-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPhysiological IRE-1-XBP-1 and PEK-1 Signaling in Larval Development and Immunity
Endoplasmic reticulum (ER) stress activates the Unfolded Protein Response, a compensatory signaling response that is mediated by the IRE-1, PERK/PEK-1, and ATF-6 pathways in metazoans. Genetic studies have implicated roles for UPR signaling in animal development and disease, but the function of the UPR under physiological conditions, in the absence of chemical agents administered to induce ER stress, is not well understood. Here, we show that in Caenorhabditis elegans XBP-1 deficiency results in constitutive ER stress, reflected by increased basal levels of IRE-1 and PEK-1 activity under physiological conditions. We define a dynamic, temperature-dependent requirement for XBP-1 and PEK-1 activities that increases with immune activation and at elevated physiological temperatures in C. elegans. Our data suggest that the negative feedback loops involving the activation of IRE-1-XBP-1 and PEK-1 pathways serve essential roles, not only at the extremes of ER stress, but also in the maintenance of ER homeostasis under physiological conditions.
Published in the journal: . PLoS Genet 7(11): e32767. doi:10.1371/journal.pgen.1002391
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002391Summary
Endoplasmic reticulum (ER) stress activates the Unfolded Protein Response, a compensatory signaling response that is mediated by the IRE-1, PERK/PEK-1, and ATF-6 pathways in metazoans. Genetic studies have implicated roles for UPR signaling in animal development and disease, but the function of the UPR under physiological conditions, in the absence of chemical agents administered to induce ER stress, is not well understood. Here, we show that in Caenorhabditis elegans XBP-1 deficiency results in constitutive ER stress, reflected by increased basal levels of IRE-1 and PEK-1 activity under physiological conditions. We define a dynamic, temperature-dependent requirement for XBP-1 and PEK-1 activities that increases with immune activation and at elevated physiological temperatures in C. elegans. Our data suggest that the negative feedback loops involving the activation of IRE-1-XBP-1 and PEK-1 pathways serve essential roles, not only at the extremes of ER stress, but also in the maintenance of ER homeostasis under physiological conditions.
Introduction
The accumulation of misfolded proteins in the endoplasmic reticulum (ER), also known as ER stress, activates the Unfolded Protein Response (UPR), which upregulates the synthesis of chaperones such as BiP and components of ER-associated degradation (ERAD), promotes ER expansion, and attenuates translation [1]–[3]. The UPR is conserved from yeast to humans and in metazoans is comprised of three branches, mediated by the transmembrane ER luminal sensors IRE-1, PERK/PEK-1, and ATF-6 [1]–[3]. In response to ER stress, IRE-1 oligomerizes, activating an endoribonuclease domain that splices the mRNA of xbp-1 to enable the generation of the activated form of the XBP-1 transcription factor [4]–[7]. PERK phosphorylates the translation initiation factor eIF-2α, causing global translational attenuation that diminishes the secretory load to the ER [8]. In addition, phosphorylation of eIF-2α selectively increases the translation of ATF4, a transcription factor that regulates stress responses [9]. ATF-6 undergoes proteolysis, releasing the cytosolic domain of ATF-6, which functions as a transcription factor that translocates to the nucleus and activates transcription of UPR genes [10].
Genetic studies suggest essential roles for UPR signaling in animal development. In mice, genetic studies focused on either the IRE-1-XBP-1 or the PERK pathway have shown that each functions in the development of specialized cell types, including plasma cells, pancreatic ß-cells, hepatocytes, and intestinal epithelial cells [3], [11]–[15]. In Caenorhabditis elegans, mutants deficient in any one of the three branches of the UPR are viable, but combining a deficiency in the IRE-1-XBP-1 pathway with loss-of-function mutations in either the ATF-6 or PEK-1 branch has been reported to result in larval lethality [6], [16]. These studies suggest that the UPR is required for animal development, but the specific essential role has not been defined. For example, UPR signaling may be required for a particular stage of development, or alternatively, constitutive UPR activity may be required. The experimental analysis of UPR signaling both in yeast and in mammalian cells has been greatly facilitated by the use of chemical agents that induce ER stress, such as the N-linked glycosylation inhibitor tunicamycin, the calcium pump inhibitor thapsigargin, and the reducing agent dithiothreitol (DTT). However, the activation of the UPR under physiological conditions is less well understood [17]. Constitutive IRE-1 activity has been observed in diverse types of mammalian cells, particularly with high secretory activity or in the setting of increased inflammatory signaling [11], [14], [15], [18]. These studies suggest critical roles for IRE-1-XBP-1 signaling in physiology and development, some of which have been proposed to be independent of its role in maintaining protein folding homeostasis in the ER [11], [19], [20].
Recently, we showed that XBP-1 is required for C. elegans larval development on pathogenic Pseudomonas aeruginosa, conferring protection to the C. elegans host against the ER stress caused by its own secretory innate immune response to infection [21]. Our study established that the innate immune response to microbial pathogens represents a physiologically relevant source of ER stress that necessitates XBP-1 function.
We sought to better understand the consequences of UPR deficiency under physiological conditions during C. elegans larval development. We describe our studies which suggest that even in the absence of ER stress induced by exogenously administered chemical agents, the IRE-1-XBP-1 pathway, in concert with the PEK-1 pathway, functions in a homeostatic loop that is under constitutive activation during C. elegans larval development. Our data implicate an essential role for the UPR in ER homeostasis, not only in the response to toxin-induced ER stress, but also under basal physiological conditions.
Results
Increased Constitutive IRE-1 Activity in C. elegans xbp-1 Mutants
The detection of IRE-1 activity provides a sensitive and responsive measure of ER stress. Most methods used to measure IRE-1 activity require functional IRE-1-XBP-1 output, relying on either detection of the activated spliced form of the xbp-1 mRNA or the transcriptional activity of the resulting XBP-1 protein. In order to follow IRE-1 activity in the absence of a functional XBP-1 protein, we utilized the C. elegans xbp-1(zc12) mutant, which has a C→T mutation that results in an early premature stop codon [4]. We reasoned that we could detect IRE-1-mediated splicing of xbp-1(zc12) mRNA by quantitative RT-PCR (qPCR), as we have done previously for wild type xbp-1 mRNA, as a measure of IRE-1 activity [21].
We anticipated, however, that the xbp-1(zc12) mRNA might be degraded by nonsense-mediated decay (NMD) [22], which would reduce the abundance of xbp-1(zc12) mRNA (Figure 1A). Thus, we constructed a strain carrying xbp-1(zc12) and a null allele of smg-2, the C. elegans homolog of the NMD component Upf1 [23]. Indeed, we observed that the level of xbp-1 mRNA in the xbp-1(zc12) mutant was markedly diminished compared with the level of xbp-1(zc12) mRNA in the smg-2(qd101); xbp-1(zc12) mutant (Figure 1B). These data confirmed that xbp-1(zc12) mRNA is a substrate for the NMD pathway, but that inhibition of NMD permits detection of xbp-1(zc12) mRNA. As expected from the predicted truncated protein product made from translation of the xbp-1(zc12) mRNA (Figure 1A), loss of NMD had no effect on the null phenotype of the xbp-1(zc12) allele, as assessed by the effect of the smg-2(qd101) mutation on expression of an xbp-1-regulated gene, the C. elegans BiP homolog hsp-4 (Figure 1B). We next examined the level of WT xbp-1 mRNA in the smg-2(qd101) mutant, and we observed that NMD inhibition increased the level of xbp-1 mRNA 2-fold relative to WT C. elegans (Figure 1C), which suggests that the NMD complex may function to decrease the level of WT xbp-1 mRNA. This observation is consistent with a prior report suggesting that stress-induced genes may be NMD targets [24], although we hypothesize that the relatively early termination codon present in the xbp-1 mRNA prior to IRE-1-mediated splicing may also contribute to recognition and degradation by the NMD pathway. Consistent with this explanation, after exposing both the WT and smg-2(qd101) strains to tunicamycin for 4 h, the level of IRE-1-spliced xbp-1 mRNA was similar between the two strains (Figure 1C). Furthermore, the loss of NMD did not increase the lethality of either the WT strain or xbp-1(zc12) mutant when grown in the presence of tunicamycin (Figure 1D).
Fig. 1. XBP-1 deficiency results in a dramatic increase in IRE-1 activity. 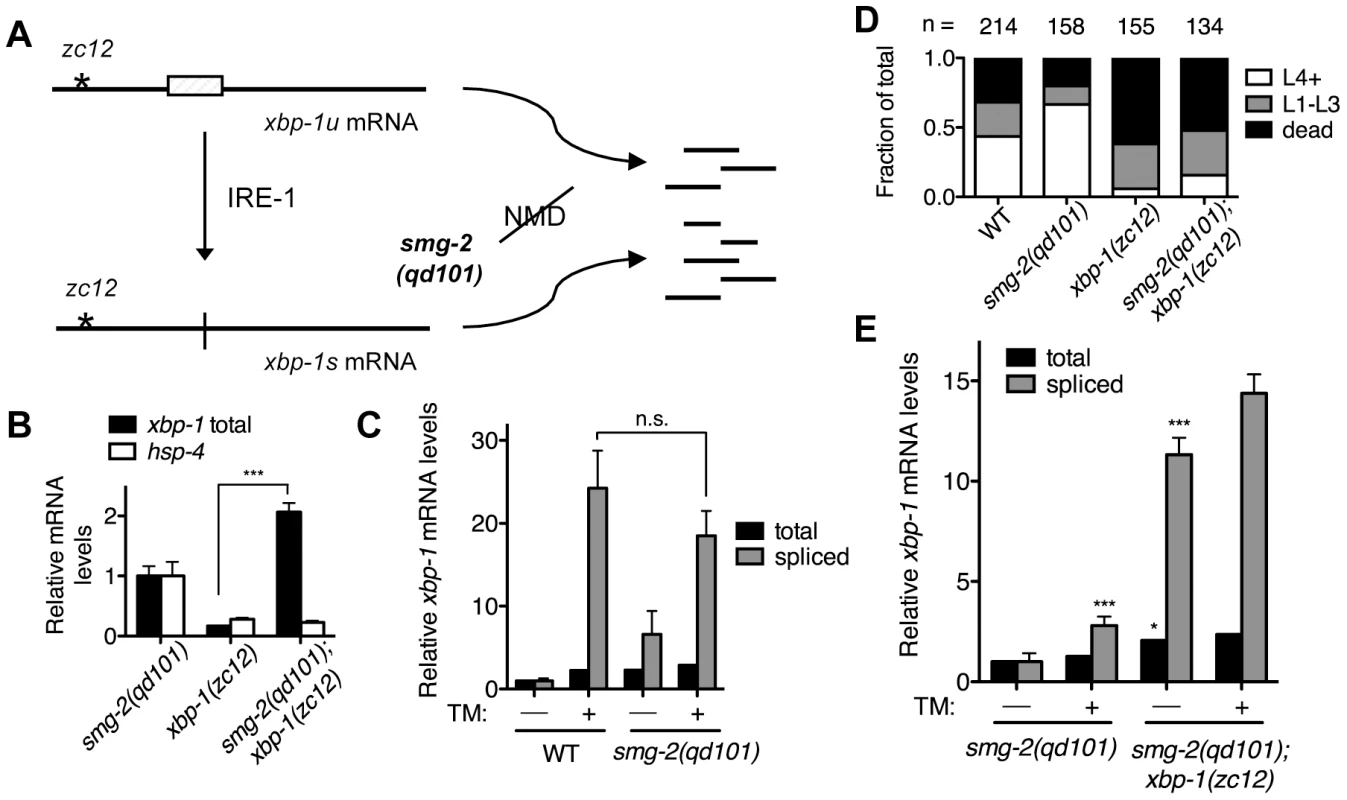
(A) Detection of xbp-1 mRNA splicing by IRE-1 in the C. elegans xbp-1(zc12) mutant. Schematic of the unspliced and spliced xbp-1 mRNA, noting the position of the premature termination codon present in the xbp-1(zc12) allele. The smg-2(qd101) mutation results in inactivation of the NMD pathway, stabilizing the xbp-1(zc12) mRNA for detection. (B) Quantitative real-time PCR measurements of mRNA levels in the xbp-1(zc12) and smg-2(qd101);xbp-1(zc12) mutants relative to the smg-2(qd101) mutant synchronized in the L3 stage. (C) Quantitative real-time PCR measurements of levels of total and spliced xbp-1 mRNA in the smg-2(qd101) strain relative to WT grown to the L3 stage and then shifted to plates with or without tunicamycin (5 µg/mL) for 4 h. (D) Larval development and survival assay showing the proportion of animals of each of the indicated strains that reach the indicated stage after 4 d of development from eggs laid on plates containing tunicamycin (2.5 µg/mL) at 16°C. (E) Quantitative real-time PCR measurements of levels of total and spliced xbp-1 mRNA in the smg-2(qd101); xbp-1(zc12) strain relative to the smg-2(qd101) strain treated as in C. (In B, C, and E, values represent fold change ± s.e.m., n = 3 independent experiments, *P<0.05, ***P<0.001, two-way ANOVA with Bonferroni post test). Comparing levels of IRE-1 activity between smg-2(qd101) and smg-2(qd101); xbp-1(zc12) animals, we observed a dramatic elevation in the level of spliced xbp-1 mRNA in the smg-2(qd101); xbp-1(zc12) strain (Figure 1E). To provide a measure for comparison, the basal elevation of spliced xbp-1 mRNA in the smg-2(qd101); xbp-1(zc12) mutant far exceeded the level of spliced xbp-1 mRNA in the smg-2(qd101) mutant even after administration of tunicamycin. Treating the smg-2(qd101); xbp-1(zc12) strain with tunicamycin resulted in only a minor additional increase in spliced xbp-1 mRNA compared with the magnitude of elevation in spliced xbp-1 in that strain under basal conditions (Figure 1E). These data show that XBP-1 deficiency results in a dramatic increase in IRE-1 activity, even in the absence of exogenously administered agents such as tunicamycin.
Increased Constitutive PEK-1 Activation in C. elegans xbp-1 Mutants
If the elevated level of IRE-1 activity observed in the smg-2(qd101); xbp-1(zc12) mutant were indicative of increased ER stress due to loss of xbp-1, we might anticipate compensatory activation of the PEK-1 and/or ATF-6 pathways in the absence of XBP-1. We therefore sought to determine levels of PEK-1 activity in an xbp-1 mutant through the detection of eIF-2α phosphorylation. In particular, these measurements would provide an additional measure of ER stress in the xbp-1 mutant that is not dependent on the inactivation of the NMD pathway and its aforementioned experimental caveats. Antibodies raised against mammalian eIF-2α and specifically phosphorylated eIF-2α (P-eIF-2α) cross-react with the highly homologous C. elegans protein [25], [26]. We detected a single band in immunoblots using these antibodies with lysates from WT C. elegans (Figure 2A). We observed that eIF-2α phosphorylation was induced by a 4 h exposure to a high dose of tunicamycin in a PEK-1-dependent manner (Figure 2A and Figure S1A). Of note, eIF-2α phosphorylation appears to be a less sensitive measure of ER stress than IRE-1-mediated xbp-1 mRNA splicing, as we did not observe a significant increase in eIF-2α phosphorylation in response to standard doses of tunicamycin sufficient to induce xbp-1 mRNA splicing.
Fig. 2. XBP-1 deficiency increases PEK-1 dependent phosphorylation of eIF2α. 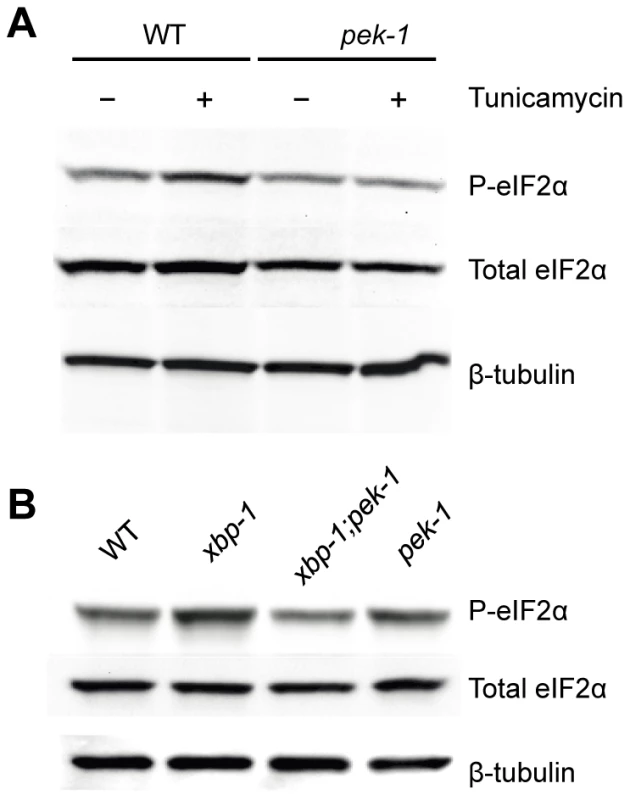
(A) PEK-1 dependent phosphorylation of eIF2α is induced by high dose (50 ug/ml) tunicamycin. Western blot of P-eIF2α, total eIF2α and tubulin in WT and pek-1(ok275) strains grown to the L4 stage and then shifted to plates with or without tunicamycin (50 µg/mL) for 4 hours. (B) PEK-1 dependent phosphorylation of eIF2α is induced by XBP-1 deficiency. Western blot of P-eIF2α, total eIF2α and tubulin in WT, xbp-1(tm2482), xbp-1(tm2482); pek-1(ok275) and pek-1(ok275) strains grown to the L4 stage. We next determined PEK-1 activity under basal physiological conditions, specifically in the xbp-1 mutant. We saw induction of PEK-1-mediated eIF-2α phosphorylation relative to WT in the absence of exogenously administered agents to induce ER stress at 16°C (Figure 2B and Figure S1B). The magnitude of the effect of XBP-1 deficiency on PEK-1 activity was comparable to the induction of PEK-1 in WT by treatment with high-dose tunicamycin. We observe no increase in eIF-2α phosphorylation in the xbp-1; pek-1 mutant relative to the pek-1 mutant, confirming that the increase in eIF-2α phosphorylation in the xbp-1 mutant relative to WT is due to activation of PEK-1. In fact, we noticed a slight decrease in eIF-2α phosphorylation in the xbp-1; pek-1 mutant relative to the pek-1 mutant, but the mechanisms underlying this difference are unclear. These data corroborate our observations of increased xbp-1 mRNA splicing in the xbp-1 mutant. Taken together, the increase in levels of IRE-1 and PEK-1 activity in the xbp-1 mutant suggests that XBP-1 deficiency is accompanied by a marked increase in constitutive ER stress under basal physiological conditions.
The Effects of Immune Activation on ER Stress Levels in the xbp-1 Mutant
Previously, we reported that the activation of innate immunity by infection with pathogenic P. aeruginosa induces ER stress, and that XBP-1 serves an essential role in protecting the host against the detrimental effects of immune activation [21]. Our prior ultrastructural analysis of the ER in xbp-1 mutants suggested that disruption of ER homeostasis contributes to this phenotype. One explanation for these observations is that ER homeostasis in the xbp-1 mutant might be minimally perturbed under basal physiological conditions but have a pronounced sensitivity to ER stress from endogenous (e.g. immune activation) or exogenous (e.g. tunicamycin) sources. However, the data in Figure 1 and Figure 2 suggest that even during physiological growth and development, XBP-1 deficiency results in a marked elevation in levels of basal ER stress. We hypothesized, therefore, that under these circumstances, the activation of innate immunity might further increase ER stress levels.
The smg-2(qd101); xbp-1(zc12) strain provided the opportunity to assess levels of ER stress caused by immune activation in the setting of XBP-1 deficiency. Whereas a 4 h exposure of the WT strain to P. aeruginosa PA14 causes a two-fold increase in spliced xbp-1 mRNA relative to exposure to the relatively non-pathogenic bacterial food Escherichia coli OP50 (Figure 3A and [21]), we observe a blunted response to P. aeruginosa infection in the smg-2(qd101) mutant (Figure 3A). This observation is likely due to the 5-fold elevation in spliced xbp-1 mRNA levels in the smg-2(qd101) mutant (Figure 1C), which may buffer the ER from the stress caused by pathogen-induced immune activation. Nevertheless, we observed that the level of spliced xbp-1 mRNA in the smg-2(qd101); xbp-1(zc12) mutant was increased by a 4 h exposure to P. aeruginosa relative to the smg-2(qd101); xbp-1(zc12) mutant treated in parallel with E. coli (Figure 3A). Specifically, under these treatment conditions, the level of spliced xbp-1 mRNA in the smg-2(qd101); xbp-1(zc12) mutant was 20-fold greater than that of the smg-2(qd101) mutant in the absence of additional stress, whereas exposure to P. aeruginosa increased the level of spliced xbp-1 mRNA to over 25-fold that of the smg-2(qd101) mutant (Figure 3A). The total amount of xbp-1 mRNA was unchanged between smg-2(qd101) and smg-2(qd101); xbp-1(zc12) strains, indicating that the increase in spliced xbp-1 mRNA is due to increased IRE-1 activation.
Fig. 3. Pathogen-induced immune activation exacerbates ER stress levels in XBP-1 deficiency in C. elegans. 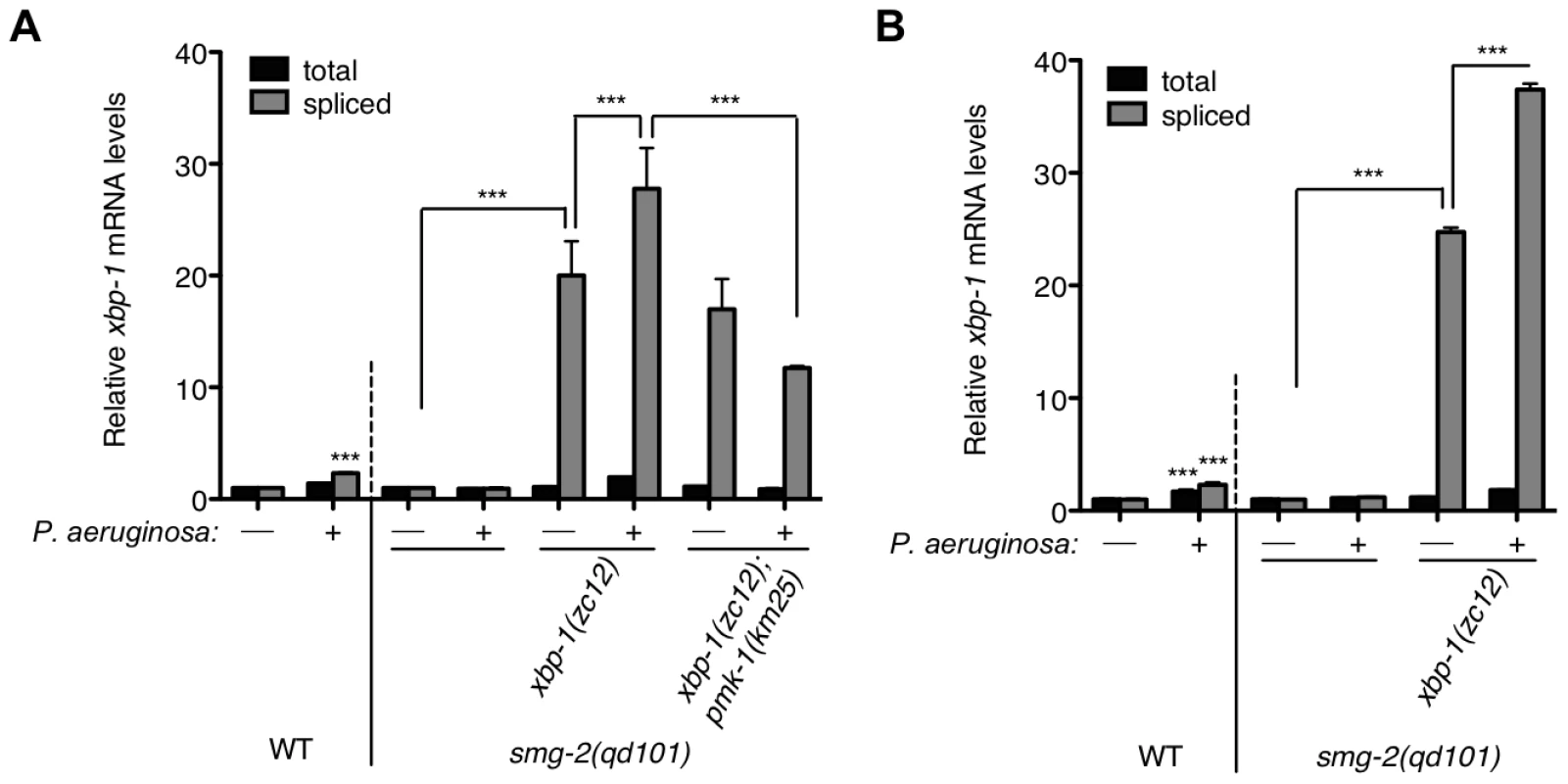
Quantitative real-time PCR measurements of levels of total and spliced xbp-1 mRNA in the indicated strains grown from synchronized L1s for 23 h at 20°C and then shifted to plates with or without P. aeruginosa PA14 at 25°C for (A) 4 h or (B) 11 h. Values represent fold change ± s.e.m. (n = 2 independent experiments, ***P<0.001, two-way ANOVA with Bonferroni post test). The WT strain exposed to either treatment was normalized to WT without P. aeruginosa; all other strains were normalized to smg-2(qd101) without P. aeruginosa. We observed persistent elevation of spliced xbp-1 mRNA after an 11 h exposure to P. aeruginosa, above the elevated basal levels of spliced xbp-1 mRNA in the xbp-1 mutant, suggesting that IRE-1 activity is not attenuated under conditions of physiological ER stress (Figure 3B).
Previously, we established that the ER stress induced by exposure to P. aeruginosa, as well as the lethality of the xbp-1 mutant during infection by P. aeruginosa, are suppressed by a loss-of-function mutation in pmk-1, which encodes a conserved p38 mitogen-activated protein kinase (MAPK) that regulates innate immunity in C. elegans [27]. Our interpretation of these data was that loss of PMK-1 activity diminished the secretory load on the ER by attenuating the innate immune response. In support of this interpretation, we found that the pathogen-induced increase in spliced xbp-1 mRNA in smg-2(qd101); xbp-1(zc12) was suppressed in the smg-2(qd101); xbp-1(zc12); pmk-1(km25) mutant, although the basal levels on E. coli OP50 nevertheless remained markedly elevated (Figure 3A). These data provide quantitative support for a model in which the activation of PMK-1-mediated innate immunity is a physiologically relevant source of ER stress, which in XBP-1-deficient animals exacerbates an already elevated level of ER stress to cause larval lethality.
A Temperature-Dependent Requirement for XBP-1 and PEK-1 Activities in C. elegans Development and Survival
What are the functional consequences of the elevated ER stress present in the xbp-1 mutant under standard growth conditions, in the absence of infection? The xbp-1 mutant, while viable, exhibits increased sensitivity to exogenously administered ER stress as well as physiological ER stress from immune activation [21], [28]. Inactivation of both xbp-1 and pek-1 was previously reported to result in larval arrest when propagated at 20°C [16]. Our observations of constitutive ER stress in the xbp-1 mutant and increased PEK-1 activity suggest a compensatory functional role for pek-1, and thus we sought to further characterize the larval arrest phenotype of the xbp-1; pek-1 mutant.
Surprisingly, we observed that the xbp-1(tm2482); pek-1(ok275) double mutant exhibited temperature-dependent viability over the physiological temperature range of C. elegans (Figure 4A). The larval development of the xbp-1(tm2482); pek-1(ok275) mutant was similar to that of WT at 16°C. At 20°C, however, approximately half of xbp-1(tm2482); pek-1(ok275) eggs developed to become gravid adults, while the remainder arrested during larval development in the L2 and L3 stages. These arrested larvae died over the course of several days with intestinal degeneration as previously described (Shen et al., 2005). At temperatures greater than 23°C, larval lethality was 100%. At 25°C, 100% of the population died in the L1 and L2 stages after just 2 days. The physiological temperature range for propagation of C. elegans in the laboratory is generally 15°C to 25°C, with optimal reproduction at 20°C. Thus, the observed temperature dependence is observed not at “heat shock” temperatures, but rather, well within the range of physiological temperatures for C. elegans.
Fig. 4. Temperature-sensitive lethality of the xbp-1;pek-1 double mutant. 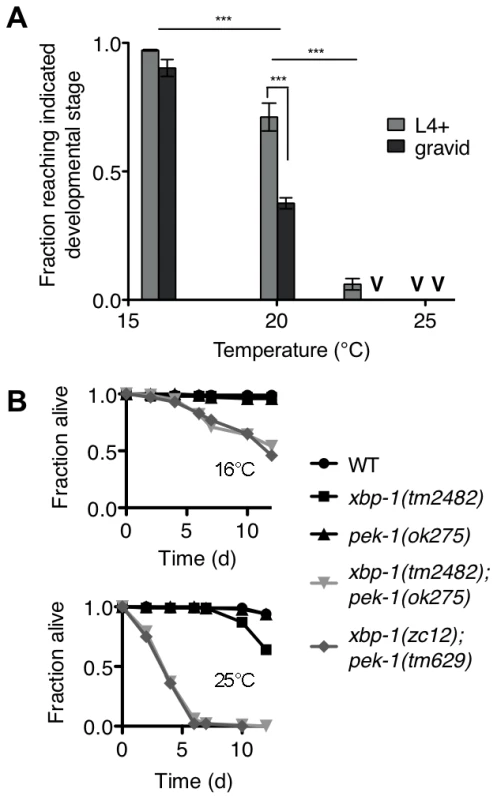
(A) Development of the xbp-1(tm2482); pek-1(ok275) mutant across physiological temperatures. Values represent average fraction of eggs developed to the indicated stage ± s.e.m. (n = 3 independent experiments at 20°C, 2 independent experiments at all other temperatures; ***P<0.001, two-way ANOVA with Bonferroni post test). (B) Lifespan of indicated strains grown at 16°C to the L4 stage, then shifted to either 16°C to 25°C. Results are representative of two independent experiments. The temperature dependence of xbp-1(tm2482); pek-1(ok275) lethality permitted the investigation of whether the larval lethality of the xbp-1; pek-1 mutant is due to a requirement for XBP-1 and PEK-1 at a specific stage of development, or whether the activities of XBP-1 and PEK-1 are required constitutively for viability at other life stages. Specifically, we propagated two xbp-1; pek-1 mutants comprised of different mutant alleles at 16°C until the animals reached the L4 larval stage, then either maintained the mutants at 16°C or shifted them to 25°C to monitor survival. When shifted to 25°C, the xbp-1; pek-1 double mutants exhibited a sharp decrease in survival as compared with the strains maintained at 16°C (Figure 4B). These observations suggest that the activity of either XBP-1 or PEK-1 is not specifically required at a particular developmental stage; instead, the constitutive activities of XBP-1 and PEK-1 are required for survival at physiological temperatures.
XBP-1 and PEK-1 Maintain Intestinal Cell Homeostasis during ER Stress Caused by Basal and Induced Innate Immunity
Although we previously observed that pek-1 and atf-6 single mutants did not exhibit larval lethality in the presence of pathogenic bacteria [21], our data presented in this paper suggest that PEK-1 functions in parallel to XBP-1 under physiological conditions in C. elegans to maintain ER homeostasis. Because the xbp-1; pek-1 mutant is viable through larval development at 16°C, we were able to ask whether PEK-1 contributes to protection against immune activation in the absence of XBP-1. Populations of synchronized eggs were grown at 16°C with P. aeruginosa as the only food source and development was monitored over time. P. aeruginosa has been shown to exhibit markedly diminished pathogenicity to C. elegans adults at 16°C relative to 25°C [29], and we found this to also be the case during larval development. Specifically, the pmk-1 mutant was able to complete larval development on P. aeruginosa at 16°C (Figure 5A), whereas only half of the pmk-1 eggs grown on P. aeruginosa develop to the L4 stage at 25°C [21], indicating that immune activation is less important for development in the presence of P. aeruginosa grown at 16°C than it is at 25°C. Likewise, the larval development of the xbp-1 mutant, which is severely compromised on P. aeruginosa at 25°C [21], was equivalent to that of WT at 16°C (Figure 5A). Both the diminished pathogenicity of P. aeruginosa at 16°C and the aforementioned temperature-sensitive requirement for UPR function may contribute the survival of the xbp-1 mutant at 16°C. Nevertheless, even under these conditions, the xbp-1(tm2482); pek-1(ok275) mutant exhibited complete larval lethality on P. aeruginosa at 16°C, reminiscent of the larval lethality of xbp-1 on P. aeruginosa grown at 25°C. Eliminating PMK-1-mediated immunity completely rescued this larval lethality (Figure 5A), demonstrating that PEK-1 functions with XBP-1 to protect against PMK-1-mediated immune activation during larval development.
Fig. 5. XBP-1 and PEK-1 each protect against elevated physiological temperature and immune activity. 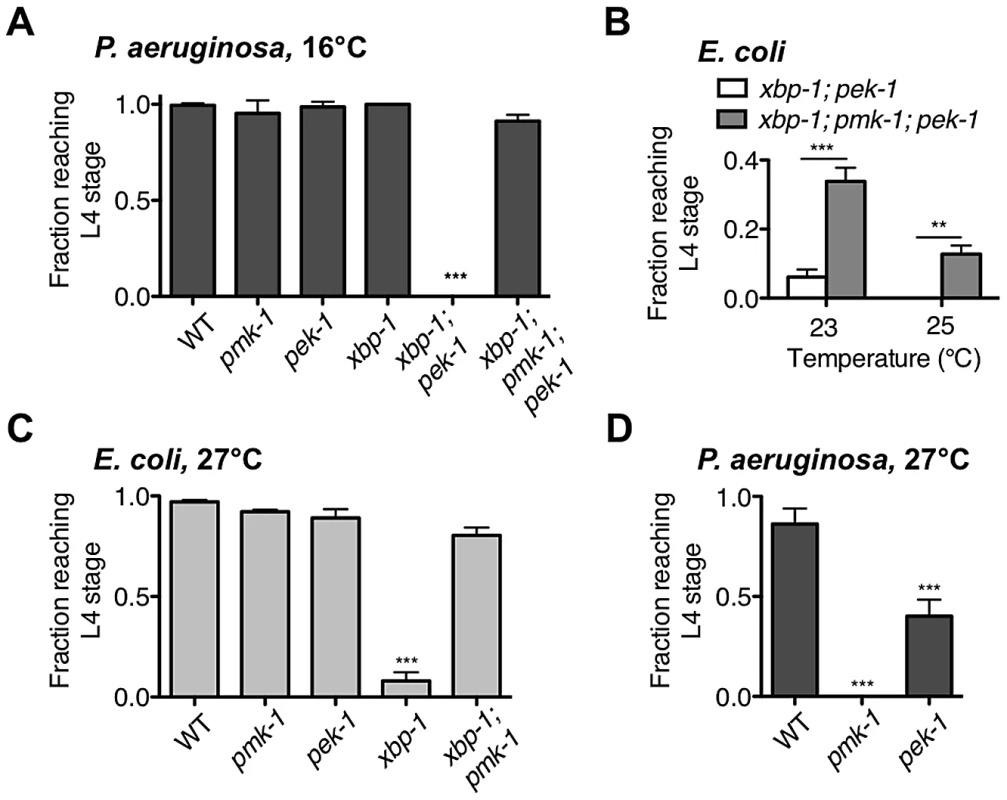
(A) Development of indicated mutants from eggs to the L4 larval stage or older after 4 d at 16°C on P. aeruginosa PA14. Values represent mean ± s.d. from 1 of 2 representative experiments (n = 4 plates with 20–50 eggs each, ***P<0.001, one-way ANOVA with Bonferroni post test). (B) Development of xbp-1(tm2482); pek-1(ok275) and xbp-1(tm2482); pmk-1(km25); pek-1(ok275) mutants from eggs on E. coli OP50. Values represent average fraction of eggs developed to the indicated stage ± s.e.m. (n = 2 independent experiments, **P<0.01, ***P<0.001, two-way ANOVA with Bonferroni post test). (C) Development of indicated mutants from eggs to the L4 larval stage or older after 2 d at 27°C on E. coli OP50. Values represent mean ± s.e.m. (n = 3 independent experiments, ***P<0.001, one-way ANOVA with Bonferroni post test). (D) Development of indicated mutants from eggs to the L4 larval stage or older after 2 d at 27°C on P. aeruginosa PA14. Values represent mean ± s.d. from 1 of 2 representative experiments (n = 3–4 plates with 20–60 eggs each, ***P<0.001, one-way ANOVA with Bonferroni post test). We next asked whether the UPR is required for survival in the presence of pathogen during adulthood. In parallel with our observation that the xbp-1; pek-1 mutant exhibits temperature-sensitive lethality both during larval development and when shifted to a higher temperature from the L4 larval stage, we found that the xbp-1; pek-1 mutant exhibits enhanced lethality relative to the WT strain or either of the single mutants when shifted at the L4 stage to P. aeruginosa at 16°C (Figure S2). These data suggest that the UPR is required for survival during immune activation both in larval development and in adulthood.
Larval arrest of xbp-1; pek-1 mutants has been reported to be accompanied by evidence of intestinal degeneration, including the appearance of vacuoles and light-reflective aggregates in intestinal cells, degradation of intestinal tissues, and distention of the intestinal lumen [16]. We observe similar morphology not only in xbp-1; pek-1 larvae at 23°C on E. coli OP50, but also at 16°C on P. aeruginosa. The similar appearance between xbp-1; pek-1 larvae dying either at 16°C on pathogenic bacteria or at 23°C on E. coli OP50 led us to consider whether ER stress arising from intestinal innate immune activation might contribute in a similar manner to both conditions. We have previously characterized PMK-1-mediated innate immunity and observed both basal and induced components to immunity regulated by PMK-1 [30]. We therefore hypothesized that basal immune activity under standard, non-pathogenic growth conditions could present a low level of ER stress that is severely exacerbated in the absence of intact physiological UPR function, leading to larval lethality of the xbp-1; pek-1 mutants. Consistent with this hypothesis, we observed that pmk-1 loss-of-function was able to partially suppress the larval lethality of the xbp-1; pek-1 double mutant at 23°C and 25°C (Figure 5B).
One explanation for the temperature-sensitive lethality of the xbp-1; pek-1 mutant is that increased temperature leads to increased PMK-1 pathway activation, perhaps as the “non-pathogenic” E. coli OP50 becomes slightly pathogenic. However, the temperature-sensitive lethality is not abrogated by loss of PMK-1; instead, the xbp-1; pmk-1; pek-1 mutant exhibits larval lethality at a temperature several degrees higher than the xbp-1; pek-1 mutant (Figure 5B). Furthermore, the temperature-sensitive larval lethality of the xbp-1; pek-1 mutant on E. coli OP50 was not suppressed by the presence of the bacteriostatic drug ampicillin (Figure S3A). These data indicate that basal immune activation and temperature are distinct contributors to ER stress that function in parallel during growth on E. coli OP50.
Thermal Stress Necessitates UPR Signaling for Survival in C. elegans
The temperature-dependent larval lethality of the xbp-1; pek-1 mutant over a physiological temperature range suggested that UPR signaling might be required for survival in response to thermal stress. Indeed, we observed that the xbp-1 mutant exhibited larval lethality when grown at 27°C, an elevated temperature at which WT N2 C. elegans exhibits a reduced brood size and increased dauer formation (Figure 5C). Similar to our observation that depletion of basal immunity rescued the development of the xbp-1; pek-1 mutant when propagated on E. coli OP50, the temperature-sensitive lethality in the xbp-1 mutant was suppressed in the xbp-1; pmk-1 double mutant (Figure 5C), but not by the presence of ampicillin (Figure S3B).
Unlike the xbp-1 mutant, the development of the pek-1 mutant at 27°C was similar to WT. This is reminiscent of our previous observation that the pek-1 mutant did not exhibit the larval lethality found in xbp-1 when grown on P. aeruginosa at 25°C [21]. However, we next grew the pek-1 mutant on P. aeruginosa at 27°C, reasoning that the elevated temperature would not only increase the ER stress caused by basal growth, but also enhance the pathogenicity of the P. aeruginosa and thereby increase the immune response relative to that at 25°C. Indeed, the pmk-1 mutant exhibited 100% larval lethality on P. aeruginosa at 27°C (Figure 5D), as compared with the 50% lethality we have previously reported for the pmk-1 mutant on P. aeruginosa at 25°C (Richardson et al., 2010). The increased susceptibility of this immune-deficient mutant to P. aeruginosa at 27°C relative to 25°C indicates that the increased temperature causes an increase in P. aeruginosa pathogenicity. On P. aeruginosa at 27°C, the pek-1 mutant exhibited larval lethality relative to the WT strain grown 27°C (Figure 5D). These data further suggest that PEK-1 functions in parallel with XBP-1 to protect C. elegans against the ER stress caused by immune activation.
The PMK-1 Pathway Protects Against Exogenous ER Stress
We showed in Figure 5B and 5C that loss of PMK-1 improves larval development of the xbp-1; pek-1 mutant and the xbp-1 mutant, respectively, in the absence of infection. We suggested that the mechanism behind this phenomenon is that the previously described basal immune activity through the PMK-1 pathway [31] contributes to ER stress. However, we also considered the possibility that the PMK-1 pathway might play an immunity-independent role in exacerbating ER stress in the setting of UPR deficiency. To test this possibility, we examined the ability of WT and UPR mutants to develop in the presence of tunicamycin with or without functional pmk-1. We found that the pmk-1 mutant actually exhibited increased sensitivity to tunicamycin during development. In fact, the pmk-1 mutant exhibited greater lethality at a lower dose of tunicamycin than either the xbp-1 or pek-1 single mutants (Figure 6). These data suggest that the PMK-1 pathway influences ER stress in two ways. First, during infection or under standard growth conditions in the setting of UPR depletion, activation of the PMK-1 pathway generates an increased secretory load that contributes to ER stress. However, when ER stress is induced exogenously with tunicamycin, the PMK-1 pathway activity serves a protective function.
Fig. 6. PMK-1 protects against exogenously induced ER stress. 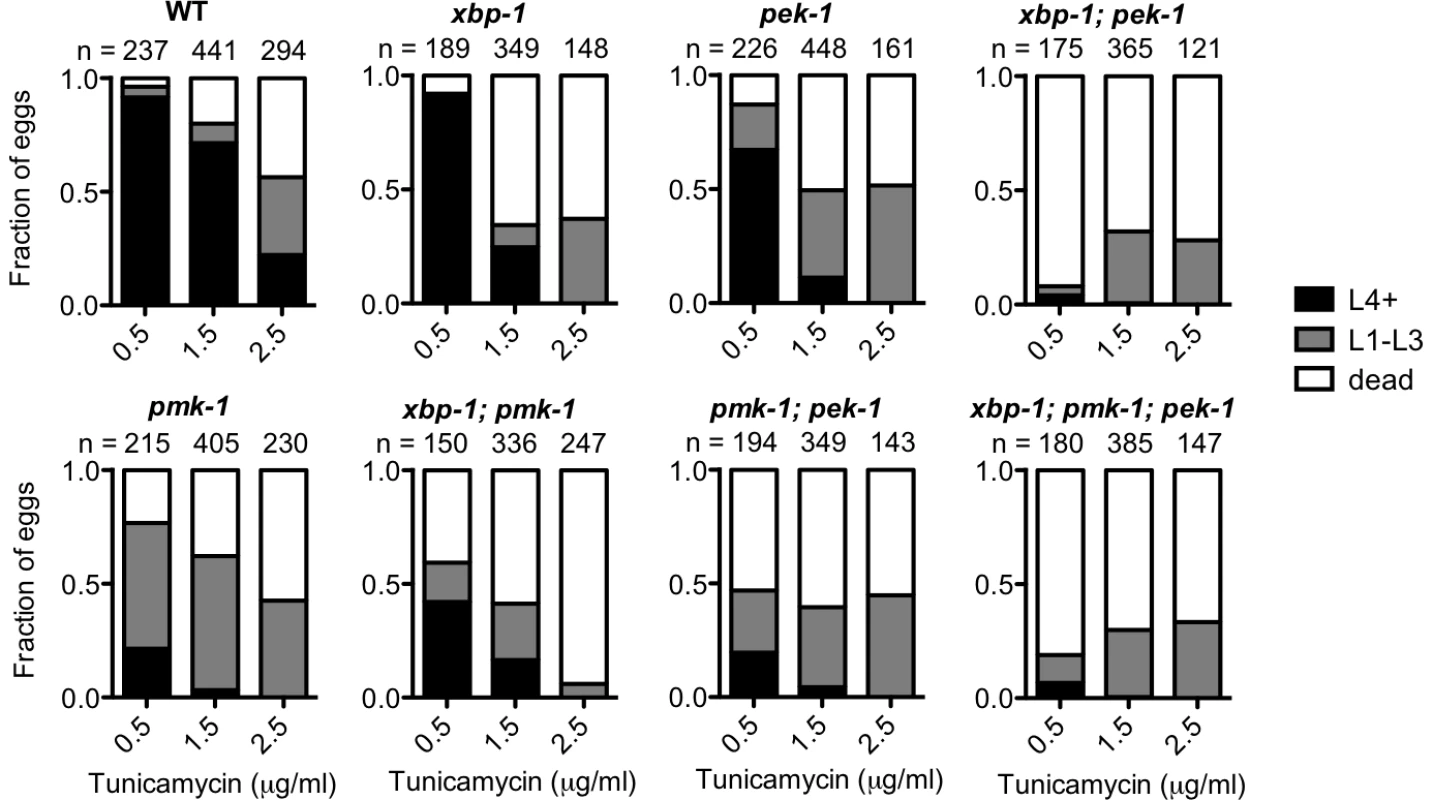
Larval development and survival assay showing the proportion of animals of each of the indicated strains that reach the indicated stage after 4 d of development from eggs laid on plates containing tunicamycin at 16°C. Values are from either one experiment (0.5 µg/ml and 2.5 µg/ml tunicamycin) or combined from two experiments with similar results (1.5 µg/ml tunicamycin). Discussion
We have shown that the IRE-1-XBP-1 and PEK-1 pathways function together to maintain ER homeostasis in C. elegans under physiological conditions. We found that XBP-1 deficiency results in marked activation of both IRE-1 and PEK-1, reflecting constitutive ER stress. Activation of innate immunity mediated by PMK-1 p38 MAPK further exacerbated the constitutive ER stress in the xbp-1 mutant. To investigate the physiological roles of UPR signaling as well as the compensatory activity between distinct UPR pathways, we examined both the individual and the combined effects of XBP-1 and PEK-1 deficiency in vivo. We found that the xbp-1; pek-1 double mutant exhibited temperature-sensitive lethality that was independent of developmental stage. Compared with the xbp-1; pek-1 mutant, the xbp-1; pmk-1; pek-1 mutant had moderately increased survival during larval development on non-pathogenic bacteria, when there is a low level of PMK-1-mediated basal immune activity, and dramatically increased survival on pathogenic P. aeruginosa, when the PMK-1-mediated immune response is induced. We further showed that both XBP-1 and PEK-1 are required for full protection against the combined stress of immune activation and that of growth at elevated physiological temperatures, confirming that these two branches of the UPR function together to protect against physiological ER stress.
Our observation of dramatically elevated levels of IRE-1 and PEK-1 activity in the setting of XBP-1 deficiency, under standard growth conditions in the absence of exogenous agents to induce ER stress, provides strong evidence for homeostatic activity of the IRE-1-XBP-1 signaling pathway under physiological conditions (Figure 7A), and not merely at the extremes of ER stress induced by pharmacological treatment or in specialized secretory cell types. Our data also reveal a dynamic requirement for UPR signaling in survival that increases with both temperature and increased secretory activity as is induced by immune activation (Figure 7B). Interestingly, the temperature-dependent role for the IRE-1 and PEK-1 pathways is manifest at physiological temperatures optimal for C. elegans development and fecundity, far from commonly utilized “heat shock” conditions (Figure 7A). We speculate that this temperature dependence may be due to altered secretory load at higher temperature or increased tendency for proteins to aggregate in the ER in the absence of intact chaperone production.
Fig. 7. Maintenance of ER homeostasis through activation of the IRE-1 and PEK-1 pathways under basal physiological conditions during development. 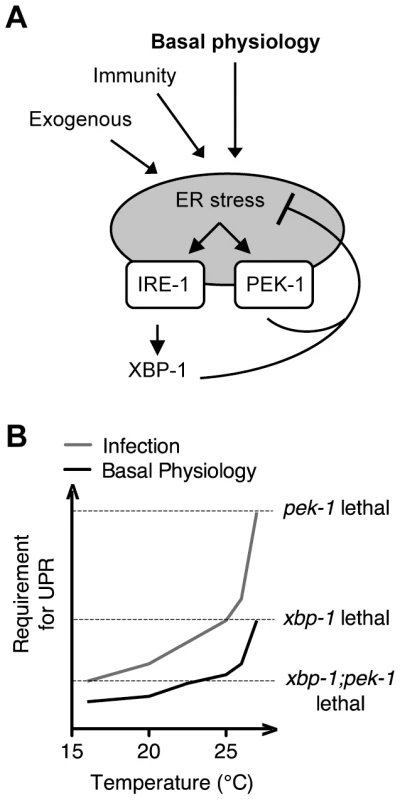
(A) The increase in both IRE-1 and PEK-1 activities in XBP-1 deficiency in the absence of exogenous compounds to impose ER stress, combined with the temperature-sensitive lethality of the UPR mutants, implies that UPR signaling maintains ER homeostasis not only in response to the extremes of ER stress, but also under basal physiological conditions. (B) Infection, basal growth and development, and elevated physiological temperature all contribute to ER stress, leading to lethality of UPR mutants as indicated by dashed lines. Importantly, our data suggest that PMK-1-mediated immune activation is one of many sources of the requirement for the UPR during larval development in the absence of infection. We found that, although loss of basal PMK-1 pathway activation partially suppressed the temperature-sensitive larval lethality of the xbp-1; pek-1 mutant, the xbp-1; pmk-1; pek-1 mutant nevertheless exhibited almost complete larval lethality at 25°C. Further, using our smg-2(qd101); xbp-1(zc12) strains, we observed high constitutive IRE-1-mediated xbp-1 splicing in the xbp-1; pmk-1 mutant that was similar under these experimental conditions to that of the xbp-1 mutant (Figure 3A). These results indicate that the UPR has an essential role during development in protection against immune activation as well as additional processes. Identification of these processes will likely lead to increased understanding of conserved physiological roles of the UPR.
We found that the PMK-1 pathway not only contributes to basal ER stress but also protects against exogenous ER stress induced by exposure to tunicamycin (Figure 6). We speculate that the mechanism underlying this dual function of the PMK-1 pathway may be differences in the PMK-1-activated transcriptional output under different circumstances. The importance of the PMK-1 pathway in protection against exogenous ER stress makes the role of the PMK-1 pathway in contributing to endogenous ER stress even more striking.
In mice, Xbp1 deficiency in intestinal epithelial cells (IEC) resulted in marked intestinal inflammation that may contribute to the observed activation of not only IRE1 but also of PERK, as measured by expression of one of its downstream effectors, CHOP [12]. In mammals, the transcription factor CHOP promotes apoptosis of mammalian cells that experience prolonged ER stress [32], and indeed, the majority of Paneth cells underwent apoptosis in the Xbp1−/− IECs. Our observations are consistent with the idea that Xbp1−/− IECs may be predisposed to detrimental consequences of additional ER stress caused by intestinal inflammation because of deregulation of basal ER homeostasis due to XBP-1 deficiency. In pancreatic ß-cells, another cell type that is specialized for high-level secretory activity, XBP1 deficiency has been observed to result in IRE1α hyperactivation, with increased degradation of mRNAs that encode insulin processing enzymes [33].
Our observations that PEK-1, in concert with XBP-1, functions to protect against ER stress from immune activation differ from observations in mouse macrophages, in which TLR stimulation was shown to activate IRE1, but PERK activation was reported to be suppressed rather than elevated [34], [20]. This difference may be due to roles for XBP-1 in macrophages that extend beyond its function in maintaining ER homeostasis. Indeed, when stimulated by TLRs in macrophages, the IRE1-XBP1 pathway was shown to induce expression of immune effectors rather than typical UPR genes, suggestive that the IRE1-XBP1 pathway may have been co-opted in macrophages to promote macrophage-specific function independent of the UPR [20].
Our data support the idea that UPR signaling does not function simply in response to the extremes of ER stress, as when induced by tunicamycin or by the elevated secretory load of specialized cells such as plasma cells, but instead, as a critical pathway in the maintenance of ER homeostasis during normal growth and development in C. elegans. The diverse and dramatic consequences of XBP-1 deficiency on development and disease, taken together with our observations on the effect of XBP-1 deficiency on basal ER stress levels, underscore the critical role of homeostatic UPR signaling in both normal physiology and disease.
Materials and Methods
Strains
C. elegans strains were constructed and propagated according to standard methods on E. coli OP50 at 16°C [35]. The smg-2(qd101) allele was isolated by K. Reddy and contains a C→T nonsense mutation at nucleotide 1189 of the spliced transcript. The following strains were used in the study: N2 Bristol, ZD627 smg-2(qd101), ZD607 smg-2(qd101);xbp-1(zc12), ZD605 smg-2(qd101);xbp-1(zc12);pmk-1(km25), KU25 pmk-1(km25), RB545 pek-1(ok275), ZD510 xbp-1(tm2482);pek-1(ok275), ZD524 xbp-1(zc12);pek-1(tm629), ZD496 xbp-1(tm2482);pmk-1(km25);pek-1(ok275). All of the alleles used are predicted to be null alleles. Specifically, xbp-1(tm2482) is a 202 bp deletion from nt 231 that causes a frame-shift. The xbp-1(zc12) allele is a nonsense mutation that changes Q34 to an ochre stop. The two alleles exhibit an equivalent phenotype in every assay tested ([21]; this work, and our unpublished data). The pek-1(ok275) allele is a 2013 bp deletion and the pek-1(tm629) allele is a 1473 bp deletion, both of which remove the PEK-1 transmembrane domain and are therefore likely null alleles [6]. Double mutants were made between xbp-1 and pek-1 by crossing strains marked with GFP: xbp-1(III);pT24B8.5::GFP(agIs220)(X) and pT24B8.5::GFP(agIs219)(III);pek-1(X). GFP-negative F2s were singled, propagated, and genotyped by PCR.
RNA Isolation and Quantitative RT-PCR
For the experiment in Figure 1B, 1C, and 1E, L1 larvae were synchronized by hypochlorite treatment, washed onto E. coli OP50 plates, and grown for 40 h at 16°C to the L3 stage, when they were washed in M9 to plates containing E. coli OP50 or E. coli OP50 with 5 µg/ml tunicamycin. For the experiments in Figure 3, strains were grown and treated as previously described [21]. Specifically, L1 larvae were synchronized by hypochlorite treatment, washed onto E. coli OP50 plates and grown at 20°C for 23 h, then washed in M9 onto treatment plates. After incubation at 25°C for indicated times, worms were washed off plates and frozen in liquid nitrogen. For P. aeruginosa treatment, P. aeruginosa strain PA14 was grown in Luria Broth (LB), and 25 µl overnight culture was seeded onto 10 cm NGM plates. Plates were incubated first at 37°C for 1 d, then at room temperature for 1 d. All RNA extraction, cDNA preparation, qRT-PCR methods and specific primers to detect xbp-1 mRNA were as described previously [21].
Immunoblotting
For all immunoblots, strains were synchronized by hypochlorite treatment and washed onto E.coli OP50 plates for growth until the L4 stage. For the experiment in Figure 2A, strains were grown at 20°C and L4 worms were then washed in M9 onto treatment plates for incubation at 25°C for 4 hours. For the experiment in Figure 2B, strains were grown at 16°C until the L4 stage and harvested without treatment. All strains were collected and rinsed 2 times in M9. Worm pellets were resuspended in an equal volume of 2× lysis buffer containing 4% SDS, 1oomM Tris Cl, pH 6.8, and 20% Glycerol. After boiling for 15 minutes with occasional vortexing to aid in dissolution, lysates were clarified by centrifugation. Protein samples (50 µg of total lysate loaded per lane) were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Bio-rad). Western blots were blocked in 5% milk in PBST and probed with (1∶10,000) anti-eIF2α [26], (1∶1,000) anti-phospho-eIFα (Cell Signaling Technology), or (1∶10,000) anti-tubulin (E7 Developmental Hybridoma Bank, Iowa City). All primary antibodies were diluted in 5% milk in PBST. Following incubation with anti-rabbit or anti-mouse IgG antibodies conjugated with horseradish peroxidase (HRP) (Cell Signaling Technology), signals were visualized with chemiluminescent HRP substrate (Amersham). Quantification of immunoblots was preformed with ImageJ [36].
Survival/Development Assays
For all development assays, strains were egg laid on 4–5 prepared plates for no more than 3 h (at least 110 eggs for each strain and treatment). Development was monitored daily for 4 d for experiments conducted at 16°C and 3 d for experiments conducted at all other temperatures. Experiments monitoring development on E. coli OP50 were performed on 6 cm NGM plates. P. aeruginosa PA14 plates were prepared as described [27]. For the data presented in Figure S2, plates were prepared as described [37], except that ampicillin was used instead of carbenicillin.
To monitor L4 survival on E. coli OP50 or P. aeruginosa PA14, strains were incubated at 16°C to the L4 stage, when they were transferred to plates containing FUDR and incubated at either 16°C or 25°C. For each strain, 30 worms were transferred to each of 3–4 plates. Alive vs. dead worms were counted, and worms that died by exploding through the vulva or desiccating on the side of plates were censored.
Supporting Information
Zdroje
1. MoriK 2009 Signaling pathways in the unfolded protein response: development from yeast to mammals. Journal of Biochemistry 146 743 750
2. RonDWalterP 2007 Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology 8 519 529
3. SchröderMKaufmanRJ 2005 The mammalian unfolded protein response. Annual Reviews in Biochemistry 74 739 789
4. CalfonMZengHQUranoFTillJHHubbardSR 2002 IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415 92 96
5. ShamuCEWalterP 1996 Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO Journal 15 3028 3029
6. ShenXEllisRELeeKLiuC-YYangK 2001 Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107 893 903
7. YoshidaHMatsuiTYamamotoAOkadaTMoriK 2001 XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107 881 891
8. HardingHPZhangYBertolottiAZengHRonD 2000 Perk is essential for translational regulation and cell survival during the unfolded protein response. Molecular Cell 5 897 904
9. HardingHPNovoaIZhangYZengHWekR 2000 Regulated translation initiation controls stress-induced gene expression in mammalian cells. Molecular Cell 6 1099 1108
10. YoshidaHOkadaTHazeKYanagiHYuraT 2000 ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Molecular and Cellular Biology 20 6755 6767
11. LeeA-HScapaEFCohenDEGlimcherLH 2008 Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320 1492 1496
12. KaserALeeA-HFrankeAGlickmanJNZeissigS 2008 XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134 743 756
13. ShafferALShapiro-ShelefMIwakoshiNNZhaoHYuX 2004 XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21 81 93
14. ToddDJLeeA-HGlimcherLH 2008 The endoplasmic reticulum stress response in immunity and autoimmunity. Nature Reviews Immunology 8 663 674
15. ZhangWFengDLiYLidaKMcGrathB 2006 PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metabolism 4 491 497
16. ShenXEllisRESakakiKKaufmanRJ 2005 Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet 1 e37 doi:10.1371/journal.pgen.0010037
17. RutkowskiDTHegdeRS 2010 Regulation of basal and cellular physiology by the homeostatic unfolded protein response. Journal of Cell Biology 189 783 794
18. ReimoldAMIwakoshiNNManisJVallabhajosyulaPSzomolanyi-TsudaE 2001 Plasma cell differentiation requires the transcription factor XBP-1. Nature 412 300 307
19. HuC-CADouganSKMcGeheeAMLoveJCPloeghHL 2009 XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO Journal 28 1624 1636
20. MartinonFChenXLeeA-HGlimcherLH 2010 TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature Immunology 11 411 418
21. RichardsonCEKooistraTKimDH 2010 An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 463 1092 1095
22. RehwinkelJRaesJIzaurraldeE 2006 Nonsense-mediated mRNA decay: target genes and functional diversification of effectors. Trends in biochemical sciences 31 639 646
23. PageMFCarrBAndersKRGrimsonAAndersonP 1999 SMG-2 is a phosphorylated protein required for mRNA surveillance in Caenorhabditis elegans and related to Upf1p of yeast. Molecular and Cellular Biology 19 5943 5951
24. GardnerLB 2008 Hypoxic inhibition of nonsense-mediated RNA decay regulates gene expression and the integrated stress response. Molecular and Cellular Biology 28 3729 3741
25. HamanakaRBBennettBSCullinanSBDiehlJA 2005 PERK and GCN2 contribute to eIF2 alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Molecular Biology of the Cell 16 5493 5501
26. NukazukaAFujisawaHInadaTOdaYTakagiS 2008 Semaphorin controls epidermal morphogenesis by stimulating mRNA translation via eIF2a in Caenorhabditis elegans. Genes and Development 22 1025 1036
27. KimDHFeinbaumRAlloingGEmersonFEGarsinDA 2002 A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297 623 626
28. BischofLJKaoC-YLosFCOGonzalezMRShenZ 2008 Activation of the Unfolded Protein Response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Path 4 e100176 doi:10.1371/journal.ppat.1000176
29. KurzCLShapiraMChenKBaillieDLTanM-W 2007 Caenorhabditis elegans pgp-5 is involved in resistance to bacterial infection and heavy metal and its regulation requires TIR-1 and p38 map kinase cascade. Biochemical and Biophysical Research Communications 363 438 443
30. TroemelERChuSWReinkeVLeeSSAusubelFM 2006 p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet 2 e183 doi:10.1371/journal.pgen.0020183
31. TroemelERFelixM-AWhitemanNKBarriereAAusubelFM 2008 Microsporidia are natural intracelular parasites of the nematode Caenorhabditis elegans. PLoS Biol 6 e309 doi:doi:10.1371/journal.pbio.0060309
32. ZinsznerHKurodaMWangXBatchvarovaNLightfootRT 1998 CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes and Development 12 982 995
33. LeeA-HHeidtmanKHotamisligilGSGlimcherLH 2011 Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proceedings of the National Academy of Sciences of the United States of America 108 8885 90 doi:10.1073/pnas.1105564108
34. WooCWCuiDArellanoJDorweilerBHardingHP 2009 Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nature Cell Biology 11 1473 1480
35. BrennerS 1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
36. RasbandWS ImageJ, U.S. National Institutes of Health, Besthesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2011
37. GariganDHsuA-LFraserAGKamathRSAhringerJ 2002 Genetic Analysis of Tissue Aging in Caenorhabditis elegans: A Role for Heat-Shock Factor and Bacterial Proliferation. Genetics 161 1101 1112
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 11
-
Všechny články tohoto čísla
- De Novo Origins of Human Genes
- Duplication Hotspots Are Associated with Late-Replicating Regions of the Genome
- De Novo Origin of Human Protein-Coding Genes
- Cyclin D/CDK4 and Cyclin E/CDK2 Induce Distinct Cell Cycle Re-Entry Programs in Differentiated Muscle Cells
- Short Day–Mediated Cessation of Growth Requires the Downregulation of AINTEGUMENTALIKE1 Transcription Factor in Hybrid Aspen
- Physiological IRE-1-XBP-1 and PEK-1 Signaling in Larval Development and Immunity
- Role of Pirh2 in Mediating the Regulation of p53 and c-Myc
- Signatures of Environmental Genetic Adaptation Pinpoint Pathogens as the Main Selective Pressure through Human Evolution
- FOXO Regulates Organ-Specific Phenotypic Plasticity In
- Heritable Epigenetic Variation among Maize Inbreds
- Foxn1 Regulates Lineage Progression in Cortical and Medullary Thymic Epithelial Cells But Is Dispensable for Medullary Sublineage Divergence
- Attenuation of the Sensing Capabilities of PhoQ in Transition to Obligate Insect–Bacterial Association
- A Novel Protein LZTFL1 Regulates Ciliary Trafficking of the BBSome and Smoothened
- Activation of Bmp2-Smad1 Signal and Its Regulation by Coordinated Alteration of H3K27 Trimethylation in -Induced Senescence
- Histone H3K56 Acetylation, CAF1, and Rtt106 Coordinate Nucleosome Assembly and Stability of Advancing Replication Forks
- The SUN Protein Mps3 Is Required for Spindle Pole Body Insertion into the Nuclear Membrane and Nuclear Envelope Homeostasis
- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- Effect of Host Species on the Distribution of Mutational Fitness Effects for an RNA Virus
- Pch2 Acts through Xrs2 and Tel1/ATM to Modulate Interhomolog Bias and Checkpoint Function during Meiosis
- SOX9 Governs Differentiation Stage-Specific Gene Expression in Growth Plate Chondrocytes via Direct Concomitant Transactivation and Repression
- from the Aphid : A Missing Link from Facultative to Obligate Insect Endosymbiont
- Recessive Antimorphic Alleles Overcome Functionally Redundant Loci to Reveal Function in Flowers and Meristems
- Over-Expression of DSCAM and COL6A2 Cooperatively Generates Congenital Heart Defects
- Consequences of Eukaryotic Enhancer Architecture for Gene Expression Dynamics, Development, and Fitness
- Distinct Genetic Architectures for Male and Female Inflorescence Traits of Maize
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- For Male , Sperm Activation Is a “Just-in-Time” Event
- PcG Complexes Set the Stage for Epigenetic Inheritance of Gene Silencing in Early S Phase before Replication
- The Gene Contains Hotspots for L1 Endonuclease-Dependent Insertion
- Relative Burden of Large CNVs on a Range of Neurodevelopmental Phenotypes
- Multiple Means to the Same End: The Genetic Basis of Acquired Stress Resistance in Yeast
- Genome-Wide Crossover Distribution in Meiosis Reveals Sex-Specific Patterns along Chromosomes
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
- Homologs of Retinoblastoma-Associated Protein 46/48 Associate with a Histone Deacetylase to Act Redundantly in Chromatin Silencing
- Genetic Interaction Maps in Reveal Functional Crosstalk among Cell Envelope Biogenesis Pathways
- The ERI-6/7 Helicase Acts at the First Stage of an siRNA Amplification Pathway That Targets Recent Gene Duplications
- PBX1 Genomic Pioneer Function Drives ERα Signaling Underlying Progression in Breast Cancer
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- De Novo Origins of Human Genes
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



