-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCorrecting Mortality for Loss to Follow-Up: A Nomogram Applied to Antiretroviral Treatment Programmes in Sub-Saharan Africa
Background:
The World Health Organization estimates that in sub-Saharan Africa about 4 million HIV-infected patients had started antiretroviral therapy (ART) by the end of 2008. Loss of patients to follow-up and care is an important problem for treatment programmes in this region. As mortality is high in these patients compared to patients remaining in care, ART programmes with high rates of loss to follow-up may substantially underestimate mortality of all patients starting ART.Methods and Findings:
We developed a nomogram to correct mortality estimates for loss to follow-up, based on the fact that mortality of all patients starting ART in a treatment programme is a weighted average of mortality among patients lost to follow-up and patients remaining in care. The nomogram gives a correction factor based on the percentage of patients lost to follow-up at a given point in time, and the estimated ratio of mortality between patients lost and not lost to follow-up. The mortality observed among patients retained in care is then multiplied by the correction factor to obtain an estimate of programme-level mortality that takes all deaths into account. A web calculator directly calculates the corrected, programme-level mortality with 95% confidence intervals (CIs). We applied the method to 11 ART programmes in sub-Saharan Africa. Patients retained in care had a mortality at 1 year of 1.4% to 12.0%; loss to follow-up ranged from 2.8% to 28.7%; and the correction factor from 1.2 to 8.0. The absolute difference between uncorrected and corrected mortality at 1 year ranged from 1.6% to 9.8%, and was above 5% in four programmes. The largest difference in mortality was in a programme with 28.7% of patients lost to follow-up at 1 year.Conclusions:
The amount of bias in mortality estimates can be large in ART programmes with substantial loss to follow-up. Programmes should routinely report mortality among patients retained in care and the proportion of patients lost. A simple nomogram can then be used to estimate mortality among all patients who started ART, for a range of plausible mortality rates among patients lost to follow-up.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(1): e32767. doi:10.1371/journal.pmed.1000390
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000390Summary
Background:
The World Health Organization estimates that in sub-Saharan Africa about 4 million HIV-infected patients had started antiretroviral therapy (ART) by the end of 2008. Loss of patients to follow-up and care is an important problem for treatment programmes in this region. As mortality is high in these patients compared to patients remaining in care, ART programmes with high rates of loss to follow-up may substantially underestimate mortality of all patients starting ART.Methods and Findings:
We developed a nomogram to correct mortality estimates for loss to follow-up, based on the fact that mortality of all patients starting ART in a treatment programme is a weighted average of mortality among patients lost to follow-up and patients remaining in care. The nomogram gives a correction factor based on the percentage of patients lost to follow-up at a given point in time, and the estimated ratio of mortality between patients lost and not lost to follow-up. The mortality observed among patients retained in care is then multiplied by the correction factor to obtain an estimate of programme-level mortality that takes all deaths into account. A web calculator directly calculates the corrected, programme-level mortality with 95% confidence intervals (CIs). We applied the method to 11 ART programmes in sub-Saharan Africa. Patients retained in care had a mortality at 1 year of 1.4% to 12.0%; loss to follow-up ranged from 2.8% to 28.7%; and the correction factor from 1.2 to 8.0. The absolute difference between uncorrected and corrected mortality at 1 year ranged from 1.6% to 9.8%, and was above 5% in four programmes. The largest difference in mortality was in a programme with 28.7% of patients lost to follow-up at 1 year.Conclusions:
The amount of bias in mortality estimates can be large in ART programmes with substantial loss to follow-up. Programmes should routinely report mortality among patients retained in care and the proportion of patients lost. A simple nomogram can then be used to estimate mortality among all patients who started ART, for a range of plausible mortality rates among patients lost to follow-up.
: Please see later in the article for the Editors' SummaryIntroduction
The World Health Organization (WHO) estimates that about 4 million people were receiving antiretroviral therapy (ART) in low - and middle-income countries by the end of 2008, with coverage reaching 42% of the estimated 9.5 million in need of ART [1]. Sub-Saharan Africa represented 70% of the estimated treatment need and 73% of the total number of people receiving treatment in low - and middle-income countries at the end of 2008 [1].
The provision of ART in resource-limited settings follows a public health approach, which is characterized by a limited number of regimens and the standardization of clinical and laboratory monitoring [2]. This approach has been shown to result in similar or superior adherence to therapy and similar virological response when compared to industrialized countries [3]–[5]. Loss of patients to follow-up and care is, however, an important problem in resource-limited settings: A systematic review of published retention rates in ART clinics in sub-Saharan Africa showed that the proportion of patients retained 2 years after starting therapy was approximately 60% [6]. Similarly, in a collaborative analysis of patients starting ART in 15 treatment programmes in Africa, Asia, and South America we found that 21% of patients were lost to follow-up 6 months after starting ART [7]. A systematic review and meta-analysis of studies tracing patients lost to follow-up found that these patients experience high mortality [8] compared to patients remaining in care [9].
The successful treatment of individual patients and the monitoring and evaluation of ART programmes both depend on regular and complete patient follow-up. Programmes with high rates of loss to follow-up and poor ascertainment of deaths in patients lost will underestimate mortality of all patients starting ART. For example, standard Kaplan-Meier survival analyses in which follow-up time in patients lost to follow-up is censored at the last visit will be biased because mortality of these patients is assumed to be identical to comparable patients remaining in care. Analyses restricted to patients remaining in care will also underestimate mortality among all patients who started ART. Biased estimates of programme-level mortality hamper the evaluation of single programmes and the comparison between different programmes and settings.
Nomograms are widely used in medicine [10]. They are graphs that allow the approximate graphical computation of a function; placing a line across its several scales immediately solves the formula [11]. We propose a nomogram and a web-based calculator to correct estimates of programme-level mortality for loss to follow-up. We illustrate its use in a case study from Kenya. We show how mortality among patients lost to follow-up can be predicted on the basis of studies that traced patients to ascertain their vital status. Finally, we apply these methods to 11 ART programmes in sub-Saharan Africa and compare uncorrected and corrected estimates of mortality at 1 year to assess the typical bias that is introduced when loss to follow-up is ignored.
Methods
Deriving the Nomogram
Mortality of all patients starting ART in a treatment programme over a defined time period is a weighted average of mortality among patients remaining in care and patients lost to follow-up. It depends on the percentage of patients lost, mortality among patients lost to follow-up, and mortality among patients not lost to follow-up. The mortality observed among patients remaining in care can be multiplied by a correction factor C to obtain an estimate of programme-level mortality that takes deaths among patients lost to follow-up into account. This correction factor can be obtained from a nomogram. The algebraic derivation of this nomogram is as follows:
Let
-
MU = Uncorrected estimates of programme-level mortality (with censoring of patients lost)
-
MC = Corrected estimate of programme-level mortality, taking deaths among patients lost into account.
-
MNL = Mortality observed in patients retained in care (not lost to follow-up)
-
ML = Mortality estimated in patients lost to follow-up
-
r = Proportion lost to follow-up
Note that
-
MNL, ML, and r need to refer to the same time period (for example the first year of ART).
ThenDividing both sides of this equation by MNL, we obtain the correction factorwhich can be used to obtain MC for a given MNL from MC = MNLC.
The nomogram (Figure 1) plots the ratio of the mortality among patients lost and not lost to follow-up (ML/MNL) on lines defined by the proportion of patients lost to follow-up (r) which are used to read off correction factor C. The broken lines refer to the case-study described in Box 1. Further details on calculations are provided in Text S1. Figures S1 and S2 provide clean versions of the nomogram. These are also available from http://www.iedea-sa.org.
Fig. 1. Nomogram for obtaining correction factors to adjust programme-level mortality estimates, based on the observed mortality among patients not lost to follow-up, the observed proportion of patients lost and an estimate of mortality among patients lost. 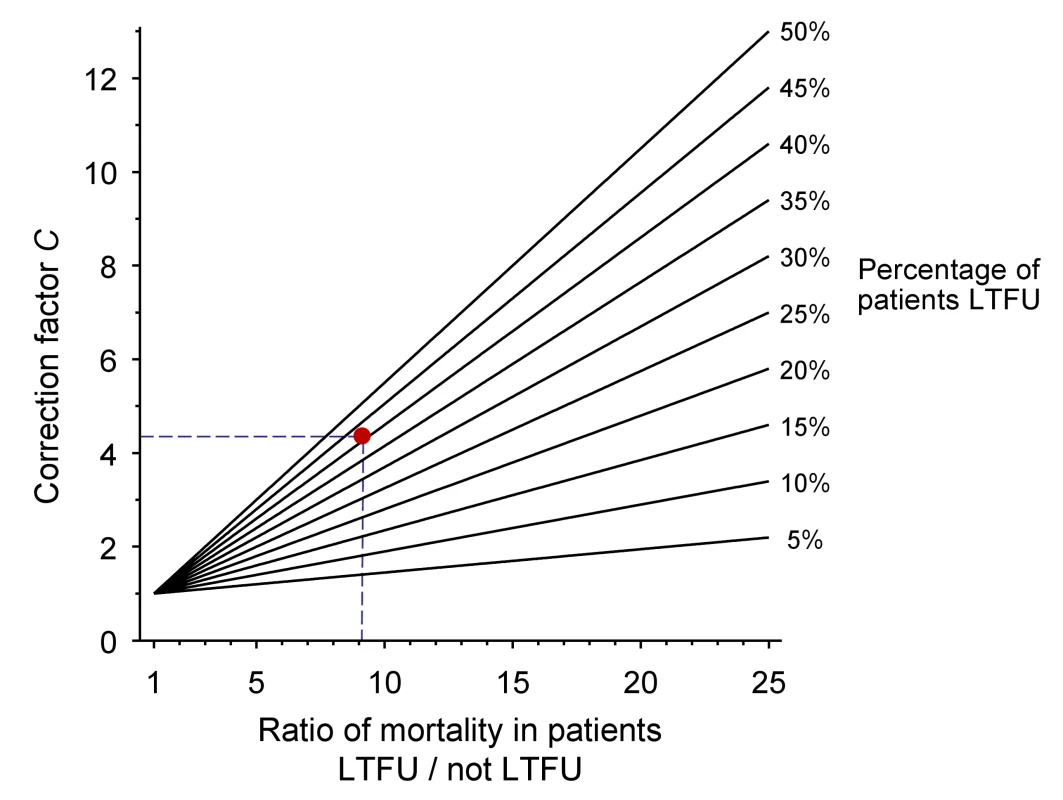
The red dot and broken lines relate to the case study described in Box 1. Box 1. Example from Western Kenya Using Tracing Method
The Academic Model Providing Access to Healthcare (AMPATH) is a large ART programme in western Kenya [21],[22]. AMPATH is based on a partnership between the Moi University School of Medicine in Eldoret and Indiana University School of Medicine in Indianapolis, Indiana, United States of America. AMPATH provides HIV care and treatment to over 70,000 adults and children living with HIV/AIDS in 18 clinics throughout western Kenya. Patients are managed according to National Kenyan protocols, which are consistent with WHO guidelines. AMPATH undertakes active outreach to patients who miss scheduled appointments (called lost to follow-up in this article). A locator card is completed for all patients enrolling in the programme, which includes contact information and a map to the patient's residence. The results of the outreach programme have been described for the Moi Teaching and Referral Hospital in the city of Eldoret and the rural health centre in Mosoriot [23]. A total of 8,977 adult patients were enrolled in the two participating sites; 3,624 patients were lost to follow-up and 5,353 remained in care between 1 January 2005 and 31 January 2007. Outreach efforts were initiated for 1,143 (31.5%) of patients lost and the vital status of 621 (54.3%) patients could be determined. The naïvely calculated Kaplan-Meier (KM) estimate of programme-level mortality (MU), ignoring loss to follow-up, was 1.7%. This estimate can now be corrected in six simple steps:
-
Determine the percentage of patients lost to follow-up (r):
3,624 of 8,977 patients were lost to follow-up: 40.5%
-
Determine mortality among patients lost to follow-up (ML):
124 of the 621 patients traced had died. The KM estimate was 20.0%
-
Determine mortality among patients not lost to follow-up (MNL):
126 of the 5,353 patients remaining in care had died. The KM estimate was 2.2%.
-
Calculate the ratio of mortality among patients lost to follow-up and patients not lost to follow-up (ML divided by MNL):
20.0 divided by 2.2 is 9.1
-
Obtain the correction factor C from the nomogram:
4.3 (see broken lines in Figure 1)
-
Calculate the corrected programme-level mortality:
4.3 times 2.2% is 9.5%
Not all patients lost to follow-up could be located and mortality among those who could not be found might have been even higher than in those who were successfully traced. Overall mortality among patients lost to follow-up may thus have been higher than 20%. The effect of assuming a higher mortality among patients lost to follow-up can be examined in a sensitivity analysis: repeating the steps assuming that 25% or 30% of patients lost to follow-up had died results in estimates of corrected programme-level mortality of 11.4% and 13.5%. Clearly, deaths in patients lost to follow-up are an important issue when estimating programme-level mortality in the AMPATH programme.
Two Ways of Estimating Mortality among Patients Lost to Follow-Up
The parameters r and MNL can be observed directly, but mortality among patients lost to follow-up must be estimated. This can be done in dedicated studies tracing patients lost to follow-up, for example by visiting the homes of patients lost to follow-up or by linking treatment programme with death registry data to ascertain the vital status of patients lost to follow-up [12]–[15]. This method will be henceforth be called “tracing method.”
In many situations, however, data from dedicated tracing studies are not available. In this case, mortality among patients lost to follow-up may be predicted on the basis of published data from similar settings. We used Brinkhof and colleagues' systematic review and meta-regression analysis of studies tracing patients lost to follow-up [8]. The meta-regression analysis was based on a total of 15 studies in patients on ART from sub-Saharan Africa. There was an inverse relation between mortality among those lost to follow-up and the rate of loss to follow-up in the programme: The higher the rate of loss to follow-up the lower the mortality among those lost. Figure 2 shows the predicted mortality among patients lost to follow-up according to the percentage of patients lost in the programme, with 95% confidence intervals (CIs). The equation is as follows:where a = 0.57287 and b = −4.04409.
Fig. 2. Predicted mortality among patients lost to follow-up according to percent of patients lost in programme (solid line) with 95% CI (limits of grey area). 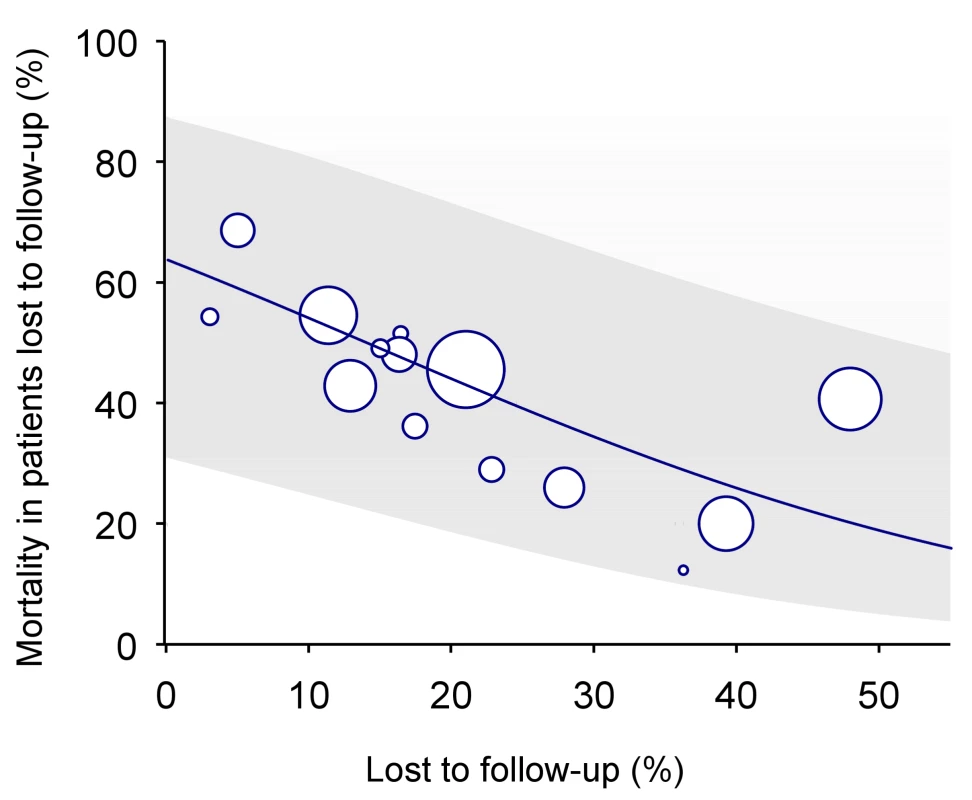
See text for regression equation. Mortality among patients lost to follow-up can be calculated using the formula above or read off Figure 2, based on the proportion of patients lost to follow-up in the programme. This method will henceforth be called “meta method.”
Allowing for Uncertainty
We used Monte Carlo simulations with 100,000 iterations to calculate 95% CIs for the corrected programme-level mortality (MC). These simulations allow for uncertainty in the estimation of (i) mortality among patients remaining in care (MNL); (ii) in the proportion of patients lost to follow-up (r); and (iii) of mortality among patients lost to follow-up (ML). Further details are provided in Text S1.
Application to ART Programmes in Sub-Saharan Africa
The International epidemiological Databases to Evaluate AIDS (IeDEA, see http://www.iedea-hiv.org) is a collaborative network of HIV/AIDS treatment programmes in seven regions of the world, including North America, Asia and the Pacific, the Caribbean and Latin America, and four regions in sub-Saharan Africa (Central Africa, East Africa, West Africa, and Southern Africa). IeDEA and its predecessor, the ART in Lower Income Countries (ART-LINC) collaboration, have been described in detail elsewhere [16]–[20]. These collaborative networks were established to address clinical and operational research questions that require large patient numbers or many treatment programmes, for example to compare outcomes of ART between different settings, delivery modes, and types of monitoring.
We included 11 ART programmes from sub-Saharan Africa. No direct evidence on the mortality of patients lost to follow-up from tracing studies was available for these programmes, and mortality was therefore predicted using the meta method. All patients aged 16 years or older with complete data on sex and date of birth, and who were treatment naïve at start of combination ART were included in the analysis. Combination ART was defined as a minimum of three antiretroviral drugs from two drug classes. Advanced stage of disease was defined as WHO stages III or IV or Centers for Disease Control and Prevention (CDC) clinical stage C. Measurements of laboratory values closest to the starting date of ART (within 6 months before up to one week after the date of starting ART) were taken as the baseline levels. The data collected at participating sites were cleaned, merged, and analysed centrally. At all sites, institutional review boards had approved the collection and transfer of data.
For each programme we determined the proportion of patients lost to follow-up at 1 year (r). A patient was considered lost to follow-up if the last visit was more than 9 months before the closure date for that site, with the closure date defined as the most recent visit date recorded in the database. This allowed for time during which a patient could have returned for a visit. Only patients who potentially had 9 months of follow-up were included in the calculations of r. We used Kaplan-Meier methods to estimate mortality (with 95% CI) at 1 year for patients remaining in care (MNL).
Web Calculator
The calculations of the nomogram, the prediction of mortality among patients lost to follow-up based on the meta-regression analysis described above, and the calculation of 95% CIs have been implemented on a dedicated website at http://www.iedea-sa.org.
Results
Case Study from Western Kenya Using the Tracing Method
Box 1 and Figure 1 illustrate the correction of naïve estimates of programme-level mortality in six simple steps. The data are from the Academic Model Providing Access to Healthcare (AMPATH), a large ART programme in western Kenya [21],[22]. Patients who miss scheduled appointments are actively traced by outreach teams, and data from the outreach programme were used to estimate mortality among patients lost to follow-up. The data are based on a previous detailed analysis of two AMPATH sites [23]. The uncorrected estimate (MU) of mortality at these two sites for the period between 1 January 2005 and 31 January 2007 was 1.7% (95% CI 1.3%–2.0%), the estimate for mortality among patients not lost to follow-up was 2.2% (95% CI 1.8%–16.6%), and the corrected estimate using the nomogram was 9.5% (Box 1). Entering the data into the web calculator at http://www.iedea-sa.org/ gives a more precise corrected estimate of programme mortality of 9.4%, with 95% CI 8.1%–10.9%. Of note, the results from the nomogram are similar to the corrected estimates that were obtained for these AMPATH sites by Yiannoutsos et al. using more complex statistical methods [23],[24].
Correction of Mortality in ART Programmes in Sub-Saharan Africa Using the Meta Method
A total of 24,257 patients from 11 ART programmes in 10 countries (Botswana, Côte d'Ivoire, Kenya, Malawi, Rwanda, Senegal, South Africa, Uganda, Zambia, Zimbabwe) were included: 1,363 deaths were observed during the first year of ART. Table 1 shows the characteristics of the 11 programmes. Four programmes were located in South Africa and one programme had sites in six different countries. The number of patients treated at each site ranged from 369 to 4,705 patients. Eight sites were public (government) clinics offering ART free of charge, two were run by a nongovernmental organisation offering free ART, one was a research site offering free ART, and one site was a private clinic operating on a fee-for-service basis. Median age across all programmes was 35 years (interquartile range 30–41 years) and 16,018 patients (66.0%) were women. The median CD4 cell count at baseline ranged from 83 to 156 cells/µL across programmes.
Tab. 1. Characteristics of ART programmes included in the study. 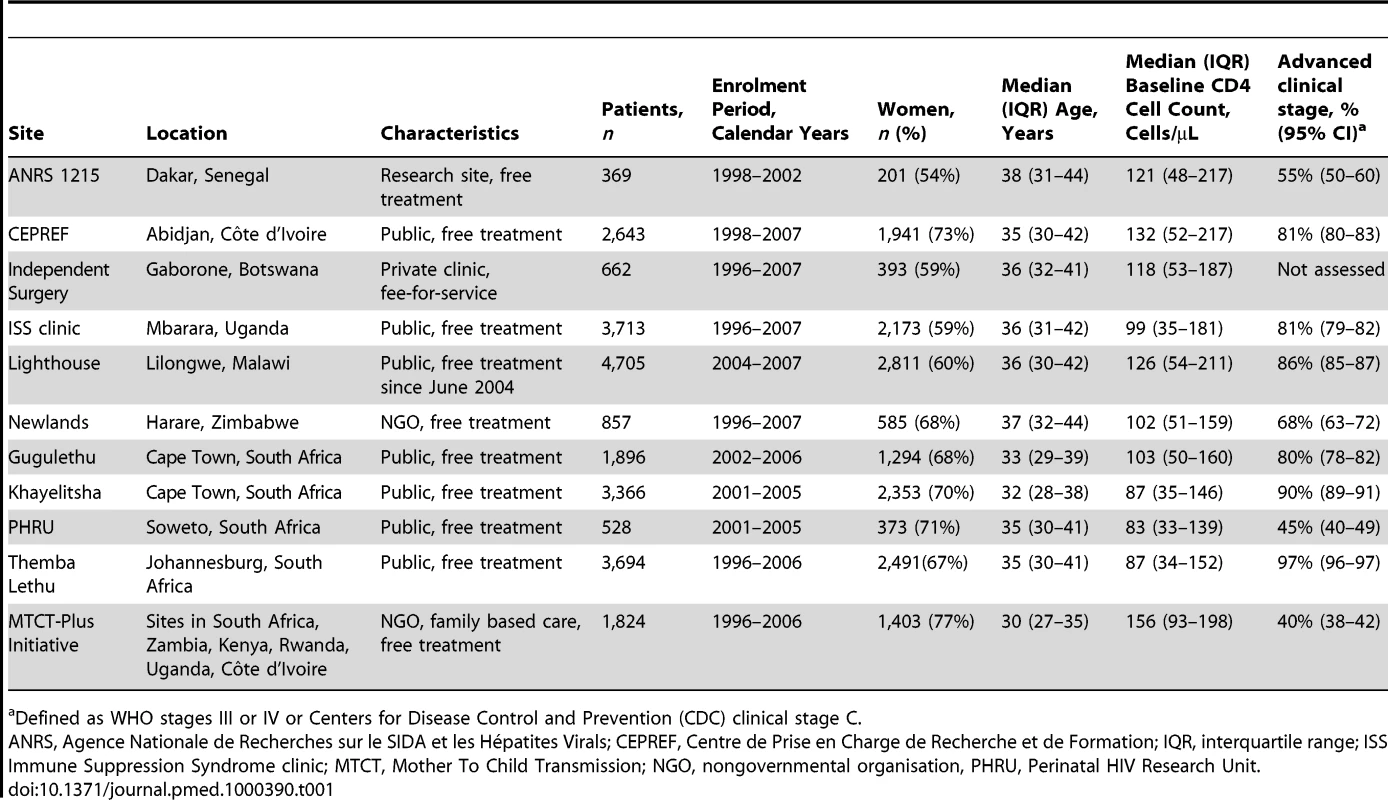
Defined as WHO stages III or IV or Centers for Disease Control and Prevention (CDC) clinical stage C. Table 2 lists the uncorrected estimates (MU) of programme-level mortality at 1 year (which do not consider mortality among patients lost to follow-up), the proportion of patients lost to follow-up at 1 year (r), the estimated 1-year mortality among patients remaining in care (MNL), the predicted mortality among patients lost to follow-up (ML, obtained from the meta-regression model of Brinkhof et al. [8]), correction factor C and the corrected estimates of programme-level mortality at 1 year (MC), which take mortality among patients lost to follow-up into account, with 95% CIs calculated as described above. Figure 3 shows the nomogram populated with the data from the 12 antiretroviral treatment programmes.
Fig. 3. Nomogram with data from 11 antiretroviral treatment programmes in sub-Saharan Africa. 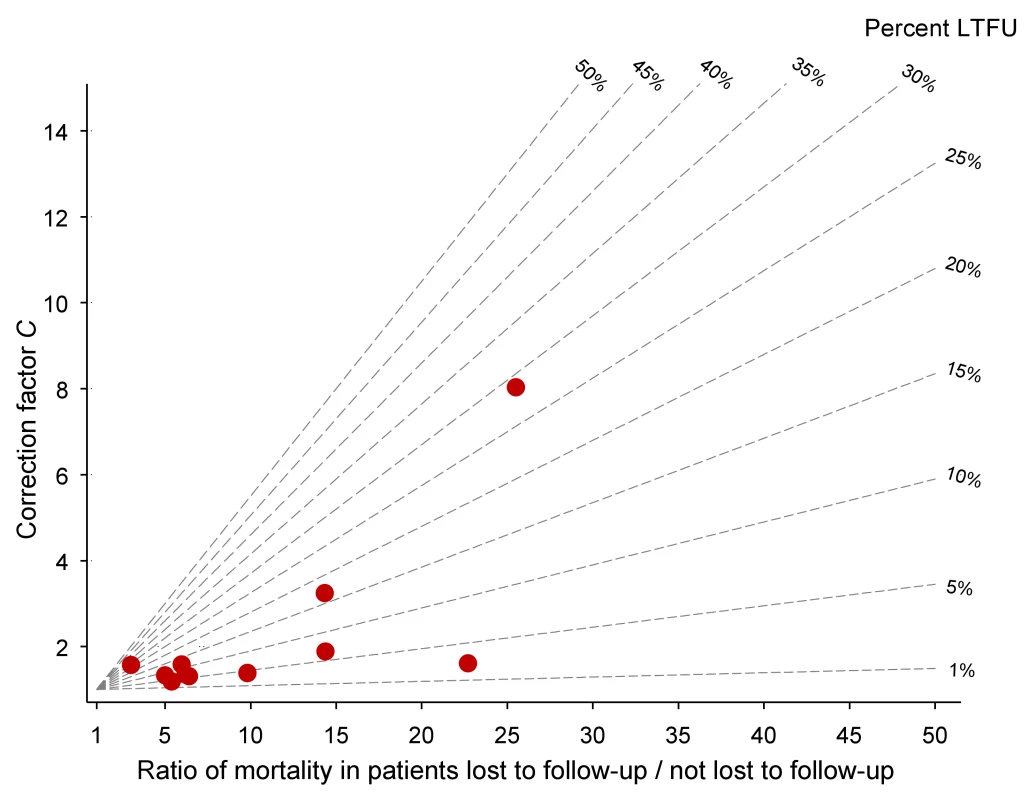
LTFU, lost to follow-up. Tab. 2. Uncorrected Kaplan-Meier estimates of programme-level mortality at 1 year for all patients starting ART, number of patients lost to follow-up, mortality estimates for patients retained in care, predicted mortality among patients lost to follow-up, correction factor C and corrected programme-level mortality at 1 year. 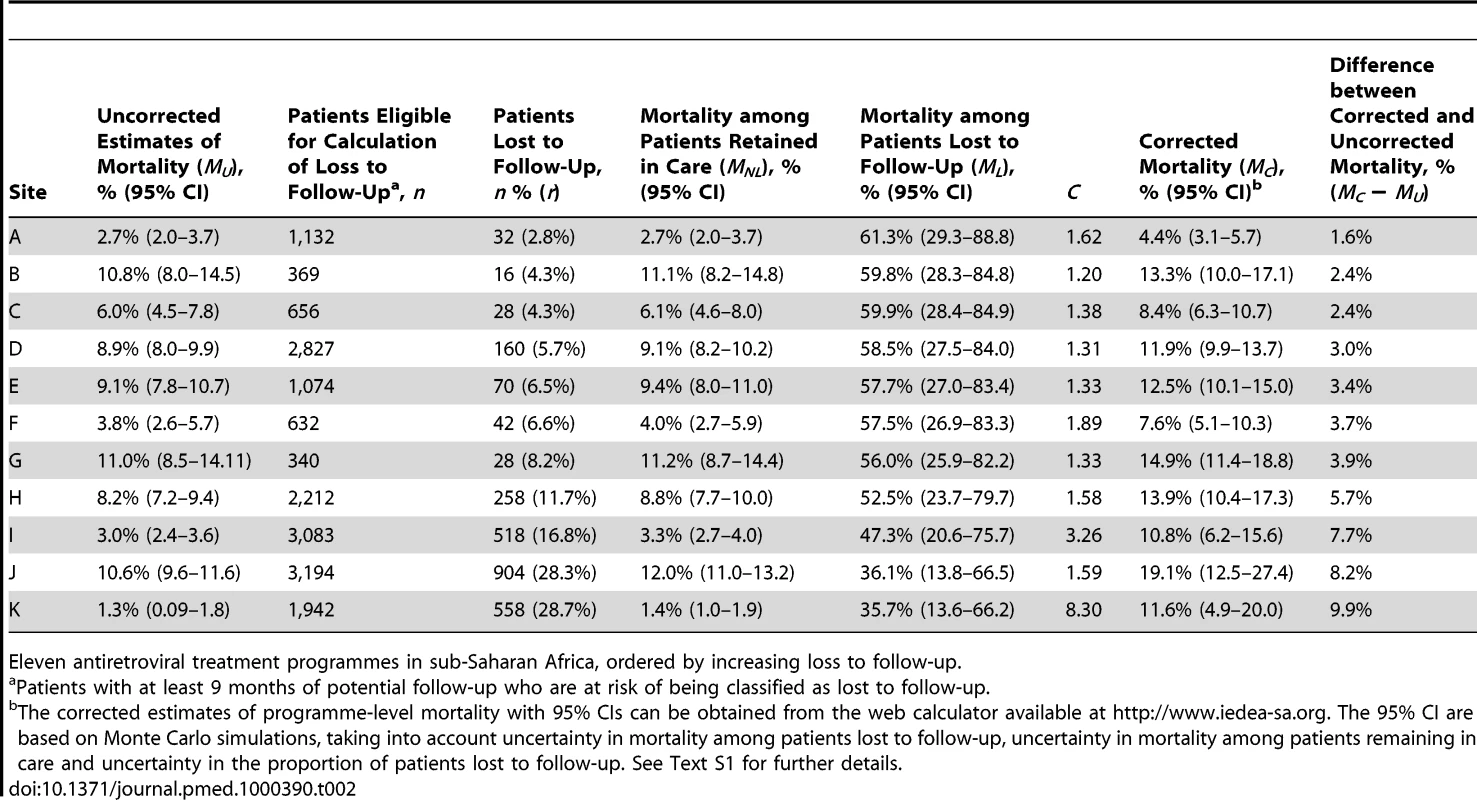
Eleven antiretroviral treatment programmes in sub-Saharan Africa, ordered by increasing loss to follow-up. The uncorrected estimates of programme-level mortality at 1 year ranged from 1.3% to 11.0%, mortality at 1 year among patients retained in care from 1.4% to 12.0%; loss to follow-up at 1 year from 2.8% to 28.7%; and correction factor C from 1.19 to 8.04. The corrected estimates of programme-level mortality ranged from 4.4% to 18.8%. Figure 4 shows a scatter plot of the uncorrected estimates of programme-level mortality (MU) against the corrected estimates (MC): the absolute difference between uncorrected and corrected mortality at 1 year was relatively small (1.7%–3.6%) in seven programmes, but more substantial in the remaining four programmes. The largest difference in mortality was 9.8%, in a programme with 28.7% of patients lost to follow-up at 1 year. The uncorrected estimate of programme-level mortality for this programme was 1.4% and the corrected estimate 11.2%.
Fig. 4. Scatterplot of uncorrected versus corrected mortality for loss to follow-up in 11 treatment programmes in sub-Saharan Africa. 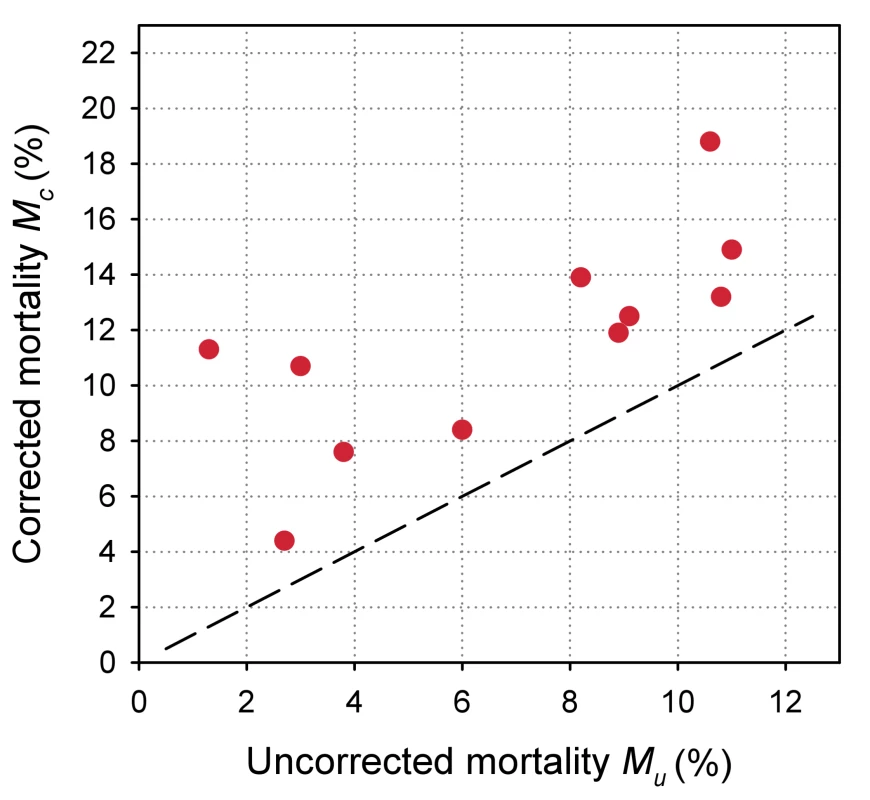
Discussion
Regular and complete patient follow-up is essential both for the care of individual patients and the monitoring and evaluation of outcomes of ART programmes. Individual treatment decisions can then be made in the light of clinical and laboratory results and the rate of complications and mortality can be accurately estimated at the programme level. Loss to follow-up is, however, an important problem in ART programmes in resource-limited settings [6],[7],[18],[25], and poor ascertainment of deaths in patients lost to follow-up may mean that programme-level mortality, i.e. mortality of all patients starting ART, is underestimated [8],[23]. Previous analyses of treatment programmes have generally censored follow-up time at the last visit to the clinic, and patients lost to follow-up therefore contributed follow-up time but no deaths [26]–[28] (here referred to as MU). We developed a simple nomogram that allows programme managers to read off a correction factor for a range of plausible mortality rates among patients lost to follow-up. This factor can then be used to assess to what extent the mortality observed among patients retained in care underestimates mortality at the programme level.
We applied the nomogram method to ART programmes in sub-Saharan Africa to estimate programme-level mortality at 1 year after starting ART and found that the bias was modest in many programmes, because loss to follow-up was relatively low. It is clear from the shape of the nomogram that if the proportion of patients lost is low (say, below 10%), the correction factor will not be greatly affected by different assumptions for mortality among patients lost to follow-up. In programmes with fewer than 10% of patients lost, the mortality observed among patients retained in care will thus generally provide a reasonable estimate, which will underestimate programme-level mortality by only a few percentage points. Conversely, if a large proportion of patients are lost, as in the case study from western Kenya (Box 1), the bias will be substantial even if the ratio of mortality between patients lost and not lost is relatively low, and the amount of bias will increase steeply with higher ratios.
Mortality among patients lost to follow-up is high: Brinkhof et al. recently reviewed studies that traced patients who became lost to ART programmes in resource-limited settings [8]. The vital status of about two-thirds of patients could be ascertained, and among these many had died: in ART programmes from sub-Saharan Africa the combined mortality from meta-analysis was 46% (95% CI 39%–54%) [8]. Patients were often lost in the first few months of ART and died soon thereafter [8].These findings are in accordance with previous analyses from the ART-LINC collaboration [27] and other treatment programmes, for example the Médecins Sans Frontières programmes in Malawi [28] and South Africa [29], or the ART programme in Mbarara, Uganda [25]. Of note, the percentage of patients lost to follow-up in these programmes was associated with estimated mortality rates in the patients lost: the estimated mortality declined from around 60% to 20% as the percentage of patients lost to the programme increased from 5% to 50%. As discussed in detail elsewhere [8], those lost to follow-up in programmes with high rates of loss to follow-up might include many low-risk patients who self-transferred to another programme, for example because of a more convenient location of the new clinic, to avoid stigma or due to work-related reasons. The negative association between the proportion lost and mortality among patients lost to follow-up will attenuate the effect of a high rate of loss to follow-up on the correction factor C.
In the absence of direct evidence, the regression analysis of Brinkhof et al. [8] can be used to predict mortality among patients lost to follow-up for a given programme. The model will provide a sensible range of estimates of mortality, which can then be used in analyses to adjust overall mortality. We used this approach when applying the nomogram to the 11 ART programmes from sub-Saharan Africa. Statistical uncertainty is, however, substantial, as documented by the wide confidence intervals around the predicted mortality among patients lost to follow-up (the grey area in Figure 2), and this translated into wide confidence intervals around the corrected estimates of programme-level mortality (see last column of Table 2).
In addition to the statistical uncertainty, the applicability of the results from the regression analysis must also be considered: The studies examined loss to follow-up and mortality in the first months after starting ART, and at present the regression model should therefore not be used to estimate mortality among patients lost to follow-up later on, for example in the second or third year of ART. The different determinants of loss to follow-up, including “silent transfer” to another programme (without notifying the programme where ART was initiated), financial constraints, and improving or deteriorating health will change with time since starting ART, and mortality among patients lost to programmes will change accordingly. In the present study we focused on loss to follow-up in the first year of ART, and the use of data from the review and meta-regression analysis was therefore appropriate. We stress that patterns of loss to follow-up and associated mortality may also change with calendar years, for example with increasing CD4 cell counts at the start of ART [17].
To obtain accurate estimates of mortality among all patients starting ART, programmes should therefore make an effort to trace patients lost to follow-up and ascertain their vital status. The results from these efforts should be made available to the scientific community so that the regression model can be updated and improved, for the benefit of programmes in settings where tracing of patients lost is not possible. Also note that results of studies tracing patients lost to follow-up can be used to directly correct estimates of mortality in the programme, for example by using double-sampling designs or weighted Kaplan-Meier methods [23],[30]. These methods make strong assumptions, however, and some require expert statisticians.
The nomogram method has the important advantage of being simple and adapted to the field. Among the data used in the nomogram the estimated or assumed mortality among patients lost to follow-up (ML) will always be associated with the greatest degree of statistical uncertainty and the greatest risk of bias. The estimates of mortality among patients remaining in care (MNL) might, however, also be biased: some patients will not meet criteria for loss to follow-up and their follow-up will be censored at the last visit. Mortality rates might be higher in these patients than in otherwise identical patients whose follow-up time was not censored. In other words, we cannot exclude the possibility that some degree of “informative censoring” might introduce some bias in our estimate of mortality among patients retained in care. Finally, the nomogram can provide only a sensitivity analysis without formal estimates of uncertainty. We overcame this by creating a web calculator with a user-friendly interface, which calculates 95% CI taking into account the statistical uncertainty in the input parameters of the nomogram.
ART programmes should strive to prevent loss to follow-up: interventions that prevent loss to follow-up in resource-limited settings can improve survival and are cost-effective by international criteria [31]. For example, outreach teams that routinely trace patients, combined with other measures, substantially reduce loss to follow-up [18]. Financial constraints are a common reason for not returning to the clinic [8], and mortality in programmes that charge user fees has been shown to be higher than in those offering free treatment [27]. Decentralization of services, task shifting to lay care providers, longer drug refill periods for stable patients, and provision of transport vouchers for those in need are some of the strategies that could address this issue. Strengthening of referral systems and regular exchange of information between clinics, together with patient education, could increase the recording of transfers and ensure continuity of care. Finally, when assessing outcomes, programmes should routinely report both mortality among patients retained in care at a given point in time and the proportion of patients lost to follow-up at that time. A simple nomogram can then be used to estimate mortality among all patients who started ART.
Supporting Information
Zdroje
1. World Health Organization 2009 Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. 2009 Progress Report. WHO, Geneva. Available: http://www.who.int/hiv/pub/2009progressreport/en/index.html. Accessed 6 December 2010
2. GilksCF
CrowleyS
EkpiniR
GoveS
PerriensJ
2006 The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 368 505 510
3. IversLC
KendrickD
DoucetteK
2005 Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis 41 217 224
4. KeiserO
OrrellC
EggerM
WoodR
BrinkhofMW
2008 Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med 5 e148 doi:10.1371/journal.pmed.0050148
5. MillsEJ
NachegaJB
BuchanI
OrbinskiJ
AttaranA
2006 Adherence to antiretroviral therapy in sub-Saharan Africa and North America: A meta-analysis. JAMA 296 679 690
6. RosenS
FoxMP
GillCJ
2007 Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med 4 e298 doi:10.1371/journal.pmed.0040298
7. BrinkhofMW
DabisF
MyerL
BangsbergDR
BoulleA
2008 Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ 86 559 567
8. BrinkhofMW
Pujades-RodriguezM
EggerM
2009 Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One 4 e5790 doi:10.1371/journal.pone.0005790
9. MayM
BoulleA
PhiriS
MessouE
MyerL
2010 Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet 376 449 457
10. GrimesDA
2008 The nomogram epidemic: resurgence of a medical relic. Ann Intern Med 149 273 275
11. PortaM
2008 A Dictionary of Epidemiology. Fifth Edition. Oxford University Press 167
12. AnglaretX
ToureS
GourvellecG
TchehyA
ZioL
2004 Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from sub-Saharan Africa. J Acquir Immune Defic Syndr 35 320 323
13. YuJK
ChenSC
WangKY
ChangCS
MakombeSD
2007 True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ 85 550 554
14. DalalRP
MacPhailC
MqhayiM
WingJ
FeldmanC
2008 Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr 47 101 107
15. FoxMP
BrennanA
MaskewM
MacPhailP
SanneI
2010 Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health 15 405 413
16. DabisF
BalestreE
BraitsteinP
MiottiP
BrinkhofWGM
2005 Antiretroviral Therapy in Lower Income Countries (ART-LINC): International collaboration of treatment cohorts. Int J Epidemiol 34 979 986
17. KeiserO
AnastosK
SchechterM
BalestreE
MyerL
2008 Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health 13 870 879
18. ForsterM
BaileyC
BrinkhofMW
GraberC
BoulleA
2008 Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Organ 86 939 947
19. McGowanCC
CahnP
GotuzzoE
PadgettD
PapeJW
2007 Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol 36 969 976
20. GangeSJ
KitahataMM
SaagMS
BangsbergDR
BoschRJ
2007 Cohort profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 36 294 301
21. InuiTS
NyandikoWM
KimaiyoSN
FrankelRM
MuriukiT
2007 AMPATH: Living proof that no one has to die from HIV. J Gen Intern Med 22 1745 1750
22. EinterzRM
KimaiyoS
MengechHN
Khwa-OtsyulaBO
EsamaiF
2007 Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med 82 812 818
23. YiannoutsosCT
AnMW
FrangakisCE
MusickBS
BraitsteinP
2008 Sampling-based approaches to improve estimation of mortality among patient dropouts: experience from a large PEPFAR-funded program in Western Kenya. PLoS One 3 e3843 doi:10.1371/journal.pone.0003843
24. FrangakisCE
RubinDB
2001 Addressing an idiosyncrasy in estimating survival curves using double sampling in the presence of self-selected right censoring. Biometrics 57 333 342
25. GengEH
BangsbergDR
MusinguziN
EmenyonuN
BwanaMB
2010 Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr 53 405 411
26. StringerJS
ZuluI
LevyJ
StringerEM
MwangoA
2006 Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA 296 782 793
27. BraitsteinP
BrinkhofMW
DabisF
SchechterM
BoulleA
2006 Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: Comparison between low-income and high-income countries. Lancet 367 817 824
28. FerradiniL
JeanninA
PinogesL
IzopetJ
OdhiamboD
2006 Scaling up of highly active antiretroviral therapy in a rural district of Malawi: An effectiveness assessment. Lancet 367 1335 1342
29. BoulleA
BockP
OslerM
CohenK
ChanningL
2008 Antiretroviral therapy and early mortality in South Africa. Bull World Health Organ 86 678 687
30. GengEH
EmenyonuN
BwanaMB
GliddenDV
MartinJN
2008 Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA 300 506 507
31. LosinaE
ToureH
UhlerLM
AnglaretX
PaltielAD
2009 Cost-effectiveness of preventing loss to follow-up in HIV treatment programs: A Cote d'Ivoire appraisal. PLoS Med 6 e1000173 doi:10.1371/journal.pmed.1000173
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- A Simple Novel Method for Determining Mortality Rates in HIV Treatment Programs Worldwide
- Setting Implementation Research Priorities to Reduce Preterm Births and Stillbirths at the Community Level
- A Research Agenda for Malaria Eradication: Monitoring, Evaluation, and Surveillance
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- A Research Agenda to Underpin Malaria Eradication
- Correcting Mortality for Loss to Follow-Up: A Nomogram Applied to Antiretroviral Treatment Programmes in Sub-Saharan Africa
- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- Setting Research Priorities to Reduce Almost One Million Deaths from Birth Asphyxia by 2015
- Predicting Live Birth, Preterm Delivery, and Low Birth Weight in Infants Born from In Vitro Fertilisation: A Prospective Study of 144,018 Treatment Cycles
- Some Lessons for the Future from the Global Malaria Eradication Programme (1955–1969)
- A Research Agenda for Malaria Eradication: Basic Science and Enabling Technologies
- A Research Agenda for Malaria Eradication: Vector Control
- The Role of Research in Viral Disease Eradication and Elimination Programs: Lessons for Malaria Eradication
- The Influence of Distance and Level of Care on Delivery Place in Rural Zambia: A Study of Linked National Data in a Geographic Information System
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
- Development of a Standardized Screening Rule for Tuberculosis in People Living with HIV in Resource-Constrained Settings: Individual Participant Data Meta-analysis of Observational Studies
- WHO/PLoS Collection “No Health Without Research”: A Call for Papers
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- A Research Agenda for Malaria Eradication: Vaccines
- A Research Agenda for Malaria Eradication: Health Systems and Operational Research
- A Research Agenda for Malaria Eradication: Diagnoses and Diagnostics
- A Research Agenda for Malaria Eradication: Drugs
- A Research Agenda for Malaria Eradication: Modeling
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



