-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaProgress in Vaccination against type b in the Americas
article has not abstract
Published in the journal: . PLoS Med 5(4): e87. doi:10.1371/journal.pmed.0050087
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.0050087Summary
article has not abstract
Worldwide, Haemophilus influenzae type b (Hib) causes at least 3 million cases of severe disease each year. Approximately 400,000 children die annually due to pneumonia or meningitis caused by Hib [1]. Severe neurological sequelae occur in 15% to 30% of those who survive Hib meningitis [2]. Other, less frequent, manifestations of Hib are epiglottitis, septic arthritis, osteomyelitis, and septicemia [1–3].
Current polysaccharide–protein conjugate Hib vaccines are highly efficacious and safe. Primary series of two or three doses protect approximately 95% of infants [4–8]. Universal infant Hib immunization has proven to dramatically reduce Hib invasive disease [9–13] through direct vaccine protection and an important herd effect related to the reduction in Hib nasopharyngeal carriage in the community [12,14–16]. Adverse events are rare, and Hib vaccine is contraindicated only for persons with hypersensitivity to any of the vaccine's components [8,17].
The World Health Organization (WHO) recommends the introduction of Hib vaccines worldwide [1]. Furthermore, the Global Alliance for Vaccines and Immunization (GAVI Alliance)—an organization that aligns public and private resources in a global effort to create greater access to the benefits of immunization—considers Hib vaccine introduction in the world's poorest countries to be a top priority [18].
Prior to vaccine introduction, an estimated 20,000 cases of Hib meningitis were estimated to occur in countries of Latin America and the Caribbean (LAC) annually, based on an overall Hib meningitis incidence of 35 per 100,000 children aged 0–4 years [19]. Another 20,000 cases of invasive Hib disease were estimated to occur in the United States annually [20]. Most cases occurred in children aged less than 24 months, with at least 60% of cases occurring in children aged 0–11 months in half of the studies. The annual mortality rate for Hib meningitis in children aged less than five years was estimated to be around two per 100,000 in the Western Hemisphere [21].
Summary Points
-
Hib vaccine introduction in countries of Latin America and the Caribbean has substantially reduced morbidity and mortality due to invasive Hib infections.
-
All Latin American and Caribbean countries but one include Hib vaccine in their routine immunization schedule for infants.
-
Factors that favored this region-wide Hib vaccine adoption include strong political will, data supporting Hib disease burden and impact in early adopting countries, and experience exchange among countries.
-
Financial sustainability when introducing the more expensive Hib vaccine was critical for ensuring successful vaccine introduction in all countries.
-
Efforts are still needed to improve vaccination coverage and to strengthen invasive bacterial disease surveillance in developing countries.
In this article, we review the progress of vaccine introduction, lessons learned, and remaining challenges regarding Hib vaccination in the Americas, with emphasis on LAC countries. We expect that this updated and summarized information on Hib vaccination will serve as a useful reference to public health officials and policy makers from other regions who face the challenge of introducing Hib and other new or underutilized vaccines.
Methods
To assess the progress accomplished by LAC countries in Hib vaccination and the remaining challenges, we reviewed the Pan American Health Organization (PAHO)'s Technical Advisory Group (TAG) on Vaccine-preventable Diseases recommendations; annual country immunization reports to PAHO; Hib-containing vaccine purchase records from PAHO's Revolving Fund; and Hib isolation data from selected countries participating in a Regional Laboratory Network for Surveillance of Bacterial Meningitis and Pneumonia (Sistema Regional de Vacunas, or SIREVA). We examined data from 1997 to 2006.
PAHO is an international public health agency with more than 100 years of experience in working to improve health and living standards of the countries of the Americas. PAHO serves as the WHO Regional Office for the Americas, and provides technical assistance to all countries and territories in the Western Hemisphere. However, most of PAHO's technical cooperation is targeted to the poorer countries of LAC [22]. PAHO's TAG is comprised of eight immunization and vaccine experts who meet biennially, in the presence of Member States' immunization representatives, to provide recommendations on vaccination policy and strategies to improve countries' vaccination efforts [22].
Since the 1980s, Member States submit annual immunization reports to PAHO, using a standardized form, the PAHO-WHO/UNICEF Joint Reporting Form (previously known as EPI tables). This form includes data on:
-
all routine vaccines included in country immunization schedules;
-
vaccine presentation;
-
annual administrative coverage rates (for Hib, number of third doses of Hib vaccine given divided by the number of children aged less than one year);
-
surveillance data (i.e., number of Hib meningitis cases);
-
immunization financing indicators, such as the percentage of all routine vaccine expenditures financed with government funds (this excludes any external financing from donors, but may include loans);
-
existence of a budget line for vaccine purchase; and
-
existence of immunization-related legislation.
The Revolving Fund (RF) is the mechanism for bulk purchase of vaccines and immunization supplies, managed by PAHO, since 1979, to serve Member States. PAHO annually consolidates vaccine orders from countries that wish to participate and conducts an international bid open to all vaccine manufacturers. PAHO procures vaccines with money drawn from the RF, and countries reimburse the RF for purchases made on their behalf [23,24].
SIREVA was started in 1993 to monitor the distribution of pneumococcal serotypes causing severe disease and the antimicrobial resistance patterns of pneumococci in six countries. Subsequently, SIREVA expanded its mandate to conduct laboratory surveillance for other agents that cause meningitis and pneumonia, such as Hib and meningococcus.
PAHO Recommendations Regarding Hib Immunization
To control Hib invasive disease and reduce Hib carriers in the Americas, PAHO's TAG recommends, since 1997, that Member States:
-
introduce Hib vaccine into the routine universal infant immunization schedule;
-
establish sustainable financial mechanisms to maintain vaccination against Hib; and
-
monitor and report vaccination coverage and Hib cases in order to evaluate the impact of the intervention.
To help ensure sustainability of Hib vaccination, TAG has also recommended purchasing Hib vaccine in combination with diphtheria-tetanuspertussis vaccine (DTP) or DTP/hepatitis B vaccine through the RF, as this can result in significant cost savings.
Progress in Hib Vaccine Introduction
By the end of 2006, all countries of the Americas, except Haiti, had included Hib in their infant immunization schedule (Table 1). As a result, over 98% of the 16 million children born annually in the Americas live in countries routinely using Hib-containing vaccines. Canada (1986) and the United States (1991) were among the first countries to introduce Hib vaccine. Bermuda (1989), the Cayman Islands (1992), and the Dutch territories (1995) were first in the Caribbean. Uruguay and Chile followed in 1994 and 1996, respectively. Uruguay conducted an aggressive catch-up introduction of Hib vaccine for all children aged one to four years, whereas Chile started vaccinating one birth cohort at a time [11,25]. Argentina followed in 1997. However, most countries and territories (n = 20) introduced Hib vaccine between 1998 and 2000, more than ten years after the vaccine was first licensed in 1987. In 2006, Dominica was the last country to introduce Hib vaccine.
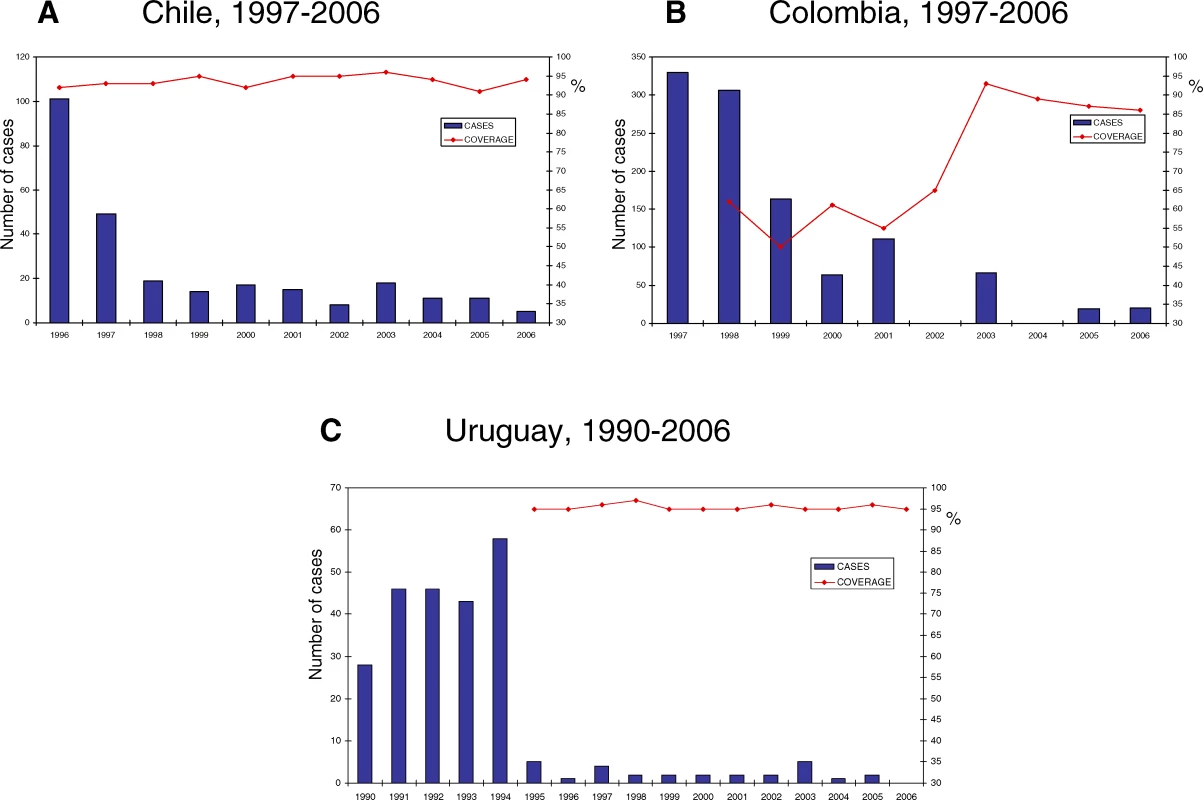
Tab. 1. Hib Vaccination in the Americas, 1991–2007 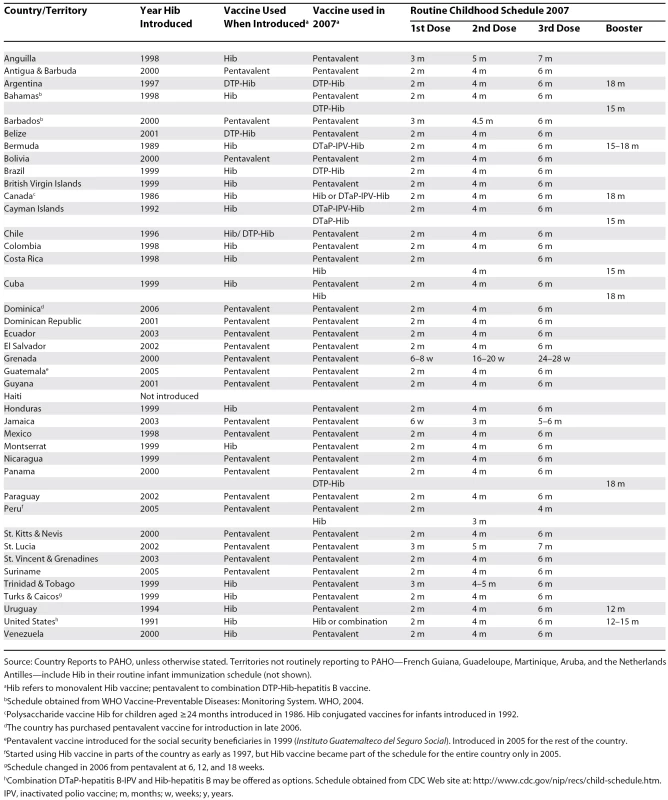
Source: Country Reports to PAHO, unless otherwise stated. Territories not routinely reporting to PAHO—French Guiana, Guadeloupe, Martinique, Aruba, and the Netherlands Antilles—include Hib in their routine infant immunization schedule (not shown). Most countries include only three doses for children aged less than one year. However, Canada, the French Departments and the Dutch territories in the Caribbean, the United States, and eight LAC countries and territories include a booster dose during the second year (Table 1).
In most countries, Hib vaccination was introduced nationwide. Due mostly to limited resources, countries such as Brazil, Guatemala, and Peru started vaccinating in selected populations or geographical areas. The use of Hib vaccine through the private sector in the region, in some cases years before introduction into the regular immunization program, has been widespread, but not quantified [11].
Lessons Learned
Logistics.
One of the lessons learned with Hib vaccine introduction in the Americas is that the selection of a vaccine formulation (combination versus monovalent) and presentation (one - versus tendose vials) is essential. It has an impact on the price, vaccine wastage percentage, cold chain space, need for reconstitution, and training activities for health care workers [11,25,26].
Currently, 34 countries use Hib in the form of a DTP-Hib-hepatitis B combination vaccine, or pentavalent (only available in one-dose vials) (Table 1). Of 17 countries and territories that started using monovalent Hib vaccine, only Canada and the United States have not switched to a combination vaccine, although in both countries, Hib combinations are often available as alternatives.
Hib vaccine in a combination formulation offers several advantages. Combination vaccines reduce the number of injections, thereby lowering the risk of injection complications and adverse events and reducing missed vaccination opportunities. In addition, parents and health care workers show greater acceptance of combination vaccines [27,28]. Combination Hib vaccine tends to be less expensive than monovalent Hib vaccine, thus contributing to the sustainability of immunization programs [27,28–30].
Financing Hib vaccination.
Although universal vaccination with Hib vaccine is highly cost-beneficial in both developed and developing settings [31–33], Hib vaccine price is higher than the traditional vaccines of the Expanded Program on Immunization (EPI). The price of Hib vaccine is more than double the measles-mumps-rubella vaccine, the most expensive among the traditional EPI vaccines (US$3.15 versus US$1.4 per dose). Therefore, financial sustainability has been a critical consideration for countries introducing Hib vaccination into their routine immunization schedule. To ensure sustainability, PAHO recommends purchasing Hib vaccine in combination with DTP or DTP/hepatitis B vaccine through the PAHO RF, and advocates for countries to issue legislation ensuring a budget line for vaccine purchase and other expenses of the immunization program.
In 2006, all but three of the LAC countries using Hib (Bolivia, the Dominican Republic, and Nicaragua) financed more than 95% of their public sector routine vaccine expenses using government funds. Also, in most LAC countries (27 of 31 with data available) at least 90% of the national immunization program's recurring costs were financed using government funds. All LAC countries using Hib vaccine have reported having a budget line for vaccine purchase (the Dominican Republic, Jamaica, and Saint Kitts and Nevis include vaccines in a budget line for medication purchase).
Of the six GAVI-eligible countries in LAC (countries with a gross national income of less than US$1,000 per capita), Guyana was the only one to introduce Hib vaccine (as pentavalent) using GAVI support. In 2006, Guyana took over the full financing of this vaccine. Other low-income countries have introduced pentavalent with support from other aid agencies.
As of 2006, 26 LAC countries reported having some immunization-related legislation, and three more were in the process of adopting such laws. In countries with legislation, laws and decrees have been used as a way to secure a budget line for vaccines and sustain vaccine introduction [34]. Among the Hib vaccine early adopters, Hib introduction followed ministerial and presidential decrees in Chile and Uruguay [25]. No information regarding the specific relation between Hib vaccine introduction and legislation is available for other countries.
Maintaining affordable prices is critical for vaccine introduction. The price of Hib vaccine purchased through the RF started at between US$6 and US$8.2 per dose in 1997 and dropped markedly, to between US$2.18 and US$2.60, at the end of 1998, as significantly more doses were purchased. For 2007, the RF price is US$3.15 per dose of the combination DTP-Hib (ten-dose vials) and US$3.92 per dose of pentavalent (one-dose vials).
In 2006, all LAC countries but four procured Hib-containing vaccines through the RF: two used Hib-containing vaccines produced in their own countries (Brazil and Cuba) and two purchased directly from a vaccine manufacturer.
Regional laboratory network.
Having a regional laboratory network has been instrumental in monitoring the circulating Hib strains that cause bacterial meningitis and pneumonias. As of 2006, SIREVA included laboratories from 19 Latin American countries plus the Caribbean Epidemiology Centre, and continued to monitor trends in the circulating serotypes and antimicrobial resistance patterns of Hib, as well as pneumococcus and meningococcus [35].
Remaining Challenges
Hib vaccination coverage.
PAHO recommends reaching coverage levels of at least 95% for all vaccines in all municipalities [36,37]. Throughout the past seven years, the overall reported administrative coverage (weighted average) with three doses of Hib vaccine (Hib3) in LAC has increased from 78% in 2000 to 94% in 2006 (with 19 and 37 countries reporting, respectively). However, the lower coverage reported in earlier years mostly reflects the partial use of Hib vaccine during the year of introduction. Hib3 coverage rates are comparable to those observed for the third dose of the diphtheria-tetanus-pertussis vaccine (DTP3). Use of combination vaccines contributes to this finding. No deterioration in DTP3 coverage during the year of Hib vaccine introduction is apparent from the coverage data available.
Coverage varies between and within countries. In 2006, reported coverage ranged from 83% to greater than 100%. Also, 42% of LAC municipalities (districts) reported coverage levels below 95%, using DTP3 as a proxy for Hib3. This finding illustrates that inequities in Hib vaccine use still exist in LAC countries.
Sustainability of immunization programs.
A major challenge for countries of the Americas is maintaining Hib vaccination while adding new, more expensive vaccines into national routine schedules. PAHO is currently developing a regional policy framework to assist countries in the following areas: exploring mechanisms to find new approaches for sustainable financing, focusing on securing longer-term and reliable funding for immunization programs, particularly through the creation of fiscal space; strengthening vaccine legislation to reduce national transaction costs; and improving the efficiency of the RF and expanding participation in its services to ensure that countries can afford new-generation vaccines [34,37–39].
“Creating fiscal space” is an expression used for mobilizing additional resources without compromising existing priorities in health. Mechanisms used include: identifying new partners; enhancing tax collection procedures; raising additional revenues through national lotteries, as was done for new vaccine purchase in Costa Rica; and exempting vaccine purchases from import tax, since import taxes can raise the vaccine purchase cost by as much as 30% in some countries.
PAHO recently described model vaccine legislation based on an analysis of existing laws [34]. Some of the legislation components aim to secure resources for immunization programs and include: a budget line for vaccines; regulations guaranteeing timely and reliable disbursement of resources; tax exemptions for vaccines and immunization supplies; flexibility to contract with suppliers, including third parties such as the RF; and streamlined customs regulations to accelerate the importation process and reduce transaction costs.
Vaccination impact.
The impact of Hib vaccine introduction has been well documented by reductions in Hib meningitis in selected countries of the Americas [9–11,25,40]. However, some countries are still lacking a surveillance of bacterial invasive disease that performs sufficiently well to document such impact.
A decrease in Hib meningitis has been well documented in Chile, Colombia, and Uruguay, in spite of differences in the introduction strategy and the coverage levels attained (Figure 1). Both Uruguay and Chile reached coverage levels of greater than 90% since vaccine introduction, but only Uruguay had an aggressive catchup strategy at vaccine introduction. The schedule in Uruguay calls for a booster dose at 12 months. Colombia initially achieved moderate coverage levels (50%–65%) that have increased to more than 86% over the last three years. Based on coverage levels achieved, 95% vaccine effectiveness, and the 20,000 cases estimated in the pre-vaccine era [19], PAHO estimates that Hib meningitis cases in LAC have decreased by approximately 85%.
In recent years, the number of Hib isolates from children aged less than two years has decreased in selected countries, from 92 in 2000 to 30 in 2005 in Brazil, from 30 in 2000 to eight in 2005 in Colombia, from 53 in 2000 to four in 2005 in the Dominican Republic, and from 44 in 2000 to two in 2005 in Venezuela. In these four countries, the number of pneumococci and menigococci isolates has remained stable over the same time period [35]. Hib isolates continue to be obtained from countries using Hib vaccine. However, neither SIREVA nor PAHO routinely collect data on the vaccination status of Hib cases reported.
Only a few LAC countries have been able to demonstrate that Hib vaccine has been effective against pneumonia in children aged less than two years. Hib vaccine has reduced the incidence of hospitalized pneumonia by about 20% in Chile [41,42]. For radiologically diagnosed pneumonia, vaccine effectiveness has been estimated at 31% in central Brazil and up to 55% in a case-control study conducted in Colombia [43,44].
Hib surveillance remains one of the most important challenges in LAC. To promote the strengthening of routine surveillance, PAHO recommends the integration of Hib into the routine surveillance of bacterial meningitis and pneumonias [3]. As done in the Central American sub-region, this will require adapting WHO's generic protocol for population-based Hib surveillance [3,45,46]. Currently, case definitions and surveillance procedures vary by country, limiting data comparability.
Monitoring the effectiveness of Hib vaccine in the routine immunization program is essential because vaccine efficacy may be affected by several factors, such as the use of booster doses, waning immunity, reduction of natural boosting due to reduced Hib transmission [47], and cold damage in storage units not maintained properly. Monitoring the changing epidemiology of H. influenzae disease in the post-vaccine era will also be important, as the role of invasive non-b type H. influenzae may become prominent [48]. The importance of continued Hib disease monitoring has been recently highlighted. In the United Kingdom, an unexpected increase in the incidence of Hib disease coincided with the distribution of combination vaccines that contain acellular pertussis (diphtheria-tetanus-acellular pertussis-Hib vaccine, or DTaP-Hib) [49–52]. In the Gambia, Hib seems to have reappeared in 2005–2006 after at least three years with no cases [53].
The added value of using a booster dose has been debated extensively because the majority of Hib disease burden in developing countries occurs before the age when the booster is usually administered (12–18 months) [1]. PAHO is currently assisting four countries to determine whether a booster dose during the second year of life is useful.
Efforts are in place to enhance the work done by SIREVA, including collecting more clinical and epidemiological data. This new initiative, known as “Network Surveillance System for the Bacterial Agents Responsible for Pneumonia and Meningitis” or SIREVA II, should provide a better understanding of the occurrence of cases in countries reporting high Hib coverage and the possibility of serotype replacement over time.
Conclusions
Although LAC countries lagged behind North America in their efforts to introduce Hib vaccine into their childhood immunization schedules, probably due mostly to financial considerations, all but Haiti had introduced this vaccine by the end of 2006. Even though the data available regarding Hib is not complete and comes from routine reporting by countries to PAHO, we believe that the main factors that favored the region-wide adoption of Hib vaccine in LAC are the safety and effectiveness of conjugated Hib vaccines; strong political will; the existence of data supporting a high Hib disease burden and vaccination impact in early adopters, and their lessons learned, shared through several publications and regional meetings; and PAHO's efforts to ensure the affordability of Hib vaccines, mainly through the RF.
Striving to use Hib in combination with other antigens and considering mechanisms to ensure sustainability, such as vaccine laws or presidential decrees whenever possible, will accelerate introduction. The main challenges remaining in LAC countries are to address low vaccination coverage in low-performing districts, ensure Hib vaccination while introducing other new and more expensive vaccines, and establish or strengthen Hib surveillance, better utilizing the well-established laboratory network. When considering the introduction of other new vaccines, LAC countries must take into account lessons learned from Hib introduction, in order to shorten the lag between vaccine development and widespread adoption in developing countries.
Supporting Information
Zdroje
1. World Health Organization
2006
WHO Position Paper on Haemophilus influenzae type b conjugate vaccines.
Wkly Epidemiol Rec
81
445
452
2. WengerJDWardJI
2004
Haemophilus
influenzae vaccine.
In
PlotkinSAOrensteinWA
editors
Vaccines
4th edition
Philadelphia
W. B. Saunders Co
229
268
3. Pan American Health Organization
2005
Control of diphtheria, pertussis, tetanus, Haemophilus influenzae type b, and
hepatitis B. Field guide.
Available http://www.ops-oms.org/English/AD/FCH/IM/fieldguide_pentavalent.pdf.
Accessed 6 March 2008
4. EskolaJPeltolaHTakalaAKKayhtyHHakulinenM
1987
Efficacy of Haemophilus
influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine
in infancy.
N Engl J Med
317
717
722
5. BooyRMoxonERMacFarlaneJAMayon-WhiteRTSlackMP
1992
Efficacy of Haemophilus
influenzae type B conjugate vaccine in Oxford region.
Lancet
340
847
6. VadheimCMGreenbergDPPartridgeSJingJWardJI
1993
Effectiveness and safety of an Haemophilus influenzae type b conjugate vaccine (PRP-T) in young
infants. Kaiser-UCLA Vaccine Study Group.
Pediatrics
92
272
279
7. MulhollandKHiltonSAdegbolaRUsenSOparaugoA
1997
Randomised trial of Haemophilus
influenzae type-b tetanus protein conjugate vaccine
[corrected] for prevention of pneumonia and meningitis in Gambian
infants.
Lancet
349
1191
1197
Erratu In: Lancet 1997;350(9076):524
8. SwinglerGFransmanDHusseyG
2003
Conjugate vaccines for preventing Haemophilus influenzae type b infections.
Cochrane Database Syst Rev
CD001729
9. MurphyTVWhiteKEPastorPGabrielLMedleyF
1993
Declining incidence of Haemophilus influenzae type b disease since introduction of
vaccination.
JAMA
269
246
248
10. Pan American Health Organization
1996
Impact of Uruguay's introduction of the Haemophilus influenzae type b (Hib)
vaccine.
EPI Newsletter
18
6
11. WengerJDDiFabioJLandaverdeJMLevineOSGaafarT
1999
Introduction of Hib conjugate vaccines in the non-industrialized world:
experience in four ‘newly adopting’ countries.
Vaccine
18
736
742
12. HeathPTMcVernonJ
2002
The UK Hib vaccine experience.
Arch Dis Child
86
396
399
13. AdegbolaRASeckaOLahaiGLloyd-EvansNNjieA
2005
Elimination of Haemophilus
influenzae type b (Hib) disease from The Gambia after the introduction
of routine immunisation with a Hib conjugate vaccine: a prospective study.
Lancet
366
144
150
14. TakalaAKEskolaJLeinonenMKayhtyHNissinenA
1991
Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in
children immunized with an Hib conjugate vaccine.
J Infect Dis
164
982
986
15. MurphyTVPastorPMedleyFOsterholmMTGranoffDM
1993
Decreased Haemophilus colonization in children vaccinated with Haemophilus influenzae type b conjugate
vaccine.
J Pediatr
122
517
523
16. HviidAMelbyeM
2004
Impact of routine vaccination with a conjugate Haemophilus influenzae type b vaccine.
Vaccine
22
378
382
17. World Health Organization
2008
Haemophilus
influenzae type b vaccine.
Available http://www.who.int/immunization/topics/hib/en/index.html. Accessed 6 March
2008
18. The Hib Initiative
2008
About the Hib Initiative: Vision.
Available: http://www.hibaction.org/abouthibinitiative.php#vision. Accessed 6 March
2008
19. PeltolaH
1997
Haemophilus
influenzae type b disease and vaccination in Latin America and the
Caribbean.
Pediatr Infect Dis J
16
780
787
20. CochiSLO'MaraDPrebludSR
1988
Progress in Haemophilus type b polysaccharide vaccine use in the United States.
Pediatrics
81
166
168
21. World Health Organization
2002
Haemophilus
influenzae type b (Hib) meningitis in the pre-vaccine era. A global
review of incidence, age distributions, and case-fatality rates.
Available http://www.who.int/immunization/documents/WHO_VB_02.18/en/index.html.
Accessed 6 March 2008
22. TambiniGAndrusJKFitzsimmonsJWRoses PeriagoM
2006
Regional immunization programs as a model for strengthening cooperation
among nations.
Rev Panam Salud Publica
20
54
59
23. DeRoeckDBawazirSACarrascoPKaddarMBrooksA
2006
Regional group purchasing of vaccines: Review of the Pan American Health
Organization EPI revolving fund and the Gulf Cooperation Council group purchasing
program.
Int J Health Plann Manage
21
23
43
24. World Health Organization
1983
EPI in the Americas: benefits from the Revolving Fund.
WHO Chronicle
37
81
85
25. LandaverdeMDi FabioJLRuoccoGLealIde QuadrosC
1999
Introduction of a conjugate vaccine against Hib in Chile and Uruguay.
Rev Panam Salud Publica
5
200
206
26. Pan American Health Organization
2004
Comparison of three alternatives for administering DTP, Haemophilus influenzae type b, and
hepatitis B vaccines through the Expanded Program on Immunization (EPI) in Bolivia.
EPI Newsletter
26
4
6
27. World Health Organization
1992
Informal discussion on quadrivalent diphtheria-tetanus-pertussis-hepatitis B
vaccine
Final report
Geneva
World Health Organization
28. SantosJILevineO
2006
Combination vaccines for childhood immunization.
In
AndrusJKde QuadrosC
editors
Recent Advances in Immunization
2nd edition
Washington (D. C.)
Pan American Health Organization
30
48
29. HadlerSC
1994
Cost benefit of combining antigens.
Biologicals
22
415
418
30. Di FabioJLde QuadrosC
2001
Considerations for combination vaccine development and use in the
developing world.
Clin Infect Dis
33
Suppl 4
S340
S345
31. HayJWDaumRS
1990
Cost-benefit analysis of Haemophilus influenzae type b prevention: Conjugate vaccination at
eighteen months of age.
Pediatr Infect Dis J
9
246
252
32. LevineOSOrtizEContrerasRLagosRVialP
1993
Cost-benefit analysis for the use of Haemophilus influenzae type b conjugate
vaccine in Santiago, Chile.
Am J Epidemiol
137
1221
1228
33. ZhouFBisgardKMYusufHRDeusonRRBathSK
2002
Impact of universal Haemophilus
influenzae type b vaccination starting at 2 months of age in the
United States: An economic analysis.
Pediatrics
110
653
661
34. AndrusJKFitzsimmonsJde QuadrosC
2006
Introduction of new and underutilized vaccines: perspectives from the
Americas.
In
AndrusJKde QuadrosC
editors
Recent Advances in Immunization
2nd edition
Washington (D. C.)
Pan American Health Organization
114
26
35. Pan American Health Organization
2007
Informe Regional de SIREVA II: Datos por país y por grupos de edad
sobre las características de los aislamientos de Streptococcus
pneumoniae, Haemophilus influenzae y Neisseria
meningitidis, en procesos invasores, 2000–2005
Washington (D. C.)
Pan American Health Organization
36. Vaccines and Immunization
2000
In the 42nd Meeting Pan American Directing Council, Washington DC, Sep
2000. Resolution CD42/R8. Pan American Health Organization.
Available: http://www.paho.org/english/gov/cd/cd42_fr-e.pdf. Accessed 6 March
2008
37. AndrusJKTambiniGdi FabioJLPeriagoMR
2004
Anticipating new vaccines in the Americas.
Rev Panam Salud Publica
16
369
370
38. Pan American Health Organization
2006
Regional strategy for sustaining National Immunization Programs in the
Americas.
Working document presented at the 138th Executive Committee. Available: http://www.paho.org/English/GOV/CE/ce138-11-e.pdf. Accessed 6 March
2008
39. AndrusJKToscanoCLewisMOliveiraLRoperoAM
2007
A model for enhancing evidence-based capacity to make informed policy
decisions on the introduction of new vaccines in the Americas: PAHO's PROVAC initiative.
Public Health Rep
122
811
816
40. RibeiroGSLimaJBReisJNGouveiaELCordeiroSM
2007
Haemophilus
influenzae meningitis 5 years after introduction of the Haemophilus influenzae type b conjugate
vaccine in Brazil.
Vaccine
25
4420
4428
41. LagosRHorwitzIToroJSan MartinOAbregoP
1996
Large scale, postlicensure, selective vaccination of Chilean infants with
PRP-T conjugate vaccine: Practicality and effectiveness in preventing invasive
Haemophilus influenzae
type b infections.
Pediatr Infect Dis J
15
216
222
42. LevineOSLagosRMunozAVillaroelJAlvarezAM
1999
Defining the burden of pneumonia in children preventable by vaccination
against Haemophilus
influenzae type b.
Pediatr Infect Dis J
18
1060
1064
43. de AndradeALde AndradeJGMartelliCMde SilvaSAde OliveiraRM
2004
Effectiveness of Haemophilus
influenzae b conjugate vaccine on childhood pneumonia: A case-control
study in Brazil.
Int J Epidemiol
33
173
181
44. de la HozFHigueraABDi FabioJLLunaMNaranjoAG
2004
Effectiveness of Haemophilus
influenzae type b vaccination against bacterial pneumonia in Colombia.
Vaccine
23
36
42
45. World Health Organization
1996
Generic protocol for population-based surveillance of Haemophilus influenzae type b.
Available http://www.who.int/vaccines-documents/DocsPDF/www9723.pdf. Accessed 6
March 2008
46. Pan American Health Organization
1998
Central America Meeting initiates surveillance system for Hib and
Streptococcus pneumoniae.
EPI Newsletter
20
4
47. KellyDFMoxonERPollardAJ
2004
Haemophilus
influenzae type b conjugate vaccines.
Immunology
113
163
174
48. Public Health Agency of Canada
2006
Invasive Haemophilus
influenzae disease in Manitoba in the post-vaccination era suggests a
changing epidemiology.
Can Commun Dis Rep
32
125
130
49. McVernonJAndrewsNSlackMPRamsayME
2003
Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with
acellular pertussis.
Lancet
361
1521
1523
50. HeathPTRamsayME
2003
Haemophilus
influenzae type b vaccine—Booster campaign.
BMJ
326
1158
1159
51. RamsayMEMcVernonJAndrewsNJHeathPTSlackMP
2003
Estimating Haemophilus
influenzae type b vaccine effectiveness in England and Wales by use of
the screening method.
J Infect Dis
188
481
485
52. JohnsonNGRuggebergJUBalfourGFLeeYCLiddyH
2006
Haemophilus
influenzae type b reemergence after combination immunization.
Emerg Infect Dis
12
937
941
53. HowieSRAntonioMAkisanyaASambouSHakeemI
2007
Re-emergence of Haemophilus
influenzae type b (Hib) disease in The Gambia following successful
elimination with conjugate Hib vaccine.
Vaccine
25
6305
6309
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Standardising Outcomes in Paediatric Clinical Trials
- Chile's Neoliberal Health Reform: An Assessment and a Critique
- Exposure to War as a Risk Factor for Mental Disorders
- Quantifying the Importance of Interleukin-6 for Coronary Heart Disease
- What Are the Implications for Childhood Pneumonia of Successfully Introducing Hib and Pneumococcal Vaccines in Developing Countries?
- Progress in Vaccination against type b in the Americas
- Research in Complex Humanitarian Emergencies: The Médecins Sans Frontières/Epicentre Experience
- A Systematic Review of Studies That Aim to Determine Which Outcomes to Measure in Clinical Trials in Children
- A Blood Test for Lung Fibrosis
- Better Reporting, Better Research: Guidelines and Guidance in
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chile's Neoliberal Health Reform: An Assessment and a Critique
- A Systematic Review of Studies That Aim to Determine Which Outcomes to Measure in Clinical Trials in Children
- Better Reporting, Better Research: Guidelines and Guidance in
- Progress in Vaccination against type b in the Americas
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání