential problems with observational studies of treatment efficacy [38], are unlikely to affect our results, since the association of statins and RA onset was not attenuated when analyses were limited only to patients with more than 5 y of follow-up data.
In addition, the present study used internal comparisons among patients who had at least one dispensed prescription of statins, reducing the threat of confounding by indication that might have occurred in previous investigations that compared statin users and nonusers [26]. For example, a recently published cohort study [39] on more than 2 million patients from 368 general practices in England and Wales found no significant association between statin use and RA. In their analysis, Hippisley-Cox and Coupland compared the risk of RA in statin users and nonusers. The study groups differed considerably in many important characteristics such as mean age (57 versus 44 y), body mass index (BMI) (28.3 versus 26), and potentially in other important variables that were not included in the multivariable analysis (e.g., cholesterol level, LDL levels, cardiovascular diseases, and other comorbid conditions, etc.). In addition, the authors reduced the statistical power of their tests by stratifying the analyses by sex and five types of statins, and did not take into account the effect of time and amount of statin purchased. All of the above mentioned aspects could have masked a significant association between persistent use of statins and RA.
This study has the following limitations. Our analysis was retrospective in nature and allocation of statin therapy was not randomized. Despite adjustment for baseline differences and an abundance of poor prognostic factors, a higher proportion of days covered with statins could still be a surrogate for other unmeasured variables that reflect a higher quality of care and more aggressive treatment strategies. In our analysis we did not address different temporal variations in patients' use of statins over the study period. However, a majority of RA patients (79%) purchased statins at least 3 mo prior to diagnosis. Also, the similarity in study results when limiting the analysis to patients with at least 5 y of follow-up reduces the possible effect of this potential limitation.
The most important methodological limitation in estimating effectiveness in observational studies is the potential for “healthy adherer” bias. Previous studies have indicated that in patients with a chronic illness, good adherence to medication, or even to placebo, is more likely to lead to better health and improved survival [40]. A recent study [41] aimed to examine whether adherence with statins is associated with a decreased risk of accidental events that were thought to be unrelated to statins (e.g., motor vehicle and workplace accidents, burns, and falls). As expected, they found a modest (10%–15%) overall reduction in accident rate among adherent patients compared to nonadherent ones. In order to evaluate this potentially important bias in our study, we conducted a similar analysis with OA as an outcome. Our results indicated that persistent use of statins was associated with a 15% lower OA risk, a relatively small difference compared to RA risk, but one that needs to be noted when considering the results of the study overall. In addition, the reduced risk for OA in patients with high PDC with statins was limited to patients with short follow-up periods and was not found in patients with a follow-up of 5 y or more. This finding supports the notion that most of the RA risk reduction is due to a real biological effect. The threat of “healthy adherer bias” in this study was further diminished by findings from our previous study [42] on the present cohort indicating that older age and the presence of chronic diseases and other risk factors for cardiovascular events are associated with higher persistence with statins.
The incidence of RA and OA in our study, based on physician diagnoses, is higher compared with previous studies [2],[43],[44]. This higher incidence can be explained by the relatively older age and the frequent visits to physicians in our study population, leading to earlier detection. Also, RA cases in most epidemiological studies were defined by the 1987 ACR criteria and not on diagnosis alone as in our study. The association between LDL levels and risk of RA is not fully understood. Some studies [45]–[47], but not all [48], suggested that patients with active untreated RA have reduced LDL levels. A recent large retrospective cohort from the Rochester Epidemiology Project showed a decrement in LDL levels during the 5-y period before RA incidence. This decrement could not be explained by usage of lipid-lowering drugs alone. This conclusion is in agreement with our finding that high LDL levels (>190 mg/dl) at index date were significantly associated with a lower short-term (<5 y) risk of RA. Since patients with higher LDL levels at index date are more likely to be persistent with statin therapy [49], a residual confounding cannot be excluded.
Mild muscle pains are one of the frequent side effects of statins documented in 5% to 10% of outpatients on statins [50],[51] and commonly result in discontinuation of treatment. Muscle symptoms frequently begin within several months after initiation of therapy [50]. Since muscle pains can be misclassified as OA symptoms and result in result in a mistaken diagnosis of OA shortly after treatment initiation. This potential differential information bias may explain the disappearance of the small negative association between statin use and OA when analysis was limited to patients with at least 5 y of follow-up. Also, one might claim that persistent use of statins may have been associated with more frequent physician visits increasing the chance of being diagnosed with RA or OA. However, the direction of this potential detection bias may only support our conclusions. In the present research, not only did the risk of RA not rise with increasing persistence, it decreased, indicating that the true association between persistent statin use and RA could be stronger than observed. Interestingly, starting statin treatment at a younger age (35–44 y) was associated with a more pronounced decline of onset of RA; this finding probably underlines the importance of inflammatory processes during this age and the potential role statins may have to block these mechanisms, in other words, the different effects that statins have at different ages once again implies that RA is a heterogeneous disease.
In conclusion, this study showed that persistence with statin treatment was associated with an ongoing decrement in the risk for contracting RA. The observed associations were greater than those that would be expected from methodological biases alone. Larger, systematic, controlled, prospective studies with high efficacy statins, particularly in younger adults who are at increased risk for RA, are needed to confirm our findings, and to elucidate the possible biological relationship between adherence to statin therapy and RA onset.
Zdroje
1. WhalleyD
McKennaS
De JongZ
Van der HeijdeD
1997
Quality of life in rheumatoid arthritis.
Br J Rheumatol
25
773
777
2. AlamanosY
VoulgariP
DrososA
2006
Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review.
Semin Arthritis Rheum
36
182
188
3. JainM
RidkerP
2005
Anti-inflammatory effects of statins: clinical evidence and basic mechanisms.
Nat Rev Drug Discov
4
977
987
4. RidkerP
BuringJ
ShihJ
MatiasM
HennekensC
1998
Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women.
Circulation
98
731
733
5. Abud-MendozaC
de la FuenteH
Cuevas-OrtaE
BarandaL
Cruz-RizoJ
2003
Therapy with statins in patients with refractory rheumatic diseases: a preliminary study.
Lupus
12
607
611
6. McCareyD
McInnesI
MadhokR
HampsonR
ScherbakovO
2004
Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial.
Lancet
363
2015
2021
7. OkamotoH
KoizumiK
KamitsujiS
InoueE
HaraM
2007
Beneficial action of statins in patients with rheumatoid arthritis in a large observational cohort.
J Rheumatol
34
964
968
8. Van DoornumS
McCollG
WicksI
2004
Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis.
Ann Rheum Dis
63
1571
1575
9. HermannF
ForsterA
Chenevard
EnseleitF
HurlimannD
2005
Simvastatin improves endothelial function in patients with rheumatoid arthritis.
J Am Coll Cardiol
45
461
464
10. LodiS
EvansS
EggerP
CarpenterJ
2010
Is there an anti-inflammatory effect of statins in rheumatoid arthritis? Analysis of a large routinely collected claims database.
Br J Clin Pharmacol
69
85
94
11. ShalevV
ChodickG
SilberH
KokiaE
JanJ
2009
Continuation of statin treatment and all-cause mortality: a population-based cohort study.
Arch Intern Med
169
260
268
12. ChodickG
HeymannA
FlashS
KokiaE
ShalevV
2010
Persistence with statins and incident cataract: a population-based historical cohort study.
Ann Epidemiol
20
136
142
13. BendelacJ
2009
Membership in sick funds 2008
Jerusalem
Israel National Insurance Institute
14. Li Wan PoA
1998
Dictionary of evidence-based medicine
Oxford
Radcliffe Medical Press
15. KopjarB
SalesA
PinerosS
SunH
LiY
2003
Adherence with statin therapy in secondary prevention of coronary heart disease in veterans administration male population.
Am J Cardiol
92
1106
1108
16. AvornJ
MonetteJ
LacourA
BohnR
MonaneM
1998
Persistence of use of lipid-lowering medications: a cross-national study.
JAMA
279
1458
1462
17. BennerJ
TierceJ
BallantyneC
PrasadC
BullanoM
2004
Follow-up lipid tests and physician visits are associated with improved adherence to statin therapy.
Pharmacoeconomics
22
Suppl 3
13
23
18. Israel Central Bureau of Statistics
1998
1995 census of population and housing
Jerusalem
Israel Central Bureau of Statistics
19. ChodickG
HeymannAD
ShalevV
KookiaE
2003
The epidemiology of diabetes in a large Israeli HMO.
Eur J Epidemiol
18
1143
1146
20. ValuckR
WilliamsS
MacArthurM
SaseenJ
NairK
2003
A retrospective cohort study of correlates of response to pharmacologic therapy for hyperlipidemia in members of a managed care organization.
Clin Ther
25
2936
2957
21. MeyerJ
SchultzJ
O'DonnellJ
PatelP
SasaneR
2005
Patterns and effectiveness of lipid-lowering therapies in a managed care environment.
Value Health
8
601
612
22. JonesP
KafonekS
LauroraI
HunninghakeD
1998
Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study).
Am J Cardiol
81
582
587
23. DujovneC
KnoppR
KwiterovichP
HunninghakeD
McBrideT
2000
Randomized comparison of the efficacy and safety of cerivastatin and pravastatin in 1030 hypercholesterolemic patients; the Cerivastatin Study Group.
May Clin Proc
75
1124
1132
24. ChengJWM
2004
Rosuvastatin in the management of hyperlipidemia.
Clin Ther
26
1368
1387
25. CoxDR
1972
Regression models and life tables (with discussion).
J R Stat Soc B
32
187
220
26. JickS
ChoiH
LiL
McInnesI
SattarN
2009
Hyperlipidaemia, statin use and the risk of developing rheumatoid arthritis.
Ann Rheum Dis
68
546
551
27. EndresM
LaufsU
HuangZ
NakamuraT
HuangP
1998
Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase.
Proc Natl Acad Sci U S A
95
8880
8885
28. EssigM
NguyenG
PrieD
EscoubetB
SraerJ
1998
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells: role of geranylgeranylation and Rho proteins.
Circ Res
83
683
690
29. del RincónI
WilliamsK
SternM
FreemanG
EscalanteA
2001
High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors.
Arthritis Rheum
44
2737
2745
30. ArnaudC
BurgerF
SteffensS
VeillardNR
NguyenTHT
2005
Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins.
Arterioscler Thromb Vasc Biol
25
1231
1236
31. KobashigawaJA
KatznelsonS
LaksHJJ
YeatmanL
WangXM
1995
Effect of pravastatin on outcomes after cardiac transplantation.
N Engl J Med
333
621
627
32. KwakB
MulhauptF
MyitS
MachF
2000
Statins as a newly recognized type of immunomodulator.
Nat Med
6
1399
1402
33. Weitz-SchmidtG
WelzenbachK
BrinkmannV
KamataT
KallenJ
2001
Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site.
Nat Med
7
687
692
34. ShimadaK
MiyauchiK
DaidaH
2004
Early intervention with atorvastatin modulates TH1/TH2 imbalance in patients with acute coronary syndrome: from bedside to bench.
Circulation
109
e213
e214
35. RidkerP
DanielsonE
FonsecaF
GenestJ
GottoAJ
2008
Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein.
N Engl J Med
359
2195
2207
36. RayK
SeshasaiS
ErqouS
SeverP
JukemaJ
2010
Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants.
Arch Intern Med
170
1024
1031
37. RidkerP
SolomonD
2009
Should patients with rheumatoid arthritis receive statin therapy?
Arthritis Rheum
60
1205
1209
38. LaupacisA
MamdaniM
2004
Observational studies of treatment effectiveness: some cautions.
Ann Intern Med
140
923
924
39. Hippisley-CoxJ
CouplandC
2010
Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database.
BMJ
340
C1297
40. GrangerB
SwedbergK
EkmanI
GrangerC
OlofssonB
2005
Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial.
Lancet
366
2005
2011
41. DormuthC
PatrickA
ShrankW
WrightJ
GlynnR
2009
Statin adherence and risk of accidents: a cautionary tale.
Circulation
119
2051
2057
42. ChodickG
ShalevV
GerberY
HeymannA
SilberH
2008
Long-term persistence with statin treatment in a not-for-profit health maintenance organization: a population-based retrospective cohort study in Israel.
Clin Ther
30
2167
2179
43. SunJ
GoochK
SvensonL
BellN
FrankC
2007
Estimating osteoarthritis incidence from population-based administrative health care databases.
Ann Epidemiol
17
51
56
44. EnglundM
JöudA
GeborekP
FelsonD
JacobssonL
2010
Prevalence and incidence of rheumatoid arthritis in southern Sweden 2008 and their relation to prescribed biologics.
Rheumatology
49
1563
1569
45. LazarevicM
ViticJ
MladenovicV
MyonesB
SkoseyJ
1992
Dyslipoproteinemia in the course of active rheumatoid arthritis.
Semin Arthritis Rheum
22
172
178
46. ChoyE
SattarN
2009
Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions.
Ann Rheum Dis
68
460
469
47. BoersM
NurmohamedM
DoelmanC
LardL
VerhoevenA
2003
Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis.
Ann Rheum Dis
62
842
845
48. Hurt-CamejoE
ParedesS
MasanaL
CamejoG
SartipyP
2001
Elevated levels of small, low density lipoprotein with high affinity for arterial matrix components in patients with rheumatoid arthritis. Possible contribution of phospholipase A2 to this atherogenic profile.
Arthritis Rheum
44
2761
2767
49. ChodickG
ShalevV
GerberY
HeymannA
SilberH
2008
Long-term persistence with statin treatment in a not-for-profit health maintenance organization: a population-based retrospective cohort study in Israel.
Clin Ther
30
2167
2179
50. BruckertE
HayemG
DejagerS
YauC
Be'gaudB
2005
Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients-the PRIMO study.
Cardiovasc Drugs Ther
19
403
414
51. NicholsG
KoroC
2007
Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients.
Clin Ther
29
1761
1770
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 9- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Examining the “Urban Advantage” in Maternal Health Care in Developing Countries
- Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: Raising the Bar
- Does It Matter Who Writes Medical News Stories?
- Drug Companies Should Be Held More Accountable for Their Human Rights Responsibilities
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Merck Perspective
- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- Effectiveness of Chest Physiotherapy in Infants Hospitalized with Acute Bronchiolitis: A Multicenter, Randomized, Controlled Trial
- Combined Impact of Lifestyle-Related Factors on Total and Cause-Specific Mortality among Chinese Women: Prospective Cohort Study
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Perspective of the Former United Nations Special Rapporteur (2002-2008)
- AIDS Vaccine for Asia Network (AVAN): Expanding the Regional Role in Developing HIV Vaccines
- Are Drug Companies Living Up to Their Human Rights Responsibilities? Moving Toward Assessment
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
- Major Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: A Population-Based Cohort Study in Ontario
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Cost-Effectiveness of Pooled Nucleic Acid Amplification Testing for Acute HIV Infection after Third-Generation HIV Antibody Screening and Rapid Testing in the United States: A Comparison of Three Public Health Settings
- Community Case Management of Fever Due to Malaria and Pneumonia in Children Under Five in Zambia: A Cluster Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



