-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
Background:
Systematic evaluations of the quality of research on a single prognostic biomarker are rare. We sought to evaluate the quality of prognostic research evidence for the association of C-reactive protein (CRP) with fatal and nonfatal events among patients with stable coronary disease.Methods and Findings:
We searched MEDLINE (1966 to 2009) and EMBASE (1980 to 2009) and selected prospective studies of patients with stable coronary disease, reporting a relative risk for the association of CRP with death and nonfatal cardiovascular events. We included 83 studies, reporting 61,684 patients and 6,485 outcome events. No study reported a prespecified statistical analysis protocol; only two studies reported the time elapsed (in months or years) between initial presentation of symptomatic coronary disease and inclusion in the study. Studies reported a median of seven items (of 17) from the REMARK reporting guidelines, with no evidence of change over time.
The pooled relative risk for the top versus bottom third of CRP distribution was 1.97 (95% confidence interval [CI] 1.78–2.17), with substantial heterogeneity (I2 = 79.5). Only 13 studies adjusted for conventional risk factors (age, sex, smoking, obesity, diabetes, and low-density lipoprotein [LDL] cholesterol) and these had a relative risk of 1.65 (95% CI 1.39–1.96), I2 = 33.7. Studies reported ten different ways of comparing CRP values, with weaker relative risks for those based on continuous measures. Adjusting for publication bias (for which there was strong evidence, Egger's p<0.001) using a validated method reduced the relative risk to 1.19 (95% CI 1.13–1.25). Only two studies reported a measure of discrimination (c-statistic). In 20 studies the detection rate for subsequent events could be calculated and was 31% for a 10% false positive rate, and the calculated pooled c-statistic was 0.61 (0.57–0.66).Conclusion:
Multiple types of reporting bias, and publication bias, make the magnitude of any independent association between CRP and prognosis among patients with stable coronary disease sufficiently uncertain that no clinical practice recommendations can be made. Publication of prespecified statistical analytic protocols and prospective registration of studies, among other measures, might help improve the quality of prognostic biomarker research.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(6): e32767. doi:10.1371/journal.pmed.1000286
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000286Summary
Background:
Systematic evaluations of the quality of research on a single prognostic biomarker are rare. We sought to evaluate the quality of prognostic research evidence for the association of C-reactive protein (CRP) with fatal and nonfatal events among patients with stable coronary disease.Methods and Findings:
We searched MEDLINE (1966 to 2009) and EMBASE (1980 to 2009) and selected prospective studies of patients with stable coronary disease, reporting a relative risk for the association of CRP with death and nonfatal cardiovascular events. We included 83 studies, reporting 61,684 patients and 6,485 outcome events. No study reported a prespecified statistical analysis protocol; only two studies reported the time elapsed (in months or years) between initial presentation of symptomatic coronary disease and inclusion in the study. Studies reported a median of seven items (of 17) from the REMARK reporting guidelines, with no evidence of change over time.
The pooled relative risk for the top versus bottom third of CRP distribution was 1.97 (95% confidence interval [CI] 1.78–2.17), with substantial heterogeneity (I2 = 79.5). Only 13 studies adjusted for conventional risk factors (age, sex, smoking, obesity, diabetes, and low-density lipoprotein [LDL] cholesterol) and these had a relative risk of 1.65 (95% CI 1.39–1.96), I2 = 33.7. Studies reported ten different ways of comparing CRP values, with weaker relative risks for those based on continuous measures. Adjusting for publication bias (for which there was strong evidence, Egger's p<0.001) using a validated method reduced the relative risk to 1.19 (95% CI 1.13–1.25). Only two studies reported a measure of discrimination (c-statistic). In 20 studies the detection rate for subsequent events could be calculated and was 31% for a 10% false positive rate, and the calculated pooled c-statistic was 0.61 (0.57–0.66).Conclusion:
Multiple types of reporting bias, and publication bias, make the magnitude of any independent association between CRP and prognosis among patients with stable coronary disease sufficiently uncertain that no clinical practice recommendations can be made. Publication of prespecified statistical analytic protocols and prospective registration of studies, among other measures, might help improve the quality of prognostic biomarker research.
: Please see later in the article for the Editors' SummaryIntroduction
What Is the Problem?
Robust research evidence on the prognostic value of circulating biomarkers is important for translational medicine and clinical decision making, but there are concerns about the quality of such evidence [1], largely based on studies in the field of cancer. Systematic reviews and meta-analyses across multiple cancer biomarkers [2]–[4] have found biases arising from selection of studies for publication, or selection of findings for inclusion within published studies. There have been few evaluations of the quality of evidence focussing on a single biomarker [5]. It is not known the extent to which such biases threaten validity of putative prognostic biomarkers among people with cardiovascular disease, because of a lack of large scale evaluations. Indeed in healthy population studies of cardiovascular disease onset [6], reliable associations largely free of such biases with a range of biomarkers have been demonstrated. We studied C-reactive protein (CRP) in the prognosis of stable coronary artery disease because it is the most widely investigated (>100 studies) novel prognostic biomarker in such patients [7], and therefore the research might be expected to have reached reliable conclusions. Furthermore, there is clinical uncertainty as to whether to measure CRP, with US [8] and European [9] clinical practice guidelines recommending measurement, but clinical practice varying widely [10].
Objectives
In evaluating the quality of published evidence on CRP in the prognosis of patients with stable coronary disease we carried out a systematic review, meta-analysis, and meta-regression [11],[12] with five specific objectives: (i) To determine the quality of study reporting based on a systematic review. In the absence of agreed criteria for measuring the quality of reporting we extended previous efforts [3], and operationalised reporting guidelines for tumour markers [12] into 17 items. A particular concern of ours [1], notably absent from reporting guidelines, was whether studies reported a reference to a study protocol or prespecified statistical analytic protocol; (ii) To determine the extent to which any association of CRP on prognosis was independent of established prognostic factors. Unlike many cancers, cardiovascular disease has numerous established markers of prognosis that are also associated with aetiology, and CRP is a good example of a prognostic biomarker that is highly correlated with these (smoking, diabetes, obesity, lipids, and other markers of inflammation, such as fibrinogen) [13],[14]. The impact of biases in incomplete adjustment for established risk factors has seldom been assessed in large meta-analyses of prognostic biomarkers; (iii) To determine the presence and magnitude of bias arising from small studies. While previous meta-analyses have highlighted that publication bias exists, here we use recently validated methods to assess the potential magnitude of such biases [15]; (iv) To explore sources of heterogeneity, particularly to assess whether aspects of study design or reporting influenced the summary estimate of effect; (v) To determine the extent to which papers report the ability of CRP to discriminate patients who do and do not experience subsequent events. Reporting such data has recently been recommended [16], but it is not known how commonly it is reported.
Methods
Search for Eligible Papers and Inclusion Criteria
We included any prospective observational study (observational cohort studies, prospective nested case control studies, observational data drawn from randomised controlled trials) that reported risk of subsequent events among patients with stable coronary disease in relation to measured CRP values. Eligible studies had to include patients with stable coronary disease, defined as clinically diagnosed angina pectoris or angiographic disease, or a history of previous acute coronary syndrome at least 2 wk prior to CRP measurement. We excluded studies where CRP was measured only during an admission with an acute coronary syndrome, or only after a coronary procedure, but before discharge. Eligible outcome events were defined as coronary (coronary death, sudden cardiac death, acute nonfatal myocardial infarction, primary percutaneous coronary intervention, unplanned emergency admissions with unstable angina), cardiovascular (where coronary events were reported in combination with heart failure, stroke, or peripheral arterial disease), and all cause mortality alone. We did not exclude any studies on the basis of methodological standards, sample size, duration of follow-up, publication year, or language of publication. We searched MEDLINE (PubMed) between 1966 and 25 November 2009 and EMBASE between 1980 and 17 December 2009 databases using a strategy developed with an expert librarian based on terms for coronary disease (from the Cochrane Library of systematic reviews and protocols), prognostic studies [17], and CRP. Three reviewers (NKF, JD, KM) reviewed article titles and abstracts for eligibility and obtained full text articles where eligibility was definite or unclear (see Figure S1).
Data Extraction for Systematic Review
The two reviewers independently abstracted data from eligible articles (n = 116) using a predefined coding protocol. Non-English articles were translated (n = 4). Individual item disagreement between the two reviewers was resolved by consensus or, rarely, adjudication by a third reviewer (HH). We extracted information on year of publication, year of study start, number of patients at baseline that were included in the analysis, their mean age and percent women, the baseline coronary morbidity (proportion with stable angina, angiographic disease, or previous myocardial infarction), average levels of biomarker at baseline (either mean [SD] or median [interquartile (IQR) range]) in the whole sample and separately among those who did and did not subsequently experience an outcome event, and type of high sensitivity CRP assay, follow-up duration, the number and type (coronary, cardiovascular, and all cause mortality) of outcome events (from which the crude annual risk was calculated).
Data Extraction for Quality of Study Reporting
We developed closed-ended questions to operationalise prognostic biomarker reporting guidelines [12] and extracted details on 17 items (see Table S1) relating to prespecified research question, population at start and end of follow up, biomarker measurement, outcome assessment, confounder measurement, and analytic choices.
Data Extraction for Relative Risks
We extracted the reported relative risk, odds ratio or hazard ratio, and 95% confidence intervals (CIs) from each study. Where one study had multiple eligible articles or one article reported multiple relative risks we extracted the relative risks for the most specific coronary outcome event (according to the hierarchy coronary, cardiovascular, all cause mortality) with the largest number of adjustment variables. Where available we extracted separate relative risk estimates with different degrees of confounder adjustment for the following prespecified conventional risk factors (age, sex, smoking status, obesity, diabetes, and one or more lipid variables [from total cholesterol, LDL cholesterol, HDL cholesterol, trigylcerides], and inflammatory markers [fibrinogen, IL-6, white cell count]).
Statistical Analysis
We converted the reported relative risk estimates onto a standard scale of effect, comparing the highest third with the lowest third of the CRP distribution, in essence giving an estimate per 2.18 SD units of CRP where 2.18 is the difference in the means of the top and bottom third of the standard normal distribution [18]. The reported comparisons included continuous measures (per SD, tertile, quartile, unit [mg/l] on original or log 10 scale), equal size groups (top versus bottom with group size 50%, 33%, or 25% for 2, 3, and 4 groups, respectively), unequal size groups (top versus bottom; 2 groups, 3 groups defined by cut-points), as well as measures on both log-transformed and untransformed CRP scales. The scaling methods assume that CRP is log normally distributed and that the association with disease risk is log-linear; both these assumptions have empirical support in healthy population studies of CRP [19],[20]. For two equal groups the difference in means is 1.59 SD units and we used a scaling factor of 1.37 (2.18/1.59). For four and five groups we used a scaling factor of 2.18/2.54 and 2.18/2.80, respectively, i.e., the difference in means between the top and bottom tertile in each case under the assumption of log normality for CRP. Unequal groups required study-specific scaling factors, which were calculated as 2.18/x where x is the difference in means between the unequal groups. The differences were found by simulating one million observations from the distribution used to report the comparison (i.e., normal or log normal). For normally transformed CRP, relative risks reported per standard deviation used a scaling factor of 2.18 and relative risks reported per unit were converted first to a SD change, using the study specific SD and thence to tertiles. For untransformed CRP, relative risks reported per standard deviation were scaled using the study-specific difference in means between the upper and lower tertiles and the SD, and those reported per mg/l were scaled using the difference in means alone.
Statistical Methods for Meta-analysis and Meta-regression
For each study, the relative risk estimate and its corresponding standard error were transformed to their natural logarithms to stabilise the variance and to normalise the distributions. Summary relative risk estimates and their 95% CIs were estimated from a random effects model that considers both within - and between-study variation [21]. Statistical heterogeneity among studies was evaluated using the I2 statistic [22].
Small study (including publication) bias was assessed with contour-enhanced funnel plots (i.e., a plot of study relative risk estimate against precision, with contours representing varying levels of statistical significance), by Begg's adjusted rank correlation test, and by Egger's regression asymmetry test [23],[24]. We used previously investigated methods to adjust the meta-analyses for the potential impact of publication bias (see Table S3) [25]. These included; (i) “trim and fill,” an iterative nonparametric method using a rank-based data augmentation technique to account for asymmetry in the funnel plot. Both the “trimming” of asymmetric studies, for which there are no counterparts, and the revised pooled estimate after “filling” (or imputing) these counterparts can be based on either a fixed or random effects meta-analysis model. Models considered here use either fixed or random effects models for both components, or fixed effect model to “trim” and random effects to “fill.” (ii) Weighted regression-based methods, which are extensions of Egger's regression asymmetry test [24],[25] and regress the outcome against a measure of study precision (standard error, variance, or sample size), weighted by either the reciprocal of either the total variance or the variance of the proportion of the number of events in a study, in order to predict the effect size in a (hypothetically) infinitely large study as a pooled estimate adjusted for publication bias. These meta-regression models can either be fixed effect or random effects models, or can allow for between-study variability via a dispersion parameter. (iii) Conditional regression-based methods, in which a test for small study bias is performed first, and then if appropriate, regression-based methods (as previously described above) are used to adjust the observed effect size [25]. A quadratic version of the original Egger regression test (using the variance rather than the standard error) and including allowing for between-study variability via a dispersion parameter has been shown in both simulation [25] and empirical [15] studies to out-perform other approaches.
To explore other potential sources of study heterogeneity, we employed a random effects meta-regression model that included study level continuous or categorical covariates. Assumptions of normality, independence, and homogeneity of residuals were verified via diagnostic plots.
Discrimination
We calculated the detection rate at different false positive rates by constructing the log-normal distributions of CRP separately for those with and without outcome events using previously reported methods [26],[27]. Calculating the detection rate for false positive rates from 0 to 100 then yields a receiver operating characteristic (ROC) curve for the outcome group, from which c-statistics can be calculated using the trapezium rule. Confidence intervals for the ROC curves and detection rate at the 10% false positive rate were obtained using large sample properties of binormal ROC curves [28] and pooled estimates of both the c-statistic and detection rate were subsequently obtained by random effects meta-analysis of the study-specific c-statistics and detection rates. All analyses were conducted using Stata, version 10.0 (StataCorp). All statistical tests were 2-sided.
Results
Systematic Review
We identified 1,566 articles of which 83 studies fulfilled our inclusion criteria (Figure S1) and are summarised in the systematic review (Table S1). There were a total of 61,684 patients and 6,485 outcome events in these studies (median per study of 53 [range 4–570]). Of these 83 studies, 72 had a unique article, and 11 were selected from studies that had multiple eligible articles reporting different CRP effects (see Table S1), but only one was included in the meta-analysis according to the rules described under “data extraction.” The mean age of patients across studies was a median (IQR) of 62.4 y (60.0–65.3 y). The median (IQR) proportion of women in studies was 24.9 (19–29). No studies reported stable angina as the sole initial presentation; the median (IQR) prevalence of previous myocardial infarction was 39% (26–50). The proportion of stable patients was 100% in 14 studies, median (IQR) of 49.8% (27.7%–67.8%) in 24 studies, and not stated in the remainder.
Quality of Study Reports
The median (IQR) number of study quality items reported was 7 (6–8) out of a possible 17 and did not change between 1997 and 2009, and was not associated with study size (correlation coefficient of 0.18, p = 0.11) (Figure 1). More than 80% of studies reported details of the healthcare setting, exclusion criteria, assay type, and manufacturer. Two studies referred to a study protocol, but no studies referred to a statistical analytic protocol. Two studies reported the time elapsed between first lifetime presentation with coronary disease and assessment of CRP. Ten different types of comparisons were used for presenting the relative risks (five based on continuous CRP measures, three with equal sized groups, and two with unequal sized groups [one two-group and one three-group comparison]); the rationale for choosing these groups was stated in 32.5% of studies.
Fig. 1. Quality of individual study reports (n = 17 items, n = 83 studies), based on the REMARK guidelines [11]. ![Quality of individual study reports (<i>n = </i>17 items, <i>n = </i>83 studies), based on the REMARK guidelines <em class="ref">[<b>11</b>]</em>.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/1a800198a783a34a48012ff220c5dec1.png)
Definitions of each item are given in Table S2. Meta-analysis Forest Plot
The pooled relative risk from the random effects model of top versus bottom third of CRP using the most highly adjusted study estimate was 1.97 (95% CI 1.78–2.17) (Figure 2). There was marked heterogeneity, with an I2 of 79.5% (95% CI 75.1–82.8). Among the 13 studies that adjusted for conventional risk factors (age, sex, smoking, obesity, diabetes, and low-density lipoprotein [LDL] cholesterol), the relative risk was 1.65 (95% CI 1.39–1.96), with a lower I2 of 33.7 (95% CI 0.0–64.6). Only three of these studies adjusted, in addition, for fibrinogen or other inflammatory markers and yielded a relative risk of 1.52 (1.25–1.85). The eight studies that adjusted for one or more markers of inflammation, irrespective of adjustment for conventional factors, yielded a relative risk of 1.99 (95% CI 1.45–2.72). Among the 25 studies reporting separate adjustments for age and sex only and for at least one (median 2) conventional risk factor the relative risk for CRP was attenuated by 39%, from 2.44 (95% CI 1.95–3.05) to 1.88 (95% CI 1.55–2.26), respectively. The median (IQR) number of adjustments not including the conventional risk factors was 4 (2–7), encompassing 78 unique risk factors (with hypertension being the most common adjustment variable, appearing in 28 studies).
Fig. 2. Forest plot of the effect of CRP on prognosis among patients with stable coronary disease. 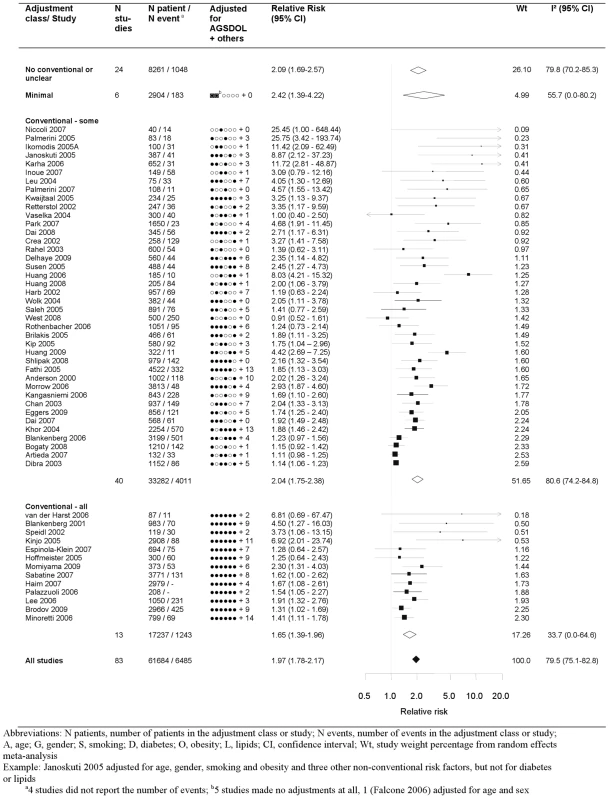
Studies are grouped according to the extent of adjustment for conventional risk factors. Publication Bias
The funnel plot was markedly asymmetrical with less precise (smaller) studies reporting higher relative risks than larger studies (Egger's test, p<0.001 and Begg's rank correlation test, p = 0.001) (Figure 3). Adjustment for the extent of publication bias reduced the estimates to between 1.03 (95% CI 0.99–1.07) and 1.63 (95% CI 1.47–1.79), depending on the method used (see Table S2). The quadratic version of the Egger test gave an adjusted estimate for the effect of CRP of 1.19 (95% CI 1.13–1.25). Using this test, there was some evidence that the publication bias was greater for studies reporting multivariate adjustments compared to those reporting only a minimally adjusted estimate (test for interaction, p<0.0001).
Fig. 3. Funnel plot with contours showing different levels of study significance. 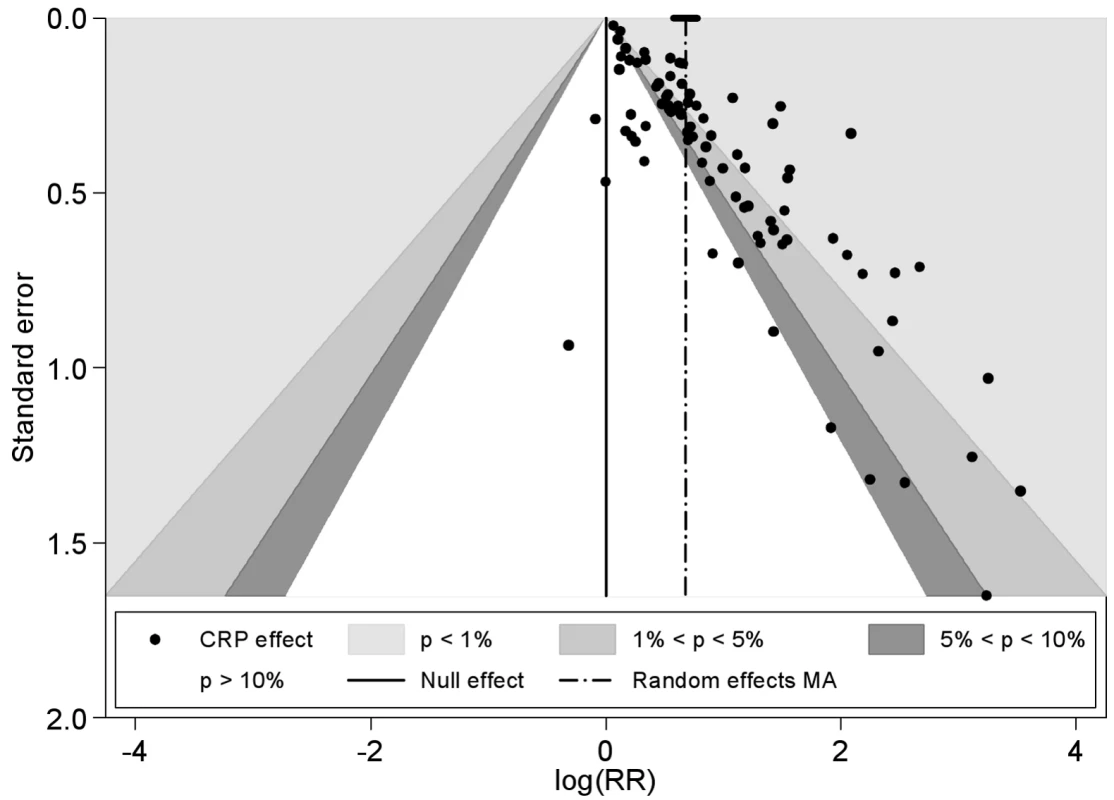
Meta-regression
Univariate random effects meta-regression analyses identified four study-level covariates that were associated with the pooled relative risk: definition of comparison group, the number of adjustment variables, the (log) number of events (p<0.01), and the proportion of patients with stable coronary disease (p = 0.02) (Figure 4). Studies originally reporting unequal CRP groups reported stronger effects than those reporting CRP on a continuous scale. Studies reported a median (IQR) of 6 (4–10) adjustment variables, and for each additional adjustment variable the relative risk decreased by 3%. The relative risk was 1.61 among studies with more than the median number of outcome events (n = 53 events), and 3.28 for smaller studies. The relative risk was 1.47 among studies confined to stable coronary disease, 2.23 in studies with a median of 48.5% stable patients, and 1.96 in the studies in which this proportion was not stated. There was no evidence that the CRP effect differed according to other continuous study level covariates (age, percent women, CRP level, percent on statins, follow-up duration, study start year, number of quality items reported) or to the categorical covariates (event type, type of relative risk). For presentation purposes the meta-regression forest plot is displayed for subgroups, with groups subsequently analysed in the meta-regression chosen for the categorical covariates and continuous covariates split above and below their respective median values. The regression coefficient, associated standard error and the I2 value, however, were obtained from random effects meta-regression. The substantial heterogeneity in the meta-analysis remained largely unchanged in the meta-regression, reflected in an I2 that stayed at around 80% and a stable random effect variance.
Fig. 4. Meta-regression of categorical and continuous study level covariates. 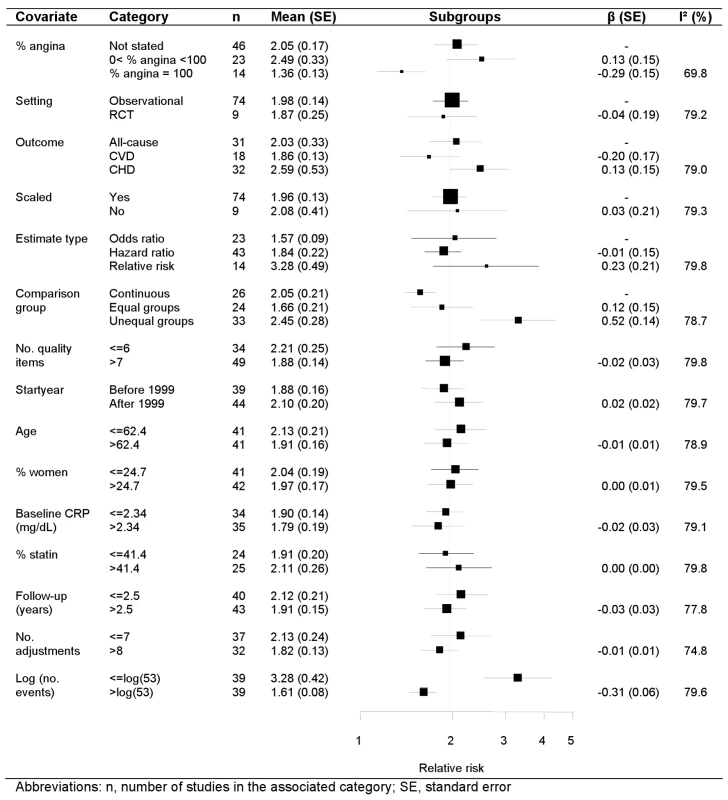
Discrimination
Only two studies reported the area under the ROC curve or equivalent c-statistic (Figure 5). Nineteen studies reported average CRP values separately among those with and without events enabling calculation of discrimination performance. We found that selecting the cut-off value of CRP that gives a 10% false positive rate (1-specificity), gave a detection rate (sensitivity) of 31% (range 6%–63%) when CRP was used alone as a screening test. Our conclusions on discrimination were based on 20 studies (2,374 events); however, the fact that these did not differ from the other studies in terms of their reported relative risks, (p = 0.49), and mean number of patients (697 versus 758, p = 0.97), and that the findings were in line with those reported for aetiologic studies, suggests that these findings are likely to be representative.
Fig. 5. Detection rates at 10% false positive rate and c-statistic for individual studies, and pooled ROC curve. 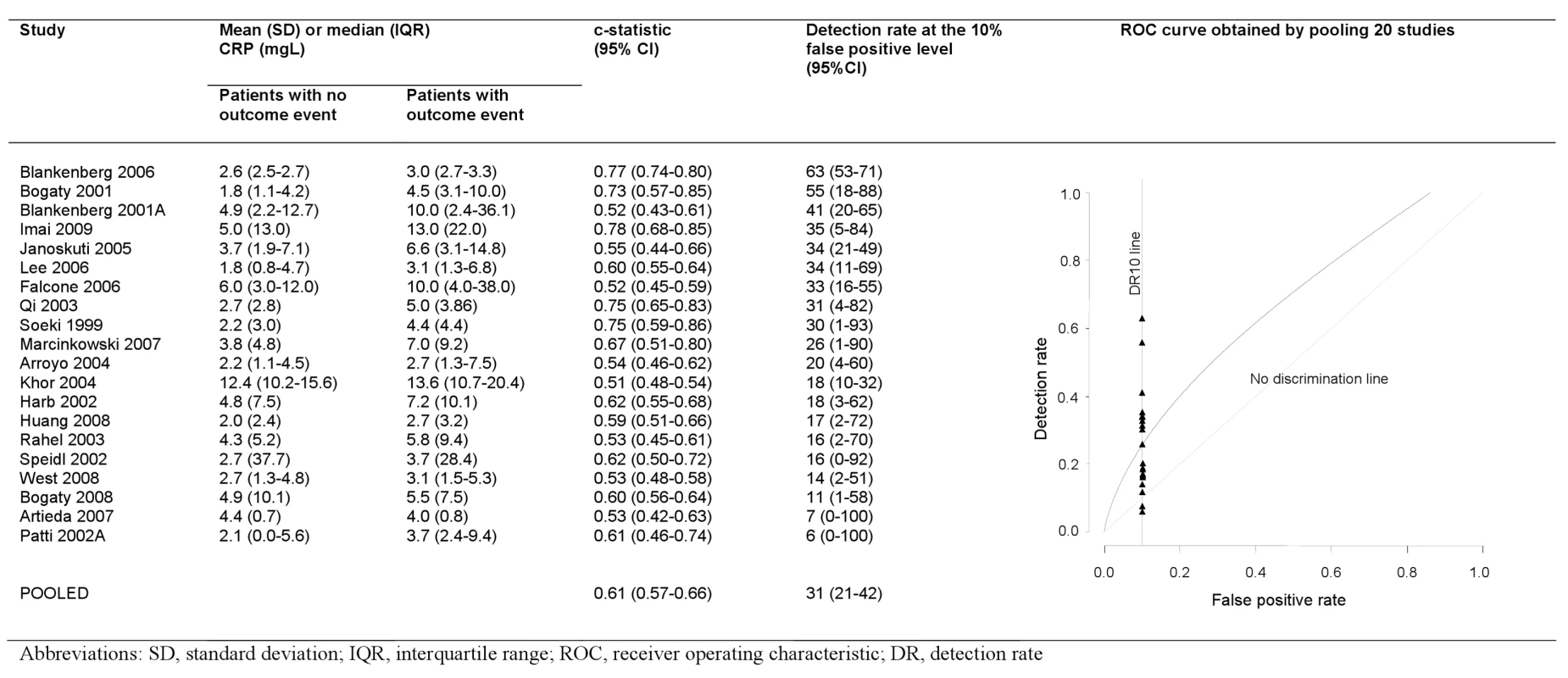
Discussion
In one of the largest (83 studies reporting over 61,000 patients) and most detailed, to our knowledge, evaluations of a single prognostic biomarker, we found the absence of prespecified statistical analytic protocols, publication bias so marked that it could potentially explain much of the association, and multiple types of reporting biases. These biases preclude firm conclusions about the magnitude and independence of the association between higher CRP levels and higher risk of subsequent death and nonfatal cardiovascular events. Taken together with evidence of biases in prognostic biomarker research in cancer [4],[5],[29], stroke [30], trauma [31], and musculoskeletal disorders [32],[33], there is a case for changing the way this type of research is designed and reported.
Quality of Reporting of Primary Studies
Arguably the most fundamental concern was that 0 studies referred to a prespecified statistical analytic protocol. Indeed only two studies referred to any kind of protocol. Thus it is difficult to know what the specific research objectives were at the start of cohort recruitment, at the time of CRP measurement, or at the onset of the statistical analysis. The rationale for comparison group definition, confounder selection, and other analytic choices, even when stated, may have been made after comparing the results of different analytic approaches. Choosing which results to select for presentation may introduce a bias towards “positive” findings.
Descriptions of study populations in the included studies were poor and potentially biased. Only two studies reported the “stage” of the disease, here operationalised as the duration since initial presentation [34]. Although all studies included patients with stable coronary disease, the magnitude of association between CRP and outcomes was greater among studies in which the percentage of stable patients was not stated.
There are no agreed comprehensive criteria for measuring the quality of study reports. The REMARK reporting guidelines for tumour prognostic markers provide a useful start, but are not currently in a form that lend themselves to measurement, and omit reference to statistical analytic protocols. We operationalised the REMARK guidelines and found that studies reported an average of seven of 17 quality items [12]. There was no increase in the average number of items reported over the 13 y since the first publication. Previous systematic reviews have assessed a smaller set of reporting items (seven items [3], three items [35]). In a systematic review of 117 studies of one prognostic biomarker, P53 in bladder cancer, only 34 studies reported sufficient data to be included in a meta-analysis [5].
Independence of CRP Effect
We graphically depict the incomplete approach to confounder adjustment. Only 13 studies adjusted for a basic set of conventional risk markers and only eight studies adjusted for fibrinogen or other measure of inflammation. Thus the available evidence does not systematically evaluate the independence of the CRP prognosis association from potential confounders, and the extent of residual confounding is not known. Such adjustments are likely to be important because: first, attenuation of the relative risk between minimally adjusted and adjusted models was about 39% in the 25 studies reporting this comparison. Second, studies including a higher number of adjustment variables reported lower relative risks, with each additional variable being associated with about a 3% reduction of the relative risk.
Publication Bias
Not only did we find strong evidence that publication bias was operating (most studies were small with a median of 53 events per study, and smaller studies were more likely to report higher relative risks), but we quantified the possible magnitude and impact of this bias. We have previously identified through simulation studies [25], and empirical [15] studies—where a gold standard of unpublished data is available—a method for adjusting for publication bias that outperforms others. This method, a quadratic version of the Egger regression test, attenuated the effect of CRP by 81%. However, all methods of adjustment produced attenuated results, with levels of attenuation ranging from 28% to 96%. It is worth noting that the funnel plot asymmetry is present even for larger studies. The degree of the bias arising from nonpublication calls into question the strength of any association between CRP and outcome.
Discrimination and Prediction
American Heart Association guidelines [16] recommend reporting measures of discrimination but only two studies in our review did. This reporting of risk prediction is of wide clinical interest because stable coronary disease has a high annual risk of fatal and nonfatal acute coronary syndromes of between 2% [36] and 5% [37],[38] and affects an increasing number of people [39] as the population ages and survival from acute coronary syndromes improves.
Because of the lack of published protocols, we do not know whether other studies carried out, but elected not to report, such analyses. Based on the 20 studies reporting CRP distributions among those with and without events, CRP on its own detected only 31% of patients who would subsequently experience events at a 10% false positive rate. We found a c-statistic of 0.61, similar to the 0.65 observed in healthy population studies [6]. Given the magnitude of the CRP relative risk, and that CRP is correlated with some of the factors (e.g. white cell count, glucose) in existing scores, it seems unlikely that CRP would add substantially to the discrimination achieved by standard clinical factors among patients with stable coronary disease [40],[41]. Even if it does add predictive information, CRP may not be cost-effective [7],[42].
Comparison with Healthy Population Studies
By contrast with the evidence among patients with coronary disease, the quality of evidence in healthy populations (aetiologic) [6],[19],[43]–[47] is not subject to the same concerns. Sufficiently unbiased and precise estimates of CRP effect have been obtained that allow assessment of confounding in mendelian randomisation approaches, which in turn have questioned the role of CRP in disease onset. A causal role in disease progression is still possible for CRP if, for example, it were associated with thrombosis and necrosis, rather than the development of atherosclerosis. The populations in our systematic review, compared to healthy population studies [6], evaluated the role of higher initial CRP levels (2.3 versus 1.28 mg/l), shorter follow up periods (median 2.5 y versus 3–20 y). and higher annual risk of events (5.5% versus <1%). Observational studies of other markers, such as body mass index are known to exhibit different aetiologic and prognostic effects [48].
Limitations
The main limitation is that we studied what authors and journal editors select for reporting and not study quality per se. However in many instances it is likely that there is a strong correlation. It is also possible that we missed published studies, although we suspect that the higher quality studies would be more likely to be detected.
Research Implications
We previously outlined ten steps for improving prognosis research [1], which include the need for prospective study registration, publication of design and analytic protocols, and prospective individual participant data meta-analysis. Pooling data from a subset of larger, higher quality, more homogeneous studies in order to make better adjustments for confounders and further investigate discrimination (e.g., with net reclassification improvement measures) is feasible in such clinical datasets [49]. But our review suggests that identifying such a subset of studies may not be easy, and there is a need for new clinical cohorts. Better reporting is required and the existing guidelines are a start [12],[50], but these require development across disease areas and formalisation into data extraction tools. The CRP–prognosis literature may be summarised as early (phase 1) stage, in which investigators aim to discover and report possible associations [51]. There is a need to move to phase 2 in which these associations are more rigorously evaluated. Such better quality observational evidence is an important basis for prioritizing other methods of addressing confounding [52] such as “mendelian randomisation” [13],[43]–[47] and randomisation to specific biomarker lowering agents [53].
Clinical Implications
Our findings suggest that clinical guidelines [8],[9] should change their recommendations. The available evidence supports a negative recommendation, i.e., that CRP should not be routinely measured among patients with stable coronary disease to quantify prognosis or to guide interventional therapies. Our findings explicitly challenge the statement for healthcare professionals made by the Centers for Disease Control that measuring CRP is both “useful” and “independent” as a marker of prognosis.
Furthermore, there is a need for a clear framework in which guideline developers can evaluate the type and quality of evidence necessary to make clinical practice recommendations on prognostic biomarkers.
Conclusion
The quality of published evidence on CRP and prognosis in stable coronary disease is poor and is not sufficient to recommend routine measurement of this biomarker. This review, and others in cancer, constitutes an indictment of the research culture in prognostic biomarkers, and highlights areas for change, the most fundamental of which is the need to register studies along with their analytic protocols.
Supporting Information
Zdroje
1. HemingwayH
RileyRD
AltmanDG
2009 Ten steps towards improving prognosis research. BMJ 339 b4184
2. KyzasPA
LoizouKT
IoannidisJP
2005 Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst 97 1043 1055
3. KyzasPA
axa-KyzaD
IoannidisJP
2007 Quality of reporting of cancer prognostic marker studies: association with reported prognostic effect. J Natl Cancer Inst 99 236 243
4. RileyRD
AbramsKR
SuttonAJ
LambertPC
JonesDR
2003 Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer 88 1191 1198
5. MalatsN
BustosA
NascimentoCM
FernandezF
RivasM
2005 P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol 6 678 686
6. KaptogeS
Di AngelantonioE
LoweG
PepysMB
Emerging Risk Factors Collaboration 2010 C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375 132 140
7. HemingwayH
HenrikksonM
ChenR
DamantJ
FitzpatrickNK
2010 The effectiveness and cost-effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision model. Health Technol Assess 14 1 151
8. PearsonTA
MensahGA
AlexanderRW
AndersonJL
CannonRO
III 2003 Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107 499 511
9. FoxK
GarciaMA
ArdissinoD
BuszmanP
CamiciPG
2006 Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 27 1341 1381
10. YoungI
RifaiN
2009 High-sensitivity C-reactive protein and cardiovascular disease. Clin Chem 55 201 202
11. StroupDF
BerlinJA
MortonSC
OlkinI
WilliamsonGD
2000 Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283 2008 2012
12. McShaneLM
AltmanDG
SauerbreiW
TaubeSE
GionM
2005 Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97 1180 1184
13. CasasJP
ShahT
HingoraniAD
DaneshJ
PepysMB
2008 C-reactive protein and coronary heart disease: a critical review. J Intern Med 264 295 314
14. TimpsonNJ
LawlorDA
HarbordRM
GauntTR
DayIN
2005 C-reactive protein and its role in metabolic syndrome: mendelian randomisation study. Lancet 366 1954 1959
15. MorenoSG
SuttonAJ
TurnerEH
AbramsKR
CooperNJ
2009 Novel methods to deal with publication biases: secondary analysis of antidepressant trials in the FDA trial registry database and related journal publications. BMJ 339 b2981
16. HlatkyMA
GreenlandP
ArnettDK
BallantyneCM
CriquiMH
2009 Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119 2408 2416
17. AltmanDG
2001 Systematic reviews of evaluations of prognostic variables. BMJ 323 224 228
18. DaneshJ
CollinsR
ApplebyP
PetoR
1998 Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279 1477 1482
19. ShahT
CasasJP
CooperJA
TzoulakiI
SofatR
2009 Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 38 217 231
20. DaneshJ
CollinsR
ApplebyP
PetoR
1998 Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279 1477 1482
21. DerSimonianR
LairdN
1986 Meta-analysis in clinical trials. Control Clin Trials 7 177 188
22. HigginsJP
ThompsonSG
2002 Quantifying heterogeneity in a meta-analysis. Stat Med 21 1539 1558
23. SterneJA
EggerM
SmithGD
2001 Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 323 101 105
24. PetersJL
SuttonAJ
JonesDR
AbramsKR
RushtonL
2006 Comparison of two methods to detect publication bias in meta-analysis. JAMA 295 676 680
25. MorenoSG
SuttonAJ
AdesAE
StanleyTD
AbramsKR
2009 Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med Res Methodol 9 2
26. WaldNJ
HackshawAK
FrostCD
1999 When can a risk factor be used as a worthwhile screening test? BMJ 319 1562 1565
27. LawMR
WaldNJ
MorrisJK
2004 The performance of blood pressure and other cardiovascular risk factors as screening tests for ischaemic heart disease and stroke. J Med Screen 11 3 7
28. ObuchowskiNA
McClishDK
1997 Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med 16 1529 1542
29. KyzasPA
Denaxa-KyzaD
IoannidisJP
2007 Almost all articles on cancer prognostic markers report statistically significant results. Eur J Cancer 43 2559 2579
30. WhiteleyW
ChongWL
SenguptaA
SandercockP
2009 Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke 40 e380 e389
31. PerelP
EdwardsP
WentzR
RobertsI
2006 Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak 6 38
32. HaydenJA
ChouR
Hogg-JohnsonS
BombardierC
2009 Systematic reviews of low back pain prognosis had variable methods and results-guidance for future prognosis reviews. J Clin Epidemiol 62 781 796
33. PengelLH
HerbertRD
MaherCG
RefshaugeKM
2003 Acute low back pain: systematic review of its prognosis. BMJ 327 323
34. SackettDL
WhelanG
1980 Cancer risk in ulcerative colitis: scientific requirements for the study of prognosis. Gastroenterology 78 1632 1635
35. Gould RothbergBE
BrackenMB
RimmDL
2009 Tissue biomarkers for prognosis in cutaneous melanoma: a systematic review and meta-analysis. J Natl Cancer Inst 101 452 474
36. BodenWE
O'RourkeRA
TeoKK
HartiganPM
MaronDJ
2007 Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 356 1503 1516
37. SekhriN
TimmisA
ChenR
JunghansC
WalshN
2008 Inequity of access to investigation and effect on clinical outcomes: prognostic study of coronary angiography for suspected stable angina pectoris. BMJ 336 1058 1061
38. SekhriN
FederGS
JunghansC
HemingwayH
TimmisAD
2007 How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart 93 458 463
39. HemingwayH
McCallumA
ShipleyM
ManderbackaK
MartikainenP
2006 Incidence and prognostic implications of stable angina pectoris among women and men. JAMA 295 1404 1411
40. ClaytonTC
LubsenJ
PocockSJ
VokoZ
KirwanBA
2005 Risk score for predicting death, myocardial infarction, and stroke in patients with stable angina, based on a large randomised trial cohort of patients. BMJ 331 869
41. DalyCA
ClemensF
Lopez SendonJL
TavazziL
BoersmaE
2005 The initial management of stable angina in Europe, from the Euro Heart Survey: a description of pharmacological management and revascularization strategies initiated within the first month of presentation to a cardiologist in the Euro Heart Survey of Stable Angina. Eur Heart J 26 1011 1022
42. HenrikssonM
PalmerS
ChenR
DamantJ
FitzpatrickNK
2010 Assessing the cost effectiveness of using prognostic biomarkers with decision models: case study in prioritising patients waiting for coronary artery surgery. BMJ 340 b5606
43. CasasJP
ShahT
CooperJ
HaweE
McMahonAD
2006 Insight into the nature of the CRP-coronary event association using Mendelian randomization. Int J Epidemiol 35 922 931
44. ElliottP
ChambersJC
ZhangW
ClarkeR
HopewellJC
2009 Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 302 37 48
45. LawlorDA
HarbordRM
TimpsonNJ
LoweGD
RumleyA
2008 The association of C-reactive protein and CRP genotype with coronary heart disease: findings from five studies with 4,610 cases amongst 18,637 participants. PLoS One 3 e3011 doi:10.1371/journal.pone.0003011
46. ZachoJ
Tybjaerg-HansenA
JensenJS
GrandeP
SillesenH
2008 Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 359 1897 1908
47. LangeLA
CarlsonCS
HindorffLA
LangeEM
WalstonJ
2006 Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA 296 2703 2711
48. Romero-CorralA
MontoriVM
SomersVK
KorinekJ
ThomasRJ
2006 Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 368 666 678
49. RileyRD
SauerbreiW
AltmanDG
2009 Prognostic markers in cancer: the evolution of evidence from single studies to meta-analysis, and beyond. Br J Cancer 100 1219 1229
50. HaydenJA
CoteP
BombardierC
2006 Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 144 427 437
51. HaydenJA
CoteP
SteenstraIA
BombardierC
2008 Identifying phases of investigation helps planning, appraising, and applying the results of explanatory prognosis studies. J Clin Epidemiol 61 552 560
52. KuperH
NicholsonA
KivimakiM
Aitsi-SelmiA
CavalleriG
2009 Evaluating the causal relevance of diverse risk markers: horizontal systematic review. BMJ 339 b4265
53. PepysMB
HirschfieldGM
TennentGA
GallimoreJR
KahanMC
2006 Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 440 1217 1221
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 6- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Studies Needed to Address Public Health Challenges of the 2009 H1N1 Influenza Pandemic: Insights from Modeling
- The Association of Factor V Leiden and Prothrombin Gene Mutation and Placenta-Mediated Pregnancy Complications: A Systematic Review and Meta-analysis of Prospective Cohort Studies
- Evaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
- Gestational Age at Delivery and Special Educational Need: Retrospective Cohort Study of 407,503 Schoolchildren
- Closing the Gaps: From Science to Action in Maternal, Newborn, and Child Health in Africa
- Sub-Saharan Africa's Mothers, Newborns, and Children: How Many Lives Could Be Saved with Targeted Health Interventions?
- Secondary Prevention of Suicide
- The Prevalence and Drug Sensitivity of Tuberculosis among Patients Dying in Hospital in KwaZulu-Natal, South Africa: A Postmortem Study
- Estimating the Global Clinical Burden of Malaria in 2007
- Long-Term Biological and Behavioural Impact of an Adolescent Sexual Health Intervention in Tanzania: Follow-up Survey of the Community-Based MEMA kwa Vijana Trial
- Where to for Sexual Health Education for Adolescents in Sub-Saharan Africa?
- Incidence and Reproduction Numbers of Pertussis: Estimates from Serological and Social Contact Data in Five European Countries
- Hospital Performance, the Local Economy, and the Local Workforce: Findings from a US National Longitudinal Study
- Sub-Saharan Africa's Mothers, Newborns, and Children: Where and Why Do They Die?
- Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT Statement
- Developing ANDI: A Novel Approach to Health Product R&D in Africa
- Maternal Health: Time to Deliver
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gestational Age at Delivery and Special Educational Need: Retrospective Cohort Study of 407,503 Schoolchildren
- Evaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
- Closing the Gaps: From Science to Action in Maternal, Newborn, and Child Health in Africa
- Secondary Prevention of Suicide
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



