-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHistone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
Two distinct Polycomb complexes, PRC1 and PRC2, collaborate to maintain epigenetic repression of key developmental loci in embryonic stem cells (ESCs). PRC1 and PRC2 have histone modifying activities, catalyzing mono-ubiquitination of histone H2A (H2AK119u1) and trimethylation of H3 lysine 27 (H3K27me3), respectively. Compared to H3K27me3, localization and the role of H2AK119u1 are not fully understood in ESCs. Here we present genome-wide H2AK119u1 maps in ESCs and identify a group of genes at which H2AK119u1 is deposited in a Ring1-dependent manner. These genes are a distinctive subset of genes with H3K27me3 enrichment and are the central targets of Polycomb silencing that are required to maintain ESC identity. We further show that the H2A ubiquitination activity of PRC1 is dispensable for its target binding and its activity to compact chromatin at Hox loci, but is indispensable for efficient repression of target genes and thereby ESC maintenance. These data demonstrate that multiple effector mechanisms including H2A ubiquitination and chromatin compaction combine to mediate PRC1-dependent repression of genes that are crucial for the maintenance of ESC identity. Utilization of these diverse effector mechanisms might provide a means to maintain a repressive state that is robust yet highly responsive to developmental cues during ES cell self-renewal and differentiation.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002774
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002774Summary
Two distinct Polycomb complexes, PRC1 and PRC2, collaborate to maintain epigenetic repression of key developmental loci in embryonic stem cells (ESCs). PRC1 and PRC2 have histone modifying activities, catalyzing mono-ubiquitination of histone H2A (H2AK119u1) and trimethylation of H3 lysine 27 (H3K27me3), respectively. Compared to H3K27me3, localization and the role of H2AK119u1 are not fully understood in ESCs. Here we present genome-wide H2AK119u1 maps in ESCs and identify a group of genes at which H2AK119u1 is deposited in a Ring1-dependent manner. These genes are a distinctive subset of genes with H3K27me3 enrichment and are the central targets of Polycomb silencing that are required to maintain ESC identity. We further show that the H2A ubiquitination activity of PRC1 is dispensable for its target binding and its activity to compact chromatin at Hox loci, but is indispensable for efficient repression of target genes and thereby ESC maintenance. These data demonstrate that multiple effector mechanisms including H2A ubiquitination and chromatin compaction combine to mediate PRC1-dependent repression of genes that are crucial for the maintenance of ESC identity. Utilization of these diverse effector mechanisms might provide a means to maintain a repressive state that is robust yet highly responsive to developmental cues during ES cell self-renewal and differentiation.
Introduction
Embryonic stem cells (ESCs) can undergo unlimited self-renewal while maintaining their pluripotent and undifferentiated states, features that are consistent with their origin within the inner cell mass of the blastocyst. Increasing evidence suggests that in addition to the core gene regulatory circuitry composed of Oct3/4, Sox2, Nanog and other transcription factors, Polycomb group proteins critically contribute to maintain the undifferentiated state of ESCs by silencing genes that are involved in development and/or transcription [1], [2], [3], [4], [5], [6]. Polycomb-mediated repression of these genes is also essential to preserve the ability of ES cells to differentiate in response to extracellular cues [7], [8], [9].
Polycomb group proteins are chromatin-modifiers that mediate transcriptional repression. They form at least two types of multimeric complexes, the Polycomb repressive complexes-1 (PRC1) and -2 (PRC2), the core components of which are conserved from Drosophila to human [10], [11], [12], [13], [14]. PRC2 contains Ezh2 or -1, which catalyze trimethylation of histone H3 lysine 27 (H3K27me3), a posttranslational modification that is thought to be recognized by the chromo-domain (CHD) protein components of PRC1 [12], [13], [14], [15], [16]. Within PRC1, Ring1B and –A act as major E3 ubiquitin ligases for histone H2A mono-ubiquitination at lysine 119 (H2AK119u1) [17], [18]. Conditional depletion of Ring1B and –A in ESCs leads to global loss of H2AK119u1 and concurrent derepression of ‘bivalent’ genes enriched for both H3K27me3 and H3K4me3 [5], [19]. H2AK119u1 deposition has been shown to localize to the inactive X chromosome (Xi), the XY body, and several silenced ‘bivalent’ loci in mouse ESCs [19], [20], [21]. Recent genome-wide H2AK119u1 analysis in MEFs (mouse embryonic fibroblast) has revealed Bmi1-dependent deposition of H2AK119u1 at the promoter regions of many repressed genes [22]. These findings suggest that H2AK119u1 could be a part of the regulatory process that is required for PRC1-mediated repression.
However, the role of H2AK119u1 in PRC1-mediated repression is still controversial. A recent study has reported that Ring1B can compact chromatin structure of the Hox loci and repress Hox expression independent of its E3 activity [23]. This idea has been supported by a previous study which showed that PRC1 components can compress nucleosomal templates assembled from tail-less histones into a form that is refractory to chromatin remodeling in vitro [24]. This hypothesis, however, needs rigorous validation because this study was performed by using Ring1B single knockout (KO) cells, in which Ring1A-catalyzed H2AK119u1 still remained in a lower level [5], [17], [20], [25]. In this experimental setup, Ring1A and associated H2AK119u1 may potentially complement Ring1B-mediated chromatin compaction of Hox genes to mediate their repression. Consistently, ESCs are capable to retain ESC-like morphology and LIF-dependent proliferation upon depletion of Ring1B but not doubly depletion of Ring1B and –A [5], [9], [20], [26]. Therefore, to properly estimate in which extent H2AK119u1 contributes to PRC1-dependent repression in ESCs, and experimental platform that excludes Ring1A is necessary.
In this study, we first determined the localization of H2AK119u1 in ESCs by ChIP-on-chip analysis and found the H2AK119u1-bound genes as core targets of PRC1-dependent repression. We further demonstrated that catalytic activity of PRC1 towards H2A is essential for silencing of target loci and maintenance of ESCs. We also found PRC1-mediated H2AK119u1 is complemented by independent functions of PRC1 that contribute to gene silencing and chromatin compaction, most notably at Hox loci. We propose that PRC1 combines diverse effecter mechanisms to mediate robust repression of target genes and stable maintenance of undifferentiated status of ESCs.
Results
Ring1-mediated H2AK119u1 demarcates central targets of PRC1 in ESCs
Global H2AK119u1 distribution has been reported only for MEFs and the human teratocarcinoma NT2 cell line [22], [27], but not for mouse ESCs. We, therefore, used ChIP-on-chip analysis to clarify H2AK119u1 deposition around transcription start sites (TSS) in mouse ESCs by using an Agilent mouse promoter array and an E6C5 monoclonal antibody (mAb) or a rabbit polyclonal antibody [28]. Ring1A/B-dKO ESCs, in which H2AK119u1 is apparently undetectable, were used as a negative control [5], [19]. Ring1A/B-dKO ESCs were induced by treating Ring1A−/−;Ring1Bfl/fl;Rosa26::CreERT2 ESCs with 4-hydroxy-tamoxifen (OHT), which rapidly activates CreERT2 and catalyzes loxP recombination at Ring1B locus [5]. Distribution of Ring1B, H3K27me3 and H2A were re-examined to obtain a reference data set.
E6C5-ChIP signals at the promoter regions of known target loci, Hoxa9, Pax9 and Tbx3 show that H2AK119u1 deposition is readily detectable in Ring1A−/− ESCs but not in Ring1A/B-dKO (dKO) ESCs (Figure S1). These results were validated by ChIP-qPCR as shown in Figure S2A. We calculated the averages of E6C5-ChIP signals in Ring1B-bound and –unbound genes and found higher H2AK119u1 deposition in Ring1B-bound genes in Ring1A−/− than in Ring1A/B-dKO (dKO) whereas little difference was noted for unbound genes (Figure 1A; Figure S3A and S3B). These data therefore appear to reflect H2AK119u1 deposition that depends on Ring1B. For detailed investigation of the genes that exhibit H2AK119u1 enrichment, we examined the gene-wise distribution of E6C5-ChIP signals after subtraction of the background enrichment value and identified 538 target genes (Figure 1B; Figure S3C; the list of genes is shown in Table S1). The results with the E6C5 mAb were re-confirmed by using a rabbit antiserum that recognizes H2AK119u1 [28]. With this reagent, 524 genes were found to be bound by H2AK119u1, and these genes significantly overlapped with those identified by the E6C5 mAb (Figure S3D; Table S1). Taken together, using the above methods we determined a set of genes in ESCs that have H2AK119u1 deposition around their TSS.
Fig. 1. Global mapping of Ring1B-dependent H2AK119u1 deposition in ESCs reveals that genes occupied by H2AK119u1 represent central targets of PRC1. 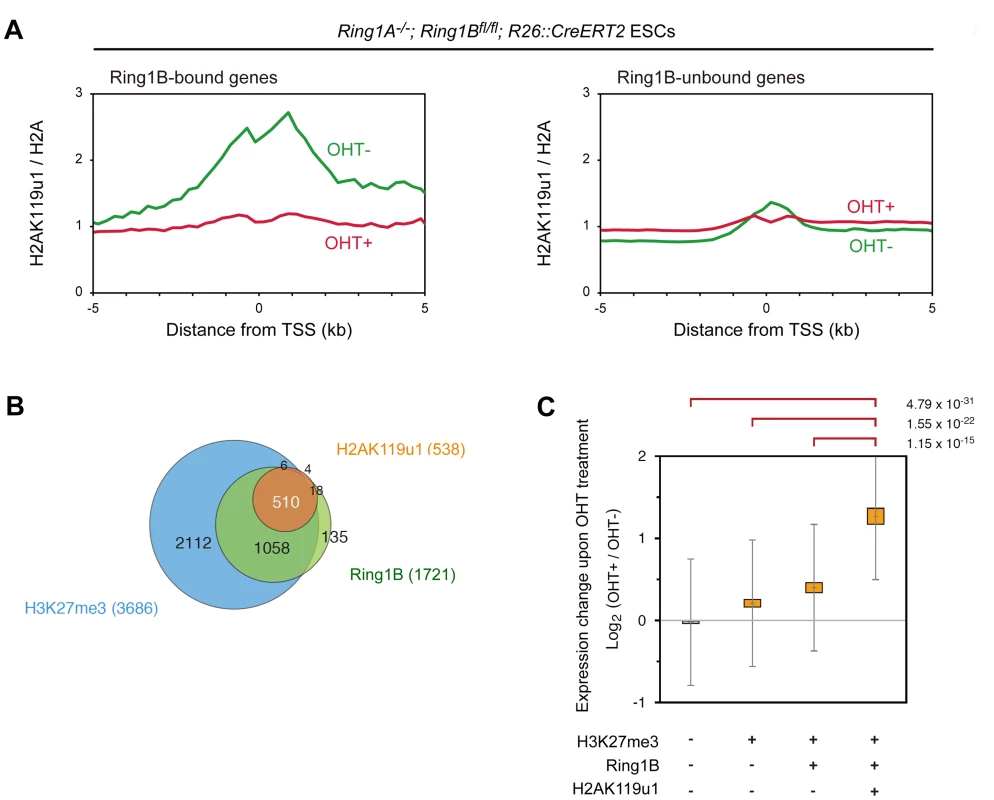
(A) ChIP-on-chip analysis showing the average of H2AK119u1 distributions at the promoter regions (from −5 kb to +5 kb relative to TSS) of Ring1B-bound and –unbound genes in Ring1A−/− (OHT−: green line) and Ring1A/B-dKO (OHT+: red line) ESCs. Enrichment of H2AK119u1 (obtained by E6C5 mAb) and H2A is expressed relative to input DNA, and H2AK119u1 is normalized to H2A. (B) Venn diagram representing the overlap among genes occupied by Ring1B, H2AK119u1 and H3K27me3. Numbers in parentheses represent the total number of genes occupied by each one. (C) Graphic representation of expression changes induced by Ring1B depletion (2 days after OHT treatment) for each subset of genes classified by the presence (+) or absence (−) of Ring1B, H2AK119u1 and H3K27me3 is shown. The average, deviation and distribution of the expression changes for the respective subsets of genes determined by microarray analysis are shown. The 95% Confidence interval (CI) and standard deviation (SD) for the average value of the expression change are indicated. Significant (P<0.001) and insignificant (P≥0.01) expression changes were determined by the Student's t-test and are indicated in orange and grey, respectively. P-values for the difference of expression changes between the indicated 2 groups are calculated by the Student's t-test and are indicated above each graph. We went on to examine the correlation of genes enriched for H2AK119u1 (H2AK119u1+) with those having Ring1B (Ring1B+) and H3K27me3 (H3K27me3+) depositions. We found that genes bound by Ring1B and H3K27me3 identified in this study were significantly overlapped with those reported in previous studies (Figure S3E, F). We identified 1721 and 3686 genes bound by Ring1B and H3K27me3, respectively, and found H2AK119u1+ genes as a subset of the Ring1B+ genes (Figure 1B; Figure S3C; Table S1). Since most Ring1B+ genes define a subset of H3K27me3+ genes, H3K27me3+ genes could be subdivided into three distinct layers, H2AK119u1+Ring1B+H3K27me3+ (Triple positive; TP), H2AK119u1-Ring1B+H3K27me3+ (Double positive; DP) and H2AK119u1-Ring1B-H3K27me3+ (Single positive; SP). We finally confirmed the quantitative difference of H2AK119u1 level at TP genes against DP or SP genes by ChIP-qPCR analysis at selected genes (Figure S2A). Although we cannot exclude a possibility that we failed to detect a low level of H2AK119u1 at some DP genes, our data demonstrate that H3K27me3+ gene promoters are not uniformly occupied by Ring1B and H2AK119u1.
We investigated functional properties of H2AK119u1+ genes among Polycomb targets. Scattered plot analysis for gene-wise deposition of H3K27me3 and Ring1B revealed that H2AK119u1 targets were significantly enriched among genes that have high levels of both Ring1B and H3K27me3 occupancy (Figure S4). This suggests that TP genes represent the central targets for Polycomb repression. We compared the impact of PRC1 loss among these subsets by examining the gene expression profiles in Ring1A/B-dKO ESCs (Figure 1C; Figure S2B). We found significant de-repression (p<0.001) of TP, DP and SP genes but no significant changes in H3K27me3-negative genes. It is worth noting that the degree of de-repression of the TP genes was significantly higher than that of the DP and SP genes (Figure 1C). Gene ontology (GO) based analyses confirmed that TP genes are most significantly enriched for functions in transcription and/or development (Figure S5). Of note, Cdx2 and Gata6, which are known to be repressed by Oct3/4 and Nanog [29], [30], are occupied by H2AK119u1, suggesting that H2AK119u1 might be involved in maintaining ESC properties by suppressing differentiation of ESCs.
E3 activity of Ring1B and H2AK119u1 are dispensable for PRC1 target binding
Above data suggest a potential importance of H2AK119u1 for repression of key developmental regulators which is required to maintain the undifferentiated status of ESCs. This however does not necessarily prove the importance of the E3 ligase activity and H2AK119u1 per se for the repression because H2A ubiquitination independent functions of PRC1 in chromatin compaction and gene silencing both in vitro [24], and in vivo [23] has been reported in previous studies. To investigate this question, we expressed mutant Ring1B proteins that are defective in the interaction with the E2 component in Ring1A/B-dKO ESCs. In this experimental setup, we have first stably expressed exogenous WT or mutant Ring1B in Ring1A−/−;Ring1Bfl/fl;R26::CreERT2 ESCs and then endogenous Ring1B was depleted by OHT treatment (Figure 2A). Similar experiments have been described previously [23] but have made use of Ring1B single KO ESCs, and therefore could not exclude the contribution of low levels of Ring1A (Figure S6A) and associated H2AK119u1 that occur in ESCs [5], [17], [20], [25]. We thus tested the role of Ring1A to mediate H2AK119u1 and repression of Polycomb targets in ESCs. We first compared global H2AK119u1 levels in Ring1B-KO ESCs with Ring1A/B-dKO and found significant amount of H2AK119u1 remained in Ring1B-KO (Figure S6B). Consistently, the expression of exogenous Ring1A obviously restored global H2AK119u1 level, ESC identity, and repression of TP genes in Ring1A/B-dKO ESCs (Figure S6B–E). We then performed ChIP-chip analysis to compare Ring1A distribution with Ring1B and found that Ring1A and Ring1B significantly shared target genes (Figure S6F, G). ChIP-qPCR analysis further confirmed Ring1A binding at promoter regions of representative TP genes such as Hoxd11 and Zic1 in the absence of Ring1B (Figure S6H). Importantly, binding of other PRC1 components such as Mel18 was concomitantly restored by the expression of exogenous Ring1A in Ring1A/B-dKO ESCs (Figure S6H). Therefore, Ring1A was shown to substitute for Ring1B functions in mediating H2AK119u1 and target gene repression. These results sufficiently justify the use of Ring1A/B-dKO ESCs instead of Ring1B-KO in following experiments.
Fig. 2. Generation of ESCs expressing catalytically inactive Ring1B. 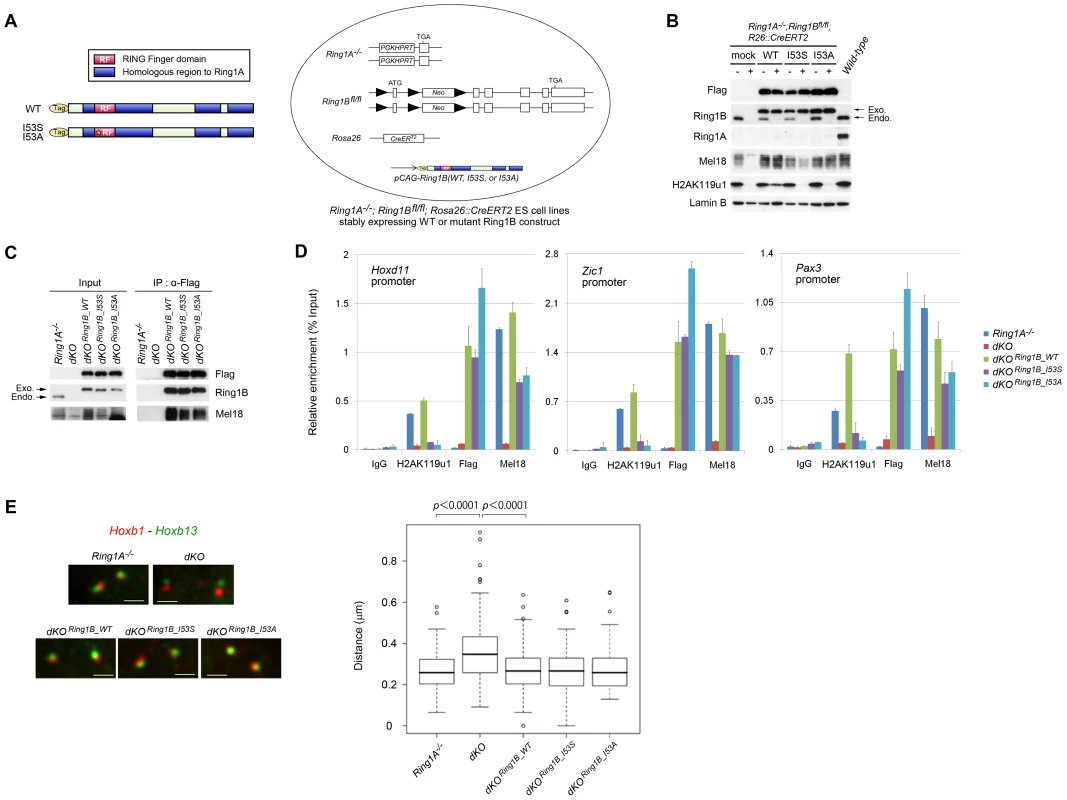
(A) Schematic representation of 3xFlag-tagged Ring1B, showing wild-type and point-mutant derivatives. Each of these construct was stably transfected into Ring1A−/−; Ring1Bfl/fl; R26::CreERT2 ESCs. (B) Immunoblot analysis of Ring1A, Ring1B, Flag, H2AK119u1 and Lamin B protein levels in whole cell lysates of wild-type and Ring1A−/−; Ring1Bfl/fl; R26::CreERT2 ESC lines expressing mock, WT, I53S, or I53A Ring1B with or without OHT treatment (OHT+ and −, respectively). (C) Immunoprecipitation (IP) analysis showing the association of exogenous Ring1B WT, I53S or I53A with an endogenous PRC1 component Mel18. Extracts of OHT-untreated (−) and -treated [(+); day 2] Ring1A−/−; Ring1Bfl/fl; R26::CreERT2 ESC lines expressing each of the constructs were immunoprecipitated with anti-Flag antibody. Resulting precipitates (IP) and lysates (Input) were immunoblotted with antibodies against Flag, Ring1B and Mel18. (D) Association of Flag-tagged proteins in Ring1A−/−; Ring1Bfl/fl; R26::CreERT2 ESC lines stably expressing mock, Flag-tagged Ring1B WT, I53S, or I53A with promoter regions of their representative target genes before (−) or after (+) OHT treatment (day 2) as determined by ChIP and site-specific real-time PCR. Error bars represent standard deviations determined from three independent experiments. (E) 3D FISH with probe pairs at Hoxb locus (Hoxb1 and Hoxb13) in PFA-fixed nuclei of Ring1A−/−; Ring1Bfl/fl; R26::CreERT2 ESC lines stably expressing mock, WT, I53S, or I53A Ring1B before (−) or after (+) OHT treatment (day 2). Scale bars indicate 1 µm. The boxes show the median and interquartile range of interprobe distances (µm) in the indicated cells. Open circles indicate outliers. The statistical significance of differences between the indicated two data was examined by the Mann-Whitney U test. We made use of the previously characterized I53S and I53A mutations located at the E2 UbcH5c binding surface that have been shown to affect the E3 activity of Ring1B both in vitro and in vivo [17], [23], [31]. We introduced expression vectors for flag-tagged wild-type (WT) or mutant Ring1B [Ring1B (I53S) or (I53A)] into Ring1A−/−;Ring1Bfl/fl;R26::CreERT2 ESCs (Figure 2A) and established stable transfectants that expressed exogenous Ring1B at similar level to the endogenous protein (Figure 2B). Expression of WT Ring1B restored global H2AK119u1 levels in Ring1A/B-dKO cells whereas Ring1B (I53S) and Ring1B (I53A) did not (Figure 2B).
We went on to check whether the levels and target binding of PRC1 could be appropriately recapitulated by exogenous wild-type or mutant Ring1B in the transfectants. Levels of other PRC1 proteins were depleted in the absence of Ring1B, presumably because complex formation stabilizes individual components [9], [26]. We found that Mel18 was clearly detectable and formed complexes with Ring1B (I53S), Ring1B (I53A) and wild-type Ring1B in the absence of endogenous Ring1 proteins in each transfectant (Figure 2C). We also confirmed that levels of Cbx2 and Phc1 were restored in these transfectants (data not shown). We next assessed the association of exogenous Ring1B with target genes in the transfectants. We used ChIP and subsequent quantitative PCR (qPCR) analysis and observed binding of Ring1B I53S or I53A to target loci. Local H2AK119u1 deposition was undetectable, confirming the impaired E3 ligase activity of the Ring1B mutant proteins (Figure 2D). We also found that Mel18 binding to these targets was considerably restored by the expression of Ring1B (I53S) or Ring1B (I53A). Finally, we tested whether condensation of Hoxb cluster could be recapitulated in the transfectants by using 3D FISH analysis with probes for Hoxb1 and Hoxb13. Consistent with a previous report using Ring1B-KO ESCs, we found that Hoxb1 and Hoxb13 were considerably separated in Ring1A/B-dKO ESCs compared to Ring1A−/− cells (Figure 2E) [23] and that condensation of the Hoxb cluster was significantly restored by the expression of Ring1B (I53S) or Ring1B (I53A). Taken together, the expression and target binding of PRC1 were sufficiently recapitulated in Ring1A/B-dKO ESCs that express catalytically inactive Ring1B. We thus concluded that these transfectants were well suited to address the role of Ring1B E3 activity in the maintenance and repression of ESC Polycomb targets. The above results also imply that E3 activity of Ring1B and H2AK119u1 are dispensable for PRC1 target binding.
E3 activity of Ring1B is required to repress Polycomb targets and maintain ESCs
We next tested the phenotypes of the transfectants after deletion of endogenous Ring1B. We observed that the expression of either Ring1B (I53S) or Ring1B (I53A) was not sufficient to maintain ESCs in an undifferentiated state (Figure 3A). We obtained similar results in the presence of three inhibitors (3i) that target FGF receptor, MEK, and GSK3 (data not shown) [32]. This implies that the E3 activity of Ring1B is required to maintain ESC identity in the absence of Ring1A. Consistently, Ring1B (I53S) failed to restore repression of differentiation markers (Kdr, Gata6, Hnf4a and Cdx2) and expression of undifferentiation markers (Pou5f1, Sox2 and Nanog) in Ring1A/B-dKO ESCs while WT Ring1B or Ring1A obviously restored (Figure S7A). We went on to examine differentiation ability of the respective ESC lines by forming embryoid bodies. We found that the progressive changes in expression of the marker genes upon induction of differentiation were considerably affected in Ring1A/B-dKO ESCs compared to wild-type or Ring1A−/− ESCs (Figure S7B). These changes were restored by WT Ring1B but not by Ring1B (I53S). Together, Ring1B catalytic activity is required for maintenance and differentiation of ESCs.
Fig. 3. H2A ubiquitination activity of Ring1B is essential for the maintenance of ESC identity and repression of target gene expression. 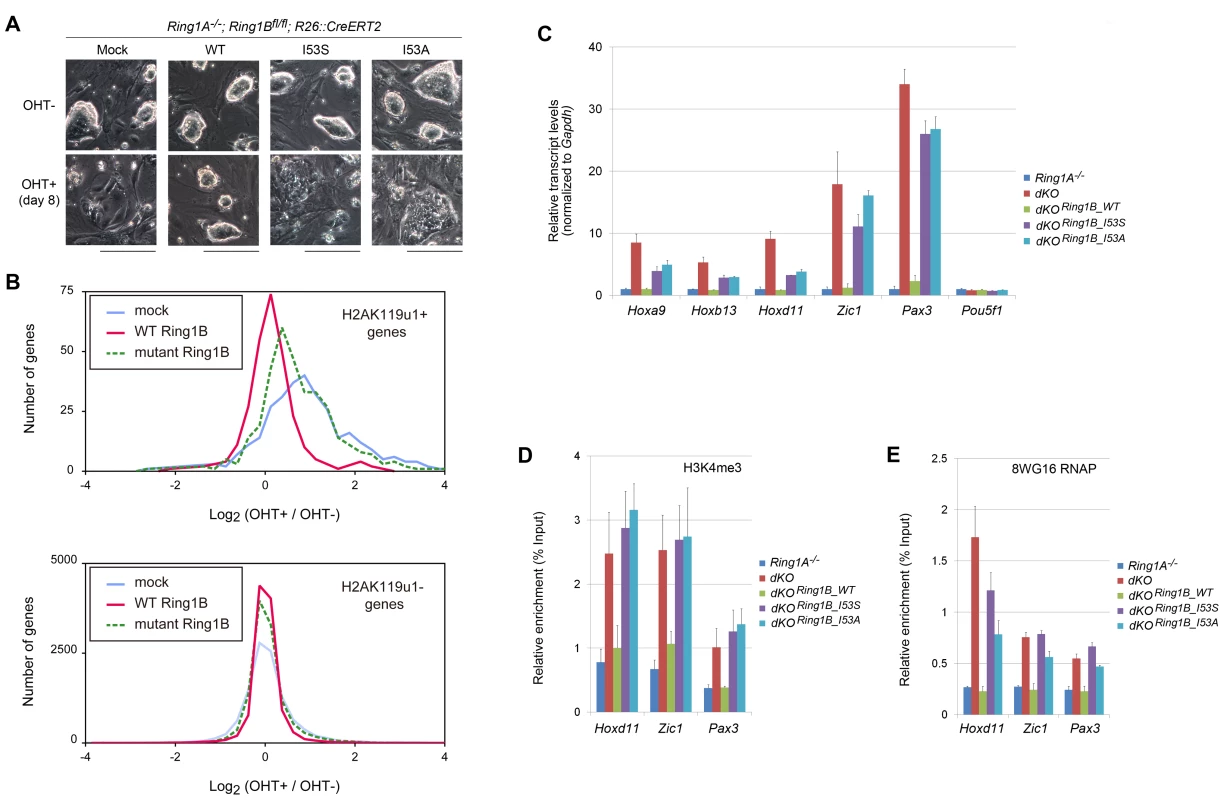
(A) Morphology of OHT-untreated and –treated (day 8) Ring1A−/−; Ring1Bfl/fl; R26::CreERT2 ESC lines expressing the indicated transgene. The images were acquired under a phase-contrast microscope. Scale bars indicate 200 µm. (B) Histograms showing the expression changes of H2AK119u1+ and H2AK119u1− genes in Ring1A−/−; Ring1Bfl/fl; Rosa26::CreERT2 ESCs expressing mock (blue line), WT Ring1B (red line), or mutant Ring1B (green dotted line) following OHT treatment. (C) Expression levels of Hoxa9, Hoxb13, Hoxd11, Zic1, Pax3 and Pou5f1 in Ring1A−/−; Ring1Bfl/fl; Rosa26::CreERT2 ESCs expressing mock, WT, I53S, or I53A Ring1B before (−) or after (+) OHT treatment (day 2). Expression levels were normalized to a Gapdh control and are depicted as fold changes relative to mock (OHT-untreated) ESCs. Error bars represent standard deviation determined from at least three independent experiments. (D) Local levels of trimethylated H3K4 (H3K4me3) at promoter regions of representative target genes in Ring1A−/−; Ring1Bfl/fl; R26::CreERT2 ESCs stably expressing mock, WT, I53S, or I53A Ring1B before (−) or after (+) OHT treatment (day 2) were determined by ChIP and site-specific real-time PCR. The relative amount of immunoprecipitated DNA is depicted as a percentage of input DNA. Error bars represent standard deviation determined from at least three independent experiments. (E) As in (D), but showing local levels of RNA polymerase II (RNAP) detected with the 8WG16 antibody. We then examined the expression of H2AK119u1+ genes in these transfectants by using expression microarrays. In the mock transfectant, we observed that H2AK119u1+ genes were significantly de-repressed by depletion of Ring1 proteins whereas expression of H2AK119u1 − genes was virtually unchanged (Figure 3B). De-repression of H2AK119u1+ genes in the Ring1A/B-dKO was mostly restored by the expression of WT Ring1B but only partially by Ring1B (I53S) and Ring1B (I53A) (Figure 3B; Figure S8). Moreover, the levels of restoration by Ring1B mutants were variable among target genes. To confirm the microarray results, we examined the expression of H2AK119u1 targets, Hoxa9, Hoxb13, Hoxd11, Zic1, and Pax3, by quantitative RT-PCR. These genes were de-repressed in Ring1A/B-dKO compared to OHT-untreated control cells (Figure 3C). WT Ring1B was shown to fully restore the repression of these genes. Ring1B (I53S) and Ring1B (I53A) could slightly restore the repression of Hoxa9, Hoxb13 and Hoxd11, but almost failed to repress Zic1 and Pax3 (Figure 3C). Therefore, the E3 activity of Ring1B is required for efficient repression of its target genes. Our results also suggest that some genes, e.g., Zic1 and Pax3 are more dependent on the E3 activity than others, notably the Hox cluster genes such as Hoxd11.
Ring1B mediates repression through H2AK119u1
The above experiments strongly suggest that repression of developmental regulators in ESCs is attributable to PRC1 mediated H2AK119u1. Previous studies report that Ring1B regulates local H3K4me3 deposition and loading of RNA polymerase II (RNAP) in ESCs [5], [19], and that H2AK119u1 has a role to suppress MLL-mediated methylation of H3 lysine 4 (H3K4) and transcriptional initiation from nucleosomal templates [28]. We, therefore, examined whether the catalytic activity of Ring1B is involved in suppressing H3K4 methylation and RNAP loading at target gene loci. Consistent with the previous reports, we found that local levels of trimethylated H3K4 (H3K4me3) and RNAP loading were considerably up-regulated at target gene promoters in Ring1A/B-dKO ESCs, which could be repressed by expression of WT Ring1B in these cells (Figure 3D and 3E). In contrast, Ring1B (I53S) and Ring1B (I53A) failed to suppress local increases of H3K4me3 and RNAP levels. Therefore, the catalytic activity of Ring1B is required to repress H3K4me3 and RNAP loading. Consistent with these observations, the profound reduction in local H3K27me3 levels in Ring1A/B-dKO ESCs could not be restored by Ring1B (I53S) or Ring1B (I53A) (Figure S9). This may also suggest the contribution of Ring1B catalytic activity to maintain repressive chromatin. Collectively, our results demonstrate that Ring1B-dependent H2AK119u1 facilitates transcriptional repression of PRC1 target genes and thereby enables the maintenance of ESC identity.
Discussion
In the present study, we present genome-wide H2AK119u1 maps in ESCs and identify a group of genes at which H2AK119u1 is deposited in a Ring1-dependent manner. These genes are a distinctive subset of genes with H3K27me3 enrichment and we suggest that these are the central targets of Polycomb silencing to maintain ESC identity. By using mutant versions of Ring1B, which can not bind to E2 components, we demonstrate the role of H2AK119u1 to facilitate the repression of these target genes. We propose that H2AK119u1 contributes to capacitate Polycomb-mediated repression in a reversible manner because recognition and de-ubiquitination of H2AK119u1 have been shown to be linked with transcriptional activation [27], [28], [33].
This conclusion is different to a recent study which suggested that the catalytic mutant Ring1B could restore repression in Ring1B mutant ES cells [23]. A key difference in that study and our analyses shown here is that we assessed the function of catalytically inactive Ring1B in a background that is null for both Ring1B and the closely related homologue Ring1A. Ring1A potentially complements loss of Ring1B in ESCs, despite the fact that the expression level of Ring1A is relatively low compared to Ring1B (Figure S6) [5], [20].
Our results are concordant with those of Eskeland et al. 2010 which reports that the ability of Ring1B to mediate the condensation of the Hoxb cluster is not dependent on its histone ubiquitination activity. In addition, in our study we have observed that the E3 activity of Ring1B contributes to the repression of Hox genes to a lesser extent than to Zic1 and Pax3 genes (Figure 3C). Based on these evidences, we propose that H2AK119u1-dependent repression is likely complemented by other PRC1-mediated mechanisms such as chromatin compaction [23]. The fact that H2AK119u1 independent repression is more prevalent at Hox loci compared to other Polycomb target genes may suggest that it is more effective when target loci are closely juxtaposed in cis. We indeed found a slight but a significant restoration of repression of H2AK119u1+ genes that are closely juxtaposed each other (<50 kb) by expression of mutant Ring1B in Ring1A/B-dKO, but this is not the case for H2AK119u1+ genes that are separated by ≥50 kb genomic regions (Figure S10). However, this tendency is not statistically significant once we excluded Hox cluster genes. Further studies are needed to elucidate the molecular nature for H2AK119u1-independent mechanisms.
Overall, our findings show that PRC1 mediates gene repression by combining multiple and different effector mechanisms, of which H2A ubiquitination is a major contributor (Figure 4). Such diverse PRC1 effector mechanisms might be required to make PRC1-mediated gene repression both flexible and robust. How H2A ubiquitination contributes to repress target gene transcription also awaits future studies, although mechanisms that antagonize against H2A ubiquitination have already been proposed [27].
Fig. 4. A schematic summary of this study demonstrating that PRC1-dependent repression of developmental genes in ES cells is mediated by multiple effector mechanisms. 
Materials and Methods
Cells and cell culture
Ring1Bfl/fl;Rosa26::CreERT2, Ring1A−/−;Ring1Bfl/fl;Rosa26::CreERT2, and Eed-KO ESCs were described previously [5], [19], [20], [26], [34]. The ESCs were cultured in DMEM with 20% fetal bovine serum, MEM nonessential amino acids (Invitrogen), sodium pyruvate (Invitrogen), L-glutamine (Invitrogen), 2-mercaptoethanol (Sigma), and ESGRO (Chemicon) either on irradiated MEF as feeder layers or directly on gelatin-coated surfaces.
Plasmids
3xFlag-tagged wild-type Ring1A, wild-type Ring1B, mutated Ring1B (I53A [17], [23] and I53S [31]) cDNAs were subcloned into the expression vector pCAG-IRES-Puro (a kind gift from Dr. Hitoshi Niwa in RIKEN CDB in Japan).
Antibodies
The following antibodies were used: Ring1B (clone #3) [35], Ring1A [36], Phc1 [37], Mel18 (Santa Cruz; sc-10744), Cbx2 [36], H3K27me3 (Millipore; 07-449), H3K4me3 (Millipore;07-473), H2AK119u1 (E6C5; Millipore; 05-678; for ChIP), H2AK119u1 (Rabbit polyclonal; for ChIP) [28], H2AK119u1 (Rabbit polyclonal; Cell Signaling Technology; #8240; for western blot), H2A (Abcam; ab18255), RNAP (8WG16; Millipore; 05-952), Lamin B (Santa Cruz; sc-6216), mouse IgM (Millipore; 12-488), and Flag-tag (M2; Sigma; F1804).
Stable transfection
Ring1A−/−; Ring1Bfl/fl; Rosa26::CreERT2 ESCs were stably transfected with tagged wild-type Ring1A, wild-type Ring1B, or mutated Ring1B. To establish stable transfectants, ESCs were electroporated (0.8 kV, 3 µF) with the respective expression vector and then selected for resistance to puromycin (1 µg/ml).
Immunoprecipitation (IP) analysis
Cells expressing each of tagged constructs were suspended in IP buffer [10 mM Tris-HCl (pH8.0), 1 mM EDTA, 140 mM NaCl, 0.4% NP-40, and 0.5 mM PMSF] and sonicated for several seconds. After centrifugation, the supernatant was collected, precleared with protein G Sepharose for 30 min at 4°C, and then incubated with anti-Flag antibody (M2) for 120 min at 4°C. The immune complexes were captured by protein G Sepharose for 60 min at 4°C. The Sepharose-bound proteins were washed with IP buffer, eluted in SDS sample buffer under reducing condition, separated on SDS-PAGE gels, and subjected to western blot analysis.
Real-time PCR
Quantitative real-time PCR was carried out with SYBR Green method and amplifications were detected with Mx3005P (Stratagene, La Jolla, CA, USA). The sequences of primers used in this study are shown in Text S1.
Chromatin immunoprecipitation (ChIP) analysis
ESCs were treated with 1% formaldehyde/PBS for 10 min at room temperature. Cells were washed with PBS, collected and resuspended in swelling buffer [20 mM Hepes (pH 7.8), 1.5 mM MgCl2, 10 mM KCl, 0.1% NP-40, and 1 mM DTT] by pipetting and then kept on ice for 10 min. After Dounce homogenizing 10–20 times, the cells were centrifuged and then the pellets were resuspended in RIPA buffer [20 mM Tis-HCl (pH 8.0), 1 mM EDTA, 140 mM NaCl, 1% Triton X-100, 0.1% SDS, and 0.1% deoxycholic acid] containing protease inhibitors and sonicated into fragments with an average length of 0.3–0.5 kb. After centrifugation, the supernatants were subjected to IP with specific antibodies as previously described [19], [38]. For H2AK119u1-ChIP, pre-cleared chromatin (400 µg) was incubated with 50 µl of E6C5 antibody (overnight, 4°C) and then the chromatin-1st antibody complexes were immunoprecipitated with 2nd antibody (rabbit anti-mouse IgM) - preconjugated protein A dynabeads (Invitrogen). Purified immunoprecipitated and input DNA was quantified by real-time PCR, and, if necessary, was subjected to the linear amplification for ChIP-chip analysis.
ChIP-on-chip experiment
ChIP-on-chip analysis was carried out using the Mouse Promoter ChIP-on-chip Microarray Set (G4490A, Agilent, Palo Alto, Calif., USA). ESCs were subjected to ChIP assay using specific antibodies as described in the previous section. Purified immunoprecipitated and input DNA was subjected to T7 RNA polymerase-based amplification as described previously [39]. Labeling, hybridization and washing were carried out according to the Agilent mammalian ChIP-on-chip protocol (ver.9.0). Scanned images were quantified with Agilent Feature Extraction software under standard conditions. All of experiments were performed by using at least two biological replicates. The obtained data were analyzed as described in Text S1.
Gene expression microarray
Total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) and purified with Qiagen RNeasy separation columns (Qiagen, Hilden, Germany). First strand cDNA was synthesized and hybridized to Affymetrix GeneChip Mouse Genome 430 2.0 arrays (Affymetrix, Santa Clara, CA, USA) to assess and compare the overall gene expression profiles. The obtained data were analyzed as described in Text S1.
Three dimensional (3D)-DNA-FISH
3D-DNA-FISH with spatial preservation of chromatin architecture was performed as described previously [40]. Experimental details are described in Text S1.
Accession numbers
ChIP-chip and microarray data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and is accessible through GEO Series accession number GSE38650.
Supporting Information
Zdroje
1. BoyerLALeeTIColeMFJohnstoneSELevineSS 2005 Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122 947 956
2. LeeTIJennerRGBoyerLAGuentherMGLevineSS 2006 Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125 301 313
3. BoyerLAPlathKZeitlingerJBrambrinkTMedeirosLA 2006 Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441 349 353
4. KimJChuJShenXWangJOrkinSH 2008 An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132 1049 1061
5. EndohMEndoTAEndohTFujimuraYOharaO 2008 Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development 135 1513 1524
6. van der StoopPBoutsmaEAHulsmanDNobackSHeimerikxM 2008 Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. PLoS ONE 3 e2235 doi:10.1371/journal.pone.0002235
7. Dahle O, Kumar A, Kuehn MR Nodal signaling recruits the histone demethylase Jmjd3 to counteract polycomb-mediated repression at target genes. Sci Signal 3 ra48
8. PasiniDBrackenAPHansenJBCapilloMHelinK 2007 The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol 27 3769 3779
9. LeebMWutzA 2007 Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells. J Cell Biol 178 219 229
10. ShaoZRaibleFMollaaghababaRGuyonJRWuCT 1999 Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98 37 46
11. MullerJHartCMFrancisNJVargasMLSenguptaA 2002 Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111 197 208
12. CzerminBMelfiRMcCabeDSeitzVImhofA 2002 Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111 185 196
13. CaoRWangLWangHXiaLErdjument-BromageH 2002 Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298 1039 1043
14. KuzmichevANishiokaKErdjument-BromageHTempstPReinbergD 2002 Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev 16 2893 2905
15. FischleWWangYJacobsSAKimYAllisCD 2003 Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev 17 1870 1881
16. BernsteinEDuncanEMMasuiOGilJHeardE 2006 Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol Cell Biol 26 2560 2569
17. BuchwaldGvan der StoopPWeichenriederOPerrakisAvan LohuizenM 2006 Structure and E3-ligase activity of the Ring-Ring complex of polycomb proteins Bmi1 and Ring1b. Embo J 25 2465 2474
18. WangHWangLErdjument-BromageHVidalMTempstP 2004 Role of histone H2A ubiquitination in Polycomb silencing. Nature 431 873 878
19. StockJKGiadrossiSCasanovaMBrookesEVidalM 2007 Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol 9 1428 1435
20. de NapolesMMermoudJEWakaoRTangYAEndohM 2004 Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell 7 663 676
21. BaarendsWMHoogerbruggeJWRoestHPOomsMVreeburgJ 1999 Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol 207 322 333
22. KallinEMCaoRJothiRXiaKCuiK 2009 Genome-wide uH2A localization analysis highlights Bmi1-dependent deposition of the mark at repressed genes. PLoS Genet 5 e1000506 doi:10.1371/journal.pgen.1000506
23. EskelandRLeebMGrimesGRKressCBoyleS 2010 Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell 38 452 464
24. FrancisNJKingstonREWoodcockCL 2004 Chromatin compaction by a polycomb group protein complex. Science 306 1574 1577
25. CaoRTsukadaYZhangY 2005 Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell 20 845 854
26. FujimuraYIsonoKVidalMEndohMKajitaH 2006 Distinct roles of Polycomb group gene products in transcriptionally repressed and active domains of Hoxb8. Development 133 2371 2381
27. RichlyHRocha-ViegasLRibeiroJDDemajoSGundemG 2010 Transcriptional activation of polycomb-repressed genes by ZRF1. Nature 468 1124 1128
28. NakagawaTKajitaniTTogoSMasukoNOhdanH 2008 Deubiquitylation of histone H2A activates transcriptional initiation via trans-histone cross-talk with H3K4 di - and trimethylation. Genes Dev 22 37 49
29. NiwaHToyookaYShimosatoDStrumpfDTakahashiK 2005 Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123 917 929
30. SinghAMHamazakiTHankowskiKETeradaN 2007 A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells 25 2534 2542
31. Ben-SaadonRZaaroorDZivTCiechanoverA 2006 The polycomb protein Ring1B generates self atypical mixed ubiquitin chains required for its in vitro histone H2A ligase activity. Mol Cell 24 701 711
32. YingQLWrayJNicholsJBatlle-MoreraLDobleB 2008 The ground state of embryonic stem cell self-renewal. Nature 453 519 523
33. JooHYZhaiLYangCNieSErdjument-BromageH 2007 Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature 449 1068 1072
34. AzuaraVPerryPSauerSSpivakovMJorgensenHF 2006 Chromatin signatures of pluripotent cell lines. Nat Cell Biol 8 532 538
35. AtsutaTFujimuraSMoriyaHVidalMAkasakaT 2001 Production of monoclonal antibodies against mammalian Ring1B proteins. Hybridoma 20 43 46
36. SchoorlemmerJMarcos-GutierrezCWereFMartinezRGarciaE 1997 Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J 16 5930 5942
37. MiyagishimaHIsonoKFujimuraYIyoMTakiharaY 2003 Dissociation of mammalian Polycomb-group proteins, Ring1B and Rae28/Ph1, from the chromatin correlates with configuration changes of the chromatin in mitotic and meiotic prophase. Histochem Cell Biol 120 111 119
38. OrlandoVStruttHParoR 1997 Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods 11 205 214
39. van BakelHvan WervenFJRadonjicMBrokMOvan LeenenD 2008 Improved genome-wide localization by ChIP-chip using double-round T7 RNA polymerase-based amplification. Nucleic Acids Res 36 e21
40. SoloveiICavalloASchermellehLJauninFScasselatiC 2002 Spatial preservation of nuclear chromatin architecture during three-dimensional fluorescence in situ hybridization (3D-FISH). Exp Cell Res 276 10 23
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



