-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEffect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Nigeria
A trial in Nigeria reveals no reduction of malnutrition in children who are treated with ready-to-use food following a bout of acute illness. Compared to reductions seen in a similar trial in Uganda, the children in this setting were more malnourished initially.
Published in the journal: . PLoS Med 13(2): e32767. doi:10.1371/journal.pmed.1001952
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001952Summary
A trial in Nigeria reveals no reduction of malnutrition in children who are treated with ready-to-use food following a bout of acute illness. Compared to reductions seen in a similar trial in Uganda, the children in this setting were more malnourished initially.
Introduction
The global burden of malnutrition among children is staggering; with an estimated 8% prevalence (51.5 million children) of moderate acute malnutrition (MAM) and 2.9% prevalence (18.7 million children) of severe acute malnutrition (SAM) [1]. Malnourished children have a higher risk of mortality, ranging from a 3-fold increased risk for the moderately malnourished to a nearly 10-fold increase for the severely malnourished [2]. However, the cause of malnutrition in most tropical countries is multi-factorial, involving not only inadequate nutrition and caring practices, but also recurrent infections. It has long been known that malnourished children have a higher risk on severe outcome of morbidity and that ill children have a higher risk on malnutrition. In 1968, almost half a century ago, the World Health Organization (WHO) suggested a synergistic relation between malnutrition and infection, but not much was known about its mechanisms [3].
In the following decades, insights developed that infections aggravate malnutrition by decreasing appetite, inducing catabolism, and increasing both the demand for nutrients as well as nutrient losses. When increased needs are not compensated by increased consumption, weight loss during an infection is frequent [4,5]. Failure to return to normal nutritional status after an illness increases a child’s susceptibility to further infections, perpetuating a cycle towards further reduced nutritional state [6–11].
It has been shown that diarrhea and malaria are associated with substantial weight loss and that supplementation could promote recovery between infections [6,12].
From the nineties onwards, it was increasingly recognized that protein–energy malnutrition has a depressing effect on the immune system and that even mild degrees of malnutrition begin to adversely affect immunity, which consequently increases morbidity and mortality [5,13].
Research advanced on the mechanisms of the immune system in general, and of the role of individual micronutrient deficiencies—such as zinc and vitamin A—in decreased immunity in particular. Further research focused on how supplementation with these individual micronutrients could prevent illness, reduce the severity of illness, or decrease morbidity and mortality [14–17].
As many micronutrients play a role in the immune system, growth and recovery, a deficiency in a few nutrients or even in one could be the limiting factor. Therefore, nutritional fortification with multiple micronutrients were tested, but the impact on diarrhea and other morbidity were mixed [18,19]. In addition, multi-micronutrients have some effect on linear growth, but the effect was small [19–21]. Reports on the effects of lipid-based nutrient supplements (LNS) on morbidity and weight gain also gave mixed results [22–24]. However, there are indications that LNS supplementation decreases mortality [25].
The question whether supplementation with micronutrients or multi-micronutrients, with or without food, is effective in preventing malnutrition and further morbidity in ill children is unanswered, as the results of research are mixed and foremost lacking. Above all, current studies targeted the general population of children, not specifically ill children.
Until now, it is the general expectation that good nutrition during convalescence of an illness improves recovery. Therefore, WHO recommends that caretakers give their children daily additional nutritious food for 14 days after the onset of illness [26–28].
In resource-poor settings—the typical context of the Médecins Sans Frontières (MSF) programs—this recommendation is likely to be ineffective, as caregivers often lack the healthy ingredients and other resources to implement it. A more effective strategy to reduce disease-related malnutrition in resource-poor areas may be to provide ill children with nutritional supplements at the point of care alongside medical treatment.
MSF treated 292,221 severely malnourished children and 71,471 moderately malnourished children in 2012. It is imperative that MSF explores appropriate ways to prevent malnutrition, of which an effective strategy might be providing nutritional supplementation alongside medical treatment to ill children.
MSF piloted the potential of supplementation to ill children in Democratic Republic of Congo (DRC). This research showed that children with malaria gained weight faster during the period of supplementation when given 14 d of ready-to-use therapeutic food (RUTF), although this difference was not seen at 4 wk follow-up [29].
Because of these promising results, MSF investigated whether supplementation of a nutritional supplement—either a lipid-based fortified food or micronutrients—to children with diarrhea, malaria, and/or lower respiratory tract infection (LRTI) during their 2-wk convalescence period is effective in reducing the incidence of malnutrition over a period of 6 mo. Two trials, one in Nigeria and one in Uganda, were conducted. Here we report on the trial that took place in Nigeria; the trial in Uganda is published elsewhere [30].
Methods
Approval
The trial was registered at clinicaltrials.gov number NCT01154803. The full protocol and the statistical analysis plan (SAP) can be accessed in the supporting information files (S1 Text and S2 Text). The study received approval, collaboration and cooperation of Nigerian health authorities (Sokoto State Director Primary Health care at 24 August 2010 and Goronyo LGA and the Sokoto State Research Ethical Committee at 24 September 2010). Approval was also obtained from the MSF Ethics Review Board (23 December 2010).
Setting
The study was implemented in the town of Goronyo, Sokoto State, in the northwest of Nigeria. Because of a high frequency of severe malnutrition among children and its contribution to high morbidity, this site was chosen to investigate whether nutritional supplementation to ill children would significantly decrease the incidence of malnutrition.
Goronyo is rural and primarily dependent on agriculture (including irrigated) and animal husbandry for its livelihood, with income also derived from trade and small-scale manufacturing. A variety of crops are grown, including millet, sorghum, rice, maize, groundnuts, cowpeas, okra, onions, spinach, and tomatoes [31]. The climate has one rainy season from May to October, which is also the traditional hunger season and malaria season, while from January to June it can be very hot.
A qualitative study in the Goronyo Local Government Area in 2009 concluded that malnutrition is linked to inappropriate infant and young child feeding practices; substandard levels of or access to health services, water supply, hygiene, and sanitation; inadequate (health) education; and a poor understanding of the importance of food quality, quantity, and diversity [32]. A high proportion of children under 5 y were not consuming protein-rich and nutrient-dense foods on a daily basis; for example, only 35% of diets included milk and dairy products, and only 8% of diets included fish and poultry [33].
Surveys conducted in March 2009 and March 2010 (during the hot, dry season) showed a global acute malnutrition (GAM) prevalence of 14.8% and 11.5%, respectively, and a SAM prevalence of 4.9% and 2.6%, respectively [33,34]. A study by Save the Children Fund concluded that a chronic existence of malnutrition is linked to a marginal economic situation and under-resourced health care system combined with gender inequality and poor weaning practices in northern Nigeria [35].
The 2009 survey found that 51% of the children reported having an illness (including fever, diarrhoea, and cough) at some time during the previous 14 d, suggesting a high burden of disease that likely exacerbates an already precarious problem of malnutrition. The prevalence of malnutrition among those children who reported an illness in the previous 14 days was 23%, compared with 7% among nonsick children (p < 0.001), illustrating the relation between malnutrition and disease [34].
Médecins Sans Frontières-Operational Centre Amsterdam (MSF-OCA) has provided medical support to Sokoto State Hospital Goronyo since 2008. The activities included outpatient clinics and hospital-based medical care for children, including a therapeutic feeding programme with intensive inpatient and four outpatient facilities. In 2012, MSF treated 9,505 children for severe malnutrition in Goronyo. MSF withdrew from Goronyo in February 2013 because of security issues.
Study Objectives and Endpoints
The aim of this three-armed, partially-blinded, randomised controlled trial was to determine the effectiveness of 14 days of nutritional supplementation with a RUTF or a micronutrient powder (MNP) given to children 6–59 mo of age and diagnosed with and treated for malaria, diarrhoea, and/or LRTI in reducing the incidence of acute malnutrition compared with a control group during a follow-up of 6 mo. Given the complexity of malnutrition in the trial’s study population, a study-specific primary endpoint event was compiled. This is called negative nutritional outcome (NNO) and, depending on nutritional status on enrolment, was defined as moderate and/or severe acute malnutrition (MAM and/or SAM). It was defined as: weight-for-height z-score <−2, MUAC <115 mm, or nutritional oedema, whichever occurred first for non-malnourished children; and weight-for-height z-score <−3, MUAC <115 mm, nutritional oedema (SAM), or weight loss >10% from baseline for moderately malnourished children (weight-for-height z-score <−2). Secondary outcomes included changes in anthropometric indicators, morbidity, and mortality.
Study Population and Randomisation
Children aged 6 to 59 mo diagnosed with one or more of the three study diseases (malaria, diarrhoea, LRTI) were included in the study when living within approximately 60 min walking distance from the clinic and intending to remain in the area for the duration of the 6-mo follow-up. Children who met the criteria for SAM (defined as weight-for-height z-score <−3, MUAC <115 mm, or nutritional oedema), were exclusively breastfed, had a severe disease (for example, severe malaria, severe pneumonia, or severe anaemia), had a sibling enrolled in the study, or were offspring of staff of the study were excluded.
Children were randomised in a 1 : 1:1 ratio to one of three intervention groups. During the 6-mo follow-up, children in the RUTF and MNP groups received nutritional supplements for 14 d whenever they were diagnosed with at least one of the three study diseases, with a maximum of 14 d of supplementation in any 28-d period. The MNP contained only micronutrients, according to the United Nations (UN) formulation [36]. The MNP MixMe (DSM Ltd, Switzerland) was mainly used, but owing to a production problem MixMe was replaced by the “vitamin and mineral powder” (Piramal Healthcare Ltd, India) for several follow-up visits from 1 October 2012 to the end of the study.
Children in the MNP group received two doses per d to ensure a micronutrient composition comparable to one sachet of RUTF (Plumpynut, Nutriset, France) (Table 1). Caretakers were instructed to mix MNP in the meal (for example, porridge) just before consumption. All groups (including the control group) received health education, including the message that following an illness, a child should eat one extra healthy meal per day for 2 wk.
Tab. 1. Nutritional supplements’ composition per serving. 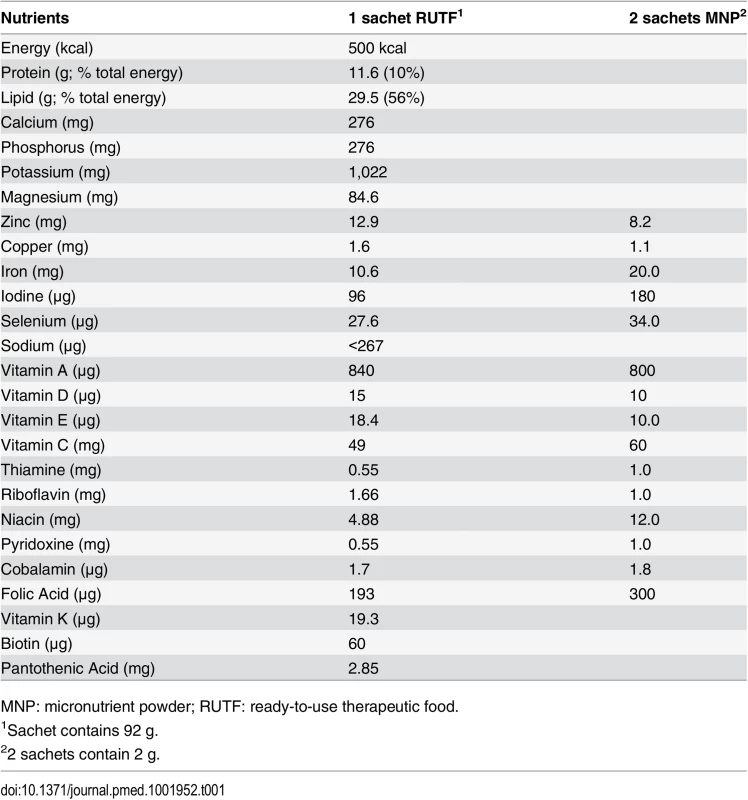
MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. The sample size was based on the assumption of a baseline/control group incidence rate of first NNO event of 0.20 within 6 mo. A 30% reduction, considered a clinically and operationally relevant improvement, would result in a targeted incidence rate of 0.14 in 6 mo in each of the two treatment groups. Using a Poisson regression model, 80% power at a 0.05 significance level, and an assumed dropout rate of approximately 10%, a sample size of 734 children was needed in each group (that is, 2,202 in total for all three groups).
Simple randomisation was based on a computer-generated randomisation list made by an expert independent of the study. Participants entering the study received a study number, and only the staff member giving the supplement was able to connect the study number to the treatment group. All other study staff (including clinicians diagnosing illnesses and deciding on giving an allocation) were blinded to allocation. The statistician was unblinded after completion of the analysis.
Procedures
Patients were screened in the regular outpatient clinic on eligibility. Potential participants were referred to the adjacent study clinic for further inclusion procedures.
Activities at inclusion included provision of information and obtaining written consent of caretakers and their husbands. In case the caretaker was illiterate the consent form was read out loud and consent was given by thumbprint in the presence of a witness. The enrolled participants were followed after the first 14 d and then monthly during 6 mo (24 wk). If a child was ill, they were invited to come whenever they felt that was needed. At every visit, the health and nutritional status of the participant was assessed.
Anthropometric measurements—weight, height, oedema, and MUAC—were taken at every visit to the study clinic. Two types of electronic weighing scales were used to measure weight: SECA model 354, with a precision of 10–20 g, and SECA 869, a mother/baby scale with a precision of 100 g. Length or height (change of measuring position at 85 cm of height) was measured using a precision height board (infant-child-adult measuring board ICAM, aluminium, precision 1 mm, Promes). MUAC was measured using a standard MUAC tape (MSF, precision 2 mm).
Malaria was diagnosed by conducting a malaria rapid diagnostic test (Paracheck). All newly enrolled participants were tested on enrollment; during the study, participants would get a malaria test upon indication (fever or history of fever), except during a malaria epidemic in 2012 when all participants were tested at every visit from 1 June onwards.
Diarrhoea was defined as three or more loose stools (bloody or nonbloody) in 24 h and was diagnosed by mothers’ report. LRTI was diagnosed by the following algorithm: children with cough or having difficulty breathing, an increased respiratory rate (for children aged 12–59 mo >50 breaths/min and for 6–11 mo >40 breaths/min), and absence of chest in-drawings were diagnosed as nonsevere LRTI; chest in-drawing was considered as a sign of severe LRTI, needing referral to the hospital.
All children received standard care and treatment according to current national protocols. Uncomplicated malaria was treated for 3 d with artemether–lumefantrine (Lumartem, Cipla, India). Acute watery diarrhoea was treated using oral rehydration therapy with low osmolarity oral rehydration salts and zinc according to WHO guidelines, regardless of the supplement received. LRTI was treated with amoxicillin 80–100 mg/kg/d for 5 d. The first treatment was given under medical supervision, and the use of and need for the drug were explained to the mother.
Participants presenting with other nonsevere diseases were treated in the study clinic according to the national protocol. Participants with measles received 2 wk of RUTF, as this is considered standard of care for measles cases; these children were kept in the study, and normal rules were applied for allocation for a follow-up supplement.
At every visit, health education was provided. This included advice on prevention and treatment of malaria and diarrhoea and the advice to give an ill child an extra nutritious meal for 14 d.
Home visitors supported the study by reminding the caretakers to come to the appointments, urging follow-up absentees to return, and reporting on deaths that occurred at home. When a participant was found to have died, the caretaker was invited to the study clinic for an interview. Otherwise, the family would be visited by a nurse for an interview. Any death was immediately reported and reviewed by a national paediatrician, principal investigator, and medical director of MSF.
Compliance was measured by questionnaires, asking how many times and for how many days the participant consumed the supplement. In addition, the caretaker was asked to return the full or empty sachets of each distribution. Finally, after the study, three focus groups discussions were held with caretakers who had completed the trial to discuss the supplements and medication used, compliance, and any barriers regarding using the supplements.
Data Analysis
The primary outcome (rates of first NNO) among the treatment groups was analysed by a Poisson regression model including the three intervention groups with contrasts for each two-group comparison. As there were three interventional groups to be compared, a hierarchical test procedure was used to account for multiplicity. If a significant result (significance level 0.05 two-sided) was observed in the RUTF group compared with control, MNP was then compared with control; if this was also statistically significant at 0.05 two-sided, RUTF was subsequently compared with MNP in a noninferiority approach with a noninferiority margin of 2% (that is, allowing a slightly worse result in the MNP than in the RUTF group, statistically significant at 0.025 one-sided). If no significant result was observed in the first or second step, all other comparisons were considered exploratory.
The incidence rate of malnutrition (NNO) is expressed in first event per 365 observation d (events/y) and is extrapolated from the study period of 168 d (24 wk).
Secondary outcomes were analysed using logistic regression or analysis of covariance with treatment group and baseline values as covariates. In addition, adjustment for nutritional status at baseline (moderate malnourished or non-malnourished on enrollment) was applied. A priori defined subgroups were analysed by including interaction terms in the main model.
Any participant attending the first and the last visit was counted as having completed the study. A participant developing severe malnutrition was referred to the therapeutic feeding programme and withdrawn from the study; all data up to the moment of withdrawal was used. When the participant was erroneously included or consent was withdrawn, the participant was withdrawn and the data was not used. When a patient was admitted to the hospital, the hospital visit was monitored and (if necessary) supplementation was temporarily suspended, but the patient was not withdrawn from the study. The primary outcome was also analysed for a per protocol population that excluded participants if they had abandoned the study, had a low compliance, or had measles (patients with measles were provided with RUTF for 2 wk regardless the supplementation arm)
Results
Participant Flow
Recruitment took place between February 2012 and February 2013. The last patient completed follow-up on 28 February 2013. 24,200 patients younger than 5 y visiting the outpatient clinic were screened on age, illness, and living area; of these, 5.4% were excluded because of severe malnutrition. 2,533 patients were assessed thoroughly for eligibility. A total of 2,213 children aged between 6 and 59 mo were included, with 25 (1.1%) participants excluded from analysis because of inclusion error (21) and withdrawal of consent (4). 160 (7.2%) participants were lost to follow-up (abandoned the study) (Fig 1). The mean number of d in the study was for RUTF, MNP and control groups was 152.3, 148.1, and 145.4 d, respectively.
Fig. 1. Flow diagram of participants of the supplementation study in Goronyo. 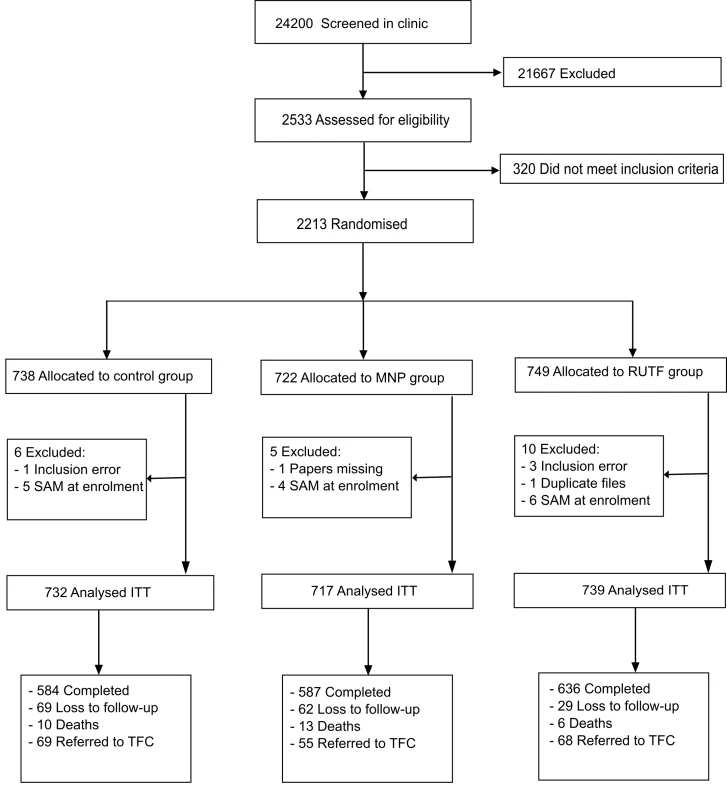
MNP, micronutrient powder; RUTF, ready-to-use therapeutic food; SAM, severe acute malnutrition; TFC, therapeutic feeding centre. Baseline Characteristics
The average household size was 10.9 people, with 29.4% children younger than 5 y. Most caretakers (2,126; 97.2%) were the mothers of the child, and 187 (8.6%) had primary school or more (completed and not completed) 1725 (78.8%) had attended only an Islamic school. More than 40% of the (male) heads of household made a living through business or retail, while 50% of the female caretakers were business owners. Caretakers also frequently (38.5%) reported being a housewife; this means that the caretakers stay at home, but they might be involved in domestic production of garments or processing foods for sale. A quarter (25%) of the heads of household made a living in agriculture or livestock, and 23.5% had a regular paid job. Three-quarters (76.2%) of the households owned livestock, 21.1% owned cows, over half (60.0%) of the households owned a motorcycle, and 24% owned a generator. Most families (79.3%) had consumed three or more meals on the day before the interview (Table 2).
Tab. 2. Baseline characteristics. 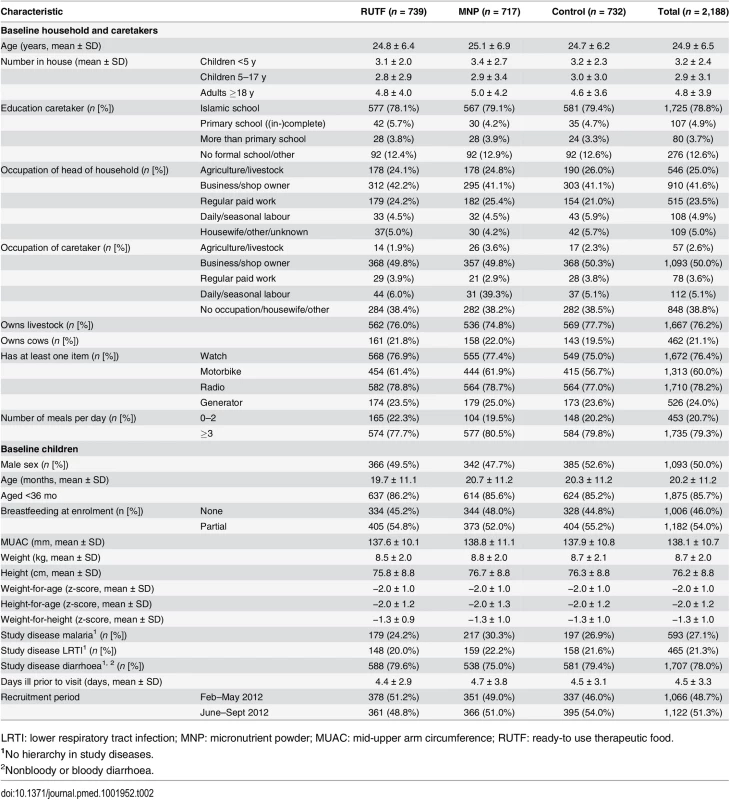
LRTI: lower respiratory tract infection; MNP: micronutrient powder; MUAC: mid-upper arm circumference; RUTF: ready-to use therapeutic food. Although children up to 5 y of age were included in the study, most (85.7%) were younger than 3 y. About half (54%) of the participants were partially breastfed. Most (78.0%) of the participants had diarrhoea on enrollment, 27.1% had malaria, and 21.3%.had LRTI (more than one disease could be reported).
Caretakers waited an average of 4.5 d after the onset of illness before seeking help at the outpatient clinic; a quarter of the mothers went to the pharmacy to seek a treatment before their clinic visit. Half of the participants (48.7%) were enrolled in the period February–May 2012, participating mostly during the dry and hot season; 51.3% were enrolled in the period June–September 2012, participating mostly during the cold/rainy season.
There was no clear relevant difference between the supplementation groups as regards baseline characteristics (Table 2).
Incidence of Malnutrition
The incidence of first NNO event in the RUTF, MNP, and control groups was 0.522, 0.495, and 0.566 events per y, respectively, during the 6-mo follow-up. There was no significant difference between study groups. The incidence rate ratio between RUTF and control was 0.922 (p = 0.471), between RUTF and MNP 1.055 (p = 0.642) and between MNP and control 0.874 (p = 0.242), resulting in a reduction of 0.044 events/y (7.8%), −0.027 events/y (−5.3%) and 0.071 events/y (12.6%) respectively. Analysis of the per protocol (PP) dataset (n = 1,748) was not different from the intention to treat (ITT) data set: 0.547, 0.517, and 0.585 events/y, respectively, for the RUTF, MNP, and control groups.
The outcome was stratified according to nutritional status on enrolment (non-malnourished and moderately malnourished). The NNO for children who were non-malnourished on enrollment comprises newly diagnosed moderate and severe malnutrition. The non-malnourished group had an incidence of NNO very close to that for the entire study group (for RUTF, MNP, and control groups: 0.522, 0.480, and 0.581 first events/y, respectively). The incidence of first NNO for children who were moderately malnourished on enrolment (comprising only severe malnutrition) was for RUTF, MNP, and control groups: 0.695, 0.705, and 0.711 first events/y, respectively. There was no significant reduction in the incidence of NNO in the RUTF or MNP group (p-value for interaction: 0.771) for non-malnourished or moderately malnourished children (Table 3).
Tab. 3. Incidence of first NNO (negative nutritional outcome) per y. 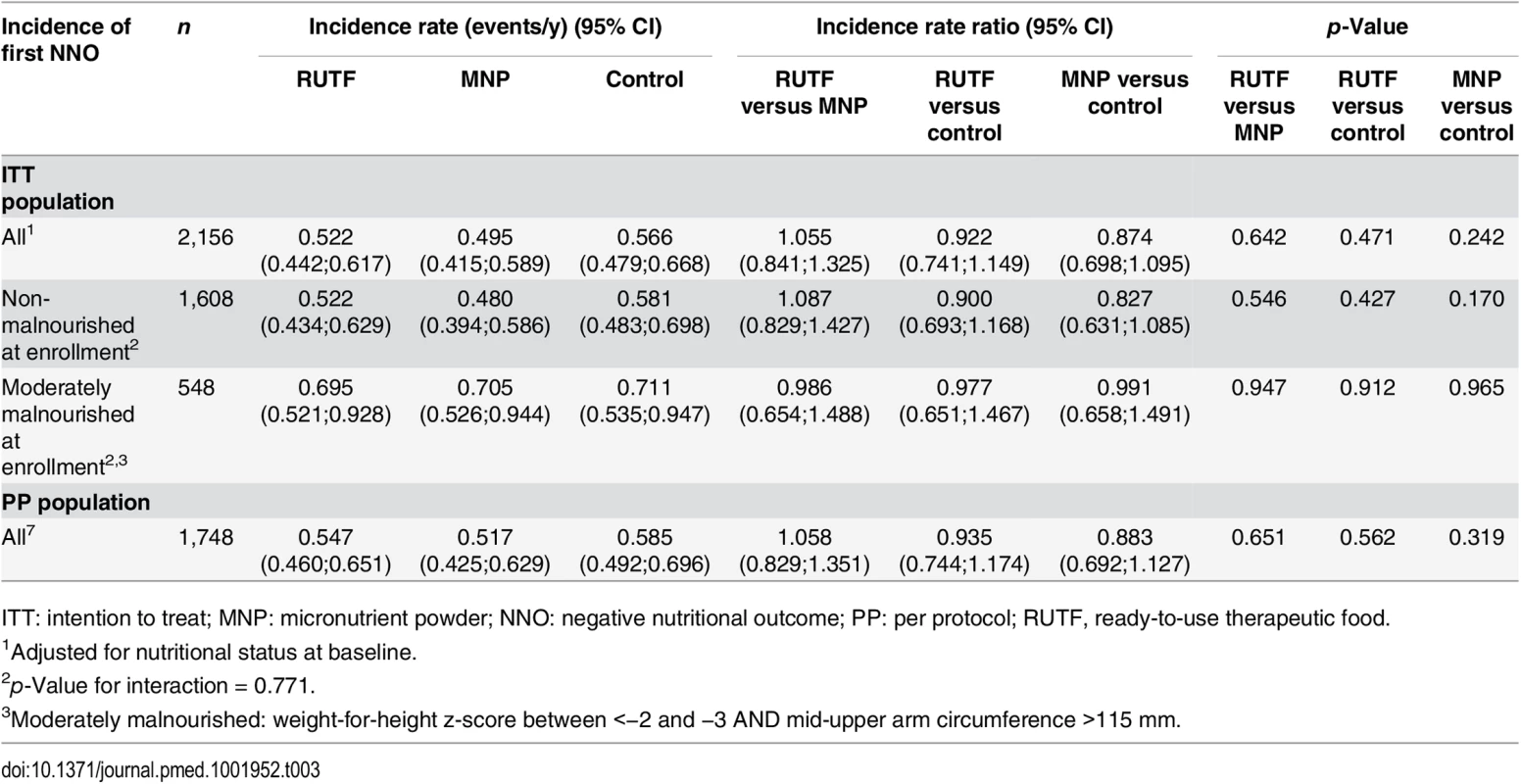
ITT: intention to treat; MNP: micronutrient powder; NNO: negative nutritional outcome; PP: per protocol; RUTF, ready-to-use therapeutic food. The NNO for children who were non-malnourished on enrollment comprises newly diagnosed moderate and severe malnutrition. The incidence rate of moderate malnutrition among non-malnourished children was 0.456, 0.439, and 0.550 first events/y for RUTF, MNP, and control groups, respectively; the differences were not significant. The incidence of severe malnutrition among non-malnourished children was 0.130, 0.080, and 0.148 first events/y for RUTF, MNP, and control groups, respectively (MNP versus control: p = 0.036) (Table 4).
Tab. 4. Incidence of first moderate and severe malnutrition event among non-malnourished at enrolment per y. 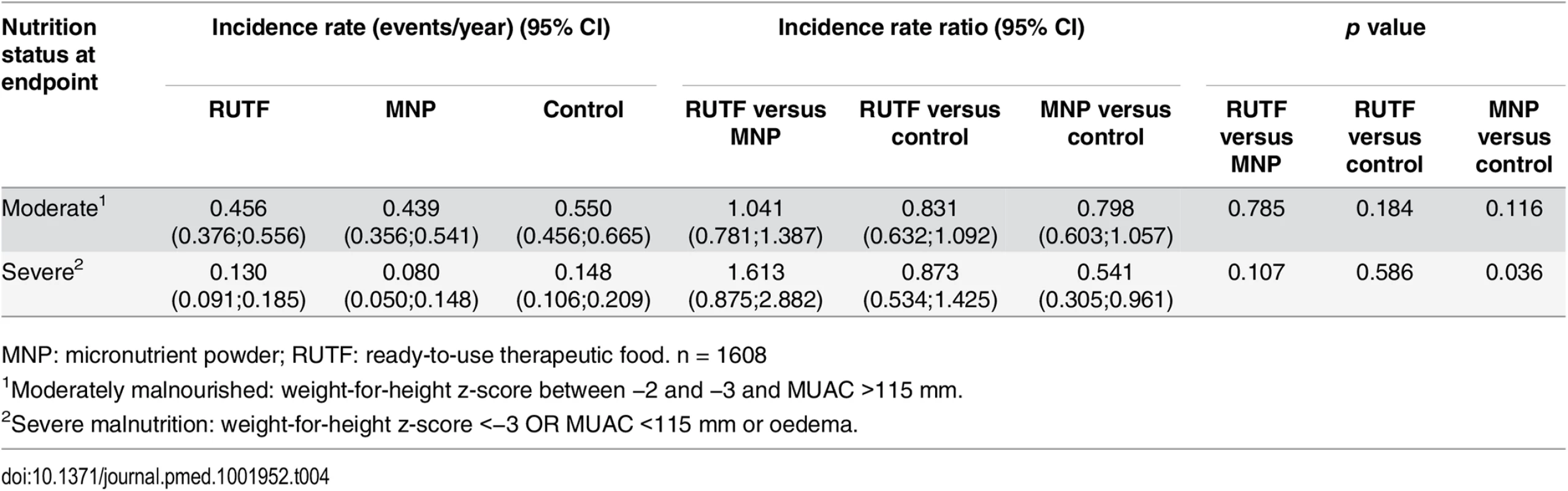
MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. n = 1608 In addition, one-quarter of the participants who were moderately malnourished upon enrollment improved to non-malnourishment without showing any difference between the supplementation groups (RUTF, MNP, and control groups: 25.4%, 25.0%, and 27.3%).
Nutritional oedema is included in the definition of NNO and the definition of SAM. A total of 18 children developed oedema (7, 4, and 7 participants in the RUTF, MNP, and control groups, respectively). In addition, all participants who were moderately malnourished at enrollment and lost 10% of their weight during the study were also diagnosed with severe malnutrition and therefore these were analysed having SAM as outcome.
Subgroup analyses (prespecified) shows that the effect of supplementation on the incidence of NNO was not modified by socioeconomic characteristics, season of enrollment, age of the participant, breastfeeding status, or study disease at enrollment (Table 5).
Tab. 5. Incidence rate of first NNO per y by subgroups, adjusted for nutritional status at baseline. 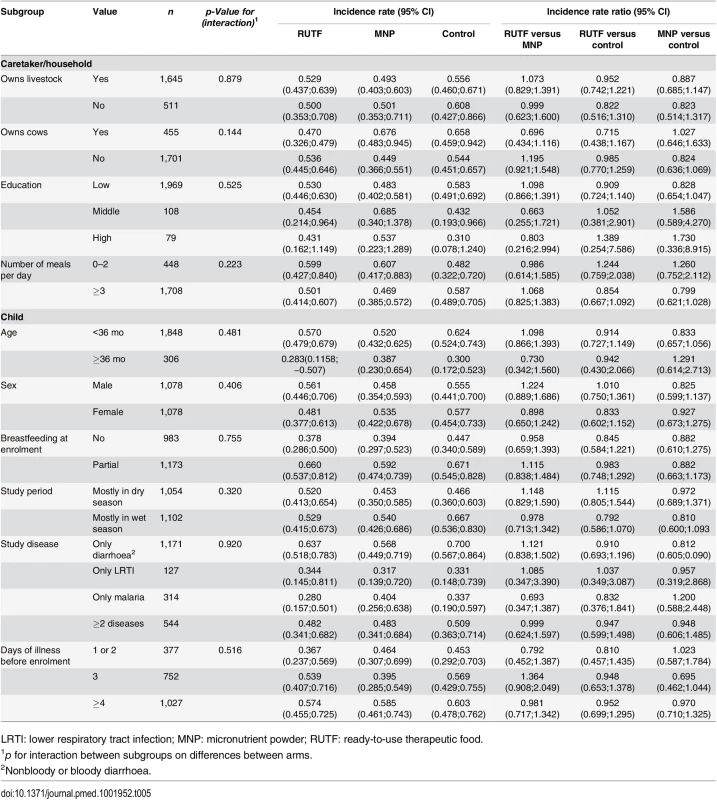
LRTI: lower respiratory tract infection; MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. This is despite the fact that in the control group, some subgroups (e.g., partially breast-fed, children younger than 36 mo, those enrolled in the wet seasons) did appear to be at higher risk of developing a NNO (Table 5).
The supplements did not have an effect at 6 mo follow up on other anthropometric indicators such as the average weight gain rate, change in MUAC, change in height, or change in weight-for-age, weight-for-height, and height-for-age of participants who completed the study.
In the first 14 d of the study, no difference in anthropometric indicators was seen between the study groups. However, regardless of the study group, the rate of weight gain was higher in the first 14 d after enrollment compared with the entire study period (for the RUTF, MNP, and control groups: 1.57, 1.47, and 1.50 g/kg/d, respectively, over the first 14 d and 0.87, 0.89, and 0.86 g/kg/d over the entire study period). Similarly, the change in MUAC was higher in the first 14 d compared with the entire study period (for the RUTF, MNP, and control groups: 0.056, 0.055, and 0.039 mm/d, respectively, over the first 14 d and 0.035, 0.036, and 0.033 mm/d over the entire study period) (Table 6).
Tab. 6. Anthropometric indicators: change from baseline to day 14 and day 168. 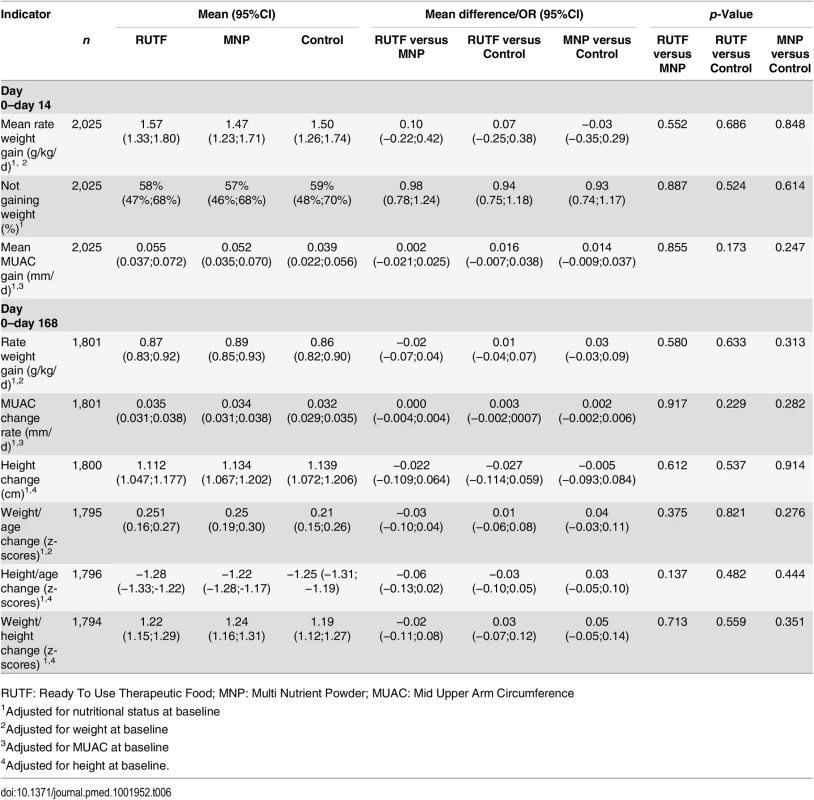
RUTF: Ready To Use Therapeutic Food; MNP: Multi Nutrient Powder; MUAC: Mid Upper Arm Circumference Disease and Mortality
More than half of the children experienced one or more new malaria episode (56%, 55%, and 56%, respectively, for the RUTF, MNP, and control groups) during their participation in the study. More than half of the participants had a one or more new LRTI episode (54%, 54%, and 55%, respectively, for RUTF, MNP, and control groups). About three-quarters of participants experienced one or more new episode of acute diarrhoea during the 6-mo follow-up period (79%, 73%, and 78%, respectively, for RUTF, MNP, and control groups). A significantly lower proportion of children in the MNP group had a new diarrhoea episode compared with both the RUTF and control groups (RUTF versus MNP, p = 0.007; MNP versus control, p = 0.029) (Table 7). The average number of episodes of study diseases during enrollment for the RUTF, MNP, and control groups, respectively, were 4.1, 3.4, and 3.6, with a significant higher number in the RUTF group compared with the MNP and control groups (RUTF versus control, p < 0.001; RUTF versus MNP, p < 0.001)
Tab. 7. Mean number of diagnosed study diseases and proportion of children with at least one newly diagnosed disease episode or mortality1. 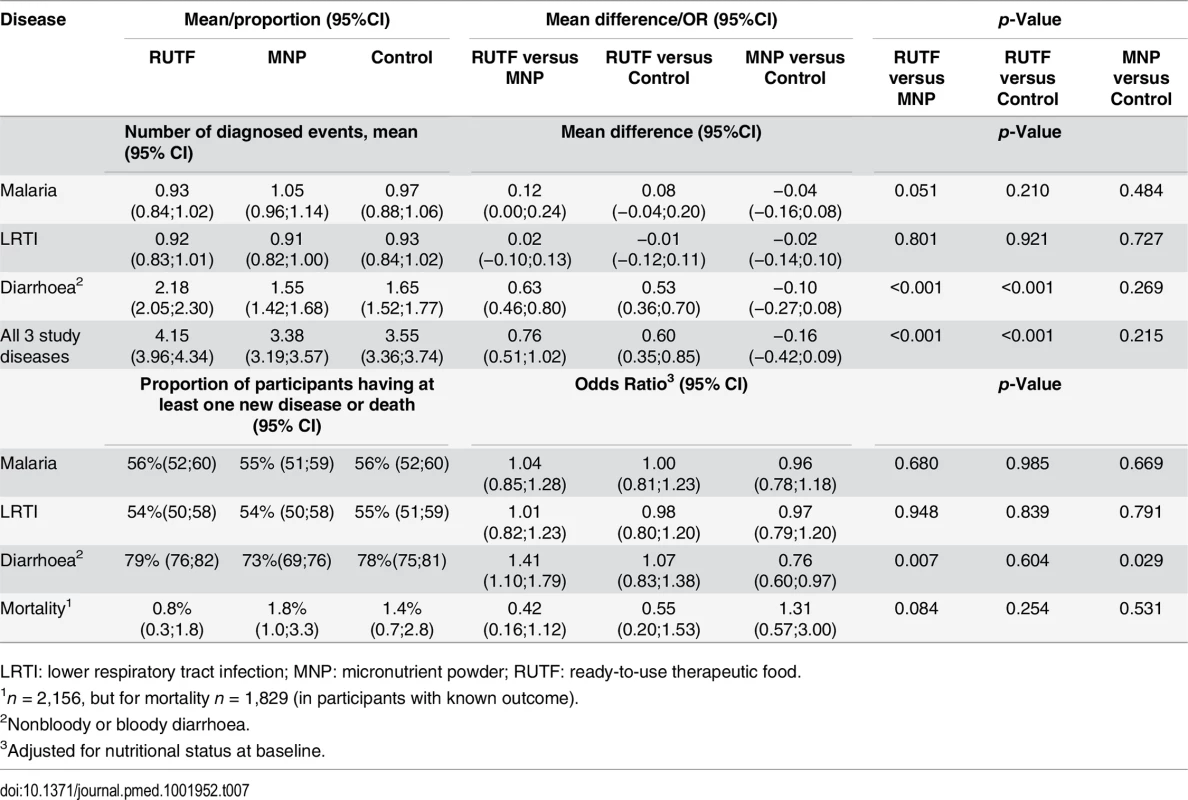
LRTI: lower respiratory tract infection; MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. A total of 29 (1.3%) children died during the study: in the RUTF, MNP, and control groups, respectively, 6, 13, and 10 children died (RUTF versus MNP, p = 0.084). The causes of death could be related to malaria (17; 58.6%), diarrhoea and dehydration (9, 31.0%) and LRTI or sepsis (3, 10.3%).
The number of patients who died from malaria in the RUTF, MNP, and control groups, respectively, was 3 (0.4%), 8 (1.1%), and 6 (0.8%); for diarrhoea, the numbers were 2 (0.3%), 4 (0.6%), and 3 (0.4%); and for LRTI, 1 (0.1%) death occurred in each intervention group. Of the 29 deceased children, 17 died at home, 7 at the hospital, 4 while hospitalised in the therapeutic feeding programme, and 1 on arrival at the hospital. In all, 54 participants needed to be admitted to hospital (21, 20, and 13 children in the RUTF, MNP, and control groups, respectively). The main cause of admission was malaria, followed by diarrhoea. The number of patients admitted due to malaria was 9 (1.2%), 10 (1.4%), and 8 (1.1%), respectively, for the RUTF, MNP, and control groups; the numbers for diarrhoea were 5 (0.7%), 7 (1.0%), and 1 (0.1%); and for LRTI they were 5 (0.7%), 3 (0.4%), and 2 (0.3%).
Causes of death and causes of hospital admissions did not show a statistically significant difference between the study arms, even when mortality and hospital admission were combined, although most admissions and deaths related to malaria and diarrhoea were in the MNP group (Table 8).
Tab. 8. Mortality and hospital admissions by intervention group. 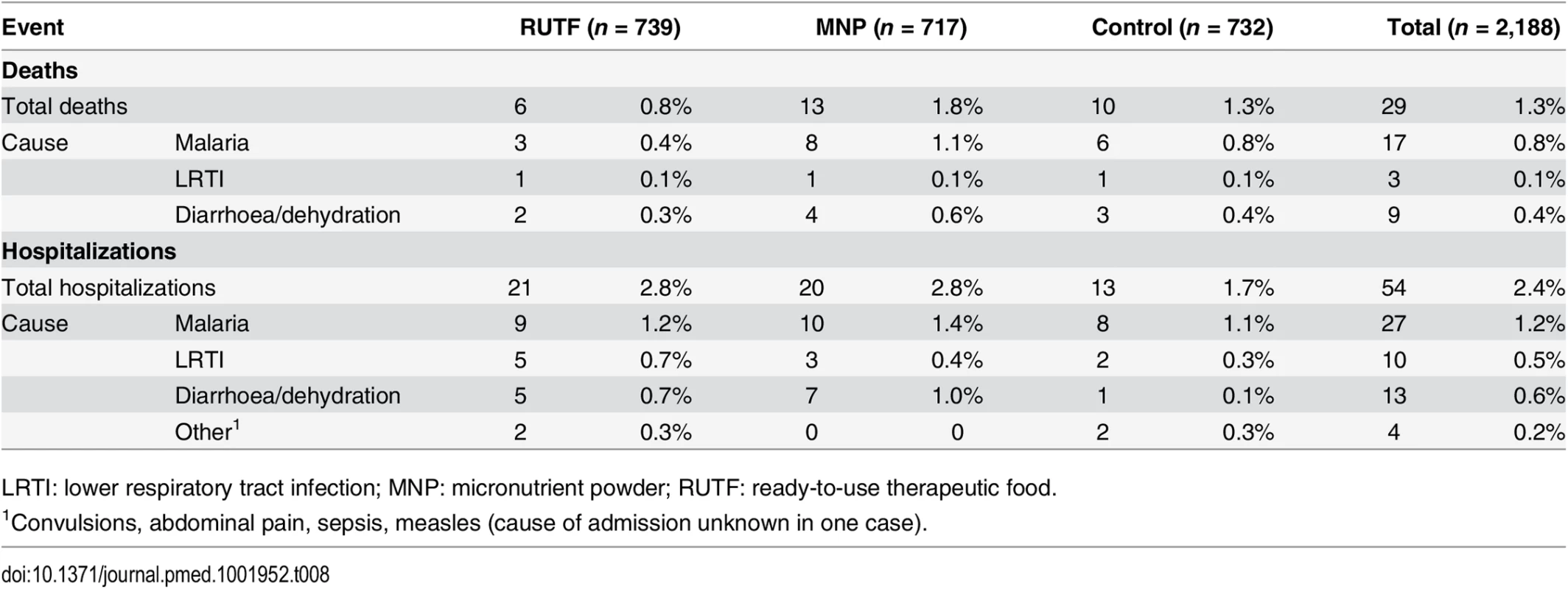
LRTI: lower respiratory tract infection; MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. Consumption and Compliance
Supplement consumption over the first 14 d after enrollment as reported by the caretaker showed that participants in the RUTF group consumed an average of 13.8 sachets (per allocation of 14 sachets) and participants in the MNP group consumed 26.9 sachets (per allocation of 28 sachets). Over the entire study period and all allocations, an average of 13.8 sachets of RUTF (out of 14 sachets) was consumed and 27.0 sachets of MNP (out of 28 sachets) (Table 9).
Tab. 9. Allocation to and compliance with supplements in intervention group. 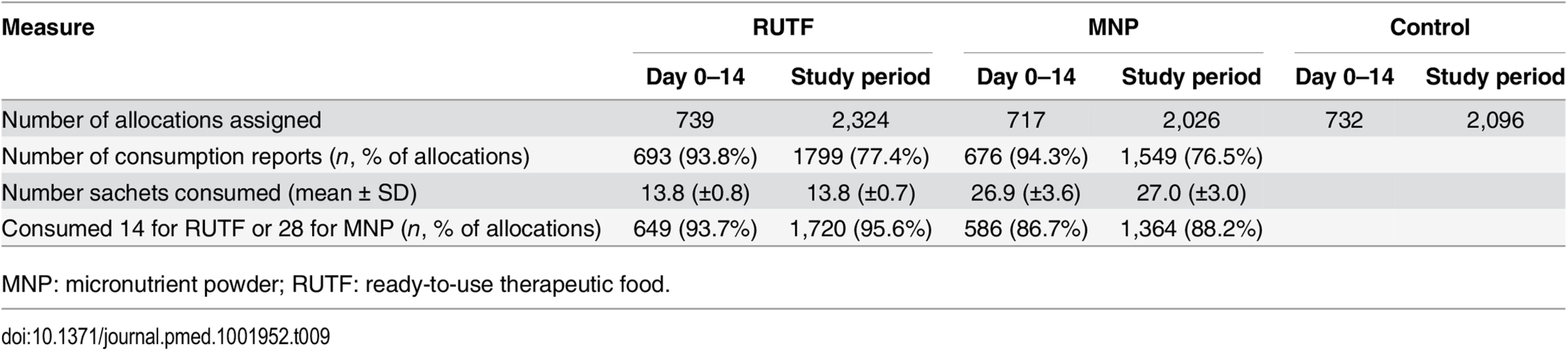
MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. Focus group discussions showed that all the mothers enjoyed giving RUTF or MNP to their children. About RUTF, they mentioned the following benefits: “Improvement of health, increased appetite, more powerful, gain the body weight.”In addition, MNP was reported to increase appetite, give more stamina, make the child stronger, and reduce diseases. The mothers expressed wanting to buy RUTF but also MNP if the price was reasonable, as they perceived it as improving their child’s health and increasing appetite and weight. Mothers reported reserving the supplement for the study child, as they saw it as part of the treatment for the disease.
In both groups, all mothers found the instructions on how to administer the supplement clear and easy to follow. The malaria treatment was also discussed; mothers said they now know how to give the medication and how important it is to finish the treatment.
Discussion
Incidence of Malnutrition
This study and its companion trial in Kaabong (Uganda) are the first large randomised controlled trials of the use of novel supplements (RUTF and MNP) for the prevention of malnutrition in children with a nonsevere illness. In our site in Nigeria with a high incidence of disease, there was no reduction in incidence of malnutrition with short-term supplementation of either RUTF or MNP compared with a control group. Supplementation had no impact on any of the anthropometric indices, and no effect of supplementation was found when comparing specific subgroups. The MNP group showed a lower number of events for diarrhoea but had a higher number of malaria events compared with the RUTF and control groups. There were fewer deaths in the RUTF group than in the control and MNP groups, this did not achieve statistical significance.
In comparison, a similar study in Kaabong (Uganda) found a significant reduction in the incidence of malnutrition in the RUTF group, but not in the MNP group (0.143, 0.185, and 0.213 first events/y for RUTF, MNP, and control, respectively; RUTF versus control, p = 0.037) [30].
We also found an overall high incidence of malnutrition in Goronyo; it was higher than anticipated at the beginning of the study. The sample size of the study was based on an incidence of first time NNO in the control group of 0.435 events/y, but the study showed 0.566 events/y in the control group, suggesting that the incidence of malnutrition among previously ill children in Goronyo was underestimated. In comparison, the incidence of malnutrition in the control group in Kaabong was only 0.213 events/y, which is low [30]. However, the study in Kaabong did not include moderately malnourished children, but the incidence of malnutrition among those who were non-malnourished in Goronyo was still high with 0.581 events/y. The incidence in Goronyo was also higher than the 0.26 events/year found in a study among the general population of children (not necessarily having been ill) in Niger [22]. This suggests that the incidence of malnutrition in Goronyo is higher than that of other countries. Goronyo is not the poorest area compared with the other surrounding countries, so other factors than poverty are likely to influence the incidence of malnutrition.
The incidence rate of severe malnutrition among participants who were moderately malnourished upon enrollment was high: a third developed severe malnutrition in the study period (incidence 0.32/168 d). The extrapolated incidence rate of 0.711/y might be misleading as it is unknown whether initial moderately malnourished children who did not develop severe malnutrition in during the first 6 mo will do so in the following 6 mo. Nevertheless, treatment of moderate malnutrition in ill children will prevent for a large part life threatening severe malnutrition in this group.
We expected that supplementation would be more effective among moderately malnourished children, as their nutritional reserves are already marginal, and supplementation could provide just the nutritional support needed. However, our study showed that supplementation was not effective in reduction of severe malnutrition in moderately malnourished ill children.
The lack of effectiveness of supplementation among moderate malnourished children could be due to the period of supplementation, which may have been too short, as moderately malnourished children need supplementation for at least 4 wk and often longer before being cured from malnutrition. Alternatively, the dose of the supplements may have been too low to be effective in supporting nutritional recovery in illness in moderately malnourished children, specifically when the morbidity is high. Finally, a potential effect of supplementation might be overshadowed by the high morbidity in Goronyo, resulting in a net loss of nutrients despite supplementation, which may be more important in the moderately malnourished group.
Morbidity and Mortality
The mean number of newly diagnosed study diseases was high. In total, more than 70% of participants reported at least one new diarrhoea episode, more than 50% a new LRTI episode, and more than 50% a new malaria episode. In total, the average number of new study diseases in the control group was 3.6 new events during the study period for the three study diseases together. In the study in Kaabong, the average number of new study diseases was 2.3 new events for the three study diseases together [30], which is lower than in Goronyo. When the illness on enrollment is included, this means that a child had an average of 4.6 disease episodes in 6 mo, which is about one illness every one and a half months.
Caretakers waited an average of 4.5 d (2.5 d in Kaabong) before presenting at a clinic when a child fell ill (as measured on enrollment). A delay in seeking treatment for diseases makes children more vulnerable to malnutrition, and in this study we found that the incidence of NNO was higher when the mothers waited longer before visiting the clinic.
The high morbidity in Goronyo is also reflected in the hospital admission and mortality rate. Fifty-five (2.5%) participants spent at least one night in the hospital, and 29 (1.3%) children died during the study. The study is unlikely to have underestimated mortality, as all participants who stopped attending the study were followed up and information from the family and their neighbours was obtained on the whereabouts of the child. The RUTF group showed the lowest mortality, and this was also observed in the Kaabong study, with no deaths in the RUTF group [30]. Our results are similar to those of a cluster randomised trial in Niger that showed a nonsignificant lower mortality in the intervention group (adjusted hazard ratio, 0.51; 95% CI, 0.25–1.05) [22]. These findings, while inconclusive, merit further research, as does the finding that the mortality was highest in the MNP group in both trial sites.
These findings show that the disease burden, the frequency of illnesses, and the delay in treatment in Goronyo was high. This could also be a reason why a supplement of short duration did not show an effect on the incidence of malnutrition. While a usual convalescence time from a disease would be at most 2 weeks, as treatment often lasts 1–2 days for diarrhoea, 3 d for malaria, and 5 d for LRTI. In addition, more than one-third of the participants had difficulties with gaining weight in the 2 wk after their illness. This lack of improvement indicates that recovery from illness was difficult. A supplementation period of 2 wk may not be enough to mitigate weight loss due to the frequent episodes of illness in Goronyo.
We compared Goronyo with other studies that supplemented all children in a population (not necessarily ill), as there is a lack of studies using modern supplements in ill children. However, this assumes that all children in the population are at equal risk of a high morbidity, and that the group of ill children in our study are comparable with all children in the population.
A longer supplementation period was effective in a study in Burkina Faso, with participants having also a considerable morbidity burden. In this study, a small quantity of LNS (with several levels of zinc) alongside morbidity surveillance and treatment and nutrition education was provided for 9 mo to children aged 9 mo. This intervention had a significant effect on all anthropometric indicators including wasting, which was 8.7% in the intervention group and 13.5% in the control group [23]. The control group did not receive morbidity surveillance and treatment, and the combination of medical care and supplementation could be essential for the significant reduction in incidence of malnutrition in Burkina Faso in the intervention group [23]. A study in Chad, where a small quantity of LNS was provided for 4 mo alongside a general food distribution, but where no specific element of treatment of morbidity was added, did not show a reduction of incidence of wasting [24]. In contrast, a research in Niger providing RUTF and morbidity surveillance and treatment for 4 months did show a significant reduction in wasting of 36% [22]. This indicates that a high quality supplement for a longer period in combination with morbidity surveillance and treatment is more effective in reducing the incidence of acute malnutrition in contexts with a high burden of morbidity then only providing a nutritional supplement.
Risks
There are reports suggesting an increased risk of severe illness when supplementing children with micronutrients. Soofi et al. [19] found a significant increase in rapid breathing and chest in-drawing in their MNP group (including zinc); however, this concern is not confirmed by our findings, as the rate of hospital admission or death due to LRTI or sepsis was low and not significantly different between the study groups, despite the fact that we were using twice the recommended dose of supplementation for MNP.
In our study, we used two doses of micronutrients resulting in twice the amount of recommended iron of 20 mg instead of 10 mg per day. Literature has suggested that iron supplements might worsen the outcome of malaria episodes [37] [38]. A review that was published before the start of our study and a later review did not confirm negative effects of iron supplementation on the outcome of malaria [39,40]. The mean number of diagnosed malaria events was higher in MNP group than in the RUTF group (p = 0.051). The mean number of morbidity events is not necessarily a new event; a child may still be ill with a previously diagnosed illness. A more robust indicator is the proportion of children with at least one new malaria episode, which as similar for all intervention groups. Also the number of hospital admissions and deaths due to malaria was higher (not significant) for MNP group compared with RUTF group. There was no difference in severe outcome between MNP and the control group; therefore, it is also possible that RUTF reduces the risk instead of MNP increasing the risk of a severe outcome.
Soofi et al. [19] also found an increased risk for severe diarrhoea and complications in their MNP groups. In contrast to their findings, in our study the MNP group had a significantly lower proportion of children having diarrhoea (non-severe) compared with the other two study groups. However, of the participants with severe diarrhoea or dehydration requiring hospital admission or resulting in death, a higher proportion came from the MNP group than the RUTF and control groups, but the numbers were small.
Overall, it seems that MNP shows a higher risk of severe disease and consequent mortality compared with RUTF but for MNP compared with the control group this is less clear. Moreover, it is also possible that RUTF lowers the risk of severe illness and outcome including severe malaria and severe diarrhoea/dehydration, which supports the observations that RUTF or lipid-based nutrient supplementation reduces mortality, as found in several studies [22,30]. As the numbers of hospital admissions and deaths were low, we cannot conclude or confirm an increased risk of micronutrient supplementation in ill children who are properly treated for their diseases.
Limitations
Despite the robust study protocols and strict implementation, this study had some limitations. First of all, the participants were not blinded for the RUTF or MNP supplementation. As the population had a positive attitude towards RUTF, this could introduce a bias to more positive reporting and compliance and a lower lost to follow up compared with MNP. However, blinding is difficult to achieve for RUTF, and efforts to produce a placebo for MNP failed. We think that, because MNP was new to the population, there were no positive or negative feelings about the product prior to participating in the study. The focus group discussions revealed that both RUTF and MNP were associated with positive health effects by the caregivers.
The sampling methodology was simple randomisation. This cannot exclude a spill-over effect of the different supplementation groups, as children in different households live relatively near to each other. Specifically, the RUTF could have been consumed by children in non-RUTF arms. It was not possible to adapt the sampling methodology towards a cluster randomised design owing to security considerations.
Diarrhoea was the only study disease that was based on caretakers’ report. As RUTF was popular among the caretakers, it is possible that participants reported diarrhoea more often in the hope of receiving RUTF; over-reporting of diarrhoea in the RUTF group cannot therefore be ruled out.
A lower compliance than measured cannot be ruled out despite the fact that the measured compliance and information from the focus group discussions suggested a high compliance and the analysis of the PP population did not give a different result. Abbeddou et al. found when doing 12-h observations in households that about 50% of supplements would be given, while measurement of compliance by questionnaires and counting of returned wrappings gave a much higher compliance [41]. This suggests that we cannot exclude a lower compliance than reported by the caretakers.
The results cannot be generalised to the general population of children. Although the participants were often ill, it cannot be assumed that all children in the population are often ill and thus that the result would apply to all children. Theoretically, it is possible that another group of children in the general population is hardly ever ill. Therefore, comparison with research implemented in the general population of children should be interpreted with caution.
Conclusions
In this randomised controlled trial of two-week supplementation with RUTF or MNP to ill children as part of routine primary medical care to children with malaria, pneumonia, or diarrhoea, we were unable to show a reduction in incidence of malnutrition. The lack of effect in Goronyo may be due to a high frequency of morbidity, which probably further affects a child’s nutritional status and children’s ability to escape from the illness–malnutrition cycle. The duration of the supplementation may have been too short or the doses of the supplements may have been too low to mitigate the effects of high morbidity and pre-existing malnutrition. An integrated approach combining prevention and treatment of diseases and treatment of moderate malnutrition, rather than prevention of malnutrition by nutritional supplementation alone, might be more effective in reducing the incidence of acute malnutrition in ill children. Further research should clarify the most cost-effective integrated approach, specifically in areas with high morbidity. Also, the extent LNS such as RUTF contributes to a lower mortality should be investigated.
Supporting Information
Zdroje
1. Black RE, Victora CG, Walker SP, Bhutta Z a, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382 : 427–51. doi: 10.1016/S0140-6736(13)60937-X 23746772
2. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371 : 243–60. doi: 10.1016/S0140-6736(07)61690-0 18207566
3. Scrimshaw NS, Tailor CE, Gordon JE. Interactions of nutrition and infection. WHO monograph series. Geneva: WHO 1968; 461–472. http://libdoc.who.int/monograph/WHO_MONO_57_(part1).pdf
4. Chen LC. Interactions of diarrhea and malnutrition: mechanisms and interactions. In: Chen LC, Scrimshaw NS, editors. Diarrhea and malnutrition: interactions, mechanisms and interventions. Plenum Publishing Corporation, New York; 1983 : 3–19.
5. Tomkins A, Watson F. Malnutrition and infection—a review—Nutrition Policy Discussion Paper No.5. Geneva; 1989. http://www.unsystem.org/scn/archives/npp05/begin.htm#Contents
6. Rowland MGM, Cole TJ, Whitehead RG. A quantitative study into the role of infection in determining nutritional status in Gambian village children. Br J Nutr. 1977;37 : 441–450. 861194
7. Tomkins AM. Protein–energy malnutrition and risk of infection. Proceedings of the Nutrition Society. Cambridge Univ Press; 1986 : 289–304. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=677892# 3099300
8. Rowland MG, Rowland SG, Cole TJ. Impact of infection on the growth of children from 0 to 2 years in an urban West African community. Am J Clin Nutr. 1988;47(1):134–138. 3337031
9. Shiff C, Checkley W, Winch P, Premij Z, Minjas J, Lubega P. Changes in weight gain and anaemia attributable to malaria in children living under holoendemic conditions. Trans R Soc Trop Med Hyg. 1996;90 : 262–265. 8758071
10. Guerrant RL, Lima AA, Davidson F. Micronutrients and infection: interactions and implications with enteric and other infections and future priorities. J Infect Dis. 2000;182 (Suppl 1):S134–8. 10944495
11. Friedman JF, Kurtis JD, Mtalib R, Opollo M, Lanar DE, Duffy PE. Malaria is related to decreased nutritional status among male adolescents and adults in the setting of intense perennial transmission. J Infect Dis. 2003;188(3):449–57. 12870128
12. Hoare S, Poppitt SD, Prentice AM, Weaver LT. Dietary supplementation and rapid catch-up growth after acute diarrhoea in childhood. Br J Nutr. 1996;76(4):479–90. 8942357
13. Scrimshaw NS, Sangiovanni JC. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66 (Suppl): S464–77.
14. Pnasziou P, Meackerras DE. Vitamin A supplementation in infectious diseases : a meta-analysis. BMJ. 1993;306 : 197–201.
15. Mahalanabis D, Bhan MK. Micronutrients as adjunct therapy of acute illness in children : impact on the episode outcome and policy implications of current findings. Br J Nut. 2001;85(Suppl 2):S151–8.
16. Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr. 2003;133(1)(Suppl):S316–21.
17. Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics. 2007;119(6):1120–30. 17545379
18. Sharieff W, Bhutta Z, Schauer C, Tomlinson G, Zlotkin S. Micronutrients (including zinc) reduce diarrhoea in children: the Pakistan Sprinkles Diarrhoea Study. Arch Dis Child. 2006;91(7):573–9. 16556612
19. Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet. 2013;382 : 29–40. doi: 10.1016/S0140-6736(13)60437-7 23602230
20. Ramakrishnan U, Aburto N, Mccabe G, Martorell R. Multimicronutrient interventions but not vitamin A or iron interventions alone improve child growth: results of 3 meta-analyses. J Nutr. 2004;134 : 2592–602. 15465753
21. Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 2013;382 : 452–77. doi: 10.1016/S0140-6736(13)60996-4 23746776
22. Isanaka S, Nombela N, Djibo A, Poupard M, Van Beckhoven D, Gaboulaud V, et al. Effect of preventive supplementation with ready-to-use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA 2009;301(3):277. doi: 10.1001/jama.2008.1018 19155454
23. Hess SY, Abbeddou S, Jimenez EY, Somé JW, Vosti SA, Ouédraogo ZP, et al. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young burkinabe children: a cluster-randomized trial. PLoS ONE. 2015;10(3):e0122242. doi: 10.1371/journal.pone.0122242 25816354
24. Huybregts L, Houngbé F, Salpéteur C, Brown R, Roberfroid D, Ait-Aissa M, et al. The effect of adding ready-to-use supplementary food to a general food distribution on child nutritional status and morbidity: a cluster-randomized controlled Trial. PLoS Med. 2012;9(9):e1001313. doi: 10.1371/journal.pmed.1001313 23028263
25. Isanaka S, Nombela N, Djibo A, Poupard M, Van Beckhoven D, Gaboulaud V, et al. Effect of preventive supplementation with ready-to-use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA. 2009;301(3):277–85. doi: 10.1001/jama.2008.1018 19155454
26. WHO. Food, water and family health: A manual for community educators. Geneva: WHO, UNDP; 1994;p19,27. http://apps.who.int/iris/bitstream/10665/62963/1/WHO_HEP_94.2.pdf?ua=1
27. Dewey KG. Guiding principles for complementary feeding of the breastfed child. WHO; 2003; p.26. http://whqlibdoc.who.int/paho/2003/a85622.pdf?ua=1
28. Dewey KG. Guiding principles for feeding non-breastfed children 6–24 months of age. WHO; 2005; p.23. http://apps.who.int/iris/bitstream/10665/43281/1/9241593431.pdf?ua=1&ua=1
29. van der Kam S, Swarthout T, Niragira O, Froud A, Sompwe ME, Mills C, et al. Ready-to-use therapeutic food for catch-up growth in children after an episode of Plasmodium falciparum malaria: an open randomised controlled trial. PLoS ONE. 2012;7(4): e35006. doi: 10.1371/journal.pone.0035006 22558108
30. van der Kam S, Roll S, Swarthout T, Edyegu-Otelu G, Matsumoto A, Kasujja FX, et al. Effect of short-term supplementation with ready-to-use therapeutic food or micronutrients for children after Illness for prevention of malnutrition: a randomised controlled trial in Uganda. PLoS Med. 2016;13(1):e1001951.
31. FEWSNET. Preliminary livelihoods zoning: Northern Nigeria. 2007; p.18. http://www.fews.net/sites/default/files/documents/reports/ng_zonedescriptions_en.pdf
32. Theuri T. Goronyo Underlying causes of Malnutrition. 2009.
33. Baily J. Nutrition survey March 2009, Goronyo, Sokoto state, Nigeria. 2009.
34. MSF-OCA. Nutrition Survey Results March 2010 Goronyo, Sokoto state, Nigeria. 2010.
35. SCF. Child malnutrition in Northern Nigeria: an illustrative case study. 2012. http://www.heawebsite.org/download/file/fid/373
36. WHO; WFP; UNICEF. Preventing and controlling micronutrient deficiencies in populations affected by an emergency. 2007. http://www.who.int/nutrition/publications/WHO_WFP_UNICEFstatement.pdf
37. Prentice AM. Iron metabolism, malaria and other infections: what is all the fuss about ? J Nutr. 2008;138 : 2537–2541. doi: 10.3945/jn.108.098806 19022986
38. Veenemans J, Milligan P, Prentice AM, Schouten LRA, Inja N, van der Heijden AC, et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 2011;8(11):e1001125.
39. Ojukwu JU, Okebe JU, Yahav D, Paul M. Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas (Review). Cochrane Database Syst Rev. 2009;(4).
40. Okebe J, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2011;(10).
41. Abbeddou S, Hess SY, Jimenez EY, Somé JW, Vosti SA, Guissou RM, et al. Comparison of methods to assess adherence to small-quantity lipid-based nutrient supplements (SQ-LNS) and dispersible tablets among young Burkinabe children participating in a community-based intervention trial. Matern Child Nutr. 2014;1–30.
Štítky
Interní lékařství
Článek 2015 Reviewer Thank You
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 2- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- 2015 Reviewer Thank You
- The Future of Diabetes Prevention: A Call for Papers
- The Case for Reforming Drug Naming: Should Brand Name Trademark Protections Expire upon Generic Entry?
- The Health Care Consequences Of Australian Immigration Policies
- Microenvironmental Heterogeneity Parallels Breast Cancer Progression: A Histology–Genomic Integration Analysis
- Hand, Foot, and Mouth Disease in China: Modeling Epidemic Dynamics of Enterovirus Serotypes and Implications for Vaccination
- Estimated Effects of Different Alcohol Taxation and Price Policies on Health Inequalities: A Mathematical Modelling Study
- Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Uganda
- Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Nigeria
- When Children Become Adults: Should Biobanks Re-Contact?
- Transforming Living Kidney Donation with a Comprehensive Strategy
- A Time for Global Action: Addressing Girls’ Menstrual Hygiene Management Needs in Schools
- The Rise of Consumer Health Wearables: Promises and Barriers
- Risk of Injurious Fall and Hip Fracture up to 26 y before the Diagnosis of Parkinson Disease: Nested Case–Control Studies in a Nationwide Cohort
- Mortality, Morbidity, and Developmental Outcomes in Infants Born to Women Who Received Either Mefloquine or Sulfadoxine-Pyrimethamine as Intermittent Preventive Treatment of Malaria in Pregnancy: A Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Hand, Foot, and Mouth Disease in China: Modeling Epidemic Dynamics of Enterovirus Serotypes and Implications for Vaccination
- A Time for Global Action: Addressing Girls’ Menstrual Hygiene Management Needs in Schools
- Transforming Living Kidney Donation with a Comprehensive Strategy
- The Rise of Consumer Health Wearables: Promises and Barriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



