-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Influence of Health Systems on Hypertension Awareness, Treatment, and Control: A Systematic Literature Review
Background:
Hypertension (HT) affects an estimated one billion people worldwide, nearly three-quarters of whom live in low - or middle-income countries (LMICs). In both developed and developing countries, only a minority of individuals with HT are adequately treated. The reasons are many but, as with other chronic diseases, they include weaknesses in health systems. We conducted a systematic review of the influence of national or regional health systems on HT awareness, treatment, and control.Methods and Findings:
Eligible studies were those that analyzed the impact of health systems arrangements at the regional or national level on HT awareness, treatment, control, or antihypertensive medication adherence. The following databases were searched on 13th May 2013: Medline, Embase, Global Health, LILACS, Africa-Wide Information, IMSEAR, IMEMR, and WPRIM. There were no date or language restrictions. Two authors independently assessed papers for inclusion, extracted data, and assessed risk of bias. A narrative synthesis of the findings was conducted. Meta-analysis was not conducted due to substantial methodological heterogeneity in included studies. 53 studies were included, 11 of which were carried out in LMICs. Most studies evaluated health system financing and only four evaluated the effect of either human, physical, social, or intellectual resources on HT outcomes. Reduced medication co-payments were associated with improved HT control and treatment adherence, mainly evaluated in US settings. On balance, health insurance coverage was associated with improved outcomes of HT care in US settings. Having a routine place of care or physician was associated with improved HT care.Conclusions:
This review supports the minimization of medication co-payments in health insurance plans, and although studies were largely conducted in the US, the principle is likely to apply more generally. Studies that identify and analyze complexities and links between health systems arrangements and their effects on HT management are required, particularly in LMICs.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(7): e32767. doi:10.1371/journal.pmed.1001490
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001490Summary
Background:
Hypertension (HT) affects an estimated one billion people worldwide, nearly three-quarters of whom live in low - or middle-income countries (LMICs). In both developed and developing countries, only a minority of individuals with HT are adequately treated. The reasons are many but, as with other chronic diseases, they include weaknesses in health systems. We conducted a systematic review of the influence of national or regional health systems on HT awareness, treatment, and control.Methods and Findings:
Eligible studies were those that analyzed the impact of health systems arrangements at the regional or national level on HT awareness, treatment, control, or antihypertensive medication adherence. The following databases were searched on 13th May 2013: Medline, Embase, Global Health, LILACS, Africa-Wide Information, IMSEAR, IMEMR, and WPRIM. There were no date or language restrictions. Two authors independently assessed papers for inclusion, extracted data, and assessed risk of bias. A narrative synthesis of the findings was conducted. Meta-analysis was not conducted due to substantial methodological heterogeneity in included studies. 53 studies were included, 11 of which were carried out in LMICs. Most studies evaluated health system financing and only four evaluated the effect of either human, physical, social, or intellectual resources on HT outcomes. Reduced medication co-payments were associated with improved HT control and treatment adherence, mainly evaluated in US settings. On balance, health insurance coverage was associated with improved outcomes of HT care in US settings. Having a routine place of care or physician was associated with improved HT care.Conclusions:
This review supports the minimization of medication co-payments in health insurance plans, and although studies were largely conducted in the US, the principle is likely to apply more generally. Studies that identify and analyze complexities and links between health systems arrangements and their effects on HT management are required, particularly in LMICs.
Please see later in the article for the Editors' SummaryIntroduction
Hypertension (HT) is common worldwide, affecting an estimated billion people, nearly three-quarters of whom live in low or middle income countries (LMICs) [1]. HT is second, after smoking, as a contributor to the Global Burden of Disease in the latest (2010) analysis [2]. In most individuals it is easily treated and controlled, with effective control reducing deaths and disability from a number of conditions, including cerebrovascular, cardiovascular, and renal disease [3]. Yet in both developed and developing countries, a significant proportion of people with HT remain unaware of their diagnosis, and of those who are aware, only a minority are treated and have their HT successfully controlled [4]. The reasons are many but, as with other chronic diseases, they include weaknesses in health systems, related to both structures and ways in which systems function [5],[6]. Health systems have been defined by the World Health Organization as “all the organizations, institutions and resources that are devoted to producing health actions” [7] and weaknesses may exist at the national, regional, district, community, and household level.
Previous systematic reviews have examined the effects of health systems interventions delivered at the community or health facility level on HT care, such as educational interventions that target providers, organisational interventions strengthening collaboration between physicians and pharmacists, and using electronic records to improve management [8]–[10]. However, we are unaware of any previous systematic review exploring the effect of actions originating at national or regional health systems level, including health policies, programs, and interventions, on HT outcomes. Actions that have been hypothesized to influence HT care include strategies for procurement of essential medications, the existence of simple national guidelines for HT management, introduction of financial incentives for health care practitioners to diagnose or treat HT, and enhanced health insurance coverage [1]. To address this gap, we systematically reviewed the literature examining the effect of national or regional health system arrangements on HT care and control, and make recommendations for future research and policy.
Methods
A protocol for this study has been published on the PROSPERO international prospective register of systematic reviews, with the record number PROSPERO 2012:CRD42012002864 [11]. We used an established framework to illustrate the health system and its elements and guide our systematic review (Figure 1). This conceptual framework, which has been found useful in understanding the systems failings that impede effective management of non-communicable diseases [12],[13], consists of four domains relating to key system level inputs that are required for effective chronic disease care: namely, physical resources (e.g., health facilities and diagnostic equipment), human resources (e.g., trained health care workers and managers), intellectual resources (e.g., treatment guidelines), and social resources (which draws on the concept of social capital and includes organizational measures to enhance collaboration). The existence of inputs is insufficient in itself, without effective systems to finance, deliver, and govern care; and these are also reflected in the framework. All of these domains influence the impact of the health system inputs on the health care outcomes of interest, which are HT awareness, treatment, control, and antihypertensive medication adherence in this case. The framework aims to capture the complex interactions and inter-relationships that exist between the elements within a health system, acknowledging that success of health systems does not simply require a “laundry list” of building blocks (as the WHO's 2007 framework is often perceived), but requires effective integration and alignment of these inputs [14],[15]. The framework also highlights the important role that context plays in shaping the relationship between health systems inputs and outcomes, recognizing the complex adaptive nature of health systems so that changes may yield different results in different settings [16].
Fig. 1. Schematic diagram of health systems conceptual framework. 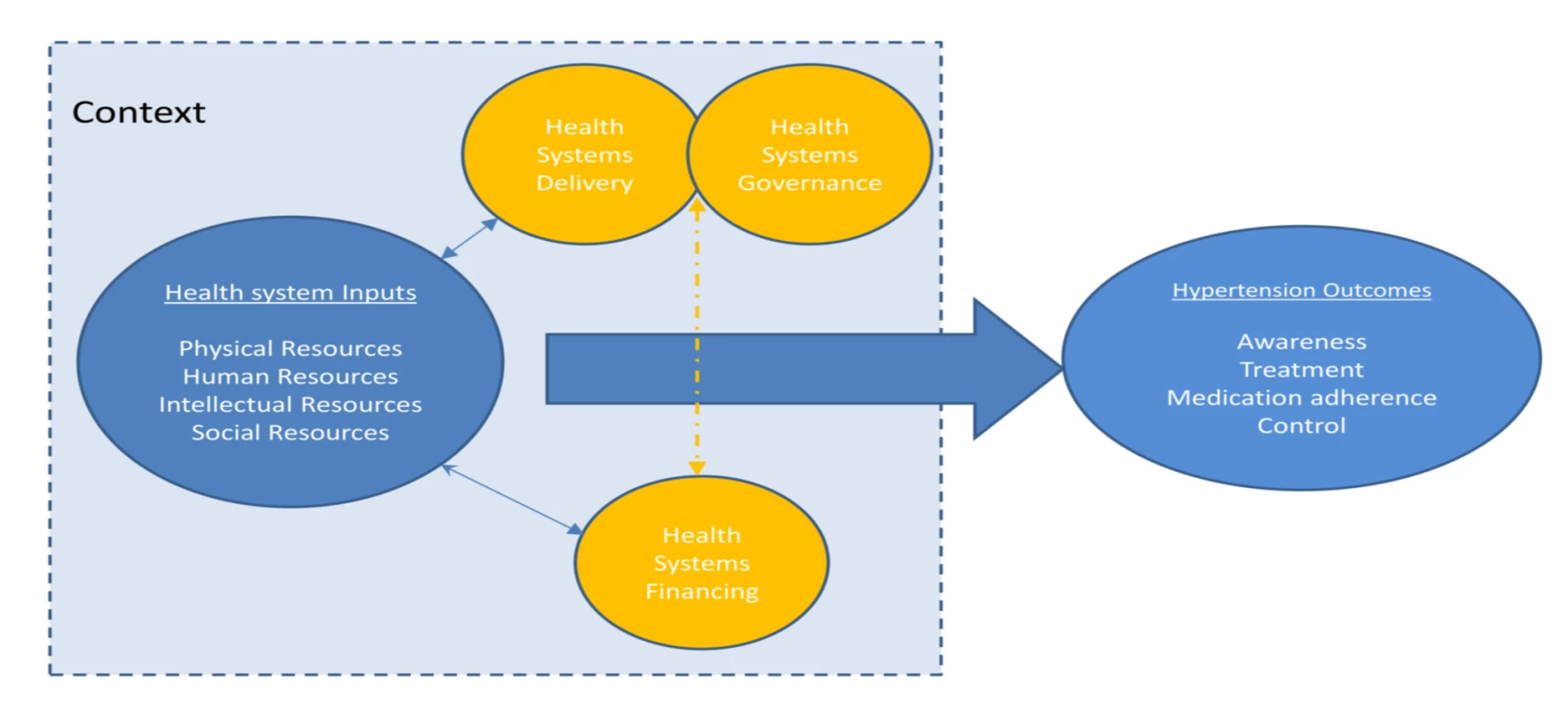
Inclusion Criteria
We included studies that reported on the effects of national or regional health system level arrangements (factors, interventions, policies, or programs) on HT control and key upstream determinants of control: HT awareness, treatment, and medication adherence. Definitions of these outcomes are given in Box 1.
We included studies looking at any adult population, including general populations, populations on treatment, and studies of people with specific co-morbidities, such as diabetes.
The following types of studies were included: (1) Studies, such as controlled trials, cohort studies, and cross-sectional studies, which quantify the effects on HT outcomes of interventions, policies, or programmes, which are enacted at national or regional health system level, acting on one or more domains of the health-system. (2) Studies, such as qualitative studies, which report on the views and experiences of actors (e.g., patients, physicians, or policy makers) on national or regional health-system level barriers to HT awareness, treatment, control, or antihypertensive medication adherence. (3) Studies reporting on the impact of national or regional HT care policies or interventions that have relevance for other disease programs or for the design of the health system more broadly, such as those that require or lead to changes in primary care provision or other general aspects of the health system.
Quantitative studies were included only if they reported a measure of association between the health system arrangement under investigation and at least one of the HT outcomes of interest (Box 1).
There were no date or language restrictions.
Studies that evaluated interventions, policies, or programs that are enacted at the individual level (e.g., provider or patient level) or organizational level of the health system (e.g., hospital or primary care organization), and do not require change at the level of the national or regional health system were excluded.
Box 1. Definitions of Included Hypertension Outcomes
(1) HT awareness. Defined as persons with clinically measured HT who have been diagnosed by a health care professional as hypertensive.
(2) HT treatment. Defined as the use of at least one antihypertensive medication in an individual with known HT.
(3) Antihypertensive medication adherence. Defined as consistently taking the antihypertensive medication regimen as prescribed by the health care provider.
(4) HT control: defined as the achievement of BP below 140/90 mmHg (or other explicitly defined threshold) in individuals being treated for HT, or, alternatively, measured by the mean BP amongst individuals with HT.
Search Strategy
The search strategy and terms were developed collaboratively with an information specialist. Key words (MeSH terms) and free text terms were identified for each domain of our health systems framework and combined with search terms for HT outcomes to generate the search strategy for the electronic databases Medline, Embase, and Global Health (Text S2). To improve the likelihood of identifying studies from LMICs, modified searches were performed on the following databases: Latin American and Caribbean Health Sciences Literature (LILACS), Africa-Wide Information, Index Medicus for the South-East Asian Region (IMSEAR), Index Medicus for the Eastern Mediterranean Region (IMEMR) Western Pacific Rim Region Index Medicus (WPRIM). All databases were searched from inception to the present day on 8th May 2013. To identify further relevant studies, reference lists of included articles were hand searched and a forward citation search was performed on included studies using Web of Science.
Study Selection
Two reviewers independently screened the search results by title and abstract for potential eligibility. Full texts of potentially suitable articles were obtained and were further screened for inclusion by two reviewers. Disagreements in the screening of full texts were resolved by a third reviewer with expertise in health systems and this was required for four of the 122 screened papers.
Data Extraction for Study Setting, Methodology, and Findings
A data extraction form was developed in Microsoft Excel. Data were extracted from each study on study design, setting, health system domains investigated, study methods, and outcomes (Table S1). Where multiple analytical models were used for HT outcomes in a study, data were taken from the analytical model that had the highest level of control for other confounding factors. Data extraction was performed independently by two reviewers and compared and checked for disparities. Erroneous or inconsistent data were identified in one of the included papers, and we attempted to contact the authors of this paper for clarification. Clarification of these data was not forthcoming, so these data were excluded from the analysis.
Risk of Bias Assessment
Included studies were independently assessed for risk of bias by two reviewers. For observational study designs, risk of bias was assessed using a simple proforma for three domains: selection bias, information bias (differential misclassification and non-differential misclassification), and confounding (Text S3). Assessment of non-differential misclassification took into consideration the reliability of the measure used to report HT outcomes, which was particularly important for medication adherence, where a variety of methods were used for measurement. Risk of bias for each domain was assessed as either low, unclear, or high. Studies that had a low risk of bias in each domain, including a low risk of confounding, were classified as having a low overall risk of bias. For randomized studies the Cochrane risk of bias tool was used [17]. Qualitative studies were evaluated for quality using an adapted version of a checklist used in a previous series of mixed methods systematic reviews incorporating both quantitative and qualitative studies (Text S4) [18],[19].
Assessment of Context and Complexity Considerations
Due to the recognized importance of context and complexity to health systems research [20], we examined the extent to which included studies describe and explore these factors. We assessed to what extent studies had described the sociodemographic, political, or economic context in which they were conducted and the wider health system setting. We also assessed whether studies demonstrated a consideration of the complexity of health systems, including addressing inter-relationships between different health systems domains, for example, those between financing arrangements and retention of skilled health care workers, as well as interactions with contextual factors, such as the level of poverty or literacy amongst the population being served. This process was performed by one reviewer and checked for consistency by a second reviewer.
Data Synthesis and Analysis
A narrative synthesis was performed, with studies categorized according to the health system domain they investigated and the setting in which the study was performed. For making causal inferences about reported associations between health systems arrangements and HT outcomes, randomized controlled trials (RCTs) were considered the strongest study design, followed by cohort studies and then case-control studies. Cross-sectional studies and ecological studies, alone, were not considered appropriate for causal inference. Meta-analysis was not conducted as we judged that the included studies were heterogeneous in important aspects, including: populations (different ages and settings), study designs (cross-sectional, case-control, cohort), variable definitions (including different definitions of exposures and outcomes), comparisons (e.g., different type of insurance schemes), and analytical strategies (adjustment for different confounders).
Results
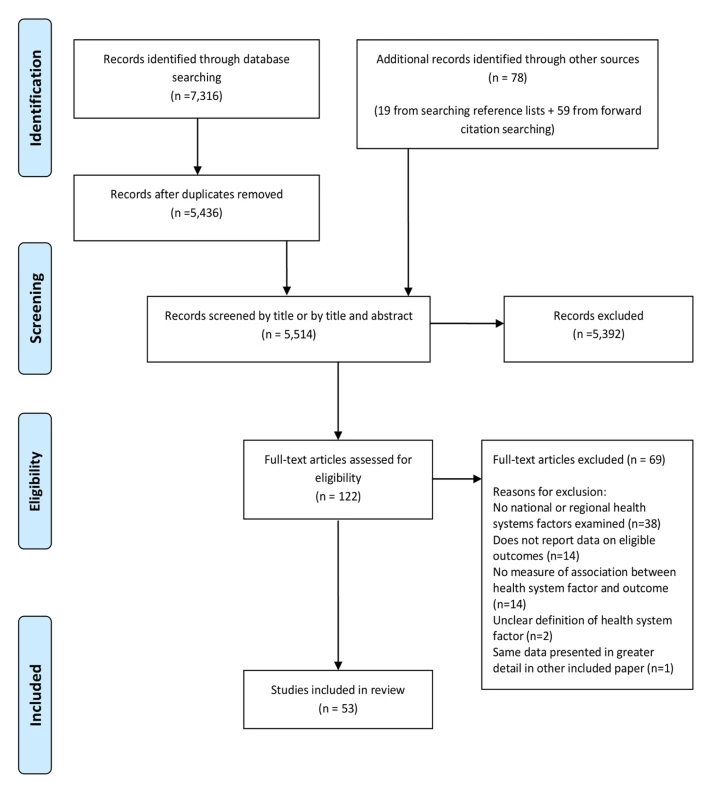
The screening process is described using an adapted Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Figure 2) [21]. 5,514 articles were screened by title and abstract for inclusion. The full text of 122 of the 5,514 articles was obtained and assessed for eligibility. 53 studies met eligibility criteria for this review. Full details of the included studies, including study design, setting, key findings, and risk of bias assessment can be found in Table S1. 51 of the included studies were quantitative and two were qualitative [22],[23]. Of the 51 quantitative studies, one was a RCT [24]; 12 were cohort studies [25]–[34], two of which were retrospective [30],[34]; three were case-control studies [35]–[37]; 32 were cross-sectional studies; and three were ecological studies [38]–[40]. 42 of the 53 studies (79%) were carried out in countries classified by the World Bank as high-income countries, 36 of which were in the US. Six studies were carried out in upper middle-income countries [38],[41]–[45], three in lower middle-income countries [23],[28],[46], and one in a low-income country [47]. Table 1 describes the health systems factors investigated, classified into the domains of the conceptual framework (Figure 1).
Tab. 1. Health system arrangements investigated by included quantitative studies, classified by health system domain. 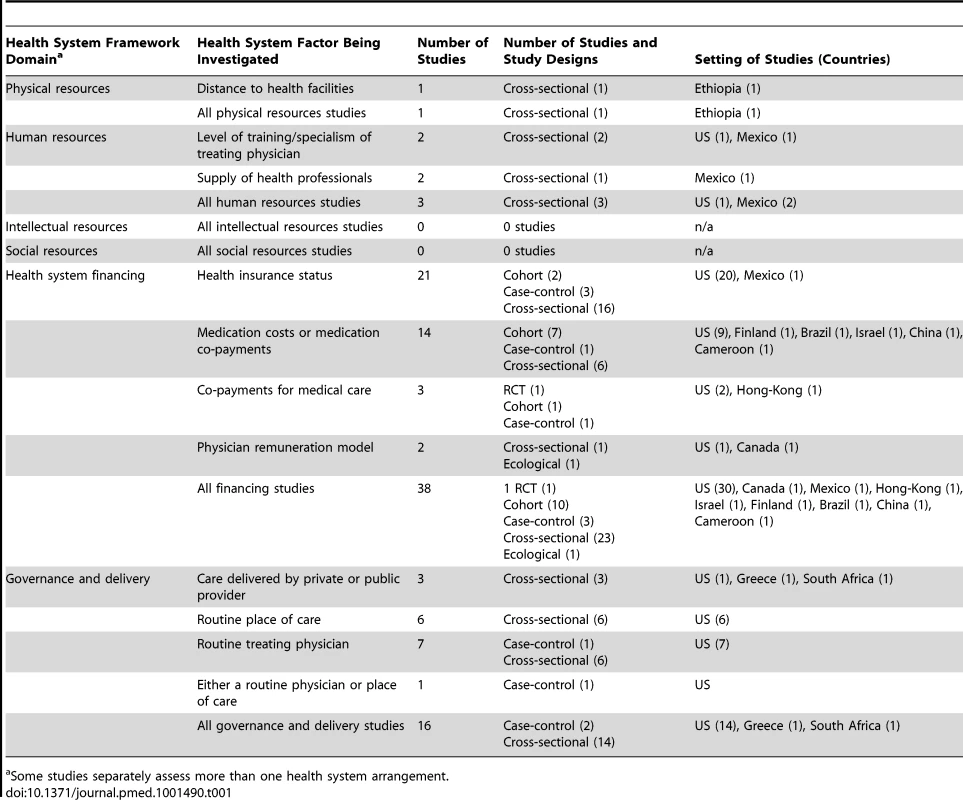
Some studies separately assess more than one health system arrangement. Effect of Health System Arrangements on Hypertension Outcomes
Physical resources
One study examined the effect of health system factors relating to physical resources (Table 2). This study was conducted in a low-income country, Ethiopia, and examined the effect of distance that patients were required to travel to health facilities providing HT care [47]. The study was cross-sectional in design and had a low risk of bias for all methodological domains assessed. The study reported a moderate positive association between a shorter distance of travel to a health facility and antihypertensive medication adherence (odds ratio [OR] for medication adherence in those with a travel time to health facilities of less than 30 min versus travel time of more than 30 min, 2.02, 95% CI 1.19–3.43).
Tab. 2. Summary of findings of studies examining the associations of arrangements relating to human or physical resources with hypertension outcomes. 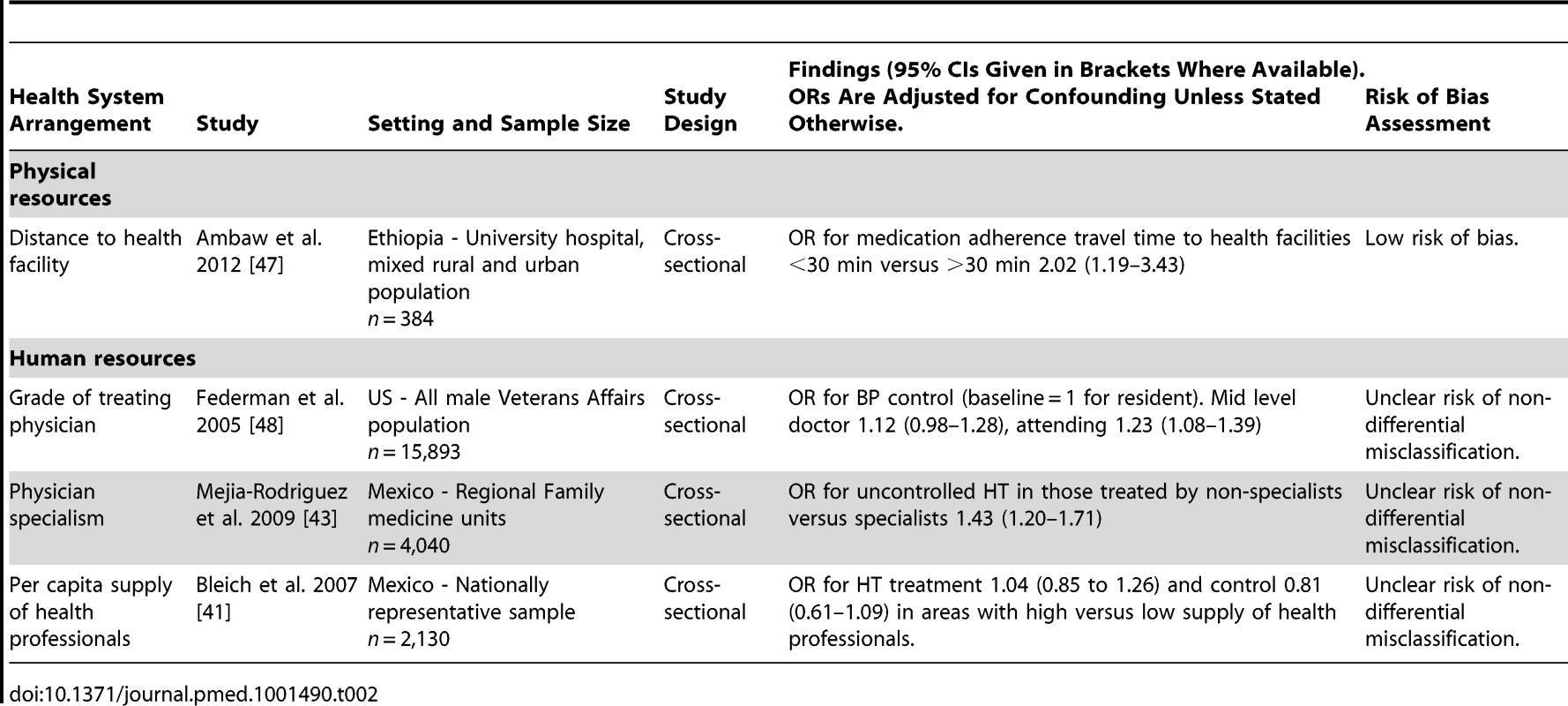
Human resources
Three studies examined the effect of health system factors relating to human resources, none of which had a low risk of bias (Table 2). Two of the three were conducted in an upper-middle income country (both in Mexico), and one was conducted in a high-income country (US).
One US cross-sectional study evaluated the effect of the treating physician's seniority on HT control [48]. This study found a small positive association between seniority of treating physician and HT control. The adjusted OR for HT control was 1.23 (95% CI 1.08–1.39) for patients treated by an attending level physician compared to those treated by a resident level physician.
One Mexican cross-sectional study evaluated the impact of being treated by a specialist on HT control [43]. This study found a moderately increased risk of uncontrolled HT in hypertensive individuals treated by non-specialist physicians (general practitioners) compared to those treated by specialists (adjusted OR 1.43, 95% CI 1.20–1.71).
Another Mexican cross-sectional study evaluated the effect of the density of health professionals and did not find an association with HT treatment or control [41].
Intellectual resources
None of the included studies evaluated the effects of health system factors relating to intellectual resources on HT outcomes.
Social resources
None of the included studies evaluated the effects of health system factors relating to social resources.
Health Systems Financing
38 quantitative studies analyzed the effects of health systems financing on HT outcomes (34 of these studies were conducted in high-income countries and four in middle-income countries). Four different health system arrangements were analyzed, with 21 studies assessing effects of health insurance coverage, 11 examining the effects of medication co-payments or costs, three analyzing co-payments for medical care, and two looking at physician remuneration models.
Twenty of 21 studies evaluating health insurance coverage were conducted in the US and one in Mexico (Table 3). Seven of the 21 studies had a low risk of bias. Two were cohort studies, three were case-control studies, and 16 were cross-sectional studies. 19 of the 21 studies evaluating health insurance reported direct comparisons of HT outcomes in insured and uninsured patients, while two studies only compared private and public insurance schemes. Two cohort studies, both set in the US, compared uninsured patients with insured patients [26],[49]. One of the cohort studies had a 9-y follow-up and found that being uninsured was associated with an increased risk of both unawareness of HT (relative risk [RR] of unawareness in uninsured versus insured patients 1.12, 95% CI 1.00–1.25) and inadequate control of HT (RR of inadequate control in uninsured versus insured patients 1.23, 95% CI 1.08–1.39) [49]. The other cohort study had a follow up period of 30 mo and found that medication adherence was lower in uninsured patients compared to insured patients (OR for medication adherence for uninsured versus insured 0.426, 95% CI 0.282–0.757) [26]. One of two US set case-control studies comparing HT outcomes in uninsured and insured patients reported that insurance was associated with an increased likelihood of HT control (OR for HT control in insured versus uninsured 2.15, 95% CI 1.02–4.52) [36]. The other case-control study reported a non-significant association between being uninsured and having severe uncontrolled HT (OR for severe uncontrolled HT in uninsured versus insured 1.9, 95% CI 0.8–4.6). [35]. Fifteen cross-sectional studies reported comparisons of HT outcomes in insured and uninsured patients. Eight of these 15 studies reported that insurance was associated with improved HT treatment, control or medication adherence [41],[50]–[56]. The seven other cross-sectional studies that compared HT outcomes in insured patients and uninsured patients, reported no significant negative or positive associations between insurance status and HT outcomes [57]–[63]. Two further studies looking at health insurance status compared HT outcomes in patients with public and private health insurance. A case-control study set in the US found increased odds of HT control in patients with private insurance compared to patients with public insurance (OR for HT control 3.40, 95% CI 1.25–9,28) [37]. A cross-sectional study, also set in the US, found no significant association between private or public insurance and HT awareness or treatment, but did report significantly lower levels of systolic blood pressure (BP) in patients with private insurance compared to public insurance (p<0.05) [64].
Tab. 3. Findings of quantitative studies examining the association of health insurance status with hypertension outcomes. 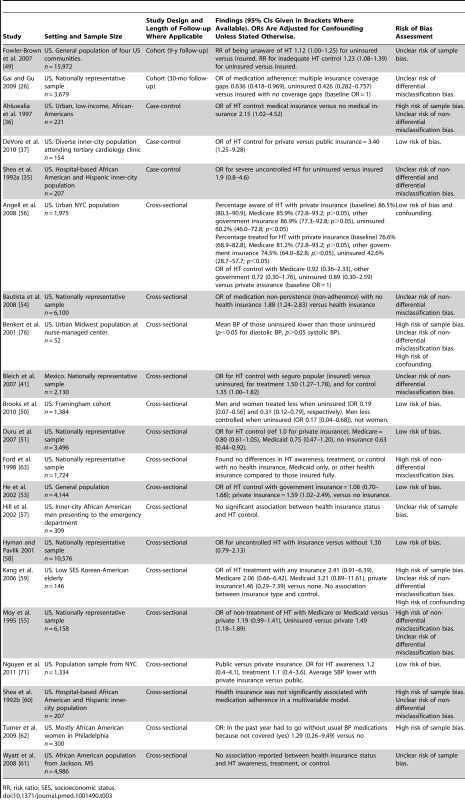
RR, risk ratio; SES, socioeconomic status. Fourteen quantitative studies measured the association of medication co-payments or costs with HT control or treatment adherence, nine of which were set in the US, and one in each of Cameroon, China, Finland, Israel, and Brazil (Table 4). Two of the 14 studies had a low risk of bias. Seven of the 14 studies were cohort studies, one was a case-control study, and six were cross-sectional studies. All seven cohort studies reported associations between increased medication costs or co-payments and reductions in HT control or reduced adherence to antihypertensive medication [25],[27],[29],[30],[32],[34],[65], although for one of these seven cohort studies, the association between increased co-payments and reduced medication adherence was only found for low medication co-payments, and at high co-payment levels medication adherence was actually found to increase (OR for medication adherence versus baseline of 1 for US$0 co-payments was 0.72 for US$1–US$9 co-payments (p<0.05), 1.02 for US$10–US$29 co-payments (p>0.05), and 1.32 for co-payments >US$30 (p<0.05)) [34]. Five cross-sectional studies and one case-control study also examined associations between medication co-payments or costs and HT control or adherence to antihypertensive medication [42],[45],[66]–[68]. All six of these studies reported significant associations between reduced co-payments or costs and improved HT control or medication adherence. One of these cross-sectional studies, set in China, also looked at the effect of medication costs on HT treatment rates. This study found that 0.0% of people given access to free antihypertensive medication remained untreated compared to 14.7% who had to pay for medication (p<0.001) [45]. One cross-sectional study set in Cameroon examined the association of medication costs with HT awareness and did not find one, although the confidence intervals were wide (OR for HT awareness for high medication cost versus low medication cost 0.44, 95% CI 0.07–2.75) [46]. Two qualitative studies, one from the US and one from Nigeria, cited cost of medications as a barrier to medication adherence [22],[23].
Tab. 4. Findings of quantitative studies examining the association of medication or medical care costs or co-payments with hypertension outcomes. 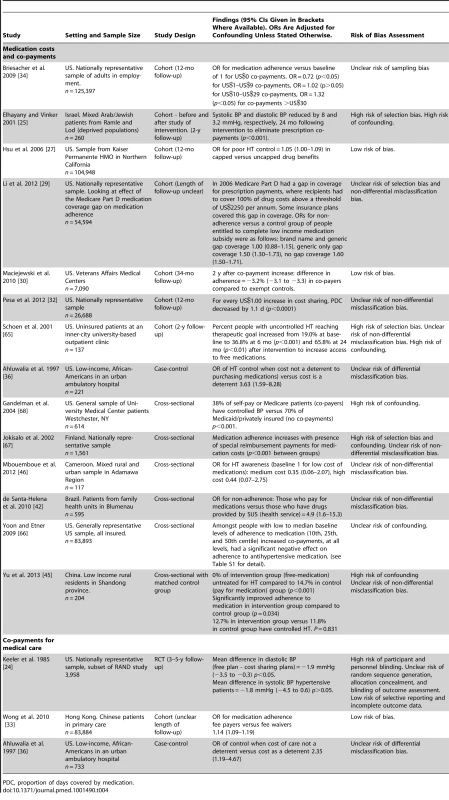
PDC, proportion of days covered by medication. Three studies assessed co-payments or costs of medical care (not simply medications), two of which were conducted in the US (an RCT and a case-control study) and one in Hong Kong (a cohort study). (Table 4) [24],[33],[36]. One of the three studies, the cohort study from Hong-Kong, had a low risk of bias [33]. The RCT reported a higher mean BP level amongst individuals with HT who had cost-sharing insurance plans compared to those with free care, although this was non-significant for systolic BP [24]. The adjusted mean difference in diastolic BP between the two groups was 1.9 mm mercury (mmHg) (95% CI 0.3–3.5 mmHg) and the adjusted mean difference in systolic BP was 1.8 mmHg (95% CI −0.6 to 4.5 mmHg). The case-control study reported that cost of care was a deterrent to BP control (adjusted OR for BP control when cost not a deterrent versus cost as a deterrent: 2.35, 95% CI 1.19–4.67) [36]. The cohort study, which was set in Hong Kong, found, conversely, that being a fee payer was associated with improved adherence to prescribed antihypertensive medications compared to fee waivers (adjusted OR for adherence fee payers versus fee waivers 1.14, 95% CI 1.09–1.19) [33].
Two studies evaluated the association of physician remuneration models with HT control or treatment adherence, one an ecological study set in Canada, and one a US cross-sectional study (Table 5). Neither study had a low risk of bias. The US study reported improved rates of HT control amongst patients treated under a capitation model compared to fee-for service patients (adjusted OR for HT control 1.82, 95% CI 1.02–3.27 for capitation versus fee-for-service patients) [69]. The Canadian study reported highest rates of HT treatment and control among practices using a capitation model, compared to fee-for-service and salary models [40]. HT awareness levels were highest in practices with a fixed salary remuneration model.
Tab. 5. Findings of quantitative studies examining the association of physician remuneration models with hypertension outcomes. 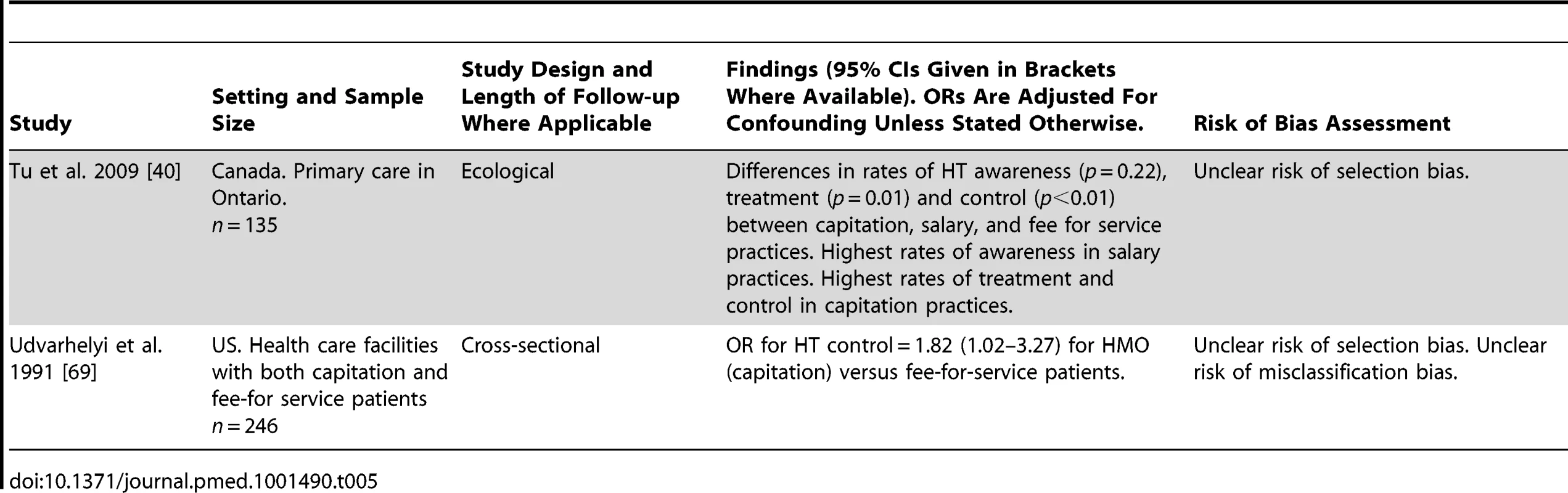
Delivery and Governance
16 studies examined the effects of health systems arrangements relating to delivery and governance on HT outcomes (Table 6). Fifteen of these studies were conducted in high-income countries and one in a LMIC. Four different health systems arrangements were analyzed, with six studies evaluating having a routine place of care for HT management, seven studies evaluating having a routine physician for HT care, one study evaluating having either a routine place or physician for HT care, and four studies assessing whether care was delivered by the private versus the public sector.
Tab. 6. Findings of studies examining health systems arrangements relating to health systems delivery and governance. 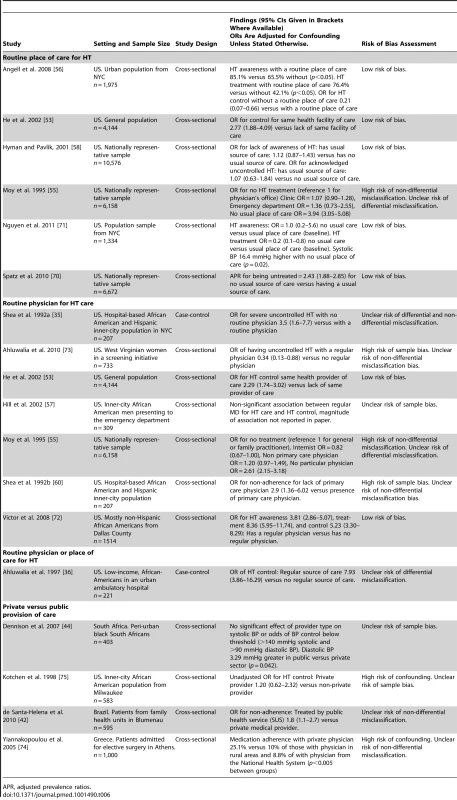
APR, adjusted prevalence ratios. All six studies analyzing having a routine place of care for HT were conducted in the US, and all were cross sectional in design, with five of the six having a low risk of bias. Five of these six studies reported a significant association between a routine place of care and improved HT awareness, treatment, or control [53],[55],[56],[70],[71]. One study found no association between a routine place of care and HT awareness or control [58]. No studies analyzed medication adherence.
Of the seven studies assessing the effects of having a routine physician for HT care, all were conducted in the US. Two of the seven studies had a low risk of bias. One was a case-control study and six were cross-sectional studies. The case-control study and five of the six cross-sectional studies found that having routine care from the same physician was significantly associated with an improvement in HT awareness, treatment control, or medication adherence [35],[53],[55],[57],[60],[72],[73]. One study did not find a significant association between a routine physician and HT control [57].
A single case-control study, which did not have a low risk of bias, analyzed having either a routine place of care or physician for HT care in the US [36]. It found that having either was strongly associated with improved HT control, with some imprecision in the effect estimate (adjusted OR for HT control 7.93, 95% CI 3.86–16.29 for a regular source of care versus no regular source of care).
Four studies assessed private versus public provision of care, with one set in each of South Africa, US, Brazil, and Greece. All four studies conducted were of cross-sectional design and none had a low overall risk of bias. One study set in Brazil found that non-adherence to antihypertensive medication was more likely in patients treated in the public versus private sector (OR for non-adherence in patients treated by public health service versus private medical provider 1.8, 95% CI 1.1–2.7) [42]. One study, set in the US, found no significant association of provider type with systolic BP or odds of blood pressure control below a threshold of 140 mmHg systolic and 90 mmHg diastolic BP, but did find that diastolic BP was 3.29 mmHg greater in patients treated by the public versus private sector (p = 0.042) [44]. The two other studies evaluating public versus private provision had a high risk of confounding, one of which was set in Greece and one in the US. The study set in Greece found increased rates of medication adherence in patients treated by a private physician compared to those treated in the National Health System (medication adherence with private physician 25.1% versus 10% of those with a physician in rural areas and 8.8% of with a physician from the National Health System, p<0.005 for between group differences) [74]. The study set in the US did not provide strong evidence of improved HT control in patients cared for by private providers (unadjusted OR for HT control for private versus non-private provider 1.20, 95% CI 0.62–2.32) [75].
Interventions Involving More Than One Health System Building Block
Four studies were included that evaluated outcomes associated with complex regional or national health policy interventions, which incorporated components from more than one health system domain (Table 7). Two of these studies were conducted in high-income countries, one in Finland and one in Trinidad and Tobago, one was conducted in a higher-middle-income country, Iran, and one was conducted in a lower middle-income country, Cameroon [28],[31],[38],[39]. None of the four studies had a low risk of bias. All four studies showed improvements in HT outcomes after the delivery of the intervention, although for one study there is a high probability that this could be due to random sampling error [38].
Tab. 7. Description and summary of findings of studies evaluating complex national or regional interventions incorporating components from more than one health system building block. 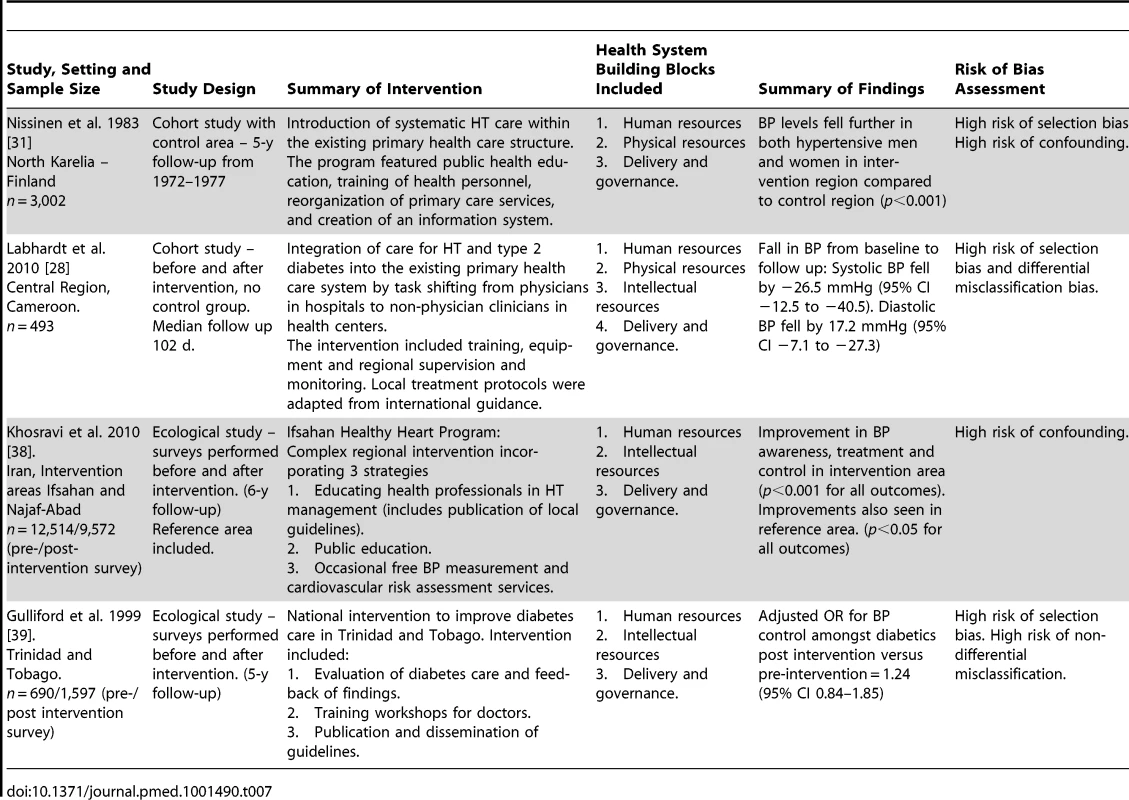
Considerations of Context
Seventeen of 53 included studies gave no information about the socio-demographic, political, or economic context in which the study was conducted [24],[26],[30]–[32],[39],[42],[47],[54],[55],[58],[63],[67],[69],[70],[74],[75]. Of the 33 studies that provided contextual information, this varied from single phrases or sentences to more detailed contextual information (Box 2). 28 of 53 included studies gave no description of the national or regional health system where the study was conducted [22],[26],[31]–[34],[37]–[39],[43],[46],[53]–[56],[58]–[62],[66]–[68],[72],[74]–[76]. Where a description of the health system was given it was in most cases limited to a brief sentence on insurance coverage or financing arrangements. A minority of studies, usually those carried out in low-income countries, gave more comprehensive descriptions of some aspects of the health system (Box 2).
Box 2. Examples of Contextual Information and Descriptions of the Health System from Included Studies
[set in a] “contemporary multi-ethnic urban community” [72], or “developing country setting” [38]
“Among industrialized countries, only the United States lacks universal healthcare.” [63]
“These cities have mixed Arab-Jewish populations and are among the poorest in Israel, defined by the Israeli Social Security Agency as having an SES in the lowest 10% of the population.” [25]
[On a regional health system in Cameroon] “When the program for hypertension and diabetes started in 2007, there were 79 peripheral clinics in the area offering nurse-led primary health care. Four of these had a physician among the staff; the remaining 75 were exclusively led by Non Physician Clinicians. Most (78%) of the peripheral clinics were public where consultations are usually free of charge but diagnostics and drugs for curative services have to be paid out-of-pocket. The area also has eight district hospitals and two missionary hospitals.” [28]
Considerations of Health Systems Complexity
Eleven of the 53 studies discussed linkages or interdependencies between health system domains [24],[28],[35],[36],[41],[57]–[59],[63],[69],[70]. Eight of these 11 studies discussed the importance of the interdependence between health system financing and structures relating to the delivery of care, with many emphasizing the link between low levels of health insurance coverage in certain US settings and a lack of structures providing access to regular high quality medical care [24],[35],[36],[58],[59],[63],[69],[70]. One study described the link between wider social factors and factors relating to health system financing in creating a barrier to care for African American men in the US: “unemployment and lack of health insurance are highly intercorrelated and constitute apparently insurmountable barriers to traditional medical care for HBP.” [57]. One study, set in Mexico, discussed the positive interaction between the presence of health insurance (financing) and the supply of health professionals (human resources) in improving HT outcomes [41].
Twenty-three of 53 studies considered how HT outcomes may be influenced by relationships between health systems factors and contextual factors, such as socioeconomic status, as well as individual factors, such as gender, age, or co-morbidities [22]–[24],[31],[36],[41],[47],[50],[54]–[57],[59],[63],[66],[70]–[72]. Five of these 23 studies used an established framework, such as the Anderson-Aday model [36],[55], or the Precede-PROCEED model to describe the multiple factors, including health system factors, that might determine HT outcomes at the individual level [44],[57],[59].
Discussion
Despite the limited scope and variable quality of literature found, as well as the context specificity of the findings, it remains possible to make inferences about the effect of some health system arrangements on HT outcomes. This is particularly the case for the characteristics of the US health system, which while unique among high income countries has features that can be found in other parts of the world. Evidence from longitudinal studies reported here suggests a small positive impact of the presence of health insurance in the US on HT awareness and control, and adherence to antihypertensive medication [26],[49]. This is supported by most, but not all case-control and cross-sectional studies [36],[41],[50]–[56]. However, these findings can be considered in relation to analogous studies, such as a 2008 systematic review of longitudinal studies that was confined to those in the US, which found improved long-term health outcomes, including reduced mortality, in insured patients compared to uninsured patients [77]. We also found an association, in both longitudinal and cross-sectional studies, between reduced co-payments or costs for medications or medical care and improved HT control or treatment adherence in multiple studies in US settings [24],[27],[29],[30],[32],[34],[36],[65],[66],[68], although in one of these studies, by Briesacher et al., the relationship between reduced co-payments and treatment adherence was only found for low levels of medication co-payments, while the highest levels of co-payments (>US$30) were, surprisingly, associated with improved medication adherence. The study authors do not provide an explanation for this result in the paper, but it could be that the subgroup of patients with co-payments of US$30 or more for medications have shared characteristics that were not analyzed in this study, such as high socioeconomic status, which may confound the association between co-payment levels and medication adherence. The association between reduced medication co-payments and improved HT outcomes was replicated in single studies from China, Finland, Israel and Brazil [25],[42],[45],[67] but not in a study of Hong Kong Chinese, which found that fee payers had improved medication adherence compared to those with fee waivers [33]. The finding of an association between reduced medication co-payments and improved HT outcomes is intuitive and suggests that costs of medications or health care consultations may act as a barrier to optimal HT care in the US, and potentially other settings, including LMICs. A relationship between increased medication co-payments and treatment discontinuation has also been reported for diabetes care in the US [78].
Although lacking longitudinal studies, we found a large positive association between having a routine physician or place of care for HT management and treatment, awareness, control, and adherence to antihypertensive treatment, again in the US [35],[36],[53],[55],[56],[60],[70]–[73]. This finding is consistent with a recent systematic review of the effect of a usual source of care, showing an association with improved preventive services and chronic disease control [79]. Although it is unclear whether having a routine physician or a place for HT care is more important, this may matter less than the implication that the absence of a consistent source of care reduces awareness, treatment, and control of HT. It is possible, however, that this effect is linked to health system financing arrangements, as those without insurance or facing high co-payments may be least likely to have consistent access to care [70]. There were no longitudinal studies looking at differences in outcomes of HT management provided by the private or public sector, and the four cross-sectional studies considering this question were all at risk of bias, were in different settings, and had different findings, so general inferences were not possible [42],[44],[75],[80].
All four included studies that evaluated complex multi-component national or regional policy interventions reported some improvement in HT care. These studies had significant methodological flaws including, in some cases, a lack of an adequate control group, precluding attribution of the improvement in HT outcomes to the intervention. However, despite their limitations, these studies may be useful for policymakers seeking to understand ways to strengthen health systems for chronic disease care, particularly in LMICs [6],[15]. Labhardt et al., for example, demonstrated the feasibility of task shifting from physicians to non-physician health care workers for HT management in Cameroon, outlining the integrated interventions across multiple health system domains required to deliver improvements in health outcomes [28].
Research on health systems factors influencing HT care is unequally distributed geographically. There is a lack of evidence from LMICs, which bear around three-quarters of the global HT burden [1]. Furthermore, even in high-income countries, health systems barriers to care have been seen mainly as financial, while the understanding of how a complex mix of other factors influence care is relatively new. Intellectual and social resources, such as the production and use of knowledge, social capital, and systems for communication have only recently emerged as distinct areas of research. As a result we found only a small number of studies examining the impact of health system factors relating to human resources or physical resources, and no studies evaluating the impact of intellectual or social resources. This meant we were unable to make firm conclusions about the effects of these factors on HT outcomes.
A number of included studies used models to conceptualize the mechanisms by which health systems factors may interact with other key variables to influence HT outcomes. For example Moy et al. and Ahluwalia et al. (1997) used the Anderson-Aday model, which illustrates how three types of population characteristics can influence medical care for HT [36],[55]. Factors relating to health systems such as the presence of a usual source of care or health insurance are seen as “enabling” factors for medical care. These “enabling” factors interact with “predisposing” factors such as ethnicity, gender, and socioeconomic status, and “need characteristics” such as health status to determine access and outcomes of medical care. Models such as Anderson-Aday are useful to the extent that they can help demonstrate how health systems factors may interact with other key factors in determining HT outcomes. However, the studies reviewed here lack quantitative and qualitative data on the nature and strength of these interactions, highlighting an important gap to be addressed by future research.
Study Limitations and Strengths
The majority of included quantitative studies were cross-sectional, and the few longitudinal studies we did find were restricted to either health system arrangements relating to financing or to evaluating the effects of complex multi-component interventions. Inferences about temporal and potentially causal relationships between health systems arrangements and HT outcomes, could, therefore, only be made for a limited number of factors. In addition, included quantitative studies were of variable methodological quality, with only one being randomized and a minority having a low risk of bias for all assessed methodological domains.
When considering the findings of this review, the risk of publication bias cannot be ruled out, particularly for the positive findings relating to health insurance status, medication and treatment costs and co-payments, and presence of a routine setting of care, where it is possible that studies with null findings are under-published. It was not possible to produce an Egger funnel plot to formally assess the risk of publication bias, for the same reasons that meta-analysis was not performed, namely the heterogeneity in the study designs, outcome measures, analysis strategies, and populations in the included studies. Reporting bias within individual studies may also be a factor, many of which might have explored the effects of multiple factors on HT outcomes, and may have failed to report results for health system arrangements which did not show significant effects. The lack of published protocols for the included studies did not allow us to estimate the magnitude of this potential bias. A strength of the review is the addition of forward and backward searching methods to the initial database search for articles. A number of additional studies were identified using these methods, before reaching a saturation point at which the only relevant studies being identified were already included. We included only two qualitative studies, which did not contribute important data about the views of policymakers and health care workers on health systems factors affecting HT care, contrary to what we had initially hoped.
The use of a conceptual health systems framework facilitated the conduct of the review, enabling systematic generation of terms for the search strategy and for classification of included studies according to the domains in the conceptual framework. However, the classification and reporting of our findings according to health system domain does not encourage the integrated view of health systems that the framework promotes. For example, classifying the effect of usual source of care into the domain of health systems governance and delivery obscures the fact that the delivery of care from a regular source is very much dependent on human and physical resources inputs to the health system. The difficulties in presenting such complexities are perhaps a reflection of the fact that few of the included studies explored inter-linkages between health system components, with the majority exploring the association between health system arrangements and HT outcomes as simple linear relationships. There were some notable exceptions, however; one study, for example, examined the interaction of insurance status and the presence of a usual source of care on HT outcomes [70].
Implications for Policy
We found an association between reduced co-payments for health care, including for medications, and improved outcomes of HT care in multiple US studies, and in single studies set in Finland, Israel, and Brazil. This is consistent with a wealth of other evidence on how co-payments reduce uptake of necessary care and has clear implications for policy makers, particularly as the balance of evidence does not suggest that reducing medication co-payments leads to an increase in overall health care expenditure [81]–[84]. On balance, we found health insurance coverage to be associated with improved outcomes of HT care in US settings, suggesting that expanded insurance coverage through The Patient Protection and Affordable Care Act (also known as Obamacare) may improve HT outcomes.
Implications for Research
This study indicates a number of possible implications for future research. Ultimately, an increase in the number of high quality, longitudinal and randomized studies identifying and analyzing the effect of health system arrangements on HT care is required, particularly in LMICs where the majority of the global burden of HT lies, and where weaknesses in health systems are thought to play a significant role in deficiencies in chronic disease care [6]. The focus on financing has highlighted important barriers to effective care and control of HT but needs to be supplemented by research examining other domains, such as delivery and governance mechanisms, production of knowledge, and the social function in the health systems. Most existing studies have a focus on independent effects of different health systems arrangements, thereby creating a “laundry list” of isolated components. Recognizing the shortcomings of this approach, it is important that future studies attempt to capture the complexities and interactions between health systems arrangements. In addition, future national or regional health systems strengthening programs that aim to improve care for chronic conditions such as HT should be robustly evaluated, using longitudinal controlled study designs where possible.
Moving forward, there is a clear need for more robust designs of studies in a much wider range of settings, especially in LMICs. This will ideally include cluster RCTs and prospective longitudinal studies with detailed data on individual and health system characteristics, complemented by qualitative studies to see inside what is often a health systems black box. Such studies also call for consistency in health systems definitions and outcome measures. A particular challenge will be to take account of the complexity of health systems and all health system domains, as well as interpreting studies by not simply as showing what works, but what works in what circumstances [85]. This review should help inform the design of such studies. In particular, the findings are being combined with multi-method appraisals of health systems to understand the barriers faced by patients with HT and their health workers to design cluster randomized trials in several LMICs [86]. Importantly, given that there are many health systems frameworks, this review has shown the practicality of using the one chosen, a framework that is also being used in the multi-method appraisals and that has been found useful in similar previous studies using diabetes as a probe to analyze health systems [12],[13]. Research such as this addresses a crucial gap in understanding of how different models of health systems contribute to health.
Supporting Information
Zdroje
1. IbrahimMM, DamascenoA (2012) Hypertension in developing countries. Lancet 380 : 611–619.
2. LimSS, VosT, FlaxmanAD, DanaeiG, ShibuyaK, et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 : 2224–2260.
3. LewingtonS, ClarkeR, QizilbashN, PetoR, CollinsR (2002) Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360 : 1903–1913.
4. PereiraM, LunetN, AzevedoA, BarrosH (2009) Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. J Hypertens 27 : 963–975.
5. PerkovicV, HuxleyR, WuY, PrabhakaranD, MacMahonS (2007) The burden of blood pressure-related disease: a neglected priority for global health. Hypertension 50 : 991–997.
6. SambB, DesaiN, NishtarS, MendisS, BekedamH, et al. (2010) Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. Lancet 376 : 1785–1797.
7. World Health Organization (2000) The World Health Report 2000: Health Systems: Improving Performance. World Health Organization: Geneva.
8. WalshJME, McDonaldKM, ShojaniaKG, SundaramV, NayakS, et al. (2006) Quality improvement strategies for hypertension management: a systematic review. Medical Care 44 : 646–657.
9. GlynnLG, MurphyAW, SmithSM, SchroederK, FaheyT (2010) Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract 60: e476–e488.
10. FaheyT, SchroederK, EbrahimS (2005) Educational and organisational interventions used to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract 55 : 875–882.
11. MaimarisW, PerelP, Legido-QuigleyH, BalabanovaD, McKeeM (2012) Health system barriers and facilitators to hypertension detection, treatment, and control: a systematic review (protocol). PROSPERO 2012: CRD42012002864.
12. BalabanovaD, McKeeM, KorolevaN, ChikovaniI, GoguadzeK, et al. (2009) Navigating the health system: diabetes care in Georgia. Health Policy Plan 24 : 46–54.
13. HopkinsonB, BalabanovaD, McKeeM, KutzinJ (2004) The human perspective on health care reform: coping with diabetes in Kyrgyzstan. Int J Health Plann Manage 19 : 43–61.
14. Gilson L (2012) Health policy and systems research: a methodology reader.World Health Organization: Geneva.
15. FrenkJ (2010) The global health system: strengthening national health systems as the next step for global progress. PLoS Med 7: e1000089 doi:10.1371/journal.pmed.1000089
16. Hoffman S, Rottingen J, Bennett S, Lavis J, Edge J, et al.. (2012) A review of conceptual barriers amd opportunities facing health systems research to inform a strategy from the World Health Organization. World Health Organization: Geneva.
17. HigginsJPT, AltmanDG, GøtzschePC, JüniP, MoherD, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928.
18. Rees R, Harden A, Brunton G, Oliver S, Oakley A (2001) Young people and physical activity: a systematic review of barriers and facilitators. London: EPPI-Centre, Social Science Research Unit, Institute of Education, University of London.
19. Harden A, Rees R, Shepherd J, Brunton G, Oliver S, et al.. (2001) Young people and mental health: a systematic review of research on barriers and facilitators. London: EPPI-Centre, Social Science Research Unit.
20. AdamT, de SavignyD (2012) Systems thinking for strengthening health systems in LMICs: need for a paradigm shift. Health Policy Plann 27: iv1–iv3.
21. MoherD, LiberatiA, TetzlaffJ, AltmanDG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535.
22. OgedegbeG, HarrisonM, RobbinsL, MancusoCA, AllegranteJP (2004) Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study. Ethn Dis 14 : 3–12.
23. OsamorPE, OwumiBE (2011) Factors associated with treatment compliance in hypertension in southwest Nigeria. J Health Popul Nutr 29 : 619–628.
24. KeelerEB, BrookRH, GoldbergGA, KambergCJ, NewhouseJP (1985) How free care reduced hypertension in the health insurance experiment. JAMA 254 : 1926–1931.
25. ElhayanyA, VinkerS (2011) Addressing healthcare inequities in Israel by eliminating prescription drug copayments. Am J Manag Care 17 : 255–259.
26. GaiY, GuNY (2009) Association between insurance gaps and continued antihypertension medication usage in a US national representative population. Am J Hypertens 22 : 1276–1280.
27. HsuJ, PriceM, HuangJ, BrandR, FungV, et al. (2006) Unintended consequences of caps on medicare drug benefits. N Engl J Med 354 : 2349–2359.
28. LabhardtND, BaloJR, NdamM, GrimmJJ, MangaE (2010) Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: a programme assessment at two years. BMC Health Serv Res 10.
29. LiP, McElligottS, BergquistH, SchwartzJS, DoshiJA (2012) Effect of the Medicare Part D coverage gap on medication use among patients with hypertension and hyperlipidemia. Ann Intern Med 156 : 776–784.
30. MaciejewskiML, BrysonCL, PerkinsM, BloughDK, CunninghamFE, et al. (2010) Increasing copayments and adherence to diabetes, hypertension, and hyperlipidemic medications. Am J Manag Care 16: E20–E34.
31. NissinenA, TuomilehtoJ, EloJ, AlasoiniA, VarvikkoP, et al. (1983) North Karelia (Finland) hypertension detection project. Five-year follow-up of hypertensive cohort. Hypertension 5 : 564–572.
32. PesaJA, Van Den BosJ, GrayT, HartsigC, McQueenRB, et al. (2012) An evaluation of the impact of patient cost sharing for antihypertensive medications on adherence, medication and health care utilization, and expenditures. Patient Prefer Adherence 6 : 63–72.
33. WongMCS, JiangJY, GriffithsSM (2010) Factors associated with antihypertensive drug compliance in 83 884 Chinese patients: a cohort study. J Epidemiol Community Health 64 : 895–901.
34. BriesacherBA, AndradeSE, FouayziH, ChanKA (2009) Medication adherence and use of generic drug therapies. Am J Manag Care 15 : 450–456.
35. SheaS, MisraD, EhrlichMH, FieldL, FrancisCK (1992) Predisposing factors for severe, uncontrolled hypertension in an inner-city minority population. N Engl J Med 327 : 776–781.
36. AhluwaliaJS, McNagnySE, RaskKJ (1997) Correlates of controlled hypertension in indigent, inner-city hypertensive patients. J Gen Intern Med 12 : 7–14.
37. DeVoreAD, SorrentinoM, ArnsdorfMF, WardRP, BakrisGL, et al. (2010) Predictors of hypertension control in a diverse general cardiology practice. J Clin Hypertens (Greenwich) 12 : 570–577.
38. KhosraviA, MehrGK, KelishadiR, ShiraniS, GharipourM, et al. (2010) The impact of a 6-year comprehensive community trial on the awareness, treatment and control rates of hypertension in Iran: experiences from the Isfahan healthy heart program. BMC Cardiovasc Disord 10.
39. GullifordMC, MahabirD (1999) A five-year evaluation of intervention in diabetes care in Trinidad and Tobago. Diabet Med 16 : 939–945.
40. TuK, Cauch-DudekK, ChenZ (2009) Comparison of primary care physician payment models in the management of hypertension. Can Fam Physician 55 : 719–727.
41. BleichSN, CutlerDM, AdamsAS, LozanoR, MurrayCJL (2007) Impact of insurance and supply of health professionals on coverage of treatment for hypertension in Mexico: population based study. BMJ 335 : 875–878.
42. de Santa-HelenaET, Battistella NemesMI, Eluf NetoJ (2010) Risk factors associated with non-adherence to anti-hypertensive medication among patients treated in family health care facilities. Cad Saude Publica 26 : 2388–2397.
43. Mejía-RodríguezO, Paniagua-SierraR, del Refugio Valencia-OrtizM, Ruiz-GarcíaJ, Figueroa-NúñezB, et al. (2009) Factores relacionados con el descontrol de la presión arterial. Salud Publica Mex 51 : 291–297.
44. DennisonCR, PeerN, SteynK, LevittNS, HillMN (2007) Determinants of hypertension care and control among peri-urban Black South Africans: The HIHI study. Ethn Dis 17 : 484–491.
45. YuB, ZhangX, WangG (2013) Full coverage for hypertension drugs in rural communities in China. Am J Manag Care 19: e22–29.
46. MbouemboueOP, YiagnigniE, KoonaAK, CackoJ, NdoboP (2012) Determinants of hypertension awareness and treatment among patients under cardiology follow-up in a Cameroonian regional hospital. International Journal of Collaborative Research on Internal Medicine and Public Health 4 : 1663–1672.
47. AmbawAD, AlemieGA, YohannesSMW, MengeshaZB (2012) Adherence to antihypertensive treatment and associated factors among patients on follow up at University of Gondar Hospital, Northwest Ethiopia. BMC Public Health 12 : 282.
48. FedermanDG, KrishnamurthyR, KancirS, GouletJ, JusticeA (2005) Relationship between provider type and the attainment of treatment goals in primary care. Am J Manag Care 11 : 561–566.
49. Fowler-BrownA, Corbie-SmithG, GarrettJ, LurieN (2007) Risk of cardiovascular events and death–does insurance matter? J Gen Intern Med 22 : 502–507.
50. BrooksEL, PreisSR, HwangSJ, MurabitoJM, BenjaminEJ, et al. (2010) Health insurance and cardiovascular disease risk factors. Am J Med 123 : 741–747.
51. DuruOK, VargasRB, KermahD, PanD, NorrisKC (2007) Health Insurance Status and Hypertension Monitoring and Control in the United States. Am J Hypertens 20 : 348–353.
52. BenkertR, BuchholzS, PooleM (2001) Hypertension outcomes in an urban nurse-managed center. J Am Acad Nurse Pract 13 : 84–89.
53. HeJ, MuntnerP, ChenJ, RoccellaEJ, StreifferRH, et al. (2002) Factors associated with hypertension control in the general population of the United States. Arch Intern Med 162 : 1051–1058.
54. BautistaLE (2008) Predictors of persistence with antihypertensive therapy: Results from the NHANES. Am J Hypertens 21 : 183–188.
55. MoyE, BartmanBA, WeirMR (1995) Access to hypertensive care. Effects of income, insurance, and source of care. Arch Intern Med 155 : 1497–1502.
56. AngellSY, GargRK, GwynnRC, BashL, ThorpeLE, et al. (2008) Prevalence, awareness, treatment, and predictors of control of hypertension in New York City. Circ Cardiovasc Qual Outcomes 1 : 46–53.
57. HillMN, BoneLR, KimMT, MillerDJ, DennisonCR, et al. (1999) Barriers to hypertension care and control in young urban black men. Am J Hypertens 12 : 951–958.
58. HymanDJ, PavlikVN (2001) Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med 345 : 479–486.
59. KangJH, HanHR, KimKB, KimMT (2006) Barriers to care and control of high blood pressure in Korean-American elderly. Ethn Dis 16 : 145–151.
60. SheaS, MisraD, EhrlichMH, FieldL, FrancisCK (1992b) Correlates of nonadherence to hypertension treatment in an inner city minority population. American J Public Health 82 : 1607–1612.
61. WyattSB, AkylbekovaEL, WoffordMR, CoadySA, WalkerER, et al. (2008) Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension 51 : 650–656.
62. TurnerBJ, HollenbeakC, WeinerMG, Ten HaveT, RobertsC (2009) Barriers to adherence and hypertension control in a racially diverse representative sample of elderly primary care patients. Pharmacoepidemiol Drug Saf 18 : 672–681.
63. FordES, WillJC, De Proost FordMA, MokdadAH (1998) Health insurance status and cardiovascular disease risk factors among 50–64-year-old U.S. women: findings from the Third National Health and Nutrition Examination Survey. J Womens Health 7 : 997–1006.
64. NguyenQC, WaddellEN, ThomasJC, HustonSL, KerkerBD, et al. (2011) Awareness, treatment, and control of hypertension and hypercholesterolemia among insured residents of New York City, 2004. Prev Chronic Dis 8: A109.
65. SchoenMD, DidomenicoRJ, ConnorSE, DischlerJE, BaumanJL (2001) Impact of the cost of prescription drugs on clinical outcomes in indigent patients with heart disease. Pharmacotherapy 21 : 1455–1463.
66. YoonJ, EttnerSL (2009) Cost-sharing and adherence to antihypertensives for low and high adherers. Am J Manag Care 15 : 833–840.
67. JokisaloE, KumpusaloE, EnlundH, HalonenP, TakalaJ (2002) Factors related to non-compliance with antihypertensive drug therapy. J Hum Hypertens 16 : 577–583.
68. GandelmanG, AronowWS, VarmaR (2004) Prevalence of adequate blood pressure control in self-pay or medicare patients versus medicaid or private insurance patients with systemic hypertension followed in a university cardiology or general medicine clinic. Am J Cardiol 94 : 815–816.
69. UdvarhelyiIS, JennisonK, PhillipsRS, EpsteinAM (1991) Comparison of the quality of ambulatory care for fee-for-service and prepaid patients. Ann Intern Med 115 : 394–400.
70. SpatzES, RossJS, DesaiMM, CanavanME, KrumholzHM (2010) Beyond insurance coverage: usual source of care in the treatment of hypertension and hypercholesterolemia. Data from the 2003–2006 National Health and Nutrition Examination Survey. Am Heart J 160 : 115–121.
71. NguyenQC, WaddellEN, ThomasJC, HustonSL, KerkerBD, et al. (2011) Awareness, treatment, and control of hypertension and hypercholesterolemia among insured residents of New York City, 2004. Prev Chronic Dis 8: A109.
72. VictorRG, LeonardD, HessP, BhatDG, JonesJ, et al. (2008) Factors associated with hypertension awareness, treatment, and control in Dallas County, Texas. Arch Intern Med 168 : 1285–1293.
73. AhluwaliaIB, TessaroI, GreenlundKJ, FordES (2010) Factors associated with control of hypertension, hypercholesterolemia, and diabetes among low-income women in West Virginia. J Womens Health 19 : 417–424.
74. YiannakopoulouEC, PapadopulosJS, CokkinosDV, MountokalakisTD (2005) Adherence to antihypertensive treatment: A critical factor for blood pressure control. Eur J Cardiovasc Prev Rehabil 12 : 243–249.
75. KotchenJM, Shakoor-AbdullahB, WalkerWE, CheliusTH, HoffmannRG, et al. (1998) Hypertension control and access to medical care in the inner city. Am J Public Health 88 : 1696–1699.
76. BenkertR, BuchholzS, PooleM (2001) Hypertension outcomes in an urban nurse-managed center. J Am Acad Nurse Pract 13 : 84–89.
77. FreemanJD, KadiyalaS, BellJF, MartinDP (2008) The causal effect of health insurance on utilization and outcomes in adults: a systematic review of US studies. Med Care 46 : 1023–1032.
78. BarronJ, WahlP, FisherM, PlauschinatC (2008) Effect of prescription copayments on adherence and treatment failure with oral antidiabetic medications. Pharmacy and Therapeutics 33 : 532–553.
79. KimMY, KimJH, ChoiIK, HwangIH, KimSY (2012) Effects of having usual source of care on preventive services and chronic disease control: a systematic review. Korean J Fam Med 33 : 336–345.
80. YiannakopoulouEC, PapadopulosJS, CokkinosDV, MountokalakisTD (2005) Adherence to antihypertensive treatment: A critical factor for blood pressure control. Eur J Cardiovasc Prev Rehabil 12 : 243–249.
81. Austvoll-DahlgrenA, AaserudM, VistG, RamsayC, OxmanAD, et al. (2008) Pharmaceutical policies: effects of cap and co-payment on rational drug use. Cochrane Database Syst Rev CD007017.
82. Newhouse J (1993) Free for all? Lessons from the RAND Health Insurance Experiment. Cambridge: Harvard University Press.
83. GemmillMC, ThomsonS, MossialosE (2008) What impact do prescription drug charges have on efficiency and equity? Evidence from high-income countries. Int J Equity Health 7 : 12.
84. LexchinJ, GrootendorstP (2004) Effects of prescription drug user fees on drug and health services use and on health status in vulnerable populations: a systematic review of the evidence. Int J Health Serv 34 : 101–122.
85. Pawson R, Tilley N (1997) Realistic Evaluation. London: Sage Publications.
86. Balabanova D, Legido-Quigley H, Perel P, McKee M (2012) Guidance for rapid appraisal of hypertension care from user and health care professional perspective: toolkit for multi-country studies. London: LSHTM.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- The Influence of Health Systems on Hypertension Awareness, Treatment, and Control: A Systematic Literature Review
- Factors Affecting the Delivery, Access, and Use of Interventions to Prevent Malaria in Pregnancy in Sub-Saharan Africa: A Systematic Review and Meta-Analysis
- Translating Translational Research into Global Health Gains
- Progress in Using Systematic Reviews of Animal Studies to Improve Translational Research
- A Comparison of Frameworks Evaluating Evidence for Global Health Interventions
- Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions
- The Effect of Tobacco Control Measures during a Period of Rising Cardiovascular Disease Risk in India: A Mathematical Model of Myocardial Infarction and Stroke
- Association of Lifecourse Socioeconomic Status with Chronic Inflammation and Type 2 Diabetes Risk: The Whitehall II Prospective Cohort Study
- Risk Prediction for Breast, Endometrial, and Ovarian Cancer in White Women Aged 50 y or Older: Derivation and Validation from Population-Based Cohort Studies
- Evaluation of Prediction Models for Decision-Making: Beyond Calibration and Discrimination
- The Growing Problem of Multidrug-Resistant Tuberculosis in North Korea
- Multiplex Identification of Gram-Positive Bacteria and Resistance Determinants Directly from Positive Blood Culture Broths: Evaluation of an Automated Microarray-Based Nucleic Acid Test
- Combatting Substandard and Falsified Medicines: A View from Rwanda
- Access to Drugs for Treatment of Noncommunicable Diseases
- Threats to Validity in the Design and Conduct of Preclinical Efficacy Studies: A Systematic Review of Guidelines for In Vivo Animal Experiments
- Recent Shifts in Global Governance: Implications for the Response to Non-communicable Diseases
- Reflections on the Global Burden of Disease 2010 Estimates
- Sickle Cell Anaemia in a Changing World
- Changes in Association between Previous Therapeutic Abortion and Preterm Birth in Scotland, 1980 to 2008: A Historical Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Changes in Association between Previous Therapeutic Abortion and Preterm Birth in Scotland, 1980 to 2008: A Historical Cohort Study
- Multiplex Identification of Gram-Positive Bacteria and Resistance Determinants Directly from Positive Blood Culture Broths: Evaluation of an Automated Microarray-Based Nucleic Acid Test
- Combatting Substandard and Falsified Medicines: A View from Rwanda
- Reflections on the Global Burden of Disease 2010 Estimates
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání