-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMeasuring the Population Burden of Injuries—Implications for Global and National Estimates: A Multi-centre Prospective UK Longitudinal Study
Background:
Current methods of measuring the population burden of injuries rely on many assumptions and limited data available to the global burden of diseases (GBD) studies. The aim of this study was to compare the population burden of injuries using different approaches from the UK Burden of Injury (UKBOI) and GBD studies.Methods and Findings:
The UKBOI was a prospective cohort of 1,517 injured individuals that collected patient-reported outcomes. Extrapolated outcome data were combined with multiple sources of morbidity and mortality data to derive population metrics of the burden of injury in the UK. Participants were injured patients recruited from hospitals in four UK cities and towns: Swansea, Nottingham, Bristol, and Guildford, between September 2005 and April 2007. Patient-reported changes in quality of life using the EQ-5D at baseline, 1, 4, and 12 months after injury provided disability weights used to calculate the years lived with disability (YLDs) component of disability adjusted life years (DALYs). DALYs were calculated for the UK and extrapolated to global estimates using both UKBOI and GBD disability weights. Estimated numbers (and rates per 100,000) for UK population extrapolations were 750,999 (1,240) for hospital admissions, 7,982,947 (13,339) for emergency department (ED) attendances, and 22,185 (36.8) for injury-related deaths in 2005. Nonadmitted ED-treated injuries accounted for 67% of YLDs. Estimates for UK DALYs amounted to 1,771,486 (82% due to YLDs), compared with 669,822 (52% due to YLDs) using the GBD approach. Extrapolating patient-derived disability weights to GBD estimates would increase injury-related DALYs 2.6-fold.Conclusions:
The use of disability weights derived from patient experiences combined with additional morbidity data on ED-treated patients and inpatients suggests that the absolute burden of injury is higher than previously estimated. These findings have substantial implications for improving measurement of the national and global burden of injury.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(12): e32767. doi:10.1371/journal.pmed.1001140
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001140Summary
Background:
Current methods of measuring the population burden of injuries rely on many assumptions and limited data available to the global burden of diseases (GBD) studies. The aim of this study was to compare the population burden of injuries using different approaches from the UK Burden of Injury (UKBOI) and GBD studies.Methods and Findings:
The UKBOI was a prospective cohort of 1,517 injured individuals that collected patient-reported outcomes. Extrapolated outcome data were combined with multiple sources of morbidity and mortality data to derive population metrics of the burden of injury in the UK. Participants were injured patients recruited from hospitals in four UK cities and towns: Swansea, Nottingham, Bristol, and Guildford, between September 2005 and April 2007. Patient-reported changes in quality of life using the EQ-5D at baseline, 1, 4, and 12 months after injury provided disability weights used to calculate the years lived with disability (YLDs) component of disability adjusted life years (DALYs). DALYs were calculated for the UK and extrapolated to global estimates using both UKBOI and GBD disability weights. Estimated numbers (and rates per 100,000) for UK population extrapolations were 750,999 (1,240) for hospital admissions, 7,982,947 (13,339) for emergency department (ED) attendances, and 22,185 (36.8) for injury-related deaths in 2005. Nonadmitted ED-treated injuries accounted for 67% of YLDs. Estimates for UK DALYs amounted to 1,771,486 (82% due to YLDs), compared with 669,822 (52% due to YLDs) using the GBD approach. Extrapolating patient-derived disability weights to GBD estimates would increase injury-related DALYs 2.6-fold.Conclusions:
The use of disability weights derived from patient experiences combined with additional morbidity data on ED-treated patients and inpatients suggests that the absolute burden of injury is higher than previously estimated. These findings have substantial implications for improving measurement of the national and global burden of injury.
: Please see later in the article for the Editors' SummaryIntroduction
Quantifying the burden placed on society is an essential component of the public health response to conditions, supporting development of policy, priority setting, and monitoring of interventions [1],[2]. The 1990 Global Burden of Diseases, Injuries and Risk Factors (GBD) Study led the way in developing population burden of disease studies [3]. This seminal study and subsequent publications revealed that injuries accounted for more than 15% of the global disability adjusted life years (DALYs) lost in 1990 and are forecasted to increase to 20% by 2020 [4]–[6]. The methodology has been used widely for the production of estimates of injury-related population burden around the world and is currently being revised with 2005-based estimates due out in 2011 [7]–[14].
Central to the GBD methodology is the concept of DALYs, a combination of premature mortality, termed years of life lost (YLLs), and years lived with disability (YLDs) [15]. YLLs are generated using mortality data. The YLD component requires establishment of disability weights and durations, for different injury types. Disability weights are multiplied by incidence and duration data to generate YLDs. The GBD Study used panel studies and expert opinion to estimate, rather than measure, weights and durations of disability for 33 injury groups [3]. Whilst the GBD was a major development, limited incidence data and the absence of disability weights for many injury types underestimated the population burden of injuries [16]. Accurate measurement of the burden of injuries is essential to ensure adequate policy responses to prevention and treatment.
Objectives
The UK Burden of Injuries (UKBOI) Study was designed to overcome the limitations of previous studies and to measure the population burden of injuries in the UK for the first time and to compare the population burden of injuries using disability and morbidity metrics from the UKBOI and GBD studies [3],[17].
Methods
Study Design
A prospective cohort study, with extrapolation to population-based data was used to establish the burden of injury. The protocol for the design of the UKBOI study has been published previously [17]. Briefly, the main component was a longitudinal study of injured individuals with extrapolation of the impact of their injuries to the UK population using routinely collected data and official statistics on emergency department (ED) attendances, hospital inpatient data, and mortality data, for the year 2005. Injuries were defined using International Classification of Diseases 10 (ICD-10) codes: S00-T73, T75, and T78 [18]. Excluded codes were T79 (certain early complications of trauma), T80–88 (complications of medical and surgical care), and T90–98 (sequelae of injuries and poisoning). For mortality data, the M80–M81 (deaths from osteoporosis) and F10–F19 (deaths from mental and behavioural disorders due to substance abuse) codes were included to ensure that all injury-related deaths due to osteoporosis and poisoning were included [19].
Setting
The prospective study recruited participants from EDs and hospital inpatients in four UK cities and towns; Swansea, Nottingham, Bristol, and Guildford [17]. Multiple sources of national and regional routine data were used to extrapolate results to the UK population.
Participants
Inclusion criteria were: participants were patients aged 5 y and over attending EDs or admitted to hospital in the four UK centres with a wide range of injuries, including fractures/dislocations, lacerations, bruises/abrasions, sprains, burns/scalds, and head, eye, thorax, and abdominal injuries (see Table 1 and published protocol [17]). The injuries had to have occurred within 2 wk for ED-treated patients and 4 wk for hospital-admitted patients. Patients had to be able to give consent and complete questionnaires or to have a suitable proxy who could assent to their inclusion and agree to complete future questionnaires.
Tab. 1. Number (%) of cases in UKBOI study by injury group using the 13-group classification. 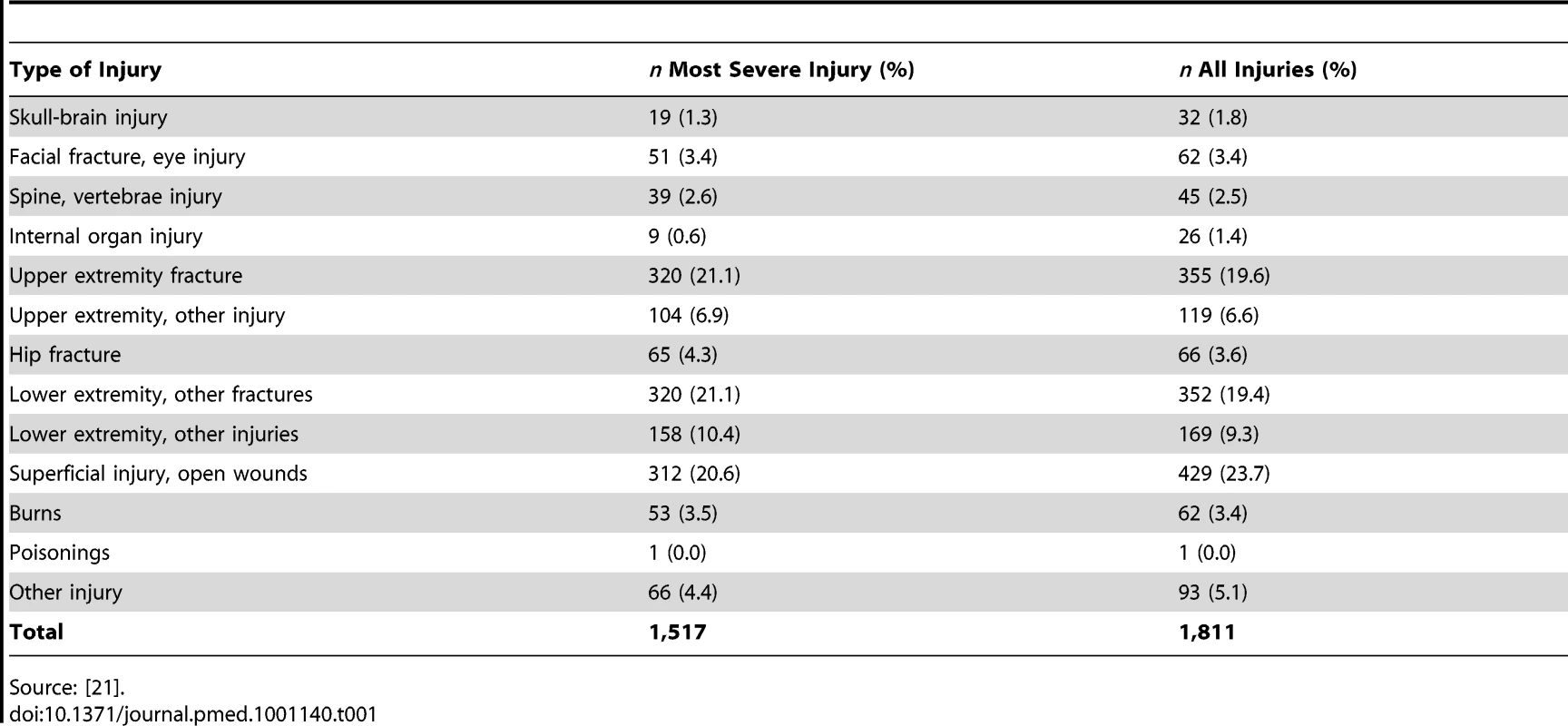
Source: [21]. Exclusion criteria were: children under 5 y (due to lack of suitable measurement instruments), those without permanent UK addresses, those who could not give consent and were without a suitable proxy, and those suffering from stings or insertion of foreign bodies in the ear.
Incentives were used to maximise recruitment and retention (£2 vouchers and a 1∶100 chance of a £100 raffle prize at each follow-up point).
Participants were recruited between September 2005 and April 2007. They were followed up until recovery or 12 mo, whichever occurred soonest. Additionally, censoring of participants occurred at refusal to complete the follow-up or death. Two reminders were sent to participants, with participants able to respond even if a previous time point was missed. Participants who did not respond to the full questionnaire were sent a shortened questionnaire, which asked whether they had recovered or not from their injuries. This study received multi-centre research ethics approval from Dyfed Powys National Health Service Local Ethics committee (number 05/WMW01/23). Sequential patients meeting the inclusion criteria and providing informed consent were recruited.
Variables
Data were collected by self-administered questionnaires at recruitment, and at three fixed times, 1, 4, and 12 mo postinjury, comprising data on socio-demographic, economic, and occupational characteristics, data on injury characteristics, use of health and social services, time off work, recovery from injury, and standardised tools to measure health-related quality of life (HRQL) EQ-5D [20], Health Utilities Index (HUI) [21], PedsQL [22],and work problems (Work Limitations Questionnaire) [23]. Data were also collected on injury mortality, hospital admissions, and ED attendances as described below.
Bias
Sequential patients meeting the inclusion criteria and providing written informed consent were recruited. In order to prevent biases due to inability to consent to participation (e.g., more serious injuries or those in substantial pain) the inclusion criteria allowed for recruitment up to 2 wk postinjury for ED-treated injuries only and 4 wk for those individuals who were hospitalised. Participants who were unable to provide consent but had a suitable proxy who could assent on their behalf were included in the overall study but were excluded from this component as quality of life measures cannot reliably be obtained by proxy [24]–[27].
The impact of participation bias was assessed by comparing subsequent health service use between recruited participants and potential participants (eligible but not recruited and with similar injuries) in the three most common categories of injury (upper extremity fractures, lower extremity fractures, and superficial injuries and open wounds), subdivided by admitted and nonadmitted patient status. Sensitivity analyses were undertaken to evaluate the effect of losses to follow-up on population estimates of the burden and to assess the impact of response biases on EQ-5D summary scores at different time points. Analysis of variables associated with response at 1 mo was undertaken but not at 4 and 12 mo as it would not be possible to determine whether variables were associated with response or earlier recovery at these time points. Analysis of the impact of missing values on EQ-5D summary scores at different time points was undertaken for a range of assumptions: (a) analysis of complete cases only; (b) analysis using baseline EQ-5D for missing cases if they had returned to normal; (c) analysis using last EQ-5D value when reported recovery for missing cases if they had returned to normal; and (d) analysis using multiple imputation of EQ-5D values in all nonresponders, including recovered cases.
Study Size
The planned study size of 1,333 (334 per centre) was designed to recruit a minimum of approximately 15–20 per age-specific injury category (see protocol [17]). This number was based on pragmatic grounds, reflecting injury incidence, a feasible recruitment time period, and available research funding rather than a formal statistical sample size calculation.
Data Sources/Measurement
Mortality data
Population mortality data are required to calculate the YLL component of DALYs. Tabulated data on registered deaths in 2005 were available separately for England and Wales, Scotland, and Northern Ireland, and individual-level data were available for Wales. These data were combined to produce population-level mortality data for the UK [17].
Hospital admission and ED data
The incidence of nonfatal injuries is necessary to quantify the YLD component of DALYs. Population data on incidence of nonfatal injuries are not available in the UK, as elsewhere, and individual-level data on attendance for treatment at free to use National Health Service EDs and admission to hospital were used with population demographic data to estimate injury incidence. Individual-level data for ED attendances were obtained from five of 13 EDs in Wales and extrapolated to provide UK estimates using a previously developed extrapolation factor [28]. Extrapolating from a sample of EDs to provide a national estimate of injury incidence is a challenging task due to difficulties in assigning catchment areas to derive appropriate denominator data [29]. Five of 13 Welsh EDs participated in an injury surveillance system in 2004. Information on aggregate counts of total attendances at all EDs is available from a Welsh Government system [28]. Comparison between the detailed information from the five hospitals (126,557 injury-related attendances among 232,211 total attendances in 2004) and the aggregate information on all attendances for all hospitals (755,070) produced an extrapolation factor of 3.25 to multiply the cases from the five hospitals to derive incidence numbers and rates for Wales and the UK, using national population denominator data [28]. One approach to validating the extrapolation factor is to compare observed and expected numbers of cases in inpatient data where the expected number comes from the sample of ED cases and the observed number from nationally collected inpatient data. However, many factors affect the likelihood of hospital admission following an injury, the majority unrelated to the severity of the injury [30]. Since ED systems do not routinely collect injury severity measures it is difficult to determine what categories of serious injuries always lead to admission, with the exception of hip fractures [31]. The 3.25 extrapolation factor estimated that there would be 4,071 hip fractures from the ED data for Wales, which compares with an observed of 4,058 recorded in the inpatient database, providing confidence in the accuracy of the extrapolation [28].
Individual-level admissions data for Wales and England were obtained from the Patient Episode Database for Wales (PEDW) and the Hospital Episode Statistics (HES) databases. Inpatient data for Scotland and Northern Ireland (11.3% of the UK population) were not available within the resources of the study and the UK incidence of hospital admissions was extrapolated from the England and Wales rates.
Development of the injury weights for injury groups for YLD calculation
The ED and ICD-10 codes were mapped to the 13, 33, and 44 nature of injury categories used in the GBD Study and in previous Dutch burden of injury studies [3],[32],[33]. A greater proportion of ED cases (91%) and inpatient cases (87%) could be mapped to the Dutch 13-group classification [32], compared with 67% and 78%, respectively, for the 33 categories of the GBD study [3], and 66% and 58% for the 44 categories in the Dutch panel study [33].
The primary outcome measure to derive disability weights was the EQ-5D HRQL measure [20], recommended for injury disability studies [34]. The EQ-5D contains five questions, producing up to 243 health state permutations. These permutations are combined with population preference values from expert-panel–based health state valuation and modelling to yield a utility score ranging from <0 (representing a state worse than death) through to 1 (1 implies perfect health), with a score of zero representing a state equivalent to death [35]. The UK EQ-5D tariffs were used to calculate the utility score [36]. The EQ-5D was administered at recruitment (baseline), and at 1, 4, and 12 mo postinjury as described above. A retrospective assessment of preinjury status was obtained at baseline by asking the participant to complete the EQ-5D considering the day prior to injury.
In the context of this study the disability weights represent the difference in EQ-5D utility summary scores derived from subtracting post from preinjury scores in participants who reported at follow-up that they were still affected by their injury. Preinjury EQ-5D scores were also compared to age - and gender-matched population norms for the UK [36]. Once participants reported recovery, no further follow-up was undertaken and recovery was assumed to be complete and permanent. Such participants were given a disability weight of zero at all subsequent follow-up points. When a participant reported recovery between assessment periods, we assumed recovery occurred midway between assessment periods. Time-weighted annualised mean disability weights were calculated for the first year following injury derived from EQ-5D differences from baseline, accounting for the different follow-up periods. The annualised means were derived by estimating the difference in EQ-5D for all participants in any injury group over each of the three time periods (0–1 mo, 1–4 mo, and 5–12 mo), then dividing that by 12 and multiplying by the number of months since the previous follow-up, and summing across the three results to provide a time-weighted average over the first year postinjury. In a small number of cases (n = 12 at baseline and n = 13 at 12 mo) EQ-5D scores were <0. These scores were included in the analysis. In a number of cases (n = 53 at 1 mo) the differences were negative, i.e., higher at follow-up than baseline. These scores were also included in the calculation of average differences for the injury groups. Residual disability at 12 mo, measured by difference between preinjury and 12 mo EQ-5D scores, was considered permanent.
Statistical Methods
The GBD Study approach for calculating YLLs was adopted, incorporating a 3% discount rate and age weighting, using the published formulae [3]. The published tables for calculating YLLs produced by the GBD were by single year of age; however, UK mortality data were published by 5-y age groups requiring calculations of YLLs based on midpoints within categories. Mean annualised disability weights for the 13 injury groups were calculated by time weighting the responses at the 1-, 4-, and 12-mo data collection points. These weights were then applied to population metrics of injury incidence to calculate YLDs. Population-level YLDs and DALYs were compared using the UKBOI-derived disability weights and the standard GBD approach [3].
In the largest centre (Swansea), data from ED and inpatient injury surveillance systems were available and were used to assess potential response biases by comparing health service use subsequent to the injury. Poisson regression was used to estimate incidence rate ratios (and 95% CI) comparing admission rates, outpatient department attendance rates, and ED attendance rates in the year following injury between participants and nonparticipants. Analysis of variance was used to compare the differences between recorded “preinjury” EQ-5D scores and UK population normative data for the same age group and gender category by duration of delay between recruitment and injury occurrence (0, 1, 2–6, 7–14, and 15–28 d) [36]. Sensitivity analyses were also undertaken, using an extreme case scenario that assumed all nonresponders had recovered at time of last follow-up and suffered no residual disability. Logistic regression was used to identify variables independently associated with response at 1 mo. Repeated measures models (generalised estimating equations) were used to compare response (yes or no) at 1, 4, and 12 mo with those who had recovered at earlier time points counted as responders at later follow-up.
Deviations from, and Extensions to, the Published Protocol
Target recruitment was exceeded by 184 participants as this was achievable in the recruitment period and increased precision of estimates. The majority of the 1,517 participants (n = 1,305, 86%) suffered a single injury. The only notable variations from this figure were for two out of the 13 injury categories: skull/brain injuries where the numbers of single injuries were nine out of 19 (47%); and three out of 9 (33%) for internal organ injuries. Among the ten patients with skull/brain injuries who had more than one injury there were 21 additional injuries, of which 16 were also injuries to the skull/brain. Among the six patients with a primary internal organ injury there were five with fractured ribs, and one case each of tibial, nasal, and orbital floor fractures. Patients were categorised by the most severe injury for analysis purposes. The ICD-10 codes were mapped to the Abbreviated Injury Scale, which provides a severity score for each injury on a scale from 1 (minor) to 6 (maximum) [37].
As the highest proportion of participants' injuries could be matched to the Dutch 13-group classification this categorisation was used to combine routine morbidity data with that from the prospective part of the study to estimate population metrics [32]. Categorisation of injuries for this purpose had not been described in detail in the original published protocol [17].
Results
Participants
A total of 1,517 participants participated in the prospective study, with a response rate of 66%. 537 (35.4%) were recruited from the Swansea site, 24.7% from Nottingham, 22.0% from Guildford, and 17.9% from the Bristol site. The median age was 37.4 y (interquartile range 20.6–60.6) and 53.9% were male. The vast majority of injuries were unintentional (91.9%) with 3.5% intentional, 1.9% of uncertain intent, and 2.7% unknown. Home was the most frequent location of injury (34.8%) followed by road traffic injuries (20.6%); 44% were admitted to hospital. Table 1 shows the distribution of cases by the 13 injury categories. It was possible to map all but 66 (4.3%) of the most severe injuries to the 12 specific categories of injury in the 13-category Dutch classification [32]. In contrast it was not possible to map injury data on 267 participants (17.6%) to the 33 GBD categories and 413 (27.2%) to the alternative 44 Dutch disability categories [3],[33].
At 1 mo, 985 participants returned questionnaires (963 full questionnaires and 22 shortened questionnaires) of whom 685 (70%) were still affected by their injury. At 4 mo the figures were 544 (79%) with 375 (69%) still affected, and at 12 mo 323 (86%) with 230 (71%) still affected. 70% (n = 691) of “preinjury” assessments of EQ-5D scores were collected within 7 d of the injury. The study sample mean was 3.3% (95% CI 1.9%–4.7%) higher than the UK population, comparing preinjury EQ-5D scores with age - and sex-matched UK population norms [36]. This difference was not affected by variations in time to collect the “preinjury” data (p = 0.4). Among the 963 responders with full questionnaires at 1 mo, 863 (90%) answered all EQ5D questions.
Outcome Data
Table 2 shows the time-weighted disability weights over the 12 mo for hospitalised and nonhospitalised cases. Hospitalised cases had substantially higher weights than those not hospitalised. A common mean weight was applied to a number of the injury groups in which the weights were all low, similar, and involved small case numbers.
Tab. 2. Time-weighted annualised disability weights for the 13-injury group classification by hospitalisation status. 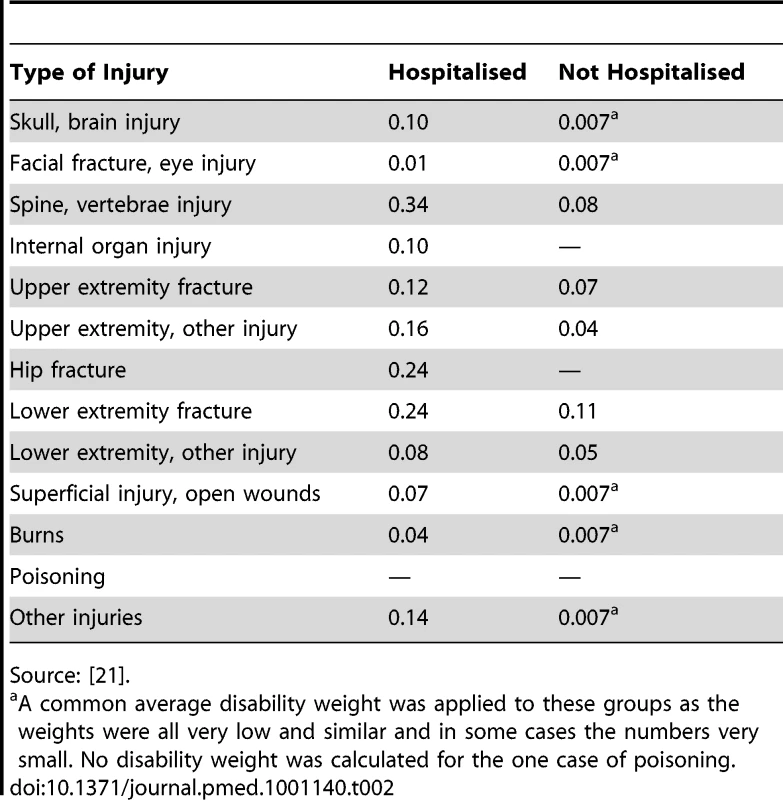
Source: [21]. A disability weight was not calculated for the single case of poisoning.
Main Results
Injury incidence
Inpatient data for England and Wales identified 665,986 injury-related admissions in the year 2005/2006. Table 3 shows the extrapolated number of admissions (750,999) and ED attendances in the UK by the 13 injury categories. Estimated numbers of hospitalised injuries were subtracted from estimated numbers of ED attendances by each injury group to approximate the number of ED injuries not admitted to hospital. The most frequent causes of admissions were fractures (32.8%), poisoning (18.4%), and superficial injuries/open wounds (19.2%).
Tab. 3. Estimated UK number and population rate of injury admissions, and ED-treated only cases extrapolated from five hospitals in the Welsh Injury Surveillance System in 2005/2006, by the 13 injury group classification. 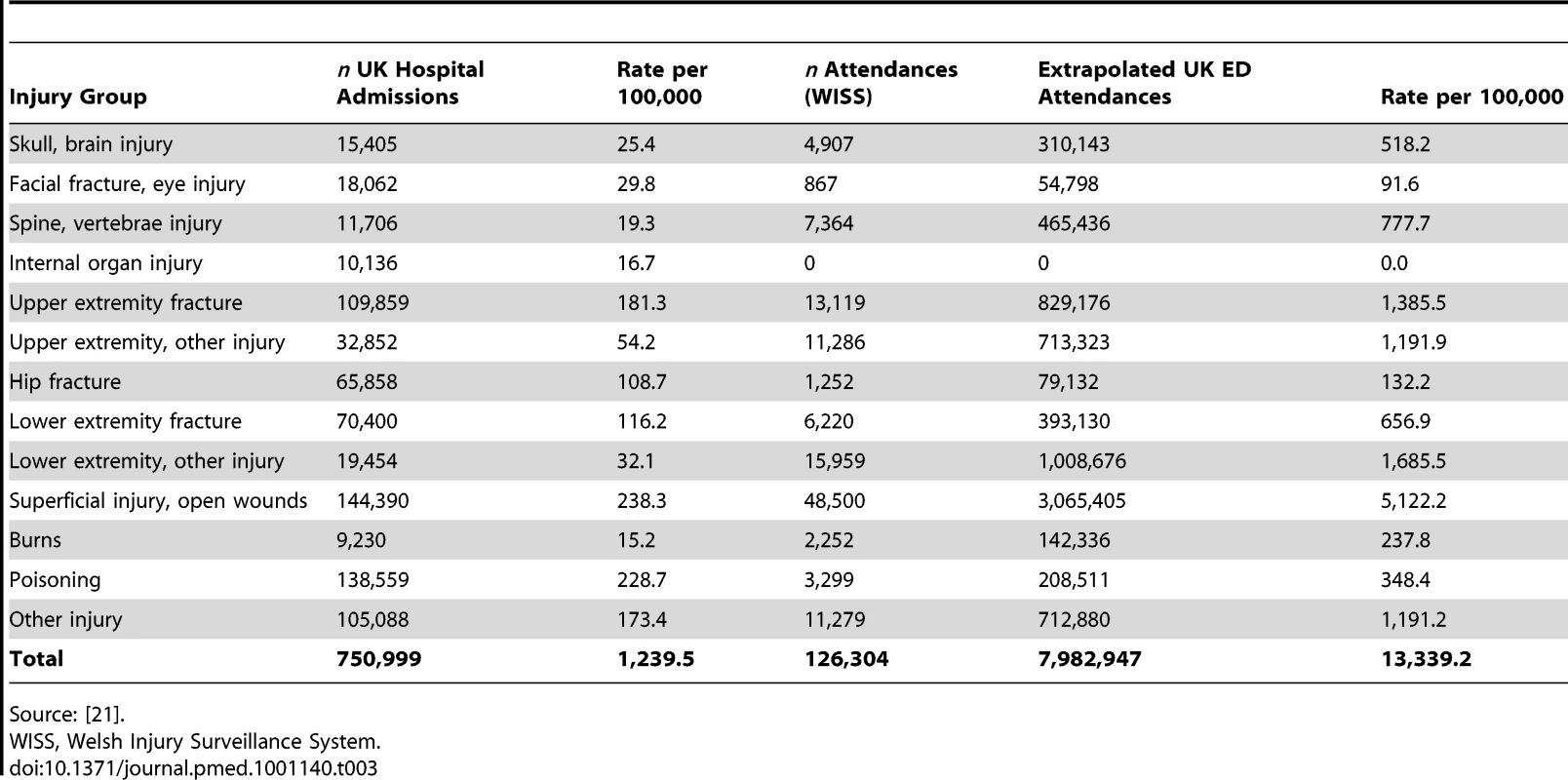
Source: [21]. YLDs
Table 4 shows the annual UK estimated population-level YLDs (1,450,765) by injury group and hospitalised status using the UKBOI disability weights; 67% were attributed to nonadmitted cases.
Tab. 4. Annual UK-estimated population-level total YLDs using UKBOI disability weights for the 13-injury classification and overall, and proportion from hospitalised cases. 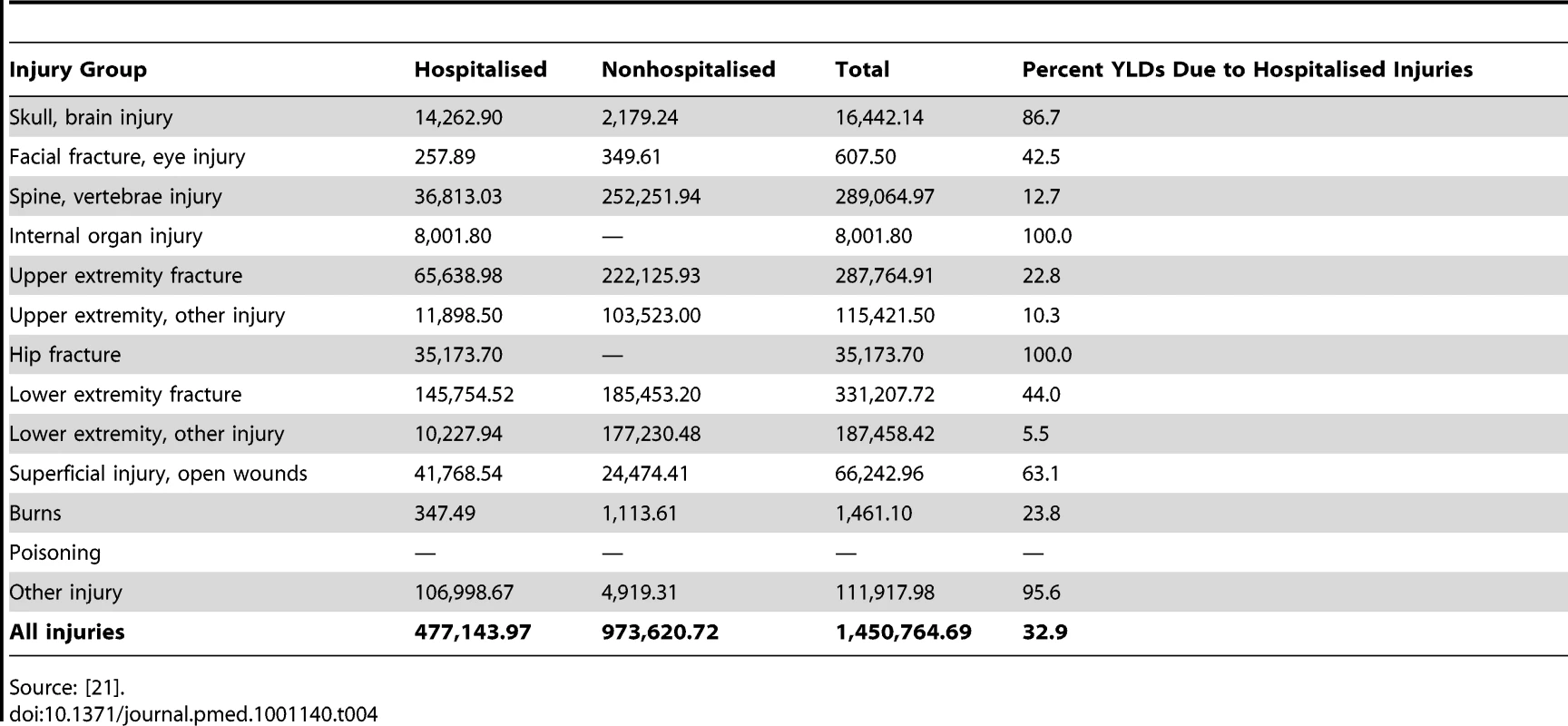
Source: [21]. Table 5 shows population-level YLDs for England and Wales by the 33 GBD injury groups using the GBD disability weights for hospital admissions only, as mapping was not possible for nonadmitted patients owing to a lack of ICD10 codes in ED datasets. The number of YLDs was 101,788 for England and Wales and extrapolated to 114,817 for UK hospitalised injuries. This number compared with a figure of 477,144 using the UKBOI disability weights. Assuming that a similar proportion of GBD and UKBOI category cases were admitted, and the relative difference in disability metrics between admitted and nonadmitted cases was the same, there should be an additional 234,284 nonadmitted YLDs in the GBD group, yielding a total of 349,101 for combined admitted and nonadmitted cases. This is a 76% lower estimate than the total of 1,450,765 YLDs using the UKBOI disability weights.
Tab. 5. Annual estimated population-level YLDs following hospital admission for injury in England and Wales by the 33 GBD injury groups, derived from GBD disability metrics. 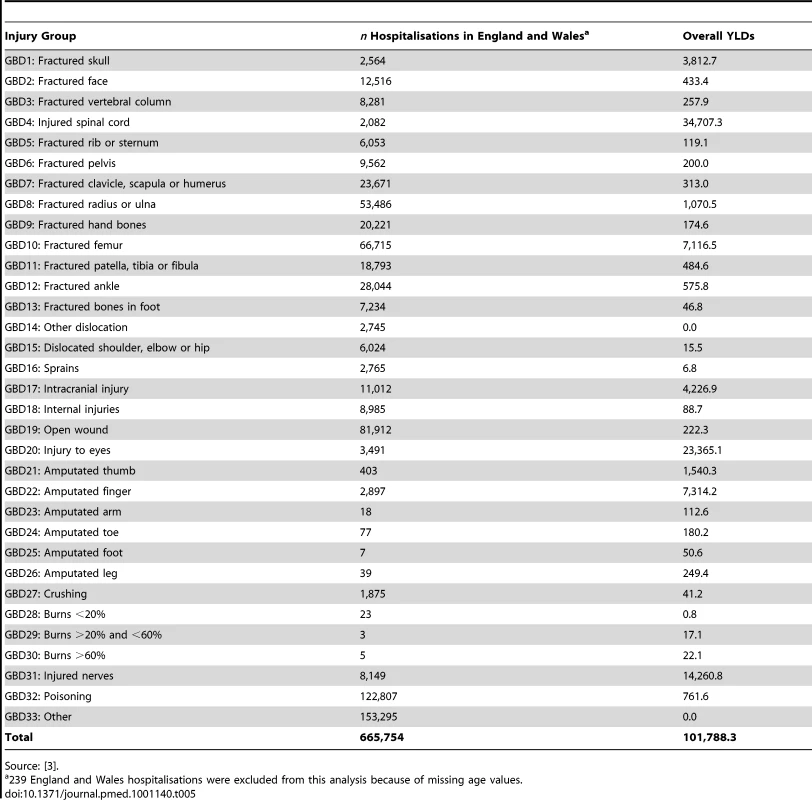
Source: [3]. DALYs
In 2005, there were 22,185 injury-related deaths across the UK, producing 320,721 YLLs. The UKBOI adopted the GBD methodology for YLL calculation and so the numbers are the same for both approaches. Summing YLLs and YLDs to produce DALYs revealed that hospital-treated injuries occurring in the UK resulted in an estimated 1,771,486 DALYs using the UKBOI approach. The GBD methodology for YLDs and YLLs for the UK produced 669,822 DALYs, a 62% lower estimate than the UKBOI approach. The 2004 World Health Organization update of the GBD estimated that injury accounted for 12.3% of 1,523 million DALYs [6]. Applying the 2.6-fold relative increase in DALYs from the UKBOI study would increase the global share of injury DALYs to approximately 27%. Sensitivity analyses adopting the conservative approach of assuming all nonresponders had recovered at time of last follow-up and suffered no residual disability reduced the estimate of UK YLDs by 48% (764,845) and DALYs by 39%.
Representativeness of the Study Sample
The results of the analyses of subsequent health service utilisation between participants and potential participants for the three commonest injury groups are shown in Tables A1–A6 in Text S1. There were no significant differences in rates of subsequent health service use between participants and nonparticipants admitted with upper or lower limb fractures. There were a number of significant differences between participants and nonparticipants with nonadmitted fractures and superficial injuries and wounds, with higher rates of subsequent health service use amongst participants than nonparticipants. For example, for nonadmitted upper arm fractures the incidence rate ratio (IRR) for subsequent admissions was 2.86 (95% CI 1.46–5.52) and 1.40 (95% CI 1.19–1.66) for outpatient attendances. For nonadmitted lower limb fractures the IRR for outpatient attendances was 1.47 (95% CI 1.13–1.90). For nonadmitted superficial injuries and open wounds the IRRs were 2.26 (95% CI 0.98–5.19) for admissions, 2.38 (95% CI 1.13–5.0) for outpatient attendances.
The results of analysis of factors associated with retention at 1 mo are shown in Table A7 in Text S1. Retention was higher for those aged 45–64 y (odds ratio [OR] 2.1) and lower (OR 0.5) for those aged 15–24 y, and lower in the more deprived communities (OR 0.48 in most deprived quintile). ORs for retention of most injury types were higher than for superficial injuries.
The results of different approaches to the analysis of the impact of missing data on EQ-5D summary scores are shown in Table A8 in Text S1. Imputed values were similar to those obtained using substitution methods, including that used for the analyses presented in this paper, but were higher than those based on a complete case analysis with EQ-5D mean summary scores being 3.2% higher at 1 mo, 8.2% higher at 4 mo, and 11.6% higher at 12 mo. The mean annualised disability weight in our main analysis, which assumed that those who had recovered returned to their baseline EQ5D, was −0.10. Imputing values for all nonresponders including recovered cases who were not sent further follow-up questionnaires resulted in a mean annualised disability weight of −0.11.
Discussion
Key Results
The results of the UKBOI study show that combining empirical data from injured individuals with multiple sources of incidence data produces much higher estimates of population burden of injuries than the GBD study methodology, the standard to date. A number of reasons are behind this finding, but key amongst them is the issue of substantially higher disability weights derived from reports of injured patients compared with those derived from uninjured panels. Despite a relatively low injury mortality rate based on international comparisons, injuries in the UK result in a substantial population burden in terms of disability and premature mortality [38]. This study is the first, to our knowledge, to report that the burden of injury from disability is larger than that from death.
Valid estimation of the burden of injury is critical for accurate ranking of injury as a global public health issue, prioritisation of prevention efforts, policy development, and health service planning [1],[2]. A notable limitation of the GBD Study methodology was the reliance on panel and expert opinion to derive disability weights rather than using empirical data from injured populations [3],[16]. While other studies have developed alternative disability weights [33],[35] and utilised disability weights derived from patient experiences to estimate YLDs, the UKBOI study has extended the application of patient-derived disability weights and multiple routine data sources to produce what appears to be the most comprehensive population-based burden of injury study to date using the DALY method [17]. We compared results with other published studies producing population-level DALYs for all injuries [6],[8],[10],[11],[13],[14] and used a recent systematic analysis of studies measuring HRQL in general injury populations to ensure we did not miss studies [39].
Combining data from the UKBOI prospective study of injured individuals with the best available routine data on ED and inpatient treatment for injuries and mortality data produced an estimated 1.77 million injury-related DALYs for the UK from injuries occurring in 2005, a rate of 2,924/100,000 population. The DALY total comprised 320,721 YLLs (18%) and 1,450,764 YLDs (82%). Nonhospitalised cases amounted to 973,620 YLDs, 67% of the YLD total. These estimates are substantially higher than published studies using the original GBD methodology [7]–[14]. Table 6 shows the YLL, YLD, and total DALYs per 100,000 population reported by published studies where sufficient information was provided to calculate a rate per 100,000 population. There are difficulties in comparing burden of injury studies as many did not declare whether age weighting and discounting were used. Nevertheless, the YLD estimate from the current study is 2.9 times higher than the next largest estimate from Austria in 1999 and the UKBOI is the first to report that the majority of the injury burden relates to disability rather than death (Table 6). There are several major differences that could explain these findings.
Tab. 6. Estimates of injury-related YLDs, YLLs, YLL;YLD ratio, and DALYs per 100,000 population from published studies and the UKBOI Study. 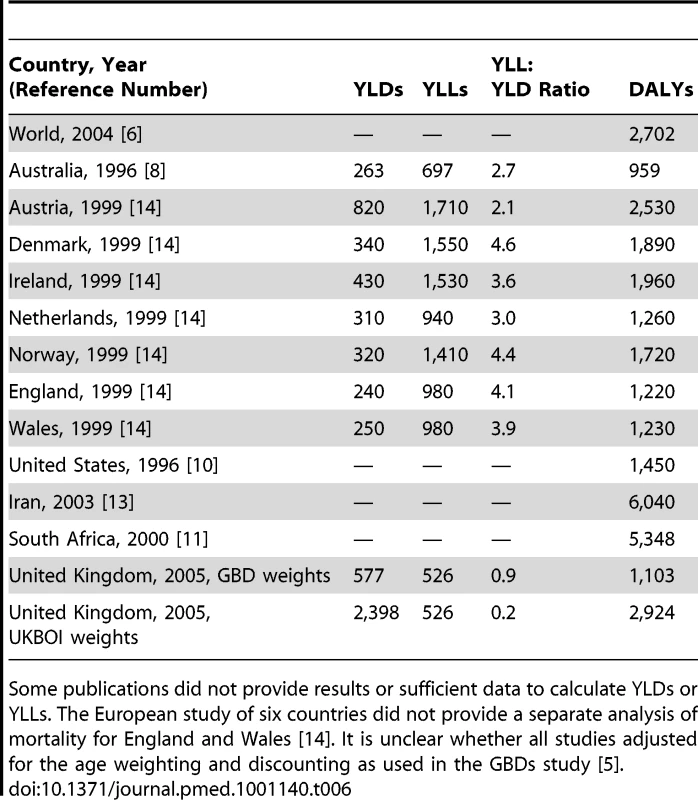
Some publications did not provide results or sufficient data to calculate YLDs or YLLs. The European study of six countries did not provide a separate analysis of mortality for England and Wales [14]. It is unclear whether all studies adjusted for the age weighting and discounting as used in the GBDs study [5]. Injury groupings
The current study mapped injuries to the 13-group classification described by Meerding et al. because a sizeable proportion (22%–40%) of injury attendance and admission data cannot be mapped to the 33 categories of the GBD Study or the 44 categories derived by another Dutch panel study, thus excluding these injuries from burden estimates [3],[32],[33]. Although 9% of ED cases and 13% of inpatient cases could not be mapped to specific categories using the 13-group classification, these could still be included in UKBOI YLD estimates under the “other injury” category, ensuring that important injury diagnoses were not lost from burden estimates.
A further related issue is the ability to map data from the longitudinal study to injury categories to derive average disability weights to be applied to the routine data to estimate the population burden. It was possible to map all but 66 (4.3%) of the most severe injuries to the 12 specific categories of injury using the Dutch 13-group classification [32]. In contrast it not possible to map injury data on 267 participants (17.6%) to the 33 GBD categories [3]. There is no GBD disability weight for “other” and, hence, these injuries do not contribute to the calculation of population-level YLDs. The fact that this study shows the difficulties of mapping to the limited GBD categories is useful in highlighting the gaps in the GBD categories and informing their revision.
Disability weight generation
Disability weights in the UKBOI study were derived from the experiences of 1,517 injured individuals, while the GBD and Dutch panel study weights were derived from panel valuation exercises [3],[33]. Panel studies require groups without the injury experience to value the impact of the injury using short vignettes of typical impact and duration provided by medical experts [33],[35]. While the Dutch panel study weights have been shown to result in greater YLDs than the GBD study [33], providing an improvement on the original GBD study weights, a comparison of disability weights derived using EQ-5D scores from 1,392 patients with lay panel-derived disability weights found that, for all but one injury health state, the patient-derived weights indicated greater disability than the panel-derived weights [35]. The authors favoured the panel-derived weights, concluding that the patient-derived weights result in “overestimation” of the disability resulting from more minor injuries, due to the potential for reporting bias and differences between self-reported health status and “actual” health status [35]. However, self-reported health status is the patient's health status as the EQ-5D and other measures of HRQL are designed to reflect the patient's perceptions of their health, making patients the most reliable witness of their health status.
Additionally, apart from fundamental differences in deriving values obtained by those who have and have not experienced injuries, there is the possibility that the standard vignettes do not portray an accurate reflection of the impact and duration of injuries, a limitation previously acknowledge by panel-based studies [35]. Summarising the course of an injury in lay terminology, while adequately addressing the variability in recovery that occurs related to personal and injury severity factors, is difficult [35]. The use of lay panels to derive disability weights is also time consuming and expensive, limiting the number of health states for which disability weights can be generated [33],[35].
Comparison of disability weights with other studies is difficult due to differing injury categories. However, the upper and lower extremity fractures subgroups were comparable in the UKBOI, GBD, and Dutch panel studies. The weights for upper extremity fractures were 0.12 and 0.07 for admitted and nonadmitted fractures in the UKBOI study, compared with 0.02 and 0.04 for the GBD and Dutch panel studies (undifferentiated by admission status) [3],[33]. For lower extremity fractures the respective figures were 0.24 and 0.11, compared to 0.02 and 0.06 (admitted cases) [3],[33]. The UKBOI figures are substantially higher than the GBD and Dutch study metrics. Consistent with the previous Dutch study the most likely explanation for this is the discrepancy between the experience of actual patients and that portrayed in the standardised vignettes of the impact and duration of injuries presented to panel studies [35]. Certainly, the duration of impact in such vignettes (often described as a number of weeks) does not appear to accurately reflect the experience of many patients [35].
Long term disability
Related to the derivation of disability weights are differences in the proportion of cases with life-long impact. The GBD Study assumed that only certain injury categories had life-long impact and these were mainly the more severe and rarer categories, such as major burns, amputations, and spinal cord injuries [3]. Head injuries and hip fractures were the exception for more common injuries and it was assumed that 15% of cases of fractured skulls, 5% of intracranial injuries, and 5% of hip fractures had life-long impact. In the UKBOI study, disability at 12 mo was assumed to be life long, which is consistent with existing literature for injury studies [40],[41]. In the UKBOI study, 15% of recruited participants were still affected at 12 mo for all injuries; 32% of hip fractures, and 23% of other lower extremity fractures. The high rate of residual impairment at 12 mo and the associated assumption of life-long disability has a substantial effect on population metrics, with 62% of population YLDs in the UKBOI occurring after the first 12 mo. There are a couple of studies limited to major trauma patients, which show some improvement in outcomes after 1 y, another that shows worsening of health status in the longer term; but it is not possible to use these selected groups to refine our estimates across a broad range of injuries [42]–[44]. There is a need for further longer term studies to clarify longer term outcomes.
Data sources and quality
Another difference between the UKBOI study and others was the greater availability of data on injury incidence, a well-recognised problem in burden of disease measurement [45]. The GBD study suffered substantially from a lack of morbidity data and thus could only produce estimates for major regions and economies of the world [3]. The Australian Burden of Disease and Injury Study used the GBD approach and estimated that injuries accounted for 7.1% of the 2.5 million DALYs in Australia in 1996, a population rate of 9.7/1,000, contrasting with the UKBOI estimate of 29.2/1,000 population [8]. Whether the difference is due to the more extensive morbidity data or the use of empirical rather than the GBD Study disability weights used in the Australian Study is unclear. A study of six western European countries adopted the GBD weights and reported a DALY rate of 12.2/1,000 for England and Wales, much lower than the UKBOI estimate [14]. Whilst there were some differences in the handling of morbidity data, the use of the GBD disability weights is likely to be the primary reason for the difference in population-level DALYs observed. The 1996 US Burden of Disease and Injury Study (USBODI) derived DALYs from a mixture of national surveys, hospital discharge data, disease registers, and extrapolation of YLDs from YLD∶YLL ratios for the established market economy (EME) countries from the GBD Study where data were unavailable [4],[10]. This study estimated a US population rate of injury DALYs of 14.5/1,000, again considerably lower than the UKBOI estimates. The difference is likely due to the use of the GBD weights, but insufficient published methodological information precludes a detailed comparison.
Both the UKBOI Study and a US study on injury costs demonstrate the importance of ED data in estimating aspects of the population burden of injuries [9]. Whilst disability weights in hospitalised cases in the UKBOI were two to ten times greater than in nonhospitalised cases (Table 2), the greater incidence of nonhospitalised cases meant that nonhospitalised cases contributed two-thirds of YLDs.
Limitations
As with every study, case selection and losses to follow-up may have introduced bias.
Recruiting patients to studies in emergency settings is challenging given ethical and other constraints [46]. The ethics committee that approved this study required potential participants to first be approached by health service staff who then sought consent for them to be approached by the researchers. The researchers were based in the ED and hospital wards. The response rate (66%) to being approached could be monitored only for the first 2 wk of the study because of the logistical difficulties of this requirement. Whilst this response rate is higher than in other studies it is far from optimal. Undoubtedly, the response rate would be higher if researchers were able to approach patients directly. Despite the use of £2 vouchers and £100 raffle prizes as incentives at each follow-up point, 20%–35% of respondents were lost at different follow-up points. However, the retention rate at 1 mo (65%) was higher than the use of mail-out recruitment in the previous Dutch study (37% at 2.5 mo), whereas losses at other time points were similar between studies [32]. However, the findings derived from the record linkage follow-up component at the Swansea site suggest that there was no major bias in the representativeness of admitted patients recruited to the study, but that nonadmitted participants tended to have more severe injuries than nonparticipants, as judged by differences in subsequent health service utilisation in comparator groups. Further research is needed to quantify the strength of the relationship between health service utilisation and disability before such information could be used to adjust results quantitatively.
We also attempted to quantify the potential impact of losses to follow-up. Adopting the very conservative approach of assuming all losses to follow-up were fully recovered would reduce our estimate of population-level YLDs by 48% and DALYs by 39%, but our estimates would still be 1.6-fold higher than those estimated using the GBD approach [4].
A variety of approaches were used to model the effects of missing data (Table A8 in Text S1). Imputed values were similar to those obtained using substitution methods but were higher than those based on a complete case analysis with EQ-5D mean summary scores being 3.2% higher at 1 mo, 8.2% higher at 4 mo, and 11.6% higher at 12 mo. There was little difference in the mean annualised disability weights resulting from assuming that those who had recovered had returned to their baseline EQ5D (−0.10) and from multiple imputation of missing values (−0.11). This finding suggests taking account of missing data would not result in substantial under - or overestimation of the burden of injury.
A further methodological choice made was to limit follow-up at additional time points to only those who reported disability at the previous time point. A previous study found deterioration in health status over time for some patient groups, but failed to quantify the proportion of patients who deteriorated and recommended cautious interpretation of these findings because of the inability to determine the impact of comorbidities and other factors on the health status of patients [47]. Where deterioration occurs, it is difficult to ascribe this to the original injury, a subsequent injury, or the development of a related or unrelated comorbidity. Overall, the impact on the disability weights and overall estimates is unlikely to be large, but warrants noting as a limitation of the study.
We also lacked individual level routine data for various aspects of the study. Estimates for ED-treated injuries were based on data from only five Welsh hospitals. Whilst the agreement between observed and expected numbers for hip fractures suggests the wider extrapolation was accurate, the possibility that injury patterns in Wales differ from the rest of the UK remains. However, as mortality rates differ by only about 5%, the bias is unlikely to be major. Whilst the inclusion of limited ED data was a strength of this study, many people with injuries do not seek ED treatment despite being associated with substantial disability [48]. Thus, the population burden will be even greater than our estimates, an issue with any study that uses medically attended injuries to measure incidence. However, response and recall biases inherent in surveys means that it may not be possible to accurately measure the true population incidence of injuries [49].
Poisoning is included in the list of ICD codes used to define injuries for international studies [18],[19]. We excluded disability from poisoning in the calculation of YLDs (but not YLLs) as there was only a single case included in the longitudinal study. Table 5 shows that poisoning accounted for 122,807 hospital admission in England and Wales in 2005, 18.4% of the total for injuries and poisoning. It is difficult to assess the effect of this exclusion but it is probably not too large in the UK context where the most common poisoning agents (e.g., paracetamol) rarely lead to mortality or long term physical damage [50]. This situation would not be the case in other settings where the use of more corrosive and toxic agents is much more prevalent.
As in many studies it was not possible to accurately determine whether death was a reason for nonresponse. In the Swansea site where a record linkage system was subsequently put in place, we recorded seven deaths within 12 mo among 468 traceable participants. Hence we would expect about 22 to 23 deaths in the entire study; however, as these are not detectable amongst all the cases of nonresponse they were treated as losses to follow-up. This finding highlights another important methodological issue common to all burden of injury studies. Measurement of injury-related DALYs assumes that population-level estimates of injury-related mortality are accurate. However, we know that mortality rates are elevated for long periods postinjury and these delayed injury-related deaths are frequently attributed to other conditions [51]. Thus, routine mortality data underestimate injury-related YLLs.
Measuring the population burden of injuries is a complex task, made particularly difficult by the heterogeneity of injury populations with respect to injuries sustained, severity, and demographic profiles. Deriving disability weights for all injury diagnoses is not feasible for panel or empirical data studies. For example, recruiting just 12 cases for each 5-y age and gender group, for each of the 1,278 ICD-10 injury diagnostic codes requires a sample size of 552,096 cases. In order to produce metrics categorisation of injuries is necessary into logical but possibly heterogeneous groups, and there will inevitably be a considerable degree of heterogeneity in the 13-group categorisation used in this study [32]. At the same time, use of greater number of injury groups, as in the GBD and Dutch panel study, replaces one problem with another in excluding high proportions of patients with injuries [3],[33]. In our study, the disability weights were generated on the basis of relatively small numbers of patients in each injury category. Each disability weight is essentially a time-weighted average of individual responses, providing a challenge for calculating the precision of the estimate of the time-weighted annualised disability weights. While measures of precision could be calculated for each time point (i.e., 1 mo, 4 mo, 12 mo), extrapolation to the time-weighted disability weight was not possible. Work on developing disability weights for a larger group of injuries is being undertaken as part of the GBD revision process. Prospective outcome studies in injury populations are still rare and more are required. Given sample size requirements, cost, and logistical challenges, there is also a need for meta-analyses of the existing individual-level data around the world.
Injured patients consistently report preinjury HRQL above population norms, although this is usually collected retrospectively [52],[53]. There is potential for recall bias in the retrospective reporting of preinjury HRQL. However, a clear consensus has not been reached as to whether the difference between population norms and retrospectively recalled scores is the result of response shift caused by the injury, or simply the injury population being healthier than population norms. A previous study has shown that the HRQL scores for injured patients reporting that they had recovered were consistent with their retrospective preinjury scores [54]. In the current study, whilst preinjury HRQL scores were 3% higher than age - and gender-weighted norms the difference was not affected by the timing of assessment of “preinjury” status within a 4-wk period postinjury and time postinjury. This finding suggests little evidence of bias in the reporting of retrospective preinjury HRQL scores when collected within weeks of injury.
Numerous instruments are available for assessing HRQL following injury. The UKBOI study used the EQ-5D, which has been recommended for injury studies [34]. A comparison of the EQ-5D and the Health Utilities Index (HUI) suggests the performance of these instruments differs according to the patient population study with the authors recommending the HUI over the EQ-5D [55]. However, the differences were noted to be small and inconsistent in pattern, and the EQ-5D resulted in higher completion rates and less expense to implement, supporting the use of the EQ-5D [56].
Interpretation
There are many challenges in estimating the population burden of injuries. The results of this study demonstrate that using disability weight data from a prospective study of injured patients with additional morbidity data sources produces estimates of the population burden of injuries that are considerably higher than previous estimates derived from the standard GBD approach [3],[4]. Whist considerable uncertainties remain, our best estimate is that injury-related DALYs are 2.6 times greater than previously thought, and even if we accept a very conservative approach of assuming no residual disability in all losses to follow-up the population estimate would be 1.6 times earlier estimates.
Generalizability
Whilst this study was carried out in the UK, the principal findings are relevant across the globe. Measurement of the population burden of injuries requires access to high quality morbidity data, which must include sources other than hospital admission data. This need will be particularly the case in less affluent countries where there is often very restricted access to health care facilities. This point has been previously highlighted by the Global Burden of Diseases Injury Expert Group, which published a call in this journal for better access to morbidity and mortality data to improve estimates of the global burden [44]. It is also clear that disability weights and durations derived from injured patients are at considerable variance from those derived from expert panels and that decisions on which to use will fundamentally affect the magnitude of the burden of injury.
Our results suggest that if the pattern of underestimation seen in the UK was mirrored across the world then injuries may account for up to a quarter of global DALYs rather than a sixth as previously estimated [6]. This estimate is not precise as it is sensitive to the relative contribution of the mortality and morbidity components of DALYs and also to improvements and refinements in data and outcome metrics for other diseases and disorders, which might change the overall attribution of DALYs between injury and illness. However, undoubtedly the global proportion of DALYs from injury is larger than previously estimated. Accurate measurement of the burden of injuries is essential to ensure appropriate policy responses to prevention and treatment. There is already evidence that policy and research responses to injury are grossly inadequate, based upon the previous estimates of the burden [57],[58].
Supporting Information
Zdroje
1. Segui-GomezMMacKenzieEJ 2003 Measuring the public health impact of injuries. Epidemiol Rev 25 3 19
2. WiebeDJNanceMLBranasCC 2006 Determining objective injury prevention priorities. Inj Prev 12 347 350
3. MurrayCJLLopezAD 1996 The global burden of disease and injury series, volume 1: the global burden of disease Geneva World Health Organization
4. MurrayCJLLopezAD 1997 Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet 349 1436 1442
5. MathersCDLopezADMurrayCJL 2006 The burden of disease and mortality by condition: data, methods, and results for 2001. LopezADMathersCDEzzatiMJamisonDTMurrayCJL Global burden of disease and risk factors Washington (D.C.) Oxford University Press and The World Bank 45 240
6. World Health Organization 2008 The Global Burden of Disease 2004 Update Geneva World Health Organization
7. HolstagHRvan BeeckEFLichtveldRALeenenLPHLindemanE 2008 Individual and population burdens of major trauma in the Netherlands. Bull World Health Organ 86 111 117
8. MathersCDVosETStevensonCEBeggSJ 2001 The burden of disease and injury in Australia. Bull World Health Organ 79 1076 1084
9. CorsoPFinkelsteinEMillerTFiebelkornIZaloshnjaE 2006 Incidence and lifetime costs of injuries in the United States. Inj Prev 12 212 218
10. MichaudCMMcKennaMTBeggSNielsTMeghnaM 2006 The burden of disease and injury in the United States 1996. Popul Health Metr 4 11
11. NormanRMatzopoulosRGroenewaldPBradshawD 2007 The high burden of injuries in South Africa. Bull World Health Organ 85 695 702
12. StevensGDiasRHThomasKJARiveraJACarvalhoN 2008 Characterizing the epidemiological transition in Mexico: national and subnational burden of diseases, injuries, and risk factors. PLoS Med 5 e125 doi:10.1371/journal.pmed.0050125
13. NaghaviMAbolhassaniFPourmalekFLakehMMJafari1N 2009 The burden of disease and injury in Iran 2003. Popul Health Metr 7 9
14. PolinderSMeerdingWJMulderSPetridouEvan BeeckE and the EUROCOST Reference Group 2007 Assessing the burden of injury in six European countries. Bull World Health Organ 85 27 34
15. World Bank 1993 World Development Report, Investing in health New York Oxford University Press
16. LyonsRA 2008 Measuring the burden of injury. Inj Prev 14 3 4
17. LyonsRATownerEMKendrickDChristieNBrophyS 2007 The UK Burden of injury study - a protocol. BMC Public Health 7 317
18. CryerCLangleyJD 2006 Developing valid indicators of injury incidence for “all injury.” Inj Prev 12 202 207
19. GriffithsCWrightORooneyC 2006 Trends in injury and poisoning mortality using the ICE on injury statistics matrix, England and Wales, 1979–2004. Office for National Statistics. Health Stat Q 32 5 18
20. BrooksR 1996 EuroQol: the current state of play. Health Policy 37 53 7221
21. FeenyDFurlongWBoyleMTorranceGW 1995 Multi-attribute health status classification systems. Health Utilities Index. Pharmacoeconomics 7 490 502
22. VarniJWSeidMRodeCA 1999 The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 37 126 139
23. LernerDAmickBCIIIRogersWHMalspeisSBungayK 2001 The work limitations questionnaire. Med Care 39 72 85
24. AndresenEMVahleVJLollarD 2001 Proxy reliability: health-related quality of life (HRQoL) measures for people with disability. Qual Life Res 10 609 619
25. GabbeBJSimpsonPMSutherlandAMPalmerCSButtW 2010 Agreement between parent and child report of health-related quality of life: Impact of time postinjury. J Trauma 69 1578 1582
26. EricksonSJMontagueEQGerstleMA 2010 Health-related quality of life in children with moderate-to-severe traumatic brain injury. Dev Neurorehabil 13 175 181
27. WeinfurtKPTruccoSMWillkeRJSchulmanKA 2002 Measuring agreement between patient and proxy responses to multidimensional health-related quality-of-life measures in clinical trials: an application of psychometric profile analysis. J Clinl Epidemiol 55 608 618
28. PockettRDLyonsRA 2006 The AWISS - SitReps injury extrapolation factor methodology July, 2006. Available: http://www.capic.org.uk/documents/060730AWISS-SitrepsMethodRPandRL.pdf. Accessed 21 December 2009
29. AlexandrescuRO'BrienSJLyonsRALeckyFE 2008 A proposed approach in defining population-based rates of major injury from a trauma registry dataset: delineation of hospital catchment areas. BMC Health Serv Res 8 80
30. LyonsRABrophySPockettRJohnG 2005 Purpose, development and use of injury indicators. Int J Inj Contr Saf Promot 4 207 211
31. CryerCGulliverPDavieGde GraafBLangleyJ 2010 Empirical validation of the New Zealand injury prevention strategy indicators: the identification of ICD diagnoses associated with a high probability of inpatient hospital admission Dunedin Injury Prevention Research Unit. ISBN: 0-908958-83-8
32. MeerdingWJLoomanCWNEssink-BotMLToetHMulderS 2004 Distribution and determinants of health and work status in a comprehensive population of injury patients. J Trauma 56 150 161
33. HaagsmaJAvan BeeckEFPolinderSHoeymansNMulderS 2008 Novel empirical disability weights to assess the burden of non-fatal injury. Inj Prev 14 5 10
34. Van BeeckEFLarsenCFLyonsRAMeerdingWJMulderS 2007 Guidelines for the conduction of follow-up studies measuring injury-related disability. J Trauma 62 534 550
35. HaagsmaJAPolinderSvan BeeckEFMulderSBonselGJ 2009 Alternative approaches to derive disability weights in injuries: do they make a difference? Qual Life Res 18 657 665
36. DolanP 1997 Modelling valuations for EuroQol health states. Med Care 35 1095 1108
37. GennarelliThomasA Elaine Wodzin (Hrsg.) 2008 The Abbreviated Injury Scale 2005. Update 2008 Des Plaines (Illinois) American Association for Automotive Medicine (AAAM)
38. PedenMOyegbiteKOzanne-SmithJHyderAABrancheC 2008 World Report on Child Injury Prevention Geneva World Health Organisation Available: http://whqlibdoc.who.int/publications/2008/9789241563574_eng.pdf. Accessed 23 October 2009
39. PolinderSHaagsmaJABeltELyonsRAErasmusV 2010 A systematic review of studies measuring health-related quality of life of general injury populations. BMC Public Health 10 783
40. HolbrookTLAndersonJPSieberWJBrownerDHoytDB 1999 Outcome after major trauma: 12-month and 18-month follow-up results from the Trauma Recovery Project. J Trauma 46 765 771
41. Baldry CurrensJCoatsT 2000 The timing of disability measurements following injury. Injury 31 93 98
42. VlesWJSteyerbergEWEssink-BotMLvan BeeckEFMeeuwisJD 2005 Prevalence and determinants of disabilities and return to work after major trauma. J Trauma 58 126 135
43. KaarlolaAPettiläVKekkiP 2003 Quality of life six years after intensive care. Intensive Care Med 29 1294 1299
44. MacKenzieEJBosseMJPollakANWebbLXSwiontkowskiMF 2005 Long-term persistence of disability following severe lower-limb trauma: results of a seven-year follow-up. J Bone Joint Surg Am 87 1801 1809
45. BhallaKHarrisonJAbrahamJBorseNNLyonsR 2009 Data sources for improving estimates of the global burden of injuries: call for contributors. PLoS Med 6 e1000001 doi:10.1371/journal.pmed.1000001
46. KendrickDLyonsRChristieNTownerEBengerJ 2007 Recruiting participants for injury studies in emergency departments. Inj Prev 13 75 77
47. PolinderSvan BeeckEFEssink-BotMLToetHLoomanCWN 2007 Functional outcome at 2.5, 5, 9, and 24 months after injury in the Netherlands. J Trauma 62 133 141
48. BarkerMPowerCRobertsI 1996 Injuries and the risk of disability in teenagers and young adults. Arch Dis Child 75 156 158
49. MockCAcheampongFAdjeiSKoepsellT 1999 The effect of recall on estimation of incidence rates for injury in Ghana. Int J Epidemiol 28 750 755
50. SheenCLDillonJFBatemanDNSimpsonKJMacdonaldTM 2002 Paracetamol toxicity: epidemiology, prevention and costs to the health-care system. QJM 95 609 619
51. CameronCMKliewerEVPurdieDMMcClureRJ 2007 Long-term mortality following injury in working-age adults: a systematic review. Int J Inj Contr Saf Promot 14 11 18
52. WatsonWLOzanne-SmithJRichardsonJ 2007 Retrospective baseline measurement of self-reported health status and health-related quality of life versus population norms in the evaluation of post-injury losses. Inj Prev 13 45 50
53. GabbeBJCameronPAGravesSEWilliamsonODEdwardsER 2007 Preinjury status: are orthopaedic trauma patients different than the general population? J Orthop Trauma 21 223 228
54. WatsonWLOzanne-SmithJRichardsonJ 2007 Retrospective baseline measurement of self-reported health status and health-related quality of life versus population norms in the evaluation of post-injury losses. Inj Prev 13 45 50
55. PolinderSHaagsmaJABonselGEssink-BotMLToetH 2010 The measurement of long-term health-related quality of life after injury: comparison of EQ-5D and the health utilities index. Inj Prev 16 147 153
56. LyonsRA 2010 Measuring quality of life after injury. Inj Prev 16 145 146
57. StucklerDKingLMcKeeM 2008 WHO's budgetary allocations and burden of disease: a comparative analysis. Lancet 372 1536 1569
58. NichollJP 2006 Health research funding: while injuries were ignored. BMJ 332 1510
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 12- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- The Toxic Effects of Cigarette Additives. Philip Morris' Project Mix Reconsidered: An Analysis of Documents Released through Litigation
- The Role of Group Dynamics in Scientific Inconsistencies: A Case Study of a Research Consortium
- Violent Crime, Epilepsy, and Traumatic Brain Injury
- Disclosure of Investigators' Recruitment Performance in Multicenter Clinical Trials: A Further Step for Research Transparency
- Shift Work as a Risk Factor for Future Type 2 Diabetes: Evidence, Mechanisms, Implications, and Future Research Directions
- Estimating the Burden of Malaria: The Need for Improved Surveillance
- Rotating Night Shift Work and Risk of Type 2 Diabetes: Two Prospective Cohort Studies in Women
- Measuring the Population Burden of Injuries—Implications for Global and National Estimates: A Multi-centre Prospective UK Longitudinal Study
- Cognitive Performance in Late Adolescence and the Subsequent Risk of Subdural Hematoma: An Observational Study of a Prospective Nationwide Cohort
- Sex-Specific Immunization for Sexually Transmitted Infections Such as Human Papillomavirus: Insights from Mathematical Models
- A Comprehensive Framework for Human Resources for Health System Development in Fragile and Post-Conflict States
- Absorbable Versus Silk Sutures for Surgical Treatment of Trachomatous Trichiasis in Ethiopia: A Randomised Controlled Trial
- Risk of Violent Crime in Individuals with Epilepsy and Traumatic Brain Injury: A 35-Year Swedish Population Study
- Poor Diet in Shift Workers: A New Occupational Health Hazard?
- The Primacy of Public Health Considerations in Defining Poor Quality Medicines
- Worldwide Incidence of Malaria in 2009: Estimates, Time Trends, and a Critique of Methods
- Surgery Versus Epilation for the Treatment of Minor Trichiasis in Ethiopia: A Randomised Controlled Noninferiority Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Violent Crime, Epilepsy, and Traumatic Brain Injury
- Rotating Night Shift Work and Risk of Type 2 Diabetes: Two Prospective Cohort Studies in Women
- Surgery Versus Epilation for the Treatment of Minor Trichiasis in Ethiopia: A Randomised Controlled Noninferiority Trial
- Worldwide Incidence of Malaria in 2009: Estimates, Time Trends, and a Critique of Methods
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



