-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe SUN Protein Mps3 Is Required for Spindle Pole Body Insertion into the Nuclear Membrane and Nuclear Envelope Homeostasis
The budding yeast spindle pole body (SPB) is anchored in the nuclear envelope so that it can simultaneously nucleate both nuclear and cytoplasmic microtubules. During SPB duplication, the newly formed SPB is inserted into the nuclear membrane. The mechanism of SPB insertion is poorly understood but likely involves the action of integral membrane proteins to mediate changes in the nuclear envelope itself, such as fusion of the inner and outer nuclear membranes. Analysis of the functional domains of the budding yeast SUN protein and SPB component Mps3 revealed that most regions are not essential for growth or SPB duplication under wild-type conditions. However, a novel dominant allele in the P-loop region, MPS3-G186K, displays defects in multiple steps in SPB duplication, including SPB insertion, indicating a previously unknown role for Mps3 in this step of SPB assembly. Characterization of the MPS3-G186K mutant by electron microscopy revealed severe over-proliferation of the inner nuclear membrane, which could be rescued by altering the characteristics of the nuclear envelope using both chemical and genetic methods. Lipid profiling revealed that cells lacking MPS3 contain abnormal amounts of certain types of polar and neutral lipids, and deletion or mutation of MPS3 can suppress growth defects associated with inhibition of sterol biosynthesis, suggesting that Mps3 directly affects lipid homeostasis. Therefore, we propose that Mps3 facilitates insertion of SPBs in the nuclear membrane by modulating nuclear envelope composition.
Published in the journal: . PLoS Genet 7(11): e32767. doi:10.1371/journal.pgen.1002365
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002365Summary
The budding yeast spindle pole body (SPB) is anchored in the nuclear envelope so that it can simultaneously nucleate both nuclear and cytoplasmic microtubules. During SPB duplication, the newly formed SPB is inserted into the nuclear membrane. The mechanism of SPB insertion is poorly understood but likely involves the action of integral membrane proteins to mediate changes in the nuclear envelope itself, such as fusion of the inner and outer nuclear membranes. Analysis of the functional domains of the budding yeast SUN protein and SPB component Mps3 revealed that most regions are not essential for growth or SPB duplication under wild-type conditions. However, a novel dominant allele in the P-loop region, MPS3-G186K, displays defects in multiple steps in SPB duplication, including SPB insertion, indicating a previously unknown role for Mps3 in this step of SPB assembly. Characterization of the MPS3-G186K mutant by electron microscopy revealed severe over-proliferation of the inner nuclear membrane, which could be rescued by altering the characteristics of the nuclear envelope using both chemical and genetic methods. Lipid profiling revealed that cells lacking MPS3 contain abnormal amounts of certain types of polar and neutral lipids, and deletion or mutation of MPS3 can suppress growth defects associated with inhibition of sterol biosynthesis, suggesting that Mps3 directly affects lipid homeostasis. Therefore, we propose that Mps3 facilitates insertion of SPBs in the nuclear membrane by modulating nuclear envelope composition.
Introduction
The hallmark feature of eukaryotic cells is the nucleus, a double membrane bound organelle that contains the genetic material. The outer nuclear membrane (ONM) of the nucleus is contiguous with the ER membrane while the inner nuclear membrane (INM) is distinct and contains a unique set of proteins that interact with chromatin and other nuclear factors. Embedded in the nuclear membrane are multiple nuclear pore complexes (NPCs) that regulate transport of macromolecules between the cytoplasm and the nucleus [1]. In organisms such as Saccharomyces cerevisiae that undergo a closed mitosis, the centrosome-equivalent organelle known as the spindle pole body (SPB) is present in the nuclear envelope throughout the life cycle [2]. The SPB organizes both cytoplasmic microtubules, which are involved in nuclear positioning, and nuclear microtubules, which are essential for chromosome segregation [3].
Both NPCs and SPBs are composed primarily of soluble proteins that partially assemble into sub-complexes in the nucleus or cytoplasm (reviewed in [1], [3]). Further assembly of both NPCs and SPBs requires insertion into the nuclear membrane at a point where the INM and ONM are joined together. Specific integral membrane proteins interact with soluble components of the NPC and SPB and are thought to anchor the complexes in the nuclear envelope. Ndc1 is essential for insertion of both the NPC and SPB [4]–[6]. At the NPC, three additional pore membrane proteins, Pom33, Pom34 and Pom152, play partially overlapping roles in NPC assembly [6]–[8], while Nbp1, Bbp1 and Mps2 are required in addition to Ndc1 for SPB insertion into the nuclear envelope [9]–[12].
The mechanism of NPC insertion has been extensively studied in both yeast and metazoan systems. Structural studies have shown that five subunits of the NPC (Nup133, Nup120, Nup85, Nup170 and Nup188) contain an ALPS motif (for ArfGAP1 lipid packing sensor), which targets them to highly curved membranes [13]. These proteins are thought to form a coat complex on the nuclear envelope to facilitate NPC insertion [14]–[17]. In addition, membrane-bending proteins of the ER such as the reticulons have been shown to play a role in de novo NPC assembly [17], [18]. Modification of lipids within nuclear membrane leaflets probably also occur at sites of NPC insertion to accommodate membrane curvature and fusion. Several proteins involved in lipid synthesis and membrane fluidity have been genetically linked to NPC assembly [19]–[21], although their role in NPC insertion is not well characterized. In vertebrates cells, the SUN (for Sad1-UNC-84 homology) protein Sun1 also is required for NPC assembly [22], [23]. A recent study suggested that hSun1 together with Pom121 is required for de novo assembly of NPCs possibly by facilitating membrane fusion [24].
The mechanism of SPB insertion into the nuclear membrane is poorly understood in comparison to insertion of NPCs. It is possible that many of the same events, such as membrane bending, curvature and lipid modification, are needed for SPB duplication since fusion of INM and ONM also must occur during SPB insertion. No specific factors that possess these functions have ever been directly implicated in the SPB duplication process with the exception of a recent report suggesting that the amphipathic alpha-helix of Nbp1 aids SPB insertion [12]. Perhaps one of the best clues as to how the SPB might insert into the nuclear membrane comes from a plethora of genetic interactions that have been identified between genes encoding SPB components and NPC subunits, including suppression of complete deletions of the SPB membrane components, MPS2 and MPS3, by POM34 or POM152 deletion [4], [25]–[27]. While it is possible that the NPC is involved in SPB insertion through its role in nuclear translocation of SPB subunits or in mRNA processing [25], [28], the fact that SPB duplication can occur in the absence of certain structural subunits points to a model in which NPCs and SPBs compete for a shared insertion factor, such as Ndc1 [4]. Alternatively, blocking NPC assembly by elimination of POM152 or POM34 could alter some aspect of the nuclear membrane that enables the SPB to duplicate in the absence of otherwise essential components. Consistent with this possibility, deletion of POM152 together with NUP170 results in nuclear membrane abnormalities [29].
In the present study, we further examine the role of the nuclear membrane in SPB duplication and show for the first time that changes in membrane composition are sufficient for SPB duplication in the absence of Mps3. In addition, we demonstrate a role for the SUN protein Mps3 in regulation of membrane architecture and provide evidence that it functions in the insertion step of SPB duplication. This role of Mps3 is distinct from the previously described functions of Mps3 in SPB duplication. As a component of the SPB substructure that templates assembly for the new SPB, known as the half-bridge, Mps3 is required for initiation of SPB duplication and for tethering the half-bridge to the soluble core SPB through its interaction with the membrane protein Mps2 [30]–[32]. This function is similar to that of other SUN domain-containing proteins, which have been shown to play a role in accurate chromosome segregation and nuclear positioning due to their role in duplication and membrane tethering of centrosomes, basal bodies and SPBs in a wide variety of systems [33]–[35]. However, our current data suggest that SUN proteins have a novel function in membrane homeostasis, which is involved in insertion of protein complexes such as the SPB into the nuclear envelope.
Results
Identification of Mps3 domains that are required for SPB duplication
A key feature of all SUN proteins, including Mps3, is the presence of a number of structural motifs, including at least one transmembrane domain, regions of coiled-coils and a C-terminal SUN domain [35]. In addition, Mps3 contains an N-terminal acidic domain, a poly-glutamine region and a putative P-loop [30] (Figure 1A). In order to determine their role in SUN protein localization and function, we created deletion alleles in each domain using site directed mutagenesis. In the case of the poly-glutamine repeat (pQ), we not only deleted this region but also expanded it two-fold since this type of mutation is often linked to disease [36], [37]. In the case of the putative P-loop, we mutated key residues anticipated to be involved in nucleotide binding to a residue with different chemical properties [38], [39]. To test if the various mutants were functional, we assayed complementation of an mps3Δ mutant using a plasmid shuffle strategy. Although Mps3 plays multiple non-essential roles in the chromosome organization within the nucleus [28], [40]–[49], the primary function of Mps3 in mitotically dividing yeast cells is at the SPB [30], [31], so this test will allow us to identify domains of Mps3 that are essential for SPB duplication.
Fig. 1. Essential regions of Mps3 for SPB duplication. 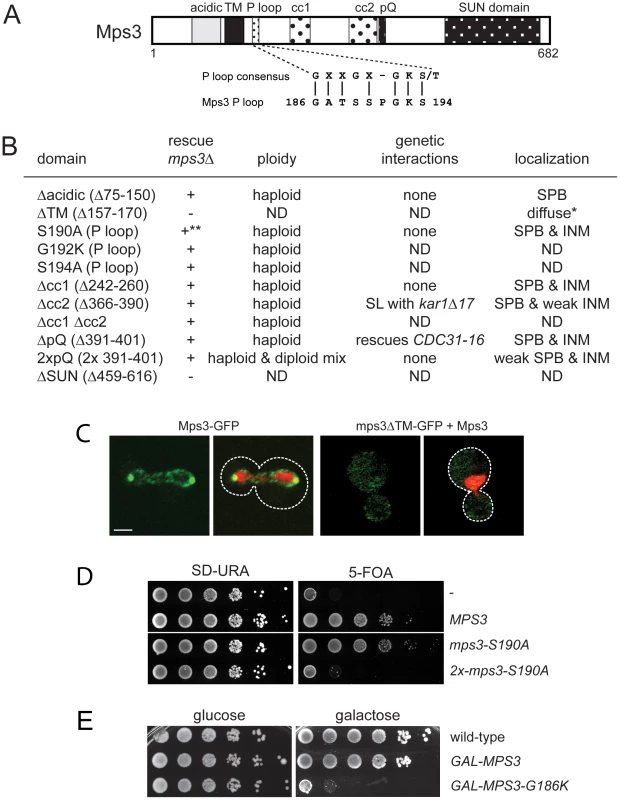
(A) Schematic of Mps3 showing the N-terminal acidic region, transmembrane domain (TM), P-loop motif, coiled-coils (cc), poly-glutamine (pQ) repeat and C-terminal SUN domain. Alignment of P-loop consensus sequence with amino acids 186–194 of Mps3 is shown below. (B) The indicated mutants within each Mps3 domain were tested for their ability to rescue a deletion of MPS3 (SLJ2039). + and − indicate growth and death, respectively, of mutants on 5-fluoroorotic acid (5-FOA) at 30°C. Viable alleles were analyzed further to determine DNA content by flow cytometry and tested for genetic interactions with other SPB mutants, including kar1Δ17 (SLJ844), cdc31-2 (SLJ894), mps2-1 (SLJ718), sfi1-3 (SLJ1558) and CDC31-16 (SLJ907). Alleles were also fused to GFP to determine the subcellular distribution of the mutant protein using confocal imaging [41]. *Non-functional mutants were localized in the presence of wild-type Mps3. ND, not determined. **The mps3-S190A is dependent of copy number: single copy integrants are viable while multiple copy integrants are lethal, as shown in the serial dilution assay in (D). (C) A representative single plane confocal image showing the localization of Mps3-GFP (SLJ2571) and mps3ΔTM-GFP (SLJ4308) (green) together with H2B-mCherry (red) in cells grown at 23°C. Bar, 2 µm. The covering plasmid was removed from SLJ2571 by plating cells to 2-amino-5-fluorobenzoic acid (5-FBA) plates immediately prior to use; because mps3ΔTM-GFP is non-functional, the wild-type untagged copy of MPS3 could not be lost. (D) Serial dilution assay of SLJ2039 containing no insert (-), wild-type MPS3, or mps3-S190A integrated in 1 copy or 2 copies on SD-URA and 5-FOA, which removes the covering plasmid containing wild-type MPS3. Plates were grown for 2 d at 30°C. (E) Wild-type (SLJ001), GAL-MPS3 (SLJ995) and GAL-MPS3-G186K (SLJ1797) cells were tested for their ability to grow on YPD (glucose) and YPGR (galactose) plates at 30°C in a serial dilution assay. mps3Δcc1, mps3Δcc2, mps3Δcc1Δcc2 and mps3ΔpQ mutants, which lack the first, second and both coiled-coil domains or the poly-glutamine region, respectively, did not exhibit any obvious growth defect at various temperatures (Figure 1B; data not shown). To further examine their role in Mps3 function at the SPB, we tested for genetic interactions with mutants in other SPB components: mps2-1, cdc31-2, CDC31-16, kar1Δ17, spc42-11, spc29-3 and sfi1-3. Each of these mutants is either inviable (synthetic lethal) or shows an enhanced growth defect (synthetic sick) with mps3-1, which has serine 472 in the SUN domain mutated to asparagine [30]. Like MPS3, KAR1 and CDC31 encode components of the SPB half-bridge that are required for the initial step of SPB duplication: Kar1 is an integral membrane protein and Cdc31 is a small calcium binding protein that also binds to Sfi1 on the cytoplasmic side of the SPB half-bridge. Spc42 and Spc29 are components of the core SPB and Mps2 is a linker protein that tethers the half-bridge to the core SPB through interactions with Mps3 [3], [32]. Surprisingly, only two genetic interactions were discovered with this new panel of mps3 alleles: synthetic lethality between mps3Δcc2 and kar1Δ17, and suppression of the temperature sensitivity of the dominant CDC31-16 mutant by mps3ΔpQ (Figure 1B). However, unlike mps3-1 mutants [30], Cdc31 and Kar1 localization to the SPB was unaffected in mps3Δcc2 or mps3ΔpQ mutants (data not shown). Most likely, this is because mps3Δcc1-GFP, mps3Δcc2-GFP and mps3ΔpQ-GFP localize to the SPB and INM in a pattern that is highly similar to that of Mps3-GFP (Figure 1B). Taken together, our data suggests that mutation of the coiled-coil domains or deletion of the poly-glutamine region have at most minor effects on the localization and function of Mps3 during SPB duplication.
Duplication of the poly-glutamine region did not affect cell growth (Figure 1B). However, analysis of DNA content by flow cytometry revealed that mps3-2xpQ mutants exhibited an increase in ploidy (Figure 1B), which is a common phenotype in SPB mutants, and it has been previously observed in a number of mps3 alleles [30], [32], [40]. The increase in ploidy was fully recessive as cells containing mps3-2xpQ and a wild-type copy of MPS3 were haploid (data not shown), indicating that although the mps3-2xpQ protein may be only partially functional, it does not form a complex that titrates out other SPB duplication factors. Our observation that mps3-2xpQ-GFP levels at the SPB are reduced compared to Mps3-GFP (Figure 1B) suggests that this mutant is unable to localize to the SPB, perhaps due to a change in binding with its receptor at the SPB.
Similar to mutants that eliminate or affect the SUN domain [30]–[32], deletion of the transmembrane domain resulted in a non-functional version of Mps3 (Figure 1B). This is most likely due to mislocalization of the mutant protein since mps3ΔTM-GFP was only visible in a diffuse pattern throughout the cytoplasm and the nucleus even in the presence of a wild-type untagged copy of Mps3 rather than at both SPBs and the peripheral nuclear envelope like Mps3-GFP (Figure 1B) [41]. Replacement of the Mps3 transmembrane domain with that of several other membrane proteins rescued the lethality of mps3Δ and restored localization to the SPB and the peripheral nuclear envelope (Figure S1A and S1B). Interestingly, some of these chimeric proteins displayed significantly different localization patterns from wild-type Mps3-GFP (Figure S1A). The fact that these proteins were sufficient to target enough Mps3 to the SPB to allow for cell proliferation and maintenance of genomic stability indicates that although membrane localization is critical for Mps3 function during SPB duplication, a specific transmembrane domain sequence is not required to target the SUN protein to the SPB.
Mutation of P-loop residues results in a novel dominant MPS3 allele
When point mutants were constructed in potential residues involved in nucleotide binding within the P-loop region, we found most alleles were able to complement mps3Δ and serve as the sole copy of MPS3 in the cell (Figure 1B; data not shown), suggesting that this domain does not function in ATP-binding in vivo. However, some mutants in the P-loop region such as mps3-S190A displayed copy number sensitivity such that cells containing a single integrated copy of the mutant gene were viable, but cells containing two or more copies of the P-loop mutant gene were dead (Figure 1D). This indicates that mps3-S190A is a weak dosage-sensitive antimorphic allele. We were unable to obtain transformants of one allele (MPS3-G186K) under a wide variety of conditions (data not shown), suggesting that it is a dominant mutant that arrests cell growth.
Using the galactose-regulatable GAL1-10 promoter, we set up a system so that we could examine the effects of MPS3-G186K on cell growth and SPB duplication. Cells containing a single integrated copy of GAL-MPS3 or GAL-MPS3-G186K in addition to the endogenous wild-type copy of MPS3 were analyzed for their effect on growth in a serial dilution assay at 30°C. Under these conditions, overexpression of wild-type MPS3 had a slight effect on cell growth while overexpression of MPS3-G186K significantly inhibited cell proliferation (Figure 1E). This confirms that MPS3-G186K is a novel dominant lethal mutant.
MPS3-G186K mutants are defective in SPB duplication
These same strains were examined following a 5 h induction with 2% galactose by flow cytometry to analyze DNA content and by indirect immunofluorescence microscopy with anti-alpha-tubulin and anti-gamma-tubulin antibodies to visualize microtubules and SPBs, respectively, to determine if the MPS3-G186K mutant affected SPB duplication and spindle assembly. We found that cells overproducing wild-type MPS3 did not arrest and underwent SPB duplication to form bipolar mitotic spindles (Figure 2A, 2B). However, MPS3-G186K overproduction resulted in an accumulation of large-budded cells with a 2N DNA content, which is suggestive of a failure in SPB duplication and/or spindle formation (Figure 2A). Examination of microtubule structures showed that 66% of MPS3-G186K mutants, but only 14% of GAL-MPS3 cells, contain monopolar spindles: a single DNA mass associated with a single SPB and microtubule array (Figure 2B; n>200). Unlike the monopolar spindle phenotype previously described for mps3 mutants where a single focus of gamma-tubulin is associated with the nuclear DNA [30], [32], 40% of MPS3-G186K cells contain two foci of gamma-tubulin, one of which does not nucleate microtubules (Figure 2B). This phenotype is indicative of a defect in SPB insertion into the nuclear envelope: the SPB that does not nucleate microtubules has not properly inserted into the nuclear envelope and assembled inner plaque components, which are necessary for the formation of a nuclear microtubule array [3]. Although the uninserted SPB may nucleate cytoplasmic microtubules, these are generally difficult to observe due to the small number that is formed at each SPB. This phenotype is highly reminiscent of that observed in mutants that are defective in SPB insertion into the nuclear envelope such as mps2-1, ndc1-1, nbp1-dg and bbp1-1 [9]–[11], [50] and suggests that like these SPB components, Mps3 has a function in the late step of SPB duplication in addition to its role in initiation of SPB duplication.
Fig. 2. MPS3-G186K expression causes a mitotic arrest due to SPB duplication defects. 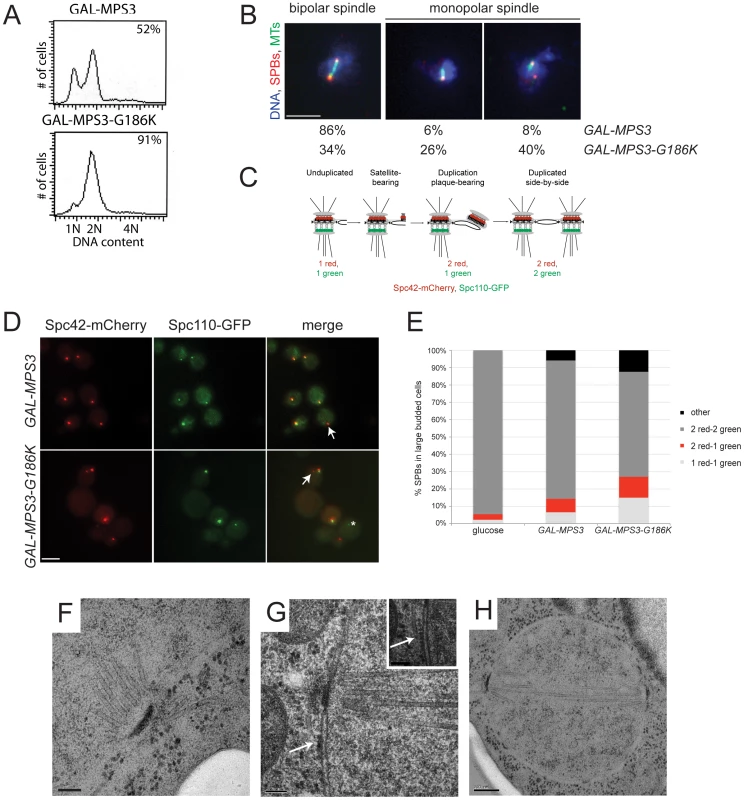
(A,B) GAL-MPS3 (SLJ995) and GAL-MPS3-G186K (SLJ1797) cells were grown overnight at 30°C in YEP plus 2% raffinose then 2% galactose was added for 5 h to induce expression. (A) DNA content was analyzed by flow cytometry and budding index was determined using phase contrast microscopy. The percentage of large-budded cells is indicated (n = 300). (B) In addition, spindle morphology was examined using indirect immunofluorescence microscopy using anti-α-tubulin (green), anti-γ-tubulin (red) and DAPI (blue). The percentage of large budded cells (n>200) with bipolar and both types of monopolar spindles is indicated for each strain. Bar, 5 µm. (C) Schematic of SPB duplication pathway showing the timing of Spc110-GFP (green) and Spc42-mCherry (red) assembly. (D) SPBs were visualized using Spc110-GFP (green) and Spc42-mCherry (red) in GAL-MPS3 (SLJ4859) and GAL-MPS3-G186K (SLJ4864) cells grown for 5 h in SC-HIS+2% galactose+2% raffinose at 30°C. Arrows point to unduplicated SPBs that contain only Spc42-mCherry, and an asterisk marks a SPB fragment seen in MPS3-G186K, which contains only Spc110-GFP. This may represent a delaminated SPB similar to that observed in some spc110 mutants [101]. Bar, 5 µm. (E) The number of SPBs containing the indicated number of Spc42-mCherry and Spc110-GFP foci in large budded cells was quantitated in two independent experiments (n>250). We also counted SPB foci in SLJ4859 cells grown in SC-HIS+2% raffinose+2% glucose for 5 h at 30°C. (F–H) GAL-MPS3-G186K (SLJ1797) mutants were also processed for thin-section EM to examine SPB morphology following induction for 5 h in YPGR at 30°C. Serial sections through nuclei of 15 cells were collected: (F) 7 nuclei contained a single unduplicated SPB, (G) 4 nuclei contained an SPB as well as electron dense material (arrow) resembling a SPB precursor on the cytoplasmic face of the nuclear envelope, and (H) 4 nuclei contained duplicated and separated SPBs, which nucleated a short bipolar spindle. The inset in (G) shows an adjacent section with a cytoplasmic microtubule emanating from the SPB precursor (position marked with an arrow). (F,G) Bar, 100 nm. (H) Bar, 200 nm. MPS3-G186K mutants are defective in SPB insertion
In order for inner plaque components of the SPB such as Spc110 to assemble onto the newly duplicated SPB, the new SPB must be inserted into the nuclear envelope (Figure 2C). Cells defective in SPB insertion due to a mutation in MPS2 or BBP1 contain two foci of the fluorescently-labeled central plaque component Spc42, which is present at the old SPB and the duplication plaque/new SPB, but a single focus of fluorescently-labeled Spc110 [11], [25]. In GAL-MPS3 cells, we found that in 80% of cells containing two Spc42-mCherry foci, those foci were coincident with two Spc110-GFP foci, indicating that these SPBs had duplicated and inserted into the nuclear envelope (Figure 2D–2E). In contrast, only 61% of GAL-MPS3-G186K cells contained two SPBs that were labeled with both Spc42-mCherry and Spc110-GFP. 15% of the remaining cells contained a single SPB with both Spc42-mCherry and Spc110-GFP, indicative of an unduplicated SPB, 12% of cells contained two SPBs labeled with Spc42-mCherry only one of which co-labeled Spc110-GFP, indicative of an arrest at an intermediate step in SPB duplication, and 12% contained multiple SPB foci (Figure 2D–2E). Thus, MPS3-G186K has pleiotropic effects on SPB duplication, including blocking SPB insertion into the nuclear envelope. Based on the fact that virtually all wild-type cells have two duplicated SPBs that co-labeled with Spc42-mCherry and Spc110-GFP, the increased frequency in aberrant pole morphologies that we observed in MPS3-G186K is striking and highly statistically significant (p<0.01). In addition, the levels of uninserted poles in MPS3-G186K is similar to that observed in other mutants such as mps2-1 and ndb1-dg, which fail in the late step of SPB duplication (see [25]).
We used electron microscopy (EM) to further evaluate the SPB duplication defect of cells overexpressing MPS3-G186K. Serial section analysis through the entire nucleus revealed that 11 out of 15 nuclei examined contained a single SPB. Of these 11 monopolar spindles, 4 had evidence of a “dead” pole characteristic of mutants defective in the late step of SPB insertion [9]–[11], [50]; that is, an electron dense structure associated with the nucleus and with cytoplasmic microtubules but not inserted into the nuclear envelope (Figure 2G). The remaining 7 SPBs appeared to be unduplicated, arresting with a terminal morphology similar to previously described mps3 alleles (Figure 2F) [30], [32]. The remaining 4 cells contained short bipolar spindles (Figure 2H). This ultrastructural analysis is consistent with our fluorescence and genetic data and suggests that MPS3-G186K affects multiple steps in SPB duplication, including SPB insertion into the nuclear envelope.
We tested the ability of overexpressed SPB components to suppress toxicity of the mutant on galactose and found that 2μ-MPS3 partially rescued growth, confirming that MPS3-G186K is an antimorphic allele (Figure S2). Most other SPB components failed to rescue the growth arrest. The notable exception was 2μ-SPC42. Spc42 is a component of the SPB central plaque that serves as a scaffold for assembly of the organelle and plays a role in anchorage in the nuclear membrane [51], [52]. The fact that its overexpression partially restores growth to MPS3-G186K mutants is consistent with a defect in membrane insertion and tethering.
MPS3-G186K cells have a membrane proliferation defect
In addition to the SPB duplication defect, several additional phenotypes were uncovered during the course of our EM analysis of MPS3-G186K cells that shed light onto the possible function of Mps3 in SPB insertion. As depicted in Figure 3C–3F, MPS3-G186K mutants appeared to have undergone massive over-proliferation of the nuclear membrane, resulting in 2–8 layers of nuclear envelope, and nuclei appear to have multiple lobes and extensions. The membrane expansion phenotype was highly specific and penetrant, occurring in 96% (48 of 50) of GAL-MPS3-G186K cells examined (Figure 3C–3F) but in none (0 of 34) of the GAL-MPS3 cells (Figure 3A–3B). Therefore, it is most probably due to an effect of the MPS3-G186K mutant and not a general result due to overexpression of MPS3 or an integral membrane protein (see also [31]). The fact that we observe excess membrane in MPS3-G186K mutant suggests that Mps3 directly or indirectly is involved in membrane homeostasis.
Fig. 3. Over-proliferation of the nuclear membrane in GAL-MPS3-G186K. 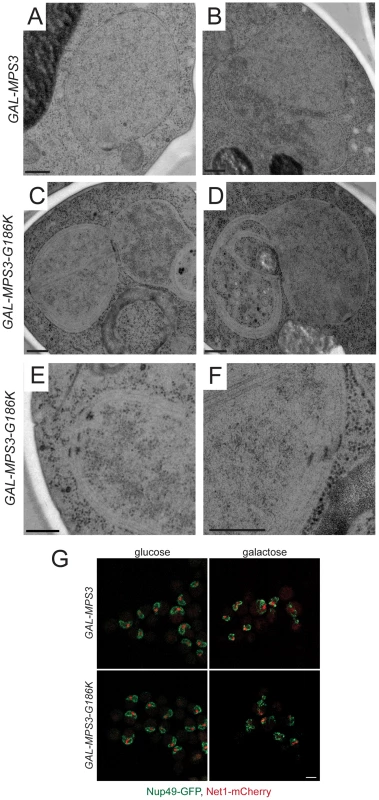
GAL-MPS3 (SLJ995) and GAL-MPS3-G186K (SLJ1797) cells were processed for thin-section EM following induction for 5 h in YPGR at 30°C. (A) Nuclei from GAL-MPS3 cells show a round or elliptical morphology (50%; n = 17) similar to that seen in wild-type cells (see [55]), or (B) an abnormal nuclear morphology (50%; n = 17). In all nuclei from GAL-MPS3 cells, only a single nuclear envelope bi-layer was observed. (C–F) Nuclei from GAL-MPS3-G186K all (n = 50) exhibited an abnormal nuclear morphology, which included multiple lobes (C), as well as highly curved projections (D). Multiple nuclear envelope layers were present, and this often resulted in (E) clustered NPCs in regions containing a single nuclear membrane layer. In other cases (F), stacked NPCs were observed. (A–F) Bar, 200 nm. (G) GAL-MPS3 (SLJ5097) and GAL-MPS3-G186K (SLJ5098) cells containing Nup49-GFP and Net1-mCherry were grown to mid-log phase in YEP+2% raffinose, then 2% glucose was added to half and 2% galactose was added to the other half and cells were allowed to grow for 5 h at 30°C. Mislocalization of Nup49-GFP (green) and Net1-mCherry (red) in MPS3-G186K in galactose are consistent with aberrant nuclear morphology seen by EM, but no NPCs were observed in the cytoplasm in either mutant. Bar, 5 µm. Membrane proliferation in GAL-MPS3-G186K was restricted to the nucleus; no excess membrane was seen on other organelles, including the ER that is contiguous with the ONM in budding yeast (Figure 4). Previous work had suggested that the membrane adjacent to the nucleolus was subject to the formation of membrane flares [53], but we found that all areas of the nuclear membrane underwent expansion, not just the nucleolar membrane. However, we did observe that the nucleolar region was often partitioned away from the main mass of the nucleus either by a membrane (Figure 4B) or by the formation of a lobe (Figure 3C). Interestingly, within an individual nucleus, membrane proliferation was not uniform in that there were regions that contained a single bilayer and other regions containing multiple bilayers (Figure 3C and 3D, Figure 4). Analysis of serial nuclear sections showed that the excess membrane begins as tubules within the nucleus, which then proliferate underneath the existing membrane and then fuse with adjacent tubules (Figure 4A) or fold back upon itself to continue proliferation within the nucleus (Figure 4B). The fact that the excess membrane forms only in tight association with existing nuclear envelope and does not form lamellae within the nucleus suggests that Mps3 is intimately involved in formation of the nuclear envelope layers.
Fig. 4. Formation of tubules and intranuclear membranes in MPS3-G186K cells. 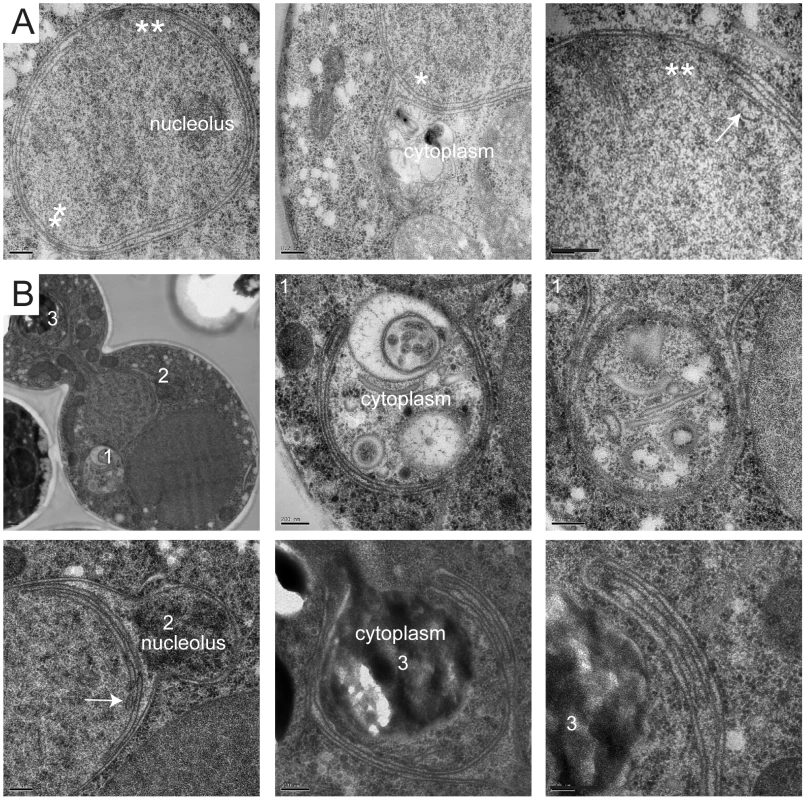
EM images from GAL-MPS3-G186K cells grown for 5 h in YPGR at 30°C showing the formation of intranuclear tubules. (A) In the cell on the left, the positions of tubule formation are indicated by a single or double asterisk, and higher magnification images of these regions are shown on the right. In the center image, the outer layers of the nuclear membrane appear to have extended into the cytoplasm to enclose a region of the cytosol. An arrow points to a tubule forming what appears to be a third nuclear layer in the right image. (B) The cell shown has at least three interesting regions of membrane expansion, which are denoted and shown in higher magnification. In region 1, at least two lipid bilayers have encircled a region of the cytosol. In region 2, expansion of membranes within the nucleus is not uniform. Here, membrane proliferation is occurring inside the nucleus to compartmentalize the nucleolus away from rest of the nucleus. In region 3, membrane is also encircling a cytoplasmic region, but it is clear that the membranes are folding back on themselves to form a tubule inside the membrane bilayers. Bars, 200 nm. While it is possible that the excess membrane in MPS3-G186K inhibits SPB insertion, we found that SPBs as well as NPCs were often associated with a membrane region containing a single bilayer (Figure 2F–2H and Figure 3C, 3E). However, we also detected NPCs in intermediate and inner layers, although these often appeared to be stacked with NPCs in adjacent layers (Figure 3F), most likely to facilitate nuclear-cytoplasmic trafficking of macromolecules, which is reduced but not completely inhibited in MPS3-G186K (Figure S3). Unlike other mutants that affect the yeast nuclear membrane [18]–[21], [54], we did not observe partially assembled NPCs in the nucleoplasm or cytoplasm of MPS3-G816K mutants that would suggest a defect in NPC insertion, perhaps due to the large number of NPCs present in the nuclear envelope [55] and the relatively short period of time in which the dominant mutant is expressed (Figure 3 and Figure 4). Because both SPBs and NPCs are observed in nuclear membrane areas that contain a single bilayer and there is no apparent defect in NPC insertion, we suspect that the SPB assembly defect associated with MPS3-G186K is the result of membrane composition rather than the excess membrane.
MPS3-G186K mutants have defects in nuclear morphology
Membrane proliferation in MPS3-G186K mutants was also accompanied by an abnormal nuclear morphology. In many cases the nuclear membrane completely encircled regions of the cytoplasm, entrapping vesicles and other components (Figure 4A–4B). Membrane extensions and protrusions were observed in all GAL-MPS3-G186K cells (n = 50) and in 50% of GAL-MPS3 cells (17 of 34), which do not over-proliferate the nuclear membrane (Figure 3B–3D). The remaining 50% of GAL-MPS3 cells had a round or oval nuclear morphology similar to wild-type, depending on the cell cycle stage (Figure 3A).
We were able to follow the formation of nuclear morphology defects in real-time in GAL-MPS3-G186K mutants and GAL-MPS3 control cells using HDEL-dsRed to visualize the nuclear and ER membranes and Pus1-GFP to visualize the nucleus. Time-lapse image analysis showed that the nuclei in MPS3 overexpressing cells underwent minor changes in nuclear shape—virtually all nuclei were round or oval shaped throughout the 3.5 h time-course, with a few extensions and protrusions forming in some nuclei only at later time points (Video S1; Figure 5B). In addition, we observed cells undergoing mitosis as the nucleus and nuclear membrane became elongated and then hour-glass shaped. In contrast, membrane extensions and nuclear deformation were easily observed in most MPS3-G186K cells by 1.5–2 h following addition of galactose (Video S2). At later time points, more severe membrane perturbation occurred in the mutant, often resulting in the formation of several masses of nuclear material within one cell (Figure 5B). Although some mutant nuclei elongated, none completed mitosis to form two distinct masses of DNA. Thus, it appears that membrane proliferation is linked to an abnormal nuclear morphology and inhibition of mitotic progression.
Fig. 5. Alteration of membrane properties suppresses MPS3-G186K. 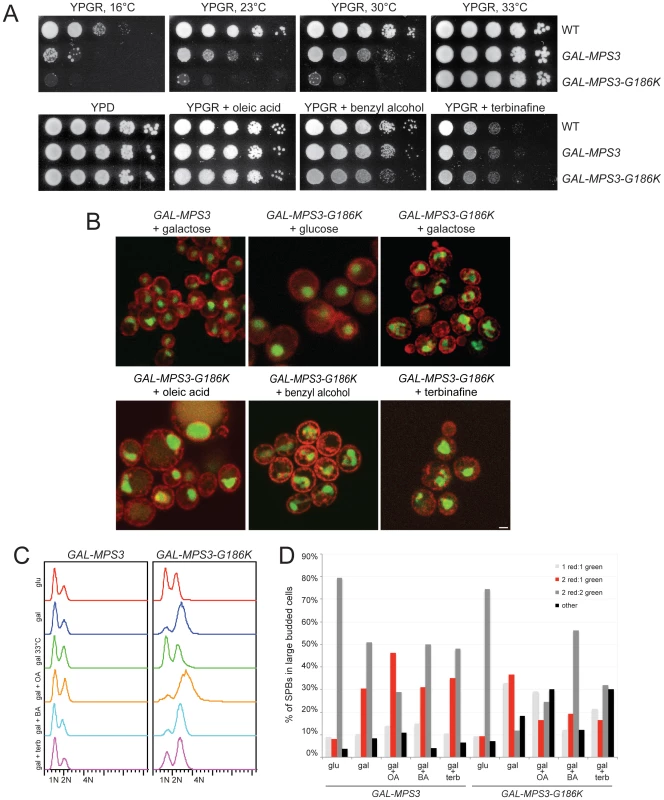
(A) Wild-type (SLJ001), GAL-MPS3 (SLJ995) and GAL-MPS3-G186K (SLJ1797) cells were tested for their ability to grow on YPGR, YPGR+0.2% benzyl alcohol, YPGR+5 mM oleic acid and YPGR+2.5 µg/ml terbinafine plates at 30°C in a serial dilution assay. Plates were grown for 2 d. Cells were also stamped to YPGR at 16°C for 7 d, 23°C for 3 d and 33°C for 2 d to test the effect of temperature on colony formation. (B) GAL-MPS3 (SLJ4963) and GAL-MPS3-G186K (SLJ4965) containing HDEL-dsRed (red) and Pus1-GFP (green) were grown overnight in SC-LEU+2% raffinose then 2% glucose, 2% galactose, 2% galactose+5 mM oleic acid, 2% galactose+0.2% benzyl alcohol, or 2% galactose+1.25 µg/ml terbinafine was added for 6 h at 30°C to visualize the effects on nuclear morphology. Bar, 5 µm. (C–D) GAL-MPS3 (SLJ4859) and GAL-MPS3-G186K (SLJ4864) cells were grown in SC-HIS+2% raffinose, then treated as in (B). A sample containing 2% galactose was also shifted to 33°C for 6 h. (C) DNA content was analyzed by flow cytometry. (D) In these same cells, the number of SPBs containing the indicated number of Spc42-mCherry and Spc110-GFP foci in large budded cells was quantitated (n>100 for all samples). Alteration of membrane composition alleviates the growth defect of MPS3-G186K
To better understand the role that Mps3 plays in SPB insertion and membrane structure, we screened the yeast deletion collection for mutants that rescued the growth defect of GAL-MPS3-G186K. If SPB duplication defects in this mutant are the result of defects in membrane composition as our cytology suggests, then it should be possible to find mutants that alter the lipid composition of the nuclear membrane to compensate for MPS3-G186K expression. In total, 93 mutants were found to grow on SC-URA+2% galactose in the presence of pURA3-GAL1-MPS3-G186K (Table S2). Because a number of these genes likely affect transcription, translation, post-translational modification or galactose-induction of the dominant allele, we used western blotting as a secondary screen to identify mutants that expressed high levels of Mps3-G186K protein, similar to that observed in wild-type cells (data not shown). 37 mutants met this secondary criterion and are shown in Figure 6A together with a wild-type and gal4Δ control. GAL4 encodes a transcription factor that is required for activation of genes in response to galactose, including expression from GAL1 [56]. This mutant was identified in our primary screen, but as expected, failed to pass our secondary test for Mps3-G186K production and serves as a control (Table S2).
Fig. 6. Suppressors of MPS3-G186K toxicity. 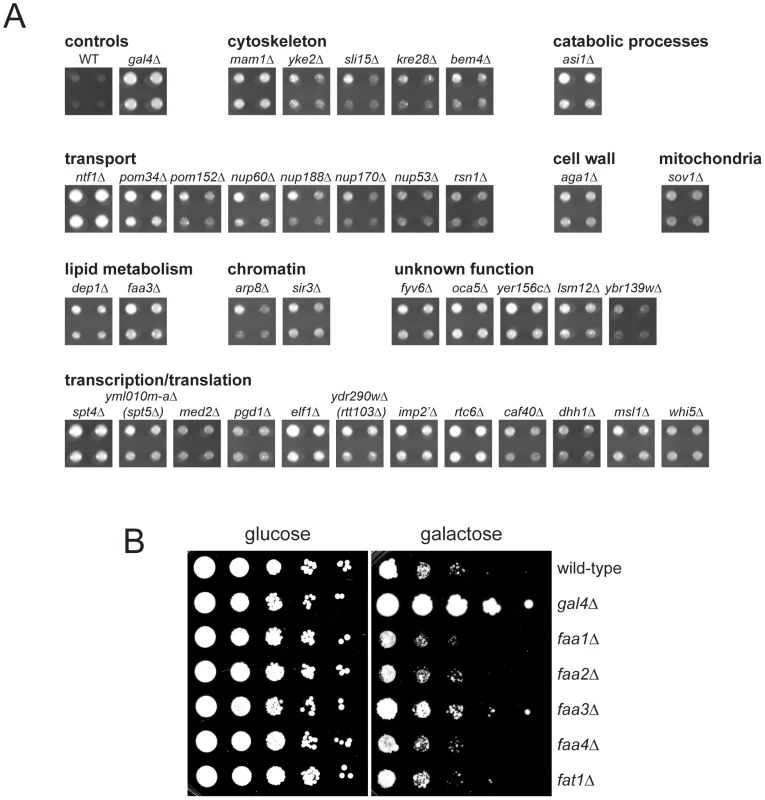
(A) Mutants from the yeast deletion collection were transformed with pURA3-GAL-MPS3-G186K, and transformants were tested for their ability to grow on SC-URA+2% galactose at for 3 d at 30°C. Possible hits were then further examined by western blotting to ensure that Mps3-G186K was expressed at high levels, similar to the wild-type control. Shown here are the hits that both rescued the growth defect of MPS3-G186K and expressed wild-type levels of Mps3-G186K. A full list of genes recovered in this screen is presented in Table S2. Also shown are the wild-type strain and the gal4Δ mutant, which we isolated in the primary screen but eliminated in the secondary screen because it does not express MPS3-G186K. Hits are organized by function using GoSlim analysis. In some cases, we isolated a mutant that deletes part of an overlapping gene, which is indicated below in parenthesis. (B) The indicated genes were deleted in GAL-MPS3-G186K (SLJ1797) and growth was tested in a serial dilution assay at 30°C on YPD (glucose) for 2 d and YPGR (galactose) for 3 d. Of the 37 deletion mutants that restore growth to MPS3-G186K, a number of genes encode proteins involved in transcription or translation. While these do not affect production of Mps3-G186K, they most likely restore growth to the mutant by affecting expression of an unknown target. One possible candidate target is POM34, which has previously been shown to be regulated by translation and whose levels influence growth of mutants defective in SPB insertion, such as mps2, bbp1 and ndc1 [4], [25], [26]. Interestingly, we also found that deletion of POM34, POM152 and several other nucleoporins suppressed MPS3-G186K mutants (Figure 6A). A number of these same deletion mutants were recently shown to rescue mps3Δ [26]. We proposed that deletion of the nucleoporins may rescue growth of cells lacking MPS3 by blocking NPC assembly, thereby liberating a shared insertion factor involved in both SPB and NPC insertion. Alternatively, their deletion may rescue growth of mps3Δ cells by changing the physical properties of the nuclear membrane, such as membrane fluidity, to facilitate SPB duplication without Mps3. Our finding that MPS3-G186K mutants, which have defects in nuclear membrane structure and SPB duplication, are suppressed by the many of the same nucleoporin deletions as mps3Δ strongly suggests that changes in nuclear membrane properties, rather than liberation of an insertion factor, alleviate the growth defect in both cases. The fact that we isolated multiple nucleoporins as MPS3-G186K suppressors suggests a common mechanism of suppression. As we demonstrate below for pom152Δ and infer for the other nucleoporins, alteration of nuclear envelope properties appears to be responsible for suppression of MPS3-G186K as well as rescue of MPS3 deletion.
In addition to nucleoporin deletions, we also found that deletion of two genes involved in lipid metabolism, FAA3 and DEP1, rescues MPS3-G186K mutants. DEP1 encodes a transcriptional regulator of many metabolic genes, including genes involved in phospholipid biosynthesis [57], [58]. FAA3 encodes one of five acyl coA synthetases that catalyze the conversion of fatty acids into activated acyl-coA intermediates in the first step in phospholipid biosynthesis. Faa1, Faa2, Faa3, Faa4 and Fat1 localize to different subcellular compartments and display distinct specificities for medium, long and very-long chain fatty acids in vitro [59]. FAA3 encodes a long chain or very long chain fatty acyl coA synthetase that is believed to be partially redundant with Fat1 in vivo [60]–[62]. However, we found that deletion of the other acyl coA synthetases, including fat1Δ, was unable to suppress the growth defect of MPS3-G186K (Figure 6B), suggesting that effects of Faa3 elimination are specific and that it may have a function in regulation of lipid composition at the nuclear membrane.
Changes in lipid composition rescues growth, nuclear morphology, and SPB duplication defects of MPS3-G186K
Deletion or overexpression of FAA3 leads to an alteration in cellular lipid content [60], [63], suggesting that changes in fatty acid levels or composition are what rescue growth of MPS3-G186K cells. To test this idea, we treated cells with chemicals that alter membrane composition or fluidity and examined the effects on cell growth and nuclear envelope morphology. We found that growth of MPS3-G186K mutants on YPGR was rescued by addition of the membrane fluidizer benzyl alcohol, the sterol biosynthesis inhibitor terbinafine and the fatty acid oleic acid (Figure 5A). In addition, increasing the temperature to 33°C, which increases membrane fluidity due to the cell's ability to adjust lipid composition by increasing levels of saturated and long chain fatty acids and altering the amount of sterols [64], [65], also suppressed the growth defect of MPS3-G186K, whereas decreasing the temperature to 23°C and 16°C, which decreases membrane fluidity, not only resulted in toxicity of MPS3-G186K but also exacerbated the phenotype associated with overexpression of wild-type MPS3 (Figure 5A). Therefore, these data are consistent with the possibility that affecting lipid composition to increase membrane fluidity is sufficient to suppress MPS3-G186K. Our observation that overproduction of wild-type Mps3 is lethal at low temperatures suggests that GAL-MPS3 cells may have an altered membrane composition due to a direct role of Mps3 in membrane homeostasis.
Further examination of cells by live cell imaging revealed that other defects associated with expression of MPS3-G186K, including abnormal nuclear morphology, cell division and SPB duplication, were at least partially suppressed by changing lipid composition. Oleic acid was particularly effective at suppressing defects in nuclear shape. The majority of nuclei appeared to maintain a round shape throughout a 3.5 h time course with few extensions or protrusions; however, few nuclei divided (Figure 5B). A rare example is shown in Video S3. In addition, analysis of DNA content by flow cytometry and examination of SPB insertion showed that most cells remained arrested in mitosis probably due to SPB duplication defects (Figure 5C–5D). It is unclear why oleic acid suppresses growth of MPS3-G186K on plates but does not rescue in liquid culture, although similar effects have been reported for numerous mutants (for example, [66], [67]).
Treatment of MPS3-G186K cells with other chemicals did not have as profound of an effect on nuclear morphology as oleic acid, perhaps because these drugs have pleiotropic effects on nuclear envelope structure (Figure 5B, Videos S4 and S5). For example, previous studies have shown that addition of benzyl alcohol affects NPC insertion [20], [21]. While it may alleviate membrane expansion caused by MPS3-G186K, benzyl alcohol addition might result in defects in NPC structure that lead to changes in nuclear morphology.
Nevertheless, despite terbinafine's partial rescue of nuclear shape, it eliminated the cell cycle arrest of MPS3-G186K and more than doubled the number of large-budded cells that contained two inserted SPBs (Figure 5C–5D). Although this could be due to a slow growth phenotype associated with terbinafine since no mitotic divisions were observed (Video S5 and Figure 5A), we favor the possibility that treatment with terbinafine affects sterol biosynthesis which in turn affects SPB duplication since we also observed that addition of other sterol inhibitors such as ketoconazole to media rescued growth of MPS3-G186K (Figure S4).
Treatment with benzyl alcohol also only partially rescued the nuclear shape and cell cycle arrest of MPS3-G186K, but it was able to restore a distribution of SPB intermediates similar to that of GAL-MPS3 cells in galactose (Figure 5B–5D), suggesting that it alleviates the SPB duplication defect associated with the dominant mutant. Consistent with this idea, we observed cells undergoing chromosome segregation and cell division in the presence of benzyl alcohol (Video S4). Importantly, addition of benzyl alcohol had virtually no effect on cell cycle progression or SPB insertion in GAL-MPS3 cells (Figure 5C–D). We also found that addition of chemicals had similar effects on MPS3 and MPS3-G186K expression (Figure S5). Thus, the effects of MPS3-G186K on growth, nuclear morphology and SPB insertion can be rescued by altering the lipid composition of the cell using both genetic and chemical methods.
mps3-1 mutants have defects in lipid homeostasis
Analysis of our mps3 deletion and point mutants showed that most are not sensitive to benzyl alcohol or sterol synthesis inhibitors (data not shown), suggesting that the synthetic effects we observe as a result of these treatments on MPS3-G186K mutants are due to a specific defect in Mps3 function that may be related to SPB insertion since MPS3-G186K, but not other mutants, display a SPB duplication defect (see Figure 1 and Figure 2). In support of this hypothesis, we found that virtually all mutants in the C-terminal SUN domain, which is required for SPB duplication [30]–[32], were resistant to terbinafine and found that a subset of these mutants, such as mps3-1, mps3-A540D and mps3-W477A, were also sensitive to benzyl alcohol (Figure 7A and 7B). Enhanced growth on terbinafine, which inhibits ergosterol biosynthesis [68], suggests that cells containing the mps3-1, mps3-A540D and mps3-W477A mutations have extremely fluid membranes; growth on membrane fluidizing agents like benzyl alcohol is toxic specifically to these mutants since they already have alterations in membrane composition. The more severe phenotype seen in some mps3 alleles presumably reflects a greater alteration in the nuclear membrane, although it is possible that some of these mutants may have other defects as well [32], [40]. It is interesting that many, but not all, of the mps3 mutants that are sensitive to benzyl alcohol are alleles that spontaneously diploidize. Perhaps the larger size of the nucleus in these mutants requires a greater need for lipid synthesis that cannot be met with two mutant copies of MPS3.
Fig. 7. mps3 mutants are sensitive to changes in membrane properties. 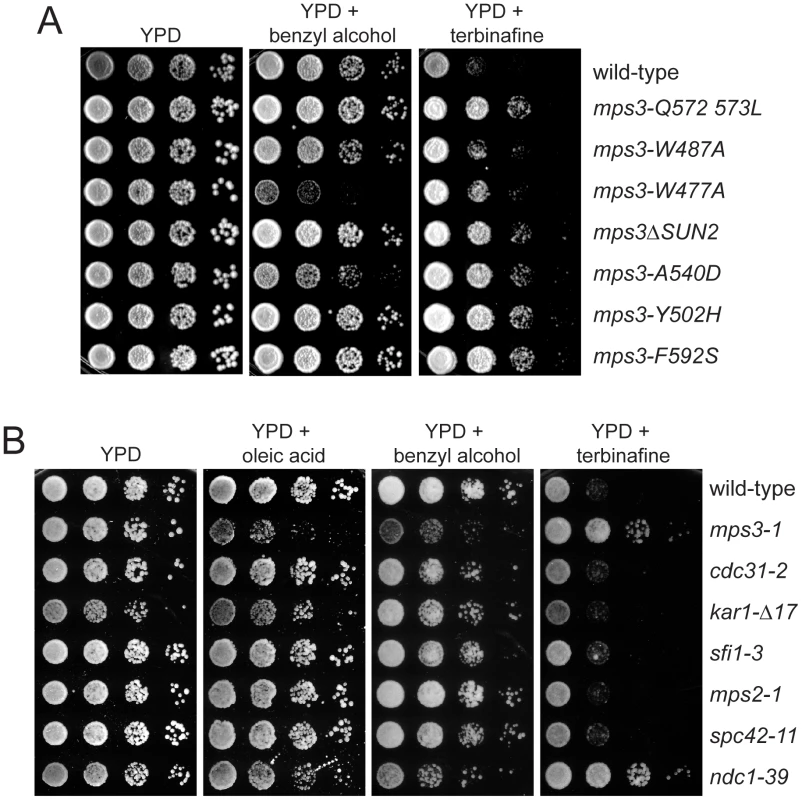
(A) Wild-type (SLJ1779), mps-Q572LQ573L (SLJ1783), mps3-W487A (SLJ1785), mps3-W477A (SLJ1793), mps3ΔSUN2 (SLJ1789), mps3-A540D (SLJ1787), mps3-Y502H (SLJ1781) and mps3-F592S (SLJ1791) cells, or (B) wild-type (SLJ001), mps3-1 (SLJ910), cdc31-2 (SLJ894), kar1Δ17 (SLJ844), sfi1-3 (SLJ1558), mps2-1 (SLJ718), spc42-11 (SLJ715) and ndc1-39 (SLJ1740) cells, were grown to log-phase in YPD, then were tested for their ability to grown on YPD, YPD+0.2% benzyl alcohol and YPD+2.5 µg/ml terbinafine plates in a serial dilution assay at 23°C for 3 d. In (B) cells were also grown on YPD+5 mM oleic acid. To determine if changes in lipid composition generally affect the SPB duplication process or are specific to mps3 mutants, we compared the growth of different recessive mutants in SPB duplication on plates containing oleic acid, benzyl alcohol and terbinafine. Most SPB alleles tested, including mutants that disrupt early (cdc31-2, kar1-Δ17, sfi1-3), intermediate (spc42-11, spc29-3) and late steps (mps2-1, bbp1-1, spc110-220) in SPB duplication and SPB regulators (mps1-1, tub4-1) [3], grew at identical rates to wild-type under all conditions tested (Figure 7B; data not shown). The notable exception was the ndc1-39 mutant, which is defective in both the late step of SPB duplication as well as in NPC assembly [5]. Like mps3-1 mutants, ndc1-39 cells exhibited increased sensitivity to oleic acid and benzyl alcohol and showed reduced sensitivity to terbinafine (Figure 7B). This suggests that membranes in ndc1-39, like mps3-1 mutants, have very fluid membranes due to changes in membrane composition. While it is possible that the basis for the membrane defect in ndc1-39 is related to its SPB insertion defect, we suspect that it is due instead to its role at the NPC since other SPB mutants, including mps2-1 and bbp1-1, do not share this property. The fact that multiple mps3 mutants, including alleles that are defective in early steps of SPB duplication and not known to cause defects in NPC assembly or function [30], [32], are specifically affected by growth on chemicals affecting lipid composition strongly supports our hypothesis that Mps3 function is intimately linked to membrane homeostasis.
Cells lacking MPS3 have aberrant lipid profiles
Our finding that MPS3-G186K mutants exhibit a severe membrane over-proliferation phenotype that is rescued by drugs and mutants that affect lipid synthesis, together with our observations that certain mps3 mutants are sensitive to changes in membrane fluidity, indicates that Mps3 may play a role either directly or indirectly in regulating lipid composition in the cell. If this were the case, then we would anticipate that cells lacking MPS3 would have an altered lipid composition compared to wild-type cells. To test this hypothesis, levels of phospholipids and neutral lipids were examined in whole cell preparations from five biological replicates of wild-type, pom152Δ and pom152Δ mps3Δ cells grown to mid-log phase in YPD at 30°C by electrospray ionization tandem mass spectrometry (ESI/MS/MS). pom152Δ mps3Δ mutants do not have an noticeable delay in cell division [26], so analysis of lipid composition in these cells will not be complicated by changes in temperature, growth media or cell cycle arrest that are associated with other mps3 alleles.
In cells lacking MPS3, we found that there was no statistically significant change in the overall levels of polar lipids compared to the controls (Figure 8A). However, examination of specific subclasses of phospholipids showed that pom152Δ mps3Δ mutants had 2.6 and 3.7 times the level of phosphatidyl serine (PS) and 2.0 and 2.3 times the level of phosphatidyl glycerol (PG) compared to wild-type and pom152Δ cells, respectively (Figure 8A; Table S3). Levels of phosphatidic acid (PA) and phosphatidyl ethanolamine (PE) were reduced 1.5 fold and increased 1.3 fold, respectively, in pom152Δ mps3Δ mutants compared with wild-type, although these values are not quite significant (p = 0.11 for PA, p = 0.37 for PE). The levels of other phospholipids such as phosphatidyl choline (PC) and phosphatidyl inositol (PI) were largely unchanged in wild-type, pom152Δ and pom152Δ mps3Δ samples. Thus, the distribution of polar lipids is affected in cells lacking Mps3 function.
Fig. 8. Aberrant lipid levels in cells lacking Mps3 function. 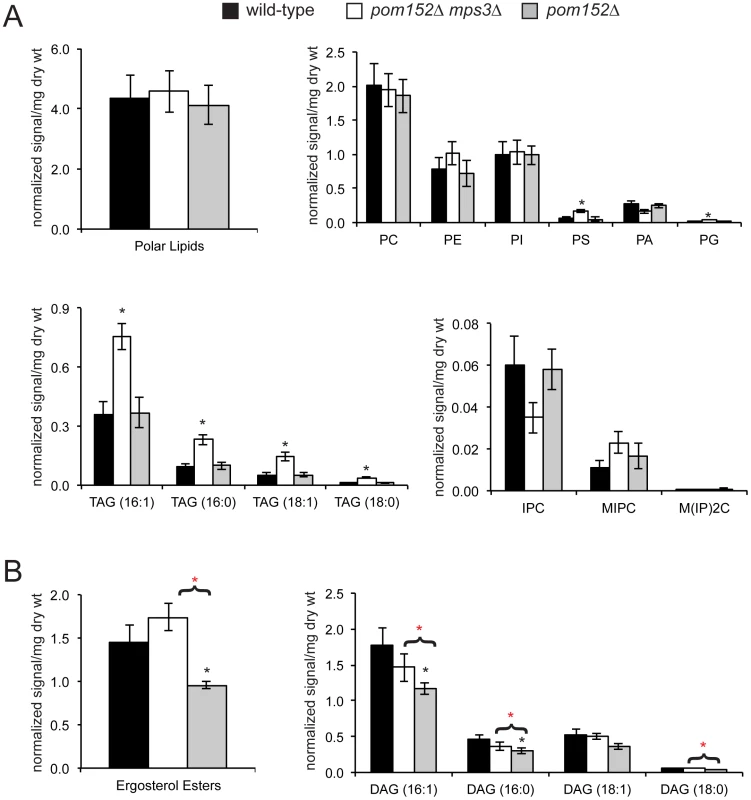
Lipids were extracted from 5 independently grown mid-log phase cultures at 30°C of wild-type (SLJ001), pom152Δ (SLJ4260) and pom152Δ mps3Δ (SLJ4259) and analyzed by ESI/MS/MS. The total amount of each type of lipid was calculated per mg weight of wet yeast cells used, and average values for each class of lipid were tabulated along with the standard deviation from the mean. A complete summary of our lipidomics analysis is presented in Table S3. (A) Although there is no change in the levels of total polar lipids, different types of phospholipids are increased or decreased in pom152Δ mps3Δ (white) compared to wild-type (black) or pom152Δ (gray). pom152Δ mps3Δ cells also show increased amounts of TAG. (B) Cells lacking POM152 only contain decreased levels of ergosterols and DAGs. Deletion of MPS3 in these cells restores the level of ergosterols and DAGs to that observed in wild-type cells. (A & B) Black asterisks indicate values that are statistically significant from wild-type and red asterisks indicate values that are statistically significant from pom152Δ (p<0.05). Examination of neutral lipids revealed that cells lacking MPS3 contained 2–3.3 times the level of triacylglycerols (TAGs) compared to wild-type and pom152Δ cells (Figure 8A; Table S3). These cells also had an altered distribution of the three yeast sphingolipids, including a 1.7 fold decrease in inositol phosphorylceramide (IPC) and a 2 fold increase in mannosylinositol phosphorylceramide (MIPC) compared to controls, although these changes were not quite statistically significant (p = 0.15 for IPC, p = 0.08 for MIPC). Even more importantly, we found that deletion of POM152 alone resulted in an 1.5 fold decrease in ergosterol levels as well as a reduction in diacylglycerol (DAG), which was eliminated by simultaneous deletion of MPS3 (Figure 8B; Table S3). This finding is critical for several reasons. First, it provides biochemical support for our theory that nucleoporin deletions result in changes in the physical properties of the nuclear membrane ([26] and above). Second, the fact that these changes in lipid composition are reversed by deletion of MPS3 is compelling evidence that Mps3 directly affects membrane homeostasis. From our lipidomics data, we would predict that Mps3 likely acts at multiple steps in lipid biosynthesis, affecting both neutral lipid and phospholipid levels.
Membrane homeostasis in cells lacking MPS3 can be rescued by FAA3
We would predict that changing properties of the nuclear membrane would result in growth defects in pom152Δ mps3Δ mutants if changes in lipid composition are required for SPB duplication and cell proliferation in the absence of MPS3. Indeed, we found that pom152Δ mps3Δ mutants were sensitive to benzyl alcohol, particularly at 37°C (Figure 9A). Although the lipid composition of pom152Δ mutants is altered, as was demonstrated in our lipidomics analysis (Figure 8; Table S3), growth of the single deletion is not affected by benzyl alcohol or other chemicals that affect membrane dynamics (Figure 9A). However, overexpression of FAA3, but not other acyl coA synthetases, partially rescued growth of pom152Δ mps3Δ mutants on benzyl alcohol-containing plates at high temperatures (Figure 9B; data not shown). Therefore, it seems that Faa3 is involved in de novo lipid synthesis required for nuclear membrane homeostasis, in particular, in membrane changes brought about by Mps3 that are essential for SPB insertion.
Fig. 9. Membrane defects in cells lacking MPS3 can be suppressed by FAA3. 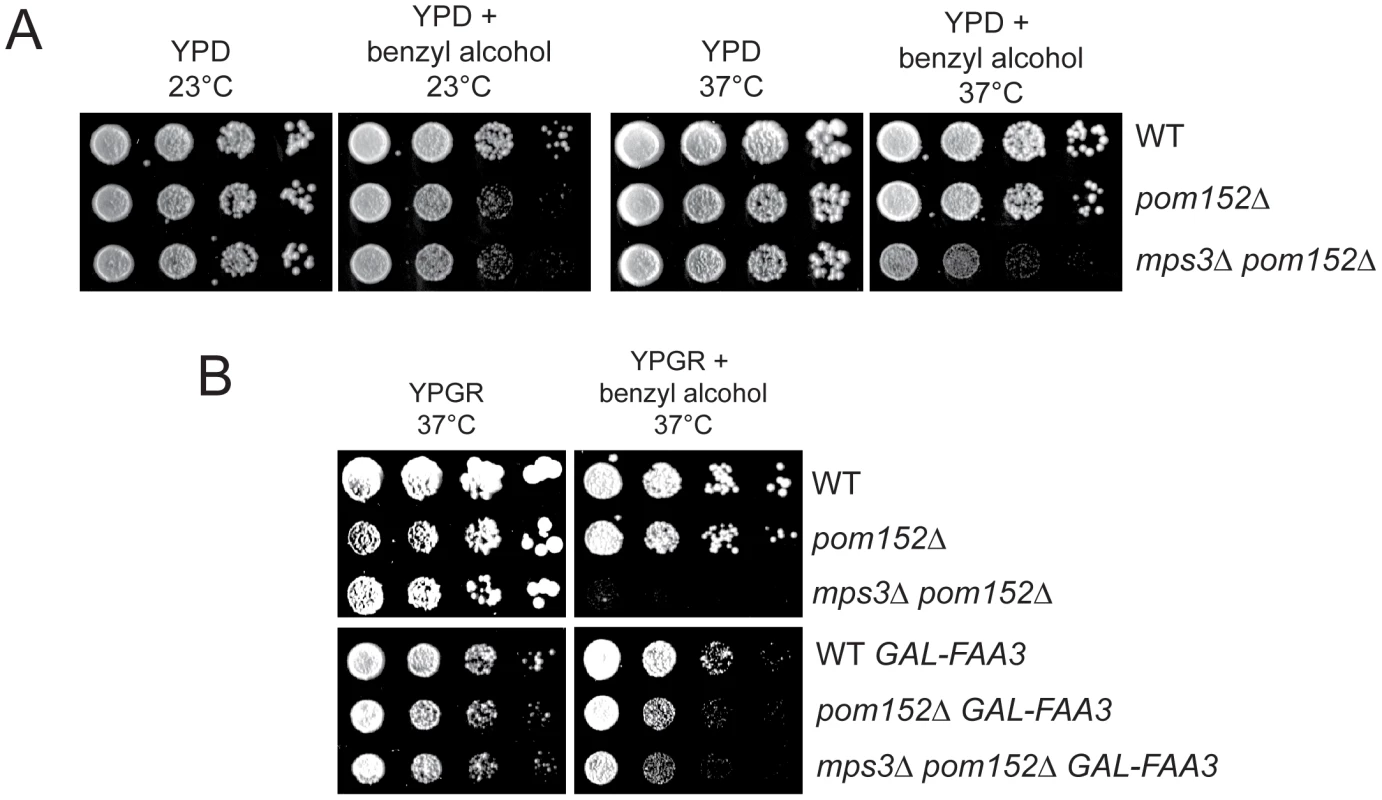
(A) Wild-type (SLJ001), pom152Δ (SLJ4260) and pom152Δ mps3Δ (SLJ5247) cells were tested for their ability to grow on YPD and YPD+0.2% benzyl alcohol at 23°C and 37°C in a serial dilution assay. Plates were grown for 2 d at 37°C and for 4 d at 23°C. (B) These same cells were transformed with an empty plasmid or with GAL-FAA3 and tested for their ability to grow on YPGR and YPGR+0.2% benzyl alcohol at 37°C for 2 d in a serial dilution assay. Discussion
Our analysis of the functional domains of Mps3 has led to new insights into its role in SPB duplication and nuclear envelope homeostasis. The requirement for the transmembrane domain in Mps3 function extends previous data suggesting that Mps3 is a structural component of the half-bridge [30], [32]. However, our observation that multiple membrane domains can substitute for the Mps3 transmembrane domain is compelling evidence that no specific intra - or intermolecular interactions occur through this region of the protein, including transport through the NPC to the INM and anchorage at the SPB. We therefore tested the requirement for other conserved domains and were surprised to find that, at least when individually mutated, most were not essential for Mps3 function at the SPB in wild-type cells. Perhaps Mps3 has multiple binding partners and interaction domains due to the critical importance of SPB duplication in the maintenance of genomic integrity.
Through mutagenesis of the putative P-loop region of Mps3, we identified a new dominant allele that revealed a role for Mps3 in insertion of the newly duplicated SPB into the nuclear envelope. Although we could detect binding between Mps3 and ATP in vitro (data not shown) and glycine 186 is a conserved residue in the P-loop, we doubt that a defect in nucleotide binding is the underlying cause of the phenotypes we observed in MPS3-G186K mutants. This is because other mutants in conserved residues in the P-loop that also block ATP binding, such as S190A, G192K and S194A, did not result in defects in SPB duplication and nuclear envelope homeostasis. Also, alignment of Mps3 with other fungal orthologs shows that this motif is not conserved even within the Saccharomyces lineage. Thus, MPS3-G186K is likely a fortuitous antimorphic allele rather than a mutant that disrupts ATP binding per se.
Although overexpression of MPS3 in our strains has few phenotypes at 30°C, its overproduction at 16°C and 23°C inhibits cell growth and leads to subtle changes in nuclear morphology (see Video S1). The simplest explanation for the temperature-dependent effect on cell viability when Mps3 is overproduced is that Mps3 plays a direct role in membrane homeostasis. Decreased amounts of saturated and long change fatty acids and changes in sterol levels, which accompany growth at lower at lower temperatures [64], [65], are incompatible with the alterations in membrane composition brought about by overproduction of Mps3. This incompatibility leads to a failure in SPB insertion as well as other changes in nuclear structure. Changes in lipid composition and nuclear architecture are exacerbated in the MPS3-G186K mutant such that this allele is toxic under most conditions, including growth at 30°C. Moreover, because the membrane pertubations are striking and growth arrest is tight, this allele is amenable to genetic and cell biological analysis.
Membrane over-proliferation in GAL-MPS3-G186K but not GAL-MPS3 strongly suggests that it is not simply due to overproduction of an integral membrane protein, but rather are a unique consequence of overexpressing a mutant version of MPS3. We propose that this altered version of Mps3 titrates out key factors required for nuclear envelope homeostasis and/or SPB duplication. The most obvious candidate is the endogenous wild-type copy of Mps3 since SUN proteins are known to oligomerize and because a reduction of Mps3 at the spindle pole leads to defects in SPB duplication [23], [32], [69]–[71]. Consistent with this idea, mild overexpression of MPS3 using a 2-micron plasmid partially rescues the growth defect of GAL-MPS3-G186K in cells grown at 30°C (Figure S2). Other candidates include the ribosome biogenesis factors Erb2 and Rrs1 that interact with Mps3 and are required for nuclear morphology [48].
Proliferation of intracellular membranes in response to increased levels of membrane proteins has been previously reported in a wide range of cell types; however, the intranuclear membranes that we observed following expression of MPS3-G186K are structurally distinct in several ways. First, unlike the flattened arrays of ER membrane known as karmallae formed following overexpression of HMG1, which encodes 3-hydroxy-3-methyl-glutaryl coenzyme A [72]–[75], the MPS3-G186K-dependent membranes appear to be exclusively intranuclear and most likely represent overproliferation of the INM. Second, unlike the intranuclear membranes formed upon overproduction of Nup53 that are devoid of NPCs [54], the MPS3-G186K membranes contain NPCs that are at least partially functional. In vertebrate cells, Sun1 localizes to NPCs and appears to play a role in their assembly [22]–[24], however, this function does not appear to be conserved in yeast despite the fact that highly curved membranes are involved in both NPC and SPB duplication since Mps3 does not co-localize with NPCs [48]. Third, the intranuclear arrays that form following MPS3-G186K expression are generally closely associated with the nuclear membrane, suggesting that proteins on the surface of the MPS3-G186K-induced membranes are able to interact with other nuclear envelope proteins. This is in contrast to intranuclear membrane arrays that form within the nucleoplasm and do not appear to associate with the nuclear membrane over large regions [76], [77]. Fourth, although the elaborate membrane extensions known as escapades formed by increasing levels of the peripheral membrane protein Esc1 also include NPCs [78], the excess membrane found in escapades originates at the nuclear vacuolar junction and results in predominant cytoplasmic extensions, whereas the MPS3-G186K membranes appear to begin as tubules inside of the nucleus that then fuse into intranuclear membranes. Fifth, unlike the membrane flares formed by disruption of proteins involved in the production of PA and DAG [53], [79]–[81], membrane proliferation in MPS3-G186K mutants was not restricted to the nucleolar region but rather appeared to occur at multiple sites inside of the nucleus. Interestingly though, the nucleolus was often partitioned away from the nucleus in a separate lobe in cells overexpressing both MPS3-G186K as well as MPS3, consistent with the idea that this region of the nuclear membrane has a unique molecular composition. In cells overexpressing MPS3 or MPS3-G186K, we observed increased levels of most types of polar lipids as well as ergosterols compared to wild-type (data not shown). Levels of DAG were also elevated in MPS3 overexpressing cells, but not in MPS3-G186K mutants, which might account for some of the differences we observed in membrane morphology and SPB duplication between these two strains.
In MPS3-G186K cells, the excess membrane appears to form as tubules within the nucleoplasm that then fuse to form the intranuclear membranes observed in the mutants. It is unclear how these tubules initiate, but they appear to be intermediates in nuclear envelope assembly. Curiously, similar tube-like structures have been detected in cells overexpressing NUP53 and during the formation of nuclear membranes around sperm chromatin in Xenopus oocytes [54], [82]. Our observation that lipid composition affects cell viability, nuclear shape and membrane morphology of MPS3-G186K mutants suggests that specific properties of intranuclear tubules are critical for their formation, fusion and stacking to form an intact nuclear envelope. At least one important parameter is the amount of fatty acids produced by Faa3. This enzyme catalyzes acylation of long and very long chain fatty acids in vitro, which are important for the formation of highly curved membranes such as those that occur at sites of NPC and SPB insertion [83], [84]. By limiting the amount of these classes of fatty acids, it may be more difficult to form a membrane bend, which could help limit the membrane extension and protrusion driven by MPS3-G186K. We did not detect a difference in chain length in fatty acids in any of our mps3 mutants, however (Table S3; data not shown). Possibly only a small pool of fatty acid side chains is modified making detection in a population assay difficult, or Faa3 may have a different substrate specificity in vivo. In addition, other membrane modifications such as sterol insertion may be a driving force in the control of nuclear envelope structure inside the cell. Many of the genes involved in sterol biosynthesis in yeast are encoded by essential genes [85] and thus, they are not present in the haploid yeast deletion collection so they would not have been discovered in our screen.
Deletion of NPC subunits suppresses the SPB duplication defect associated with mutation of several genes encoding SPB components [4], [25]–[27]. Our finding that deletion of POM152 results in reduced levels of ergosterols and DAG compared to wild-type cells points to the possibility that the nucleoporin deletions affect the lipid composition of the nuclear envelope. This might occur through reduced transport of genes involved in lipid biosynthesis or through decreased export of the associated mRNAs. These changes in membrane composition could also account for genetic interactions between pom152Δ and genes involved in membrane fluidity, sterol synthesis, organelle integrity and membrane bending [18], [20], [21], [86].
In pom152Δ mps3Δ mutants, lipidomics analysis shows that the balance of ergosterol and DAG is reset to near wild-type levels, which could explain why SPB duplication and nuclear morphology defects were not observed in the double delete [26]. Other classes of lipids such as TAG are elevated in cells lacking MPS3, suggesting that Mps3 plays a direct role in regulation of membrane homeostasis. Our observation of allele specific defects in membrane proliferation and drug sensitivity in mps3 mutants that was not detected in other SPB mutants further supports this hypothesis. The fact that these same alleles of MPS3 are defective in SPB duplication indicates that Mps3-dependent changes in the nuclear envelope are important for formation of the new SPB, perhaps at multiple steps in SPB assembly. Studies in yeast, Dictyostelium, C. elegans and mammalian cells have shown that loss of SUN protein function leads to aberrant nuclear morphology [30], [32], [41], [48], [87]–[89]. Whether they are directly involved in the synthesis or maintenance of the nuclear membrane or are indirectly involved through binding to the nuclear lamina is not well understood [90]. Perhaps the best hint as to how SUN proteins might function in membrane homeostasis comes from two-hybrid analysis of the fission yeast SUN protein Sad1, which pointed to an interaction with the acetyl coA carboxylase enzyme Cut6 that is needed for de novo biosynthesis of long-chain fatty acids and mitotic progression [91], [92]. Much like the binding between SUN proteins and their ONM binding partners, known as KASH proteins, in the perinuclear space [35], we envision that SUN proteins like Mps3 may also associate with factors involved in membrane structure. Mps3 binding to enzymes or other proteins involved in membrane remodeling could be a mechanism to promote membrane bending and fusion at specific sites in the nuclear envelope, such as at the duplicating SPB or the NPC. However, Mps3 could also control membrane synthesis at the transcriptional level, by sequestering factors involved in lipid synthesis at the nuclear periphery. Future studies aimed at identification of Mps3 binding partners will help us better understand the role SUN proteins in membrane dynamics and nuclear morphology as well as elucidate the mechanism by which Mps3-dependent changes in the nuclear membrane enable duplicated SPBs to insert into the nuclear envelope.
Materials and Methods
Yeast strains and plasmids
All strains are derivatives of W303 (ade2-1 trp1-1 leu2-3,112 ura3-1 his3-11,15 can1-100) and are listed in Table S1 except those used in Figure 6 and Table S2, which are in BY4741 (Mata his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and were taken from the yeast deletion collection (Open Biosystems). Standard techniques were used for DNA and yeast manipulations.
Deletion of MPS3, fusion of SPC110 and NUP49 to GFP and fusion of HTB2 (which encodes one of the two copies of histone 2B), SPC42 or NET1 to mCherry was done by PCR-based methods [93], [94]. Correct integration was confirmed by PCR.
The MPS3 ORF and ∼500 bp of promoter were amplified by PCR and cloned into the XhoI and BamHI sites of a pRS306 vector containing GFP to construct pSJ650 (pRS306-MPS3-GFP) [41]. Construction of pSJ148 (pRS305-MPS3) has been previously described [32]. To overexpress MPS3, the entire open reading frame was inserted immediately adjacent to GAL1 in pRS306 to create pSJ146 (pRS306-GAL1-MPS3). Plasmids were digested with BstEII or ApaI to target integration to LEU2 or URA3, respectively. The number of copies of MPS3 integrated was determined by Southern blotting. Deletion and point mutants, as well as transmembrane insertions, were generated in these plasmids using the Quick Change Mutagenesis Kit (Stratagene). Each deletion mutant contains an in-frame deletion of the indicated amino acids; in the case of 2xpQ mutant, an extra copy of the pQ domain was inserted immediately after the pQ coding sequence. Sequencing was performed to confirm correct base pair substitutions or deletions were made.
To test for rescue of MPS3-G186K, plasmids containing the indicated SPB genes were taken from the yeast tiling library [95] and transformed into SLJ1797. For dilution assays, 5 OD600 of cells were serially-diluted 10-fold in sterile growth media and stamped onto agar plates. YPD has 2% glucose and YPGR has 2% galactose and 2% raffinose as the carbon source. Chemicals were purchased from Sigma and were added to media in the following amounts: 5 mM oleic acid, 0.2% benzyl alcohol, 1.25 µg/ml terbinafine and 1 µg/ml ketoconazole.
Cytological techniques
Localization of Mps3-GFP, H2B-mCherry, the nuclear localization sequence (NLS) reporter, HDEL-dsRed and Pus1-GFP were visualized as previously described [41]. Briefly, 1 ml of culture was centrifuged, washed with 1 ml ddH2O, resuspended in approximately 100 µL of ddH2O and 10 µL was placed on a 25% gelatin pad for image analysis. Fluorescence was performed using a confocal microscope (LSM-510-META; Zeiss) equipped with a ConforCor 3 module with avalanche photodiode detectors, which allow single photon counting, with a 100× 1.46 NA α-Plan Fluar objective (Zeiss). GFP was excited using a 488-nm Argon laser line, while mCherry was excited with a 561-nm HeNe laser line with the appropriate filter sets. Emitted photons were collected through BP 505–540 nm and LP 580 nm filters, with a pinhole size of 1.03 Airy units according to the green channel. Data was acquired using AIM v.4.2 software (Zeiss, Inc.). Images were collected with 8–10 image stacks with a 0.3 micron step size through the cells at room temperature. Images were processed using ImageJ software (NIH). At least two independent transformants of each genotype were analyzed by fluorescence microscopy in at least three independent experiments. N∶C ratios were calculated as previously described [28].
Time-lapse image analysis of GAL-MPS3 or GAL-MPS3-G186K strains was performed on cultures grown overnight in YEP plus 2% raffinose at 30°C then diluted back to an OD600 of 0.2 and grown in the same media for 2 h. 2% galactose and nothing, 0.2% benzyl alcohol, 5 mM oleic acid, 1.25 µg/ml terbinafine or 1 µg/ml ketoconazole were added at 30°C for 30 min. One ml of culture was centrifuged to concentrate the cells and 5 µL was placed on a 1% agarose pad containing the same media and chemical treatment. Fluorescent images were taken from 1 h to 3 h 30 min after galactose induction using the same microscope configuration but with a 40× 1.3 NA oil objective (Zeiss). Emitted photons were collected through BP 505–540 nm and LP 580 nm filters, with a pinhole size of 1.03 Airy units according to the green channel. Images were collected with 17 image stacks with a 0.7 micron step size through the cells at room temperature. The 512×512 images were binned spatially 2×2 and a max projection was applied for each Z-slice. All data processing and video generation were done in ImageJ (NIH).
For the characterization of the SPB duplication using the red/green foci assay, images were acquired with a 100× 1.4 NA oil objective on an inverted Zeiss 200 m equipped with a Yokagawa CSU-10 spinning disc. 488 nm excitation and 568 nm excitation were used for GFP and mCherry, respectively, and emission was collected through BP 500–550 nm and BP 590–650 nm filters, respectively, onto a Hamamatsu EMCCD (C9000-13). For each channel, a Z-stack was acquired using 0.6 or 0.7 micron spacing. 13 total slices were acquired, and a max projection image was created for analysis of foci using ImageJ (NIH).
Analysis of DNA content by flow cytometry, EM, and protein localization by indirect immunofluorescence microscopy were performed as previously described [30], [32], [96]. Poly(A)+ in situ hybridization was performed using an Cy3-labeled oligo(dT)50 probe as described [97]. Cells were examined with a Zeiss Axioimager using a 100× Zeiss Plan-Fluar lens (NA = 1.45), and images were captured with a Hamamatsu Orca-ER digital camera and processed using ImageJ (NIH).
Western blotting
The following primary antibody dilutions were used: 1∶1000 anti-Flag (Sigma) and 1∶1000 anti-glucose-6-phosphate dehydrogenase (G6PDH; Sigma). Fluorescently conjugated secondary antibodies were used at 1∶10000 for analysis and quantification using the Odyssey system (Li-Cor).
ESI-MS/MS lipid profiling
Yeast cells were grown overnight at 30°C in YPD, diluted to OD600 of 0.2 in the same media and allowed to grow for 6–8 h at 30°C. The wet weight of the sample was recorded and lipids were extracted by the following procedure. Pelleted cells were homogenized using glass beads and water and the aqueous homogenized cells were transferred to 15 ml Teflon-lined glass tubes. To 0.8 parts (1.6 ml) of homogenized cells in aqueous solution, 1 part (2 ml) of chloroform and 2 parts (4 ml) of methanol were added. The slurry was shaken, 1 part (2 ml) of chloroform and 1 part of water (2 ml) were added. After shaking well, the slurry was centrifuged at low speed for 5–10 min and the lower layer was removed. One part of chloroform was added, shaken, centrifuged and the lower layer was again removed. This was repeated, and the combined lower layers were washed once with a small volume of 1 M KCl and once with a small volume of water. The combined and washed chloroform extracts were dried under liquid nitrogen and stored at −20°C in 2 ml Teflon-lined glass vials.
An automated electrospray ionization-tandem mass spectrometry (ESI-MS/MS) approach was used to analyze lipid composition in these samples, and data acquisition and analysis were carried out as described previously [98] with modifications. A 5 to 20 µl of aliquot of extract in chloroform was used from each 1 ml lipid sample. Precise amounts of internal standards, obtained and quantified as previously described [99], were added in the following quantities: 0.3 nmol di12 : 0-PC, 0.3 nmol di24 : 1-PC, 0.3 nmol 13 : 0-lysoPC, 0.3 nmol 19 : 0-lysoPC, 0.3 nmol di12 : 0-PE, 0.3 nmol di23 : 0-PE, 0.3 nmol 14 : 0-lysoPE, 0.3 nmol 18 : 0-lysoPE, 0.3 nmol di14 : 0-PG, 0.3 nmol di20 : 0(phytanoyl)-PG, 0.3 nmol di14 : 0-PA, 0.3 nmol di20 : 0(phytanoyl)-PA, 0.2 nmol di14 : 0-PS, 0.2 nmol di20 : 0(phytanoyl)-PS, 0.46 nmol 16 : 0-18 : 0-PI, 0.33 nmol di18 : 0-PI, 3.1 nmol tri17 : 1 TAG, and 4.6 nmol di15 : 0 DAG. The sample and internal standard mixture was combined with solvents, such that the ratio of chloroform/methanol/300 mM ammonium acetate in water was 300/665/35, and the final volume was 1.4 ml.
Unfractionated lipid extracts were introduced by continuous infusion into the ESI source on a triple quadrupole MS (4000QTrap, Applied Biosystems). Samples were introduced using an autosampler (LC Mini PAL, CTC Analytics AG, Zwingen, Switzerland) fitted with the required injection loop for the acquisition time and presented to the ESI needle at 30 µl/min.
Sequential precursor and neutral loss scans of the extracts produce a series of spectra with each spectrum revealing a set of lipid species containing a common head group fragment. Lipid species were detected with the following scans: PC and lysoPC, [M+H]+ ions in positive ion mode with Precursor of 184.1 (Pre 184.1); PE and lysoPE, [M+H]+ ions in positive ion mode with Neutral Loss (NL) of 141.0 (NL 141.0); PG, [M+NH4]+ in positive ion mode with NL 189.0 for PG; PI, [M+NH4]+ in positive ion mode with NL 277.0; PS, [M+H]+ in positive ion mode with NL 185.0; PA, [M+NH4]+ in positive ion mode with NL 115.0; IPC, [M−H]− in negative ion mode with Precursor of 259.0; MIPC, [M−H]− in negative ion mode with Precursor of 421.0; M(IP)2C, [M−H]− in negative ion mode with Precursor of 663.1; (lysoPG, [M−H] − in negative ion mode with Precursor of 152.9 used as a standard for IPC, MIPC, M(IP)2C). The collision gas pressure was set at 2 (arbitrary units). The collision energies, with nitrogen in the collision cell, were +28 V for PE, +40 V for PC, +25 V for PA, PI and PS, +20 V for PG, −72 for IPC, −80 for MIPC, −75 for M(IP)2C, −57 for lysoPG. Declustering potentials were +100 V for all positive mode scans, −180 for IPC, MIPC, and M(IP)2C, −100 for lysoPG. Entrance potentials were +15 V for PE and +14 V for PC, PA, PG, PI, and PS, −13 for IPC, MIPC, and M(IP)2C, −10 for lysoPG. Exit potentials were +11 V for PE and +14 V for PC, PA, PG, PI, PS, −10 for IPC, MIPC, and M(IP)2C, −14 for lysoPG. The scan speed was 50 or 100 u per sec. The mass analyzers were adjusted to a resolution of 0.7 u full width at half height. For each spectrum, 9 to 150 continuum scans were averaged in multiple channel analyzer (MCA) mode. A series of neutral loss scans were used to detect ergosterol esters, DAG, and TAG species as [M+NH4]+ ions. The scans targeted losses of various fatty acids as neutral ammoniated fragments: NL 285.2 (17∶1, for the TAG internal standard); NL 259.2 (15∶0, for the DAG internal standard); NL 271.2 (16∶1); NL 273.2 (16∶0); NL 299.2 (18∶1); and NL 301.2 (18∶0). The scan speed was 100 u per sec. The collision energy was +25 V, entrance potential was +14 V, and exit potential was +14 V. Fifty continuum scans were averaged in MCA mode. The source temperature (heated nebulizer) was 100°C, the interface heater was on, +5.5 kV was applied to the electrospray capillary, the curtain gas was set at 20 (arbitrary units), and the two ion source gases were set at 45 (arbitrary units).
The background of each spectrum was subtracted, the data were smoothed, and peak areas integrated using a custom script and Applied Biosystems Analyst software, and the data were corrected for overlap of isotopic variants (A+2 peaks). The first and typically every 11th set of mass spectra were acquired on the internal standard mixture only. Peaks corresponding to the target lipids in these spectra were identified and molar amounts calculated in comparison to the two internal standards on the same lipid class. To correct for chemical or instrumental noise in the samples, the molar amount of each lipid metabolite detected in the “internal standards only” spectra was subtracted from the molar amount of each metabolite calculated in each set of sample spectra. The data from each “internal standards only” set of spectra was used to correct the data from the following 10 samples. Finally, the data were corrected for the fraction of the sample analyzed and normalized to the mg wet weight to produce data in the units nmol/mg, except DAG, TAG, and ergosterol esters. For analysis of these classes, there is variation in ionization efficiency [100]. Thus, the ratio of the MS response of the internal standard does not provide a value that is directly proportional to the content of each species. DAG, TAG, and ergosterol ester amounts are thus expressed as relative mass spectral signal/mg wet weight, where a signal of 1.0 represents a signal equal to the signal of 1 nmol tri17 : 1 TAG, or 1 nmol di15 : 0 DAG (the internal standards).
Supporting Information
Zdroje
1. Strambio-De-CastilliaCNiepelMRoutMP 2010 The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol 11 490 501
2. ByersBGoetschL 1975 Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol 124 511 523
3. JaspersenSLWineyM 2004 The budding yeast spindle pole body: structure, duplication, and function. Annu Rev Cell Dev Biol 20 1 28
4. ChialHJRoutMPGiddingsTHWineyM 1998 Saccharomyces cerevisiae Ndc1p is a shared component of nuclear pore complexes and spindle pole bodies. J Cell Biol 143 1789 1800
5. LauCKGiddingsTHJrWineyM 2004 A novel allele of Saccharomyces cerevisiae NDC1 reveals a potential role for the spindle pole body component Ndc1p in nuclear pore assembly. Eukaryotic Cell 3 447 458
6. OnischenkoEStantonLHMadridASKieselbachTWeisK 2009 Role of the Ndc1 interaction network in yeast nuclear pore complex assembly and maintenance. J Cell Biol 185 475 491
7. MakioTStantonLHLinCCGoldfarbDSWeisK 2009 The nucleoporins Nup170p and Nup157p are essential for nuclear pore complex assembly. J Cell Biol 185 459 473
8. ChadrinAHessBSan RomanMGattiXLombardB 2010 Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution. The Journal of Cell Biology 189 795 811
9. WineyMGoetschLBaumPByersB 1991 MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol 114 745 754
10. ArakiYLauCKMaekawaHJaspersenSLGiddingsTHJr 2006 The Saccharomyces cerevisiae spindle pole body (SPB) component Nbp1p is required for SPB membrane insertion and interacts with the integral membrane proteins Ndc1p and Mps2p. Mol Biol Cell 17 1959 1970
11. SchrammCElliottSShevchenkoASchiebelE 2000 The Bbp1p-Mps2p complex connects the SPB to the nuclear envelope and is essential for SPB duplication. Embo J 19 421 433
12. KupkeTDi CeccoLMullerHMNeunerAAdolfF 2011 Targeting of Nbp1 to the inner nuclear membrane is essential for spindle pole body duplication. EMBO J 30 3337 3352
13. DrinGCasellaJFGautierRBoehmerTSchwartzTU 2007 A general amphipathic alpha-helical motif for sensing membrane curvature. Nature Struct Mol Biol 14 138 146
14. AlberFDokudovskayaSVeenhoffLMZhangWKipperJ 2007 Determining the architectures of macromolecular assemblies. Nature 450 683 694
15. AlberFDokudovskayaSVeenhoffLMZhangWKipperJ 2007 The molecular architecture of the nuclear pore complex. Nature 450 695 701
16. DrinGAntonnyB 2010 Amphipathic helices and membrane curvature. FEBS Letters 584 1840 1847
17. DoucetCMTalamasJAHetzerMW 2010 Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell 141 1030 1041
18. DawsonTRLazarusMDHetzerMWWenteSR 2009 ER membrane-bending proteins are necessary for de novo nuclear pore formation. J Cell Biol 184 659 675
19. SchneiterRHitomiMIvessaASFaschEVKohlweinSD 1996 A yeast acetyl coenzyme A carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol Cell Biol 16 7161 7172
20. ScarcelliJJHodgeCAColeCN 2007 The yeast integral membrane protein Apq12 potentially links membrane dynamics to assembly of nuclear pore complexes. J Cell Biol 178 799 812
21. HodgeCAChoudharyVWolyniakMJScarcelliJJSchneiterR 2010 Integral membrane proteins Brr6 and Apq12 link assembly of the nuclear pore complex to lipid homeostasis in the endoplasmic reticulum. J Cell Sci 123 141 151
22. LiuQPanteNMisteliTElsaggaMCrispM 2007 Functional association of Sun1 with nuclear pore complexes. J Cell Biol 178 785 798
23. LuWGotzmannJSironiLJaegerVMSchneiderM 2008 Sun1 forms immobile macromolecular assemblies at the nuclear envelope. Biochim Biophys Acta 1783 2415 2426
24. TalamasJAHetzerMW 2011 POM121 and Sun1 play a role in early steps of interphase NPC assembly. J Cell Biol 194 27 37
25. SezenBSeedorfMSchiebelE 2009 The SESA network links duplication of the yeast centrosome with the protein translation machinery. Genes Dev 23 1559 1570
26. WitkinKLFriederichsJMCohen-FixOJaspersenSL 2010 Changes in the nuclear envelope environment affect spindle pole body duplication in Saccharomyces cerevisiae. Genetics 186 867 883
27. GreenlandKBDingHCostanzoMBooneCDavisTN 2010 Identification of Saccharomyces cerevisiae spindle pole body remodeling factors. PLoS ONE 5 e15426 doi:10.1371/journal.pone.0015426
28. GardnerJMSmoyerCJStensrudESAlexanderRGogolM 2011 Targeting of the SUN protein Mps3 to the inner nuclear membrane by the histone variant H2A.Z. J Cell Biol 193 489 507
29. AitchisonJDRoutMPMarelliMBlobelGWozniakRW 1995 Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J Cell Biol 131 1133 1148
30. JaspersenSLGiddingsTHJrWineyM 2002 Mps3p is a novel component of the yeast spindle pole body that interacts with the yeast centrin homologue Cdc31p. J Cell Biol 159 945 956
31. NishikawaSTerazawaYNakayamaTHirataAMakioT 2003 Nep98p is a component of the yeast spindle pole body and essential for nuclear division and fusion. J Biol Chem 278 9938 9943
32. JaspersenSLMartinAEGlazkoGGiddingsTHJrMorganG 2006 The Sad1-UNC-84 homology domain in Mps3 interacts with Mps2 to connect the spindle pole body with the nuclear envelope. J Cell Biol 174 665 675
33. RazafskyDHodzicD 2009 Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J Cell Biol 186 461 472
34. FridkinAPenknerAJantschVGruenbaumY 2009 SUN-domain and KASH-domain proteins during development, meiosis and disease. Cell Mol Life Sci 66 1518 1533
35. StarrDAFridolfssonHN 2010 Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol 26 421 444
36. WilliamsAJPaulsonHL 2008 Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci 31 521 528
37. van HamTJBreitlingRSwertzMANollenEA 2009 Neurodegenerative diseases: Lessons from genome-wide screens in small model organisms. EMBO Mol Med 1 360 370
38. SarasteMSibbaldPRWittinghoferA 1990 The P-loop–a common motif in ATP - and GTP-binding proteins. Trends Biochem Sci 15 430 434
39. MaBGChenLJiHFChenZHYangFR 2008 Characters of very ancient proteins. Biochem Biophys Res Commun 366 607 611
40. AntoniacciLMKennaMAUetzPFieldsSSkibbensRV 2004 The spindle pole body assembly component Mps3p/Nep98p functions in sister chromatid cohesion. J Biol Chem 279 49542 49550
41. BuppJMMartinAEStensrudESJaspersenSL 2007 Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J Cell Biol 179 845 854
42. AntoniacciLMKennaMASkibbensRV 2007 The nuclear envelope and spindle pole body-associated Mps3 protein bind telomere regulators and function in telomere clustering. Cell Cycle 6 75 79
43. ConradMNLeeCYWilkersonJLDresserME 2007 MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 104 8863 8868
44. OzaPJaspersenSLMieleADekkerJPetersonCL 2009 Mechanisms that regulate localization of a DNA double-strand break to the nuclear periphery. Genes Dev 23 912 927
45. SchoberHFerreiraHKalckVGehlenLRGasserSM 2009 Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev 23 928 938
46. ConradMNLeeCYChaoGShinoharaMKosakaH 2008 Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell 133 1175 1187
47. KoszulRKimKPPrentissMKlecknerNKameokaS 2008 Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell 133 1188 1201
48. HorigomeCOkadaTShimazuKGasserSMMizutaK 2011 Ribosome biogenesis factors bind a nuclear envelope SUN domain protein to cluster yeast telomeres. EMBO J 30 3799 811
49. ChanJNPoonBPSalviJOlsenJBEmiliA 2011 Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Developmental cell 20 867 879
50. WineyMHoytMAChanCGoetschLBotsteinD 1993 NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J Cell Biol 122 743 751
51. DonaldsonADKilmartinJV 1996 Spc42p: a phosphorylated component of the S. cerevisiae spindle pole body (SPD) with an essential function during SPB duplication. J Cell Biol 132 887 901
52. BullittERoutMPKilmartinJVAkeyCW 1997 The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell 89 1077 1086
53. CampbellJLLorenzAWitkinKLHaysTLoidlJ 2006 Yeast nuclear envelope subdomains with distinct abilities to resist membrane expansion. Mol Biol Cell 17 1768 1778
54. MarelliMLuskCPChanHAitchisonJDWozniakRW 2001 A link between the synthesis of nucleoporins and the biogenesis of the nuclear envelope. J Cell Biol 153 709 724
55. WineyMYararDGiddingsTHJrMastronardeDN 1997 Nuclear pore complex number and distribution throughout the Saccharomyces cerevisiae cell cycle by three-dimensional reconstruction from electron micrographs of nuclear envelopes. Mol Biol Cell 8 2119 2132
56. CampbellRNLeverentzMKRyanLAReeceRJ 2008 Metabolic control of transcription: paradigms and lessons from Saccharomyces cerevisiae. Biochem J 414 177 187
57. LampingELucklJPaltaufFHenrySAKohlweinSD 1994 Isolation and characterization of a mutant of Saccharomyces cerevisiae with pleiotropic deficiencies in transcriptional activation and repression. Genetics 137 55 65
58. CarrozzaMJFlorensLSwansonSKShiaW-JAndersonS 2005 Stable incorporation of sequence specific repressors Ash1 and Ume6 into the Rpd3L complex. Biochim Biophys Acta 1731 77 87
59. BlackPNDiRussoCC 2007 Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta 1771 286 298
60. KnollLJJohnsonDRGordonJI 1994 Biochemical studies of three Saccharomyces cerevisiae acyl-CoA synthetases, Faa1p, Faa2p, and Faa3p. J Biol Chem 269 16348 16356
61. ChoiJYMartinCE 1999 The Saccharomyces cerevisiae FAT1 gene encodes an acyl-CoA synthetase that is required for maintenance of very long chain fatty acid levels. J Biol Chem 274 4671 4683
62. WatkinsPALuJFSteinbergSJGouldSJSmithKD 1998 Disruption of the Saccharomyces cerevisiae FAT1 gene decreases very long-chain fatty acyl-CoA synthetase activity and elevates intracellular very long-chain fatty acid concentrations. J Biol Chem 273 18210 18219
63. KamisakaYTomitaNKimuraKKainouKUemuraH 2007 DGA1 (diacylglycerol acyltransferase gene) overexpression and leucine biosynthesis significantly increase lipid accumulation in the Deltasnf2 disruptant of Saccharomyces cerevisiae. Biochem J 408 61 68
64. MurataNLosDA 1997 Membrane Fluidity and Temperature Perception. Plant Physiology 115 875 879
65. ZhangYMRockCO 2008 Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6 222 233
66. HardwickKGWeissELucaFCWineyMMurrayAW 1996 Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science 273 953 956
67. JaspersenSLCharlesJFTinker-KulbergRLMorganDO 1998 A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol Biol Cell 9 2803 2817
68. PatranyGRyderNSStutzA 1984 Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science v224 p1239(1233)
69. MaloneCJFixsenWDHorvitzHRHanM 1999 UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development 126 3171 3181
70. CrispMLiuQRouxKRattnerJBShanahanC 2006 Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 172 41 53
71. HaqueFLloydDJSmallwoodDTDentCLShanahanCM 2006 SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol 26 3738 3751
72. WrightRBassonMD'AriLRineJ 1988 Increased amounts of HMG-CoA reductase induce “karmellae”: a proliferation of stacked membrane pairs surrounding the yeast nucleus. J Cell Biol 107 101 114
73. ParrishMLSengstagCRineJDWrightRL 1995 Identification of the sequences in HMG-CoA reductase required for karmellae assembly. Mol Biol Cell 6 1535 1547
74. ProfantDARobertsCJWrightRL 2000 Mutational analysis of the karmellae-inducing signal in Hmg1p, a yeast HMG-CoA reductase isozyme. Yeast 16 811 827
75. KoningAJRobertsCJWrightRL 1996 Different subcellular localization of Saccharomyces cerevisiae HMG-CoA reductase isozymes at elevated levels corresponds to distinct endoplasmic reticulum membrane proliferations. Mol Biol Cell 7 769 789
76. BastosRLinAEnarsonMBurkeB 1996 Targeting and function in mRNA export of nuclear pore complex protein Nup153. J Cell Biol 134 1141 1156
77. RalleTGrundCFrankeWWStickR 2004 Intranuclear membrane structure formations by CaaX-containing nuclear proteins. J Cell Sci 117 6095 6104
78. HattierTAndrulisEDTartakoffAM 2007 Immobility, inheritance and plasticity of shape of the yeast nucleus. BMC Cell Biol 8 47
79. SiniossoglouSSantos-RosaHRappsilberJMannMHurtE 1998 A novel complex of membrane proteins required for formation of a spherical nucleus. EMBO J 17 6449 6464
80. Santos-RosaHLeungJGrimseyNPeak-ChewSSiniossoglouS 2005 The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J 24 1931 1941
81. O'HaraLHanGSPeak-ChewSGrimseyNCarmanGM 2006 Control of phospholipid synthesis by phosphorylation of the yeast lipin Pah1p/Smp2p Mg2+-dependent phosphatidate phosphatase. J Biol Chem 281 34537 34548
82. DreierLRapoportTA 2000 In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J Cell Biol 148 883 898
83. ZimmerbergJKozlovMM 2006 How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol 7 9 19
84. Lippincott-SchwartzJPhairRD 2010 Lipids and cholesterol as regulators of traffic in the endomembrane system. Annu Rev of Biophy 39 559 578
85. LeesNDSkaggsBKirschDRBardM 1995 Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae–a review. Lipids 30 221 226
86. CostanzoMBaryshnikovaABellayJKimYSpearED 2010 The genetic landscape of a cell. Science 327 425 431
87. LeiKZhangXDingXGuoXChenM 2009 SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci U S A 106 10207 10212
88. SchulzIBaumannOSamereierMZoglmeierCGrafR 2009 Dictyostelium Sun1 is a dynamic membrane protein of both nuclear membranes and required for centrosomal association with clustered centromeres. Eur J Cell Biol 88 621 638
89. ZhouKRollsMMHallDHMaloneCJHanna-RoseW 2009 A ZYG-12-dynein interaction at the nuclear envelope defines cytoskeletal architecture in the C. elegans gonad. J Cell Biol 186 229 241
90. BurkeBRouxKJ 2009 Nuclei take a position: managing nuclear location. Dev Cell 17 587 597
91. MikiFKurabayashiATangeYOkazakiKShimanukiM 2004 Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics 270 449 461
92. SaitohSTakahashiKNabeshimaKYamashitaYNakasekoY 1996 Aberrant mitosis in fission yeast mutants defective in fatty acid synthetase and acetyl CoA carboxylase. J Cell Biol 134 949 961
93. LongtineMSMcKenzieA3rdDemariniDJShahNGWachA 1998 Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14 953 961
94. SheffMAThornKS 2004 Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 21 661 670
95. JonesGMStalkerJHumphraySWestACoxT 2008 A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat Methods 5 239 241
96. LangCGravaSFinlaysonMTrimbleRPhilippsenP 2010 Structural mutants of the spindle pole body cause distinct alteration of cytoplasmic microtubules and nuclear dynamics in multinucleated hyphae. Mol Biol Cell 21 753 766
97. WindgassenMSturmDCajigasIJGonzalezCISeedorfM 2004 Yeast shuttling SR proteins Npl3p, Gbp2p, and Hrb1p are part of the translating mRNPs, and Npl3p can function as a translational repressor. Mol Cell Biol 24 10479 10491
98. DevaiahSPRothMRBaughmanELiMTamuraP 2006 Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dalpha1 knockout mutant. Phytochemistry 67 1907 1924
99. WeltiRLiWLiMSangYBiesiadaH 2002 Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J Biol Chem 277 31994 32002
100. HanXGrossRW 2001 Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem 295 88 100
101. YoderTJMcElwainMAFrancisSEBagleyJMullerEG 2005 Analysis of a spindle pole body mutant reveals a defect in biorientation and illuminates spindle forces. Mol Biol Cell 16 141 152
102. HofmannKStoffelW 1993 TMbase-A database of membrane spanning protein segments. Biol Chem 374 166
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 11
-
Všechny články tohoto čísla
- De Novo Origins of Human Genes
- Duplication Hotspots Are Associated with Late-Replicating Regions of the Genome
- De Novo Origin of Human Protein-Coding Genes
- Cyclin D/CDK4 and Cyclin E/CDK2 Induce Distinct Cell Cycle Re-Entry Programs in Differentiated Muscle Cells
- Short Day–Mediated Cessation of Growth Requires the Downregulation of AINTEGUMENTALIKE1 Transcription Factor in Hybrid Aspen
- Physiological IRE-1-XBP-1 and PEK-1 Signaling in Larval Development and Immunity
- Role of Pirh2 in Mediating the Regulation of p53 and c-Myc
- Signatures of Environmental Genetic Adaptation Pinpoint Pathogens as the Main Selective Pressure through Human Evolution
- FOXO Regulates Organ-Specific Phenotypic Plasticity In
- Heritable Epigenetic Variation among Maize Inbreds
- Foxn1 Regulates Lineage Progression in Cortical and Medullary Thymic Epithelial Cells But Is Dispensable for Medullary Sublineage Divergence
- Attenuation of the Sensing Capabilities of PhoQ in Transition to Obligate Insect–Bacterial Association
- A Novel Protein LZTFL1 Regulates Ciliary Trafficking of the BBSome and Smoothened
- Activation of Bmp2-Smad1 Signal and Its Regulation by Coordinated Alteration of H3K27 Trimethylation in -Induced Senescence
- Histone H3K56 Acetylation, CAF1, and Rtt106 Coordinate Nucleosome Assembly and Stability of Advancing Replication Forks
- The SUN Protein Mps3 Is Required for Spindle Pole Body Insertion into the Nuclear Membrane and Nuclear Envelope Homeostasis
- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- Effect of Host Species on the Distribution of Mutational Fitness Effects for an RNA Virus
- Pch2 Acts through Xrs2 and Tel1/ATM to Modulate Interhomolog Bias and Checkpoint Function during Meiosis
- SOX9 Governs Differentiation Stage-Specific Gene Expression in Growth Plate Chondrocytes via Direct Concomitant Transactivation and Repression
- from the Aphid : A Missing Link from Facultative to Obligate Insect Endosymbiont
- Recessive Antimorphic Alleles Overcome Functionally Redundant Loci to Reveal Function in Flowers and Meristems
- Over-Expression of DSCAM and COL6A2 Cooperatively Generates Congenital Heart Defects
- Consequences of Eukaryotic Enhancer Architecture for Gene Expression Dynamics, Development, and Fitness
- Distinct Genetic Architectures for Male and Female Inflorescence Traits of Maize
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- For Male , Sperm Activation Is a “Just-in-Time” Event
- PcG Complexes Set the Stage for Epigenetic Inheritance of Gene Silencing in Early S Phase before Replication
- The Gene Contains Hotspots for L1 Endonuclease-Dependent Insertion
- Relative Burden of Large CNVs on a Range of Neurodevelopmental Phenotypes
- Multiple Means to the Same End: The Genetic Basis of Acquired Stress Resistance in Yeast
- Genome-Wide Crossover Distribution in Meiosis Reveals Sex-Specific Patterns along Chromosomes
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
- Homologs of Retinoblastoma-Associated Protein 46/48 Associate with a Histone Deacetylase to Act Redundantly in Chromatin Silencing
- Genetic Interaction Maps in Reveal Functional Crosstalk among Cell Envelope Biogenesis Pathways
- The ERI-6/7 Helicase Acts at the First Stage of an siRNA Amplification Pathway That Targets Recent Gene Duplications
- PBX1 Genomic Pioneer Function Drives ERα Signaling Underlying Progression in Breast Cancer
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- De Novo Origins of Human Genes
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



