-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaArtemisinin-Naphthoquine versus Artemether-Lumefantrine for Uncomplicated Malaria in Papua New Guinean Children: An Open-Label Randomized Trial
Background:
Artemisinin combination therapies (ACTs) with broad efficacy are needed where multiple Plasmodium species are transmitted, especially in children, who bear the brunt of infection in endemic areas. In Papua New Guinea (PNG), artemether-lumefantrine is the first-line treatment for uncomplicated malaria, but it has limited efficacy against P. vivax. Artemisinin-naphthoquine should have greater activity in vivax malaria because the elimination of naphthoquine is slower than that of lumefantrine. In this study, the efficacy, tolerability, and safety of these ACTs were assessed in PNG children aged 0.5–5 y.Methods and Findings:
An open-label, randomized, parallel-group trial of artemether-lumefantrine (six doses over 3 d) and artemisinin-naphthoquine (three daily doses) was conducted between 28 March 2011 and 22 April 2013. Parasitologic outcomes were assessed without knowledge of treatment allocation. Primary endpoints were the 42-d P. falciparum PCR-corrected adequate clinical and parasitologic response (ACPR) and the P. vivax PCR-uncorrected 42-d ACPR. Non-inferiority and superiority designs were used for falciparum and vivax malaria, respectively. Because the artemisinin-naphthoquine regimen involved three doses rather than the manufacturer-specified single dose, the first 188 children underwent detailed safety monitoring. Of 2,542 febrile children screened, 267 were randomized, and 186 with falciparum and 47 with vivax malaria completed the 42-d follow-up. Both ACTs were safe and well tolerated. P. falciparum ACPRs were 97.8% and 100.0% in artemether-lumefantrine and artemisinin-naphthoquine-treated patients, respectively (difference 2.2% [95% CI −3.0% to 8.4%] versus −5.0% non-inferiority margin, p = 0.24), and P. vivax ACPRs were 30.0% and 100.0%, respectively (difference 70.0% [95% CI 40.9%–87.2%], p<0.001). Limitations included the exclusion of 11% of randomized patients with sub-threshold parasitemias on confirmatory microscopy and direct observation of only morning artemether-lumefantrine dosing.Conclusions:
Artemisinin-naphthoquine is non-inferior to artemether-lumefantrine in PNG children with falciparum malaria but has greater efficacy against vivax malaria, findings with implications in similar geo-epidemiologic settings within and beyond Oceania.Trial registration:
Australian New Zealand Clinical Trials Registry ACTRN12610000913077
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(12): e32767. doi:10.1371/journal.pmed.1001773
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001773Summary
Background:
Artemisinin combination therapies (ACTs) with broad efficacy are needed where multiple Plasmodium species are transmitted, especially in children, who bear the brunt of infection in endemic areas. In Papua New Guinea (PNG), artemether-lumefantrine is the first-line treatment for uncomplicated malaria, but it has limited efficacy against P. vivax. Artemisinin-naphthoquine should have greater activity in vivax malaria because the elimination of naphthoquine is slower than that of lumefantrine. In this study, the efficacy, tolerability, and safety of these ACTs were assessed in PNG children aged 0.5–5 y.Methods and Findings:
An open-label, randomized, parallel-group trial of artemether-lumefantrine (six doses over 3 d) and artemisinin-naphthoquine (three daily doses) was conducted between 28 March 2011 and 22 April 2013. Parasitologic outcomes were assessed without knowledge of treatment allocation. Primary endpoints were the 42-d P. falciparum PCR-corrected adequate clinical and parasitologic response (ACPR) and the P. vivax PCR-uncorrected 42-d ACPR. Non-inferiority and superiority designs were used for falciparum and vivax malaria, respectively. Because the artemisinin-naphthoquine regimen involved three doses rather than the manufacturer-specified single dose, the first 188 children underwent detailed safety monitoring. Of 2,542 febrile children screened, 267 were randomized, and 186 with falciparum and 47 with vivax malaria completed the 42-d follow-up. Both ACTs were safe and well tolerated. P. falciparum ACPRs were 97.8% and 100.0% in artemether-lumefantrine and artemisinin-naphthoquine-treated patients, respectively (difference 2.2% [95% CI −3.0% to 8.4%] versus −5.0% non-inferiority margin, p = 0.24), and P. vivax ACPRs were 30.0% and 100.0%, respectively (difference 70.0% [95% CI 40.9%–87.2%], p<0.001). Limitations included the exclusion of 11% of randomized patients with sub-threshold parasitemias on confirmatory microscopy and direct observation of only morning artemether-lumefantrine dosing.Conclusions:
Artemisinin-naphthoquine is non-inferior to artemether-lumefantrine in PNG children with falciparum malaria but has greater efficacy against vivax malaria, findings with implications in similar geo-epidemiologic settings within and beyond Oceania.Trial registration:
Australian New Zealand Clinical Trials Registry ACTRN12610000913077
Please see later in the article for the Editors' SummaryIntroduction
Malaria control programs incorporating artemisinin combination therapy (ACT) have contributed to a decline in malaria morbidity and mortality worldwide, renewing interest in global eradication [1]. However, in geo-epidemiologic settings outside sub-Saharan Africa where there is transmission of multiple Plasmodium species, P. vivax remains a major obstacle to eradication because of its complex life cycle and transmission biology [2]. There is the potential for late relapses from liver-stage hypnozoites, but also growing evidence that the burden of disease and the potential for death from acute P. vivax infections have both been underestimated [3],[4].
Transmission of both P. falciparum and P. vivax can complicate the choice of antimalarial treatment. A recent trial of ACTs involving children from Papua New Guinea (PNG) with uncomplicated malaria showed that the most efficacious were artemether-lumefantrine for P. falciparum and dihydroartemisinin-piperaquine for P. vivax infections [5]. Because of the impracticality of species-specific treatment where there are limited diagnostic facilities, and because falciparum malaria more often progresses to complications and death, artemether-lumefantrine was chosen as the first-line therapy under 2009 PNG national guidelines [6]. This recommendation means that potentially preventable morbidity and mortality due to vivax malaria remain of concern [7].
There is a need for assessment of other ACTs that might replicate the high cure rate of artemether-lumefantrine in falciparum malaria while ensuring that vivax malaria is also effectively treated in PNG and similar epidemiologic settings. Of the few available alternatives, artemisinin-naphthoquine has been marketed in a range of countries in Africa, Asia, and Oceania as single-dose treatment based on limited pharmacologic, efficacy, and safety data in adults [8],[9] and without World Health Organization (WHO) prequalification. Because of its commercial availability in PNG, and as a prelude to a formal safety and efficacy comparison with artemether-lumefantrine, we performed pharmacokinetic dose-finding studies in PNG children aged 5–10 y, which provided evidence that a 3-d daily artemisinin-naphthoquine regimen was well tolerated, safe, and efficacious for uncomplicated malaria [10],[11]. This regimen satisfies the WHO requirement that ACTs be administered over 3 d to retard parasite drug resistance and improve efficacy [12]. In addition, the elimination half-life of naphthoquine [10] is longer than that of piperaquine [13], suggesting that it should be more effective in suppressing P. vivax relapses in patients treated for vivax or falciparum malaria [5].
The aim of the present study was to compare the efficacy of three daily artemisinin-naphthoquine doses to that of a six-dose, 3-d artemether-lumefantrine regimen in PNG children aged 0.5–5 y with uncomplicated malaria. We hypothesized that artemisinin-naphthoquine would be well tolerated and safe in this younger vulnerable pediatric population, and that its efficacy would be (i) non-inferior to that of artemether-lumefantrine for falciparum malaria and (ii) superior to that of artemether-lumefantrine for vivax malaria.
Methods
Study Design, Setting, and Approvals
This open-label, parallel-group trial was conducted at the Mugil and Alexishafen Health Centers, Madang Province, PNG, from 28 March 2011 to 22 April 2013. The primary efficacy endpoints were (i) reappearance of P. falciparum within 42 d of treatment after correction for reinfections identified by polymerase chain reaction (PCR) genotyping of polymorphic parasite loci, and (ii) appearance of any P. vivax parasitemia within 42 d after treatment for vivax malaria. Secondary endpoints for falciparum malaria, assessed over 42 d, were (i) reappearance of PCR-uncorrected P. falciparum parasitemia, (ii) appearance of P. vivax parasitemia, and (iii) persistence/appearance of P. falciparum gametocytes. For vivax malaria, secondary endpoints were (i) persistence/appearance of P. vivax gametocytes over 42 d and (ii) reappearance of PCR-corrected P. vivax over 42 d (added post hoc when genotyping techniques became available [14]). Although the WHO recommendation is 28 d of post-treatment monitoring [15], monitoring can be extended to 42 d in the case of ACT partner drugs with a long half-life [12]. Analyses of key outcomes were thus performed at both 28 d and 42 d. Safety assessment included conventional clinical and laboratory monitoring as well as electrocardiographic indices.
Since artemether-lumefantrine has a high (>95%) day-42 adequate parasitologic and clinical response (ACPR) for falciparum malaria in the PNG pediatric population [5], a non-inferiority study design was selected. A sample size of 220 per falciparum malaria treatment arm was chosen based on the assumption that the P. falciparum day-42 PCR-corrected ACPR for artemether-lumefantrine would be 95.2% (as in our previous study [5]), a 5% non-inferiority margin for artemisinin-naphthoquine or artesunate-pyronaridine (approximating the 10% failure rate recommended by WHO for change in treatment policy [12]), 25% attrition, 5% type 1 error (one-tailed), and 80% power. For vivax malaria, given that artemether-lumefantrine has a low cure rate and that dihydroartemisinin-piperaquine was twice as efficacious as artemether-lumefantrine in PNG children in our previous study [5], a superiority study design was used. A sample size of 60 children per arm was selected under the assumption that the day-42 uncorrected P. vivax ACPR for artemisinin-naphthoquine or artesunate-pyronaridine would be superior (at least double the 30.3% ACPR for artemether-lumefantrine [5]), with 25% attrition, 5% type 1 error (two-tailed), and 90% power.
The original study design involved a comparison of conventional artemether-lumefantrine with both artemisinin-naphthoquine and artesunate-pyronaridine. Artesunate-pyronaridine was not initially available because concerns regarding hepatotoxicity were being addressed [16]. The subsequent recommendation that this ACT be limited to a single lifetime dose made its use untenable for malaria treatment programs in countries such as PNG where children are at risk of repeated episodes of malaria [17], and, as a result, the trial was conducted as a two-arm comparison. A prespecified artemisinin-naphthoquine safety assessment was scheduled after 50 patients, as well as further interim efficacy analyses and safety assessments that were scheduled primarily to determine whether artemisinin-naphthoquine violated the non-inferiority margin in the case of falciparum malaria or unexpected toxicity had emerged.
The study was approved by the PNG Institute of Medical Research Review Board, the Medical Research Advisory Committee of PNG, and the University of Western Australia Human Research Ethics Committee. Written informed consent was obtained from parents/guardians of all children.
Patients
Children aged 0.5–5 y presenting with an axillary temperature >37.5°C or a history of fever during the previous 24 h were screened using on-site blood film microscopy. Those with P. falciparum (>1,000 asexual parasites/µl whole blood) or P. vivax (>250/µl) were eligible if (i) there were no features of severity [18], (ii) they had not taken a study drug in the previous 14 d, (iii) there was no history of allergy to study drugs, and (iv) there was no evidence of another infection or co-morbidity.
Clinical Procedures
An initial standardized clinical assessment was performed, and blood was drawn for measurement of hemoglobin and blood glucose (Hemocue 201+, Hemocue, Ängelholm, Sweden), as well as hepatic and renal function (Vitros DT60 II system, Ortho Clinical Diagnostics, Mulgrave, Victoria, Australia) and a full blood count (Coulter Ac·T diff, Beckman Coulter, Brea, California, US). A 12-lead electrocardiograph was taken for measurement of the QT interval. Each QT interval was corrected for heart rate using Bazett's formula () to allow comparisons with other studies in the PNG pediatric population involving antimalarial drugs with effects on ventricular repolarization in which this correction has been applied [11],[19],[20].
Based on computer-generated block randomization (24 children per site), eligible patients were allocated 1∶1 to artemether-lumefantrine (1.7 mg/kg artemether plus 10 mg/kg lumefantrine; Novartis Pharma, Basel, Switzerland) twice daily for 3 d or to artemisinin-naphthoquine (20 mg/kg artemisinin plus 8 mg/kg naphthoquine; Kunming Pharmaceutical Corporation, Yunnan, China) daily for 3 d. Randomization was independent of Plasmodium species. Allocated treatments were concealed in sealed numbered envelopes that were opened in sequence by study medical or nursing staff, and the specified treatment was administered. As recommended by the respective manufacturers, artemether-lumefantrine was administered as 1–3 whole tablets per dose with 250 ml of milk to optimize bioavailability [21], and artemisinin-naphthoquine as 1–4 whole tablets per dose with water.
Treatments were not blinded, primarily because the endpoints were based on objective clinical and parasitologic criteria. In addition, we sought to simplify drug administration as much as possible to maximize patient adherence and retention, including only once daily dosing and the avoidance of potential gastrointestinal side effects with the unnecessary ingestion of milk in children allocated artemisinin-naphthoquine [10]. Only the evening artemether-lumefantrine dose was unsupervised, being administered at home by parents/guardians who were instructed as to the importance of dosing with milk, which was supplied for this purpose. Adherence was verified by study staff the following morning in each case, and a day-7 plasma lumefantrine concentration was determined for each child. Children vomiting within 30 min of drug administration were to be retreated.
Standardized assessment, including axillary temperature and blood film microscopy, was repeated on days 1, 2, 3, 7, 14, 28, and 42. Blood was drawn for full blood count and hepatorenal function, and an electrocardiograph was taken, on days 0, 3, and 7. An additional electrocardiograph was performed 4 h after the day-2 dose (at the predicted peak plasma naphthoquine concentration [10]) in artemisinin-naphthoquine-treated children and in a convenience sample of 30 children at the same time after the morning dose of artemether-lumefantrine. All blood films were reexamined independently by two skilled microscopists who were blind to allocated treatment. Parasite density was calculated from the number of parasites per 200–500 leucocytes and an assumed leucocyte count of 8,000/µl. Slides discrepant for positivity/negativity, speciation, or density (>3× difference) were adjudicated by a senior microscopist.
Efficacy was assessed using WHO definitions [15]. Early treatment failure (ETF) was defined as the development of signs of severity or an inadequate parasitologic response by day 3. Any child developing parasitemia between days 4 and 42 was considered a late parasitologic failure (LPF) or, if febrile, late clinical failure (LCF). An ACPR was recorded otherwise. P. falciparum reinfection and recrudescence were distinguished using PCR genotyping of merozoite surface protein 2 (MSP2) and MSP1 based on paired samples collected on day 0 and at reappearance [22],[23]. P. vivax recrudescence was determined based on genotyping of MSP1F3 and Microsatellite 16 [14]. Parasite clearance time (PCT) and fever clearance time (FCT) were defined as the times to the first of two consecutive assessments at which the child was afebrile and slide-negative, respectively.
Day-7 plasma lumefantrine concentrations were determined using a validated ultra-high-performance liquid chromatography–tandem mass spectrometry assay as previously described [24],[25]. The inter-day and intra-day variability were ≤11.2% and ≤9.6%, respectively, at concentrations over the linear range between 20 and 20,000 ng/ml.
Statistical Analysis
Per-protocol prespecified analyses included children with complete follow-up or a confirmed treatment failure, and excluded those treated for malaria without confirmatory microscopy, those for whom the alternative Plasmodium species was detected, and those who defaulted from follow-up despite repeated attempts at contact. These excluded patients were retained in prespecified modified intention-to-treat analyses utilizing (i) a worst-case approach (ETF assumed for day-3 exclusions, LPF/LCF otherwise) and (ii) a best-case approach (all missing follow-up blood films assumed parasite-negative) [5].
Kaplan-Meier estimates were computed for each endpoint defined by parasite species. Statistical analysis was performed using IBM SPSS Statistics version 20 (IBM Corporation, Somers, New York, US) and STATA/IC 11.2 (StataCorp, College Station, Texas, US). Two-sample comparisons for normally distributed variables were by Student's t test, for non-normally distributed variables by Mann-Whitney U test, and for proportions by Fisher's exact test. Safety and tolerability were assessed from the incidence of symptoms/signs to day 7 using Poisson regression. Kaplan-Meier estimates were computed for each endpoint by Plasmodium species, and treatments compared by log-rank test. Differences in parasitemia, gametocytemia, and hemoglobin concentration by treatment type and time were assessed using generalized linear modeling with Bonferroni correction to adjust for multiple pairwise comparisons, and with adjustment for parasitemia in the case of hemoglobin. Unless otherwise stated, all p-values are two-tailed, with p<0.05 taken as significant.
Results
Trial Profile
The first patient was recruited on 28 March 2011, and the last follow-up was completed on 22 April 2013. No adverse events occurred in the first 50 patients. A further safety assessment was scheduled when one-third of the total planned sample size of 560 had been recruited. Safety data were available for 91 artemether-lumefantrine-treated and 97 artemisinin-naphthoquine-treated children at that time (including those excluded post hoc; see Figure 1). No significant drug-related adverse events had occurred. Detailed safety monitoring was therefore stopped, but scheduled visits still included basic clinical/laboratory parameters including axillary temperature, blood film microscopy, and hemoglobin concentration. A prespecified interim efficacy analysis was performed when nearly 50% of the sample had been recruited (267 of 560). This analysis showed that artemisinin-naphthoquine was non-inferior for falciparum malaria and superior for vivax malaria compared with artemether-lumefantrine (see below), justifying cessation of the trial.
Fig. 1. Trial profile showing numbers of patients remaining in the study from screening to day-42 assessment. 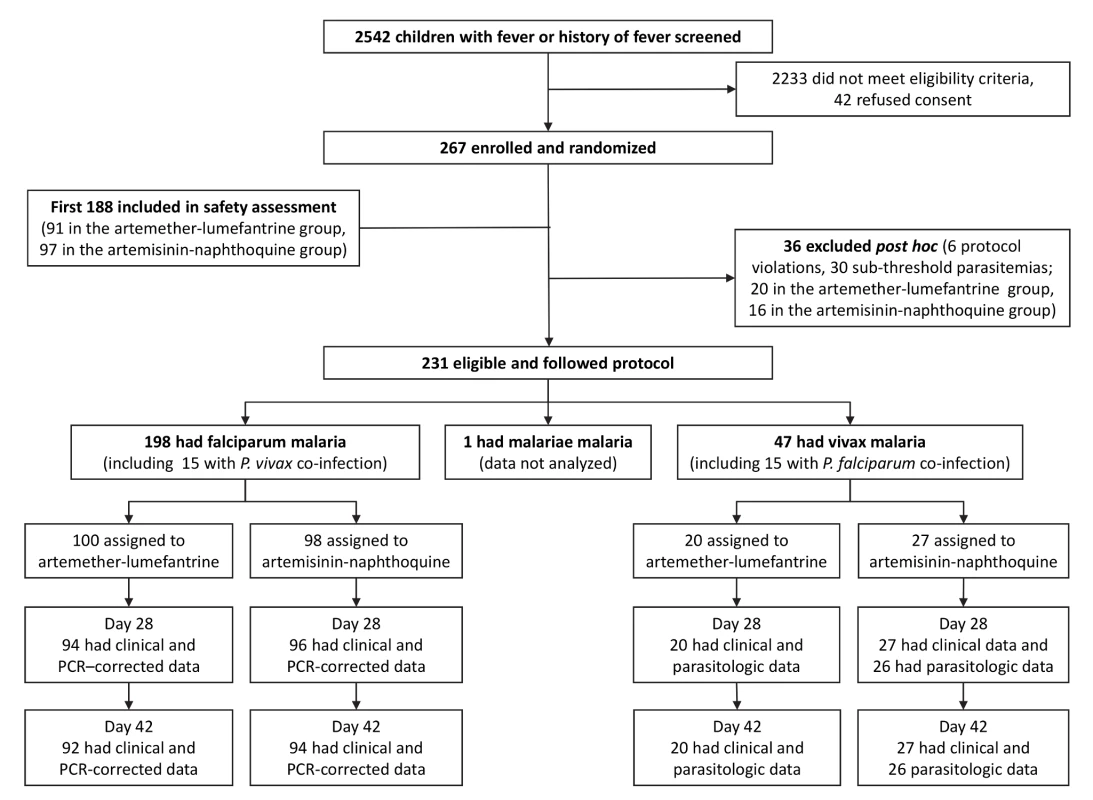
PCR-corrected denotes correction for reinfections identified by polymerase chain reaction genotyping of polymorphic parasite loci. Of 267 randomized children, 36 (13.5%) were excluded post hoc because of protocol violations (20 allocated to artemether-lumefantrine and 16 to artemisinin-naphthoquine; see Figure 1). Six children had not taken study treatment as stipulated (three allocated to each treatment), seven were slide-negative by expert microscopy (four allocated to artemether-lumefantrine and three to artemisinin-naphthoquine), and 23 had sub-threshold parasite densities by expert microscopy (13 allocated to artemether-lumefantrine and ten to artemisinin-naphthoquine). There were no significant differences between treatment allocations for the proportions of total exclusions or individual reasons for exclusion (p>0.47 in each case). In addition, there were no differences between the treatments allocated to the 23 excluded patients who had falciparum malaria or between the treatments allocated to the six with vivax malaria (p>0.67). The 15 children (6.5% of the randomized sample) presenting with mixed P. falciparum/P. vivax infections at densities above each species-specific threshold were included in both species arms [5]. A single patient with P. malariae infection completed all study procedures but was not included in analyses.
Of the remaining 230 children, the day-42 retention was ≥92% for all species/treatment groups. Ten children (six allocated to artemether-lumefantrine and four to artemisinin-naphthoquine, all with falciparum malaria) withdrew during follow-up. None of these children had experienced adverse effects; they had all relocated outside the study catchment areas. Two children with P. falciparum infections in the artemether-lumefantrine arm were found to have symptomatic vivax malaria on day 28 with parasitemias of 21,137/µl and 1,609/µl, respectively. These patients were retreated with artemether-lumefantrine under national guidelines [6] at that time and were excluded from the per-protocol analysis. The remaining children with P. vivax detected during follow-up after treatment for falciparum malaria were mostly asymptomatic and had lower parasitemias, and were either not treated or given artemether-lumefantrine on day 42 after assessment. One child with P. vivax at recruitment who was allocated to artemisinin-naphthoquine attended all follow-up visits but did not have parasitologic data at day 28 and was excluded from the per-protocol analysis. All children allocated artemether-lumefantrine had detectable lumefantrine in plasma on day 7, with a median concentration (192 ug/l [interquartile range 122–352]) consistent with all six doses having been administered successfully [24],[25].
Safety Assessment
There were no significant differences in baseline demographic and anthropometric characteristics (age, sex, weight, height, and mid-upper-arm circumference), vital signs (axillary temperature, heart rate, and respiratory rate), hematologic parameters (white and red blood cell counts, platelet count, and hemoglobin concentration), or blood glucose concentrations between the 91 children allocated to artemether-lumefantrine and the 97 who received artemisinin-naphthoquine in the detailed safety assessment (p≥0.21; see Table 1). No child in either group had a baseline serum creatinine concentration more than three times the upper limit of the reference range (ULRR) for PNG children [26] (≥112 µmol/l), but two children in the artemether-lumefantrine group had a baseline serum alanine aminotransferase (ALT) concentration more than three times the ULRR (≥130 U/l; see Table 2). Consistent with malaria-associated hemolysis, approximately one-third of the children in both groups had a baseline serum total bilirubin concentration more than three times the ULRR (≥24 µmol/l), but there was no between-group difference in this proportion (p = 0.60) and no children were considered to have jaundice.
Tab. 1. Demographic, clinical, and laboratory parameters as part of safety monitoring. 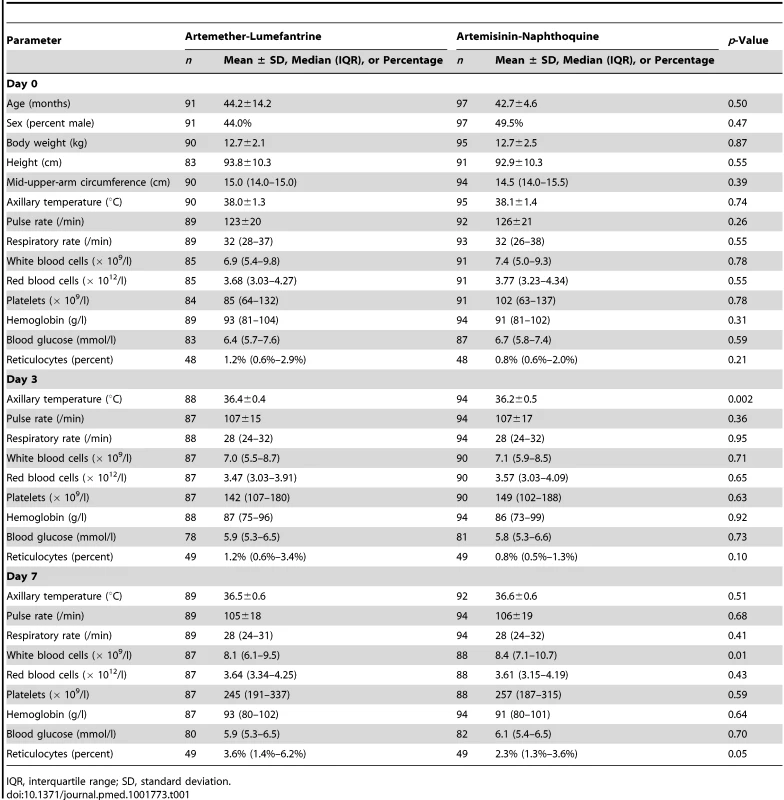
IQR, interquartile range; SD, standard deviation. Tab. 2. Biochemical measurements in the safety sub-study by allocated treatment. 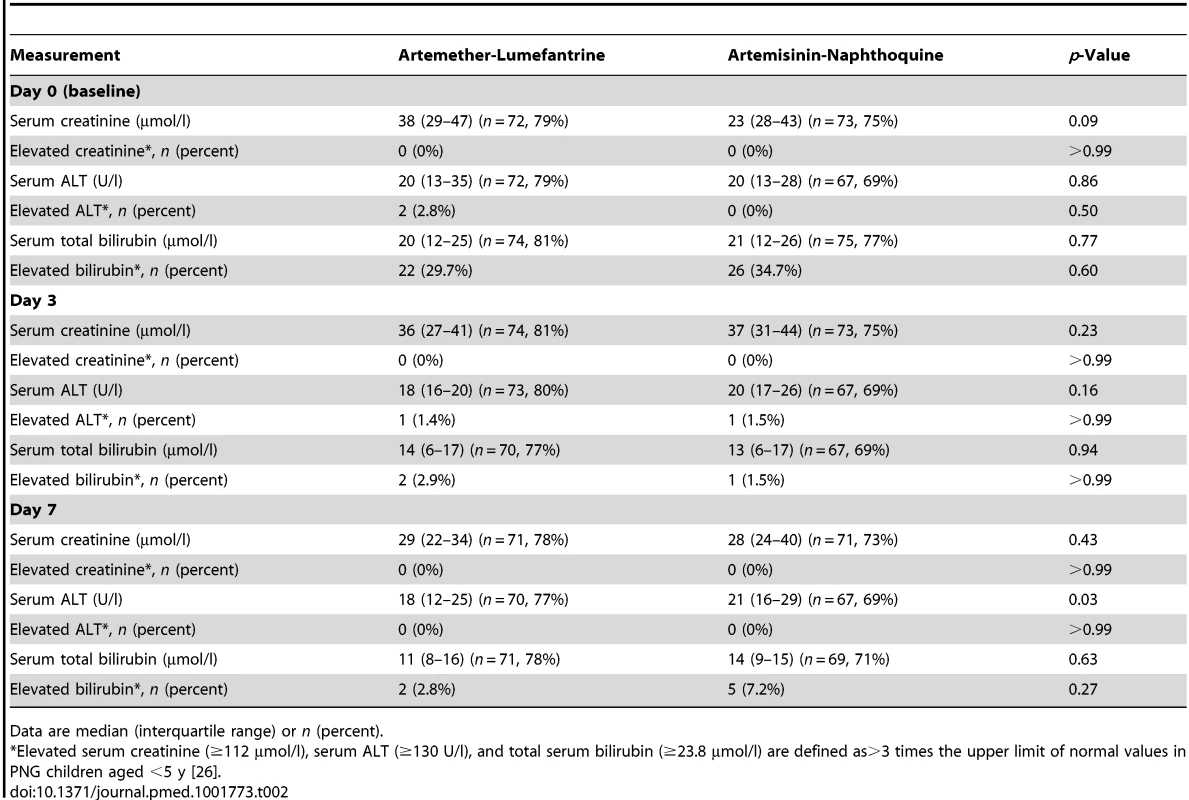
Data are median (interquartile range) or n (percent). Both treatments were well tolerated, and there were no between-group differences in the incidence rates of reported/observed signs and symptoms during the first 7 d of follow-up, apart from a significantly greater incidence of abdominal pain in the artemisinin-naphthoquine-treated patients (incidence rate 11.6% versus 7.8% in the artemether-lumefantrine group; incidence rate ratio 1.48 [95% CI 1.03–2.13], p = 0.033). This symptom was mild and short-lived in all cases (see Table 3). On day 3, one child in each group had a serum ALT more than three times the ULRR, but both were afebrile and asymptomatic, and this abnormality had resolved in both cases by day 7 (see Table 2). Most children had a normal serum bilirubin by day 7. No child became hypoglycemic. Other statistically significant between-group differences in vital signs and hematologic parameters on days 3 and 7 were not clinically significant.
Tab. 3. Incidence rate of main reported or observed signs and symptoms during the first 7 d of follow-up in randomized children, expressed as reports per 100 observations. 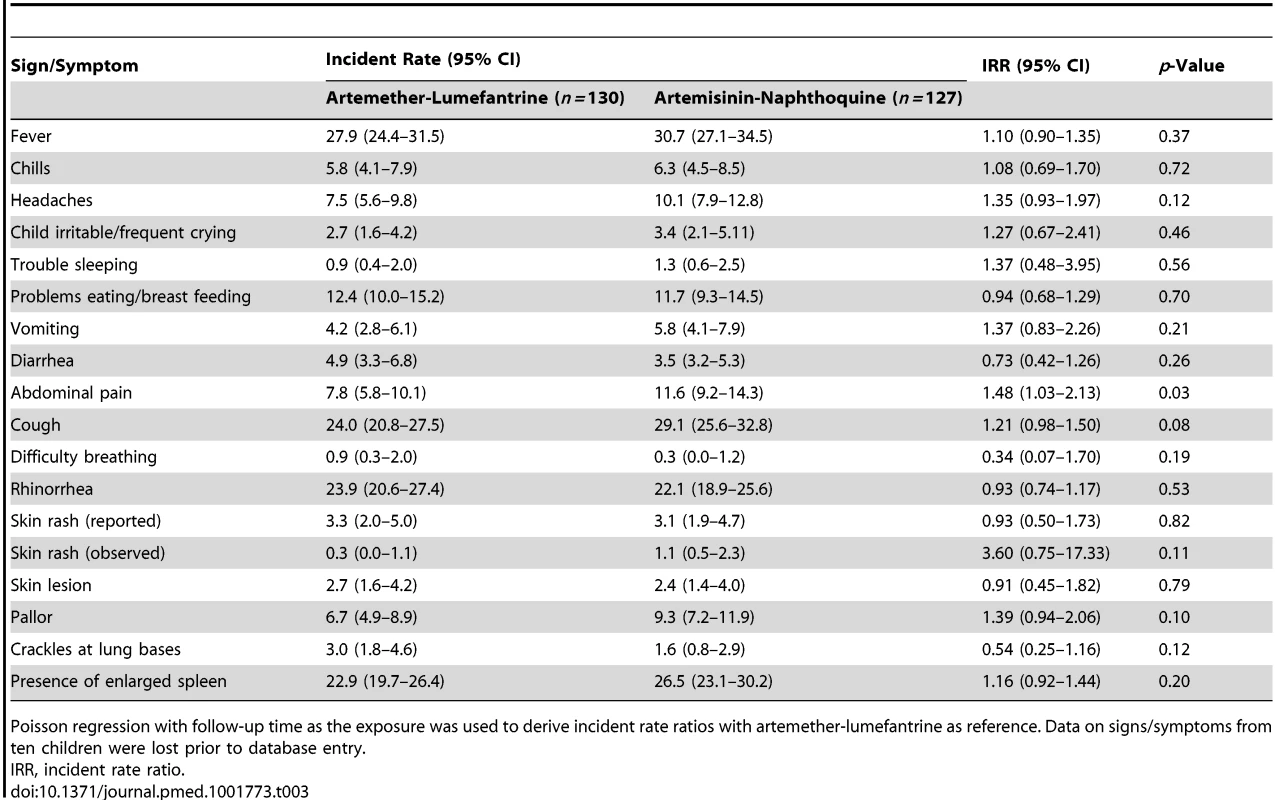
Poisson regression with follow-up time as the exposure was used to derive incident rate ratios with artemether-lumefantrine as reference. Data on signs/symptoms from ten children were lost prior to database entry. Despite the technical challenges associated with performing electrocardiography in unwell children in a rural clinic, interpretable traces were obtained from between 68% and 88% of the children in each group at each time point. The QTc increased from baseline to 4 h after the last dose of artemisinin-naphthoquine by a median of 28 msec0.5 (interquartile range 18–36) (p<0.01 versus artemether-lumefantrine-treated children; see Figure 2 and Table 4). Most values had returned to baseline by day 7, with a prolongation relative to baseline of 5 msec0.5 (interquartile range 2–9). There were no significant changes in QTc in the artemether-lumefantrine group. Twenty-six of 102 children (25.5%, all but one in the much larger artemisinin-naphthoquine group) with an interpretable electrocardiograph on day 2 had QTc>460 msec0.5. The longest QTc at this time point was 505 msec0.5, in an artemisinin-naphthoquine-treated child. No dysrhythmias, dyspnea, syncope, or other transient neurologic events were recorded during follow-up.
Fig. 2. Electrocardiographic QTc intervals after treatment for uncomplicated malaria. 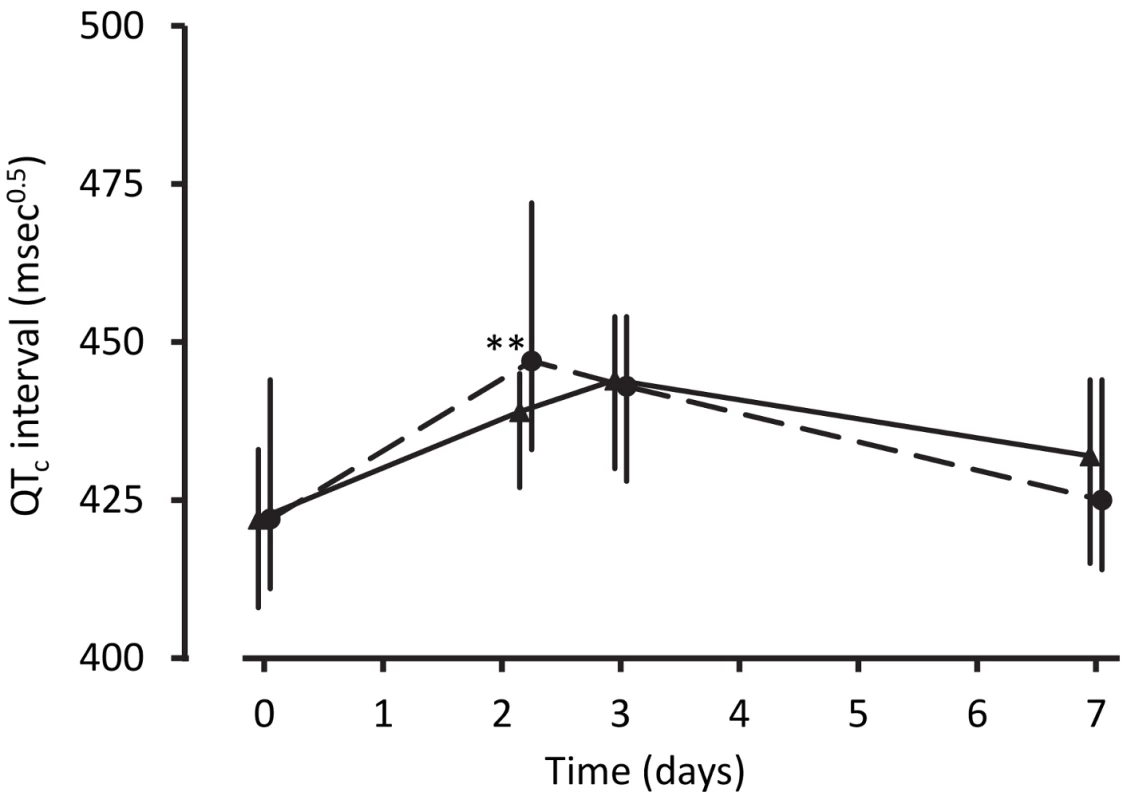
Data are median and interquartile range (vertical bars) for artemether-lumefantrine (▴, solid lines) and artemisinin-naphthoquine (•, dashed lines). **p<0.01 for between-treatment comparisons. Tab. 4. Electrocardiographic QTc measurements by allocated treatment. 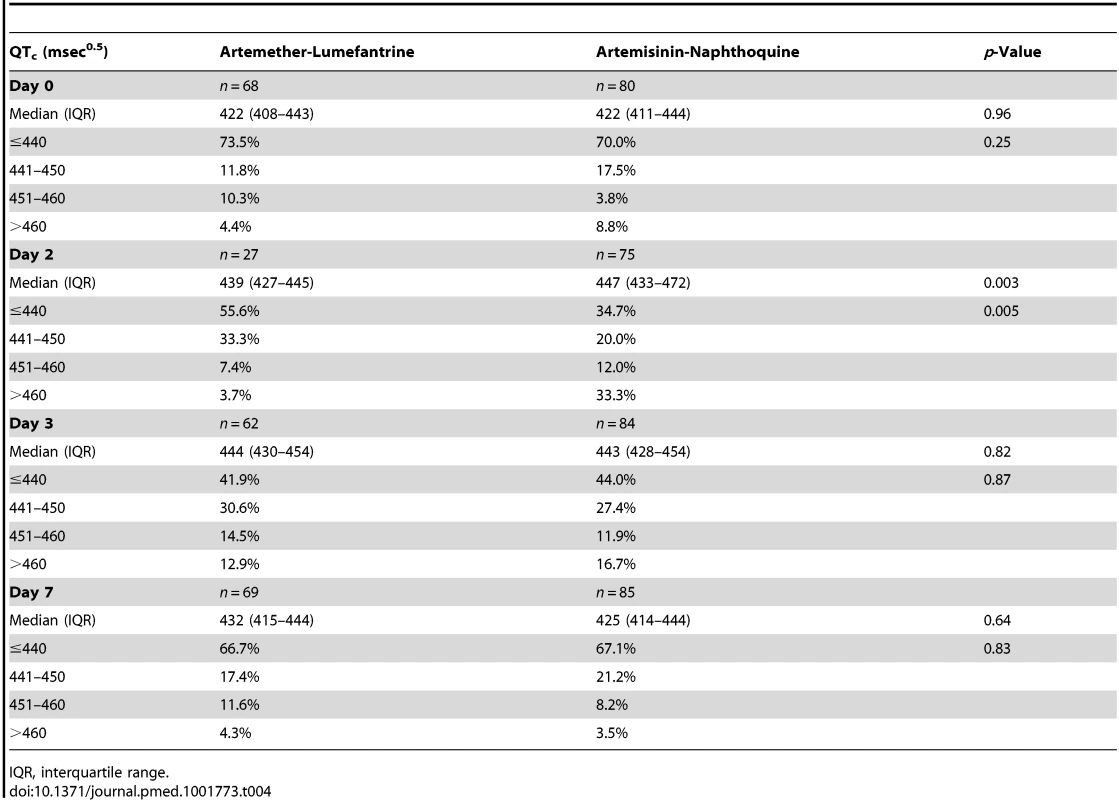
IQR, interquartile range. The only severe adverse event was considered non-drug related. A 48-mo-old child allocated to artemisinin-naphthoquine was hospitalized with, and treated successfully for, lobar pneumonia diagnosed on day 23 of follow-up. This patient was negative by both microscopy and PCR for malaria at the time of re-presentation.
Antimalarial Efficacy
The two falciparum malaria treatment groups were well matched for age, sex, anthropometric measures, vital signs, and other characteristics (see Table 5). Initial fever and parasite clearance were prompt. Most children in each group (≥88.0%) were afebrile, and approximately one-third were parasite negative, by day 1, and almost all had cleared their fever (100.0% in the artemether-lumefantrine arm versus 98.0% in the artemisinin-naphthoquine arm, p = 0.25) and parasitemia (99.0% versus 98.0%, p>0.99) by day 3 (see Table 6).
Tab. 5. Baseline characteristics of patients classified by Plasmodium species and allocated treatment. 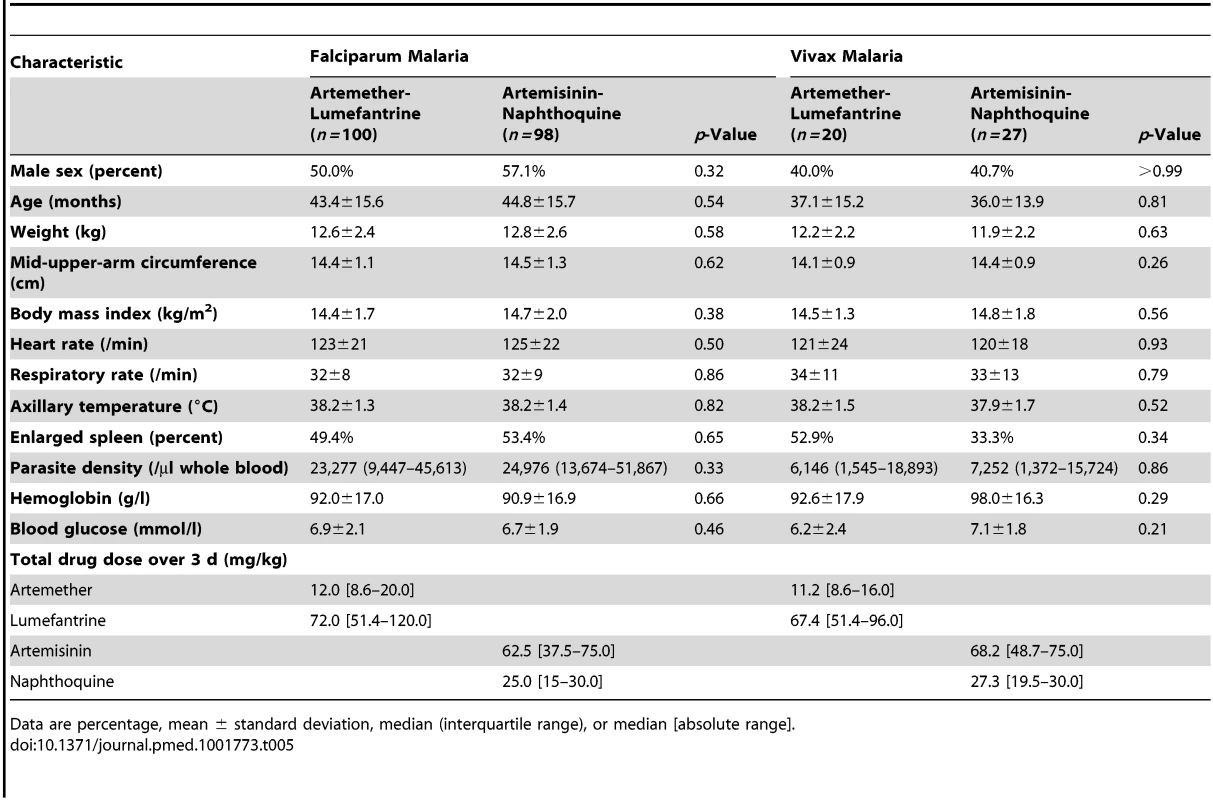
Data are percentage, mean ± standard deviation, median (interquartile range), or median [absolute range]. Tab. 6. Fever and parasite clearance times by Plasmodium species and allocated treatment. 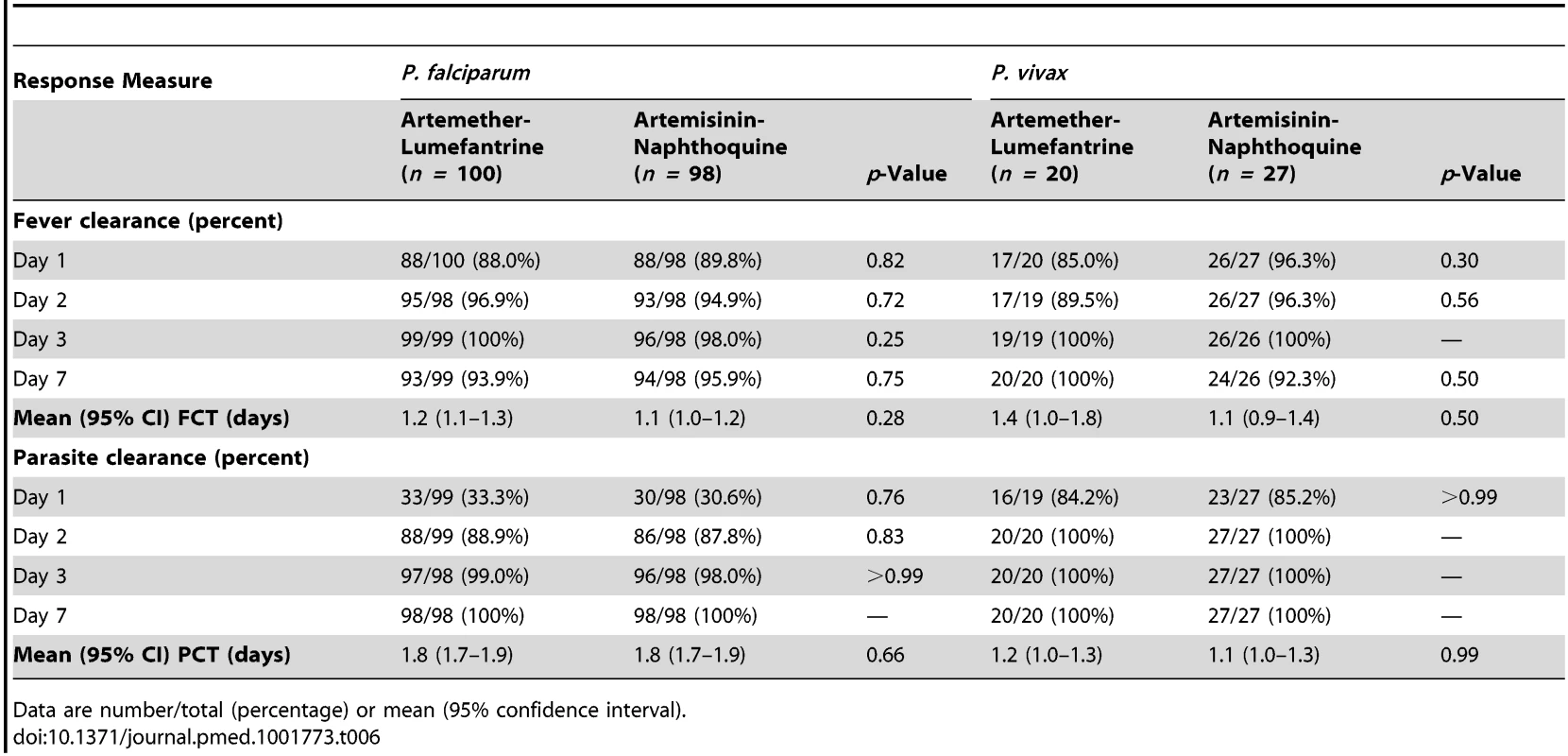
Data are number/total (percentage) or mean (95% confidence interval). The PCR-corrected P. falciparum ACPR was 98.9% (95% CI 94.3%–100%; 93 of 94 children) and 100% (95% CI 95.2%–100%; 96 of 96 children) in the artemether-lumefantrine and artemisinin-naphthoquine groups, respectively, on day 28 (a secondary endpoint), and 97.8% (95% CI 91.6%–99.6%; 90 of 92 children) and 100% (95% CI 95.1%–100%; 94 of 94 children), respectively, on day 42 (a primary endpoint, p = 0.15 by log-rank test; see Figure 3 and Table 7). Using these latter data and the same 5.0% non-inferiority margin, type 1 error probability, and power as in the original sample size estimates, the 186 children who completed follow-up exceeded the total of 52 required to show non-inferiority of artemisinin-naphthoquine for this primary endpoint [27]. In addition, the non-inferiority margin of −5.0% fell below the confidence interval for the difference between treatments (2.2% [95% CI −3.0% to 8.4%]), further evidence of non-inferiority.
Fig. 3. Kaplan-Meier plots showing percentages of patients positive for PCR-corrected P. falciparum and for PCR-uncorrected P. vivax after treatment. 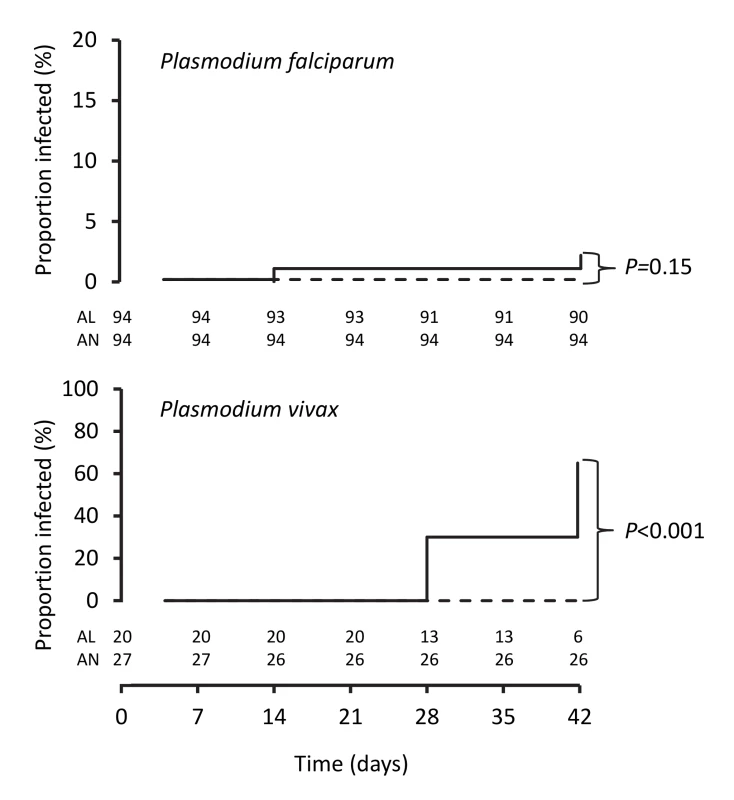
Data are for artemether-lumefantrine (solid lines) and artemisinin-naphthoquine (dashed lines). Numbers of children at risk at each time point are shown for artemether-lumefantrine (AL) and artemisinin-naphthoquine (AN). p-Values are for log-rank tests. Tab. 7. Per-protocol analysis of treatment responses in children with falciparum (after PCR correction) or vivax (PCR-uncorrected) malaria. 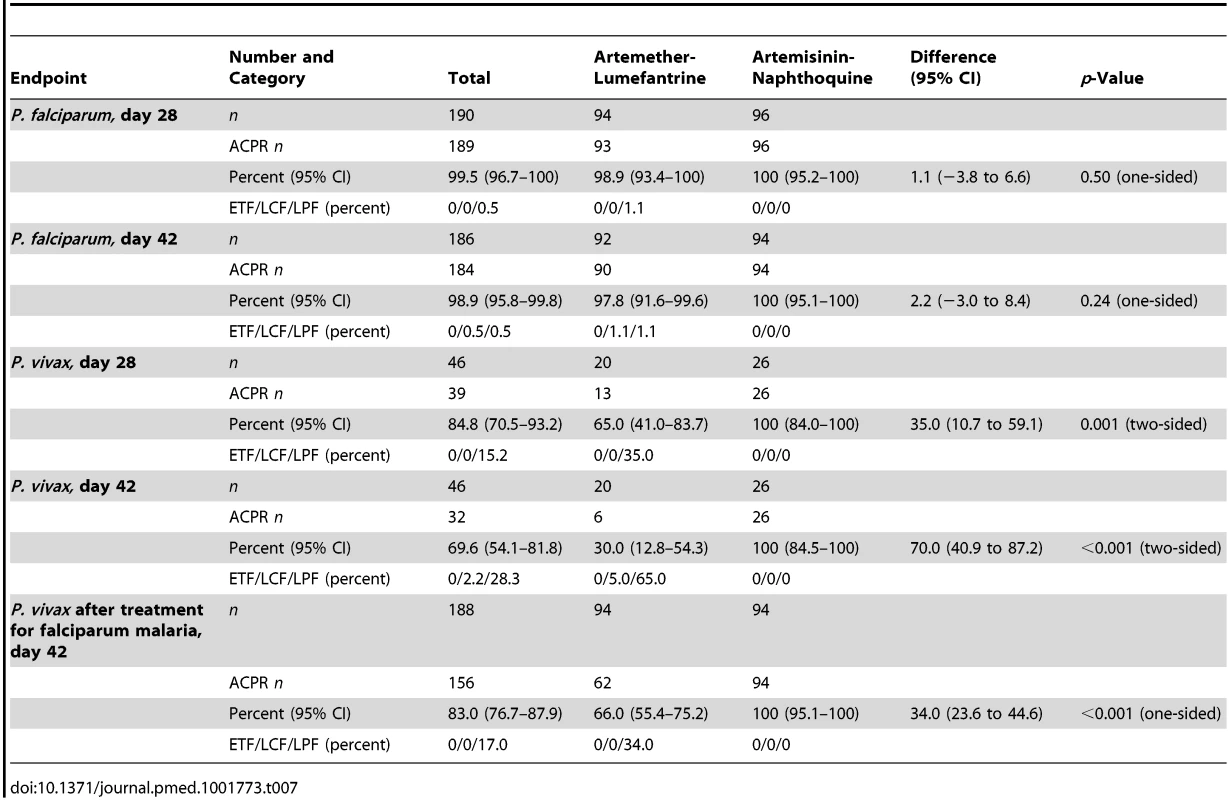
The prespecified secondary endpoint of day-42 uncorrected ACPR was significantly greater in the artemisinin-naphthoquine group (100% [95% CI 95.1%–100%] versus 94.6% [95% CI 87.2%–98.0%] in the artemether-lumefantrine-treated children, p = 0.028; see Table 8). In addition, the appearance of P. vivax after treatment for P. falciparum, also a secondary endpoint, was significantly less frequent in the artemisinin-naphthoquine group (0% versus 34.0% in in the artemether-lumefantrine-treated children, p<0.001; see Table 7). The modified intention-to-treat analyses showed results that were consistent with these findings (see Tables 9 and 10).
Tab. 8. Per-protocol secondary endpoint analysis of response in children with PCR-uncorrected <i>P. falciparum</i> reinfections or PCR-corrected <i>P. vivax</i> recrudescence by treatment allocation. 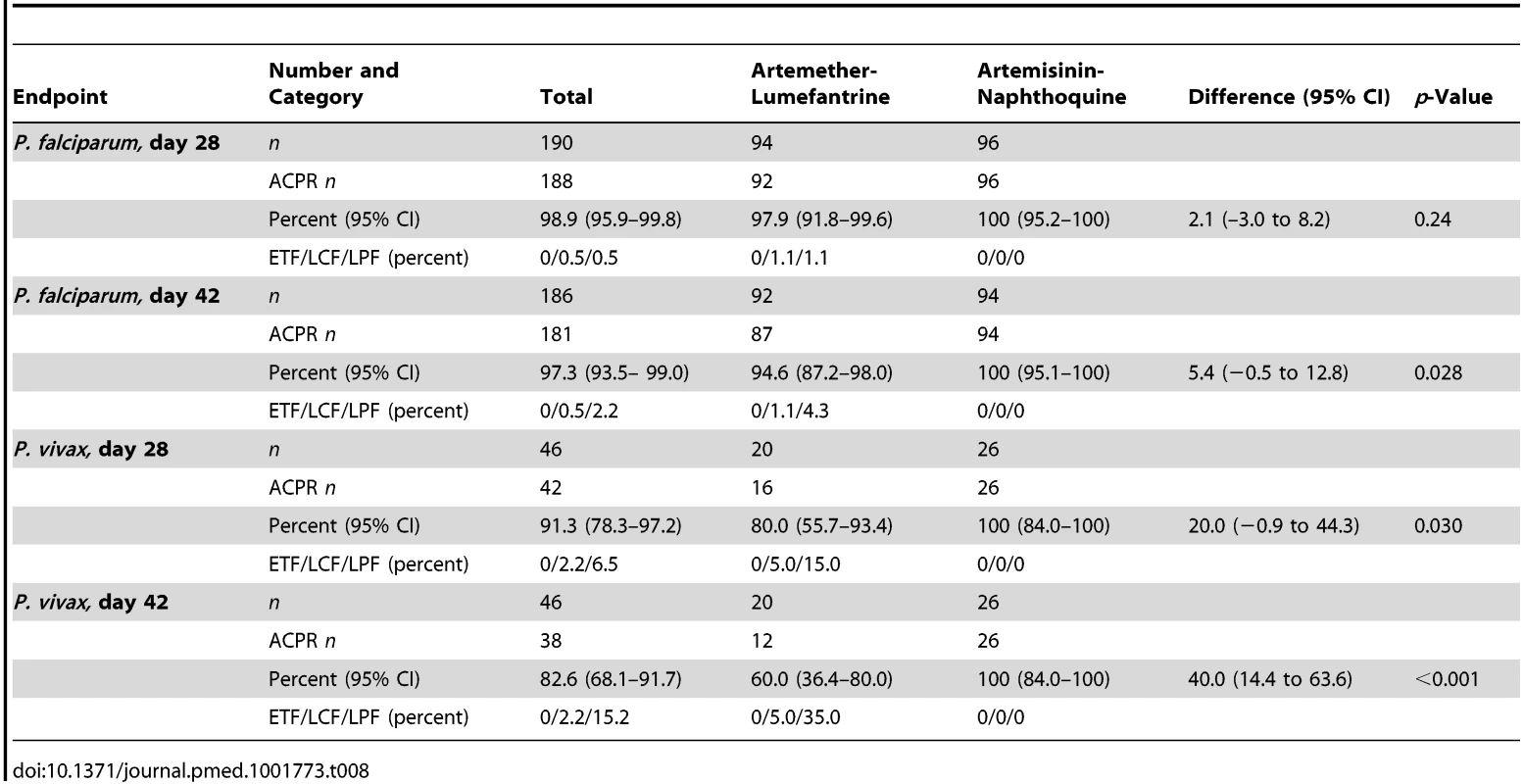
Tab. 9. Modified intention-to-treat analysis of treatment response by treatment allocation utilizing a worst-case scenario (i.e., all instances of missing data during follow-up scored as treatment failure). 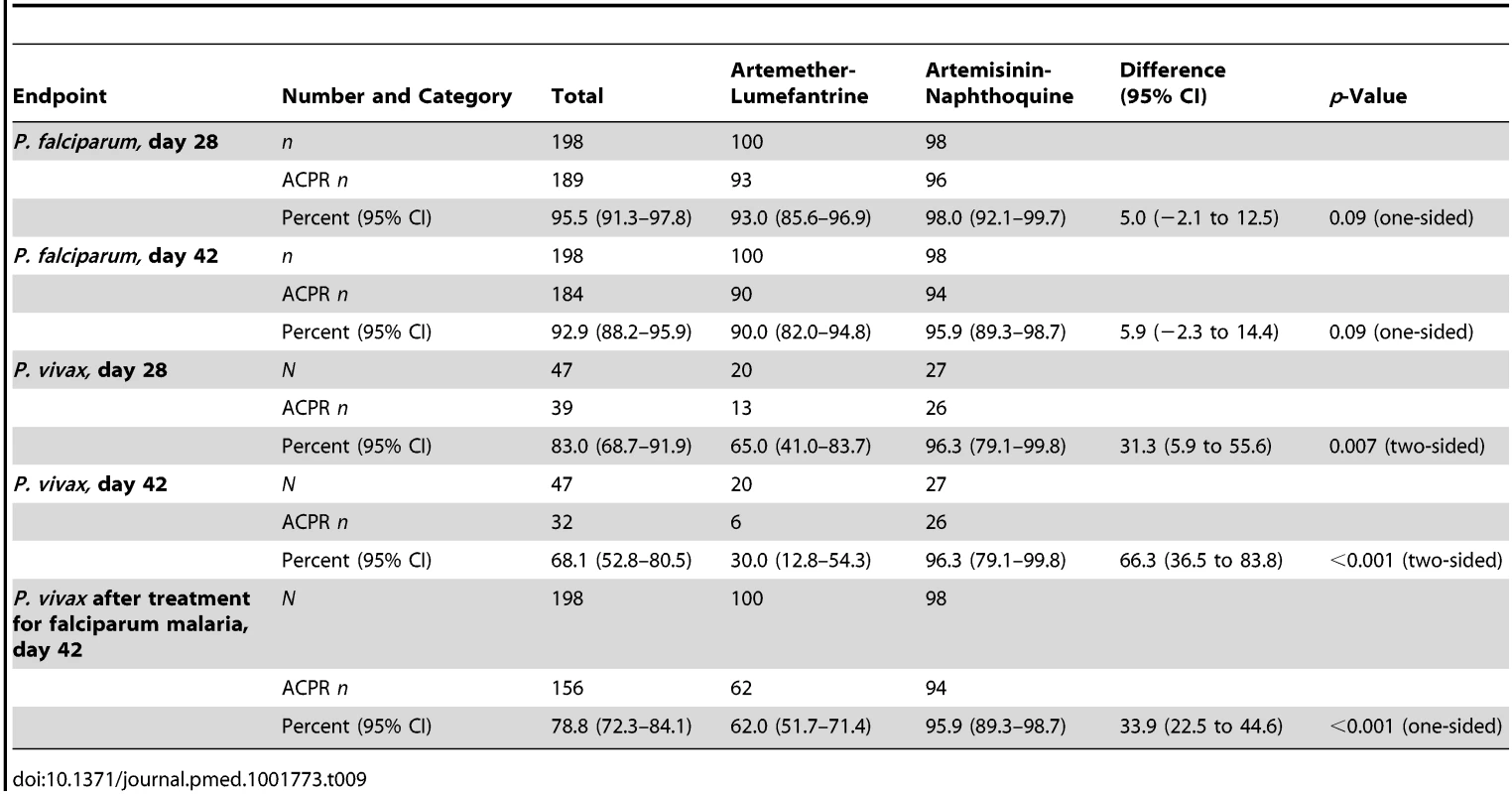
Tab. 10. Modified intention-to-treat analysis of treatment response by treatment allocation utilizing a best-case scenario (i.e., all instances of missing data during follow-up scored as parasite-negative). 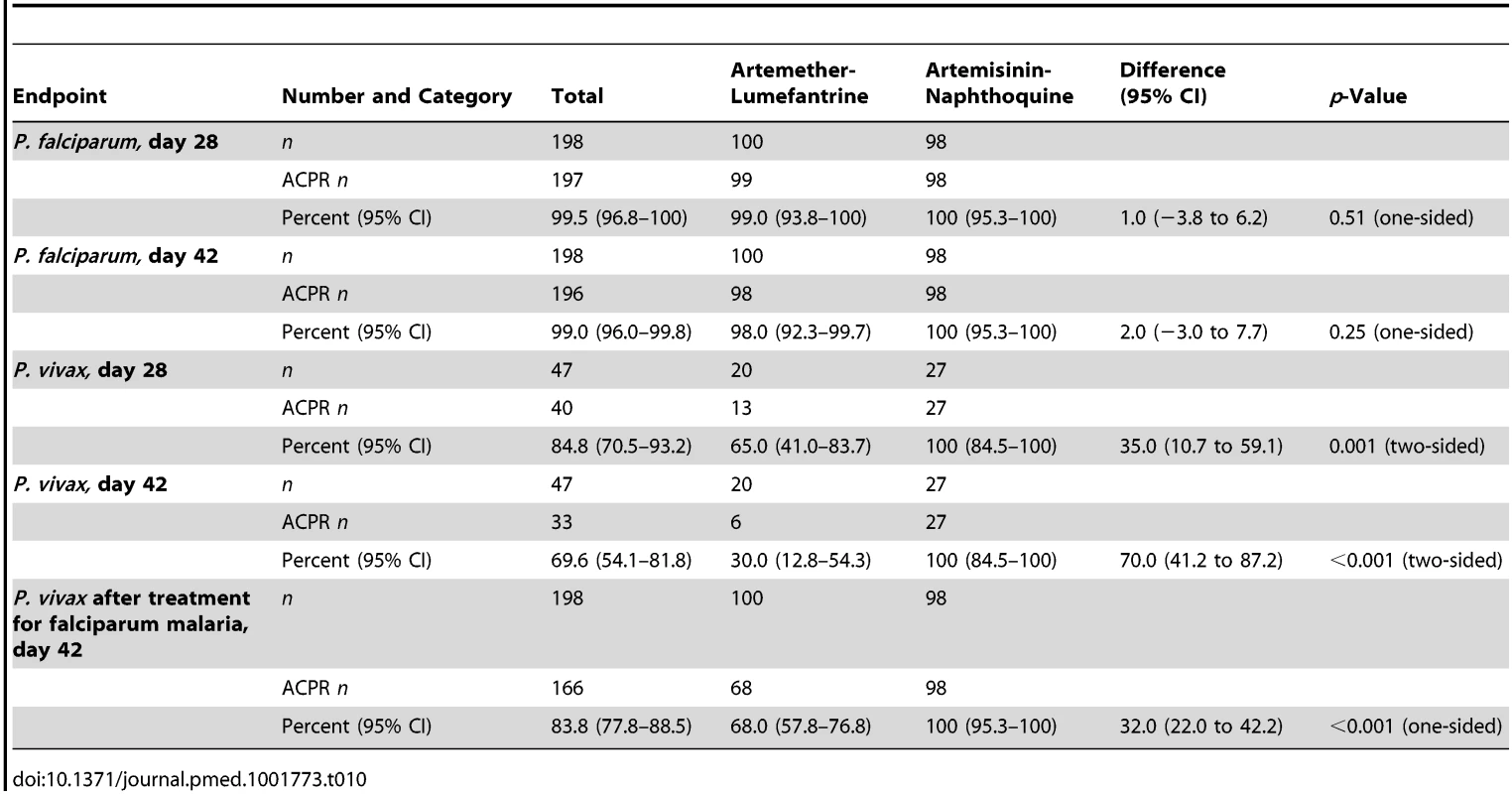
The two vivax malaria treatment groups were also well matched for baseline characteristics (see Table 5). Parasite clearance was more rapid in children with vivax malaria than in the children with falciparum malaria, with >84% of children with vivax malaria in both treatment groups slide-negative for asexual forms of P. vivax by day 1, and 100.0% in both treatment groups both afebrile and aparasitemic by day 3 (see Table 6).
The day-42 PCR-uncorrected ACPR for P. vivax infections was 30.0% (95% CI 12.8%–54.2%; six of 20 children) in the artemether-lumefantrine group and 100% (95% CI 84.5%–100%; 26 of 26 children) in the artemisinin-naphthoquine group (see Figure 3 and Table 7). Using these data and the same type 1 error probability and power as in the original sample size estimates, the 46 children who completed the trial exceeded the total of ten children required to show superiority of artemisinin-naphthoquine for this primary endpoint [28]. The significantly greater day-42 ACPR for artemisinin-naphthoquine remained after PCR correction of post-treatment responses for infections with different genetic profiles than those at baseline (100% [95% CI 84.0%–100%] versus 60.0% [95% CI 36.4%–80.0%] in the artemether-lumefantrine-treated children, p<0.001; see Table 8). The modified intention-to-treat analyses showed results that were consistent with these findings (see Tables 9 and 10).
The single patient with P. malariae infection was randomized to artemether-lumefantrine and responded with prompt parasite clearance and an ACPR at days 28 and 42.
Gametocyte Carriage and Hemoglobin Concentration
The percentage of children carrying P. falciparum gametocytes based on microscopy was not significantly different between groups at baseline (p = 0.36), but artemisinin-naphthoquine-treated patients were more likely to have gametocytemia between days 3 and 14 (see Figure 4). The odds ratio for carriage in the artemisinin-naphthoquine group was 3.2 (95% CI 1.5–6.8) that of children in the artemether-lumefantrine group on day 7, and 15.1 (95% CI 1.9–118.1) on day 14. When only those children who were gametocyte positive at baseline were considered (n = 32 in the artemether-lumefantrine group and n = 39 in the artemisinin-naphthoquine group), the equivalent odds ratios for gametocyte carriage on days 7 and 14 were 2.6 (95% CI 1.0–6.8) and 10.7 (95% CI 1.3–88.8).
Fig. 4. Proportions of patients with gametocytes during follow-up after treatment for P. falciparum and for P. vivax. 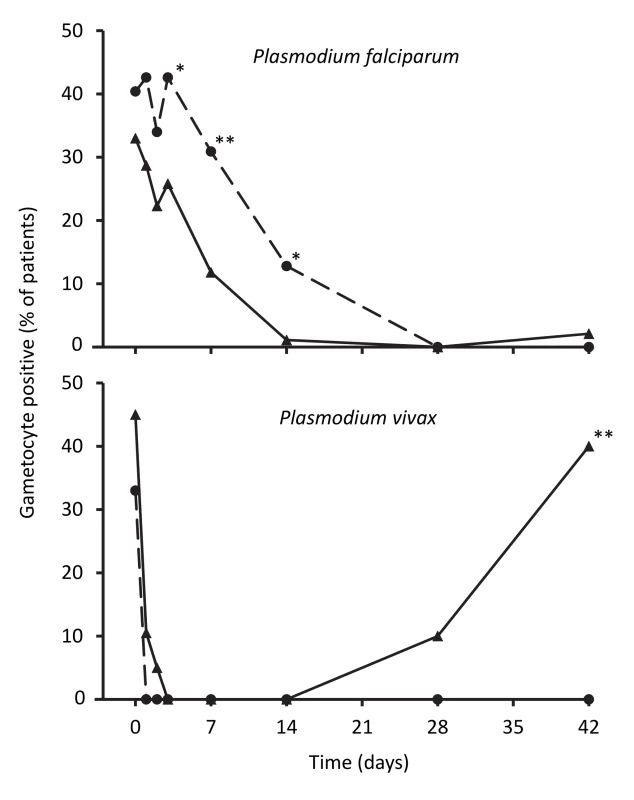
Data are for artemether-lumefantrine (▴, solid lines) and artemisinin-naphthoquine (•, dashed lines). *p<0.05, **p<0.01 for between-treatment comparisons. In patients with vivax malaria, there was prompt gametocyte clearance in both groups by day 4, but, consistent with more frequent treatment failure in the artemether-lumefantrine group, a significant proportion of patients (40.0%) had become positive for gametocytes by day 42, while none of the artemisinin-naphthoquine-treated children were gametocytemic at that time (see Figure 4).
Hemoglobin concentrations in children treated for falciparum malaria were similar in the two treatment groups until day 42 after treatment, when the artemisinin-naphthoquine-treated children had a significantly higher mean concentration (109±13 g/l versus 102±12 g/l in the artemether-lumefantrine group, p<0.001; see Figure 5). In patients with vivax malaria, the hemoglobin concentrations had diverged significantly at day 7, but this difference was not sustained.
Fig. 5. Hemoglobin concentrations during follow-up after treatment for P. falciparum and for P. vivax. 
Data are mean and standard deviation (vertical bars) for artemether-lumefantrine (▴, solid lines) and artemisinin-naphthoquine (•, dashed lines). **p<0.01 for between-treatment comparisons. Discussion
Three daily doses of artemisinin-naphthoquine proved a highly efficacious treatment for both falciparum and vivax malaria in PNG children living in an area of intense transmission of multiple Plasmodium species. This efficacy profile addresses recognized species-specific shortcomings associated with artemether-lumefantrine and dihydroartemisinin-piperaquine in this patient group [5]. Artemisinin-naphthoquine was well tolerated, and there were no significant safety concerns identified through detailed post-treatment monitoring. Artemisinin-naphthoquine treatment was associated with a higher day-42 hemoglobin concentration than artemether-lumefantrine treatment, with potential implications for attenuated morbidity associated with subsequent malarial episodes and other illnesses. A further advantage of artemisinin-naphthoquine is that it is taken once daily and without the need for co-administration with fat [10], which should improve adherence compared with the relatively complex six-dose artemether-lumefantrine schedule with milk co-administration. Artemisinin-naphthoquine was, however, associated with greater post-treatment gametocyte carriage and, as is the case for a number of other ACT partner drugs, with prolongation of the QTc interval.
There were no reappearances of asexual forms of either P. falciparum or P. vivax during 42 d of follow-up in any child treated with three daily doses of artemisinin-naphthoquine in the present study. Treatment failures were observed in our previous single - and two-dose studies in PNG children aged 5–10 y [11], and most studies in adults utilizing similar regimens have fallen short of a 100% ACPR, even with only 28 d of follow-up [8],[9],[29],[30], including an Indonesian study with a 28-d ACPR of 79% in mixed P. falciparum/P. vivax infections [8],[9]. Only one other study, in Chinese adults with vivax malaria, has utilized a 3-d artemisinin-naphthoquine regimen (17.5 mg/kg artemisinin plus 7 mg/kg naphthoquine versus 20 mg/kg artemisinin plus 8 mg/kg artemisinin in the present study), and this regimen was associated with a day-42 ACPR of 98.4% [31]. Overall, these data support a 3-d regimen to maximize the efficacy of artemisinin-naphthoquine. The present artemisinin-naphthoquine outcomes are clearly superior to the outcomes of both artemether-lumefantrine and dihydroartemisinin-piperaquine in our previous multi-arm trial in young PNG children, since these regimens were associated with P. falciparum day-42 PCR-corrected ACPRs of 95.2% and 87.9%, respectively, and P. vivax day-42 uncorrected ACPRs of 30.3% and 69.4%, respectively [5].
We hypothesize that the better efficacy of artemisinin-naphthoquine reflects naphthoquine's relatively long terminal elimination half-life and wide therapeutic index. The mean elimination half-life of naphthoquine in PNG children is 21–25 d [10] compared with 14–21 d for piperaquine in this patient group [32]–[34]. This indicates that naphthoquine can suppress reappearances of both P. falciparum and P. vivax for a longer period than piperaquine and especially lumefantrine, which has an elimination half-life of only 4–5 d [35]. In addition, the magnitude of piperaquine dosing is limited by gastrointestinal side effects including nausea [13]. Currently recommended doses of dihydroartemisinin-piperaquine may be inadequate in young children [36] such as those who participated in our present and previous [5] studies, but higher doses may have reduced tolerability. Gastrointestinal and other symptoms in our artemisinin-naphthoquine-treated children were mild and short-lived despite the use of the manufacturer's recommended single-dose regimen on three consecutive days.
There are theoretical reasons why artemisinin-naphthoquine may be a suboptimal ACT. As shown by ourselves [10] and others [37], auto-induction of artemisinin metabolism results in declining plasma concentrations over the 3 d of treatment. Artemether also induces its own metabolism [38], but unlike artemisinin, which has an inactive metabolite, artemether is converted to dihydroartemisinin, which has the greatest activity of all derivatives against P. falciparum [39]. These pharmacokinetic/pharmacodynamic factors reflect the relatively low efficacy of artemisinin-naphthoquine given for less than 3 d [8],[9],[11] and may have contributed to the greater percentage of gametocytemic children during the first 2 wk after artemisinin-naphthoquine treatment in the present study. The persistence of P. falciparum gametocytemia has implications for malaria control and could be managed by administration of single-dose primaquine, provided that cost-effective point-of-care screening for glucose-6-phosphate dehydrogenase deficiency is available [40]. It is possible that the need for longer courses of primaquine for radical cure of P. vivax may be less with artemisinin-naphthoquine given the lack of appearance of asexual forms of this species during the 42-d follow-up period in children treated for acute malaria with this ACT, but this possibility needs to be evaluated in further studies with longer-term follow-up.
Despite potential pharmacokinetic/pharmacodynamic shortcomings, initial asexual parasite clearance was as rapid with artemisinin-naphthoquine as with artemether-lumefantrine. There is in vitro evidence of cross-resistance between chloroquine and naphthoquine in P. falciparum isolates from PNG, as is also the case for piperaquine [41]. Whether this has clinical implications for recrudescence in areas of chloroquine-resistant P. falciparum and P. vivax is uncertain, but it did not appear influential in the present study, even though the majority of P. falciparum strains in Madang Province (>80%) show in vitro evidence of chloroquine resistance with near fixation of the resistance-associated pfcrt allele [40]. The three-dose artemisinin-naphthoquine combination regimen that complied with WHO recommendations for all ACTs [12] and that was developed for the present study to maximize efficacy against asexual and sexual parasite forms [11] could have unmasked significant adverse effects such as the hepatotoxicity that prevented inclusion of artesunate-pyronaridine in the present study [16],[17],[42], but the present safety data are reassuring.
Naphthoquine is a tetra-aminoquinoline drug, and our previous preliminary safety study of artemisinin-naphthoquine in older PNG children did not find evidence of aminoquinoline-specific side effects such as hearing loss, orthostatic hypotension, and hypoglycemia [11]. Similarly, the artemisinin-naphthoquine-treated children in the present study did not complain of deafness, tinnitus, dizziness, or posture-related symptoms during follow-up, and no cases of hypoglycemia were recorded. The lack of hematologic and hepatorenal toxicity in the present study is also consistent with our previous findings [11]. An important outcome variable in the present study was electrocardiographic effects, and there was significant QTc prolongation between baseline and 4 h after the final dose in our artemisinin-naphthoquine-treated children. Since there were no significant between-group differences in pulse rate and fever clearance during initial monitoring, this QTc prolongation is consistent with a naphthoquine-specific effect rather than reflecting a disproportionately larger reduction in sympathetic activity with recovery in artemisinin-naphthoquine-treated children that could have independently promoted an increase in the QTc interval in this group [43].
Many aminoquinoline drugs cause QTc prolongation. Chloroquine increases the QTc by a mean of up to 30 msec0.5 in healthy volunteers [44], but no malignant arrhythmias or sudden deaths have been reported with recommended antimalarial doses [43]. Piperaquine also has this effect, with studies in children in PNG and Cambodia showing QTc prolongation similar to that in the present study for children treated with artemisinin-naphthoquine [19],[20]. Repeated piperaquine dosing with the potential for accumulation is not associated with definite cardiotoxicity [45], and there is no in vitro evidence of torsadogenic or other pro-arrhythmic effects [46]. In the case of halofantrine (now withdrawn), cardiotoxicity is plasma-concentration-dependent [43], but the day-2 QTc prolongation observed in our artemisinin-naphthoquine-treated children had resolved by day 7, when plasma naphthoquine concentrations would still have been high [10].
This latter observation, amongst other considerations, suggests that the artemisinin-naphthoquine regimen used in the present study was not pro-arrhythmic. Relatively frequent QTc prolongation has been observed in young children presenting with untreated malaria [43] and other serious illnesses [47], but it is transient and not associated with adverse outcomes. There has been a lack of clinically evident cardiotoxicity in the present and other studies of naphthoquine [8], including three daily doses for vivax malaria in adults [31]. Nevertheless, given concerns that piperaquine-containing ACTs should not be used in people who have, or are at risk of, QTc prolongation or cardiac arrhythmias (including those with electrolyte disturbance such as hypokalemia and hypocalcemia), and that they should not be taken with other drugs that prolong the QTc interval [48], it may be appropriate to recommend similar restrictions for three-dose artemisinin-naphthoquine regimens.
Our study had limitations. Baseline microscopy by study staff under field conditions led to 11% of the 267 randomized patients being included when they had sub-threshold parasitemia based on expert microscopy, while electrocardiography proved difficult in a significant minority of children. We did not directly observe all artemether-lumefantrine doses, but parents/guardians were instructed as to the importance of all doses given at home, adherence was assessed at the next follow-up visit, and day-7 plasma lumefantrine concentrations were consistent with full adherence in each case. The children screened and recruited were drawn from coastal PNG communities that are typical of those in Oceania where hyper - or holo-endemic transmission of multiple Plasmodium species is found. It is possible that treatment responses in children with uncomplicated malaria who have been raised in areas with lower-level transmission may be attenuated because of their reduced or absent malarial immunity. Nevertheless, ACTs such as artemether-lumefantrine are considered efficacious where there is unstable transmission [12], while most of the studies evaluating artemisinin-naphthoquine—which had mostly acceptable efficacy—were conducted in this epidemiologic setting [8],[9]. The strengths of the study are the high rates of laboratory (including parasitologic) data collection and the ≥92% retention rate of children in each of the four species/treatment arms, which was greater than anticipated when sample sizes were determined and greater than in our previous multi-arm trial in PNG children (with, for example, only 77% in one of the arms [5]).
The efficacy, tolerability, and safety of three daily doses of artemisinin-naphthoquine suggest that this regimen should be considered together with other currently available effective ACTs for treatment of uncomplicated malaria in PNG and similar epidemiologic settings with transmission of multiple Plasmodium species. Artemisinin-naphthoquine may have advantages where P. vivax predominates. Although pharmaco-economic analyses were beyond the scope of the present study, it seems likely that the additional cost of three doses compared to the single dose currently recommended will be more than offset by the substantial reduction in post-treatment vivax malaria.
Supporting Information
Zdroje
1. MurrayCJ, RosenfeldLC, LimSS, AndrewsKG, ForemanKJ, et al. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379 : 413–431.
2. MendisK, SinaBJ, MarchesiniP, CarterR (2001) The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64 : 97–106.
3. BairdJK (2008) Real-world therapies and the problem of vivax malaria. N Engl J Med 359 : 2601–2603.
4. PriceRN, TjitraE, GuerraCA, YeungS, WhiteNJ, et al. (2007) Vivax malaria: neglected and not benign. Am J Trop Med Hyg 77 : 79–87.
5. KarunajeewaHA, MuellerI, SennM, LinE, LawI, et al. (2008) A trial of combination antimalarial therapies in children from Papua New Guinea. N Engl J Med 359 : 2545–2557.
6. Papua New Guinea National Department of Health (2009) National malaria treatment protocol. Port Moresby: National Department of Health.
7. ManningL, LamanM, LawI, BonaC, AipitS, et al. (2011) Features and prognosis of severe malaria caused by Plasmodium falciparum, Plasmodium vivax and mixed Plasmodium species in Papua New Guinean children. PLoS ONE 6: e29203.
8. WangJY, CaoWC, ShanCQ, ZhangM, LiGF, et al. (2004) Naphthoquine phosphate and its combination with artemisinine. Acta Trop 89 : 375–381.
9. HombhanjeFW, HuangQ (2010) Artemisinin-naphthoquine combination (ARCO®): an overview of the progress. Pharmaceuticals 3 : 3581–3593.
10. BattyKT, SalmanS, MooreBR, BenjaminJ, LeeST, et al. (2012) Artemisinin-naphthoquine combination therapy for uncomplicated pediatric malaria: a pharmacokinetic study. Antimicrob Agents Chemother 56 : 2472–2484.
11. BenjaminJ, MooreB, LeeST, SennM, GriffinS, et al. (2012) Artemisinin-naphthoquine combination therapy for uncomplicated pediatric malaria: a tolerability, safety, and preliminary efficacy study. Antimicrob Agents Chemother 56 : 2465–2471.
12. World Health Organization (2010) Guidelines for the treatment of malaria, 2nd edition. Geneva: World Health Organization
13. DavisTM, HungTY, SimIK, KarunajeewaHA, IlettKF (2005) Piperaquine: a resurgent antimalarial drug. Drugs 65 : 75–87.
14. BarnadasC, KoepfliC, KarunajeewaHA, SibaPM, DavisTM, et al. (2011) Characterization of treatment failure in efficacy trials of drugs against Plasmodium vivax by genotyping neutral and drug resistance-associated markers. Antimicrob Agents Chemother 55 : 4479–4481.
15. World Health Organization (2003) Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: World Health Organization.
16. BukirwaH, UnnikrishnanB, KramerCV, SinclairD, NairS, et al. (2014) Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev 3: CD006404.
17. European Medicines Agency (2012) Assessment report. Pyramax—pyronaridine tetraphosphate/artesunate. Procedure No.: EMEA/H/W/002319. Available: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/06/WC500129290.pdf. Accessed 19 November 2014.
18. World Health Organization (2000) Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg 94 (Suppl 1)S1–S90.
19. KarunajeewaH, LimC, HungTY, IlettKF, DenisMB, et al. (2004) Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br J Clin Pharmacol 57 : 93–99.
20. MooreBR, BenjaminJM, SalmanS, GriffinS, GinnyE, et al. (2014) Effect of co-administered fat on the tolerability, safety and pharmacokinetic properties of dihydroartemisinin-piperaquine in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother 58 : 5784–5794.
21. AshleyEA, StepniewskaK, LindegardhN, AnnerbergA, KhamA, et al. (2007) How much fat is necessary to optimize lumefantrine oral bioavailability? Trop Med Int Health 12 : 195–200.
22. CattamanchiA, KyabayinzeD, HubbardA, RosenthalPJ, DorseyG (2003) Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of MSP-1, MSP-2, and GLURP. Am J Trop Med Hyg 68 : 133–139.
23. FelgerI, BeckHP (2002) Genotyping of Plasmodium falciparum. PCR-RFLP analysis. Methods Mol Med 72 : 117–129.
24. WongRP, SalmanS, IlettKF, SibaPM, MuellerI, et al. (2011) Desbutyl-lumefantrine is a metabolite of lumefantrine with potent in vitro antimalarial activity that may influence artemether-lumefantrine treatment outcome. Antimicrob Agents Chemother 55 : 1194–1198.
25. SalmanS, Page-SharpM, GriffinS, KoseK, SibaPM, et al. (2011) Population pharmacokinetics of artemether, lumefantrine, and their respective metabolites in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother 55 : 5306–5313.
26. ManningL, LamanM, TownsendMA, ChubbSP, SibaPM, et al. (2011) Reference intervals for common laboratory tests in Melanesian children. Am J Trop Med Hyg 85 : 50–54.
27. BlackwelderWC (1982) “Proving the null hypothesis” in clinical trials. Control Clin Trials 3 : 345–353.
28. Pocock SJ (1983) Clinical trials: a practical approach. Chichester: John Wiley.
29. KrudsoodS, ChalermrutK, PengruksaC, SrivilairitS, SilachamroonU, et al. (2003) Comparative clinical trial of two-fixed combinations dihydroartemisinin-napthoquine-trimethoprim (DNP) and artemether-lumefantrine (Coartem/Riamet) in the treatment of acute uncomplicated falciparum malaria in Thailand. Southeast Asian J Trop Med Public Health 34 : 316–321.
30. TjitraE, HasugianAR, SiswantoroH, PrasetyoriniB, EkowatiningsihR, et al. (2012) Efficacy and safety of artemisinin-naphthoquine versus dihydroartemisinin-piperaquine in adult patients with uncomplicated malaria: a multi-centre study in Indonesia. Malar J 11 : 153.
31. LiuH, YangHL, XuJW, WangJZ, NieRH, et al. (2013) Artemisinin-naphthoquine combination versus chloroquine-primaquine to treat vivax malaria: an open-label randomized and non-inferiority trial in Yunnan Province, China. Malar J 12 : 409.
32. HungTY, DavisTM, IlettKF, KarunajeewaH, HewittS, et al. (2004) Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br J Clin Pharmacol 57 : 253–262.
33. KarunajeewaHA, IlettKF, MuellerI, SibaP, LawI, et al. (2008) Pharmacokinetics and efficacy of piperaquine and chloroquine in Melanesian children with uncomplicated malaria. Antimicrob Agents Chemother 52 : 237–243.
34. SalmanS, Page-SharpM, BattyKT, KoseK, GriffinS, et al. (2012) Pharmacokinetic comparison of two piperaquine-containing artemisinin combination therapies in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother 56 : 3288–3297.
35. EzzetF, MullR, KarbwangJ (1998) Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol 46 : 553–561.
36. WorldWide Antimalarial Resistance Network (WWARN) DP Study Group (2013) The effect of dosing regimens on the antimalarial efficacy of dihydroartemisinin-piperaquine: a pooled analysis of individual patient data. PLoS Med 10: e1001564.
37. Hassan AlinM, AshtonM, KihamiaCM, MteyGJ, BjorkmanA (1996) Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans R Soc Trop Med Hyg 90 : 61–65.
38. van AgtmaelMA, Cheng-QiS, QingJX, MullR, van BoxtelCJ (1999) Multiple dose pharmacokinetics of artemether in Chinese patients with uncomplicated falciparum malaria. Int J Antimicrob Agents 12 : 151–158.
39. SkinnerTS, ManningLS, JohnstonWA, DavisTM (1996) In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int J Parasitol 26 : 519–525.
40. MooreBR, SalmanS, BenjaminJ, Page-SharpM, RobinsonLJ, et al. (2014) Pharmacokinetic properties of single-dose primaquine in Papua New Guinean children: feasibility of abbreviated high-dose regimens for radical cure of vivax malaria. Antimicrob Agents Chemother 58 : 432–439.
41. WongRP, LautuD, TavulL, HackettSL, SibaP, et al. (2010) In vitro sensitivity of Plasmodium falciparum to conventional and novel antimalarial drugs in Papua New Guinea. Trop Med Int Health 15 : 342–349.
42. KurthF, BelardS, BasraA, RamharterM (2011) Pyronaridine-artesunate combination therapy for the treatment of malaria. Curr Opin Infect Dis 24 : 564–569.
43. WhiteNJ (2007) Cardiotoxicity of antimalarial drugs. Lancet Infect Dis 7 : 549–558.
44. BustosMD, GayF, DiquetB, ThomareP, WarotD (1994) The pharmacokinetics and electrocardiographic effects of chloroquine in healthy subjects. Trop Med Parasitol 45 : 83–86.
45. LwinKM, PhyoAP, TarningJ, HanpithakpongW, AshleyEA, et al. (2012) Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother 56 : 1571–1577.
46. BorsiniF, CrumbW, PaceS, UbbenD, WibleB, et al. (2012) In vitro cardiovascular effects of dihydroartemisin-piperaquine combination compared with other antimalarials. Antimicrob Agents Chemother 56 : 3261–3270.
47. Van DornCS, JohnsonJN, TaggartNW, ThorkelsonL, AckermanMJ (2011) QTc values among children and adolescents presenting to the emergency department. Pediatrics 128: e1395–1401.
48. European Medicines Agency (2011) Annex I: summary of product characteristics. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001199/WC500118113.pdf. Accessed 25 November 2014.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 12- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- A Stronger Post-Publication Culture Is Needed for Better Science
- From Joint Thinking to Joint Action: A Call to Action on Improving Water, Sanitation, and Hygiene for Maternal and Newborn Health
- Metabolic Signatures of Adiposity in Young Adults: Mendelian Randomization Analysis and Effects of Weight Change
- Artemisinin-Naphthoquine versus Artemether-Lumefantrine for Uncomplicated Malaria in Papua New Guinean Children: An Open-Label Randomized Trial
- Home-Based Versus Mobile Clinic HIV Testing and Counseling in Rural Lesotho: A Cluster-Randomized Trial
- Genomic Predictors for Recurrence Patterns of Hepatocellular Carcinoma: Model Derivation and Validation
- Efficacy of Neonatal HBV Vaccination on Liver Cancer and Other Liver Diseases over 30-Year Follow-up of the Qidong Hepatitis B Intervention Study: A Cluster Randomized Controlled Trial
- Impact of Replacing Smear Microscopy with Xpert MTB/RIF for Diagnosing Tuberculosis in Brazil: A Stepped-Wedge Cluster-Randomized Trial
- World Health Organization Guidelines for Management of Acute Stress, PTSD, and Bereavement: Key Challenges on the Road Ahead
- Tracking Rural Health Facility Financial Data in Resource-Limited Settings: A Case Study from Rwanda
- Evaluation of the Lung Cancer Risks at Which to Screen Ever- and Never-Smokers: Screening Rules Applied to the PLCO and NLST Cohorts
- Alcohol Harm Reduction: Corporate Capture of a Key Concept
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Artemisinin-Naphthoquine versus Artemether-Lumefantrine for Uncomplicated Malaria in Papua New Guinean Children: An Open-Label Randomized Trial
- Home-Based Versus Mobile Clinic HIV Testing and Counseling in Rural Lesotho: A Cluster-Randomized Trial
- A Stronger Post-Publication Culture Is Needed for Better Science
- Metabolic Signatures of Adiposity in Young Adults: Mendelian Randomization Analysis and Effects of Weight Change
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



