-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaExposure to Second-Hand Smoke and the Risk of Tuberculosis in Children and Adults: A Systematic Review and Meta-Analysis of 18 Observational Studies
Background:
According to WHO Global Health Estimates, tuberculosis (TB) is among the top ten causes of global mortality and ranks second after cardiovascular disease in most high-burden regions. In this systematic review and meta-analysis, we investigated the role of second-hand smoke (SHS) exposure as a risk factor for TB among children and adults.Methods and Findings:
We performed a systematic literature search of PubMed, Embase, Scopus, Web of Science, and Google Scholar up to August 31, 2014. Our a priori inclusion criteria encompassed only original studies where latent TB infection (LTBI) and active TB disease were diagnosed microbiologically, clinically, histologically, or radiologically. Effect estimates were pooled using fixed - and random-effects models. We identified 18 eligible studies, with 30,757 children and 44,432 adult non-smokers, containing SHS exposure and TB outcome data for inclusion in the meta-analysis. Twelve studies assessed children and eight studies assessed adult non-smokers; two studies assessed both populations. Summary relative risk (RR) of LTBI associated with SHS exposure in children was similar to the overall effect size, with high heterogeneity (pooled RR 1.64, 95% CI 1.00–2.83). Children showed a more than 3-fold increased risk of SHS-associated active TB (pooled RR 3.41, 95% CI 1.81–6.45), which was higher than the risk in adults exposed to SHS (summary RR 1.32, 95% CI 1.04–1.68). Positive and significant exposure–response relationships were observed among children under 5 y (RR 5.88, 95% CI 2.09–16.54), children exposed to SHS through any parent (RR 4.20, 95% CI 1.92–9.20), and children living under the most crowded household conditions (RR 5.53, 95% CI 2.36–12.98). Associations for LTBI and active TB disease remained significant after adjustment for age, biomass fuel (BMF) use, and presence of a TB patient in the household, although the meta-analysis was limited to a subset of studies that adjusted for these variables. There was a loss of association with increased risk of LTBI (but not active TB) after adjustment for socioeconomic status (SES) and study quality. The major limitation of this analysis is the high heterogeneity in outcomes among studies of pediatric cases of LTBI and TB disease.Conclusions:
We found that SHS exposure is associated with an increase in the relative risk of LTBI and active TB after controlling for age, BMF use, and contact with a TB patient, and there was no significant association of SHS exposure with LTBI after adjustment for SES and study quality. Given the high heterogeneity among the primary studies, our analysis may not show sufficient evidence to confirm an association. In addition, considering that the TB burden is highest in countries with increasing SHS exposure, it is important to confirm these results with higher quality studies. Research in this area may have important implications for TB and tobacco control programs, especially for children in settings with high SHS exposure and TB burden.
Published in the journal: . PLoS Med 12(6): e32767. doi:10.1371/journal.pmed.1001835
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001835Summary
Background:
According to WHO Global Health Estimates, tuberculosis (TB) is among the top ten causes of global mortality and ranks second after cardiovascular disease in most high-burden regions. In this systematic review and meta-analysis, we investigated the role of second-hand smoke (SHS) exposure as a risk factor for TB among children and adults.Methods and Findings:
We performed a systematic literature search of PubMed, Embase, Scopus, Web of Science, and Google Scholar up to August 31, 2014. Our a priori inclusion criteria encompassed only original studies where latent TB infection (LTBI) and active TB disease were diagnosed microbiologically, clinically, histologically, or radiologically. Effect estimates were pooled using fixed - and random-effects models. We identified 18 eligible studies, with 30,757 children and 44,432 adult non-smokers, containing SHS exposure and TB outcome data for inclusion in the meta-analysis. Twelve studies assessed children and eight studies assessed adult non-smokers; two studies assessed both populations. Summary relative risk (RR) of LTBI associated with SHS exposure in children was similar to the overall effect size, with high heterogeneity (pooled RR 1.64, 95% CI 1.00–2.83). Children showed a more than 3-fold increased risk of SHS-associated active TB (pooled RR 3.41, 95% CI 1.81–6.45), which was higher than the risk in adults exposed to SHS (summary RR 1.32, 95% CI 1.04–1.68). Positive and significant exposure–response relationships were observed among children under 5 y (RR 5.88, 95% CI 2.09–16.54), children exposed to SHS through any parent (RR 4.20, 95% CI 1.92–9.20), and children living under the most crowded household conditions (RR 5.53, 95% CI 2.36–12.98). Associations for LTBI and active TB disease remained significant after adjustment for age, biomass fuel (BMF) use, and presence of a TB patient in the household, although the meta-analysis was limited to a subset of studies that adjusted for these variables. There was a loss of association with increased risk of LTBI (but not active TB) after adjustment for socioeconomic status (SES) and study quality. The major limitation of this analysis is the high heterogeneity in outcomes among studies of pediatric cases of LTBI and TB disease.Conclusions:
We found that SHS exposure is associated with an increase in the relative risk of LTBI and active TB after controlling for age, BMF use, and contact with a TB patient, and there was no significant association of SHS exposure with LTBI after adjustment for SES and study quality. Given the high heterogeneity among the primary studies, our analysis may not show sufficient evidence to confirm an association. In addition, considering that the TB burden is highest in countries with increasing SHS exposure, it is important to confirm these results with higher quality studies. Research in this area may have important implications for TB and tobacco control programs, especially for children in settings with high SHS exposure and TB burden.Introduction
According to recent global health estimates, tuberculosis (TB) is among the top ten causes of global mortality and ranks second after cardiovascular disease (CVD) in most high-burden regions [1,2]. In the latest WHO Global Tuberculosis Report [3], it was estimated that approximately one-third of the world’s population is infected with Mycobacterium tuberculosis (Mtb), and that each year 8.6 million people develop TB disease and 1.3 million die of the disease. Children contribute a substantial burden of new TB cases (6%) and deaths (8%) globally [3,4]. The international community of TB researchers has recognized the urgent need to address pediatric TB, and is now working toward reducing child deaths from TB to zero [5].
Among the many risk factors for TB, smoking of tobacco products has recently been restudied for its association with TB [6,7]. Biologically plausible mechanisms for the effect of smoking on risk of TB infection include decrease in ciliary function, alterations in macrophage number and response [8,9], and decrease in CD4 and CD8 cells that produce interferon gamma and TNF alpha [9]. There is some evidence indicating damaging effects of second-hand smoke (SHS; also referred to as involuntary smoking, passive smoking, or environmental tobacco smoke [ETS]) on the innate immune system of the lungs as well as the adaptive immune response, leading to a greater susceptibility to Mtb infection and progression to active TB [10,11]. However, the link between SHS exposure and TB is not fully understood. In an attempt to fill this gap, we conducted a systematic review and meta-analysis to examine the association of SHS exposure with the risk of latent TB infection (LTBI) and active TB disease in children and adult non-smokers.
Methods
Search Strategy
We did a systematic review (according to PRISMA guidelines) to identify studies related to SHS exposure and TB. We searched Medline (via PubMed) and Embase for studies published between January 1, 1946, and August 31, 2014 (for search terms see Box 1). Articles resulting from these searches and relevant references cited in those articles were reviewed.
Box 1. Search Strategy and Terms Used to Identify Studies on Second-Hand Smoke Exposure and TB
Medline term search:
“tuberculosis”
“second-hand smoking”
“environmental exposure”
“passive smoking”
“tobacco pollution”
“cohort studies” OR “case-control studies” OR “cross-sectional studies” OR “epidemiologic studies” OR “prospective studies” OR “ratio” OR “risk”
“(1) AND (2) AND (6)” OR “(1) AND (3) AND (6)” OR “(1) AND (4) AND (6)” OR “(1) AND (5) AND (6)”
Embase term search:
“tuberculosis”
“second-hand smoking”
“environmental exposure”
“passive smoking”
“tobacco pollution”
“cohort studies” OR “case-control studies” OR “cross-sectional studies” OR “epidemiologic studies” OR “prospective studies” OR “ratio” OR “risk”
“(8) AND (9) AND (13)” OR “(8) AND (10) AND (13)” OR “(8) AND (11) AND (13)” OR “(8) AND (12) AND (13)”
Google Scholar term search:
“tuberculosis”
“second-hand smoking”
“environmental exposure”
“passive smoking”
“tobacco pollution”
“cohort studies” OR “case-control studies” OR “cross-sectional studies” OR “epidemiologic studies” OR “prospective studies” OR “ratio” OR “risk”
“(15) AND (16) AND (20)” OR “(15) AND (17) AND (20)” OR “(15) AND (18) AND (20)” OR “(15) AND (19) AND (20)”
The searches and studies included were not limited by publication date, country, or language. Database searches were conducted independently by two authors (J. P. and M. B.). To ensure comprehensive acquisition of literature, independent supplemental manual searches were performed on the reference lists of relevant articles and other databases, including Scopus, Web of Science, and Google Scholar. The contents of the abstracts or full-text articles identified during the literature search were reviewed independently by two reviewers (J. P. and M. B.) to determine whether they met the criteria for inclusion.
Inclusion and Exclusion
Articles were independently identified by two reviewers for inclusion and in-depth examination. Our inclusion criteria selected only (1) original studies where LTBI or active TB were diagnosed with clinical, radiological, histological, and/or microbiological diagnostic tools; (2) cohort studies, case-control studies, or cross-sectional surveys (case reports, review articles, and editorials were excluded); and (3) studies on human participants aged ≤15 y, (i.e., children) or >15 y (i.e., adults). For included articles reporting on the same study population, the more detailed article was selected. Discrepancies in article inclusion between reviewers were resolved by consensus. We categorized exposures as SHS or ETS exposure, parental smoking, household smoking or presence of household smoker(s), and regular contact with smokers. Study selection is summarized in Fig 1. The exclusion criteria disallowed studies in which active smoking was not distinguished from passive smoking, active smoking was the only exposure, or prenatal exposure was included. We excluded studies that explored high-risk populations with HIV or other immune-compromising conditions.
Fig. 1. Flowchart of study identification and inclusion. 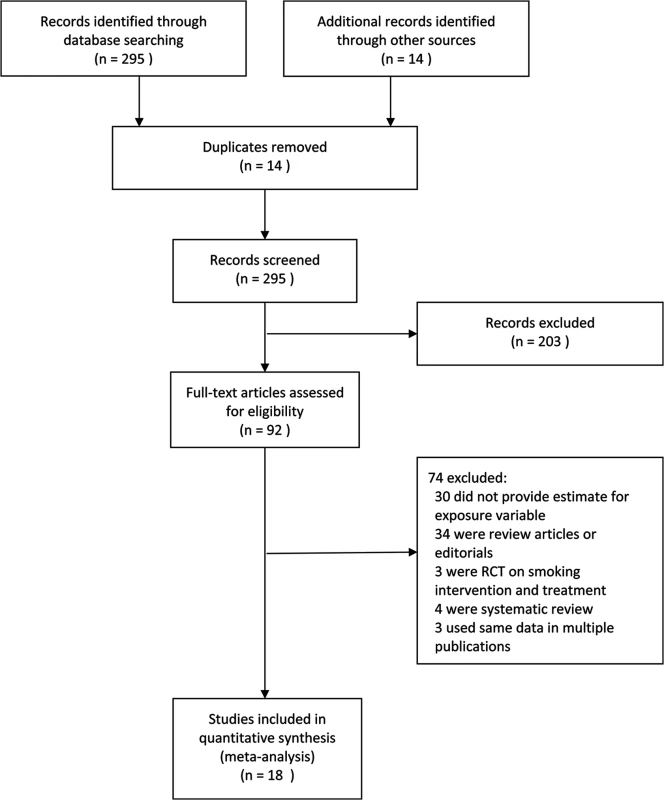
RCT, randomized controlled trial. Data Extraction and Synthesis
Data extracted included study location, setting (e.g., community, hospital, etc.), participant age, number of participants, exposure definition and levels, diagnosis of outcome, crude and adjusted effect sizes, and 95% confidence intervals (CIs). The presence of appropriate controls and the covariates used for adjustment in multivariate analysis were extracted as study design quality indicators. Study authors were contacted as necessary to obtain pertinent data not published in the articles. When necessary, we manually calculated the unadjusted odds ratio from raw data in an article for inclusion in the meta-analysis, or excluded the article if this was not possible. We assessed the quality of the included studies using the Newcastle–Ottawa scale (S1 Table) [12].
Statistical Analysis
We conducted meta-analyses of risk estimates for LTBI and active TB disease for exposure to SHS compared with non-exposure to SHS in non-smoking children and adults, and we report pooled estimates and 95% CIs. For data extraction, analysis, and reporting, we used the PRISMA guidelines for meta-analysis of observational studies (S1 Text) [13]. We tested for and quantified heterogeneity with the Cochran Q statistic (p < 0.05) and I2 statistic, respectively, to describe the variation in effect size attributable to heterogeneity across studies [14,15]. We used the I2 statistic to select the pooling method: fixed-effects models for I2 < 50% and random-effects models for I2 ≥ 50% [14,15]. CIs of I2 were calculated by the method suggested by Higgins and Thompson [15]. We summarized pooled odds ratios using the inverse-variance method for fixed-effects models and the DerSimonian and Laird method for random-effects models [14]. We used Galbraith plots to visualize the impact of individual studies on the overall homogeneity test statistic [16]. We used meta-regression to evaluate whether effect size estimates were significantly different by specific study characteristics and quality factors, specifically, adjustment for covariates and for laboratory-confirmed TB diagnosis (most rigorous method) versus a mix of clinical and laboratory-confirmed diagnosis (less rigorous). We also re-estimated the effect size stratified on the same study characteristics and quality factors to produce separate estimates. Subgroup analysis was conducted on all relevant study characteristics regardless of statistical significance. We investigated the presence and the effect of publication bias using a combination of the Begg’s test [15] and Egger’s test [17]. Statistical analyses were performed using Stata 12.1 (StataCorp). The metan, heterogi, metareg, metabias, galbr, metatrim, and metafunnel macros were used for meta-analytic procedures. p-Values < 0.05 were considered statistically significant. An overview of the study protocol is provided in S1 Protocol.
Results
Our search strategy resulted in 309 studies, of which 92 were deemed relevant upon initial inspection of study titles. Eighteen studies, with 30,757 children and 44,432 adult non-smokers, met all of the inclusion criteria and were included in the meta-analysis (Fig 1). There was only one non-English (Spanish) study included in the analysis [18]. Six studies [19–24] had LTBI as an outcome of interest, and the remaining 12 studies [18,25–35] had active TB as an outcome of interest. Twelve studies [18–22,24,26,29,31,33–35] assessed children and eight studies [22,23,25,27,28,30–32] assessed adult non-smokers; two studies [22,31] assessed both populations. All studies with active TB as an outcome had information on dose–response by a variety of variables, including age [18,25–35], amount of smoke exposure in the household [21,26,33], contact with a TB patient [20,21,24,26,35], and household crowdedness [18,26,35], with at least three exposure categories. Table 1 summarizes the study characteristics. Given the significant heterogeneity among the effect estimates of these studies, we conducted sensitivity analyses with important study characteristics for each outcome for both children and adults.
Tab. 1. Study characteristics. 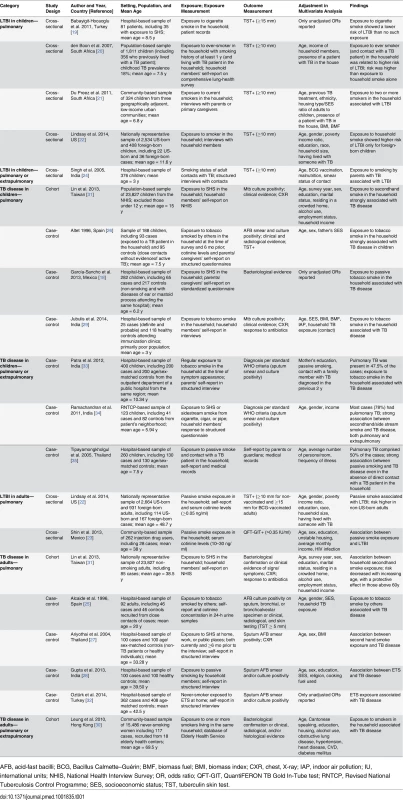
AFB, acid-fast bacilli; BCG, Bacillus Calmette–Guérin; BMF, biomass fuel; BMI, biomass index; CXR, chest, X-ray; IAP, indoor air pollution; IU, international units; NHIS, National Health Interview Survey; OR, odds ratio; QFT-GIT, QuantiFERON TB Gold In-Tube test; RNTCP, Revised National Tuberculosis Control Programme; SES, socioeconomic status; TST, tuberculin skin test. Second-Hand Smoke Exposure and Latent TB Infection
We identified six studies [19–24] reporting the relative risk (RR) of LTBI upon exposure to SHS (Table 2). All six studies were cross-sectional, and half [20–22] were assessed to be of good quality. These studies were conducted in diverse WHO regions including Europe [19], South-East Asia [24], Africa [20,21], and the Americas [22,23]. Overall, LTBI was positively associated with SHS exposure (pooled RR 1.67, 95% CI 1.12–2.48, p for heterogeneity = 0.001, I2 = 65.3%) (see Fig 2A). Relative risk was higher in the South-East Asia Region (RR 2.93, 95% CI 1.97–4.36) and African Region (RR 2.11, 95% CI 1.00–4.49) than in the other regions. Our analysis was limited since only a few studies adjusted for potentially confounding variables. However, among the studies that did show adjusted estimates, effects were significant after adjustment for age (RR 1.78, 95% CI 1.19–2.68), biomass fuel (BMF) use (RR 2.66, 95% CI 1.31–5.39), and contact with a TB patient in the household (RR 2.03, 95% CI 1.25–3.28) (Table 2). There was no significant association between SHS exposure and LTBI after a combined adjustment for age and socioeconomic status (SES) (RR 1.11, 95% CI 0.54–2.31) or after adjustment for just SES (RR 1.50, 95% CI 0.93–2.43), although age - and SES-adjusted estimates were significant in the subgroup analysis for adults (pooled RR 1.58, 95% CI 1.03–2.43, p for heterogeneity = 0.466, I2 = 0.0%) (S2 Table) [22,23]. Meta-regression analysis showed significant effect size modification for studies adjusted for SES (p = 0.033) and marginal significance for studies adjusted for presence of a TB patient in the household (p = 0.105).
Fig. 2. Risk of latent TB infection and active TB disease for second-hand smoke exposure compared to non-exposure in children and adults. 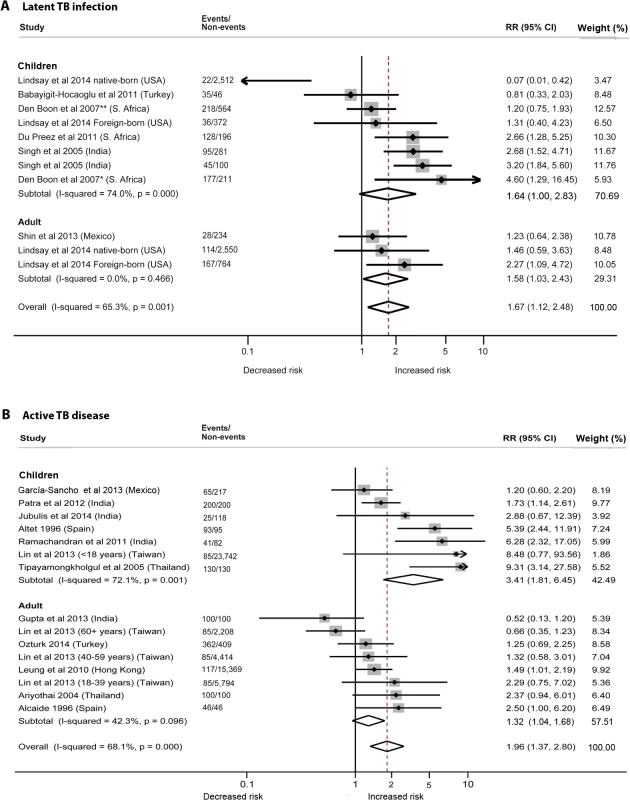
(A) LTBI; (B) active TB disease. Singh et al. [24] reported SHS risks for children with contacts with sputum-negative (95/281) and sputum-positive TB patients (45/100). The effect estimate (diamond) for US-born children in the study by Lindsay et al. [22] is not displayed due to its smaller size and weight. Lin et al. [31] did not report age-stratified TB cases. Weights are from random-effects analysis. *Patient with TB living in house. **No patient with TB living in house. Tab. 2. Quality assessment and subgroup analysis: second-hand smoke exposure and latent TB infection. 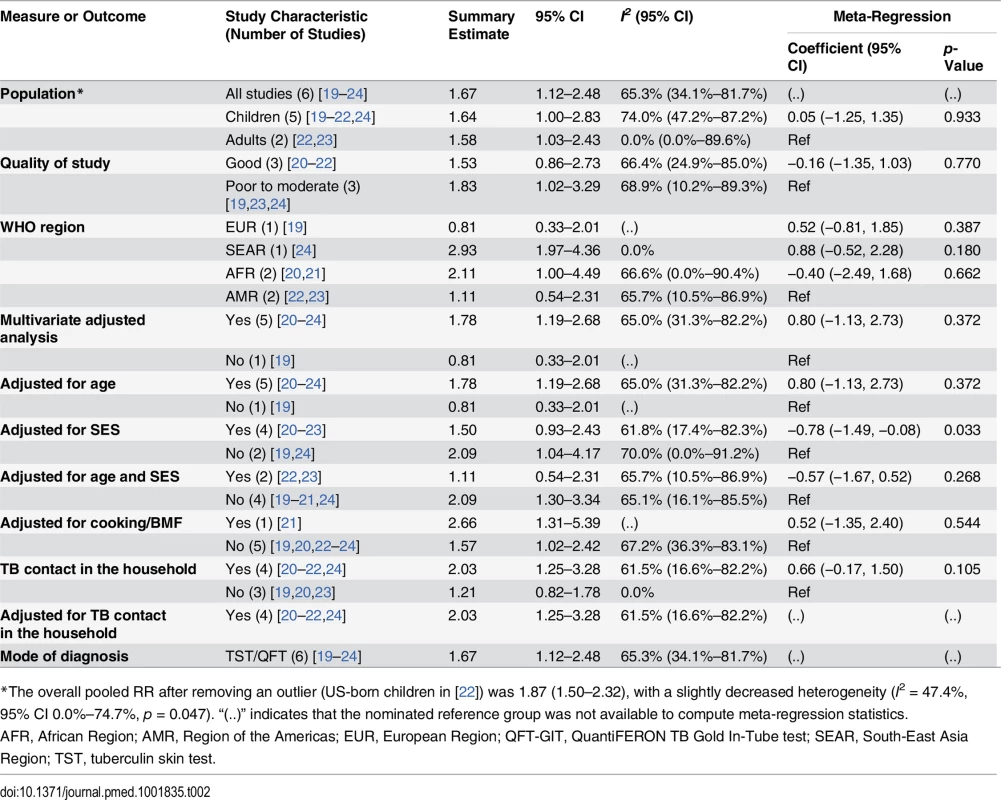
*The overall pooled RR after removing an outlier (US-born children in [22]) was 1.87 (1.50–2.32), with a slightly decreased heterogeneity (I2 = 47.4%, 95% CI 0.0%–74.7%, p = 0.047). “(..)” indicates that the nominated reference group was not available to compute meta-regression statistics. The summary relative risk of LTBI associated with SHS exposure in children derived from five studies [19–22,24] was similar to the overall effect size (pooled RR 1.64, 95% CI 1.00–2.83), but substantial heterogeneity was observed (I2 = 74.0%). Both the Galbraith plot and funnel plot showed that one study estimate from US [22] was a potential source of heterogeneity. The distinct findings (very low effect size) in this study may have resulted from a difference in the methodology: stratification of the sample in the pediatric population by their birth place (US-born versus foreign-born). When this single study estimate (RR = 0.07) was excluded, the overall effect estimate increased and the heterogeneity score improved (pooled RR 1.87, 95% CI 1.50–2.32, p for heterogeneity = 0.047, I2 = 47.4%); therefore, in a subsequent LTBI analysis for children we presented a sensitivity analysis without the outlier (see S2 Table for pooled and subgroup analysis). With this outlier removed, summary relative risk of LTBI associated with SHS exposure in children was higher than the overall effect size, with modest heterogeneity (pooled RR 2.00, 95% CI 1.30–3.08, p for heterogeneity = 0.022, I2 = 49.5%) (S2 Table). Three [20,21,24] of the five studies in children reported a positive and significant association between SHS exposure and LTBI (Fig 2A). Four of the five studies in children [20–22,24] had adjusted for presence of a TB contact in the household, and the association was found to be stronger (RR 2.79, 95% CI 2.02–3.84); meta-regression analysis indicated that the difference between presence and absence of TB contact had statistical significance (p = 0.045) (S2 Table). Meta-regression analysis did not show any other significant effect size modification by the specific study characteristics considered, possibly because of a relatively small number of studies.
Second-Hand Smoke Exposure and Active TB Disease
Twelve of the 18 studies [18,25–35] discussed the risk of active TB with exposure to SHS (Table 3). The pooled estimate for all 12 studies showed a nearly 2-fold increased risk of TB disease from SHS exposure (pooled RR 1.96, 95% CI 1.37–2.80). The majority of the studies were rated as good quality (10/12, with RR 2.18, 95% CI 1.43–3.32), were case-control studies (10/12, RR 2.32, 95% CI 1.45–3.70), discussed pulmonary TB only (8/12, RR 1.66, 95% CI 1.07–2.57), and used microbiological diagnosis (8/12, RR 1.74, 95% CI 1.15–2.64). Half of the studies [27–29,33–35] were from the South-East Asia Region (RR 2.61, 95% CI 1.26–5.40). Further subgroup analysis revealed that case-control studies with community-based controls showed a notably a stronger association of SHS exposure with active TB (RR 4.90, 95% CI 2.15–11.17, p for heterogeneity = 0.387, I2 = 0.0%) compared to studies with hospital-based controls (RR 1.96, 95% CI 1.16–3.31, p for heterogeneity = 0.017, I2 = 66.8%) or other controls (RR 2.01, 95% CI 0.56–7.20, p for heterogeneity = 0.004, I2 = 82.3%). The stratified analysis for the presence of a TB patient in the household (7/12 studies [26,27,29,30,33–35]) showed a significant association of SHS with active TB disease (RR 3.07, 95% CI 1.82–5.16). After adjusting for TB contacts in the household (3/7 studies [27,29,33]), risk attenuated but remained statistically significant (RR 1.87, 95% CI 1.30–2.69, p for heterogeneity = 0.693, I2 = 0.0%). The association remained positive and statistically significant even after adjustments for both age and SES (RR 2.13, 95% CI 1.18–3.83, p for heterogeneity < 0.001, I2 = 71.4%) and for BMF (RR 2.03, 95% CI 1.13–3.63, p for heterogeneity = 0.004, I2 = 73.9%), although significant heterogeneities were observed. Of study characteristics assessed in meta-regression, studies with a pediatric population (p = 0.028) and presence of a TB patient in the household (p = 0.029) were statistically significant (Table 3). Meta-regression also showed marginal significance (p = 0.105) when a microbiological mode of diagnosis was used for TB outcome compared with other modes of diagnosis.
Tab. 3. Quality assessment and subgroup analysis: second-hand smoke exposure and active TB disease. 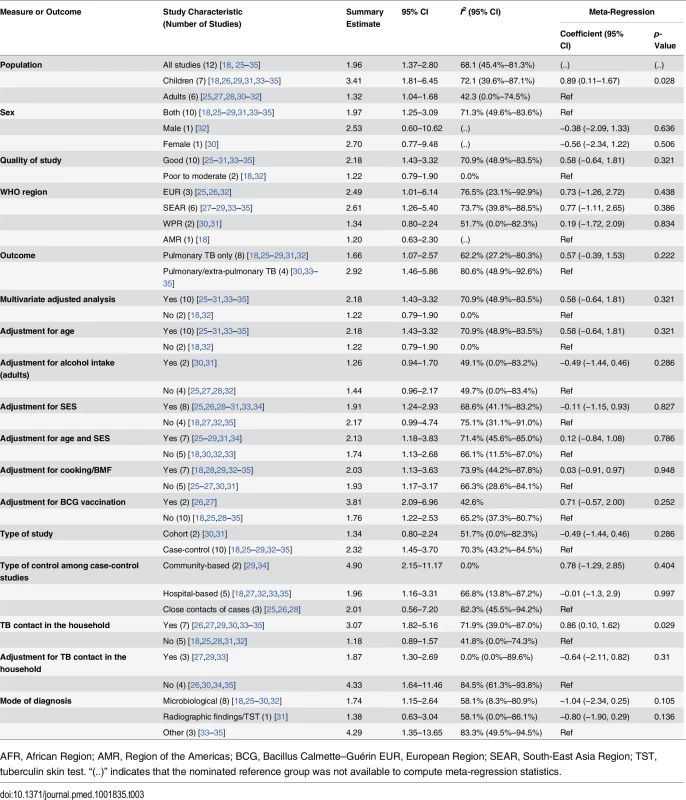
AFR, African Region; AMR, Region of the Americas; BCG, Bacillus Calmette–Guérin EUR, European Region; SEAR, South-East Asia Region; TST, tuberculin skin test. “(..)” indicates that the nominated reference group was not available to compute meta-regression statistics. In subgroup analysis, children showed a more than 3-fold increased risk of SHS-associated active TB (RR 3.41, 95% CI 1.81–6.45), which was also higher than the risk in adults (RR 1.32, 95% CI 1.04–1.68) (S3 Table). In children exposed to SHS, risk of pulmonary TB was three times as high (RR 2.96, 95% CI 1.11–7.85) as in those not exposed to SHS. Risk of pulmonary or extra-pulmonary TB disease was even greater (RR 4.29, 95% CI 1.35–13.65) and was also higher than in adults exposed to SHS (RR 1.49, 95% CI 1.01–2.19). We found strong associations between SHS exposure and active TB, particularly for children, in the stratified analysis by outcome definition, study design, control population (for case-control studies), multivariate adjustment, and mode of diagnosis (S3 Table). Stratification by most of these study-specific variables did not fully explain the variability but partially accounted for the heterogeneity. The association with SHS exposure was stronger for pulmonary or extra-pulmonary TB disease than for pulmonary TB alone in both men and women. However, effect estimates for pulmonary TB were not statistically different from those for any TB disease (p = 0.222) (see Table 3). Among studies that reported pulmonary/extra-pulmonary TB, pulmonary TB represented 86% of total active TB cases (72% among children and 97% among adults). The heterogeneity among these studies was largely explained by the age of the populations. Four of seven studies among children [26,33–35] showed strong associations between SHS exposure and active TB, although there was significant heterogeneity among studies (I2 = 72.1%) (Fig 2B). Among adults, five out of six studies [25,27,28,30,32] used microbiological testing as the mode of diagnosis (RR 1.47, 95% CI 1.11–1.94) and showed low heterogeneity (I2 = 33.1%). Meta-regression for adult and child subgroup analyses showed no significant effect size modification by most study-specific variables, except for adjusted estimates in children living with a TB contact in the household (p = 0.033).
Factors Showing a Dose–Response Relation with Risk of Active TB Disease
Among those exposed to SHS who developed active TB disease, several socio-demographic and household factors showed a dose–response relation. For children, these factors included age, frequency and closeness of contact with a TB patient in the household, household crowdedness, and relationship with the smoker. For adults, these factors were age, contact with a TB patient in the household, number of smokers in the household, and number of cigarettes smoked by household members.
While each age group showed an increased risk of TB disease with exposure to SHS, children aged 0–5 y showed the highest relative risk (RR 5.88, 95% CI 2.09–16.54) (Fig 3). Relative risk decreased progressively with age (p trend < 0.001). Within the adult population, those aged 19–40 y were at a higher risk than older adults (RR 1.76, 95% CI 1.06–2.90).
Fig. 3. Dose–response relationship between second-hand smoke exposure and active TB disease in children and adults. 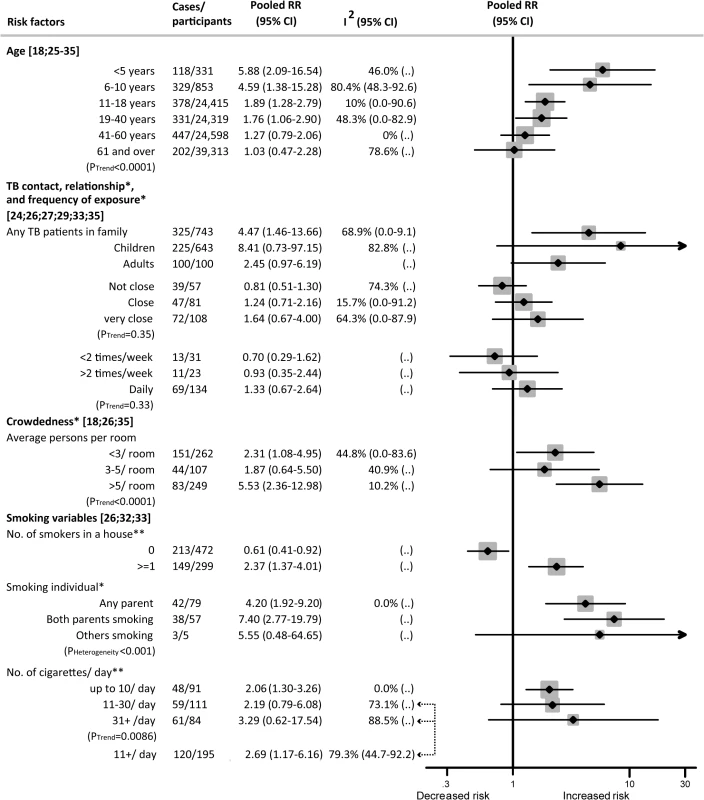
Weights are from random-effects analysis. “(..)” indicates that not enough studies were available to compute I2 or confidence intervals around I2. *Children only. **Adults only. Those exposed to SHS and in contact with a TB patient in the household had a greater than four times higher risk of developing active TB (RR 4.47, 95% CI 1.46–13.66) than those unexposed to SHS and without a TB patient in the household. When stratified by sub-populations, SHS exposure was associated with an 8-fold increase in the risk of active TB disease in children (RR 8.41, 95% CI 0.73–97.15) and a 2.5-fold increase in adults (RR 2.45, 95% CI 0.97–6.19), with marginal significance (Fig 3). For children exposed to SHS versus those not exposed, the relative risk of active TB increased progressively, but not significantly, with closeness of contact to a TB patient (RR 1.64, 95% CI 0.67–4.00) and daily exposure to a TB contact (RR 1.33, 95% CI 0.67–2.64) compared to those with less closeness or less frequent exposure. A clear dose–response was found when stratified by both crowdedness and exposure intensity to SHS. SHS exposure in children living in households with more than five people per room showed a significantly higher association with active TB (RR 5.53, 95% CI 2.36–12.98, p trend < 0.001) than SHS exposure in those living in households with fewer people per room. Similar risk was observed for children with one parent (RR 4.20, 95% CI 1.92–9.20) or both parents (RR 7.40, 95% CI 2.77–19.79) smoking (p for heterogeneity < 0.001).
Among non-smoking adults, a linear, increasing dose–response was observed for the frequency of smokers in their household and the smoking quantity of those smokers (p trend = 0.0086). Any number of smokers in the household was strongly associated with an increased risk of TB (RR 2.37, 95% CI 1.37–4.01), while having no smokers in the household was not (RR 0.61, 95% CI 0.41–0.92). SHS exposure to those smoking ten cigarettes or more per day was significantly associated with a higher risk of TB (RR 2.69; 95% CI 1.17–6.16) (Fig 3).
Publication Bias
When we plotted the natural logarithms of the effect estimates against their standard errors (Fig 4), we detected very little asymmetry of effect estimates among small studies on TB disease [26,28,34,35]. We also noted that one data point for a large study on LTBI [22] fell outside the boundary of the funnel plot and Galbraith plot. Exclusion from the analysis decreased heterogeneity scores overall and among children. No evidence for substantial publication bias was found by the Begg’s test (p = 0.37) or Egger’s test (p = 0.79).
Fig. 4. Funnel plot with pseudo 95% confidence limits. 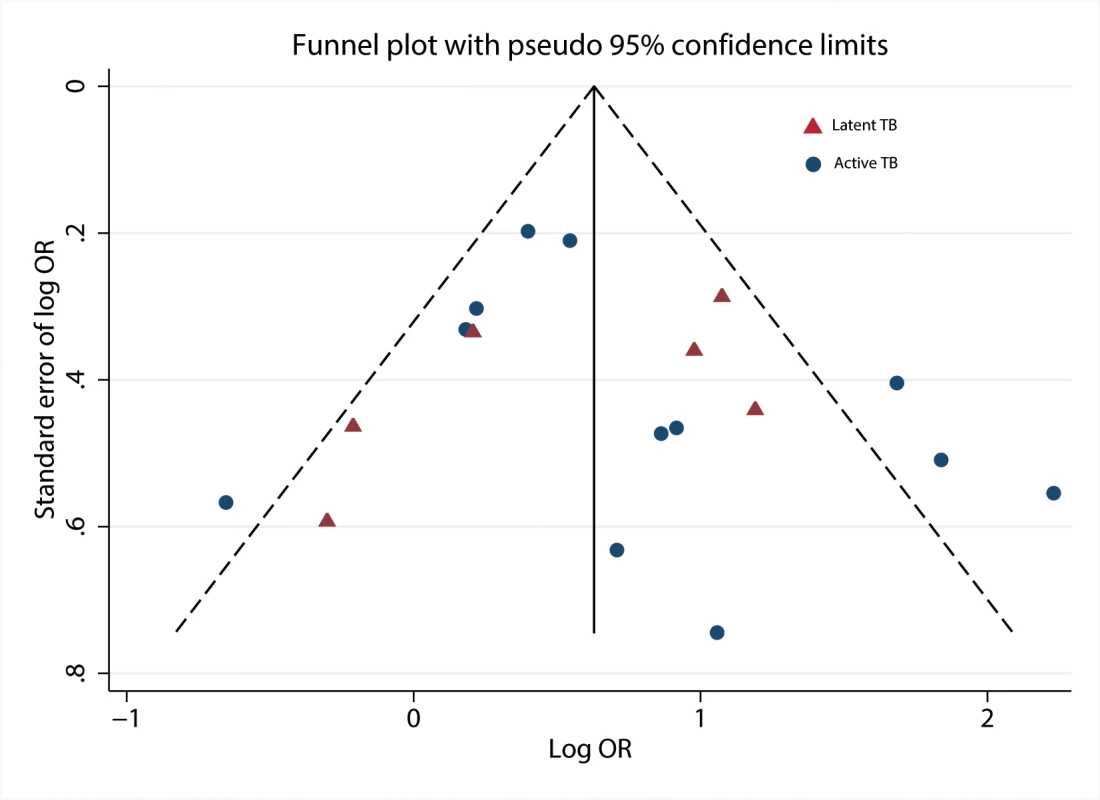
OR, odds ratio. Discussion
In this meta-analysis, SHS exposure appeared to be associated with LTBI and active TB after adjustment for a limited number of factors, although one caveat is that the possibility of confounding could not be completely ruled out, especially in the stratified analyses for SES and the study quality. Among the studies with adjusted measures, we found that SHS exposure was associated with an increased relative risk of TB and LTBI infection after controlling for age, BMF use, contact with a TB patient, SES, and study quality; the association of SHS exposure with LTBI was significant after adjustments for the above factors, except for SES and study quality. Much of the evidence on the biological mechanism linking SHS exposure to TB development concerns the effects of mainstream cigarette smoke, i.e., residual smoke exhaled upon cigarette smoking [10]. However, sidestream smoke that is released from a burning cigarette can also adversely affect the pulmonary mechanical barriers and immune system to influence TB disease development [10,11]. Prolonged exposure to cigarette smoke damages the first line of defense against Mtb by inducing growth of abnormal cilia on the pulmonary lining. These cilia may impair the mucociliary clearance process that is responsible for trapping contaminants such as Mtb in the respiratory tract [10]. The pulmonary immune system in smokers is also weakened because of reduced T cell proliferation and suboptimal functioning of alveolar macrophages [10], which can also lead to increased susceptibility to Mtb infection and, subsequently, progression to TB disease.
Regional variations were found in the risk of LTBI and active TB. The highest risk was observed in South-East Asia and sub-Saharan Africa. This is not surprising considering that certain countries comprising these regions and represented in this analysis, specifically India, South Africa, and Thailand, are among those with the highest burden of TB disease [3,4]. It is interesting to note that a high risk of active TB due to SHS exposure was also found in the studies conducted in Spain. Spain also has a high prevalence of SHS exposure in both children and adults compared to the US, indicating that even in the setting of a developed economy, exposure to SHS may be related to the risk of developing active TB [36].
The high risk for children under 5 y, and the increase in risk for both children and adults, with number of smokers in the household and household crowdedness shows that when exposed to SHS, household/environmental factors may increase the risk of TB. Mainstream and sidestream smoke in households is the major source of SHS exposure for children, and living in a crowded house with adult smokers, especially parents, prolongs children’s exposure. The increased risk of TB disease presented in this meta-analysis supports the association between SHS exposure and active TB disease [37].
Strengths and Limitations
To our knowledge, this is the first systematic review and meta-analysis of the epidemiological evidence on the association between SHS exposure and latent/active TB in children and adults. We assessed sensitivity to study design, methods, and quality indicators using meta-regression and subgroup analysis, and we searched multiple databases without language limitation for our systematic review. The major limitation of this analysis is the high heterogeneity in outcomes among the studies investigating pediatric cases of TB disease. Sputum is difficult to obtain in children, and TB disease is largely paucibacillary, which makes microbiological diagnosis in children a challenge. The studies in this meta-analysis are highly variable in terms of the diagnostic tests (microbiological, clinical, and/or radiological) used to diagnose TB in children. There is a possibility of confounding in our meta-analysis since all the studies we included were observational. The major potential confounding factors included age, SES, BMF use, and contact with a TB patient in the household. Newborns, young adults, and socioeconomically disadvantaged individuals, including those frequently using BMF, share a larger burden of TB disease [38,39] and a higher exposure to SHS [36,40]. To control for the effect of these potential confounders, we conducted sensitivity analyses of studies that adjusted for these variables. The association between SHS exposure and TB disease remained after adjustment for a limited number of factors (age, BMF use, and TB contact in the household). However, the effect size attenuated after adjustment for SES; this highlights the confounding role of SES, and is also consistent with the existing evidence showing a higher burden of TB in low - and middle-income countries [35]. In addition, these studies do not capture the variability in household air pollution, which is affected by the type of BMF used, kitchen ventilation, and indoor versus outdoor tobacco smoking—factors that affect exposure to SHS and are linked to TB disease [38]. It is challenging to decipher the exact nature of the association between SHS exposure and TB given the relatively few studies that have been published on this topic; however, this only indicates a greater need to further explore the impact of SHS exposure, whether as an independent risk factor or as a factor that works in conjunction with other risk factors, such as BMF exposure and contact with a TB patient, to exacerbate their impact on the risk of TB disease.
Implications
Despite low comparability between studies, this meta-analysis lends to future hypotheses exploring the role of SHS exposure in TB disease, particularly in children and in low - and middle-income countries that have a greater prevalence of SHS exposure. Future studies should aim to use nationally representative datasets to rigorously explore the effect of BMF use on SHS exposure, diverse (and perhaps more reliable) measures of SHS exposure such as hair cotinine levels [41], and pediatric populations with standardized TB diagnostic measures. Exposure to SHS and its impact on TB should be explored further, given that TB deaths are projected to increase from 2010 to 2050 by as much as 167%, as found in a mathematical modelling analysis [42]. Further examination of this association and the potential for subsequent efforts to reduce SHS exposure along with TB control efforts will have important implications for reducing TB incidence and deaths. Probing potential links between SHS exposure and TB may have important implications for TB and tobacco control programs, especially for children in settings with high SHS exposure and TB burden [42,43].
Supporting Information
Zdroje
1. Lozano R, Laghavi M, Foreman K, Lim S, Shibuya K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380 : 2095–2128. doi: 10.1016/S0140-6736(12)61728-0 23245604
2. World Health Organization.Health statistics and information systems: estimates for 2000–2012—cause specific mortality. Geneva: World Health Organization; 2015. http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html. Accessed May 4, 2015.
3. World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization; 2014. http://www.who.int/tb/publications/global_report/en/. Accessed May 4, 2015.
4. Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2:e453–e459. doi: 10.1016/S2214-109X(14)70245-1 25103518
5. World Health Organization. Roadmap for childhood tuberculosis: towards zero deaths. Geneva: World Health Organization; 2013. http://apps.who.int/iris/bitstream/10665/89506/1/9789241506137_eng.pdf. Accessed November 10, 2014.
6. Patra J, Jha P, Rehm J, Suraweera W. Tobacco smoking, alcohol drinking, diabetes, low body mass index and the risk of self-reported symptoms of active tuberculosis: individual participant data (IPD) meta-analyses of 72,684 individuals in 14 high tuberculosis burden countries. PLoS ONE. 2014;9:e96433. doi: 10.1371/journal.pone.0096433 24789311
7. Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4:e20. 17227135
8. Hodge S, Hodge G, Ahem J, Jersmann H, Holmes M, Reynolds PN. Smoking alters alveolar macrophage recognition and phagocytic ability implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2007;37 : 748–755. 17630319
9. Shang S, Ordway O, Henao-Tamayo M, Bai X, Oberley-Deegan R, Shanley C, et al. Cigarette smoke increases susceptibility to tuberculosis—evidence from in vivo and in vitro models. J Infect Dis. 2011;203 : 1240–1248. doi: 10.1093/infdis/jir009 21357942
10. Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2 : 372–377. 12033743
11. Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9 : 377–384. doi: 10.1038/nri2530 19330016
12. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa Hospital Research Institute; 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 2, 2015.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151 : 264–269. 19622511
14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7 : 177–188. 3802833
15. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21 : 1539–1558. 12111919
16. Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7 : 889–894. 3413368
17. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315 : 629–634. 9310563
18. García-Sancho C, Fernandez-Plata R, Martinez-Briseno D, Torre-Bouscoulet L, Gochicoa-Rangel L, Franco-Marina F, et al. Exposure to wood smoke and tuberculosis in children. Neumol Cir Torax. 2013;72 : 281–286.
19. Babayigit-Hocaoglu A, Olmez-Erge D, Anal O, Makay B, Uzuner N, Karaman O. Characteristics of children with positive tuberculin skin test. Tuberk Toraks. 2011;59 : 158–163. 21740391
20. den Boon S, Verver S, Marais BJ, Enarson DA, Lombard CJ, Bateman ED, et al. Association between passive smoking and infection with Mycobacterium tuberculosis in children. Pediatrics. 2007;119 : 734–739. 17403844
21. Du Preez K., Mandalakas AM, Kirchner HL, Grewal HM, Schaaf HS, van Wyk SS, et al. Environmental tobacco smoke exposure increases Mycobacterium tuberculosis infection risk in children. Int J Tuberc Lung Dis. 2011;15 : 1490–1496. doi: 10.5588/ijtld.10.0759 22008762
22. Lindsay RP, Shin SS, Garfein RS, Rusch MLA, Novotny TE. The association between active and passive smoking and latent tuberculosis infection in adults and children in the United States: results from NHANES. PLoS ONE. 2014;9:e93137. doi: 10.1371/journal.pone.0093137 24664240
23. Shin SS, Laniado-Laborin R, Moreno PG, Novotny TE, Strathdee SA, Garfein RS. Dose-response association between salivary cotinine levels and Mycobacterium tuberculosis infection. Int J Tuberc Lung Dis. 2013;17 : 1452–1458. doi: 10.5588/ijtld.13.0311 24125450
24. Singh M, Mynak ML, Kumar L, Mathew JL, Jindal SK. Prevalence and risk factors for transmission of infection among children in household contact with adults having pulmonary tuberculosis. Arch Dis Child. 2005;90 : 624–628. 15908630
25. Alcaide J, Altet MN, Plans P, Parron I, Folguera L, Salto E, et al. Cigarette smoking as a risk factor for tuberculosis in young adults: a case-control study. Tuber Lung Dis. 1996;77 : 112–126. 8762844
26. Altet MN, Alcaide J, Plans P, Taberner JL, Salto E, Folguera L, et al. Passive smoking and risk of pulmonary tuberculosis in children immediately following infection. A case-control study. Tuber Lung Dis. 1996;77 : 537–544. 9039447
27. Ariyothai N, Podhipak A, Akarasewi P, Tornee S, Smithtikarn S, Thongprathum P. Cigarette smoking and its relation to pulmonary tuberculosis in adults. Southeast Asian J Trop Med Public Health. 2004;35 : 219–227. 15272772
28. Gupta D, Vinay N, Agarwal R, Agarwal AN. Socio-demographic profile of patients with sarcoidosis vis-a-vis tuberculosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30 : 186–193. 24284291
29. Jubulis J, Kinikar A, Ithape M, Khandave M, Dixit S, Hotalkar S, et al. Modifiable risk factors associated with tuberculosis disease in children in Pune, India. Int J Tuberc Lung Dis. 2014;18 : 198–204. doi: 10.5588/ijtld.13.0314 24429313
30. Leung CC, Lam TH, Ho KS, Yew WW, Tam CM, Chan WM, et al. Passive smoking and tuberculosis. Arch Intern Med. 2010;170 : 287–292. doi: 10.1001/archinternmed.2009.506 20142576
31. Lin HH, Chiang YT, Chuang JH, Yang SL, Chang HY, Ezzati M, et al. Exposure to secondhand smoke and risk of tuberculosis: prospective cohort study. PLoS ONE. 2013;8:e77333. doi: 10.1371/journal.pone.0077333 24204811
32. Oztürk AB, Kilicaslan Z, Issever H. Effect of smoking and indoor air pollution on the risk of tuberculosis: smoking, indoor air pollution and tuberculosis. Tuberk Toraks. 2014;62 : 1–6. 24814071
33. Patra S, Sharma S, Behera D. Passive smoking, indoor air pollution and childhood tuberculosis: a case control study. Indian J Tuberc. 2012;59 : 151–155. 23362712
34. Ramachandran R, Indu PS, Anish TS, Nair S, Lawrence T, Rajasi RS. Determinants of childhood tuberculosis—a case control study among children registered under revised national tuberculosis control programme in a district of South India. Indian J Tuberc. 2011;58 : 204–207. 22533171
35. Tipayamongkholgul M, Podhipak A, Chearskul S, Sunakorn P. Factors associated with the development of tuberculosis in BCG immunized children. Southeast Asian J Trop Med Public Health. 2005;36 : 145–150. 15906658
36. Oberg M, Jaakkola M, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011;377 : 139–146. doi: 10.1016/S0140-6736(10)61388-8 21112082
37. Seddon JA, Shingadia D. Epidemiology and disease burden of tuberculosis in children: a global perspective. Infect Drug Resist. 2014;18 : 153–165.
38. Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102 : 843–851. doi: 10.1016/j.trstmh.2008.05.028 18639310
39. Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam KB, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2 : 823–860. doi: 10.1016/S2213-2600(14)70168-7 25193349
40. Pisinger C, Helmich LH, Andreasen AH, Jogensen T, Glumer C. Social disparities in children’s exposure to second hand smoke at home: a repeated cross-sectional survey. Environ Health. 2012;11 : 65. doi: 10.1186/1476-069X-11-65 22984822
41. Al-Delaimy WK, Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health. 2002;56 : 66–71. 11801622
42. Basu S, Stuckler D, Bitton A, Glantz SA. Projected effects of tobacco smoking on worldwide tuberculosis control: mathematical modelling analysis. BMJ. 2011;343:d5506. doi: 10.1136/bmj.d5506 21972295
43. World Health Organization. A WHO/The Union monograph on TB and tobacco control: joining efforts to control two related global epidemics. World Health Organization; 2007. http://www.who.int/tobacco/resources/publications/tb_tob_control_monograph/en. Accessed Feb 19, 2015.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 6- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Inequitable and Ineffective: Exclusion of Mental Health from the Post-2015 Development Agenda
- Mistreatment of Women in Childbirth: Time for Action on This Important Dimension of Violence against Women
- HIV Programs for Sex Workers: Lessons and Challenges for Developing and Delivering Programs
- Efficacy of Handwashing with Soap and Nail Clipping on Intestinal Parasitic Infections in School-Aged Children: A Factorial Cluster Randomized Controlled Trial
- Assessing Development Assistance for Mental Health in Developing Countries: 2007–2013
- Achieving Systemic and Scalable Private Sector Engagement in Tuberculosis Care and Prevention in Asia
- Maximizing the Impact of Training Initiatives for Health Professionals in Low-Income Countries: Frameworks, Challenges, and Best Practices
- Shifts in the Antibiotic Susceptibility, Serogroups, and Clonal Complexes of in Shanghai, China: A Time Trend Analysis of the Pre-Quinolone and Quinolone Eras
- Transmission of Multidrug-Resistant and Drug-Susceptible Tuberculosis within Households: A Prospective Cohort Study
- The Potential for Reducing the Number of Pneumococcal Conjugate Vaccine Doses While Sustaining Herd Immunity in High-Income Countries
- Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS–Cognitive and Affective Study
- The Mistreatment of Women during Childbirth in Health Facilities Globally: A Mixed-Methods Systematic Review
- Exposure to Second-Hand Smoke and the Risk of Tuberculosis in Children and Adults: A Systematic Review and Meta-Analysis of 18 Observational Studies
- Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Mistreatment of Women during Childbirth in Health Facilities Globally: A Mixed-Methods Systematic Review
- The Potential for Reducing the Number of Pneumococcal Conjugate Vaccine Doses While Sustaining Herd Immunity in High-Income Countries
- Mistreatment of Women in Childbirth: Time for Action on This Important Dimension of Violence against Women
- Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



