-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Prevalence and Drug Sensitivity of Tuberculosis among Patients Dying in Hospital in KwaZulu-Natal, South Africa: A Postmortem Study
Background:
Tuberculosis is the leading cause of death in South Africa by death notification, but accurate diagnosis of tuberculosis is challenging in this setting of high HIV prevalence. We conducted limited autopsies on young adults dying in a single public hospital in the province of KwaZulu-Natal between October 2008 and August 2009 in order to estimate the magnitude of deaths attributable to tuberculosis.Methods and Findings:
We studied a representative sample of 240 adult inpatients (aged 20–45 years) dying after admission to Edendale Hospital. Limited autopsies included collection of respiratory tract secretions and tissue by needle core biopsies of lung, liver, and spleen. Specimens were examined by fluorescent microscopy for acid-fast bacilli and cultured in liquid media; cultures positive for M. tuberculosis were tested for drug susceptibility to first - and second-line antibiotics. Ninety-four percent of our study cohort was HIV seropositive and 50% of decedents had culture-positive tuberculosis at the time of death. Fifty percent of the participants were on treatment for tuberculosis at the time of death and 58% of these treated individuals remained culture positive at the time of death. Of the 50% not receiving tuberculosis treatment, 42% were culture positive. Seventeen percent of all positive cultures were resistant to both isoniazid and rifampin (i.e., multidrug resistant); 16% of patients dying during the initiation phase of their first ever course of tuberculosis treatment were infected with multidrug-resistant bacilli.Conclusions:
Our findings reveal the immense toll of tuberculosis among HIV-positive individuals in KwaZulu-Natal. The majority of decedents who remained culture positive despite receiving tuberculosis treatment were infected with pan-susceptible M. tuberculosis, suggesting that the diagnosis of tuberculosis was made too late to alter the fatal course of the infection. There is also a significant burden of undetected multidrug-resistant tuberculosis among HIV-coinfected individuals dying in this setting. New public health approaches that improve early diagnosis of tuberculosis and accelerate the initiation of treatment are urgently needed in this setting.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(6): e32767. doi:10.1371/journal.pmed.1000296
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000296Summary
Background:
Tuberculosis is the leading cause of death in South Africa by death notification, but accurate diagnosis of tuberculosis is challenging in this setting of high HIV prevalence. We conducted limited autopsies on young adults dying in a single public hospital in the province of KwaZulu-Natal between October 2008 and August 2009 in order to estimate the magnitude of deaths attributable to tuberculosis.Methods and Findings:
We studied a representative sample of 240 adult inpatients (aged 20–45 years) dying after admission to Edendale Hospital. Limited autopsies included collection of respiratory tract secretions and tissue by needle core biopsies of lung, liver, and spleen. Specimens were examined by fluorescent microscopy for acid-fast bacilli and cultured in liquid media; cultures positive for M. tuberculosis were tested for drug susceptibility to first - and second-line antibiotics. Ninety-four percent of our study cohort was HIV seropositive and 50% of decedents had culture-positive tuberculosis at the time of death. Fifty percent of the participants were on treatment for tuberculosis at the time of death and 58% of these treated individuals remained culture positive at the time of death. Of the 50% not receiving tuberculosis treatment, 42% were culture positive. Seventeen percent of all positive cultures were resistant to both isoniazid and rifampin (i.e., multidrug resistant); 16% of patients dying during the initiation phase of their first ever course of tuberculosis treatment were infected with multidrug-resistant bacilli.Conclusions:
Our findings reveal the immense toll of tuberculosis among HIV-positive individuals in KwaZulu-Natal. The majority of decedents who remained culture positive despite receiving tuberculosis treatment were infected with pan-susceptible M. tuberculosis, suggesting that the diagnosis of tuberculosis was made too late to alter the fatal course of the infection. There is also a significant burden of undetected multidrug-resistant tuberculosis among HIV-coinfected individuals dying in this setting. New public health approaches that improve early diagnosis of tuberculosis and accelerate the initiation of treatment are urgently needed in this setting.
: Please see later in the article for the Editors' SummaryIntroduction
The emergence of HIV in South Africa has resulted in a dramatic rise in the incidence of tuberculosis (TB). Between 1990 and 2007, as the adult HIV prevalence rose to 18.1% [1] the incidence of tuberculosis increased more than 3-fold from 301 to 948 cases/100,000 persons/year [2]. Approximately 80% of incident TB cases in South Africa are HIV-seropositive [2]. These coinfected individuals are more likely to have sputum smear–negative pulmonary or extrapulmonary TB [3],[4], which can be difficult to diagnose clinically. The World Health Organization recommends mycobacterial culture for patients diagnosed with sputum smear–negative tuberculosis. However, the utility of this investigation is limited by cost and long laboratory turn-around times.
The HIV epidemic has undermined TB control in South Africa, and the appearance of multidrug-resistant tuberculosis (MDRTB: resistant to the two most effective first-line drugs, isoniazid and rifampin) and extensively drug resistant tuberculosis (XDRTB: MDRTB with additional resistance to at least one fluoroquinolone and one injectable drug) has further compromised the effectiveness of standard interventions [5]. The interaction between drug-resistant TB and HIV was highlighted by an outbreak of nosocomial XDRTB among HIV-positive adults in Tugela Ferry, KwaZulu-Natal (KZN), South Africa; the outbreak was associated with a mortality rate of 98% and a median time of death at 16 days after diagnosis [6]. The diagnosis of MDRTB is laboratory-dependent and additional costs and time are both required for a definitive result.
TB has become the leading cause of death in South Africa by clinically determined death notification [7]; however, the precise role of TB in adult mortality is difficult to determine from death notifications due to the inherent clinical difficulties in securing an accurate diagnosis. In our study area, HIV seroprevalence is highest among adults in the 20 - to 40-y age range, and the average life expectancy is 43 y [8]. In order to assess the magnitude of deaths associated with TB in young adults in KZN, we conducted a limited autopsy study of patients dying in a single public hospital between October 2008 and August 2009.
Methods
Ethics Statement
The biomedical research ethics committee at the University of KwaZulu-Natal and the KwaZulu-Natal Department of Health approved the protocol for this study.
Study Setting
Edendale Hospital is an 860-inpatient-bed district and regional facility located near the city of Pietermaritzburg in the province of KwaZulu-Natal, South Africa. A previous study in this hospital found that 28% of admitted patients were diagnosed with active TB [9]. In 2006, the local incidence of TB was estimated at 1,094 cases/100,000 persons/year and the HIV prevalence among women in antenatal clinics was more than 39% [10],[11]. The total estimated number of prevalent MDRTB cases was 2,390 in 2006 and 2,799 in 2007 (unpublished data).
Study Population
We recruited eligible decedents from a cohort of adult inpatients at Edendale until we reached our target sample size of 240. Eligibility criteria included age between 20 and 45 y at time of death, death occurring after admission to either the medical or surgical ward for inpatient treatment at Edendale Hospital, and the consent of a family member for inclusion in the study. Inpatients dying from trauma or obstetric complications were excluded.
A trained research nurse approached family members of recently deceased patients and, after informing them of the aims and protocol of the study, invited these families to participate. For logistical reasons, family members were approached between 08 : 00 and 14 : 00 three days a week. A detailed informed consent document was provided in both isiZulu and English. Family members were counseled on TB symptoms and how to access TB testing, and were encouraged to test for HIV infection in accordance with South African government guidelines. Close family contacts of decedents diagnosed with MDRTB were referred for specialist examination. HIV status of the decedent was not routinely reported to the family. Any close contact who signed a confidential form reporting a sexual relationship with the deceased was informed of the HIV status of the decedent. Family members were provided with compensation to defray the costs of travel to and from the hospital.
Data Collection and Diagnostic Procedures
We collected demographic and medical history data from each participant's medical record. Information collected included age at time of death, date of hospital admission, inpatient wards occupied, inpatient medical diagnoses at time of death, current TB treatment status, history of TB antibiotic exposure (“new TB cases” are those with less than one month of previous exposure to TB drugs and “retreatment cases” are those with at least one month of previous TB treatment before the initiation of current TB therapy), other antibiotics received (including antiretroviral therapy), and known MDRTB exposure prior to admission or contact with a TB patient who had died within the previous 5 y.
The HIV status of decedents was collected from the medical record. For individuals without a documented HIV test result, a postmortem HIV test was done on blood or serum aspirated from the heart or great vessels, using a dried blood spot methodology [12]–[14]; accordingly, we were able to definitively classify the HIV status of every decedent included in the study.
A limited autopsy was performed to obtain specimens for microscopic examination for acid-fast bacilli and for M. tuberculosis culture. Respiratory tract secretions were obtained by saline lavage through a cricothyroid membrane puncture, and tissue was obtained by needle core biopsies of the lung, liver, and spleen. The liver and spleen were located using percussion and standard anatomical surface markings [15]. Lung biopsies were obtained through both second intercostal spaces, in the mid-clavicular line, with the needle directed posteriorly and inferiorly, and from areas that were dull to percussion. As greater amounts of tissue have been demonstrated to improve yield, we obtained multiple cores from each organ sampled [16]–[19]. If full-length core samples were successfully retrieved (at least 3 cm), then only three samples were taken, but if smaller samples were retrieved, up to five specimens were collected from each organ. Biopsy specimens were combined into a single pooled specimen. Samples were transported to the NHLS referral mycobacteriology laboratory at Inkosi Albert Luthuli Central Hospital. Standard decontamination procedures (using N-acetyl-L-cysteine and 4% sodium hydroxide with a final concentration of 1% for decontamination and exposure for 15–20 min) were used for mucolysis and to minimize contamination rates. Specimens were examined by fluorescent microscopy for acid-fast bacilli and cultured in liquid culture media (BACTEC MGIT 960 Becton Dickinson). Positive cultures were identified as M. tuberculosis using the niacin-nitrate test. Drug sensitivity testing for first - and second-line agents (isoniazid, rifampin, ethambutol, and streptomycin, kanamycin, ofloxacin) were performed on Middlebrook 7H10 solid media using standardized protocols [20],[21].
In order to evaluate the generalizability of our findings we reviewed medical records to assess the HIV seropositivity rate, the antiretroviral usage, and TB treatment status of a randomly sampled group of decedents who were eligible but not included in the study.
Statistical Analysis
We used bivariate and multivariate logistic regression to identify independent associations between measured covariates and (1) the probability of TB and (2) the probability of MDRTB among those with TB at the time of death. We included all variables examined in the bivariate analysis in our multivariate models and also assessed whether or not antiretroviral therapy modified the association between HIV and each dependent variable. Associations with p-values <0.05 were considered statistically significant. Data were recorded on standard paper forms and entered into a Microsoft Access database and checked against original paper records by two different individuals. Analysis was done using Stata version 9.2 (College Station, TX, USA).
Results
Two hundred and forty decedents were recruited into the study with dates of death beginning on 28 October 28 2008 and ending on 12 August 12 2009. Over this entire time period, a total of 997 deaths occurred among inpatients who were eligible for inclusion in the study. Table 1 displays selected characteristics of individuals included in this sample. We found no significant difference in the age and sex distribution of those included in the sample and those eligible to be in the sample. The median age was 33 y with an interquartile range (IQR) of 28–38 y for those actually included in the study and also 33 y (IQR 28–38 y) for those eligible to be in the study, p = 0.31; the sex distribution was 44% male in the study compared with 46% male among those eligible to be in the study) (p = 0.60). Two hundred and twenty six (94%) of the decedents in the study were HIV-positive; 200 of the seropositive individuals had been diagnosed with HIV before death. As such, the apparent HIV prevalence (not including those whose HIV infections were diagnosed after death) among those included in the study (200/240 = 83%) was not statistically significantly different than the observed HIV prevalence among a random selection of eligible decedents who were not included in the study (126/164 = 77%; p = 0.13). In total, 17% of HIV-positive decedents included in the study were receiving antiretroviral therapy at the time of death; of the subset of participants known to be HIV positive before autopsy, 20% were receiving antiretroviral drugs. Antiretroviral usage was lower among participants than among the random selection of known HIV positive decedents that were not included in the study (38/126 = 30%; p-value = 0.03). 15% of decedents had at least one previous hospitalization within the past 6 mo. Decedents died a median of 4 d (IQR 1–7 d) after admission to the hospital.
Tab. 1. Study population characteristics. 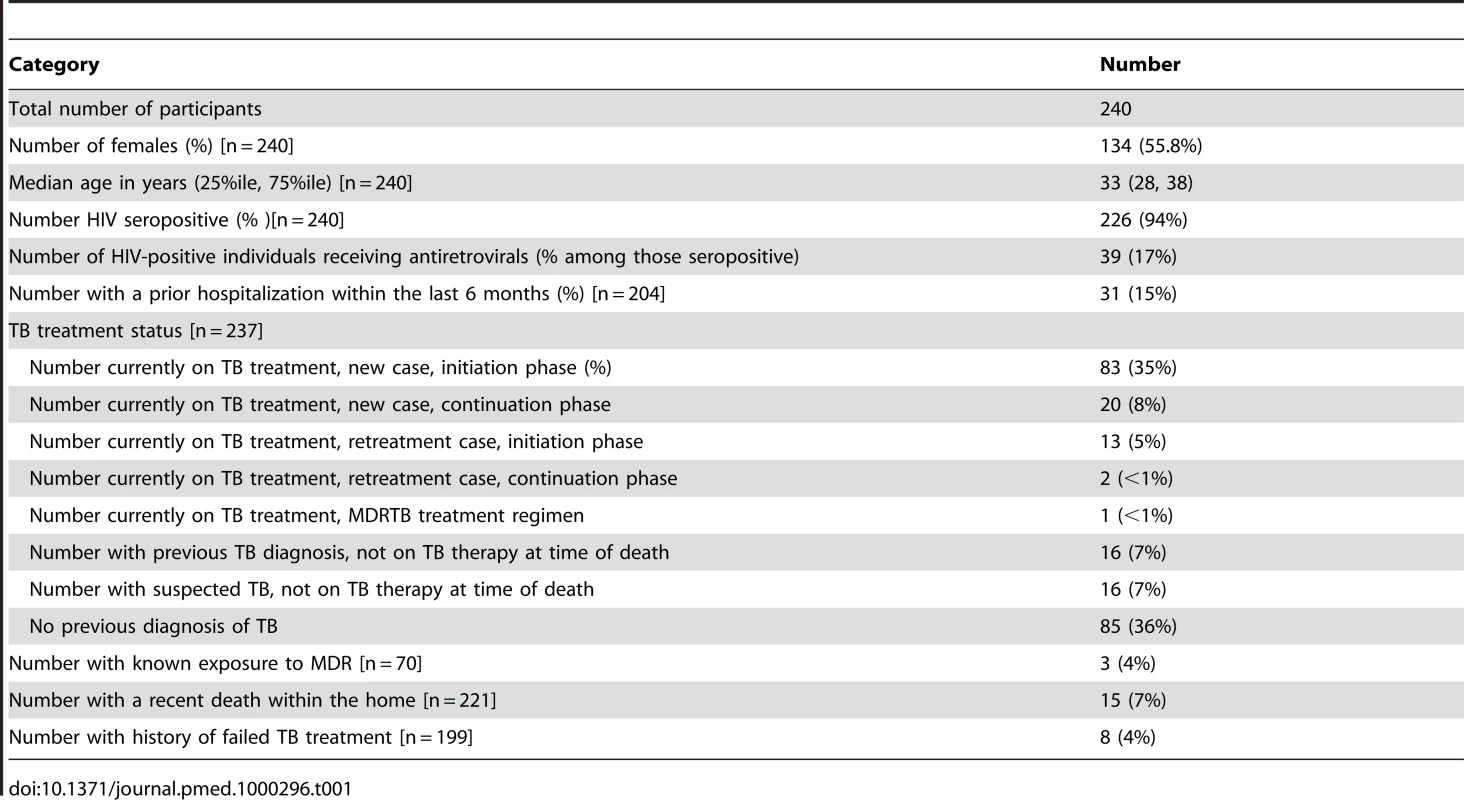
One hundred and nineteen decedents (50%) were being treated for TB in accordance with national guidelines at the time of death; this was not statistically significantly different than the proportion receiving tuberculosis treatment among the random selection of eligible decedents that were not included in the study (77/164 = 47%; p = 0.55). Sixty-one percent of patients on TB treatment were diagnosed during their final hospital admission. The median length of hospitalization before death did not differ significantly (p = 0.85) between those diagnosed with TB and started on anti-TB drugs during this final admission (a median of 5 d between hospitalization and death, IQR 1–9 d) and those suspected of having TB but were not started on anti-TB regimens and were found to be culture positive only after death (a median of 4 d between hospitalization and death, IQR 3–6 d). Fewer than 10% of decedents had either known exposures to MDRTB, recent unexplained deaths among household contacts, or a history of previous failed TB treatment.
Culture-Positive Tuberculosis Disease
A total of 236 specimens were examined in the laboratory; of these, 11 (4.7%) were contaminated; this is consistent with expected laboratory performance [19]. Smear examination of autopsy specimens was positive in 79 (34%) of those tested, and 110 (50%) were culture-positive for M. tuberculosis (Table 2). Three specimens (<3%) were shown to be non-tuberculous mycobacteria.
Tab. 2. Postmortem smear and culture status among participants, categorized by TB treatment status. 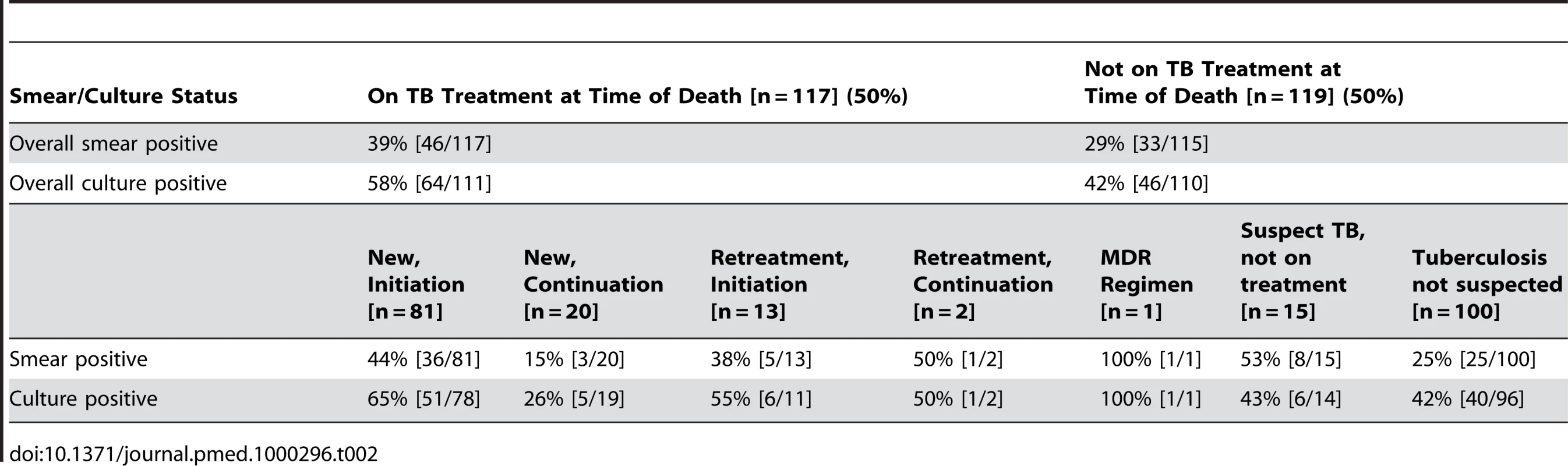
Among decedents receiving or not receiving antitubercular therapy, 64 (58%) and 46 (45%) were culture positive, respectively. Six (43%) TB suspects who were not yet on treatment had a positive culture. Among those participants in whom TB was not suspected at the time of death, 40 (42%) were culture positive; 39 of these patients (98%) were HIV seropositive. The top three leading inpatient diagnoses for these unsuspected M. tuberculosis culture-positive individuals were: pneumonia/lower respiratory infection (25%), meningitis (18%), and gastroenteritis (15%).
In bivariate analysis, individuals receiving TB treatment and males were significantly over-represented among those with positive cultures at time of death (Table 3). No other measured factors were associated with culture positivity. Among the decedents who were positive for HIV, antiretroviral use did not modify the probability of TB. Adjusting for each of the other factors, males had nearly twice the odds of having a positive culture for M. tuberculosis than females (odds ratio [OR] 1.98; 95% confidence interval [CI] 1.12–3.52) and individuals on TB treatment at the time of death had over 80% increased odds of culture positivity (OR 1.82; 95% CI 1.05–3.18).
Tab. 3. Bivariate and multivariate association with culture positive TB at time of death. 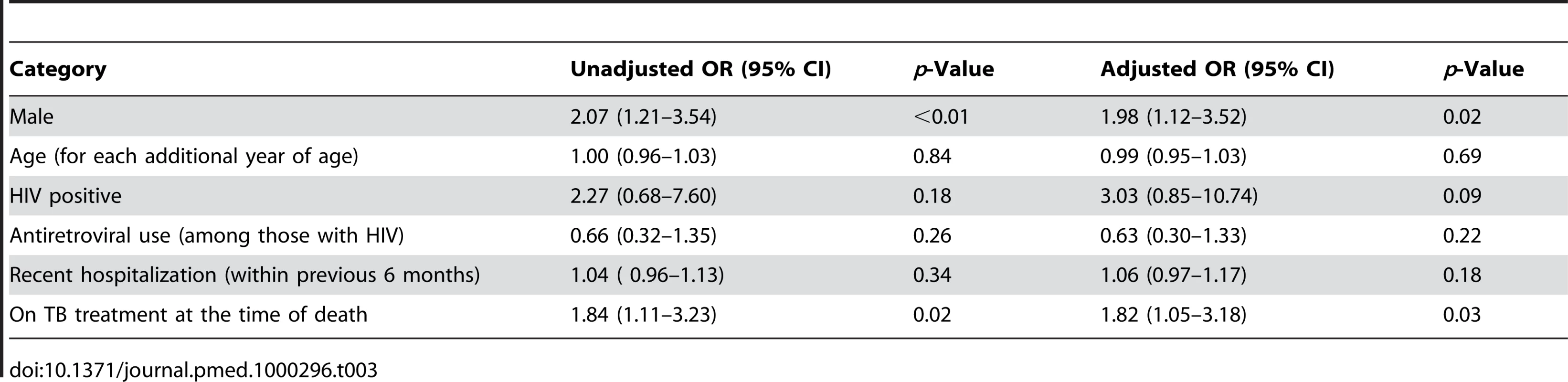
Drug Resistance
Seventeen (17%) of the M. tuberculosis isolates were MDR. Only one XDR isolate was identified; it was found in an HIV-positive patient with no recent history of hospitalization who was in the initiation phase of his first course of TB chemotherapy. Only one specimen was monoresistant to isoniazid and one monoresistant to rifampin; all other samples that were resistant to one of these drugs were resistant to both drugs. Table 4 displays resistance profiles categorized by TB treatment status.
Tab. 4. Resistance profiles for those with positive cultures, categorized by TB treatment status. 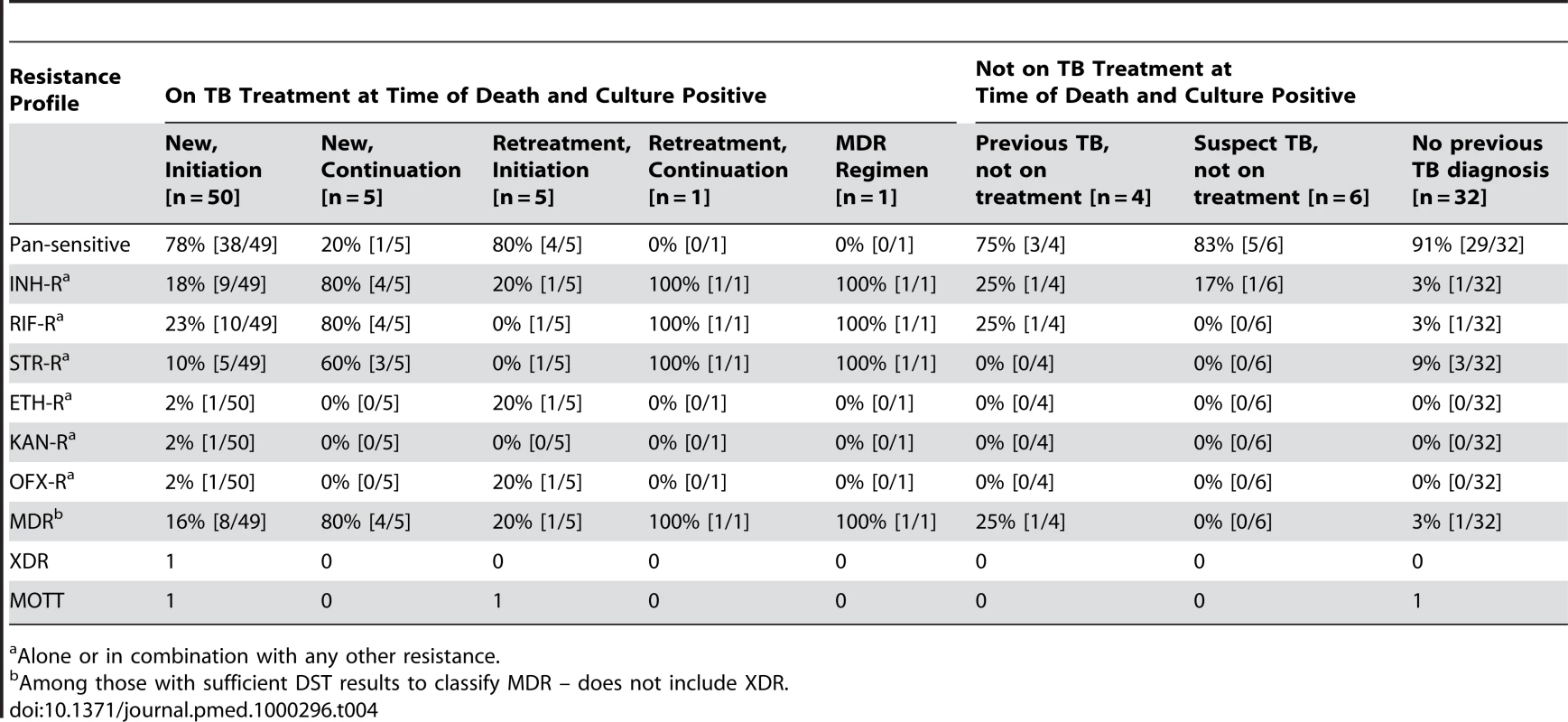
Alone or in combination with any other resistance. Bivariate analyses revealed that a recent previous hospitalization and current tuberculosis therapy were each associated with an increased probability of detecting MDR among those with disseminated M. tuberculosis (Table 5). Multivariate analyses revealed that among those who were culture positive, being on current TB treatment was associated with a greater than 6-fold increase in the odds of MDR (OR 6.56; 95% CI 1.31–32.75). Adjusting for other factors, recent hospitalization did not remain statistically significantly associated with MDR (OR 1.16; 95% confidence interval 0.99–1.36).
Tab. 5. Bivariate and multivariate associations with MDR (among those that were culture positive and with sufficient DST) at time of death. 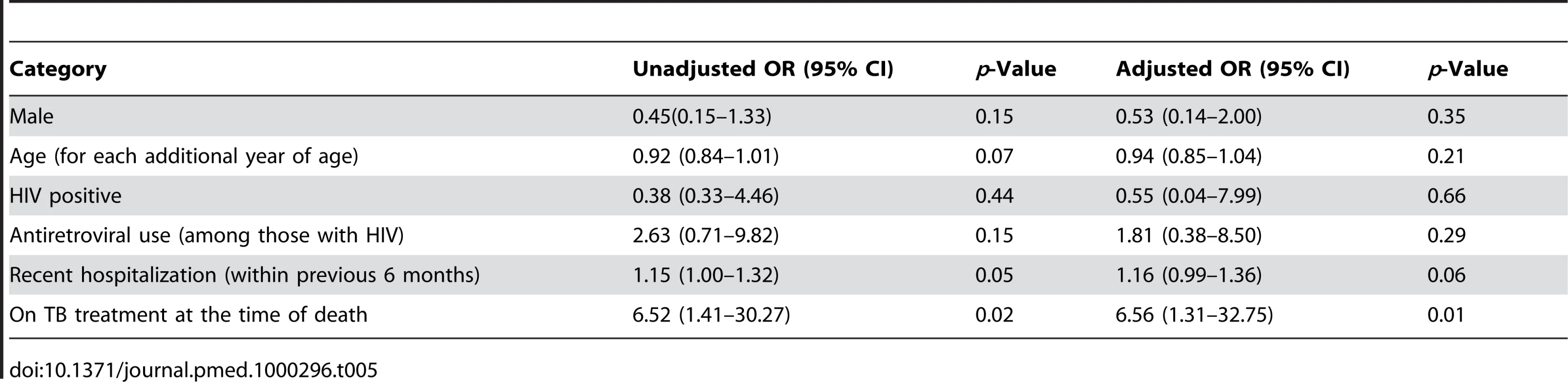
Discussion
While TB is recognized as a major cause of early death in KwaZulu-Natal, South Africa, our findings reveal that the toll of TB is far larger than has been previously reported. Despite efforts to prioritize diagnosis and treatment of TB, especially among those coinfected with HIV, we found that half of the inpatients who died at a single hospital had evidence of TB at the time of death. Although almost half of those who died were on TB treatment at the time of death, more than half of those on treatment still had evidence of viable M. tuberculosis in their postmortem specimens. Nearly half of the decedents who were not on TB treatment at the time of death also had evidence of TB. Since needle core biopsies of organs and respiratory specimens are unlikely to detect all cases of disease, the proportion of decedents from which we could grow M. tuberculosis should be considered a minimum estimate of the actual fraction with TB at the time of death.
Our data are consistent with a growing body of literature that shows extraordinarily high rates of TB in young HIV-positive adults dying in African hospitals [22]–[24]. Although we did not preferentially target HIV-positive individuals for inclusion, nearly all (94%) of the decedents who were eligible and entered our study were HIV seropositive. By the time this study had begun, more than 7,000 adults had initiated antiretroviral therapy at Edendale Hospital. Antiretroviral use was not associated with the probability of culture-positive TB in our study population; however, we were not able to determine the duration of antiretroviral therapy prior to death.
Since we cultured all samples collected, we were able to perform speciation and assess the drug sensitivity of the M. tuberculosis isolates that we detected. Forty-three (70%) culture positive patients who died while receiving TB treatment had pan-sensitive M. tuberculosis isolated. This suggests either that the diagnosis of TB was made too late to alter the fatal course of the infection, that adherence was not adequate, or that drug malabsorption [25] played some role in these poor outcomes among patients who would otherwise be expected to respond well to standard TB treatment [26],[27].
Although previous hospitalization was not found to be independently associated with an increased risk of MDR, the association almost reached the level of statistical significance (p = 0.06). If there is a relationship between previous hospitalization and risk of dying with MDR, this could be consistent with either a longer course of TB (either causing resistance or as a result of resistance) or nosocomial transmission of resistant disease. An increased risk of MDR among those on first-line TB treatment may indicate either an increased risk of acquired resistance or that individuals with MDR are more likely to remain culture positive despite this treatment. That nearly one in six TB patients dying during the initiation phase of a first TB treatment regimen were MDR provides support for the latter hypothesis.
It is startling that 17% of the M. tuberculosis isolates we detected were MDR and that more than 16% of patients dying during the initiation phase of their first ever course of TB therapy have MDRTB. These patients are quite likely to have been primarily infected with drug-resistant M. tuberculosis and, since many had no recent history of hospitalization, they provide additional evidence of community transmission of drug-resistant TB [6]. The scarcity of XDRTB in our data confirms that the Tugela Ferry outbreak has not extended to the Edendale Hospital catchment area. Nevertheless, these findings point to a hidden burden of MDR disease among HIV-coinfected individuals in KZN, and the contribution of MDRTB to early mortality in this highly vulnerable population must be further investigated.
This study has several limitations. We obtained the clinical data and epidemiological data on decedents from two sources: (1) their medical charts and (2) interviews with family members. While the medical charts were the most comprehensive clinical record available, these data were not collected prospectively for the purpose of the study. The accuracy of information obtained from family members about potential TB exposures within the home may not be reliable. Because of cost and human resource concerns, we pooled all specimens collected from each participant for culture; this limits our ability to identify the site of disease involvement among those with culture-positive TB. We also did not have resources to carry out additional histological examination of tissues gathered at autopsy; previous studies [22]–[25] have shown that additional diagnoses would likely have been made in this population that is highly exposed to and possibly infected with multiple opportunistic pathogens.
The prevalence of TB detected in our study should serve as an alarm call in this setting of high HIV prevalence, antiretroviral scale-up, and the emergence of MDR and XDRTB. The fact that nearly half of all cases included in our survey had culture-positive TB at the time of death, and that this proportion was essentially the same regardless of TB treatment status or antiretroviral use among those with HIV infection, mandates the implementation of early, more efficacious interventions against TB and HIV. Decreasing patient and provider delays in the diagnosis of TB in this high HIV burden setting is essential to avert unnecessary early deaths [28],[29]. Physicians caring for HIV-positive or status-unknown patients should be aware that TB may occur together with other, more acute conditions. Symptom screening, physical examination, and evaluation of the chest radiograph for active TB is essential [30]. Acutely ill hospitalized patients should routinely submit sputum for TB screening with laboratory-based approaches that increase the rapidity and/or sensitivity of diagnosis of TB such as front-loaded (same day) smear microscopy [31], sputum concentration methods [32], and LED fluorescence microscopy [33]. Antiretroviral therapy prevents TB ; however, less than a fifth of the HIV-positive decedents in this study had started treatment. Expanded access to, and timely initiation of, antiretroviral therapy is essential in order to reduce TB mortality [5],[34].
The substantial proportion of patients dying with MDRTB during the initiation phase of their first course of TB therapy highlights the difficulty of selecting appropriate treatment in locations where drug resistance is emerging, even among those patients for whom a diagnosis of TB is actually made. Promising molecular tools for the rapid diagnosis of drug-resistant TB have been developed [35],[36], and their adoption in these settings has already begun [37]. The support of the Stop TB Partnership for field evaluation of a line probe assay for MDRTB is a significant advance. However, the assay is recommended only for sputum smear-positive or culture-positive specimens, which limits usage in resource-limited high-HIV prevalent settings such as KZN. The availability of rapid and accurate drug resistance testing must also be accompanied by expansion of access to second-line drugs and support to enable these patients to adhere to the lengthy treatment regimens that are required to treat highly drug resistant TB.
The large burden of unrecognized disease among hospitalized patients also emphasizes the need to improve infection control both within nosocomial settings and within the community in locations where transmission is likely to occur. Efforts to increase the availability of TB screening for those with HIV and to conduct intensified case finding will have the dual benefit of getting individuals more quickly onto appropriate therapy and reducing the opportunity for onward transmission of TB.
Zdroje
1. UNAIDS/WHO Epidemiological Fact Sheet on HIV and AIDS, 2008 Update.
Available: http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_ZA.pdf
2. World Health Organization
2009
Global tuberculosis control – epidemiology, strategy, financing
Geneva
World Health Organization (WHO/HTM/TB/2009.411)
3. ColebundersR
BastianI
2000
A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis.
Int J Tuberc Lung Dis
4
97
107
4. HarriesAD
MaherD
NunnP
1998
An approach to the problems of diagnosing and treating adult smear-negative pulmonary tuberculosis in high-HIV-prevalence settings in sub-Saharan Africa.
Bull World Health Organ
76
651
662
5. Abdool KarimSS
ChurchyardGJ
Abdool KarimQ
LawnSL
2009
HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response.
Lancet
374
921
933
6. GandhiNR
MollA
SturmAW
PawinskiR
GovenderT
2006
Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa.
Lancet
368
1575
1580
7. Statistics South Africa
2009
Mortality and causes of death in South Africa 2007: finding from death notification.
Available: http://www.statssa.gov.za/publications/statsdownload.asp?PPN=P0309.3&SCH=4507
8. South African Institute of Race Relations
South Africa Survey 2008/2009.
Press Report available online http://www.sairr.org.za/press-office/press-releases/027-2009-survey-12-press-release-12-11-2009.pdf; accessed Dec 12, 2009
9. AlvarezGG
ThembelaBL
MullerFJ
ClinchJ
SinghalN
CameronDW
2004
Tuberculosis at Edendale Hospital in Pietermaritzburg, Kwazulu Natal, South Africa.
Int J Tuberc Lung Dis
8
1472
1478
10. The Italian Cooperation in South Africa, Demographic and Health Indicators KwaZulu-Natal.
Public document available online http://italcoop.co.za/PublicDocuments/Demographic_Health_Statisticaldata_KW_EC_SA%20_EN.pdf (Accessed 01 March 2010)
11. National Department of Health, South Africa
2007
The national HIV and syphilis survey, South Africa.
http://www.doh.gov.za/docs/reports/2007/hiv/part1.pdf (Accessed 01 March 2010)
12. Le CouerS
HalembokakaG
KhlatM
BrouardN
PurhuenceF
2005
Impact of AIDS on adult mortality: a morgue-based study in Pointe-Noire, Republic of Congo.
AIDS
19
1683
1687
13. WebberLM
SwanevelderC
GrabowWO
FourieP
2000
Evaluation of a rapid test for HIV antibodies in saliva and blood.
SA Med
90
1004
1007
14. ZehnerR
BratzkeH
MebsD
1995
Evaluation of a rapid assay system, HIV 1/HIV 2 Testpack, Abbott, to detect human immunodeficiency virus antibodies in postmortem blood.
J Forensic Sci
40
113
115
15. WilliamsPL
WarwickR
DysonM
BannisterL
1989
Gray's Anatomy, thirty seventh edition
Edinburgh
Churchill Livingstone
16. GuerraI
OrtizE
PortuJ
AtaresB
Aldamiz-EtxebarriaM
2001
Value of limited necropsy in HIV-positive patients.
Pathol Res Pract
197
165
168
17. SatyanarayanaS
KalghatgiAT
MalaviyaAK
BhardwajJR
MuralidharA
2003
Needle necropsy in AIDS.
Indian J Pathol Microbiol
46
416
419
18. GutierrezEB
ZanettaDM
SaldivaPH
CapelozziVL
2002
Autopsy-proven determinants of death in HIV-infected patients treated for pulmonary tuberculosis in Sao Paulo, Brazil.
Pathol Res Pract
198
339
346
19. HannaBA
2004
Laboratory diagnosis.
RomWN
GaraySM
Tuberculosis (2nd Edition)
Philadelphia
Lippincott Williams and Wilkins
20. KentPT
KubicaGP
1985
Public Health Mycobacteriology.
A Guide For the Level III Laboratory. Atlanta, U.S department of Health and Human Services, Public Health Service, CDC
21. IsenbergHD
2004
Clinical Microbiology Procedures Handbook, vol(1) Chapter 5.13.1
Washington DC
ASM Press
22. RanaFS
HawkenMP
MwachariC
BhattSM
AbdullahF
2000
Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi, Kenya.
J Acquir Immune Defic Syndr
24
23
29
23. EchejohGO
MandongBM
TankoMN
ManassehAN
OkekeEN
2006
Hepatic histopathological findings in HIV patients at postmortem in Jos university teaching hospital, Nigeria.
Tropical doctor
36
228
231
24. AnsariNA
KombeAH
KenyonTA
HoneNM
TapperoJW
2002
Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998.
Int J Tuberc Lung Dis
6
55
63
25. MartinsonNA
KarstaedtA
VenterF
OmarT
KingP
2007
Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study.
AIDS
21
2043
2050
26. ChideyaS
WinstonCA
PeloquinCA
BradfordWZ
HopewellPC
2009
Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana.
Clin Infect Dis
48
1685
1694
27. BwireR
BorgdorffMW
Sticht-GrohV
RiederHL
KawumaHJ
1999
Tuberculosis chemotherapy and sputum conversion among HIV-seropositive and HIV-seronegative patients in south-eastern Uganda.
East Afr Med J
76
307
313
28. MeintjesG
SchoemanH
MorroniC
WilsonD
MaartensG
2008
Patient and provider delay in tuberculosis suspects from communities with a high HIV prevalence in South Africa: a cross-sectional study.
BMC Infect Dis
25
8
72
29. MillenSJ
UysPW
HargroveJ
van HeerdenPD
WilliamsBJ
2008
The effect of diagnostic delays on the drop-out rate and the total delay to diagnosis of tuberculosis.
PLoS ONE
3
e1933
30. CainKP
McCarthyKD
HeiligCM
MonkongdeeP
2010
An algorithm for tuberculosis screening and diagnosis in people with HIV.
N Eng J Med
362
707
16
31. HiraoS
YassinMA
KhamofuHG
LawsonL
CambanisA
2007
Same-day smears in the diagnosis of tuberculosis.
Trop Med Int Health
12
1459
1463
32. SteingartKR
NgV
HenryM
HopewellPC
RamsayA
2006
Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review.
Lancet Infectious Diseases
6
664
674
33. MaraisBJ
BrittleW
PainczykK
HesselingAC
BeyersN
2008
Use of light-emitting diode fluorescence microscopy to detect acid-fast bacilli in sputum.
Clin Infect Dis
47
203
207
34. LawnSD
KranzerK
WoodR
2009
Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings.
Clin Chest Med
30
685
99
35. PaiM
KalantriS
DhedaK
2006
New tools and emerging technologies for the diagnosis of tuberculosis: part II. Active tuberculosis and drug resistance.
Expert Rev Mol Diagn
6
423
432
36. Mäkinenj
MarttilaHJ
MarjamäkiM
ViljanenMK
SoiniH
2006
Comparison of two commercially available DNA line probe assays for detection of multidrug-resistant Mycobacterium tuberculosis.
J Clin Microbiol
44
350
352
37. BarnardM
AlbertH
CoetzeeG
O'BrienR
BosmanME
2008
Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa.
Am J Respir Crit Care Med
177
787
792
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 6- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Studies Needed to Address Public Health Challenges of the 2009 H1N1 Influenza Pandemic: Insights from Modeling
- The Association of Factor V Leiden and Prothrombin Gene Mutation and Placenta-Mediated Pregnancy Complications: A Systematic Review and Meta-analysis of Prospective Cohort Studies
- Evaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
- Gestational Age at Delivery and Special Educational Need: Retrospective Cohort Study of 407,503 Schoolchildren
- Closing the Gaps: From Science to Action in Maternal, Newborn, and Child Health in Africa
- Sub-Saharan Africa's Mothers, Newborns, and Children: How Many Lives Could Be Saved with Targeted Health Interventions?
- Secondary Prevention of Suicide
- The Prevalence and Drug Sensitivity of Tuberculosis among Patients Dying in Hospital in KwaZulu-Natal, South Africa: A Postmortem Study
- Estimating the Global Clinical Burden of Malaria in 2007
- Long-Term Biological and Behavioural Impact of an Adolescent Sexual Health Intervention in Tanzania: Follow-up Survey of the Community-Based MEMA kwa Vijana Trial
- Where to for Sexual Health Education for Adolescents in Sub-Saharan Africa?
- Incidence and Reproduction Numbers of Pertussis: Estimates from Serological and Social Contact Data in Five European Countries
- Hospital Performance, the Local Economy, and the Local Workforce: Findings from a US National Longitudinal Study
- Sub-Saharan Africa's Mothers, Newborns, and Children: Where and Why Do They Die?
- Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT Statement
- Developing ANDI: A Novel Approach to Health Product R&D in Africa
- Maternal Health: Time to Deliver
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gestational Age at Delivery and Special Educational Need: Retrospective Cohort Study of 407,503 Schoolchildren
- Evaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
- Closing the Gaps: From Science to Action in Maternal, Newborn, and Child Health in Africa
- Secondary Prevention of Suicide
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



