-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenomic Data Reveal a Complex Making of Humans
In the last few years, two paradigms underlying human evolution have crumbled. Modern humans have not totally replaced previous hominins without any admixture, and the expected signatures of adaptations to new environments are surprisingly lacking at the genomic level. Here we review current evidence about archaic admixture and lack of strong selective sweeps in humans. We underline the need to properly model differential admixture in various populations to correctly reconstruct past demography. We also stress the importance of taking into account the spatial dimension of human evolution, which proceeded by a series of range expansions that could have promoted both the introgression of archaic genes and background selection.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002837
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1002837Summary
In the last few years, two paradigms underlying human evolution have crumbled. Modern humans have not totally replaced previous hominins without any admixture, and the expected signatures of adaptations to new environments are surprisingly lacking at the genomic level. Here we review current evidence about archaic admixture and lack of strong selective sweeps in humans. We underline the need to properly model differential admixture in various populations to correctly reconstruct past demography. We also stress the importance of taking into account the spatial dimension of human evolution, which proceeded by a series of range expansions that could have promoted both the introgression of archaic genes and background selection.
Introduction
Until recently, the out-of-Africa model of human evolution was favoured by most genetic analyses, but this model collapsed when the sequencing of the Neanderthal genome revealed that 1%–3% of the genome of Eurasians was of Neanderthal origin. At the same time, refined analyses of modern human genomic data [1]–[3] have changed our view of evolutionary forces acting on our genome. While most people assumed that the out-of-Africa expansion had been characterized by a series of adaptations to new environments [4]–[6] leading to recurrent selective sweeps [7], our genome actually contains little trace of recent complete sweeps [2], [3], [8] and the genetic differentiation of human population has been very progressive over time, probably without major adaptive episodes [9]. In this review, we detail these changes of paradigm and we discuss their implication for future studies of human diversity.
Interbreeding between Modern and Archaic Humans
In line with previous studies [10]–[12] which suggested that some aspects of human genomic diversity were incompatible with a complete replacement of archaic hominins, evidence for admixture between humans and Neanderthals emerged from the first analysis of a complete Neanderthal genome [13]. Indeed, the presence of a significant excess of Neanderthal-derived alleles in Eurasian populations as compared to Africans has been interpreted as resulting from an admixture episode between the ancestors of Eurasians and Neanderthals somewhere in the Middle East [13] (Figure 1A). Even though the existence of a very ancient population subdivision in Africa from which both Neanderthals and Eurasians would have emerged could lead to similar patterns [14], the maintenance of such a subdivision over tens of thousands of generations seems unlikely. The sequencing of another archaic hominin from the Denisova cave in the Altaï mountains in Siberia has further revealed that Papua New Guineans showed signs of introgression from this archaic human [15]. Further studies of 33 populations from Southeast Asia and Oceania [16] showed that Denisovan admixture was actually present in other Oceanians, Melanesians, Polynesians, and east Indonesians but was virtually absent in mainland east Asians (but see [17] for evidence of possible Denisovan introgression on the Asian continent). Overall, these genomic analyses of admixture suggest that 1%–3% of the genome of all Eurasians and native Amerindians is of Neanderthal origin [15], and that Papua New Guineans and Australians have another 3.5% of their genome of Denisovan origin [16]. The out-of-Africa model of human evolution, which posited a complete replacement of archaic by modern humans in Eurasia, thus needs to be modified to include a limited assimilation of archaic genes, but the fact that most of the genetic variation observed in extant non-African populations comes from Africa remains true.
Fig. 1. Sketches of different scenarios of human dispersal and admixture with archaic human populations during their range expansion out of Africa. 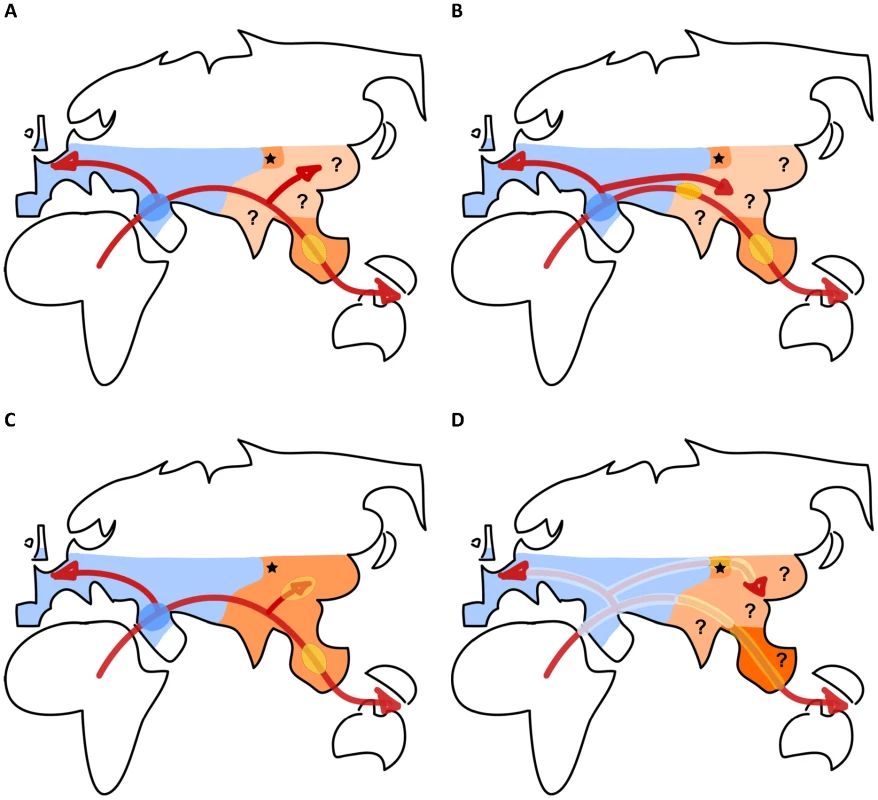
Red arrows indicate approximate migration routes. Neanderthal range is in blue, Denisovan range(s) in orange, and the location of the Denisova site is indicated as a black star. Question marks in the Denisovan range indicate uncertainty on Denisovan hominin presence. Filled ellipses indicate potential places of admixture in scenarios (A–C). (A) Scenario of Reich et al. [15], [16] with pulses of admixture between modern humans and Neanderthals (dark blue ellipse) and between modern humans and Denisovans (yellow ellipse). (B) Scenario of Rasmussen et al. [24] with two waves into Asia. Denisovan admixture in Oceanians would have occurred during the first wave, possibly at different places during the migration. (C) Scenario of Skoglund and Jakobsson [17], with distinct Denisovan admixture events in Oceanians and East Asians. (D) Extension of the spatially explicit scenario of Currat and Excoffier [25] postulating a continuous admixture between modern humans and archaic hominins along migration routes overlapping with archaic hominin ranges. Different shades of orange indicate potentially different archaic hominin populations in Asia. The finding of archaic admixture in Eurasia gives credit to previous statistical analyses, which have suggested the presence of archaic material in Eurasian and African populations [11]. In order to better assess the possibility of admixture in Africa, Hammer and colleagues [18] recently looked for signals of archaic admixture in two African hunter-gatherer populations and in a West African farmer population using a set of 61 non-coding autosomal loci. They found that an absence of admixture could not explain observed patterns of linkage disequilibrium in the hunter-gatherer populations, suggesting that they were potentially admixed with a yet unknown archaic hominin. A model including admixture suggested a recent admixture event (10–40 Kya) with a very divergent archaic population. While the confidence intervals of the archaic admixture rate are extremely broad (ranging from 0% to 100%), point estimates suggest that admixture was low and limited to 0.5%–2%. It remains to be shown if this estimate would be sensitive to other forms of admixture (e.g., with Bantu recent input into Pygmies and San [19]–[21]).
Where and How Did Admixture Occur?
There is thus both direct [13], [15] and indirect [11], [18] evidence for archaic admixture on four continents, suggesting that modern humans have not been totally genetically isolated since their emergence, some 150–200 Kya in East Africa [22], [23]. However, there is still quite some discussion about the place, the timing, the exact numbers of admixture events, and the biological implications of these interbreeding events (see Figure 1). The finding of almost equal levels of Neanderthal introgression in all Eurasians has been interpreted as evidence for a unique pulse of admixture in the Middle East between Neanderthals and the ancestors of Eurasians [13] (Figure 1A). The fact that Denisovan admixture had been first evidenced in Papua New Guineans suggested that admixture had occurred as a single pulse in Southeast Asia, after the separation of the ancestors of Oceanians and other Asians [15], [16] (Figure 1A). The analysis of an Australian genome has confirmed the presence of Denisovan admixture in Australians [24] and suggested that admixture occurred during a first early wave of colonization towards Oceania, either in Southeast Asia or earlier in Eurasia (Figure 1B). A reanalysis of a large human SNP database and its comparison with Denisovan-derived alleles has suggested the presence of Denisovan admixture in East Asians, albeit at lower levels than in Oceanians [17], which could have occurred at a different place than for Oceanians, somewhere in East Asia (Figure 1C). Contrastingly, Currat and Excoffier [25] introduced a spatially explicit model of interbreeding between Neanderthals and Eurasians that could occur over the whole Neanderthal range (Figure 1D). They obtained similarly low levels (1%–3%) of Neanderthal introgression in both Europe and China if interspecific exchanges were locally extremely limited (only 200–400 interbreeding events over the >6,000 years of co-existence between the two species). An extension of this scenario to Denisovan admixture would imply that modern humans could have hybridized along all migration routes overlapping with the range(s) of archaic humans (Figure 1D). The fact that the largest levels of Denisovan introgression are found in Oceanians raises the question of a potential discontinuity in the Denisovan range (Figure 1A, 1B) or of a genetic differentiation of archaic hominins living in different ecosystems (Figure 1D). Alternatively, modern humans could have admixed with other hominins [26], and/or inferred hominin introgression could result from the sharing of some derived sites between Neanderthals, Denisovans, and unidentified archaic hominins. A scenario involving an unsampled Eurasian archaic hominin has received support from a recent study [27] showing the presence of a highly divergent (>3 Mya) haplotype of the innate immune gene OAS1. This deep lineage is found at high frequencies in Oceania (and at lower frequencies up to Pakistan). This DNA segment is more closely related (0.6 Mya divergence) to the Denisova sequence than to the Neanderthal sequence, which is itself closer to the human reference sequence. It has been speculated [27] that this fragment had introgressed from a more archaic hominin than Denisovans, who could have been themselves introgressed earlier.
Genomic Distribution of Archaic Admixture Is Still Lacking
Our understanding of the exact sequence and location of admixture events would highly benefit from a more precise knowledge of the nature and the distribution of Neanderthal segments in our genome. Unfortunately, current estimations of introgression levels are based on a statistic measuring a genome-wide difference in the proportion of archaic-derived alleles between two human populations [13], [14], so that the genomic distribution of introgressed segments is still unknown. However, in addition to the OAS1 segment mentioned above [27], several authors have recently argued they had identified candidate regions harboring archaic haplotypes [13], [28], [29]. These regions usually show highly divergent haplotypes with very little evidence for recombination [30]. A dozen genomic regions where Eurasians have haplotypes much more divergent than Africans and a high proportion of derived Neanderthal alleles have been proposed as candidates for Neanderthal introgression [13]. More recently, an X-linked haplotype (B006) in an intron of the dystrophin (dys44) gene, almost absent from Africa but with 9% average frequency outside Africa, has been proposed to be of Neanderthal origin [29]. It is close to the ancestral X haplotype, shares 2/3 of derived alleles with Neanderthals, and has little associated diversity, suggesting a recent origin in humans. Another study has also suggested that several immune-related HLA class I alleles in humans could be of Denisovan origin and that they helped Eurasian populations build their immunity [28]. Whereas the hypothesis of an adaptive introgression is highly seductive, its support is relatively thin. “Denisovan” HLA class I alleles are currently not confined to Oceania but are found widespread in Asia. Moreover, the strongest argued case of Denisovan allelic ancestry (HLA-B*73) is actually not found at all in the Denisovan genome and is presently distributed in western Asia, well in the former Neanderthal range. One should therefore be extremely cautious not to assume that each very divergent haplotype found in humans is necessarily of archaic origin, as cases of incomplete lineage sorting are not rare between higher primates [31], especially in the HLA system where trans-specific polymorphism is facilitated by balancing selection [32]. However, if some introgressed genes were really advantageous, they should have spread and fixed in the human population, but as discussed below there is no widespread signature of strong selective sweeps in Eurasia.
It may nevertheless be valuable to identify further genomic regions of potential archaic origin. Previous candidate regions have been identified, as they showed a much larger time to the most recent common ancestor (TMRCA) in Eurasia than in Africa. This signal may, however, not be optimal, since if Neanderthals and modern humans diverged only 270–440 Kya [13], the presence of some Neanderthal lineages in a Eurasian population should not greatly affect the TMRCA unless Eurasian ancestors had gone through a very drastic bottleneck, which does not seem the case [33]. Indeed, modern human segments show a TMRCA modal value around 1.5 Mya [34], well beyond the divergence with Neanderthals/Denisovans. Assuming that large TMRCA is a true signal of admixture, one would expect to see many more regions of potential archaic origin in Oceanians, which show higher levels of archaic introgression than mainland Eurasians (5% versus 1.5%, respectively, [16]). Until the diversity of archaic haplotypes along the chromosomes is better assessed, other signals of introgression might be more discriminant to find archaic segments in our genomes, like spikes of positive Tajima's D or measures of tree imbalance [35].
Can We Still Analyse Human Genetic Data without Taking Admixture into Account?
If human populations do not all have the same level of archaic introgression, the current genetic structure of human populations might be partly shaped by differential admixture. Estimates of population sizes and divergence times between human populations should thus be affected by past admixture events. The divergence time between an admixed and a non-admixed population should be overestimated if admixture is not properly modelled. Similarly, the effective size of admixed populations should be overestimated as archaic lineages inflate genetic diversity. In Figure 2, we report a simulation study of this bias in a very simple case of population divergence without migration. The overestimations of divergence time and admixed population size are almost linearly increasing with admixture rate (Figure 2). For instance, a divergence time of 1,600 generations (40,000 y assuming a 25-y generation time) is perfectly recovered if none of the populations is admixed, but is overestimated by 100 generations (2,500 y) with 1% admixture in one population, and already by 350 generations (8,750 y) with 5% admixture. Even though our simulated scenario is unrealistically simple, it is likely that differential admixture should affect population genetic affinities under more complex models of population differentiation. The proper interpretation of human genetic affinities should thus probably be re-evaluated in the light of these results. In particular, the divergence between Africans and Oceanians (showing up to 5% archaic admixture [16]) could be more recent than previously reported (62–75 Kya [24]). It remains unclear whether the method used by Rasmussen et al. [24] to date this divergence is also sensitive to differential introgression, but, if that was the case, the colonization wave to Oceania thought to well predate that towards East Asia [24] could have occurred at roughly the same time once differential admixture had been taken into account.
Fig. 2. Biased estimation of divergence time and population sizes in case of admixture. 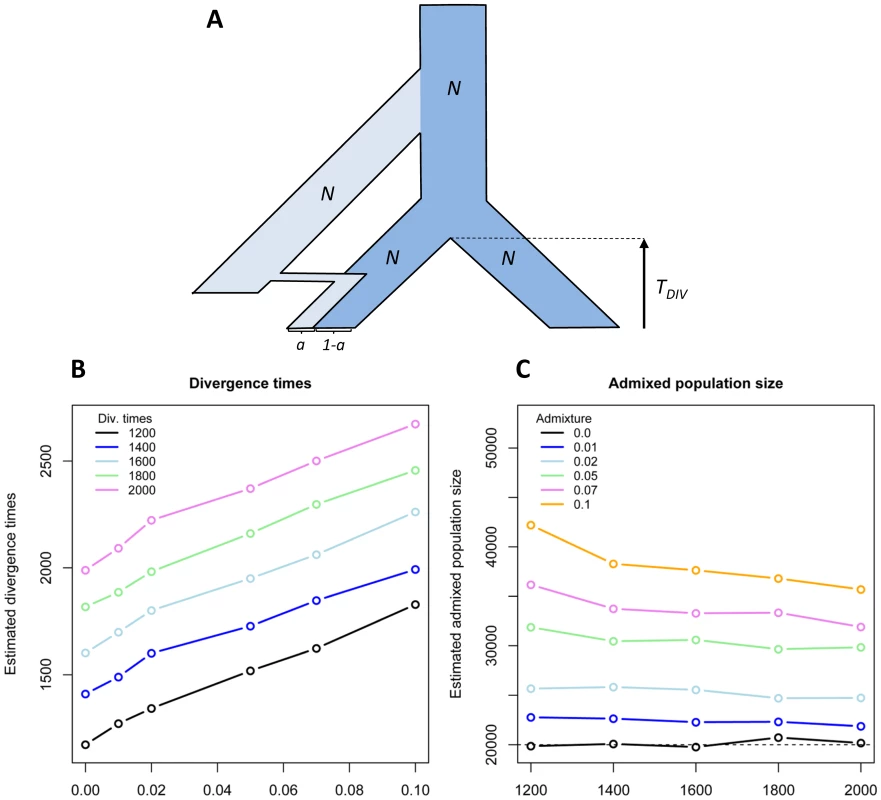
(A) Model of population divergence and admixture: one of two populations having diverged TDiv generations ago has received a fraction a of its genes from another unsampled population that diverged 14,000 generations ago (350,000 y assuming a generation time of 25 y). All populations sizes are assumed to consist of N = 20,000 haploids. (B) Estimated divergence time as a function of initial admixture rate a. (C) Estimated admixed population size for different divergence times and admixture rates. Simulated data consisted of 400,000 segments of 50 bp, thus totalling a 20-Mb DNA sequence. Parameters are estimated by maximizing the probability of the observed joint site frequency spectrum (SFS) [68], where the expected SFS is estimated by simulation following the approach of Nielsen et al. [69]. Missing Signals of Adaptation in Our Genome
Most methods aiming at detecting recent episodes of selection in humans have been designed under the paradigm that adaptations were mainly driven by classical positive selection: beneficial alleles should go to fixation, strongly reducing diversity and increasing levels of linkage disequilibrium in the surrounding regions. Such selective sweeps would thus strongly affect various aspects of molecular diversity within and between populations (e.g., [36]). Several lines of evidence support the past action of positive selection, such as increased levels of population differentiation in or close to genic regions [3], [37], increased diversity with distance from coding regions [38], or lower diversity and increased population differentiation in regions of low recombination where selective sweep should be more efficient [8], [39]–[41]. However, this paradigm has been recently eroded as it has been realized that our genome does not show many sites that are fixed between human populations [2], [38], and that fixed differences are always between populations from different continents [3], suggesting that strong adaptive events rarely occurred in response to local adaptation.
Background Selection Can Explain Most Observed Patterns of Polymorphism
Three recent observations have further shaken the paradigm of positive selection. First, it has been realized that regions showing high levels of differentiation between continents (high FST) were not associated with large levels of linkage disequilibrium, suggesting that allele frequency shifts occurred long ago and not because of recent adaptive events [3], [9]. Second, it was shown that the reduction in diversity is practically identical around non-synonymous or synonymous sites [2], suggesting that the diversity trough in genic regions is not due to positive selection acting on amino-acid changing mutations, but better fits a model of background selection, which eliminates strongly deleterious mutations in functional regions (see e.g., [42], [43] for recent reviews on background selection). Finally, models with selective sweeps have been shown to lead to an overly strong negative correlation between levels of synonymous polymorphism and non-synonymous divergence [8], whereas models of background selection fit the observed correlation. Evidence is thus building that background selection can explain most aspects of observed patterns of polymorphism. As illustrated in Figure 3, background selection lowers levels of diversity at linked sites [44], increases levels of both linkage disequilibrium [45] and population differentiation [46], and has an effect similar to a reduction of the effective population size [47], which locally lowers coalescence times [48] but also distorts the site frequency spectrum, which shows an excess of rare variants [45]. The effects of background selection on associated diversity should also be more pronounced in regions of low recombination [42] and thus provide an alternative explanation for the positive correlation between recombination rates and levels of diversity [44]. Because background selection can explain most aspects of human genetic diversity, it does not mean that adaptive events driven by positive selection have not occurred in recent or past human evolution (e.g., [49]), but they might not be that widespread and detecting their signal might be more difficult than anticipated. However, while we emphasize here the potentially important role of background selection, it is clear that other forms of selection (see e.g., [9], [50]) or other purely demographic factors (e.g., [3], [51], [52]) have certainly played an important role in shaping human genetic diversity.
Fig. 3. Effect of background selection (BGS) on molecular diversity within and between populations. 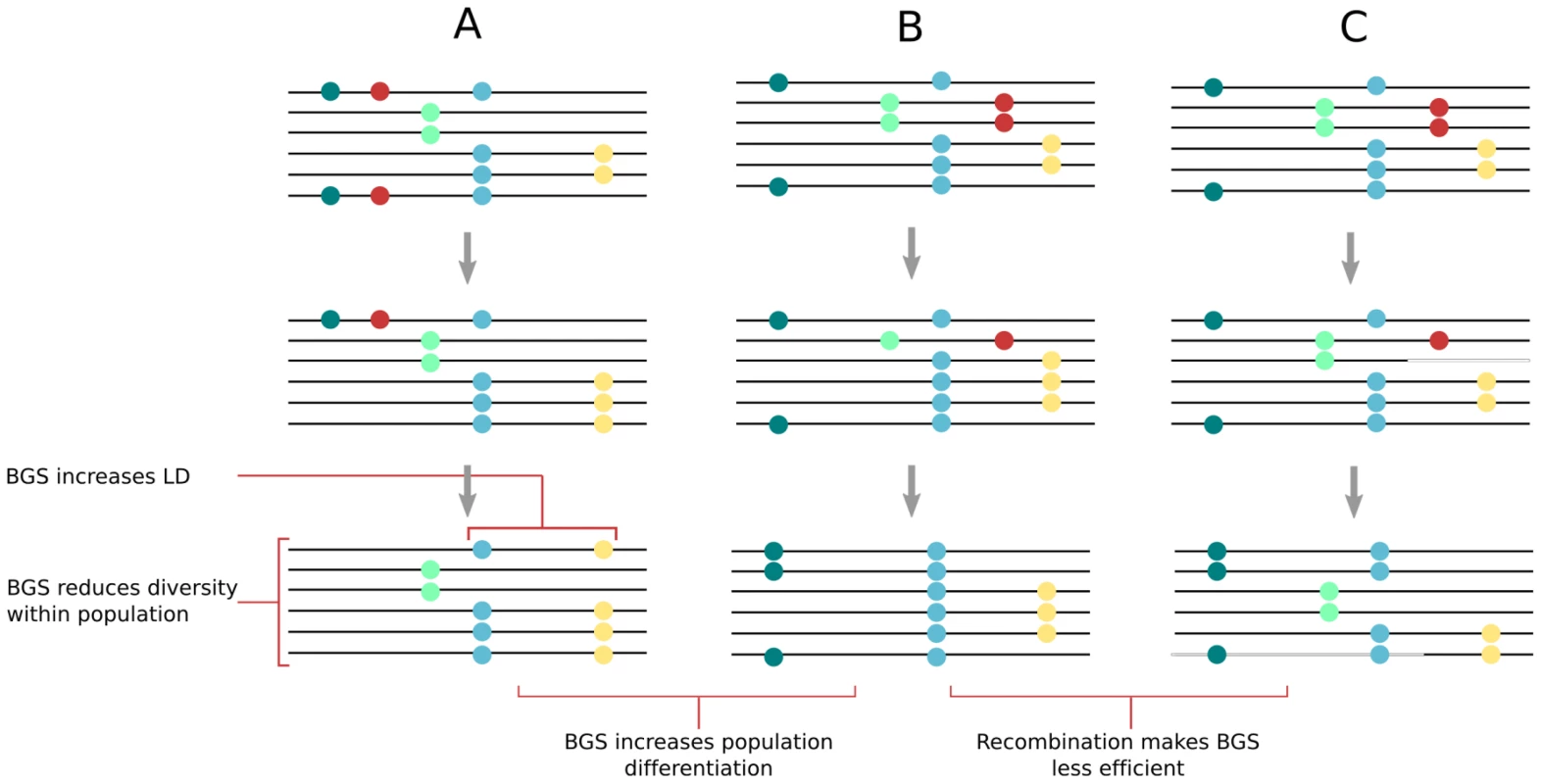
After a BGS episode, deleterious mutations (shown in red) are eliminated together with neutral mutations on the same chromosome, leading to reduced diversity. For illustrative purposes, initial neutral diversity is identical in all cases (A–C). Comparison of cases (A) and (B) shows that different BGS episodes will contribute to populations' genetic differentiation. Comparison of cases (B) and (C) shows that recombination reduces the effect of BGS, maintaining diversity, and reducing linkage disequilibrium (LD) as well as population differentiation (compare final states in [A] and [C]). Alternative Explanation for a Lack of Complete Sweeps
At a single locus, selection on standing variation [53] as well as recurrent mutation or migration [54] can result in soft sweeps where a given beneficial mutation is fixed on different chromosomal backgrounds. Positive selection acting simultaneously on several alleles [55] or sequentially over time on different alleles can lead to incomplete sweeps, where beneficial mutations are not necessarily fixed. However, most phenotypic traits are controlled by several loci, so that Pritchard and colleagues [9], [50] have argued that an absence of hard sweeps in humans could be due to polygenic adaptation from standing variation. This model assumes that most traits are controlled by multiple genes and that an adaptive event will result in the simultaneous increase in frequency of different alleles at multiple unlinked loci. After a selective event shifting the phenotype distribution around a new optimum, several selected alleles would have increased in frequency without any one being necessarily fixed.
Necessity and Benefits of Spatial Scenarios of Human Evolution
A proper scenario of human evolution should explain both the current distribution of archaic introgression given the past distribution of archaic hominins and the likely migration routes of modern humans. Spatially explicit methods simultaneously modeling range expansions and interbreeding use observed levels of admixture to assess migration and demographic processes, and thus bring additional information on the biology of our species. Whereas the surfing of neutral polymorphism during range expansions has been shown to lead to molecular signatures similar to selective sweeps [52], [56], the spread of deleterious alleles during range expansions could make background selection more potent. Spatially explicit scenarios of evolution can thus make better use of available information and provide new explanations for observed molecular diversity patterns.
Implications of Spatial Models of Admixture
Scenarios of pulses of admixture do not provide any explanation for why interbreeding only occurred in some places and why archaic hominins disappeared in regions where no admixture took place. Contrastingly, scenarios of continuous admixture during range expansion explicitly posit that archaic hominins disappeared due to their interaction or competition [57], [58] with the first human invaders. This is not very flattering for our species, but it provides a hypothesis framework that could be tested with archaeological and future genomic data. Moreover, a spatially explicit model of admixture has provided information on the frequency of interbreeding events [25], and it predicts an asymmetric introgression from archaic to modern humans [13], even if archaic populations have been much less numerous than invading modern humans [59]. High levels of introgression from the local population are indeed expected if on average more than one gene introgresses the newly invading population at any given location on the wave front [60], [61]. Had this happened, modern humans would have become archaic and the expansion would have stopped. Note also that the large levels of introgression expected after a range expansion with interbreeding argue against a complete replacement of the European Palaeolithic people by Neolithic populations expanding from the Middle East [62]. It implies that the presence of any European-specific component of Neanderthal admixture should not have been totally erased by later Neolithic expansions in Europe. A Palaeolithic introgression signal should thus be still visible in Europe, allowing one to distinguish between hypotheses of single pulses of admixture (Figure 1A; [13]) and of continuous admixtures with different archaic populations (Figure 1D).
Colonization Routes through Eurasia Mapped by Admixture?
The patterns and levels of archaic admixture in current Eurasians should be informative about modern humans' migration routes in Eurasia if they had hybridized with genetically distinct archaic populations or species. For instance, Europeans and Asians could show distinct components of Neanderthal admixture if they had admixed with European and central Asian Neanderthals [25], respectively. A detailed inventory of the genomic diversity of archaic hominins should not only allow us to better define their past range, but also make it possible to geographically map the most likely places of past admixture events, test the hypothesis of pulses of admixture, and reconstruct the migration trajectories of the ancestors of human populations from different continents. Additional statistical analyses of extant data could also allow us to date past admixture events (e.g., [63]), which could help us distinguish between scenarios of ancient admixture pulses in the Middle East and more recent interbreedings in peripheral regions.
Spatial Expansions Can Promote Background Selection
Taking into account the fact that human populations went through recurrent range expansions could also help us understand the prevalence of background selection. It has indeed been shown that in addition to beneficial and neutral mutations, deleterious mutations could surf during range expansions and thus temporarily increase in frequency at the wave front [64], [65]. This spread of deleterious alleles during spatial expansions is made possible by low population densities on wave fronts and a high growth rate favoured by a relaxation of competition for resources [66], which increases the role of drift and limits that of selection. Deleterious mutations can thus behave as neutral mutations and accumulate on expanding wave fronts. Once population densities increase in the range core, selection can become stronger than drift: purifying and background selection can progressively operate. If confirmed, this phenomenon could explain the observation in European populations of an excess of slightly deleterious alleles [67], which could have accumulated during Palaeolithic and Neolithic range expansions, but more work is needed to fully understand the interaction of beneficial and deleterious mutations in expanding populations.
Conclusions
As James F. Crow would have put it, in human evolution the questions have remained the same but the answers have changed. Genomics has revealed that the genome of Eurasians is partly of archaic origin, and genome-wide patterns of diversity have not revealed expected signals of adaptive selection in humans. The sequencing of additional archaic hominins should be helpful to distinguish between alternative scenarios of admixture, infer the timing and the geographic location of admixture events, and assess human migration routes over Eurasia. Archaic admixture can also seriously impact estimated human demography, which should be revisited to account for differential introgression among human populations. Scenarios of human evolution need to be geographically coherent and integrate range expansions during which deleterious mutations can readily surf and accumulate on wave fronts, giving later fuel to background selection. Whereas our view of human evolution has drastically changed over the past few years, it would be pretentious to believe we now know the true history of modern humans and that we have identified all selective forces that have shaped the diversity of our genome. However, progress in the analysis of modern and ancient genomes is likely to soon provide the data that will allow us to test complex scenarios of human evolution and contrast the role of various selective forces that are currently or were acting in our genome.
Zdroje
1. PickrellJKCoopGNovembreJKudaravalliSLiJZ 2009 Signals of recent positive selection in a worldwide sample of human populations. Genome Res 19 826 837
2. HernandezRDKelleyJLElyashivEMeltonSCAutonA 2011 Classic selective sweeps were rare in recent human evolution. Science 331 920 924
3. CoopGPickrellJKNovembreJKudaravalliSLiJ 2009 The role of geography in human adaptation. PLoS Genet 5 e1000500 doi:10.1371/journal.pgen.1000500
4. KayserMBrauerSStonekingM 2003 A genome scan to detect candidate regions influenced by local natural selection in human populations. Mol Biol Evol 20 893 900
5. StorzJFPayseurBANachmanMW 2004 Genome scans of DNA variability in humans reveal evidence for selective sweeps outside of Africa. Mol Biol Evol 21 1800 1811
6. VoightBFKudaravalliSWenXPritchardJK 2006 A map of recent positive selection in the human genome. PLoS Biol 4 e72 doi:10.1371/journal.pbio.0040072
7. WilliamsonSHHubiszMJClarkAGPayseurBABustamanteCD 2007 Localizing recent adaptive evolution in the human genome. PLoS Genet 3 e90 doi:10.1371/journal.pgen.0030090
8. LohmuellerKEAlbrechtsenALiYKimSYKorneliussenT 2011 Natural selection affects multiple aspects of genetic variation at putatively neutral sites across the human genome. PLoS Genet 7 e1002326 doi:10.1371/journal.pgen.1002326
9. PritchardJKPickrellJKCoopG 2010 The genetics of human adaptation: hard sweeps, soft sweeps, and polygenic adaptation. Curr Biol 20 R208 R215
10. EswaranVHarpendingHRogersAR 2005 Genomics refutes an exclusively African origin of humans. J Hum Evol 49 1 18
11. WallJDLohmuellerKEPlagnolV 2009 Detecting ancient admixture and estimating demographic parameters in multiple human populations. Mol Biol Evol 26 1823 1827
12. LabudaDZietkiewiczEYotovaV 2000 Archaic lineages in the history of modern humans. Genetics 156 799 808
13. GreenREKrauseJBriggsAWMaricicTStenzelU 2010 A draft sequence of the Neandertal genome. Science 328 710 722
14. DurandEYPattersonNReichDSlatkinM 2011 Testing for ancient admixture between closely related populations. Mol Biol Evol 28 2239 2252
15. ReichDGreenREKircherMKrauseJPattersonN 2010 Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 468 1053 1060
16. ReichDPattersonNKircherMDelfinFNandineniMR 2011 Denisova admixture and the first modern human dispersals into Southeast Asia and Oceania. Am J Hum Genet
17. SkoglundPJakobssonM 2011 Archaic human ancestry in East Asia. Proc Natl Acad Sci U S A 108 18301 18306
18. HammerMFWoernerAEMendezFLWatkinsJCWallJD 2011 Genetic evidence for archaic admixture in Africa. Proc Natl Acad Sci U S A 108 15123 15128
19. Destro-BisolGDonatiFCoiaVBoschiIVerginelliF 2004 Variation of female and male lineages in sub-Saharan populations: the importance of sociocultural factors. Mol Biol Evol 21 1673 1682
20. BatiniCFerriGDestro-BisolGBrisighelliFLuiselliD 2011 Signatures of the preagricultural peopling processes in sub-Saharan Africa as revealed by the phylogeography of early Y chromosome lineages. Mol Biol Evol 28 2603 2613
21. PatinELavalGBarreiroLBSalasASeminoO 2009 Inferring the demographic history of African farmers and pygmy hunter-gatherers using a multilocus resequencing data set. PLoS Genet 5 e1000448 doi:10.1371/journal.pgen.1000448
22. WhiteTDAsfawBDeGustaDGilbertHRichardsGD 2003 Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature 423 742 747
23. McDougallIBrownFHFleagleJG 2005 Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature 433 733 736
24. RasmussenMGuoXWangYLohmuellerKERasmussenS 2011 An Aboriginal Australian genome reveals separate human dispersals into Asia. Science 334 94 98
25. CurratMExcoffierL 2011 Strong reproductive isolation between humans and Neanderthals inferred from observed patterns of introgression. Proc Natl Acad Sci U S A 108 15129 15134
26. LiangMNielsenR 2011 Q&A: who is H. sapiens really, and how do we know? BMC Biol 9 20
27. MendezFLWatkinsJCHammerMF 2012 Global genetic variation at OAS1 provides evidence of archaic admixture in Melanesian populations. Mol Biol Evol 29 1513 1520
28. Abi-RachedLJobinMJKulkarniSMcWhinnieADalvaK 2011 The shaping of modern human immune systems by multiregional admixture with archaic humans. Science 334 89 94
29. YotovaVLefebvreJFMoreauCGbehaEHovhannesyanK 2011 An X-linked haplotype of Neandertal origin is present among all non-African populations. Mol Biol Evol 28 1957 1962
30. HawksJCochranGHarpendingHCLahnBT 2008 A genetic legacy from archaic Homo. Trends Genet 24 19 23
31. HobolthADutheilJYHawksJSchierupMHMailundT 2011 Incomplete lineage sorting patterns among human, chimpanzee, and orangutan suggest recent orangutan speciation and widespread selection. Genome Res 21 349 356
32. TakahataNNeiM 1990 Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics 124 967 978
33. GutenkunstRNHernandezRDWilliamsonSHBustamanteCD 2009 Inferring the joint demographic history of multiple populations from multidimensional SNP frequency data. PLoS Genet 5 e1000695 doi:10.1371/journal.pgen.1000695
34. BlumMGJakobssonM 2011 Deep divergences of human gene trees and models of human origins. Mol Biol Evol 28 889 898
35. BlumMGHeyerEFrancoisOAusterlitzF 2006 Matrilineal fertility inheritance detected in hunter-gatherer populations using the imbalance of gene genealogies. PLoS Genet 2 e122 doi:10.1371/journal.pgen.0020122
36. BiswasSAkeyJM 2006 Genomic insights into positive selection. Trends Genet 22 437 446
37. BarreiroLBLavalGQuachHPatinEQuintana-MurciL 2008 Natural selection has driven population differentiation in modern humans. Nat Genet 40 340 345
38. DurbinRMAbecasisGRAltshulerDLAutonABrooksLD 2010 A map of human genome variation from population-scale sequencing. Nature 467 1061 1073
39. KeinanAReichD 2010 Human population differentiation is strongly correlated with local recombination rate. PLoS Genet 6 e1000886 doi:10.1371/journal.pgen.1000886
40. CaiJJMacphersonJMSellaGPetrovDA 2009 Pervasive hitchhiking at coding and regulatory sites in humans. PLoS Genet 5 e1000336 doi:10.1371/journal.pgen.1000336
41. McVickerGGordonDDavisCGreenP 2009 Widespread genomic signatures of natural selection in hominid evolution. PLoS Genet 5 e1000471 doi:10.1371/journal.pgen.1000471
42. CharlesworthB 2012 The effects of deleterious mutations on evolution at linked sites. Genetics 190 5 22
43. StephanW 2010 Genetic hitchhiking versus background selection: the controversy and its implications. Philos Trans R Soc Lond B Biol Sci 365 1245 1253
44. CharlesworthBMorganMTCharlesworthD 1993 The effect of deleterious mutations on neutral molecular variation. Genetics 134 1289 1303
45. ZengKCharlesworthB 2011 The joint effects of background selection and genetic recombination on local gene genealogies. Genetics 189 251 266
46. HuXSHeF 2005 Background selection and population differentiation. J Theor Biol 235 207 219
47. CharlesworthDCharlesworthBMorganMT 1995 The pattern of neutral molecular variation under the background selection model. Genetics 141 1619 1632
48. HudsonRRKaplanNL 1995 The coalescent process and background selection. Philos Trans R Soc Lond B Biol Sci 349 19 23
49. CrisciJLWongAGoodJMJensenJD 2011 On characterizing adaptive events unique to modern humans. Genome Biol Evol 3 791 798
50. PritchardJKDi RienzoA 2010 Adaptation - not by sweeps alone. Nat Rev Genet 11 665 667
51. HoferTFollMExcoffierL 2012 Evolutionary forces shaping genomic islands of population differentiation in humans. BMC Genomics 13 107
52. ExcoffierLFollMPetitRJ 2009 Genetic consequences of range expansions. Annual Review in Ecology, Evolution, and Systematics 40 481 501
53. HermissonJPenningsPS 2005 Soft sweeps: molecular population genetics of adaptation from standing genetic variation. Genetics 169 2335 2352
54. PenningsPSHermissonJ 2006 Soft sweeps II–molecular population genetics of adaptation from recurrent mutation or migration. Mol Biol Evol 23 1076 1084
55. RalphPCoopG 2010 Parallel adaptation: one or many waves of advance of an advantageous allele? Genetics 186 647 668
56. KlopfsteinSCurratMExcoffierL 2006 The fate of mutations surfing on the wave of a range expansion. Mol Biol Evol 23 482 490
57. FabreVCondemiSDegioanniAHerrscherE 2011 Neanderthals versus modern humans: evidence for resource competition from isotopic modelling. Int J Evol Biol 2011 689315
58. BanksWEd'ErricoFPetersonATKageyamaMSimaA 2008 Neanderthal extinction by competitive exclusion. PLoS ONE 3 e3972 doi:10.1371/journal.pone.0003972
59. MellarsPFrenchJC 2011 Tenfold population increase in Western Europe at the Neandertal-to-modern human transition. Science 333 623 627
60. CurratMRuediMPetitRJExcoffierL 2008 The hidden side of invasions: massive introgression by local genes. Evolution 62 1908 1920
61. CurratMExcoffierL 2004 Modern humans did not admix with Neanderthals during their range expansion into Europe. PLoS Biol 2 e421 doi:10.1371/journal.pbio.0020421
62. CurratMExcoffierL 2005 The effect of the Neolithic expansion on European molecular diversity. Proc Biol Sci 272 679 688
63. PugachIMatveyevRWollsteinAKayserMStonekingM 2011 Dating the age of admixture via wavelet transform analysis of genome-wide data. Genome Biol 12 R19
64. TravisJMMunkemullerTBurtonOJBestADythamC 2007 Deleterious mutations can surf to high densities on the wave front of an expanding population. Mol Biol Evol 24 2334 2343
65. HallatschekONelsonDR 2010 Life at the front of an expanding population. Evolution 64 193 206
66. MoreauCBhererCVezinaHJompheMLabudaD 2011 Deep human genealogies reveal a selective advantage to be on an expanding wave front. Science 334 1148 1150
67. LohmuellerKEIndapARSchmidtSBoykoARHernandezRD 2008 Proportionally more deleterious genetic variation in European than in African populations. Nature 451 994 997
68. AdamsAMHudsonRR 2004 Maximum-likelihood estimation of demographic parameters using the frequency spectrum of unlinked single-nucleotide polymorphisms. Genetics 168 1699 1712
69. NielsenRPaulJSAlbrechtsenASongYS 2011 Genotype and SNP calling from next-generation sequencing data. Nat Rev Genet 12 443 451
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



