-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
Embryonic stem cells and induced pluripotent stem cells represent potentially important therapeutic agents in regenerative medicine. Complex interlinked transcriptional and signaling networks control the fate of these cells towards maintenance of pluripotency or differentiation. In this study we have focused on how mouse embryonic stem cells begin to differentiate and lose pluripotency and, in particular, the role that the ERK MAP kinase and GSK3 signaling pathways play in this process. Through a genome-wide siRNA screen we have identified more than 400 genes involved in loss of pluripotency and promoting the onset of differentiation. These genes were functionally associated with the ERK and/or GSK3 pathways, providing an important resource for studying the roles of these pathways in controlling escape from the pluripotent ground state. More detailed analysis identified MAP kinase phosphatases as a focal point of regulation and demonstrated an important role for these enzymes in controlling ERK activation kinetics and subsequently determining early embryonic stem cell fate decisions.
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003112
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003112Summary
Embryonic stem cells and induced pluripotent stem cells represent potentially important therapeutic agents in regenerative medicine. Complex interlinked transcriptional and signaling networks control the fate of these cells towards maintenance of pluripotency or differentiation. In this study we have focused on how mouse embryonic stem cells begin to differentiate and lose pluripotency and, in particular, the role that the ERK MAP kinase and GSK3 signaling pathways play in this process. Through a genome-wide siRNA screen we have identified more than 400 genes involved in loss of pluripotency and promoting the onset of differentiation. These genes were functionally associated with the ERK and/or GSK3 pathways, providing an important resource for studying the roles of these pathways in controlling escape from the pluripotent ground state. More detailed analysis identified MAP kinase phosphatases as a focal point of regulation and demonstrated an important role for these enzymes in controlling ERK activation kinetics and subsequently determining early embryonic stem cell fate decisions.
Introduction
Embryonic stem cells and induced pluripotent stem cells (iPS cells) are currently generating intense interest due to their potential therapeutic roles in regenerative medicine (reviewed in [1]). We are beginning to understand the rules governing the establishment and maintenance of the pluripotent state and, in particular, the signaling and transcriptional networks which define this state (reviewed in [2]–[3]). A number of genome-wide si/shRNA screens have been instrumental in deciphering these networks [4]–[6]. In contrast, less attention has been directed towards understanding how embryonic stem cells lose their pluripotency and begin to differentiate.
Mouse embryonic stem cells can be maintained in a pluripotent state by culturing under a variety of defined conditions (reviewed in [7]). Traditionally, these cells are cultured in medium containing serum and the cytokine leukaemia inhibitory factor (LIF) [8]–[9]. However, more recently, it was demonstrated that mouse embryonic stem cells can be maintained in a pluripotent ground state by using two specific protein kinase inhibitors (known as “2i” conditions) which target the ERK pathway component MEK and glycogen synthase kinase (GSK3) ([10]; reviewed in [11]). Removal of these two inhibitors promotes exit from the naïve ground state. These studies therefore revealed an important role for the ERK and GSK3 pathways to enter into lineage commitment (reviewed in [12]). Moreover, the suppression of ERK signalling in the mouse embryo is sufficient to expand the pluripotent compartment in the early mouse embryo [13] and can enhance the efficiency of iPS cell generation by promoting completion of reprogramming [14]–[15]. Importantly, the same pathways may operate in a functionally analogous manner in human pluripotent stem cells that have been genetically manipulated [16]–[17]. The ERK pathway has previously been shown to trigger mouse ES cell differentiation [18]–[19] and is implicated in numerous developmental processes (reviewed in [20]) in addition to playing an important role in a variety of different stem cell types (reviewed in [21]). Less is known about GSK3 function in development and stem cell biology and the role for GSK3 is usually attributed to its ability to regulate β-catenin stability and hence limit the responses to Wnt pathway signalling (reviewed in [11], [22]). Recently, a β-catenin-dependent mode of action has been demonstrated for GSK3 in the context of mouse embryonic stem cells, although this mode of action is not sufficient to explain all the effects of GSK3 signalling in this context ([23]–[24]; reviewed in [25]).
One major function of ERK MAP kinase signalling, is to orchestrate gene expression programmes in the cell. In particular, this pathway directly targets a number of transcription and chromatin regulators and thereby controls their activities (reviewed in [26]–[27]). However, which of the ERK targets are important in embryonic stem cell differentiation are unknown. It is also unclear how the canonical ERK pathway is controlled in these cells. In this study, we took advantage of the fact that the combinatorial use of ERK pathway and GSK3 inhibitors maintains mouse embryonic stem cell pluripotency [10] and carried out a genome-wide siRNA screen to identify regulators and mediators of these pathways that influence the exit from pluripotency. This has led to the identification of over 400 genes whose functions are required for efficient embryonic stem cell differentiation away from the pluripotent ground state. The vast majority of these genes have not previously been implicated in this process; therefore our study provides an important new resource for the community. Moreover, further downstream analysis has partitioned these genes into classes that functionally interact with the ERK and/or GSK3 pathways and has revealed an important role for MAP kinase phosphatases in controlling embryonic stem cell fate.
Results
An RNAi screen for genes required for ERK/GSK3-mediated ES cell differentiation
To identify the programme of genes involved in the loss of pluripotency and subsequent differentiation of embryonic stem cells, a genome-wide RNAi screen was performed using E14Tg2a mouse ES cells which are engineered to express an unstable version of GFP from the endogenous rex1 (also known as zfp42) locus. This reporter gene is regulated in an analogous manner to endogenous rex1 [23] and provides a convenient readout for the loss of a naieve pluripotent stem cell marker Rex1 [28] (reviewed in [11]). Rex1GFPd2 ES cells were maintained in media containing MEK and GSK inhibitors (2i) to maintain their ES cell status and treated with siRNAs pools targeting ∼17,000 individual genes. After 24 hrs, cells were exchanged into fresh media lacking these inhibitors and the levels of GFP in each cell were assessed over time (Figure 1A). A gradual loss of GFP expression occurred upon inhibitor withdrawal over a ∼2 day time period, with conversion of the majority of cells to low expression (1A). We wanted to conduct the screen at the earliest possible time point to maximise the chances of detecting genes directly involved in the exit from pluripotency rather than secondary effectors. The control siRNAs for fgf4 and gsk3β both significantly reduced GFP loss at 27–30 hrs (Figure S1B). Therefore we monitored the ratio of cells expressing high and low levels of GFP at this time point. siRNAs were scored as positive hits when this ratio increased by more than two standard deviations (SD) above the mean of all siRNAs on each plate. A conservative threshold was selected at this stage to be more inclusive before further downstream validation was performed. This led to the identification of 792 siRNAs that delayed the loss of GFP expression, and hence target genes potentially involved in promoting pluripotency loss and/or cell differentiation (Figure 1B; Table S1A). Examples, include 2400001e08rik, raf1 and jarid2 (Figure 1C; Figure S2A–S2D, left panels). Importantly, this primary screen identified RNAi pools targeting nras, raf1 and gsk3β, as would be expected due to their known roles in the ERK and GSK3 pathways. Moreover, further validation of the efficacy of our screen was demonstrated by the identification of a large number of siRNAs targeting genes encoding proteosomal proteins, as would be expected due to the subsequent increased half-life of the unstable GFP protein used as a readout in these assays. In addition, this primary screen also revealed 130 siRNAs that accelerate the loss of GFP expression and hence target genes that function to maintain pluripotency and/or inhibit cell differentiation including known effectors such as esrrb, stat3, and ctr9 [4], [29]–[30](Figure 1B; Figure S2E; Table S2). Furthermore, several of genes identified in our screen in this category were also identified in other screens designed to identify genes required for pluripotency [4]–[5], [31]–[33], including stat3 and smc1a (both identified in 2 and 3 additional screens, respectively) (Table S3). As our primary interest was on the mechanisms of escape from the pluripotent ground state rather than the maintenance of pluripotency, we subsequently focussed on genes that were required for modulating the onset of differentiation. Two secondary screens were performed with a different set of siRNA pools targeting the genes identified in the primary screen and either the same reporter cells (ie Rex1GFPd2) or ES cells containing an alternative reporter gene, where GFP is instead driven by the oct4 (also known as pou5fl) promoter, thereby providing an independent readout for the loss of pluripotency (Figure 1A; Figure S1C). These screens gave rise to 398 and 420 positive hits respectively, and 316 of these siRNAs scored positive in both secondary screens (Figure 1A, 1D and 1E; Figure S2A–S2D, Table S1B and S1C). These 316 siRNAs therefore define a high confidence dataset of genes that are required for the efficient loss of pluripotency and/or promoting the onset of differentiation of ES cells. A number of these genes have already been implicated in embryonic stem cell differentiation control including tcf7l1(tcf3), jarid2, and dpy30 [34]–[37] (Table S4) further supporting the quality of our dataset. Moreover, comparisons to other RNAi and overexpression screens performed on mouse ES cells [4], [31]–[33], [38] identified several genes in common, including jun and mbd3 which were both identified in two of these screens in addition to our own (Table S3). However, the vast majority of genes we have identified here, have not been previously implicated in controlling the escape from the pluripotent ground state. To assess the types of biological processes and potential mechanisms of actions of these 316 genes, gene ontology (GO) analysis was performed and prominent terms identified included a number of signalling pathways and also genes encoding transcriptional regulators (Figure 1F and 1G; Figure S3). Thus cellular signalling events and subsequent gene expression control appear to play prominent roles in the early events associated with ES cell differentiation.
Fig. 1. A genome-wide RNAi library screen identifies factors involved in signal-dependent embryonic stem cell differentiation. 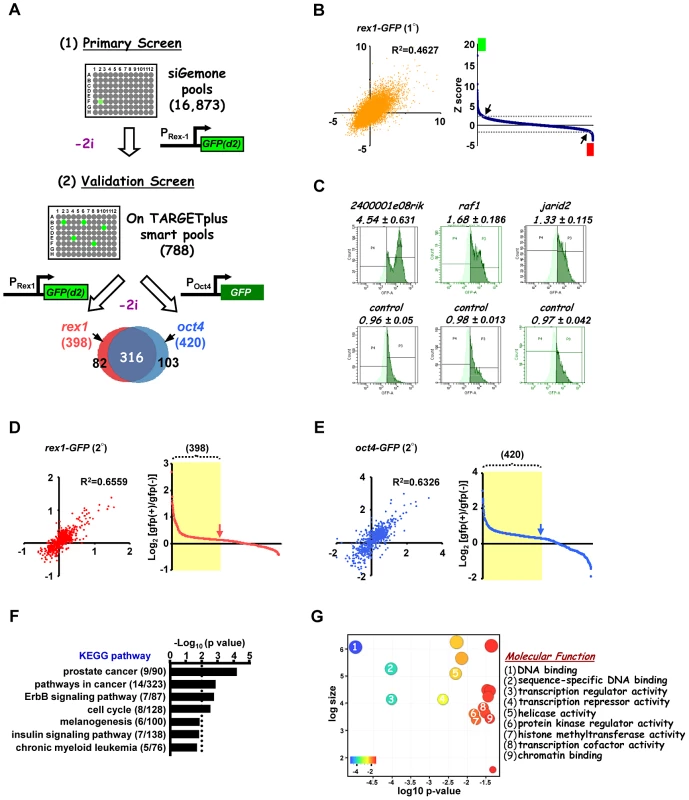
(A) Schematic representation of primary screen and secondary validation screens using mouse embryonic stem cell lines containing GFP reporter constructs under the control of either the endogenous rex1 (Rex1GFPd2) and/or oct4 (Oct4GFP) promoters. “−2i” indicates that the two kinase inhibitors (CHIR99021 and PD0325901) were removed for 28 hrs (Rex1GFPd2 cells) and 72 hrs (Oct4GFP cells) before quantifying the GFP-positive population of cells. The Venn diagram shows 316 high confident hits resulting from the overlap of both validation screens. (B) The z-score of each of two biological replicates from the primary screens are plotted against each other (left panel). The average of ranked z-scores from the knockdown of individual siRNA pools is shown (right panel). The arrows indicate the z-score threshold (2 and −2). The green and red boxes mark the hits with z-score >2 and <−2, respectively. (C) Representative FACS profiles from control non-targeting and positive siRNA hits from the primary rex1-GFP(d2) screen. The high GFP expressing population is depicted in dark green and numbers above each graph are the corresponding GFP high/GFP low ratios. (D and E) The log2 ratio of GFP(+)/GFP(−) of each of the two biological replicates are plotted against each other in either the rex1-GFP(d2) (D) or oct4-GFP (E) validation screens (left panels) and graphical representations of the ranked log2 values of these ratios from the average of two independent experiments upon knockdown of individual genes are shown (right panels). The yellow shaded boxes indicate the positive hits which scored as a GFP(+)/GFP(−) ratio above 1.25× standard deviation of the controls in each of the sub-screens. (F and G) Enriched KEGG (F) and “molecular function” level GO terms (G) amongst the high confidence hits identified in both of the secondary validation screens. Distinct groups of genes are required for the action of the ERK and GSK3 signalling pathways
Having established the core network of genes working in concert with the GSK3 and ERK pathways we wanted to discover the relative contributions of these genes to the actions of the individual pathways. First we performed a counter screen in the presence of both pathway inhibitors (“+2i”) to eliminate siRNAs which promoted accumulation of GFP in the cells irrespective of the activity of the ERK and GSK3 pathways (Figure 2A). This eliminated a further 42 siRNAs, including 14 that targeted proteosomal components and hence stabilised the GFP (Table S5). This left 274 siRNAs which define genes required for efficient signal-dependent loss of pluripotency and the onset of differentiation. The differentiation of ES cells away from pluripotency is maximally promoted by removing inhibitors of both GSK3 and the ERK pathway. However, the removal of a single inhibitor permits ES cell differentiation and loss of Rex1-GFP signal, albeit with delayed kinetics (Figure S4). We took advantage of this to partition our dataset and identify genes whose functions are specifically required for differentiation driven by either the ERK pathway or the GSK3 pathway alone. siRNAs targeting the genes constituting the high confidence data set from the “2i” withdrawal screens were tested for their effect on Rex1-GFP loss upon single inhibitor withdrawal (ie “1i” withdrawal screens; Figure 2A). Of the 274 siRNAs tested, 133 delayed GFP loss upon withdrawal of the MEK inhibitor and 168 upon withdrawal of the GSK3 inhibitor. Amongst these, 106 were in common. A further 79 siRNAs had no effect on Rex1-GFP expression under either condition (Figure 2A and 2B; Figure S5). Thus there are four functionally distinct classes of hits identified that are involved in promoting the onset of differentiation: (i) in the context of the ERK pathway (“ERK only hits” eg nras, Figure S2A; identified upon MEK inhibitor withdrawal only); (ii) in the context of the GSK3 pathway (“GSK only hits” eg dmbx1, Figure S2B; identified upon GSK3 inhibitor withdrawal only); (iii) in the context of either pathway (“ERK/GSK hits” eg jun, Figure S2C; identified upon GSK3 or MEK inhibitor withdrawal); and (iv) in the context of both pathways together (“ERK and GSK hits” eg gli3, Figure S2D; no effect when either inhibitor is withdrawn). Next to gain an insight into how the ERK and GSK3 pathways might function in the context of embryonic stem cells, we used gene ontology analysis to determine whether different groups of genes identified from the single inhibitor (“1i”) screens are associated with different biological processes. Generally, the enriched GO terms for the genes from the initial 2i screen closely resemble those enriched in the “GSK” dataset (Figure S6). However, closer inspection of the data revealed enriched GO terms that are more specific for genes which were associated with either the ERK or the GSK3 pathway, thereby revealing functionally distinct contributions of these pathways to the exit from pluripotency (Figure 2C and 2D; Figure S7A–S7D). For example, genes associated with either the ERK or GSK3 pathways are enriched in different signalling pathways (Figure 2C) and a number of terms associated with mitochondrial function are preferentially enriched in the genes associated with the GSK pathway (Figure S7C). However, other groups of GO terms were identified with generally high enrichment for genes associated with both the GSK and the ERK pathways. This is typified by a large number of GO terms associated with transcriptional control (Figure 2D). Weaker enrichment of specific terms could be discerned for genes functionally associated with either the ERK or the GSK pathways (Figure 2D).
Fig. 2. Secondary screening and association of genes to the ERK and/or GSK3 pathways. 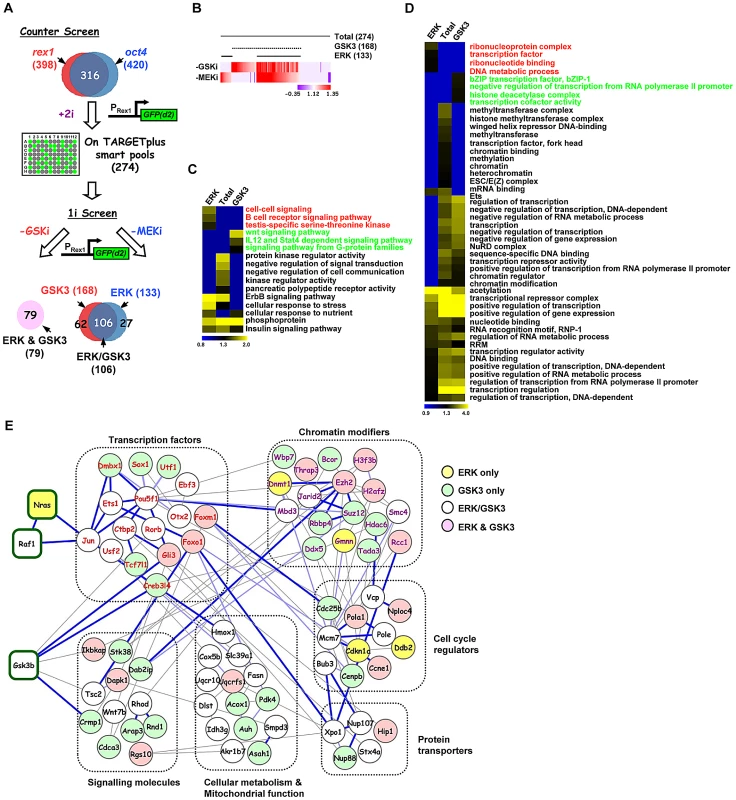
(A) Schematic representation of the strategy used to stratify the high confidence positive hits via either the ERK and/or GSK3 pathways (1i-screens) using the rex1-GFP reporter system. A counter screen was performed in the presence of the two kinase inhibitors (CHIR99021 and PD0325901)(“+2i”) and remaining hits were tested when either the GSK3 inhibitor (CHIR99021; -GSKi) or the MEK inhibitor (PD0325901; -MEKi) was withdrawn. The Venn diagram illustrates the four different hit categories. (B) Heatmap summary depicts the stratification of the hits according to their effects on the GFP(+)/GFP(−) ratio upon withdrawal of the GSK3 inhibitor (-GSK3i) or MEK inhibitor (-MEKi). The numbers of genes in each category are indicated. The colour scale represents the ratio of high to low GFP expressing cells for each siRNA pool in each of the “1i” screens. (C and D) Heatmaps of the enriched GO terms identified for genes corresponding to hits specific to the total dataset from the “2i” screen (274), or hits from the “1i” screens; ERK (133), or GSK3 (168) pathways. Each GO term is scored by −log10(P-value). The associated GO term descriptions are indicated (the GO terms enriched in only the ERK or GSK3 categories are indicated in red or green font respectively). Distinct functional groups corresponding to terms associated with cell signaling (C) and gene expression (D) are manually clustered. (E) STRING network analysis of the core network formed by the 274 genes associated with signal-dependent loss of pluripotency and promoting early differentiation processes in the mouse embryonic stem cells. Genes are grouped according to common biological processes. The coloured lines of edges represent confidence scores of interconnectivity. Dark blue lines represent 0.8–1, light blue lines represent 0.6–0.8, and light grey lines represent 0.4–0.6 confidence levels, respectively. We then created a network out of the genes from the high confidence dataset identified in the “2i” screen based on previous knowledge of physical and functional interactions. Functionally related subnetworks could be identified, two of the most prominent of which are composed of genes encoding proteins associated with regulating chromatin modifications and sequence-specific DNA binding transcription factors (Figure 2E; Figure S8A). These genes showed strong interconnectivities with the rest of the network as might be expected from their regulatory functions. Although only a limited number of connections between ERK and GSK3 signalling pathway components identified in the screen were revealed during network construction, these connections are made to transcription and chromatin regulators associated with the correct respective pathways (eg Jun is connected to the Ras pathway and Gli3 is connected to Gsk3β; Figure 2E; Figure S8B).
In summary, by comparing single inhibitor assays, we have been able to subcategorise the genes required for embryonic stem cell differentiation and tentatively assign them to mediating or regulating the effects of either the ERK pathway, or the GSK3 pathway or both. Each pathway appears to require genes associated with overlapping and yet distinct biological processes.
Functional dissection of genes associated with ERK pathway signalling
Our RNAi screen identified genes belonging to many functionally related categories and they are potentially involved in many biological processes. However, to begin to understand the roles of the genes we have identified in controlling the loss of pluripotency and subsequent differentiation, we decided to focus mainly on the genes which were required for ERK-mediated differentiation as this pathway has a well established role in triggering mouse ES cell differentiation [18]–[19]. The majority of “ERK only” genes and a subset of “ERK/GSK” genes were taken for further investigation alongside several control genes from the “GSK only” hits (Figure 3A). The relative strength of the effect of the knockdown of each gene in the context of the “1i” screens is illustrated in Figure 3A. First we validated the roles of these genes by using RT-qPCR to monitor the loss of the pluripotency markers rex1 and nanog and the appearance of the early differentiation marker fgf5. The majority of the siRNAs tested showed increased rex1 and nanog expression relative to control siRNAs upon “2i” withdrawal (Figure 3B; Figure S9B). Importantly, an excellent correlation was observed between effects on rex1 and nanog expression (Figure 3B; R2 = 0.81). Conversely, more than half of the siRNAs tested reduced the accumulation of fgf5 mRNA (Figure S9C). However, there was generally reduced concordance between the severity of the effects on fgf5 and rex1 (Figure 3C) or fgf5 and nanog (Figure S9D) expression. For example depletion of jarid2 and pabpc1 causes some of the largest effects in maintaining rex1 expression but has no effect on reducing fgf5 accumulation. Conversely, reductions in ets1 and dmbx1 limit fgf5 expression while having only a small effect on rex1 expression. Nevertheless, a group of siRNAs can be identified that limit the loss of rex1 expression and show reduced accumulation of fgf5 (Figure 3C; quadrant 1) and hence have effects on both loss of naive pluripotency and the onset of differentiation. In contrast, there is another large group of genes that appear to affect pluripotency status but have little effect on the onset of early differentiation (Figure 3C, quadrant 2). It is unclear why this occurs but it might reflect that although individual siRNAs promote retention of pluripotency, they might also trigger the activation of subsets differentiation markers, thus the two processes need not be tightly linked. To extend the analysis of differentiation events, we focused on the two of the top hits attributed to ERK signalling, gmnn and 3830406c13rik, and also asked whether the appearance of markers of the three embryonic cell lineages was affected. First we determined whether pluripotent cells remained in the population by alkaline phosphatase staining. Increased numbers of alkaline phosphatase stained cells were identified 5 days after “2i” withdrawal upon depletion of either gene, confirming their importance for escape from the pluripotent ground state (Figure 3D). Depletion of gmnn caused reductions in the expression of all three lineage markers at both 3 and 5 days following “2i” withdrawal, consistent with a general role in regulating the escape from the pluripotent ground state (Figure 3E). Similarly, depletion of 3830406c13rik, caused reduced expression of all three markers at day 3 (albeit only marginally for tbx6), and reduced levels of nestin after 5 days (Figure 3E). However, increased expression of gata4 and tbx6 was observed at this later timepoint, suggesting a lineage specific role for this gene. Thus, the contributions of individual genes identified in our screen towards individual lineage commitment are likely complex. In summary, the use of marker genes allows us to further validate the hits in our screen, although the effects of depleting individual genes on the loss of naive pluripotency and/or differentiation vary according to the gene involved.
Fig. 3. The role of ERK pathway-specific hits in the expression of pluripotency and early differentiation marker genes. 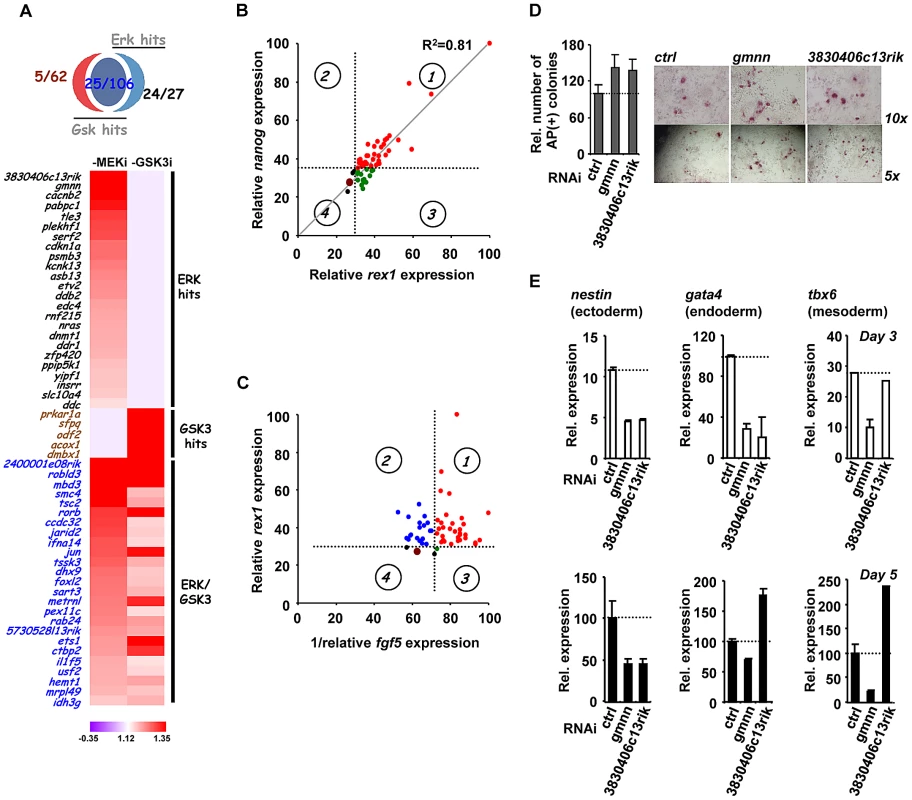
(A) Venn diagram (top) and heatmap summary (bottom; see Figure 2B for details) illustrating the number and a list of selected screen hits used in the subsequent studies. These selected hits are distributed within three categories as indicated on the heatmap. The colour scale represents the ratio of high to low GFP expressing cells for each siRNA pool in each of the “1i” screens. (B) rex1 (x-axis) and nanog (y-axis) mRNA expression levels following 2i withdrawal for 36 hrs are plotted upon knockdown of individual genes (see Figure S9B for details). Data are shown for each siRNA duplex relative to the maximal expression exhibited in the presence of an siRNA pool (taken as 100). Dotted lines represent the expression values >2 standard deviations above the mean of the negative control siRNAs. Red dots represent siRNA duplexes which promote elevated expression of both genes (quadrant 1), whereas green (quadrant 3) and black (quadrant 4) dots represent siRNAs that cause changes at or below this threshold cut-off value for only one gene. The brown dot represents the negative control siRNAs. (C) rex1 (y-axis) and the reciprocal of fgf5 (x-axis) mRNA expression levels upon 2i withdrawal for 36 hrs and 48 hrs, respectively, are plotted following knockdown of individual genes (see Figure S9B and C for details). The labeling is as indicated in (B), except that red dots represent siRNA duplexes which promote elevated expression of rex1 and lower levels of fgf5 (quadrant 1). Blue dots (quadrant 2) represent siRNAs that cause elevated rex1 expression but fail to show reductions in fgf5 expression. (D) Alkaline phosphatase staining of Rex1GFPd2 ES cells following treatment of cells with siRNAs against gmnn or 3830406c13rik or a non-targeting control (ctrl) and release from “2i” for 5 days. Data are means ± SEM (n = 2) (E) RT-PCR analysis of the expression of the indicated lineage marker genes following treatment of cells with siRNAs against gmnn or 3830406c13rik or a non-targeting control (ctrl) and release from “2i” for 3 (top) or 5 days (bottom). Data are presented as means ± SEM (n = 2). Next, to further investigate the function of the hits identified in our screen, we investigated how this subset of genes impacted on ERK pathway regulation and function. In theory, genes might act to control ERK pathway activity or alternatively might mediate the effects of ERK pathway signaling. Therefore as a first step to partition genes as acting up or downstream of ERK, we used western blotting to monitor the active phosphorylated form of ERK (Figure 4A, Figure S10). Using this assay, siRNAs targeting 21 different genes were identified as upstream regulators of ERK. Importantly, none of the “GSK3 only” hits affected ERK activation, further validating our partitioning of the data (Figure 4A; Figure S10). Furthermore, while “ERK only” hits are partitioned evenly as acting up and downstream of ERK activation, the “ERK/GSK” hits are more prominent downstream of ERK (Figure 4B), as might be expected for genes which are important for GSK-mediated differentiation when ERK signaling is inhibited. To further delineate their point of action, we then tested the subset of siRNAs which acted upstream of ERK for their effects on Ras activation by an ELISA-based assay (Figure 4C). Eleven genes were identified whose point of action is upstream of both Ras and ERK (Figure 4C; Figure S11). Importantly, one of these genes was nras itself. These assays therefore enabled us to position genes from the “ERK only”, and “ERK/GSK” datasets at different points in the ERK pathway, either acting upstream of Ras eg plekh1 or on the core pathway downstream from Ras (Figure 4D). The rest of the genes analysed appear to act downstream from ERK and hence are likely mediators of ERK pathway function. Interestingly, transcription factors are over-represented in the subgroup of genes which act downstream of ERK (Figure 4E), in keeping with the known major role of ERK signalling in controlling gene expression programmes (reviewed in [26]–[27]). Together, these findings indicate that we have identified groups of genes which affect either signalling through the ERK pathway and/or the downstream consequences of ERK activation.
Fig. 4. Association of siRNA screen hits with the ERK signaling pathway. 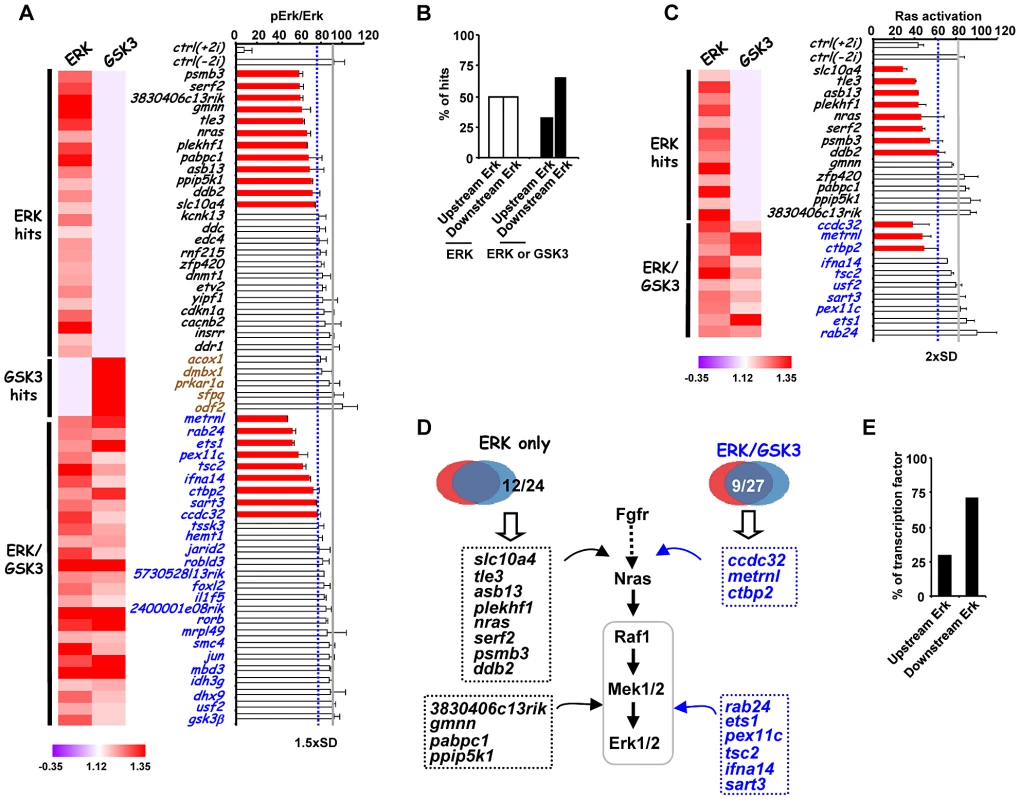
(A) ERK activation levels following 2i withdrawal (−2i) for 20 mins are plotted as the ratio of phospho-ERK2 and ERK2 signals upon depletion of selected genes as indicated. The blue dashed line indicates the threshold level (1.5× SD above the mean of the negative controls) and levels below this are indicated by red bars. The average activity in the presence of control siRNA (ctrl) is shown by the solid grey line and data are plotted relative to the siRNA giving the highest levels of phospho-Erk (taken as 100). The data are presented as means ± SEM and are the average of three biological replicates (n = 3). A heatmap summary of the effect of each siRNA duplex in the “1i” screens is shown on the left. (B) Summary of the points of action of the siRNA screen hits with respect to the ERK pathway. Genes are partitioned according to which class of siRNA hits they belong. (C) Ras activity levels upon depletion of the indicated genes upon 2i withdrawal (−2i) for 2 mins. The blue dashed line indicates the threshold level (2× SD above the mean of the negative controls) and levels below this are indicated by red bars. The average activity in the presence of control (ctrl) siRNA is shown by the solid grey line and data are plotted relative to the siRNA giving the highest levels of Ras activity (taken as 100). Data are presented as means ± SEM and are the average of three biological replicates (n = 3). A heatmap summary of the effect of each siRNA duplex in the “1i” screens is shown on the left. (D) Summary diagram illustrating the point of action of upstream ERK effectors in the ERK pathway as either upstream of Ras or between Ras and Erk. The hit lists are grouped into the ERK-unique or ERK/GSK-shared hit categories. (E) Summary of the points of action of genes encoding transcriptional regulators respect to the ERK pathway. While in this study we have focussed on studying genes which affect escape from pluripotency, and are associated with the ERK and GSK3 pathways, it is likely that many of the genes we have identified might also play a more general role in controlling stem cell pluripotency. Indeed, several genes identified in our study were also identified previously in other siRNA screens conducted in cells maintained in the presence of serum and LIF rather than the “2i” conditions we used (Table S3). To investigate this further, we tested 8 genes for their role in escape from pluripotency in Rex1GFPd2 ES cells maintained in serum and LIF and induced to differentiate by withdrawal of LIF. Depletion of three of these genes, otx2, etv5 and mbd3, caused an increased retention of rex1 promoter-driven GFP expression, consistent with a disruption in escape from pluripotency (Figure S12). Thus, it is likely that many of the genes we have identified in this screen will play a more general role in controlling cell fate decisions in ES cells maintained in serum plus LIF or “2i” conditions.
Dual specificity phosphatases are key regulators of ES cell differentiation
A group of 10 genes was identified which acted downstream of Ras but affected ERK phosphorylation levels and hence ERK activity (Figure 4D). To further probe the point of action of these genes, we tested MEK activation levels following their depletion but saw little difference (data not shown). Next, we therefore focussed on MAP kinase phosphatases (also known as dual specificity phosphatases [DUSPs]), and hypothesised that increases in the levels and/or activity of these enzymes might be responsible for the reduced ERK activation that we observed and consequent effects on embryonic stem cell differentiation.
First we examined the set of genes we identified which accelerated differentiation in our primary siRNA screen for candidate dusp genes as we expected the loss of DUSPs would be predicted to enhance ERK phosphorylation and promote exit from pluripotency. Dusp1, dusp3 and dusp15 were amongst this category of genes (Table S2). We therefore determined the expression of these genes and a range of additional phosphatases in embryonic stem cells before and after “2i” removal. Amongst the genes tested, dusp1, dusp5 and dusp6 levels all increased following “2i” withdrawal while dusp14 levels were fairly constant (Figure 5A; Figure S13A). The increased expression of all these phosphatases was dependent on active ERK pathway signalling as expected from other cellular systems (reviewed in [39]) but in the case of dusp1 combinatorial inhibition of ERK and GSK signalling was required for maximal inhibition (Figure 5B; Figure S13B). However, at the protein level, Dusp1 levels gradually declined following “2i” withdrawal while Dusp6 levels increased in line with the increases in their mRNA levels (Figure S13C). Due to their dynamic expression, we focussed on Dusp1, Dusp5 and Dusp6 as these have the potential for controlling ERK pathway activity during embryonic stem cell differentiation. We therefore asked whether depletion of any of the genes identified in our screen would affect Dusp1, Dusp5 and Dusp6 expression at the mRNA or protein levels. Almost all the siRNAs tested (9/10) caused an increase in basal dusp1 mRNA levels and the same was observed on dusp6 levels for 4/10 genes (Figure 5C). In contrast, none of the siRNAs caused increases in dusp5 levels under these conditions (Figure S13D). Similarly, the levels of these dusps followed a similar pattern in response to siRNA treatment after release from “2i” for 40 mins (Figure S13E). Importantly, increases in Dusp1 and Dusp6 at the protein level were also observed which generally correlated with the effects of these siRNAs on mRNA levels (Figure S13F) although there were exceptions typified by Rab24 whose depletion does not affect dusp1 mRNA levels but instead appears to act post-transcriptionally to cause increased levels of Dusp1 protein.
Fig. 5. The regulation of Dusp1 and Dusp6 activity and ES cell differentiation. 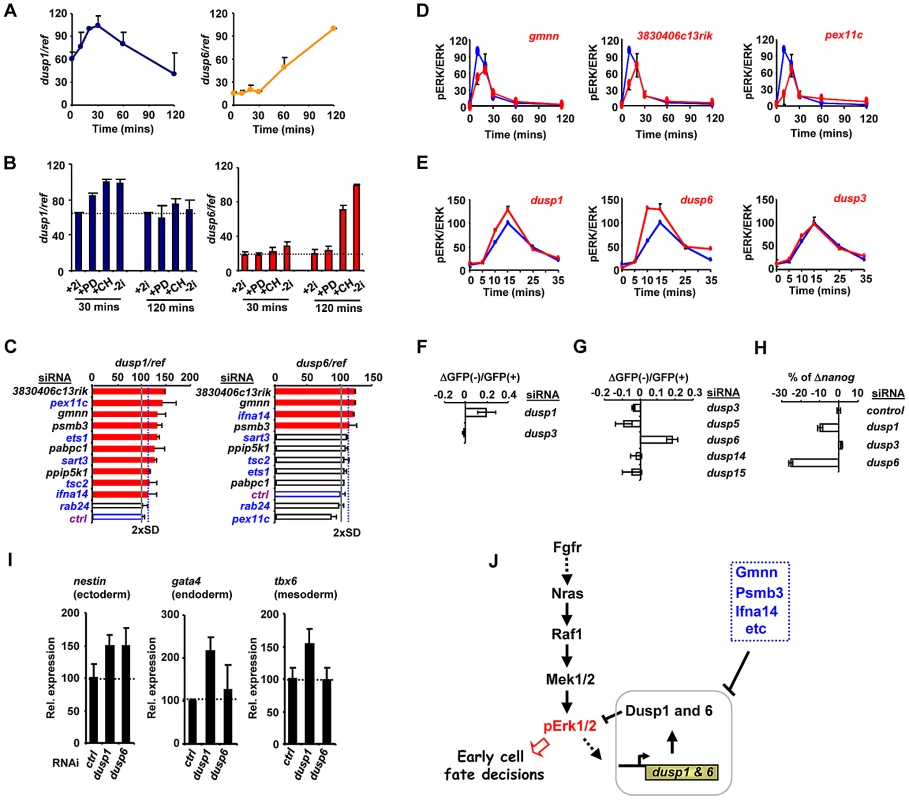
(A–C) RT-qPCR analysis of dusp1 and dusp6 mRNA expression in mouse ES cells. Data are normalised by the average values of three reference genes (ref) and are presented as means ± SEM and are the average of three biological replicates (n = 3). (A) The kinetics of dusp1 and dusp6 expression at the indicated times following 2i withdrawal. (B) The effects of the indicated inhibitors, either alone or in combination, on the expression of dusp1 and dusp6 at the indicated times following inhibitor withdrawal. (C) The effects of depletion of the indicated genes on dusp1 and dusp6 mRNA expression in the presence of 2i. The blue dashed line indicates the threshold level (2× SD above the mean of the negative controls) and levels below this are indicated by red bars. The average activity in the presence of control siRNA (ctrl) is shown by the solid grey line (taken as 100). (D and E) Active ERK levels were determined by the ratio of phospho-ERK (pERK)/total ERK (ERK) levels at the indicated times following 2i release in the presence of the indicated siRNAs (red lines) or control siRNA (blue lines). The data are plotted relative to maximal levels with the control siRNA (taken as 100) and are presented as means ± SEM from the average of two biological replicates (n = 2). (F and G) The change in the ratio of GFP negative to GFP positive Rex1GFPd2 cells 28 hrs after 2i withdrawal in the presence of the siRNAs against the indicated dusps relative to control siRNAs is shown. Data are the average of two biological replicates. (H) RT-qPCR analysis of the changes in nanog mRNA expression in Rex1GFPd2 cells upon 2i withdrawal for 28 hrs and depletion of the indicated dusps. The data are normalised by the average of three reference genes, and presented relative to control siRNAs. Data are presented as means ± SEM and are the average of two biological replicates (n = 2). (I) RT-qPCR analysis of the expression of the indicated lineage marker genes following treatment of cells with siRNAs against dusp1 or dusp6 or a non-targeting control (ctrl) and release from “2i” for 3 (top) or 5 days (bottom). Data are presented as means ± SEM (n = 2). (J) Summary diagram illustrating the key regulatory role of the Dusps in mediating the action of the ERK pathway in early cell fate decisions during loss of pluripotency and onset of differentiation. An increase in the basal levels of MAP kinase phosphatases would likely lead to changes in the ERK activation kinetics, leading to the decreases in phosphorylated ERK levels we observed previously (Figure 4A). Indeed all of the siRNAs tested which promote increases in Dusp levels also cause a delay in peak activation of ERK and a subsequent reduction in the magnitude of this activation (Figure 5D; Figure S13G). Importantly other control siRNAs do not elicit this effect (Figure S14A). This suggests a causative link between the genes we identified in our screen, their effects on dusp gene expression and subsequent changes in ERK activity and downstream differentiation. Two key predictions of this model are that reductions in Dusp levels should first increase the rate and level of ERK pathway activation, and secondly, promote differentiation of embryonic stem cells. Indeed, depletion of Dusp6 and Dusp1 levels caused premature and higher amplitude activation of ERK whereas depletion of Dusp3 and a range of other Dusps had little effect on ERK activity levels (Figure 5E; Figure S14A). Importantly, while depletion of dusp1 and dusp6 caused increased levels of ERK activation, no increases could be detected on the low levels of Jnk and p38 phosphorylation, demonstrating a specific effect on the ERK pathway (Figure S14B). In our primary siRNA screen, we found that dusp1 depletion enhanced the loss of rex1 promoter-driven GFP expression (Figure 5F). We therefore depleted other Dusps to examine whether might function in an analogous manner and found that amongst these, only reductions in dusp6 levels triggered more efficient inactivation of the rex1-GFP reporter gene (Figure 5G). Similarly, dusp1 and dusp6 depletion caused increased loss of mRNA expression of the pluripotency marker nanog whereas dusp3 depletion had little effect (Figure 5H). Thus Dusp1 and Dusp6 appear to play an important role in maintaining pluripotency.
We extended this analysis to examine whether depletion of dusp1 or dusp6 affected lineage commitment by examining the expression of different marker genes 5 days after “2i” withdrawal. The depletion of dusp1 caused increased expression of all three lineage markers, consistent with a general role for this gene in inhibiting loss of pluripotency (Figure 5I). In contrast, depletion of dusp6 only caused increased levels of the ectoderm marker nestin, suggesting a more specific role in controlling differentiation into this lineage (Figure 5I).
Together, these results therefore demonstrate that our RNAi screen has enabled us to identify an important role for a subset of MAP kinase phosphatases in determining the rate and efficiency of ERK pathway activation in embryonic stem cells, and hence influence their ability to escape from pluripotency and begin to differentiate.
Discussion
The derivation of pluripotent iPS cells and the controlled differentiation of embryonic stem cells into defined cell fates are two of the most important areas of research in the area of regenerative medicine. Numerous studies have helped build up a view of the complex signaling and transcriptional networks involved in maintaining the pluripotent state of embryonic stem cells (reviewed in [2]–[3]) but in contrast, much less is known about the pathways leading to the loss of pluripotency. Here we have conducted a genome-wide siRNA screen and identified over 400 genes which play a role in the onset of differentiation which allows ES cells to initiate escape from pluripotency. The vast majority of these genes have not previously been implicated in this process. This dataset therefore provides an important resource for the community and is a rich source of information for further investigating this phenomenon and also for a more basic understanding of the mechanisms governing the regulation and action of the core ERK and GSK3 signaling pathways.
Due to the controlled conditions used in our screen, we were able to link the genes which we identified to either the ERK and/or the GSK3 pathways as potential regulators or mediators of pathway functions. Importantly, it appears likely that many genes we have identified might also be important in the context of different culture conditions such as the commonly used serum and LIF-containing media (see Figure S12). However, further analysis on a case by case basis is required to substantiate a role for individual genes under these conditions. It is important to emphasise that ES cells grown in LIF and “2i” conditions exhibit very different epigenetic landscapes, so only a partial overlap in regulatory factors is expected when comparing these conditions [40]. Indeed, this is not unexpected considering that RNAi screens, including our own, commonly identify chromatin and transcriptional regulators as major important functionally enriched categories (see Figure 2E). Here we focused on genetic interactions with the ERK pathway, and we were able to place a large number of genes as acting upstream or downstream from ERK (Figure 4). Further subpartitioning of the dataset enabled us to identify genes which functioned upstream of Ras or between Ras and ERK (Figure 4D; Table S6). A surprising finding was that all of the genes which acted downstream of Ras, controlled ERK activation levels through controlling the levels of the MAP kinase phosphatases Dusp1 and/or Dusp6. The major point of control was at the transcriptional level. MAP kinase phosphatases are known regulators of MAP kinases activity in different cellular contexts, and Dusp6 in particular operates as part of a feedback loop in response to ERK activation (reviewed in [39]). While Dusp6 is able to specifically dephosphorylate and inactivate ERK in vitro, Dusp1 can also target the stress activated MAP kinases, JNK and p38 (reviewed in [39]). However, we saw no evidence for elevated levels of phosphorylated Jnk and p38 in mouse embryonic stem cells upon depletion of Dusp1, indicating its effects are likely via ERK. Fluctuations in both Dusp1 and Dusp6 levels occur upon ERK pathway activation in ES cells, suggesting that they play an important feedback regulatory role in this system. It appears likely that the combined amounts of these phosphatases helps set the threshold for ERK activation and hence ERK-mediated loss of pluripotency (Figure 5J). Indeed, tampering with this threshold control switch, either by depleting genes that control Dusp levels, or by directly depleting dusp1 or dusp6, alters this threshold and changes the activation kinetics of the ERK pathway. This in turn accelerates the loss of pluripotency and increases the expression of lineage-specific markers, indicating that Dusps help control the equilibrium between pluripotency and differentiation by maintaining the correct levels of ERK activity. Our demonstration of a key role for Dusps in early ES cell differentiation, adds to the literature demonstrating the role of these enzymes in controlling developmental processes (reviewed in [41]) and illustrates the importance of establishing signaling thresholds by balancing activating and inactivating mechanisms which converge on ERK pathway signaling. Indeed, a recent study demonstrated a role for a different phosphatase, Dusp9, in maintaining pluripotency in mouse embryonic stem cells maintained in the presence of LIF and BMP4 [42]. In this study, BMP4 was implicated in upregulating dusp9 expression through Smad pathway activation and hence leading to a dampening down of ERK activity. Importantly, they demonstrated that Dusp9 was not relevant to ERK control in stem cells maintained in 2i conditions, and rather as we have demonstrated, Dusp1 and Dusp6 are more important under these conditions. Reciprocally, we have shown that depletion of either dusp1 or dusp6 does not affect escape from pluripotency in ES cells released from maintenance in serum plus LIF conditions (Figure S12). Together these studies emphasise the critical importance of Dusps in controlling ERK signaling levels in stem cells to regulate the decisions about escape from pluripotency. Importantly, two of the top hits we identified in our screen, gmnn and 3830406c13rik, which act to control Dusp levels and hence ERK activation kinetics, are not only involved in the loss of pluripotency but also in the appearance of differentiation markers for all three lineages (Figure 3). At this stage, it is unclear how these proteins impact on ERK pathway regulation at the molecular level but it points to a pivotal role of these proteins in controlling this key cellular fate decision.
In addition to regulating ERK activation, it is clear that many of the genes identified in our screen contribute to other molecular and biological processes. For example, there are a large numbers of genes encoding transcription and chromatin regulators identified (Figure 2E; Figure S6). This is not unexpected as cells must make wholesale changes in their gene expression programmes as they lose pluripotency and begin to differentiate (reviewed in [43]). There is also enrichment in our screen of functional categories of genes associated with core cellular metabolism and cell cycle control, which presumably reflects the changing anabolic, catabolic and proliferative requirements of the cells as they receive altered signaling input which might contribute to their change in identity (Figure 2E). In addition to enrichments of specific functional categories of genes, many of the genes show strong interconnectivities, implying that we have also uncovered functionally interdependent networks of genes which are important in specifying stem cell fate. This is particularly apparent amongst cell cycle regulators, transcription factors and chromatin modifiers where functionally distinct subnetworks can be observed but also clear interactions between the different subnetworks are apparent. Future studies are required to probe the functional relevance of the networks we have uncovered.
One of the future challenges will be to connect the genes identified in our screen with the ERK and GSK3 signalling pathways. We have begun to do this by focusing on a subset of genes associated with the ERK pathway. However, even though we have implicated many genes in controlling ERK activity, the only information we have for 35 of these genes, is that their point of action is downstream from ERK activation. ERK signaling might be needed to activate their expression (either directly or indirectly) or alternatively the genes might encode proteins which are directly phosphorylated by ERK. For example, it is known that transcriptional regulators such as Ets1, Jun and FoxO1 can all be phosphorylated by ERK in other situations [44]–[46]. More complicated mechanisms can also be envisaged where, for example, ERK and/or GSK3 signalling might converge on the activation of a key target gene, in parallel to one of the other regulators identified in this screen. Additional methodologies will need to be applied to help provide these links.
Another key issue to address is whether the ERK and GSK3 pathways work together or in parallel manner, to target different substrates and ultimately control different gene expression programmes and biological functions in ES cells. The two pathway inhibitors have both distinct and overlapping affects on ES cells (reviewed in [11]). Consistent with this, our study suggests that there may well be specific biological functions associated with GSK3 and ERK pathway signaling as different GO terms are enriched in hits from our screen. However, for the most part, the GO terms are often shared by genes associated with both pathways (see Figure 2 and Figure S6), suggesting that there might be a high degree of cooperativity. Indeed, it is well established that ERK-dependent phosphorylation often acts as a priming event for GSK3-mediated phosphorylation of substrates as exemplified by Smad1 [47]. Thus it appears likely that the pathways might act more generally in a combinatorial manner, either at the level of phosphorylation of common substrates or through convergence in activating gene expression through targeting distinct regulatory factors.
In summary, this study has identified an important role for the precise modulation of ERK MAP kinase signaling levels in the ability of a cell to exit the pluripotent ground state. Furthermore, we have identified a large number of genes that potentially impact on the function of the ERK pathway and GSK3 function in embryonic stem cells. It is becoming increasingly obvious that modulating these pathways has a potential impact on the reprogramming of somatic cells to the iPS cell state (reviewed in [3]) and reciprocally in promoting the differentiation of ES and iPS cells down defined lineages. Thus, the resource we have generated has paved the way for designing alternative strategies to either promote pluripotency or the subsequent generation of new cell identities for therapeutic purposes.
Materials and Methods
Tissue culture, RNA interference, and RT–PCR
ES cells were generally maintained in NDiff N2B27 media (Stem Cells, Inc.; scs-sf-nb-02) in the presence of the GSK3 inhibitor CHIR99021 (Stemgent, 04-0004; 3 µM) and MEK inhibitor PD0325901 (Stemgent, 04-0006; 1 µM) (“+2i” media) and were routinely passaged using Accutase (Sigma, A6964) every other day. For differentiation, the media containing inhibitor was removed and replaced with NDiff N2B27 media. Where indicated, ES cells were maintained in serum/LIF conditions in media containing knockout DMEM (Invitrogen 10829-018), 15% heat inactivated FBS (Invitrogen 10082-147), 2 mM of Glutamax-1 supplement (Invitrogen 35050-038), 1% non-essential amino acids (Invitrogen 11140-035), 50 µM 2-mercaptoethanol (Invitrogen 31350-010) and 5×105 U of LIF (Millipore 103 U/ml). The ES cells cultured under “+2i” conditions were adapted in serum/LIF culture conditions for at least 8 passages before the experiments were performed. Cells were stained for alkaline phosphatase expression using an alkaline phosphatase detection kit as described by the manufacturer's (Millipore).
For RNAi, 4×104/cm2 cells (ie 1.28×104 cells/well of 96 well plate) were plated out into a mixture of 0.3 µl of RNAi Max (Invitrogen) and 100 nM siRNA in 100 µl of “+2i” media for 24 hrs. All validation experiments used ON-TARGETplus siRNA SMART pools from Dharmacon.
Real time RT-qPCR was carried out as described previously [48]. For assays in 96 well plate format, the same basic protocol was followed except the RNA was obtained using a Fastlane cell RT-PCR kit (QIAGEN). Data were normalized for the average expression of the control genes gapdh, hmbs and tbp. The primer-pairs used for RT-PCR experiments are listed in Table S7.
Western blot analysis
Western blotting was carried out with the primary antibodies; Erk2 (137F5; Cell Signalling, 4695), phospho-ERK (E10; Cell Signalling, 9106), Dusp1 (MKP-1; Upstate, 07535), Dusp6 (MKP-3; Epitomics, 2138-1) and Pou5f1 (Oct-3/4; Santa Cruz, sc-8628). All experiments were carried out in 96-well plates. The lysates were directly harvested in the 2×SDS sample buffer followed by sonication (Bioruptor, Diagenode). The proteins were detected using infrared dye-conjugated secondary antibodies (LI-COR Bioscience, IRDye 800CW [1 in 10,000] and IRDye 680LT [1 in 20,000]), and the signal was collected with a LI-COR Odyssey Infrared Imager and quantified using Odyssey software (LI-COR Bioscience, Odyssey Infrared Imaging system application software version 3.0.25).
Ras activation assay
The Ras activities were examined using Ras activation ELISA assay kit (Millipore) as described in the manufacturers' instructions. The total lysates used in the ELISA assay was normalised with the quantity of the proteins assayed by the BCA protein assay kit (ThermoScientific).
Flow cytometry analysis
Flow cytometric analysis was carried out using a LSRII flow cytometer and samples were loaded using HTS loader (BD Biosciences). For sampling, media was removed from each well. Single cell suspensions were generated by treating cells with accutase at 37°C for 7 mins followed by resuspendion in 0.03% BSA/PBS. Dead cells were stained by Sytox Red dead cell stain (Invitrogen, 5 nM). The cells were analysed immediately after sampling. Each sample was analysed with 10,000 event counts with the flow rate at 1 µl/s. The resulting GFP profile (green channel) was created by gating with the right ranges of cell sizes based on forward and reverse scatter plot (ssc vs fsc; blue channel) and dead cells were gated away based on the Sytox Red stain profile (red [APC] channel).
siRNA library screening
All liquid handling processes were performed using Biomek robotic system (Beckman Coulter). For the primary screen, Rex1GFPd2 ES were grown in 96 well plates in the presence of “2i” and reverse transfection was performed using siGENOME siRNA pools (Dharmacon; mouse protein kinase [G-013500], GPCR [G-013600], druggable [G-014600] and genome [G-015000] libraries). 24 hrs later, the “2i” media was removed and replaced with fresh NDiff N2 B27 media. After 28 hrs, the levels of GFP in the cells were determined by flow cytometry as described above. Each plate contained 8 control non-targeting siRNAs, and the positive control siRNAs against gsk3β and fgf4. To take into account slight variations in the timing of pluripotency loss, the ratio of high GFP to low GFP expressing cells was established on each plate based on the non-targeting controls, allowing a threshold to be set as 1 (ie 50% high GFP and 50% low GFP). This threshold was used to determine the ratio of high to low GFP expressing cells in the other wells. The mean plus/minus standard deviation (SD) was calculated for each plate, and individual wells were scored positive if they exceeded 2×SD above or below this mean. The screen was performed in duplicate, with duplicate plates being analysed on different days. A final list of positive hits was determined by taking siRNAs which scored an average of 2×SD across both plates (or on a single plate where the duplicate well was defective in the case of 30 siRNAs), generally with both plates scoring >1.5×SD above the mean. However, an additional small number of siRNAs were scored as positive where the average score was >2.5×SD above the mean where only one plate had to score >1.5×SD above the mean, and also for seven siRNAs where the average score was >1.9×SD above the mean and both plates scored >1.9×SD above the mean.
For the validation screens, either Rex1GFPd2 or Oct4GFP ES cells were used and screens were performed as above except that ON-TARGETplus siRNA duplexes were used and GFP levels in Oct4GFP ES cells were determined 72 hrs after release from “2i”. Individual wells in each screen were scored as positive if the average GFP(+)/GFP(−) ratio exceeded 1.25×SD above the non-targeting controls across both duplicate plates. Additional hits were considered as positive if they scored >0.8×SD above the mean in one validation screen and also scored >1.0×SD above the mean in the other.
For the “1i” screens, Rex1GFPd2 were used as for the validation screens but only one inhibitor (ie either CHIR99021 or PD0325901) was withdrawn. Wells were scored as positive if the average GFP(+)/GFP(−) ratio exceeded 1.5×SD above the mean of the non-targeting controls.
Bioinformatics analysis
For constructing networks, lists of gene names were uploaded into STRING [49] with the confidence score set high (0.40). The resulting networks were saved as *.txt files and then uploaded into Cytoscape (v. 2.7.0) choosing coexpression, textmining, knowledge and experimental data as proximity criteria. yFiles→organic network layouts were applied and the positioning and graphic representation of nodes were adjusted manually for increased clarity.
GO term analysis was carried out using DAVID Bioinformatics Resources 6.7 (NIH) [50]. The enriched terms from the functional annotation chart were extracted and manually clustered. Heat maps of GO terms were generated by MultiexperimentViewer (MeV 4_7_4). GO term summary and visualization was carried out by REVIGO [51].
Supporting Information
Zdroje
1. YamanakaS, BlauHM (2010) Nuclear reprogramming to a pluripotent state by three approaches. Nature 465 : 704–712.
2. YoungRA (2011) Control of the embryonic stem cell state. Cell 144 : 940–954.
3. SangesD, CosmaMP (2010) Reprogramming cell fate to pluripotency: the decision-making signalling pathways. Int J Dev Biol 54 : 1575–1587.
4. DingL, Paszkowski-RogaczM, NitzscheA, SlabickiMM, HeningerAK, et al. (2009) A genome-scale RNAi screen for Oct4 modulators defines a role of the Paf1 complex for embryonic stem cell identity. Cell Stem Cell 4 : 403–415.
5. HuG, KimJ, XuQ, LengY, OrkinSH, et al. (2009) A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev 23 : 837–848.
6. ChiaNY, ChanYS, FengB, LuX, OrlovYL, et al. (2010) A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 468 : 316–320.
7. BlairK, WrayJ, SmithA (2011) The liberation of embryonic stem cells. PLoS Genet 7: e1002019 doi:10.1371/journal.pgen.1002019.
8. SmithAG, HeathJK, DonaldsonDD, WongGG, MoreauJ, et al. (1988) Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336 : 688–690.
9. WilliamsRL, HiltonDJ, PeaseS, WillsonTA, StewartCL, et al. (1988) Myeloid leukemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336 : 684–687.
10. YingQL, WrayJ, NicholsJ, Batlle-MoreraL, DobleB, et al. (2008) The ground state of embryonic stem cell self-renewal. Nature 453 : 519–523.
11. WrayJ, KalkanT, SmithAG (2010) The ground state of pluripotency. Biochem Soc Trans 38 : 1027–1032.
12. SmithA (2009) Design principles of pluripotency. EMBO Mol Med 1 : 251–254.
13. NicholsJ, SilvaJ, RoodeM, SmithA (2009) Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136 : 3215–3222.
14. SilvaJ, BarrandonO, NicholsJ, KawaguchiJ, TheunissenTW, et al. (2008) Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol 6: e253 doi:10.1371/journal.pbio.0060253.
15. SridharanR, TchieuJ, MasonMJ, YachechkoR, KuoyE, et al. (2009) Role of the murine reprogramming factors in the induction of pluripotency. Cell 136 : 364–377.
16. HannaJ, ChengAW, SahaK, KimJ, LengnerCJ, et al. (2010) Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA 107 : 9222–9227.
17. WangW, YangJ, LiuH, LuD, ChenX, et al. (2011) Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc Natl Acad Sci USA 108 : 18283–18288.
18. KunathT, Saba-El-LeilMK, AlmousailleakhM, WrayJ, MelocheS, et al. (2007) FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134 : 2895–2902.
19. StavridisMP, LunnJS, CollinsBJ, StoreyKG (2007) A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development 134 : 2889–2894.
20. DoreyK, AmayaE (2010) FGF signalling: diverse roles during early vertebrate embryogenesis. Development 137 : 3731–3742.
21. DreesenO, BrivanlouAH (2007) Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev 3 : 7–17.
22. HurEM, ZhouFQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11 : 539–551.
23. WrayJ, KalkanT, Gomez-LopezS, EckardtD, CookA, et al. (2011) Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol 13 : 838–845.
24. YiF, PereiraL, HoffmanJA, ShyBR, YuenCM, et al. (2011) Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol 13 : 762–770.
25. SokolSY (2011) Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 138 : 4341–4350.
26. WhitmarshAJ (2007) Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta 1773 : 1285–1298.
27. YangSH, SharrocksAD, WhitmarshAJ (2003) Transcriptional regulation by the MAP kinase signaling cascades. Gene 320 : 3–21.
28. ToyookaY, ShimosatoD, MurakamiK, TakahashiK, NiwaH (2008) Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135 : 909–918.
29. ChenX, XuH, YuanP, FangF, HussM, et al. (2008) Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133 : 1106–1117.
30. NiwaH, BurdonT, ChambersI, SmithA (1998) Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 12 : 2048–2060.
31. AbujarourR, EfeJ, DingS (2010) Genome-wide gain-of-function screen identifies novel regulators of pluripotency. Stem Cells 28 : 1487–1497.
32. FazzioTG, HuffJT, PanningB (2008) An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell 134 : 162–174.
33. KageyMH, NewmanJJ, BilodeauS, ZhanY, OrlandoDA, et al. (2010) Mediator and cohesin connect gene expression and chromatin architecture. Nature 467 : 430–435.
34. ColeMF, JohnstoneSE, NewmanJJ, KageyMH, YoungRA (2008) Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev 22 : 746–755.
35. GuoG, HuangY, HumphreysP, WangX, SmithA (2011) A PiggyBac-based recessive screening method to identify pluripotency regulators. PLoS ONE 6: e18189 doi:10.1371/journal.pone.0018189.
36. PengJC, ValouevA, SwigutT, ZhangJ, ZhaoY, et al. (2009) Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139 : 1290–1302.
37. JiangH, ShuklaA, WangX, ChenWY, BernsteinBE, et al. (2011) Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell 144 : 513–525.
38. WestermanBA, BraatAK, TaubN, PotmanM, VissersJH, et al. (2011) A genome-wide RNAi screen in mouse embryonic stem cells identifies Mp1 as a key mediator of differentiation. J Exp Med 208 : 2675–2689.
39. OwensDM, KeyseSM (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26 : 3203–3213.
40. MarksH, KalkanT, MenafraR, DenissovS, JonesK, et al. (2012) The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149 : 590–604.
41. BermudezO, PagèsG, GimondC (2010) The dual-specificity MAP kinase phosphatases: critical roles in development and cancer. Am J Physiol Cell Physiol 299: C189–C202.
42. LiZ, FeiT, ZhangJ, ZhuG, WangL, et al. (2012) BMP4 Signaling Acts via Dual-Specificity Phosphatase 9 to Control ERK Activity in Mouse Embryonic Stem Cells. Cell Stem Cell 10 : 171–182.
43. JaenischR, YoungR (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132 : 567–582.
44. SeidelJJ, GravesBJ (2002) An ERK2 docking site in the Pointed domain distinguishes a subset of ETS transcription factors. Genes Dev 16 : 127–137.
45. LeppäS, SaffrichR, AnsorgeW, BohmannD (1998) Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J 17 : 4404–4413.
46. AsadaS, DaitokuH, MatsuzakiH, SaitoT, SudoT, et al. (2007) Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal 19 : 519–527.
47. SapkotaG, AlarcónC, SpagnoliFM, BrivanlouAH, MassaguéJ (2007) Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell 25 : 441–454.
48. O'DonnellA, YangSH, SharrocksAD (2008) MAP kinase-mediated c-fos regulation relies on a histone acetylation relay switch. Mol Cell 29 : 780–785.
49. SnelB, LehmannG, BorkP, HuynenMA (2000) STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res 28 : 3442–3444.
50. HuangDW, ShermanBT, LempickiRA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37 : 1–13.
51. SupekF, BošnjakM, ŠkuncaN, ŠmucT (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6: e21800 doi:10.1371/journal.pone.0021800.
Štítky
Genetika Reprodukční medicína
Článek Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction DiseaseČlánek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



