-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Blood Test for Lung Fibrosis
article has not abstract
Published in the journal: . PLoS Med 5(4): e98. doi:10.1371/journal.pmed.0050098
Category: Perspective
doi: https://doi.org/10.1371/journal.pmed.0050098Summary
article has not abstract
Idiopathic pulmonary fibrosis (IPF) is a disease of unknown aetiology and uncertain pathogenesis, and there are no effective therapies. IPF, also known as usual interstitial pneumonia and cryptogenic fibrosing alveolitis, is a chronic progressive disease in which excessive deposition of extracellular matrix leads to irreversible scarring of interstitial lung tissue with consequent reduction in gas diffusion and loss of lung volumes. This is a devastating disease with an average survival of less than three years from the time of diagnosis [1]. There is therefore a pressing need to understand more about the underlying cellular, molecular, and genetic mechanisms in order to more effectively monitor the disease, follow its progression, and most importantly to develop effective treatments. Understanding the fibrotic process also has implications for other chronic fibrotic diseases, such as cirrhosis, and for fibrosis of small airways, which is the main lesion of chronic obstructive pulmonary disease.
Pathology of IPF
Pulmonary fibrosis results from injury to alveolar epithelial cells and represents a repair process that is inappropriate. The cause of the original injury is unknown, and there may be several causal mechanisms and pathways that all end up with excessive and progressive scarring of the lung. A major problem in clinical research in this disease is the late presentation of clinical symptoms, as shortness of breath on exertion only becomes apparent when less than 50% of lung capacity remains. By this stage the disease is essentially untreatable and has a poor prognosis.
The disease is patchy, and the characteristic histological feature of IPF is the presence of fibroblastic foci with areas of fibrosis. There is usually a low-grade inflammation, but the role of inflammation is controversial as there are often few inflammatory cells and there is no significant therapeutic benefit of anti-inflammatory treatments, such as corticosteroids and azathioprine. The absence of response to anti-inflammatory treatment suggests that fibrosis may develop as the consequence of an abnormal wound healing response to some type of chronic alveolar epithelial injury [2]. However, inflammation may play a more important role in the early stages of disease and may then disappear.
Linked Research Article
This Perspective discusses the following new study published in PLoS Medicine:
Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, et al. (2008) MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 5(4): e93. doi:10.1371/journal.pmed.0050093
Naftali Kaminski and colleagues find increased levels of specific proteins in the bloodstream of individuals with idiopathic pulmonary fibrosis, and suggest that these proteins may ultimately provide a biomarker for the disease.
The trigger that initiates the alveolar injury in IPF is unknown, but there is increasing recognition that there may be many causes and that many different interacting pathways may be activated to produce the abnormal healing response, based in part on genetic susceptibility [3]. The genes that determine susceptibility to IPF have not yet been identified, but there is particular interest in genes that regulate tissue repair [4]. Important clues may be provided by familial pulmonary fibrosis, which is a rare dominant trait with incomplete penetrance.
The Challenge of Identifying Possible Biomarkers
In view of the complexity of IPF it has been difficult to identify useful biomarkers in bronchoalveolar lavage or blood, particularly ones that might be clinically useful in differentiating IPF from other pulmonary fibrotic diseases such as hypersensitivity pneumonitis (HP) and non-specific interstitial pneumonia (NSIP). Gene microarray of lung biopsies from patients with IPF reveals a distinct pattern, with increased expression of tissue remodelling, epithelial, and myofibroblast genes, whereas HP shows a greater expression of inflammatory and immune genes [5]. Matrix metalloproteinases (MMPs), especially MMP7, also show increased gene expression in IPF lungs [6]. Interestingly, NSIP, which is often difficult to differentiate from IPF and HP, shows a different pattern of gene expression, although some cases resemble the profile of either IPF or HP. These studies imply that different patterns of biomarkers might distinguish these different types of pulmonary fibrosis, and they suggest that blood markers could be identified for this purpose.
There has been an extensive search for such biomarkers of IPF, with interest in the increased plasma concentrations of surfactant proteins and the fibrogenic cytokine osteopontin [7,8]. In a new study published in this issue of PLoS Medicine, Naftali Kaminski and colleagues assayed 49 plasma proteins in a relatively large number of patients with IPF (74 patients with IPF and 53 healthy controls) and showed that five of these proteins distinguished IPF from controls [9]. The concentrations of two of these proteins, MMP1 (collagenase) and MMP7 (matrilysin), were also raised in lung tissue and bronchoalveolar lavage of patients with IPF. Furthermore, MMP1 and MMP7 plasma concentrations were greater in patients with IPF than in patients with other chronic fibrotic lung diseases, including chronic obstructive pulmonary disease, sarcoidosis, and HP. Patients with NISP were not included in this study, but it is likely that such patients would show similar changes in MMP1 and MMP7 to those seen in patients with IPF, as increased expression of MMP1 and MMP7 has previously been reported in lung tissue and bronchoalveolar lavage fluid of patients with both IPF and NSIP [10,11]. Elevated plasma MMP7 was found in patients with IPF with asymptomatic disease, albeit at lower concentrations than in symptomatic disease, and correlated with reduced gas diffusion and lung volumes. These findings suggest that MMP7 levels may be used as an early marker of disease and may be useful in monitoring disease progression.
Kaminski and colleagues' findings have biological plausibility, as abnormal expression of MMPs have previously been reported in lungs of patients with IPF [10,12], and this family of more than 20 enzymes regulates the turnover of all extracellular matrix proteins. MMP1 is not normally expressed and degrades fibrillar collagen, whereas MMP7 degrades other extracellular matrix proteins and is involved in tissue remodelling. These MMPs may be produced by alveolar epithelial cells in response to injury, or perhaps to inflammatory mediators and oxidative stress in parallel with fibrogenic mediators such as transforming growth factor-β (TGF-β) (Figure 1). Whether they play a role in counteracting fibrosis or may contribute to pathogenesis is unclear, but it is likely that they are a signature of tissue remodelling. MMPs may generate neutrophil chemotactic peptides from extracellular matrix proteins [13], which may link neutrophilic inflammation to IPF, and this may further stimulate fibrosis through the release of reactive oxygen species and TGF-β. However, in mice with targeted disruption of the MMP7 gene there is a reduction in the fibrotic response to bleomycin, suggesting that it may have a profibrotic role [6].
Fig. 1. Mechanisms of IPF 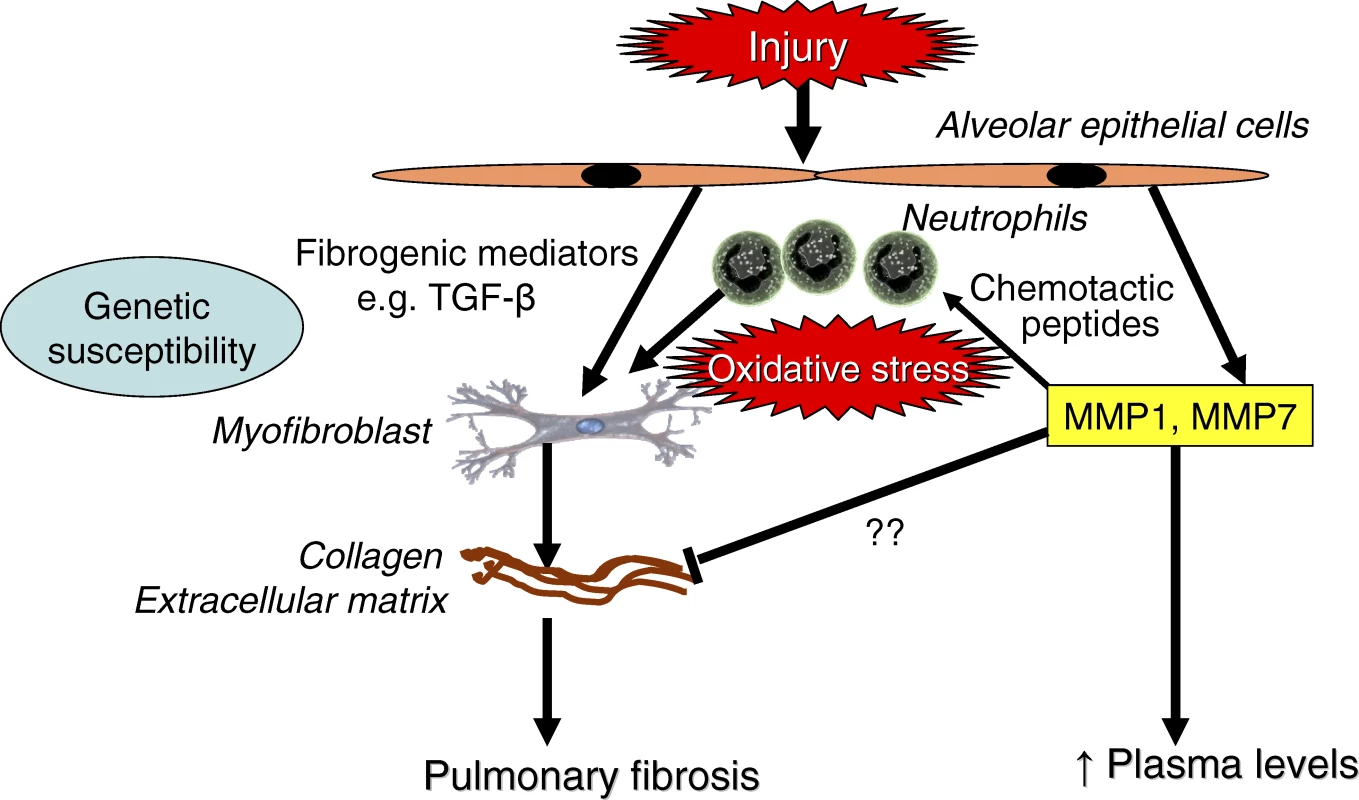
Injury of alveolar epithelial cells releases fibrogenic mediators, such as TGF-β, which stimulate myofibroblasts to produce extracellular matrix proteins. Matrix metalloproteinases MMP1 and MMP7 are also produced and may reflect tissue remodelling or contribute to fibrosis through recruitment of neutrophils, which release reactive oxygen species and TGF-β. Increased plasma concentrations of MMPs may therefore reflect increased extracellular matrix turnover and disease activity. Moving Towards Clinical Application
What is the clinical relevance of these findings? Elevation of plasma MMP1 and MMP7 may help to confirm a diagnosis of IPF and probably NSIP, and help to distinguish these diseases from HP, sarcoidosis, and perhaps other fibrotic lung diseases. However, a normal individual value does not exclude these diagnoses, as there is considerable overlap between levels of these MMPs in healthy people and patients with other chronic lung diseases. However, the plasma concentrations of MMP7 may be useful in following disease progression and even in facilitating early diagnosis. This marker is unlikely to be useful in screening for IPF, as it is an uncommon disease. It is not yet known whether plasma MMP1 and MMP7 are useful indicators of rapid disease progression or may be used for early detection of acute exacerbations. What this study tells us about disease mechanisms and future therapy is uncertain, as the roles of MMPs in the pathogenesis of IPF are far from clear.
Zdroje
1. StrieterRM
2005
Pathogenesis and natural history of usual interstitial pneumonia: The whole story or the last chapter of a long novel.
Chest
128
526S
532S
2. SelmanMKingTEPardoA
2001
Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy.
Ann Intern Med
134
136
151
3. MaherTMWellsAULaurentGJ
2007
Idiopathic pulmonary fibrosis: Multiple causes and multiple mechanisms.
Eur Respir J
30
835
839
4. LawsonWELoydJE
2006
The genetic approach in pulmonary fibrosis: Can it provide clues to this complex disease.
Proc Am Thorac Soc
3
345
349
5. SelmanMPardoABarreraLEstradaAWatsonSR
2006
Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis.
Am J Respir Crit Care Med
173
188
198
6. ZuoFKaminskiNEuguiEAllardJYakhiniZ
2002
Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans.
Proc Natl Acad Sci U S A
99
6292
6297
7. TakahashiHShiratoriMKanaiAChibaHKurokiY
2006
Monitoring markers of disease activity for interstitial lung diseases with serum surfactant proteins A and D.
Respirology
11
Suppl
S51
S54
8. KadotaJMizunoeSMitoKMukaeHYoshiokaS
2005
High plasma concentrations of osteopontin in patients with interstitial pneumonia.
Respir Med
99
111
117
9. RosasIORichardsTJKonishiKZhangYGibsonK
2008
MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis.
PLoS Med
5
e93
doi:10.1371/journal.pmed.0050093
10. CosgroveGPSchwarzMIGeraciMWBrownKKWorthenGS
2002
Overexpression of matrix metalloproteinase-7 in pulmonary fibrosis.
Chest
121
25S
26S
11. VuorinenKMyllarniemiMLammiLPiirilaPRytilaP
2007
Elevated matrilysin levels in bronchoalveolar lavage fluid do not distinguish idiopathic pulmonary fibrosis from other interstitial lung diseases.
APMIS
115
969
975
12. PardoASelmanM
2006
Matrix metalloproteases in aberrant fibrotic tissue remodeling.
Proc Am Thorac Soc
3
383
388
13. WeathingtonNMvan HouwelingenAHNoeragerBDJacksonPLKraneveldAD
2006
A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation.
Nat Med
12
317
323
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Standardising Outcomes in Paediatric Clinical Trials
- Chile's Neoliberal Health Reform: An Assessment and a Critique
- Exposure to War as a Risk Factor for Mental Disorders
- Quantifying the Importance of Interleukin-6 for Coronary Heart Disease
- What Are the Implications for Childhood Pneumonia of Successfully Introducing Hib and Pneumococcal Vaccines in Developing Countries?
- Progress in Vaccination against type b in the Americas
- Research in Complex Humanitarian Emergencies: The Médecins Sans Frontières/Epicentre Experience
- A Systematic Review of Studies That Aim to Determine Which Outcomes to Measure in Clinical Trials in Children
- A Blood Test for Lung Fibrosis
- Better Reporting, Better Research: Guidelines and Guidance in
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chile's Neoliberal Health Reform: An Assessment and a Critique
- A Systematic Review of Studies That Aim to Determine Which Outcomes to Measure in Clinical Trials in Children
- Better Reporting, Better Research: Guidelines and Guidance in
- Progress in Vaccination against type b in the Americas
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



