-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
The meiotic recombination checkpoint is a signalling pathway that blocks meiotic progression when the repair of DNA breaks formed during recombination is delayed. In comparison to the signalling pathway itself, however, the molecular targets of the checkpoint that control meiotic progression are not well understood in metazoans. In Drosophila, activation of the meiotic checkpoint is known to prevent formation of the karyosome, a meiosis-specific organisation of chromosomes, but the molecular pathway by which this occurs remains to be identified. Here we show that the conserved kinase NHK-1 (Drosophila Vrk-1) is a crucial meiotic regulator controlled by the meiotic checkpoint. An nhk-1 mutation, whilst resulting in karyosome defects, does so independent of meiotic checkpoint activation. Rather, we find unrepaired DNA breaks formed during recombination suppress NHK-1 activity (inferred from the phosphorylation level of one of its substrates) through the meiotic checkpoint. Additionally DNA breaks induced by X-rays in cultured cells also suppress NHK-1 kinase activity. Unrepaired DNA breaks in oocytes also delay other NHK-1 dependent nuclear events, such as synaptonemal complex disassembly and condensin loading onto chromosomes. Therefore we propose that NHK-1 is a crucial regulator of meiosis and that the meiotic checkpoint suppresses NHK-1 activity to prevent oocyte nuclear reorganisation until DNA breaks are repaired.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001179
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001179Summary
The meiotic recombination checkpoint is a signalling pathway that blocks meiotic progression when the repair of DNA breaks formed during recombination is delayed. In comparison to the signalling pathway itself, however, the molecular targets of the checkpoint that control meiotic progression are not well understood in metazoans. In Drosophila, activation of the meiotic checkpoint is known to prevent formation of the karyosome, a meiosis-specific organisation of chromosomes, but the molecular pathway by which this occurs remains to be identified. Here we show that the conserved kinase NHK-1 (Drosophila Vrk-1) is a crucial meiotic regulator controlled by the meiotic checkpoint. An nhk-1 mutation, whilst resulting in karyosome defects, does so independent of meiotic checkpoint activation. Rather, we find unrepaired DNA breaks formed during recombination suppress NHK-1 activity (inferred from the phosphorylation level of one of its substrates) through the meiotic checkpoint. Additionally DNA breaks induced by X-rays in cultured cells also suppress NHK-1 kinase activity. Unrepaired DNA breaks in oocytes also delay other NHK-1 dependent nuclear events, such as synaptonemal complex disassembly and condensin loading onto chromosomes. Therefore we propose that NHK-1 is a crucial regulator of meiosis and that the meiotic checkpoint suppresses NHK-1 activity to prevent oocyte nuclear reorganisation until DNA breaks are repaired.
Introduction
Meiosis is a specialised form of cell division that differs from mitosis in many respects, particularly during the exchange of genetic information between homologous chromosomes in recombination. In early meiotic prophase, DNA double-strand breaks (DSBs) are introduced into meiotic chromosomes by the conserved enzyme Spo11 to initiate recombination [1]–[4]. An elaborate structure, the synaptonemal complex, then forms between homologous chromosomes stabilising their pairing and recombination [5]. Once recombination is complete and DSBs have been repaired, the synaptonemal complex is disassembled. As these events are meiosis-specific, molecular mechanisms of meiotic prophase progression need to be established beyond our understanding of mitotic cell cycle control.
Eukaryotes have a surveillance-signalling system, the so-called meiotic recombination checkpoint (hereafter referred to as the meiotic checkpoint), which prevents meiotic progression until DSBs generated during recombination are repaired [6]–[8]. Many advances have been made recently in determining the mechanisms involved in the detection of and signalling downstream from DSBs [9]. In contrast, little is known about how the checkpoint signal blocks meiotic progression, except in yeast. In yeast, the Cdc28 (Cdk1)-Cyclin complex is suppressed in various ways by the meiotic checkpoint to delay or block meiotic division [10]–[12].
In Drosophila, the meiotic checkpoint was first revealed by the study of a class of mutants collectively called spindle (spn) mutants. These spn mutants were originally identified based on their abnormal dorsal-ventral oocyte polarity [13]–[16]. They also share abnormalities in a meiosis-specific organisation of chromosomes called the karyosome [14], [17], [16].
The meiotic checkpoint pathway is activated in spn mutants by persistent DSBs caused either by defects in DNA repair during recombination [18], [19] or in processing of repeat-associated siRNA that suppress germline retrotransposition [20]–[22]. Signalling downstream of DSBs in the meiotic checkpoint requires the successive activation of two conserved kinases, Mei-41 (an ATM/ATR homologue) and Mnk/Chk2 [17], [23]. Their activation blocks both oocyte polarisation and karyosome formation. Vasa was proposed to act downstream of the meiotic checkpoint to mediate both oocyte polarisation and karyosome formation [17], [23], but a more recent study suggests that Vasa acts upstream of the checkpoint through involvement in processing of repeat-associated siRNA [24]. Gurken has been shown to be a downstream effector required for oocyte polarisation which is inhibited by the meiotic checkpoint [25], [16], but an effector required for karyosome formation has not been identified.
The karyosome is a compact cluster of meiotic chromosomes formed within the Drosophila oocyte nucleus [26] and similar structures are also found in human oocytes [27]. In addition to the successful completion of recombination, recent studies by us and others have shown that nucleosomal histone kinase-1 (NHK-1) is essential for karyosome formation [28], [29]. NHK-1 is a Histone 2A kinase conserved from nematodes to humans (Vrk-1 in C. elegans, and Vrk1-3 in mammals) [30]. We showed that NHK-1 also phosphorylates Barrier-to-Autointegration Factor (BAF) to release meiotic chromosomes from the oocyte nuclear envelope during karyosome formation [31]. However nothing is known about how NHK-1 activity itself might be controlled during meiosis.
In this report, we have investigated the functional relationship between NHK-1 and the meiotic checkpoint. We found that the meiotic checkpoint suppresses NHK-1 activity to prevent reorganisation of the oocyte nucleus, including karyosome formation, synaptonemal complex disassembly and condensin loading, until DNA breaks are repaired. Therefore, we propose that NHK-1 is a critical meiotic regulator controlled by the meiotic checkpoint.
Results/Discussion
The meiotic checkpoint pathway is not activated in an nhk-1 mutant
In the wild-type oocyte nucleus, meiotic chromosomes are clustered together to form a spherical body called the karyosome [26] (Figure 1C). Female sterile nhk-1 mutations show an abnormal morphology of the karyosome, which is less compact and often attached to the nuclear envelope [28], [29], [31] (Figure 1B and Figure S1). A similar karyosome abnormality is also observed in the spn class of mutants, which were originally identified based on their abnormal oocyte polarity [13], [14], [16], [17] (Figure 1A and Figure S1). Most spn mutants contain persistent DNA double stranded breaks (DSBs) in meiotic chromosomes and activate the meiotic checkpoint pathway [18]–[22]. Both the karyosome and polarity defects in these spn mutants can be rescued by inactivation of the meiotic checkpoint [17], [21], [23] (Figure 1D).
Fig. 1. Inactivation of the meiotic checkpoint did not suppress nhk-1 karyosome defects. 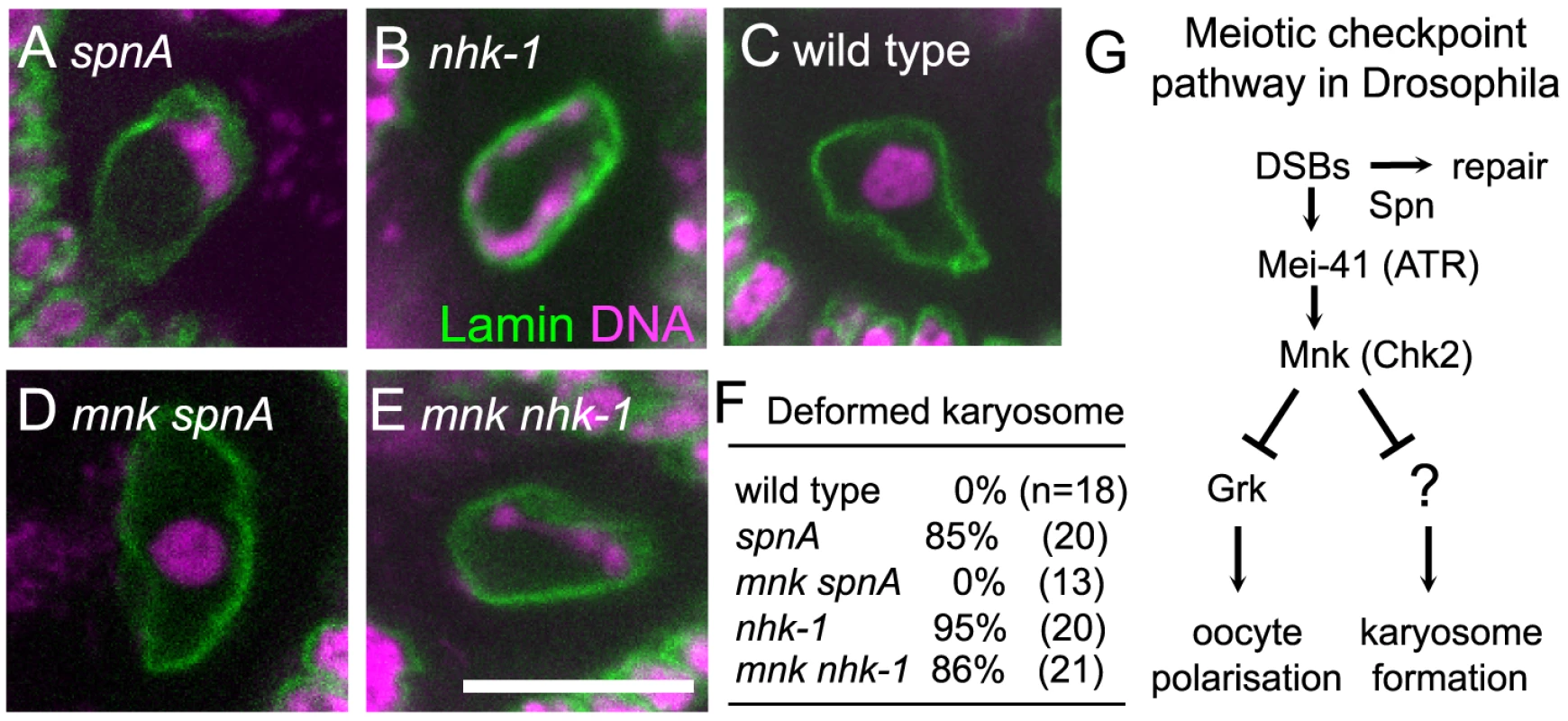
The karyosome morphology in an oocyte from spnA1 (A), nhk-1E24/Df (B), wild type (C), an mnkp6 spnA1 double mutant (D), and an mnkp6 nhk-1E24/Df double mutant (E). Bar = 10 µm. (F) The frequency of deformed karyosomes in oocytes with various genotypes. Inactivation of the meiotic checkpoint by the mnkp6 mutation rescued the karyosome defect in spnA1 (p<0.01), but not in nhk-1E24/Df (G) The meiotic recombination checkpoint pathway in Drosophila oocytes (modified from 23). A possible explanation for this similarity in the karyosome defects between nhk-1 and spn mutants is that nhk-1 mutations lead to an activation of the meiotic checkpoint pathway. To test this possibility, we assessed the activation of the meiotic checkpoint pathway in an nhk-1 mutant by examining for the persistence of DSBs on meiotic chromosomes and the presence of oocyte/embryo polarity defects. The nhk-1Z3-0437 mutant has been previously shown to have no delay in DSB repair or polarity defects [28]. However, as this allele contains a mis-sense mutation in a residue with unknown function in the kinase domain, the phenotype may be due to the specific nature of this allele. To exclude this possibility, we examined another female sterile allele, nhk-1E24/Df, that expresses a reduced amount of wild-type NHK-1 protein and shows karyosome defects [29], [31]. To assess oocyte polarity, we examined the dorsal appendages of eggs laid by females, whose formation depends on correct dorsal-ventral axis specification in the oocyte [32]. Dorsal appendages of eggs laid by the nhk-1E24/Df mutant did not show abnormalities, indicating that oocyte polarity was properly established. Furthermore, immunostaining using an antibody against the phosphorylated form of the Drosophila H2AX variant (γ-H2Av) which accumulates at DSB sites [33] showed no detectable DSB foci at late oogenesis stages, indicating DSBs were repaired in the nhk-1E24/Df mutant (Figure S2). These results indicated that, unlike spn mutants, the meiotic checkpoint pathway is not activated in the nhk-1E24/Df mutant, despite the clear karyosome defect observed in this mutant.
The karyosome defect in an nhk-1 mutant does not require meiotic checkpoint activation
To further confirm that the karyosome defect in the nhk-1E24/Df mutant arises without meiotic checkpoint activation, we examined whether inactivating the checkpoint rescues the karyosome defect in the nhk-1E24/Df mutant. The meiotic checkpoint signalling pathway contains two kinases, Mei-41 and Mnk, which are homologues of ATM/ATR and Chk-2 respectively (Figure 1G). A mutation in either of these genes has been shown to rescue the karyosome defect caused by unrepaired DSBs in spn mutants (Figure 1D) [17], [21], [23], although mei-41 mutations have been shown to be less proficient at rescuing the karyosome defect probably due to the presence of a second ATM/ATR homologue [21].
We constructed a double mutant between nhk-1E24/Df and mnk by successive genetic crosses, and immunostaining of oocytes was carried out to visualise the oocyte nucleus and the karyosome. This showed that inactivation of the meiotic checkpoint failed to rescue the karyosome defect in the nhk-1E24/Df mutant (Figure 1E and 1F). In an mnk nhk-1 double mutant, 86% of oocytes showed deformed karyosome morphology, similar to the nhk1 single mutant in which 95% of oocytes showed deformed karyosomes (p>0.3). In a control analysis done in parallel, no oocytes from an mnk spnA double mutant showed deformed karyosome morphology (Figure 1D and 1F), in comparison to 85% of oocytes from a spnA single mutant (p<0.01).
In conclusion, these results demonstrated that the karyosome defect in the nhk-1E24/Df mutant is not caused by activation of the meiotic checkpoint pathway.
Unrepaired DSBs suppress NHK-1 kinase activity
The above results demonstrated that the nhk-1E24/Df mutation induces karyosome defects without activation of the meiotic checkpoint pathway. Therefore, this places NHK-1 function either downstream or in parallel to the meiotic checkpoint pathway. One way to distinguish between these two possibilities would be to examine the kinase activity of NHK-1 in oocytes under conditions activating the meiotic checkpoint pathway (ie. in spn mutants). It is known that NHK-1 directly phosphorylates Histone 2A (H2A) at threonine 119 (T119; 30), and this phosphorylation in the oocyte nucleus has been shown to depend on NHK-1 activity [28]. Therefore we decided to examine the level of H2A T119 phosphorylation in the oocyte nucleus as a readout of NHK-1 activity in vivo by immunostaining using a phospho-specific antibody (anti-dH2ApT119) [30].
Ovaries from spn mutants (spnA, spnB, spnD and vasa) were dissected and immunostained with the anti-dH2ApT119 antibody. As a control, we also examined wild type and the nhk-1E24/Df mutant in parallel. Compared to wild type, we found that the H2ApT119 signal was greatly reduced on meiotic chromosomes in oocytes from spn mutants, as well as in oocytes from the nhk-1E24/Df mutant (Figure 2A and Figure S3A).
Fig. 2. Unrepaired DNA breaks suppress NHK-1 kinase activity. 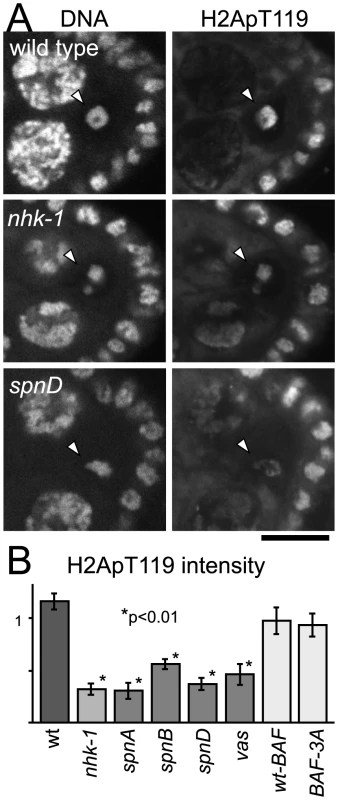
(A) H2A T119 phosphorylation in wild type, nhk-1E24/Df and spnD2. Ovaries at stage 5–7 were immunostained with anti-dH2ApT119 antibody and propidium iodide. Arrowheads indicate meiotic chromosomes in oocytes. Bar = 10 µm. (B) The H2ApT119 signal intensity on the chromosomes in oocytes was measured relative to that in follicle cells. The bars on the graph represent standard error of the mean (SEM). A minimum of eight oocytes from each genotype were quantified. NHK-1 activity measured by H2A T119 phosphorylation was significantly reduced in nhk-1 and spn mutant oocytes (p<0.01; marked with asterisks). H2A T119 phosphorylation in oocytes expressing wild-type BAF and non-phosphorylatable BAF (BAF-3A) was comparable to that in wild type, indicating the karyosome abnormality itself is not the cause of low dH2ApT119 signals in spn mutants. To quantify the level of the H2ApT119 signal reproducibly and comparably between different oocytes, we measured the H2ApT119 signal in the oocyte nucleus relative to that in follicle cell nuclei, in which H2A T119 phosphorylation has been shown to be independent of NHK-1 activity [28]. H2ApT119 signals in spn mutants were significantly reduced (p<0.01; Figure 2B).
We considered the possibility that the reduction of H2ApT119 signal under meiotic checkpoint activation was simply due to abnormal karyosome morphology itself (and we were in fact measuring an artefact or a secondary consequence). First of all, this is unlikely because H2ApT119 signals were also reduced in karyosomes which retained relatively normal morphology in the spn mutants (Figure 2A and Figure S3A). To exclude this possibility further, we took advantage of our previous study showing that expressing a non-phosphorylatable version of BAF (a substrate of NHK-1) disrupts the karyosome in these oocytes [31]. Under these conditions, although the karyosome was disrupted, the level of H2ApT119 signal in the oocyte nucleus was comparable to that in oocytes expressing wild-type BAF (that show normal karyosome morphology) or wild-type oocytes (Figure 2B and Figure S3B). Furthermore, to exclude the possibility that the apparent reduction in H2ApT119 was simply due to reduced chromosome condensation or DNA density, we re-quantified H2ApT119 signal intensity relative to DNA staining signal intensity in oocyte nuclei (Figure S3C). The H2ApT119 signal in the oocyte nucleus relative to that in follicle cell nuclei was divided by the DNA staining signal which had been measured using the same method. The result still showed a significant reduction in the H2ApT119 signal relative to DNA signal in spn mutant oocytes. The possibility that a simple reduction in H2A levels or its occupancy on DNA accounted for the decrease in H2Ap119 signal was further excluded by immunostaining using a phospho-independent antibody against H2A which did not show reduction in H2A signal in spn mutant oocytes (Figure S3D, S3E). These results confirm the genuine suppression of H2A T119 phosphorylation (which infers the suppression of NHK-1 activity) in these mutants.
Therefore we conclude that, judged by the phosphorylation level of one of its substrates, unrepaired DSBs in spn mutants suppress NHK-1 kinase activity in the oocyte nucleus.
The meiotic checkpoint mediates suppression of NHK-1 activity
To confirm whether this suppression of NHK-1 activity by unrepaired DSBs is mediated by the meiotic checkpoint, we tested whether inactivation of the checkpoint (as shown in Figure 1) could abolish this suppression. Inactivation of the checkpoint was achieved by introduction of a mutation in mnk, which encodes the crucial checkpoint kinase Chk2. Examination of double mutants between mnk and spnA and between mnk and spnD showed that the H2ApT119 signal on meiotic chromosomes in spn mutants is restored by inactivation of the checkpoint (Figure 3 and Figure S4). This confirmed that the suppression of NHK-1 activity in the presence of DSBs is mediated by the meiotic checkpoint.
Fig. 3. The meiotic recombination checkpoint suppresses NHK-1 activity. 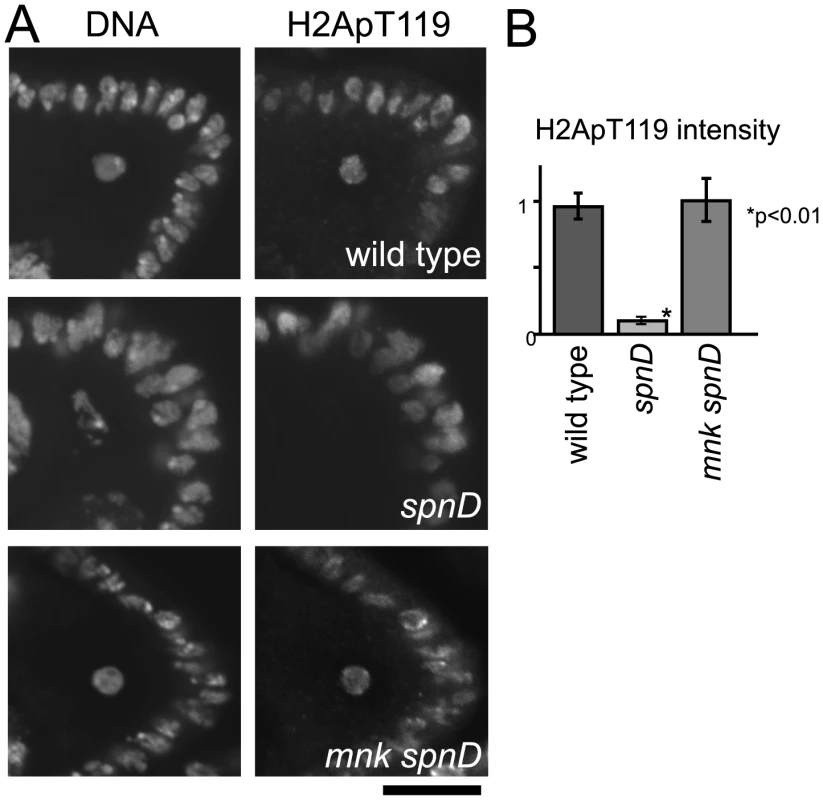
(A) H2A T119 phosphorylation in oocytes of wild type, spnD, and mnk spnD. Mnk (the Chk2 orthologue) is an essential kinase in the meiotic checkpoint. Ovaries at stage 5–7 were immunostained with anti-dH2ApT119 antibody and DAPI. (B) The H2ApT119 signal intensity on the chromosomes in oocytes was measured relative to that in follicle cells. The bars on the graph represent standard error of the mean (SEM). At least ten oocytes from each genotype were quantified. These samples were processed in parallel and compared only with each other, as exact values vary over time due to changes in factors including the conditions of the antibodies and fixative. NHK-1 activity measured by H2A T119 phosphorylation was significantly reduced in oocytes of a spnD mutant (p<0.01; marked with an asterisk), but not in those of an mnk spnD double mutant. Inactivation of the meiotic checkpoint rescued the suppression of NHK-1 activity in the presence of DSBs. DNA breaks suppress NHK-1 kinase activity in Drosophila cultured cells
Our cytological study showed that DSBs suppress the kinase activity of NHK-1, judged by phosphorylation of its substrate H2A at T119. We wished to confirm this suppression of NHK-1 activity by biochemical means. As biochemical measurements of oocyte-specific NHK-1 activity is challenging, we wondered whether similar suppression of NHK-1 may be observed when DSBs are induced in Drosophila cultured cells, without involvement of meiosis-specific factors.
To aid purification of NHK-1 from cultured cells (S2 cells), the NHK-1 gene was fused to GFP in frame and placed under the control of the metallothionein promotor. After transfection into S2 cells, a stable cell line inducibly expressing NHK-1-GFP was established. These cells were irradiated with X-rays at 1 Gy/min for 5 minutes. Immunostaining using a γ-H2Av antibody confirmed that this dose of X-rays efficiently induced DSBs without damaging the ability of cells to repair DSBs (data not shown). Fifteen minutes after X-ray irradiation, cells were collected and NHK-1-GFP was immunoprecipitated from cell extract using a GFP antibody in the presence of phosphatase inhibitors. The kinase activity of immunoprecipitated NHK-1-GFP was assayed in vitro by adding radioactive ATP without inclusion of exogenous substrates, as the NHK-1 substrate BAF is co-immunoprecipitated with NHK-1 [31].
Interestingly, we found that in vitro phosphorylation of co-immunoprecipitated BAF was greatly reduced in irradiated cells in comparison to non-irradiated cells processed in parallel (Figure 4A). This phosphorylation was dependent on NHK-1 kinase activity, as it was abolished by a mutation in NHK-1 [31] that eliminates its kinase activity but does not interfere with its binding to BAF (Figure 4A). Immunoblotting confirmed that comparable amounts of NHK-1 were immunoprecipitated from irradiated and non-irradiated samples (Figure 4A). When cells were collected 15 minutes after irradiation, their mitotic indexes were comparable (1.2% irradiated vs 1.4% non-irradiated), their nuclei still had unrepaired DSBs and nuclear localisation of NHK-1 was unaffected (Figure 4B). This indicates that the reduction in NHK-1 kinase activity in irradiated cells was not due to a reduction of mitotic cells or a change in NHK-1 localisation.
Fig. 4. DSBs suppress NHK-1 activity in S2 cells. 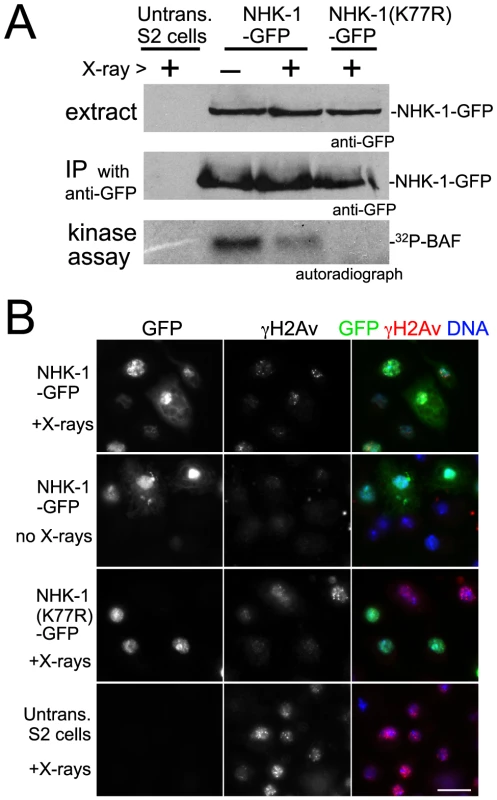
(A) Kinase activity of NHK-1-GFP was reduced after X-ray irradiation. S2 cells stably expressing NHK-1-GFP or NHK-1(K77R)-GFP, together with untransfected S2 cells, were irradiated with X-rays. Cells were collected 15 minutes later and NHK-1-GFP was immunoprecipitated from cell extracts by a GFP antibody. For the kinase assays, 32P-γATP was added and phosphorylation of co-immunoprecipitated BAF by NHK-1 was detected by autoradiograph. Cell extracts and immunoprecipitates used for kinase assays were immunoblotted with a GFP antibody. (B) DSBs were retained and the nuclear localisation of NHK-1-GFP was unaffected when cells were collected after X-ray treatment. DSBs and NHK-1-GFP were detected by immunostaining using antibodies against γH2Av and GFP, respectively. Bar = 10 µm. The suppression of NHK-1 kinase activity after X-ray irradiation was observed in three independent experiments. These biochemical results in S2 cells give further support to our observation in oocytes that NHK-1 kinase activity is suppressed in response to DSBs.
Activation of the meiotic checkpoint delays other NHK-1 dependent events
In addition to karyosome formation, NHK-1 has been shown to be required for the disassembly of the synaptonemal complex and loading of the condensin complex onto chromosomes during meiosis [28]. Our results showed that the meiotic checkpoint suppresses NHK-1 activity when DSBs are not repaired. From these observations, a prediction is that these other NHK-1 dependent events would also be blocked or delayed when the meiotic checkpoint pathway is activated. Indeed, a previous report showed that disassembly of the synaptonemal complex is delayed in a spnA mutant [18].
To test how universal this is, we examined the disassembly of the synaptonemal complex during oogenesis in various spn mutants. Immunostaining using an antibody against the synaptonemal complex protein C(3)G [34] showed that synaptonemal complex disassembly was significantly delayed in spn mutants. In wild-type oocytes, synaptonemal complex disassembly was completed by oogenesis stage 6. However, the characteristic filamentous structure of the synaptonemal complex or its remnants were still detected by the C(3)G antibody on meiotic chromosomes even at stage 6 or later in most oocytes of spn mutants (spnA, spnB, spnD; Figure 5A and Figure 4B). This delay in synaptonemal complex disassembly in spn mutants, but not in the nhk-1Z3-0437/Df mutant, was reversed by inactivation of the meiotic checkpoint using an mnk mutation (Figure S5).
Fig. 5. Disassembly of the synaptonemal complex and loading of the condensin complex is delayed by meiotic checkpoint activation. 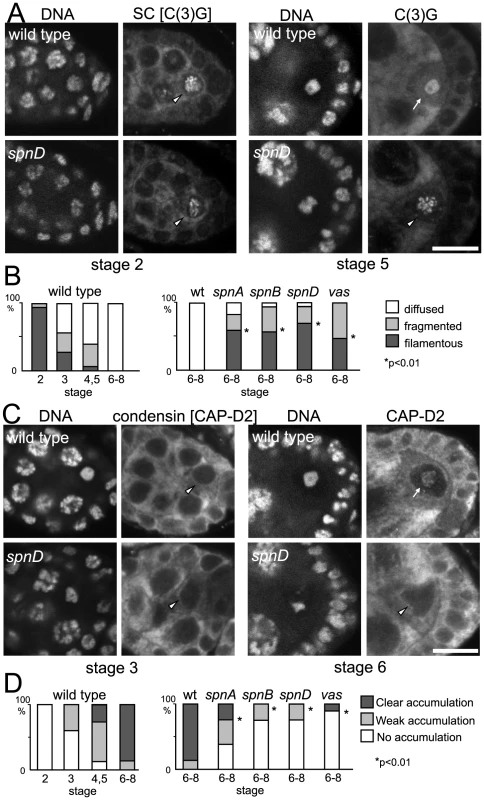
(A) Synaptonemal complex in wild-type and spn mutant oocytes. Ovaries were immunostained for the tranverse filament protein C(3)G and DNA. Bar = 10 µm. (B) The C(3)G staining pattern was classified as filamentous (arrowheads in A), fragmented or diffused (arrow in A). spn mutants significantly delayed disassembly of the synaptonemal complex (p<0.01; marked with asterisks). A minimum of thirteen oocytes were counted. (C) Condensin in wild-type and spn mutant oocytes. Ovaries were immunostained for the condensin subunit CAP-D2 and DNA. Bar = 10 µm. (D) Chromosome accumulation of CAP-D2 staining was classified into clear (arrow in C), weak or absent (arrowheads in C). spn mutants significantly delayed condensin loading (p<0.01; marked with asterisks). A minimum of nine oocytes were counted. Next we examined condensin loading onto meiotic chromosomes in wild type and spn mutants by immunostaining. In wild-type oocytes, the conserved condensin subunit CAP-D2 [35] is fully recruited onto meiotic chromosomes by stage 6 of oogenesis. In spn mutants, the protein had not fully accumulated onto meiotic chromosomes in most oocytes even at stage 6 or later (Figure 5C and 5D), indicating that the condensin complex was not fully loaded onto meiotic chromosomes.
These results showed that unrepaired DSBs not only disrupt karyosome formation but also other NHK-1 dependent events. This suggests that suppression of NHK-1 activity plays wider roles in delaying meiotic progression in response to DSBs.
The meiotic checkpoint suppresses NHK-1 activity to delay nuclear reorganisation in meiosis
These NHK-1 dependent events, disassembly of the synaptonemal complex, loading of condensin and karyosome formation, represent an important transition in oocyte nuclear organisation during meiosis. Temporally karyosome formation takes place at the transition between oogenesis stage 2 and 3 [26] and in fact our quantitative study of synaptonemal complex disassembly and condensin loading in wild-type meiosis (Figure 5B and 5D) indicated that the initiation of these other two NHK-1 dependent events also occurs between oogenesis stage 2 and 3. As these events depend on the presence of a functioning NHK-1 kinase [28], it suggests that NHK-1 is a key meiotic regulator of this important transition in nuclear organisation in oocytes.
It has long been known that activation of the meiotic recombination checkpoint disrupts karyosome formation, and additionally we show here that synaptonemal complex disassembly and condensin loading are delayed by the presence of unrepaired DSBs and an activated checkpoint. It has previously been shown that the meiotic checkpoint blocks oocyte polarisation by suppressing Gurken translation or localisation [16], [17], [23], but it was not previously known how the checkpoint affects karyosome formation or any other nuclear events in oocytes. Our study has shown that the meiotic checkpoint suppresses phosphorylation of an NHK-1 substrate, H2A, when DSBs are not repaired. Furthermore, we found that DSBs induced by X-rays suppress the kinase activity of NHK-1 in S2 cells. These results indicate that NHK-1 is a downstream effector of the meiotic recombination checkpoint, whose suppression is responsible for blocking karyosome formation and other meiotic events until DSBs are repaired.
Based on this evidence, we propose a model in which DSBs formed during recombination suppress the activity of the conserved kinase NHK-1 through the meiotic recombination checkpoint to delay oocyte nuclear reorganisation from a recombination to a post-recombination phase (Figure 6). Although the evidence is mostly genetic or cytological, all data are so far consistent with this model. Nevertheless, the model is likely to be too simplistic and to represent only a part of the whole picture. For example, we do not exclude the possibility that other checkpoint effectors are also involved in delaying meiotic progression. We hope that our proposed model will prompt further investigation to fully uncover how the meiotic checkpoint is linked to meiotic progression.
Fig. 6. A central role for NHK-1 kinase in meiotic progression. 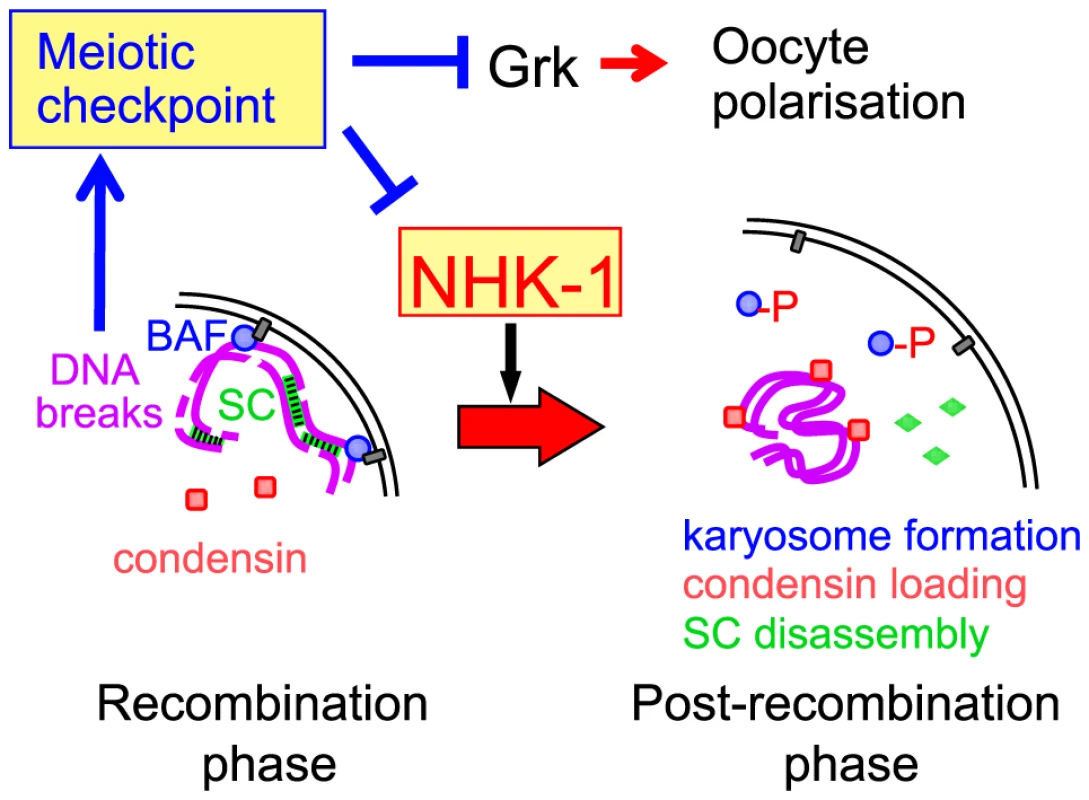
NHK-1 promotes nuclear reorganisation from a recombination phase to a post-recombination phase, including karyosome formation, synaptonemal complex disassembly and condensin loading. NHK-1 directly phosphorylates BAF that anchors meiotic chromosomes to the nuclear envelope. DSBs activate the meiotic recombination checkpoint that suppresses NHK-1 kinase to prevent nuclear reorganisation. How does NHK-1 kinase control this critical transition in meiosis? Our previous study showed that NHK-1 directly controls karyosome formation through phosphorylation of BAF, a linker between the nuclear envelope and chromatin [31]. Phosphorylation of BAF by NHK-1 releases meiotic chromosomes from tethering at the nuclear envelope to allow karyosome formation. Expression of non-phosphorylatable BAF disrupts karyosome formation, but not synaptonemal complex disassembly or condensin loading (Figure S6). Therefore, NHK-1 appears to control two independent pathways during nuclear reorganisation. This is consistent with a recent study [36] showing that condensin is required for synaptonemal complex disassembly but not for karyosome formation. Karyosome formation and condensin loading are therefore likely to be two primary targets of NHK-1 activity.
Finally, this study in Drosophila is likely to have significant implications for our understanding of meiotic regulation at a molecular level in other organisms, since the processes we studied here are conserved among eukaryotes. The meiotic checkpoint that coordinates recombination events with meiotic progression is universally found across eukaryotes. Furthermore, NHK-1 is well conserved among animals, and karyosome-like clustering of meiotic chromosomes, as well as synaptonemal complex disassembly and condensin loading, is widely found in oocytes of various species including humans. In addition, this study has also suggested an involvement of NHK-1 in the DNA damage response during the mitotic cell cycle.
Materials and Methods
Drosophila genetics
Standard techniques of fly manipulation were followed [37]. All stocks were grown at 25°C on standard cornmeal media except in some case where females were matured at 18°C. General details of mutations, chromosome aberrations and common vectors can be found in [38] or at Flybase [39]. w1118 was used as wild type. The following mutant alleles were used in this study: nhk-1E24 and nhk-1Z3-0437 analysed as a hemizygote over the deficiency Df(3R)ro-80b [29], [28]; spnA1 [13], spnB1 [13] analysed as a hemizygote over the deficiency Df(3R)red3I and spnD2 [13] that were obtained from the Bloomington Drosophila Stock Centre; vas4 [40]; mnkp6 [21]. Flies containing both spnA1 and mnkp6 mutations were obtained by standard successive genetic crosses. BAF and non-phosphorylatable BAF-3A were expressed from pUASp-BAF and pUASp-BAF-3A transgenes using a maternal Gal4 driver (V2H) under the α-tubulin67C promotor, as previously described [31].
Immunological and cytological techniques
Standard immunological techniques were used throughout [41]. Drosophila ovaries were immunostained essentially as described [42]. Briefly, ovaries were dissected from mature females in Robb's medium (100 mM HEPES, pH7.4, 55 mM sodium acetate, 40 mM potassium acetate, 100 mM sucrose, 10 mM glucose, 1.2 mM MgCl2, 1 mM CaCl2) before being fixed in formaldehyde (8% paraformaldehyde, 100 mM potassium cacodylate, pH7.2, 100 mM sucrose, 40 mM potassium acetate, 10 mM sodium acetate, 10 mM EGTA). Following a blocking and permeabilization step (in PBS containing 10% foetal bovine serum and 1% Triton X-100), ovaries were successively incubated in primary and secondary antibody solutions before mounting on coverslips in mounting medium (85% glycerol, 2.5% propyl gallate). The primary antibodies used in this study were those against H2ApT119 [30] (1/200), γ-H2Av (this study; 1/100), H2A (Ab13923, Abcam; 1/250), Lamin [43] (1/250), C(3)G [44] (1/3000) and CAP-D2 [35] (1/5000). The antibody against γ-H2Av was generated by Eurogentec using a phospho-peptide (CQRKGNVILpSQAY-COOH), and differentially purified using the phospho-peptide and an equivalent non-phospho-peptide. The antibody gave punctate staining in oocyte nuclei during early oogenesis and in X-ray irradiated nuclei in S2 cells, but not during late oogenesis or non-irradiated S2 nuclei. Secondary antibodies conjugated with Cy3 or Alexa488 (Jackson Lab or Molecular Probes) were used at a 1/100 dilution. DNA was counterstained with DAPI (0.4 µg/ml; Sigma) or propidium iodide (2 µg/ml, Sigma). A series of 1 µm optical sections were taken using a Plan-Apochromat lens (63X, 1.4NA; Zeiss) attached to an Axiovert 200M (Zeiss) with a confocal scan head (LSM510meta; Zeiss) or to an Axioimager (Zeiss) with an LSM5 Exciter (Zeiss). A single mid-section of the oocyte nucleus has been presented. All digital images were imported to Photoshop (Adobe) and adjusted for brightness and contrast uniformly across entire fields.
Expression of NHK-1-GFP in S2 cells and in vitro kinase assay
The culture and transfection of S2 cells were performed as previously described [45]. NHK-1-GFP under the metallothionein promoter was generated by a Gateway-based method (Invitrogen) and NHK-1(K77R)-GFP was made by site-directed mutagenesis. Stable cell lines were created through selection by inclusion of blasticidin in the culture medium. Untransfected S2 cells were used as a control. NHK1-GFP expression from the metallothionein promoter was induced by culturing in medium containing 0.7 mM copper sulfate for 72 h. Aliquotes of cells were adhered to coverlips coated with Concanavalin A for immunostaining for γ-H2Av and GFP. Cells, together with adhered cells, were irradiated with X-rays (1 Gy/minute) for 5 minutes. Subsequently, 2.5×107 cells were lysed in 500 µl of buffer (20 mM Tris pH 7.5, 150 mM NaCl, 5 mM EGTA, 1 mM DTT, 1 mM PMSF, Complete EDTA-free protease inhibitor cocktail (Roche)) supplemented with 10× Protein Phosphatase Inhibitor Cocktail 2 (Sigma).
The cleared lysate was then incubated with 5µl of mouse-anti-GFP antibody (3E6, Molecular Probes) for 1h at 4°C before the addition of 50 µl of 1∶1 protein G beads (Invitrogen) in lysis buffer for 1h at 4°C. The beads were washed with the lysis buffer and kinase reaction buffer (10 mM HEPES pH 7.6, 50 mM KCl, 5 mM MgCl2, Complete EDTA-free protease inhibitor cocktail (Roche)) supplemented with 10× Protein Phosphatase Inhibitor Cocktail 2 (Sigma).
In a typical kinase reaction, the suspension of beads and kinase buffer was mixed with 5 µCi of γ-[32P]ATP (EasyTides, Perkin Elmer) and incubated at room temperature (20°C) for 60 min before the addition of 20 µl of 2× protein sample buffer. The samples were analyzed by SDS-PAGE, and dried gels were exposed to x-ray films (high performance autoradiography films, GE Healthcare).
Karyosome image analysis
Analysis of karyosome morphology was performed for images of oocytes stained for lamin and DNA. For each series of images through an oocyte nucleus, the optical sections within which the karyosome was visible were determined. Of these, the mid-optical section was selected for analysis (or the lower of the middle two optical sections where this was the case), and the karyosome morphology categorised. Relative intensities of H2ApT119 or H2A signal on the karyosome in the oocyte nucleus were calculated using images of oocytes stained for H2ApT119 or H2A and DNA. As described above, the mid-optical section within which the karyosome was visible was selected for analysis. Using ImageJ software (NIH), the area corresponding to the karyosome was selected and the maximum H2ApT119 signal intensity on the karyosome was obtained and divided by that in surrounding follicle cell nuclei (an average of maximum signal intensity measurements in three nuclei at similar focal planes) after both intensities had been normalized by subtracting the background maximum signal intensity for a randomly selected area in the oocyte cytoplasm. Relative intensities of DNA staining signal on the karyosome were obtained using the same analysis method, and a measurement of H2ApT119 or H2A signal relative to DNA staining signal on the karyosome was made by dividing the two values for each image. We always compare samples processed in parallel or within a short time frame, as the exact values can vary over time due to a change in various factors including the conditions of the fixative and antibodies. Karyosome staining patterns for oocytes stained for C(3)G or CAP-D2 and counterstained for DNA were categorised as described in ‘Results and Discussion’ and Figure 5. A student t test or χ2 test was used for statistical analysis.
Supporting Information
Zdroje
1. KeeneyS
GirouxCN
KlecknerN
1997 Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88 375 384
2. DernburgAF
McDonaldK
MoulderG
BarsteadR
DresserM
1998 Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94 387 398
3. McKimKS
Hayashi-HagiharaA
1998 mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev 12 2932 2942
4. KeeneyS
BaudatF
AngelesM
ZhouZH
CopelandNG
1999 A mouse homolog of the Saccharomyces cerevisiae meiotic recombination DNA transesterase Spo11p. Genomics 61 170 182
5. PageSL
HawleyRS
2004 The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 20 525 558
6. BishopDK
ParkD
XuL
KlecknerN
1992 DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69 439 456
7. GartnerA
MilsteinS
AhmedS
HodgkinJ
HengartnerMO
2000 A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol Cell 5 435 443
8. Di GiacomoM
BarchiM
BaudatF
EdelmannW
KeeneyS
2005 Distinct DNA-damage-dependent and –independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci U S A 102 737 742
9. HochwagenA
AmonA
2006 Checking your breaks: surveillance mechanisms of meiotic recombination. Curr Biol 16 R217 R228
10. HepworthSR
FriesenH
SegallJ
1998 NDT80 and the meiotic recombination checkpoint regulate expression of middle sporulation-specific genes in Saccharomyces cerevisiae. Mol Cell Biol 18 5750 5761
11. ChuS
HerskowitzI
1998 Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol Cell 1 685 696
12. LeuJY
RoederGS
1999 The pachytene checkpoint in S. cerevisiae depends on Swe1-mediated phosphorylation of the cyclin-dependent kinase Cdc28. Mol Cell 4 805 814
13. TearleR
Nüsslein-VolhardC
1987 Tübingen mutants stocklist. Drosophila Inform Service 66 209 226
14. Gonzalez-ReyesA
ElliottH
St JohnstonD
1997 Oocyte determination and the origin of polarity in Drosophila: the role of the spindle genes. Development 124 4927 4937
15. GhabrialA
RayRP
SchüpbachT
1998 okra and spindle-B encode components of the RAD52 DNA repair pathway and affect meiosis and patterning in Drosophila oogenesis. Genes Dev 12 2711 2723
16. StyhlerS
NakamuraA
SwanA
SuterB
LaskoP
1998 vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development 125 1569 1578
17. GhabrialA
SchüpbachT
1999 Activation of a meiotic checkpoint regulates translation of Gurken during Drosophila oogenesis. Nat Cell Biol 1 354 357
18. Staeva-VieiraE
YooS
LehmannR
2003 An essential role of DmRad51/SpnA in DNA repair and meiotic checkpoint control. EMBO J 22 5863 5874
19. MehrotraS
McKimKS
2006 Temporal analysis of meiotic DNA double-strand break formation and repair in Drosophila females. PLoS Genet 2 e200 10.1371/journal.pgen.0020200
20. ChenY
PaneA
SchüpbachT
2007 cutoff and aubergine mutations result in retrotransposon upregulation and checkpoint activation in Drosophila. Curr Biol 17 637 642
21. KlattenhoffC
BratuDP
McGinnis-SchultzN
KoppetschBS
CookHA
2007 Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev Cell 12 45 55
22. PaneA
WehrK
SchüpbachT
2007 zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline. Dev Cell 12 851 862
23. AbduU
BrodskyM
SchüpbachT
2002 Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr Biol 12 1645 1651
24. MaloneCD
BrenneckeJ
DusM
StarkA
McCombieWR
2009 Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 137 522 535
25. TomancakP
GuichetA
ZavorszkyP
EphrussiA
1998 Oocyte polarity depends on regulation of gurken by Vasa. Development 125 1723 1732
26. KingRC
1970 Ovarian development in Drosophila melanogaster New York Academic Press 227
27. ParfenovV
PotchukalinaG
DudinaL
KostyuchekD
GruzovaM
1988 Human antral follicles: oocyte nucleus and the karyosphere formation (electron microscopic and autoradiographic data). Gamete Res 22 219 231
28. IvanovskaI
KhandanT
ItoT
Orr-WeaverTL
2005 A histone code in meiosis: the histone kinase, NHK-1, is required for proper chromosomal architecture in Drosophila oocytes. Genes Dev 19 2571 2582
29. CullenCF
BrittleAL
ItoT
OhkuraH
2005 The conserved kinase NHK-1 is essential for mitotic progression and unifying acentrosomal meiotic spindles in Drosophila melanogaster. J Cell Biol 171 593 602
30. AiharaH
NakagawaT
YasuiK
OhtaT
HiroseS
2004 Nucleosomal histone kinase-1 phosphorylates H2A Thr 119 during mitosis in the early Drosophila embryo. Genes Dev 18 877 888
31. LancasterOM
CullenCF
OhkuraH
2007 NHK-1 phosphorylates BAF to allow karyosome formation in the Drosophila oocyte nucleus. J Cell Biol 179 817 824
32. RiechmannV
EphrussiA
2001 Axis formation during Drosophila oogenesis. Curr Opin Genet Dev 11 374 383
33. HarrisonJC
HaberJE
2006 Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet 40 209 235
34. PageSL
HawleyRS
2001 c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev 15 3130 3143
35. SavvidouE
CobbeN
SteffensenS
CotterillS
HeckMM
2005 Drosophila CAP-D2 is required for condensin complex stability and resolution of sister chromatids. J Cell Sci 118 2529 2543
36. ResnickTD
DejKJ
XiangY
HawleyRS
AhnC
2009 Mutations in the chromosomal passenger complex and the condensin complex differentially affect synaptonemal complex disassembly and metaphase I configuration in Drosophila female meiosis. Genetics 181 875 887
37. AshburnerM
GolicKG
HawleyRS
2005 Drosophila: a laboratory handbook New York Cold Spring Harbor Laboratory Press 1409
38. DrysdaleRA
CrosbyMA
The FlyBase Consortium 2005 FlyBase: genes and gene models. Nuc Acids Res 33 D390 D395
39. LindsleyDL
ZimmGG
1992 The genome of Drosophila malanogaster New York Academic Press 1133
40. LaskoPF
AshburnerM
1990 Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev 4 905 921
41. HarlowE
LaneD
1988 Antibodies: a laboratory manual New York Cold Spring Harbor Laboratory Press 726
42. TheurkaufWE
SmileyS
WongML
AlbertsBM
1992 Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development 115 923 936
43. StuurmanN
MausN
FisherPA
1995 Interphase phosphorylation of the Drosophila nuclear lamin: site-mapping using a monoclonal antibody. J Cell Sci 108 3137 3144
44. AndersonLK
RoyerSM
PageSL
McKimKS
LaiA
2005 Juxtaposition of C(2)M and the transverse filament protein C(3)G within the central region of Drosophila synaptonemal complex. Proc Natl Acad Sci U S A 102 4482 4487
45. BrittleAL
OhkuraH
2005 Mini spindles, the XMAP215 homologue, suppresses pausing of interphase microtubules in Drosophila. EMBO J 24 1387 1396
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



