-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaVitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: A Mendelian randomization study
In a Mendelian randomization analysis, J. Brent Richards and colleagues investigate possible relations between genetic elements associated with vitamin D levels and atopic susceptibility.
Published in the journal: . PLoS Med 14(5): e32767. doi:10.1371/journal.pmed.1002294
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002294Summary
In a Mendelian randomization analysis, J. Brent Richards and colleagues investigate possible relations between genetic elements associated with vitamin D levels and atopic susceptibility.
Introduction
Atopy refers to the shared predisposition to develop allergic diseases, such as asthma and atopic dermatitis, and is characterized by increased serum immunoglobulin E (IgE) levels. Observational studies have identified a controversial association between low vitamin D status (as measured by serum 25-hydroxyvitamin D [25OHD] levels) and risk of asthma, atopic dermatitis, and elevated serum IgE levels [1–9]. If low vitamin D were a causal risk factor for atopic diseases, this would be important for public health because vitamin D insufficiency is common, affecting 42% of Americans [10], and correctable using vitamin D supplementation.
The immune system appears to play an important role in atopy pathogenesis, and vitamin D, a proposed modulator of the immune system response, may influence the development of atopic susceptibility [11]. Although two randomized controlled trials (RCTs) [12,13] and a recent Cochrane meta-analysis of RCTs for asthma [14] showed a role for vitamin D supplementation in the reduction of atopic exacerbations, other recent data do not support the benefits of vitamin D supplementation for asthma [12,13,15,16], atopic dermatitis, or IgE levels [17–19]. Given this lingering controversy, clinical practice guidelines do not support vitamin D supplementation to prevent atopic disease [1,4]. However, RCT evidence is typically of low quality in vitamin D trials because of their small sample size and limited duration; since vitamin D therapy cannot be patented, large-scale trials would rely upon the limited means of the public purse.
Given the inconclusive results from the existing RCTs and observational studies, the principles of Mendelian randomization (MR) can be applied to test the role of biomarkers in disease etiology [20]. MR uses genetic data to ascertain whether a given biomarker, such as 25OHD, is implicated in disease etiology, relying on a simple tenet: if a biomarker is etiologically involved in a disease process, then the genetic factors that influence the biomarker will influence disease risk. This established technique greatly limits confounding, since genotypes are expected to be randomly assorted at conception; further, it is free of reverse causation since genotypes are always assigned prior to the onset of disease. Thus, MR studies overcome some of the limitations of observational studies and are conceptually similar to RCTs, but provide a lifelong assessment of exposure to a biomarker, such as low 25OHD levels. Further, recent advances in genotyping enable the application of MR methods in sample sizes that are not realistic for RCTs of vitamin D therapy.
In the present study, we adopted an MR design to estimate the effect of genetically lowered 25OHD levels on atopic susceptibility, combining retrospective data from several large-scale studies of people of European descent. Our MR instruments were single-nucleotide polymorphisms (SNPs) identified by the Study of Underlying Genetic Determinants of Vitamin D and Highly Related Traits (SUNLIGHT) Consortium, the largest genome-wide association study (GWAS) published to date for 25OHD levels (n = 33,995) [21]. We then applied MR to test whether genetically lowered 25OHD levels influenced asthma and atopic dermatitis susceptibility and total serum IgE levels, using data from the largest GWAS meta-analyses to date—the asthma GABRIEL consortium [22] combined with the UK Biobank [23]—for a total sample size of 25,471 cases and 121,290 controls for asthma; childhood asthma in 7,047 cases and 7,961 controls from the GABRIEL consortium; 10,788 cases and 30,047 controls for atopic dermatitis in the Early Genetics and Lifecourse Epidemiology (EAGLE) Eczema Consortium [24]; and the GABRIEL consortium for IgE levels (5,888 asthma cases and 6,965 controls).
Methods
Ethical approval
All human studies were approved by their institutional ethics review committees, and all participants provided written consent.
SNP selection and data sources
This study did not have a prospective analysis plan, since it was based on already available data from large GWAS meta-analyses. Specifically, we selected the lead genome-wide significant SNPs (p-values < 5 x 10−8) associated with 25OHD from the SUNLIGHT Consortium [21] as instruments in the present MR analysis.
The estimates of the effect of each SNP on 25OHD levels were obtained using data from the Canadian Multicentre Osteoporosis Study (CaMos) [25], since the effect of each SNP on 25OHD levels was not reported in the SUNLIGHT Consortium (because of the different 25OHD measurement methods used among individual cohorts) and because CaMos was among the largest replication cohorts in the SUNLIGHT Consortium, thereby providing more accurate effects of SNPs on 25OHD. The effects of these SNPs in CaMos have been previously reported [26].
To obtain precise estimates of the genome-wide significant SNPs for vitamin D levels on asthma, we tested the effect of each of these SNPs in a meta-analysis of the UK Biobank Asthma GWAS [27] with the GABRIEL consortium (S1 Text) [22].
The association between vitamin D levels and asthma may be stronger in children [28] than in adults [29]. Therefore, we conducted a separate analysis in a subsample of the GABRIEL consortium, including 15,008 individuals (7,047 cases / 7,961 controls) with childhood-onset asthma.
For atopic dermatitis, we obtained the effects of the selected SNPs from the EAGLE Eczema Consortium [24]. For naturally log-transformed total serum IgE levels, the same estimates were obtained from the GABRIEL consortium. A description of the participating studies and definitions of the asthma, atopic dermatitis, and IgE phenotypes appear in S1 Text.
SNP validation
To validate the four SUNLIGHT 25OHD SNPs as instruments for our MR analysis, we tested them for the three MR assumptions: strong association with the exposure (25OHD); absence of association with known confounders of the exposure—outcome association; and absence of pleiotropy, where the genetic variant influences the atopic outcome through mechanisms that are independent of the vitamin D. Bias due to linkage disequilibrium (LD) and population stratification has been previously tested for the four 25OHD-associated SNPs [26].
Because of randomization of alleles at conception, confounding is greatly minimized in MR studies; however, we have examined if 25OHD-associated SNPs may influence important known confounders that may link vitamin D to common disease [30]. Specifically, these SNPs were not associated with sun exposure, time outside, physical activity, smoking, or body mass index (BMI) [30].
Pleiotropy may bias results if the chosen SNPs exert effects on asthma, atopic dermatitis, and IgE levels independently of the 25OHD levels. In this study, pleiotropy is less likely since all 25OHD-associated SNPs map to genes strongly implicated in 25OHD physiology. Nonetheless, we conducted a PubMed literature search to identify possible pleiotropic mechanisms (S1 Text).
Statistical analysis
Association of SUNLIGHT SNPs with asthma, atopic dermatitis, and IgE level
We first assessed whether each SNP was associated with risk of asthma, atopic dermatitis, or elevated IgE level, applying a Bonferroni correction, where statistical significance was declared at p ≤ 0.05 / 4 since four SNPs were used as instruments.
MR estimates
We assessed the effects of the SNPs upon the four outcomes, weighting the effect of each SNP by the magnitude of its effect upon 25OHD levels. In the absence of available data on 25OHD levels in the GWAS assessing the four outcomes, the instrumental variable estimates of genetically determined odds ratios and betas were calculated by using the two-sample MR approach [31]. To provide a summary measure for the effect including all SNPs genome-wide significant for 25OHD, we combined weighted estimates using fixed effects models and used the I2 estimate as a measure of heterogeneity [32,33]. The effect size for the meta-analysis is reported in our results as the effect of a standard deviation (SD) change in natural log-transformed 25OHD levels, since this metric is more interpretable than an arbitrary difference. Finally, we undertook power calculations [34] to test whether our study was adequately powered to detect a clinically relevant change in the outcomes.
Sensitivity analyses
Our MR estimates were recalculated after exclusion of SNPs potentially influenced by pleiotropy or population stratification. We also performed a stratified MR analysis in which SNPs involved in either 25OHD synthesis or metabolism were analyzed separately [30].
Results
SNP selection and validation
SNP selection
The SUNLIGHT Consortium identified four genome-wide significant vitamin-D associated SNPs [21]: rs2282679 in GC (vitamin D binding protein), rs12785878 near DHCR7 (7-dehydrocholesterol reductase), rs10741657 near CYP2R1 (cytochrome P450 family 2 subfamily R member 1), and rs6013897 in CYP24A1 (cytochrome P450 family 24 subfamily A member 1) (Table 1). All four SNPs map near or in genes implicated in mechanisms modulating 25OHD levels, and, more specifically, transport (GC), synthesis (DHCR7), hepatic hydroxylation (CYP2R1), and catabolism (CYP24A1) [35].
Tab. 1. Characteristics of Single-Nucleotide Polymorphisms (SNPs) used as instrumental variables and their association with asthma, atopic dermatitis, and Immunoglobulin E (IgE) levels. 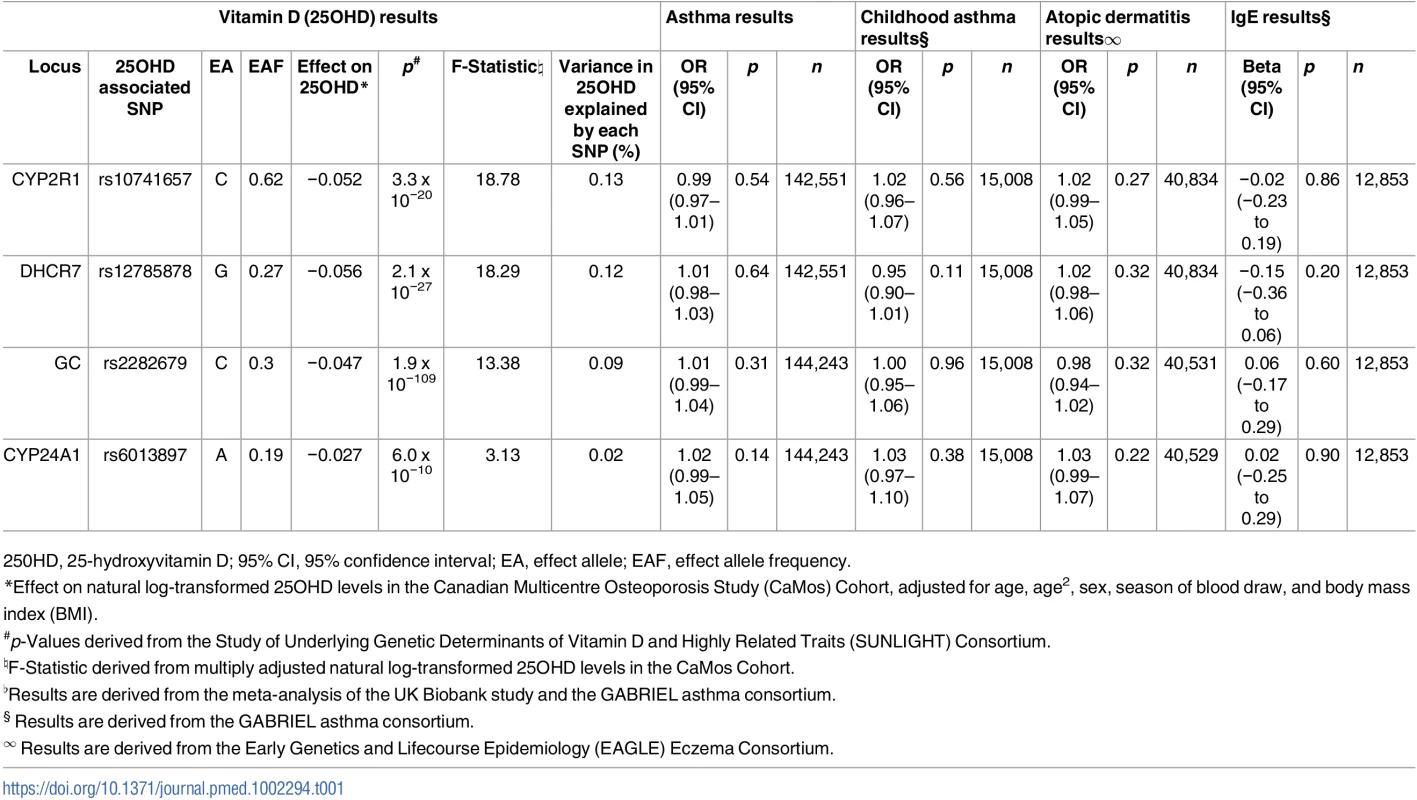
250HD, 25-hydroxyvitamin D; 95% CI, 95% confidence interval; EA, effect allele; EAF, effect allele frequency. LD, confounding, and pleiotropy assessment
We found no evidence of LD between any of these SNPs (all pairwise r2 ≤ 0.01).
In our literature search for potential confounders, obesity and smoking were identified as risk factors for asthma [36] that have been associated with vitamin D levels [30]. We found no association between these SNPs and BMI (all p-values ≥ 0.29) in the Genetic Investigation of Anthropometric Traits (GIANT) [37] consortium or with smoking in the Tobacco and Genetics Consortium [38] (all p-values ≥ 0.18) (S1 Table).
The 25OHD-associated SNPs may also influence risk of atopic disease, independently of 25OHD, through pleiotropy (Figs 1, 2, and 3). Two CYP2R1 SNPs (rs2060793 and rs1933064) have been associated with increased eosinophil counts, while the GC SNP rs7041 and the CYP2R1 SNP rs7935792 may be associated with changes in total IgE levels [39]. Therefore, to investigate possible pleiotropy, we tested the CYP2R1 SNP rs10741657 for LD with the aforementioned CYP2R1 SNPs. We found evidence for strong LD between the SUNLIGHT SNP rs10741657 and the eosinophil-related rs2060793 (r2 = 0.96), but no evidence for linkage between rs10741657 and the two other SNPs (r2 < 0.2). We also found weak LD between the SUNLIGHT GC SNP (rs2282679) and rs7041, which is associated with IgE levels (r2 = 0.5). Additionally, our literature review found evidence of an association in children between CYP24A1 mRNA and LL-37, an immunomodulating peptide potentially related to asthma [29]. Therefore, we performed sensitivity analyses excluding the CYP2R1 and CYP24A1 SNPs (rs10741657 and rs6013897, respectively) from our MR instruments in our asthma analysis and excluding the CYP2R1 SNP (rs10741657) in our atopic dermatitis and IgE analysis.
Fig. 1. Direct Acyclic Graph (DAG) of the Mendelian randomization analysis for asthma. 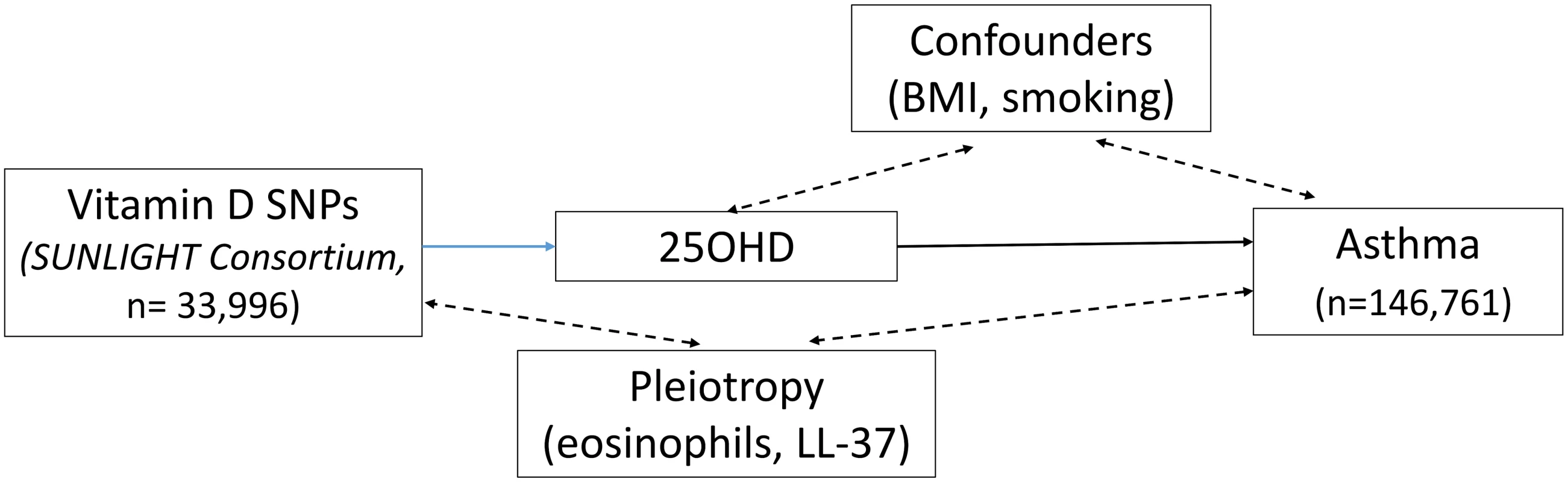
The effect of single-nucleotide polymorphisms (SNPs) on the change in natural log-transformed 25-hydroxyvitamin D (25OHD) levels. BMI, body mass index; SUNLIGHT, Study of Underlying Genetic Determinants of Vitamin D and Highly Related Traits. Fig. 2. Direct Acyclic Graph (DAG) of the Mendelian randomization analysis for atopic dermatitis. 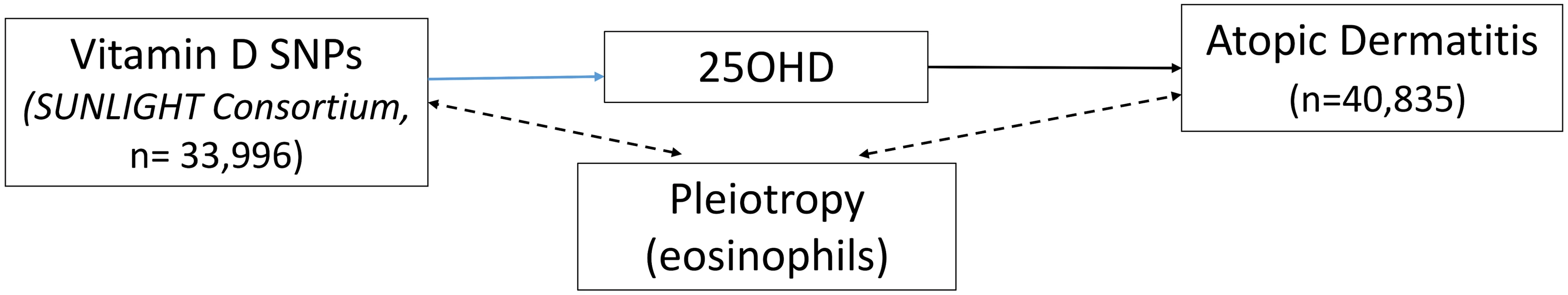
The effect of single-nucleotide polymorphisms (SNPs) on the change in natural log-transformed 25-hydroxyvitamin D (25OHD) levels. SUNLIGHT, Study of Underlying Genetic Determinants of Vitamin D and Highly Related Traits. Fig. 3. Direct Acyclic Graph (DAG) of the Mendelian randomization analysis for Immunoglobulin E (IgE) levels. 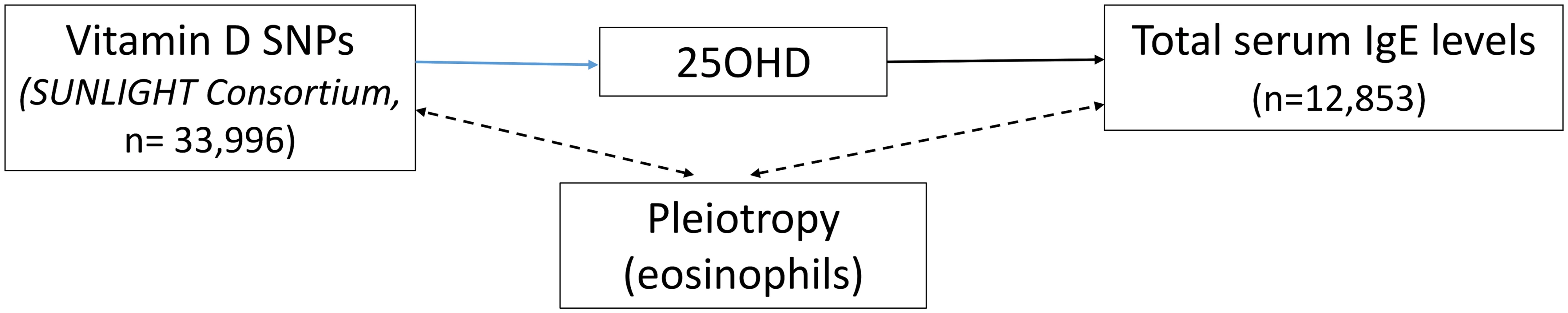
The effect of single-nucleotide polymorphisms (SNPs) on the change in natural log-transformed 25-hydroxyvitamin D (25OHD) levels. SUNLIGHT, Study of Underlying Genetic Determinants of Vitamin D and Highly Related Traits. Population stratification assessment
Based on our previously published results [26], only rs12785878 at DHCR7 was strongly associated with non-European ancestry. Given that the prevalence of both asthma and atopic dermatitis is increased in the individuals of African ancestry [40], we undertook sensitivity analyses excluding this SNP.
Association of SUNLIGHT SNPs with 25OHD levels
The association of the four genome-wide significant SNPs from SUNLIGHT with 25OHD levels is described in Table 1. The proportion of the population-level variance in 25OHD levels explained by the four SNPs is reflected by the F-statistics. Although the low F-statistic of the CYP24A1 SNP suggests that this might be a rather “weak” MR instrument, it is important to note that these F-statistics are derived from CaMos, a subsample of the SUNLIGHT study, and consequently the F-statistics would tend to increase if they were tested in the entire SUNLIGHT study. We have previously shown [41] that the count of 25OHD decreasing alleles across these four SNPs was strongly associated with lower total 25OHD levels in CaMos (p = 2.4 x 10−12) (Table 1).
Association of SUNLIGHT SNPs with asthma and atopic dermatitis susceptibility and IgE levels
Summary statistics for the four 25OHD-associated SNPs and their associations with asthma were taken from the fixed-effects meta-analysis of the UK Biobank and GABRIEL studies. Since the CYP24A1 SNP was absent in the UK Biobank genotypic dataset, we used the estimate of its perfect proxy rs17217119 (r2 = 1.0). None of the four 25OHD-decreasing alleles were associated with risk of asthma, childhood asthma, atopic dermatitis, or elevated IgE levels (Table 1), and the 95% confidence intervals were generally tight around the null.
MR analysis for the association of 25OHD with asthma risk
In order to estimate the association of genetically lowered 25OHD with asthma, we used a fixed-effects model including all four 25OHD-decreasing alleles. A decrease in 25OHD levels by one SD on the natural log scale was not associated with asthma (odds ratio [OR] = 1.03, 95% CI 0.90–1.19; p = 0.63, I2 = 0%) (Table 2 and Fig 4). Since our model included only four SNPs, the 95% CIs of the I2 statistic were wide (0%–85%) and, consequently, heterogeneity could not be accurately measured using this parameter. In addition, because of potential population stratification, we undertook sensitivity analyses by excluding the DHCR7 SNP and again observed no association with asthma (Table 3). To assess the effect of the independent vitamin D pathways on risk of asthma, we analyzed SNPs near genes implicated in 25OHD synthesis (DHCR7 and CYP2R1) and metabolism (GC and CYP24A1) separately and found that none were associated with increased risk of asthma (Table 3). Also, because of evidence of possible pleiotropy for the CYP2R1 and CYP24A1 SNPs, we performed a sensitivity analysis after removing these two variants. The results again showed no evidence of an effect (Table 3).
Fig. 4. Mendelian randomization estimate of the association of 25-hydroxyvitamin D (25OHD) levels with risk of asthma. 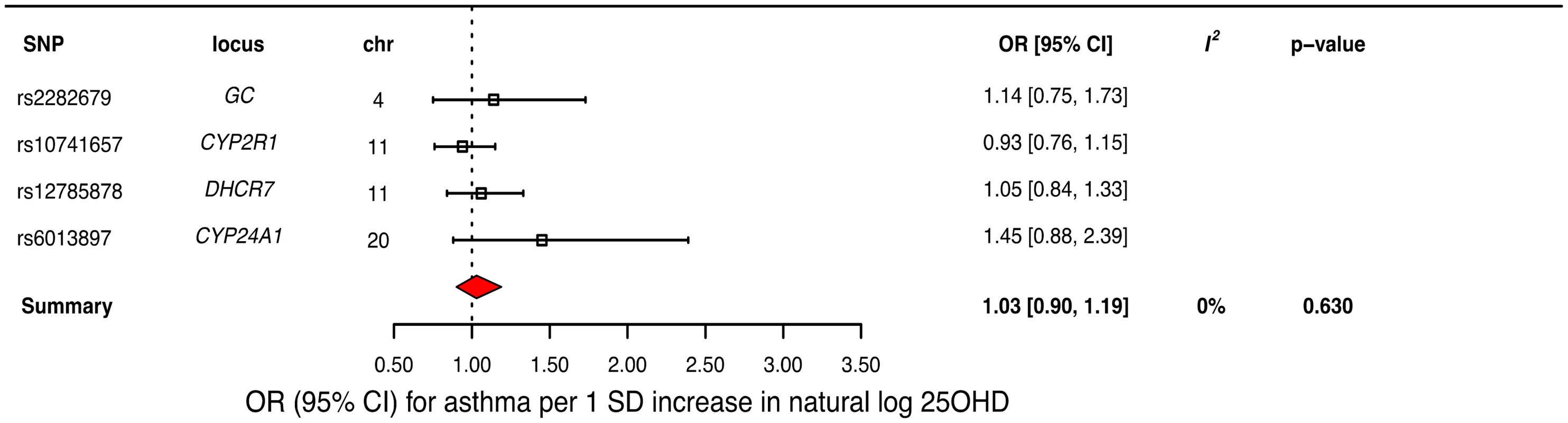
Estimates obtained from using a fixed-effects model. 95% CI, 95% confidence interval; chr, chromosome, OR, odds ratio; SD, standard deviation; SNP, single-nucleotide polymorphism. Tab. 2. Mendelian Randomization (MR) estimates of the association of decreased 25-hydroxyvitamin D (25OHD) on the risk of asthma, atopic dermatitis, and elevated Immunoglobulin E (IgE) levels. 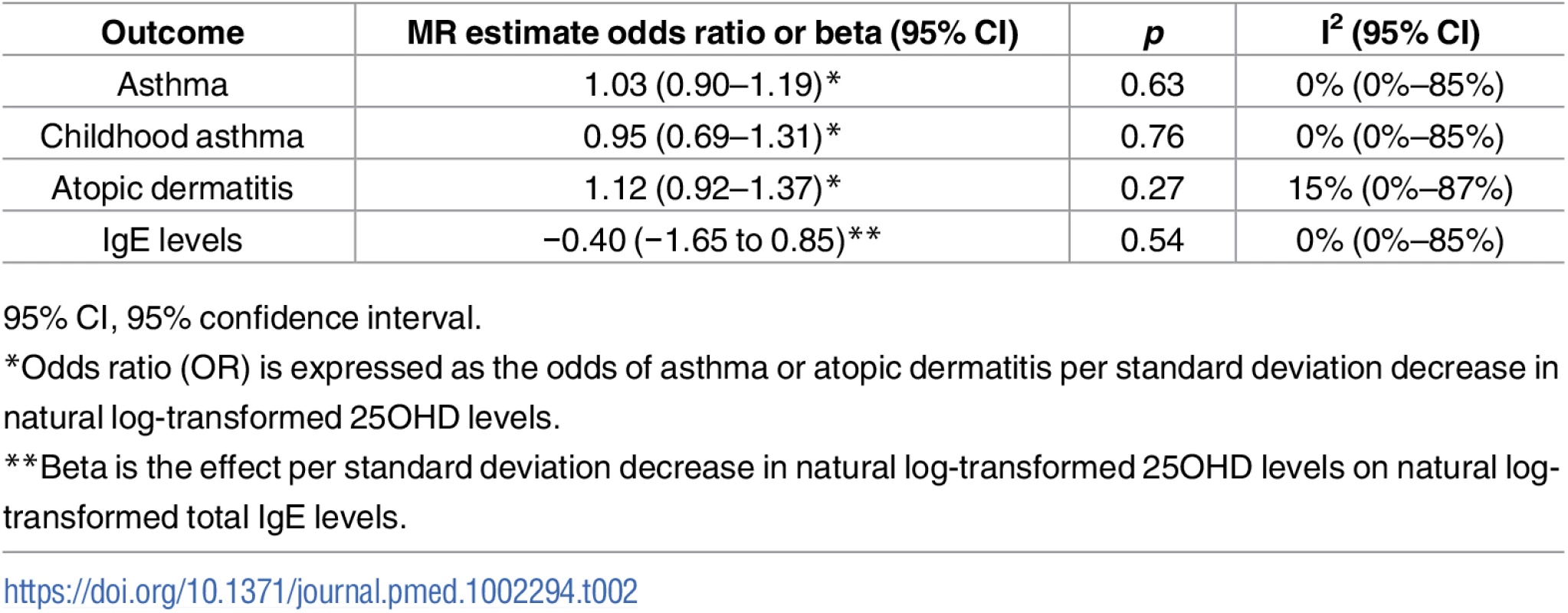
95% CI, 95% confidence interval. Tab. 3. Sensitivity analyses testing Mendelian Randomization (MR) assumptions. 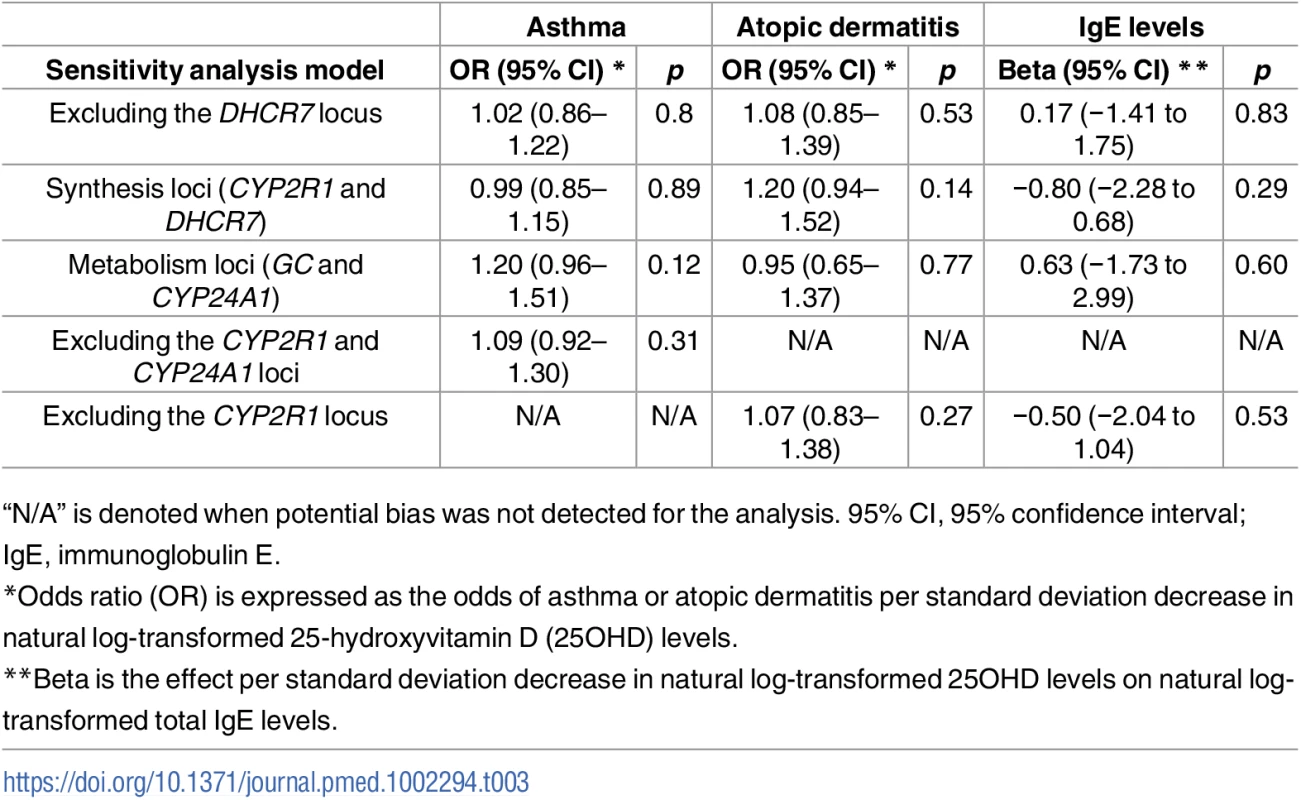
“N/A” is denoted when potential bias was not detected for the analysis. 95% CI, 95% confidence interval; IgE, immunoglobulin E. Testing the effect of genetically reduced 25OHD on risk of childhood asthma, we found that each SD decrease in natural log-transformed 25OHD was not associated with risk of childhood asthma (OR = 0.95, 95% CI 0.69–1.31; p = 0.76, I2 = 0%).
Given these null results, we undertook a power calculation [34]. Based on a clinically relevant effect of an OR of 1.6 for asthma [1], a sample size of 144,243 individuals, and setting alpha to 0.05, our study had a power of 100% to detect an OR of 1.6 for a 1 SD change in log-transformed 25OHD levels on asthma risk, and an 80% power to exclude effects as small as an OR of 1.12. The same power calculation for a sample size of 15,008 individuals for childhood asthma gave to our study 100% power to detect an OR of 1.6 for a 1 SD change in log-transformed 25OHD levels on childhood asthma risk, and an 80% power to exclude effects as small as an OR of 1.33.
MR analysis of the association of 25OHD with atopic dermatitis
A decrease in 25OHD levels by 1 SD on the natural log scale was not associated with atopic dermatitis (OR = 1.12, 95% CI 0.92–1.37; p = 0.27, I2 = 15%) (Table 2 and Fig 5). Similar to the previous analysis for asthma, we undertook sensitivity analyses by excluding the DHCR7 SNP to control for possible population stratification and by removing the CYP2R1 SNP, because of potential pleiotropy, and the results were similar (Table 3). Analyzing SNPs near genes implicated in 25OHD synthesis and metabolism separately, again no association was found (Table 3). Based on a previously reported OR of 1.5 for atopic dermatitis in vitamin D insufficient individuals [5], a sample size of 40,835 individuals, and setting alpha to 0.05, our study had a power of 100% to detect an OR of 1.5 for 1 SD decrease in log-transformed 25OHD levels on atopic dermatitis risk, and an 80% power to observe effects down to an OR of 1.21.
Fig. 5. Mendelian randomization estimate of the association of 25-hydroxyvitamin D 25OHD levels with risk of atopic dermatitis. 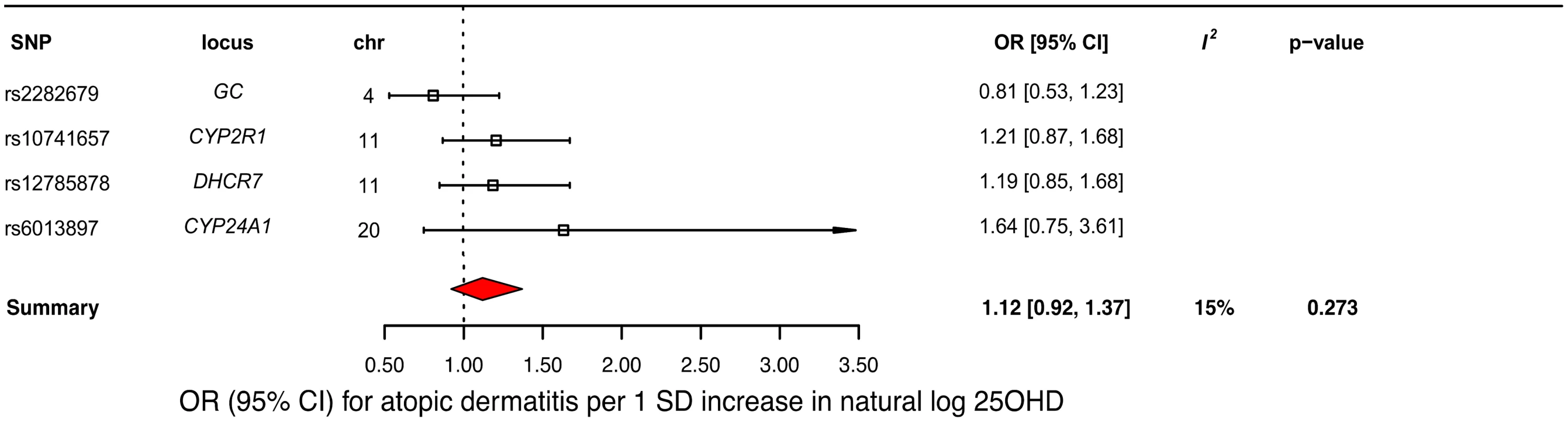
Estimates obtained from using a fixed-effects model. 95% CI, 95% confidence interval; chr, chromosome, OR, odds ratio; SD, standard deviation; SNP, single-nucleotide polymorphism. MR analysis of the association of 25OHD with IgE levels
MR analyses for IgE levels showed that a decrease in 25OHD levels by 1 SD on the natural log scale was not associated with an increase in naturally log-transformed total serum IgE levels (beta = −0.40 natural log-transformed units, 95% CI −1.65 to 0.85, p = 0.54, I2 = 0) (Table 2 and Fig 6). After sensitivity analyses excluding the DHCR7 or the CYP2R1 SNP, and analyzing separately 25OHD synthesis and metabolism SNPs, the results still included the null (Table 3). Based on a previously reported beta of −0.43 for log-transformed total IgE levels per SD increase in log-transformed 25OHD levels [42], our sample size of 12,853 individuals, and setting alpha to 0.05, our study had a power of 86%.
Fig. 6. Mendelian randomization estimate of the association of 25-hydroxyvitamin D (25OHD) levels with Immunoglobulin E (IgE) levels. 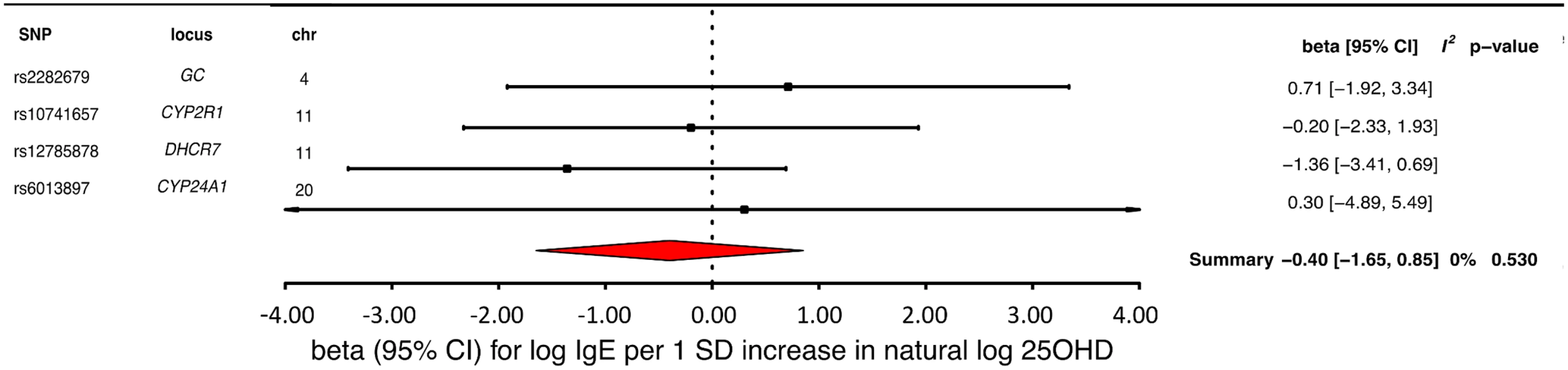
Estimates obtained from using a fixed-effects model. 95% CI, 95% confidence interval; chr, chromosome, OR, odds ratio; SD, standard deviation; SNP, single-nucleotide polymorphism. Discussion
Using large populations of individuals of European descent, our study failed to provide evidence supporting a role for vitamin D in adult and childhood onset asthma, atopic dermatitis, and IgE levels, although small effects cannot be excluded. These findings provide no rationale for the use of vitamin D supplementation for prevention of these conditions in populations of similar ethnicity.
Residual confounding may account for a large part of the discrepancy between our findings and those of observational studies. For instance, adiposity predisposes to asthma [43] and lowers 25OHD levels [44]. While previous studies have controlled for BMI, it is a poor measure of adiposity [45]. Physical activity might also be a strong confounder in observational studies, because it is associated with asthma [46] and sunlight exposure, which in turn influences vitamin D status. Another possible explanation of the discrepancy between MR results and observational studies could be reverse causation, since asthmatic individuals tend to be less active [46] and are therefore less exposed to sunlight. Further, steroid therapies used in asthma may result in low vitamin D levels [47]. Last, individuals with darker skin are at increased risk of atopic dermatitis and asthma [40], while they are also more susceptible to develop vitamin D insufficiency [48].
Our results are in accordance with two recent meta-analyses of RCTs [15,16], which conclude that evidence is lacking to support regular use of vitamin D supplements for prevention of asthma exacerbations, but are in contrast with the findings of a recent Cochrane meta-analysis of RCTs [14]. Our findings for atopic dermatitis and IgE agree with recent observational studies and RCTs [3,4,9]. In contrast, we have used the same methods to provide evidence supporting a causal role for 25OHD in risk of multiple sclerosis [41]. Thus, it would appear that immunomodulatory effects of 25OHD do not uniformly influence immune-mediated diseases.
Previous studies have explored the effects of vitamin D-related genes and risk on atopic phenotypes, without applying an MR approach [7,39,49–52]. SNPs in the vitamin D pathway (CYP27A1, CYP27B1, CYP2R1, CYP24A1, and GC) affecting 25OHD levels demonstrated moderate effects on risk of asthma in a prior adult study [49], but the role of these variants on asthma risk later in life is unknown. A recent study provided evidence of an association between the vitamin D receptor genes and asthma in adolescents with normal 25OHD levels [50]. Another study reported an association between a CYP2R1 variant and FEV1 in children, and between specific haplotypes on CYP2R1 and CYP27A1 and asthma phenotypes [51]. With regard to atopic dermatitis, a polymorphism in the CYP24A1 gene has been associated with severe atopic dermatitis in adults [52]. A vitamin D-related SNP on CYP27A1 was also found to be protective against eczema, whereas CYP2R1 and VDR haplotypes appear to alter eczema susceptibility. In regard to IgE levels, carrying a rare variant on CYP27A1 appears to increase the risk of elevated total serum IgE levels (above 1000 IU/ml), and a CYP27B1 allele has also been associated to IgE levels [53]. Nevertheless, other than testing genetic associations, the above studies were not designed to test the causal relationship between 25OHD levels and atopy.
Other MR studies have been carried out in asthma and have provided evidence supporting a causal role for increased BMI in asthma [43], and evidence against a causal role of prenatal alcohol exposure in asthma and atopy in childhood [54]. A recent MR study used a smaller sample of 1,208 cases and 3,877 controls for childhood asthma in individuals of European and non-European ancestry [55] and did not find any evidence for a causal role of vitamin D in asthma. The instruments used in this childhood MR asthma study were only the CYP2R1 and GC SNPs and did not include the SNPs near DHCR7 and CYPR24A1. Our study thus provides a more thorough examination of the effects of 25OHD on asthma risk by using a substantially larger sample size, including all 25OHD-associated loci, in both adult and childhood asthma.
Strengths of this study include the large sample size of the adult cohorts, which enabled us to more precisely test our study hypothesis than if we had used individual-level data from small studies. Although the findings from this study were null, the high statistical power and tight confidence intervals exclude most clinically relevant effects of 25OHD on risk of asthma, atopic dermatitis, and IgE levels. Importantly, our findings represent the association of a life-long exposure to reduced vitamin D levels in the general population.
This study also has limitations. While we controlled for pleiotropy, residual bias is possible since the exact function of the SNPs studied is unknown. However, all the SNPs lie in, or near, genes well validated for their role in vitamin D physiology. The null result could also be explained by canalization, which is a phenomenon resulting in compensatory feedback mechanisms [20]. Our childhood asthma MR study was underpowered to exclude effects of vitamin D on pediatric asthma smaller than an OR less than 1.33 per SD change in log vitamin D. There was heterogeneity in the definition of the different atopic outcomes, since in some GWAS used for this MR study the outcomes were self-reported, and in others physician-diagnosed (see S1 Text). Our MR analysis might also be limited in its ability to elucidate a possible role of biologically active vitamin D, reflected by the levels of the active metabolite 1,25-dihydroxyvitamin D. Although genetically lowered total 25OHD levels do not appear to be associated with increased risk of the studied atopic phenotypes, we have not assessed whether reduced lifelong 1,25-dihydroxyvitamin D could influence these outcomes, since levels of total 25OHD and 1,25-dihydroxyvitamin D are weakly correlated [56]. Also, our study can only address the role of circulating 25OHD levels, and not on the action of 25OHD at the cellular level. Our analyses were restricted to white populations of European ancestry, and further work will be required to investigate their relevance in populations of different ethnicity or in those with frank vitamin D deficiency.
In conclusion, our MR study provides no support for an unconfounded relationship between 25OHD levels and risk of atopic disease in individuals of European descent. Instead, association of 25OHD levels with atopic diseases in the general population is more likely to be attributable to confounding by lifestyle factors such as obesity and physical inactivity.
Supporting Information
Zdroje
1. Man L, Zhang Z, Zhang M, Zhang Y, Li J, Zheng N, et al. Association between vitamin D deficiency and insufficiency and the risk of childhood asthma: evidence from a meta-analysis. Int J Clin Exp Med. 2015;8(4):5699–706. 26131154
2. Cassim R, Russell MA, Lodge CJ, Lowe AJ, Koplin JJ, Dharmage SC. The role of circulating 25 hydroxyvitamin D in asthma: a systematic review. Allergy. 2015;70(4):339–54. doi: 10.1111/all.12583 25631639
3. Robl R, Uber M, Abagge KT, Lima MN, Carvalho VO. Serum Vitamin D Levels Not Associated with Atopic Dermatitis Severity. Pediatr Dermatol. 2016.
4. Debinska A, Sikorska-Szaflik H, Urbanik M, Boznanski A. The role of vitamin D in atopic dermatitis. Dermatitis. 2015;26(4):155–61. doi: 10.1097/DER.0000000000000128 26172483
5. Cheng HM, Kim S, Park GH, Chang SE, Bang S, Won CH, et al. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol. 2014;133(4):1048–55. doi: 10.1016/j.jaci.2013.10.055 24388009
6. Wang SS, Hon KL, Kong AP, Pong HN, Wong GW, Leung TF. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatr Allergy Immunol. 2014;25(1):30–5. doi: 10.1111/pai.12167 24383670
7. Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE—a significant but nonlinear relationship. Allergy. 2009;64(4):613–20. doi: 10.1111/j.1398-9995.2008.01865.x 19154546
8. Kang JW, Kim JH, Kim HJ, Lee JG, Yoon JH, Kim CH. Association of serum 25-hydroxyvitamin D with serum IgE levels in Korean adults. Auris Nasus Larynx. 2016;43(1):84–8. doi: 10.1016/j.anl.2015.06.010 26209260
9. Dogru M, Kirmizibekmez H, Yesiltepe Mutlu RG, Aktas A, Ozturkmen S. Clinical effects of vitamin D in children with asthma. Int Arch Allergy Immunol. 2014;164(4):319–25. doi: 10.1159/000366279 25277142
10. Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001 21310306
11. Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30(3):397–409. doi: 10.1016/j.iac.2010.05.005 20670821
12. Arshi S, Fallahpour M, Nabavi M, Bemanian MH, Javad-Mousavi SA, Nojomi M, et al. The effects of vitamin D supplementation on airway functions in mild to moderate persistent asthma. Ann Allergy Asthma Immunol. 2014;113(4):404–9. doi: 10.1016/j.anai.2014.07.005 25091714
13. Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311(20):2083–91. doi: 10.1001/jama.2014.5052 24838406
14. Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511. doi: 10.1002/14651858.CD011511.pub2 27595415
15. Riverin BD, Maguire JL, Li P. Vitamin D Supplementation for Childhood Asthma: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10(8):e0136841. doi: 10.1371/journal.pone.0136841 26322509
16. Luo J, Liu D, Liu CT. Can Vitamin D Supplementation in Addition to Asthma Controllers Improve Clinical Outcomes in Patients With Asthma?: A Meta-Analysis. Medicine (Baltimore). 2015;94(50):e2185.
17. Kim G, Bae JH. Vitamin D and atopic dermatitis: A systematic review and meta-analysis. Nutrition. 2016.
18. Bath-Hextall FJ, Jenkinson C, Humphreys R, Williams HC. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
19. Hata TR, Audish D, Kotol P, Coda A, Kabigting F, Miller J, et al. A randomized controlled double-blind investigation of the effects of vitamin D dietary supplementation in subjects with atopic dermatitis. J Eur Acad Dermatol Venereol. 2014;28(6):781–9. doi: 10.1111/jdv.12176 23638978
20. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63. doi: 10.1002/sim.3034 17886233
21. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. doi: 10.1016/S0140-6736(10)60588-0 20541252
22. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–21. doi: 10.1056/NEJMoa0906312 20860503
23. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 25826379
24. Paternoster L, Standl M, Chen CM, Ramasamy A, Bonnelykke K, Duijts L, et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. 2012;44(2):187–92.
25. Faye LL, Sun L, Dimitromanolakis A, Bull SB. A flexible genome-wide bootstrap method that accounts for ranking and threshold-selection bias in GWAS interpretation and replication study design. Stat Med. 2011;30(15):1898–912. doi: 10.1002/sim.4228 21538984
26. Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Goltzman D, et al. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2015;12(8):e1001866. doi: 10.1371/journal.pmed.1001866 26305103
27. Ollier W, Sprosen T, Peakman T. UK Biobank: from concept to reality. Pharmacogenomics. 2005;6(6):639–46. doi: 10.2217/14622416.6.6.639 16143003
28. Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J. 2011;38(6):1320–7. doi: 10.1183/09031936.00029011 21565922
29. Goleva E, Searing DA, Jackson LP, Richers BN, Leung DY. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J Allergy Clin Immunol. 2012;129(5):1243–51. doi: 10.1016/j.jaci.2012.01.044 22330698
30. Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10(2):e1001383. doi: 10.1371/journal.pmed.1001383 23393431
31. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65. doi: 10.1002/gepi.21758 24114802
32. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 12958120
33. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–40. doi: 10.1002/sim.6522 25950993
34. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–501. doi: 10.1093/ije/dyt179 24159078
35. Dastani Z, Li R, Richards B. Genetic regulation of vitamin D levels. Calcif Tissue Int. 2013;92(2):106–17. doi: 10.1007/s00223-012-9660-z 23114382
36. Thomsen SF. Epidemiology and natural history of atopic diseases. Eur Clin Respir J. 2015;2.
37. Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–69. doi: 10.1038/ng.2274 22581228
38. Tobacco, Genetics C. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–7. doi: 10.1038/ng.571 20418890
39. Wang SS, Hon KL, Kong AP, Tang MF, Sy HY, Chan JC, et al. Eczema phenotypes are associated with multiple vitamin D pathway genes in Chinese children. Allergy. 2014;69(1):118–24. doi: 10.1111/all.12337 24730053
40. Silverberg JI. Atopic dermatitis. JAMA Dermatol. 2014;150(12):1380. doi: 10.1001/jamadermatol.2014.2757 25493472
41. Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Leong A, et al. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2015;12(8):e1001866. doi: 10.1371/journal.pmed.1001866 26305103
42. Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765–71. doi: 10.1164/rccm.200808-1361OC 19179486
43. Granell R, Henderson AJ, Evans DM, Smith GD, Ness AR, Lewis S, et al. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS Med. 2014;11(7):e1001669. doi: 10.1371/journal.pmed.1001669 24983943
44. Vimaleswaran KS, Cavadino A, Berry DJ, LifeLines Cohort Study investigators, Jorde R, Dieffenbach AK, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–29. doi: 10.1016/S2213-8587(14)70113-5 24974252
45. Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2(3):141–7. 12120099
46. Lochte L, Nielsen KG, Petersen PE, Platts-Mills TA. Childhood asthma and physical activity: a systematic review with meta-analysis and Graphic Appraisal Tool for Epidemiology assessment. BMC Pediatr. 2016;16(1):50.
47. Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125(5):995–1000. doi: 10.1016/j.jaci.2010.03.008 20381849
48. Wang S. Epidemiology of vitamin D in health and disease. Nutr Res Rev. 2009;22(2):188–203. doi: 10.1017/S0954422409990151 19860998
49. Bosse Y, Lemire M, Poon AH, Daley D, He JQ, Sandford A, et al. Asthma and genes encoding components of the vitamin D pathway. Respir Res. 2009;10 : 98. doi: 10.1186/1465-9921-10-98 19852851
50. Papadopoulou A, Kouis P, Middleton N, Kolokotroni O, Karpathios T, Nicolaidou P, et al. Association of vitamin D receptor gene polymorphisms and vitamin D levels with asthma and atopy in Cypriot adolescents: a case-control study. Multidiscip Respir Med. 2015;10(1):26. doi: 10.1186/s40248-015-0025-0 26346690
51. Leung TF, Wang SS, Tang MF, Kong AP, Sy HY, Hon KL, et al. Childhood asthma and spirometric indices are associated with polymorphic markers of two vitamin D 25-hydroxylase genes. Pediatr Allergy Immunol. 2015;26(4):375–82. doi: 10.1111/pai.12392 25845986
52. Hallau J, Hamann L, Schumann RR, Worm M, Heine G. A Promoter Polymorphism of the Vitamin D Metabolism Gene Cyp24a1 is Associated with Severe Atopic Dermatitis in Adults. Acta Derm Venereol. 2016;96(2):169–72. doi: 10.2340/00015555-2226 26315479
53. Suzuki H, Makino Y, Nagata M, Furuta J, Enomoto H, Hirota T, et al. A rare variant in CYP27A1 and its association with atopic dermatitis with high serum total IgE. Allergy. 2016;71(10):1486–9. doi: 10.1111/all.12950 27259383
54. Shaheen SO, Rutterford C, Zuccolo L, Ring SM, Davey Smith G, Holloway JW, et al. Prenatal alcohol exposure and childhood atopic disease: a Mendelian randomization approach. J Allergy Clin Immunol. 2014;133(1):225–32 e1–5. doi: 10.1016/j.jaci.2013.04.051 23806636
55. Hysinger EB, Roizen JD, Mentch FD, Vazquez L, Connolly JJ, Bradfield JP, et al. Mendelian randomization analysis demonstrates that low vitamin D is unlikely causative for pediatric asthma. J Allergy Clin Immunol. 2016.
56. Zittermann A, Schleithoff SS, Frisch S, Gotting C, Kuhn J, Koertke H, et al. Circulating calcitriol concentrations and total mortality. Clin Chem. 2009;55(6):1163–70. doi: 10.1373/clinchem.2008.120006 19359534
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 5- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Rotavirus vaccine will have an impact in Asia
- Towards control of the global HIV epidemic: Addressing the middle-90 challenge in the UNAIDS 90–90–90 target
- Ebola exposure, illness experience, and Ebola antibody prevalence in international responders to the West African Ebola epidemic 2014–2016: A cross-sectional study
- Long-term inpatient disease burden in the Adult Life after Childhood Cancer in Scandinavia (ALiCCS) study: A cohort study of 21,297 childhood cancer survivors
- Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: An exploratory multi-omic analysis
- Association between expansion of primary healthcare and racial inequalities in mortality amenable to primary care in Brazil: A national longitudinal analysis
- Vitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: A Mendelian randomization study
- Maternal age and severe maternal morbidity: A population-based retrospective cohort study
- Measuring personal beliefs and perceived norms about intimate partner violence: Population-based survey experiment in rural Uganda
- A universal testing and treatment intervention to improve HIV control: One-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial
- Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: A model-based analysis
- First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: A meta-analysis of observational studies
- Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of infection in Ethiopia: A randomized controlled trial
- Impact evaluation of different cash-based intervention modalities on child and maternal nutritional status in Sindh Province, Pakistan, at 6 mo and at 1 y: A cluster randomised controlled trial
- Tobacco control: Developing an innovative and effective global strategy
- Data sharing in clinical trials: An experience with two large cancer screening trials
- Mortality and kidnapping estimates for the Yazidi population in the area of Mount Sinjar, Iraq, in August 2014: A retrospective household survey
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mortality and kidnapping estimates for the Yazidi population in the area of Mount Sinjar, Iraq, in August 2014: A retrospective household survey
- Vitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: A Mendelian randomization study
- Maternal age and severe maternal morbidity: A population-based retrospective cohort study
- Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: A model-based analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



