-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDefining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
article has not abstract
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003154
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003154Summary
article has not abstract
Atrial fibrillation (AF) is the most commonly observed arrhythmia, and it is estimated to affect more than 2 million adults annually in the United States alone [1]. The risk of stroke, heart failure, and sudden death are all increased in patients with AF. The frequency of AF increases with age and most often underlies other overt cardiomyopathies or cardiovascular diseases such as ischemic heart disease, congestive heart failure, or hypertension [2], [3]. However, for nearly 70 years AF has also been known to be an inherited, Mendelian disorder [4]. Indeed, work from the Framingham Heart Study reported a significant increase in the risk of AF in patients who had just one parent with AF [5]. The genetic mechanisms that lead to AF vary widely as do the degrees of penetrance [6], [7].
Atrioventricular (AV) conduction disease is characterized by a prolonged PR interval in surface electrocardiogram (ECG) recordings. Importantly, a prolonged PR interval is a strong predictor of AF; and like AF, variability in the PR interval has a genetic component. However, pinpointing the genetic factors underlying AV conduction has been wrought with difficulty and remarkably little is known about the genes leading to increased susceptibility to PR interval variability. In this issue of PLOS Genetics, Lodder and colleagues provide compelling evidence that increased expression of the Tnni3k gene (which encodes Troponin I-interacting kinase 3) is significantly correlated with increased PR interval [8].
Previous findings from this research team identified a quantitative trait locus for PR interval variability on chromosome 3 within a mouse model that harbors a variant in the cardiac voltage-gated sodium channel (Scn5a1798D/+) [9], [10]. In the process of engineering this experimental mouse model, the investigators noted that the ECGs of the parental strains, 129P2-Scn5a1798insD/+ and FVBN/J-Scn5a1798insD/+, both phenocopied many of the ECG characteristics of human patients harboring the homologous SCN5A-1795insD variant, namely conduction defects manifested in the PR interval. These two mouse strains displayed variation in the severity of the PR interval variation, indicating that while the root genetic cause is likely the same, the degree of penetrance diverges owing to differing haplotypes. In their new study, the team cleverly exploits this natural variation to precisely pinpoint the underlying genetic locus, Tnni3k, that is shared by the mouse strains and is responsible for PR interval variability. Importantly, the investigators perform in vivo functional studies using mice overexpressing human troponin I-interacting kinase 3 to confirm the role of this gene product in regulating PR interval in atrioventricular conduction.
Tnni3k belongs to the MAPKKK family of polypeptides [11]. Interestingly, PLOS Genetics recently published work by Wheeler et al. that identified Tnni3k as a strong modifier of disease progression in a mouse model of cardiomyopathy [12]. In this work, the evidence implied that Tnni3k actively participates only in the stress response pathways within the heart, as mice with minimal Tnni3k expression at baseline showed no cardiac abnormalities. Furthermore, and importantly, Tnni3k expression is restricted to cardiac tissue. Taken together, this evidence increases the prominence of Tnni3k as a potential therapeutic target for multiple cardiac electrical and structural pathologies.
While the genetic and in vivo findings are compelling, the new data from Lodder and colleagues raises questions regarding the exact functional role(s) that Tnni3k polypeptides play in the heart (Figure 1). In this study, phosphorylation targets of this kinase were not identified. Indeed, our current understanding of how this kinase functions is broadly lacking. As this work is focused upon AV conduction disease, the authors appropriately speculate that gap junctions and/or cardiac ion channels involved in action potential propagation may be direct targets of Tnni3k. Investigations that identify the phosphorylation targets of Tnni3k are warranted. Furthermore, a clear link to human heart disease has yet to be established: does Tnni3k modify the progression towards heart failure or regulate PR interval duration in conduction diseases as it is now known to in mice? While Tnni3k expression is expected to be cardiac-specific, its particular distribution within the heart is unknown. Establishing differential expression in the various chambers, structures (i.e., sinoatrial and atrioventricular nodes), or tissues will further inform us on the functional role(s) of Tnni3k.
Fig. 1. The development of a reentry circuit due to slowed action potential velocity. 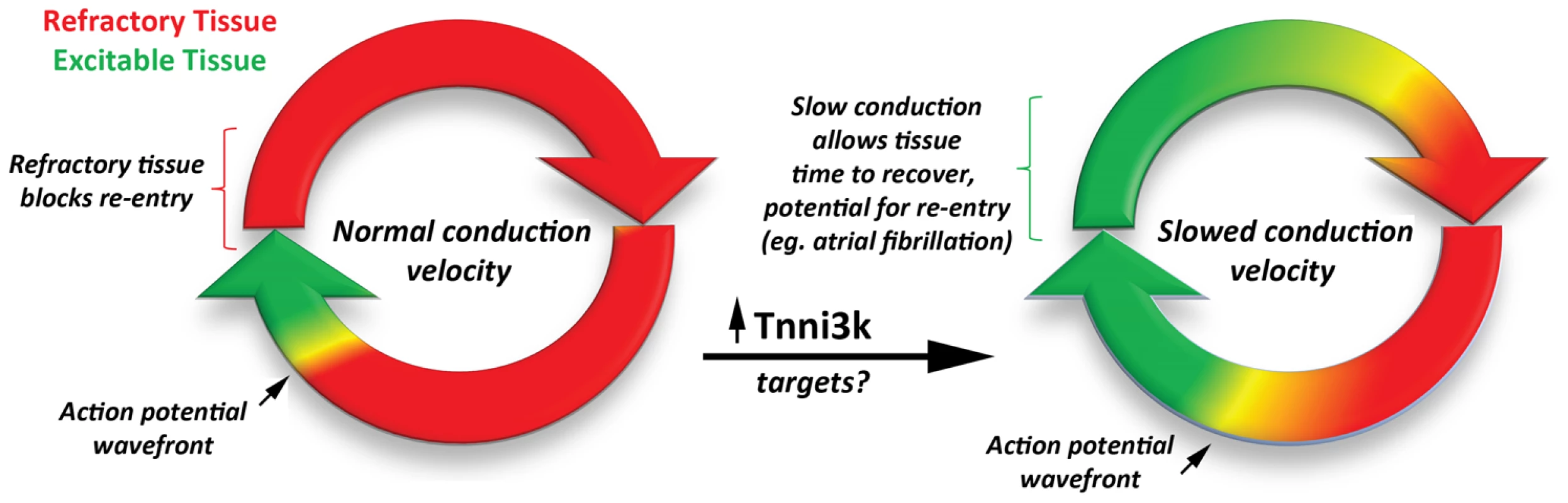
Increased expression of Tnni3k (and downstream targets) slows conduction velocity of the propagating action potential. Slowed conduction, in turn, favors the formation of stable reentrant propagation, a common mechanism for sustained arrhythmia. In summary, Lodder et al. have provided strong evidence that Tnni3k regulates PR interval variability in a mouse model of atrioventricular conduction disease. Importantly, this new work highlights the potential for original insights into gene function through creative, varied genetic and physiological approaches, leading to a more educated translation of basic science into new therapeutic targets.
Zdroje
1. FeinbergWM, BlackshearJL, LaupacisA, KronmalR, HartRG (1995) Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 155 : 469–473.
2. GoAS, HylekEM, PhillipsKA, ChangY, HenaultLE, et al. (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285 : 2370–2375.
3. KrahnAD, ManfredaJ, TateRB, MathewsonFA, CuddyTE (1995) The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 98 : 476–484.
4. WolfL (1943) Familial auricular fibrillation. N Engl J Med 229 : 396–397.
5. FoxCS, PariseH, D'AgostinoRBSr, Lloyd-JonesDM, VasanRS, et al. (2004) Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA 291 : 2851–2855.
6. SinnerMF, EllinorPT, MeitingerT, BenjaminEJ, KaabS (2011) Genome-wide association studies of atrial fibrillation: past, present, and future. Cardiovasc Res 89 : 701–709.
7. DarbarD, HerronKJ, BallewJD, JahangirA, GershBJ, et al. (2003) Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol 41 : 2185–2192.
8. LodderEM, SciclunaBP, MilanoA, SunAY, TangH (2012) Dissection of a quantitative trait locus for PR interval duration identifies Tnni3k as a novel modulator of cardiac conduction. PLoS Genet 8: e1003113 doi:10.1371/journal.pgen.1003113.
9. RemmeCA, SciclunaBP, VerkerkAO, AminAS, van BrunschotS, et al. (2009) Genetically determined differences in sodium current characteristics modulate conduction disease severity in mice with cardiac sodium channelopathy. Circ Res 104 : 1283–1292.
10. SciclunaBP, WildeAA, BezzinaCR (2008) The primary arrhythmia syndromes: same mutation, different manifestations. Are we starting to understand why? J Cardiovasc Electrophysiol 19 : 445–452.
11. LaiZF (2009) TNNI3K could be a novel molecular target for the treatment of cardiac diseases. Recent Pat Cardiovasc Drug Discov 4 : 203–210.
12. WheelerFC, TangH, MarksOA, HadnottTN, ChuPL, et al. (2009) Tnni3k modifies disease progression in murine models of cardiomyopathy. PLoS Genet 5: e1000647 doi:10.1371/journal.pgen.1000647.
Štítky
Genetika Reprodukční medicína
Článek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



