-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
TDP-43 is a multifunctional nucleic acid binding protein linked to several neurodegenerative diseases including Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia. To learn more about the normal biological and abnormal pathological role of this protein, we turned to Caenorhabditis elegans and its orthologue TDP-1. We report that TDP-1 functions in the Insulin/IGF pathway to regulate longevity and the oxidative stress response downstream from the forkhead transcription factor DAF-16/FOXO3a. However, although tdp-1 mutants are stress-sensitive, chronic upregulation of tdp-1 expression is toxic and decreases lifespan. ALS–associated mutations in TDP-43 or the related RNA binding protein FUS activate the unfolded protein response and generate oxidative stress leading to the daf-16–dependent upregulation of tdp-1 expression with negative effects on neuronal function and lifespan. Consistently, deletion of endogenous tdp-1 rescues mutant TDP-43 and FUS proteotoxicity in C. elegans. These results suggest that chronic induction of wild-type TDP-1/TDP-43 by cellular stress may propagate neurodegeneration and decrease lifespan.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002806
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002806Summary
TDP-43 is a multifunctional nucleic acid binding protein linked to several neurodegenerative diseases including Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia. To learn more about the normal biological and abnormal pathological role of this protein, we turned to Caenorhabditis elegans and its orthologue TDP-1. We report that TDP-1 functions in the Insulin/IGF pathway to regulate longevity and the oxidative stress response downstream from the forkhead transcription factor DAF-16/FOXO3a. However, although tdp-1 mutants are stress-sensitive, chronic upregulation of tdp-1 expression is toxic and decreases lifespan. ALS–associated mutations in TDP-43 or the related RNA binding protein FUS activate the unfolded protein response and generate oxidative stress leading to the daf-16–dependent upregulation of tdp-1 expression with negative effects on neuronal function and lifespan. Consistently, deletion of endogenous tdp-1 rescues mutant TDP-43 and FUS proteotoxicity in C. elegans. These results suggest that chronic induction of wild-type TDP-1/TDP-43 by cellular stress may propagate neurodegeneration and decrease lifespan.
Introduction
TDP-1 is the Caenorhabditis elegans orthologue of the multifunctional DNA/RNA binding protein TDP-43 (TAR DNA Binding Protein 43). Mutations and accumulations of TDP-43 have been found in patients with Amyotrophic Lateral Sclerosis (ALS), Frontotemporal Dementia, and in a growing number of neurodegenerative disorders [1]. As part of its numerous roles in RNA metabolism, TDP-43 is a component of the cytoplasmic ribonucleoprotein complexes known as stress granules that form in response to environmental stresses like heat shock, oxidative and osmotic stress among others [2], [3]. ALS is an age-dependent neurodegenerative disorder and given that TDP-43 is a stress responsive protein we hypothesized that TDP-1 may regulate longevity and the cellular stress response.
In worms a major axis of stress-response signaling and longevity is the Insulin/IGF-signaling (IIS) pathway. IIS follows an evolutionarily conserved and genetically regulated pathway that regulates numerous processes including development, metabolism, fecundity, cellular stress resistance and aging [4]. In C. elegans, IIS initiates a phosphorylation cascade through the DAF-2/Insulin-IGF receptor that phosphorylates the forkhead transcription factor DAF-16 and retains it in the cytoplasm [5]–[8]. Hypomorphic daf-2 mutants relieve DAF-16 phosphorylation causing DAF-16 to translocate to the nucleus where it activates a large number of genes resulting in increased lifespan and augmented stress resistance [9]. daf-2 mutants are also resistant to numerous stresses including oxidative, osmotic, thermal and proteotoxicity [10]. The IIS pathway likely employs multiple mechanisms to combat a variety of cellular insults but little is known about how these separate functions are regulated by DAF-16.
We attempted to address this issue by asking whether TDP-1 participated in the cellular stress response and longevity pathways in C. elegans. We asked if TDP-1 participated in the IIS pathway to specify developmental, stress response, and longevity outcomes. Given the importance of human TDP-43 to neurodegeneration we also asked if tdp-1 regulated age-dependent proteotoxicity. Our work points to TDP-1 as a key stress response protein at the crossroads of IIS, proteotoxicity and endoplasmic reticulum (ER) stress. Moreover, persistent induction of TDP-1 may actively promote neurodegeneration.
Results
tdp-1 Regulates Lifespan
Downregulation of DAF-2 extends lifespan and promotes stress resistance via regulation of DAF-16 transcriptional activity [9]. To determine if tdp-1 participated in the IIS pathway we used a deletion mutant tdp-1(ok803) that removes the two RNA Recognition Motifs of TDP-1 (Figure S1A). Looking directly at the IIS pathway and longevity, daf-2(e1370) animals were long-lived but this phenotype was significantly reduced in a daf-2(e1370);tdp-1(ok803) double mutant strain (Figure 1A, Table S1). Conversely, daf-16(mu86) mutants were short-lived compared to wild type worms and the inclusion of tdp-1(ok803) did not further reduce daf-16 mutants' lifespan (Figure 1A, Table S1). We confirmed that tdp-1 regulated daf-2 mediated longevity with RNA interference and observed that tdp-1(RNAi) reduced the extended lifespan of daf-2 mutants (Figure 1B, Table S1). These data suggest that tdp-1 regulates the lifespan phenotypes of reduced IIS and the inability of tdp-1 to further reduce daf-16 lifespan suggests that this effect is dependent on daf-16.
Fig. 1. tdp-1 regulates lifespan. 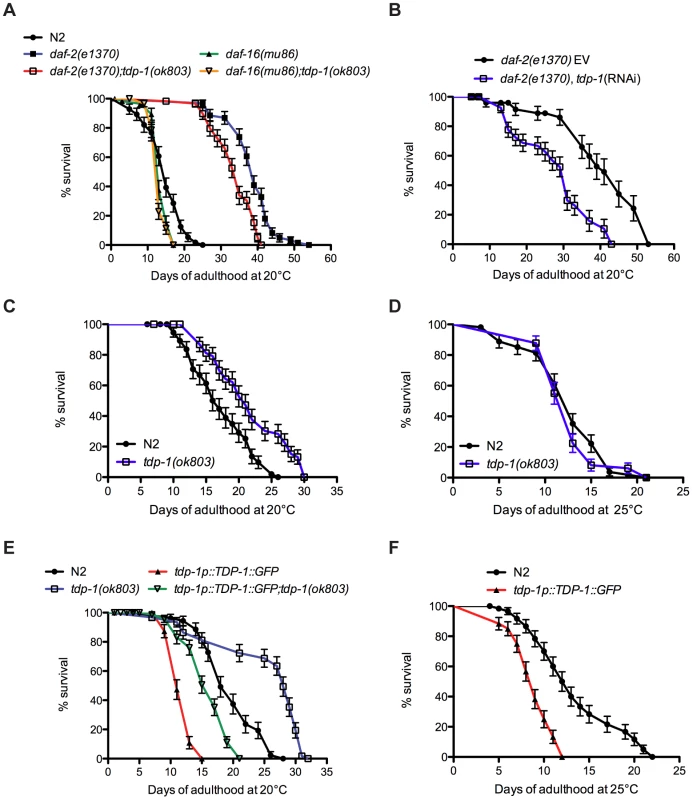
(A) Mutation in tdp-1 reduced the lifespan of daf-2(e1370) mutants but did not further reduce the lifespan of daf-16(mu86) mutants. (B) RNAi against tdp-1 reduced the lifespan of daf-2(e1370) mutants. EV is the empty vector control. (C) tdp-1(ok803) mutants had increased lifespan compared to N2 worms at 20°C. (D) tdp-1(ok803) mutants and N2 worms had comparable lifespan at 25°C. (E) TDP-1::GFP overexpression strains had reduced lifespans compared to N2 worms or tdp-1(ok803) mutants at 20°C. (F) TDP-1::GFP transgenic had reduced lifespans compared to N2 worms when grown at 25°C. Please also see Table S1. Surprisingly, despite tdp-1(ok803) limiting the extreme lifespan of daf-2 mutants we observed that at 20°C tdp-1(ok803) mutants had a small but significant increase in lifespan versus N2 controls (Figure 1C, Table S1). However this effect was lost when the worms were grown at 25°C (Figure 1D, Table S1) suggesting that tdp-1 may respond to stressful environmental conditions. Consistently, opposite to reducing tdp-1 function, overexpression of TDP-1 fused to GFP from the endogenous tdp-1 promoter (tdp-1p::TDP-1::GFP) greatly reduced lifespan compared to wild type N2 worms and tdp-1 deletion mutants at 20°C (Figure 1E). The lifespan of a tdp-1(ok803);TDP-1::GFP strain was greater than TDP-1::GFP alone, but less than either N2 worms or the tdp-1(ok803) deletion mutant (Figure 1E, Table S1). Finally, TDP-1::GFP transgenics showed a further decrease in mean and maximum lifespan when grown at 25°C compared to N2 worms (Figure 1F, Table S1). These data show that tdp-1 has a dose-dependent effect on lifespan in worms and that lifespan is sensitive to tdp-1 expression levels, which is consistent with studies of TDP-43 in flies [11] and mice [12] where overexpression also reduces lifespan.
tdp-1 Is Essential for Resistance against Oxidative and Osmotic Stress
Intimately linked to IIS regulation of lifespan in worms is dauer formation and stress resistance [4]. daf-2(e1370) mutant larvae show near complete dauer formation when grown at 25°C, but this phenotype was not altered in daf-2(e1370);tdp-1(ok803) mutants (Figure S2A) suggesting that tdp-1 does not function in the dauer formation axis of IIS in worms.
To further define the role of tdp-1 in the in vivo stress response, we challenged worms against juglone induced oxidative stress. Juglone is a natural product derived from the black walnut tree that raises intracellular oxide levels [13]. Adult wild type N2 worms transferred to juglone plates showed complete mortality after 14 hours, while daf-2(e1370) worms were completely resistant (Figure 2A). If tdp-1 were required for protection against stress we would expect the mutant tdp-1 worms to be hypersensitive to juglone-induced toxicity. Interestingly, tdp-1(ok803) mutants were more sensitive to juglone than N2 worms, and tdp-1(ok803) completely abolished daf-2's resistance to juglone in the daf-2(e1370);tdp-1(ok803) double mutant strain (Figure 2A). To corroborate the role of tdp-1 in resistance to oxidative stress we used hydrogen peroxide, which is another oxidative stress enhancer. Tested on hydrogen peroxide plates we observed that tdp-1(ok803) animals were more sensitive than wild type N2 worms, and tdp-1(ok803) sensitized the normally resistant daf-2 animals to stress-induced mortality (Figure 2B). These data show that tdp-1 is required for daf-2 mediated resistance to oxidative stress. Given the effect of tdp-1(ok803) on daf-2 mutants' stress resistance compared to the lack of effect on dauer phenotypes we wondered if tdp-1 might have a more specific role in the stress response axis of IIS.
Fig. 2. tdp-1 specifies stress response signaling. 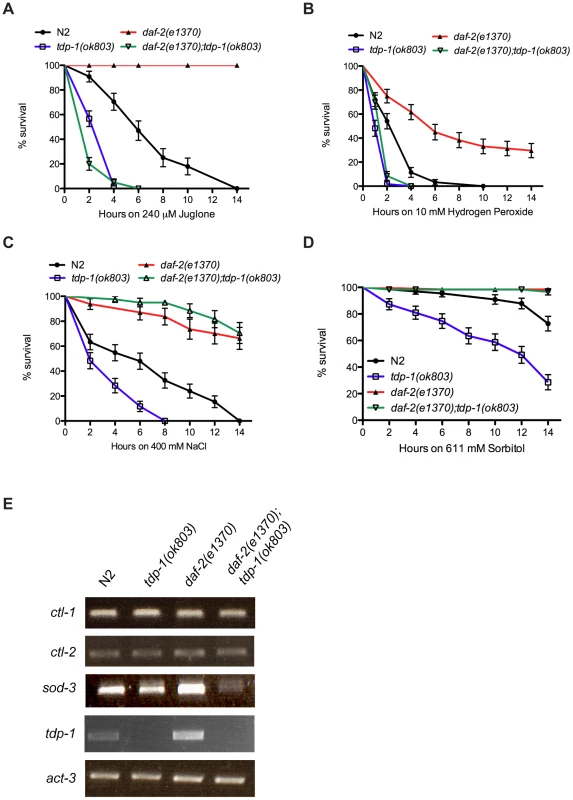
(A) tdp-1(ok803) mutants were more sensitive to juglone than N2 worms (P<0.001). daf-2(e1370) mutants were completely resistant to juglone-induced mortality after 14 hours of exposure. daf-2(e1370);tdp-1(ok803) mutants were highly sensitive to juglone toxicity compared to N2 or daf-2(e1370) controls (P<0.001). (B) tdp-1(ok803) mutants were more sensitive to hydrogen peroxide than N2 worms (P<0.001). daf-2(e1370) mutants were more resistant to hydrogen peroxide-induced mortality after 14 hours of exposure than N2 worms (P<0.001). daf-2(e1370);tdp-1(ok803) mutants were highly sensitive to hydrogen peroxide toxicity compared to N2 or daf-2(e1370) controls (P<0.001). (C) tdp-1(ok803) mutants were more sensitive to high NaCl levels than N2 worms (P<0.001), while daf-2(e1370) and daf-2(e1370);tdp-1(ok803) mutants were more resistant to NaCl-induced mortality than N2 worms after 14 hours of exposure (P<0.001). (D) tdp-1(ok803) mutants were more sensitive to high sorbitol levels than N2 worms (P<0.001), while daf-2(e1370) and daf-2(e1370);tdp-1(ok803) mutants were completely resistant to Sorbitol-induced mortality after 14 hours of exposure (P<0.001). (E) RT-PCR revealed no change in the expression of the catalases ctl-1 and ctl-2 by deletion of tdp-1. Expression of the superoxide dismutase sod-3 was diminished in daf-2(e1370);tdp-1(ok803) mutants. No expression of tdp-1 was observed in tdp-1(ok803) or daf-2(e1370);tdp-1(ok803) mutants. act-3 was used as an endogenous control. Please see Figure S2 and Table S2. Not all forms of stress are equal at the cellular level so we investigated several additional forms of cellular stress including hypertonic stress with elevated sodium chloride (NaCl) or sorbitol, increased temperature, low oxygen and ultraviolet irradiation. Compared to N2 worms, tdp-1 mutants were sensitive to hypertonic stress from NaCl (Figure 2C). Interestingly, both daf-2(e1370) and daf-2(e1370);tdp-1(ok803) mutants were resistant to this hypertonic stress suggesting that tdp-1 may not function, or may function in parallel to the IIS pathway to regulate the response to osmotic stress. To confirm this hypothesis we used sorbitol, another hypertonic stress producer, and similarly observed that tdp-1(ok803) mutants were sensitive to osmotic stress but daf-2(e1370) and daf-2(e1370);tdp-1(ok803) mutants were both resistant after either 14 hours (Figure 2D) or 48 hours of exposure (Figure S2B). Finally, tdp-1 had no discernable role in the response to thermal stress, hypoxia, or radiation since there was no difference between N2 and tdp-1(ok803) worms, as well as daf-2(e1370) and daf-2(e1370);tdp-1(ok803) mutants in response to these stresses (Figure S2C–S2E). Thus, tdp-1 functions in the IIS pathway to specify the response to oxidative stress and lifespan, but tdp-1 is dispensable for the regulation of hypertonic stress by the IIS pathway. A second tdp-1 deletion allele, ok781, was available for investigation and although we observed that tdp-1(ok781) animals had a modest increase in lifespan, they were statistically indistinguishable from wild type N2 worms in stress assays (Figure S3).
A component of DAF-2's response to cellular stress is induction of DAF-16 stress response genes [9]. Thus we examined if DAF-16 targets implicated in oxidative stress signaling were affected by tdp-1. We used RT-PCR to examine expression levels of three DAF-16 genes including the catalases ctl-1 and ctl-2, as well as the superoxide dismutase sod-3 in various genetic backgrounds. First of all, no expression of tdp-1 was observed in strains containing the deletion allele tdp-1(ok803) (Figure 2E). Next we observed that ctl-1 and clt-2 expression levels were unaltered by the deletion of tdp-1, either alone or in combination with daf-2(e1370) (Figure 2E). However, we observed that sod-3 expression was greatly reduced in daf-2(e1370);tdp-1(ok803) animals (Figure 2E). These data suggest that under low IIS conditions tdp-1 is required for the expression of certain DAF-16 targets implicated in oxidative stress and may partially explain the sensitivity of tdp-1 mutants to juglone and hydrogen peroxide.
Stress and Insulin/IGF Signaling Induce TDP-1 Expression
Given the remarkable sensitivity of tdp-1(ok803) mutants to oxidative and osmotic stresses we next wanted to see if there were in vivo changes in TDP-1 expression in response to these two stress conditions. In wild type unstressed animals TDP-1::GFP is lowly expressed (Figure 3A), primarily nuclear and is expressed in most tissues (Figure 3B–3D). We next tested if shifting TDP-1::GFP animals to either NaCl or juglone plates affected TDP-1::GFP expression. Young adult TDP-1::GFP worms were exposed to either NaCl or juglone for 90 minutes and live animals were examined for changes in TDP-1::GFP expression. Strikingly, exposure to hypertonic or oxidative stress greatly increased TDP-1::GFP expression compared to untreated control worms as detected by visual inspection and quantification of images (Figure 3E and Figure S4A). Endogenous tdp-1 was not required for expression of the TDP-1::GFP transgene since expression was induced by stress in tdp-1(ok803) deletion mutants (Figure 3F and Figure S4B). As a control for generic effects of stress on transgene expression we tested two other strains and observed no induction with a neuronal GFP reporter, while juglone and NaCl induced expression of the well-characterized sod-3p::GFP reporter (Figure S5).
Fig. 3. Insulin/IGF signaling and cellular stress regulate TDP-1 expression. 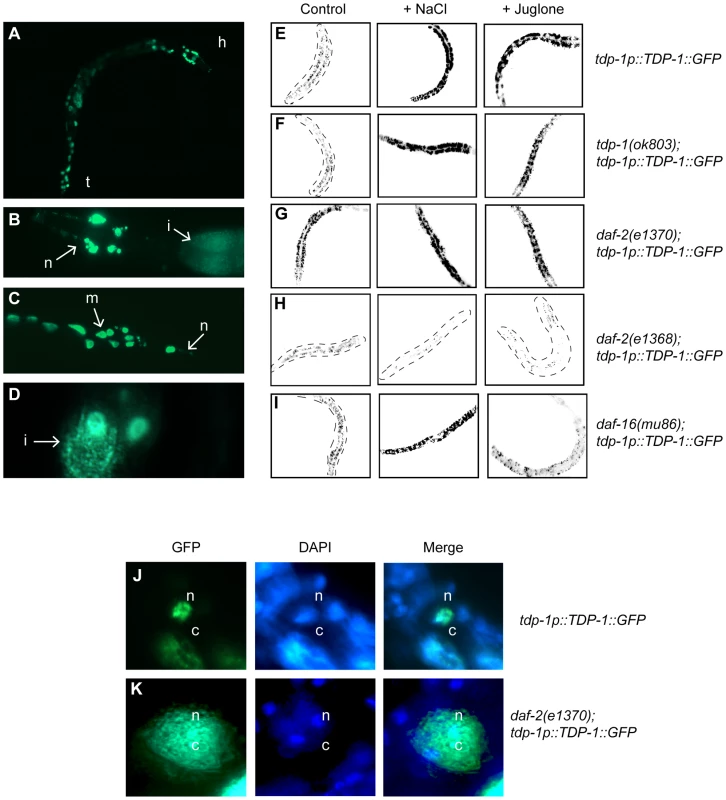
(A) Fluorescent signals observed under low magnification in an adult transgenic worm expressing a full-length TDP-1::GFP fusion under control of the endogenous tdp-1 promoter. The head (h) and tail (t) of the animal are indicated. GFP is observed in many tissues and appears to be primarily nuclear. (B) High magnification image of the head of a TDP-1::GFP transgenic revealing expression in head neurons (n) as well as the intestine (i). (C) High magnification image of the tail region of a TDP-1::GFP transgenic revealing expression in tail neurons (n) as well as muscle cells (m). (D) High magnification image revealing the expression of TDP-1::GFP in intestinal (i) cells. (E–I) are low-resolution photographs of young adult TDP-1::GFP transgenic worms. The images have been taken at the same exposure, converted to black and white and photo-reversed to aid visualization. Difficult to see worms are outlined. (E) TDP-1::GFP is widely expressed and primarily nuclear under normal conditions. TDP-1::GFP expression was highly induced by osmotic stress with NaCl or oxidative stress with juglone compared to untreated worms as seen in the left panel. (F) TDP-1::GFP expression was observed in tdp-1(ok803) mutants and was induced by osmotic and oxidative stress. (G) Compared to untreated controls in (A) TDP-1::GFP expression was elevated in daf-2(e1370) mutants and further upregulated by stress conditions. (H) TDP-1::GFP remained lowly expressed in daf-2(e1368) mutants under normal and stress conditions. (I) TDP-1::GFP showed faint expression and nuclear localization in daf-16(mu86) mutants. Osmotic stress induced TDP-1::GFP expression in daf-16(mu86) mutants. TDP-1::GFP was not induced by oxidative stress in daf-16(mu86) mutants. (J) TDP-1::GFP is primarily expressed in the nuclei (n, marked with DAPI) of non-stressed transgenics. (K) TDP-1::GFP expression is increased and is now also found in the cytoplasm (c) of daf-2 animals. Please also see Figure S4. Having shown that our TDP-1::GFP transgenics are potent stress reporters, using daf-2 and daf-16 mutants we tested if IIS regulated this effect. We observed that TDP-1::GFP was highly induced in the daf-2(e1370);TDP-1::GFP strain (Figure 3G) compared to TDP-1::GFP controls (Figure 3A) and was further elevated in daf-2(e1370) mutants exposed to stress (Figure 3G and Figure S4C). Given the pleiotropic phenotypic effects of daf-2 mutations we tested another allele and observed that opposite to e1370, TDP-1::GFP expression remained low under normal and stress conditions in the presence of the daf-2(e1368) mutation (Figure 3H and Figure S4C) suggesting a complex role for IIS in TDP-1 expression.
Looking deeper into the IIS pathway, previous research has shown that the increased stress resistance of daf-2 mutants is dependent on daf-16 mediated nuclear transcription [14]. TDP-1::GFP was not upregulated by mutation in daf-16 under normal conditions (Figure 3I). Next, to directly test if daf-16 was essential for the stress dependent induction of tdp-1 we exposed daf-16(mu86);TDP-1::GFP transgenics to stress. Exposure to NaCl continued to induce TDP-1::GFP expression in daf-16 mutants (Figure 3I) while treatment with juglone failed to induce TDP-1::GFP expression in the daf-16 mutants (Figure 3I and Figure S4D). These data suggest that the expression of TDP-1 in response to oxidative stress is dependent on daf-16, while induction of TDP-1 by osmotic stress is independent, which is consistent with our data showing that tdp-1 is not required for daf-2's resistance to hypertonic stress (Figure 2C and 2D). Additionally, the upregulation of TDP-1::GFP in daf-2(e1370) mutants was abolished by daf-16(RNAi) directly linking the IIS pathway to tdp-1 expression (Figure S6A and S6B, and Figure S4E). Finally, stress resistance and lifespan phenotypes from low IIS requires nuclear localization of DAF-16 and transcriptional activation of target genes [6], [9], [14] but we observed that tdp-1(ok803) had no effect on DAF-16::GFP stress-induced nuclear localization (Figure S6C–S6F).
Since TDP-43 is known to shuttle between the nucleus and cytoplasm we wondered if the subcellular distribution of TDP-1::GFP was altered under stress conditions. Examining TDP-1::GFP animals under high magnification we observed that when exposed low IIS from the daf-2(e1370) mutation TDP-1::GFP was no longer restricted to the nucleus and was observed in the cytoplasm (Figure 3J and 3K). These data suggest stress and/or low IIS significantly influences the expression and cellular distribution of TDP-1 proteins.
Endogenous TDP-1 Is Induced by Stress
To make certain that the induction of TDP-1 by stress was not a phenomenon restricted to our transgenic reporter strain we examined endogenous TDP-1 protein levels under normal and stress conditions. Using a monoclonal antibody against C. elegans TDP-1 (Figure S7) and western blotting we observed a significant increase in TDP-1 protein levels in N2 worms exposed to hyperosmotic or oxidative stress compared to untreated controls (Figure 4A). To confirm the opposing effects of daf-2 mutations on TDP-1 expression we examined daf-2(e1370) and daf-2(e1368) mutants under normal and stressed conditions. As seen with our TDP-1::GFP reporter strain, we observed that TDP-1 protein levels were elevated in daf-2(e1370) animals, and we observed a further increase in TDP-1 levels in daf-2(e1370) animals exposed to oxidative stress (Figure 4B). Consistent with what we observed in the daf-2(e1368);TDP-1::GFP animals, we observed that TDP-1 protein levels remained low in daf-2(e1368) animals under normal and stress conditions (Figure 4C). Finally, endogenous TDP-1 protein was lowly expressed in daf-16 mutants, greatly increased upon exposure to osmotic stress, but again remained low in daf-16 animals treated with juglone (Figure 4D) in agreement with our findings with the daf-16(mu86);TDP-1::GFP reporter strain. In summary, TDP-1 is a stress responsive protein whose expression is greatly influenced by the IIS pathway especially in the context of oxidative stress.
Fig. 4. Endogenous TDP-1 protein levels influenced by stress and Insulin/IGF signaling. 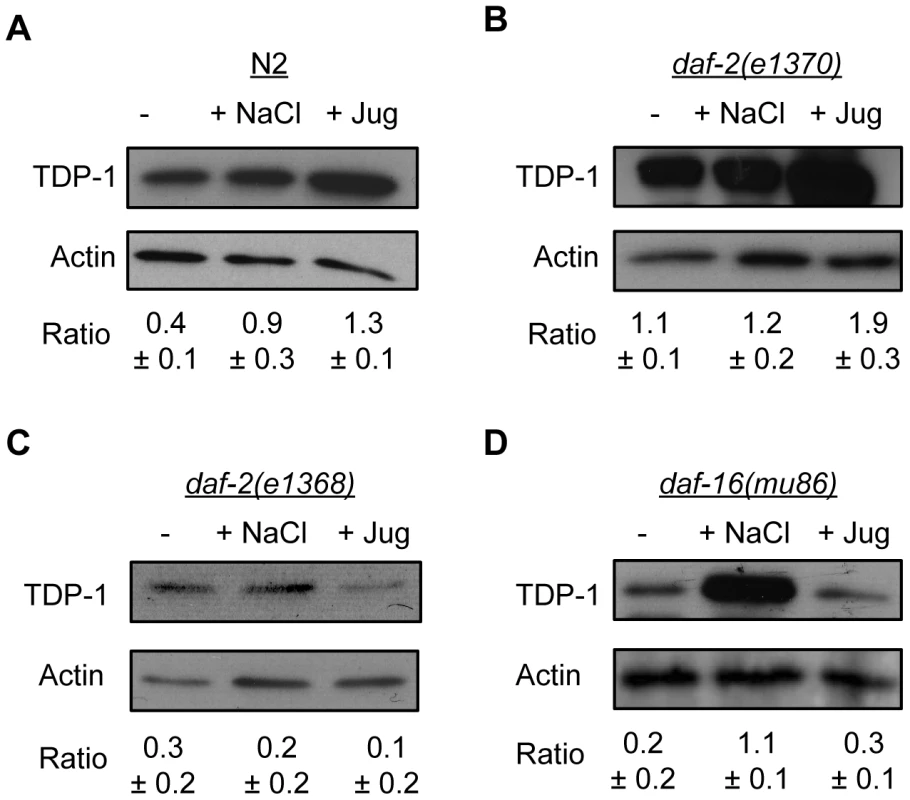
Western blotting of worm extracts from different strains under normal and stress conditions with an anti-TDP-1 antibody. (A) Osmotic and oxidative stress significantly increased the expression of TDP-1 in N2 worms. (B) The level of TDP-1 protein was comparable in untreated and osmotically stressed daf-2(e1370) worms. TDP-1 protein expression was significantly increased by exposure to oxidative stress in daf-2(e1370) worms. (C) TDP-1 protein levels remained low in daf-2(e1368) animals with or without stress. (D) TDP-1 protein expression was significantly increased by osmotic stress in daf-16(mu86) animals. Oxidative stress did not induce TDP-1 expression in daf-16 mutants. Proteotoxicity Induces TDP-1 Expression
Our findings begin to outline a complex role for TDP-1 in lifespan and the cellular stress response in relation to the IIS pathway. As one of our main interests is understanding the role of aging and stress signaling in the context of age-dependent neurodegeneration we next investigated how proteotoxicity contributed to these processes.
A feature of many late neurodegenerative disorders is proteotoxic stress caused by misfolded proteins and mutations in human TDP-43 are believed to cause proteotoxicity leading to neuronal dysfunction and cell death [15]. We examined this directly with transgenic worm strains expressing human wild type and mutant TDP-43 in worm motor neurons [16]. HSP-4 is a C. elegans Hsp70 protein orthologous to mammalian Grp78/BiP, and the transgenic C. elegans hsp-4p::GFP reporter is activated in response to misfolded proteins within the endoplasmic reticulum (ER), including chemically by compounds like tunicamycin (Figure 5A and 5B) [17]. Using this reporter we observed that transgenic strains expressing wild type TDP-43 did not induce reporter expression whereas transgenics expressing mutant TDP-43 strongly induced hsp-4p::GFP expression (Figure 5C and 5D). These data indicate that mutant TDP-43 toxicity may activate the ER unfolded protein response (UPRER). We did not observe induction of GFP in reporter strains for the mitochondrial chaperones hsp-6/Hsp70 and hsp-60/Hsp10/60 or the cytoplasmic chaperone hsp-16.2/Hsp16 (Figure S8).
Fig. 5. Mutant TDP-43 induces HSP-4/BiP expression. 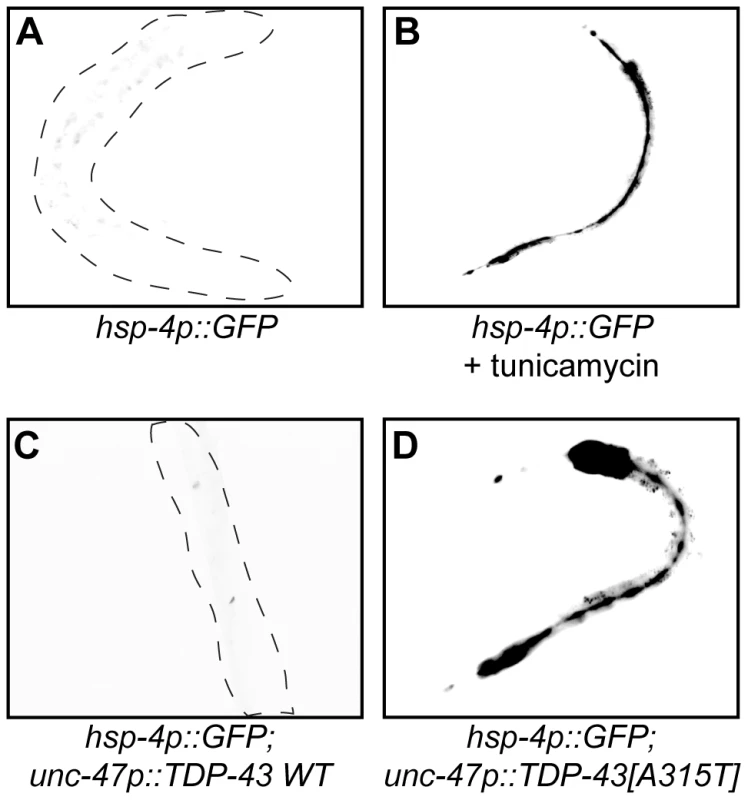
(A to D) are low-resolution photographs of young adult transgenic worms. The images have been converted to black and white and photo-reversed to aid visualization. Difficult to see worms are outlined. (A) Representative image of a young adult worm containing an integrated hsp-4p::GFP transgene under non-stressed conditions. (B) hsp-4p::GFP expression was induced by the chemical ER stressor tunicamycin. (C) hsp-4p::GFP expression was not induced by wild type TDP-43 in a double transgenic TDP-43 WT;hsp-4p::GFP strain. (D) hsp-4p::GFP expression was induced by mutant TDP-43 in a double transgenic TDP-43[A315T];hsp-4p::GFP strain. Since we identified TDP-1 as a stress responsive protein we wondered if it also responded to ER and proteotoxic stress. We noticed increased expression of TDP-1::GFP in worms grown on plates with the ER stress inducing compound tunicamycin compared to untreated controls (Figure 6A and 6B). Looking at proteotoxic stress with our TDP-43 transgenics we observed that wild type TDP-43 had no effect on TDP-1::GFP expression while mutant TDP-43 strongly induced TDP-1 expression (Figure 6C and 6D, and Figure S4F). To confirm that induction of TDP-1::GFP was due to protein misfolding and proteotoxicity and not to an artifact of mutant TDP-43 transgenes we examined another proteotoxicity model based on the expression of wild type and mutant human FUS in motor neurons [16]. FUS is a nucleic acid binding protein related to TDP-43 that has also been implicated in ALS and dementia and the expression of an ALS-linked FUS allele in C. elegans motor neurons produces degenerative phenotypes similar to mutant TDP-43 [16]. Similar to the TDP-43 model, we observed no effect on TDP-1 expression from wild type FUS but mutant FUS greatly induced TDP-1 expression (Figure 6E and 6F, and Figure S4F). These data suggest that misfolded mutant TDP-43 and FUS initiate the UPRER, which in turn activates expression of TDP-1. Finally, activation of TDP-1 by ER stress converged on the IIS since induction of TDP-1::GFP expression by tunicamycin was blocked by a null mutation in daf-16 (Figure 6G).
Fig. 6. TDP-1 expression is induced by proteotoxicity. 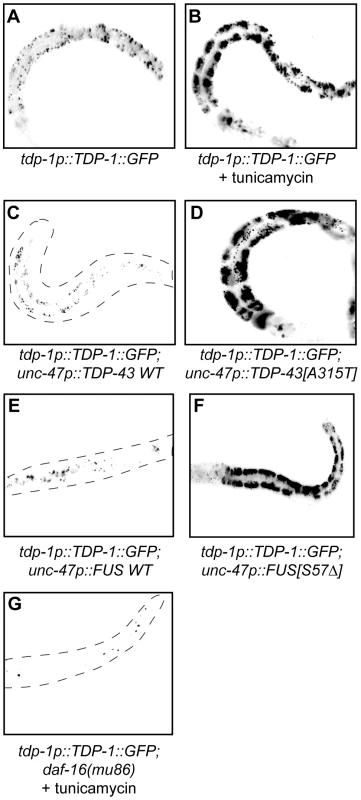
(A–G) are low-resolution photographs of young adult transgenic worms. The images have been converted to black and white and photo-reversed to aid visualization. Difficult to see worms are outlined. (A) Low expression and nuclear localization of TDP-1::GFP under normal growth conditions. (B) The ER stressor tunicamycin highly induced TDP-1::GFP expression. (C) Wild type TDP-43 did not induce TDP-1::GFP expression in a TDP-1::GFP;TDP-43 WT transgenic strain. (D) Mutant TDP-43 induced TDP-1::GFP expression in a TDP-1::GFP;TDP-43[A315T] transgenic strain. (E) Wild type FUS did not induce TDP-1::GFP expression in a TDP-1::GFP;FUS WT transgenic strain. (F) Mutant FUS induced TDP-1::GFP expression in a TDP-1::GFP;FUS[S57Δ] transgenic strain. (G) Mutation in daf-16 blocked the induction of TDP-1::GFP expression by tunicamycin treatment. Please also see Figure S4. Oxidative Stress Links Mutant TDP-43 and FUS Proteotoxicity to DAF-16
A consequence of processing misfolded proteins within the ER is the production of reactive oxygen species as part of what is termed the integrated stress response [18]. Our data show that tdp-1 responds to oxidative stress in a daf-16 dependent matter so we hypothesized that the ER stress produced from mutant TDP-43 and FUS may generate oxidative stress thus linking proteotoxicity to the IIS pathway. To test this hypothesis we stained our transgenic TDP-43 and FUS strains with dihydrofluorescein diacetate (DHF), a compound that fluoresces when exposed to intracellular peroxides associated with oxidative stress [18]. We observed no DHF signal from wild type TDP-43 and FUS transgenics but strong fluorescence from mutant TDP-43 and transgenics (Figure 7A–7D). These data suggest that in addition to activating the UPRER mutant TDP-43 and FUS generate oxidative stress. To further establish the link between proteotoxicity and the IIS we examined the subcellular localization of DAF-16. DAF-16::GFP is typically cytoplasmic under non-stressed and/or low insulin signaling conditions (Figure 7E). Consistently we observed nuclear localization of DAF-16 in a TDP-43[A315T];DAF-16::GFP strain indicating that mutant TDP-43 causes cellular stress that is transmitted by the IIS pathway (Figure 7F).
Fig. 7. Mutant TDP-43 and FUS increase oxidative stress. 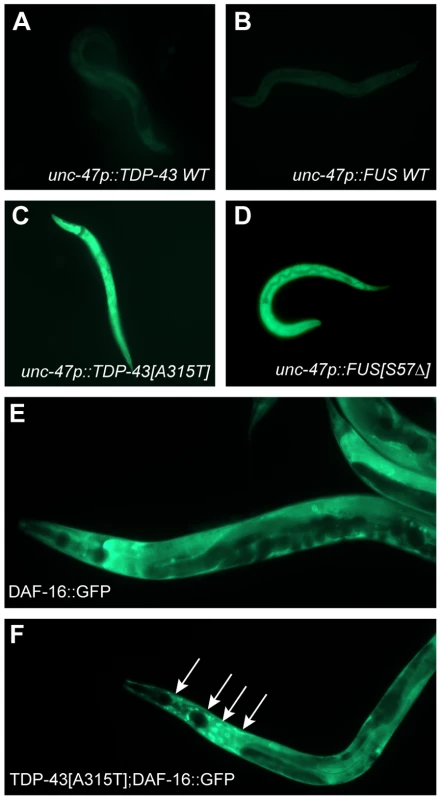
(A–D) are low-resolution images of young adult transgenic worms stained with dihydrofluorescein diacetate. Transgenics expressing wild type (A) TDP-43 or (B) wild type FUS showed no fluorescence compared to the bright fluorescent signals observed in animals expressing (C) mutant TDP-43 or (D) mutant FUS. (E to F) are higher magnification images of DAF-16::GFP transgenics. (E) Diffuse appearance of predominantly cytoplasmic DAF-16::GFP. (F) Introduction of mutant TDP-43 causes nuclear localization (arrows) of DAF-16::GFP. Opposing Effects of DAF-2 on Proteotoxicity
Several studies have shown that reduced daf-2 function suppresses proteotoxicity [19]–[22]. However we found that daf-2 mutations have opposite effects on TDP-1 expression and if elevated TDP-1 expression were cytotoxic then we would expect to see opposing effects of daf-2(e1368) and daf-2(e1370) on TDP-43 toxicity in our models. To examine this directly we created daf-2(e1368);TDP-43[A315T] and daf-2(e1370);TDP-43[A315T] strains and scored paralysis phenotypes. Interestingly, we observed that daf-2(e1368) suppressed paralysis while daf-2(e1370) enhanced paralysis compared to TDP-43[A315T] alone (Figure 8A). This intriguing finding suggests that daf-2 can have variable effects on proteotoxicity. Furthermore, daf-2(e1368) significantly reduced the motor neuron degeneration caused by mutant TDP-43 [16] while daf-2(e1370) enhanced degeneration (Figure 8B). Since the IIS pathway is believed to regulate the expression of numerous protein quality control genes we examined if the two daf-2 alleles had an effect on misfolded mutant TDP-43 with a biochemical assay to detect protein aggregation. Here, homogenized protein extracts from transgenic worms are separated into two fractions, supernatant (detergent-soluble) and pellet (detergent-insoluble) and by western blotting with human TDP-43 antibodies we have previously shown that mutant TDP-43 is prone to aggregation and is highly insoluble [16]. Looking at protein extracts from TDP-43[A315T], daf-2(e1368);TDP-43[A315T], and daf-2(e1370);TDP-43[A315T] animals we observed that daf-2(1368) greatly reduced the amount of insoluble TDP-43 compared to control transgenics while daf-2(e1370) had no effect (Figure 8C). In summary these data suggest that different daf-2 alleles have widely variable effects on proteotoxicity but are consistent for each allele: e1368 reduces mutant TDP-43 insolubility, suppresses mutant TDP-43 induced paralysis, neurodegeneration, and keeps TDP-1 expression low while e1370 has no effect on protein insolubility, enhances paralysis, neurodegeneration and induces TDP-1 expression.
Fig. 8. Opposing effects of daf-2 on proteotoxicity. 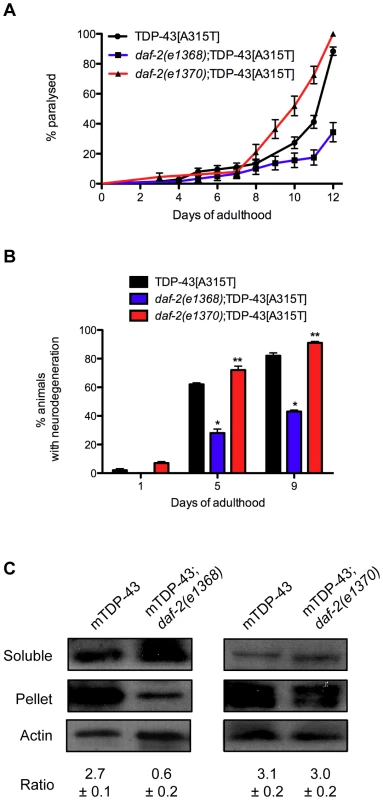
(A) daf-2(e1368);TDP-43[A315T] animals had significantly deceased rates of paralysis, while daf-2(e1370);TDP-43[A315T] animals had enhanced paralysis compared to TDP-43[A315T] transgenics alone (P<0.001). (B) daf-2(e1368);TDP-43[A315T] animals had significantly deceased motor neuron degeneration, while daf-2(e1370);TDP-43[A315T] animals had enhanced neurodegeneration compared to TDP-43[A315T] transgenics alone (*P<0.001, **P<0.05). (C) Western blotting revealed a significant reduction in the amount of insoluble TDP-43 protein in extracts from daf-2(e1368);TDP-43[A315T] animals compared to TDP-43 alone. daf-2(e1370) had no effect on mutant TDP-43 solubility. Please also see Table S3. TDP-1 Regulates Mutant TDP-43 and FUS Neuronal Toxicity
Although loss of tdp-1 sensitizes worms to oxidative and osmotic stress, elevated and chronic expression of TDP-1 leads to decreased lifespan suggesting that the induction of TDP-1 by proteotoxicity, oxidative stress or the IIS pathway may exacerbate neuronal toxicity and decrease neuronal survival. We directly tested this hypothesis by crossing TDP-1::GFP worms with our mutant TDP-43[A315T] and FUS[S57Δ] transgenics and scored for survival and the onset of paralysis. We observed that TDP-43[A315T] and FUS[S57Δ] strains containing the TDP-1::GFP transgene had significantly decreased lifespans compared to control transgenics (Figure 9A and 9B, Table S1). The expression of either mutant TDP-43[A315T] or FUS[S57Δ] causes motility defects leading to progressive paralysis and strains also expressing TDP-1::GFP had accelerated rates of paralysis compared to single transgenic controls (Figure 9C and 9D). These data suggest that induction of wild type TDP-1 expression by proteotoxicity has negative consequences on survival and neuronal function.
Fig. 9. TDP-1 regulates mutant TDP-43 and FUS proteotoxicity in C. elegans. 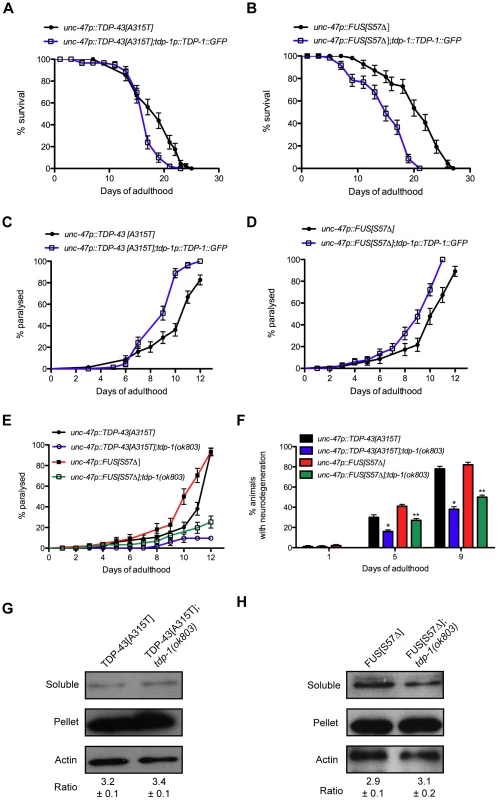
(A) TDP-43[A315T];TDP-1::GFP transgenics had shorter lifespans than transgenics expressing mutant TDP-43 alone (P<0.01). (B) FUS[S57Δ];TDP-1::GFP transgenics had shorter lifespans than transgenics expressing mutant FUS alone (P<0.001). Transgenics expressing (C) mutant TDP-43 or (D) mutant FUS along with TDP-1::GFP had accelerated rates of paralysis compared to transgenics expressing either mutant TDP-43 or mutant FUS alone (P<0.001 for mutant TDP-43 or FUS compared to mutant TDP-43;TDP-1::GFP or mutant FUS;TDP-1::GFP respectively). (E) Transgenics expressing mutant TDP-43 or FUS show age-dependent paralysis that is greatly reduced in worms harboring a deletion mutation of endogenous tdp-1 (P<0.001). (F) Age-dependent motor neuron degeneration was reduced in tdp-1(ok803);TDP-43[A315T] and tdp-1(ok803);FUS[S57Δ] strains compared to TDP-43[A315T] or FUS[S57Δ] transgenics respectively (*, **P<0.001). Deletion of tdp-1 did not affect the proportion of insoluble (G) mutant TDP-43 or (H) FUS proteins in extracts from whole worms. Please also see Tables S1 and S3. To rule out the possibility that the negative effects observed were simply due to transgene effects from TDP-1 overexpression we predicted that removing endogenous TDP-1 would reduce proteotoxicity. To test this we constructed TDP-43[A315T];tdp-1(ok803) and FUS[S57Δ];tdp-1(ok803) double mutant strains and observed that these animals had a significantly lower rate of paralysis compared to single transgenic TDP-43[A315T] or FUS[S57Δ] worms (Figure 9E). The expression of mutant TDP-43[A315T] or FUS[S57Δ] is accompanied by the age-dependent degeneration of motor neurons that was reduced in tdp-1(ok803) mutants (Figure 9F). Finally, to examine if protein misfolding was reduced in tdp-1(ok803) strains co-expressing mutant TDP-43 or mutant FUS, we examined the solubility of mutant TDP-43 and FUS proteins with our biochemical assay [16]. We observed no change in protein solubility after deletion of the endogenous tdp-1 suggesting that the protective effects are not due to down-regulation or clearance of mutant proteins (Figure 9G and 9H).
Discussion
TDP-1 Regulates Lifespan and the Cellular Stress Response
This work identified tdp-1 as a key stress responsive gene at the interface of longevity, stress resistance and neurodegeneration (Figure 10). The role of TDP-1 in lifespan is complex and suggests that worms like other species are sensitive to TDP-1/TDP-43 levels [23]–[26]. TDP-1 overexpression reduces lifespan while deletion of tdp-1 in unstressed worms promotes a modest increase in lifespan but leaves worms sensitive to specific environmental stresses.
Fig. 10. Integrated model for stress-induced TDP-1 expression. 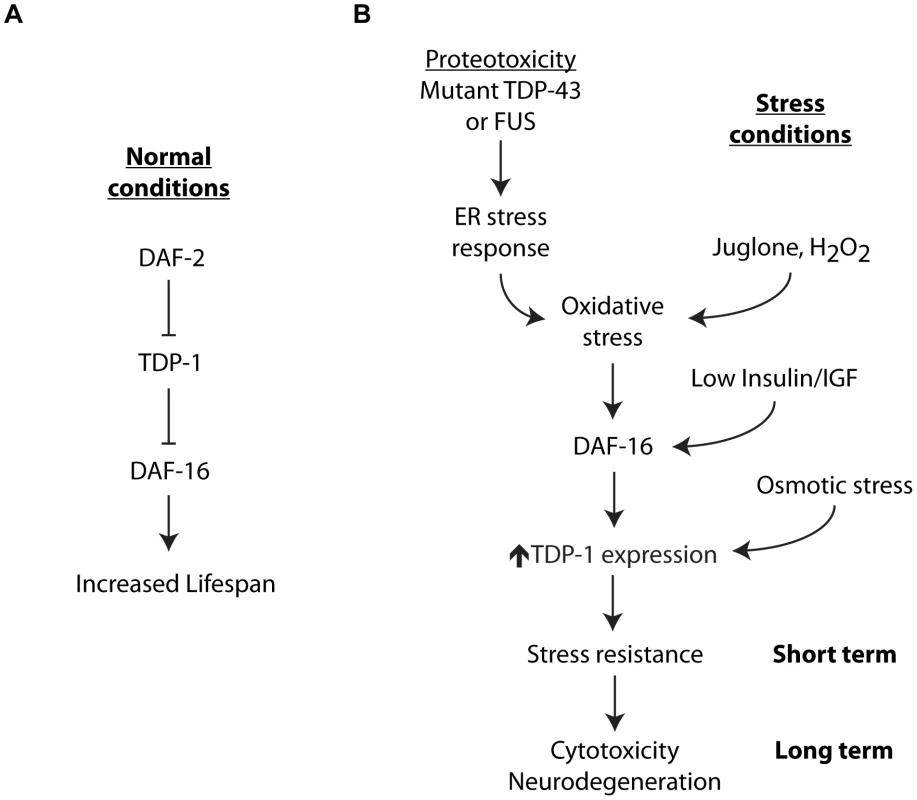
TDP-1 has multiple contributions to lifespan and the cellular stress response. (A) Under normal conditions TDP-1 may function in the Insulin/IGFP pathway upstream from DAF-16 to regulate lifespan. (B) A variety of cellular stresses induce TDP-1 expression. TDP-1 induction by oxidative stress and/or the Insulin/IGF pathway is dependent on DAF-16, while induction by osmotic stress is independent of DAF-16. Misfolded proteins activate the unfolded protein response and a secondary consequence of this is the generation of oxidative stress that in turn can induce TDP-1 expression via DAF-16. The downstream consequences of tdp-1 expression are dependent on the length and strength of induction. tdp-1 mutants are sensitive to stress suggesting that TDP-1 is essential for protection against acute stress. Genetically encoded proteotoxicity from proteins like mutant TDP-43 leads to chronic induction of TDP-1 expression with negative consequences including enhanced neurodegeneration. This model also suggests that mutant proteins may act as a seed for the induction of pathological TDP-1 expression. TDP-1 also has a complex role in the cellular stress response. We showed here that TDP-1 specifies the response to cellular stress with roles in oxidative, osmotic and protein-misfolding stress, but independent of high temperature, low-oxygen and damage from radiation. TDP-43 is a component of the ribonucleoprotein complexes known as stress granules that form under stressful conditions where they perform molecular mRNA triage where mRNAs are sorted for storage, degradation, or translation during stress and recovery [3]. The stress-inducible aspect of TDP-1/TDP-43 function likely reflects an ancient mechanism for enduring acute environmental stress until conditions improve. While the role of TDP-43 in response to acute stress is being actively studied [27]–[29], its role in response to chronic stresses like protein misfolding during aging is unknown.
Although the tdp-1 alleles ok803 and ok781 are predicted to be molecular null alleles they did not show identical phenotypes. Both alleles extended lifespan but ok781 did not affect several stress phenotypes. The difference between the two alleles is that ok781 still maintains the putative Nuclear Localization Signal (Figure S1) and any potential protein product may behave differently than ok803. Furthermore our predictions show that the ok781 allele may also produce an amino acid sequence with no known homology thus limiting its biological relevance. We believe the lifespan and stress phenotypes observed in the tdp-1(ok803) mutants are truly linked to this gene based on several lines of experimental evidence.
Concerning the lifespan phenotypes, tdp-1(RNAi) reduces the long-lived phenotype of daf-2(e1370) worms in agreement with the tdp-1(ok803);daf-2(e1370) strain mutant. We also tested if introducing wild type tdp-1 DNA sequence could rescue the long-lived phenotype of tdp-1(ok803) mutants. Using a strain expressing a full length TDP-1 open reading frame driven by the endogenous tdp-1 promoter we observed that it could partially rescue the extended lifespan phenotype of tdp-1(ok803) mutants. This is a direct proof that the lifespan phenotypes we observe in tdp-1(ok803) mutants is due to loss of the sequence and that we can correct this phenotype by introduction of wild type tdp-1 sequence.
We corroborated the role of tdp-1 in the cellular stress response with several experimental approaches. First, tdp-1(ok803) mutants are sensitive to oxidative and osmotic stress and we observed that our TDP-1::GFP reporter is induced by these same stresses. Second, western blotting with a TDP-1 antibody showed that endogenous TDP-1 protein levels are also induced by oxidative and osmotic stress. Third, our genetic experiments with tdp-1(ok803) showed that these mutants are sensitive to oxidative stress via the IIS pathway, but sensitivity to osmotic stress is independent of the IIS pathway. Our experiments with the TDP-1::GFP reporter or immunoblotting with the TDP-1 antibody fully support the observations made with the tdp-1(ok803) mutant. Finally, tdp-1(ok803) suppresses proteotoxicity while TDP-1::GFP enhances toxicity. Again, this is akin to a classic genetic rescue experiment and provides direct evidence that the phenotype observed by deletion of tdp-1 can be modified by the introduction of wild type tdp-1 sequence. In total we believe our hypothesis that tdp-1 has roles in lifespan and stress is well supported by multiple approaches. It is not clear why we see very little effect for the ok781 allele in stress assays, but at the same time there is not yet general consensus for any of the phenotypes observed for the tdp-1 mutants.
A Feed-Forward Model of TDP-1–Mediated Neurodegeneration
An early location for mutant TDP-43 and FUS toxicity may be within the ER. The ER has critical cellular functions including protein folding, and misfolded proteins within the ER cause stress leading to activation of the UPRER to restore homeostasis [30]. Cellular insults can lead to increased protein misfolding as can the expression of genetically encoded proteins like mutant TDP-43 or FUS [16]. Early phases of the UPRER are protective but ER stress also stimulates the clearance of misfolded proteins from the ER through ER-associated degradation (ERAD) by transporting misfolded proteins from the ER lumen to the cytoplasm for degradation by the ubiquitin-proteasome. ERAD is energetically costly, redox intense and leads to substantial production of oxidative stress and if the ER stress is not resolved it can lead to cell death [30]. We hypothesize that protein misfolding is an early step in neurodegeneration that may result in at least three overlapping mechanisms of toxicity: primary toxicity from misfolded proteins, secondary toxicity from increased oxidative stress and tertiary toxicity propagated by stress induced TDP-1 expression (Figure 10). This feed forward mechanism originating in the ER may drive cytotoxicity and neurodegeneration. If this mechanism is conserved, these data may help explain why the intracellular accumulation of wild type TDP-43 is observed in a growing number of neurodegenerative disorders. Furthermore, given TDP-43's propensity to aggregate and its inherent cytotoxicity, wild type TDP-43 may actively contribute to neurodegeneration. Indeed recent hypotheses suggest that mutant proteins may act as seeds for the accumulation of wild type TDP-43 into pathogenic conformations as described for prion toxicity [31].
Our model complements the “two-hits” hypothesis for sporadic diseases that highlights the role of environmental stresses in combination with cytoplasmic accumulations of TDP-43 as part of the trigger for pathogenesis [32]. Proteostasis is essential to survival and healthy aging but gradually becomes less efficient as organisms age and may contribute to the accumulation of TDP-43 [33]–[35]. Add to this the stress-induced expression of TDP-43 and the cell is faced with increased cytoplasmic aggregation leading to pathogenesis.
Many TDP-43 models have been described and they all show toxicity from the over expression of wild type TDP-43, which is sometimes less toxic than mutant but not always [11], [12], [21], [24], [36]–[41]. These findings again demonstrate that control of TDP-43 levels is important for cell survival and that wild type TDP-43 may contribute to neuronal toxicity. We genetically tested this premise in C. elegans by creating transgenic strains that either overexpressed stress-activated TDP-1 or were missing the worm's endogenous tdp-1. Paralysis and lifespan phenotypes of TDP-43 and FUS transgenics were worsened by increased expression of TDP-1. Consistently, deletion of endogenous tdp-1 from strains expressing mutant TDP-43 or FUS remarkably reduced paralysis and motor neuron degeneration phenotypes. Taken together we directly showed that wild type tdp-1 plays an important role in neurodegeneration caused by mutant TDP-43 and FUS proteins.
However two studies in C. elegans have shown no effect on TDP-43 toxicity in animals mutant for endogenous tdp-1 [24], [37] while another study has shown that deleting tdp-1 reduces TDP-43 and SOD1 toxicity [42]. The reason for the differences are not clear but may be due to differences between models, where in our model animals express mutant TDP-43 in only 26 GABAergic motor neurons [16] while the other models described rely on the expression of TDP-43 transgenes throughout the worms entire nervous system [24], [37], [42]. In our model we observe adult-onset, progressive motility defects and neurodegeneration [16], while the other models describe uncoordinated motility problems from earlier stages. It may be that the pan neuronal expression of TDP-43 in some models causes phenotypes that are too severe for modulation by reducing endogenous tdp-1. Additionally, the fact that we see a reduction of mutant FUS toxicity by deleting tdp-1 bolsters the notion that proteotoxic induction of TDP-1 propagates toxicity and this may not be a phenomenon restricted to mutant TDP-43.
A surprising finding is that the UPRER appears to be activated in a cell non-autonomous manner. The hsp-4p::GFP reporter is expressed primarily in the worms intestinal cells while mutant TDP-43 and FUS are expressed in motor neurons. Thus ER stress generated within neurons is capable of signaling to other cells and tissue types perhaps as part of a coordinated organism wide response. Cell non-autonomous signaling has been described in C. elegans for mitochondrial stress regulating longevity [43] and the heat shock response [44]. Whether mutant TDP-43 and FUS similarly induce a system wide ER stress response in mammals awaits investigation.
TDP-1 and the Insulin/IGF Pathway
Concerning the regulation of lifespan, our work describes a complex relationship between tdp-1 and daf-2. tdp-1(ok803) mutants have extended lifespan but are stress sensitive, while daf-2(e1370);tdp-1(ok803) mutants are stress sensitive and have decreased lifespan compared to daf-2(e1370) alone. In all systems examined increased TDP-43 expression is toxic [45] and there is widespread speculation in the field that wild type TDP-43 may contribute to cytotoxicity and neurodegeneration over long term settings [31]. Thus deleting tdp-1 from worms leaves them sensitive to stress but frees them from potential long-term cytotoxic effects. We are not alone in the observation that removing tdp-1 increases lifespan [42] but we are the first to look at tdp-1's role in the cellular stress response. Looking at daf-2, the difference here may be that tdp-1 is essential for the stress resistance aspects of daf-2 mutants and concomitant long-lived phenotype. Thus removing tdp-1 renders daf-2 animals sensitive to stress induced damage limiting their extended lifespan.
A surprising finding was the opposing effects of daf-2 mutations on proteotoxicity and TDP-1 expression. Several publications have reported that reduced daf-2 function suppresses proteotoxicity [19]–[21]. Our experiments are not fully comparable to these studies since some studies rely solely on daf-2(RNAi) or on a single daf-2 allele (e1370) to investigate proteotoxicity while we looked at two different daf-2 alleles, e1368 and e1370. Further complicating the matter is the fact that some models are based on muscle-cell expression vectors and/or have severe developmental effects like the recently described TDP-43 model [21].
daf-2 mutations are grouped into a complex allelic series comprising two classes. Class 1 alleles are less severe and mutations fall within the extracellular regions of the DAF-2 receptor protein [46]. Class 2 alleles tend to be more severe, display pleiotropic effects and the mutations are found within the ligand binding area of the receptor or the tyrosine kinase domain [46]. Using the class 1 allele daf-2(e1368) and the class 2 allele daf-2(e1370) we observed that the two alleles had opposing effects on mutant TDP-43 toxicity and TDP-1 expression. In our mutant TDP-43 worms we observed that daf-2(e1368) suppressed mutant TDP-43 induced paralysis, while the class 2 allele daf-2(e1370) enhanced paralysis. Furthermore, daf-2(e1368) reduced the insolubility of mutant TDP-43 protein, while daf-2(e1370) had no effect on mutant TDP-43 solubility. Using a TDP-1::GFP reporter or western blotting with a TDP-1 antibody we observed that daf-2(e1370) increased the expression of TDP-1 while daf-2(e1368) had no effect. Given that the IIS pathway regulates both the toxicity of mutant TDP-43 proteins and the expression of endogenous TDP-1/TDP-43, altered IIS may directly contribute to ALS pathogenesis.
The pleiotropic and sometimes opposite effects of daf-2 are known and they are consistent within each class [46]. Regarding stress, a notable example is that class 1 daf-2(e1368) mutants are sensitive to hypoxia while class 2 daf-2(e1370) mutants are highly resistant to hypoxia [47]. Here we observe opposing effects for daf-2 on proteotoxicity and the cytotoxic induction of TDP-1 expression both of which are consistent for each allele. Our data are in agreement with studies showing that reduced IIS diminishes proteotoxicity but the question remains as to why the more severe class 2 daf-2(e1370) allele enhances toxicity in our TDP-43 transgenics. Part of the reason may lie with intrinsic differences between models. Our transgenics are based on the expression of TDP-43 in 26 GABAergic neurons and the worms do not show motor impairment until well into adulthood [16] while the other models show early effects and the animals are severely impaired by the time they reach adulthood [19], [21]. Thus it may be that stronger daf-2 mutations are required to suppress severe phenotypes but are too strong for the milder, late-onset toxicity in our TDP-43 transgenics. There may be an optimal rate of IIS unique to each situation that has been referred to the Insulin Signalling Paradox [10]. Our data begin to shed light on this phenomenon where changes in signaling can have dramatically different effects and suggests that the role of IIS in neurodegeneration is more complicated that currently appreciated and requires further investigation.
Conclusion
It is still unclear if mutant TDP-43 results in a gain, a loss of function or both but work from zebrafish and fly TDP-43 models suggest that it may be both [11], [41]. Our data introduce a novel gain of function mechanism where the increased expression of wild type TDP-1 is induced by proteotoxic stress. Several strategies come to mind to alleviate the neuronal toxicity caused by wild type and mutant TDP-43 including reducing levels of wild type TDP-43, mutant TDP-43, and/or reducing UPRER stress by promoting protein folding. In the future it will be important to determine if strategies to reduce TDP-43 neuronal toxicity may be applicable to additional neurodegenerative disease as a shared mechanism of cell death in the development of new therapeutics.
Materials and Methods
C. elegans Strains
Nematode strains used were described previously [16] or received from the Caenorhabditis elegans Genetics Center CGC (St Paul, MN). All strains were maintained following standard methods on OP50 bacteria plates. Strains used in this study include: tdp-1(ok803) and tdp-1(ok781) both outcrossed 5 times to N2 prior to use, daf-2(e1368), daf - 2(e1370), daf-16(mu86), gpIs1[hsp-16.2::GFP], oxIs12[unc-47p::GFP;lin-15(+)], xqIs93[tdp-1p::TDP-1::GFP], xqIs132[unc-47p::TDP-43-WT;unc-119(+)], xqIs133[unc-47p::TDP-43[A315T];unc-119(+)], xqIs173[unc-47p::FUS-WT;unc-119(+)], xqIs98[unc-47p::FUS[S57Δ];unc-119(+)], zIs356[daf-16p::daf-16-gfp; rol-6], zcIs4[hsp-4p::GFP], zcIs9[hsp-60p::GFP],gpIs1[hsp-16.2p::GFP] and zcIs13[hsp-6p::GFP].
Lifespan Assays
Sixty synchronized L4 were grown on OP50 bacteria plates (20 animals/plate) and three independent assays were performed. Lifespan analyses were performed at 20°C and 25°C and worms were scored every 1–2 days from adult day 1 until death. Worms were scored dead if they didn't respond to tactile or heat stimulus.
RNAi Experiments
RNAi-treated strains were fed with E. coli (HT115) containing an Empty Vector (EV), daf-16 (R13H8.1), or tdp-1 (F44G4.4) RNAi clones from the ORFeome RNAi library (Open Biosystems). RNAi experiments were performed at 20°C. Worms were grown on NGM enriched with 1 mM Isopropyl-b-D-thiogalactopyranoside (IPTG). For lifespan, worms were transferred to RNAi 5-fluorodexyuridine (FUDR, 12.5 mg/L) plates at adult day 1 until death. Worms were declared dead if they didn't respond to tactile or heat stimulus. Experiments were conducted with 20 animals/plate by triplicates.
Dauer Formation Assay
Young adult daf-2(e1370) were allowed to lay eggs overnight at 20°C. The eggs were then transferred to 25°C and scored for dauer formation 5 days later. Three different trials on different days were performed.
Stress Assays
Stress tests were performed at 20°C (oxidative, osmotic and UV stress), 25°C (hypoxia) and 37°C (thermal stress). Worms were grown on NGM and transferred to NGM plates + 240 µM juglone (oxidative stress), or NGM plates + 10 mM Hydrogen peroxide (oxidative stress), or NGM plates + 400 mM NaCl (osmotic stress), or NGM plates + 611 mM Sorbitol (osmotic stress), all at adult day 1. For the oxidative, osmotic and thermal stress assays, worms were evaluated for survival every 30 minutes for the first 2 hours and every 2 hours after up to 14 hours; for sorbitol we also performed a test over 48 hours, starting the counts after 14 hours on the compound. For UV irradiation, adult day 1 worms were transferred to NGM plates without any food source and exposed to UV (1200 J/m2). Worms were then transferred to NGM plates with OP50 bacteria and died animals were counted every 2 hours till 14 hours after irradiation. For hypoxia experiments young adult animals were transferred to a new plate and subjected to low oxygen conditions with the AnaeroPack system (Mitsubishi Gas Chemical America) for 24 hours and assayed for survival. For all experiments nematodes were scored as dead if they were unable to move in response to heat or tactile stimuli. For all tests worms, 20 animals/plate by triplicates were scored.
Strain Construction
Gateway system (Invitrogen) compatible tdp-1 promoter and open reading frame plasmid clones were obtained from Open Biosystems and recombined with plasmid pDES-MB14 (kindly donated by M. Vidal, Harvard), and verified by sequencing to create a tdp-1p::TDP-1::GFP plasmid, which was injected at 5 ng/µl into unc-119(ed3) animals along with myo-3p::mCherry, myo-2p::mCherry comarkers at 5 ng/µl and wild type transformants expressing GFP were kept. The transgene was integrated using UV radiation and wild type, GFP positive animals were kept for further study. Multiple stable transgenics were isolated and outcrossed to N2 4 times before use. Strain XQ93 xqIs93[tdp-1p::TDP-1::GFP] was used in this study.
Fluorescence Microscopy
For visualization of TDP-1::GFP animals, M9 buffer with 5 mM levamisole was used for immobilization. Animals were mounted on slides with 2% agarose pads. TDP-1::GFP expression was visualized with a Leica CTR 6000 and a Leica DFC 480 camera. L4 animals were grown on NGM plates and transferred to NGM plates + 240 µM juglone (oxidative stress) or NGM plates + 400 mM NaCl (osmotic stress) for 90 minutes, and examined for fluorescence with the Leica system described above. Some animals were also stained with DAPI (1∶1000, diluted in 1× PBS). Image processing was done with Adobe Photoshop. For images of TDP-1::GFP alone, images were converted to black and white and the images reversed to allow for better contrast and visualization. Quantification of TDP-1::GFP levels was done with ImageJ (NIH) and the mean and SD was calculated from 5 images for each strain and experimental condition. For visualization of DAF-16::GFP, hsp-4p::GFP, hsp-6p::GFP, hsp-16.2p::GFP and hsp-60p::GFP animals, M9 buffer with 5 mM levamisole was used for immobilization. Animals were mounted on slides with 2% agarose pads and examined for fluorescence with the Leica system described above.
Dihydrofluorescein Diacetate Assay
For visualization of oxidative damage in the transgenic strains the worms were incubated on a slide for 30 minutes with 5 mM dihydrofluorescein diacetate dye (Sigma-Aldrich) and then washed with 1× PBS three times. After the slide was fixed fluorescence was observed with the Leica system described above.
RT–PCR
RNA was extracted with an RNAeasy kit (Qiagen) and reverse transcribed with QuantiTect (Qiagen). Primers used include: ctl-1 forward, AGGTCACCCATGACATCACCAAGT; ctl-1 reverse, GAT TGCGCTTCAGGGCATGAATGA; ctl-2 forward, TTCGCTGAGTTGAACAATCCG; ctl-2 reverse, GTTGCTGATTGTCATAAGCCATTGC; tdp-1 forward, AAAGTGGGATCGAGTGACGAC; tdp-1 reverse, GACAGCGTAACGAATGCAAAGC; sod-3 forward, CGAGCTCGAACCTGTAATCAGCCATG; sod-3 reverse, GGGGTACCGCTGATATTCTTCCACTTG; act-3 forward, GTTGCCGCTCTTGTTGTAGAC; act-3 reverse, GGAGAGGACAGCTTGGATGG.
Worm Lysates
Worms were collected in M9 buffer, washed 3 times with M9 and pellets were placed at −80°C overnight. Pellets were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris pH 7.4, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate) + 0.1% protease inhibitors (10 mg/ml leupeptin, 10 mg/ml pepstatin A, 10 mg/ml chymostatin LPC;1/1000). Pellets were passed through a 271/2 G syringe 10 times, sonicated and centrifuged at 16000 g. Supernatants were collected.
Protein Solubility
For TDP-43 and FUS transgenics, soluble/insoluble fractions, worms were lysed in Extraction Buffer (1 M Tris-HCl pH 8, 0.5 M EDTA, 1 M NaCl, 10% NP40 + protease inhibitors (LPC;1/1000). Pellets were passed through a 271/2 G syringe 10 times, sonicated and centrifuged at 100000 g for 5 minutes. The soluble supernatant was saved and the remaining pellet was resuspended in extraction buffer, sonicated and centrifuged at 100000 g for 5 minutes. The remaining pellet was resuspended into 100 µl of RIPA buffer, sonicated until the pellet was resuspended in solution and saved.
Protein Quantification
All supernatants were quantified with the BCA Protein Assay Kit (Thermo Scientific) following the manufacturer instructions.
Immunoblot
Worm RIPA samples (175 µg/well for transgenic worms; 15 µg/well for non transgenics) were resuspended directly in 1× Laemmli sample buffer, migrated in 10% polyacrylamide gels, transferred to nitrocellulose membranes (BioRad) and immunoblotted. Antibodies used: rabbit anti-TDP-43 (1∶200, Proteintech), rabbit anti-FUS/TLS (1∶200, Abcam), rabbit anti-TDP-1 (1∶500, Petrucelli laboratory) and mouse anti-Actin (1∶10000, MP Biomedical). Blots were visualized with peroxidase-conjugated secondary antibodies and ECL Western Blotting Substrate (Thermo Scientific). Densitometry was performed with Photoshop (Adobe).
Statistics
For lifespan and stress-resistance tests, survival curves were generated and compared using the Log-rank (Mantel-Cox) test, and 20–30 animals were tested per genotype and repeated at least three times. For progeny counts, dauer-formation assays and hypoxia tests the mean and SEM were calculated for each trial and two-tailed t-tests were used for statistical analysis.
Supporting Information
Zdroje
1. Lagier-TourenneCPolymenidouMClevelandDW 2010 TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet 19 R46 64
2. AndersonPKedershaN 2009 Stress granules. Curr Biol 19 R397 398
3. AndersonPKedershaN 2009 RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol 10 430 436
4. KenyonC 2005 The plasticity of aging: insights from long-lived mutants. Cell 120 449 460
5. KenyonCChangJGenschERudnerATabtiangR 1993 A C. elegans mutant that lives twice as long as wild type. Nature 366 461 464
6. LibinaNBermanJRKenyonC 2003 Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115 489 502
7. LinKDormanJBRodanAKenyonC 1997 daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278 1319 1322
8. LinKHsinHLibinaNKenyonC 2001 Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet 28 139 145
9. MurphyCTMcCarrollSABargmannCIFraserAKamathRS 2003 Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 277 283
10. CohenEDillinA 2008 The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci 9 759 767
11. EstesPSBoehringerAZwickRTangJEGrigsbyB 2011 Wild-type and A315T mutant TDP-43 exert differential neurotoxicity in a Drosophila model of ALS. Hum Mol Genet
12. XuYFGendronTFZhangYJLinWLD'AltonS 2010 Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci 30 10851 10859
13. Van RaamsdonkJMHekimiS 2009 Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet 5 e1000361 doi:10.1371/journal.pgen.1000361
14. HendersonSTJohnsonTE 2001 daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol 11 1975 1980
15. DouglasPMDillinA 2010 Protein homeostasis and aging in neurodegeneration. J Cell Biol 190 719 729
16. VaccaroATauffenbergerAAggadDRouleauGDrapeauP 2012 Mutant TDP-43 and FUS Cause Age-Dependent Paralysis and Neurodegeneration in C. elegans. PLoS ONE 7 e31321 doi:10.1371/journal.pone.0031321
17. UranoFCalfonMYonedaTYunCKiralyM 2002 A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol 158 639 646
18. HardingHPZhangYZengHNovoaILuPD 2003 An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11 619 633
19. CohenEBieschkeJPerciavalleRMKellyJWDillinA 2006 Opposing activities protect against age-onset proteotoxicity. Science 313 1604 1610
20. Teixeira-CastroAAilionMJallesABrignullHRVilacaJL 2011 Neuron-specific proteotoxicity of mutant ataxin-3 in C. elegans: rescue by the DAF-16 and HSF-1 pathways. Hum Mol Genet 20 2996 3009
21. ZhangTMullanePCPerizGWangJ 2011 TDP-43 neurotoxicity and protein aggregation modulated by heat shock factor and insulin/IGF-1 signaling. Hum Mol Genet
22. ParkerJAArangoMAbderrahmaneSLambertETouretteC 2005 Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37 349 350
23. AyalaYMDe ContiLAvendano-VazquezSEDhirARomanoM 2011 TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J 30 277 288
24. AshPEZhangYJRobertsCMSaldiTHutterH 2010 Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet
25. WegorzewskaIBellSCairnsNJMillerTMBalohRH 2009 TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A 106 18809 18814
26. JohnsonBSMcCafferyJMLindquistSGitlerAD 2008 A yeast TDP-43 proteinopathy model: Exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci U S A
27. ColombritaCZennaroEFalliniCWeberMSommacalA 2009 TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem 111 1051 1061
28. BoscoDALemayNKoHKZhouHBurkeC 2010 Mutant FUS Proteins that Cause Amyotrophic Lateral Sclerosis Incorporate into Stress Granules. Hum Mol Genet
29. McDonaldKKAulasADestroismaisonsLPicklesSBeleacE 2011 TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet
30. WalkerAKAtkinJD 2011 Stress signaling from the endoplasmic reticulum: A central player in the pathogenesis of amyotrophic lateral sclerosis. IUBMB Life
31. PolymenidouMClevelandDW 2011 The Seeds of Neurodegeneration: Prion-like Spreading in ALS. Cell 147 498 508
32. PesiridisGSTripathyKTanikSTrojanowskiJQLeeVM 2011 A “two-hit” hypothesis for inclusion formation by carboxyl-terminal fragments of TDP-43 protein linked to RNA depletion and impaired microtubule-dependent transport. J Biol Chem 286 18845 18855
33. MakridesSC 1983 Protein synthesis and degradation during aging and senescence. Biol Rev Camb Philos Soc 58 343 422
34. PartridgeLGemsD 2002 Mechanisms of ageing: public or private? Nat Rev Genet 3 165 175
35. RattanSIClarkBF 1996 Intracellular protein synthesis, modifications and aging. Biochem Soc Trans 24 1043 1049
36. WilsHKleinbergerGJanssensJPeresonSJorisG 2010 TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A 107 3858 3863
37. LiachkoNFGuthrieCRKraemerBC 2010 Phosphorylation Promotes Neurotoxicity in a Caenorhabditis elegans Model of TDP-43 Proteinopathy. J Neurosci 30 16208 16219
38. LiYRayPRaoEJShiCGuoW 2010 A Drosophila model for TDP-43 proteinopathy. Proc Natl Acad Sci U S A 107 3169 3174
39. BarmadaSJSkibinskiGKorbERaoEJWuJY 2010 Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci 30 639 649
40. ZhangYJXuYFCookCGendronTFRoettgesP 2009 Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A 106 7607 7612
41. KabashiELinLTradewellMLDionPABercierV 2009 Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum Mol Genet 19 671 683
42. ZhangTHwangHYHaoHTalbotCJrWangJ 2012 Caenorhabditis elegans RNA-processing Protein TDP-1 Regulates Protein Homeostasis and Lifespan. J Biol Chem
43. DurieuxJWolffSDillinA 2011 The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144 79 91
44. PrahladVCorneliusTMorimotoRI 2008 Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320 811 814
45. Da CruzSClevelandDW 2011 Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr Opin Neurobiol 21 904 919
46. PatelDSGarza-GarciaANanjiMMcElweeJJAckermanD 2008 Clustering of genetically defined allele classes in the Caenorhabditis elegans DAF-2 insulin/IGF-1 receptor. Genetics 178 931 946
47. ScottBAAvidanMSCrowderCM 2002 Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296 2388 2391
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



