-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAntiretroviral Therapy for Prevention of HIV Infection: New Clues From an Animal Model
article has not abstract
Published in the journal: . PLoS Med 5(2): e30. doi:10.1371/journal.pmed.0050030
Category: Perspective
doi: https://doi.org/10.1371/journal.pmed.0050030Summary
article has not abstract
Background
The introduction of antiretroviral therapy (ART) in the early 1990s profoundly changed the face of HIV infection by improving survival rates [1]. But ART has equal potential for prevention, since it reduces the probability of HIV transmission from an infected person to their sexual partner(s). Although there have been no randomized controlled clinical trials on the subject, antiretroviral drugs are currently used in clinical practice for post-exposure prophylaxis after inadvertent occupational exposure (based on the results of a case control study [2]) or after sexual exposure to the virus [3]. Pre - and post-exposure prophylaxis (PrEP and PEP, respectively) have been used successfully to interrupt transmission of HIV from infected mothers to their babies [4].
Investigators at the United States Centers for Disease Control and Prevention have conducted a series of studies in rhesus macaques to explore antiretroviral prophylaxis. First, they developed a rectal inoculation model using concentrations of simian HIV (SHIV) representative of human exposure [5]. Using this model, the investigators showed that tenofovir disoproxil fumarate (TDF, a nucleotide analogue reverse transcriptase inhibitor) delayed, but did not prevent, acquisition of SHIV in these animals (seven out of eight animals infected over 14 weeks) [6]. A new study in this issue of PLoS Medicine by Walid Heneine and colleagues [7] extends earlier observations and will certainly affect the direction of human clinical trials and public health policy.
The Results
In the new study, macaques were exposed to weekly rectal virus challenges for up to 14 weeks. The authors compared infections observed in 18 untreated macaques to infections in macaques that received a variety of antiretroviral PrEP regimens containing the nucleotide reverse transcriptase inhibitor emtricitabine (FTC) alone or in combination with TDF. With subcutaneous FTC alone (at a human-equivalent dose), four out of six animals became infected. With a combination of oral FTC and TDF at a dose equivalent to Truvada (FTC 200 mg + TDF 300 mg) in humans, two out of six animals became infected. With subcutaneous FTC and a supratherapeutic subcutaneous dose of tenofovir (given either daily or in a two-dose regimen before and after exposure), complete protection from infection was observed (none of the 12 animals became infected).
For animals that became infected during treatment, the investigators noted that infection was delayed, and all animals had blunted acute viremia, suggesting the possibility of reduced immune damage during acute HIV infection [8]. Resistance to FTC was observed in two out of six animals that failed therapy.
This Perspective discusses the following new study published in PLoS Medicine:
García-Lerma JG, Otten RA, Qari SH, Jackson E, Cong M, et al. (2008) Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med 5(2): e28. doi:10.1371/journal.pmed.0050028
Using a repeat-exposure macaque model, Walid Heneine and colleagues find that pre-exposure prophylaxis with combination antiretroviral drugs provides protection against rectal challenge with a SHIV virus.
The Implications
These and earlier animal studies have provided the basis for human clinical trials with PrEP. The observation of FTC resistance during therapy emphasizes the risk of PrEP to the individual and the community. PrEP continued in the face of unrecognized infection might be expected to promote replication of a resistant variant, which could be transmitted widely [9]. Tenofovir and FTC resistance are common in populations receiving ART, including in sub-Saharan Africa [10]. In addition, clade C HIV (predominant in sub-Saharan Africa) may be more susceptible to the evolution of a tenofovir resistance mutation (the K65R mutation) [11].
Strengths and Limitations of the New Study
This new report [7] represents the culmination of a series of recent studies specifically designed to guide and inform human clinical PrEP trials [5,6]. In addition, the new study shows protection from SHIV by intermittent dosing with tenofovir and FTC, a regimen that is closer to true PrEP than continuous daily dosing.
The study had four weaknesses. First, it included only small numbers of animals. Second, nine out of the 18 controls used were historical in nature. Third, FTC and tenofovir doses chosen as human-equivalent were based on first-dose pharmacokinetics in a limited number of animals, and they represent higher drug exposures than seen in humans (FTC and tenofovir areas under the concentration-time curves in macaques were approximately 30% and 40% higher, respectively, than exposures in humans [12]). In addition, intracellular pharmacokinetics of the active agents also differ between macaques and humans [13–15]. Finally, complete protection from HIV acquisition was only observed with a subcutaneous tenofovir dose that provided concentrations greater than can be achieved with oral therapy [16].
The Future
These results highlight an exciting and potentially important use of ART to prevent sexual transmission of HIV [3], and offer further support for human clinical trials in progress or planned. Optimistic modeling experiments suggest an important role for PrEP in HIV prevention [17]. But the application of PrEP highlights a unique tension between prevention and treatment; widespread usage of ART for prevention in communities where ART for treatment is still being rationed might cause conflict [18]. Also, PrEP has the potential to accelerate transmitted drug resistance [9], thereby limiting the utility of drugs critical to combination ART. Human PrEP trials must address these concerns. One PrEP safety trial has been completed in women at high risk of acquiring HIV in Africa [19]; other current trials designed to measure PrEP safety and efficacy are summarized in Table 1. These PrEP trials will shine a light on the potential of ART for prevention, and help physicians to think more broadly about the public health implications of these life-saving drugs.
Tab. 1. Current and Proposed Pre-Exposure Prophylaxis Trials, October 2007 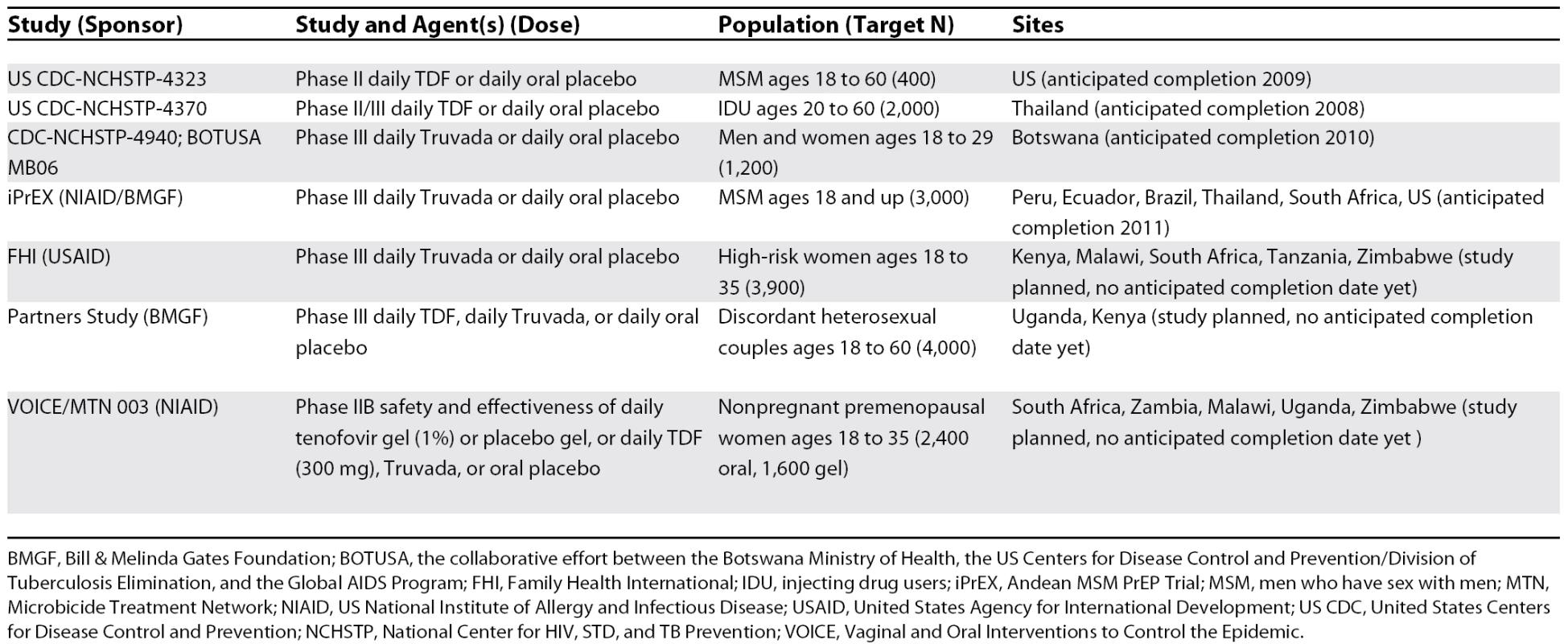
BMGF, Bill & Melinda Gates Foundation; BOTUSA, the collaborative effort between the Botswana Ministry of Health, the US Centers for Disease Control and Prevention/Division of Tuberculosis Elimination, and the Global AIDS Program; FHI, Family Health International; IDU, injecting drug users; iPrEX, Andean MSM PrEP Trial; MSM, men who have sex with men; MTN, Microbicide Treatment Network; NIAID, US National Institute of Allergy and Infectious Disease; USAID, United States Agency for International Development; US CDC, United States Centers for Disease Control and Prevention; NCHSTP, National Center for HIV, STD, and TB Prevention; VOICE, Vaginal and Oral Interventions to Control the Epidemic.
Zdroje
1. PalellaFJJrDelaneyKMMoormanACLovelessMOFuhrerJ
1998
Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators.
N Engl J Med
338
853
860
2. CardoDMCulverDHCiesielskiCASrivastavaPUMarcusR
1997
A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group.
N Engl J Med
337
1485
1490
3. CohenMSGayCKashubaADBlowerSPaxtonL
2007
Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1.
Ann Intern Med
146
591
601
4. FowlerMGLampeMAJamiesonDJKourtisAPRogersMF
2007
Reducing the risk of mother-to-child human immunodeficiency virus transmission: past successes, current progress and challenges, and future directions.
Am J Obstet Gynecol
197
S3
S9
5. OttenRAAdamsDRKimCNJacksonEPulliumJK
2005
Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates.
J Infect Dis
191
164
173
6. SubbaraoSOttenRARamosAKimCJacksonE
2006
Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges.
J Infect Dis
194
904
911
7. García-LermaJGOttenRAQariSHJacksonECongM
2008
Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir.
PLoS Med
5
e28
doi:10.1371/journal.pmed.0050028
8. BrenchleyJMSchackerTWRuffLEPriceDATaylorJH
2004
CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract.
J Exp Med
200
749
759
9. BoothCLGerettiAM
2007
Prevalence and determinants of transmitted antiretroviral drug resistance in HIV-1 infection.
J Antimicrob Chemother
59
1047
1056
10. FerradiniLJeanninAPinogesLIzopetJOdhiamboD
2006
Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment.
Lancet
367
1335
1342
11. BrennerBGOliveiraMDoualla-BellFMoisiDDNtemgwaM
2006
HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture.
AIDS
20
F9
F13
12. Gilead Sciences
2007
Truvada package insert. 21-752-GS-20.
Available: http://www.gilead.com/pdf/truvada_pi.pdf. Accessed 3 January 2008
13. WangLHBegleyJSt ClaireRL3rdHarrisJWakefordC
2004
Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection.
AIDS Res Hum Retroviruses
20
1173
1182
14. HawkinsTVeikleyWSt ClaireRL3rdGuyerBClarkN
2005
Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens.
J Acquir Immune Defic Syndr
39
406
411
15. KingTBushmanLKiserJAndersonPLRayM
2006
Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells.
J Chromatogr B Analyt Technol Biomed Life Sci
843
147
156
16. Van RompayKKBrignoloLLMeyerDJJeromeCTararaR
2004
Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy) propyl]adenine (tenofovir) to newborn and infant rhesus macaques.
Antimicrob Agents Chemother
48
1469
1487
17. AbbasULAndersonRMMellorsJW
2007
Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings.
PLoS ONE
2
e875
doi:10.1371/journal.pone.0000875
18. GrantRMBuchbinderSCatesWJrClarkeECoatesT
2005
AIDS. Promote HIV chemoprophylaxis research, don't prevent it.
Science
309
2170
2171
19. PetersonLTaylorDRoddyRBelaiGPhillipsP
2007
Tenofovir disoproxil fumarate for prevention of HIV infection in women: A phase 2, double-blind, randomized, placebo-controlled trial.
PLOS Clin Trial
2
e27
doi:10.1371/journal.pctr.0020027
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 2- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Antiretroviral Therapy for Prevention of HIV Infection: New Clues From an Animal Model
- A Collaborative Epidemiological Investigation into the Criminal Fake Artesunate Trade in South East Asia
- Does Preventing Obesity Lead to Reduced Health-Care Costs?
- Solving the Mystery of Myelodysplasia
- The Evolution of Norovirus, the “Gastric Flu”
- Maternal Death, Autopsy Studies, and Lessons from Pathology
- Soft Targets: Nurses and the Pharmaceutical Industry
- Eliminating Human African Trypanosomiasis: Where Do We Stand and What Comes Next?
- Should Data from Demographic Surveillance Systems Be Made More Widely Available to Researchers?
- New Formulation of Paraquat: A Step Forward but in the Wrong Direction?
- The Neglected Diseases Section in : Moving Beyond Tropical Infections
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Eliminating Human African Trypanosomiasis: Where Do We Stand and What Comes Next?
- Solving the Mystery of Myelodysplasia
- The Neglected Diseases Section in : Moving Beyond Tropical Infections
- Should Data from Demographic Surveillance Systems Be Made More Widely Available to Researchers?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



