-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPhenyl salicylates – a new group of potential antituberculotics
Fenylsalicyláty – nová skupina potenciálních antituberkulotik
V souvislosti se studiem protituberkulózní aktivity salicylových derivátů jsme studovali antimykobakteriální aktivitu fenyl-salicylátů (saluolů). Fenylsalicyláty jsou estery. Naše pozornost byla dříve zaměřena na amidy. Fenyl-salicyláty (saluoly) tvoří novou skupinu antimykobakteriálních látek. Jejich aktivita je menší než u odpovídajících amidů. Nejaktivnější látka ve studované skupině měla v salicylové části molekuly 4-methoxy skupinu. Studie přináší nové informace o antimykobakteriálních salicylových derivátech.
Klíčová slova:
saloly • mykobakterie • antimykobakteriální aktivita • fenyl-salicyláty
Došlo 10. října 2012 / Přijato 13. listopadu 2012
Authors: Karel Waisser 1; Eva Novotná 2; Jiří Kuneš 1; Jiřina Solaříková 3
Authors place of work: Charles University in Prague, Faculty of Pharmacy in Hradec Králové 1; Department of Biochemical Sciences 2; Regional Institute of Public Health, Ostrava 3
Published in the journal: Čes. slov. Farm., 2012; 61, 282-284
Category: Krátká sdělení
Summary
Antimycobacterial activity of phenyl salicylates (salols) was studied in connection with antituberculotic activity of salicylic derivatives. Phenyl salicylates are esters. Our attention was previously oriented on amides. Phenyl salicylates (salols) represent a new group of antimycobacterial compounds. They are less active than the corresponding amides. The most active compound in the group under study is substituted on phenyl in the salicyl moiety with a 4-methoxy group. The study reports a new item of information about antimycobacterial salicylic derivatives.
Keywords:
salols • mycobacterium • antimycobacterial activity • phenyl salicylateIntroduction
The recent reappearance of tuberculosis and other infectious diseases is a serious problem worldwide. According to the World Health Organization, there were 9.4 million new tuberculosis cases in 2008 included 1.4 million cases among HIV positive people. In the year 2008 WHO also reported the highest rates of MDR-TB ever recorded
1). Therefore, there is a great need to develop new antituberculotics and other antibiotics. For many years, our research has been oriented on exploring new active compounds. Our attention was predominantly oriented on salicylamides. Phenyl salicylate (salol) is known for its biological activity2). Previously we used phenyl salicylates for the synthesis of antimycobacterially active salicyl amides with heterocyclic substituents on nitrogen3). The goal of the paper consists in the preparation of some substituted phenyl salicylates and investigation of their antimycobacterial properties. Variation of substituents was on phenyl in the salicylic part of molecules.
Experimental part
Chemistry
General information
The melting points were determined on a Kofler apparatus. The samples for the analyses and antimycobacterial tests were dried over P2O5 at 61 °C and 66 Pa for 24 h. Elemental analyses (C, H, N) were performed on a CHNS-O CE elemental analyzer (Fisions EA 1110, Milan) and were within ± 0.4 % of the theoretical values. The IR spectra were measured in KBr pellets on a Nicolet Impact 400 apparatus; the wavenumbers are given in cm‑1. Crystallization of products was carried out from ethanol. The 1H NMR and 13C NMR spectra of new compounds were recorded in DMSO‑d6 solutions at ambient temperature on a Varian Mercury-Vx BB 300 spectrometer operating at 300 MHz for 1H NMR, and 75 MHz for 13C NMR. Chemical shifts were recorded as δ values in parts per million (ppm) and were indirectly referenced to tetramethylsilane via the solvent signal (2.49 for 1H or 39.7 for 13C).
Synthetic procedures for the preparation of phenyl salicylates
A mixture of substituted salicylic acid (1 mol) and phenol (1 mol) was heated with the presence of phosphorus oxychloride (0.38 mol) at 75–80 °C for 4 hours under a reflux condenser. Then, the reaction mixture was reduced to a molten mass and poured slowly into a solution of sodium carbonate with continuous stirring. The precipitated ester was collected on a filter and washed four times with 20 ml portions of water. The crude product was crystallized from ethanol.
1.2.1 Phenyl 3-methylsalicylate (2), yield 64 %, mp. 42–43 °C, IR (ννCO) 1685 cm-1; 1H NMR (300 MHz, DMSO) δ 10.54 (1H, bs, OH), 7.93–7.86 (1H, m, H6), 7.56–7.44 (3H, m, H4, H3’, H5’), 7.38–7.27 (3H, m, H2’, H4’, H6’), 6.94 (1H, t, J=7,7 Hz, H5), 2.22 (3H, s, CH3); 13C NMR (75 MHz, DMSO) δ 168.6, 159.5, 150.1 137.4, 129.8, 128.1, 126.6, 126.4, 122.1, 119.4, 111.4, 15.6; Anal. Calcd for C14H12O3 (228.25): C 73.67; H 5.30 %; Found: C 73.53; H 5.48 %.
1.2.2. Phenyl 3-methoxysalicylate (3), yield 56 %, mp. 67–69 °C, IR (ννCO) 1684 cm-1; 1H NMR (300 MHz, DMSO) δ 10.15 (1H, bs, OH), 7.60–7.43 (3H, m, H6, H3’, H5’), 7.36–7.26 (4H, m, H4, H2’, H4’, H6’), 6.95 (1H, t, J=8,0 Hz, H5), 3.83 (3H, s, OCH3); 13C NMR (75 MHz, DMSO) δ 167.4, 150.8, 150.3, 148.6, 129.8, 126.4, 122.1, 121.7, 119.2, 117.6, 113.4, 56.2; Anal. Calcd for C14H12O4 (244.25): C 68.85; H 4.95 %; Found: C 68.47; H 5.03 %.
1.2.3. Phenyl 4-methylsalicylate (4), yield 52 %, mp. 44–45 °, IR (ννCO) 1770, 1700 cm-1; 1H NMR (300 MHz, DMSO) δ 10.25 (1H, bs, OH), 7.89 (1H, d, J=8,3 Hz, H6), 7.52–7.42 (2H, m, H3’, H5’), 7.36–7.25 (3H, m, H2’, H4’, H6’), 6.90–6.81 (2H, m, H3, H5), 2.49 (3H, s, CH3); 13C NMR (75 MHz, DMSO) δ 167.3, 160.7 150.3, 147.5, 130.7, 129.8, 126.4, 122.2, 120.9, 117.9, 110.2, 21.6; Anal. Calcd for C14H12O3 (228.25): C 73.67; H 5.30 %; Found: C 73.70; H 5.51 %.
1.2.4. Phenyl 4-chlorosalicylate (5), yield 54 %, mp. 57 °C, IR (ννCO) 1672 cm-1; 1H NMR (300 MHz, DMSO) δ 10.47 (1H, bs, OH), 8.04 (1H, d, J=2,5 Hz, H3), 7.70 (1H, dd, J=8,8 Hz, J=2,5 Hz, H5), 7.53–7.42 (2H, m, H3’, H5’), 7.36–7.26 (3H, m, H2’, H4’, H6’), 7.02 (1H, d, J=8,8 Hz, H6); 13C NMR (75 MHz, DMSO) δ 165.7, 160.7, 150.3, 139.9, 132.8, 129.8, 126.4, 122.1, 119.9, 117.5, 113.2; Anal. Calcd for C13H9ClO3 (248.67): C 62.79; H 3.65 %; Found: C 62.97; H 3.80 %.
1.2.5. Phenyl 4-methoxysalicylate (6), yield 51 %, mp. 62 °C. IR (ννCO) 1672 cm-1; 1H NMR (300 MHz, DMSO) δ 10.50 (1H, bs, OH), 7.94 (1H, d, J=8,8 Hz, H6), 7.51–7.42 (2H, m, H3’, H5’), 7.36–7.23 (3H, m, H2’, H4’, H6’), 6.63–6.57 (2H, m, H3, H5), 3.83 (3H, s, OCH3); 13C NMR (75 MHz, DMSO) δ 167.4 165.9, 163.2, 150.3, 132.3, 129.8, 126.3, 122.2, 107.9, 105.2, 101.3, 55.9; Anal. Calcd for C14H12O4 (244.25): C 68.85; H 4.95 %; Found: C 68.88; H 4.99 %.
1.2.6. Phenyl 5-fluorosalicylate (7), yield 43 %, mp. 84–85 °C, IR (ννCO) 1679 cm-1; 1H NMR (300 MHz, DMSO) δ 10.20 (1H, bs, OH), 7.72 (1H, dd, J=9,1 Hz, J=3,3 Hz, H6), 7.54–7.41 (3H, m, H4, H3’, H5’), 7.37–7.27 (3H, m, H2’, H4’, H6’), 7.07 (1H, dd, J=9,1 Hz, J=4,4 Hz, H3); 13C NMR (75 MHz, DMSO) δ 165.7 (d, J=2,9 Hz), 156.5, 154.8 (d, J=236,2 Hz), 150.3, 129.8, 126.5, 123.3 (d, J=23,5 Hz), 122.1, 119.4 (d, J=7,8 Hz), 116.2 (d, J=24,4 Hz), 114.2 (d, J=7,5 Hz); Anal. Calcd for C13H9FO3 (232.21): C 67.24; H 3.91 %; Found: C 67.00; H 3.77 %.
1.2.7. Phenyl 5-chlorosalicylate (8), yield 67 %, mp. 95–97 °C, IR (ννCO) 1685 cm-1; 1H NMR (300 MHz, DMSO) δ 10.45 (1H, bs, OH), 8.93 (1H, d, J=2,8 Hz, H6), 7.59 (1H, dd, J=8,8 Hz, J=2,8 Hz, H4), 7.53–7.42 (2H, m, H3’, H5’), 7.36–7.26 (3H, m, H2’, H4’, H6’), 7.07 (1H, d, J=8,8 Hz, H3); 13C NMR (75 MHz, DMSO) δ 165.2, 158.6, 150.3, 135.4, 130.1, 129.8, 126.4, 122.9, 122.1, 119.9, 115.6; Anal. Calcd for C13H9ClO3 (248.67): C 62.79; H 3.65 %; Found: C 62.90; H 3.81 %.
1.2.8. Phenyl 5-bromosalicylate (9), yield 40 %, mp. 112–113 °C, IR (ννCO) 1692 cm-1; 1H NMR (300 MHz, DMSO) δ 10.47 (1H, bs, OH), 8.04 (1H, d, J=2,5 Hz, H6), 7.70 (1H, dd, J=8,8 Hz, J=2,5 Hz, H4), 7.53–7.42 (2H, m, H3’, H5’), 7.36–7.26 (3H, m, H2’, H4’, H6’), 7.02 (1H, d, J=8,8 Hz, H3); 13C NMR (75 MHz, DMSO) δ 165.1, 159.0, 150.3, 138.2, 133.0, 129.8, 126.4, 122.1, 120.2, 116.2, 110.2; Anal. Calcd for C13H9BrO3 (293.12): C 53.27; H 3.09 %; Found: C 53.42; H 2.96 %.
Microbiology
For the in-vitro evaluation of the antimycobacterial activity of the substances, the following strains were used: M. tuberculosis CNCTC My 331/88 (identical with H37RV and ATCC 27294), M. kansasii CNCTC My 235/80 (identical with ATCC 12 478), M. avium CNCTC My 330/88 (identical with – ATCC 25291), obtained from the Czech National Collection of Type Cultures (CNCTC), National Institute of Public Health, Prague, and a clinical isolate of M. kansasii 6509/ 96. The antimycobacterial activity of the compounds was determined in the Šula semisynthetic medium (SEVAC, Prague). Each strain was simultaneously inoculated into a Petri dish containing the Löwenstein-Jensen medium for the control of sterility of the inoculum and its growth. The compounds were added to the medium in DMSO solutions. The final concentrations were 1000, 500, 250, 125, 62.5, 32, 16, 8, 4, 2, 1, 0.5 and 0.25 ∝mol/l. The MICs were determined after incubation at 37 °C for 14 days and 21days. The MIC was the lowest concentration of the antimycobacterially effective substance (on the above concentration scale), at which the inhibition of the growth of mycobacteria occurred. The evaluation was repeated three times and the values of the MIC were the same. Result are summarised in Table 1.
Tab. 1. Overview of the phenyl salicylates and their antimycobacterial activity 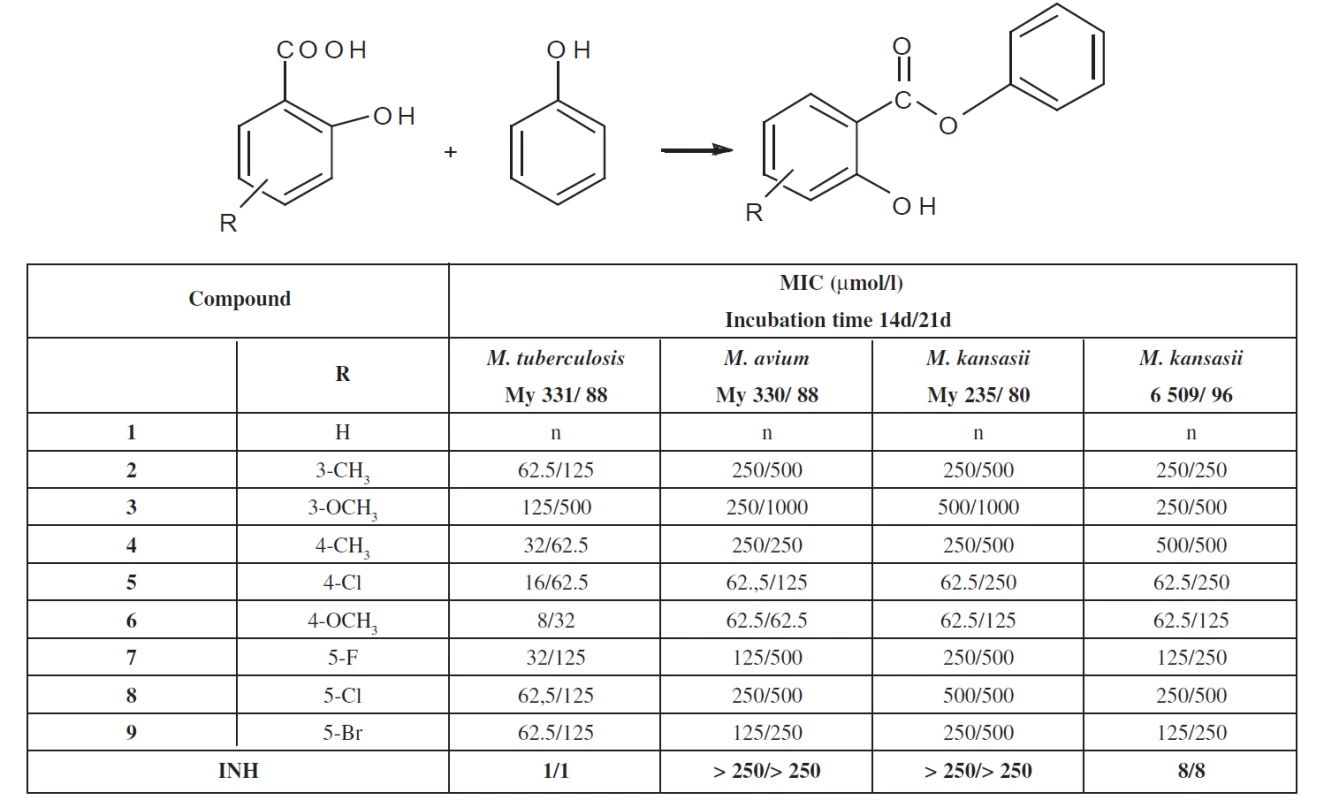
Results
Antimycobacterial activity of phenyl salicylates (salols) was studied in connection with antituberculotic activity of salicylic derivatives. Phenyl salicylates are esters. Our attention was previously oriented on amides. Phenyl salicylates (salols) represent a new group of antimycobacterial compounds. They are less active than the corresponding amides. The significance of the paper consists in the preparation of some substituted phenyl salicylates and investigation of their antimycobacterial properties. Variation of substituents was on phenyl in the salicylic part of molecules. The most active compound in the group under study is substituted on phenyl in the salicyl moiety with a 4-methoxy group. The study reports new information about antimycobacterial substances.
Conflicts of interest: none
Received 10 October 2012 / Accepted 13 November 2012
Karel Waisser1, Eva Novotná2, Jiří Kuneš1, Jiřina Solaříková3
2Department of Biochemical Sciences
3Regional Institute of Public Health, Ostrava
Address:
prof. RNDr. Karel Waisser, DrSc.
1Charles University in Prague, Faculty of Pharmacy in Hradec Králové
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: waisser@faf.cuni.cz
Zdroje
1. WHO (2010) Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response, 2010. [cit. 2010-10-14]. Available from: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pd.
2. Rainford K. D. Aspirin and Related Drugs. London and New York: Taylor & Francis 2004.
3. Matyk J., Waissr K., Dražková K., Kuneš J., Klimešová V., Palát K. Jr., Kaustová J. Heterocyclic isosters of antimycobacterial salicylamides. Farmaco 2005; 60, 399–408.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2012 Číslo 6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Halloysite – interesting nanotubular carrier for drugs
- Effects of zinc and cadmium ions on cell growth and production of coumarins in cell suspension cultures of Angelica archangelica L.
- Comparison of sanguinarine production in suspension cultures of the Papaveraceae plants
- Evaluation of content uniformity of tablets with a low content of the active ingredient with a narrow therapeutic index
- Analysis of pharmaceutical care in dispensing of over-the-counter orlistat
- Phenyl salicylates – a new group of potential antituberculotics
-
Study of local anaesthetics – Part 200
Choice of the optimal type of chitosan for the formulation of local anaesthetics of the carbamate type into hydrogels - FIP oslavila 100. výročí
-
TEACHING COMMUNICATION SKILLS IN PHARMACY PRACTISE
TO KNOW AND TO DO ARE TWO DIFFERENT THINGS -
PATIENT COUNSELING IN PHARMACY PRACTICE
OPTIMIZATION OF DRUG THERAPY - The Czech Pharmaceutical Society 1871 - 2012
- Za doc. DrPh. PhMr. Jozefom Hegerom
- Príručka vybratých pojmov v analytickej chémii
- INSTRUCTIONS FOR THE AUTHORS SUBMITTING PAPERS TO THE JOURNAL CZECH AND SLOVAK PHARMACY
- AUTORSKÝ REJSTŘÍK
- Věcný rejstřík
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evaluation of content uniformity of tablets with a low content of the active ingredient with a narrow therapeutic index
- Analysis of pharmaceutical care in dispensing of over-the-counter orlistat
- Za doc. DrPh. PhMr. Jozefom Hegerom
- Halloysite – interesting nanotubular carrier for drugs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



