-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAIDS Vaccine for Asia Network (AVAN): Expanding the Regional Role in Developing HIV Vaccines
article has not abstract
Published in the journal: . PLoS Med 7(9): e32767. doi:10.1371/journal.pmed.1000331
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1000331Summary
article has not abstract
Summary Points
-
The HIV/AIDS pandemic continues to spread and an AIDS vaccine is urgently needed.
-
Regional alliances and international collaborations can foster the development and evaluation of the next generation of AIDS vaccine candidates.
-
The importance of coordinating and harmonizing efforts across regional alliances has become abundantly clear.
-
We recently formed the AIDS Vaccine for Asia Network (AVAN) to help facilitate the development of a regional AIDS vaccine strategy that accelerates research and development of an AIDS vaccine through government advocacy, improved coordination, and harmonization of research; develops clinical trial and manufacturing capacity; supports ethical and regulatory frameworks; and ensures community participation.
The Challenge
The HIV/AIDS pandemic continues to spread and an AIDS vaccine is urgently needed. While facing unprecedented challenges, AIDS vaccine development activities are continuing around the globe [1],[2]. Recent results of the Thai Phase III vaccine trial are renewing such efforts [3]. In accordance with the goals of the Global HIV Vaccine Enterprise (the Enterprise) [4], there is now clear recognition of the role that regional alliances can play in fostering and facilitating AIDS vaccine development [5], and there is broad agreement that international collaborations are the most effective way forward to develop and evaluate the next generation of AIDS vaccine candidates [6].
In response to these challenges, the Asian region has recently formed the AIDS Vaccine for Asia Network (AVAN), with a clear vision and mission (Box 1).
Box 1. AVAN's Vision and Mission
Vision
-
To develop a safe and effective AIDS vaccine and ensure its access as a part of a comprehensive public health strategy for the control of new HIV infections across the Asian region.
Goals
-
To accelerate the development of an AIDS vaccine through expanding capacity for all aspects of AIDS vaccine research and development.
-
To build up regional resource centres and collaborative platforms to promote innovative AIDS vaccine research strategies.
-
To enlarge the pipeline of candidate AIDS vaccines suitable for use among Asian populations.
-
To strengthen capacity and harmonize regulatory and ethical frameworks for the conduct of clinical trials that comply with internationally recognized standards.
-
To actively involve community partners at all stages of AIDS vaccine development, clinical trials, and future use.
-
To promote and share manufacturing and production capacity in the region, in compliance with GMP standards, both for clinical trials and for the potential of high demand for an AIDS vaccine in Asia.
-
To engage with and advocate to governments and the private sector to commit to and provide political and financial support for all phases of AIDS vaccine research and development, including licensing and future access.
-
To facilitate collaboration between Asian scientists and industry with the rest of the world.
-
To align with the values and strategic goals of the Global HIV Vaccine Enterprise.
The Need for AVAN: The HIV Epidemic in the Asian Region
The total Asia population is approximately 4 billion people—just over 60% of the world population. More than 500 million people are considered to be at-risk for HIV exposure and infection, including youth, injecting drug users (IDUs), sex workers, men who have sex with men (MSM), and mobile populations. Close to 5 million people have already been infected with HIV across the region, although the epidemic is highly variable across different countries, communities and populations [7].
Asian HIV sub-epidemics are driven primarily by unsafe sex and drug injection and are therefore currently concentrated in groups with higher risk for HIV; however, the epidemic is slowly spreading into the general population. Although impressive results have been achieved following the scaling up of available HIV prevention strategies in some countries [8], the level of prevention, care, and treatment coverage among risk-associated groups remains unacceptably low.
The main factors influencing HIV vaccine development in the region include circulation of multiple HIV subtypes and circulating recombinant forms (CRFs) transmitted through the various modes of transmission, diverse host genetics, and disparate social, cultural, and political contexts in the region. While IDUs were the initial driving force of many HIV epidemics in Asian countries, sexual transmission has gradually taken over. Significant increases in the number of new HIV infections are also expected to occur across the region among MSM. The geographic distribution of HIV subtypes and CRFs is relatively homogeneous but varies by subcontinent: CRF_07B′/C, B′, and CRF01_AE in China [9]–[11]; subtype C in India [12]; and CRF01_AE in Thailand [13] (Figure 1). Unfortunately, HIV incidence data remain incomplete and need to be bolstered for optimal planning of vaccine studies. These interrelated factors may affect AIDS vaccine research and development in the region and therefore should be given due consideration in planning future studies. The complexity of the epidemic in the region poses substantial challenges but also affords tremendous opportunities to accelerate AIDS vaccine development considering the relatively good infrastructure of doing epidemiology study in the region.
Fig. 1. Regional distribution of HIV-1 subtypes and recombinants. 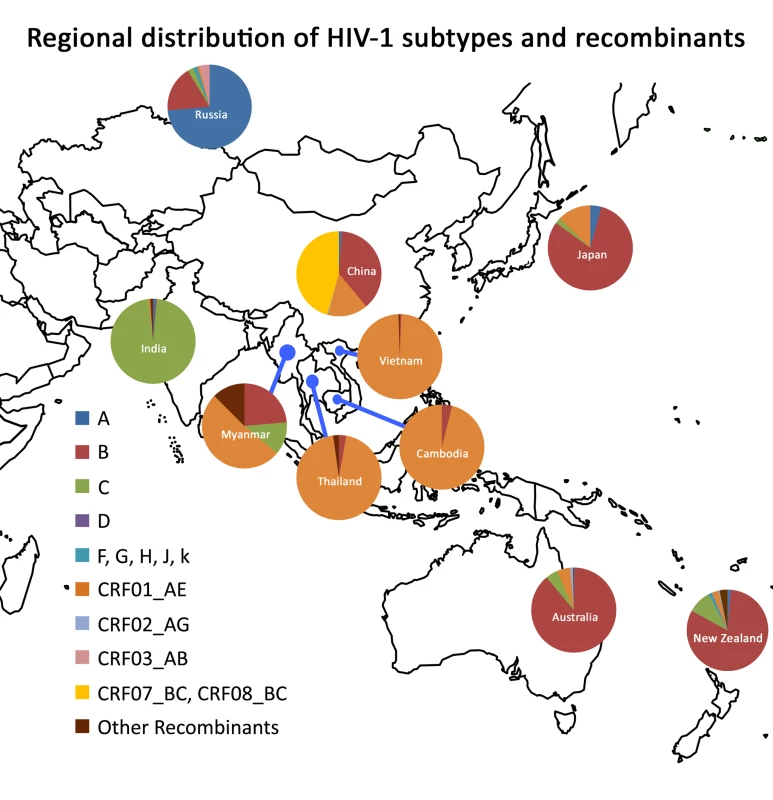
The relative proportion of each subtype/recombinant is shown in the pie chart for each country. The data on Figure 1 are from the former studies in the region and estimates made by AVAN Task Force members of the manuscript based on the unpublished data of their own research projects. Current Efforts in HIV Vaccine Research and Development in Asia
Significant basic and clinical HIV vaccine research and development efforts are already underway across the region, with notable achievements (see Table 1). Several Asian countries now have National AIDS Vaccine Plans and Strategies. Further harmonization and consolidation of efforts will result in more productive associations and acceleration of AIDS vaccine development.
Tab. 1. AIDS Vaccine Trials across Asia to Date. 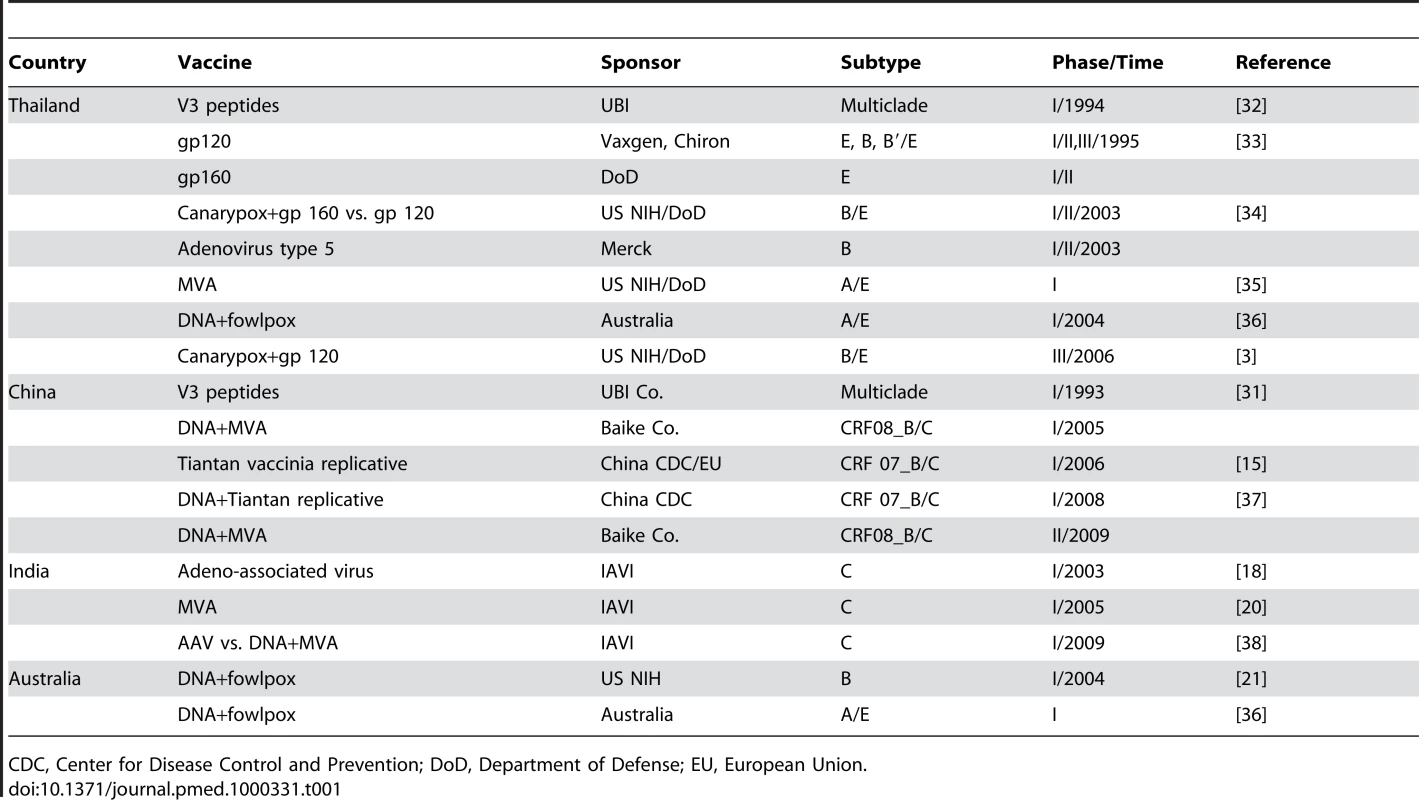
CDC, Center for Disease Control and Prevention; DoD, Department of Defense; EU, European Union. Thailand
AIDS vaccine activities have a long-standing history in Thailand, beginning in the mid-1990s [14], reflecting a strong political will to invest in AIDS vaccine research and development to stem the epidemic in Thailand. Thailand has conducted Phase I, II, and III trials with the AIDSVax gp120 B′/E vaccine, as well as studies of other candidate vaccines (Table 1).
Most notably, Phase III trials of the ALVAC (Canarypox, vCP1521) and AIDSVAX gp120 B/E prime-boost regimens have also been completed. This landmark trial, the largest AIDS vaccine efficacy trial conducted to date involving over 16,000 volunteers, showed a modest (31%) but significant reduction in new HIV infections and is the first time that an AIDS vaccine has exhibited protection against HIV acquisition [3].
The Thai experience in successfully conducting two Phase III efficacy trials has provided world-class expertise in collaborative teamwork, community engagement activities, good clinical practice, ethical compliance, sample repositories, immunology studies, good laboratory practices, volunteer retention strategies, and large-scale data management approaches. These efforts and investments resulted in an extraordinary volunteer retention rate of 96% during the latest Phase III trial. The Thai experience offers a good example of what can be achieved through international collaboration. In addition, several preclinical studies with promising HIV vaccine candidates are now ongoing in Thailand. Researchers in Chulalongkorn University are working on a HIV-1 AE/B mosaic DNA vaccine as well as techniques to improve vaccine delivery system. The Thailand Regional Laboratories in the Comprehensive Antibody-Vaccine Immune Monitoring Consortium are working with researchers from the Los Alamos National Library and the National Cancer Institute on a cocktail of the mosaic DNA vaccine and DNA/vaccinia primed-boosted strategy. These candidates were tested in mice and have shown a promising immunogenicity result.
China
The Chinese government has significantly invested in AIDS vaccine research. China's vaccine scientists have recently formed the Chinese AIDS Vaccine Initiative (CAVI), supported by the government of China. The initiative includes projects aimed at cohort development, vaccine vector design, establishment of clinical trial units, a manufacturing facility using principles of good manufacturing practice (GMP), a primate centre, a humanized mouse centre, and technical platforms encompassing both neutralizing antibody and T cell expertise.
Clinical studies conducted so far include three Phase I trials: V3 peptide vaccine in 1993, DNA/MVA (modified vaccinia Ankara) in 2005, and a DNA/Tiantan vaccinia strain (replication-competent) in 2007 (Table 1). Pre-clinical studies with DNA and modified Tiantan vaccinia, as well as new versions of DNA, vaccinia, and adenovirus vector approaches for inducing mucosal immunity show considerable promise [15]–[17]. China's AIDS vaccine programme now has substantial capacity in primate centres, vaccine production, and clinical trial sites development that will all help to facilitate further research and development in the region.
India
AIDS vaccine trials have accelerated significantly in India in recent years. Activities include an adeno-associated virus vector Phase I trial, modified MVA vector vaccine trials [18],[19], and a recent DNA/MVA Phase I prime-boost trial initiated in 2009 [20]. The International AIDS Vaccine Initiative (IAVI) is supporting Indian AIDS vaccine trials and applied research on neutralizing antibody immunogens [21]. Exploration of several novel concepts, including prime-boost regimens with CD40L adjuvants, as well as research towards development of improved Env-based immunogens, is also progressing. Improved awareness of AIDS vaccine issues, training of staff, and development of trial sites have substantially improved the capacity of conducting AIDS vaccine research and clinical trials within India.
Australia
Australia has long-standing capacities in both fundamental HIV research and clinical trial activities. A consortium, termed the Australia-Thai HIV Vaccine Consortium, conducted two recent trials, first in Sydney and then in Bangkok, of a DNA-prime and Fowlpox virus boost vaccine using both subtype B and CRF01_AE strains [22]. Australia was a clinical trial site of the adenovirus-based efficacy (STEP) trial that was not efficacious. Through collaborations, several new vaccine candidates are emerging. These include peptide-based vaccines, gp140 immunogens, recombinant influenza vectors, and particle-based vaccine strategies [23],[24]. In addition, improved and simpler assays to measure T cell immunity and antibody-dependent cellular cytotoxicity (ADCC) are emerging [25],[26]. These scientific approaches could be accelerated into expanded clinical trials in future collaborations across the Asian region.
Japan
Japan has developed a pipeline of promising AIDS vaccine candidates. These include recombinant Mycobacterium bovis/Bacillus Calmette-Guérin (BCG) vector-based regimens that are moving towards clinical trial development in collaboration with Thai groups [27]. In addition, novel Sendai virus vectors are being developed with the opportunity to deliver vaccines mucosally to induce mucosal immunity [28]. Sendai virus vectors combined with DNA vaccine candidates are moving towards clinical trials in collaboration with IAVI.
The Evolution, Mission, and Strategic Plan Development of AVAN
The first Asian meetings devoted to AIDS vaccines took place in Japan, China, and Thailand in the late 1990s. The initial efforts were followed by a World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (UNAIDS) regional consultation on AIDS vaccines in Japan in 2006 and in Beijing in 2009 [29]–[31]. The challenges for engaging countries and communities in expanding pre-clinical and clinical trials, and in enhancing regulatory and manufacturing capacity to accelerate the development of AIDS vaccines in the region, led to the establishment of AVAN, announced at the 2009 AIDS Vaccine Conference in Paris. AVAN has had regular consultations towards developing an Asia-specific Strategic AIDS Vaccine Plan in alignment with the Enterprise Scientific Strategic Plan [6].
An AVAN Task Force was recently established to foster further dialogue and set the stage for the development of principles and priorities for a focussed, forward-looking and effective strategic plan for the network. Eventually, the Task Force will be replaced by a steering committee to guide implementation of a strategic plan. An initial AVAN Task Force secretariat has been established, currently based in China, with strong support from the Chinese AIDS Vaccine Initiative, WHO, the Enterprise, and the Chinese government. The Task Force and secretariat foster forums for consultation and networking around many of the most critical future issues. Regional workshops are planned to further implement the emerging strategic plan in June 2010 in Bangkok, Thailand, and in August/September 2010 in Sapporo, Japan. AVAN plans to support and promote the development of regional resource centres to provide information, a reagents repository, protocols, and training on the many aspects of AIDS vaccine research and development. Coordination and harmonization of various aspects of AIDS vaccine development, particularly regional vaccine immunology evaluation laboratories and regulatory reviews, is a high priority in moving AIDS vaccine research forward across Asia. AVAN shall ultimately become the key advocate for AIDS vaccine research in the region, building upon the trust created through working together in collaborative partnerships in the region and with support from the Enterprise.
Past Collaborations and Future Opportunities
AVAN aims to make a significant impact as the region undertakes a stronger role in AIDS vaccine research and development. Asian investigators have substantial capacity to conduct basic research, which could be significantly enhanced through better collaboration. The current pipeline of new vaccine candidates needs further expansion with more innovative concepts and trans-national efforts to accelerate research. Clinical trial capacity could be quickly exhausted with multiple, concurrent efficacy trials in the future. As partially efficacious AIDS vaccines are identified either in Asia or around the world, determining their effectiveness in regional epidemics involving differing HIV virus subtypes will be critical. Considering the large number of IDUs in many countries of the region still with a high incidence of HIV infection, Asia may serve as the ideal site suitable for testing a vaccine to prevent parental HIV transmission.
Building upon and maintaining the substantial efficacy trials expertise in Thailand is both an opportunity and a challenge. Substantial opportunities exist to harmonize the regulatory and ethical review of AIDS vaccine trials across the region—too often, delays experienced in initiating trials with vectors of known safety profile are very lengthy. Data management and data sharing across the region can also be enhanced. Opportunities for training young immunologists and virologists in the region to foster the next generation of scientists need to be encouraged and supported. Technology transfer from Western countries to Asian countries also needs to be strengthened. Finally, tapping into the large cost-effective vaccine manufacturing capacity in several Asian countries is an opportunity and priority.
International groups have previously provided high level support for AIDS vaccine activities in the Asian region, including research and technical transfer, funding and policy. In the past two decades, the US National Institutes of Health (NIH) has provided extensive support for basic and clinical research across many Asian countries and funded the majority of the RV 144 trial. The Walter Reed Army Institute of Research and the US Military HIV Research Program (MHRP), in collaboration with the Royal Thai Army, sponsored several clinical trials in Thailand, including the RV144 trial. IAVI is supporting clinical trial capacity building along with policy and preparedness activities. The EuroVacc AIDS Vaccine network has included the Chinese HIV strains in their development work in view of future plans of conducting trials in the region. The Collaboration for AIDS Vaccine Discovery (CAVD), supported by the Bill & Melinda Gates Foundation, is also looking towards enhancing research on AIDS vaccines in Asia in coming years. WHO-UNAIDS and the Global HIV Vaccine Enterprise are actively engaging in policy development, assisting the development of national AIDS vaccine plans, and providing a global umbrella for AIDS vaccine development activities in Asia. Further investment in AIDS vaccine development by the most developed nations in the Asian region would be welcome. The consolidation of this collaborative support will be critical in progressing future vaccine development efforts.
Conclusions
The development of an effective AIDS vaccine has never been more urgent, particularly for the hundreds of millions of people across Asia at substantial risk of acquiring HIV. Similarly, the necessity of coordinating and harmonizing efforts across regional alliances has become abundantly clear. AVAN has been initiated to meet these needs and actively facilitate the development of a regional AIDS vaccine strategy that accelerates research and development of an AIDS vaccine through government advocacy, improved coordination and harmonization of research; develops clinical trial and manufacturing capacity; supports ethical and regulatory frameworks; and ensures community participation.
Zdroje
1. BuchbinderSP
MehrotraDV
DuerrA
FitzgeraldDW
MoggR
2008 Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372 1881 1893
2. FauciAS
JohnstonMI
DieffenbachCW
BurtonDR
HammerSM
2008 HIV vaccine research: the way forward. Science 321 530 532
3. Rerks-NgarmS
PitisuttithumP
NitayaphanS
KaewkungwalJ
ChiuJ
2009 Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med 361 2209 2220
4. KlausnerRD
FauciAS
CoreyL
NabelGJ
GayleH
2003 Medicine. The need for a global HIV vaccine enterprise. Science 300 2036 2039
5. KaleebuP
AbimikuA
El-HalabiS
Koulla-ShiroS
MamotteN
2008 African AIDS vaccine programme for a coordinated and collaborative vaccine development effort on the continent. PLoS Med 5 e236 doi:10.1371/journal.pmed.0050236
6. Coordinating Committee of the Global HIV/AIDS Vaccine Enterprise 2005 The Global HIV/AIDS Vaccine Enterprise: scientific strategic plan. PLoS Med 2 e25 doi:10.1371/journal.pmed.0020025
7. UNAIDS 2010 Joint United Nations Programme on HIV/AIDS homepage. Available: http://www.unaids.org/en/CountryResponses/Regions/Asia.asp. Accessed 17 August 2010
8. Commission on AIDS in Asia 2008
MaraisH
Redefining AIDS in Asia: crafting an effective response. Report of the Commission on AIDS in Asia. New Delhi Oxford University Press
9. LuL
JiaM
MaY
YangL
ChenZ
2008 The changing face of HIV in China. Nature 455 609 611
10. WangW
JiangS
LiS
YangK
MaL
2008 Identification of subtype B, multiple circulating recombinant forms and unique recombinants of HIV type 1 in an MSM cohort in China. AIDS Res Hum Retroviruses 24 1245 1254
11. HemelaarJ
GouwsE
GhysPD
OsmanovS
2006 Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS 20 W13 W23
12. DeshpandeA
JauvinV
PinsonP
JeannotAC
FleuryHJ
2009 Phylogenetic analysis of HIV-1 reverse transcriptase sequences from 382 patients recruited in JJ Hospital of Mumbai, India, between 2002 and 2008. AIDS Res Hum Retroviruses 25 633 635
13. WirachsilpP
KantakamalakulW
FoongladdaS
ChuenchitraT
KohriangudomS
2007 Surveillance of subtype and genetic variation of the circulating strains of HIV-1 in Thailand. Southeast Asian J Trop Med Public Health 38 814 827
14. PitisuttithumP
2008 HIV vaccine research in Thailand: lessons learned. Expert Rev Vaccines 7 311 317
15. ShaoY
LiT
ShenX
ZhangJ
ZhuH
2008 The safety and immunogenicity of the replicative Tiantan vaccinia HIV vaccine in phase I clinical trial. Poster No P13-18, AIDS Vaccine 2008, Cape Town, South Africa, Oct 13–16
16. HuangX
LuB
YuW
FangQ
LiuL
2009 A novel replication-competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination. PLoS ONE 4 e4180 doi:10.1371/journal.pone.0004180
17. DaiK
LiuY
LiuM
XuJ
HuangW
2008 Pathogenicity and immunogenicity of recombinant Tiantan Vaccinia Virus with deleted C12L and A53R genes. Vaccine 26 5062 5071
18. MehendaleS
van LunzenJ
ClumeckN
RockstrohJ
VetsE
2008 A phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C adeno-associated virus vaccine. AIDS Res Hum Retroviruses 24 873 880
19. RamanathanVD
KumarM
MahalingamJ
SathyamoorthyP
NarayananPR
SolomonS
2009 A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV-1 subtype C modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res Human Retroviruses 25 1107 1116
20. BerkleyS
2008 HIV vaccine trials in India. Nat Biotechnol 26 495 author reply 496
21. International AIDS Vaccine Initiative 2010 Working with communities: India. Available: http://www.iavi.org/working-with-communities/country-programs/Pages/india.aspx. Accessed 17 August 2010
22. KelleherAD
PulsRL
BebbingtonM
BoyleD
FfrenchR
2006 A randomized, placebo-controlled phase I trial of DNA prime, recombinant fowlpox virus boost prophylactic vaccine for HIV-1. AIDS 20 294 297
23. De RoseR
FernandezCS
SmithMZ
BattenCJ
AlcantaraS
2008 Control of viremia following immunotherapy of SIV-infected macaques with peptide pulsed blood. PLoS Pathog 4 e1000055 doi:10.1371/journal.ppat.1000055
24. De RoseR
ZelikinA
JohnstonAPR
SextonA
ChongSF
2008 Binding, internalisation and antigen presentation of vaccine-loaded nanoengineered capsules in blood. Advanced Materials 20 4698 4703
25. StratovI
ChungA
KentSJ
2008 Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol 82 5450 5459
26. ZaundersJJ
MunierML
SeddikiN
PettS
IpS
2009 High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J Immunol 183 2827 2836
27. HiroiT
GotoH
SomeyaK
YanagitaM
HondaM
2001 HIV mucosal vaccine: nasal immunization with rBCG-V3J1 induces a long term V3J1 peptide-specific neutralizing immunity in Th1 - and Th2-deficient conditions. J Immunol 167 5862 5867
28. KawadaM
TsukamotoT
YamamotoH
IwamotoN
KuriharaK
2008 Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J Virol 82 10199 10206
29. EsparzaJ
1999 AIDS vaccine research in Asia: needs and opportunities. Report from a UNAIDS/WHO/NIID meeting Tokyo, 28–30 October 1998. AIDS 13 UNAIDS 1 UNAIDS 13
30. ExclerJL
PitisuttithumP
Rerks-NgarmS
ShaoY
ZhangL
2008 Expanding research capacity and accelerating AIDS vaccine development in Asia. Southeast Asian J Trop Med Public Health 39 766 784
31. Rerks-NgarmS
PitisuttithumP
GangulyN
ZhangLi
TamashiroH
2010 Defining the objectives of the AIDS Vaccine for Asia Network: WHO-UNAIDS/Global HIV Vaccine Enterprise regional consultation on expanding AIDS vaccine research and development capacity in Asia. Curr Opin HIV AIDS 5 435 452
32. LenihanF
1993 China and Thailand to start trials of AIDS vaccines. BMJ 306 1564 1565
33. PitisuttithumP
GilbertP
GurwithM
HeywardW
MartinM
2006 Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 194 1661 1671
34. ThongcharoenP
SuriyanonV
ParisRM
KhamboonruangC
de SouzaMS
2007 A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. J Acquir Immune Defic Syndr 46 48 55
35. EarlPL
CotterC
MossB
VanCottT
CurrierJ
2009 Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine 27 5885 5895
36. De RoseR
CheaS
DaleCJ
ReeceJ
FernandezCS
2005 Subtype AE HIV-1 DNA and recombinant Fowlpoxvirus vaccines encoding five shared HIV-1 genes: safety and T cell immunogenicity in macaques. Vaccine 23 1949 1956
37. ShaoY
LiT
WolfH
LiuY
WangH
2009 The safety and immunogenicity of HIV-1 vaccines based on DNA and replication competent vaccinia vector in phase I clinical trial. Poster NoP14-15LB, AIDS Vaccine 2009, Paris, Oct 19–22
38. KumarS
AggarwalP
VajpayeeM
PandeyRM
SethP
2006 Development of a candidate DNA/MVA HIV-1 subtype C vaccine for India. Vaccine 24 2585 2593
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 9- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Examining the “Urban Advantage” in Maternal Health Care in Developing Countries
- Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: Raising the Bar
- Does It Matter Who Writes Medical News Stories?
- Drug Companies Should Be Held More Accountable for Their Human Rights Responsibilities
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Merck Perspective
- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- Effectiveness of Chest Physiotherapy in Infants Hospitalized with Acute Bronchiolitis: A Multicenter, Randomized, Controlled Trial
- Combined Impact of Lifestyle-Related Factors on Total and Cause-Specific Mortality among Chinese Women: Prospective Cohort Study
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Perspective of the Former United Nations Special Rapporteur (2002-2008)
- AIDS Vaccine for Asia Network (AVAN): Expanding the Regional Role in Developing HIV Vaccines
- Are Drug Companies Living Up to Their Human Rights Responsibilities? Moving Toward Assessment
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
- Major Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: A Population-Based Cohort Study in Ontario
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Cost-Effectiveness of Pooled Nucleic Acid Amplification Testing for Acute HIV Infection after Third-Generation HIV Antibody Screening and Rapid Testing in the United States: A Comparison of Three Public Health Settings
- Community Case Management of Fever Due to Malaria and Pneumonia in Children Under Five in Zambia: A Cluster Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



