-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
Zn2+-finger proteins comprise one of the largest protein superfamilies with diverse biological functions. The ATM substrate Chk2-interacting Zn2+-finger protein (ASCIZ; also known as ATMIN and ZNF822) was originally linked to functions in the DNA base damage response and has also been proposed to be an essential cofactor of the ATM kinase. Here we show that absence of ASCIZ leads to p53-independent late-embryonic lethality in mice. Asciz-deficient primary fibroblasts exhibit increased sensitivity to DNA base damaging agents MMS and H2O2, but Asciz deletion or knock-down does not affect ATM levels and activation in mouse, chicken, or human cells. Unexpectedly, Asciz-deficient embryos also exhibit severe respiratory tract defects with complete pulmonary agenesis and severe tracheal atresia. Nkx2.1-expressing respiratory precursors are still specified in the absence of ASCIZ, but fail to segregate properly within the ventral foregut, and as a consequence lung buds never form and separation of the trachea from the oesophagus stalls early. Comparison of phenotypes suggests that ASCIZ functions between Wnt2-2b/ß-catenin and FGF10/FGF-receptor 2b signaling pathways in the mesodermal/endodermal crosstalk regulating early respiratory development. We also find that ASCIZ can activate expression of reporter genes via its SQ/TQ-cluster domain in vitro, suggesting that it may exert its developmental functions as a transcription factor. Altogether, the data indicate that, in addition to its role in the DNA base damage response, ASCIZ has separate developmental functions as an essential regulator of respiratory organogenesis.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001170
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001170Summary
Zn2+-finger proteins comprise one of the largest protein superfamilies with diverse biological functions. The ATM substrate Chk2-interacting Zn2+-finger protein (ASCIZ; also known as ATMIN and ZNF822) was originally linked to functions in the DNA base damage response and has also been proposed to be an essential cofactor of the ATM kinase. Here we show that absence of ASCIZ leads to p53-independent late-embryonic lethality in mice. Asciz-deficient primary fibroblasts exhibit increased sensitivity to DNA base damaging agents MMS and H2O2, but Asciz deletion or knock-down does not affect ATM levels and activation in mouse, chicken, or human cells. Unexpectedly, Asciz-deficient embryos also exhibit severe respiratory tract defects with complete pulmonary agenesis and severe tracheal atresia. Nkx2.1-expressing respiratory precursors are still specified in the absence of ASCIZ, but fail to segregate properly within the ventral foregut, and as a consequence lung buds never form and separation of the trachea from the oesophagus stalls early. Comparison of phenotypes suggests that ASCIZ functions between Wnt2-2b/ß-catenin and FGF10/FGF-receptor 2b signaling pathways in the mesodermal/endodermal crosstalk regulating early respiratory development. We also find that ASCIZ can activate expression of reporter genes via its SQ/TQ-cluster domain in vitro, suggesting that it may exert its developmental functions as a transcription factor. Altogether, the data indicate that, in addition to its role in the DNA base damage response, ASCIZ has separate developmental functions as an essential regulator of respiratory organogenesis.
Introduction
Pathways that maintain genome integrity by responding to spontaneous DNA damage are crucial for normal development and ageing, and act as tumor suppressors to prevent the onset of cancer [1]. While DNA damage signaling is rather generic in that structurally diverse lesions eventually lead to activation of one or both of the central checkpoint kinases ATM or ATR [2], DNA repair pathways are believed to be highly lesion-specific [1]. In addition to environmental DNA damage, eukaryotic cells incur a high level of spontaneous DNA damage as a consequence of normal metabolism, most notably abasic sites that are generated as repair intermediates of the base excision repair (BER) pathway with an estimated incidence of ∼10,000 per cell per day [3]. Abasic sites can emanate from various base modifications (e.g. oxidation, methylation), which for experimental purposes are most commonly generated by treatment with methylmethane sulfonate (MMS) or H2O2, and key BER enzymes for their repair include apyrimidinic/apurinic endonuclease (APE) and DNA polymerase beta (Polß) [4], [5].
The importance of the BER pathway is indicated by findings that absence of any of the BER genes acting downstream of abasic sites results in embryonic or perinatal lethality in mice [6], [7]. However, some of the key BER enzymes also seem to have DNA damage-independent functions; for example, APE1 has a separate role as a redox regulator of several transcription factors [8]. Similarly, increased apoptotic cell death during development of Polß-null mice can be suppressed by deletion of p53 (TRP53), indicating that this part of the phenotype is indeed due to defective base damage repair. On the other hand, the perinatal lethality of these mice that is associated with defective neuronal and lung development as a DNA damage-independent defect is not rescued by p53 deletion [9]–[11].
While the DNA damage processing enzymes of the BER pathway are clearly defined, new accessory factors that regulate the activity or stability of Polß and other BER enzymes keep emerging, including the non-histone DNA-binding protein HMGB1 [12], arginine methyl-transferases [13], and ubiquitin ligases [14]. We recently identified ASCIZ ( = ATM substrate Chk2-interacting Zn2+-finger) as a new Zn2+-finger (ZnF) protein with roles in the DNA base damage response. In human cells, ASCIZ forms DNA damage-induced nuclear foci specifically in response to DNA damaging agents that generate lesions repaired by the BER pathway (MMS and H2O2) in a manner that is enhanced by the BER inhibitor methoxyamine, and Asciz depletion by siRNA leads to increased MMS sensitivity [15]. Likewise, Asciz deletion in the chicken DT40 B lymphocyte line leads to increased sensitivity to MMS and H2O2, but not to ionizing radiation (IR), UV irradiation and other DNA lesions, as well as increased erroneous repair of enzyme-generated DNA base damage consistent with a role in the BER pathway [16]. Moreover, Asciz deletion suppresses the dramatic MMS hypersensitivity of Polß-deficient DT40 cells [16], reminiscent of the protective effect of simultaneous deletion of the relevant upstream methyl-purine-glycosylase (MPG) in Polß-deficient murine embryonic fibroblasts [17]. ASCIZ contains a large number of conserved ATM/ATR kinase phosphorylation sites in an SQ/TQ cluster domain [18], and consistent with its original classification as an ATM substrate, ASCIZ was subsequently re-isolated as an ATM-interacting protein (thus also called ATMIN)[19]. It was proposed that ASCIZ acts as an essential co-factor of ATM that was required for ATM stability (and vice versa ATM was required for ASCIZ stability) as well as for ATM activation by some stimuli, though surprisingly not by canonical DNA damaging ATM activators such as IR [19].
To better understand the role of ASCIZ in vivo, we have here generated a mouse line that lacks the vast majority of the Asciz protein-coding sequence in the germline. Our results confirm that Asciz-deficient cells are specifically hypersensitive to DNA lesions that are processed by the BER pathway, but challenge the proposed interdependence between ASCIZ and ATM levels. In contrast to Atm-deficient mice that overall develop normally [20], Asciz deletion results in late embryonic lethality with severe respiratory defects reminiscent of mouse mutants in Wnt2/2b and FGF10 signaling pathways. The data indicate that Asciz has an unexpected DNA damage-independent developmental function as an essential regulator of pulmonary organogenesis.
Results
Generation of Asciz gene-targeted mice
Human and mouse Asciz have a similar gene structure where exons A–C encode the N-terminal ZnF region of about 220 amino acid residues, and exon D encodes the bulk of the protein (601 of 823 or 818 residues) including the nuclear localization signal, core domain and SQ/TQ cluster domain (Figure 1A, 1B; NCBI Gene ID 23300). Because there is evidence for expression of alternative isoforms that differ in the number of N-terminal ZnFs (http://www.uniprot.org/uniprot/O43313), we integrated loxP sites flanking exon D into the murine Asciz locus to remove the majority of the protein-coding sequence (Figure 1B). Germline deletion of this exon after crossing with PGK-Cre knock-in mice, followed by outcrossing of PGK-Cre (all on a pure C57BL/6 background), was confirmed by Southern blot and PCR genotyping (Figure 1C). In over 600 offspring from Asciz+/− heterozygote intercrosses genotyped at weaning (∼3 weeks of age), we failed to detect any homozygous Asciz-deleted mice (Figure 1C and Table 1). However, homozygous Asciz-deleted embryos were readily detectable even at relatively late stages of gestation (Figure 1D; and more detail below).
Fig. 1. Generation of Asciz-deficient mice. 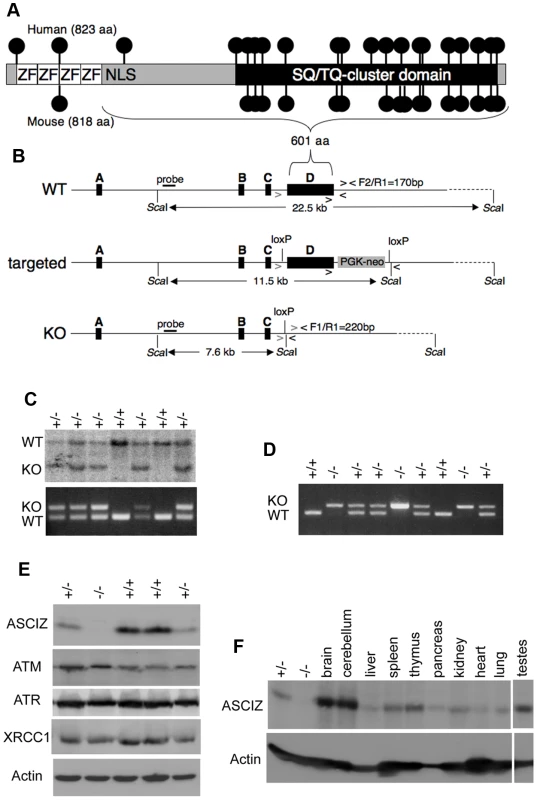
(A) Schematic comparison of human and mouse ASCIZ. ZF, Zn2+ finger; NLS, nuclear localization signal. Lollipops indicate predicted ATM/ATR phosphorylation sites. (B) Asciz gene structure and targeting strategy, drawn approximately to scale. The four exons (A–D) are indicated by black boxes, as are locations of oligonucleotide primers, ScaI restriction sites and the probe for genotyping, and the positions of loxP sites. (C) Southern blot (top) and PCR genotyping (bottom) of a randomly chosen litter from a heterozygote intercross at weaning. (D) PCR genotyping of a randomly chosen litter at E15.5. (E) Western blot analysis of head extracts of a randomly chosen litter at E12.5 using the indicated antibodies. (F) Western blot analysis of the indicated tissues of an 8-week old male WT mouse, and E15.5 Asciz+/− and Asciz−/− head extracts as antibody specificity controls. Tab. 1. Genotypes of Asciz+/− intercross litters. 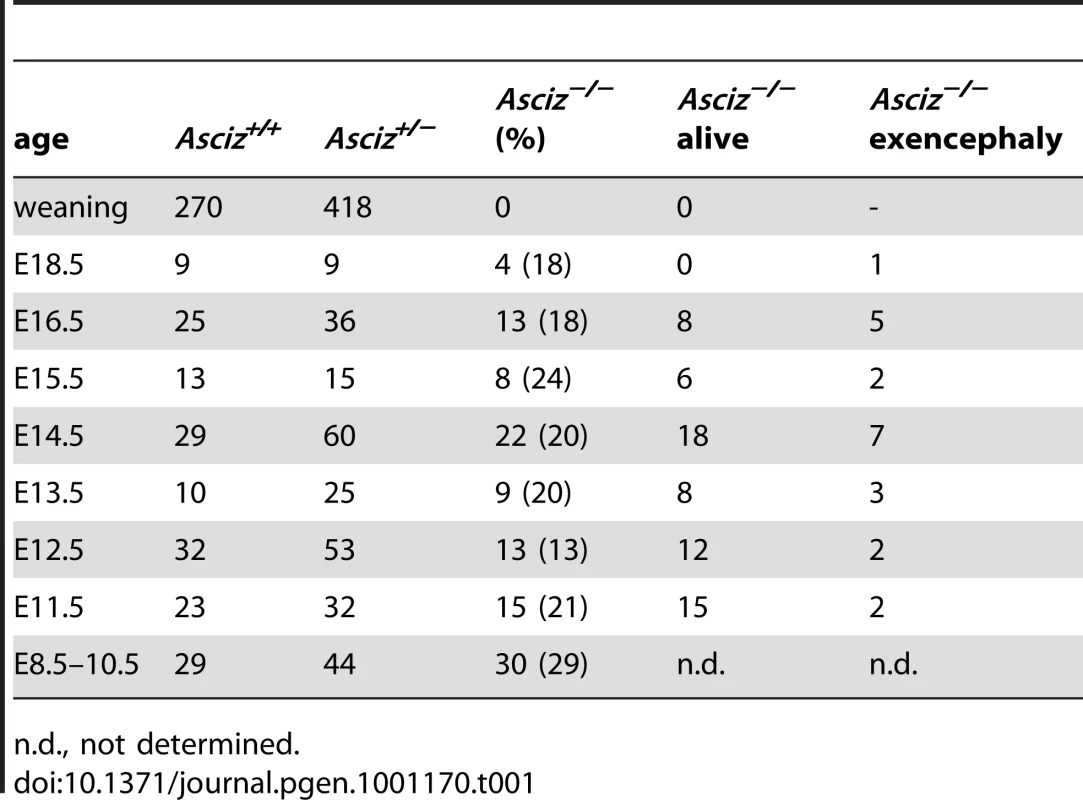
n.d., not determined. Western blotting of head extracts confirmed the absence of ASCIZ protein in Asciz−/− embryos, and a ∼50% reduction of protein levels in heterozygotes compared to wildtype (WT) littermates (Figure 1E). Levels of other DNA damage response proteins (including ATM) appeared to be normal in Asciz-deficient embryos (Figure 1E and below). In Northern blots using a probe for the non-deleted exon C, the residual exon D-deleted Asciz transcript was present in homozygous targeted embryos at <15% of wildtype (WT) mRNA levels (Figure S1), indicating that the mutated mRNA is highly unstable. Using Asciz null embryo lysates as an antibody specificity control, we found that ASCIZ is ubiquitously expressed in adult mice, with overall similar levels relative to the loading control in all tissues except for somewhat higher levels in the brain, cerebellum and testes (Figure 1F).
Absence of Asciz leads to p53-independent late-embryonic lethality
The absence of homozygous Asciz−/− mice at weaning prompted us to investigate the development of ASCIZ-deficient embryos in more detail. Asciz−/− embryos were recovered at near-Mendelian ratios at all time points analysed (Table 1). Based on peripheral circulation scored during uterine dissections, Asciz−/− embryos appeared to lose viability around embryonic day 16.5 post conception (E16.5) (Table 1), at which point they were considerably growth-retarded compared to littermates (Figure 2A, 2B). Embryonic lethality due to DNA damage response gene deletions can often be suppressed by p53 deletion [6]. To test if p53 status affects the essential requirement for Asciz, we intercrossed compound Asciz+/−/p53+/− heterozygous mice. However, we could again not detect any viable Asciz−/− mice amongst >300 genotyped offspring at weaning (Table 2). Altogether, these data indicate that absence of Asciz leads to progressively impaired development during late gestation and becomes absolutely incompatible with life a few days before term.
Fig. 2. Late gestational growth defect of Asciz-deficient embryos. 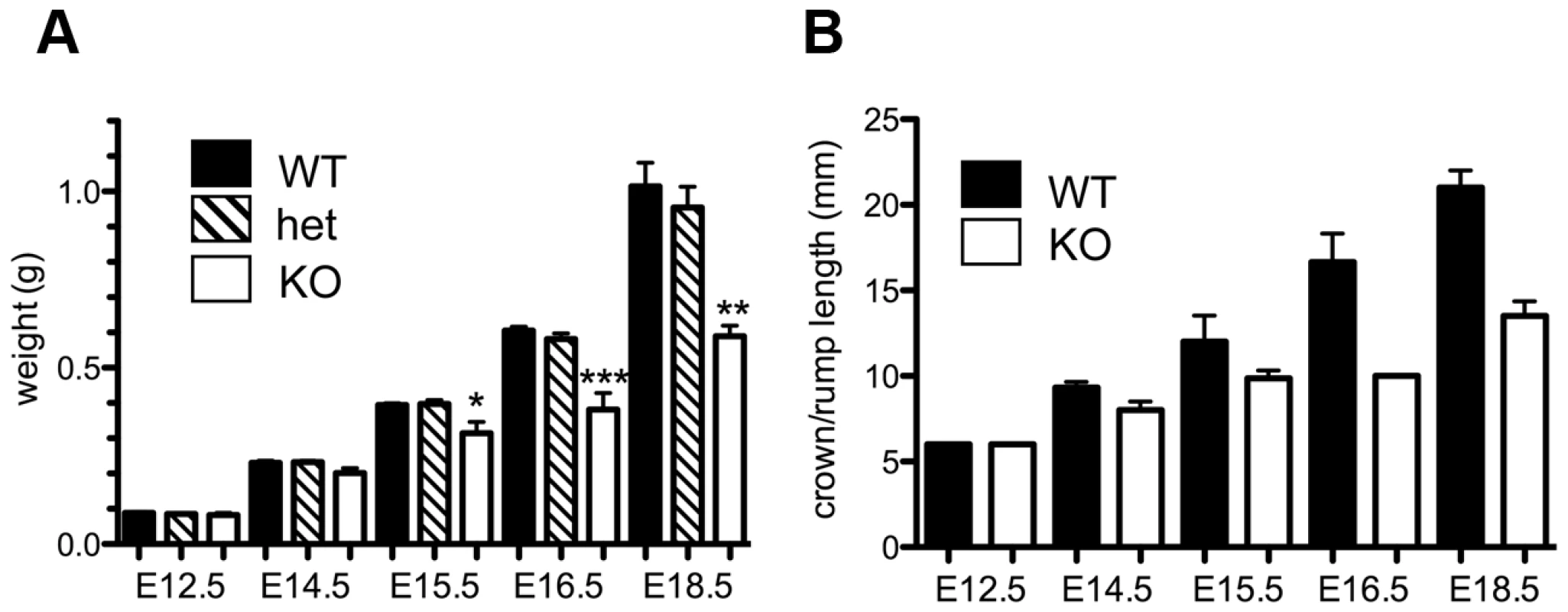
(A) Embryo weights of WT, heterozygotes and Asciz-deficient embryos at the indicated times post-conception. Data are the mean ± standard error; 22–55 embryos were analysed per timepoint (KOs: n = 8 (E12.5), 12 (E14.5), 8 (E15.5), 11 (E16.5), and 4 (E18.5). (B) Crown-rump length of embryos determined by histomorphometry. Data are mean ± standard error, n = 3–9 per data point. *p<0.05, **p<0.01, ***p<0.001. Tab. 2. Genotypes of offspring from <i>Asciz<sup>+/−</sup> p53<sup>+/−</sup></i> intercrosses at weaning. 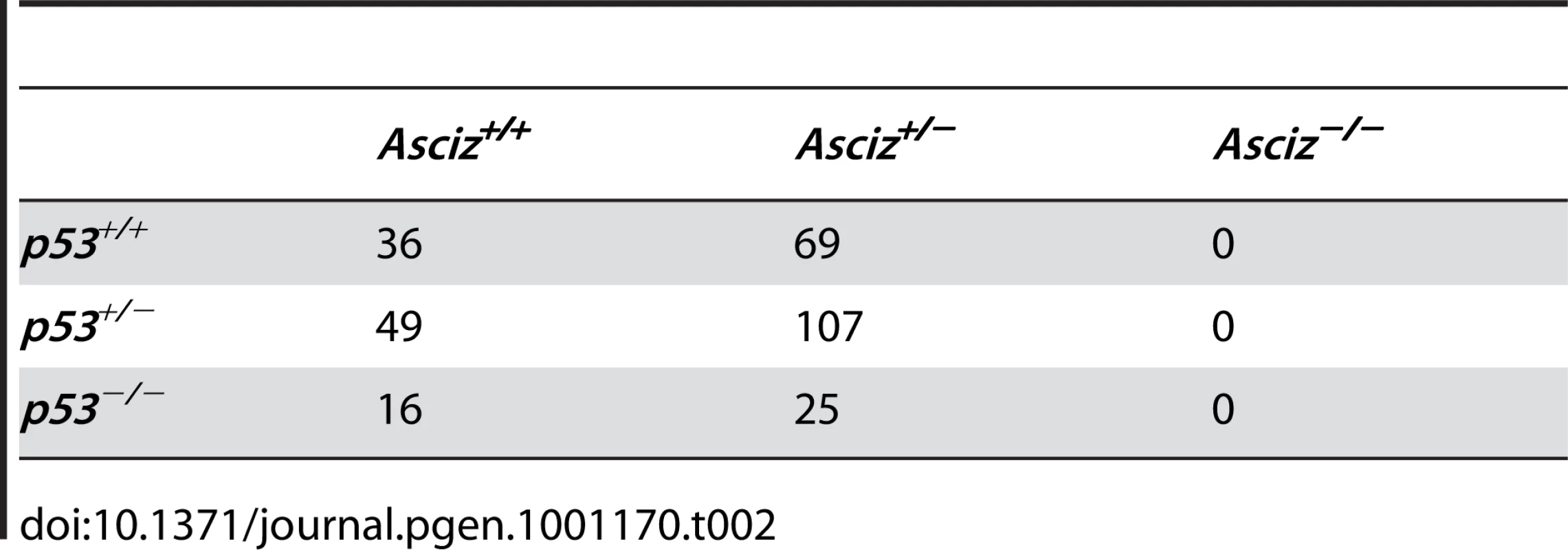
Asciz deficiency leads to modestly accelerated senescence and BER-like DNA damage hypersensitivity in primary fibroblasts
To monitor DNA damage sensitivity of primary Asciz-deficient cells, we isolated murine embryonic fibroblasts (MEFs) from viable Asciz−/− embryos and matched WT littermate controls between E12.5–E14.5 (i.e., before growth retardation was apparent). Standardized proliferation assays using a 3T3 protocol under normoxic conditions (20% O2) revealed a modest premature senescence phenotype of Asciz-deficient MEFs compared to WT controls, with growth arrest after ∼20% fewer population doublings (Figure 3A). When normalized to the maximum population doublings within each litter, Asciz−/− MEFs always senesced earlier than the matched WT cultures (Figure 3B). As senescence of MEFs under these conditions is believed to involve an oxygen-induced DNA damage response [21], these results indicated a role of ASCIZ in the response to oxidative base damage in primary cells. To corroborate this, we treated early-passage MEFs (P2–P3, when proliferation differences between genotypes were minimal) with a panel of DNA damaging agents. In these assays, Asciz-deficient MEFs were significantly more sensitive to MMS and H2O2, which cause damage that is predominantly repaired by the BER pathway, compared to matched WT littermate controls (Figure 3C, 3D), but they were not hypersensitive to agents such as UV or hydroxyurea (HU) whose damage is repaired by other pathways (Figure 3E, 3F). MMS hypersensitivity of Asciz−/− MEFs was less pronounced than that of WT cells co-treated with methoxyamine (Figure S2), which blocks the single-nucleotide BER pathway through partial inhibition of APE1 and Polß [5]. In addition, MMS hypersensitivity of ASCIZ-deficient cells was further enhanced by methoxyamine (Figure S2), indicating that absence of ASCIZ only partially impairs BER. Altogether, these results are consistent with a role of Asciz as an accessory factor in the BER pathway in primary cells.
Fig. 3. Cellular phenotypes of Asciz-deficient primary fibroblasts. 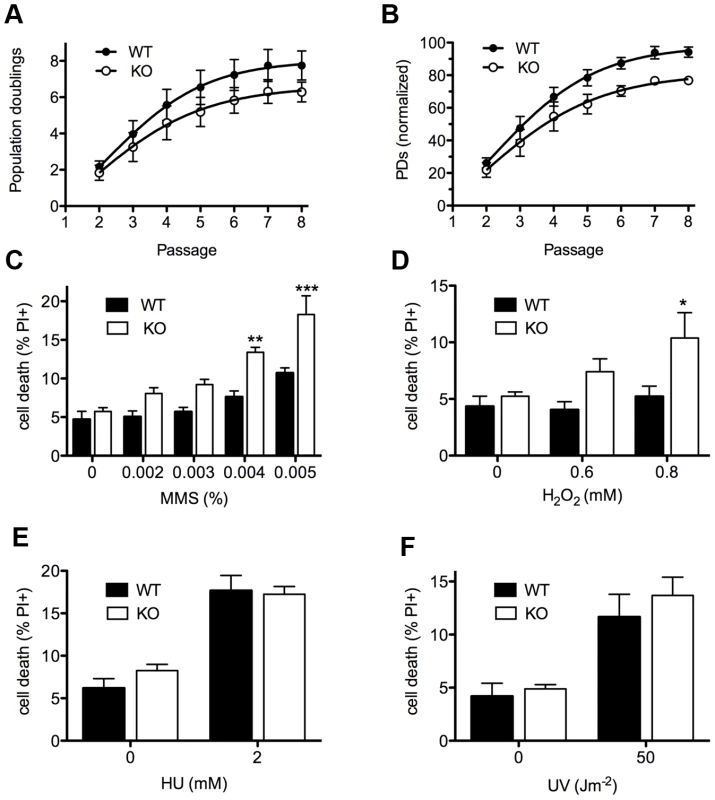
(A) Cumulative population doublings (PDs) of Asciz-deficient and matched WT littermate MEFs in a standardized 3T3 assay. Data are mean ± standard error, n = 6. (B) Data from panel A normalized to the PD maximum in the relevant litter. (C–F) DNA damage sensitivity assays. Cells were treated with the indicated doses of MMS, H2O2, 2 mM HU or 50 J/m2 UV, and viability was determined by propidium iodide exclusion and FACS. *p<0.05, **p<0.01, ***p<0.001. Because we had originally identified ASCIZ based on its interaction with Chk2 [15], and because ASCIZ was later proposed to be sometimes required for ATM activation [19], we tested if the MMS hypersensitivity of Asciz−/− MEFs could be due to defective ATM signaling. However, there was no reduction in MMS-dependent ATM activation (detected by an antibody against mouse pS1987-ATM; human pS1981-ATM) and phosphorylation of key ATM/ATR targets γH2AX and pS18-p53 in Asciz-deficient MEFs compared to WT littermate controls (Figure S3A, S3B). MMS-induced p53-S18 phosphorylation was completely abolished by the highly specific synthetic ATM kinase inhibitor KU55933 [22] (Figure S3C), indicating that it is a genuinely ATM-dependent process, whereas H2AX phosphorylation under these conditions was ATM-independent and thus likely ATR-mediated. Because our antibody for Chk2 phosphorylation on T68, widely considered to be one of the most ATM-specific phosphorylation sites, did not detect this site in MEF extracts (data not shown), we monitored Chk2-T68 phosphorylation in human U2OS cells following Asciz depletion by siRNA treatment. However, Chk2 was still efficiently phosphorylated in response to MMS (as well as IR) in Asciz-depleted cells (Figure S4A, S4B). Altogether, these data indicate that the increased MMS sensitivity of Asciz-deficient cells is not caused by impaired ATM signaling.
ASCIZ and ATM protein levels are not interdependent in mouse, human, or chicken cells
During analyses of DNA damage signaling in Asciz-depleted human cells (Figure S4B and data not shown) and initial protein blots of embryo extracts (Figure 1E), we could not detect any meaningful reduction of ATM levels in Asciz-deficient cells. Because this contradicted the recent report that ASCIZ and ATM levels were mutually dependent on each other [19], we explored this discrepancy first in additional embryos. Again, we saw no reduction of ATM protein levels in any of the Asciz−/− or heterozygous samples compared to WT littermate controls (Figure 4A, left panel, and data not shown). Likewise, we also did not see a reduction of ATM levels in human cells after almost complete depletion of ASCIZ by siRNA-treatment (Figure 4B, left panel, compare control siRNA treated to si-ASCIZ treated U2OS cells, lanes 1 and 2; and data not shown), or in two independently generated ASCIZ knockout clones [16] in the chicken DT40 B cell line (Figure 4C, left panel).
Fig. 4. Reciprocal independence of ASCIZ and ATM protein levels. 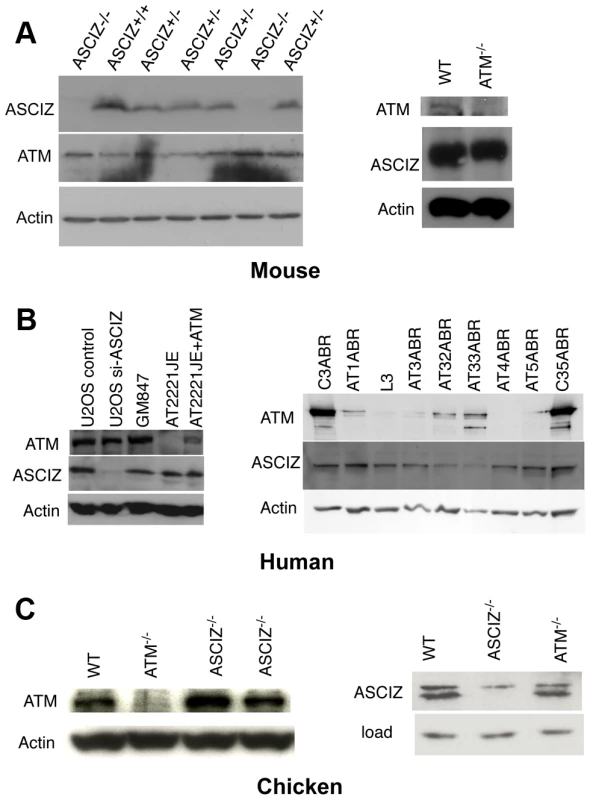
(A) Protein levels in mouse tissues. Left panel, Western blot analysis of head extracts of a randomly chosen litter from an Asciz heterozygote intercross at E12.5. Right panel, brain extracts of WT and Atm-null littermate mice [20]. (B) Protein levels in human cell lines. Left panel, adherent cells: U2OS osteosarcoma cells treated with GL2 control or Asciz siRNA; GM847 control fibroblasts, Atm-deficient AT2221JE fibroblasts containing an empty-vector control (FTY pEBS7) or reconstituted with WT Atm (FTYZ5) [23]. Right panel, lymphoblastoid cell lines from healthy donors (C3ABR, C35ABR) and seven separate AT patients (L3 and AT1ABR–AT33ABR); note that ATM was immunoprecipitated before blotting as described [24]. (C) Protein levels in chicken DT40 B cell lysates. Left panel, comparison of ATM levels in two independent Asciz-deleted clones using the anti-chicken ATM antibody and the ATM-deleted DT40 clone as specificity control. Right panel, comparison of ASCIZ levels in WT and an Atm-deleted clone [25] with an Asciz-deficient clone [16] as antibody specificity control (NB, anti-human ASCIZ was used at 1∶100 dilution rather than 1∶2000–1∶4000 for mouse or human samples). We also revisited the proposal that ATM was in turn required for ASCIZ stability [19]. In contrast to the severe reduction of ASCIZ levels in an ataxia telangiectsia (AT) fibroblast line reported by Kanu and Behrens (2007), we did not detect any loss of ASCIZ in another human AT patient-derived fibroblast cell line (Figure 4B, left panel, AT2221JE) that is considered to be bona fide ATM-deficient [23] compared to control fibroblasts (Figure 4B, left panel, GM847) or the isogenic AT cell line reconstituted with WT Atm (Figure 4B, left panel, AT2221JE+ATM [23]). We expanded this analysis to a panel of seven independent human AT-patient derived lymphoblastoid cell lines [24]. When adjusted for loading (actin), there was no reduction of ASCIZ levels in four of these lines that contained no or extremely low levels of residual ATM protein (AT1ABR, L3, AT4ABR, AT5ABR) compared to two independent healthy donor control cell lines (C3ABR, C35ABR)(Figure 4B, right panel); ASCIZ levels were also unaffected in two further AT lines that contained intermediate ATM protein levels (AT1ABR, AT33ABR), and there was only a modest reduction of ASCIZ levels in a third AT cell line with intermediate ATM levels (AT32ABR). Similarly, there was also no reduction of ASCIZ protein levels in brain lysates of Atm null mice [20] compared to WT littermates (Figure 4A, right panel), or in an Atm-deleted chicken DT40 clone [25] compared to the WT control (Figure 4C, right panel). Taken together, these data demonstrate that ASCIZ and ATM are not required for each other's stability in three different vertebrate species.
Asciz is essential for pulmonary organogenesis
Because of the overall relatively mild DNA damage phenotypes of Asciz-deficient primary cells (Figure 3) and the absence of a p53-effect on viability (Table 2), we wondered whether the underlying cause for the late gestational lethality of Asciz−/− embryos could be DNA damage-independent, and performed histological analyses of litters between E12.5 and E18.5. The most striking defect at all time points was the complete absence of lungs in all Asciz-deficient embryos analyzed (n>30; Figure 5A–5C) and apparent lack of tracheal tissue in all but one of these (Figure 5C and data not shown); consistently, in all cases where absence of lungs was subsequently noticed during routine MEF or protein preparations, this phenotype was 100% predictive of the Asciz−/− genotype (not shown).
Fig. 5. Histological analysis of Asciz-null embryos. 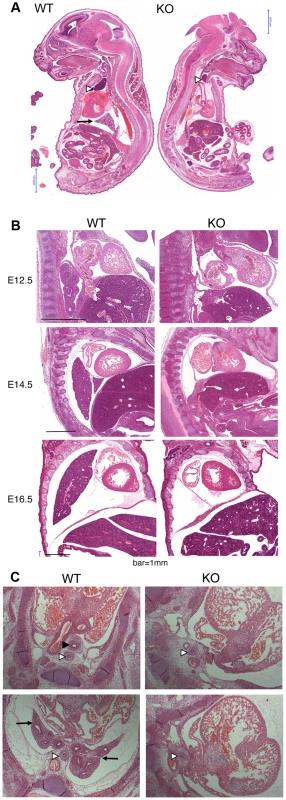
(A) Sagittal sections of comparable levels of WT and Asciz−/− littermates at E18.5. Note the absence of lung (arrow), hypoplastic thymus (arrowhead), compressed thorax, steep ascending aorta, and exencephaly in the Asciz-null embryo. This embryo also represents an isolated case of omphalocele. Scale bars = 2 mm. (B) Micrographs of comparable sagittal sections of WT and Asciz−/− littermates at E12.5–16.5. Note the apparent caudal drop of the atrium relative to the ventricle in Asciz null embryos compared to WT littermates where the atrium seems to be propped up by the developing left lung. Scale bars = 1 mm. (C) Micrographs of comparable transverse sections of E12.5 WT and Asciz−/− littermates at the upper (top panels) and lower levels (bottom panels) of the thorax. Open arrowheads point to the oesophagus, the filled arrowhead and arrows point at the trachea and lungs respectively that are only present in the WT. Interestingly, the absence of lungs seemed to lead to topological alterations in the position of the heart and its axis within the thoracic cavity, with an apparent drop of the atrium in Asciz null embryos into the space otherwise occupied by the lung in WT littermates (Figure 5B, 5C). In addition, the thymus appeared hypoplastic in all Asciz−/− embryos analyzed (Figure 5A), which could also be a secondary consequence of the defective respiratory system as the thymus descends into the mediastinum from its common origin with the parathyroid gland in close proximity of the upper trachea [26]. Besides these thoracic defects, ∼25% of Asciz−/− embryos exhibited already macroscopically obvious exencephaly (Figure 5A and Table 1), indicating that ASCIZ also contributes to neural tube development, but histologically other organs seemed to be developing normally.
ASCIZ is required for separation of respiratory progenitors from the ventral foregut
The combined trachea and lung defects in Asciz−/− embryos were interesting because both organs originate at the same time but presumably independently of each other from the common respiratory endoderm in the ventral foregut [27], [28]. Shortly after specification of respiratory precursors that are characterized by expression of the Nkx2.1 transcription factor, bilateral lung buds and, just rostral of these, a central tracheal primordium emerge from the ventral foregut around E9.5 in the mouse. The junction of these primordia marks the bifurcation of the trachea into the two main bronchi, and the lung buds expand caudally into the surrounding mesoderm to form the bronchial tree and pulmonary epithelium by branching morphogenesis, whereas the trachea septates from the common foregut lumen in an upwards “unzipping” motion [27], [28]. Thus, in a simplified view, the origin of the respiratory system can be traced back to the projection of the tracheo-bronchial bifurcation onto the ventral oesophagus.
To more clearly assess the developing trachea and lungs in three dimensions, we performed optical projection tomography (OPT) on whole-mount E-cadherin stained embryos. WT embryos showed clear separation of oesophagus and trachea at the larynx, bifurcation of the trachea into two bronchi and advanced branching of the developing pulmonary epithelium (Figure 6A, 6C). As expected, all five Asciz−/− embryos analyzed again lacked developing pulmonary epithelium (Figure 6B, 6D, Figure S5, and data not shown). One Asciz null embryo contained a very short incompletely separated tracheal stump that ended bluntly where it would normally connect to the main bronchi (Figure 6B). Interestingly, the other Asciz null embryos contained single centrally located bud-like structures that emerged from the ventral oesophagus near the level where the trachea bifurcates into bronchi in the relevant WT littermates (Figure 6D, Figure S5); the central location suggested that this bud-like structure represented tracheal primordium.
Fig. 6. Defective pulmonary and tracheal development in Asciz-null embryos. 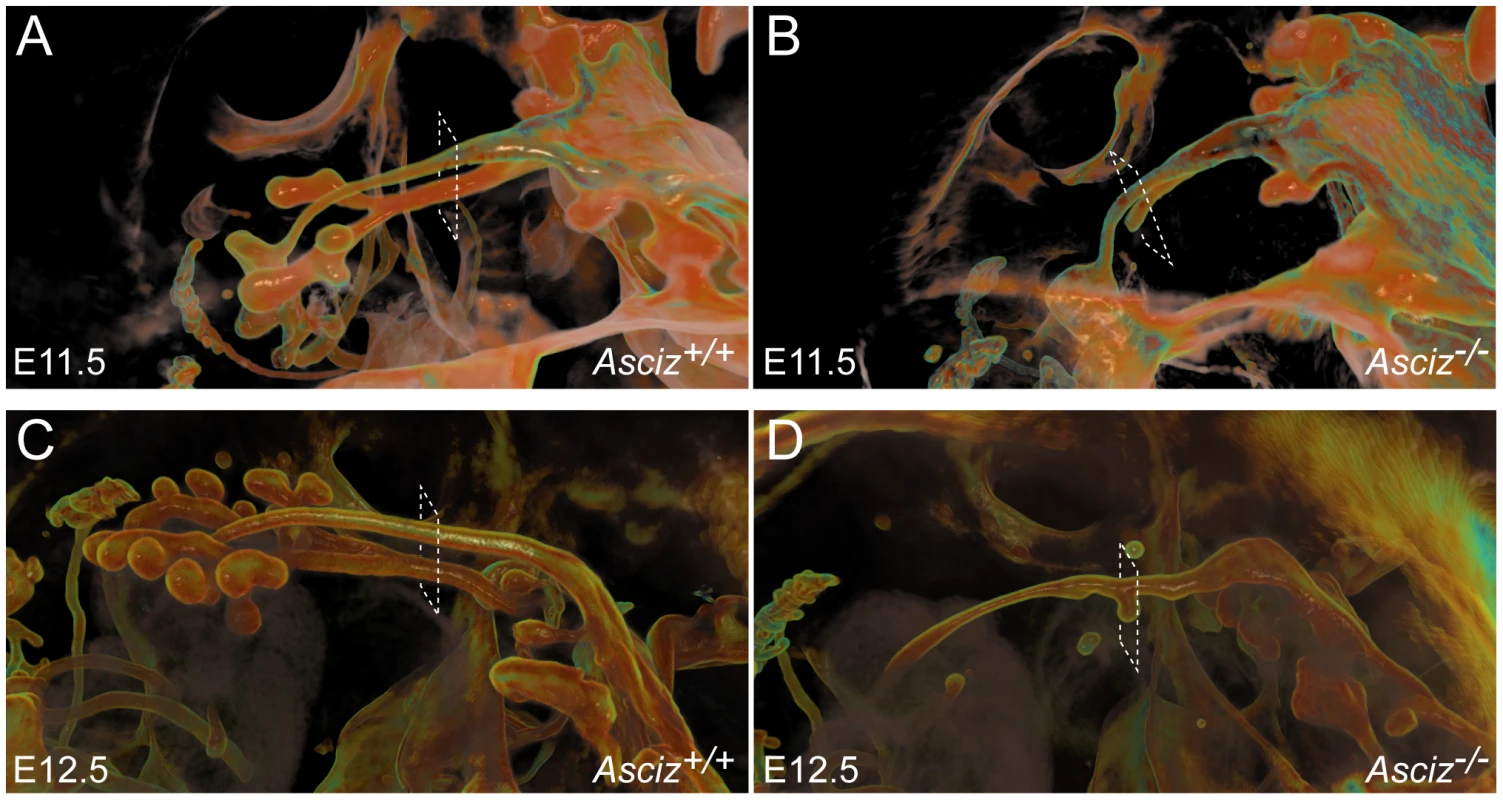
Optical projection tomography of whole-mount E-cadherin stained of E11.5 (A, B) and E12.5 (C, D) littermates. Stippled boxes indicate the approximate plane of sections chosen for immunofluorescence analysis in Figure 7. Panels are arranged with the oesophagus on top. Two of the Asciz−/− whole-mount embryos and littermate controls were sectioned at the level of the truncated trachea (Figure 7B, 7B′) or tracheal bud-like structure (Figure 7D, 7D′) for immunofluorescence staining with the respiratory marker Nkx2.1. The tracheal stump in the mutant stained homogenously with Nkx2.1 (Figure 7B, bottom panel), similar to the trachea in the WT littermate (Figure 7A), and the ventral part of the tracheal bud-like structure in the other Asciz−/− embryo was also enriched for Nkx2.1 (Figure 7D′) with staining intensity similar to the separated trachea in the matched WT littermate control (Figure 7C′). Interestingly, in stark contrast to the WT oesophagus, some ectopic Nkx2.1-positive cells remained in the ventral part of the oesophagus in the mutant where the trachea had partially separated (Figure 7B, top panel).
Fig. 7. Expression analysis of markers of foregut development. 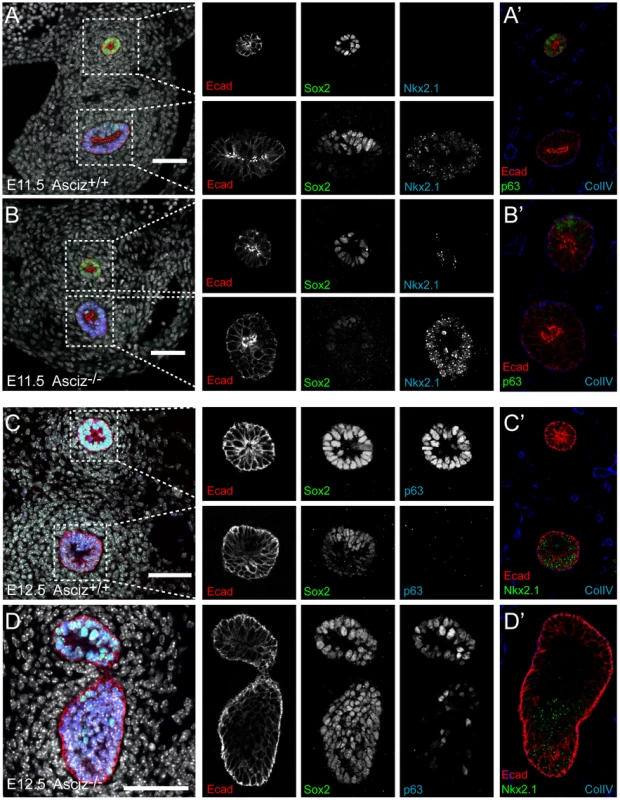
Sections from the levels indicated in Figure 6 were stained with the indicated antibodies. All panels are oriented with the oesophagus or dorsal foregut on top. A′–D′ are sections adjacent to the ones shown in A–D. In the merged panel on the left, nuclei are counterstained with DAPI. Scale bars = 20 µm. We also analysed these sections for expression of p63, a p53-like transcription factor that is normally highly expressed in the oesophagus, but also present in basal cells of the trachea [29]. Under our staining conditions at the developmental stages studied here, p63 seemed only to be present in the oesophagus but not in the trachea in WT embryos (Figure 7A′, 7C). However, p63-positive cells were readily detectable in the ventral part of the tracheal bud-like structure in the Asciz−/− embryo (Figure 7D), suggesting defective partitioning of specified cells between trachea and oesophagus. As ectopic p63 expression can result from increased Sox2 levels [29], [30], a transcription factor involved in foregut separation that is normally highly expressed in the oesophagus and dorsal part of the trachea but downregulated in the ventral part of the developing trachea, we also monitored Sox2 expression in these sections. WT tracheas (Figure 7A, 7C, bottom panels) and the partially separated Asciz−/− trachea (Figure 7B, bottom panel) exhibited the expected dorsally polarized Sox2 expression pattern; in contrast, Sox2 was still expressed at high levels throughout the ventral part of the bud-like structure in the Asciz−/− embryo (Figure 7D). Thus, while impaired local down-regulation of Sox2 could contribute to the Asciz−/− phenotype, it is interesting to note that most of the ectopic Sox2-positive cells in the tracheal bud-like structure were still able to downregulate p63. We also observed aberrantly high Sox2 levels in the ventral foregut in Asciz−/− embryos around E10.25, i.e. before oesophagus and trachea were separated in the matched littermate control with appropriately down-regulated Sox2 (Figure S6), indicating that impaired dorso-ventral patterning of Sox2 expression is not merely a secondary consequence of impaired foregut separation in our mutant.
Altogether, these analyses indicate that Asciz-deficient mice are able to initially specify the respiratory endoderm, based on Nkx2.1 expression, but then fail to remodel the endoderm in a manner required for initiation of lung budding and efficient separation of the trachea.
The ASCIZ SQ/TQ-cluster domain has the propensity to activate transcription
When ASCIZ was originally isolated in a yeast two-hybrid screen [15], we noticed during vector-swapping control experiments that ASCIZ could very strongly activate yeast two-hybrid reporter genes on its own once it was fused to the Gal4 DNA-binding domain (Gal4-DBD). As a large proportion of genes that regulate foregut development function as transcription factors (e.g., Sox2, p63, Nkx2.1 mentioned above), and because the modular domain composition of ASCIZ resembles some ZnF transcription factors (see below), we revisited the yeast reporter system to explore the potential of ASCIZ to function as a transcriptional regulator. Both the four-ZnF 823-residue and the two-ZnF 667-residue splice isoforms of human ASCIZ were able to activate the GAL1-HIS3 and GAL2-ADE2 reporter genes in these one-hybrid assays (Figure 8A). Importantly, similar dual luciferase reporter assays in human U2OS cells using the 667-residue isoform demonstrated that ASCIZ also has an intrinsic ability to activate gene expression in mammalian cells when tethered to promoters (Figure 8B). Interestingly, truncation analysis revealed that the SQ/TQ-cluster domain - but not the ZnF or core domains - of ASCIZ was sufficient for reporter gene activation (Figure 8A).
Fig. 8. ASCIZ has transcription activating function in reporter assays. 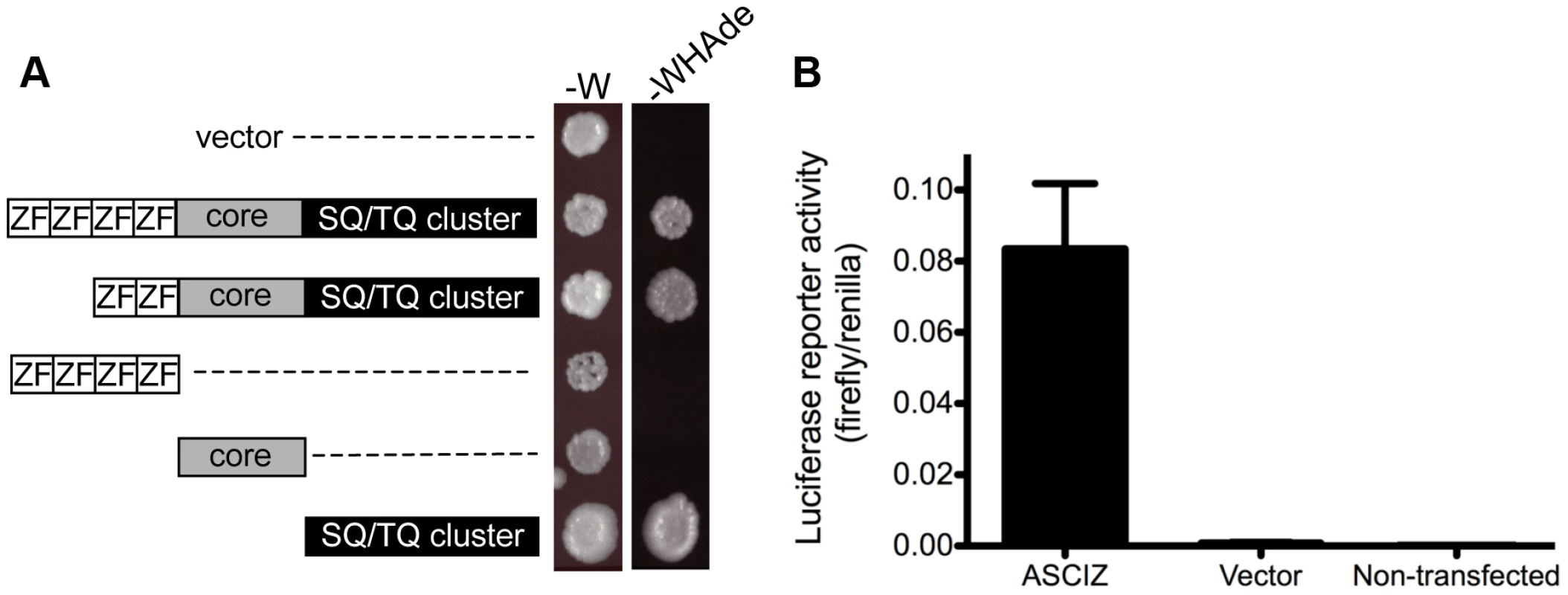
(A) Yeast one-hybrid assay. Yeast strains containing the empty vector expressing the Gal4-DBD only or the indicated human ASCIZ constructs (“long form”, residues 1–823; “short form”, residues 156–823; ZnF domain, residues 67–223; “core domain”, residues 230–442; SQ/TQ cluster domain, residues 432–823) were spotted onto -W plates as a loading control and -WHAde plates as an assay for activation of the GAL1-HIS3 and GAL2-ADE2 reporter genes. (B) Dual luciferase reporter assay of human U2OS cells transfected with pCDNA3-Gal4DBD or Gal4DBD-ASCIZ667. Discussion
DNA damage and ATM-related functions of ASCIZ
Here we have shown that Asciz is essential for pulmonary organogenesis during embryonic development in mice, and required for proper DNA base damage responses in primary cells. Although the lung defect is mechanistically most likely unrelated to defective DNA damage responses, the overall phenotype - MMS and H2O2 hypersensitivity and embryonic lethality - is consistent with a role of ASCIZ as an accessory BER factor downstream of glycosylases, as proposed by previous work in human and chicken cells [15], [16]. Although Asciz null embryos die a few days earlier and their lung defect is considerably more severe than in case of Polß-deficient embryos, the latter also seem to have a very comparable late gestational growth retardation [10], [11], and furthermore, the essential requirement for Polß is also not suppressed by deletion of p53 [9]. Likewise, embryos deficient in Yb-1, another protein recently linked to accessory functions in the BER pathway [31], [32], also share overall similar late embryonic growth retardation and lethality, frequent exencephaly and modestly increased cellular oxidative stress-induced senescence phenotypes [33].
In contrast to similarities with BER-related genes, the phenotype of Asciz-deficient mice differs fundamentally from the phenotype of Atm-deficient mice. For example, the key phenotype of Asciz-deficient mice - embryonic lethality with absence of lungs - is not shared by Atm-null mice [20], and the key phenotype of Atm-deficiency - dramatically increased ionizing radiation sensitivity - is not shared by Asciz-deficient cells [16], [19]. Consistent with normal ATM protein levels in human, mouse or chicken cells in the absence of ASCIZ, ATM signaling was also unaffected in our Asciz-deficient MEFs or Asciz-depleted human cell lines (Figures S3, S4, and data not shown), including in response to HU, hypotonic NaCl and chloroquine, that required ASCIZ for ATM activation according to Kanu and Behrens [19]. Thus, the completely different phenotypes and absence of ASCIZ effects on ATM stability and activation question the classification of ASCIZ as an “essential co-factor” and regulator of ATM [19]. It is not clear why the other group obtained different results, as our gene targeting strategy was identical to theirs. Kanu and Behrens did not provide genetic background information for their mice, but given that we consistently observed unimpaired ATM levels in Asciz-deficient human, chicken or mouse cells, it seems unlikely that the differing effects could be mouse strain-dependent. As we have confirmed normal ATM levels directly in freshly prepared tissue extracts, we can also exclude the possibility that we may have missed differences in protein levels as a result of variable cell culture conditions. Likewise, given that we did not see a meaningful correlation between ATM and ASCIZ levels in numerous independent AT cell lines, including isogenic AT cell controls reconstituted with WT Atm, as well as genuine mouse and chicken Atm gene deletions (Figure 4), we can only speculate that the previously reported dramatic loss of ASCIZ may be a peculiarity of that particular AT cell line, possibly due to increased genome instability of AT cells. Considering that the positions of 15 potential ATM phosphorylation sites are exactly conserved from chicken to human and mouse ASCIZ, we favour a model where DNA damage-related functions of ASCIZ may be modulated by its direct phosphorylation by ATM. Indeed, our preliminary data that ASCIZ can be directly phosphorylated by ATM in vitro and that its MMS-induced focus formation in vivo seems to be at least partially regulated by ATM (to be reported elsewhere in detail) are consistent with a functional interaction between the two proteins.
Role of ASCIZ in lung development
As early lung development is unlikely to be specifically affected by DNA damage signaling, the finding of complete pulmonary agenesis and severe tracheal atresia in Asciz null embryos was surprising, particularly as there are very few mouse mutants with comparable respiratory defects (reviewed in [27], [28], [34], [35]). Specification and early development of the respiratory tract is regulated by extensive signaling crosstalk between the foregut endoderm and surrounding mesoderm [27], [28], and mouse mutants have revealed major signaling pathways involved in these processes (Figure 9). Double-knockout mice lacking the Gli2 and Gli3 transcription factors of the hedgehog pathway also seem to lack lungs as well as the trachea; however, they also lack the oesophagus indicating a more severe foregut defect [36](NB, these defects are considerably less severe in sonic hedgehog (Shh) null embryos [37]). Foregut development appears overall normal in Wnt2/Wnt2b double-null embryos as well as Shh-Cre driven conditional ß-catenin (Ctnnb1) KO mice, but these never establish the Nkx2.1-positive respiratory endoderm and consequently exhibit complete lung and tracheal agenesis [38], [39]. Mice lacking FGF-10 [40], [41] or its cognate FGF-receptor 2b [42] also lack lungs, but seem to contain a grossly normal trachea (and are also characterized by a complete absence of limbs in contrast to the Asciz−/− phenotype). Conversely, FoxG1-Cre driven conditional Bmp4 deletion results in selective tracheal agenesis, where the main bronchi and primitive lungs emerge directly from the oesophagus [43].
Fig. 9. Comparison of the Asciz−/− phenotype to other mouse mutants with pulmonary agenesis. 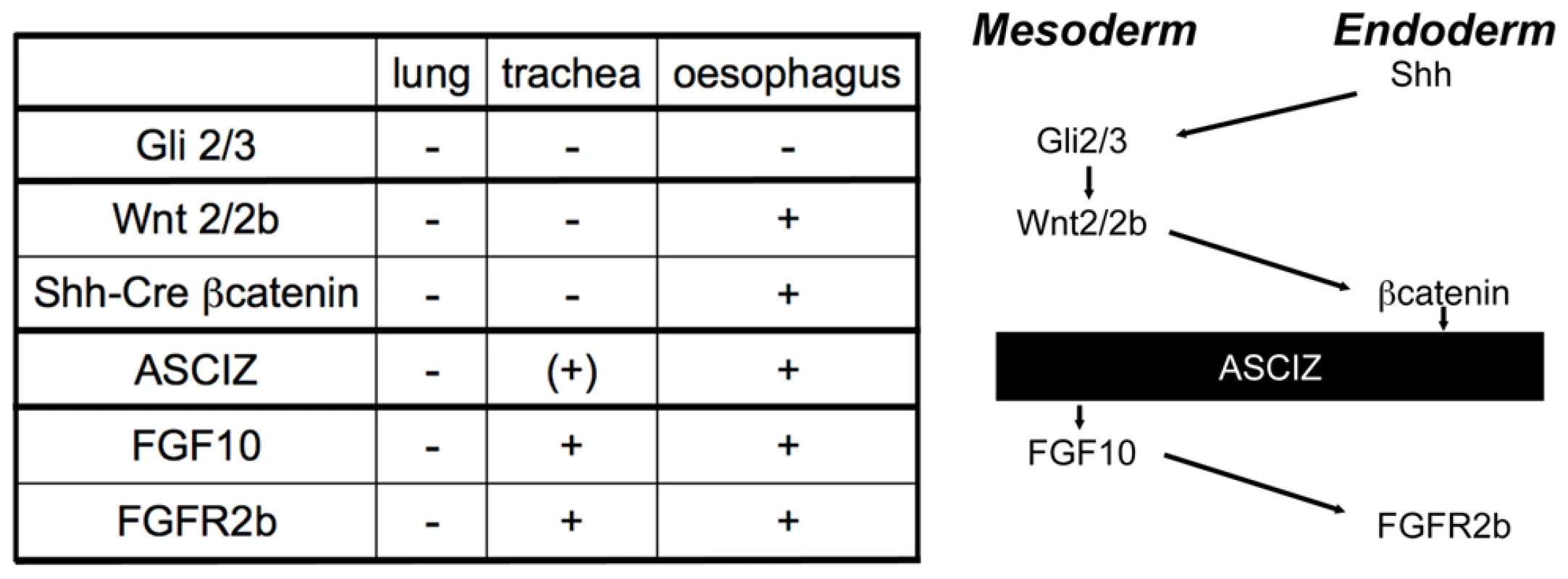
Table summarizing comparable phenotypes and schematic diagram of their role in the crosstalk between endodermal and mesodermal signaling pathways regulating early respiratory tract development; see discussion for details. Based on these comparisons (Figure 9), the Asciz−/− phenotype is less severe than the complete respiratory precursor defect with absence of Nkx2.1 expression and combined agenesis of lungs and trachea in Wnt2/2b and Shh-Cre/ß-catenin mutants, but more severe than the respiratory tract defect in Fgf10 or FGF-receptor 2b mutants with selective pulmonary agenesis yet preserved tracheal development. Genetically, these data thus suggest a crucial regulatory function for ASCIZ in the regulation of respiratory organogenesis at a level between endodermal ß-catenin and mesodermal FGF10 signaling pathways (Figure 9). As FGF10 has been proposed to regulate the downregulation of Sox2 expression in respiratory precursors [30], our finding of impaired dorso-ventral patterning of Sox2 expression in Asciz−/− embryos before foregut separation (Figure S6), and when tracheal separation stalls early (Figure 7D), are also consistent with a role of ASCIZ upstream of FGF10.
The signaling pathways discussed here ultimately regulate developmentally important gene expression programs during specification, morphogenesis and differentiation of the respiratory system. ASCIZ is a predominantly nuclear protein [15], [19], its ZnF structure is generally reminiscent of transcriptional regulators [44], and we have shown here that ASCIZ has the propensity to function as a transcriptional activator via its SQ/TQ cluster domain (Figure 8). In some regards, ASCIZ can be considered as a mirror image of the Sp1 transcription factor. Whereas ASCIZ contains a ZnF domain at the N-terminus and an extended SQ/TQ cluster towards the C-terminus, Sp1 that also is essential for murine development [45] contains an SQ/TQ rich N-terminal transcription activation domain and a triple-ZnF domain at the C-terminus. While Sp1 has been extensively studied as a transcription factor, it is now becoming apparent that it also has transcription-independent roles as an ATM substrate that relocates into DNA damage-induced foci [46], [47], somewhat similar to our original interest in ASCIZ. Altogether, these analogies make it tempting to speculate that ASCIZ may regulate pulmonary development as a transcription factor.
In conclusion, we have shown here that ASCIZ has dual functions with a role in the response to DNA lesions that are repaired by the BER pathway, as well as pleiotropic functions during murine embryonic development, most notably as a member of a very select group of essential regulators of respiratory organogenesis. Nkx2.1-positive respiratory precursors seem to still be specified in the absence of Asciz, but then fail to properly segregate within the foregut. Impaired foregut separation in Asciz−/− embryos seems to correlate with an inability to downregulate Sox2 expression in the ventral foregut, but the exact mechanism responsible for this defect remains to be determined. Asciz null embryos die a few days before birth rather than perinatally from an acute inability to breathe, indicating that additional developmental defects beyond the respiratory system may contribute to the lethality. Our study provides a basis to further investigate how exactly ASCIZ regulates respiratory organogenesis and possibly other developmental processes by expanding the analysis to tissue-specific or temporally regulated conditional knockout systems.
Methods
Ethics statement
All mouse procedures were approved by the St. Vincent's Hospital Animal Research Ethics Committee.
Generation and genotyping of Asciz gene-targeted mice
The mouse Asciz gene contains four exons spread over ∼15 kbp on chromosome 8 and was targeted in C57BL/6 ES cells by integrating loxP sites on both sides of exon D (Figure 1B) using standard homologous recombination, ES cell and blastocyst manipulation techniques as a contracted service by Ozgene Pty Ltd, Perth. A diagnostic ScaI restriction site was integrated just 3′-terminal of the downstream loxP site. Gene targeting was confirmed by Southern blotting using 5′ - and 3′-probes located outside the targeting vector. The 5′-probe can be used for Southern blot analysis of ScaI-digested DNA to distinguish between the WT (22.5 kbp), targeted (11.5 kbp), and Asciz-KO (7.6 kbp) alleles (Figure 1B). The germline Asciz KO allele was generated by crossing the targeted line with C57BL/6 mice containing a PGK-Cre knockin in the ROSA locus, followed by two C57BL/6 backcrosses to remove PGK-Cre and for embryo transfer into the St. Vincent's Hospital Biological Resources Centre. Thus, the Asciz KO line is on a pure C57BL/6 genetic background. Animals were housed in SPF microisolators. Genotyping can also be performed by PCR using primers F1 (5′-CATGGAATTGTTAAAAGCTC-3′), F2 (5′-CCGACTGGGGATGTAGTCAG-3′) and R1 (5′-AAAAGATAGAATAGCTACAC-3′), which result in bands of 170 bp for the WT and 220 bp for the KO allele.
Asciz+/− mice were crossed with germline p53-targeted mice (deletion of exon 2–10 [48], [49]) to generate compound heterozyotes, and offspring were genotyped at weaning using primers above and p53 primers Trp53-1F (5′-CACAAAAAACAGGTTAAACCCAG-3′), Trp53-1R (5′-AGCACATAGGAGGCAGAGAC-3′) and Trp53-10R (5′-GAAGACAGAAAAGGGAGGG-3′), which result in bands of 290 bp for the WT and 612 bp for the KO allele. Recovery of p53−/− mice at weaning was approximately half of the expected Mendelian ratios, a known phenomenon for p53 null homozygosity on inbred backgrounds [50].
Embryo analyses
The time of pregnancies was defined as E0.5 on the morning vaginal plugs were observed in Asciz+/− intercrosses. Embryos were dissected from the uterus in cold PBS, weighed after blotting off excess fluid and immediately fixed for histology, or processed for protein extraction or MEF isolation, and genoyped by PCR using yolk sac or tail DNA. For histology, whole embryos were fixed in Bouin's solution or paraformaldehyde, processed to paraffin and sagittal sections were stained using haematoxylin-eosin and scanned using a Zeiss Mirax Digital Slide Scanner by the Australian Phenomics Network Histopathology and Organ Pathology Service, University of Melbourne, or manually processed and photographed as described [51].
Cell lines and MEF cultures
Human and chicken cell lines were cultured as described [15], [16], [24], [52]. MEFs were prepared by dissecting embryos in cold PBS, heads and internal organs were removed, and remaining corpses were sliced into smaller pieces and trypsinized cells were cultured in Dulbecco's Modified Eagles medium containing 10% fetal calf serum for 2 days, trypsinized, Coulter-counted, and re-seeded at 106 cells per 10 cm dish. This passage, defined as P1, was incubated for 3 days, trypsinized, counted and re-seeded at 106 cells per 10 cm dish (P2), and this process was repeated for 8 passages. For DNA damage sensitivity assays, 5×104 MEFs (P2–P3) were seeded per 35 mm well, grown in Dulbecco's modified Eagle's medium and treated as indicated in the figure legend, and after 18 hours cell viability was determined by propidium iodide exclusion using flow cytometry. Each set of DNA damage sensitivity experiments was performed in parallel with MEFs from at least three independent embryos per genotype.
Optical projection tomography (OPT)
Staged embryos were stained for OPT [53] with an antibody to E-cadherin (ECCD2, Invitrogen, 1/200 dilution) as described [54], with 48 hour primary and secondary antibody incubations interspersed with extensive 12 hour washes to remove unbound antibody. Samples were imaged on a Bioptonics 3001 OPT machine (Bioptonics, UK) and datasets reconstructed by NRecon (Skyscan, Belgium) and visualized using Drishti (http://anusf.anu.edu.au/Vizlab/drishti/). Embryos were rescued from agarose after imaging, processed to paraffin and sectioned, or directly prepared for cryo-sectioning. After antigen retrieval in citrate buffer sections were stained with antibodies to Nkx2.1 (anti-TTF1, Zymed, 1/200), p63 (Abcam, 1/200), and Sox2 (Chemicon) to examine differentiation.
Blot analyses
Southern, Northern and Western blots were performed as described [15], [51]. Antibodies against ASCIZ ([15], available from Millipore) and chicken ATM [52] were described before. Other antibodies: Actin (MAB1501, Millipore), ATM (5C2, Abcam), ATR (sc-1887, Santa Cruz Biotechnology), human p53 (sc-126, Santa Cruz Biotechnology), mouse p53 (1C12, Cell Signaling Technology, PML (sc-5621, Santa Cruz), XRCC1 (sc-11429, Santa Cruz), γH2AX (05-636, Millipore), pS1981(mouse: pS1987)-ATM (200-301-400, Rockland; or 10H11.E12, Cell Signaling Technology), pS15(mouse: pS18)-p53 (9284, Cell Signaling Technology), pT68-Chk2 (2661 or 2197, Cell Signaling Technology).
Transcription reporter assays
For yeast assays, ASCIZ constructs were cloned in pAS2.1 and transformed into PJ69-4A, except the isolated SQ/TQ cluster domain that was cloned into the low-level expression vector pGBT9 because its high level expression was toxic in yeast. One-hybrid reporter assays were performed essentially as described previously for two-hybrid assays in our laboratory [55], [56] except that plates were supplemented with leucine. For mammalian dual luciferase reporter assays, the 667-residue ASCIZ isoform was cloned into pCDNA3-Gal4DBD for transient transfection of U2OS cells with equal amounts of the reporter vectors pFR-Luc and pRL-CMV for use with the Dual-Luciferase Reporter Assay kit (Promega) according to the manufacturer's instructions and measurement of luminescence using a Polarstar Optima (BMG Labtechnologies).
Supporting Information
Zdroje
1. JacksonSP
BartekJ
2009 The DNA-damage response in human biology and disease. Nature 461 1071 1078
2. ShilohY
2003 ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3 155 168
3. BarnesDE
LindahlT
2004 Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet 38 445 476
4. AlmeidaKH
SobolRW
2007 A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair (Amst) 6 695 711
5. HortonJK
WilsonSH
2007 Hypersensitivity phenotypes associated with genetic and synthetic inhibitor-induced base excision repair deficiency. DNA Repair (Amst) 6 530 543
6. HakemR
2008 DNA-damage repair; the good, the bad, and the ugly. Embo J 27 589 605
7. XuG
HerzigM
RotreklV
WalterCA
2008 Base excision repair, aging and health span. Mech Ageing Dev 129 366 382
8. XanthoudakisS
MiaoGG
CurranT
1994 The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc Natl Acad Sci U S A 91 23 27
9. SugoN
NiimiN
ArataniY
Takiguchi-HayashiK
KoyamaH
2004 p53 Deficiency rescues neuronal apoptosis but not differentiation in DNA polymerase beta-deficient mice. Mol Cell Biol 24 9470 9477
10. SugoN
ArataniY
NagashimaY
KubotaY
KoyamaH
2000 Neonatal lethality with abnormal neurogenesis in mice deficient in DNA polymerase beta. EMBO J 19 1397 1404
11. EspositoG
TexidoG
BetzUA
GuH
MullerW
2000 Mice reconstituted with DNA polymerase beta-deficient fetal liver cells are able to mount a T cell-dependent immune response and mutate their Ig genes normally. Proc Natl Acad Sci U S A 97 1166 1171
12. PrasadR
LiuY
DeterdingLJ
PoltoratskyVP
KedarPS
2007 HMGB1 is a cofactor in mammalian base excision repair. Mol Cell 27 829 841
13. El-AndaloussiN
ValovkaT
ToueilleM
SteinacherR
FockeF
2006 Arginine methylation regulates DNA polymerase beta. Mol Cell 22 51 62
14. ParsonsJL
TaitPS
FinchD
DianovaII
AllinsonSL
2008 CHIP-mediated degradation and DNA damage-dependent stabilization regulate base excision repair proteins. Mol Cell 29 477 487
15. McNeesCJ
ConlanLA
TenisN
HeierhorstJ
2005 ASCIZ regulates lesion-specific Rad51 focus formation and apoptosis after methylating DNA damage. Embo J 24 2447 2457
16. OkaH
SakaiW
SonodaE
NakamuraJ
AsagoshiK
2008 DNA damage response protein ASCIZ links base excision repair with immunoglobulin gene conversion. Biochem Biophys Res Commun 371 225 229
17. SobolRW
KartalouM
AlmeidaKH
JoyceDF
EngelwardBP
2003 Base excision repair intermediates induce p53-independent cytotoxic and genotoxic responses. J Biol Chem 278 39951 39959
18. TravenA
HeierhorstJ
2005 SQ/TQ cluster domains: concentrated ATM/ATR kinase phosphorylation site regions in DNA-damage-response proteins. Bioessays 27 397 407
19. KanuN
BehrensA
2007 ATMIN defines an NBS1-independent pathway of ATM signalling. Embo J 26 2933 2941
20. ElsonA
WangY
DaughertyCJ
MortonCC
ZhouF
1996 Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci U S A 93 13084 13089
21. Di MiccoR
CicaleseA
FumagalliM
DobrevaM
VerrecchiaA
2008 DNA damage response activation in mouse embryonic fibroblasts undergoing replicative senescence and following spontaneous immortalization. Cell Cycle 7 3601 3606
22. HicksonI
ZhaoY
RichardsonCJ
GreenSJ
MartinNM
2004 Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res 64 9152 9159
23. ZivY
Bar-ShiraA
PeckerI
RussellP
JorgensenTJ
1997 Recombinant ATM protein complements the cellular A-T phenotype. Oncogene 15 159 167
24. KozlovSV
GrahamME
PengC
ChenP
RobinsonPJ
2006 Involvement of novel autophosphorylation sites in ATM activation. EMBO J 25 3504 3514
25. FukaoT
ChenP
RenJ
KanekoH
ZhangGX
2004 Disruption of the BLM gene in ATM-null DT40 cells does not exacerbate either phenotype. Oncogene 23 1498 1506
26. KaufmanM
BardJ
1999 The anatomical basis of mouse development San Diego Academic Press
27. CardosoWV
LuJ
2006 Regulation of early lung morphogenesis: questions, facts and controversies. Development 133 1611 1624
28. MorriseyEE
HoganBL
2010 Preparing for the First Breath: Genetic and Cellular Mechanisms in Lung Development. Dev Cell 18 8 23
29. QueJ
OkuboT
GoldenringJR
NamKT
KurotaniR
2007 Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134 2521 2531
30. QueJ
LuoX
SchwartzRJ
HoganBL
2009 Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136 1899 1907
31. MarensteinDR
OcampoMT
ChanMK
AltamiranoA
BasuAK
2001 Stimulation of human endonuclease III by Y box-binding protein 1 (DNA-binding protein B). Interaction between a base excision repair enzyme and a transcription factor. J Biol Chem 276 21242 21249
32. DasS
ChattopadhyayR
BhakatKK
BoldoghI
KohnoK
2007 Stimulation of NEIL2-mediated oxidized base excision repair via YB-1 interaction during oxidative stress. J Biol Chem 282 28474 28484
33. LuZH
BooksJT
LeyTJ
2005 YB-1 is important for late-stage embryonic development, optimal cellular stress responses, and the prevention of premature senescence. Mol Cell Biol 25 4625 4637
34. ChenF
DesaiTJ
QianJ
NiederreitherK
LuJ
2007 Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 134 2969 2979
35. RamasamySK
MailleuxAA
GupteVV
MataF
SalaFG
2007 Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol 307 237 247
36. MotoyamaJ
LiuJ
MoR
DingQ
PostM
1998 Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet 20 54 57
37. LitingtungY
LeiL
WestphalH
ChiangC
1998 Sonic hedgehog is essential to foregut development. Nat Genet 20 58 61
38. GossAM
TianY
TsukiyamaT
CohenED
ZhouD
2009 Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell 17 290 298
39. Harris-JohnsonKS
DomyanET
VezinaCM
SunX
2009 beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A 106 16287 16292
40. MinH
DanilenkoDM
ScullySA
BolonB
RingBD
1998 Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev 12 3156 3161
41. SekineK
OhuchiH
FujiwaraM
YamasakiM
YoshizawaT
1999 Fgf10 is essential for limb and lung formation. Nat Genet 21 138 141
42. De MoerloozeL
Spencer-DeneB
RevestJM
HajihosseiniM
RosewellI
2000 An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127 483 492
43. LiY
GordonJ
ManleyNR
LitingtungY
ChiangC
2008 Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol 322 145 155
44. GamsjaegerR
LiewCK
LoughlinFE
CrossleyM
MackayJP
2007 Sticky fingers: zinc-fingers as protein-recognition motifs. Trends Biochem Sci 32 63 70
45. MarinM
KarisA
VisserP
GrosveldF
PhilipsenS
1997 Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell 89 619 628
46. OlofssonBA
KellyCM
KimJ
HornsbySM
Azizkhan-CliffordJ
2007 Phosphorylation of Sp1 in response to DNA damage by ataxia telangiectasia-mutated kinase. Mol Cancer Res 5 1319 1330
47. IwahoriS
YasuiY
KudohA
SatoY
NakayamaS
2008 Identification of phosphorylation sites on transcription factor Sp1 in response to DNA damage and its accumulation at damaged sites. Cell Signal 20 1795 1803
48. JonkersJ
MeuwissenR
van der GuldenH
PeterseH
van der ValkM
2001 Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 29 418 425
49. WalkleyCR
QudsiR
SankaranVG
PerryJA
GostissaM
2008 Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 22 1662 1676
50. SahVP
AttardiLD
MulliganGJ
WilliamsBO
BronsonRT
1995 A subset of p53-deficient embryos exhibit exencephaly. Nat Genet 10 175 180
51. DuX-J
ColeTJ
TenisN
GaoX-M
KöntgenF
2002 Impaired cardiac contractility response to hemodynamic stress in S100A1-deficient mice. Mol Cell Biol 22 2821 2829
52. KobayashiM
OnoH
MiharaK
TauchiH
KomatsuK
2006 ATM activation by a sulfhydryl-reactive inflammatory cyclopentenone prostaglandin. Genes Cells 11 779 789
53. SharpeJ
AhlgrenU
PerryP
HillB
RossA
2002 Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296 541 545
54. ShortKM
HodsonMJ
SmythIM
2010 Tomographic quantification of branching morphogenesis and renal development. Kidney Int
55. PikeBL
YongkiettrakulS
TsaiMD
HeierhorstJ
2004 Mdt1, a novel Rad53 FHA1 domain-interacting protein, modulates DNA damage tolerance and G2/M cell cycle progression in Saccharomyces cerevisiae. Mol Cell Biol 24 2779 2788
56. HammetA
PikeBL
HeierhorstJ
2002 Posttranscriptional regulation of the RAD5 DNA repair gene by the Dun1 kinase and the Pan2-Pan3 poly(A)-nuclease complex contributes to survival of replication blocks. J Biol Chem 277 22469 22474
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



