-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe First Use of the Global Oral Cholera Vaccine Emergency Stockpile: Lessons from South Sudan
Andrew Azman and colleagues describe their experience of deploying >250,000 doses of oral cholera vaccine in South Sudan in 2014
Published in the journal: . PLoS Med 12(11): e32767. doi:10.1371/journal.pmed.1001901
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1001901Summary
Andrew Azman and colleagues describe their experience of deploying >250,000 doses of oral cholera vaccine in South Sudan in 2014
Summary Points
A global oral cholera vaccine (OCV) stockpile was established in 2013 to improve rapid access to the vaccine in outbreaks and emergencies in which cholera risk is high. The first deployment from the global OCV stockpile was to South Sudan in 2014 because of high cholera risk from massive population displacements within the civil war.
256,700 doses of OCV were delivered, with high coverage, throughout the country as part of a comprehensive cholera prevention strategy by multiple agencies, some of which had little to no previous experience with this vaccine.
A cholera epidemic began during vaccination, and a basic comparison of epidemic curves in vaccinated and unvaccinated areas suggests little to no transmission occurred in vaccinated areas, though more in depth analysis is needed.
This deployment highlights the feasibility of effective deployments from the OCV stockpile and the importance of strong coordination between governmental and nongovernmental agencies in cholera prevention and control planning from the assessment of cholera risk to the deployment of the vaccines.
A larger global supply of OCV is urgently needed to cover those most in need. With limited vaccine availability now and likely in the upcoming years, more work is needed on deciding how to most efficiently use the vaccine, which may include alternative dosing regimens and targeting specific subpopulations.
Background
In December 2013, violence erupted in South Sudan’s capital, Juba, and quickly spread throughout the country. The crisis exacerbated an already dire humanitarian situation in the youngest and one of the poorest countries in the world, where 40% of the population had access to basic health services, 70% to an improved water supply, and only 13% to adequate sanitation facilities [1,2]. Continued fighting and the threat of escalating violence resulted in displacement of more than one in five people throughout the country, many of them residing in Protection of Civilian (PoC) sites inside United Nations Mission bases and spontaneous settlements of internally displaced persons (IDPs).
The Decision to Use Oral Cholera Vaccine (OCV)
The appalling conditions of the PoC and IDP camps throughout the country led to early discussions between the South Sudan Ministry of Health (MoH), the World Health Organization (WHO), and other partners about the possibility of a cholera outbreak exploding within the camps. Based on the perceived success of a preventative cholera vaccination campaign in Maban County, South Sudan, the previous year [3], the MoH and partners agreed that oral cholera vaccination should be part of an emergency preventative health package and commissioned a rapid risk assessment. The assessment, conducted by the MoH and WHO in January 2014, confirmed that the poor water and sanitation conditions, poor hygiene behaviors, low nutritional status of IDPs, overcrowded camps, and high acute watery diarrhea rates all pointed to an increased likelihood of a cholera epidemic with significant morbidity and mortality if cholera were to be introduced to the camps.
Within six weeks from the start of the emergency (Fig 1, S1 Fig), the MoH made the first-ever request to the International Coordinating Group (ICG) to access the global OCV stockpile (http://www.who.int/cholera/vaccines/ocv_stockpile_2013/en/) while at the same time planning for increased water sanitation and hygiene activities through the National Cholera Taskforce. The emergency stockpile, established in 2013, currently manages most of the world’s (limited) supply of Shanchol (Shantha Biotechnics, a Sanofi Company), one of the two internationally licensed OCVs, with 2–3 million doses available in 2014 and 2015 and more projected for future years. This stockpile follows a similar model to other existing emergency vaccine stockpiles like those for yellow fever and meningitis to allocate vaccines rapidly for outbreaks and other situations in which disease risk is high [4].
Fig. 1. Timeline of key vaccination events in South Sudan in 2014. 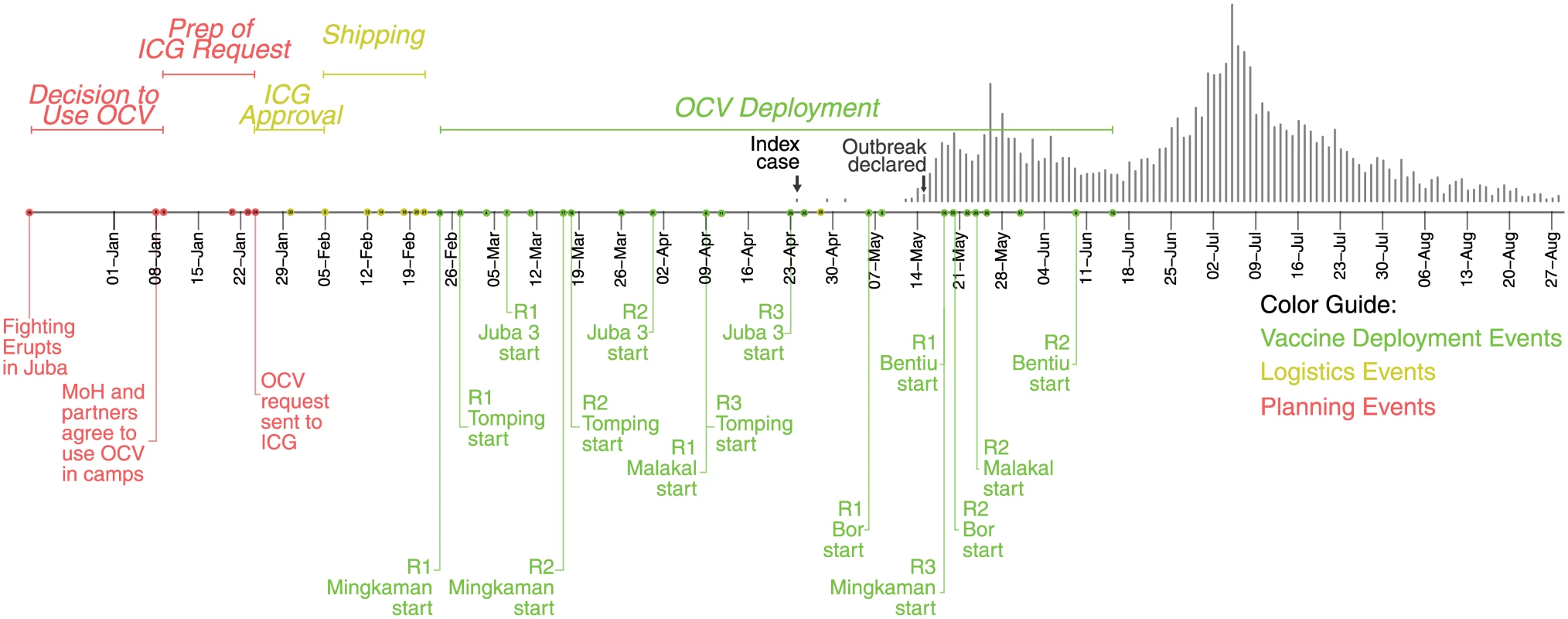
The dots on the timeline represent key vaccination-related events throughout the country. Labels are shown for a selection of events, with the complete timeline shown in S1 Fig. Colors represent different phases of the vaccination campaigns, with red representing planning events, yellow representing logistical activities leading up to the vaccine deployment, and green representing vaccination deployment activities. The epidemic curve is shown as grey vertical bars above. The target population for this first request included 163,000 IDPs but did not include the broader host communities because of limited vaccine availability and the assessed lower risk of spread in these areas. Within one week, part of the request (188,000 doses) was approved with a second approval for other locations (including Juba) provided a week after (64,000 doses). The first batch of OCV arrived in country 30 days after the initial request was made and 24 days after initial approval because of shipping and logistical constraints (Fig 1, S1 Fig).
Multisite Multipartner Oral Cholera Vaccination Campaigns
This intervention targeted all nonpregnant IDPs who were at least one year old in PoC camps in Juba (Juba 3 and Tongping), Bentiu, Bor, and Malakal, in addition to Mingkaman camp (Fig 2). Unlike other OCV campaigns [3,5,6], this first deployment from the stockpile involved multiple agencies with shared roles and responsibilities. The WHO coordinated the vaccine request and the overall strategy and ensured coherence among agencies participating in the immunization sessions. United Nations Children's Fund (UNICEF) kept the central stock of vaccine and guaranteed storage under adequate cold chain. The campaigns were implemented by four different agencies: Médecins Sans Frontières (MSF), Medair, International Organization for Migration, and International Medical Corps. Only MSF had prior experience leading an OCV campaign.
Fig. 2. Epidemic curves of suspected cholera cases in key vaccinated (green) and unvaccinated areas (blue). 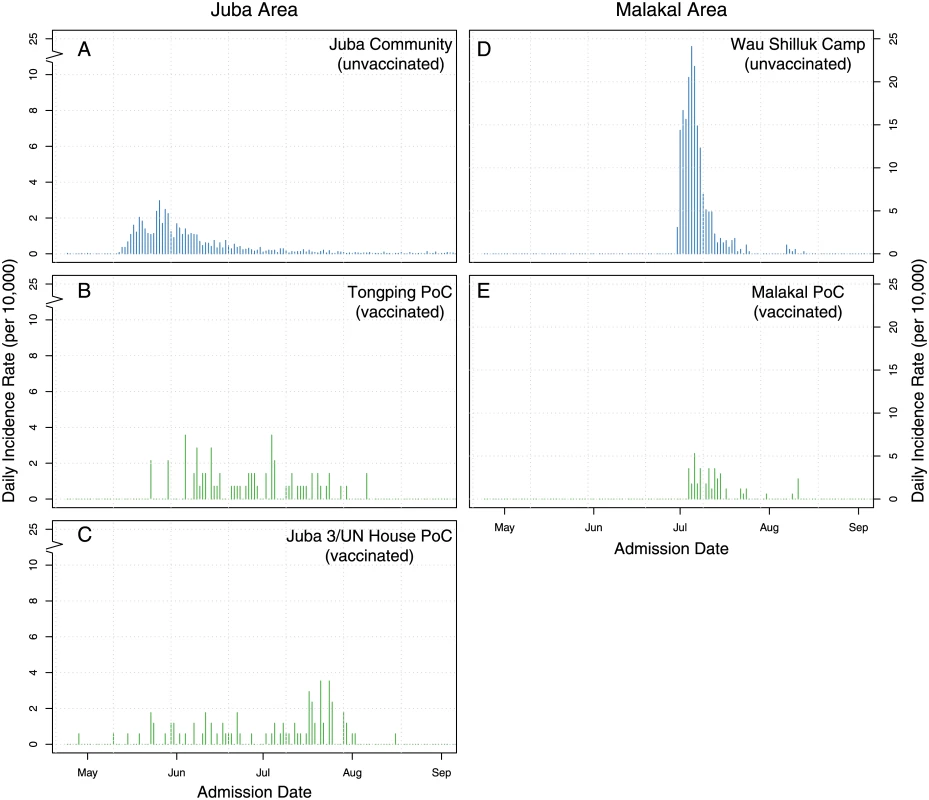
Panels show the daily incidence rate of suspected cholera cases in the Juba community (A), the Juba PoC camps (B–C), Wau Shilluk camp (D), and Malakal PoC camp (E). Note that panels A–C have a break in the y-axis, as they had a much smaller incidence rate. The first campaign started on 23 February in Mingkaman, and the last one on 19 May in Bentiu; thus, nearly continuous vaccination activities occurred in different parts of the country for three months (Fig 1). In three of the six sites, an additional (third) vaccination round was conducted to provide an additional opportunity for new arrivals to receive the second and final dose of OCV (Table 1). Ultimately, 256,700 doses were delivered through fixed vaccination posts and mobile vaccination teams, with interdose timing ranging from 12 to 56 days (Table 1, S1 Table, Fig 1, and S1 Fig).
Tab. 1. Details of OCV deployments in South Sudan in 2014 by site. 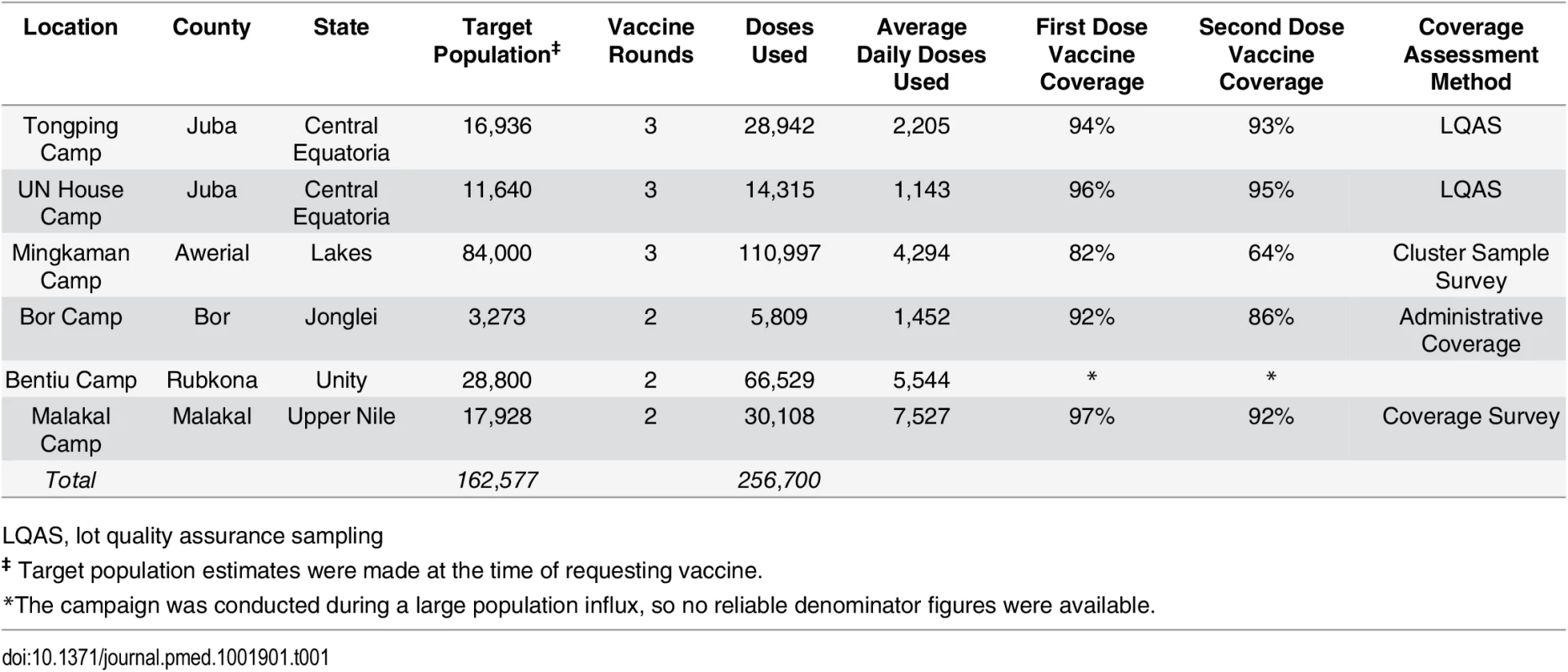
LQAS, lot quality assurance sampling Vaccine coverage was estimated using different methods between locations (S1 Table). Lot quality assurance sampling (LQAS, [7]) was conducted in Juba camps, and a cluster-based household survey was conducted in Mingkaman; in the other sites, only administrative vaccine coverage was available (i.e., doses delivered/target population size). First dose coverage estimates ranged from 82% in Mingkaman to 97% in Malakal, with second-dose coverage estimates ranging from 64% (Mingkaman) to 95% (Juba, UN House) (Table 1, S1 Table). Though the coverage estimates were generally high, each comes with a different degree of uncertainty because of the methods used; thus, comparisons should be made with caution.
Although costing data was not systematically collected from each agency involved, these campaigns allow for some new insight into the costs of vaccine delivery and quality control. From two agencies (MSF and Medair) that delivered the majority of the vaccines, the delivery cost per vaccine was US$0.63 and US$0.73 (excluding international staff). While these costs can likely be reduced as agencies gain more experience with OCV, the cost of each dose (currently US$1.85 through the stockpile) will likely continue to dominate campaign costs in the near term.
Cholera Strikes South Sudan
After a period of five years without confirmed cholera in South Sudan, a suspected cholera case was reported from UN House/Juba 3 camp in Juba on 29 April (approximately two months from the start of vaccination activities, Fig 1). Although the origins of this reported infection are unknown, a cholera epidemic had been reported in a neighboring area of Uganda the previous week [8]. Following field investigations and laboratory confirmation, on 15 May, the MoH declared a cholera outbreak in Juba caused by Vibrio cholerae O1 Inaba sensitive to tetracycline and ciprofloxacin. By the end of October, 6,269 suspected cases had been reported throughout the country (Fig 3), including 105 deaths in health facilities and 51 community deaths (case fatality ratio 2.4%).
Fig. 3. Illustration of the county-level attack rates in the 2014 cholera epidemic with respect to the vaccination locations (orange dots). 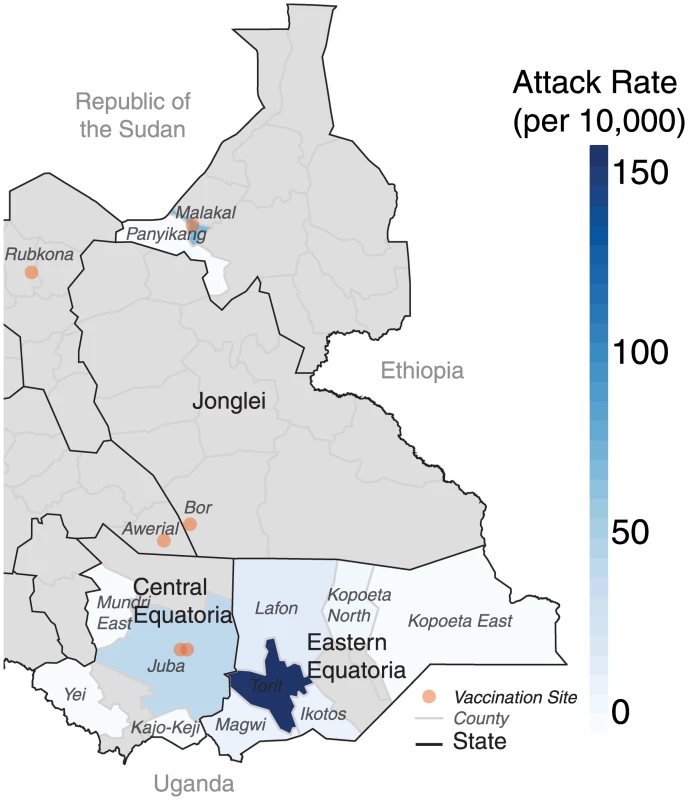
Grey areas represent counties with no suspected cholera cases. From Juba and its surrounding areas, where most cases occurred outside of the camps, the epidemic spread east to Torit (Eastern Equatoria) in June (Fig 3). In Eastern Equatoria, the slow outbreak response and relatively poor baseline health care infrastructure led to high case fatality ratios around the state, reaching as high as 11% (21 deaths) in Ikotos county. The epidemic then jumped over 1,000 kilometers north (presumably from an infected traveler), over a large area with no confirmed cholera, to the states of Upper Nile and Jonglei, where it almost exclusively affected displaced persons living outside the vaccinated PoC camps. Of the five areas vaccinated countrywide, only two (the Malakal and Juba PoC camps) experienced cases either within the population targeted for vaccination or the surrounding area (i.e., the host community or nearby informal settlements).
Some cholera cases were reported in vaccinated camps, but there was little indication of transmission. The epidemic curves in the vaccinated camps did not have the typical epidemic curve observed during cholera outbreaks (i.e., only sporadic cases reported without a clear peak followed by a decline, Fig 2B, 2C and 2E). In comparison, a more typical epidemic curve, suggestive of transmission, was observed in the unvaccinated surrounding areas (Fig 2A and 2D). This is especially striking in the comparison of Malakal and Wau Shilluck, two relatively similar camps just across a river from one another. Despite the suggestive visual evidence that vaccination slowed transmission in the vaccinated areas, more rigorous analyses are needed (analyses progress at the time of writing this manuscript) to assess the full impact of this vaccination campaign on the population.
Lessons Learned
This first deployment of OCV through the global stockpile provided ~250,000 doses of OCV to high-risk populations months before the onset of a cholera epidemic within the country. Strong leadership by the MoH and WHO, pre-existing coordination structures (e.g., the UN Health Cluster), clearly defined roles and responsibilities of the different actors (i.e., one lead agency per site), and previous use of the vaccine played crucial roles in ensuring the success of these campaigns during a humanitarian emergency. This experience involved non-medically focused agencies without prior OCV experience, which points towards the possibility of diversifying the actors involved in future campaigns in order to avoid missed opportunities for OCV use and to improve its integration into cholera control and prevention programs.
Despite the preventative vaccination campaign, it was not enough to prevent an outbreak within the country, and several issues prevented additional reactive use of OCV. The competing priorities for finite (financial and human) resources in complex emergencies are exacerbated during outbreaks, and difficult decisions must be made through weighing the costs and benefits of different interventions. When the cholera outbreak was declared in Juba, an analysis of (limited) historical data and a risk assessment conducted early on in the epidemic pointed to some unvaccinated areas at high risk for cholera introduction and spread (e.g., Torit and Wau Shilluk). However, despite these warnings, additional vaccination was not used because of limited resources within South Sudan and the global stockpile and limited access to key populations. Even with interested policy makers, it was difficult to motivate operational organizations to engage in reactive vaccination, as they were consumed with other outbreak response activities, though the spatiotemporal pattern of spread within this epidemic suggests that reactive vaccination in many of these (at the time) unaffected high-risk areas surrounding the outbreak may have been an efficient mechanism to limit its spread.
The vaccines arrived in South Sudan about 30 days after the request to the stockpile. In this preemptive deployment, the delay likely had no impact on the overall vaccine-attributable reductions in cholera cases once the epidemic started. However, in other settings, particularly where OCV is being used to fight an ongoing epidemic, a quicker timeline from submission of the vaccine request to delivery in country is crucial. While some delays are inevitable and out of the control of the ICG, lessons from this and future stockpile deployments should be used to improve the lead times between the submission of the ICG request and the delivery of the vaccines.
The limited global supply of OCV made it difficult to decide on the target population for reactive campaigns and to advocate for an expanded preemptive target population. If more doses would have been available, it is possible that the host communities, including Juba, where over 2,000 cases and 40 deaths were reported, and informal IDP settlements would have received OCV. The WHO cholera stockpile was designed to have 2 million doses (subject to global production constraints) for outbreak response and emergencies, which if fully available to a single epidemic, would often eliminate challenges in deciding what population to target. Nonetheless, the imbalance between the number of doses available and the population at risk (2.8 billion [9]) means that difficult choices will likely be confronted. Thus, tools to identify populations in which the health benefits might be maximized in a timely manner (e.g., populations with the highest mortality risk or disease risk) are a clear priority that could facilitate the OCV decision-making process.
Despite the fact that many (still) displaced people are today protected by vaccine, the water sanitation and hygiene conditions in the camps remain dismal and conducive to the spread of cholera and/or other waterborne diseases. With the continued population movements and a high birth rate, vaccine alone will likely not protect the entire population from another outbreak. Investments in vaccine must be coupled with long-term commitments to improving water sanitation and hygiene conditions if sustainable gains in cholera-attributable morbidity and mortality are to be made.
Way Forward
This first deployment of OCV through the global stockpile highlighted how this mechanism can be efficiently used to protect individuals and slow the transmission of cholera, even in complex emergencies.
More work is needed in understanding how to target appropriate populations if OCV is to become more integrated into standard cholera control and prevention packages. With enhancement of cholera surveillance systems and analyses of historical epidemiologic data, target populations may be identified more precisely—whether based on geography [10], age [11], or other criteria [12]. More evidence is needed on alternative dosing strategies, and field-adapted variants of currently licensed OCV should be fast-tracked. Changes to the ways in which the current vaccine is administered and/or development of better vaccines could greatly increase the feasibility of OCV campaigns, including changes to the packaging, reduction in the cold-chain requirements (i.e., a controlled temperature chain strategy), and the possibility of using a reduced one-dose regimen [13]. Above all, the dissemination of the evidence supporting OCV use in cholera epidemics will be important for its adoption by countries and humanitarian actors alike. The global OCV stockpile is an asset to the public health community, and we must continue to improve awareness of the vaccine as an outbreak prevention and control tool. At the same time, we must seek smarter deployment strategies and the development of better vaccines in order to maximize the benefits and minimize the costs.
Supporting Information
Zdroje
1. United States Agency for International Development. Southern Sudan Health System Assessment. In: healthsystemassessment.com [Internet]. Jul 2007 [cited 27 Apr 2015]. http://www.healthsystemassessment.com/wp-content/uploads/2012/06/Sudan_GAVI_Version_9_08_FIN_.pdf
2. WHO/UNICEF. Joint Monitoring Programme for Water Supply and Sanitation. Progress on Sanitation and Drinking-Water: 2014 Update; World Health Organization: Geneva, Switzerland, 2014.
3. Porta MI, Lenglet A, de Weerdt S, et al. Feasibility of a preventive mass vaccination campaign with two doses of oral cholera vaccine during a humanitarian emergency in South Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene 108(12):815. doi: 10.1093/trstmh/tru153
4. Yen C, Hyde TB, Costa AJ, et al. The development of global vaccine stockpiles. The Lancet Infectious Diseases 2015;15(3):340–7. doi: 10.1016/S1473-3099(14)70999-5 25661473
5. Ciglenecki I, Sakoba K, Luquero FJ, Heile M, Itama C, Mengel M, et al. Feasibility of Mass Vaccination Campaign with Oral Cholera Vaccines in Response to an Outbreak in Guinea. PLoS Med 2013 Sep;10(9):e1001512. doi: 10.1371/journal.pmed.1001512
6. Luquero FJ, Grout L, Ciglenecki I, Sakoba K, Traore B, Heile M, et al. Use of Vibrio cholerae Vaccine in an Outbreak in Guinea. N Engl J Med. 2014 May 29;370(22):2111–20. doi: 10.1056/NEJMoa1312680 24869721
7. Lanata Cf, George Stroh Jr, Black Re, Gonzales H. An Evaluation of Lot Quality Assurance Sampling to Monitor and Improve Immunization Coverage. International Journal of Epidemiology 1990;19(4):1086–90. 2083994
8. Agaba J and Amoli L (2014, May 1). Cholera—One more dead as patients’ toll hits 92. New Vision. http://www.newvision.co.ug/news/655136-cholera-one-more-dead-as-patients-toll-hits-92.html
9. Ali M, Lopez AL, You YA, et al. The global burden of cholera. Bull World Health Org 2012; 90(3):209–218A. 22461716
10. Azman AS, Lessler J. Reactive vaccination in the presence of disease hotspots. Proceedings of the Royal Society B: Biological Sciences 2015;282(1798):20141341–1. doi: 10.1098/rspb.2014.1341 25392464
11. Dimitrov DT, Troeger C, Halloran ME, Longini IM, Chao DL. Comparative Effectiveness of Different Strategies of Oral Cholera Vaccination in Bangladesh: A Modeling Study. PLoS Negl Trop Dis 2014;8(12):e3343. doi: 10.1371/journal.pntd.0003343 25473851
12. Ivers LC, Charles RC, Hilaire IJ, et al. Immunogenicity of the Bivalent Oral Cholera Vaccine Shanchol in Haitian Adults With HIV Infection. J Infect Dis 2015;jiv108.
13. Azman AS, Luquero FJ, Ciglenecki I, Grais RF, Sack DA, Lessler J. The Impact of a One-Dose versus Two-Dose Oral Cholera Vaccine Regimen in Outbreak Settings: A Modeling Study. PLoS Med 2015;12(8):e1001867 doi: 10.1371/journal.pmed.1001867 26305226
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Dispersion of the HIV-1 Epidemic in Men Who Have Sex with Men in the Netherlands: A Combined Mathematical Model and Phylogenetic Analysis
- Venous Thrombosis Risk after Cast Immobilization of the Lower Extremity: Derivation and Validation of a Clinical Prediction Score, L-TRiP(cast), in Three Population-Based Case–Control Studies
- From Checklists to Tools: Lowering the Barrier to Better Research Reporting
- Care that Matters: Quality Measurement and Health Care
- The Missing Men: HIV Treatment Scale-Up and Life Expectancy in Sub-Saharan Africa
- The First Use of the Global Oral Cholera Vaccine Emergency Stockpile: Lessons from South Sudan
- The PneuCarriage Project: A Multi-Centre Comparative Study to Identify the Best Serotyping Methods for Examining Pneumococcal Carriage in Vaccine Evaluation Studies
- Mass HIV Treatment and Sex Disparities in Life Expectancy: Demographic Surveillance in Rural South Africa
- The HIV Treatment Gap: Estimates of the Financial Resources Needed versus Available for Scale-Up of Antiretroviral Therapy in 97 Countries from 2015 to 2020
- Selection of an HLA-C*03:04-Restricted HIV-1 p24 Gag Sequence Variant Is Associated with Viral Escape from KIR2DL3+ Natural Killer Cells: Data from an Observational Cohort in South Africa
- Shortening Turnaround Times for Newborn HIV Testing in Rural Tanzania: A Report from the Field
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venous Thrombosis Risk after Cast Immobilization of the Lower Extremity: Derivation and Validation of a Clinical Prediction Score, L-TRiP(cast), in Three Population-Based Case–Control Studies
- The First Use of the Global Oral Cholera Vaccine Emergency Stockpile: Lessons from South Sudan
- The HIV Treatment Gap: Estimates of the Financial Resources Needed versus Available for Scale-Up of Antiretroviral Therapy in 97 Countries from 2015 to 2020
- Selection of an HLA-C*03:04-Restricted HIV-1 p24 Gag Sequence Variant Is Associated with Viral Escape from KIR2DL3+ Natural Killer Cells: Data from an Observational Cohort in South Africa
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



