-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStrategies to Prevent Cholera Introduction during International Personnel Deployments: A Computational Modeling Analysis Based on the 2010 Haiti Outbreak
A cholera outbreak modeling study reveals that chemprophylaxis treatment for deployed peacekeepers in Haiti would have reduced the risk of disease introduction by 90%.
Published in the journal: . PLoS Med 13(1): e32767. doi:10.1371/journal.pmed.1001947
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001947Summary
A cholera outbreak modeling study reveals that chemprophylaxis treatment for deployed peacekeepers in Haiti would have reduced the risk of disease introduction by 90%.
Introduction
An estimated 1.4 billion people are at risk for cholera in countries across Africa, Asia, and South and Central America where transmission is endemic [1]. In addition, explosive epidemics can occur when cholera is introduced to non-endemic populations. One of the most severe cholera epidemics of the modern era began in Haiti in 2010, causing over 700,000 reported cases and nearly 9,000 deaths to date [2]. Prior to the outbreak, cholera had been absent from Haiti for over a century [3]. Several pieces of evidence have contributed to widespread acceptance that the epidemic resulted from contamination of the Artibonite watershed with infected sewage from a United Nations Peacekeeping Mission in Haiti (MINUSTAH) base [4]. The causative Vibrio cholerae strain was imported from Nepal and diverged from strains circulating in that country around the time 454 Nepalese troops were deployed to Haiti [5], and the first cholera cases in Haiti were seen downstream from the base days after troops arrived [6].
Although preventing V. cholerae introduction is paramount for avoiding epidemics, there are no established protocols utilizing biomedical interventions to prevent cholera importation from endemic settings. An independent expert report to the United Nations (UN) following the Haiti outbreak [7] advocated for three pre-deployment interventions to limit transmission risk from peacekeepers: V. cholerae diagnostic screening, prophylaxis with antimicrobial chemotherapies, and immunization using oral cholera vaccines (OCVs). Diagnostic screening and antimicrobial chemoprophylaxis (a controversial control strategy amidst emerging drug resistance [8,9]) aim to decrease the risk of an infected person traveling and shedding V. cholerae on arrival. Antimicrobial drugs can also provide indirect protection against transmission by hastening bacterial clearance or preventing infection among individuals exposed to V. cholerae. OCVs, similarly, confer indirect protection by reducing bacterial output in stool [10,11].
Uncertainty about the extent to which these approaches reduce the risk for epidemic introduction is a contributing factor in the recent decision by the UN against implementing the recommended interventions [12,13]. To provide support for policymakers weighing the benefits of different approaches, we estimated how the proposed interventions would have influenced the likelihood of the 2010 cholera epidemic in Haiti, compared the costs associated with their implementation, and assessed how the various approaches would benefit peacekeepers by reducing their likelihood of suffering cholera.
Methods
Study Design
We compared interventions according to the probability for a symptomatic cholera case to occur in the host community. To estimate this, we developed a stochastic model simulating the arrival of asymptomatically infected peacekeepers (or those with mild illness that would not prevent them from being deployed) and dynamics of cholera transmission from the MINUSTAH base to the community under protocols in place as of October 2010. We used the model to assess the extent to which the proposed interventions alter the likelihood and dynamics of an introduced epidemic. Specifically, we quantified the impact of the interventions on the probability for two events requisite to the establishment of an epidemic: (1) undetected importation of V. cholerae from the endemic source country (Nepal) to Haiti by an asymptomatically infected peacekeeper and (2) transmission of V. cholerae from peacekeepers to the general public.
We provide an expanded description of the methods, including relevant equations, in S1 Text. R (version 3.2.1) scripts for implementing the model and related analyses are publicly available at https://github.com/joelewnard/choleraHaiti.git.
Probability of Cholera Importation
Background incidence rate
The number of peacekeepers who were infected upon arrival at the MINUSTAH base is unknown. We inferred probability distributions for the prevalence of asymptomatic V. cholerae infection and incubation among peacekeepers at time of deployment based on background incidence rates reflecting potential transmission exposures experienced during their 10-d leave period preceding deployment (S1 Text §1.1). The annual, underreporting-adjusted incidence of cholera among Nepalese adults was previously estimated to be 1.8 cases per 1,000 persons [1]. In view of uncertainty associated with such estimates resulting from seasonal and geographic variation in incidence, we additionally modeled scenarios considering background incidence rates of 0.5, 1, 2, 5, and 10 cases annually per 1,000 persons.
Estimating symptom probability in an endemic setting
We inferred the prevalence of asymptomatic infection among peacekeepers via a meta-analysis of six epidemiological field studies that monitored the onset of symptomatic and asymptomatic V. cholerae shedding among household and community contacts of cholera patients (S1 Text §1.2) [14–19]. Since studies reported the number of contacts who experienced symptomatic and asymptomatic infections, the Beta distribution provided a direct way to compare study-level symptom probabilities based on available data; for each study i, we took the symptom probability σi to be Beta-distributed with the parameters αi and βi representing the number of new symptomatic and asymptomatic V. cholerae infections reported among contacts of index cases. The expected values and variances of the log-transformed symptom probability for each study were obtained as
for the digamma function ψ(x), and for the trigamma function ψ1(x). We pooled the log-transformed means in an inverse variance-weighted random effects model to fit the distribution of the population parameter ln(σ) across studies using the metafor (version 1.9–4) package in R [20]. This approach resulted in an estimate that 24.2% (95% credible interval [CrI]: 14.4%, 40.7%) of cholera infections in an endemic setting were symptomatic (S1 Table).Modeling importation
The peacekeeping battalion departed Nepal on October 7, arriving in Haiti on October 8 and entering the MINUSTAH base on October 9 [6,7]. We accounted for natural clearance of infection and intervention effects over the 2 d peacekeepers were in transit when estimating the prevalence of infection upon arrival at the MINUSTAH base. We modeled the time spent incubating and shedding V. cholerae as exponentially-distributed random variables. The probability for an individual peacekeeper to shed V. cholerae following arrival was equivalent to the probability that clearance had not occurred during transit, conditioned on having departed while incubating or asymptomatically shedding V. cholerae (S1 Text §1.3, 1.4).
We calibrated the model to replicate epidemiological observations following the arrival of differing numbers of infected peacekeepers, and we pooled outcome estimates according to a binomial probability distribution for each initial number of infected peacekeepers under the assumed background cholera incidence rates (S1 Text §1.1).
Model Outline
Following peacekeepers’ arrival at a MINUSTAH base that disposed untreated sewage into a tributary of the Artibonite River, the early spread of cholera in central Haiti was mediated by downstream transport of V. cholerae to the lower Artibonite basin. The majority of early cases in this area were among individuals who farmed in rice fields adjacent to the Artibonite, drank untreated water from the river and its canals, and practiced open defecation [6,21,22]. Traditional routes of local interpersonal spread facilitated slower dissemination of the epidemic beyond the Artibonite basin [6,7]. Consequently, we modeled V. cholerae transmission via river-mediated and local pathways, partitioning the population of Haiti into several groups according to transmission exposures. The first group included persons in Artibonite River-adjacent communes situated downstream from the MINUSTAH base, among whom we considered a small subgroup coming into direct contact with the river for water and sanitation needs. The remaining population of Haiti, among whom we assumed no Artibonite exposure, was affected only by local transmission.
Our model tracked a population of susceptible, latently infected, infectious, and recovered persons, as well as an environmental reservoir of V. cholerae in the Artibonite watershed (S1 Text §3.1). We used a quantitative dose-response relation (S1 Text §3.2, 3.4) linking V. cholerae exposure to individuals’ likelihood for infection and symptoms to account for variability in the distribution of symptomatic cases during cholera epidemics [19], exemplified in the Haiti epidemic by the precipitous rise in symptomatic cases 2 wk following peacekeepers’ arrival (Fig 1).
Fig. 1. Epidemiological dynamics. 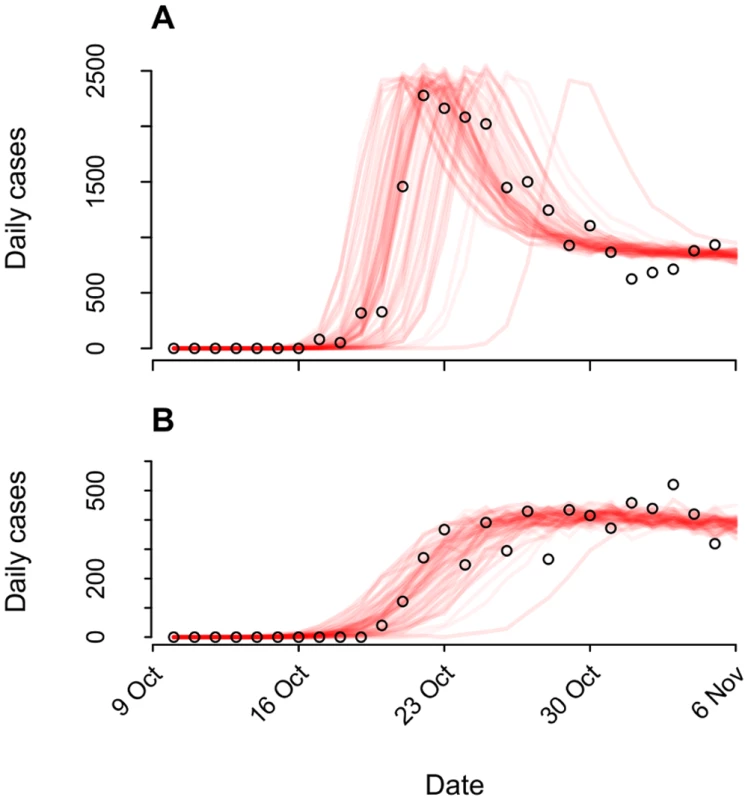
We illustrate model-data concordance by plotting case observations (points) along with sample paths from stochastic realizations of the model (n = 100) under the status-quo scenario used for fitting. We plot instances (n = 79) in which transmission ensued and omit those where no epidemic occurred (n = 21). (A): Cases in the Artibonite-adjacent communes; (B): cases in all other communes. The relative infectiousness of symptomatic and asymptomatic cases in the context of local transmission is a source of uncertainty in cholera modeling [23,24]. Consequently, we calibrated the model using different log-linear relationships between stool output and infectiousness and verified that outcomes were not sensitive to our assumptions (S1 Text §3.4).
Model Calibration
We derived model parameters for cholera natural history and disease based on data from clinical and epidemiological studies (Table 1). We propagated uncertainty in remaining parameters by sampling from possible values using a Bayesian Markov Chain Monte Carlo (MCMC) approach, calibrating model output to data on suspected and confirmed cholera cases from all ambulatory patients, hospital admissions, and deaths in Haiti from 16 October to 5 November 2010 (Table 2; S1 Text §4). The time period comprised the initial outbreak and its immediate spread within and outside the Artibonite Valley, prior to a second phase in the epidemic associated with transmission increases attributed to Hurricane Tomas [25,26].
Tab. 1. Fixed model parameters for cholera progression. 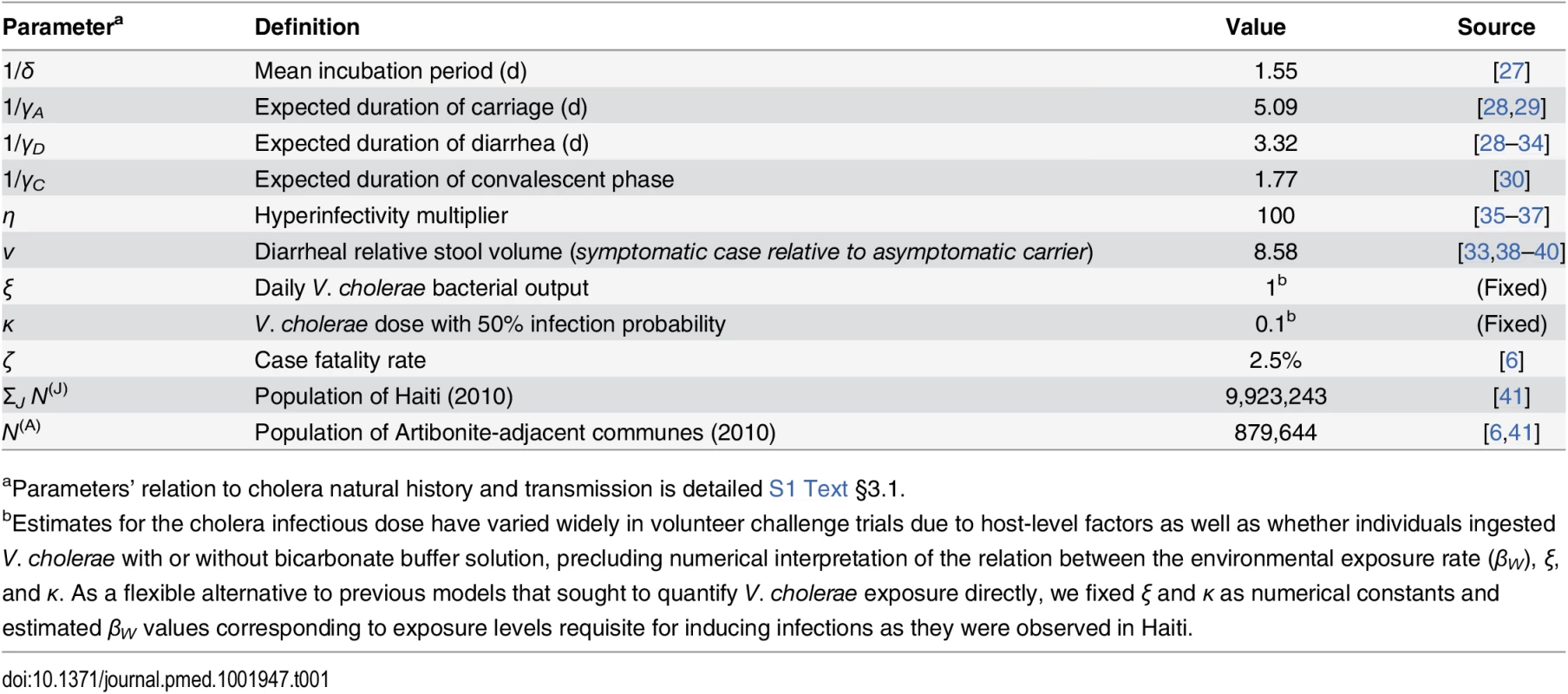
aParameters’ relation to cholera natural history and transmission is detailed S1 Text §3.1. Tab. 2. Estimated parameters for transmission dynamics in Haiti. 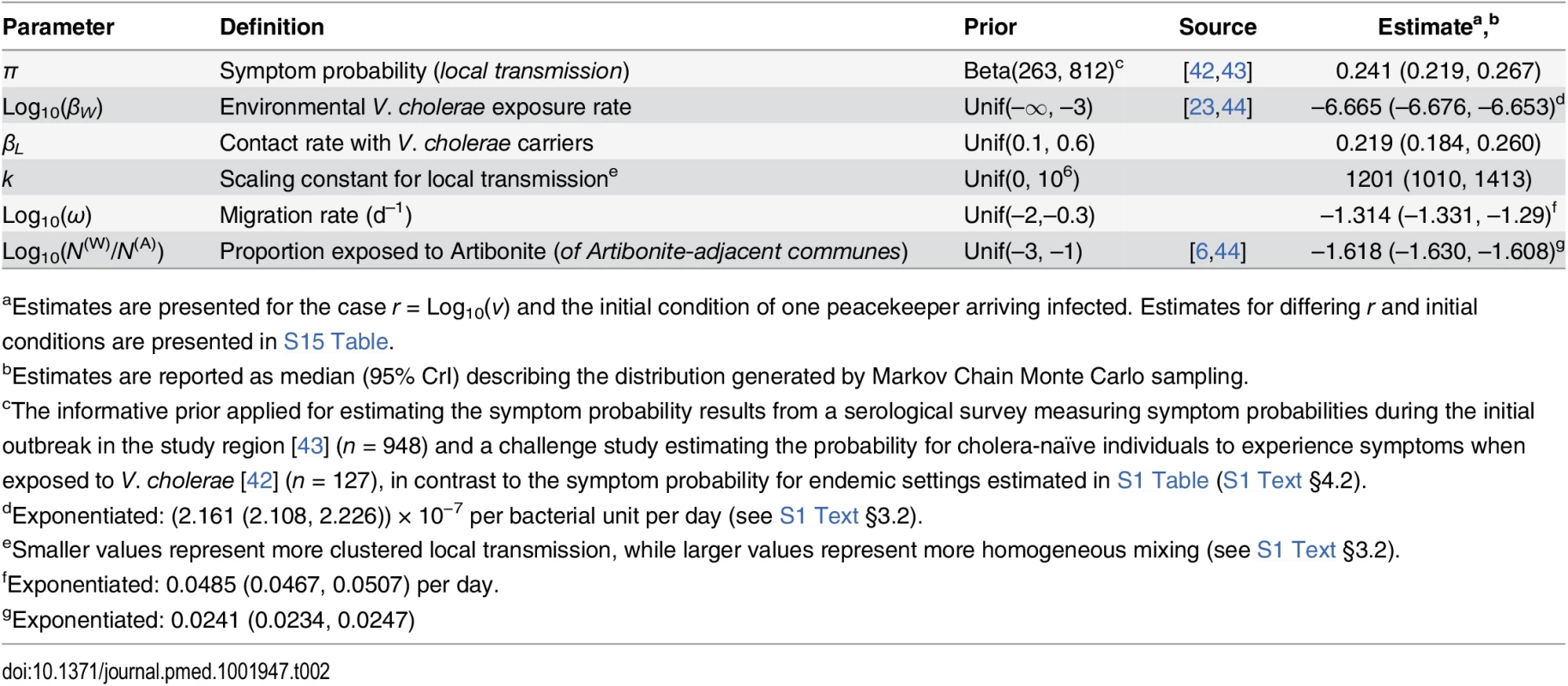
aEstimates are presented for the case r = Log10(ν) and the initial condition of one peacekeeper arriving infected. Estimates for differing r and initial conditions are presented in S15 Table. Interventions
We compared four interventions against status-quo protocols: (I) screening peacekeepers for V. cholerae carriage at their time of departure from Nepal using a licensed, commercially-available immunochromatographic rapid diagnostic test (RDT) following an enrichment step; (IIa) administering antimicrobial chemotherapy at time of departure (time-of-departure prophylaxis) or (IIb) beginning 7 d prior to departure (early-initiated prophylaxis); (III) immunizing peacekeepers using OCV administered beginning 5 wk before departure; and (IV) combined immunization and chemoprophylaxis following the time-of-departure (IVa) and early-initiated (IVb) schedules (Fig 2, Table 3).
Fig. 2. Intervention timeline. 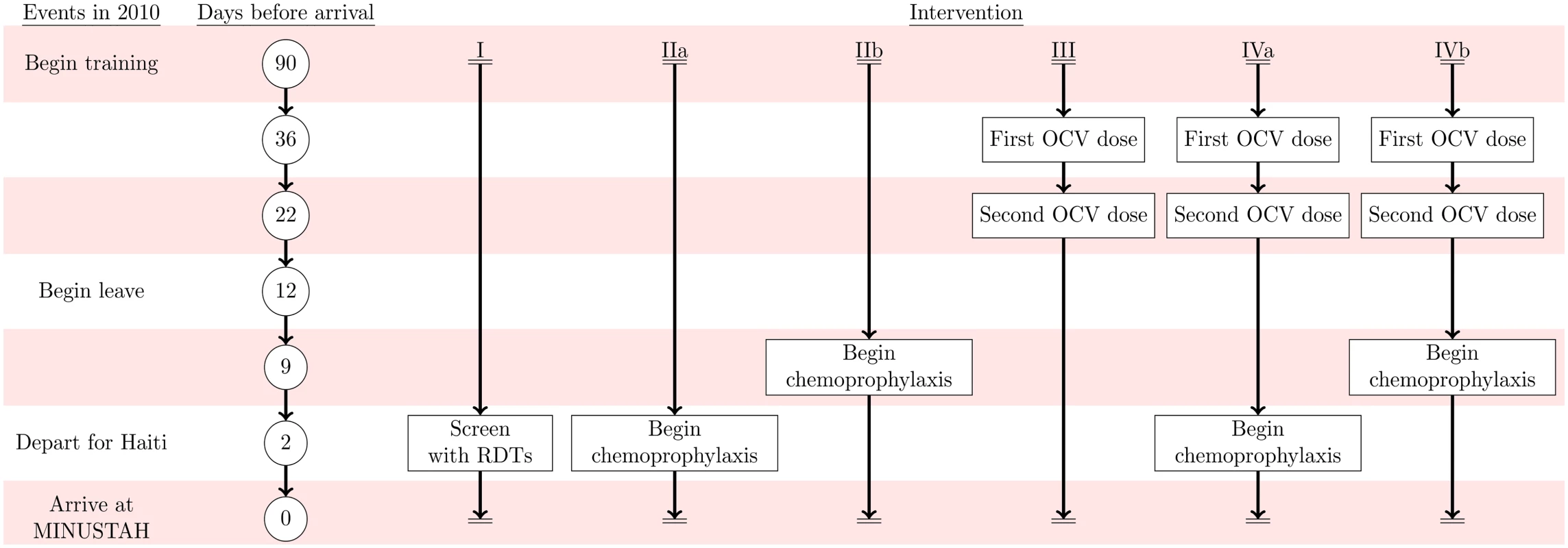
Events in 2010 are listed on the left alongside the timeframe for the simulated interventions: (I) RDT screening, (IIa) time-of-departure prophylaxis with antimicrobial drugs, (IIb) early-initiated prophylaxis with antimicrobial drugs beginning 7 d prior to deployment, (III) two-dose OCV immunization at 36 and 22 d prior to deployment, (IVa) two-dose OCV immunization combined with time-of-departure chemoprophylaxis, and (IVb) two-dose OCV immunization combined with early-initiated chemoprophylaxis. Tab. 3. Individual-level intervention efficacy. 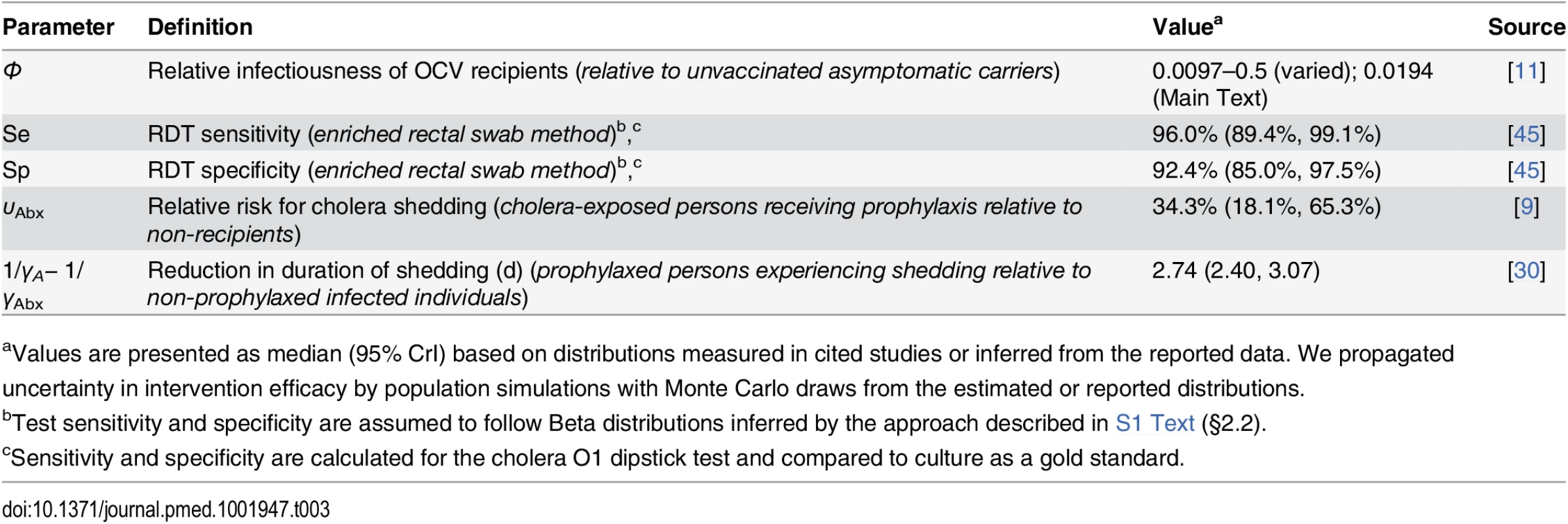
aValues are presented as median (95% CrI) based on distributions measured in cited studies or inferred from the reported data. We propagated uncertainty in intervention efficacy by population simulations with Monte Carlo draws from the estimated or reported distributions. We assessed the impact of the interventions on the probability for a symptomatic cholera case to occur in the host community in Haiti.
Status quo scenario
We assume the observed epidemic was representative of a status-quo scenario entailing no testing, antimicrobial chemotherapy, or immunization among asymptomatic peacekeepers, as per current (and 2010) pre-departure indications [7,12,13]. Existing protocols include the isolation and clinical management of diarrheal cases. We assumed that these measures are effective in limiting the risk for peacekeepers experiencing symptomatic cholera during deployment to transmit infection under status quo. We consequently modeled the impact of interventions on the probability for transmission by asymptomatically infected peacekeepers only.
Rapid diagnostic screening
Recommendations for the UN to screen departing peacekeepers are most likely to be implemented using rapid diagnostic tests (RDTs), which are capable of detecting V. cholerae at low densities obtainable via rectal swabs of asymptomatic carriers following brief (4–6h) enrichment [45,46]. Alternative methods for identifying V. cholerae among asymptomatic carriers, such as culture or polymerase chain reaction, may be impractical by comparison for routine peacekeeping operations because individuals may acquire or clear infection in the time required for diagnosis (24–72h), and/or because of limited laboratory facilities in many cholera-endemic settings [47]. We used previous sensitivity and specificity estimates for the leading licensed, commercially-available RDT (Crystal VC, Span Diagnostics, Surat, India) following an enrichment step (“confirmation method” [45]) to quantify the impact of screening on the probability for infected cholera-infected peacekeepers to be deployed (S1 Text §2.2). The confirmation method yields higher specificity than direct stool screening via the dipstick. We conducted further analyses considering sensitivities and specificities ranging from 50% to 99% to examine the range of plausible outcomes under different point-of-departure screening protocols.
Antimicrobial chemoprophylaxis
Numerous antimicrobial drugs are efficacious for cholera treatment and prophylaxis. We used estimates from meta-analyses [9,30] aggregated across antimicrobial drug classes to quantify two correlates of protection: (1) reduced risk for infection, and (2) shortened duration of shedding given infection (S1 Text §2.3). We considered two drug administration schedules. The first was a typical prophylaxis approach beginning at the time of departure from Nepal (IIa; “time-of-departure” prophylaxis), intended to hasten clearance of infection among peacekeepers who acquired infection during their leave period. Shown to be particularly effective in an early trial [48], the second (IIb; “early-initiated” prophylaxis) was to administer prophylaxis beginning 7 d prior to deployment, which would both hasten clearance and prevent V. cholerae infection in the days leading up to departure.
Emergence of resistance to conventional cholera therapies, such as tetracycline, doxycycline, and ciprofloxacin, undermines interpretation of antimicrobial drug efficacies reported in earlier trials [9,30]. We therefore assessed how externalities such as reduced antimicrobial susceptibility may limit the effectiveness of chemoprophylaxis interventions by conducting a sensitivity analysis, considering that conventional antimicrobial agents conferred 10% to 50% lower-than-reported efficacy. While resistance to azithromycin is less prevalent and single-dose regimens of this drug have shown superiority over conventional therapies for cholera treatment [30,49], the efficacy of azithromycin prophylaxis has not been studied. We also assessed the potential utility of azithromycin under a time-of-departure single-dose regimen assuming 10%–50% superior prophylactic efficacy over conventional therapies.
Oral cholera vaccination
Although OCVs do not necessarily protect recipients against cholera infection, they reduce density of V. cholerae in stool and reduce an individual’s likelihood for experiencing disease given that infection occurs [10,11]. We modeled reductions in bacterial shedding among asymptomatic peacekeepers resulting from immunization with two doses of killed bivalent whole-cell OCV (Shanchol, Shantha Biotechnics, Hyderabad, India), administered beginning 5 wk before departure, to compare transmission risk from immunized and non-immunized peacekeepers who experience asymptomatic infection (S1 Text §2.4). While numerical reductions in excreted V. cholerae density among OCV recipients have been reported in a challenge trial [11], there is uncertainty in the quantitative relation between total excreted V. cholerae and transmission risk [23]. We therefore conducted a sensitivity analysis comparing effectiveness estimates assuming differing levels of protection against transmission conferred by OCV.
Current and next-generation OCVs, including recombinant live vaccines (e.g., CVD 103-HgR), may confer some protection against infection in addition to reducing bacterial shedding among recipients [11,50,51]. Considering this possibility, we performed an additional sensitivity analysis considering potential outcomes of OCV interventions when vaccines were modeled to prevent a proportion (5%, 10%, 25%, or 50%) of recipients from experiencing infection, in addition to conferring reductions in shedding among recipients who become infected.
Combined antimicrobial chemoprophylaxis and immunization
We assessed the effectiveness of a combined intervention where peacekeepers received two doses of OCV beginning 5 wk before departure as well as either early-initiated or time-of-departure antimicrobial chemotherapy. Under such an intervention, peacekeepers’ likelihood for experiencing shedding upon arrival and rate of clearing V. cholerae infection were determined using the parameters for antimicrobial chemoprophylaxis interventions described above. Peacekeepers were assumed to shed V. cholerae at reduced densities due to vaccine receipt, as for the OCV interventions.
Benefit to peacekeepers
In addition to assessing how the interventions under consideration affect the probability for asymptomatic peacekeepers to import and transmit V. cholerae, we estimated how antimicrobial chemoprophylaxis and OCV immunization benefit peacekeeping forces by lowering individuals’ risk for experiencing symptomatic cholera disease. We estimated these reductions using outcomes of previous studies that assessed direct effects of antimicrobials and OCV in preventing (1) V. cholerae infection, and (2) cholera symptoms given that infection occurs [9,11,52].
Intervention costs
We estimated the direct costs of the interventions in terms of the cost per peacekeeper of purchasing the necessary tests, drugs, or vaccine doses, excluding additional indirect costs associated with delivery and implementation. We considered the most recently-reported manufacturer prices for RDTs and generic antibiotics [53]. We assessed OCV costs based on reported per-dose pricing for the global OCV stockpile [54].
Model Implementation
To incorporate measures of uncertainty resulting from model parameterization and the stochastic nature of transmission, we implemented the model using the Gillespie algorithm (S1 Text §3.8), sampling fitted parameter values from their posterior distribution inferred by MCMC [55]. We propagated uncertainty in screening and chemoprophylaxis intervention efficacy via Monte Carlo sampling from the fitted distributions in stochastic model realizations. We computed probabilities for a symptomatic cholera case in the Haitian population (after which a large epidemic was likely to occur) as the proportion of model realizations in which a case occurred prior to the extinction of V. cholerae transmission. We calculated relative risk estimates comparing probabilities under intervention scenarios against status quo. Due to the computationally-intensive nature of the model, we generated 95% credible intervals surrounding effect size estimates via bootstrap resampling. We carried out computations on the Louise High-Performance Computing Cluster at Yale University.
Results
Infectious Arrivals
The estimated prevalence of asymptomatic V. cholerae infection and incubation among peacekeepers at their time of deployment ranged from 2.9 (1.3, 5.5) to 57.4 (26.2, 110.6) per 100,000 for background incidence rates of 0.5 to 10 cases per 1,000 person-years at risk (PYAR) (S2 Table). Allowing for cholera progression during transit to the MINUSTAH base, we estimated the prevalence of V. cholerae carriage among arrivals to range from 1.8 (0.8, 3.4) to 35.4 (16.2, 68.2) per 100,000 (S2 Table). These estimates corresponded to a probability of between 0.8% (0.4%, 1.6%) and 14.8% (7.0%, 26.9%), respectively, for at least one of the 454 peacekeepers to have been infected upon arrival at the MINUSTAH base (Table 4).
Tab. 4. Importation probabilities under status quo and intervention scenarios, with estimated effectiveness against importation. 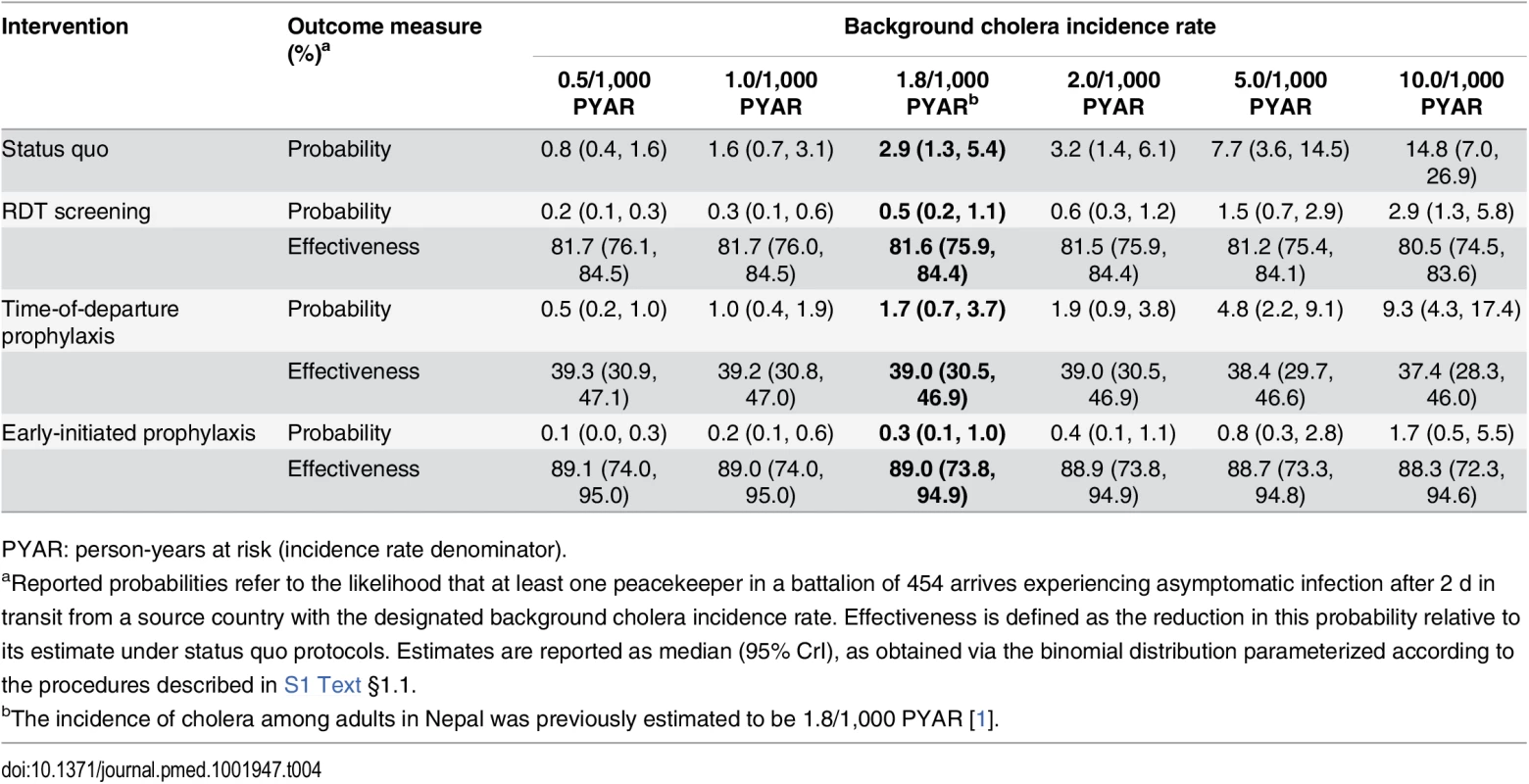
PYAR: person-years at risk (incidence rate denominator). Epidemiological Dynamics
We estimated the basic reproductive number (R0) at the outset of the epidemic to have been 1.80 (1.64, 2.00) within Artibonite-adjacent communes and 1.13 (0.98, 1.34) nationwide assuming a background cholera incidence rate of 1.8 per 1,000 PYAR in Nepal (S1 Text §3.9) (Table 5). This value defined the number of secondary cases an index case was expected to cause in the fully susceptible population, and did not vary significantly across the incidence rates considered (Table 5) or among fitted models that assumed a different relationship between stool output and infectiousness (S13 Table). Quantifying the contributions of the modeled transmission pathways, we estimated the reproductive number for Artibonite-mediated V. cholerae transport was 30.24 (30.03, 30.44), while most transmission occurred via slower local spread with a reproductive number equal to 1.07 (0.91, 1.27). Cholera cases were expected to occur in the community with 82.2% (81.2%, 83.2%) probability following the arrival of one infected peacekeeper, and with over 95% probability following the arrival of two or more infected peacekeepers (Table 6).
Tab. 5. Estimated basic reproduction numbers (R0). 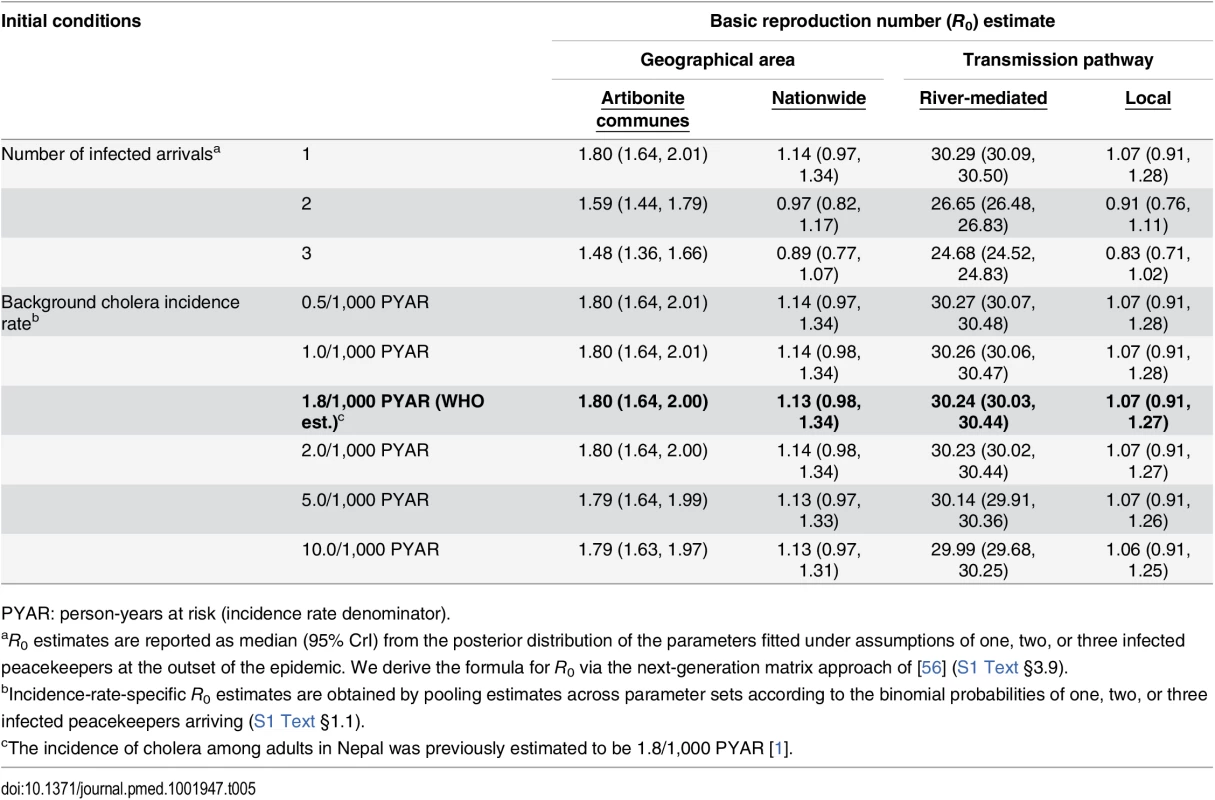
PYAR: person-years at risk (incidence rate denominator). Tab. 6. Case probabilities following importation by one, two, or three peacekeepers. 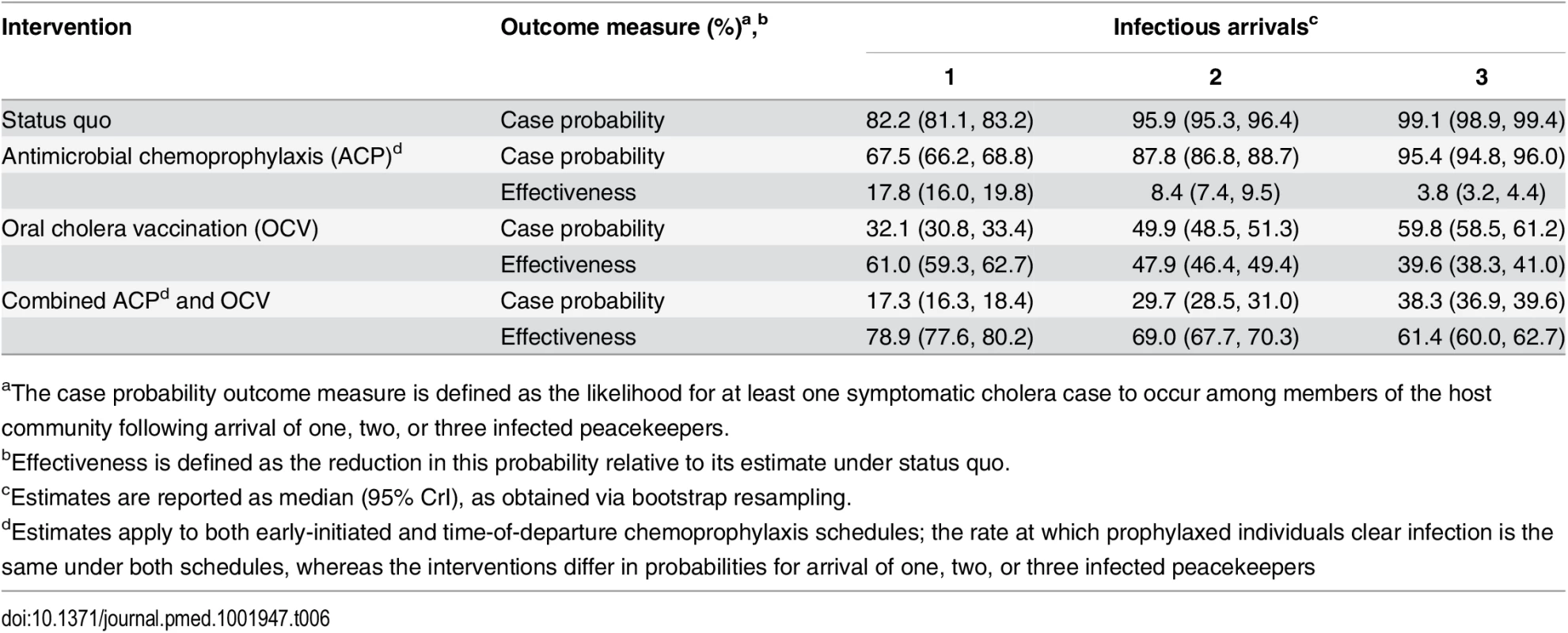
aThe case probability outcome measure is defined as the likelihood for at least one symptomatic cholera case to occur among members of the host community following arrival of one, two, or three infected peacekeepers. Pooling case probabilities according to the likelihood for one, two, or three peacekeepers arriving infected (S3 Table; S1 Text §1.1), we estimated the total probability for community cases ranged from 0.7% (0.3%, 1.3%) to 12.4% (5.8%, 22.4%) under the different background incidence rates modeled. This outcome did not depend on the modeled relationship between shedding rates and infectiousness among symptomatic and asymptomatic peacekeepers (S14 Table). The timing of the first symptomatic case was subject to variability across stochastic realizations of the model (95% CrI: October 11 to October 20) (Fig 1). The first symptomatic case is reported to have occurred October 12 [57]; our model predicts a case on or before this date in 24% of simulations, whereas 71% of simulations predict a case on or before October 14, which is the date of symptoms onset for the first culture-confirmed cholera cases identified by a Cuban medical brigade that investigated the outbreak early in its course (S1 Text §4.5) [6].
Intervention Effects
Using RDTs with an enrichment step to screen for and exclude potential cholera carriers from transiting to Haiti conferred an 81.6% (75.9%, 84.4%) reduction in the probability for V. cholerae importation at the assumed background incidence rate of 1.8 cases per 1,000 PYAR (Table 4, Table 7). However, under such a screening program, 7% (2%, 15%) of tests administered (33 [10, 70] peacekeepers in a battalion of 454) were expected to result in false positive outcomes. Reductions in importation probabilities and expected false-positive rates did not vary significantly across the range of background incidence rates considered (S4 and S5 Tables). A point-of-departure screening approach with 99% sensitivity and specificity would reduce the importation probability by up to 83% relative to status quo, with 0.9% of tests (4 of 454) returning false-positive results.
Tab. 7. Total reduction in case probability afforded by interventions, with estimated intervention effectiveness. 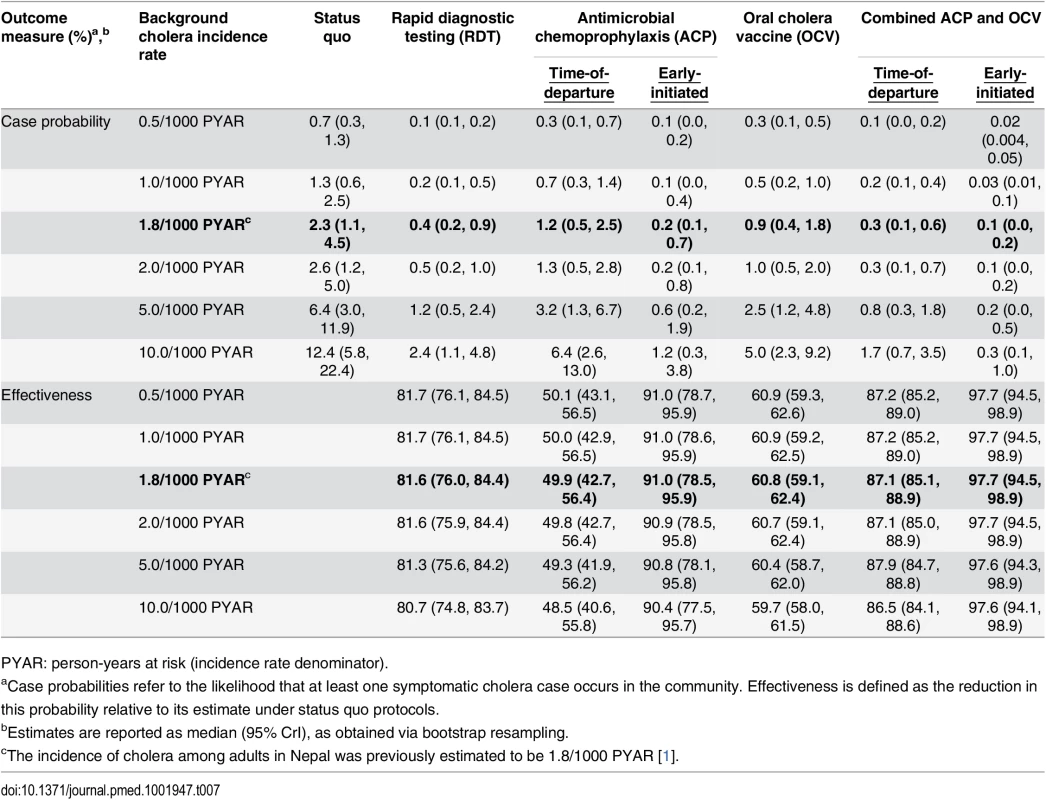
PYAR: person-years at risk (incidence rate denominator). Early-initiated antimicrobial chemoprophylaxis offered marginally superior protection against importation, reducing the probability of at least one infected peacekeeper arriving by 89.0% (73.8%, 94.9%). In contrast, prophylaxis delivered at time of departure reduced the importation probability by only 39.0% (30.5%, 46.9%) (Table 4). Accounting for both protection against importation as well as reduced opportunities for transmission due to faster clearance of infection among treated peacekeepers, antimicrobial chemoprophylaxis was expected to reduce the probability for a case by 49.9% (42.7%, 56.4%) when administered at time of departure, or by 91.0% (78.5%, 95.9%) if treatment were initiated 1 wk before departure (Table 7).
OCV immunization reduced the probability for a case in the community by up to 60.8% (59.1%, 62.4%). Concurrent OCV immunization and antimicrobial chemoprophylaxis resulted in up to 87.1% (85.1%, 88.9%) and 97.7% (94.5%, 98.9%) reductions in the probability for a case under time-of-departure and early-initiated treatment regimens, respectively.
We found that the effectiveness of immunization was highly sensitive to the modeled level of protection resulting from OCV-mediated reductions in shedding. If vaccination reduces the risk of transmission from an asymptomatically infected individual by less than 96% (ϕ = 0.0388), OCV interventions cease to be more effective than time-of-departure chemoprophylaxis. Even assuming reductions in transmission risk greater than 99% (ϕ = 0.0097), OCV interventions offered lower effectiveness than screening and early-initiated prophylaxis (S7 Table). At reported levels of reductions in bacterial shedding (ϕ = 0.0194) [11], OCV interventions offered comparable effectiveness (>80% reduction in case probability) to screening and early-initiated prophylaxis only if we assumed vaccination reduces the probability of any infection by 50% (S16 Table).
In contrast, antimicrobial chemoprophylaxis remained effective when considering potential limitations that could undermine this approach. Early-initiated chemoprophylaxis showed superior effectiveness over RDT screening when we assumed up to 25% lower-than-reported efficacy of antimicrobial drugs, as might be expected if resistance to first-line chemotherapies or poor compliance with drug regimens limited this approach (S6 Table). Early-initiated chemoprophylaxis remained superior to OCV interventions even when we assumed 50% lower-than-reported efficacy for antimicrobial drugs. Under such conditions, we expected a 69.7% (34.6%, 85.1%) reduction in the probability for a symptomatic cholera case.
Administering a single dose of azithromycin at time of departure was expected to offer superior effectiveness over screening and early-initiated prophylaxis with conventional therapies if azithromycin offers a 50% improvement in protection against shedding onset and a 50% greater reduction in the duration of shedding relative to conventional drugs (S8 Table). Even if the efficacy of azithromycin is only 10% superior to conventional agents, time-of-departure prophylaxis with this treatment offers a 56.6% (49.3%, 64.1%) reduction in the probability for a case, nearly equal to the estimated effectiveness of OCV.
Benefit to Peacekeepers
Antimicrobial chemoprophylaxis and immunization with OCV directly benefit peacekeepers from cholera-endemic settings by reducing their risk for experiencing symptomatic cholera illness. These benefits would also be afforded to peacekeepers traveling to endemic countries where they may be exposed to cholera, with OCV offering longer-lasting protection. Based on available studies (Table 8), we estimated that antimicrobial drugs lower the probability for experiencing cholera diarrhea by 95.5% (70.4%, 99.9%) among peacekeepers exposed to V. cholerae before deployment. OCV interventions are expected to reduce exposed peacekeepers’ probability for cholera diarrhea by 62.8% (22.2%, 90.0%). Combining chemoprophylaxis with vaccination provides up to 98.0% (84.9%, 99.9%) protection against cholera diarrhea.
Tab. 8. Benefit to peacekeepers. 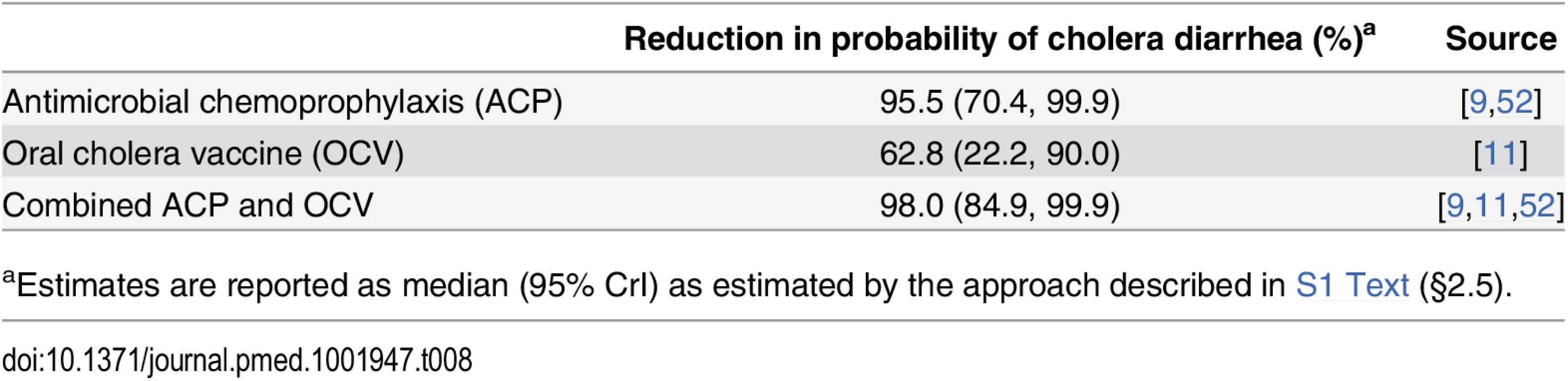
aEstimates are reported as median (95% CrI) as estimated by the approach described in S1 Text (§2.5). Intervention Costs
Antimicrobial chemoprophylaxis is the lowest-cost strategy under consideration (Table 9). Early-initiated and time-of-departure chemoprophylaxis with conventional cholera therapies each cost under US$1 per peacekeeper, while single-dose prophylaxis using azithromycin would cost between US$0.52 and US$1.32 per peacekeeper. Although the direct cost of purchasing RDTs is only moderately more expensive at US$2.54 per peacekeeper, there are additional indirect costs associated with training and employing personnel to take rectal swabs, perform sample enrichment, and administer tests, which together may exceed the direct costs of assays [58]. Delaying the deployment of peacekeepers who test positive for V. cholerae imposes additional indirect costs and logistical burden, particularly if tests have low specificity (S5 Table). Administering OCV is expected to cost US$3.70 per peacekeeper for the bivalent vaccine without B subunit (Shancol), or between US$9.40 and US$18.80 for the vaccine containing B subunit (Dukoral). Additional indirect costs associated with maintaining a cold chain for shipment and storage of the vaccine [54] are not reflected in the unit price of vaccine doses.
Tab. 9. Intervention costs. 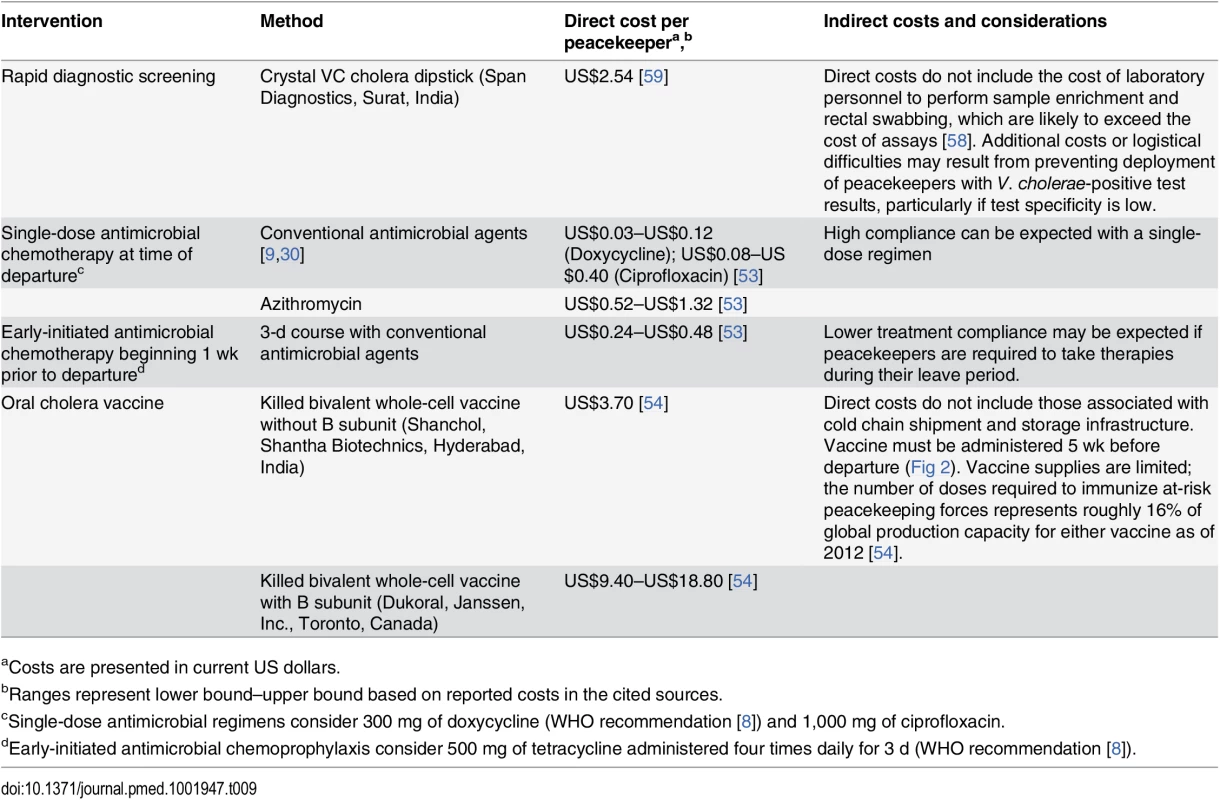
aCosts are presented in current US dollars. Vaccine availability also presents a limitation to implementing OCV interventions. Of 104,928 active-duty UN peacekeeping personnel in 2015, 74% were deployed from WHO-classified cholera-endemic countries. Considering most deployments are 6 mo long, over 310,000 OCV doses would be required to immunize peacekeepers departing endemic countries each year, representing 16% of total annual production capacity for either Shanchol or Dukoral (2 million doses each as of 2012 [54]).
Discussion
The cholera outbreak in Haiti arose from a confluence of preventable circumstances. Systemic inadequacies in sanitation infrastructure made Haiti vulnerable to water-borne disease [60], like other disaster-affected settings where peacekeeping operations are undertaken. Mass personnel movements from a cholera-endemic country and deficient waste management practices at a MINUSTAH base led to the introduction of V. cholerae to a susceptible population. Prior to the outbreak, there were no biomedical interventions in place to prevent its occurrence despite the recognized risk for spread of infectious diseases from military to civilian populations [61]. While the UN has been reluctant to implement interventions in the wake of the epidemic in part due to uncertainties surrounding their effectiveness [12,13], our findings suggest antimicrobial chemoprophylaxis reduces the risk of disease introduction by over 90%. The low costs and minimal logistical burden of chemoprophylaxis relative to the other interventions suggest this approach warrants consideration as a strategy to limit risk for cholera introduction in future peacekeeping operations.
Prospective recommendations for OCV use during peacekeeping deployments had been in place prior to the outbreak [62]. Our analysis suggests antimicrobial chemoprophylaxis of peacekeepers beginning 1 wk prior to deployment is more effective than OCV in preventing epidemic introduction, and is also less expensive and easier to implement. While screening via RDTs offers similar levels of protection in comparison to early-initiated antimicrobial chemoprophylaxis, logistical and cost constraints must be considered in evaluating the two intervention strategies. In contrast to conventional culture, administering RDTs using enriched rectal swab samples is simple to perform outside laboratories and requires minimal personnel time. However, false positive results are expected in large battalions even if the screening procedure has high specificity, suggesting confirmatory diagnoses or provision of antimicrobial drugs may be warranted for individuals with positive test outcomes. This logistical constraint was acknowledged in a follow-up report by the UN [12,13]. Our analysis provides quantitative support for comparing the different interventions in terms of their impact on cholera transmission, and demonstrates that screening and OCV interventions would not surpass early-initiated chemoprophylaxis in effectiveness.
Although effective, prophylactic use of antimicrobial drugs for cholera is controversial [8,9,13]. Intensive population-wide tetracycline prophylaxis has been associated with local emergence of drug resistance [63]. In addition, resistance to tetracycline derivatives is prevalent in V. cholerae depending on geographical region [64]. Azithromycin is an attractive alternative: a single-dose regimen is efficacious in resolving cholera diarrhea and pathogen excretion [49], and resistance in V. cholerae is comparatively rare [65]. We found single-dose azithromycin to be an optimal strategy if its superiority for cholera treatment translates to high prophylactic efficacy. Furthermore, targeted chemoprophylaxis among strictly-defined groups such as peacekeeping personnel imposes low or negligible selective pressure for drug resistance relative to historical population-based approaches, and has been advocated as a cholera control strategy [19,60]. To date, chemoprophylaxis studies have used differing drug classes and dosing, and have measured bacteriological outcomes inconsistently [9]. Trials are needed to define optimal antimicrobial regimens for cholera chemoprophylaxis. In view of prevalent resistance to conventional therapies, assessing the prophylactic efficacy of azithromycin is of particular priority.
It is uncertain how many infected peacekeepers introduced cholera to Haiti. Considering a wide range of possible incidence rates in Nepal, we identified statistical support for the hypothesis that one infected peacekeeper initiated the outbreak (S3 Table), consistent with genomic analyses supporting a single-source introduction [5,66,67]. There is no record of any peacekeeper experiencing symptoms in Haiti or before departure, and V. cholerae was not detected in personnel or camp sewage in October 2010 [6,7]. These factors suggest secondary generations of infection did not occur among peacekeepers. The high estimated R0 for river-mediated transmission, underlying the observed incidence of over 2,000 symptomatic cases per day nationwide by 22 October [6], distinguishes the Artibonite watershed as a high-risk environment for cholera introduction [25,60].
Previously-reported transmission models have not accounted for the dose-response relationship between V. cholerae exposure and the probability of symptoms in addition to infection [38]. Our model links dose-response observations from human challenge experiments to temporal variability in the distribution of symptomatic and asymptomatic infections [23]. This approach provides a novel explanation for the explosive nature of cholera epidemics such as the Haiti outbreak, and addresses previous uncertainty [6] as to how a small number of asymptomatic peacekeepers could instigate a large outbreak despite low initial levels of V. cholerae contamination. A quiescent initial increase in asymptomatic cases, which result from lower infectious doses, precedes the precipitous rise in observed cases in our model after a threshold level of environmental contamination is reached.
We consider how the interventions affect transmission from asymptomatic peacekeepers under the assumption that existing protocols for isolation and clinical management of diarrheal cases are effective in preventing symptomatic peacekeepers from transmitting. Because deficient sanitation infrastructure at peacekeeping bases could undermine the effectiveness of existing protocols, proper sewage disposal must be implemented in tandem with biomedical interventions to limit risk for cholera introduction.
Our analysis is subject to several limitations. First, external factors affecting V. cholerae concentration in the environment mediate the relationship between bacterial shedding and transmission risk [68], limiting our ability to infer how lower levels of V. cholerae shedding among asymptomatic OCV recipients impact the relative transmission risk of immunized and non-immunized asymptomatic carriers. Thus, our finding that the effectiveness of OCV varies widely depending on the relationship between reduced bacterial shedding and transmission risk undermines support for OCV as a preventive strategy (S8 Table). Our analysis did not consider single-dose OCV administration [69,70], which may be more logistically feasible than two-dose schedules given costs and limited vaccine stockpiles, or in the event that peacekeepers are deployed on short notice during acute crises. In addition, evidence that the vaccine does not confer direct protection against infection comes from limited studies; one recruited healthy subjects with no previous cholera exposure [11], and the other reported incomplete details on follow-up of asymptomatic individuals [10]. We found that OCV would deliver comparable effectiveness to prophylaxis only if vaccines prevent 50% of infections in addition to reducing bacterial shedding among recipients experiencing infection. Protection against infection therefore merits attention in studies of next-generation cholera vaccines including CVD 103-HgR. Unlike antimicrobials that reduce overall risk of infection, OCV could marginally increase peacekeepers’ risk of unknowingly importing V. cholerae by increasing the fraction of cases that are asymptomatic.
Last, the UN has previously acknowledged that commercial RDTs have only been field-validated among symptomatic cholera cases [12,13]. Validation of RDTs for detecting V. cholerae in enriched specimens from asymptomatic carriers is a research priority, as this approach could greatly expand low-cost options for screening individuals at risk of transmission. Together with cost and logistical considerations, uncertainty regarding the effectiveness of OCV and screening interventions strengthens the case for chemoprophylaxis as the preferred preventive strategy.
While the role of large-scale international personnel deployments in the global spread of cholera and other infectious diseases is not known, exposure to infectious disease agents in low-income countries that deploy and receive the majority of peacekeeping forces has motivated previous calls for enhanced infectious disease surveillance among troops [61,71]. Whereas the previous absence of cholera from Haiti contributed to interest in ascertaining the geographic origin of the 2010 epidemic [4], importation events affecting peacekeeper-receiving countries where sporadic, epidemic, or endemic cholera transmission already occurs may be less readily detected.
Inadequate water and sanitation facilities and the lack of population immunity to cholera in Haiti put this country at exceptionally high risk for an epidemic following contamination of the Artibonite watershed. However, UN peacekeeping missions serve populations affected by geopolitical and humanitarian emergencies whose vulnerable living circumstances bring about high risk for cholera transmission [60,72,73]. Although our model primarily offers a retrospective analysis of how several interventions might have led to different outcomes in Haiti, inferences about the comparative effectiveness of the interventions are generalizable to other settings. In particular, estimated reductions in importation probability through screening and prophylaxis hold for peacekeepers departing any country with endemic cholera transmission. In this respect, we identify that these interventions offer at minimum 81% and 89% effectiveness, respectively, against asymptomatic V. cholerae importation during future global deployments. As of 2015, over 75,000 UN peacekeeping personnel actively serving at international missions were deployed from WHO-classified cholera-endemic countries [1,74]. With operations at this scale, the risk for further importation events is high under existing protocols even if individual peacekeepers or battalions have a low probability of carrying V. cholerae.
Troop-deploying countries are currently encouraged to provide OCVs to protect peacekeepers against cholera during deployments to cholera-endemic countries [7,12,13]. Our finding that biomedical interventions serve the dual purpose of reducing risk for cholera introduction in troop-receiving countries suggests existing UN protocols are suboptimal. Chemoprophylaxis with antimicrobial drugs, targeted judiciously to peacekeepers deployed from cholera-endemic settings, offers superior protection against cholera introduction relative to immunization and imposes lower costs and logistical burden than RDT-based screening or immunization with OCV. Combined interventions utilizing OCV with chemoprophylaxis offer particularly high effectiveness. Quantitative assessments provide support and guidance for policymakers weighing the merits and cost-sharing of different interventions.
Supporting Information
Zdroje
1. Ali M, Lopez AL, Ae You Y, Eun Kim Y, Sah B, et al. The global burden of cholera. Bull World Health Organ. 2012;90 : 209–218. doi: 10.2471/BLT.11.093427 22461716
2. Ministère de la santé publique et de la population. Rapport cholera 01 mars 2015. http://bit.ly/1ArOsuT.
3. Jenson D, Szabo V. Cholera in Haiti and other Caribbean regions, 19th century. Emerg Infect Dis. 2011;17 : 2130–2135. doi: 10.3201/eid1711.110958 22099117
4. Orata FD, Keim PS, Boucher Y. The 2010 cholera outbreak in Haiti: how science solved a controversy. PLoS Pathog. 2014;10: e1003967 doi: 10.1371/journal.ppat.1003967 24699938
5. Katz LS, Petkau A, Beaulaurier J, Tyler S, Antonova ES, et al. Evolutionary dynamics of Vibrio cholerae O1 following a single-source introduction to Haiti. mBio. 2013;4:e00398–13. doi: 10.1128/mBio.00398-13 23820394
6. Piarroux R, Barrais R, Faucher BB, Haus R, Piarroux M, et al. Understanding the cholera epidemic, Haiti. Emerg Infect Dis. 2011;17 : 1161–1167. doi: 10.3201/eid1707.110059 21762567
7. Lantagne D, Balakrish Nair G, Lanata CF, Cravioto A. The cholera outbreak in Haiti: Where and how did it begin? Curr Top Microbiol Immunol. 2013;379 : 145–164.
8. Nelson EJ, Nelson DS, Salam MA, Sack DA. Antibiotics for both moderate and severe cholera. N Engl J Med. 2011;364 : 5–7. doi: 10.1056/NEJMp1013771 21142691
9. Reveiz L, Chapman E, Ramon-Pardo P, Koehlmoos TP, Cuervo LG, et al. Chemoprophylaxis in contacts of patients with cholera: systematic review and meta-analysis. PLoS ONE. 2011;6:e2760.
10. Sanchez JL, Vasquez B, Begue RE, Meza R, Castellares G, et al. Protective efficacy of oral whole-cell/recombinant-B-subunit cholera vaccine in Peruvian military recruits. Lancet. 1994;344 : 1273–1276. 7967990
11. Black RE, Levine MM, Clements ML, Young CR, Svennerholm AM, et al. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun. 1987;55 : 1116–1120. 3552989
12. United Nations follow-up to the recommendations of the Independent Panel of Expertos on the Cholera Outbreak in Haiti. http://www.un.org/News/dh/infocus/haiti/Follow-up-to-Recommendations-of-IPE.pdf.
13. Medrano Rojas P. Assistant Secretary General’s response. http://bit.ly/1Sw6O3z.
14. Hughes JM, Boyce JM, Levine RJ, Khan M, Aziz KM, et al. Epidemiology of El Tor cholera in rural Bangladesh: importance of surface water in transmission. Bull World Health Organ. 1982;60 : 395–404. 6982775
15. Bart KJ, Huq Z, Khan M, Mosley WH. Seroepidemiologic studies during a simultaneous epidemic of infection with El Tor Ogawa and classical Inaba Vibrio cholerae. J Infect Dis. 1970;121 : 17–24. 4912068
16. Woodward WE, Mosley WH. The spectrum of cholera in rural Bangladesh: comparison of El Tor Ogawa and classical Inaba infection. Am J Epidemiol. 1972;96 : 342–351. 4564735
17. Tamayo JF, Mosley WH, Alvero MG, Joseph PR, Gomez CZ, et al. Studies of cholera El Tor in the Philippines. Bull World Health Organ. 1965;33 : 645–649. 5295145
18. Glass RI, Holmgren J, Haley CE, Khan MR, Svennerholm AM, et al. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol. 1985;121 : 791–796. 4014172
19. Weil AA, Khan AI, Chowdhury F, Larocque RC, Faruque ASG, et al. Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin Infect Dis. 2009;49 : 1473–1479. doi: 10.1086/644779 19842974
20. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36 : 1–48.
21. Ernst S, Weinrobe C, Bien-Aime C, Rawson I. Cholera management and prevention at Hôpital Albert Schweitzer, Haiti. Emerg Infect Dis. 2011;17 : 2155–2157. doi: 10.3201/eid1711.110815 22099123
22. Update: outbreak of cholera—Haiti, 2010. Morbid Mortal Wkly Rep. 2010;59 : 1586–1590.
23. Grad YH, Miller JC, Lipsitch M. Cholera modeling: challenges to quantitative analysis and predicting the impact of interventions. Epidemiology. 2012;23 : 523–530. doi: 10.1097/EDE.0b013e3182572581 22659546
24. Chao DL, Longini IM, Morris JG. Modeling cholera outbreaks. Curr Top Microbiol Immunol. 2014;379 : 195–209. doi: 10.1007/82_2013_307 23412687
25. Gaudart J, Rebaudet S, Barrais R, Boncy J, Faucher B, et al. Spatio-temporal dynamics of cholera during the first year of the epidemic in Haiti. PLoS Negl Trop Dis. 2013;7: e0002145.
26. Barzilay EJ, Schaad N, Magloire R, Mung KS, Boncy J, et al. Cholera surveillance during the Haiti epidemic—the first 2 years. N Engl J Med. 2013;368 : 599–609. doi: 10.1056/NEJMoa1204927 23301694
27. Azman AS, Rudolph KE, Cummings DAT, Lessler J. The incubation period of cholera: A systematic review. J Infect. 2013;66 : 432–438. doi: 10.1016/j.jinf.2012.11.013 23201968
28. Alam NH, Ashraf H, Khan WA, Karim MM, Fuchs GJ. Efficacy and tolerability of racecadotril in the treatment of cholera in adults: a double blind, randomised, controlled clinical trial. Gut. 2003;52 : 1419–1423. 12970133
29. Dutta D, Bhattacharya SK, Bhattacharya MK, Deb A, Deb M, et al. Efficacy of norfloxacin and doxycycline for treatment of Vibrio cholerae O139 infection. J Antimicrob Chemother. 1996;37 : 575–581. 9182114
30. Leibovici-Weissman Y, Neuberger A, Bitterman R, Sinclair D, Salam MA, et al. Antimicrobial drugs for treating cholera. Cochrane database Syst Rev. 2014;6: CD008625 doi: 10.1002/14651858.CD008625.pub2 24944120
31. Patra FC, Majumder RN, Eeckels R, Desjeux JF, Mahalanabis D. Sacolene in cholera: a double blind randomized controlled trial. Scand J Infect Dis. 1999;31 : 151–154. 10447324
32. Rabbani GH, Islam MR, Butler T, Shahrier M, Alam K. Single-dose treatment of cholera with furazolidone or tetracycline in a double-blind randomized trial. Antimicrob Agents Chemother. 1989;33 : 1447–1450. 2684006
33. Lindenbaum J, Greenough WB, Islam MR. Antibiotic therapy of cholera. Bull World Health Organ. 1967;36 : 871–883. 4865453
34. Shahadat Hossain M, Salam MA, Rabbani GH, Kabir I, Biswas R, et al. Tetracycline in the treatment of severe cholera due to Vibrio cholerae O139 Bengal. J Heal Popul Nutr. 2002;20 : 18–25.
35. Hartley DM, Morris JG, Smith DL. Hyperinfectivity: A critical element in the ability of V. cholerae to cause epidemics?. PLoS Med. 2006;3 : 63–69.
36. Merrell DS, Butler SM, Qadri F, Dolganov Na, Alam A, et al. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417 : 642–645. 12050664
37. Alam A, Larocque RC, Harris JB, Vanderspurt C, Ryan ET, et al. Hyperinfectivity of Human-Passaged Vibrio cholerae can be modeled by growth in the infant mouse. Infect Immun. 2005;73 : 6674–6679. 16177344
38. Cash RA, Music SI, Libonati JP, Snyder MJ, Wenzel RP, et al. Response of man to infection with Vibrio cholerae. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974;129 : 45–52. 4809112
39. Sack DA, Tacket CO, Cohen MB, Sack RB, Losonsky Ga., et al. Validation of a volunteer model of cholera with frozen bacteria as the challenge. Infect Immun. 1998;66 : 1968–1972. 9573077
40. Rendtorff RC, Kashgarian M. Stool patterns of healthy adult males. Dis Colon Rectum. 1953;10 : 222–228.
41. Haiti 2009 population data, section estimates, stratified by age and sex. Centers for Disease Control and Prevention 2012. http://www.cdc.gov/haiticholera/.
42. Clements ML, Levine MM, Young CR, Black RE, Lim YL, et al. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis. 1982;145 : 465–473. 7069227
43. Jackson BR, Talkington DF, Pruckler JM, Fouché MDB, Lafosse E, et al. Seroepidemiologic survey of epidemic cholera in Haiti to assess spectrum of illness and risk factors for severe disease. Am J Trop Med Hyg. 2013;89 : 654–664. doi: 10.4269/ajtmh.13-0208 24106192
44. Chao DL, Halloran ME, Longini IM. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci U S A. 2011;108 : 7081–7085. doi: 10.1073/pnas.1102149108 21482756
45. Bhuiyan NA, Qadri F, Faruque ASG, Malek MA, Salam MA, et al. Use of dipsticks for rapid diagnosis of cholera caused by Vibrio cholerae O1 and O139 from rectal swabs. J Clin Microbiol. 2003;41 : 3939–3941. 12904424
46. Chakraborty S, Alam M, Scobie HM, Sack DA. Adaptation of a simple dipstick test for detection of Vibrio cholerae O1 and O139 in environmental water. Front Microbiol. 2013;4 : 1–3.
47. Dick MH, Guillerm M, Moussy F, Chaignat CL. Review of two decades of cholera diagnostics: how far have we really come? PLoS Negl Trop Dis. 2012;6: e0001845.
48. McCormack WM, Chowdhury AM, Jahangir N. Tetracycline prophylaxis in families of cholera patients. Bull World Health Organ. 1967;38 : 787–792
49. Saha D, Karim MM, Khan WA, Ahmed S, Salam MA, et al. Single-dose azithromycin for the treatment of cholera in adults. N Engl J Med. 2006;354 : 2452–2462. 16760445
50. Levine M, Herrington D, Losonsky G, Tall B, Kaper J, et al. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;332 : 467–470.
51. Shin S, Desai SN, Sah BK, Clemens JD. Oral vaccines against cholera. Clin Infect Dis. 2011;52 : 1343–1349. doi: 10.1093/cid/cir141 21498389
52. Echevarría J, Seas C, Carrillo C, Mostorino R, Ruiz R, et al. Efficacy and tolerability of ciprofloxacin prophylaxis in adult household contacts of patients with cholera. Clin Infect Dis. 1995;20 : 1480–1484. 7548495
53. International Drug Price Indicator Guide. Medford, Massachusetts, US: Management Sciences for Health. 2014.
54. Guidance on how to access the Oral Cholera Vaccine (OCV) from the ICG emergency stockpile. http://www.who.int/cholera/vaccines/Guidance_accessing_OCV_stockpile.pdf?ua=1.
55. Gillespie DT. Exact stochastic simulation of coupled chemical reactions. J Phys Chem. 1977;81 : 2340–2361.
56. Van Den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci. 2002;180 : 29–48. 12387915
57. Ivers LC, Walton DA. The “first” case of cholera in Haiti: Lessons for global health. Am J Trop Med Hyg. 2012;86 : 36–38. doi: 10.4269/ajtmh.2012.11-0435 22232448
58. Harris JR, Cavallaro EC, De Nóbrega AA, Jean JCB, Bopp C, et al. Field evaluation of crystal VC rapid dipstick test for cholera during a cholera outbreak in Guinea-Bissau. Trop Med Int Heal. 2009;14 : 1117–1121.
59. Crystal VC (Dipstick) immunochromatographic test for detection of V. cholerae in stool (rapid test). SPAN Diagnostics. http://www.span.co.in/ProductList/ProductDetails/REAGENTS/279-Crystal_VC_(Dipstick).HTM.
60. Farmer P, Almazor CP, Bahnsen ET, Barry D, Bazile J, et al. Meeting Cholera’s challenge to Haiti and the world: A joint statement on cholera prevention and care. PLoS Negl Trop Dis. 2011;5: e0001145.
61. Chretien JP, Blazes DL, Coldren RL, Lewis MD, Gaywee J, et al. The importance of militaries from developing countries in global infectious disease surveillance. Bull World Health Organ. 2007;85 : 174–180. 17486207
62. Steffen R. Immunization of peacekeeping forces. Emerg Infect Dis. 1999;5 : 485–486. 10341196
63. Okeke IN, Aboderin OA, Byarugaba DK, Ojo KK, Opintan JA. Growing problem of multidrug-resistant enteric pathogens in Africa. Emerg Infect Dis. 2007;13 : 1640–1646. doi: 10.3201/eid1311.070674 18217545
64. Faruque ASG, Alam K, Malek Ma, Khan MGY, Ahmed S, et al. Emergence of multidrug-resistant strain of Vibrio cholerae OI in Bangladesh and reversal of their susceptibility to tetracycline after two years. J Heal Popul Nutr. 2007;25 : 241–243.
65. Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012;379 : 2466–2476. doi: 10.1016/S0140-6736(12)60436-X 22748592
66. Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, et al. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364 : 33–42. doi: 10.1056/NEJMoa1012928 21142692
67. Ali A, Chen Y, Johnson JA, Redden E, Mayette Y, et al. Recent clonal origin of cholera in Haiti. Emerg Infect Dis. 2011;17 : 699–701. doi: 10.3201/eid1704.101973 21470464
68. Constantin de Magny G, Murtugudde R, Sapiano MRP, Nizam A, Brown CW, et al. Environmental signatures associated with cholera epidemics. Proc Natl Acad Sci U S A. 2012;105 : 17676–17681.
69. Azman AS, Luquero FJ, Ciglenecki I, Grais RF, Sack Da., et al. The Impact of a One-Dose versus Two-Dose Oral Cholera Vaccine Regimen in Outbreak Settings: A Modeling Study. PLoS Med. 2015;12: e1001867. doi: 10.1371/journal.pmed.1001867 26305226
70. Reyburn R, Deen JL, Grais RF, Bhattacharya SK, Sur D, et al. The case for reactive mass oral cholera vaccinations. PLoS Negl Trop Dis. 2011;5: e952. doi: 10.1371/journal.pntd.0000952 21283614
71. Houston S, Houston A. Screening and Treating UN Peacekeepers to Prevent the Introduction of Artemisinin-Resistant Malaria into Africa. PLoS Med. 2015;12: e1001822. doi: 10.1371/journal.pmed.1001822 25942008
72. Watson JT, Gayer M, Connolly MA. Epidemics after natural disasters. Emerg Infect Dis. 2007;13 : 1–5. 17370508
73. Dowell SF, Braden CR. Implications of the introduction of cholera to Haiti. Emerg Infect Dis. 2011;17 : 1299–1300. 21762593
74. United Nations Peacekeeping. Troop and police contributors. http://www.un.org/en/peacekeeping/resources/statistics/contributors.shtml.
Štítky
Interní lékařství
Článek Sharing Clinical Trial Data: A Proposal from the International Committee of Medical Journal EditorsČlánek Data Sharing as Part of the Normal Scientific Process: A View from the Pharmaceutical Industry
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Léčba bolesti u seniorů
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Sharing Clinical Trial Data: A Proposal from the International Committee of Medical Journal Editors
- Pharmaceutical Industry Off-label Promotion and Self-regulation: A Document Analysis of Off-label Promotion Rulings by the United Kingdom Prescription Medicines Code of Practice Authority 2003–2012
- Can Data Sharing Become the Path of Least Resistance?
- Between Openness and Privacy in Genomics
- Developing Global Norms for Sharing Data and Results during Public Health Emergencies
- Advancing Medical Professionalism in US Military Detainee Treatment
- Resuscitating the Dying Autopsy
- Data Sharing as Part of the Normal Scientific Process: A View from the Pharmaceutical Industry
- Evidence for Community Transmission of Community-Associated but Not Health-Care-Associated Methicillin-Resistant Strains Linked to Social and Material Deprivation: Spatial Analysis of Cross-sectional Data
- Intimate Partner Violence and Depression Symptom Severity among South African Women during Pregnancy and Postpartum: Population-Based Prospective Cohort Study
- Intramuscular Artesunate for Severe Malaria in African Children: A Multicenter Randomized Controlled Trial
- Strategies to Prevent Cholera Introduction during International Personnel Deployments: A Computational Modeling Analysis Based on the 2010 Haiti Outbreak
- Preventing Weight Gain in Women in Rural Communities: A Cluster Randomised Controlled Trial
- Cotrimoxazole Prophylaxis Discontinuation among Antiretroviral-Treated HIV-1-Infected Adults in Kenya: A Randomized Non-inferiority Trial
- Sharing Individual Participant Data (IPD) within the Context of the Trial Reporting System (TRS)
- “Asymptomatic” Malaria: A Chronic and Debilitating Infection That Should Be Treated
- “Real-Time” Monitoring of Under-Five Mortality: A Vision Tempered by Reality
- “Real-Time” Monitoring of Under-Five Mortality: Lessons for Strengthened Vital Statistics Systems
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cotrimoxazole Prophylaxis Discontinuation among Antiretroviral-Treated HIV-1-Infected Adults in Kenya: A Randomized Non-inferiority Trial
- Sharing Individual Participant Data (IPD) within the Context of the Trial Reporting System (TRS)
- Pharmaceutical Industry Off-label Promotion and Self-regulation: A Document Analysis of Off-label Promotion Rulings by the United Kingdom Prescription Medicines Code of Practice Authority 2003–2012
- Between Openness and Privacy in Genomics
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





