-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFetal Growth and Risk of Stillbirth: A Population-Based Case–Control Study
Background:
Stillbirth is strongly related to impaired fetal growth. However, the relationship between fetal growth and stillbirth is difficult to determine because of uncertainty in the timing of death and confounding characteristics affecting normal fetal growth.Methods and Findings:
We conducted a population-based case–control study of all stillbirths and a representative sample of live births in 59 hospitals in five geographic areas in the US. Fetal growth abnormalities were categorized as small for gestational age (SGA) (<10th percentile) or large for gestational age (LGA) (>90th percentile) at death (stillbirth) or delivery (live birth) using population, ultrasound, and individualized norms. Gestational age at death was determined using an algorithm that considered the time-of-death interval, postmortem examination, and reliability of the gestational age estimate. Data were weighted to account for the sampling design and differential participation rates in various subgroups. Among 527 singleton stillbirths and 1,821 singleton live births studied, stillbirth was associated with SGA based on population, ultrasound, and individualized norms (odds ratio [OR] [95% CI]: 3.0 [2.2 to 4.0]; 4.7 [3.7 to 5.9]; 4.6 [3.6 to 5.9], respectively). LGA was also associated with increased risk of stillbirth using ultrasound and individualized norms (OR [95% CI]: 3.5 [2.4 to 5.0]; 2.3 [1.7 to 3.1], respectively), but not population norms (OR [95% CI]: 0.6 [0.4 to 1.0]). The associations were stronger with more severe SGA and LGA (<5th and >95th percentile). Analyses adjusted for stillbirth risk factors, subset analyses excluding potential confounders, and analyses in preterm and term pregnancies showed similar patterns of association. In this study 70% of cases and 63% of controls agreed to participate. Analysis weights accounted for differences between consenting and non-consenting women. Some of the characteristics used for individualized fetal growth estimates were missing and were replaced with reference values. However, a sensitivity analysis using individualized norms based on the subset of stillbirths and live births with non-missing variables showed similar findings.Conclusions:
Stillbirth is associated with both growth restriction and excessive fetal growth. These findings suggest that, contrary to current practices and recommendations, stillbirth prevention strategies should focus on both severe SGA and severe LGA pregnancies.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(4): e32767. doi:10.1371/journal.pmed.1001633
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001633Summary
Background:
Stillbirth is strongly related to impaired fetal growth. However, the relationship between fetal growth and stillbirth is difficult to determine because of uncertainty in the timing of death and confounding characteristics affecting normal fetal growth.Methods and Findings:
We conducted a population-based case–control study of all stillbirths and a representative sample of live births in 59 hospitals in five geographic areas in the US. Fetal growth abnormalities were categorized as small for gestational age (SGA) (<10th percentile) or large for gestational age (LGA) (>90th percentile) at death (stillbirth) or delivery (live birth) using population, ultrasound, and individualized norms. Gestational age at death was determined using an algorithm that considered the time-of-death interval, postmortem examination, and reliability of the gestational age estimate. Data were weighted to account for the sampling design and differential participation rates in various subgroups. Among 527 singleton stillbirths and 1,821 singleton live births studied, stillbirth was associated with SGA based on population, ultrasound, and individualized norms (odds ratio [OR] [95% CI]: 3.0 [2.2 to 4.0]; 4.7 [3.7 to 5.9]; 4.6 [3.6 to 5.9], respectively). LGA was also associated with increased risk of stillbirth using ultrasound and individualized norms (OR [95% CI]: 3.5 [2.4 to 5.0]; 2.3 [1.7 to 3.1], respectively), but not population norms (OR [95% CI]: 0.6 [0.4 to 1.0]). The associations were stronger with more severe SGA and LGA (<5th and >95th percentile). Analyses adjusted for stillbirth risk factors, subset analyses excluding potential confounders, and analyses in preterm and term pregnancies showed similar patterns of association. In this study 70% of cases and 63% of controls agreed to participate. Analysis weights accounted for differences between consenting and non-consenting women. Some of the characteristics used for individualized fetal growth estimates were missing and were replaced with reference values. However, a sensitivity analysis using individualized norms based on the subset of stillbirths and live births with non-missing variables showed similar findings.Conclusions:
Stillbirth is associated with both growth restriction and excessive fetal growth. These findings suggest that, contrary to current practices and recommendations, stillbirth prevention strategies should focus on both severe SGA and severe LGA pregnancies.
Please see later in the article for the Editors' SummaryIntroduction
One in 160 births at ≥20 wk gestation in the United States is stillborn, resulting in over 25,000 stillbirths each year [1], a rate similar to the rate of infant death [2]. Stillbirth also constitutes one of the main causes of mortality worldwide [3],[4]. Fetal growth restriction, and its proxy small for gestational age (SGA) fetus, is one of the most important predictors of stillbirth because of its strong association with stillbirth and relatively high prevalence. Fetal growth restriction is a pathological decrease in the rate of fetal growth that frequently results in an adverse outcome, but is difficult to define because it requires serial evaluation of fetal growth. Conversely, SGA is easier to define—a birth weight smaller than expected—but includes a proportion of small but normal pregnancies. The overlap between fetal growth restriction and SGA depends on the norm and cutoff used. Approximately a quarter of stillbirths are associated with SGA fetus, almost double the proportion associated with any other risk factor [5],[6]. For these reasons, standards of clinical practice recommend evaluation of fetal growth during each prenatal visit and further evaluation and possible intervention if the fetal growth rate is poor [7]–[10]. Despite ubiquitous use of fetal growth monitoring in clinical practice and extensive research, the relationship between fetal growth and stillbirth is not completely understood, for the following reasons.
First, birth weight percentile is a function of birth weight and age of the fetus. Thus, studies using gestational age (GA) at delivery rather than GA at death, as an estimate of the age of the fetus, systematically overestimate the GA of stillbirths. This leads to overestimation of the proportion of birth weights considered SGA and underestimation of birth weights considered large for GA (LGA) among stillbirths.
Second, a number of maternal characteristics such as weight, height, age, race/ethnicity, parity, exposures during pregnancy, smoking, and measures of placental function (such as maternal blood concentrations of placental hormones) have been observed to influence the magnitude of both fetal growth and risk of stillbirth [5],[6],[11]–[14]. Specific ranges of these characteristics are also associated with birth weight among uncomplicated live birth pregnancies and are accounted for by using individualized birth weight norms [15]. Thus, to accurately assess fetal growth and identify fetal growth abnormalities, an accurate estimate of GA and accounting for the effect of physiologic determinants of fetal growth are needed. This is especially important in stillbirths, because their exact GA is mostly not known, and GA at delivery overestimates GA of stillbirths. Moreover, many characteristics that affect the growth of live and normal pregnancies, such as maternal race/ethnicity, body mass index (BMI), and parity, are also associated with the risk of stillbirth. The effect of these characteristics on fetal growth and risk of stillbirth is complex, because within a certain range their effect on fetal growth is observed among normal live pregnancies without complications [15].
Therefore, we hypothesized that accounting for time of death in determining fetal age and birth weight percentile, and determining percentiles of birth weight based on norms that account for factors affecting birth weight in normal pregnancies, will more accurately identify the proportion of stillbirths associated with abnormal growth than using traditional methods.
Methods
Ethics Statement
This study was approved by the institutional review boards at each of the clinical recruiting sites and at the data coordinating center. All mothers participating in the study gave written informed consent.
Study Design
The Stillbirth Collaborative Research Network (SCRN) conducted a population-based case–control study of stillbirths in the United States. The study design has been previously described [16]. Briefly, the population consisted of residents of five geographic areas defined a priori by state and county lines: (1) the state of Rhode Island and Bristol County, Massachusetts; (2) DeKalb County, Georgia; (3) Galveston and Brazoria Counties, Texas; (4) Bexar County, Texas; and (5) Salt Lake County, Utah. Participants were recruited at delivery between March 1, 2006, and September 30, 2008, from 59 community and academic, urban, and rural hospitals in the five areas, with a cumulative average of 80,000 deliveries per year. The investigators selected the 59 hospitals to ensure access to at least 90% of all pregnancies ending in either stillbirth or live birth within each geographic area based on estimates of the number of hospital deliveries from vital statistics data and hospital medical records available during study planning. All women whose pregnancies resulted in stillbirth and a representative sample of women with live births, oversampled for those delivering at <32 wk gestation and those of African descent delivering at ≥32 wk gestation, were approached for enrollment. Terminations of pregnancy were excluded.
A stillbirth was defined by Apgar scores of 0 at 1 and 5 min, and no signs of life by direct observation. The protocol included an in-hospital maternal interview, medical record abstraction, placental pathology examination, and biospecimen collection for stillbirths and live births. For stillbirths, a standardized postmortem examination was also performed.
Fetal Age
Fetal age was based on an estimated due date and the date of delivery for live births and an estimated due date and estimated date of death for stillbirths, using an algorithm developed by the SCRN investigators [17]. For both stillbirths and live births, the due date was estimated using (1) menstrual dating criteria that agreed with ultrasound or ultrasound dating criteria (when the last menstrual period date was unknown or uncertain, or menstrual dating did not agree with ultrasound dating within specified constraints) in 88.3% of pregnancies, (2) menstrual dating alone when ultrasound was not available in 6.1%, and (3) review of all clinical information available in 5.6%.
The SCRN algorithm for estimating time of death and fetal age at death in stillbirths considered the following: the reliability of the estimated due date; the length of the interval between the time the fetus was last documented alive and the time fetal demise was first recorded based on information from prenatal care visits, hospitalizations, and ultrasound examinations (the time-of-death interval); and information available from postmortem examination, including degree of fetal maceration and foot length measurement. The estimated due date was considered reliable if estimated by ultrasound or by certain menstrual dating that agreed with ultrasound (within specified constraints). Briefly, (1) if the estimated due date was reliable, the fetal age at death was estimated using the due date and the date fetal demise was diagnosed (if the interval during which death occurred was ≤1 d) or the date at the midpoint of the time-of-death interval (if the interval was >1 and ≤7 d); (2) if the estimated due date was unreliable, or the time-of-death interval was >7 d, GA at death was estimated using foot length, or using GA reported by the study site at screening based on clinical criteria when foot length information was not available. Precise estimates of GA at death, defined as meeting reliable dating criteria and having an interval of 1 wk or less during which the demise could have occurred, were possible for 47% of stillbirths.
The fetal age at death estimated by the SCRN algorithm was used for all stillbirths in the primary analysis. For comparison, GA at stillbirth delivery, calculated using the estimated due date and the delivery date, and GA at delivery minus 2 d were also used as estimates of fetal age at death to examine the impact on birth weight percentiles. For live births, fetal age at delivery was estimated using the estimated due date if reliable and the delivery date, or if the estimated due date was unreliable, the GA reported by the study site at screening was used.
Fetal Weight
Fetal weight percentiles were determined based on birth weight and the fetal age estimate for stillbirths and live births. Birth weight was obtained from medical records or postmortem examination and was compared to expected weight for GA based on three types of norms: population, ultrasound, and individualized norms [15],[18],[19]. Alexander et al.'s population norms reported percentiles of birth weight for completed weeks of GA, 20–44 wk, based on data from the population of over 3 million US single live births in 1991 [18]. SCRN live birth and stillbirth birth weights were compared to Alexander et al.'s population norms to determine a percentile category. Hadlock et al.'s ultrasound norms used fetal weight estimated in utero by ultrasound in 392 uncomplicated pregnancies progressing to term to develop an equation to predict fetal weight as a function of GA [19]. The observed SCRN birth weight was compared to the fetal weight predicted by Hadlock et al.'s equation, with a percentile computed under normality assumptions. Bukowski et al.'s individualized norms used data from 9,818 uncomplicated pregnancies resulting in singleton births to develop an equation to predict expected term birth weight based on a number of maternal and fetal characteristics affecting normal fetal growth [15]. A proportion derived using Hadlock et al.'s equation [19] was then used to adjust the prediction at term to a prediction at the GA of the SCRN live birth or stillbirth, and the observed birth weight was then compared to this individualized predicted birth weight, with a percentile computed under normality assumptions. Steps used to estimate percentiles are summarized in Figure 1, and details of estimation are provided for each norm below. SGA birth weight was defined as birth weight less than the 10th percentile, and LGA as birth weight above the 90th percentile for GA, while severe SGA and LGA were defined by the 5th and 95th percentiles respectively. Birth weights in the 10th–90th percentile range were classified as appropriate for age (AGA).
Fig. 1. Summary of steps used to assign infants to a birth weight percentile category. 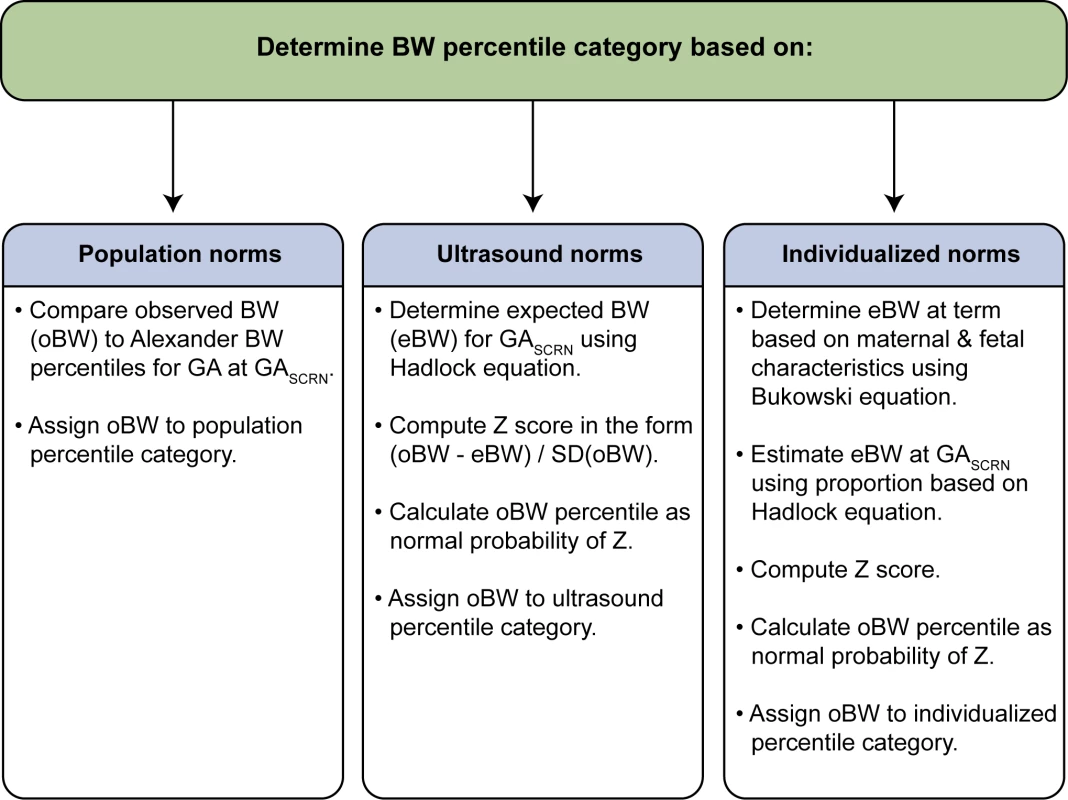
GASCRN is the fetal age at death (for stillbirths) or delivery (for live births) estimated by the SCRN algorithm [17]. BW, birth weight; SD, standard deviation. References: Alexander et al. [18], Hadlock et al. [19]; Bukowski et al. [15]. Population norms
The estimated GA at death (for stillbirths) or delivery (for live births) using the SCRN algorithm (GASCRN) was recorded in weeks, with days retained as a fractional component. Alexander et al. reported 5th, 10th, 50th, 90th, and 95th percentiles of birth weight for completed weeks of GA derived using data from over 3 million US 1991 single live births [18]. Linear interpolation was used with Alexander et al.'s reported percentiles for each completed week of GA, 20–44 wk, to derive birth weight percentiles for GASCRN. For interpolation, each percentile reported by Alexander et al. was taken to represent birth weight at the midpoint of the GA week range (0–6 d). For example, the 10th percentile of birth weight reported for an infant of 30 wk GA was considered the 10th percentile for 30.5 wk GA.
The observed birth weight of each infant was compared to the interpolated 5th, 10th, 50th, 90th, and 95th percentiles of birth weight for GASCRN and assigned to a percentile category (e.g., <10th percentile). Three infants with GASCRN>44.5 wk were assigned to the 10th–90th percentile category based on birth weight between the 10th and 90th percentiles reported for 44 wk.
Ultrasound norms
Hadlock et al. used in utero fetal weight, estimated with ultrasound measurements, from 392 uncomplicated pregnancies progressing to term among predominantly middle-class white women to develop a best-fitting equation for fetal weight as a function of GA [19]. Furthermore, they estimated fetal weight percentiles by GA, assuming a Gaussian distribution for fetal weight on the natural log scale. In like manner, a birth weight percentile for GASCRN was estimated for each infant in the SCRN cohort as the normal probability of a Z-score, ZULTRASOUND, calculated as follows:
where ln(oBW) is the natural log of the observed birth weight of the SCRN infant, SD(ln[oBW]) is the standard deviation of birth weight on the natural log scale reported by Hadlock et al. ( = 0.12), and ln(eBW) is the natural log of the expected birth weight for GASCRN by Hadlock et al.'s equation:Individualized norms
Bukowski et al. developed a regression model for birth weight in a normal population using singleton births from 9,818 women with uncomplicated pregnancies [15]. The model equation accounts for maternal and other characteristics affecting birth weight (see Table 2 of [15]). This equation, with some modifications, was used to compute an individualized expected birth weight at 280 d for each SCRN infant. The modifications were as follows. (1) Maternal weight in the first trimester was not available, and maternal pre-pregnancy weight was substituted. (2) Fetal heart rate in the first trimester was not available and could not be included. (3) GA was not needed in the prediction equation when estimating birth weight at 280 d, as Bukowski et al.'s regression model used GA minus 280. (4) SCRN marital status categories were “not married,” “cohabitating,” and “married.” For the purposes of modeling, “not married” was given the coefficient for “single,” and “cohabitating” was given the coefficient for “other” in Table 2 of [15]. (5) Values for continuous variables outside the range of values observed in the population in which the model equation was developed were truncated to conform to those ranges. Values were truncated for nine of the 19 variables included in the model equation. For eight of these nine variables—maternal weight, cigarettes/day, nuchal translucency size, and maternal blood concentrations of five placental hormones—values were truncated for between 1 and 21 infants (representing <1% to 3.8%). DeltaGA (the difference between GA estimated by first trimester ultrasound and age estimated by last menstrual period date) was truncated for 190 infants with measures outside the −7 to 7 range of the data included in the modeling (representing 29%). (6) Accommodations were made for a number of variables that were missing. Missing values for ordinal variables, with the exception of education, were set to mean or median values (maternal weight: 64 kg; maternal height: 165 cm; number of prior term pregnancies: 0; number of prior abortions: 0; number of cigarettes/day: 0; first and second trimester test results in multiples of the median: 1; DeltaGA: 0). For each categorical variable, and for education, the coefficient for “missing” values was derived as a weighted average of the coefficients of the non-missing levels, with weights taken as the proportion of the corresponding level in the SCRN singleton cohort.
Tab. 1. Variables used to compute individualized expected birth weight at 280(unweighted <i>n</i> = 2,348). 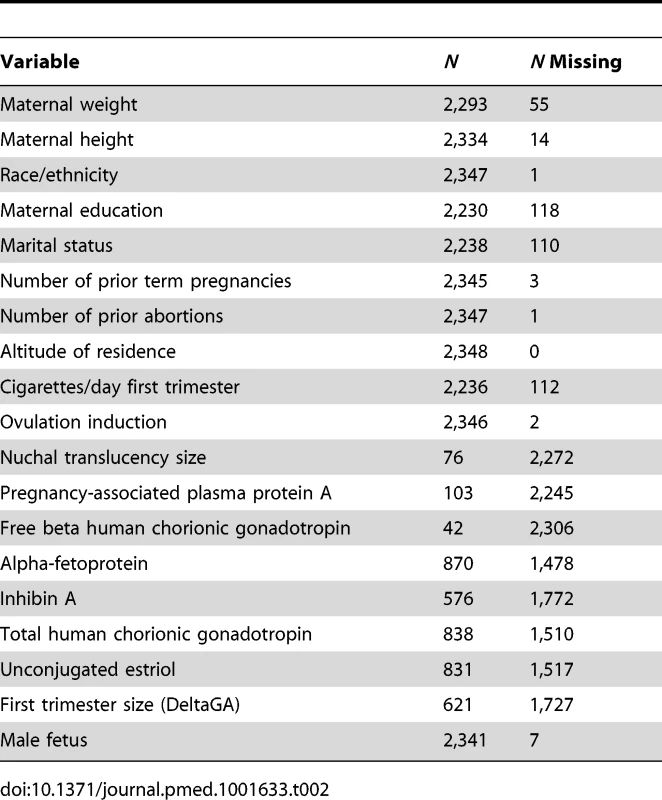
After computing an individualized expected birth weight at 280 d (BWINDIV280) for each SCRN infant, an individualized expected birth weight for GASCRN (eBW) was calculated using a proportion derived using Hadlock et al.'s equation [19]:
where BWHADLOCK is the predicted birth weight for GASCRN using Hadlock et al.'s equation and BWHADLOCK280 is the predicted birth weight for GA at 280 d using Hadlock et al.'s equation ( = 3,619.17 g).Finally, an individualized birth weight percentile was estimated as the normal probability of an individualized Z-score, ZINDIV, of the form:
where oBW is the observed birth weight of the SCRN infant, eBW is the individualized expected birth weight at GASCRN, and SD(oBW) is an estimate of the standard deviation of the observed birth weight. The standard deviation was estimated using an estimate of the coefficient of variation at 280 d (CVINDIV280) multiplied by eBW (CVINDIV280×eBW). CVINDIV280 was taken as the reported square root of the residual mean square from the model from Bukowski et al. (358.479 g) divided by the expected birth weight at 280 d from Hadlock et al. (BWHADLOCK280 = 3,619.17 g). Thus, CVINDIV280 = 358.479/3,619.17.In sensitivity analyses, individualized norm percentiles were recalculated based on three different reduced prediction equations that included subsets of variables largely non-missing in the SCRN cohort, instead of the original 19 variables. First, percentiles were estimated based on a prediction equation that included the 11 variables with largely non-missing values in the SCRN cohort (maternal weight, height, race/ethnicity, education, marital status, number of prior term pregnancies, number of prior abortions, altitude of residence, use of ovulation induction to become pregnant, cigarettes smoked per day during the first trimester, and male fetus), with coefficients derived using data from the original Bukowski et al. population. Second, percentiles were estimated based on a prediction equation that included the six variables suggested previously [20] (maternal weight, height, race/ethnicity, number of prior term pregnancies, cigarettes smoked per day during the first trimester, and male fetus), with coefficients derived using data from the original Bukowski et al. population. Third, percentiles were estimated based on a prediction equation that included five of the six variables listed previously (cigarette smoking was excluded), with coefficients derived using data from the original Bukowski et al. population.
Statistical Analysis
Data were weighted for the analysis. The analysis weights were constructed in steps. First, weights were constructed that took into account the sampling design, including staggered enrollment starts across the 59 site hospitals, and different sampling probabilities associated with the oversampling of live births <32 wk gestation and live births at ≥32 wk gestation to women of African descent. Some women screened and determined eligible for the study were not approached, and others were approached but did not consent. Additional weight adjustments were constructed to account for this nonresponse and utilized information collected at screening to determine characteristics associated with the likelihood of participating. The final analysis weights were constructed as the product of these weighting factors. Details of the live birth sample selection procedure and construction of the analysis weights have been reported previously [16]. The weighted sample was intended to approximate a random selection of live births in the five geographic areas during the enrollment period, with proportions according to GA and maternal race reflective of the population, and to reduce potential bias associated with failing to include all women eligible for participation.
The analysis was restricted to singleton stillbirths and live births with non-missing birth weight and GA≥20 wk at death or delivery. Statistical significance for comparisons of characteristics between stillbirths and live births was determined by the median or chi-square test, and for comparisons between proportions among stillbirths by the McNemar test. Crude and adjusted odds ratios (ORs) and 95% confidence intervals were calculated from logistic regression models. Primary results used all stillbirths and all live births. Term stillbirth and live birth were defined as GA of 37 wk 0 d or more. To assess the association of SGA and LGA with preterm stillbirth, the logistic model was restricted to preterm stillbirth versus all live births. Multivariable models used to estimate adjusted ORs included study site (five geographic areas) and stillbirth risk factors known at the beginning of pregnancy [21]. Approximately 10% of observations were missing values of covariates included in the multivariable models and were omitted from computations of adjusted ORs. Weighted analyses were conducted using SUDAAN software version 10.0.1.
Results
The SCRN identified 953 women with stillbirths and 3,088 women with live births eligible for participation in the study. Of these, 663 (70%) women with stillbirths and 1,932 (63%) women with live births consented to participate. Women with stillbirths enrolled in the study did not differ from those not enrolled on maternal age, maternal race/ethnicity, insurance/method of payment, or GA at delivery [21]. Women with live births enrolled in the study did not differ from those not enrolled on maternal age and insurance/method of payment. However, the proportion of Hispanic women was larger among those enrolled than among those not enrolled (34% versus 27%), and the proportions of non-Hispanic black and non-Hispanic white women were smaller (21% versus 25% and 41% versus 42%, respectively). Enrolled and non-enrolled women with live births also differed on GA at delivery, with a larger proportion of births ≥37 wk among those enrolled (75% versus 69%) [21]. From the pregnancies of women enrolled, 527 singleton stillbirths and 1,821 singleton live births were included in these analyses (Figure 2).
Fig. 2. Study enrollment and inclusion in analysis. 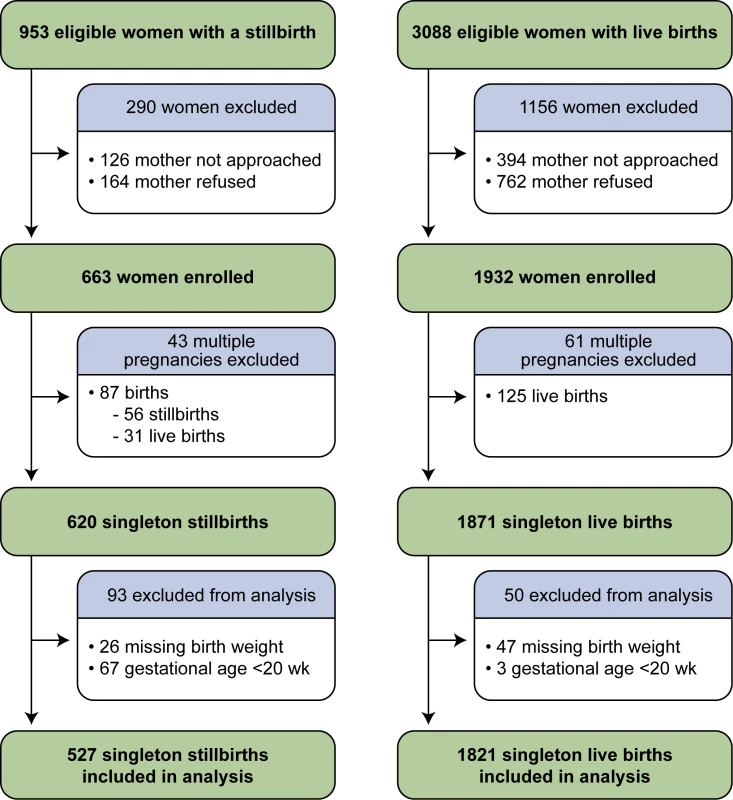
Women who delivered stillbirths were more likely than women who delivered live births to be non-Hispanic black (23% versus 11%, p<0.001), overweight (BMI≥25 kg/m2) (57% versus 46%, p<0.01), single (52% versus 39%, p<0.001), smokers before pregnancy (21% versus 14%, p<0.01), and less educated (less than 12 y of school) (24% versus 19%, p<0.001). They were more likely to have hypertension (11% versus 5%, p<0.001), to have pregestational diabetes (5% versus 2%, p<0.001), and to be nulliparous or to have had a prior stillbirth (p<0.001) (Table 1). As expected, stillborn infants had lower GA (median 28 versus 39 wk, p<0.001) and lower birth weight (p<0.001) and were more likely to have congenital malformations than live born infants (13% versus 3%, p<0.001) (Table 1).
Tab. 2. Characteristics of stillbirth and live birth pregnancies. 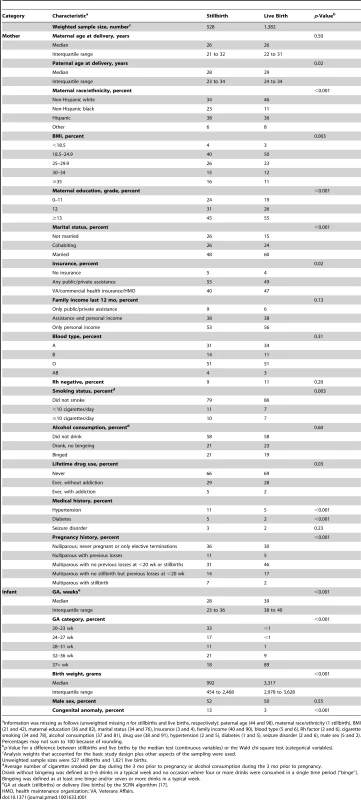
Information was missing as follows (unweighted missing n for stillbirths and live births, respectively): paternal age (44 and 98), maternal race/ethnicity (1 stillbirth), BMI (21 and 42), maternal education (36 and 82), marital status (34 and 76), insurance (3 and 4), family income (40 and 90), blood type (5 and 6), Rh factor (2 and 6), cigarette smoking (34 and 78), alcohol consumption (37 and 81), drug use (38 and 91), hypertension (2 and 5), diabetes (1 and 5), seizure disorder (2 and 6), male sex (5 and 2). Some variables used to compute individualized norms were missing in a proportion of pregnancies and were set to mean, median, or weighted average (Table 2). However, in sensitivity analyses, individualized norm percentiles derived using a subset of 11 variables largely non-missing in the cohort, or derived from a subset of six or five variables suggested previously [20] (with or without smoking), yielded patterns of association with stillbirth similar to those derived using the full model (Table 3). Thus, accounting for maternal smoking in computing individualized norms did not affect the association between fetal growth abnormalities and stillbirth. The five-variable model without smoking also showed that the association between SGA and LGA and risk of stillbirth was observed when individualized norms were derived without including factors with potentially adverse effects on pregnancy. However, it could be argued that smoking should not be used in individualization of fetal growth because of its potential adverse effect on a given pregnancy.
Tab. 3. Birth weight percentiles among stillbirths and live births using different equations to estimate individualized expected weight. 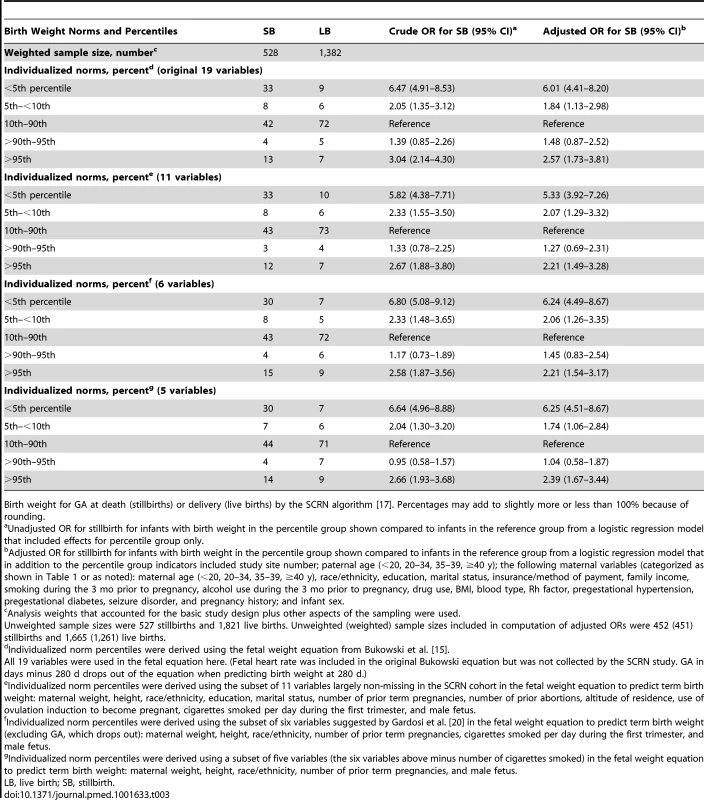
Birth weight for GA at death (stillbirths) or delivery (live births) by the SCRN algorithm [17]. Percentages may add to slightly more or less than 100% because of rounding. SGA pregnancies were associated with a statistically significant 3 - to 4-fold increased risk of stillbirth compared to AGA pregnancies using percentiles based on population, ultrasound, and individualized norms (OR [95% CI]: 3.0 [2.2 to 4.0]; 4.7 [3.7 to 5.9]; 4.6 [3.6 to 5.9], respectively). LGA birth weight was associated with a significantly increased risk of stillbirth using percentiles derived from the ultrasound and individualized norms (OR [95% CI]: 3.5 [2.4 to 5.0]; 2.3 [1.7 to 3.1], respectively), but not using percentiles based on the population norms (OR [95% CI]: 0.6 [0.4 to 1.0]). Abnormal fetal growth (SGA or LGA) was identified in 25% of stillbirths using population norms and in twice as many stillbirths using ultrasound (57%, p<0.001) and individualized norms (58%, p<0.001). The association between stillbirth and SGA and LGA infants was mainly due to the high risk of stillbirth among infants with birth weights in the <5th and >95th percentiles and was not substantially changed when adjusted for stillbirth risk factors (Table 4).
Tab. 4. Birth weight percentiles among stillbirths and live births. 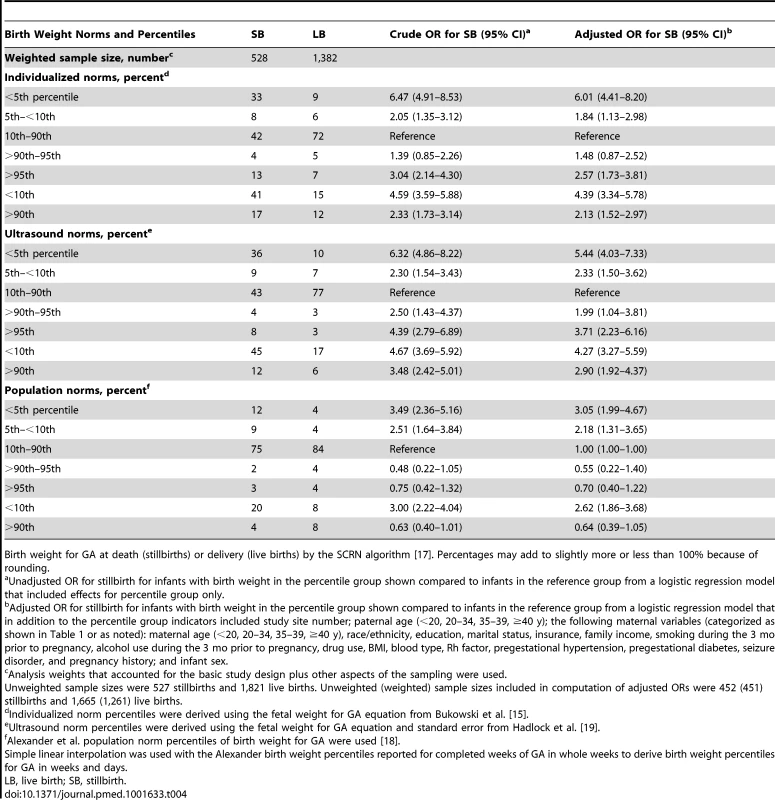
Birth weight for GA at death (stillbirths) or delivery (live births) by the SCRN algorithm [17]. Percentages may add to slightly more or less than 100% because of rounding. SGA and LGA defined using the ultrasound and individualized norms were also associated with significantly increased risk of stillbirth in the subsets of pregnancies without pregestational diabetes, gestational diabetes, hypertension, or preeclampsia; non-anomalous births at more than 24 wk of gestation; and pregnancies with optimal estimates of GA and time of death (Table 5). Fetuses classified as SGA based on the population reference were also at significantly increased risk of stillbirth in these subsets, but those classified as LGA were not.
Tab. 5. Birth weight percentiles among subsets of stillbirths and live births. 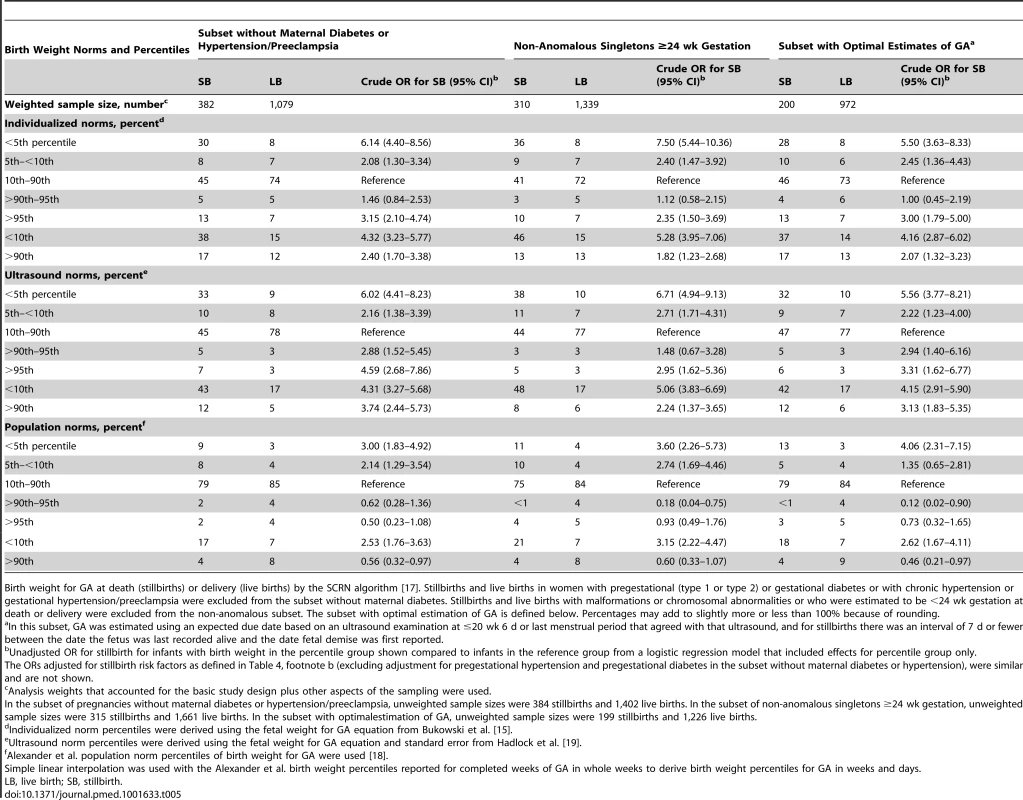
Birth weight for GA at death (stillbirths) or delivery (live births) by the SCRN algorithm [17]. Stillbirths and live births in women with pregestational (type 1 or type 2) or gestational diabetes or with chronic hypertension or gestational hypertension/preeclampsia were excluded from the subset without maternal diabetes. Stillbirths and live births with malformations or chromosomal abnormalities or who were estimated to be <24 wk gestation at death or delivery were excluded from the non-anomalous subset. The subset with optimal estimation of GA is defined below. Percentages may add to slightly more or less than 100% because of rounding. Among stillbirths identified as LGA by each of the norms, only one had a GA of more than 40 wk, and LGA was observed among preterm as well as term stillbirths. Hydrops was diagnosed among 11% (10/91), 14.5% (9/62), and 12.5% (3/24) of stillbirths identified as LGA using the individualized, ultrasound, and population norms, respectively. LGA was associated with stillbirth in the subset of non-anomalous pregnancies, which excluded the pregnancies with hydrops (Table 5).
Accounting for the time of death in stillbirths to determine fetal age did influence the proportion of SGA and LGA infants (Table 6). Increasing the accuracy of the fetal age estimate—from using GA at delivery to GA at delivery minus 2 d to GA at estimated time of fetal death—to determine weight-for-age percentiles decreased the proportion of stillbirths classified as SGA and increased the proportion classified as LGA using each of the three norms of fetal growth. ORs for stillbirth associated with SGA increased, and those associated with LGA decreased, when GA at delivery was used to determine birth weight percentiles for stillbirths instead of GA at death, regardless of which norms were used.
Tab. 6. Birth weight percentiles in relation to different GA estimates among singleton stillbirths. 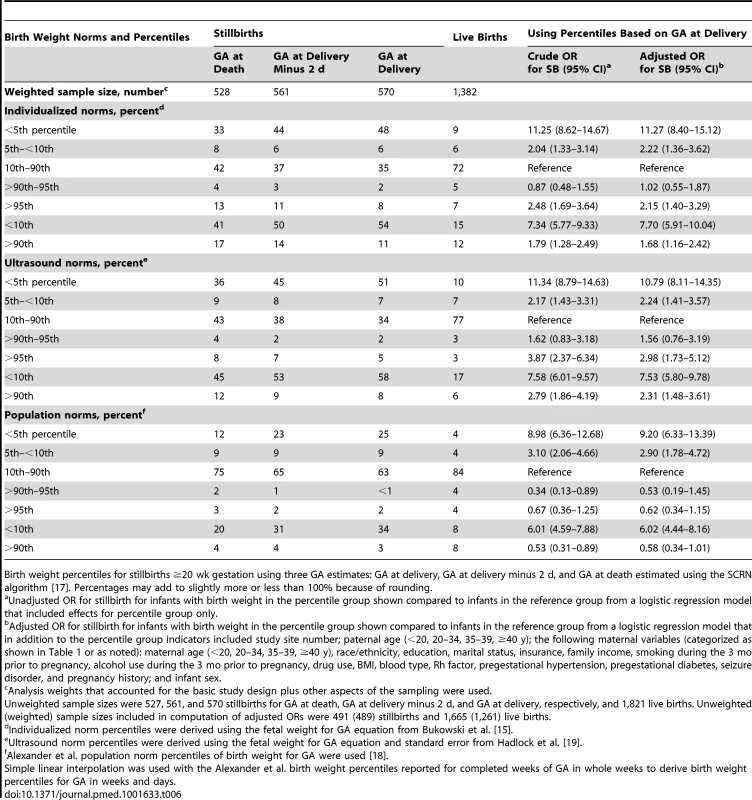
Birth weight percentiles for stillbirths ≥20 wk gestation using three GA estimates: GA at delivery, GA at delivery minus 2 d, and GA at death estimated using the SCRN algorithm [17]. Percentages may add to slightly more or less than 100% because of rounding. SGA and LGA birth weights based on ultrasound and individualized norm percentiles were significantly associated with an increased risk of preterm as well as term stillbirth. Using population norms, only SGA pregnancies were significantly associated with preterm and term stillbirth (Table 7). There was no significant difference in the GA distributions of stillbirths classified as LGA versus AGA or SGA using ultrasound or individualized norms.
Tab. 7. Birth weight percentiles among preterm and term stillbirths and live births. 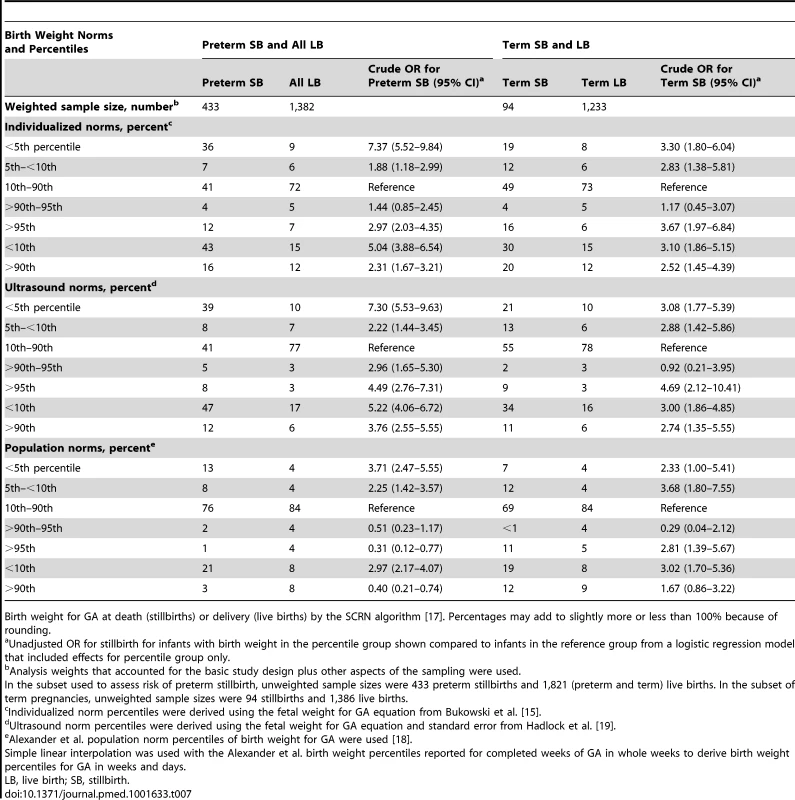
Birth weight for GA at death (stillbirths) or delivery (live births) by the SCRN algorithm [17]. Percentages may add to slightly more or less than 100% because of rounding. Discussion
This study demonstrates that stillbirth is associated with both growth restriction and excess growth. The extremes of SGA and LGA (<5th and >95th percentiles) were associated with the highest risk of stillbirth.
Strengths
The strengths of this study lie in its geographically defined population-based design capturing live births and stillbirths, the large number of stillbirths evaluated, the accurate estimation of GA at death in stillbirths, the assessment of fetal growth using different fetal growth standards, and the ability to examine the contribution of factors affecting both birth weight and the risk of stillbirth. The systematic and standardized estimation of time of death and thus GA at death for stillbirths allowed for more accurate assessment of the association between birth weight and stillbirth and, consequently, of the association of stillbirth with LGA.
Prior studies have either not accounted for the interval between time of death and time of delivery of stillbirths [22]–[27] or have assigned an arbitrary interval of 2 d from GA at death to GA at delivery [28]–[30]. These approaches substantially overestimate GA at death [17],[31]–[33] and thus result in overestimation of the proportion of SGA infants and underestimation of the proportion of LGA infants.
Studies evaluating the interval between death and delivery have shown that in 25%–50% of stillbirths the interval was longer than 7 d [31]–[33]. Using the algorithm defined by the SCRN, over 40% of deaths were estimated to have occurred at least a week before delivery [17]. Thus, increasing the accuracy of the fetal age estimate in stillbirths, from GA at delivery to GA at delivery minus 2 d to GA at estimated time of death, decreased the proportion of stillbirths classified as SGA and increased the proportion classified as LGA.
Many prior studies of stillbirth have focused exclusively on the association of stillbirth with SGA [22]–[24],[28]–[30]. They compared risk of stillbirth in women with and without SGA infants, including LGA infants in the non-SGA comparison group. This approach resulted in failure to identify the association between LGA and stillbirth and also decreased the strength of the association of stillbirth with SGA. A retrospective study analyzed the association between both SGA and LGA and stillbirth, using population norms derived from the study population [27]. In that study, the proportion of SGA infants was 25% among stillbirths and 10% among live births, but the proportions of LGA infants were the same among stillbirths and live births, both 10%. That study was also limited by incomplete account of the confounding effects of stillbirth risk factors such as diabetes, maternal weight, hypertension, and smoking. Another study of the association between birth weight and risk of stillbirth found that birth weight in excess of 4,500 g was associated with increased risk of stillbirth [34]. However, infants with birth weight greater than 4,500 g are almost exclusively post-term and thus at risk of stillbirth due to prolonged duration of pregnancy [18],[19],[35].
A recent case series reported a higher than expected proportion of LGA among stillbirths. However, the majority of the LGA stillbirths in this series were related to fetal hydrops or maternal diabetes [36].
In our study, abnormal fetal growth was identified in twice as many stillbirths using ultrasound and individualized norms as when using population norms. Although SGA was associated with stillbirth based on all three norms, the association of stillbirth with LGA was observed only when using ultrasound or individualized norms. Differences in design may account for these results. The population norms by Alexander et al. are commonly used and were developed using birth weights from all pregnancies resulting in single live births, including those with complications associated with growth abnormalities, resulting in a wide range of birth weights between the 10th and 90th percentiles, classified as AGA [18]. In contrast, both the ultrasound and the individualized norms were derived from a cohort of uncomplicated pregnancies. Furthermore, the individualized norms account for maternal and pregnancy characteristics to the extent they affect birth weight in uncomplicated live born pregnancies, which may result in more accurate assessment of the expected fetal weight and deviations from expected size.
In a large population of uncomplicated pregnancies, population reference percentiles and, to a lesser degree, ultrasound norms were shown to overestimate the proportion of AGA and to underestimate the proportion of LGA infants [15]. Consistent with those findings, in this study only 8% of live births and 4% of stillbirths were classified as LGA by population norms. Using individualized norms, 12% of live births and 17% of stillbirths were classified as LGA. The underestimation of LGA and the high proportion of stillbirths classified as AGA by population norms may explain the observed lack of association between stillbirth and LGA based on population norms.
In individualized norms, the predictors of fetal growth, the sizes of their effects, and the ranges of their values were derived from a carefully selected population of almost 10,000 pregnancies without pregnancy or neonatal complications [15]. Among those predictors are ones with known association with SGA, such as smoking. This is likely because the relationship between smoking and fetal growth is complex. Smoking is associated with SGA, but the majority of those SGA pregnancies will not have adverse outcomes [37]. Smoking is also associated with a decreased risk of preeclampsia, a major risk factor for SGA [38]. Because of the opposing effects of smoking on birth weight, smoking was taken into account in individualization of fetal growth, despite its clear adverse effect on pregnancy outcome. Consistent with these observations, our sensitivity analysis showed that individualized norms with and without smoking, as well as with all the predictors, had similar patterns of association with stillbirth (Table 3). However, an argument might be made in general against accounting for maternal smoking in predicting optimal individualized expected birth weight, as smoking may have an adverse effect on a particular pregnancy, and the effect of smoking on an individual pregnancy is difficult to determine. The effect of smoking on fetal growth and adverse pregnancy outcome is complex because of its dual effect on both SGA and pregnancy complications due to a positive association of SGA directly with smoking, but a negative association indirectly through lower risk of preeclampsia. Additionally, the effect of smoking on SGA and complications of pregnancy is confounded by co-exposures and other characteristics, and is unclear with very low levels of exposure.
Consistent with prior studies, the findings of this study show that population norms are inferior to norms derived from uncomplicated populations, either ultrasound or individualized norms [39]. The ultrasound and individualized norms performed similarly in identifying pregnancies at risk of stillbirth. Both similarly detected SGA pregnancies associated with stillbirth in this study, and both, in a prior study, classified approximately 10% of uncomplicated pregnancies as SGA [15]. Ultrasound norms appear to underestimate the proportion of LGA pregnancies, both among stillbirth in this study and among uncomplicated pregnancies [15]. The association of fetal growth with other pregnancy outcomes, such as neonatal mortality and morbidity, was not investigated in this study. There is substantial uncertainty about the performance of different norms adjusting for fetal growth determinants [40],[41]. This study was not designed to compare customized norms. A previous study comparing customized and individualized norms showed that the latter performed better in identifying pregnancies with various complications [15]. An advantage of individualized norms is that the fetal growth predictors, their effect sizes, and the ranges of their values were derived from a large population of uncomplicated pregnancies rather than arbitrarily chosen.
The strength and pattern of the association between fetal growth and risk of stillbirth was similar in term and preterm pregnancies. SGA and LGA birth weights were associated with increased risk of stillbirth in preterm as well as term pregnancies using ultrasound or individualized norms. Using population norms, only SGA pregnancies had an increased risk of stillbirth, both preterm and term. The distribution of GA at death was similar among stillbirths classified as SGA, AGA, and LGA using ultrasound norms and also when classified using individualized norms, and the association of stillbirth with SGA and with LGA was observed when using both of these norms.
The association of LGA with stillbirth in this study was not related to post-term GA or known conditions that increase fetal weight and risk of stillbirth. Among stillbirths classified as LGA, only one pregnancy was greater than 40 wk. LGA was also associated with stillbirth among the subset of pregnancies that excluded hydrops and other congenital abnormalities. Because the relationship between congenital abnormalities and birth weight is complex and depends on the type of abnormality, we conducted analyses in pregnancies with and without congenital abnormalities. Both showed similar patterns of associations. Although the effect of maceration on a stillbirth's birth weight is uncertain, if birth weight is decreased in macerated stillbirths, this would decrease the strength of the association with LGA. However, the associations between SGA and LGA and risk of stillbirth were also observed in the subset with optimal estimates of fetal age that included non-macerated stillbirth infants with a short interval of less than 7 d between the time they were last reported alive and the time they were first identified as demised. Results of the subset analysis of pregnancies without pregestational or gestational diabetes or hypertension also showed similar associations with LGA.
Screening for gestational diabetes is performed in the US at 24 to 28 wk. Women at increased risk with a history of gestational diabetes, impaired glucose metabolism, or obesity are additionally screened in early pregnancy [42]. It is possible that a small proportion of women without those risk factors and delivering before routine screening was performed were not diagnosed, although those with LGA stillbirth are also recommended to be screened after stillbirth. However, it is unlikely that any misclassification would significantly affect the study findings, as the patterns of association between fetal growth and risk of stillbirth observed in the subset without pregestational or gestational diabetes and in the entire study population, including women with diabetes, were very similar.
Limitations
A limitation of this study is that retrospective review of medical records was used to obtain birth weight, criteria for GA estimation, and certain maternal characteristics. However, these variables were recorded in medical records prospectively and thus were unlikely to be subject to substantial bias. Many of the characteristics used to determine individualized expected birth weight were missing and were replaced with reference values. However, individualized norms based on subsets of non-missing variables showed very similar findings (Table 3). In this study 70% of cases and 63% of controls agreed to participate. Analysis weights accounted for the observed differences between consenting and non-consenting women [16].
The mechanism of stillbirth in LGA pregnancies is not known. However, it has been suggested that LGA stillbirths may have relatively insufficiently large placentae, which, although not small per se, may be inadequate to support the metabolic demands of a large fetus, rendering it vulnerable to insults during pregnancy [36]. Burmeister et al. found that almost half of LGA stillbirths had placentae smaller than expected for fetal weight, and none larger than expected [36].
Summary
In summary, when accounting for time of death and using norms developed in normal pregnancies, both SGA and LGA birth weights were associated with stillbirth in our study. The association is mainly related to severe SGA and LGA pregnancies, with birth weights either below the 5th or above the 95th percentile. Thus, classifying 10% of pregnancies as abnormally grown has the potential to identify 44%–46% of future stillbirths. This would provide an opportunity for prevention of stillbirth, especially in term pregnancies, when delivery is associated with relatively low neonatal mortality and morbidity. However, the effectiveness of stillbirth prevention would be expected to be decreased by inaccuracy of the fetal growth estimates. The association between large birth weights and stillbirth cannot be captured by population norms that include pregnancies with complications associated with growth abnormalities.
Implications
Our results suggest that, contrary to current practices and recommendations, the most effective approach to identifying fetal growth abnormalities for prediction and prevention of stillbirth would focus on both severe SGA and LGA (<5th and >95th percentile) pregnancies and would use norms developed from normal pregnancies, rather than population norms, for fetal growth surveillance. This strategy would identify as at risk almost half of the pregnancies that would result in stillbirth. However, the majority of stillbirths would remain unidentified either because of inaccuracy of the fetal growth assessment or because they are not associated with growth abnormalities.
Supporting Information
Zdroje
1. MacDormanMF, KirmeyerS (2009) Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep 57 : 1–19.
2. MacDormanMF, MathewsTJ (2008) Recent trends in infant mortality in the United States. NCHS Data Brief 1–8.
3. LawnJE, BlencoweH, PattinsonR, CousensS, KumarR, et al. (2011) Stillbirths: Where? When? Why? How to make the data count? Lancet 377 : 1448–1463 doi:10.1016/S0140-6736(10)62187-3
4. StantonC, LawnJE, RahmanH, Wilczynska-KetendeK, HillK (2006) Stillbirth rates: delivering estimates in 190 countries. Lancet 367 : 1487–1494 doi:10.1016/S0140-6736(06)68586-3
5. FlenadyV, KoopmansL, MiddletonP, FroenJF, SmithGC, et al. (2011) Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 377 : 1331–1340 doi:10.1016/S0140-6736(10)62233-7
6. SmithGC, FrettsRC (2007) Stillbirth. Lancet 370 : 1715–1725 doi:10.1016/S0140-6736(07)61723-1
7. ACOG practice bulletin. Antepartum fetal surveillance. Number 9, October 1999 (replaces Technical Bulletin Number 188, January 1994). Clinical management guidelines for obstetrician-gynecologists. Int J Gynaecol Obstet 68 : 175–185.
8. Royal College of Obstetricians and Gynaecologists (2010) Late intrauterine fetal death and stillbirth. RCOG Green-top Guideline No. 55. London: Royal College of Obstetricians and Gynaecologists.
9. ListonR, SawchuckD, YoungD (2007) Fetal health surveillance: antepartum and intrapartum consensus guideline. Society of Obstetricians and Gynaecologists of Canada. J Obstet Gynaecol Can 29: S3–56.
10. Royal Australian and New Zealand College of Obstetricians and Gynaecologists (2009) Intrapartum fetal surveillance clinical guidelines—second edition. East Melbourne: Royal Australian and New Zealand College of Obstetricians and Gynaecologists.
11. SmithGC, ShahI, WhiteIR, PellJP, CrossleyJA, et al. (2007) Maternal and biochemical predictors of antepartum stillbirth among nulliparous women in relation to gestational age of fetal death. BJOG 114 : 705–714 doi:10.1111/j.1471-0528.2007.01343.x
12. SmithGC, CrossleyJA, AitkenDA, PellJP, CameronAD, et al. (2004) First-trimester placentation and the risk of antepartum stillbirth. JAMA 292 : 2249–2254 doi:10.1001/jama.292.18.2249
13. SpencerK (2000) Second-trimester prenatal screening for Down syndrome and the relationship of maternal serum biochemical markers to pregnancy complications with adverse outcome. Prenat Diagn 20 : 652–656.
14. YaronY, CherryM, KramerRL, O'BrienJE, HallakM, et al. (1999) Second-trimester maternal serum marker screening: maternal serum alpha-fetoprotein, beta-human chorionic gonadotropin, estriol, and their various combinations as predictors of pregnancy outcome. Am J Obstet Gynecol 181 : 968–974.
15. BukowskiR, UchidaT, SmithGC, MaloneFD, BallRH, et al. (2008) Individualized norms of optimal fetal growth: fetal growth potential. Obstet Gynecol 111 : 1065–1076 doi:10.1097/AOG.0b013e3181704e48
16. ParkerCB, HogueCJ, KochMA, WillingerM, ReddyUM, et al. (2011) Stillbirth Collaborative Research Network: design, methods and recruitment experience. Paediatr Perinat Epidemiol 25 : 425–435 doi:10.1111/j.1365-3016.2011.01218.x
17. ConwayDL, HansenNI, DudleyDJ, ParkerCB, ReddyUM, et al. (2013) An algorithm for the estimation of gestational age at the time of fetal death. Paediatr Perinat Epidemiol 27 : 145–157 doi:10.1111/ppe.12037
18. AlexanderGR, HimesJH, KaufmanRB, MorJ, KoganM (1996) A United States national reference for fetal growth. Obstet Gynecol 87 : 163–168 doi:10.1016/0029-7844(95)00386-X
19. HadlockFP, HarristRB, Martinez-PoyerJ (1991) In utero analysis of fetal growth: a sonographic weight standard. Radiology 181 : 129–133.
20. GardosiJ, ChangA, KalyanB, SahotaD, SymondsEM (1992) Customised antenatal growth charts. Lancet 339 : 283–287.
21. Stillbirth Collaborative Research Network (2011) Association between stillbirth and risk factors known at pregnancy confirmation. JAMA 306 : 2469–2479 doi:10.1001/jama.2011.1798
22. BorrellC, CireraE, RicartM, PasarinMI, SalvadorJ (2003) Social inequalities in perinatal mortality in a southern European city. Eur J Epidemiol 18 : 5–13.
23. CanterinoJC, AnanthCV, SmulianJ, HarriganJT, VintzileosAM (2004) Maternal age and risk of fetal death in singleton gestations: USA, 1995–2000. J Matern Fetal Neonatal Med 15 : 193–197 doi:10.1080/14767050410001668301
24. CnattingiusS, HaglundB, KramerMS (1998) Differences in late fetal death rates in association with determinants of small for gestational age fetuses: population based cohort study. BMJ 316 : 1483–1487.
25. HutcheonJA, ZhangX, CnattingiusS, KramerMS, PlattRW (2008) Customised birthweight percentiles: does adjusting for maternal characteristics matter? BJOG 115 : 1397–1404 doi:10.1111/j.1471-0528.2008.01870.x
26. ZhangX, PlattRW, CnattingiusS, JosephKS, KramerMS (2007) The use of customised versus population-based birthweight standards in predicting perinatal mortality. BJOG 114 : 474–477 doi:10.1111/j.1471-0528.2007.01273.x
27. RayJG, UrquiaML (2012) Risk of stillbirth at extremes of birth weight between 20 to 41 weeks gestation. J Perinatol 32 : 829–836 doi:10.1038/jp.2012.60
28. ClaussonB, GardosiJ, FrancisA, CnattingiusS (2001) Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG 108 : 830–834.
29. FroenJF, GardosiJO, ThurmannA, FrancisA, Stray-PedersenB (2004) Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand 83 : 801–807 doi:10.1111/j.0001-6349.2004.00602.x
30. GardosiJ, MulT, MongelliM, FaganD (1998) Analysis of birthweight and gestational age in antepartum stillbirths. Br J Obstet Gynaecol 105 : 524–530.
31. GenestDR, SingerDB (1992) Estimating the time of death in stillborn fetuses: III. External fetal examination; a study of 86 stillborns. Obstet Gynecol 80 : 593–600.
32. GenestDR (1992) Estimating the time of death in stillborn fetuses: II. Histologic evaluation of the placenta; a study of 71 stillborns. Obstet Gynecol 80 : 585–592.
33. GenestDR, WilliamsMA, GreeneMF (1992) Estimating the time of death in stillborn fetuses: I. Histologic evaluation of fetal organs; an autopsy study of 150 stillborns. Obstet Gynecol 80 : 575–584.
34. ZhangX, DeckerA, PlattRW, KramerMS (2008) How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol 198 : 517.e1–6 doi:10.1016/j.ajog.2007.12.005
35. SmithGC (2001) Life-table analysis of the risk of perinatal death at term and post term in singleton pregnancies. Am J Obstet Gynecol 184 : 489–496 doi:10.1067/mob.2001.109735
36. BurmeisterB, ZaleskiC, ColdC, McPhersonE (2012) Wisconsin Stillbirth Service Program: analysis of large for gestational age cases. Am J Med Genet A 158A: 2493–2498 doi:10.1002/ajmg.a.35578
37. PolakowskiLL, AkinbamiLJ, MendolaP (2009) Prenatal smoking cessation and the risk of delivering preterm and small-for-gestational-age newborns. Obstet Gynecol 114 : 318–325 doi:10.1097/AOG.0b013e3181ae9e9c
38. CastlesA, AdamsEK, MelvinCL, KelschC, BoultonML (1999) Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med 16 : 208–215.
39. GibbonsK, ChangA, FlenadyV, MahomedK, GardenerG, et al. (2013) Customised birthweight models: do they increase identification of at-risk infants? J Paediatr Child Health 49 : 380–387 doi:10.1111/jpc.12189
40. CarberryAE, GordonA, BondDM, HyettJ, Raynes-GreenowCH, et al. (2011) Customised versus population-based growth charts as a screening tool for detecting small for gestational age infants in low-risk pregnant women. Cochrane Database Syst Rev 2011: CD008549 doi:10.1002/14651858.CD008549.pub2
41. GibbonsKS, ChangAM, FlenadyVJ, MahomedK, GrayPH, et al. (2013) A test of agreement of customised birthweight models. Paediatr Perinat Epidemiol 27 : 131–137 doi:10.1111/ppe.12041
42. Committee on Practice Bulletins—Obstetrics (2013) Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol 122 : 406–416 doi:10.1097/01.AOG.0000433006.09219.f1
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- What We Know and What We Don't Know About Preventing Stroke
- Safety of Pediatric HIV Elimination: The Growing Population of HIV- and Antiretroviral-Exposed but Uninfected Infants
- The Use of Preliminary Scientific Evidence in Public Health: A Case Study of XMRV
- A Physicians' Wish List for the Clinical Application of Intestinal Metagenomics
- HIV Monoclonal Antibodies: A New Opportunity to Further Reduce Mother-to-Child HIV Transmission
- Fialuridine Induces Acute Liver Failure in Chimeric TK-NOG Mice: A Model for Detecting Hepatic Drug Toxicity Prior to Human Testing
- Regional Changes in Charcoal-Burning Suicide Rates in East/Southeast Asia from 1995 to 2011: A Time Trend Analysis
- Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis
- Changes in HIV Incidence among People Who Inject Drugs in Taiwan following Introduction of a Harm Reduction Program: A Study of Two Cohorts
- Burden of Total and Cause-Specific Mortality Related to Tobacco Smoking among Adults Aged ≥45 Years in Asia: A Pooled Analysis of 21 Cohorts
- Fetal Growth and Risk of Stillbirth: A Population-Based Case–Control Study
- Indoor Residual Spraying in Combination with Insecticide-Treated Nets Compared to Insecticide-Treated Nets Alone for Protection against Malaria: A Cluster Randomised Trial in Tanzania
- Association between Prenatal Exposure to Antiretroviral Therapy and Birth Defects: An Analysis of the French Perinatal Cohort Study (ANRS CO1/CO11)
- Optimal Evidence in Difficult Settings: Improving Health Interventions and Decision Making in Disasters
- Modifiable Etiological Factors and the Burden of Stroke from the Rotterdam Study: A Population-Based Cohort Study
- Geographical Inequalities in Use of Improved Drinking Water Supply and Sanitation across Sub-Saharan Africa: Mapping and Spatial Analysis of Cross-sectional Survey Data
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Burden of Total and Cause-Specific Mortality Related to Tobacco Smoking among Adults Aged ≥45 Years in Asia: A Pooled Analysis of 21 Cohorts
- What We Know and What We Don't Know About Preventing Stroke
- Safety of Pediatric HIV Elimination: The Growing Population of HIV- and Antiretroviral-Exposed but Uninfected Infants
- Regional Changes in Charcoal-Burning Suicide Rates in East/Southeast Asia from 1995 to 2011: A Time Trend Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání







