-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
Reklamaβ-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
Hair cell stereocilia structure depends on actin filaments composed of cytoplasmic β-actin and γ-actin isoforms. Mutations in either gene can lead to progressive hearing loss in humans. Since β-actin and γ-actin isoforms are 99% identical at the protein level, it is unclear whether each isoform has distinct cellular roles. Here, we compared the functions of β-actin and γ-actin in stereocilia formation and maintenance by generating mice conditionally knocked out for Actb or Actg1 in hair cells. We found that, although cytoplasmic actin is necessary, neither β-actin nor γ-actin is required for normal stereocilia development or auditory function in young animals. However, aging mice with β-actin – or γ-actin–deficient hair cells develop different patterns of progressive hearing loss and distinct pathogenic changes in stereocilia morphology, despite colocalization of the actin isoforms. These results demonstrate overlapping developmental roles but unique post-developmental functions for β-actin and γ-actin in maintaining hair cell stereocilia.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001158
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001158Summary
Hair cell stereocilia structure depends on actin filaments composed of cytoplasmic β-actin and γ-actin isoforms. Mutations in either gene can lead to progressive hearing loss in humans. Since β-actin and γ-actin isoforms are 99% identical at the protein level, it is unclear whether each isoform has distinct cellular roles. Here, we compared the functions of β-actin and γ-actin in stereocilia formation and maintenance by generating mice conditionally knocked out for Actb or Actg1 in hair cells. We found that, although cytoplasmic actin is necessary, neither β-actin nor γ-actin is required for normal stereocilia development or auditory function in young animals. However, aging mice with β-actin – or γ-actin–deficient hair cells develop different patterns of progressive hearing loss and distinct pathogenic changes in stereocilia morphology, despite colocalization of the actin isoforms. These results demonstrate overlapping developmental roles but unique post-developmental functions for β-actin and γ-actin in maintaining hair cell stereocilia.
Introduction
Actin reversibly polymerizes to form stiff, strong and polarized filaments, a process that is modulated by numerous actin binding proteins. Polymerization itself can provide force for diverse functions including membrane protrusion and cell motility, endocytosis or propelling objects within the cytoplasm. Actin filaments are substrates for myosin motors and also form structural elements of certain cellular organelles including microvilli and stereocilia.
Actb and Actg1 encode β-actin and γ-actin, respectively, which are the two ubiquitously expressed cytoplasmic members of the actin family that includes four additional genes that are predominately expressed in muscle [1]. β-Actin and γ-actin are closely related proteins, with each amino acid sequence exactly conserved across vertebrates. The isoforms differ by only 4 biochemically-similar residues clustered in the N-terminal 10 amino acids [2]. However, these subtle differences were recently shown to confer distinct biochemical properties, with β-actin exhibiting more dynamic polymerization properties than γ-actin [3]. Interestingly, β-actin and γ-actin can co-polymerize with the overall dynamics reflecting the composition of the mixture [3]. In addition to these biochemical differences, β-actin and γ-actin are reported to have different localization patterns in certain cell types [4], [5] and are subject to different post-translational modifications [6]. Finally, more severe phenotypes result from loss of β-actin than γ-actin in vivo. Mice lacking β-actin are embryonic lethal [7], [8] while Actg1 knockout mice (Actg1−/−) are viable but develop progressive hearing loss [9]. In humans, a mutation in ACTB results in severe syndromic phenotypes that include developmental malformations and deafness [10]. In contrast, several different mutations in human ACTG1 cause dominant progressive hearing loss without other syndromic phenotypes [11]–[15].
Auditory function seems to be particularly sensitive to perturbations of cytoplasmic actins, perhaps because actin is a key structural component of auditory hair cells, which convert sound waves to neural signals. Hair cells are housed in the organ of Corti, both of which feature an intricate architecture that is required for proper function. The organ of Corti consists of three rows of outer hair cells (OHCs) and one row of inner hair cells (IHCs) together with several types of support cells. This ribbon-like structure runs longitudinally along the length of the cochlea. OHCs function to improve sensitivity to sound while IHCs are the auditory receptors [16]. Both cell types are topped with specialized structures called stereocilia, which are elaborated microvilli formed from a mixture of β-actin and γ-actin filaments that are organized in a tightly bundled paracrystalline array [17]–[20]. In mouse OHCs, around 90 stereocilia are arranged in three staircase-like rows within a V-shaped bundle. Stereocilia are connected by a variety of proteinaceous links, including tip links that connect the tops of stereocilia in shorter rows to the sides of stereocilia in the adjacent taller row (reviewed in [21]). Tip links are composed of cadherin23 and protocadherin15 and are required for mechanoelectric transduction that occurs when sound pressure waves displace the hair bundle [22], [23]. Hair cells, together with their stereocilia, are not regenerated and must therefore be preserved for the life of the organism. Hearing maintenance is affected by the composition of stereocilia and tip links. Dimorphic alleles of the gene encoding cadherin23 confer either susceptibility or resistance to age-related hearing loss in several different inbred mouse strains [24], [25].
We previously demonstrated that whole body Actg1−/− mice initially have normal hearing and stereocilia morphology while young. However, these mice develop progressive hearing loss together with stereocilia degradation [9]. Therefore, γ-actin is dispensable for stereocilia formation but is required for maintenance. Here, we used conditional gene ablation in mice to show that hair cell stereocilia development requires at least one cytoplasmic actin, but proceeds normally in the absence of either isoform. Mice with β-actin or γ-actin deficient hair cells each have normal hearing at young ages but subsequently develop distinct forms of adult-onset, progressive hearing loss along with different pathogenic changes in stereocilia morphology. Fluorescently-labeled primary antibodies specific for β-actin or γ-actin each uniformly label the stereocilia actin core. Together, these data show that β-actin and γ-actin, despite exhibiting nearly identical protein sequences and inner hair cell stereocilia localization patterns, perform distinct functions in maintaining auditory hair cell stereocilia.
Results
β-actin is not required for stereocilia formation
β-actin might be essential for stereocilia development because it is the only cytoplasmic actin detected at the earliest stages of stereocilia formation [9]. To test this idea, we used Foxg1-cre in combination with a newly generated floxed allele of Actb (Figure S1) to knock out β-actin in the developing ear. Consistent with the early onset of Foxg1-cre, which is expressed throughout the otic vesicle from E9.5 [26], Actb-flox Foxg1-cre inner ear tissue at postnatal day 2 (P2) was devoid of β-actin as detected by immunofluorescent staining (Figure 1A and 1C). Surprisingly, phalloidin staining revealed stereocilia with apparently normal morphology and organization (Figure 1B and 1D). Furthermore, phalloidin-stained stereocilia were still detectable at P21 (Figure 1E and 1F).
Fig. 1. β-actin is not required for stereocilia formation. 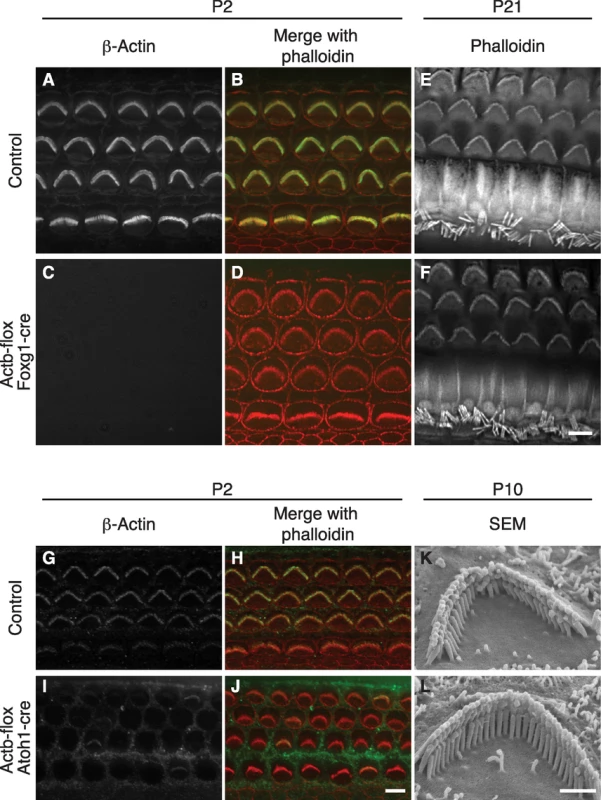
(A–F) Hair cells from control or Actb-flox Foxg1-cre mice. β-Actin staining of stereocilia in control pup overlays with phalloidin staining (A–B), but is absent from all cell types in Actb-flox Foxg1-cre mice (C) while phalloidin staining of stereocilia appears normal (D). At P21, phalloidin stained stereocilia are similar in control (E) and Actb-flox Foxg1-cre (F) hair cells. (G–L) Analysis of Actb-flox Atoh1-cre hair cells. In control hair cells (G–H), anti-β-actin stained stereocilia overlay with phalloidin staining. In Actb-flox Foxg1-cre hair cells β-actin is stained in support cells but is mostly absent from hair cell stereocilia (I), while phalloidin staining reveals normal stereocilia (J). (K–L) SEM analysis shows that control (K) and Actb-flox Atoh1-cre (L) stereocilia have similar architecture. In merged images, phalloidin staining is red and β-actin is green. Bar in A–J is 5 µm; K–L is 1 µm. Because Actb-flox Foxg1-cre mice rarely survived to adulthood, we also used Atoh1-cre to mediate Actb knockout. In the organ of Corti, Atoh1-cre expression is limited to IHCs and OHCs with expression beginning before E18.5 [27]. Correspondingly, β-actin immunofluorescent staining is retained in neighboring support cells but is largely absent from both inner and outer hair cells (Figure 1G and 1I). Residual β-actin expression at early postnatal stages is likely due to a long β-actin protein half-life. Consistent with our observations in Actb-flox Foxg1-cre mice, Actb-flox Atoh1-cre stereocilia appear to develop normally as judged by phalloidin staining at P2 (Figure 1H and 1J), as well as by scanning electron microscopy (SEM) at P10 (Figure 1K and 1L).
β-actin and γ-actin colocalize
β-actin and γ-actin have been shown to have distinct localization patterns in a variety of cell types [4], [5], [9], [18], [28]; however, owing to species differences or experimental design, conflicting localization patterns have been reported. Here, we assessed whether primary-secondary antibody combinations report the same localization pattern as dye-conjugated primary antibodies when staining the densely packed actin array of IHC stereocilia.
Using paraformaldehyde-fixed tissue that was post-fixed in methanol, we found that a monoclonal anti-γ-actin primary antibody in combination with a secondary antibody gave a previously described peripheral localization pattern (Figure 2A–2D) [9]. In contrast, the same anti-γ-actin antibody directly coupled to a fluorescent dye was observed to label stereocilia uniformly (Figure 2E–2H). Moreover, unconjugated β-actin antibodies detected with a secondary antibody produced the same peripheral localization pattern as observed with unlabeled antibodies to γ-actin (Figure S2). Finally, β-actin and γ-actin appeared to colocalize in cells simultaneously labeled with dye-conjugated antibodies to each isoform (Figure 2I and 2K). Therefore, at the level of light microscopy, we did not detect differential localization of β-actin and γ-actin in IHC stereocilia. These data further suggest that secondary antibodies are either unable to fully penetrate densely packed stereocilia actin, or are sterically prevented from binding their primary antibody targets.
Fig. 2. β-actin and γ-actin colocalize in hair cells. 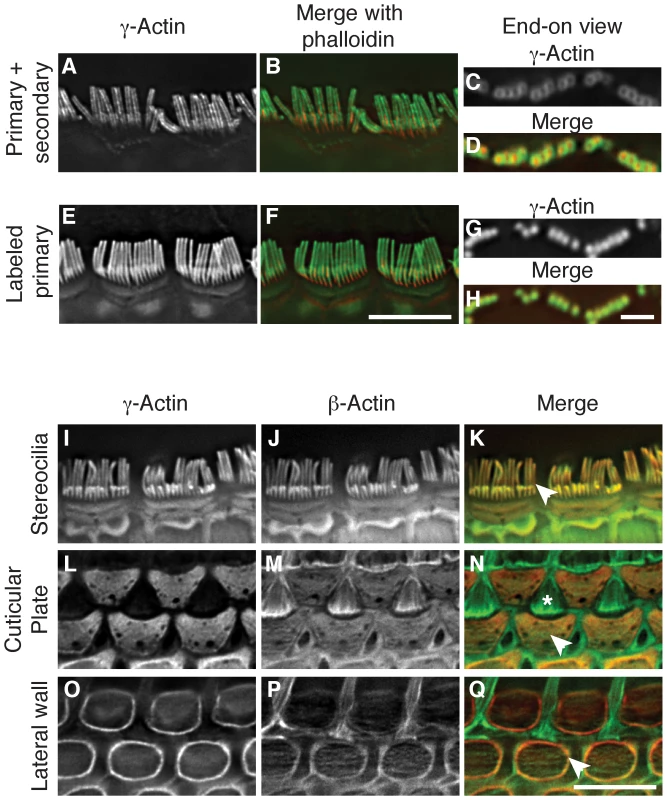
(A–H) Comparison of γ-actin localization using unlabeled anti-γ-actin antibody with a fluorescent secondary antibody (A–D) or using the same anti-γ-actin antibody coupled directly to a fluorescent dye (E–H). Detection with a secondary antibody results in a peripheral localization pattern when viewed laterally (A–B) or end-on (C–D). Dye-conjugated anti-γ-actin antibody labels stereocilia uniformly and completely overlays with phalloidin in lateral (E–F) or end-on (G–H) views. In merged images, γ-actin is green and phalloidin is red. (I–Q) Localization of β-actin and γ-actin using primary antibodies conjugated to two different fluorescent dyes. γ-Actin and β-actin colocalize in stereocilia (I–K), cuticular plate (L–N) and lateral wall (O–Q). In merged images, β-actin is green and γ-actin is red. Arrowheads indicate the structure of interest and the asterisk in (N) marks the top of a support cell that borders outer hair cells. Bars in F and Q, 5 µm; H, 2 µm. Hair cells have other actin-rich structures including the cuticular plate, which is an actin meshwork where stereocilia are anchored, and the lateral wall, which features an actin-spectrin lattice. In agreement with previous studies [18], [29], we found both isoforms in the cuticular plate (Figure 2L–2N) and in the lateral wall, where γ-actin appears to be more abundant (Figure 2O–2Q). Although the ratio of β-actin to γ-actin may vary, our immunofluorescent studies did not identify any structure in hair cells that contained only a single cytoplasmic actin isoform.
Stereocilia formation requires cytoplasmic actin
The finding that neither cytoplasmic actin is individually essential for stereocilia formation raised the possibility that cytoplasmic actins are entirely dispensable for auditory development. Therefore, we generated Actb Actg1 Atoh1-cre mediated double knockout hair cells. In P5 mice, double knockout hair cells were largely devoid of phalloidin-stained stereocilia (Figure 3A and 3B). The few remaining bundles immunostained for either β-actin or γ-actin (Figure 3C–3H) and likely persisted because of incomplete cre-mediated recombination at this age. We conclude that either β-actin or γ-actin is required for stereocilia development even though each is dispensable, suggesting that other members of the actin family are unable to compensate for loss of cytoplasmic actin function in hair cells.
Fig. 3. Actb Actg1 double knockout cells do not develop stereocilia. 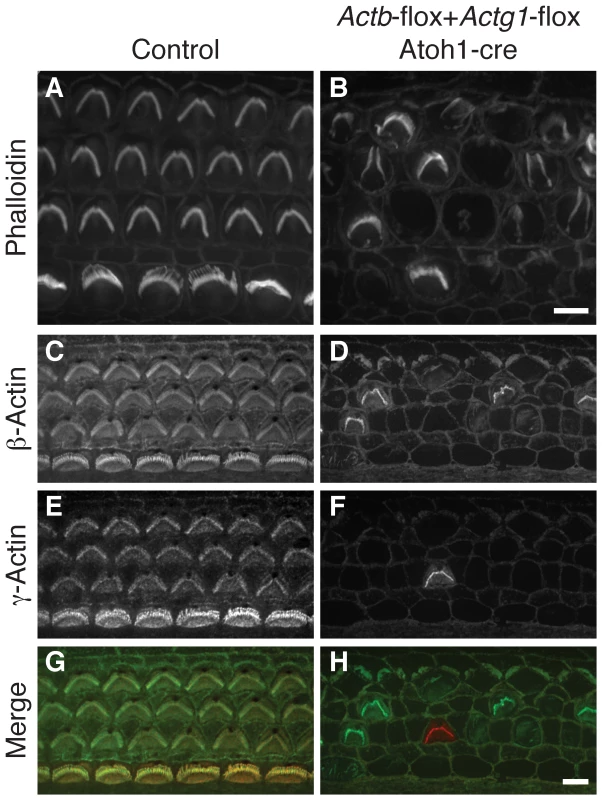
(A, B) Phalloidin staining of organ of Corti from control (A) or Actb-flox Actg1-flox Atoh1-cre (double knockout) (B) P5 pups shows normal stereocilia in control but generally absent stereocilia in the double knockout. (C–H) double label with dye-conjugated antibodies to β-actin or γ-actin show that remaining stereocilia contain one of the actin isoforms. In merged images in (G–H) β-actin is green and γ-actin is red. Bars, 5 µm. Actb and Actg1 hair cell knockout mice develop distinct patterns of progressive hearing loss
Because of the ubiquitous expression pattern of cytoplasmic actins, aspects of the slowly progressive hearing loss recently observed in whole-body Actg1−/− mice may be unrelated to hair cell or stereocilia function [9]. Therefore, we measured auditory brainstem response (ABR) in Actg1-flox Atoh1-cre mice, where cre expression in the auditory system is limited to inner hair cells, outer hair cells and spiral ganglion cells [27]. Correspondingly, in the organ of Corti, we observed loss of γ-actin in both inner and outer hair cells (Figure S3). ABR uses subdural electrodes to record synchronous neural activity from an anesthetized mouse in response to a short tone burst of a given frequency and sound level. The lowest sound intensity level that produces a neural response is the hearing threshold.
Similar to Actg1−/− mice, we found that 6 week-old Actg1-flox Atoh1-cre mice had thresholds that were indistinguishable from control mice at frequencies between 4 kHz and 22 kHz, while the 32 kHz threshold was elevated (Figure 4A). However, by 18 weeks-of-age, Actg1-flox Atoh1-cre mice had significantly elevated thresholds at all frequencies tested (Figure 4A). Although this pattern of hearing loss corresponds very well with that observed for Actg1−/− mice of the same age [9], differences between the two models were apparent at later stages. While 24 week-old Actg1-floxed Atoh1-cre mice had thresholds at 22 kHz and 32 kHz that were above 90 dB SPL, the maximum sound level tested, hearing between 4 and 16 kHz was not markedly worse than at 18 weeks-of-age (Figure 4A). In contrast, hearing function worsened in Actg1−/− mice over the same interval [9], suggesting additional functions for γ-actin outside of hair cells during this time frame. Together, these data demonstrate that Actg1-flox Atoh1-cre mice have nearly normal hearing as young adults, but develop progressive loss of hearing at all frequencies. The early stages of hearing loss recapitulate that of Actg1−/− mice and suggest critical hair-cell specific functions for γ-actin.
Fig. 4. Mice with hair cells deficient for either β-actin or γ-actin develop distinct forms of progressive hearing loss. 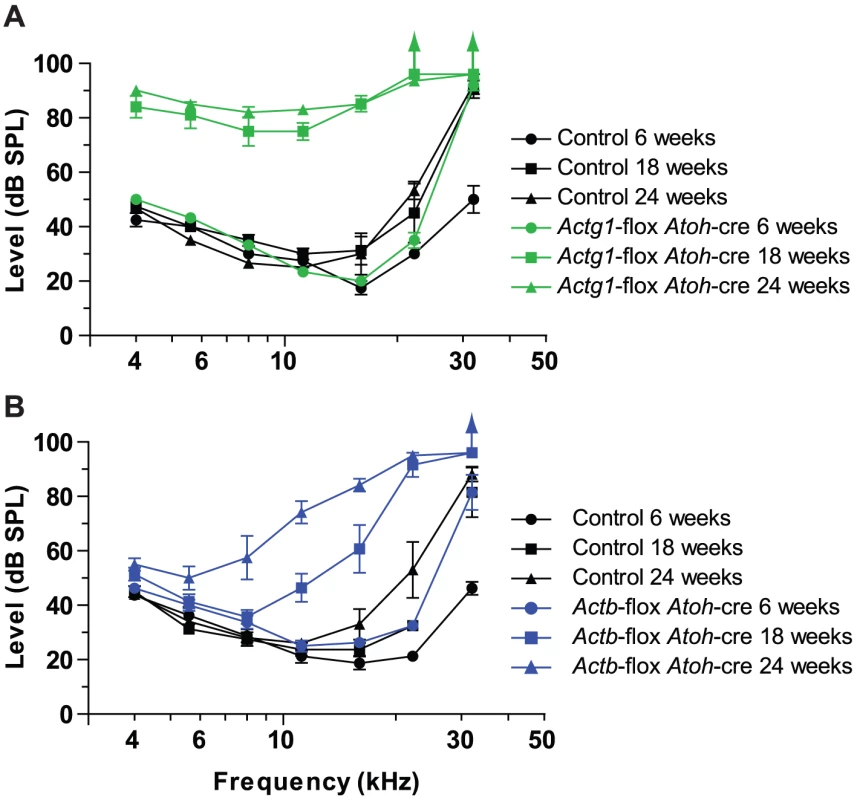
Auditory brainstem response (ABR) thresholds at 6, 18 and 24 weeks of age. (A) Actg1-flox Atoh1-cre mice have near normal ABR thresholds for sound tones between 4 and 22 kHz at 6 weeks of age, but elevated thresholds at all frequencies at 18 and 24 weeks of age. (B) Actb-flox-Atoh1-cre mice have progressive loss of hearing starting with higher frequency sounds. Actb-flox Atoh1-cre mice provide an excellent system for comparing β-actin function to γ-actin function in hair cells. In these mice at 6 weeks-of-age, ABR thresholds between 4 kHz and 16 kHz were similar to control littermates, while the 22 kHz threshold was slightly elevated and the 32 kHz threshold was markedly elevated (Figure 4B). By 18 weeks-of-age, hearing loss had progressed to include drastic elevation of the 22 and 32 kHz thresholds and moderate elevation of 11 and 16 kHz thresholds. These thresholds were further elevated at 24 weeks-of-age. In addition, lower frequencies, most prominently 8 kHz, were also higher than in control animals (Figure 4B).
Therefore the loss of β-actin preferentially affects high frequency hearing while the loss of γ-actin affects all frequencies more uniformly and progresses more rapidly. These different patterns of hearing loss suggest that β-actin and γ-actin have different and non-redundant functions in hair cells.
β-actin and γ-actin deficient stereocilia have distinct pathology
Different frequencies of sound are detected at discrete locations along the length of the cochlea, with the highest frequencies detected at the basal end and the lowest frequencies at the apical end. Since hearing loss in mice with γ-actin or β-actin deficient hair cells is frequency dependent, we examined the morphology of OHC stereocilia from apical, middle and basal regions. At 18 and 24 weeks-of-age, hair bundles from all regions of Actg1-flox Atoh1-cre cochlea were degraded with a significant loss of individual stereocilia (Figure 5E–5H, 5N, 5Q). Other stereocilia in the same bundle were shortened, but many retained apparently normal morphology (Figure 5N). The consistent stereocilia pathology in apical, middle and basal regions corresponds with the uniform elevation of ABR thresholds across the frequency range (Figure 4A). In contrast, hair bundles from Actb-flox Atoh1-cre mice had less severe morphological defects at 18 weeks-of-age (Figure 5I–5L, 5Q). In keeping with normal ABR thresholds at low and middle frequencies, we did not observe obvious stereocilia defects in the apical or middle regions (Figure 5I–5K). Outer hair cells from the basal region, which detects high frequency sounds, had an altered morphology characterized by shortened stereocilia with heights that were variable and not in register with neighboring stereocilia (Figure 5L).
Fig. 5. β-actin and γ-actin deficient stereocilia develop distinct pathology. 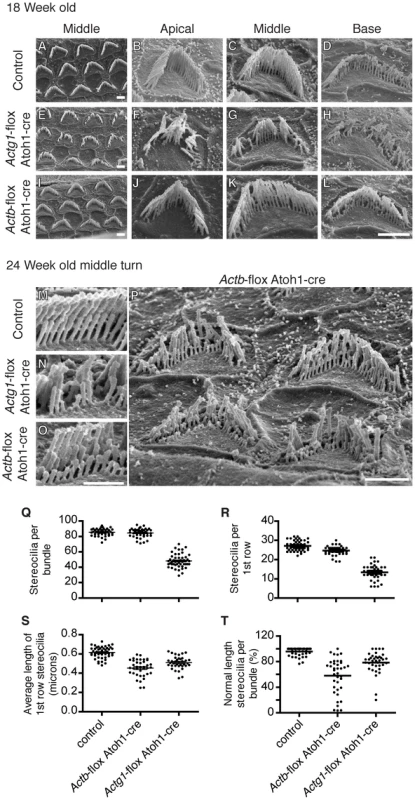
Analysis of stereocilia morphology by scanning electron microscopy at 18 weeks-of-age (A–L) and 24 weeks-of-age (M–P). (A–D) 18 Week-old control stereocilia. (E–H) 18 Week-old γ-actin deficient stereocilia from all cochlear locations are degraded with significant numbers of individual stereocilia missing (Q). (I–L) β-Actin deficient stereocilia at 18 weeks of age from the apical (J) and middle turns (I,K) are similar to control, while stereocilia from the basal turn are abnormally short (L). Bars, 2 µm. (M–O) Comparison of stereocilia pathology at 24 weeks-of-age. γ-Actin deficient bundles are missing individual stereocilia while β-actin deficient stereocilia are uniformly shortened. Bar, 1 µm. (P) β-Actin deficient stereocilia from the middle turn have developed pathology ranging from mild to severe shortening of most members of whole rows in the bundle. Bars, 2 µm. (Q) Number of stereocilia in 18 week-old hair cell bundles, only Actg1-flox Atoh1-cre stereocilia numbers are statistically different. 40 OHC bundles from 4 ears of each genotype were analyzed. (R–T) Analysis of the first row of stereocilia of bundles from 24 week-old mice. The number (R) and average length (S) of first row stereocilia were determined. The percent of these stereocilia that were of normal length (within 2 standard deviations of the control average) was calculated (T). In (R–T), each dot plotted represents one cell (more than 35 cells from 3 mice of each genotype) and the bar is the mean. All groups are statistically different from all other groups. At 24 weeks-of-age, pathology of β-actin-deficient stereocilia progressed, now affecting stereocilia from the middle cochlear turn, corresponding with the progression of hearing loss in Actb-flox Atoh1-cre mice (Figure 5O–5P). β-Actin-deficient stereocilia lengths varied considerably from cell to cell. In some cases, stereocilia were of normal length but lacked the precise height registration with neighboring stereocilia that is a hallmark of normal morphology. In other cases individual stereocilia and whole rows of stereocilia were severely shortened (Figure 5O–5P). Nevertheless, β-actin-deficient and γ-actin-deficient stereocilia had distinct morphologies (Figure 5M–5O).
To compare β-actin deficient stereocilia to γ-actin deficient stereocilia, we quantified morphological changes in 24 week-old stereocilia by measuring the lengths of the first (shortest) row of stereocilia in bundles from all rows of OHCs in the middle region of the cochlea. Consistent with our previous observations, γ-actin-deficient hair cells had markedly fewer stereocilia (Figure 5R) that had a shorter average length than control stereocilia (Figure 5S). In contrast, β-actin deficient hair cells had only slightly fewer stereocilia than control cells (Figure 5R), and these stereocilia were shorter than either control or γ-actin deficient stereocilia (Figure 5S). The difference in average length between β-actin and γ-actin deficient stereocilia was statistically significant but appeared slight, perhaps due to a few very short γ-actin-deficient stereocilia mixed in with remaining members of normal height (Figure 5N). Therefore, we also compared the percentage of stereocilia that were of normal height, defined here as measured heights within two standard deviations of the average length in controls. Using this criterion, we found that appreciably more γ-actin deficient bundles than β-actin deficient bundles retained a high fraction of normal length stereocilia (Figure 5T). This data demonstrates that γ-actin deficient hair cells have lost a large percentage of stereocilia, with most remaining stereocilia retaining normal length. In contrast, β-actin deficient stereocilia are largely retained but are more consistently shortened in length.
Hearing loss and stereocilia pathology depend on γ-actin concentration
β-actin and γ-actin-deficient stereocilia develop different phenotypes, suggesting that each actin isoform has a distinct role in maintaining stereocilia morphology and auditory function. We reasoned that the severity of the phenotype should depend on cellular concentration of each isoform. We generated a series of mice with variable levels of γ-actin protein by taking advantage of a hypomorphic allele (Actg1O) caused by insertion of a neomycin cassette into intron 1 [30]. Based on quantitative immunoblot analysis of cochlear extracts, we determined that Actg1+/−, Actg1O/O and Actg1O/− mice express 44%, 15% and 5%, respectively, of wild-type γ-actin levels (Figure 6A). We previously found that Actg1+/+ and Actg1+/− mice had similar ABR thresholds [9]. Here, we found that hearing loss onset and progression depend on the concentration of γ-actin. Actg1O/O and Actg1O/− mice both had a normal 22 kHz ABR threshold at 16 weeks-of-age, a time point at which Actg1−/− mice have profound hearing loss (Figure 6B) [9]. At subsequent time points, both lines progressively lost hearing sensitivity, with the lower γ-actin level in Actg1O/− mice correlating with a faster rate of hearing loss (Figure 6B). By 28 weeks-of-age, Actg1O/− mice had marked hearing loss across the range of tested frequencies, recapitulating the pattern of impairment observed in Actg1-flox Atoh1-cre mice (Figure 6C). Finally, SEM analysis demonstrated that Actg1O/− stereocilia morphology at 28 weeks phenocopied the pathology observed in γ-actin-deficient hair cells, where in both cases hair bundles appear degraded with individual stereocilia missing (Figure 6D). Therefore, these distinct phenotypes depend on the γ-actin concentration, further suggesting that γ-actin has a specific cellular function.
Fig. 6. Onset and progression of hearing loss depends on γ-actin concentration. 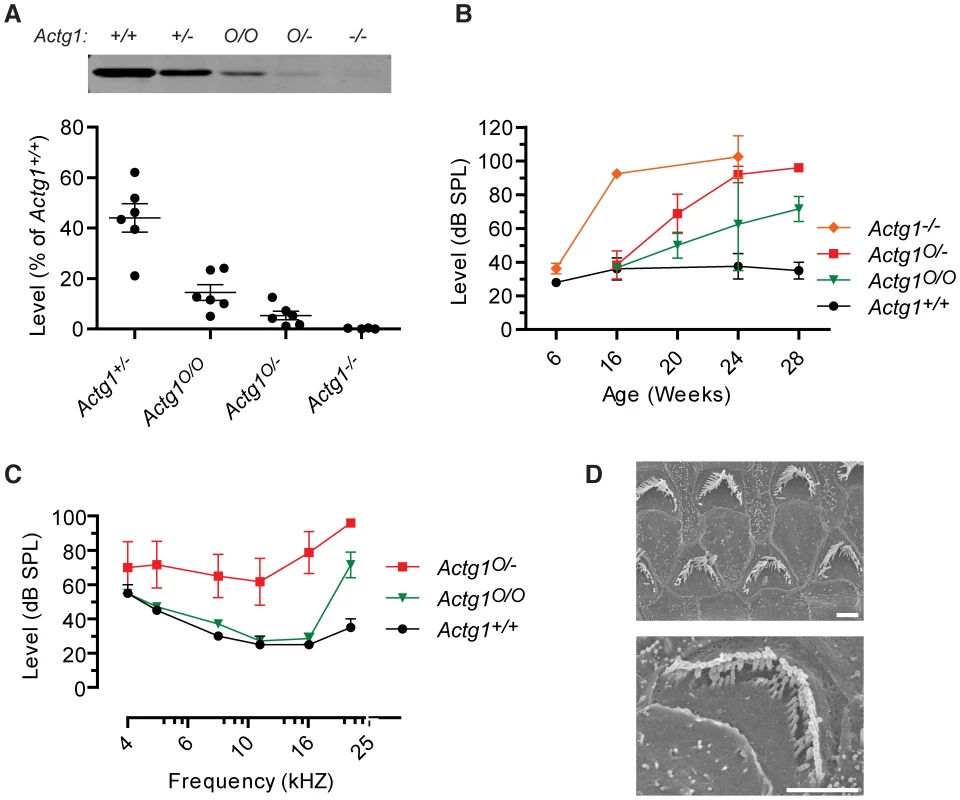
(A) γ-actin protein levels in cochlea from Actg1+/+, Actg1+/−, Actg1O/O, Actg1O/− and Actg1−/− mice were quantified using immunoblot analysis. (B) 22 kHz auditory brainstem response (ABR) thresholds were determined at the indicated ages, demonstrating that the onset and rate of hearing loss progression depend on the concentration of γ-actin. Actg1−/− data is replotted from [9]. (C) ABR thresholds from 28 week-old Actg1+/+, Actg1O/O and Actg1O/− mice. (D) Scanning electron microscopy of outer hair cells from Actg1O/− mice, demonstrating that mice with hypomorphic γ-actin expression develop a phenotype similar to that of hair cells devoid of γ-actin. Bars, 2 um. Progressive hearing loss in Actg1 hair cell knockout mice is not rescued by cadherin23
The Actg1-floxed Atoh1-cre mice were characterized on the C57Bl/6 inbred genetic background, which is predisposed to progressive hearing loss by the age-related hearing loss (AHL) susceptible allele of the cadherin23 gene (Cdh23ahl) [25]. Cdh23ahl transcripts lack exon 7, while the dominant-acting resistance allele, CdhAhl+, encodes the full-length transcript and protects against AHL [24], [25]. Since cadherin23-mediated tip link stability might impact stereocilia maintenance, we next determined if the hearing loss and stereocilia pathology that we observed in Actg1-floxed Atoh1-cre mice depends on the Cdhahl allele. We crossed mice harboring Cdh23Ahl+ on an otherwise C57Bl/6 background [24] to Actg1-floxed Atoh1-cre mice. At six weeks of age, Actg1-floxed Atoh1-cre Cdh23Ahl+/ahl mice had ABR thresholds similar to control Actg1-floxed Cdh23Ahl+/ahl mice. However, ABR thresholds in these γ-actin-deficient mice were markedly increased at 18 weeks of age at all frequencies tested, and were further elevated at 24 weeks of age (Figure 7A). As expected, both control and Actg1-floxed Atoh1-cre mice carrying Cdh23Ahl+ had lower ABR thresholds at high frequencies (Figure 7A) compared to mice on a homozygous Cdh23ahl/ahl background (Figure 4A). In addition, Cdh23AHL+ generally slowed the progression of hearing loss in Actg1-floxed Atoh1-cre mice (Figure 7A). Nevertheless, SEM analysis of OHC stereocilia from 18 week old Actg1-floxed Atoh1-cre Cdh23Ahl+/ahl mice revealed the characteristic γ-actin-deficient pathology, including hair bundle degradation with loss of individual stereocilia (Figure 7B–7G). Together, these data demonstrate that progressive hearing loss and stereocilia degradation associated with γ-actin deficiency occurs regardless of defects in Cdh23.
Fig. 7. Cdh23Ahl+ does not rescue progressive hearing loss in mice with γ-actin–deficient hair cells. 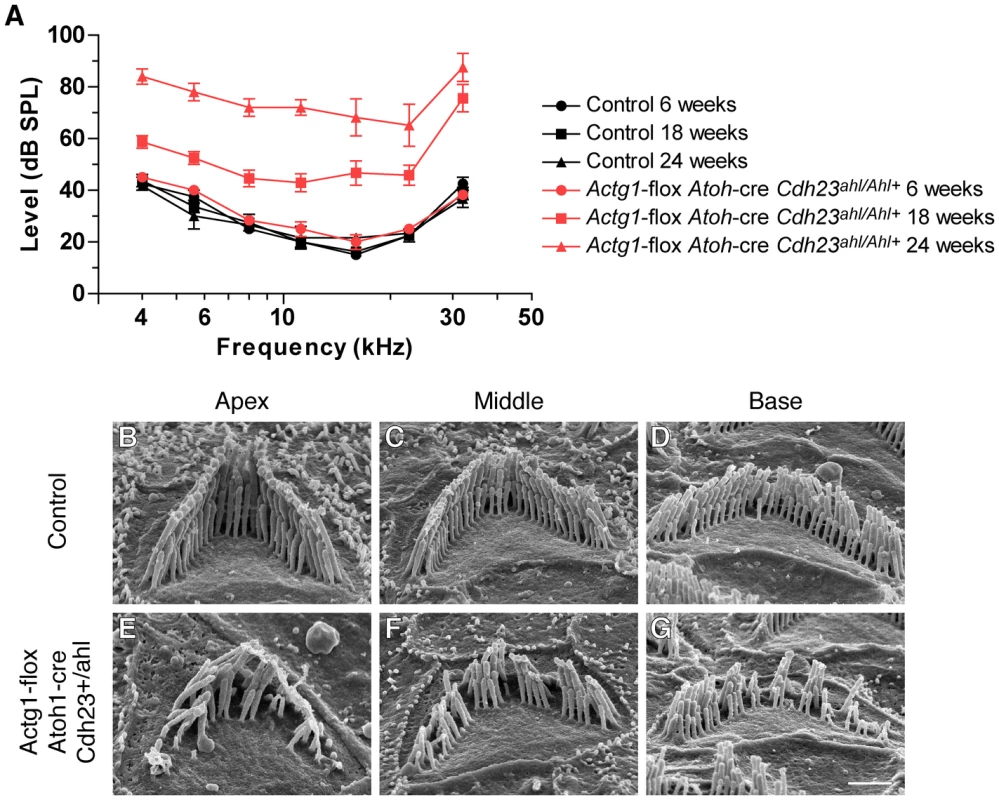
(A) Actg1-flox Atoh1-cre CdhAhl+/ahl mice have elevated ABR thresholds at 18 and 24 weeks of age. (B–G) SEM analysis of stereocilia morphology at 18 weeks of age. OHC hair bundles from control Actg1-flox CdhAhl+/ahl mice (B–D) have normal stereocilia morphology while OHC hair bundles from Actg1-flox Atoh1-cre CdhAhl+/ahl mice have a degraded appearance with missing individual stereocilia. Bar is 1 µm. Discussion
Mice with β-actin or γ-actin deficient auditory hair cells form functional, morphologically normal stereocilia and have normal hearing at young ages. However, when both isoforms are ablated, stereocilia are absent. Together, these data demonstrate that cytoplasmic actin is required during hair cell development, but that β-actin and γ-actin are redundant. Following development and the onset of hearing, hair cells and their stereocilia must be maintained for the life of the organism. During this phase, β-actin and γ-actin are both required because each knockout develops distinct stereocilia pathology and each has a distinct pattern of progressive hearing loss.
Actb and Actg1 gene products, rather than gene regulation, are important for stereocilia maintenance
The mammalian cytoplasmic actins are 99% identical; consequently, it has been unclear whether the proteins have different functions. Instead, two actin genes might be necessary to properly regulate the level of cellular actin, as is the case in Drosophila. Flies have two essential, closely related cytoplasmic actin genes. Lethality is rescued by expressing only one actin protein sequence from both promoters, demonstrating that gene regulation, rather than protein function, is the critical factor [31]. The analogous experiment has not been done in any mammalian system, but several lines of evidence suggest that gene regulation is not a limiting factor in hair cells. First, both isoactins can be expressed in hair cells at sufficient levels to independently support normal development. Second, knockout of Actg1 does not change the level of total actin in the cochlea because other isoforms are upregulated [9], arguing that changes in the composition, but not the concentration, of actin results in the observed pathology. Finally, Actb or Actg1 ablation results in different phenotypes. Furthermore, varying the dose of γ-actin changes the age of onset and the rate of progression of hearing loss, but results in the same end γ-actin-specific phenotype. These relationships strongly imply that each cytoplasmic actin gene product has distinct cellular functions.
Actb transcripts have a “zipcode” sequence element in their 3′UTR that mediates interactions with binding proteins that both regulate expression and transport the transcript to specific regions of the cell, such as neuronal growth cones or the leading edge of fibroblasts [32]–[34]. γ-Actin mRNA lack the zipcode sequence, and resulting differences in transcript localization and regulation likely contribute to the evolutionary conservation of both cytoplasmic actin genes. The zipcode sequence has not been studied in hair cells. Although β-actin and γ-actin seem to be colocalized in hair cells, transcript localization may nevertheless contribute to phenotypic differences seen in this study.
β-actin and γ-actin colocalize in hair cells but knockouts have distinct phenotypes
Differential subcellular localization of β-actin and γ-actin in hair cells could indicate that each protein has distinct functions and explain why each knockout phenotype is different. However, we detected both actin isoforms colocalized in inner hair cell stereocilia, cuticular plate and lateral wall. γ-Actin may be more abundant than β-actin in the cuticular plate [18] and more strikingly so in the lateral wall (Figure 2O–2P) [18]. Therefore, loss of γ-actin may affect these structures more than the loss of β-actin if the local actin concentration is not maintained.
Alternatively, colocalized and copolymerized β-actin and γ-actin may have distinct functions based on unique biochemical properties of each isoform, an argument bolstered by a recent biochemical study of recombinant γ-actin and β-actin [3]. γ-Actin, particularly in the calcium bound form, was shown to polymerize, depolymerize and treadmill significantly more slowly than β-actin. In addition, γ-actin and β-actin readily copolymerize, supporting our immunofluorescence data suggesting that stereocilia actin filaments are copolymers of both isoforms. Critically, the polymerization properties of the copolymer reflect the ratio of β-actin to γ-actin [3]. In this way, ablating or altering the concentration of one actin isoform in stereocilia likely alters the biochemical properties of the actin array.
We observed differences in the length of β-actin-deficient stereocilia and the stability γ-actin-deficient stereocilia, which could be explained in part by different polymerization kinetics of the actin isoforms. Stereocilia actin undergoes continuous treadmilling, with new monomers adding to actin filaments at stereocilia tips and depolymerizing at the stereocilia base [35]. Critically, the rate of actin treadmilling is precisely coupled to stereocilia length, suggesting that actin polymerization must be tightly regulated in order to maintain the proper hair bundle architecture [35]. Changes in the concentration of β-actin and γ-actin may have more significant consequences in hair cells than in other tissues. As Rubenstein and colleagues point out, the uniquely high calcium concentration in stereocilia may result in calcium-bound β-actin and γ-actin, which have dramatically different polymerization properties than the more commonly found magnesium-bound species [3]. Since stereocilia must be maintained for the life of the organism, it is conceivable that changes in the actin polymerization rate could account for the observed, slowly developing morphological defects.
We found that stereocilia develop normally in β-actin or γ-actin-deficient hair cells. Therefore, if actin isoform dependent polymerization kinetics contributes to the pathology we observed, then actin dynamics must be different in developing stereocilia and adult stereocilia. The rate of actin treadmilling in stereocilia has thus far only been measured in perinatal hair cell explants, leaving open the possibility that the treadmilling rate may be significantly different in adult stereocilia. Such differences may explain why β-actin or γ-actin deficient stereocilia develop normally but deteriorate in adults.
In addition to changes in polymerization properties, β-actin and γ-actin may have different affinities for a subset of actin binding proteins. Indeed, the excellent specificity of actin isoform specific antibodies suggests that each protein can be distinguished on the basis of protein-protein interactions. In keeping with this, cofilin [36], ezrin [37], l-plastin [38], βCAP73 [39], Thymosin b4 [40] and profilin [41] differentially interact with cytoplasmic and muscle actin isoforms while Annexin V is reported to bind γ-actin, but not β-actin [42]. Recent advances in recombinant cytoplasmic actin expression and more sophisticated assays of biochemical function should facilitate the discovery of additional isoform specific binding proteins.
Cytoplasmic actins in progressive hearing loss
Progressive hearing loss is common in the aging human population, resulting from a combination of environmental and genetic factors. Genetic mapping studies using inbred mouse lines have identified several genes that predispose mice for age-related hearing loss (AHL), including Cdh23ahl and ahl8, which encodes the R109H variant of fascin2 [25], [43], [44]. Assembling the products of genes that promote AHL into molecular pathways may provide useful insight into maintenance of auditory function. Along these lines, fascin2 and γ-actin are likely connected because hair cells that are γ-actin-deficient or carry fascin2 R109H develop phenotypically similar stereocilia degradation [44]. Furthermore, Johnson and colleagues have recently noted that in mice the respective genes (Actg1 and Fscn2) are separated by only 13kb and that transcript expression is coordinately regulated [44]. However, in contrast to the fascin2 R109H phenotype [43], progressive hearing loss due to γ-actin-deficiency does not require homozygosity for Cdh23ahl. Therefore, γ-actin likely also functions in pathways distinct from cadherin23 and fascin2 that are important for hair cell maintenance. This is consistent with the finding that γ-actin is required for normal growth and viability of isolated fibroblasts [45]. Finally, since mouse models lacking either β-actin or γ-actin develop distinct forms of progressive hearing loss, we conclude that the unique properties of each cytoplasmic actin are necessary to maintain proper structure and function of long-lived auditory hair cells.
Materials and Methods
Ethics statement
The experimental protocols in this study were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Generation of the Actbflox allele
A targeting vector containing loxP sites flanking exons 2 and 3, as well as a neomycin cassette, was electroporated into 129S6 murine embryonic stem cells at the Gene Targeting Mouse Service Core (University of Cincinnati, Cincinnati, OH). A clone with successful recombination was identified by Southern blot analysis, karyotyped and injected into C57Bl/6 blastocysts at the University of Wisconsin-Madison (Figure S1B). Chimeric males were bred to C57Bl/6 females to determine germline transmission of the floxed allele. The neomycin cassette was removed by crossing to a line expressing EIIa-cre [46] as described [30], generating Actbflox mice, which were backcrossed to C57Bl/6 for 10 generations. Details of the vector construction, Southern blot and genotyping procedures can be found in Text S1.
Immunofluorescent microscopy
Adult mice were perfused with 4% paraformaldehyde (PFA) in PBS, cochlea were dissected, additional fixative was gently perfused through the round and oval windows and then incubated in the same fixative solution for 2 hours at room temperature. Cochlea were washed in PBS and then decalcified in 170 mM EDTA in PBS at 4°C for 16 hours. Cochlea from post-natal mice at the indicated ages, where P0 is the day of birth, were dissected and immersed in fixative for 16 hours at 4°C and were not decalcified. The organ of Corti was dissected, postfixed in 100% methanol at −20°C for 10 minutes, rinsed in PBS and permeablized in 0.5% triton X-100 in PBS for 20 minutes at room temperature. Tissue was blocked for 1 hour in 5% goat serum in PBS prior to incubation with the indicated antibodies. Samples were mounted in ProLong anti-fade reagent and viewed on a Deltavision PersonalDV microscope equipped with a 100×1.4 NA objective (Applied Precision). Stacks of images were collected at 0.20 µm intervals and subsequently deconvolved using Resolve3d software (Applied Precision).
Scanning electron microscopy
Cochlea were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer with 1 mM CaCl2 by perfusing dissected cochlea through the round and oval windows followed by incubation in the same solution at room temperature for 4 hours. Following decalcification in 170 mM EDTA at 4°C for 16 hours, the organ of Corti was dissected and processed for SEM as described [9]. Briefly, tissue was successively incubated in 2% each arginine, glycine, glutamic acid and sucrose in water, 2% each tannic acid and guanidine-HCl in water and then 1% osmium tetroxide. Samples were critical point dried from CO2 and sputter coated with platinum before viewing on a cold field emission scanning electron microscope (Hitachi S4700). Stereocilia were measured using ImageJ software and statistical analysis (one-way ANOVA with Tukey post-test) was performed using GraphPad Prism software.
Mice
The Actg1flox alleles, mice expressing cre recombinase from either the Foxg1 locus (Jackson labs, on the C57Bl/6 background, stock number 006084 [26]) or from a Atoh1-cre transgene [27], and mice carrying the Cdh23Ahl+ allele on an otherwise C57Bl/6 background [24] (Jackson labs, stock number 002756) have been previously described. Standard mouse husbandry practices were used to generate the indicated lines. Actb-flox Atoh1-cre and Actg1-flox Atoh1-cre mice were backcrossed to C57Bl/6 mice for 5 generations. Animals were housed and treated in accordance with the standards set by the University of Minnesota Institutional Animal Care and Use Committee.
Antibodies
Monoclonal mouse anti-γ-actin antibody clone 1–37 [47] IgG was purified from ascites using a T-gel Purification kit (Pierce) and conjugated to either Alexa-488 or Alexa-568 fluorescent dyes using a Monoclonal Antibody Labeling Kit (Invitrogen) following the manufacturer's instructions. Unlabeled antibodies were detected with goat anti-mouse secondary antibodies labeled with Alexa-488 (Invitrogen). Unlabeled (clone AC-74) and FITC labeled anti-β-actin antibodies (clone AC-15) was obtained from Abcam.
Auditory Brainstem Response (ABR)
ABR waveforms were collected as previously described [9] for frequencies between 4 kHz and 32 kHz at half-octave intervals, starting at supra-threshold levels and decreasing in 5 dB steps to a sub-threshold level. A Tucker-Davis Technologies System 3 was used to generate symmetrically shaped tone bursts 1 ms in duration with 300 µs raised cosine ramps that were delivered to a calibrated magnetic speaker. Mice were anesthetized with Avertin and scalp potentials were recorded with subdermal electrodes with signals amplified 20,000 times, bandpass filtered between 0.03 and 10 kHz, digitized using a 20,000 kHz sampling rate and subjected to artifact rejection. Stacked waveforms were compared and the lowest level of stimulation that evoked an unambiguous ABR waveform was designated as the threshold.
Immunoblot analysis
As previously described [9], cochlea were dissected from mice of the indicated genotypes, frozen in liquid nitrogen, ground into powder, boiled in 1% SDS buffer and centrifuged to remove insoluble material. Protein concentration in the resulting lysate was determined by A280 measurement. Equal amounts of protein were separated by SDS-PAGE, transferred to nitrocellulose membranes and probed with anti-γ-actin antibody pAb7577 [47]. Fluorescently labeled secondary antibodies were detected and quantified using an Odyssey infrared scanner and software (Li-Cor Biosciences).
Supporting Information
Zdroje
1. HermanIM
1993 Actin isoforms. Curr Opin Cell Biol 5 48 55
2. RubensteinPA
1990 The functional importance of multiple actin isoforms. Bioessays 12 309 315
3. BergeronSE
ZhuM
ThiemSM
FridericiKH
RubensteinPA
2010 Ion-dependent polymerization differences between mammalian beta - and gamma-nonmuscle actin isoforms. J Biol Chem 285 16087 16095
4. BassellGJ
ZhangH
ByrdAL
FeminoAM
SingerRH
1998 Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci 18 251 265
5. DuginaV
ZwaenepoelI
GabbianiG
ClementS
ChaponnierC
2009 Beta - and gamma-cytoplasmic actins display distinct distribution and functional diversity. Journal of Cell Science 122 2980 2988
6. KarakozovaM
KozakM
WongCCL
BaileyAO
YatesJR
2006 Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science 313 192 196
7. ShmerlingD
DanzerC-P
MaoX
BoisclairJ
HaffnerM
2005 Strong and ubiquitous expression of transgenes targeted into the beta-actin locus by Cre/lox cassette replacement. Genesis 42 229 235
8. ShawlotW
DengJM
FohnLE
BehringerRR
1998 Restricted beta-galactosidase expression of a hygromycin-lacZ gene targeted to the beta-actin locus and embryonic lethality of beta-actin mutant mice. Transgenic Res 7 95 103
9. BelyantsevaIA
PerrinBJ
SonnemannKJ
ZhuM
StepanyanR
2009 Gamma-actin is required for cytoskeletal maintenance but not development. Proc Natl Acad Sci USA 106 9703 9708
10. ProcaccioV
SalazarG
OnoS
StyersML
GearingM
2006 A mutation of beta-actin that alters depolymerization dynamics is associated with autosomal dominant developmental malformations, deafness, and dystonia. Am J Hum Genet 78 947 960
11. MorellRJ
FridericiKH
WeiS
ElfenbeinJL
FriedmanTB
2000 A new locus for late-onset, progressive, hereditary hearing loss DFNA20 maps to 17q25. Genomics 63 1 6
12. RendtorffND
ZhuM
FagerheimT
AntalTL
JonesM
2006 A novel missense mutation in ACTG1 causes dominant deafness in a Norwegian DFNA20/26 family, but ACTG1 mutations are not frequent among families with hereditary hearing impairment. Eur J Hum Genet 14 1097 1105
13. van WijkE
KriegerE
KempermanMH
De LeenheerEM
HuygenPL
2003 A mutation in the gamma actin 1 (ACTG1) gene causes autosomal dominant hearing loss (DFNA20/26). J Med Genet 40 879 884
14. ZhuM
YangT
WeiS
DeWanAT
MorellRJ
2003 Mutations in the gamma-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26). Am J Hum Genet 73 1082 1091
15. LiuP
LiH
RenX
MaoH
ZhuQ
2008 Novel ACTG1 mutation causing autosomal dominant non-syndromic hearing impairment in a Chinese family. J Genet Genomics 35 553 558
16. DallosP
1992 The active cochlea. J Neurosci 12 4575 4585
17. TilneyLG
DerosierDJ
MulroyMJ
1980 The organization of actin filaments in the stereocilia of cochlear hair cells. J Cell Biol 86 244 259
18. FurnessDN
KatoriY
MahendrasingamS
HackneyCM
2005 Differential distribution of beta - and gamma-actin in guinea-pig cochlear sensory and supporting cells. Hear Res 207 22 34
19. SlepeckyNB
SavageJE
1994 Expression of actin isoforms in the guinea pig organ of Corti: muscle isoforms are not detected. Hear Res 73 16 26
20. FrolenkovGI
BelyantsevaIA
FriedmanTB
GriffithAJ
2004 Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet 5 489 498
21. GoodyearRJ
MarcottiW
KrosCJ
RichardsonGP
2005 Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol 485 75 85
22. AhmedZM
GoodyearR
RiazuddinS
LagzielA
LeganPK
2006 The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. Journal of Neuroscience 26 7022 7034
23. KazmierczakP
SakaguchiH
TokitaJ
Wilson-KubalekEM
MilliganRA
2007 Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449 87 91
24. KeithleyEM
CantoC
ZhengQY
Fischel-GhodsianN
JohnsonKR
2004 Age-related hearing loss and the ahl locus in mice. Hear Res 188 21 28
25. Noben-TrauthK
ZhengQY
JohnsonKR
2003 Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet 35 21 23
26. HébertJM
McConnellSK
2000 Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol 222 296 306
27. MateiV
PauleyS
KaingS
RowitchD
BeiselKW
2005 Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn 234 633 650
28. HöferD
NessW
DrenckhahnD
1997 Sorting of actin isoforms in chicken auditory hair cells. J Cell Sci 110 Pt 6 765 770
29. FurnessDN
MahendrasingamS
OhashiM
FettiplaceR
HackneyCM
2008 The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high - and low-frequency cells and evidence for a connection to the lateral membrane. Journal of Neuroscience 28 6342 6353
30. SonnemannKJ
FitzsimonsDP
PatelJR
LiuY
SchneiderMF
2006 Cytoplasmic gamma-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Developmental Cell 11 387 397
31. WagnerCR
MahowaldAP
MillerKG
2002 One of the two cytoplasmic actin isoforms in Drosophila is essential. Proc Natl Acad Sci USA 99 8037 8042
32. HuttelmaierS
ZenklusenD
LedererM
DictenbergJ
LorenzM
2005 Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature 438 512 515
33. KislauskisEH
ZhuX
SingerRH
1994 Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol 127 441 451
34. LeungKM
van HorckFP
LinAC
AllisonR
StandartN
2006 Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat Neurosci 9 1247 1256
35. SchneiderME
BelyantsevaIA
AzevedoRB
KacharB
2002 Rapid renewal of auditory hair bundles. Nature 418 837 838
36. De La CruzEM
2005 Cofilin binding to muscle and non-muscle actin filaments: isoform-dependent cooperative interactions. J Mol Biol 346 557 564
37. YaoX
ChengL
ForteJG
1996 Biochemical characterization of ezrin-actin interaction. J Biol Chem 271 7224 7229
38. NambaY
ItoM
ZuY
ShigesadaK
MaruyamaK
1992 Human T cell L-plastin bundles actin filaments in a calcium-dependent manner. J Biochem 112 503 507
39. ShusterCB
LinAY
NayakR
HermanIM
1996 Beta cap73: a novel beta actin-specific binding protein. Cell Motil Cytoskeleton 35 175 187
40. WeberA
NachmiasVT
PenniseCR
PringM
SaferD
1992 Interaction of thymosin beta 4 with muscle and platelet actin: implications for actin sequestration in resting platelets. Biochemistry 31 6179 6185
41. LarssonH
LindbergU
1988 The effect of divalent cations on the interaction between calf spleen profilin and different actins. Biochim Biophys Acta 953 95 105
42. TzimaE
TrotterPJ
OrchardMA
WalkerJH
2000 Annexin V relocates to the platelet cytoskeleton upon activation and binds to a specific isoform of actin. Eur J Biochem 267 4720 4730
43. JohnsonKR
Longo-GuessC
GagnonLH
YuH
ZhengQY
2008 A locus on distal chromosome 11 (ahl8) and its interaction with Cdh23 ahl underlie the early onset, age-related hearing loss of DBA/2J mice. Genomics 92 219 225
44. ShinJB
Longo-GuessCM
GagnonLH
SaylorKW
DumontRA
2010 The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci 30 9683 9694
45. BunnellTM
ErvastiJM
2010 Delayed embryonic development and impaired cell growth and survival in Actg1 null mice. Cytoskeleton 67 564 572
46. HolzenbergerM
LenznerC
LeneuveP
ZaouiR
HamardG
2000 Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a target gene. Nucleic Acids Res 28 E92
47. HanftLM
RybakovaIN
PatelJR
Rafael-FortneyJA
ErvastiJM
2006 Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc Natl Acad Sci USA 103 5385 5390
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



