-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvidence for a Xer/ System for Chromosome Resolution in Archaea
Homologous recombination events between circular chromosomes, occurring during or after replication, can generate dimers that need to be converted to monomers prior to their segregation at cell division. In Escherichia coli, chromosome dimers are converted to monomers by two paralogous site-specific tyrosine recombinases of the Xer family (XerC/D). The Xer recombinases act at a specific dif site located in the replication termination region, assisted by the cell division protein FtsK. This chromosome resolution system has been predicted in most Bacteria and further characterized for some species. Archaea have circular chromosomes and an active homologous recombination system and should therefore resolve chromosome dimers. Most archaea harbour a single homologue of bacterial XerC/D proteins (XerA), but not of FtsK. Therefore, the role of XerA in chromosome resolution was unclear. Here, we have identified dif-like sites in archaeal genomes by using a combination of modeling and comparative genomics approaches. These sites are systematically located in replication termination regions. We validated our in silico prediction by showing that the XerA protein of Pyrococcus abyssi specifically recombines plasmids containing the predicted dif site in vitro. In contrast to the bacterial system, XerA can recombine dif sites in the absence of protein partners. Whereas Archaea and Bacteria use a completely different set of proteins for chromosome replication, our data strongly suggest that XerA is most likely used for chromosome resolution in Archaea.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001166
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001166Summary
Homologous recombination events between circular chromosomes, occurring during or after replication, can generate dimers that need to be converted to monomers prior to their segregation at cell division. In Escherichia coli, chromosome dimers are converted to monomers by two paralogous site-specific tyrosine recombinases of the Xer family (XerC/D). The Xer recombinases act at a specific dif site located in the replication termination region, assisted by the cell division protein FtsK. This chromosome resolution system has been predicted in most Bacteria and further characterized for some species. Archaea have circular chromosomes and an active homologous recombination system and should therefore resolve chromosome dimers. Most archaea harbour a single homologue of bacterial XerC/D proteins (XerA), but not of FtsK. Therefore, the role of XerA in chromosome resolution was unclear. Here, we have identified dif-like sites in archaeal genomes by using a combination of modeling and comparative genomics approaches. These sites are systematically located in replication termination regions. We validated our in silico prediction by showing that the XerA protein of Pyrococcus abyssi specifically recombines plasmids containing the predicted dif site in vitro. In contrast to the bacterial system, XerA can recombine dif sites in the absence of protein partners. Whereas Archaea and Bacteria use a completely different set of proteins for chromosome replication, our data strongly suggest that XerA is most likely used for chromosome resolution in Archaea.
Introduction
In Bacteria, homologous recombination is essential during DNA replication to resume stalled replication forks and to repair DNA double and single strand breaks. Odd numbers of homologous recombination events between circular chromosomes generate dimers, which need to be resolved to ensure proper segregation in daughter cells. In Escherichia coli two paralogous site-specific tyrosine recombinases XerC and XerD were shown to convert chromosome dimers to monomers [1] by acting at a specific DNA recombination site, dif, located close to the replication termination region [2]–[4]. Homologues of XerCD are widespread in the bacterial domain, and dif sites have been characterized in several Proteobacteria and Firmicutes [5]–[10]. dif sites are semi-conservative inverted repeats formed by two arms (Xer protein binding sites) of 11 base pairs [11], separated by a spacer of 6 bp and are fairly conserved among Bacteria [5]. In Proteobacteria the XerCD activity is tightly regulated by the cell division protein FtsK, a DNA translocase anchored at the division septum [12]–[15]. In E. coli, 8 bp G-rich polar sequence elements (KOPS) direct FtsK translocation on DNA [16]–[19]. KOPS are oriented from the origin of replication towards dif where their polarity is precisely inverted. FtsK DNA translocation is therefore always oriented towards the dif site and dif sites carried on a chromosome dimer are brought together at midcell. FtsK further controls chromosome dimer resolution by activating XerD activity through protein-protein interactions [20], [21]. In several Lactococcus and Streptococcus strains, the canonical bacterial XerCD-dif system has been replaced by a single tyrosine recombinase, XerS (distantly related to XerCD) whose gene is located next to its specific dif-like site and localized at the terminus of replication [22]. Strikingly, this XerS-dif-like system still depends on the KOPS-oriented FtsK activity to form the synaptic complex for recombination [22].
Archaea harbour circular chromosomes and have an active homologous recombination system [23]. Therefore, they are expected to resolve chromosomal dimers to ensure proper chromosome segregation. It was previously reported that most archaeal genomes encode a single protein homologous to bacterial XerCD [24]; however, none encode a FtsK homologue. It is thus unclear whether archaeal Xer-like proteins (hereafter called XerA) are involved in chromosome resolution in Archaea, as in Bacteria.
In order to determine whether XerA is involved in chromosome resolution, we performed an in silico search for XerA specific recombination dif-like sites in four closely related archaeal genomes from Thermococcales. We identified a highly conserved 28 bp sequence that shares 14 out of 28 bases with characterized bacterial dif sites. The predicted dif sites are systematically located in the replication termination regions of the four genomes. The same analysis performed on three Sulfolobales genomes revealed that a similar site is also present in this crenarchaeotal species. We further identified short polarized sequences that point towards the predicted dif sites in Thermococcales genomes. We validated the in silico predictions by showing that a purified recombinant XerA protein from Pyrococcus abyssi specifically recombines plasmids carrying the predicted dif site of this archaeon. The recombination activity did not require the presence of any protein partner, in contrast to bacterial Xer-mediated recombination. Our data strongly suggest that XerA is most likely used for chromosome resolution in Archaea.
Results
The majority (88%) of archaeal genomes sequenced so far (KEGG database [25]) harbour single orthologues of the bacterial XerCD recombinases. Alignments of several bacterial XerCD proteins with XerA proteins from different Archaea revealed that they share a conserved C-terminal domain where the catalytic site (Figure S1) is located. The six catalytic residues (R-K-H-R-[H/W]-Y) characteristic of tyrosine recombinases [26] are perfectly conserved in archaeal XerA proteins [24].
The more variable outer sequences of the bacterial dif sites are the place of specific amino-acids/bases contacts that drive protein-DNA interaction specificity. Several amino acids residues involved in these contacts were identified, which led to the definition of a dif-binding region within the C-terminal domain of the Xer recombinases [27]–[29]. The dif binding motif is also conserved in XerA proteins. Notably, the key residues that define binding specificity for the XerC or XerD binding sites are distinct from XerC or XerD in XerA (Figure 1A and Figure S1).
Fig. 1. Alignments of Xer dif-binding motifs and dif sites from Archaea and Bacteria. 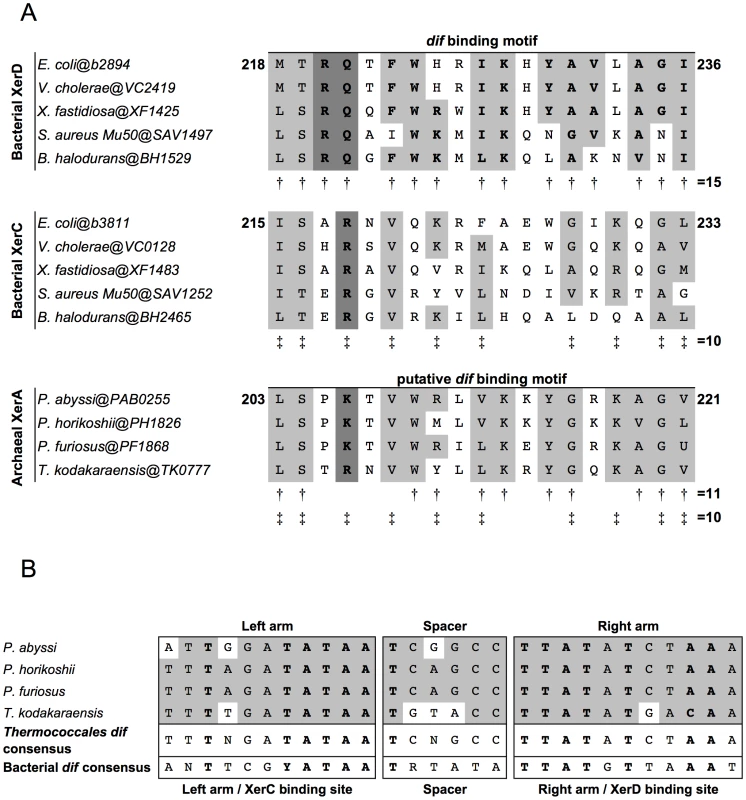
A. Alignments of the dif binding motifs from bacterial XerC and XerD and archaeal XerA proteins. Amino acids shared between XerD dif binding motifs, XerC dif binding motifs and putative dif binding motifs of archaeal XerA proteins from Thermococcales are boxed. Conserved positions in XerD dif binding motifs are indicated by ‘†’ and conserved positions in XerC dif binding motifs are indicated by ‘‡’. The putative dif binding motifs of XerA proteins share several positions with XerD and/or XerC proteins. The key motif for each family (XRX for XerC, XKX for XerA and and RQX for XerD) are shown in bold and shaded in dark gray. B. Predicted Thermococcales dif sites are highly conserved, show very few or no mismatches between the two arms of the inverted repeat and share several positions with the bacterial dif consensus (bold letters). Consensus sequences are indicated according to the IUPAC code. In silico identification of dif-like sites in archaeal genomes
To search for conserved putative archaeal dif sites, we selected as first candidates four closely related genomes of Thermococcales since their XerA proteins [Pyrococcus abyssi (Pab0255), Pyrococcus horikoshii (PH1826), Pyrococcus furiosus (PF1868) and Thermococcus kodakaraensis (TK0777)] are the most similar to bacterial XerCD (35%–39% identity; Figure S2) among archaeal XerA. The putative dif-binding motif of these XerA proteins shares numerous conserved positions with both XerC (10 out of 19 positions) and XerD (11 out of 19 positions) proteins (Figure 1A). These remarkable sequence similarities suggest that one may expect to identify conserved dif-like sequences in these four closely related archaeal genomes based on known properties of bacterial dif sites. Finally, XerA proteins are well conserved between these four species (above 85% similarity, Figure S2). Thermococcales XerA proteins are thus expected to recognize similar dif sites.
We built an algorithm to search for any potential tyrosine recombinase-binding site. We searched for imperfect inverted repeats of 11 to 15 bp separated by spacers ranging from 4 to 10 bp. A total of 481,319 sequences were recovered after this analysis. In order to reduce the sequences to one single most likely dif candidate, we selected only sequences that were conserved above 80% similarity in the four genomes. We found six sequences fulfilling this criterion: two were shawn to be spacer sequences of CRISPRs [30], [31], three were imperfect inverted repeats of 11 bp separated by long spacers (one of 8 bp and two of 10 bp), and only one sequence in each genome was composed of 11 bp imperfect inverted repeats separated by a 6 bp spacer (Figure 1B). Strikingly, these sequences are 100% conserved between P. horikoshii and P. furiosus, have three mismatches with that of Pyrococcus abyssi and seven with that of T. kodakaraensis. Moreover, these four predicted sites share many positions with the bacterial dif consensus sites (Figure 1B). The three Pyrococcus sites show 14 out of 28 conserved positions of the bacterial dif-consensus, and the T. kodakaraensis site shows 18 out of 28 (Figure 1B). The same site search was performed on the T. gammatolerans, T. onnurineus and T. sibiricus genomes and led to the identification of unique sites showing the same level of conservation with the bacterial dif-consensus (Figure S3). Further analysis of the dif sites environment in Thermococcales genomes revealed that all dif sites are surrounded by conserved flanking sequences (Figure S3).
As in the canonical bacterial model, Thermococcales dif sites predicted by our analysis were not located next to the xerA genes (Figure 2 and Figure S4). The position of the xerA genes relative to the replication origins (oriC) was highly variable (Table S1), whereas the predicted dif sites were located within the second quarter of the genome for P. horikoshi, P. furiosus and T. kodakaraensis (135°, 122° and 130° from oriC, respectively) and in the third quarter (−142° from oriC) for P. abyssi (Figure 2). In the latter case, the difference in position could be a consequence of the large fragment inversion containing oriC that recently occurred in this species [32]. The conservation of dif site positions relative to oriC in the four Thermococcales (between 122° and 142°) is especially striking since these genomes have been extensively rearranged by chromosome recombination, as indicated by the patterns obtained from whole genome alignments (Figure S5).
Fig. 2. Genomic localization of dif sites and xer genes. 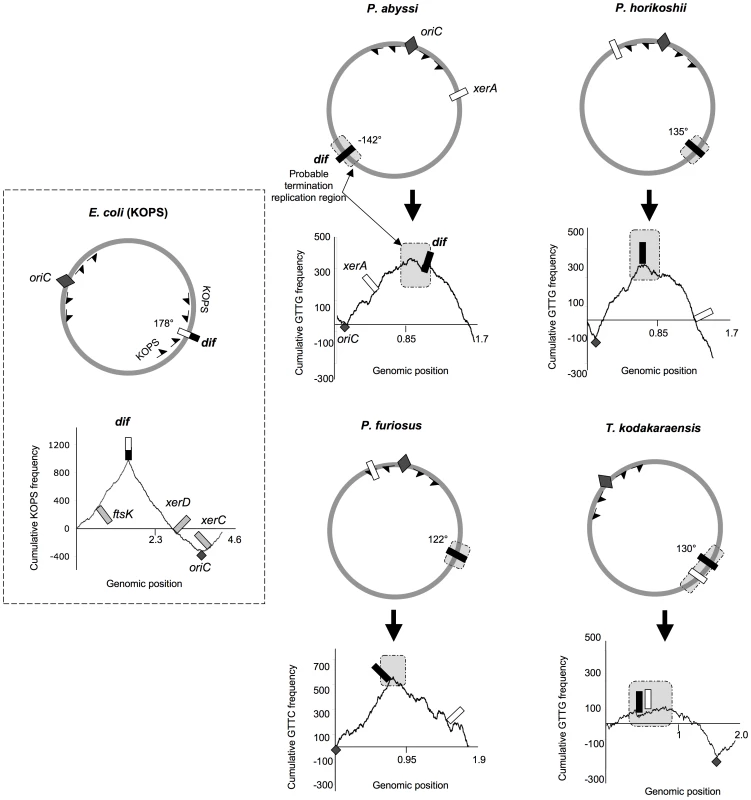
The E. coli KOPS cumulative skew produces a pyramidal shaped graphic with the dif site (black and white rectangle) located at the summit (178° from oriC). xerC, xerD and ftsK genes are located in the oriC region. ASPS skew graphics from Thermococcales show rounded shapes where oriC (dark gray diamond) can be localized precisely and where putative regions of replication termination (light gray rectangles) can be broadly determined. GTTG is the most skewed 4 nt sequence in P. abyssi, P. horikoshii and T. kodakaraensis genomes whereas GTTC is the most skewed 4 nt sequence in P. furiosus genome. Thermococcales dif sites (black rectangles) are all located at the summit of the ASPS skews graphics, at 120–142° from oriC, in putative replication termination regions. The xerA gene (white rectangles) positions are more variable. Genomic coordinates of oriC, dif and xerA genes can be found in Table S1. The dif-like sites identified in our analyses do not localize precisely at 180° from oriC. However, the predicted dif site of P. abyssi is located into a late replicating fragment of the genome [33]. dif sites positions are therefore compatible with a localization in the terminus region of chromosome replication. It is not known if the two replication forks always meet at the same point in Thermococcales. A precise site for the terminus of DNA replication in Thermococcales genomes cannot be predicted by using GC skew analysis because, in contrast to the sharp peak observed at oriC, the potential termination region appears as a broad distribution [33], [34]. The terminus region appears to be especially prone to chromosomal rearrangement in Thermococcales [32], possibly explaining this lack of resolution.
We next extended our analysis to archaeal genomes outside of the Thermococcales group. Using the dif sites and flanking sequences found in Thermococcales, we constructed a Hidden Markov Model with HMMER2 [35] and searched other archaeal genomes for potential dif sequences. As an example, a single statistical-significant sequence matching the Thermococcales dif sites was found in the Methanosphaera stadtmanae genome. This site is located at about 180°C from oriC (Figure S6), and is surrounded by imperfect inverted repeats. We then selected three Sulfolobales genomes to search for dif sites in Crenarchaeota. Sulfolobus species possess multiple replication origins [36], [37] raising the possibility that an alternative to the canonical Xer recombination system may occur in these organisms. We used the same initial methodology that was applied to Thermococcales genomes. Unique dif sites were found for S. acidocaldarius and S. tokodaï, whereas two copies of this site were found at the same chromosomal location in the S. solfataricus genome (Figure S7). As opposed to Euryarchaeota, the predicted dif sites localized close to xerA genes, and were flanked on only one side by a short conserved sequence of 13 bp.
Identification of polarized sequences that point to the predicted dif sites
In Bacteria, the directionality of FtsK-mediated DNA translocation is determined by octamers that are polarized on each arm of the chromosome with their orientation switching at the dif site [16]–[18]. Although Archaea lack an FtsK homologue, we searched for the most frequent and skewed sequences in Thermococcales genomes by using the R'MES program ([38], see Materials and Methods). We used the E. coli genome to validate this methodology and, as expected, we found that KOPS are the most over-represented skewed 8nt-long sequences. The corresponding diagram shows a sharp peak corresponding to the position of the E. coli dif site (Figure 2). We then analyzed all possible sequences of 4 to 8 nucleotide-long in the four Thermococcales genomes. We identified GTTG as the most over-represented and skewed sequence in the P. abyssi, P. horikoshi and T. kodakaraensis genomes, and GTTC in the P. furiosus genome. For Thermococcales genomes, the cumulative frequency of these 4 nucleotide sequences (Archaea Short Polarized Sequences, ASPS) does not give a perfect triangle-shaped diagram as observed for Proteobacteria (Figure 2) or Firmicutes where dif sites locate exactly at the KOPS skew inversion. Nevertheless, in the case of the three Pyrococcus genomes, the cumulative ASPS skews diagrams displayed a sharp optima precisely located next to the dif-like sites identified in silico (Figure 2). The optimum was located very close to the dif sites in the cases of P. horikoshi and P. furiosus, whereas it was located more to the left in the case of P. abyssi (around 160° instead of 142°). This shift could be due to a recent transposition of two chromosomal segments that occurred in the terminus region of this species [32]. In the case of T. kodakaraensis, we only obtained the sharp minimum corresponding to the replication origin, whereas the opposite region appeared as a broad peak containing the predicted dif site. The same analysis was extended to other archaeal genomes where dif sites were predicted, and revealed that both euryarchaeal and crenarchaeal genomes harbour ASPS (Figure 2; Figures S4, S6, S7). Remarkably, the M. stadtmanae ASPS skew displays the triangle-shaped diagram observed in Bacteria, with the predicted dif site precisely located at the skew inversion (Figure S6).
P. abyssi XerA protein binds to the predicted dif site
To test our in silico predictions, we purified to homogeneity the P. abyssi XerA protein (Pab0255) as a recombinant protein. Recognition of the predicted dif site by the P. abyssi XerA protein was first evaluated by Electrophoretic Mobility Shift Assay (EMSA). Double stranded oligonucleotides corresponding to the P. abyssi dif site were incubated with increasing amounts of P. abyssi XerA protein at 20° and 65°C (Figure 3). As a control, P. abyssi XerA protein was also incubated in presence of a non-specific DNA site corresponding to the minimal recombination site (attP) of another archaeal tyrosine recombinase, the SSV1 integrase [39]. XerC and XerD from E. coli have only been shawn to bind to an oligonucleotide containing the E. coli dif site [40]. In contrast, P. abyssi XerA was able to bind to both substrates, with two protein-DNA complexes detected in each case (Figure 3). However, complex migration was different between the two DNA substrates, with P. abyssi XerA/dif complexes showing a higher mobility than P. abyssi XerA/attP complexes. Furthermore, the P. abyssi XerA protein presented a preference for the P. abyssi dif site as compared to the non-specific substrate, with a 4 fold increase in complex formation at 20°C and an 8 fold increase at 65°C (Figure 3). At the P. abyssi optimal growth temperature (90°C), XerA binding to the dif site should therefore be highly specific. Competition experiments further confirmed that XerA has a much higher affinity for its dif site than for a heterologous tyrosine recombinase binding site (Figure S8).
Fig. 3. Binding of P. abyssi XerA to specific and non-specific DNA substrates. 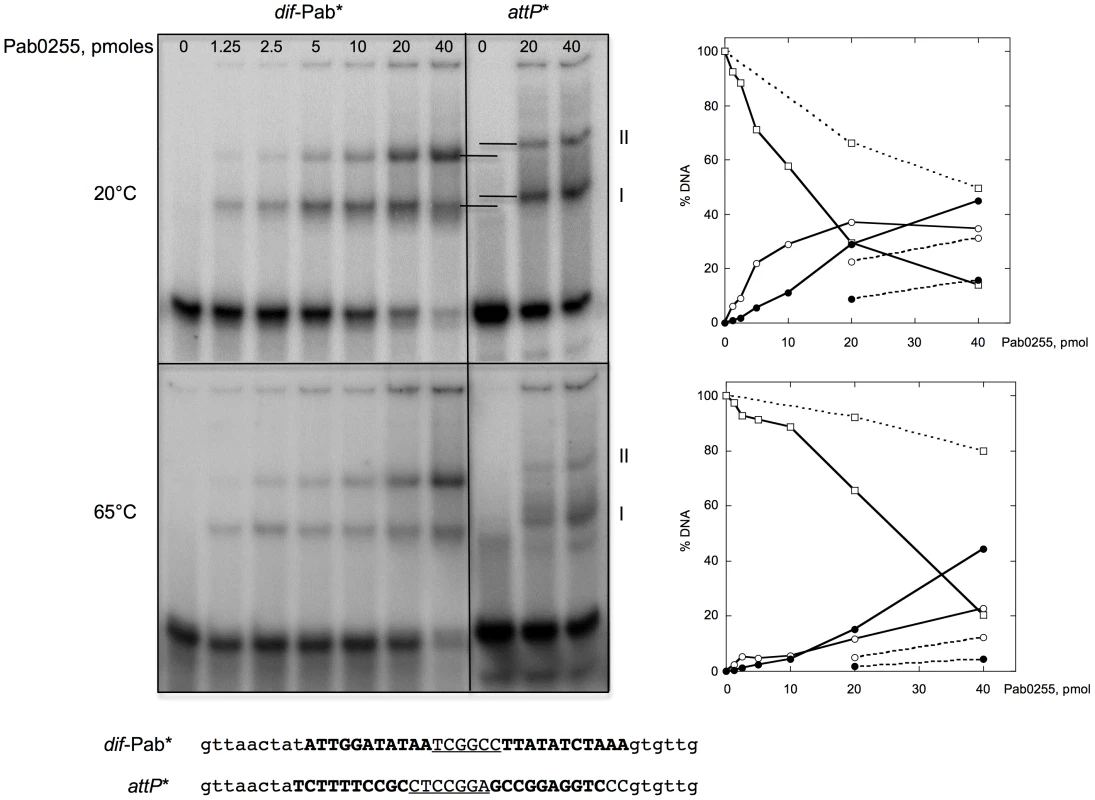
Increasing amounts of XerA were incubated at 20°C or 65°C with two different double-stranded oligonucleotides 5′end-labeled on their top strand. Reactions were loaded onto 5% non denaturing acrylamide gels containing 5% glycerol. Electrophoresis was performed in TGE buffer (50 mM Tris, pH 7.5, 8 mM glycine, 0.1 mM EDTA) at 4°C for 4 h at 7.5 V/cm. The DNA-protein complexes were visualized by phosphore-imaging. Right panel: quantification of free and bound DNA as a function of Pab0255 amount. Plain lines, dif-Pab substrate; dotted lines, attP substrate. Free DNA, square; complex I, white circle; complex II, black circle. P. abyssi XerA protein specifically recombines plasmids containing the predicted dif site
We next searched for full or partial site-specific recombination activity. In the case of XerCD, in vitro recombination at dif sites requires the C-terminal domain of FtsK [15]. However, dif-dependent DNA relaxation has been observed for XerC and XerD [41]. In order to test for such activity, we cloned an oligonucleotide containing the predicted dif site into a plasmid vector. After incubation of XerA with this substrate, reactions products were analyzed by agarose gel electrophoresis. Whereas incubation of the control plasmid (without dif site) with P. abyssi XerA protein did not reveal any reaction product, addition of the protein to the dif-containing plasmid led to the appearance of several new bands of lower mobility than the open circular (Moc) form of the substrate (Figure 4A). The migration of the major product suggested that it may correspond to the supercoiled form of a dimeric plasmid (Dsc), while the other products may correspond to increasing multimers of the dif-containing plasmid. The reaction was strongly dependent on temperature (Figure 4A), as expected for a reaction catalyzed by a protein from a hyperthermophilic organism. A single product, migrating slightly above the open circular substrate form appeared when the incubation was performed at 20° or 35°C. The amount of product strongly increased when the incubation was performed at 50° and 65°C, reaching an amount roughly equivalent to that of the remaining monomeric supercoiled (Msc) substrate. Several new products of low mobility were detected when the reaction was performed above 50°C, and their relative amounts increased from 50° to 65°C. To determine whether the reaction products generated by the P. abyssi XerA were indeed multimers of the dif-containing plasmid, we took advantage of a unique HindIII restriction site present on the plasmid. We assumed that partial HindIII digestion of the reaction products would produce linear multimeric plasmids that could be identified by their size. The products of a P. abyssi XerA catalyzed reaction performed for 20 minutes at 65°C were incubated with one unit of HindIII for one hour, either at 37°C for full digestion or at 20°C for partial digestion (Figure 4B). At 37°C, digestion of reaction products produced only linear DNA of the monomeric size (LM, 2.6 kb), indicating that all reaction products were multimers of dif-containing plasmids which were cleaved at all available HindIII sites (Figure 4B). HindIII digestion at 20°C produced two additional bands of linear DNA with the expected molecular weight for linear dimers (LD, 5.2 kb) or linear trimers (LT, 7.8 kb) of the dif-containing plasmid. This result indicates that P. abyssi XerA can recombine dif-containing plasmids in the absence of protein partners, producing multimeric forms of the initial substrate. The specificity of the reaction was further controlled by using as substrate a plasmid containing the attP site (Figure S9). No recombination activity could be detected on this substrate, further indicating that binding of XerA to the attP site is non specific.
Fig. 4. Recombination activity of P. abyssi XerA. 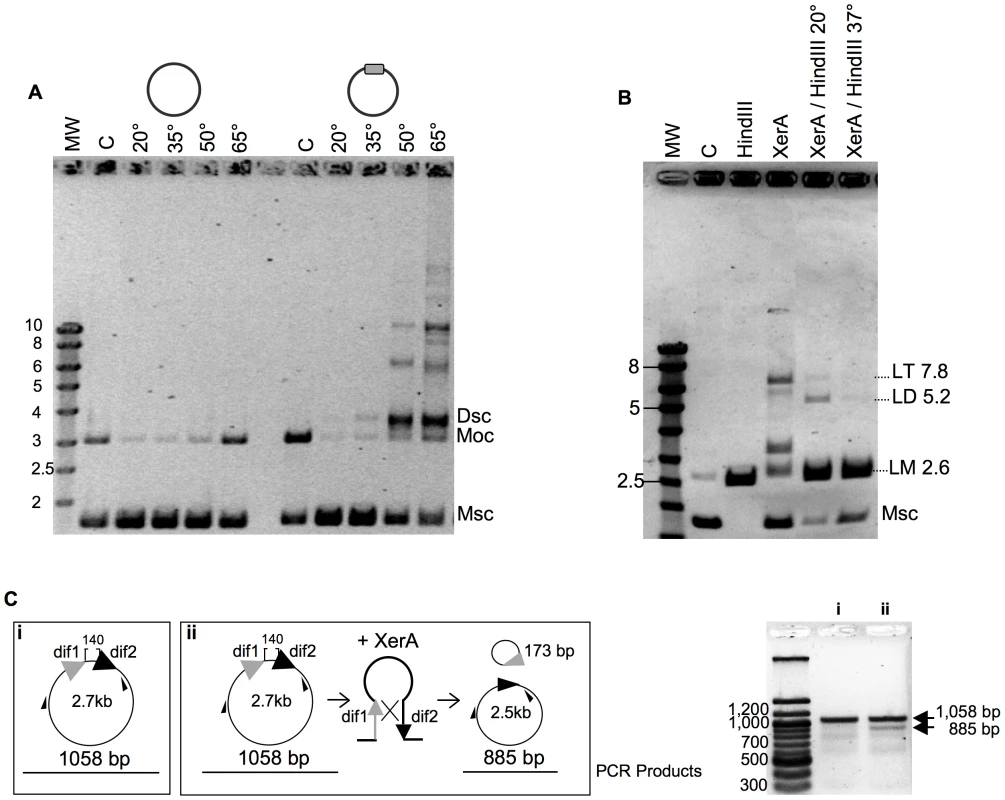
A. Temperature dependence of the reaction. XerA was incubated at different temperatures with a plasmid containing or not the predicted dif site (gray box). MW: molecular weight marker (identified in Kbp on the left). C: control without protein. The control assay was performed at 65°C. Msc: supercoiled monomer. Moc: open circular monomer. Dsc: supercoiled dimer. B. Recombination product analysis. C: control without protein. HindIII, pBend2dif full restriction by HindIII. XerA: recombination reaction, 1h at 65°C. XerA/HindIII 20°C: Recombination reaction followed by restriction with one unit of HindIII, for 1 h at 20°C. XerA/HindIII 37°C: same as before, but restriction performed at 37°C. Msc: supercoiled monomer. LM: linearized monomer (2.6 kbp). LD: linearized dimer (5.2 kbp). LT: linearized trimer (7.8 kbp). C. Resolution reaction. The substrate used is diagramed in (i) and the product in (ii). Small black arrows correspond to the hybridization position of the oligonucleotides used in the PCR assay. PCR fragments obtained on the substrate (i) or on the reaction products (ii) were analyzed by agarose gel electrophoresis (right). We followed the time course formation of multimeric plasmids by P. abyssi XerA on the dif-containing plasmid at 65°C. Plasmid dimers were obtained after five minutes incubation and plasmid multimers were detected after 10 minutes (Figure S9). After 30 minutes reaction time, the relative intensity of all bands remained fairly constant suggesting either that the recombination activity had reached enzymatic equilibrium, or that the protein was rapidly inactivated upon incubation at 65°C. However, pre-incubating the protein alone for up to 40 min at 65°C did not reduce the extent of recombination (Figure S9) thus ruling out protein denaturation during the time course assay. This suggests that at the reaction equilibrium, production of multimers from monomers is equivalent to multimer resolution into monomers.
To further validate the resolution activity of the P. abyssi XerA protein, we constructed a substrate with two dif sites in direct repeat (Figure 4C). The reaction products were analyzed by PCR as both integration and resolution events can occur on this substrate. Resolution events reduced the distance between the two primers from 1058 bp to 885 bp (Figure 4C). Even though integration events were still favoured, as attested by the appearance of plasmid multimers (not shown), a PCR product with a size around 900 bp was detected (Figure 4C). This product indicates that XerA was able to assemble a recombination proficient synaptic complex and that although at low level resolution events also occurred. The P. abyssi XerA protein is therefore able to catalyse both resolution and integration depending on the substrate provided in the reaction.
Discussion
We have shown that the P. abyssi XerA protein (homologous to the bacterial XerCD recombinases) specifically recombines a plasmid containing a predicted dif sequence present in the P. abyssi genome. This dif sequence was identified in silico, taking into account known features of tyrosine recombinase recombination sites and searching for sequences present in the genomes of four closely related Thermococcales. Importantly, whereas the location of xerA genes relative to the replication origin (oriC) varies from one genome to the other, the positions of the dif sites with respect to oriC is relatively conserved in all four genomes. These observations strongly suggest that archaeal XerA proteins may be involved in the resolution of chromosome dimers at the terminus of replication.
Interestingly, although Archaea lack a FtsK homologue, we could identify polarized sequences of four nucleotides (ASPS) that define the replication termination region and point towards the predicted dif sites in Euryarchaeota. The ASPS are shorter than the KOPS used by FtsK in Bacteria. Strikingly, in most genomes the predicted dif site localized at the summit of curves obtained by ASPS cumulative skew analyses. Archaea may therefore use a functional analogue of the bacterial FtsK-KOPS mechanism to produce a dif-synaptic complex in vivo. It was suggested that the archaeal bipolar DNA helicase HerA, which is probably involved in the processing of double-strand breaks for homologous recombination [42]–[44] may also be a functional analogue of FtsK in Archaea on the basis of their common ATPase domain used for DNA translocation [45]. Even though our results show that P. abyssi XerA does not require an accessory protein to catalyse recombination in vitro, as opposed to bacterial XerCD which only recombine dif sites in the presence of FtsK [12], [15], they do not exclude that a protein partner could either regulate synaptic complex assembly or XerA activity in vivo. Indeed, in Thermococcales genomes, dif sites are flanked by inverted repeats (Figure S4) that may be binding sites for such partner.
Using different in silico methodologies, we were able to predict dif sequences in other euryarchaeal genomes and crenarchaeal genomes that harbour xerA genes. Archaea lacking a xerA gene, such as Thaumarchaea and Pyrobaculum species, may have recruited another tyrosine recombinase, as happened in some bacterial groups [5], [22].
Although the archaeal and bacterial Xer/dif systems use similar dif sites, they differ in terms of biochemical properties and reaction mechanisms. Whereas E. coli and B. subtilis XerCD do not bind a DNA fragment without dif site [8], the P. abyssi XerA protein can bind with a lower affinity to a heterologous tyrosine recombinase site. However this site is not recognized as a recombination substrate. More significantly, the P. abyssi XerA protein can recombine plasmids carrying the dif sequence in the absence of protein partner. In contrast, the XerCD activity depends in vitro and in vivo on FtsK to recombine the chromosomal dif site or on other partners (PepA and ArcA or ArgR) to recombine the plasmidic recombination sites psi on pSC101 or cer on ColE1 [46]–[49]. The ability of P. abyssi XerA to recombine in vitro a dif-containing plasmid without any accessory protein suggests that XerA may also work alone in vivo. However we cannot rule out that a protein partner may bind the conserved dif-flanking sequences. Such a partner may either control the directionality of the reaction towards resolution or coordinate the recombination activity with the progression of the cell cycle. Alternatively, XerA activity may be limited to replication termination and chromosome segregation by regulating XerA expression at the transcriptional level. In agreement with this view, the xerA gene of the crenarchaeon Sulfolobus acidocaldarius (Saci 1490) is induced during the G1/S phase and reaches its maximal expression level in the G2 phase of the cell cycle [50].
Our results show that Archaea possess a Xer/dif system similar to its bacterial counterpart that is likely involved in chromosome dimer resolution. However, the archaeal Xer system displays differences, such as the involvement of a unique protein and the ability to perform site-specific recombination in vitro in the absence of accessory proteins. To get a better view of the evolution of the Xer system, we performed a phylogenetic analysis including bacterial and archaeal Xer proteins and a subset of bacteriophage encoded tyrosine recombinases (Figure 5). Unfortunately, the resulting tree is not resolved at most basal nodes, preventing a clear view of the evolutionary relationships of these proteins. However, it shows that bacterial and archaeal homologues are not intermixed, indicating that no recent horizontal gene transfer has occurred between domains. Our data thus suggest that a Xer/dif system was present in the common ancestor of Archaea and Bacteria, suggesting that this ancestor had a circular double-stranded DNA genome. However, this raises further issues such as why the replication machinery of Archaea and Bacteria are now composed of non homologous proteins [51]. Alternatively, homologous viral tyrosine recombinases may have been recruited independently in Archaea and Bacteria to be used as Xer/dif systems after transition from RNA to DNA genomes [52]. In any case, the presence of a shared Xer-dif system in Bacteria and Archaea illustrates the complex origin of modern DNA genomes. Further studies of Xer-dif systems in different archaeal and bacterial groups will be now necessary to test alternative scenarios for the origin and evolution of Xer proteins.
Fig. 5. Xer phylogeny. 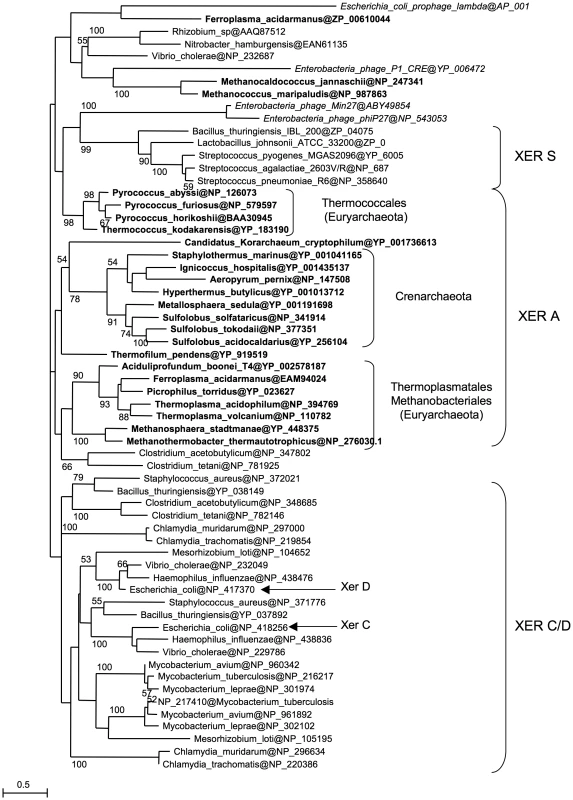
Unrooted maximum likelihood tree of a selection of bacterial and archaeal (bold) Xer homologues and viral tyrosine recombinases (italics). Sequences are identified by their NCBI Reference Sequence. Numbers at nodes represent bootstrap values. The scale bar represents the average number of substitutions per site. Materials and Methods
Identification of dif candidates
A model searched for all inverted repeats (11 to 15bp) separated by a spacer (4 to 10bp) present in non-coding genomic regions. A consensus sequence was deduced from the alignment of predicted dif sites and represented as a sequence logo [53].
Skew analyses
Statistical significant skewed words in the four Thermococcales genomes were determined using the R'MES program (http://mig.jouy.inra.fr/logiciels/rmes/) [38]. Since R'MES reads only one single strand of DNA at time, we artificially defined several ends of replication, starting at 120°, and then moving by 5° steps up to 200° from oriC. From these artificially-defined ends of replication to oriC we reverse complemented the genomic sequence. The most skewed word for each analyzed genome was selected by comparing the R'MES results. Cumulative skews were calculated using the formula:
Genome alignments
Genomes of the four Thermococcales where aligned by dot-plot analyses based on BlastP searches (e-value of 1×10−10).
Homology searches and phylogenetic analysis
Xer homologues were searched by BLASTP at the NCBI (http://www.ncbi.nlm.nih.gov/) against complete sequenced archaeal genomes using E. coli XerC and XerD proteins as query (threshold of 1×e−10). The retrieved homologues were aligned using Muscle and a specific HMM profile was calculated for exhaustive detection of homologues. Using the HMMER program (http://hmmer.janelia.org/) we performed iterative searches until no new homologues were detected in the archaeal genomes. From the resulting dataset, a preliminary phylogenetic analysis allowed to select 62 representative XerC and XerD sequences from several bacterial species and several tyrosine recombinases from plasmids/viruses or from mobile elements integrated into cellular genomes. The final alignment was trimmed to remove ambiguously aligned positions, leading to 222 conserved residues for phylogenetic analysis. A maximum likelihood tree was obtained by using PHYML [54], with the WAG evolutionary model including correction for heterogeneity of evolutionary rates (4 categories+invariant) and statistical support at nodes was calculated by non parametric bootstrap on 100 resampling of the original dataset by PHYML.
Cloning, expression, and purification of Pab0255
The Pab0255 gene was amplified by PCR using P. abyssi genomic DNA as a template, Phusion high-fidelity DNA polymerase (Finnzyme) and the following primers:
5′-GGGAACATATGCACCATCACCATCACCATGAGGAGAGGGAGGAGAGAGTGAGGGATGATACAATTG-3′
5′-TTTTTGCGGCCGCTTAGGAACCCCCGATG-3′
The forward primer allows the addition of six histidine codons in frame with the ATG start codon (underlined). The PCR product was digested by NdeI and NotI and cloned into a derivative of a pET vector (Novagen). The resulting recombinant plasmid was sequenced prior to being transformed into the E. coli expression strain Rosetta(DE3)pLys (Novagen).
Cells were grown in 2xYT medium (BIO101Inc.) at 37°C to A600nm = 1 and expression of Pab0255 was induced by the addition of 0.5 mM IPTG (final concentration). Four hours after induction, cells were harvested by centrifugation, and the pellets resuspended in 40 ml of 50 mM Tris-HCl, pH 8.0, 1 M NaCl and 5 mM β-mercaptoethanol and stored at −20°C. Cells were lysed by sonication and centrifuged at 13, 000× g for 30 min at 25°C. The supernatant was collected and heated for 15 minutes at 70°C. After centrifugation at 13,000× g, the supernatant was loaded onto a Ni2+ affinity column (Ni-NTA agarose, Qiagen) pre-equilibrated in the same buffer. The His-tagged Pab0255 was eluted at 20 mM imidazole, and loaded onto a 2 ml HiTrap Heparin (Amersham Biosciences) column pre-equilibrated in a buffer containing 50 mM Tris-HCl pH 7.0, 200 mM NaCl, 1 mM DTT. A NaCl linear gradient (200 mM to 2 M) was developed, and the protein eluted at about 800 mM NaCl. Finally, the protein was loaded onto a cation-exchange SP Sepharose column (Amersham Biosciences) pre-equilibrated in the same buffer, and eluted by a NaCl linear gradient. The purified protein was dialysed against 50 mM Tris pH7.0, 300 mM NaCl, 50% glycerol prior to being stored at −20°C.
Substrate preparation
The following 43 nt long oligonucleotides containing the top and bottom strands of the predicted dif site from P. abyssi (Pab) or minimal attP site from SSV1 [39] were purchased from Eurogentec:
Pab-Dif-Top 5′-gttaactatATTGGATATAATCGGCCTTATATCTAAAgtgttg-3′
Pab-Dif-Bottom 5′-caacacTTTAGATATAAGGCCGATTATATCCAATatagttaac-3′
attP-Top 5′-gttaactaTCTTTTCCGCCTCCGGAGCCGGAGGTCCCgtgttg-3′
attP-Bottom 5′-caacacGGGACCTCCGGCTCCGGAGGCGGAAAAGAtagttaac-3′
For binding assays, top strand oligonucleotides were 5′-end labeled by using [g-32P]ATP and T4 polynucleotide kinase. Unincorporated nucleotides were removed by spin dialysis, and the labeled oligonucleotide was then hybridized with a 2-fold excess of unlabeled complementary strand in TE buffer (10 mM Tris, pH 8.0, 1 mM EDTA).
Electrophoretic mobility shift assays
The DNA binding reactions were carried out in 20 µl of a mixture composed of 0.5 µM 5′-end labeled dif substrate or attP substrate, increasing amounts of Pab0255 in a binding buffer composed of 50 mM Tris pH 7.5, 30 mM NaCl and 0.5 µg poly(dIdC).poly(dIdC). Incubation was performed for 30 min at either 20°C or 65°C, and then 5 µl of 5× loading buffer (10 mM Tris pH 7.5, 1 mM EDTA, 20% glycerol, 0.1 mg/ml BSA, 0.1% xylene cyanol) was added to the binding reactions. The samples were loaded onto 8% polyacrylamide gels (30∶0.5 acrylamide∶bisacrylamide), and electrophoresis performed in 1× TGE buffer (50 mM Tris, 8 mM Glycine, 0.1 mM EDTA) at 4°C for 4 h at 7 V/cm. The DNA-protein complexes were visualized by autoradiography and phosphorimaging.
Recombination assays
The 43 bp double stranded oligonucleotide containing the predicted P. abyssi dif and the 43 bp double stranded oligonucleotide containing the attP site were cloned into the pBend2 (2.6 Kbp) vector [55]. pBend2, pBend2-dif and pBend2-attP plasmids were purified on CsCl gradients. Recombination reactions were performed in 20 µl of reaction mixture consisting of 30 mM Tris pH 7.5, 50 µg/ml bovine serum albumin, 50 mM NaCl, 500 ng of plasmid and 40 pmol of XerA protein. The reaction mixture was incubated at 65°C (unless otherwise stated) and at the times indicated, quenched with SDS (0.5% final) and 10× loading buffer (100 mM EDTA, 5% SDS, 40% glycerol, 0.35% Bromphenol blue) was added. Reaction mixes were loaded on a 1.2% agarose gel, and electrophoresis performed in 1× TAE buffer at room temperature for 3 hr at 4 V/cm with buffer circulation. DNA was visualized by staining with ethidium bromide.
Supporting Information
Zdroje
1. BlakelyG
MayG
McCullochR
ArciszewskaLK
BurkeM
1993 Two related recombinases are required for site-specific recombination at dif and cer in E. coli K12. Cell 75 351 361
2. BlakelyG
CollomsS
MayG
BurkeM
SherrattD
1991 Escherichia coli XerC recombinase is required for chromosomal segregation at cell division. New Biol 3 789 798
3. ClergetM
1991 Site-specific recombination promoted by a short DNA segment of plasmid R1 and by a homologous segment in the terminus region of the Escherichia coli chromosome. New Biol 3 780 788
4. KuempelPL
HensonJM
DircksL
TecklenburgM
LimDF
1991 dif, a recA-independent recombination site in the terminus region of the chromosome of Escherichia coli. New Biol 3 799 811
5. CarnoyC
RotenCA
2009 The dif/Xer recombination systems in proteobacteria. PLoS One 4 e6531 doi:10.1371/journal.pone.0006531
6. JensenRB
2006 Analysis of the terminus region of the Caulobacter crescentus chromosome and identification of the dif site. J Bacteriol 188 6016 6019
7. NeilsonL
BlakelyG
SherrattDJ
1999 Site-specific recombination at dif by Haemophilus influenzae XerC. Mol Microbiol 31 915 926
8. SciochettiSA
PiggotPJ
BlakelyGW
2001 Identification and characterization of the dif Site from Bacillus subtilis. J Bacteriol 183 1058 1068
9. ValME
KennedySP
El KarouiM
BonneL
ChevalierF
2008 FtsK-dependent dimer resolution on multiple chromosomes in the pathogen Vibrio cholerae. PLoS Genet 4 e1000201 doi:10.1371/journal.pgen.1000201
10. YenMR
LinNT
HungCH
ChoyKT
WengSF
2002 oriC region and replication termination site, dif, of the Xanthomonas campestris pv. campestris 17 chromosome. Appl Environ Microbiol 68 2924 2933
11. BlakelyGW
SherrattDJ
1994 Interactions of the site-specific recombinases XerC and XerD with the recombination site dif. Nucleic Acids Res 22 5613 5620
12. AusselL
BarreFX
AroyoM
StasiakA
StasiakAZ
2002 FtsK is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell 108 195 205
13. BarreFX
AroyoM
CollomsSD
HelfrichA
CornetF
2000 FtsK functions in the processing of a Holliday junction intermediate during bacterial chromosome segregation. Genes Dev 14 2976 2988
14. IpSC
BreguM
BarreFX
SherrattDJ
2003 Decatenation of DNA circles by FtsK-dependent Xer site-specific recombination. EMBO J 22 6399 6407
15. SteinerW
LiuG
DonachieWD
KuempelP
1999 The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol Microbiol 31 579 583
16. BigotS
SalehOA
LesterlinC
PagesC
El KarouiM
2005 KOPS: DNA motifs that control E. coli chromosome segregation by orienting the FtsK translocase. EMBO J 24 3770 3780
17. CorreJ
LouarnJM
2002 Evidence from terminal recombination gradients that FtsK uses replichore polarity to control chromosome terminus positioning at division in Escherichia coli. J Bacteriol 184 3801 3807
18. LevyO
PtacinJL
PeasePJ
GoreJ
EisenMB
2005 Identification of oligonucleotide sequences that direct the movement of the Escherichia coli FtsK translocase. Proc Natl Acad Sci U S A 102 17618 17623
19. PeasePJ
LevyO
CostGJ
GoreJ
PtacinJL
2005 Sequence-directed DNA translocation by purified FtsK. Science 307 586 590
20. SivanathanV
AllenMD
de BekkerC
BakerR
ArciszewskaLK
2006 The FtsK gamma domain directs oriented DNA translocation by interacting with KOPS. Nat Struct Mol Biol 13 965 972
21. YatesJ
ZhekovI
BakerR
EklundB
SherrattDJ
2006 Dissection of a functional interaction between the DNA translocase, FtsK, and the XerD recombinase. Mol Microbiol 59 1754 1766
22. Le BourgeoisP
BugarelM
CampoN
Daveran-MingotML
LabonteJ
2007 The unconventional Xer recombination machinery of Streptococci/Lactococci. PLoS Genet 3 e117 doi:10.1371/journal.pgen.0030117
23. HaldenbyS
WhiteMF
AllersT
2009 RecA family proteins in archaea: RadA and its cousins. Biochem Soc Trans 37 102 107
24. SerreMC
DuguetM
2003 Enzymes that cleave and religate DNA at high temperature: the same story with different actors. Prog Nucleic Acid Res Mol Biol 74 37 81
25. KanehisaM
GotoS
KawashimaS
NakayaA
2002 The KEGG databases at GenomeNet. Nucleic Acids Res 30 42 46
26. SherrattDJ
WigleyDB
1998 Conserved themes but novel activities in recombinases and topoisomerases. Cell 93 149 152
27. CaoY
HalletB
SherrattDJ
HayesF
1997 Structure-function correlations in the XerD site-specific recombinase revealed by pentapeptide scanning mutagenesis. J Mol Biol 274 39 53
28. SpiersAJ
SherrattDJ
1997 Relating primary structure to function in the Escherichia coli XerD site-specific recombinase. Mol Microbiol 24 1071 1082
29. SubramanyaHS
ArciszewskaLK
BakerRA
BirdLE
SherrattDJ
1997 Crystal structure of the site-specific recombinase, XerD. EMBO J 16 5178 5187
30. LillestolRK
RedderP
GarrettRA
BruggerK
2006 A putative viral defence mechanism in archaeal cells. Archaea 2 59 72
31. MakarovaKS
GrishinNV
ShabalinaSA
WolfYI
KooninEV
2006 A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol Direct 1 7
32. ZivanovicY
LopezP
PhilippeH
ForterreP
2002 Pyrococcus genome comparison evidences chromosome shuffling-driven evolution. Nucleic Acids Res 30 1902 1910
33. MyllykallioH
LopezP
Lopez-GarciaP
HeiligR
SaurinW
2000 Bacterial mode of replication with eukaryotic-like machinery in a hyperthermophilic archaeon. Science 288 2212 2215
34. LopezP
PhilippeH
MyllykallioH
ForterreP
1999 Identification of putative chromosomal origins of replication in Archaea. Mol Microbiol 32 883 886
35. EddySR
1998 Profile hidden Markov models. Bioinformatics 14 755 763
36. LundgrenM
AnderssonA
ChenL
NilssonP
BernanderR
2004 Three replication origins in Sulfolobus species: synchronous initiation of chromosome replication and asynchronous termination. Proc Natl Acad Sci U S A 101 7046 7051
37. RobinsonNP
DionneI
LundgrenM
MarshVL
BernanderR
2004 Identification of two origins of replication in the single chromosome of the archaeon Sulfolobus solfataricus. Cell 116 25 38
38. HoebekeM
SchbathS
2006 R'MES: Finding Exceptional Motifs, version 3. User guide http://genome.jouy.inra.fr/ssb/rmes
39. SerreMC
LetzelterC
GarelJR
DuguetM
2002 Cleavage properties of an archaeal site-specific recombinase, the SSV1 integrase. J Biol Chem 277 16758 16767
40. HayesF
SherrattDJ
1997 Recombinase binding specificity at the chromosome dimer resolution site dif of Escherichia coli. J Mol Biol 266 525 537
41. CornetF
HalletB
SherrattDJ
1997 Xer recombination in Escherichia coli. Site-specific DNA topoisomerase activity of the XerC and XerD recombinases. J Biol Chem 272 21927 21931
42. ConstantinescoF
ForterreP
KooninEV
AravindL
ElieC
2004 A bipolar DNA helicase gene, herA, clusters with rad50, mre11 and nurA genes in thermophilic archaea. Nucleic Acids Res 32 1439 1447
43. QuaiserA
ConstantinescoF
WhiteMF
ForterreP
ElieC
2008 The Mre11 protein interacts with both Rad50 and the HerA bipolar helicase and is recruited to DNA following gamma irradiation in the archaeon Sulfolobus acidocaldarius. BMC Mol Biol 9 25
44. ZhangS
WeiT
HouG
ZhangC
LiangP
2008 Archaeal DNA helicase HerA interacts with Mre11 homologue and unwinds blunt-ended double-stranded DNA and recombination intermediates. DNA Repair (Amst) 7 380 391
45. IyerLM
MakarovaKS
KooninEV
AravindL
2004 Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res 32 5260 5279
46. CollomsSD
AlenC
SherrattDJ
1998 The ArcA/ArcB two-component regulatory system of Escherichia coli is essential for Xer site-specific recombination at psi. Mol Microbiol 28 521 530
47. CornetF
MortierI
PatteJ
LouarnJM
1994 Plasmid pSC101 harbors a recombination site, psi, which is able to resolve plasmid multimers and to substitute for the analogous chromosomal Escherichia coli site dif. J Bacteriol 176 3188 3195
48. StirlingCJ
CollomsSD
CollinsJF
SzatmariG
SherrattDJ
1989 xerB, an Escherichia coli gene required for plasmid ColE1 site-specific recombination, is identical to pepA, encoding aminopeptidase A, a protein with substantial similarity to bovine lens leucine aminopeptidase. EMBO J 8 1623 1627
49. StirlingCJ
SzatmariG
StewartG
SmithMC
SherrattDJ
1988 The arginine repressor is essential for plasmid-stabilizing site-specific recombination at the ColE1 cer locus. EMBO J 7 4389 4395
50. LundgrenM
BernanderR
2007 Genome-wide transcription map of an archaeal cell cycle. Proc Natl Acad Sci U S A 104 2939 2944
51. ForterreP
1999 Displacement of cellular proteins by functional analogues from plasmids or viruses could explain puzzling phylogenies of many DNA informational proteins. Mol Microbiol 33 457 465
52. ForterreP
2002 The origin of DNA genomes and DNA replication proteins. Curr Opin Microbiol 5 525 532
53. CrooksGE
HonG
ChandoniaJM
BrennerSE
2004 WebLogo: a sequence logo generator. Genome Res 14 1188 1190
54. GuindonS
GascuelO
2003 A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52 696 704
55. KimJ
ZwiebC
WuC
AdhyaS
1989 Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene 85 15 23
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



