-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Relationship between Anti-merozoite Antibodies and Incidence of Malaria: A Systematic Review and Meta-analysis
Background:
One of the criteria to objectively prioritize merozoite antigens for malaria vaccine development is the demonstration that naturally acquired antibodies are associated with protection from malaria. However, published evidence of the protective effect of these antibodies is conflicting.Methods and Findings:
We performed a systematic review with meta-analysis of prospective cohort studies examining the association between anti-merozoite immunoglobin (Ig) G responses and incidence of Plasmodium falciparum malaria. Two independent researchers searched six databases and identified 33 studies that met predefined inclusion and quality criteria, including a rigorous definition of symptomatic malaria. We found that only five studies were performed outside sub-Saharan Africa and that there was a deficiency in studies investigating antibodies to leading vaccine candidates merozoite surface protein (MSP)-142 and erythrocyte binding antigen (EBA)-175. Meta-analyses of most-studied antigens were conducted to obtain summary estimates of the association between antibodies and incidence of P. falciparum malaria. The largest effect was observed with IgG to MSP-3 C terminus and MSP-119 (responders versus nonresponders, 54%, 95% confidence interval [CI] [33%–68%] and 18% [4%–30%] relative reduction in risk, respectively) and there was evidence of a dose-response relationship. A tendency towards protective risk ratios (RR<1) was also observed for individual study estimates for apical membrane antigen (AMA)-1 and glutamate-rich protein (GLURP)-R0. Pooled estimates showed limited evidence of a protective effect for antibodies to MSP-1 N-terminal regions or MSP-1-EGF (epidermal growth factor-like modules). There was no significant evidence for the protective effect for MSP-2 (responders versus nonresponders pooled RR, MSP-2FC27 0.82, 95% CI 0.62–1.08, p = 0.16 and MSP-23D7 0.92, 95% CI 0.75–1.13, p = 0.43). Heterogeneity, in terms of clinical and methodological diversity between studies, was an important issue in the meta-analysis of IgG responses to merozoite antigens.Conclusions:
These findings are valuable for advancing vaccine development by providing evidence supporting merozoite antigens as targets of protective immunity in humans, and to help identify antigens that confer protection from malaria. Further prospective cohort studies that include a larger number of lead antigens and populations outside Africa are greatly needed to ensure generalizability of results. The reporting of results needs to be standardized to maximize comparability of studies. We therefore propose a set of guidelines to facilitate the uniform reporting of malaria immuno-epidemiology observational studies.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(1): e32767. doi:10.1371/journal.pmed.1000218
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000218Summary
Background:
One of the criteria to objectively prioritize merozoite antigens for malaria vaccine development is the demonstration that naturally acquired antibodies are associated with protection from malaria. However, published evidence of the protective effect of these antibodies is conflicting.Methods and Findings:
We performed a systematic review with meta-analysis of prospective cohort studies examining the association between anti-merozoite immunoglobin (Ig) G responses and incidence of Plasmodium falciparum malaria. Two independent researchers searched six databases and identified 33 studies that met predefined inclusion and quality criteria, including a rigorous definition of symptomatic malaria. We found that only five studies were performed outside sub-Saharan Africa and that there was a deficiency in studies investigating antibodies to leading vaccine candidates merozoite surface protein (MSP)-142 and erythrocyte binding antigen (EBA)-175. Meta-analyses of most-studied antigens were conducted to obtain summary estimates of the association between antibodies and incidence of P. falciparum malaria. The largest effect was observed with IgG to MSP-3 C terminus and MSP-119 (responders versus nonresponders, 54%, 95% confidence interval [CI] [33%–68%] and 18% [4%–30%] relative reduction in risk, respectively) and there was evidence of a dose-response relationship. A tendency towards protective risk ratios (RR<1) was also observed for individual study estimates for apical membrane antigen (AMA)-1 and glutamate-rich protein (GLURP)-R0. Pooled estimates showed limited evidence of a protective effect for antibodies to MSP-1 N-terminal regions or MSP-1-EGF (epidermal growth factor-like modules). There was no significant evidence for the protective effect for MSP-2 (responders versus nonresponders pooled RR, MSP-2FC27 0.82, 95% CI 0.62–1.08, p = 0.16 and MSP-23D7 0.92, 95% CI 0.75–1.13, p = 0.43). Heterogeneity, in terms of clinical and methodological diversity between studies, was an important issue in the meta-analysis of IgG responses to merozoite antigens.Conclusions:
These findings are valuable for advancing vaccine development by providing evidence supporting merozoite antigens as targets of protective immunity in humans, and to help identify antigens that confer protection from malaria. Further prospective cohort studies that include a larger number of lead antigens and populations outside Africa are greatly needed to ensure generalizability of results. The reporting of results needs to be standardized to maximize comparability of studies. We therefore propose a set of guidelines to facilitate the uniform reporting of malaria immuno-epidemiology observational studies.
: Please see later in the article for the Editors' SummaryIntroduction
Malaria caused by Plasmodium falciparum is a leading cause of mortality and morbidity globally, particularly among young children. After repeated exposure, individuals develop effective immunity that controls blood-stage parasitaemia, thereby reducing clinical symptoms and life-threatening complications (reviewed in [1]). Antibodies are important mediators of acquired immunity to malaria as evidenced by experimental animal models and, most importantly, passive transfer studies in which antibodies from malaria-immune adults were successfully used to treat patients with severe malaria [2],[3]. Antibodies to merozoite antigens are considered important targets of protective antibodies and are thought to function in vivo by inhibiting merozoite invasion of erythrocytes, opsonizing merozoites for phagocytosis, and antibody-dependent cellular inhibition [4]–[7]. However, it is unclear which merozoite antigens are important targets of naturally acquired immunity.
A number of merozoite antigens have established roles in erythrocyte invasion and some have been identified as targets of human invasion-inhibition antibodies or antibody-dependent cellular inhibition in vitro [8]–[15]. Merozoite surface proteins (MSPs) are thought to be involved in the initial attachment of the merozoite to the erythrocyte surface (e.g., MSP-1) and apical membrane antigen-1 (AMA-1) has been implicated in apical reorientation of the merozoite prior to invasion. Two invasion ligand families present in the apical organelles, the erythrocyte binding antigens (e.g., EBA175, EBA181, EBA140) and P. falciparum reticulocyte-binding homologues are also required for invasion [16]. There are numerous surface proteins with no known function including MSP-2, MSP-3, MSP-4, and glutamate-rich protein (GLURP) [16]. Genetic polymorphisms exist in most antigens and some can be grouped into major allelic types. Many of these antigens are currently being evaluated or developed as candidates for inclusion in an erythrocytic-stage malaria vaccine [17].
There are several criteria that can be used to objectively prioritize known and predicted antigens for vaccine development [17]. These include the demonstration that antibodies against these antigens inhibit P. falciparum growth in vitro, or are protective in animal models, and the demonstration that naturally acquired antibodies are associated with protection from symptomatic disease in malaria endemic populations. Consequently, numerous epidemiological studies have investigated the role of merozoite surface antigens as targets of human immunity. However, the epidemiological evidence of the protective effect of naturally acquired anti-merozoite responses is conflicting. There are numerous potential reasons for the inconsistencies in the estimates of protection. In malaria endemic areas the rate at which natural immunity develops is dependent on the intensity and stability of exposure to P. falciparum, with immunity to severe and mild disease developing more rapidly in areas with higher transmission [1],[18]. Differences in the acquisition of immunity may influence associations between specific responses and immunity. Furthermore, the prevalence of the major allelic types of specific antigens and subsequent acquisition of allele-specific immunity may be different across populations. The alleles represented in recombinant proteins used for determining antibody responses varies between studies in addition to the preparation of antigens used in immunoassays. Most importantly, the study designs used to investigate the associations between antibody responses and P. falciparum malaria studies vary considerably among the published literature. Evidence quoted in the literature regarding the protective role of antigen-specific antibodies is often based on data from cross-sectional or case-control studies. Examining the association of antibody responses with parasitological and clinical outcomes determined at a single time point, or in individuals who have already developed disease, makes the establishment of causality problematic. The highest level of evidence of causality in observational studies comes from prospective cohort studies in which a temporal relationship can be established between exposure and outcome.
We performed a systematic review, with meta-analyses, of cohort studies to determine the association of antibody responses to merozoite surface antigens with incidence of P. falciparum malaria in naturally exposed populations, and to identify factors that may account for differences in reported findings. The broad aim of this study was to advance our understanding of naturally acquired immunity to malaria and to contribute to rational vaccine development.
Materials and Methods
We performed a systematic review of the published literature according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines for the conduct of meta-analyses of observational studies [19]. Results are reported according to the recently published PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses; http://www.prisma-statement.org; Text S1). The study protocol was developed by FJIF, JAS, and JGB.
Search Methods for Identification of Studies
PubMed, Web of Science, Scopus, Google Scholar, African Index Medicus, and LILACS (Latin American and Caribbean Health Sciences Literature) (all years, ending 31 January 2009) were searched for studies examining the association of antibody responses to merozoite antigens with P. falciparum malaria. Key words included: MSP, merozoite surface protein, MSA, merozoite surface antigen, GLURP, glutamate-rich protein, serine repeat antigen, SERA, S-Antigen, ABRA, AMA, apical membrane antigen, EBA, erythrocyte binding antigen, rhoptry, malaria, P. falciparum, immunity, antibodies, IgG, cohort, longitudinal, incidence, risk, epidemiology, vaccine. The key words variant surface antigen (VSA) were also used because merozoite antigens are sometimes used as comparative antigens in studies investigating VSAs. The reference lists of obtained papers were searched for further studies. Studies reported in languages other than English were included.
Criteria for Considering Studies for This Review
Study designs
The criteria for inclusion of studies were population-based prospective studies and population-based treatment to reinfection studies. Population-based cross-sectional studies to determine prevalence were excluded because causality cannot be established. Case-control studies, hospital-based studies, and vaccine efficacy trials of blood-stage vaccines were also excluded because of the rigorous inclusion and exclusion criteria applied during these studies, such that the participants would not be representative of the general population.
Study participants
The criterion for inclusion of participants was individuals living in malaria endemic areas. Studies restricted to pregnant women and/or children <1 y (including maternal transfer studies) were excluded to remove the confounding effect of maternal transferred immunity. Studies where individuals were selected according to their P. falciparum status and studies investigating returned travellers or transmigrants were also excluded as they would not be representative of the general population.
Antibody measures
Total immunoglobulin G (IgG) responses to recombinant or synthetic defined merozoite antigens measured at baseline (i.e., time 0) were considered. IgG responses to full length proteins, processing products, and defined regions of merozoite antigens were included, but IgG responses to peptides that represent undefined regions or incomplete domains or subdomains of antigens were excluded.
Malaria outcome measures
The following malaria outcome measures during follow-up were included: high density P. falciparum infection (≥5,000/µl), symptomatic P. falciparum malaria, severe P. falciparum malaria, and P. falciparum malaria-associated mortality. In treatment-to-reinfection studies P. falciparum reinfection was also included as an outcome. Newly established blood-stage infection must have been differentiated from treatment failure by either PCR or documented clearance of infection within a specified time frame appropriate for the chosen antimalarial.
Quality criteria
The minimum quality criteria for inclusion in the review were that: detection of malaria was by active case detection (ACD) and/or passive case detection (PCD); parasitaemia was confirmed by slide microscopy, rapid detection kit, or PCR; symptomatic malaria was defined as fever and/or history of fever (within the past 72 h) plus a high density parasitaemia threshold (to increase specificity because low-grade parasitaemia is common in most settings); severe malaria was defined by the World Health Organization criteria and other causes of morbidity excluded; and other common causes of mortality excluded before a diagnosis of malaria-associated mortality [20],[21].
Selection of Studies
Review authors (FJIF, JSR, and JGB) identified possible studies, FJIF and JSR assessed the methodological quality of included studies independently, with discrepancies resolved by discussion with JGB.
Effort to include all available studies and data
Authors of studies that had defined a case of symptomatic malaria as fever and/or history of fever plus a P. falciparum parasitaemia of any density (i.e., did not meet quality criteria of fever plus a high density threshold) were invited to provide estimates or data meeting the quality criteria. Some studies had analysed antibody levels at baseline as the outcome variable, comparing baseline levels in those who had or did not have P. falciparum malaria during follow-up. For these studies, data was extracted and reanalysed so that malaria was the outcome variable and related to antibodies at baseline. If the raw data were not presented, authors of the study were invited to reanalyse or provide data for the inclusion of their study in the systematic review. In addition, we contacted several authors whose studies did not meet the inclusion criteria yet contained data that were eligible for the systematic review. These were the authors of studies that had measured antibody responses after baseline (i.e., examined the association of antibody responses with malaria cases diagnosed both retrospectively and prospectively) and invited them to provide estimates or data concerning prospective P. falciparum incidence only. We also contacted authors who had restricted analysis to individuals who were parasite positive at baseline and invited them to provide estimates or data on the whole cohort where possible. If authors were unable to provide estimates or data, the study was classified as not meeting inclusion and/or quality criteria and excluded from the systematic review.
Data Analysis
Data collection
Measures of association (odds ratios [ORs], risk ratios [RRs], incidence rate ratios [IRRs], or hazard ratios [HRs]) and their 95% confidence intervals (CIs) were extracted or derived using data reported in the publications. Data extraction was performed independently by FJIF and JSR, using proforma designed by FJIF, JAS, and JGB. The investigators of the original studies were contacted if relevant information on eligibility or key study data were not available in the published report. An email was sent to authors explaining the nature of the systematic review and the information required together with proforma. If the author did not respond within three email attempts then no further action was taken. Where a study does not provide measures of association (or they could not be calculated with the information provided), the study results will be described only in narrative terms.
Standardization of antibody measures
A major issue in reviewing the published results of different epidemiologic studies examining the relation between an exposure variable and risk of the outcome is that the results are presented in many different ways. Determining antibody levels by enzyme-linked immunosorbent assay (ELISA) does not produce a common metric measurement among studies. Individuals can be classified as “responders” or “nonresponders” relative to a negative control (unexposed sera) within each study. Study-specific comparisons of these exposure variables can then be pooled. However, categories based on arbitrary cut-offs (including categories of responders based on statistical rankings) cannot be pooled across studies.
For studies where the antibody measures were analysed as continuous exposure variables we either asked the authors to reanalyse their data by collapsing the antibody data into categories or asked them to provide the standard deviation of the data so we could calibrate the estimate to represent the relative change in the risk of malaria associated with a change of one standard deviation of the antibody level. For log transformed antibody data we used log base 2 so that the relative change in malaria risk corresponds to a doubling of antibody level.
Standardization of malaria outcome measures
ORs considerably overestimate the RR, if the incidence risk is >20%, which is often the case in highly malaria endemic areas [22]. Thus, RR, HR, and IRR were extracted or calculated where possible, or unadjusted ORs were converted to RR using the method of Zhang and Yu [23]. RR, HR, and IRR are hereinafter denoted as RR. A RR equal to 1 occurs when the incidence risk of malaria is equal for those with antibody responses (responders) and those without (nonresponders), and when the incidence risk is unchanged for 2-fold increases in the antibody levels. Where possible, estimates adjusted for demographic variables, spatial confounders, P. falciparum parasitaemia (at baseline or preseason), and/or bed net use are reported. Estimates adjusted for other anti-merozoite antibodies (including antibodies to schizont protein extract) are not reported because antibody responses are typically highly correlated making it difficult to estimate their individual regression coefficients reliably; in these cases unadjusted estimates are reported. For all malaria outcomes the study-assigned P. falciparum definitions were used.
Our aim was to obtain a single RR estimate for each study. If antibody responses to the same antigen, in the same population-based study, were reported in several publications, results from the largest sample size were used. Separate estimates were obtained for the RR associated with AMA-1 (full-length ectodomains of FVO [pro-DI-DII-DIII] and 3D7 [DI-DII-DIII]), EBA-175 (all regions including F1 and F2), GLURP (R0, R1, and R2 fragments), MSP-2 (full length 3D7, full length FC27, and C terminus), MSP-3 (full length 3D7, full length K1, and the conserved C terminus). For MSP-1, separate analyses were done for each region and allelic type (MSP-1-block 1 [MAD20], MSP-1-block 2 [K1-like (3D7), MAD20-like [MAD20], and RO33-like [RO33]), and processing fragments (MSP-142, MSP-119 [including MSP-1-EGF1, MSP-1-EGF2]). Estimates from the above-mentioned regions/alleles were used to ensure maximum comparability between studies. Separate analyses were not done for MSP-3-Ct or MSP-119 alleles because of the conserved nature of MSP-3-Ct and MSP-119 (similarly EGF domains). For these antigens, if responses to multiple alleles were investigated in the same study, the most common circulating allele in the population was included in the meta-analysis.
Meta-analysis
Where there were sufficient data, a pooled summary statistic for each malaria outcome was calculated using either a fixed-effect or random-effects model. The standard error of the natural logarithm (ln) of the RR was calculated using the formula (ln[upper limit of CI]−ln[RR])/1.96. Heterogeneity between studies was tested with the I2 statistic [24]. If the I2 statistic was ≤30%, a meta-analysis based on a fixed-effect model was conducted; otherwise the random-effects model was used. When the I2 statistic was >75% and/or the lower 95% confidence limit was between 50%–100%, the studies were not combined [25]. When statistical heterogeneity was noted it was evaluated by fitting meta-regression models to the log-transformed individual study RRs.
Clinical and methodological heterogeneity was explored using prespecified variables to minimize spurious findings. Variables evaluated included study design (prospective cohort, treatment-to-reinfection), length of follow-up, age of study participants (dichotomous variable: adults and children, children only), malaria endemicity (perennial, seasonal, perennial with seasonal peaks), source of malaria cases (dichotomous variable: ACD only, PCD, and ACD), definition of symptomatic malaria, preparation of antigen (allele, expression vector, tag), and method of antibody determination (ELISA, microarray). Influence analysis was also performed whereby pooled estimates were calculated omitting one study at a time. Where possible, publication bias was assessed visually by plotting a funnel plot [26]; publication bias is unlikely if the funnel plots shows a symmetrical inverted V shape [27]. All analyses were performed using the open source statistical package, R 2.9.0 (R Foundation for Statistical Computing).
Results
Identification and Description of Included Studies
Figure 1 outlines identification of studies for this systematic review. The literature search identified 73 potentially relevant studies, of which only 30 fulfilled the inclusion and quality criteria (details of excluded studies can be found in Text S2) [28]–[57]. We obtained further data from three studies after contacting authors (Figure 1), giving a total of 33 studies to be included in the systematic review [58]–[60].
Fig. 1. Flow chart of study identification. 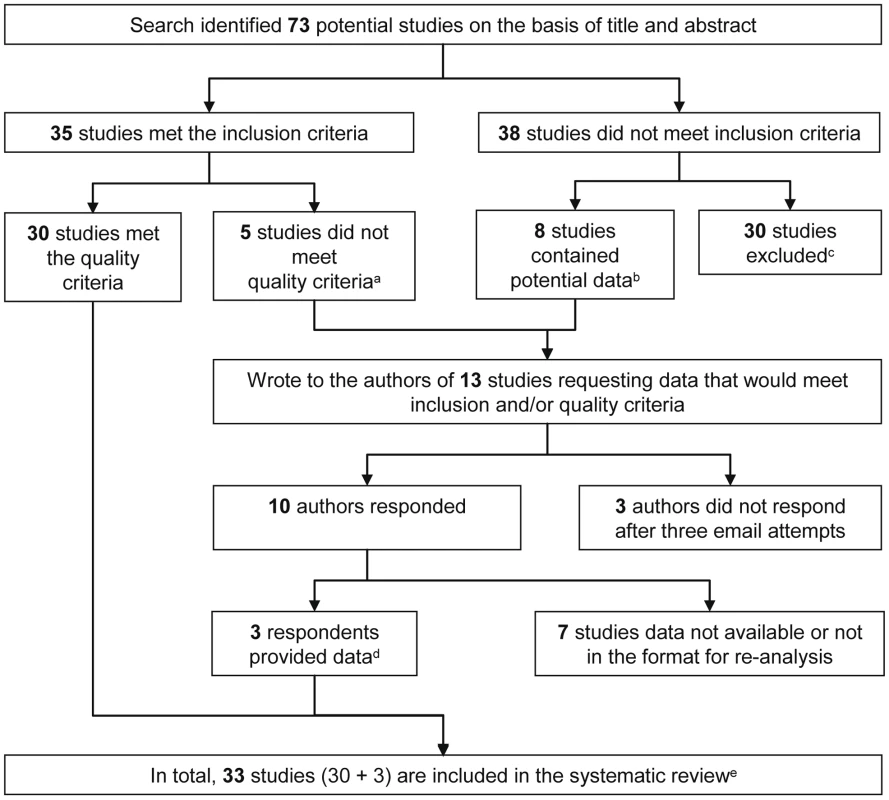
Details of excluded studies can be found in Text S2. aDefinition of symptomatic malaria did not meet protocol definition; bAnalysed retro- and prospectively collected clinical data (n = 3), analysed antibody levels as outcome (n = 4,) and data presented on P. falciparum positive individuals only (n = 1); cReasons for exclusion: Data from seroprevalence surveys (n = 15); hospital-based study/recruited cases based on clinical/parasitemic status (n = 6); did not include malaria outcome of interest (n = 5); mother/infant studies (n = 3); measured IgG responses to undefined regions of antigens (n = 1); dScopel et al. (2007) provided data using a definition of symptomatic malaria that met our quality criteria, Sarr et al. (2006) provided data so P. falciparum could be analysed as outcome, and Osier et al. (2008) provided estimates for the whole cohort, whereas the manuscript originally presented data from P. falciparum-positive individuals only [58]–; eThe characteristics of included studies are given in Table 1. The 33 studies reported data obtained from 14 separate prospective cohort studies and six separate treatment-to-reinfection population studies (Tables 1 and 2, respectively) indicating that multiple publications arise from a single population-based study. For the purpose of this review we shall refer to each publication as a study. The majority of studies report data from Africa (28/33; 84.8%), with three in Papua New Guinea, one in Asia, and one from South America. Study size ranged from 80 to 1,071 participants (median = 280) and duration of participant follow-up ranged from 3 to 18 mo (median = 6). The association of antibody responses to MSP-1 (including processing fragments and defined blocks), MSP-2, and MSP-3, AMA-1, EBA-175, and GLURP with incidence risk of P. falciparum malaria was examined in 19, eight, seven, five, three, and six studies, respectively. Details of recombinant proteins and sero-prevalences can be found in Text S2 (Tables A and B). All studies measured total IgG by ELISA with the exception of Gray et al. (2007), who measured IgG by microarray [40]. Symptomatic P. falciparum malaria during follow-up was the most common outcome, examined in 29 studies; with reinfection and high density infection during follow-up examined in five and three studies, respectively. No study examined the association of anti-merozoite responses with incidence risk of severe P. falciparum malaria or P. falciparum malaria-associated mortality.
Tab. 1. Characteristics of prospective studies included in the systematic review by country. 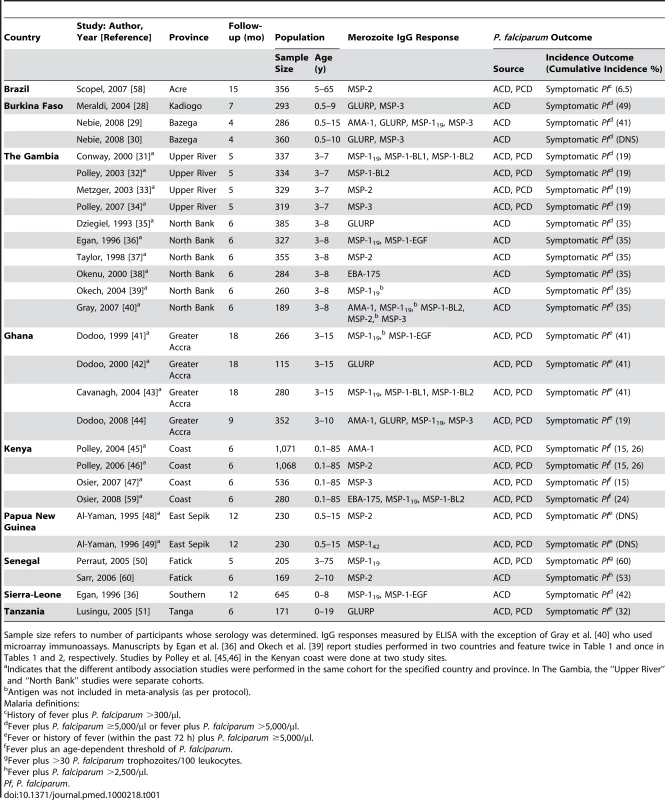
Sample size refers to number of participants whose serology was determined. IgG responses measured by ELISA with the exception of Gray et al. [40] who used microarray immunoassays. Manuscripts by Egan et al. [36] and Okech et al. [39] report studies performed in two countries and feature twice in Table 1 and once in Tables 1 and 2, respectively. Studies by Polley et al. [45],[46] in the Kenyan coast were done at two study sites. Tab. 2. Characteristics of prospective treatment-to-reinfection studies included in the systematic review by country. 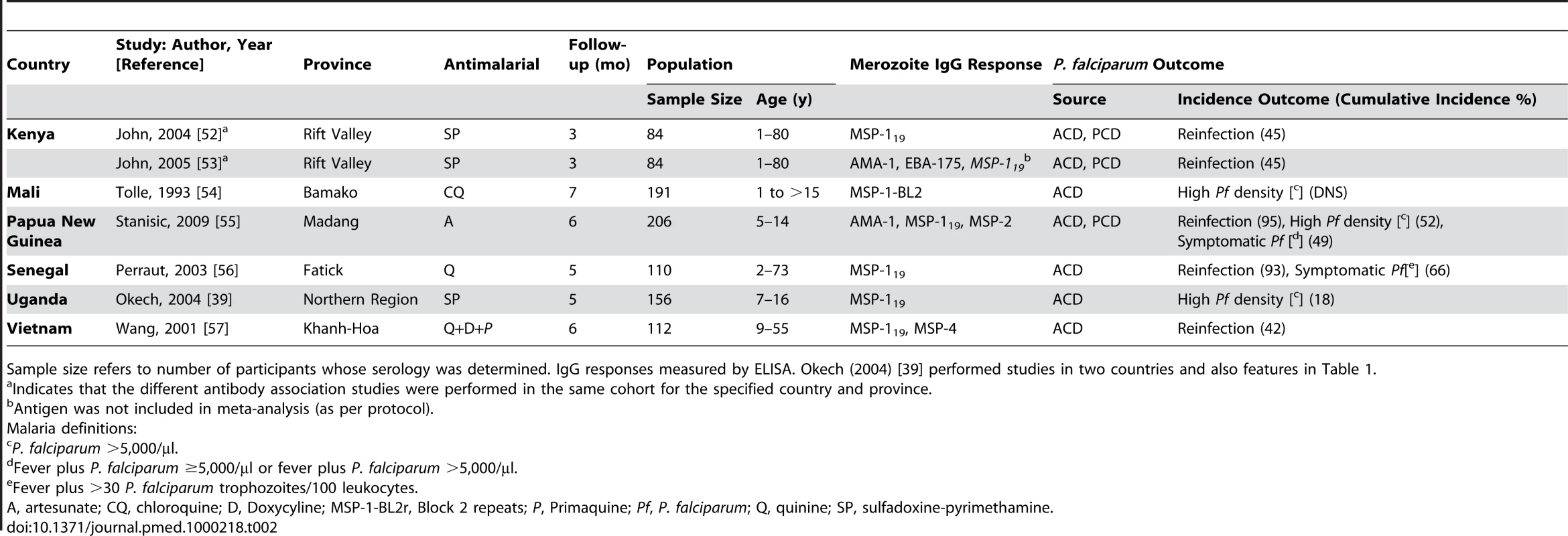
Sample size refers to number of participants whose serology was determined. IgG responses measured by ELISA. Okech (2004) [39] performed studies in two countries and also features in Table 1. Association between Anti-MSP-1 Responses and Incidence of P. falciparum Malaria
MSP-1 C-terminal (Ct)–processing fragments
MSP-1 is a high molecular mass protein (Mr≈180 kDa) that is proteolytically processed into 83 kDa, 30 kDa, 38 kDa, and C-terminal 42 kDa (MSP-142) fragments [61]. During invasion, MSP-142 is further processed into MSP-119 and MSP-133 fragments. Both MSP-119 and MSP-142 are regarded as potential vaccine candidates and have been shown to be protective in animal models [17]. Meta-analysis of five studies showed that MSP-119 IgG responders had an 18% reduction in the risk of symptomatic P. falciparum malaria compared to nonresponders (pooled RR using random-effects [reRR] 0.82, 95% CI 0.7–0.96, p = 0.012; Figure 2) [31],[36][43][50][59]. Meta-regression analysis revealed heterogeneity between allelic groups (p = 0.0223) with the greatest magnitude of effect seen with MAD20 and Palo Alto alleles (29% and 33% relative reduction in symptomatic disease, respectively; Figure 2). Because the methods for the preparation of each antigen was the same for each allelic variant (Text S2, Table A) similar results were obtained when grouping according to expression system and tag used to make the recombinant antigen. Other methodological and clinical characteristics of the studies did not influence estimates and there was no evidence of publication bias.
Fig. 2. Forest plot of the association of MSP-119 responses with incidence of symptomatic P. falciparum malaria. 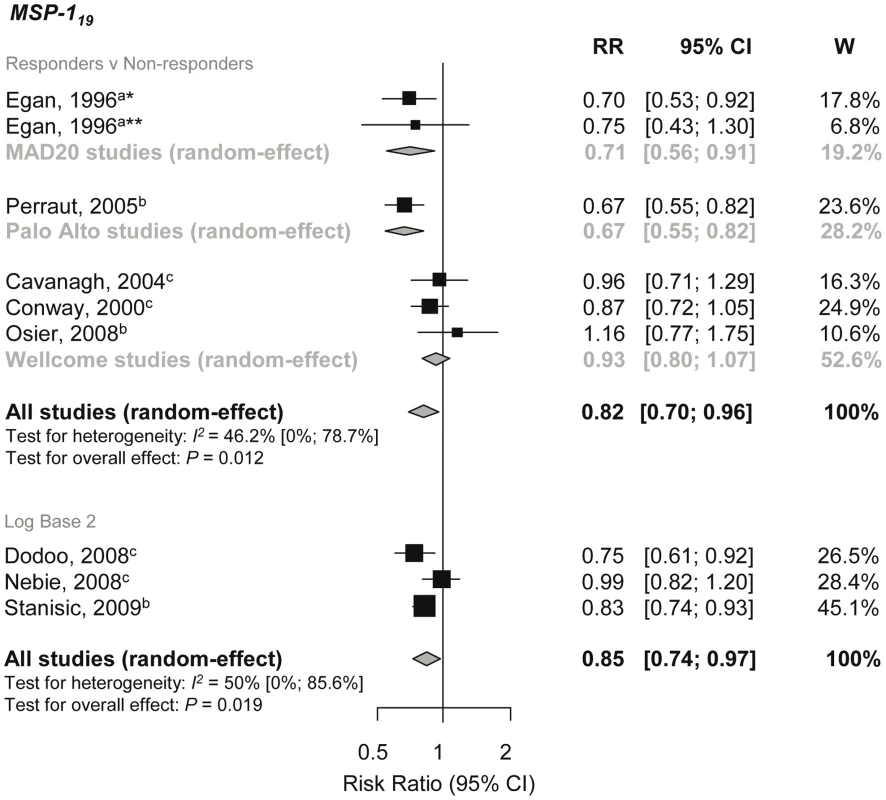
RRs correspond to risk of symptomatic P. falciparum malaria for MSP-119 responders versus nonresponders and per doubling of antibody responses (log base 2). RR<1 indicate that antibody responses are protective against symptomatic P. falciparum whereas RR>1 indicate susceptibility. aEstimates are calculated by authors from data in the paper; bdata supplied by original authors and calculated by current authors; cestimates are published estimates. All estimates are unadjusted with the exception of estimates from Nebie et al. (2008) and Dodoo et al. (2008), which are adjusted for age, and estimates from Stanisic (2009) are adjusted for age and spatial confounders [29],[44],[55]. W, weight. Note: Egan, 1996 had two study sites *Sierra-Leone and **The Gambia, and their analysis only included those with clinical disease versus asymptomatics, i.e., excluded those uninfected as they were assumed to be unexposed [36]. Data were obtained from a further three studies and pooled to examine the dose-response association of MSP-119 levels (log base 2) and the risk of malaria [29],[44],[55]. A 15% reduction in symptomatic P. falciparum per doubling of antibody levels was observed (reRR 0.85, 95% CI 0.74–0.97, p = 0.019; Figure 2). With only three studies in the meta-analysis, further subgroup analysis was not feasible. One additional study examined the association of antibody levels (excluded from meta-analysis because transformation, if any, was not stated in the original manuscript) with symptomatic P. falciparum and found weak evidence of a protective effect (RR 0.97, 95% CI 0.94–1.00, p = 0.0713) [56]. There was no conclusive evidence to support an association between anti-MSP-119 responses with P. falciparum high density infection or reinfection (see Text S2).
MSP-119 is made up of two epidermal growth factor-like modules (EGF-1 and EGF-2). Meta-analyses showed no association between the presence of responses to either MSP-1-EGF1 or MSP-1-EGF2 with protection against symptomatic P. falciparum (RR 1.06, 95% CI 0.88–1.26, p = 0.56 and reRR 0.59, 95% CI 0.19–1.84, p = 0.37; I2 = 71.4%, 95% CI 2.8–91.6%, respectively) [36],[41]. For individual study estimates see Text S2.
Only one study examined the association of MSP-142 levels (log base 2) with incidence risk of symptomatic P. falciparum and found a reduced risk (RR 0.76, 95% CI data not shown in original manuscript [DNS], p≤0.001) [49].
MSP-1 polymorphic N-terminal regions
MSP-1 block 2 can be grouped into three allelic types, K1-like, RO33-like, and MAD20 like. The association of incidence risk of symptomatic P. falciparum with allelic specific MSP-1 block 2 responders compared to nonresponders was examined in four studies [31],[40],[43],[59]. Pooled results were done separately for each allelic type. Meta-analysis revealed no evidence of an association with the K1-like (reRR 0.88, 95% CI 0.67, 1.17, p = 0.39) or RO33-like allele (RR 0.99, 95% CI 0.81, 1.21, p = 0.91) (Figure 3). There was weak evidence of a protective effect of MAD20-like responses with incidence risk of symptomatic P. falciparum (reRR 0.79, 95% CI 0.6, 1.04, p = 0.093; Figure 3).
Fig. 3. Forest plot of the association of MSP-1 block 2 and block 1 responses with incidence of symptomatic P. falciparum malaria. 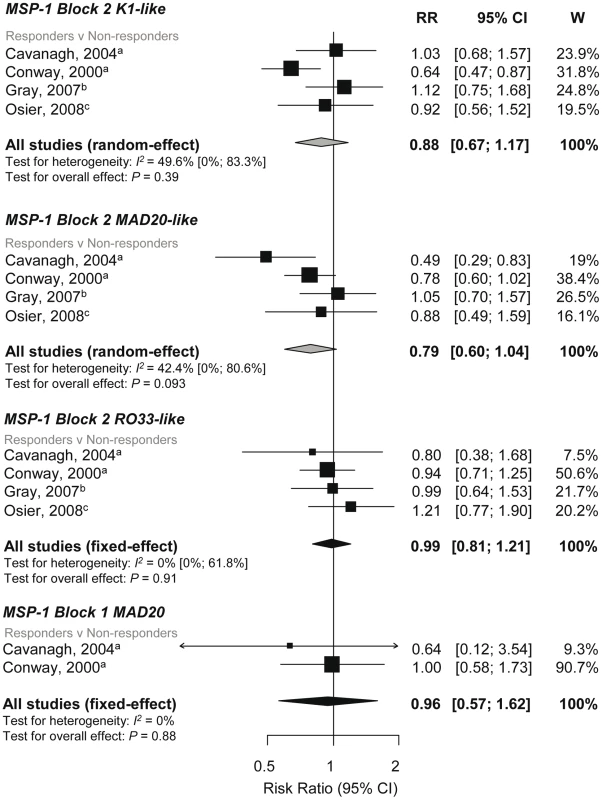
RRs represent the risk of symptomatic P. falciparum malaria in IgG responders relative to nonresponders. RR<1 indicate that responders are protected from symptomatic P. falciparum whereas RR>1 indicate susceptibility. aEstimates are published estimates; bestimates are calculated by authors from data in the paper; cdata supplied by original authors and calculated by current authors. All reported estimates are unadjusted. W, weight. The K1-like and MAD20-like types of MSP-1 block 2 differ in the length of tri-peptide repeats in the middle of the block as well as the flanking nonrepetitive sequences. Meta-analysis was performed on three studies investigating the association between responses to MSP-1 block 2 repeats and flanking regions (responders versus nonresponders) and incidence risk of symptomatic P. falciparum [32],[40],[43]. There was some evidence of an association for MSP-1 block 2 K1-like repeats (RR 0.72, 95% CI 0.54–0.97, p = 0.031) but not MSP-1 block 2 MAD20-like repeats (reRR 0.79, 95% CI 0.48–1.3, p = 0.35) (Figure 4). There was also no evidence of an association between MSP-1 block 2 flanking regions with risk of symptomatic P. falciparum (K1-like RR 0.87, 95% CI 0.66–1.14, p = 0.31 and MAD20-like reRR 0.84, 95% CI 0.52–1.34, p = 0.46; Figure 4).
Fig. 4. Forest plot of the association of MSP-1-block 2 repeats and flanking region responses with incidence of symptomatic P. falciparum malaria. 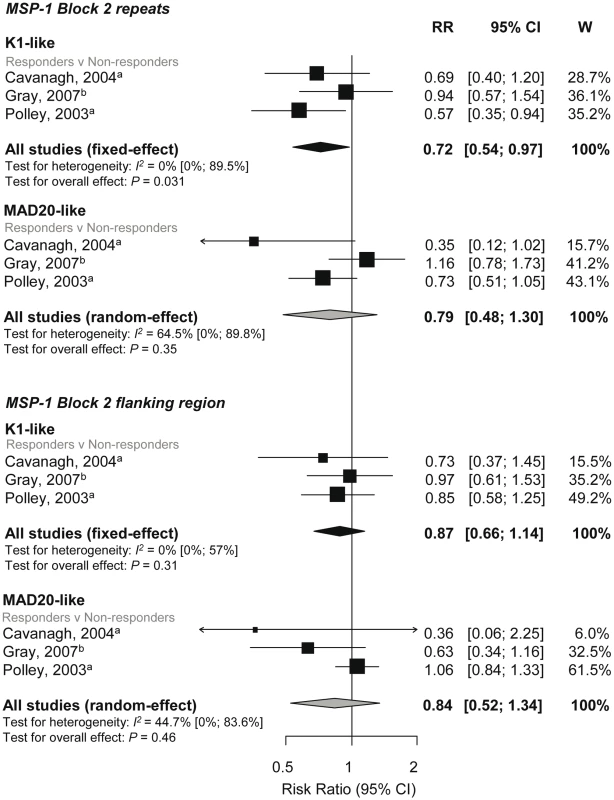
RRs represent the risk of symptomatic P. falciparum malaria in IgG responders relative to nonresponders. RR<1 indicate that responders are protected from symptomatic P. falciparum whereas RR>1 indicate susceptibility. aEstimates are published estimates; bestimates are calculated by authors from data in the paper. All reported estimates are unadjusted. W, weight. Combined results from two studies showed no evidence of an association of MSP-1 block 1 responses with risk of symptomatic falciparum malaria (responders versus nonresponders RR 0.96, 95% CI 0.57–1.62, p = 0.88; Figure 4) [31],[43].
Association between Anti-MSP-2 Responses and Incidence of P. falciparum Malaria
The single msp2 locus of P. falciparum is highly polymorphic but can be grouped into two major allelic types, 3D7 and FC27. Meta-analysis of six studies investigating MSP-23D7 and MSP-2FC27 showed no evidence of a reduced risk of symptomatic P. falciparum in those with responses compared to those without responses (MSP-23D7, reRR 0.92, 95% CI 0.75–1.13, p = 0.43; MSP-2FC27, reRR 0.82, 95% CI 0.62–1.08, p = 0.16, Figure 5) [33],[37],[46],[55],[58],[60]. Methodological and clinical characteristics of the studies did not influence estimates and there was no evidence of publication bias. One additional study found a dose-dependent response with MSP-23D7 antibody levels (log base 2) and risk of symptomatic P. falciparum (RR 0.81, 95% CI DNS, p = 0.003) but not MSP-2FC27 (RR 0.99, 95% CI DNS, p = 0.86) [48]. Another study examined the effect of MSP-2-Ct (responders versus nonresponders) and found no evidence of an association with symptomatic P. falciparum (RR 0.55, 95% CI 0.27, 1.14, p = 0.11) [33]. Only one study examined the association of MSP-2 antibodies with reinfection and high density infection and found no association (see Text S2) [55].
Fig. 5. Forest plot of the association of MSP-2 responses with incidence of symptomatic P. falciparum malaria. 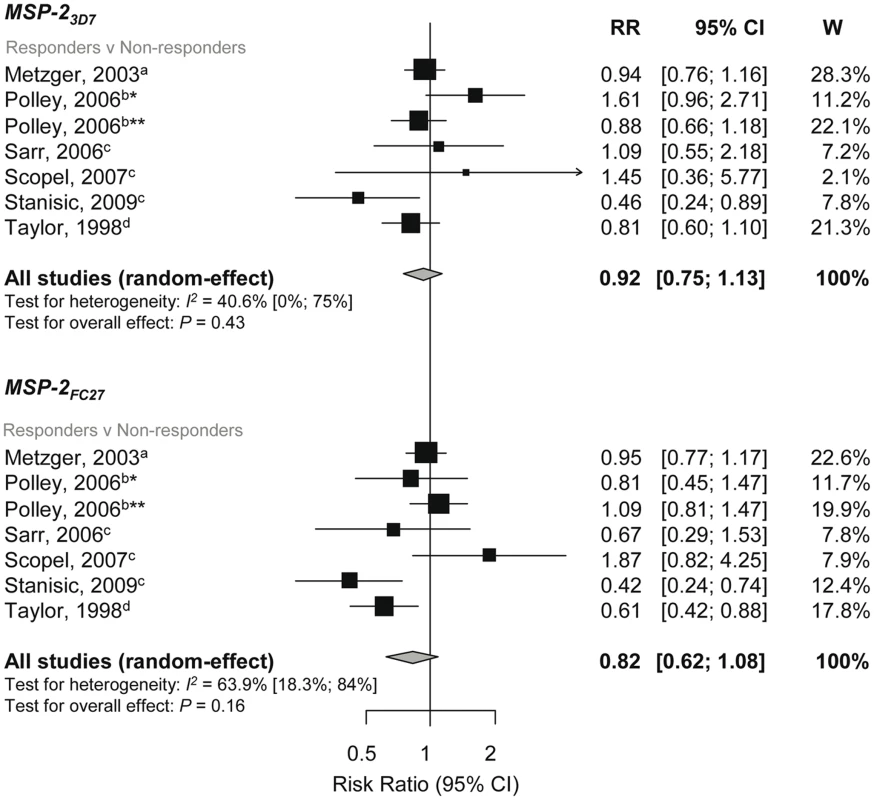
RR<1 indicate that responders are protected from symptomatic P. falciparum compared to nonresponders whereas RR>1 indicate susceptibility. aEstimates are published estimates; bconverted published estimate; cestimates are calculated by authors from data supplied by original author; destimates are calculated by authors from data in the paper. W, weight. Estimates reported are unadjusted with the exception of Stanisic (2009) (adjusted for spatial confounders and age) and Metzger (2003) (adjusted for age and preseason parasitaemia) [33],[55]. Note that estimates for Taylor (1998) are based on clinical and asymptomatic cases only (i.e., those uninfected were excluded on the basis they were unexposed) [37]. Polley (2006) stratified for two study sites in Coastal Kenya, *Chonyi and **Ngerenya [46]. Association between Anti-MSP-3 Responses and Incidence of P. falciparum Malaria
The C-terminal region of MSP-3 (MSP-3-Ct) is highly conserved whereas the remainder of the sequence is defined by two major allelic types, 3D7 and K1 [62]. Meta-analyses of four studies [28],[30],[34],[47] examining antibodies to MSP-3-Ct responses showed a 54% reduction in symptomatic P. falciparum in responders versus nonresponders (RR 0.46, 95% CI 0.32–0.67, p<0.0001; Figure 6). Meta-analyses of two studies also showed a decreased incidence risk per doubling of MSP-3-Ct antibody levels (RR 0.73, 95% CI 0.6–0.88, p = 0.001, Figure 6) [29],[44].
Fig. 6. Forest plot of the association of MSP-3 responses with incidence of symptomatic P. falciparum malaria. 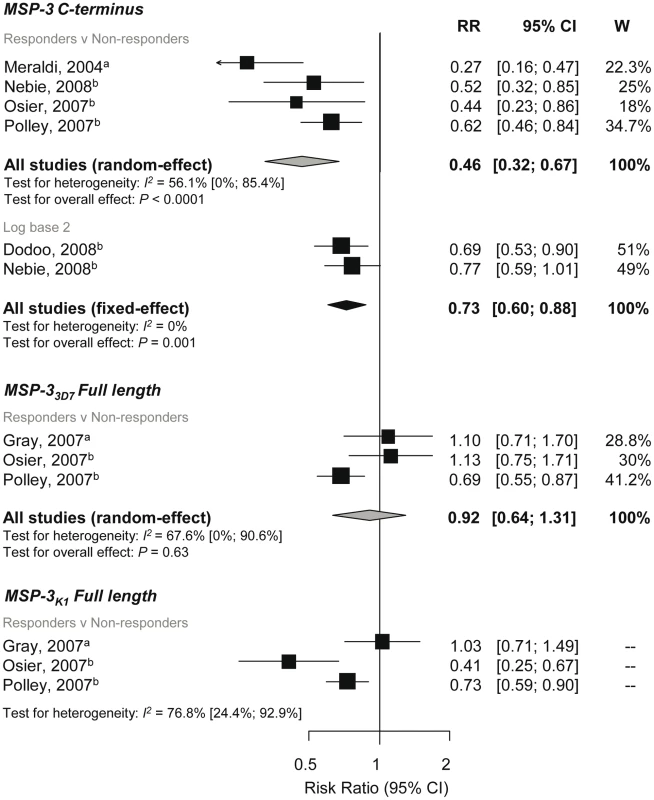
RR<1 indicate protection from symptomatic P. falciparum whereas RR>1 indicate susceptibility in responders versus nonresponders or per doubling of antibody responses. Estimates reported are unadjusted with the exception of Nebie (2008) (adjusted for age, sex, and village) [30] and Nebie (2008) and Dodoo (2008) (adjusted for age) [29],[44]. aEstimates are calculated by authors from data in the paper; bestimates are published estimates. All reported estimates are unadjusted. W, weight. Three studies examined the association of full length MSP-33D7 and MSP-3K1 responses (responders versus nonresponders) with risk of symptomatic P. falciparum [34],[40],[47]. Meta-analysis showed no evidence of an association with anti-MSP-33D7 responses (reRR 0.92, 95% CI 0.64–1.31, p = 0.63; Figure 6), but a large amount of heterogeneity was observed (I2 = 67.6%, 95% CI 0–90.6%). A high degree of heterogeneity was also seen for MSP-3K1 associations (I2 = 76.8%, 95% CI 24.4–92.9) so results were not combined (Figure 6). Due to the small number of studies in these meta-analyses, exploration of heterogeneity by subgroup analysis was not feasible.
Association between anti-AMA-1 Responses and Incidence of P. falciparum Malaria
There are currently two different AMA-1 strains of the full-length ectodomain under development as vaccine candidates (FVO and 3D7) [17]. There was evidence of reduced risk of symptomatic P. falciparum with AMA-13D7 responders versus nonresponders (RR 0.79, 95% CI 0.65–0.96, p = 0.015), and there was also a tendency towards a protective effect in the study that examined tertiles (Figure 7) [40],[45],[55]. For AMA-1FVO, one study showed a reduced risk of symptomatic P. falciparum in AMA-1FVO responders compared to nonresponders (RR 0.66 95% CI 0.52–0.84, p = 0.0007), but combined results of two studies showed no association of anti-AMA-1FVO levels (log base 2) with incidence risk of symptomatic P. falciparum (RR 0.99, 95% CI 0.9–1.08, p = 0.76; Figure 7) [29],[44],[45]. There was insufficient evidence to show an association between AMA-1 responses with risk of reinfection and high density P. falciparum (see Text S2).
Fig. 7. Forest plot of the association of AMA-1 responses with incidence of symptomatic P. falciparum malaria. 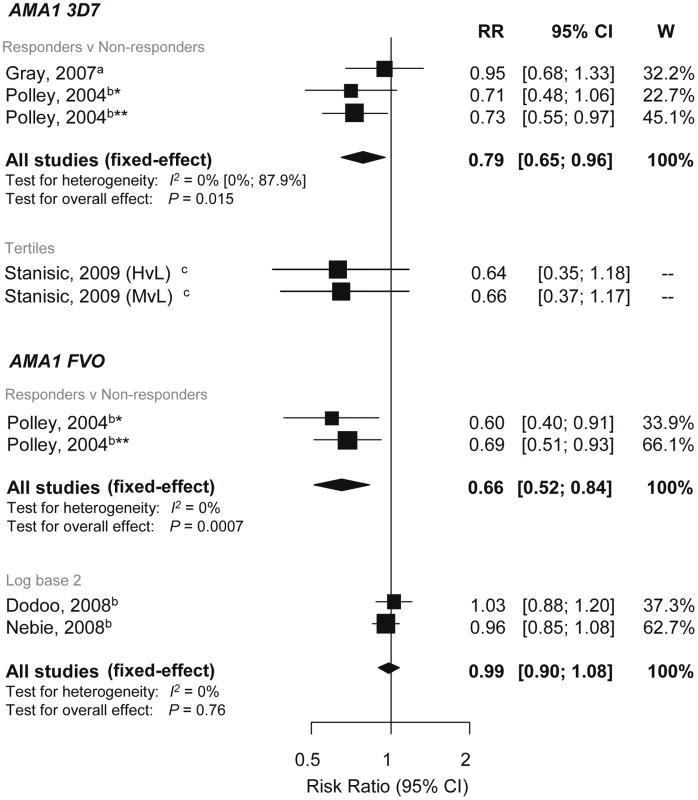
RRs correspond to risk of symptomatic P. falciparum malaria for AMA1 responders versus nonresponders, High (H) and medium (M) versus low (L) responders (based on tertiles because sero-prevalence was high) and per doubling of antibody responses (log base 2). RR<1 indicate that antibody responses are protective against symptomatic P. falciparum whereas RR>1 indicate susceptibility. aEstimates are calculated by authors from data in the paper; bestimates are published estimates; cestimates supplied by the original authors. All estimates are unadjusted with the exception of Dodoo (2008) and Nebie (2008) with adjustments for age and Stanisic (2009) with adjustments for age and spatial confounders [29],[44],[55]. Polley (2004) stratified for two study sites in Coastal Kenya, *Chonyi and **Ngerenya. Association between Anti-GLURP Responses and Incidence of P. falciparum Malaria
GLURP can be divided into an N-terminal nonrepeat region (R0), a central repeat region (R1), and a C-terminal repeat region (R2). A reduced risk of symptomatic P. falciparum was shown in GLURP-R0 responders compared to nonresponders (RR 0.69, 95% CI 0.48–0.97, p = 0.032) and per doubling of antibody levels (RR 0.79, 95% CI 0.69–0.91, p = 0.0006; Figure 8) [29],[30],[44]. Dodoo et al. (2000) also reported that anti-GLURP-R0 levels were associated with protection (p<0.005), but no estimates or 95% CI were given [42]. Conversely, Lusingu et al. (2005) reported no association with anti-GLURP-R0 responders with odds (RR were incalculable) of symptomatic episode (OR 1.13, 95% CI 0.5–2.53, p = 0.77) [51].
Fig. 8. Forest plot of the association of GLURP responses with incidence of symptomatic P. falciparum malaria. 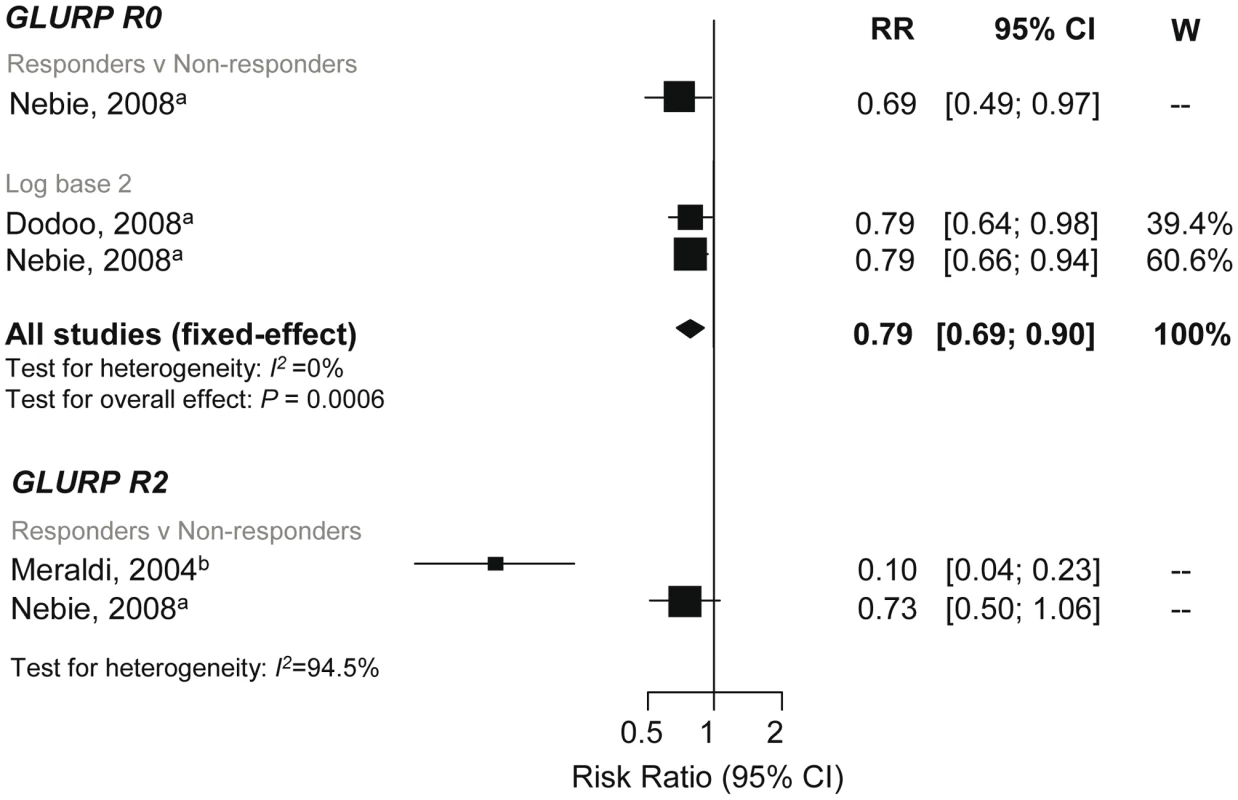
RRs correspond to risk of symptomatic P. falciparum malaria for GLURP responders versus nonresponders and per doubling of antibody responses (log base 2). RR<1 indicate that antibody responses are protective against symptomatic P. falciparum whereas RR>1 indicate susceptibility. aEstimates are published estimates with adjustments for age, Nebie (2008) responder versus nonresponder analysis also adjusted for sex and village [30]; bestimates are calculated by authors from data in the paper. GLURP-R2 estimates were not combined because I2>75%. W, weight. GLURP-R2 was associated with protection to varying degrees. Meraldi et al. and Nebie et al. showed a 90% (RR 0.1, 95% CI 0.05–0.23, p<0.001) and 27% (RR 0.73, 95% CI 0.5–1.06, p = 0.1; Figure 8) reduction in symptomatic malaria in responders versus nonresponders [28],[30]. Estimates from these two studies were not combined (I2 = 94.5%). Another study found no evidence of an association between anti-GLURP-R2 (p = 0.2) or GLURP-R1 (p = 0.3) levels (estimates and 95% CI, DNS) [42]. One study examined the association of GLURP-R1-R2 with malaria, which showed a reduced risk of symptomatic P. falciparum (RR 0.73, 95% CI 0.55–0.97, p = 0.03) [35].
Association between Other Responses and Incidence of P. falciparum Malaria
Only three studies meeting our inclusion and quality criteria measured anti-EBA-175 responses [38],[53],[59]. Osier et al. (2008) (used recombinant F2 domain) and Okenu et al. (2000) (used recombinant region II) showed no association of anti-EBA-175 antibodies with risk of symptomatic P. falciparum (responders versus nonresponders, RR 1.36, 95% CI 0.81–2.3, p = 0.246 for Osier et al.; RR 0.96, 95% CI 0.71–1.29, p = 0.77 for Okenu et al.). John et al. (2005) showed no association between antibodies (to region II) and risk of reinfection (>75 percentile versus <75% percentile 1.25, 95% CI 0.66–2.36, p = 0.49). One study investigated the relationship between MSP-4 and MSP-4-EGF1 with risk of reinfection and showed no association (RR 0.9, 95% CI 0.58–1.39, p = 0.64 and RR 0.8, 95% CI 0.56–1.15, p = 0.22, respectively) [57].
Discussion
This systematic review strongly supports the protective effect of total IgG responses to particular merozoite surface antigens against symptomatic P. falciparum malaria in humans. Meta-analyses showed that individuals who have IgG to MSP-3-Ct and MSP-119 have a risk of symptomatic P. falciparum that is 54% and 18%, respectively, less than those without detectable IgG. Moreover, there was evidence of a dose-response relationship such that the magnitude of association with these antigens increased per doubling of antibody levels. A tendency towards protective RR was also observed when individual estimates for AMA-13D7 and GLURP-R0 were examined, but pooled estimates of more than two studies could not be determined owing to heterogeneity among studies. Pooled estimates showed limited evidence of a protective effect of IgG responses towards MSP-2, MSP-1 N-terminal region, or MSP-1-EGF subregion with symptomatic P. falciparum malaria. Importantly, this systematic review revealed a paucity of studies examining the association of IgG responses towards the vaccine candidates MSP-142 and EBA-175 with incidence of P. falciparum malaria.
Heterogeneity, in terms of both clinical and methodological diversity between studies, was an important issue in the meta-analyses. Clinical heterogeneity was noted in MSP-119 meta-analyses whereby the magnitude of effect varied with allelic group. However, given that MSP-119 is relatively conserved and that the different alleles are based on four amino acid changes, the biological relevance of this observation is unknown. Methodological heterogeneity was most evident across studies investigating AMA-1 and GLURP responses. Antibody variables were defined differently across studies and estimates with errors and/or raw data were not presented. Subsequently the standardization of antibody variables and pooling of results was problematic. Statistical heterogeneity (I2 value) was greatest for GLURP R2 and the full length MSP-3 antigen meta-analyses.
There are many factors influencing the selection of antigens for vaccine development and testing in clinical trials, and evidence from observational studies can provide valuable knowledge to inform this process. MSP-119 was the most featured merozoite surface antigen and meta-analyses showed that antibody responses to MSP-119 were indicative of protection. It is thus surprising that MSP-119 has only featured in one vaccine in humans, in which it was used in combination with AMA-1 (PfCP2.9/ISA720) in phase I trials [63],[64]. Conversely, only one study has demonstrated evidence of protection for antibodies to MSP-142, but this antigen has been tested in a phase II vaccine trial where it was not protective [65]. The reasons for the failure of this vaccine remain unclear, but may relate to antigen polymorphism or the nature of the vaccine-induced response, or instead may indicate that MSP-142 antibodies are not protective. Further studies of this antigen are clearly needed. Other merozoite surface antigens currently undergoing phase II trials in malaria endemic countries include AMA-1 (AMA-C1, which includes 3D7 and FVO strains) and MSP-3 (as a long synthetic peptide and a MSP-3/GLURP chimera), which were shown to be protective against symptomatic malaria in this review [17]. There are currently no vaccines with MSP-1-block 1 and 2 proteins, and data from this systematic review does not support the development of these antigens as vaccine targets.
The aim of this systematic review was to be as comprehensive and inclusive as possible and fulfil guidelines for meta-analyses [19]. We performed an extensive search of six different databases and did not limit our searches by language to remove the potential for bias due to exclusion of non-English studies [66]. Furthermore, we identified and contacted the investigators for the studies that did not meet our initial inclusion and quality criteria but contained potential data. In addition, instead of excluding studies that did not provide estimates, we contacted authors and asked them to provide estimates or data. We did not limit our review to IgG subclasses as it would substantially decrease the number of studies included. Examining subclass-specific responses to merozoite antigens has provided further insights into protective targets and mechanisms of acquired immunity [55],[67]. However, differences in the specificity and sensitivity of subclass-typing reagents between studies makes comparisons between studies difficult. We also assessed publication bias where possible, although in some cases where only a few studies were combined this assessment was difficult. The extent to which the selective publication of studies based on the direction and magnitude of findings within malaria epidemiological research is unknown. The publication of all studies regardless of findings should be encouraged.
Determining a causal relationship between antibodies and protection against P. falciparum malaria is one of the main challenges in malaria immuno-epidemiology. Study designs used in the published literature include cross-sectional studies, case-control studies, and cohort studies. To ensure the best inference of causality from the published literature we did two things. Firstly, we only included studies that examined the association of antibodies with prospectively collected P. falciparum data to establish a temporal relationship between antibody responses and risk of P. falciparum malaria. Secondly, we included a parasitaemia density cut-off in our definition of symptomatic malaria as part of our quality criteria to improve specificity and ensure that P. falciparum was the causative agent of the febrile episode. The prevailing view in the field is that a cut-off level of parasitaemia is needed to improve the specificity of clinical malaria diagnosis in most populations [68]–[70]. The population-specific definitions of a high P. falciparum density cut-off in the studies ranged from >300 parasites/µl to >5,000 parasites/µl and the sensitivity of these definitions would vary across populations. In addition, we would expect reduced specificity of the definition for the one study that reanalysed data with a high density cut-off for inclusion in our review [58].
A causal relationship between anti-merozoite antibodies and P. falciparum malaria is strengthened by the consistent demonstration of findings under different circumstances. Consistent findings were demonstrated for some antigens despite differences in the preparation of antigens, malaria endemicity, study participants, and study area. Interestingly, we found very few published studies that were performed outside Africa. Of the 32 included studies, only one was performed in Asia (excluding Papua New Guinea) and only one in South America (see Table 1). The generalizability of our findings to populations living in these less-represented regions is unknown. Additionally, we only identified two studies that investigated allele-specific immunity (both studies MSP-2 only), whereby the allele-specific antibody response was related to the strain causing the malaria episode [55],[58]. If protection is purely allele-specific then the true causal protective effect will be underestimated in studies that do not use allele-specific P. falciparum outcomes.
Another important limitation in published literature is that data generated by ELISA does not produce a common metric measurement thereby restricting the standardization of exposure variables. In meta-analyses we were able to pool RR for responders versus nonresponders and RR derived from log base 2 antibody levels, which represent the change in risk per doubling of antibody levels. However, antibody concentrations vary across populations according to the level of exposure to malaria. Therefore the magnitude of effect according to quantified responses may vary significantly across studies. This was evident by the dose-dependent relationships between some antibody responses and level of protection and would suggest that antibody responses need to be quantified. Furthermore, knowledge on how long specific merozoite antibody responses last, how they are boosted, and the duration of any protection from responses is presently limited. The duration of the follow up in observational studies may therefore have an impact on the strength and direction of an association, an effect we explored in meta-regression. Further studies that measure responses at multiple time points are needed to better understand these issues.
The definition of “protected” individuals (i.e., those who did not have symptomatic malaria) varied across studies. For most studies this definition included all participants who had no recorded episodes of symptomatic P. falciparum malaria. Three studies excluded individuals who did not have any detected parasitaemia during follow-up from the “protected” group, on the basis that these individuals were unexposed [35]–[37]. Only the six treatment-to-reinfection studies had regular blood collection for detection of parasitaemia; all other included studies only collected blood slides during follow-up when an individual was febrile, so accurately determining true “unexposed” individuals in areas where asymptomatic parasitaemia is prevalent will be problematic. Recent analyses by Bejon et al. (2009) of anti-VSA antibodies in individuals living in Kilifi, Kenya, showed that by removing unexposed children from conventional analyses, the magnitude of effect was greater between those with high and low responses [71]. This is consistent with other studies in Kilifi that showed that associations between specific merozoite antibody responses and protection were stronger in children who were asymptomatic at baseline [45],[46],[59]. A further consideration is that studies in malaria-endemic areas typically compare individuals with different levels of immunity, not individuals with complete immunity versus individuals with no immunity. Therefore, the reported effect size may not accurately reflect the true magnitude of the response in the study population.
Conclusion and Guidelines for Future Research
IgG responses to some, but not all, merozoite surface antigens were associated with protection against symptomatic P. falciparum in malaria endemic areas. We identified very few antigens that had been well studied and a deficiency of studies done outside Africa. More studies in different populations, examining multiple antigens at multiple time-points, are needed to better determine the role of anti-merozoite antibodies in protection against malaria, with prospective cohort studies as the preferred study design to establish temporal causality. In the future, there should be as much uniformity between studies as possible to ensure maximum comparability. This could be improved by the quantification and standardization of IgG responses, which could be achieved by establishing a reference reagent for determining antibody concentrations. Furthermore, the protective effects of anti-merozoite responses observed epidemiologically must also be supported by evidence of the function of the antibodies. Development and application of functional assays rather than standard immunoassays would also be highly valuable. Presently, data on the function of antibodies against merozoite antigens is very limited [8],[12],[15],[72]. Lastly, there is a need to incorporate strain-specific responses and endpoints to address whether protective responses against particular antigens are strain-transcending or strain-specific.
A challenging aspect of this systematic review was the standardization of exposure and outcome measurements as there is no consistent approach to reporting of data. To facilitate the standardization of results in future studies, we propose guidelines for the reporting of malaria immuno-epidemiology studies adapted from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Table 3) [73],[74]. Standardizing studies, and removing as much methodological heterogeneity as possible, will help obtain more comparable results in the future. By doing so, we will then be in a more favourable position to assess the relative contribution of responses to certain antigens, thereby informing vaccine candidate choices.
Tab. 3. Proposed guidelines of the reporting of Malaria Immuno-epidemiology Observational Studies (MIOS guidelines). 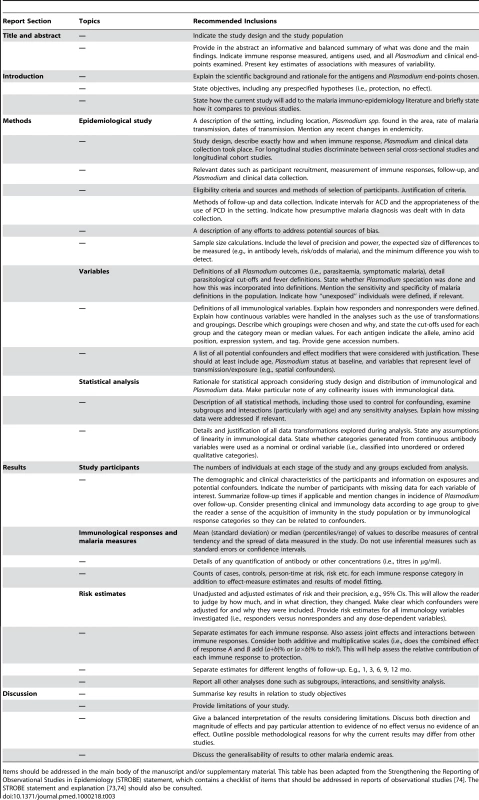
Items should be addressed in the main body of the manuscript and/or supplementary material. This table has been adapted from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement, which contains a checklist of items that should be addressed in reports of observational studies [74]. The STROBE statement and explanation [73],[74] should also be consulted. Supporting Information
Zdroje
1. MarshK
KinyanjuiS
2006 Immune effector mechanisms in malaria. Parasite Immunol 28 51 60
2. CohenS
McGI
CarringtonS
1961 Gamma-globulin and acquired immunity to human malaria. Nature 192 733 737
3. SabchareonA
BurnoufT
OuattaraD
AttanathP
Bouharoun-TayounH
1991 Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45 297 308
4. CohenS
ButcherGA
CrandallRB
1969 Action of malarial antibody in vitro. Nature 223 368 371
5. BrownGV
AndersRF
MitchellGF
HeywoodPF
1982 Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. Nature 297 591 593
6. McCallumFJ
PerssonKE
MugyenyiCK
FowkesFJ
SimpsonJA
2008 Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS One 3 e3571 doi:10.1371/journal.pone.0003571
7. Bouharoun-TayounH
AttanathP
SabchareonA
ChongsuphajaisiddhiT
DruilheP
1990 Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 172 1633 1641
8. HodderAN
CrewtherPE
AndersRF
2001 Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun 69 3286 3294
9. GaurD
MayerDC
MillerLH
2004 Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol 34 1413 1429
10. O'DonnellRA
de Koning-WardTF
BurtRA
BockarieM
ReederJC
2001 Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J Exp Med 193 1403 1412
11. ClarkJT
DonachieS
AnandR
WilsonCF
HeidrichHG
1989 46–53 kilodalton glycoprotein from the surface of Plasmodium falciparum merozoites. Mol Biochem Parasitol 32 15 24
12. OeuvrayC
Bouharoun-TayounH
Gras-MasseH
BottiusE
KaidohT
1994 Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84 1594 1602
13. TheisenM
SoeS
OeuvrayC
ThomasAW
VuustJ
1998 The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun 66 11 17
14. DuttaS
HaynesJD
MochJK
BarbosaA
LanarDE
2003 Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites. Proc Natl Acad Sci USA 100 12295 12300
15. PerssonKE
McCallumFJ
ReilingL
ListerNA
StubbsJ
2008 Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest 118 342 351
16. CowmanAF
CrabbBS
2006 Invasion of red blood cells by malaria parasites. Cell 124 755 766
17. RichardsJS
BeesonJG
2009 The future for blood-stage vaccines against malaria. Immunol Cell Biol 87 377 390
18. GuptaS
SnowRW
DonnellyCA
MarshK
NewboldC
1999 Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5 340 343
19. StroupDF
BerlinJA
MortonSC
OlkinI
WilliamsonGD
2000 Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283 2008 2012
20. World Health Organization 1990 Severe and complicated malaria. World Health Organization, Division of Control of Tropical Diseases. Trans R Soc Trop Med Hyg 84 Suppl 2 1 65
21. World Health Organization 2000 Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg 94 Suppl 1 S1 S90
22. EggerM
SmithGD
PhillipsAN
1997 Meta-analysis: principles and procedures. BMJ 315 1533 1537
23. ZhangJ
YuKF
1998 What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280 1690 1691
24. HigginsJP
ThompsonSG
DeeksJJ
AltmanDG
2003 Measuring inconsistency in meta-analyses. BMJ 327 557 560
25. IoannidisJP
PatsopoulosNA
EvangelouE
2007 Uncertainty in heterogeneity estimates in meta-analyses. BMJ 335 914 916
26. SterneJA
EggerM
2001 Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54 1046 1055
27. EggerM
Davey SmithG
SchneiderM
MinderC
1997 Bias in meta-analysis detected by a simple, graphical test. BMJ 315 629 634
28. MeraldiV
NebieI
TionoAB
DialloD
SanogoE
2004 Natural antibody response to Plasmodium falciparum Exp-1, MSP-3 and GLURP long synthetic peptides and association with protection. Parasite Immunol 26 265 272
29. NebieI
DiarraA
OuedraogoA
SoulamaI
BougoumaEC
2008 Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun 76 759 766
30. NebieI
TionoAB
DialloDA
SamandoulougouS
DiarraA
2008 Do antibody responses to malaria vaccine candidates influenced by the level of malaria transmission protect from malaria? Trop Med Int Health 13 229 237
31. ConwayDJ
CavanaghDR
TanabeK
RoperC
MikesZS
2000 A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat Med 6 689 692
32. PolleySD
TettehKK
CavanaghDR
PearceRJ
LloydJM
2003 Repeat sequences in block 2 of Plasmodium falciparum merozoite surface protein 1 are targets of antibodies associated with protection from malaria. Infect Immun 71 1833 1842
33. MetzgerWG
OkenuDM
CavanaghDR
RobinsonJV
BojangKA
2003 Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol 25 307 312
34. PolleySD
TettehKK
LloydJM
AkpoghenetaOJ
GreenwoodBM
2007 Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J Infect Dis 195 279 287
35. DziegielM
RoweP
BennettS
AllenSJ
OlerupO
1993 Immunoglobulin M and G antibody responses to Plasmodium falciparum glutamate-rich protein: correlation with clinical immunity in Gambian children. Infect Immun 61 103 108
36. EganAF
MorrisJ
BarnishG
AllenS
GreenwoodBM
1996 Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis 173 765 769
37. TaylorRR
AllenSJ
GreenwoodBM
RileyEM
1998 IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg 58 406 413
38. OkenuDM
RileyEM
BickleQD
AgomoPU
BarbosaA
2000 Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect Immun 68 5559 5566
39. OkechBA
CorranPH
ToddJ
Joynson-HicksA
UthaipibullC
2004 Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect Immun 72 1557 1567
40. GrayJC
CorranPH
MangiaE
GauntMW
LiQ
2007 Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin Chem 53 1244 1253
41. DodooD
TheanderTG
KurtzhalsJA
KoramK
RileyE
1999 Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect Immun 67 2131 2137
42. DodooD
TheisenM
KurtzhalsJA
AkanmoriBD
KoramKA
2000 Naturally acquired antibodies to the glutamate-rich protein are associated with protection against Plasmodium falciparum malaria. J Infect Dis 181 1202 1205
43. CavanaghDR
DodooD
HviidL
KurtzhalsJA
TheanderTG
2004 Antibodies to the N-terminal block 2 of Plasmodium falciparum merozoite surface protein 1 are associated with protection against clinical malaria. Infect Immun 72 6492 6502
44. DodooD
AikinsA
Asamoah KusiK
LampteyH
RemarqueE
2008 Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J 7 142
45. PolleySD
MwangiT
KockenCH
ThomasAW
DuttaS
2004 Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23 718 728
46. PolleySD
ConwayDJ
CavanaghDR
McBrideJS
LoweBS
2006 High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine 24 4233 4246
47. OsierFH
PolleySD
MwangiT
LoweB
ConwayDJ
2007 Naturally acquired antibodies to polymorphic and conserved epitopes of Plasmodium falciparum merozoite surface protein 3. Parasite Immunol 29 387 394
48. Al-YamanF
GentonB
AndersR
TaraikaJ
GinnyM
1995 Assessment of the role of the humoral response to Plasmodium falciparum MSP2 compared to RESA and SPf66 in protecting Papua New Guinean children from clinical malaria. Parasite Immunol 17 493 501
49. Al-YamanF
GentonB
KramerK
ChangS
HuiG
1996 Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papa New Guinean children from malaria morbidity. Am J Trop Med Hyg 54 443 448
50. PerrautR
MarramaL
DioufB
SokhnaC
TallA
2005 Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J Infect Dis 191 264 271
51. LusinguJP
VestergaardLS
AlifrangisM
MmbandoBP
TheisenM
2005 Cytophilic antibodies to Plasmodium falciparum glutamate rich protein are associated with malaria protection in an area of holoendemic transmission. Malar J 4 48
52. JohnCC
O'DonnellRA
SumbaPO
MoormannAM
de Koning-WardTF
2004 Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-1 19) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J Immunol 173 666 672
53. JohnCC
MoormannAM
PregibonDC
SumbaPO
McHughMM
2005 Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg 73 222 228
54. TolleR
FruhK
DoumboO
KoitaO
N'DiayeM
1993 A prospective study of the association between the human humoral immune response to Plasmodium falciparum blood stage antigen gp190 and control of malarial infections. Infect Immun 61 40 47
55. StanisicDI
RichardsJS
McCallumFJ
MichonP
KingCL
2009 IgG subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 77 1165 1174
56. PerrautR
MarramaL
DioufB
FontenilleD
TallA
2003 Distinct surrogate markers for protection against Plasmodium falciparum infection and clinical malaria identified in a Senegalese community after radical drug cure. J Infect Dis 188 1940 1950
57. WangL
RichieTL
StowersA
NhanDH
CoppelRL
2001 Naturally acquired antibody responses to Plasmodium falciparum merozoite surface protein 4 in a population living in an area of endemicity in Vietnam. Infect Immun 69 4390 4397
58. ScopelKK
da Silva-NunesM
MalafronteRS
BragaEM
FerreiraMU
2007 Variant-specific antibodies to merozoite surface protein 2 and clinical expression of Plasmodium falciparum malaria in rural Amazonians. Am J Trop Med Hyg 76 1084 1091
59. OsierFH
FeganG
PolleySD
MurungiL
VerraF
2008 Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 76 2240 2248
60. SarrJB
PelleauS
TolyC
GuitardJ
KonateL
2006 Impact of red blood cell polymorphisms on the antibody response to Plasmodium falciparum in Senegal. Microbes Infect 8 1260 1268
61. KoussisK
Withers-MartinezC
YeohS
ChildM
HackettF
2009 A multifunctional serine protease primes the malaria parasite for red blood cell invasion. Embo J 28 725 735
62. HuberW
FelgerI
MatileH
LippsHJ
SteigerS
1997 Limited sequence polymorphism in the Plasmodium falciparum merozoite surface protein 3. Mol Biochem Parasitol 87 231 234
63. HuJ
ChenZ
GuJ
WanM
ShenQ
2008 Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults. PLoS One 3 e1952 doi:10.1371/journal.pone.0001952
64. MalkinE
HuJ
LiZ
ChenZ
BiX
2008 A phase 1 trial of PfCP2.9: an AMA1/MSP1 chimeric recombinant protein vaccine for Plasmodium falciparum malaria. Vaccine 26 6864 6873
65. OgutuBR
ApolloOJ
McKinneyD
OkothW
SianglaJ
2009 Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE 4 e4708 doi:10.1371/journal.pone.0004708
66. MoherD
FortinP
JadadAR
JuniP
KlassenT
1996 Completeness of reporting of trials published in languages other than English: implications for conduct and reporting of systematic reviews. Lancet 347 363 366
67. RoussilhonC
OeuvrayC
Muller-GrafC
TallA
RogierC
2007 Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med 4 e320 doi:10.1371/journal.pmed.0040320
68. MabundaS
AponteJJ
TiagoA
AlonsoP
2009 A country-wide malaria survey in Mozambique. II. Malaria attributable proportion of fever and establishment of malaria case definition in children across different epidemiological settings. Malar J 8 74
69. MwangiTW
RossA
SnowRW
MarshK
2005 Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis 191 1932 1939
70. SmithT
SchellenbergJA
HayesR
1994 Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med 13 2345 2358
71. BejonP
WarimweG
MackintoshCL
MackinnonMJ
KinyanjuiSM
2009 Analysis of immunity to febrile malaria in children that distinguishes immunity from lack of exposure. Infect Immun 77 1917 1923
72. EganAF
BurghausP
DruilheP
HolderAA
RileyEM
1999 Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol 21 133 139
73. VandenbrouckeJP
von ElmE
AltmanDG
GotzschePC
MulrowCD
2007 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 147 W163 194
74. von ElmE
AltmanDG
EggerM
PocockSJ
GotzschePC
2007 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370 1453 1457
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Quantifying the Number of Pregnancies at Risk of Malaria in 2007: A Demographic Study
- The Global Health System: Actors, Norms, and Expectations in Transition
- Microscopy Quality Control in Médecins Sans Frontières Programs in Resource-Limited Settings
- The Global Health System: Strengthening National Health Systems as the Next Step for Global Progress
- Meeting the Demand for Results and Accountability: A Call for Action on Health Data from Eight Global Health Agencies
- Relationship between Vehicle Emissions Laws and Incidence of Suicide by Motor Vehicle Exhaust Gas in Australia, 2001–06: An Ecological Analysis
- The Global Health System: Lessons for a Stronger Institutional Framework
- Geographic Distribution of Causing Invasive Infections in Europe: A Molecular-Epidemiological Analysis
- The Global Health System: Linking Knowledge with Action—Learning from Malaria
- The Relationship between Anti-merozoite Antibodies and Incidence of Malaria: A Systematic Review and Meta-analysis
- Neonatal Circumcision for HIV Prevention: Cost, Culture, and Behavioral Considerations
- “Working the System”—British American Tobacco's Influence on the European Union Treaty and Its Implications for Policy: An Analysis of Internal Tobacco Industry Documents
- The Evolution of the Epidemic of Charcoal-Burning Suicide in Taiwan: A Spatial and Temporal Analysis
- Mapping the Distribution of Invasive across Europe
- Science Must Be Responsible to Society, Not to Politics
- Are Patents Impeding Medical Care and Innovation?
- Male Circumcision at Different Ages in Rwanda: A Cost-Effectiveness Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Evolution of the Epidemic of Charcoal-Burning Suicide in Taiwan: A Spatial and Temporal Analysis
- Male Circumcision at Different Ages in Rwanda: A Cost-Effectiveness Study
- Geographic Distribution of Causing Invasive Infections in Europe: A Molecular-Epidemiological Analysis
- “Working the System”—British American Tobacco's Influence on the European Union Treaty and Its Implications for Policy: An Analysis of Internal Tobacco Industry Documents
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



