-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEducational Outreach with an Integrated Clinical Tool for Nurse-Led Non-communicable Chronic Disease Management in Primary Care in South Africa: A Pragmatic Cluster Randomised Controlled Trial
In a cluster-randomized trial done in South Africa, Lara Fairall and co-workers investigate the effectiveness of a clinical management tool for non-communicable diseases in primary care.
Published in the journal: . PLoS Med 13(11): e32767. doi:10.1371/journal.pmed.1002178
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002178Summary
In a cluster-randomized trial done in South Africa, Lara Fairall and co-workers investigate the effectiveness of a clinical management tool for non-communicable diseases in primary care.
Introduction
South Africa is facing a quadruple burden of disease: HIV and tuberculosis (TB); non-communicable diseases (NCDs), including mental health conditions; injury and violence; and maternal, neonatal, and childhood illnesses [1]. The past 15 years have seen concentrated efforts to strengthen the capacity of the public health system to treat HIV and TB. These investments seem at last to be paying off, with a rise in life expectancy, a decline in mortality [2], and fewer new HIV infections [3]. Yet the burden of NCDs and mental health remains unchecked; cardiovascular disease is now the second leading cause of death in South Africans after communicable diseases [4,5].
In South Africa, responsibility for the detection and treatment of NCDs lies at the primary care level, with nurses seeing nine out of ten patients, most of whom have more than one presenting condition [6]. However, the quality of NCD care is generally poor, characterised by under-diagnosis, under-treatment, and poor clinical control [1,7,8]. We have previously successfully piloted and trialled task-sharing interventions for communicable diseases, increasing the capacity of nurses to take on assessment and prescribing roles for HIV and TB previously restricted to doctors [9–15]. This programme has been scaled up throughout South Africa as part of the national government’s accelerated response to HIV and TB launched in 2010 [16]. A similar programme has been developed for use in other countries including Malawi, Botswana, Brazil, and Mexico [17]. We have since expanded this programme, now called Primary Care 101 (PC101), to include NCDs and mental health, hoping to leverage the health system reforms that accompanied the scale-up of antiretroviral therapy (ART) to improve the quality of primary care for other priority conditions.
These integrated programmes of care seek to overcome the limitations of vertical services that tend to neglect multimorbidity [18–23], and to expand the roles of nurses, increasing the number and distribution of health workers providing treatment for common NCD conditions.
While the benefits of nurse substitution and supplementation for a limited number of NCDs in high-income settings are well recognised [24], evidence from low - and middle-income countries (LMICs) is sparse and limited to a few pilot studies [25–28]. Fewer studies still have sought to improve care across several NCDs simultaneously. Meta-analyses of complex interventions in health systems confirm only small effect sizes (ranging from 0.4% to 6.3%) for carer behaviour (improved care), but given the size of the populations affected, these effect sizes are considered important, provided the interventions are introduced without harm. We report here the findings of the PC101 Trial, a pragmatic cluster randomised study evaluating the effectiveness of the PC101 intervention, which combines provision of an integrated management tool with educational outreach to nurses. The primary outcomes of interest were intensification of treatment for hypertension, diabetes, and chronic respiratory disease and case detection of depression in overlapping cohorts of patients with these conditions.
Methods
Ethical approval for the trial was obtained from the University of Cape Town Human Research Ethics Committee (reference number 119/2010) and the Western Cape Department of Health.
Study Design
This was a pragmatic, parallel-group cluster randomised controlled trial performed in the Eden and Overberg districts of the Western Cape Province. Clusters were public sector primary healthcare clinics randomised within six sub-district strata. Outcome measures in each of four cohorts were assessed in individual patients. Patient cohorts overlapped; patients with more than one condition of interest were included in each applicable cohort, and cohorts were powered and analysed separately. This study design, with multiple cohorts, each with its own primary outcome evaluated simultaneously, aimed to reflect the realities in primary care clinics that nurses are required to diagnose and manage a wide range of conditions, that NCDs are associated with multimorbidity, and that a focus on one condition may compromise the management of others [29]. The Western Cape Department of Health provided consent for the inclusion and randomisation of clinics, before randomisation was performed. Patients provided written consent for data collection after randomisation of clinics and prior to data collection.
Participants
Clinics
The study was conducted in the predominantly rural districts of Eden and Overberg, where public sector clinics serve a population of around 800,000, mainly people with lower socio-economic status. Busy town clinics had fulltime doctors, but most clinics were nurse led, with doctors in attendance on a sessional basis (Table A in S1 Appendix).
Eligible clinics provided services, including for NCDs, at least five days a week and reported more than 10,000 attendances per year, so were likely able to contribute sufficient numbers of patients to the study. Of 124 clinics in the Eden district, 33 clinics in five sub-districts met these criteria. We supplemented this sample with five clinics from a sub-district in an adjacent district (Overberg), to increase the number of clinics available for randomisation and strengthen the study’s power.
The health districts in the study are representative of health services offered to more than 80% of the population of South Africa, comprising clinics both from medium-sized towns and rural areas [6].
Patients
The study population comprised patients with one or more of the following: hypertension, diabetes, chronic respiratory disease, or depression. Initial eligibility criteria were being 18 y or older, likely to reside in the area for the next year, and capable of actively engaging in an interviewer-administered questionnaire at the time of recruitment. Inclusion criteria for the four cohorts were as follows (Table 1): for the hypertension and diabetes cohorts, if patients reported being on medication for hypertension or diabetes, respectively; for the respiratory disease cohort, if they reported receiving medication for chronic airway disease (asthma or chronic obstructive pulmonary disease [COPD]) or reported a cough and/or difficult breathing for 2 wk or more prior to enrolment and were not on treatment for TB [30]; for the depression cohort, if they scored ten or more on the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10) [31,32]. We selected this instrument because the 20-item version has been validated in a similar setting in South Africa [33], and the 10-item version in primary care populations elsewhere [34–36]. The shorter version was necessary to limit the length of the screening process for all four conditions. Patients were eligible even if at the time of enrolment they had no record of current treatment for their condition.
Tab. 1. Eligibility criteria, primary outcome definitions, and required sample size estimates for each cohort. 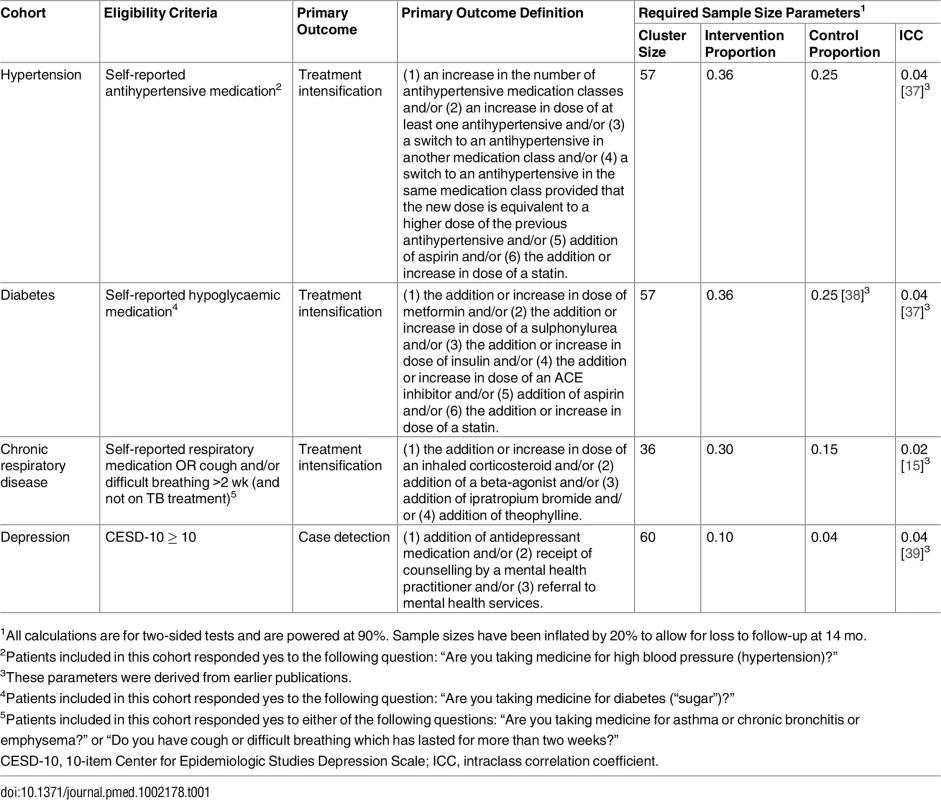
1All calculations are for two-sided tests and are powered at 90%. Sample sizes have been inflated by 20% to allow for loss to follow-up at 14 mo. In keeping with the study’s pragmatic design, enrolment was not restricted to patients with uncontrolled disease or to patients considered to be adherent to current treatment [40]. Although encouraging adherence was included in the management tool, it was not monitored.
Randomisation
Clinics within each of six health sub-district strata were randomised to avoid potential confounding resulting from geographically determined differences in management of clinical services. Two strata contained equal numbers of clinics, meaning that randomisation could be done in a 1 : 1 ratio. The four strata containing odd numbers of clinics were randomly allocated to have either one more or one fewer intervention clinic than control clinics, to achieve an equal number of clinics in each group (19 per group, 38 in total).
Randomisation was completed by the trial statistician using nQuery Advisor after recruitment of clinics, independently of the managers giving permission for the clinics to be included in the trial, and prior to patient recruitment and implementation of the intervention.
Setting and Programme
Usual care for non-communicable and communicable diseases (control group)
South African primary care clinics provide free services for communicable disease and NCDs. Patients are seen by a clinician, usually a nurse, and stable patients with NCDs are seen at intervals of 3–6 mo, but are required to collect medications each month either from the clinic or from an off-site medication pick-up point. The clinical load borne by nurses is great; in 2008 the median number of patients per nurse seen each working day in the clinics studied was 25. Although all clinics were attended by doctors, in more than half this was on a part-time basis rather than daily (Table A in S1 Appendix). National regulations require that prescriptions be renewed and co-signed by a doctor at least every 6 mo, a process that is time-consuming, reducing opportunities for the doctor to review complex cases and mentor nurses. The selection of medications and level of prescribing provisions (nurse versus doctor) are governed by the South African national essential medicines list and standard treatment guidelines [41], which are revised by the National Department of Health every 5 y. Nurse prescribing provisions differ by province and, prior to the trial, were limited in the Western Cape to first-line medications such as thiazide diuretics for hypertension, metformin for diabetes, and low-dose inhaled corticosteroids for asthma. Prescription of antidepressants, which is governed by regulatory conditions for high schedule medications, is restricted to doctors.
Guidelines and policies for communicable diseases change more frequently than those for NCDs. Guidelines for both tend to be lengthy and text-heavy, at times containing confusing differences in recommendations for the same condition. To address this issue, we developed and implemented the Practical Approach to Lung Health and HIV/AIDS in South Africa (PALSA PLUS), a management tool that integrates guidelines and configures them concisely and simply in an algorithmic format that more closely aligns with presentations in primary care (symptoms and follow-up for chronic conditions) and ensures harmonisation of disease-specific guidelines. It also clarifies prescriber levels (nurse versus doctor) [13,17,42]. It was implemented in two provinces in 2006 (Western Cape and Free State), and in all nine provinces of South Africa between 2010 and 2011. Since 2007 it has included provision for nurse initiation and re-prescription of ART. This inclusion was based on the results of a large pragmatic randomised controlled trial performed in the Free State Province that showed that nurses were as effective as doctors in providing ART care [9,11,12], and on a second trial in the Western Cape and Gauteng Provinces that evaluated nurse re-prescription of ART [43]. This development was prompted by the urgent need to expand ART services in South Africa. Use of the latest (2011/2012) version of PALSA PLUS was the standard of care in control clinics during the PC101 Trial. Prior to the development of PC101, nurses were required to manage NCDs, but they received relatively little training and support, resources for NCD management were not user-friendly, and initiation or intensification of NCD medications was largely dependent on the availability of doctors. The introduction of PALSA PLUS was the first attempt to change this pattern; providing more user-friendly management tools that expanded nurses’ scope of practice and prescribing with increasing “diagonal” integration.
A second key component of the PALSA PLUS programme is training clinicians to use the management tool. This component employs an educational outreach model [44] in which facility trainers, typically nurse middle managers, are trained and equipped to deliver repeated short (1.5 h), onsite, interactive training sessions using carefully constructed case scenarios [42]. Clinicians are trained to use the tool as a care pathway in case management and to use it during each consultation. Follow-up “refresher” training accompanied distribution of the revised management tool each year. By 2011, around 70% of all nurses working in the trial districts had received initial training in PALSA PLUS, and training continued as usual in the control clinics.
An unanticipated change in usual care in the health districts under study was a shift in focus from communicable disease care to NCD care. Midway through the trial, the district health department launched a 3-mo campaign called Chronic Disease Season in all clinics to improve NCD recognition and care. Chronic Disease Season focused on hypertension and diabetes and involved both community and clinic health workers. The community-level interventions included several “health screening days” in which free blood pressure and finger-prick glucose measurements were offered at venues such as shopping centres and town halls. People with high values were referred to local clinics. In addition, around 10% of community health workers (33 in total) were equipped to provide basic education on lifestyle measures including diet and physical activity.
Intervention rationale
The PC101 intervention was a further development of the PALSA PLUS programme, aimed to include all common symptoms and conditions, including NCDs, among adults attending primary care services. This expanded scope was strongly motivated by input from primary care nurses and managers, who reported that coverage for NCDs in PALSA PLUS, particularly hypertension and diabetes, would greatly improve its usefulness. The implementation of PC101 aimed to use the same educational outreach approach as used for PALSA PLUS. This educational approach was shown in three pragmatic trials to be effective for the management of communicable diseases. Beneficial effects included reproducible and substantial improvements in TB case detection [9,13,15]; increases in appropriate prescribing, including inhaled corticosteroids for asthma [15], co-trimoxazole prophylaxis for HIV [13], and appropriate switching to second-line ART [9]; and appropriate referral of severe [15] and complex cases [9]. Changes in healthcare utilisation included fewer and shorter hospital admissions and a higher number of primary care visits [9,13,15]. The impact on health worker morale was also documented in parallel qualitative evaluations, with nurses reporting a sense of empowerment and emphasising the value of combining simplified diagnostic and treatment algorithms, onsite training, and expansions in prescribing provisions [12,42]. No harmful effects of the intervention were noted.
Intervention materials
The main intervention material was a 101-page evidence - and policy-informed algorithmic management tool. Based on PALSA PLUS, it was developed over a period of 5 y (2006–2011) with input from specialist clinicians, primary care doctors and nurses, allied health professionals, managers, and representatives of patient advocacy groups. The selection of content was based on the results of a cross-sectional survey in 18,000 consultations in primary care clinics across four provinces in South Africa of the most common reasons for attendance. The first half of PC101 covers 40 of the most common symptoms in adults attending primary care and prompts screening for the 20 chronic conditions included in the second half of the tool [6]. The selection of chronic conditions took a health services approach, including those that required regular planned follow-up in primary care. Included were communicable diseases (HIV, TB, sexually transmitted infections), NCDs (diabetes, hypertension, asthma, COPD, epilepsy), mental health conditions (depression, substance abuse, schizophrenia, dementia), and women’s health (contraception, antenatal care). Content was extracted from existing disease and policy guidelines and structured in a simple summative form: one page for “diagnosis” and one to two pages for follow-up “routine care” (organised under the headings of “assess”, “advise”, and “treat”) for each condition. Promotion of integrated care was a key objective. Extensive use was made of algorithms and checklists to optimise presentation of content, and provide actionable support that is readily applied during consultations. Content for diverse conditions was organised in a standard format; symptom pages prompted screening for multiple chronic conditions, and pages on the routine care of chronic conditions included screening for common comorbidities. In addition, care was taken to ensure that recommendations that were applicable to multiple pages of the tool, such as blood pressure thresholds for diagnosis, treatment, and lifestyle advice, were harmonised and consistently reflected. The management tool was provided as a ring-bound, high-quality, full-colour illustrated booklet to every clinician (nurse and doctor) responsible for primary care in the 19 intervention clinics. The tool is updated annually to reflect changes in evidence, policy, and feedback from clinicians and managers. For examples of updated content, see http://knowledgetranslation.co.za/programmes/pack-adult/.
The case scenarios used for training built on a set that had been extensively used during PALSA PLUS implementation. An illustration of a typical waiting room scene provided a cast of characters, each of whom was fleshed out in a case scenario (Table B in S1 Appendix). The cases were carefully constructed to build familiarity with use of the management tool, grow knowledge specifically related to NCDs and depression, and scaffold development of knowledge and skills [45], moving from straightforward clinical presentations toward greater complexity and multimorbidity. The cases formed the basis for the educational training sessions (Table B in S1 Appendix). A desk-blotter with a calendar illustrating key messages for priority conditions was provided to all staff in intervention clinics, to facilitate booking of follow-up appointments and to remind clinicians of essential elements of care.
Training
Six health department nurse trainers with experience in primary care and with responsibility for existing training initiatives within the study districts—including Integrated Management of Childhood Illness, PALSA PLUS, and ad hoc training in the TB programme—and with a support role for nurses were employed as facility trainers for the study. They were initially trained in PC101 during a 5-d live-in training course in May 2011. This course was led by an experienced adult education practitioner with a background in nursing (G. F.) and the family doctor who had led the expansion of the management tool (R. C.). The programme adopted a strong experiential focus, and gave as much attention to equipping the nurse trainers to be educators as it did to the expanded content of the management tool. It included multiple practice sessions during which the nurse trainers facilitated case-scenario-based training sessions with their peers, followed by critical feedback. It included exercises to help each trainer understand their own learning style [46] and to learn reflective practice. Facility trainers delivered eight short (1.5 h), on-site, interactive educational outreach sessions using the PC101 management tool and case scenarios to all clinical staff at intervention clinics over several weeks. In all, 155 face-to-face educational outreach sessions were held at the 19 intervention clinics, eight sessions in each clinic. Owing to clinical demands and absences due to night duties or annual sick or study leave, not all staff were able to attend every session. In total, 81 nurses (who each participated in a median of six sessions), five pharmacists, and four doctors were trained. The trainers received no payment from the research team. In addition to on-site training, nurse trainers provided support to staff through regular visits during which they would discuss difficult cases, review folders of patients whose care nurses had changed using PC101, or jointly see patients.
The nurse trainers themselves were supported through quarterly 1-d workshops, facilitated by G. F. These workshops included opportunities to report back on training at the clinic, troubleshoot difficulties in scheduling or completing educational outreach sessions, resolve queries related to the clinical content of the management tool, and practise facilitation skills. They also aimed to continue the community of practice that had been established during the initial live-in training.
Expanded prescribing provisions
Professional nurses who successfully completed the educational outreach were authorised by the district manager to prescribe an additional seven medications for NCDs previously restricted to doctors: enalapril and amlodipine for hypertension, glibenclamide and gliclazide for diabetes, simvastatin for increased cardiovascular risk, inhaled budesonide for asthma, and short courses of oral prednisone for exacerbations of COPD (Table C in S1 Appendix). These expansions were clearly reflected in the management tool, which colour-coded all medications to reflect whether they could be prescribed by a doctor or a nurse or only by a doctor, and were also communicated to clinic managers by way of a circular from the district managers. The expanded prescribing provisions initially resulted in some tensions between nurses, doctors, and pharmacists. These were resolved through a facilitated group session and informal communication within clinics, sometimes involving the nurse trainer. This intervention was the only modification to the training during the trial.
Intervention monitoring
The integrity of the intervention was assessed in several ways. Nurse trainers were observed during the initial live-in course and at quarterly follow-up workshops. Two nurse trainers were interviewed, and, in December 2011, focus group discussions were held with nurses in four intervention clinics. Nurses representing both rural and small town locations were enthusiastic about the management tool and recognised that it was a new way of strengthening care for NCDs. In particular they appreciated the format and the standardised framework for providing routine care, and the familiar features shared with PALSA PLUS. Consistent with our previous experience with PALSA PLUS, some variation in uptake of the management tool by nurses was reported. There was a tendency for nurses who formerly used PALSA PLUS to adopt PC101 and use it regularly, whereas nurses who had not used PALSA PLUS were less likely to begin to use the new management tool routinely [11,12]. Uptake by the trainers was considered excellent, and trainers completed planned sessions in all intervention clinics, some repeating sessions to ensure coverage of most staff.
Data Collection
Fieldworkers recruited from local communities were trained to collect the trial data. They invited patients seated in the waiting rooms to be considered for the study and screened them using a structured questionnaire. Patients who met the eligibility criteria (Table 1) and provided informed consent were enrolled in the trial and completed the baseline questionnaire in Afrikaans, isiXhosa, or English, administered by the fieldworker using a handheld electronic device. Anthropometry (weight, height, waist circumference) and blood pressure were recorded [47]. Patients were asked to attend a follow-up interview 14 mo after their baseline interview. The lengthy period between interviews was intended to allow adequate opportunity for health workers to intervene in the care of trial patients, given that chronic disease patients are seldom reviewed at clinics more often than every 3–6 mo.
The questionnaire included questions on medical history, smoking status, mental health, health-related quality of life, and socio-economic status. The severity of respiratory symptoms among patients in the respiratory cohort was assessed using the symptom and activity domains of the St George’s Respiratory Questionnaire [48]. Patients who chose to complete the interview in isiXhosa were excluded from this section of the interview as there is no tested isiXhosa translation of this instrument. The presence of symptoms of depression was assessed with the CESD-10, administered to all patients enrolled in the study [32].
Depression treatment was defined as having received counselling, having been referred to psychiatric services, or being on an antidepressant at a therapeutic dose. Low-dose amitriptyline and imipramine are widely prescribed in South Africa for pain management or insomnia. We therefore defined antidepressant use at a therapeutic dose as prescription of amitriptyline or imipramine ≥50 mg daily and/or any other antidepressant. Counselling was defined as “talking with someone in a way that helps to find solutions to problems, or receive emotional support, and not just receiving advice on how to take medication.”
Fieldworkers extracted and photocopied patients’ prescription charts from their folders, clinic stores, and pharmacies for the year preceding the baseline interview. The medically qualified trial manager (N. F.) analysed all prescription charts and recorded prescriptions of chronic medication for each patient at the time of their interview. A data capturer entered the prescription data (medication, dose, and frequency) into a database, and the total daily dose for each medication was calculated. Prescription, interview, and laboratory data were imported and stored in a SQL server database, and a single longitudinal record constructed for every patient by the study database scientist (V. T.).
Reminder letters and cell phone text messages were sent to patients in the month preceding their scheduled follow-up interview. Patients who failed to attend this appointment were traced by phone or home visit. Patients received a gift voucher for a local grocery store with a value of ZAR100 (US$12.25) on completion of the follow-up interview, to compensate for travel costs and time. The follow-up questionnaire was similar to the baseline questionnaire, and fieldworkers repeated the anthropometry and blood pressure measurements. At follow-up, prescription data for the period since baseline were extracted, photocopied, analysed, and documented in the same way as at baseline.
Quality control measures included supervision of fieldworkers, electronic alert messages for fieldworkers if unusually high or low values were entered into the electronic questionnaire, monitoring of the data to identify unusual values or trends, and double entry of prescription data. At follow-up, prescription data were queried if they were missing, if the date of the prescription fell outside of a 1-mo window period based on the scheduled re-interview date, or if cohort-specific medications were excluded.
Blinding of the intervention was not possible at the clinic level due to the nature of the intervention.
Outcome Measures
The primary outcome for hypertension, diabetes, and chronic respiratory disease was treatment intensification, reflected by an increase in dose or number of medications or change in medication class. This outcome was chosen after considering research identifying clinician inertia as a key reason for failure to control these conditions [49,50]; treatment intensification is associated with improved control [51–53]; was likely appropriate for the study population, where under-treatment was highly prevalent [1,7,8]; fitted well with the focus of the intervention on the clinical practices of nurses and the expansion of their prescribing with training; and could be applied across three of the four chronic conditions of interest. Definitions of treatment intensification by cohort are summarised in Table 1. For the depression cohort, case detection was selected as the primary outcome because depression is recognised to be under-diagnosed and under-treated in primary care [54].
Secondary outcome measures were as follows: disaggregation of primary outcomes by type of medication; cardiovascular disease risk and risk factors such as blood pressure, body mass index (BMI), and smoking status; health-related quality of life measured using the EuroQol-5D [55] and the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) [56]; mortality; and healthcare utilisation. These last four outcomes were designed to detect evidence of harm resulting from shifting clinical responsibility from doctors to nurses, an often overlooked consideration in evaluations of task-shifting [57].
Sample Size and Statistical Power
The study was powered to detect clinically important differences in primary outcomes within each cohort, accounting for the cluster randomisation design. With 38 clinics available for randomisation, we calculated the number of patients needed per clinic for each cohort to detect differences in primary outcomes of between 10% and 15%, with 90% power, 5% significance, and intraclass correlations of outcome based on previous studies, and assuming 20% loss to follow-up (Table 1). Baseline rates of treatment intensification were not available in South Africa, and so we used rates from studies completed in high-income settings [50,58].
HbA1c was measured as part of the pre-planned blood sampling strategy in a subgroup of clinics because resource limitations meant that we could not measure it in all diabetic patients in all 38 clinics. We estimated that HbA1c tests were needed from 30 diabetic patients in 10 clinics in each group (i.e., 600 diabetic patients from 20 clinics in total) in order to a show a difference of 0.5% (HbA1c of 8.8% in the control group versus HbA1c of 8.3% in the intervention group, assuming a standard deviation of 3.4%).
Analysis
We compared baseline clinic and patient characteristics between treatment groups. All clinics and patients were analysed in the treatment group to which they were randomly assigned. Primary and secondary outcomes were analysed at the patient level, separately within each cohort. No adjustment was made for the multiple disease-specific primary outcomes. The cluster randomisation design was accounted for using robust cluster variance-covariance estimates. Intervention effects were estimated using binomial regression models with treatment as the main effect, adjusted for stratification, and are reported with 95% confidence intervals. Secondary analyses were further adjusted for potentially confounding baseline characteristics such as treatment status and disease control at baseline, smoking status, age, sex, and co-morbidity with one of the study diseases.
We carried out pre-specified subgroup analyses of the primary outcomes stratified by baseline level of disease control using binomial regression models including baseline disease control as a covariate. Baseline disease control of hypertension was defined as blood pressure < 140/90 (or, in patients with diabetes or a history of cardiovascular disease, <130/80), and for diabetes, as HbA1c < 7%. For depression, since the outcome was detection, “control” was defined as any patient receiving treatment for depression as follows: being on antidepressant medication at therapeutic dosage or having received counselling in the past year or having been referred to psychiatric services in the last year. No definition of disease control was applied to patients with chronic respiratory disease. Heterogeneity of the intervention effect was assessed by looking at the interaction between treatment and baseline disease control. In addition, we pre-specified secondary analyses of the primary outcomes disaggregated by component. For the primary outcomes, missing data were considered not to have occurred.
We used linear regression to compare changes between baseline and follow-up in blood pressure, waist circumference, weight, BMI, HbA1c, and health status measures between the treatment groups, adjusted for stratification. Similarly, we used ordinal logistic regression to compare readiness to quit smoking, and Poisson regression to compare rates of healthcare utilisation between the treatment groups. Stata version 13.0 statistical software was used for all analyses.
Results
Fig 1 shows the trial profile. All 38 randomised clinics completed the trial. In all, 4,904 patients were screened, of whom 4,393 patients met the eligibility criteria and were enrolled in the trial. Recruitment targets were exceeded for all cohorts except for diabetes, where recruitment fell short of targets. Enrolment of patients took place between 28 March 2011 and 10 November 2011 and was completed in intervention clinics before educational outreach sessions to nurses began. Follow-up data collection began on 21 May 2012 and ended on 13 December 2012.
Fig. 1. Trial profile. 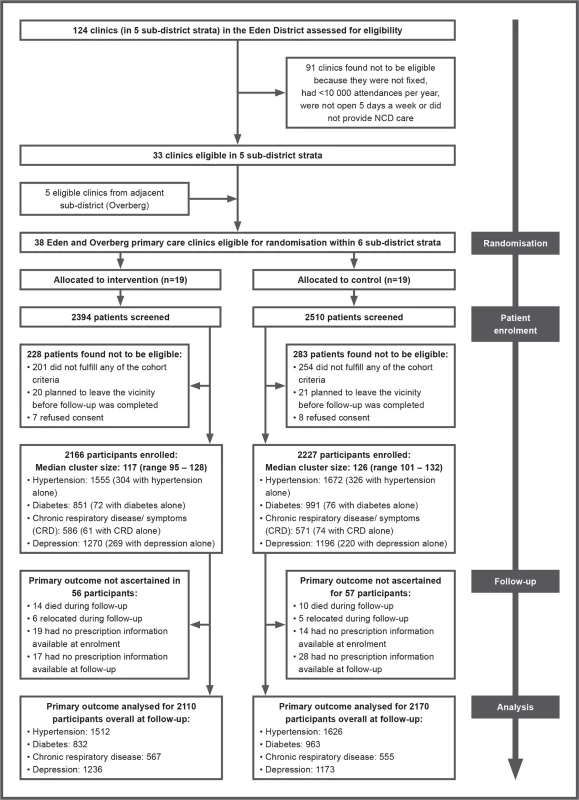
NCD, non-communicable disease. In all, 1,927 patients in the intervention group were interviewed at follow-up (1,927/2,166; 89%), and 2,050 in the control group (2,050/2,227; 92%). Reasons for not being re-interviewed were similar between groups: death (63 in the intervention group versus 54 in the control group); relocation (42 in the intervention group versus 26 in the control group); too ill to be re-interviewed (two in the intervention group versus zero in the control group); and could not be traced (132 in the intervention group versus 97 in the control group). Prescription charts could be traced, and thus the primary outcome ascertained, for 206 patients who were not re-interviewed in the intervention group, and 151 in the control group, accounting for the very high rates of patients contributing data to the primary endpoint analysis (Fig 1).
Baseline patient characteristics are presented in Table 2 and detailed in a separate publication [47]. Baseline clinic characteristics are provided in Table A in S1 Appendix. Intervention and control clinics had similar numbers of nurses and doctors. Control clinics tended to be larger and, by chance, had more psychiatric services and on-site pharmacy facilities.
Tab. 2. Characteristics of patients allocated to an educational outreach programme (intervention group) or no new training (control group). 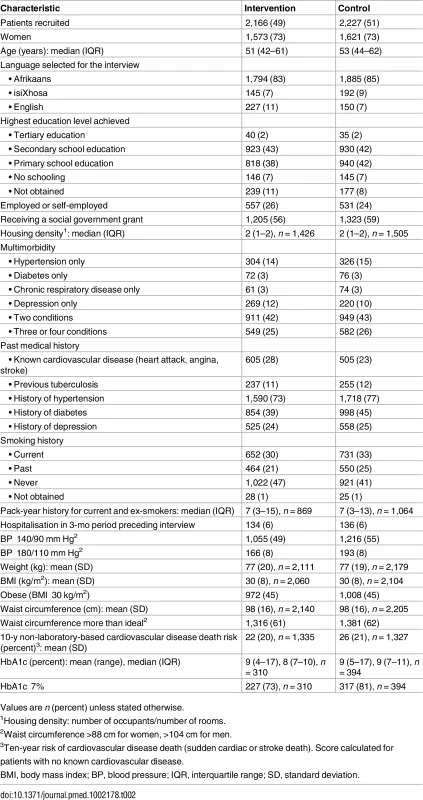
Values are n (percent) unless stated otherwise. Baseline patient characteristics were generally well balanced between arms. Seventy-three percent of patients were women, and the median age was 52 y. There were high levels of unemployment and receipt of social welfare grants. Multimorbidity was common: 42% of patients had two conditions, and 26% more than two. The percentage of patients with a single condition of interest was as follows: hypertension, 20% (630 of 3,227); depression, 20% (489 of 2,466); diabetes, 8% (148 of 1,842); and chronic respiratory disease, 12% (135 of 1,157). A quarter of patients reported established cardiovascular disease. Eleven percent reported previous TB, and 2% reported being on ART. There were signs of under-treatment and under-diagnosis, with 18% of hypertensive patients reporting no or only one current antihypertensive medication, only 51% of diabetic patients receiving statins, only 50% of those with chronic respiratory disease or symptoms receiving any respiratory medication, and only 25% of those who screened positive for depression reporting some form of relevant treatment for the condition.
There was poor control of hypertension and diabetes despite treatment: blood pressure was ≥140/90 mm Hg in 59% of hypertensive patients, and HbA1c was ≥7% in 77% of those with diabetes in whom HbA1c was measured at baseline (704/1,842; 38%).
Treatment intensification in the hypertension and diabetes cohorts across both the intervention and control groups was common during the study period (Table 3), slightly favouring the intervention group (44% versus 40% for hypertension and 57% versus 50% for diabetes), although these differences were not significant when adjusted for stratification by sub-district and clustering. For hypertension, the risk ratio (RR) was 1.08 (95% CI 0.94 to 1.24; p = 0.252); for diabetes, the RR was 1.10 (95% CI 0.97 to 1.24; p = 0.126). Rates of treatment intensification in the chronic respiratory disease cohort were low (14% in the intervention group versus 12% in the control group) and not significantly different between groups (RR 1.08; 95% CI 0.75 to 1.55; p = 0.674). Fewer patients who screened positive for depression in the intervention group reported receiving treatment for depression at follow-up than their control group counterparts (18% versus 24%), but there was no difference between groups after adjustment for the trial’s design (RR 0.76; 95% CI 0.53 to 1.10; p = 0.142). Adjustment for baseline characteristics (Table 2) did not materially alter these results. The full regression models are presented in Table D in S1 Appendix.
Tab. 3. Primary outcomes for each disease cohort. 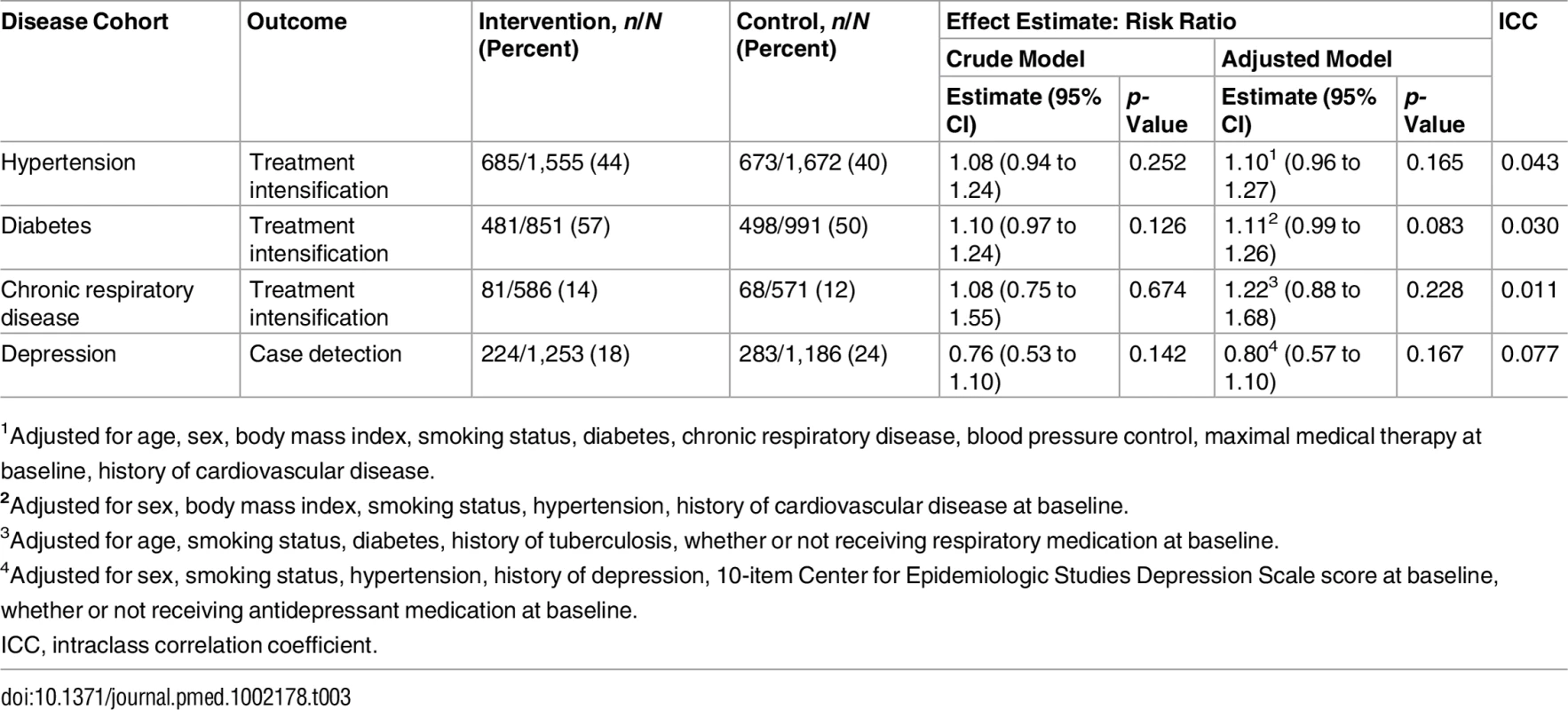
1Adjusted for age, sex, body mass index, smoking status, diabetes, chronic respiratory disease, blood pressure control, maximal medical therapy at baseline, history of cardiovascular disease. Pre-specified subgroup analyses by baseline level of disease control (Table 4) showed that, in the diabetic cohort, the intervention was associated with treatment intensification only among patients with baseline HbA1c of 7%–10% (RR 1.30; 95% CI 1.16 to 1.47; p-value for interaction = 0.010). In the other cohorts, there were no significant differences in effectiveness between subgroups. However, treatment intensification tended to be more common, in both arms, in subgroups with poorer control at baseline. The non-significant difference in depression treatment, which favoured the control group, was mostly among those already receiving treatment for depression at baseline.
Tab. 4. Subgroup analyses: primary outcomes stratified by level of disease control at baseline. 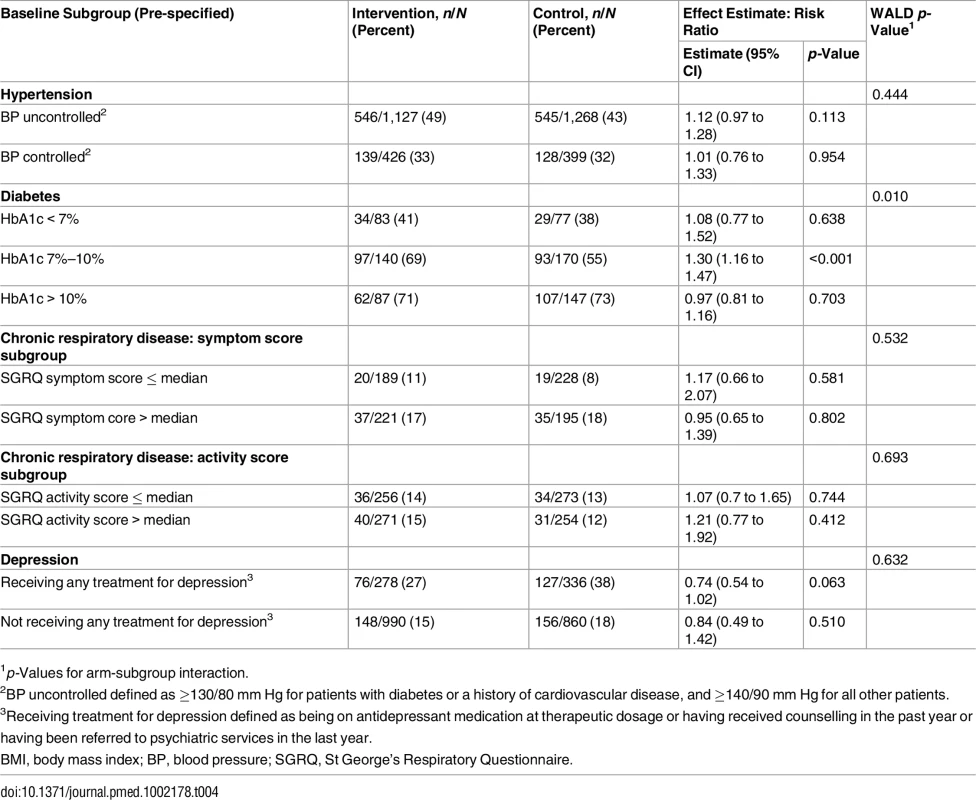
1p-Values for arm-subgroup interaction. Disaggregated primary outcomes are presented in Table E in S1 Appendix. Notable findings include apparently significantly higher rates of aspirin initiation among patients with hypertension and diabetes attending intervention clinics, even though aspirin prescribing was restricted to doctors. Angiotensin-converting enzyme (ACE) inhibitor use was significantly higher among intervention group patients with known cardiovascular disease, as was sulphonylurea use among intervention group diabetic patients with BMI ≥ 30 kg/m2. In the depression cohort, the higher rate of depression treatment in the control arm was because more control group patients reported receiving counselling (15% in the intervention arm versus 22% in the control arm) and referral to psychiatric services (5% in the interventional arm versus 9% in the control arm). There was no significant difference between groups in the use of antidepressants, which was very low (<5%).
Table 5 reports differences in cardiovascular risk factors between baseline and follow-up. There were no differences between groups in terms of blood pressure, waist circumference, BMI, or HbA1c. Smoking quit rates were high overall, but similar between groups. However, readiness to quit smoking was significantly higher in the intervention group (odds ratio 1.73; 95% CI 1.17 to 2.57).
Tab. 5. Effect on cardiovascular disease risk and risk factors; all four cohorts pooled. 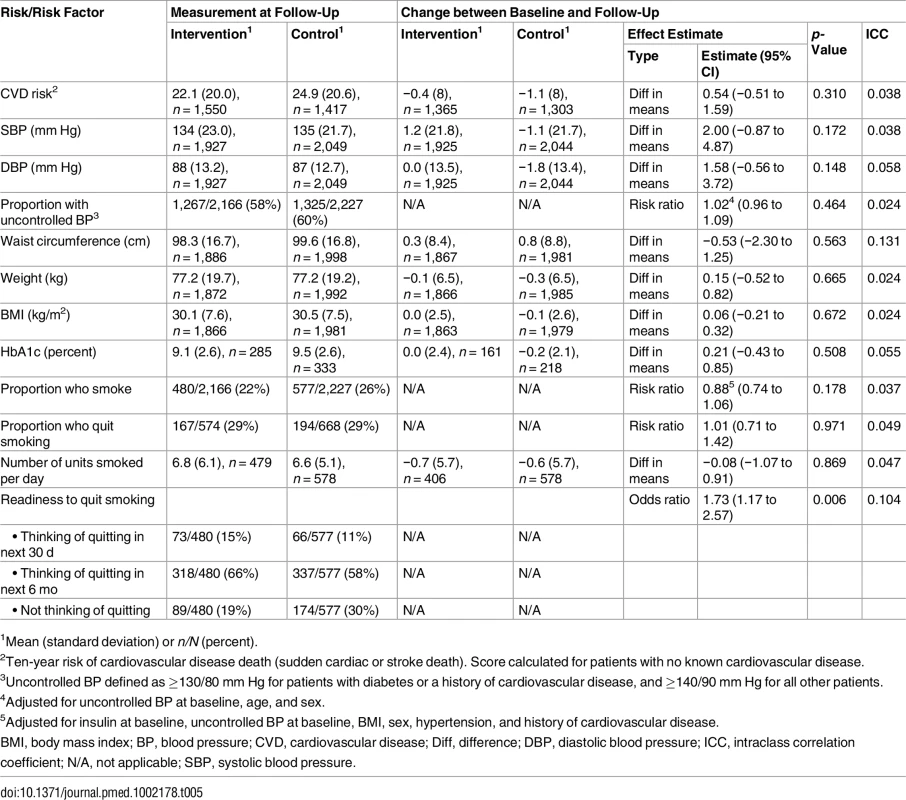
1Mean (standard deviation) or n/N (percent). There were no differences between groups in health outcomes measured with the EuroQol-5D [55], CESD-10 [32], or World Health Organization Disability Assessment Schedule 2.0 [56] (Table 6). Mortality did not differ between groups (Table 6). Healthcare utilisation, as measured by clinic visits and hospital admissions during the 3 mo before the follow-up visit, was similar between groups, but there was a statistically non-significant higher number of hospital admissions in the intervention group (Table 7).
Tab. 6. Effect on quality of life, depression, and mortality. 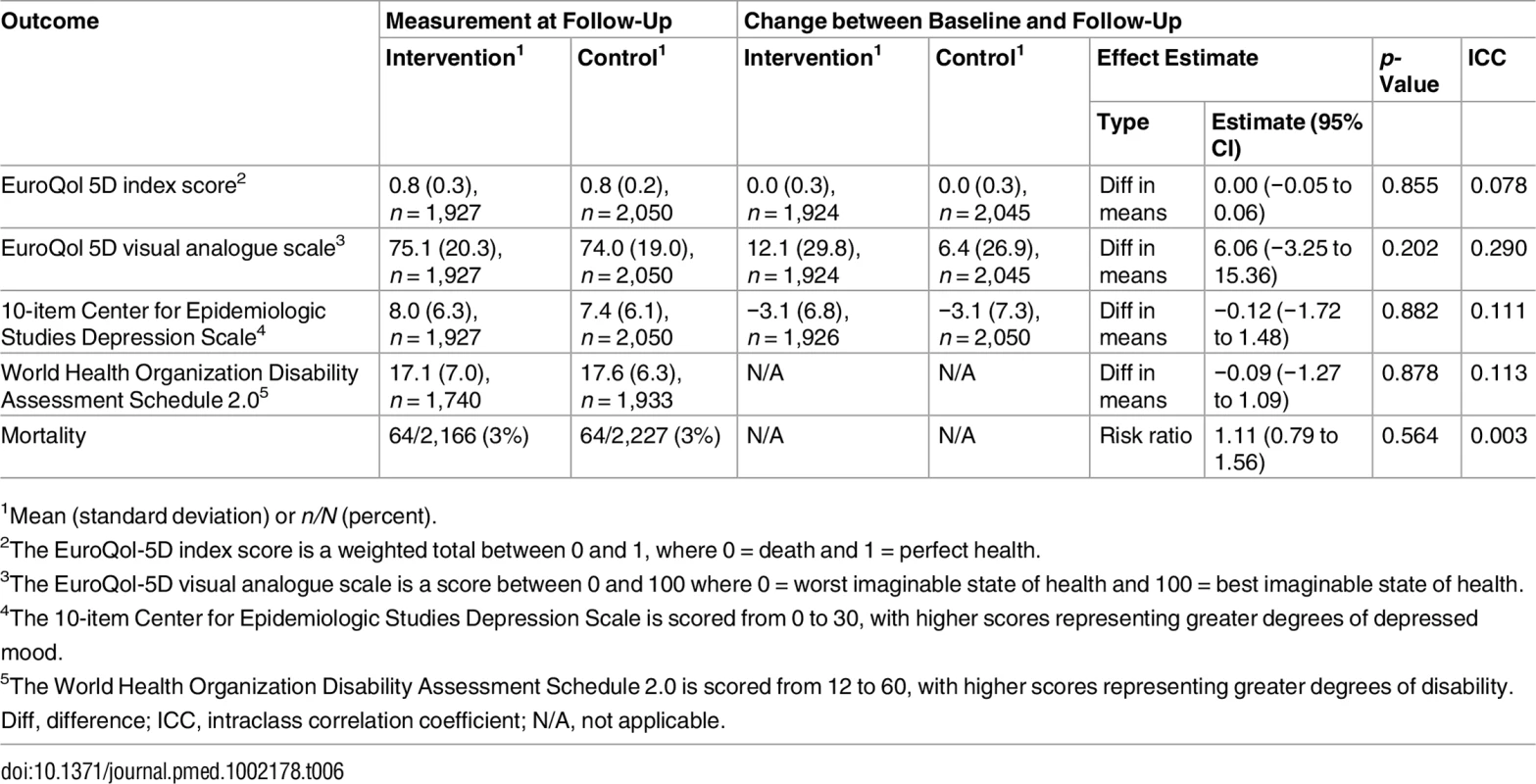
1Mean (standard deviation) or n/N (percent). Tab. 7. Effect on healthcare utilisation. 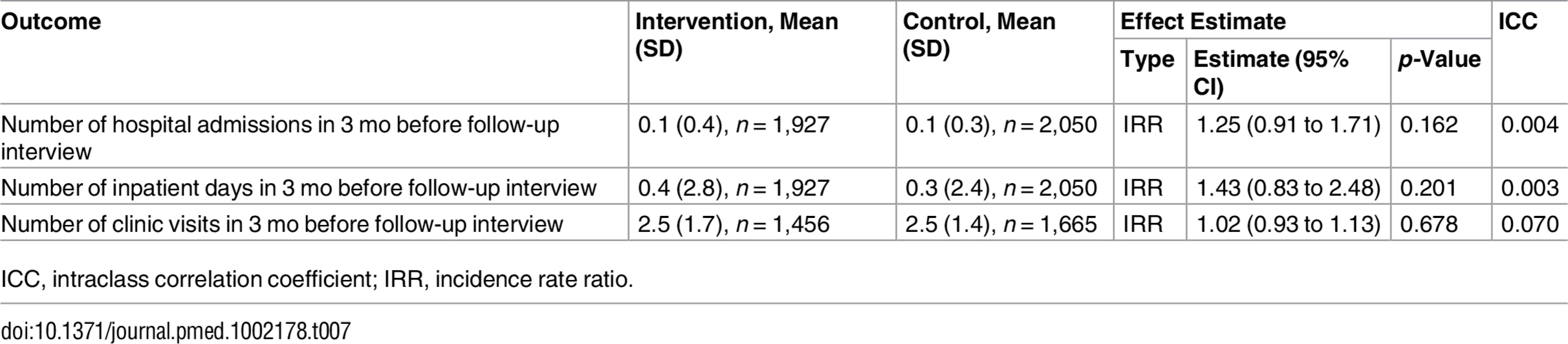
ICC, intraclass correlation coefficient; IRR, incidence rate ratio. Discussion
This paper reports our evaluation of the clinical effectiveness of a complex health systems intervention, based on task-shifting by adding nurse-led NCD and depression care to a proven effective, and scalable, integrated care model for nurse-led care of communicable diseases, in the context of limited availability of physicians to treat a high burden of multimorbid and poorly controlled NCDs in a middle-income country.
The primary analyses found no statistically significant effects of the intervention on the primary outcomes for any of the four disease cohorts. These cohorts were analysed separately, equivalent to four parallel trials; adjustment for having four primary outcomes instead of one would only have decreased statistical differences. Health status outcomes also did not differ between the intervention and control groups. But neither was there evidence of harm for any of these endpoints, or in terms of reduced well-being or excess hospitalisations or deaths. In addition, the intervention was not associated with higher healthcare utilisation at the primary care or hospital level. A pre-planned subgroup analysis by baseline level of diabetes control showed a benefit of the intervention in the subgroup of patients with moderately uncontrolled diabetes (HbA1c 7%–10% at baseline), but the two other pre-specified subgroup analyses (for hypertension and depression by baseline level of disease control) did not show a significant difference between groups.
While no primary outcomes showed a significant benefit of the intervention, the upper confidence limits included the possibility of meaningful clinical improvements, and the direction of results in three of the four primary endpoints in the study was consistent and positive. Also, the pre-specified secondary analysis of patients with diabetes and uncontrolled HbA1c measurements at baseline demonstrated a positive effect. After disaggregation of the disease groups, other significant findings were higher rates of aspirin initiation among patients with hypertension and diabetes, higher use of ACE inhibitors in patients with known cardiovascular disease, and more prescriptions of sulphonylureas in patients with diabetes and a high BMI (Table E in S1 Appendix).
The non-significant findings for the primary outcomes contrast with positive findings in our three previous pragmatic randomised controlled trials using a similar integrated management tool and the same training approach, focused on a narrower range of mainly communicable conditions [9–15,30,42,59]. These trials showed modest, but consistent, improvements across a range of process indicators and health and healthcare utilisation outcomes.
There are several potential reasons for the non-significant findings on the primary outcomes of our study. One is the level of uptake of PC101 into daily clinical practice. Owing to limited research funding, a complete and suitably detailed process evaluation of the uptake of PC101 into clinical practice was not possible. However, limited focus group discussions and observations in clinics by members of the research team confirmed heterogeneous uptake of PC101 within and between clinics, as might be expected in a pragmatic trial intervention. Overall low levels of uptake would seem unlikely, given the enthusiastic response and high uptake of the method by clinic staff reported in our previous implementation studies with the PALSA PLUS management tool [12,42]. Other factors should be considered, such as training. The addition of NCD care to the training programme may have proved a step too far—the content of the PC101 management tool was twice as substantial as that of the PALSA PLUS tool—and potentially overwhelming for nurses who were still learning to implement nurse-initiated and -managed antiretroviral treatment when the trial started. Furthermore, NCDs have long been managed by nurses in primary care clinics throughout South Africa, albeit with minimal training or intervention. As seen in the baseline characteristics, poor NCD care may have become entrenched, and markers of poor disease control routinely ignored [60]. The challenge of “undoing” these clinical habits and effecting a change in clinical behaviour is well described and may take repeated training sessions to achieve. Although training was provided throughout the trial, the comprehensive nature of PC101 made it difficult to cover the curriculum for NCDs sufficiently within the time frame of the study. Owing to limited research funding, but consistent with a pragmatic trial design, formal assessments of adequacy of training and uptake (use) of PC101 were not performed.
A further potential reason for the failure to show differences between groups was the effect of a co-intervention, the concurrent Chronic Disease Season campaign, instituted by the clinic managers in both control and intervention clinics. The impact of this unforeseen development is seen in the higher rates of treatment intensification for hypertension and diabetes (the focus of the campaign) than for chronic respiratory disease or depressive symptoms in both the intervention and control clinics. Whereas only 13% of patients with chronic respiratory disease and 3% of those with depression had medication intensified at follow-up, nearly half of those with hypertension and diabetes had intensified treatment (42% and 53%, respectively). These rates of intensification of antihypertensive and diabetic medications are similar to or slightly higher than those reported in high-income country settings [50,58].
Another consideration concerns methodology. We recruited all patients with the diseases of interest rather than only those requiring treatment intensification, and failed to assess adherence and exclude patients who did not adhere to previously prescribed medications and who might therefore have been less likely to have been prescribed additional treatment. However, the eligibility criteria were adopted on the assumption that decision-makers wanted evidence of effectiveness of the intervention across broad groups of patients, rather than for subgroups, and that, as lack of disease control was highly prevalent at baseline, the majority of patients would qualify for treatment intensification.
Other limitations of the study design include dependence on self-reported diagnoses for inclusion in the patient cohorts, reliance on process outcomes, and insufficient resources to measure important health outcomes, such as HbA1c, at follow-up. Also, the duration and timing of the follow-up data collection might not have been optimal for a study of chronic diseases, where follow-up visits being only every 3–6 mo limited opportunities for treatment intensification. This is illustrated by the low number of clinic visits during the follow-up period, a mean of around 2.5 per patient over a period of 14 mo (Table 7).
The main strength of the study was that it was a pragmatic trial, implemented under routine circumstances in a real-world setting with the intervention delivered by usual health department trainers, with minimal research-related distortions of care delivery. Observing this real-world implementation appears to have given relevant policy-makers sufficient confidence to make a decision on the suitability of the intervention for their health systems. Other strengths of the study include the cluster randomised design (appropriate to reduce the risk of contamination in an intervention directed at groups of nurses working in clinics), high follow-up rates for both patient interviews and prescription data, the inclusion of four different chronic diseases in a context characterised by high rates of multimorbidity, and identification and follow-up of patient participants by fieldworkers independent of clinical care.
So what are the implications of the trial for decision-makers in South Africa and other LMICs who are faced with overstretched health services and the need to address NCDs and mental health? In October 2013, even before the trial results were finalised, decision-makers were increasingly enthusiastic about the PC101 intervention, and both the Western Cape Department of Health and the National Department of Health in South Africa elected to commence implementation. Later dissemination of the trial findings on the effectiveness of this intervention to these local and national policy-makers did not change this decision. The decision, we were told, was much more influenced by demand from frontline clinicians and managers for what was perceived to be a highly feasible and acceptable approach to expanding skills for NCDs. Further factors that may have influenced decision-makers were the benefits of the new mode of clinician training reported in our prior studies [9,13,15], an independent report supporting the integrated Chronic Care Model as a feasible component of health system reform in South Africa [61], and the findings of a non-randomised evaluation of PC101 performed in 42 primary care clinics in three additional health districts [62]. The PC101 management tool is correctly seen as a means of overcoming the “silo” approach to individual disease management in which recommendations for different conditions may vary and even conflict and, more importantly, ensures that NCDs and mental health are not overlooked because of prioritisation of communicable diseases. For us, as researchers who look to rigorous research methods to guide health system development, this has been a powerful lesson in understanding that evidence of effectiveness is only one element under consideration by decision-makers [63]. Given clinicians’ strong attraction to the ease of integrating PC101 into clinic practice and the positive system effects of our intervention mentioned above, it might have been more useful to focus our primary analysis on lack of harm. For example, the study was not powered to test for differences in healthcare utilisation and reasons for referrals and hospitalisations. Thus, it is not possible to evaluate the significance of the small imbalance in numbers of hospital admissions between the intervention group and the control group, since an increase in hospitalisations reflecting more appropriate referrals from primary care may be interpreted as favourable rather than as a treatment failure. Specifically designed trials are required.
We now consider that it is our responsibility as health system researchers to invest in improving the effectiveness of this intervention. There are patterns in the data from the trial that provide reassurance that the intervention is not harmful and that, with further optimisation, might demonstrate improvements in effectiveness. Several adjustments have been made to the programme that is being scaled up with the aim of increasing its impact on skills, clinician confidence, and quality of care. The PC101 content has been broken down into four training modules (communicable diseases, NCDs, mental health, and women’s health) to allow staff to become familiar with one area at a time and embed changes into their clinical practice before moving to the next. We now also explicitly aim PC101 training at doctors, through dedicated workshops for professionals who would otherwise miss regular onsite training due to the sessional nature of their work. Implementation workshops, with an extra day aimed at meeting the needs of facility and middle managers, are included in the training of nurse trainers, and appointment of clinical governance teams within sub-districts allows local troubleshooting of barriers to implementation and inclusion of non-clinicians in the day-to-day running of the programme. A further cluster randomised trial in the North West province of South Africa (ClinicalTrials.gov NCT02407691) is currently evaluating the effect of the mental health module when combined with the provision of manualised depression counselling by lay health workers delivered to ART patients with co-morbid depression. A second study is evaluating this mental health module in patients with hypertension and co-morbid depression [64]. This expansion of human resources to include lay health workers is based on our experience from the PC101 trial that nurse training alone is insufficient to close the gap in depression care when there is limited access to treatment in the form of counselling services or antidepressant prescriptions (prescribing currently restricted to doctors).
Although it will not be possible to conduct another randomised controlled trial of the adapted PC101 implementation as it is scaled up, we plan to conduct such trials for future national and international adaptations of this programme [17]. Ease of implementability appears to be a major feature for policy-makers, and we will include proxies, such as acceptability to frontline clinicians, as outcome measures in future trials.
In conclusion, this pragmatic cluster randomised trial of the effects of an integrated management tool implemented using educational outreach to nurses showed no effect on treatment intensification in patients with NCDs or on case detection of depression. But neither was there evidence of harm. Despite this lack of positive clinical outcomes, decision-makers were disposed to view PC101 as a coherent, feasible, and acceptable extension of a programme of integrated care previously shown to be effective in the South African health system, and health authorities have committed to a national rollout of an improved version of the PC101 programme. The disjuncture between the clinical outcomes of our study and the policy choice exposes the different responsibilities of researchers and decision-makers in a health system. For us, as intervention developers, this focuses our attention on longer term improvements to strengthen components of the programme in order to achieve clinical impact on care for NCDs, while, as evaluators, we see the need for ongoing audit and further randomised pragmatic controlled trials to evaluate the effectiveness of these improvements.
Health systems research and development is an interactive and deliberative process. Perhaps the greatest contribution of this study lies in the relationships developed between our team and health system decision-makers, during a series of five large randomised evaluations of health systems interventions that responded to decision-maker-defined health systems needs over 16 years [17]. To this process we have each brought our different skills and perspectives, and together have developed, and are scaling up, an iteratively improved, evidence-informed approach to nurse-led primary care that strengthens human resources and health systems, and brings better care to South Africans, as well as models that can be applied in other low - and middle-income country settings.
Supporting Information
Zdroje
1. Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374 : 934–47. doi: 10.1016/S0140-6736(09)61087-4 19709736
2. Bradshaw D, Dorrington RE, Laubscher R. Rapid mortality surveillance report 2011. Cape Town: South African Medical Research Council; 2012.
3. Rehle TM, Hallett TB, Shisana O, Pillay-van Wyk V, Zuma K, Carrara H, et al. A decline in new HIV Infections in South Africa: estimating HIV incidence from three national HIV surveys in 2002, 2005 and 2008. PLoS ONE. 2010;5:e11094. doi: 10.1371/journal.pone.0011094 20559425
4. Bradshaw D, Groenewald P, Laubscher R, Nannan N, Nojilana B, Norman R, et al. Initial burden of disease estimates for South Africa, 2000. S Afr Med J. 2003;93 : 682–88. 14635557
5. Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Matzopoulos R, et al. Second national burden of disease study South Africa: national and subnational mortality trends, 1997–2009. Lancet. 2013;381:S113.
6. Mash B, Fairall L, Adejayan O, Ikpefan O, Kumari J, Mathee S, et al. A morbidity survey of South African primary care. PLoS ONE. 2012;7:e32358. doi: 10.1371/journal.pone.0032358 22442666
7. Steyn K, Levitt NS, Patel M, Fourie J, Gwebushe N, Lombard C, et al. Hypertension and diabetes: poor care for patients at community health centres. S Afr Med J. 2008;98 : 618–22. 18928041
8. Williams DR, Herman A, Stein DJ, Heeringa SG, Jackson PB, Moomal H, et al. Twelve-month mental disorders in South Africa: prevalence, service use and demographic correlates in the population-based South African Stress and Health Study. Psychol Med. 2008;38 : 211–20. doi: 10.1017/S0033291707001420 17903333
9. Fairall L, Bachmann MO, Lombard C, Timmerman V, Uebel K, Zwarenstein M, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380 : 889–98. doi: 10.1016/S0140-6736(12)60730-2 22901955
10. Colvin CJ, Fairall L, Lewin S, Georgeu D, Zwarenstein M, Bachmann MO, et al. Expanding access to ART in South Africa: the role of nurse-initiated treatment. S Afr Med J. 2010;100 : 210–2. 20459957
11. Uebel KE, Fairall LR, van Rensburg DH, Mollentze WF, Bachmann MO, Lewin S, et al. Task shifting and integration of HIV care into primary care in South Africa: the development and content of the streamlining tasks and roles to expand treatment and care for HIV (STRETCH) intervention. Implement Sci. 2011;6 : 86. doi: 10.1186/1748-5908-6-86 21810242
12. Georgeu D, Colvin CJ, Lewin S, Fairall L, Bachmann MO, Uebel K, et al. Implementing nurse-initiated and managed antiretroviral treatment (NIMART) in South Africa: a qualitative process evaluation of the STRETCH trial. Implement Sci. 2012;7 : 66. doi: 10.1186/1748-5908-7-66 22800379
13. Zwarenstein M, Fairall LR, Lombard C, Mayers P, Bheekie A, English RG, et al. Outreach education for integration of HIV/AIDS care, antiretroviral treatment, and tuberculosis care in primary care clinics in South Africa: PALSA PLUS pragmatic cluster randomised trial. BMJ. 2011;342:d2022. doi: 10.1136/bmj.d2022 21511783
14. Barton GR, Fairall L, Bachmann MO, Uebel K, Timmerman V, Lombard C, et al. Cost-effectiveness of nurse-led versus doctor-led antiretroviral treatment in South Africa: pragmatic cluster randomised trial. Trop Med Int Health. 2013;18 : 769–77. doi: 10.1111/tmi.12093 23480523
15. Fairall LR, Zwarenstein M, Bateman ED, Bachmann MO, Lombard C, Majara BP, et al. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. BMJ. 2005;331 : 750–4. doi: 10.1136/bmj.331.7519.750 16195293
16. Department of Health. National Department of Health strategic plan 2010/11–2012/13: Pretoria: Department of Health; 2010 [cited 2016 Oct 19]. Available from: http://www.nationalplanningcycles.org/sites/default/files/country_docs/South%20Africa/south_africa_strategic_health_plan_2010-2013.pdf.
17. Fairall L, Bateman E, Cornick R, Faris G, Timmerman V, Folb N, et al. Innovating to improve primary care in less developed countries: towards a global model. BMJ Innov. 2015;1 : 196–203. doi: 10.1136/bmjinnov-2015-000045 26692199
18. Coventry PA, Small N, Panagioti M, Adeyemi I, Bee P. Living with complexity; marshalling resources: a systematic review and qualitative meta-synthesis of lived experience of mental and physical multimorbidity. BMC Fam Pract. 2015;16 : 171. doi: 10.1186/s12875-015-0345-3 26597934
19. Déruaz-Luyet A, N’Goran AA, Tandjung R, Frey P, Zeller A, Haller DM, et al. Multimorbidity in primary care: protocol of a national cross-sectional study in Switzerland. BMJ Open. 2015;5:e009165. doi: 10.1136/bmjopen-2015-009165 26510730
20. Foguet-Boreu Q, Violán C, Rodriguez-Blanco T, Roso-Llorach A, Pons-Vigués M, Pujol-Ribera E, et al. Multimorbidity patterns in elderly primary health care patients in a south Mediterranean European region: a cluster analysis. PLoS ONE. 2015;10:e0141155. doi: 10.1371/journal.pone.0141155 26524599
21. Luijks H, Lucassen P, van Weel C, Loeffen M, Lagro-Janssen A, Schermer T. How GPs value guidelines applied to patients with multimorbidity: a qualitative study. BMJ Open. 2015;5:e007905. doi: 10.1136/bmjopen-2015-007905 26503382
22. Moffat K, Mercer SW. Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam Pract. 2015;16 : 129. doi: 10.1186/s12875-015-0344-4 26462820
23. Nicholson K, Terry A, Fortin M, Williamson T, Thind A. Understanding multimorbidity in primary health care. Can Fam Physician. 2015;61 : 918. 26472799
24. Laurant M, Harmsen M, Wollersheim H, Grol R, Faber M, Sibbald B. The impact of nonphysician clinicians: do they improve the quality and cost-effectiveness of health care services? Med Care Res Rev. 2009;66(6 Suppl):36S–89S. doi: 10.1177/1077558709346277 19880672
25. Labhardt ND, Balo J-R, Ndam M, Grimm J-J, Manga E. Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: a programme assessment at two years. BMC Health Serv Res. 2010;10 : 339. doi: 10.1186/1472-6963-10-339 21144064
26. Kengne AP, Awah PK, Fezeu LL, Sobngwi E, Mbanya J-C. Primary health care for hypertension by nurses in rural and urban sub-Saharan Africa. J Clin Hypertens (Greenwich). 2009;11 : 564–72.
27. Coleman R, Gill G, Wilkinson D. Noncommunicable disease management in resource-poor settings: a primary care model from rural South Africa. Bull World Health Organ. 1998;76 : 633–40. 10191559
28. Gill GV, Price C, Shandu D, Dedicoat M, Wilkinson D. An effective system of nurse-led diabetes care in rural Africa. Diabet Med. 2008;25 : 606–11. doi: 10.1111/j.1464-5491.2008.02421.x 18445175
29. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655 18824488
30. English RG, Bateman ED, Zwarenstein MF, Fairall LR, Bheekie A, Bachmann MO, et al. Development of a South African integrated syndromic respiratory disease guideline for primary care. Prim Care Respir J. 2008;17 : 156–63. doi: 10.3132/pcrj.2008.00044 18701971
31. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1 : 385–401.
32. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10 : 77–84. 8037935
33. Myer L, Smit J, Roux LL, Parker S, Stein DJ, Seedat S. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STDS. 2008;22 : 147–58. doi: 10.1089/apc.2007.0102 18260806
34. Zhang W, O’Brien N, Forrest JI, Salters KA, Patterson TL, Montaner JSG, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS ONE. 2012;7:e40793. doi: 10.1371/journal.pone.0040793 22829885
35. Miller WC, Anton HA, Townson AF. Measurement properties of the CESD scale among individuals with spinal cord injury. Spinal Cord. 2008;46 : 287–92. doi: 10.1038/sj.sc.3102127 17909558
36. Cheung YB, Liu KY, Yip PSF. Performance of the CES-D and its short forms in screening suicidality and hopelessness in the community. Suicide Life Threat Behav. 2007;37 : 79–88. doi: 10.1521/suli.2007.37.1.79 17397282
37. O’Connor PJ, Desai J, Solberg LI, Reger LA, Crain AL, Asche SE, et al. Randomized trial of quality improvement intervention to improve diabetes care in primary care settings. Diabetes Care. 2005;28 : 1890–7. 16043728
38. Grant R, Adams AS, Trinacty CM, Zhang F, Kleinman K, Soumerai SB, et al. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care. 2007;30 : 807–12. doi: 10.2337/dc06-2170 17259469
39. Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372 : 902–9. doi: 10.1016/S0140-6736(08)61400-2 18790313
40. Zwarenstein M, Treweek S, Gagnier J, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. doi: 10.1136/bmj.a2390 19001484
41. Department of Health. Standard treatment guidelines and essential medicines list for South Africa: Primary Health Care. Pretoria: Department of Health; 2008.
42. Stein J, Lewin S, Fairall L, Mayers P, English R, Bheekie A, et al. Building capacity for antiretroviral delivery in South Africa: a qualitative evaluation of the PALSA PLUS nurse training programme. BMC Health Serv Res. 2008;8 : 240. doi: 10.1186/1472-6963-8-240 19017394
43. Sanne I, Orrell C, Fox M, Conradie F, Ive P, Zeinecker J, et al. Nurse management is not inferior to doctor management of antiretroviral patients: the CIPRA South Africa randomised trial. Lancet. 2010;376 : 33–40. doi: 10.1016/S0140-6736(10)60894-X 20557927
44. O’Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;4:CD000409.
45. Van Der Stuyf RR. Scaffolding as a teaching strategy. SlideShare; 2002 [cited 2016 Oct 19]. Available from: http://www.slideshare.net/valms1/vygotsky-17272530.
46. Swinton L. Kolb’s Learning Style Inventory and Kolb’s Learning Cycle explained—no fluff, no filler, just facts. Mftrou.com; 2016 [cited 2016 Oct 19]. Available from: http://www.mftrou.com/kolb-learning-style-inventory.html.
47. Folb N, Timmerman V, Levitt NS, Steyn K, Bachmann MO, Lund C, et al. Multimorbidity, control and treatment of non-communicable diseases among primary healthcare attenders in the Western Cape, South Africa. S Afr Med J. 2015;105 : 642–7. 26449692
48. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31.
49. Van Bruggen R, Gorter K, Stolk R, Klungel O, Rutten G. Clinical inertia in general practice: widespread and related to the outcome of diabetes care. Fam Pract. 2009;26 : 428–36. doi: 10.1093/fampra/cmp053 19729401
50. Schmittdiel JA, Uratsu CS, Karter AJ, Heisler M, Subramanian U, Mangione CM, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23 : 588–94. doi: 10.1007/s11606-008-0554-8 18317847
51. Berlowitz DR, Ash AS, Hickey EC, Friedman RH, Glickman M, Kader B, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339 : 1957–63. doi: 10.1056/NEJM199812313392701 9869666
52. Berlowitz DR, Ash AS, Glickman M, Friedman RH, Pogach LM, Nelson AL, et al. Developing a quality measure for clinical inertia in diabetes care. Health Serv Res. 2005;40 : 1836–53. doi: 10.1111/j.1475-6773.2005.00436.x 16336551
53. Carter BL, Bergus GR, Dawson JD, Farris KB, Doucette WR, Chrischilles EA, et al. A cluster-randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10 : 260–71.
54. Seedat S, Stein DJ, Herman A, Kessler R, Sonnega J, Heeringa S, et al. Twelve-month treatment of psychiatric disorders in the South African Stress and Health Study (World Mental Health Survey Initiative). Soc Psychiatry Psychiatr Epidemiol. 2008;43 : 889–97. doi: 10.1007/s00127-008-0399-9 18677573
55. Bachmann MO, Louwagie G, Fairall L. Quality of life and financial measures in HIV/AIDS in Southern Africa. In: Preedy VR, Watson RR, editors. Handbook of disease burdens and quality of life measures. New York: Springer Science + Business Media; 2010. p. 3223–3243.
56. Garin O, Ayuso-Mateos JL, Almansa J, Nieto M, Chatterji S, Vilagut G, et al. Validation of the ‘World Health Organization Disability Assessment Schedule, WHODAS-2’ in patients with chronic diseases. Health Qual Life Outcomes. 2010;19 : 51.
57. Sheikh A, Panesar SS, Larizgoitia I, Bates DW, Donaldson LJ. Safer primary care for all: a global imperative. Lancet Glob Health. 2013;1:e182–3. doi: 10.1016/S2214-109X(13)70030-5 25104342
58. Billue KL, Safford MM, Salanitro AH, Houston TK, Curry W, Kim Y, et al. Medication intensification in diabetes in rural primary care: a cluster-randomised effectiveness trial. BMJ Open. 2012;2:e000959. doi: 10.1136/bmjopen-2012-000959 22991217
59. Fairall L, Bachmann MO, Zwarenstein M, Bateman ED, Niessen LW, Lombard C, et al. Cost-effectiveness of educational outreach to primary care nurses to increase tuberculosis case detection and improve respiratory care: economic evaluation alongside a randomised trial. Trop Med Int Health. 2010;15 : 277–86. doi: 10.1111/j.1365-3156.2009.02455.x 20070633
60. Lewin S, Green J. Ritual and the organisation of care in primary care clinics in Cape Town, South Africa. Soc Sci Med. 2009;68 : 1464–71. doi: 10.1016/j.socscimed.2009.02.013 19278764
61. Mahomed OH, Asmall S. Development and implementation of an integrated chronic disease model in South Africa: lessons in the management of change through improving the quality of clinical practice. Int J Integr Care. 2015;15:e038. 26528101
62. Mahomed OH, Naidoo S, Asmall S, Taylor M. Evaluation of PC 101 training for professional nurses in 3 districts in South Africa. Pretoria: National Department of Health; 2015.
63. Kruk ME, Yamey G, Angell SY, Beith A, Cotlear D, Guanais F, et al. Transforming global health by improving the science of scale-up. PLoS Biol. 2016;14:e1002360. doi: 10.1371/journal.pbio.1002360 26934704
64. Lund C, Tomlinson M, De Silva M, Fekadu A, Shidhaye R, Jordans M, et al. PRIME: a programme to reduce the treatment gap for mental disorders in five low - and middle-income countries. PLoS Med. 2012;9:e1001359. doi: 10.1371/journal.pmed.1001359 23300387
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Effectiveness of Seasonal Malaria Chemoprevention in Children under Ten Years of Age in Senegal: A Stepped-Wedge Cluster-Randomised Trial
- Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial
- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Projected Impact of Mexico’s Sugar-Sweetened Beverage Tax Policy on Diabetes and Cardiovascular Disease: A Modeling Study
- Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050?
- Educational Outreach with an Integrated Clinical Tool for Nurse-Led Non-communicable Chronic Disease Management in Primary Care in South Africa: A Pragmatic Cluster Randomised Controlled Trial
- Measures of Malaria Burden after Long-Lasting Insecticidal Net Distribution and Indoor Residual Spraying at Three Sites in Uganda: A Prospective Observational Study
- Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults
- Under-prescribing of Prevention Drugs and Primary Prevention of Stroke and Transient Ischaemic Attack in UK General Practice: A Retrospective Analysis
- The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
- Improving the Pipeline for Developing and Testing Pharmacological Treatments in Pregnancy
- Seasonal Malaria Chemoprevention: An Evolving Research Paradigm
- Exposure Patterns Driving Ebola Transmission in West Africa: A Retrospective Observational Study
- Minimally Invasive Autopsy: A New Paradigm for Understanding Global Health?
- Towards Equity in Health: Researchers Take Stock
- Willingness to Know the Cause of Death and Hypothetical Acceptability of the Minimally Invasive Autopsy in Six Diverse African and Asian Settings: A Mixed Methods Socio-Behavioural Study
- Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels
- Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis
- Validity of a Minimally Invasive Autopsy for Cause of Death Determination in Adults in Mozambique: An Observational Study
- The Dengue Vaccine Dilemma: Balancing the Individual and Population Risks and Benefits
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



