-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMarkers of Dysglycaemia and Risk of Coronary Heart Disease in People without Diabetes: Reykjavik Prospective Study and Systematic Review
Background:
Associations between circulating markers of dysglycaemia and coronary heart disease (CHD) risk in people without diabetes have not been reliably characterised. We report new data from a prospective study and a systematic review to help quantify these associations.Methods and Findings:
Fasting and post-load glucose levels were measured in 18,569 participants in the population-based Reykjavik study, yielding 4,664 incident CHD outcomes during 23.5 y of mean follow-up. In people with no known history of diabetes at the baseline survey, the hazard ratio (HR) for CHD, adjusted for several conventional risk factors, was 2.37 (95% CI 1.79–3.14) in individuals with fasting glucose ≥7.0 mmol/l compared to those <7 mmol/l. At fasting glucose values below 7 mmol/l, adjusted HRs were 0.95 (0.89–1.01) per 1 mmol/l higher fasting glucose and 1.03 (1.01–1.05) per 1 mmol/l higher post-load glucose. HRs for CHD risk were generally modest and nonsignificant across tenths of glucose values below 7 mmol/l. We did a meta-analysis of 26 additional relevant prospective studies identified in a systematic review of Western cohort studies that recorded fasting glucose, post-load glucose, or glycated haemoglobin (HbA1c) levels. In this combined analysis, in which participants with a self-reported history of diabetes and/or fasting blood glucose ≥7 mmol/l at baseline were excluded, relative risks for CHD, adjusted for several conventional risk factors, were: 1.06 (1.00–1.12) per 1 mmol/l higher fasting glucose (23 cohorts, 10,808 cases, 255,171 participants); 1.05 (1.03–1.07) per 1 mmol/l higher post-load glucose (15 cohorts, 12,652 cases, 102,382 participants); and 1.20 (1.10–1.31) per 1% higher HbA1c (9 cohorts, 1639 cases, 49,099 participants).Conclusions:
In the Reykjavik Study and a meta-analysis of other Western prospective studies, fasting and post-load glucose levels were modestly associated with CHD risk in people without diabetes. The meta-analysis suggested a somewhat stronger association between HbA1c levels and CHD risk.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(5): e32767. doi:10.1371/journal.pmed.1000278
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000278Summary
Background:
Associations between circulating markers of dysglycaemia and coronary heart disease (CHD) risk in people without diabetes have not been reliably characterised. We report new data from a prospective study and a systematic review to help quantify these associations.Methods and Findings:
Fasting and post-load glucose levels were measured in 18,569 participants in the population-based Reykjavik study, yielding 4,664 incident CHD outcomes during 23.5 y of mean follow-up. In people with no known history of diabetes at the baseline survey, the hazard ratio (HR) for CHD, adjusted for several conventional risk factors, was 2.37 (95% CI 1.79–3.14) in individuals with fasting glucose ≥7.0 mmol/l compared to those <7 mmol/l. At fasting glucose values below 7 mmol/l, adjusted HRs were 0.95 (0.89–1.01) per 1 mmol/l higher fasting glucose and 1.03 (1.01–1.05) per 1 mmol/l higher post-load glucose. HRs for CHD risk were generally modest and nonsignificant across tenths of glucose values below 7 mmol/l. We did a meta-analysis of 26 additional relevant prospective studies identified in a systematic review of Western cohort studies that recorded fasting glucose, post-load glucose, or glycated haemoglobin (HbA1c) levels. In this combined analysis, in which participants with a self-reported history of diabetes and/or fasting blood glucose ≥7 mmol/l at baseline were excluded, relative risks for CHD, adjusted for several conventional risk factors, were: 1.06 (1.00–1.12) per 1 mmol/l higher fasting glucose (23 cohorts, 10,808 cases, 255,171 participants); 1.05 (1.03–1.07) per 1 mmol/l higher post-load glucose (15 cohorts, 12,652 cases, 102,382 participants); and 1.20 (1.10–1.31) per 1% higher HbA1c (9 cohorts, 1639 cases, 49,099 participants).Conclusions:
In the Reykjavik Study and a meta-analysis of other Western prospective studies, fasting and post-load glucose levels were modestly associated with CHD risk in people without diabetes. The meta-analysis suggested a somewhat stronger association between HbA1c levels and CHD risk.
: Please see later in the article for the Editors' SummaryIntroduction
Diabetes is an established risk factor for coronary heart disease (CHD). There is considerable interest in whether circulating markers of glucose metabolism are associated with risk of CHD in people without diabetes. Various measures of dysglycaemia have been assessed in long-term studies of CHD, notably: fasting glucose concentration (an indicator of steady-state glucose metabolism at the time of measurement); post-load glucose concentration (an indicator of immediate response to glycaemic stress); and glycated haemoglobin (HbA1c, an indicator of average blood glucose concentration over the previous 1–3 mo) [1],[2]. It has been proposed that markers of dysglycaemia may be log-linearly and importantly associated with risk of subsequent vascular disease at all levels (including below the thresholds defining diabetes) [3]–[5], but available data are not conclusive. For example, the US Preventive Services Task Force recently stated that published prospective data on fasting glucose and CHD were “inconsistent” and had “serious limitations” [6].
We report new data from the population-based Reykjavik prospective study on associations of fasting and post-load glucose levels with CHD incidence across the range of glucose values. We also did a systematic review and meta-analysis of tabular data from 26 additional relevant Western cohorts [7]–[31]. In total, the current report considers data on 303,961 participants, including 16,982 incident CHD cases.
Methods
Participants in the Reykjavik Study
The Reykjavik study has been described in detail previously [32]. Men born during 1907–1934 and women born during 1908–1935 who were resident in Reykjavik, Iceland and its adjacent communities on 1 December 1966 were identified in the national population register and invited to participate during five stages of recruitment between 1967 and 1991. A total of 8,888 male and 9,681 female participants without a history of myocardial infarction agreed to take part (72% response rate). Nurses administered questionnaires, made physical measurements, recorded an electrocardiogram, and collected fasting blood samples (taken after ≥8 h of fasting) at baseline. All participants have been monitored subsequently by central registries for occurrence of major cardiovascular morbidity (based on WHO MONICA [Multinational Monitoring of Trends and Determinants in Cardiovascular Disease] or similar criteria) or cause-specific mortality (based on a death certificate with International Classification of Diseases [ICD] 9 codes 410–414), with a loss to follow-up of only about 0.6% to date. During mean follow-up of 23.5 y, nonfatal MI or fatal CHD was recorded in a total of 4,664 participants, of whom 4,490 (including 3,088 men and 1,402 women) had no history of diabetes at baseline. Data were not available on incidence of stroke, diabetes, or microvascular disease. The National Bioethics Committee and the Data Protection Authority of Iceland approved the study protocol, and participants gave informed consent.
Laboratory Methods
Fasting glucose measurement was carried out in fresh capillary whole blood within hours of blood sampling at the initial examination by a micro method on a Technicon Autoanalyzer using a modification of the W. S. Hoffman method (coefficient of variation 4%) and standardised to the Hyland Normal Clinical Chemistry Control Serum (Nygaard A/S, Oslo) [33]. Post-load glucose levels were measured 60 and 90 min after ingestion of a 50 g glucose load. Lipid and other measurements involved standard assays, as previously described [32]. HbA1c measurements were not done.
Statistical Methods
The shapes of associations with CHD risk in the Reykjavik study were characterized by calculation of hazard ratios (HRs) across tenths of glucose values, including the full range of glucose values observed. Cox proportional hazards regression models were adjusted for age, sex, smoking status, systolic blood pressure, total cholesterol, and body mass index (BMI). Ninety-five percent confidence intervals (95% CIs) were estimated from the variances that reflect the amount of information underlying each group (including the reference group) [34]. Subsidiary analyses corrected for regression dilution using serial glucose measurements made in 370 of the participants (mean interval: 12 y) [35]. In 18,333 participants without evidence of diabetes at baseline (i.e., no self-reported history and fasting blood glucose <7 mmol/l), HRs for CHD were calculated per 1 mmol/l higher glucose concentration. Impaired fasting glucose was defined using published guidelines [36]. Effect-modification was investigated by formal tests of interaction.
Systematic Review
Three of the current investigators (NS, RG, and SRKS) sought prospective studies published between January 1970 and September 2009 that had reported on associations of fasting blood glucose, post-load glucose, and/or HbA1c with incident CHD. Details of the search strategies and a flow diagram are provided in Text S1. Published studies were identified through electronic searches not limited to the English language (using MEDLINE, EMBASE, BIOSIS, and the Science Citation Index), by scanning reference lists of articles identified for all relevant studies (including review articles), and by hand searching of selected general medical journals (i.e., BMJ, JAMA, Lancet, NEJM, PLoS Medicine, Annals of Internal Medicine, Archives of Internal Medicine), cardiovascular/diabetes journals (i.e., Circulation, Diabetes, Diabetes Care, European Heart Journal, Journal of the American College of Cardiology), and epidemiological journals (i.e., American Journal of Epidemiology, International Journal of Epidemiology). Studies were eligible for inclusion if they: (1) did not select participants on the basis of having pre-existing vascular disease; (2) were located in Western Europe, North America, or Australasia (a restriction to reduce potential heterogeneity due to factors related to geographical location, e.g., ethnicity; see Discussion); (3) had more than 1 y of follow-up; and (4) reported on nonfatal MI (as defined by WHO MONICA or equivalent criteria, i.e., involving diagnosis based on clinical symptoms, electrocardiographic abnormalities, and/or cardiac biomarkers) and/or fatal CHD (defined by ICD criteria). Eligibility for inclusion of identified studies was considered by two investigators (RG and SRKS). Any disagreement was resolved by discussion and, if necessary, by the deciding vote of a third reviewer (NS).
A request for tabular data was sent to investigators of every eligible study identified. The following information was sought (excluding participants with a self-reported history of diabetes and/or fasting blood glucose ≥7 mmol/l at baseline), according to a uniform protocol: number of incident CHD outcomes recorded; relative risk (RR with 95% CI for CHD per unit higher dysglycaemia marker, initially after adjustment for age and sex only and then after additional adjustment for smoking status, systolic blood pressure, lipid concentrations, and BMI); the content of the glucose load and interval before post-load venipuncture; number of participants with resurvey measurements; interval between such measurements; and degree of long-term within-person variability of dysglycaemia markers (Table S1). Accuracy of the information supplied was cross-checked against published data. In the few instances of apparent discrepancy, resolution was achieved through consultation with study investigators.
Meta-analysis
Summary RRs for CHD per unit higher dysglycaemia marker were calculated by pooling study-specific estimates using a random effects model. Analyses involved only within-study comparisons. As regards units of analysis used, 1 mmol/l higher fasting glucose corresponds approximately to 1-standard deviation (SD) higher levels; 1 mmol/l higher post-load glucose corresponds approximately to 2-SD higher levels; and 1% higher HbA1c corresponds approximately to 1-SD higher levels. Consistency of findings was assessed by standard χ2 tests and the I2 statistic [37]. Diversity at the study level was investigated by grouping studies by recorded characteristics and by meta-regression (including study size and duration of follow-up as continuous variables). Small-study effects were investigated [38].
Results
Reykjavik Study
Baseline conventional risk factors were significantly higher in those who subsequently recorded incident CHD than in non-cases, as were fasting and 1-h post-load glucose levels (Table S2). Fasting and 1-h post-load glucose levels were each significantly correlated with several conventional risk factors and with each other (Table S3). In people without diabetes at baseline, serial measurements yielded intra-class correlation coefficients of 0.61 (95% CI 0.54–0.67) for fasting blood glucose, 0.50 (0.42–0.57) for 1-h post-load glucose, 0.59 (0.51–0.67) for total cholesterol, and 0.65 (0.54–0.77) for systolic blood pressure. In people who had no history of diabetes at baseline, HR for CHD—adjusted for age, sex, recruitment period, smoking status, systolic blood pressure, total cholesterol, and BMI (henceforth, “adjusted HR”)—was 2.37 (1.79–3.14) in those with fasting glucose ≥7.0 mmol/l compared with those <7.0 mmol/l. (HR was 1.67 [1.36–2.02] with fasting glucose ≥6.1 mmol/l compared with those <6.1 mmol/l, a definition of diabetes proposed for studies involving capillary whole blood samples [39].) In analyses across tenths of fasting glucose values, adjusted HRs for CHD were generally weak and nonsignificant at levels below 7 mmol/l (Figure 1). Findings were broadly similar for 1-h post-load glucose levels (Figure 2) and in analyses that: adjusted for age and sex only (Figure S1); corrected for regression dilution (Figure S2); assessed 90-min post-load glucose (available upon request from NS).
Fig. 1. Risk of CHD across tenths of baseline fasting glucose levels in the Reykjavik Study. 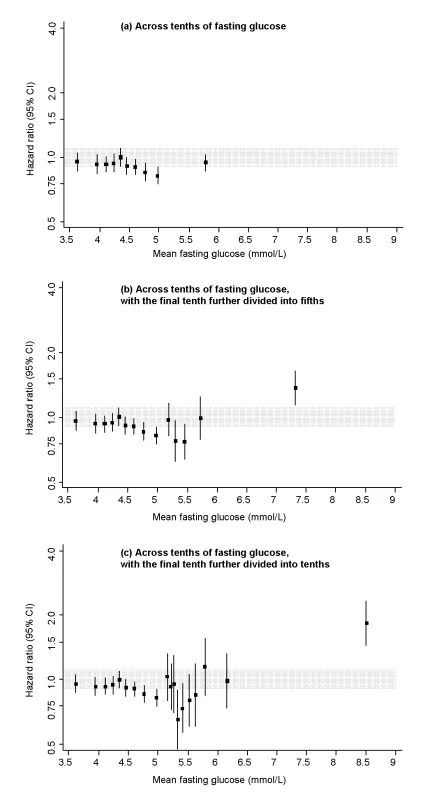
All hazard ratios are adjusted for age, sex, recruitment period, smoking status, systolic blood pressure, total cholesterol, and BMI, and all are compared to individuals in the middle tenth of the distribution. The grey area denotes the 95% CI of the reference group. Analyses involved the full range of glucose values (i.e., including individuals with glucose levels in the diabetic range). To limit any bias related to having a diagnosis of diabetes (e.g., medication use, lifestyle changes), however, individuals with a known history of diabetes at the baseline survey were excluded. Fig. 2. Risk of CHD across tenths of baseline 1 h post-load glucose levels in the Reykjavik Study. 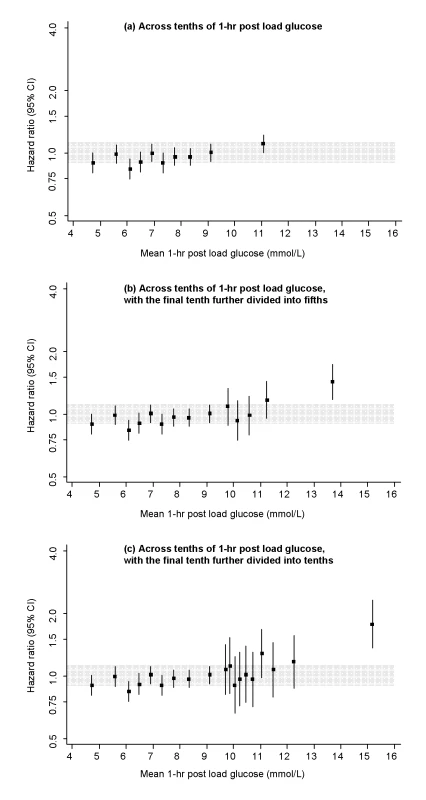
All hazard ratios are adjusted for age, sex, recruitment period, smoking status, systolic blood pressure, total cholesterol, and BMI, and all are compared to individuals in the middle tenth of the distribution. The grey area denotes the 95% CI of the reference group. Analyses involved the full range of glucose values (i.e., including individuals with glucose levels in the diabetic range). To limit any bias related to having a diagnosis of diabetes (e.g., medication use, lifestyle changes), however, individuals with a known history of diabetes at the baseline survey were excluded. In people who had no history of diabetes and fasting glucose <7.0 mmol/l at baseline, adjusted HR for CHD was 0.95 (0.89–1.01) per 1 mmol/l higher fasting glucose and 1.03 (1.01–1.05) per 1 mmol/l higher 1-h post-load glucose. Similar findings were observed in analyses that: explored HRs in a range of clinically relevant subgroups (Figure S3); assessed HRs in 5-y intervals of follow-up (available upon request); or excluded people with post-load glucose levels ≥11.1 mmol/l (available upon request). Compared with individuals with fasting glucose <5.6 mmol/l, adjusted HRs for CHD were: 1.27 (0.96–1.68) with fasting glucose levels 6.1–7.0 mmol/l and 1.08 (0.87–1.33) with fasting glucose levels 5.6–6.1 mmol/l (i.e., corresponding to categories of fasting glucose concentration used to define impaired fasting glucose).
Meta-analysis
Thirty-five potentially eligible prospective studies were identified, including the present Reykjavik study. Of these studies, 27 contributed data to the current analysis, yielding 303,961 participants, including 16,982 incident CHD cases (Table S4). This information constitutes >85% of the relevant incident CHD cases identified (Text S1 lists the noncontributing studies). Fourteen of the contributing studies were based in Western Europe, ten in North America, and three in Australia. All recruited participants from population registers or occupational settings. Fifty-six percent of the participants were male, and most were middle-aged and of European descent. For fasting glucose, there were 23 contributing studies, yielding 255,171 participants and 10,808 incident CHD cases. All studies used standard glucose assay methods and reported generally similar mean glucose values (Table S4), with four studies involving whole blood samples and 19 plasma or serum. For post-load glucose, there were 15 contributing studies, yielding 102,382 participants and 12,652 cases. Ten of these studies measured glucose 2 h after a 75 g load and five studies used other methods. For HbA1c, there were nine contributing studies, yielding 49,099 participants and 1,639 cases.
Seven studies [8],[11],[13],[17],[23],[30] provided information on long-term serial measurements, yielding weighted intra-class correlation coefficients of 0.67 (0.66–0.69) for fasting glucose (7,834 participants, mean interval 3.5 y), 0.48 (0.46–0.50) for post-load glucose (5,617 participants, 3.5 y), and 0.69 (0.68–0.70) for HbA1c (6,370 participants, 4 y). Combined RRs, adjusted for age and sex only, were: 1.12 (1.06–1.18) per 1 mmol/l higher fasting glucose; 1.08 (1.04–1.11) per 1 mmol/l higher post-load glucose; and 1.34 (1.24–1.44) per 1% higher HbA1c. Corresponding RRs adjusted for several conventional risk factors were: 1.06 (1.00–1.12) with fasting glucose; 1.05 (1.03–1.07) with post-load glucose; and 1.20 (1.10–1.31) with HbA1c (Figure 3). There was heterogeneity in each RR (I2 of 82% [74–88], 88% [83–93], and 42% [0–69], respectively), but little of it was explained by characteristics recorded (Figure 4). For fasting glucose, RRs were very similar in studies involving whole blood samples and in those with plasma or serum (1.05 versus 1.06). For post-load glucose, RRs were very similar in studies measuring glucose 2 h after a 75 g glucose load and in those in studies using other methods (1.05 versus 1.05). Subsidiary analyses that omitted any particular study did not materially alter the findings (e.g., omission of the Reykjavik study yielded adjusted RRs of 1.06 [1.00–1.12] with fasting glucose and 1.05 [1.03–1.08] with post-load glucose; and omission of the San Antonio Heart Study, which had unusually narrow CIs given its size, yielded adjusted RRs of 1.02 [0.99–1.06] with fasting glucose and 1.04 [1.02–1.07] with post-load glucose). There was no good evidence of small study effects (e.g., Egger test p>0.1 for each marker assessed). Literature-based analyses yielded broadly similar RRs with fasting glucose in the non-contributing studies to those reported here (Text S1).
Fig. 3. Prospective studies of markers of dysglycaemia and CHD risk in individuals without diabetes in Western populations. 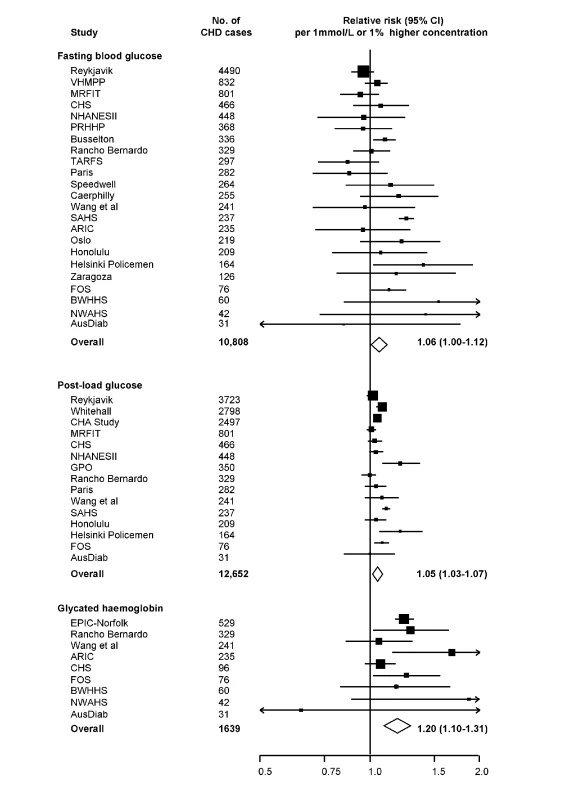
Analyses were restricted to individuals who did not have a self-reported history of type 2 diabetes or had a fasting blood glucose <7.0 mmol/l at baseline. Risk estimates are presented per 1 mmol/l higher fasting and post-load glucose, and per 1% higher HbA1c. Abbreviations as listed in Table S2. Fig. 4. Prospective studies of fasting and post-load glucose and CHD risk in individuals without diabetes in Western population, grouped by study characteristics. 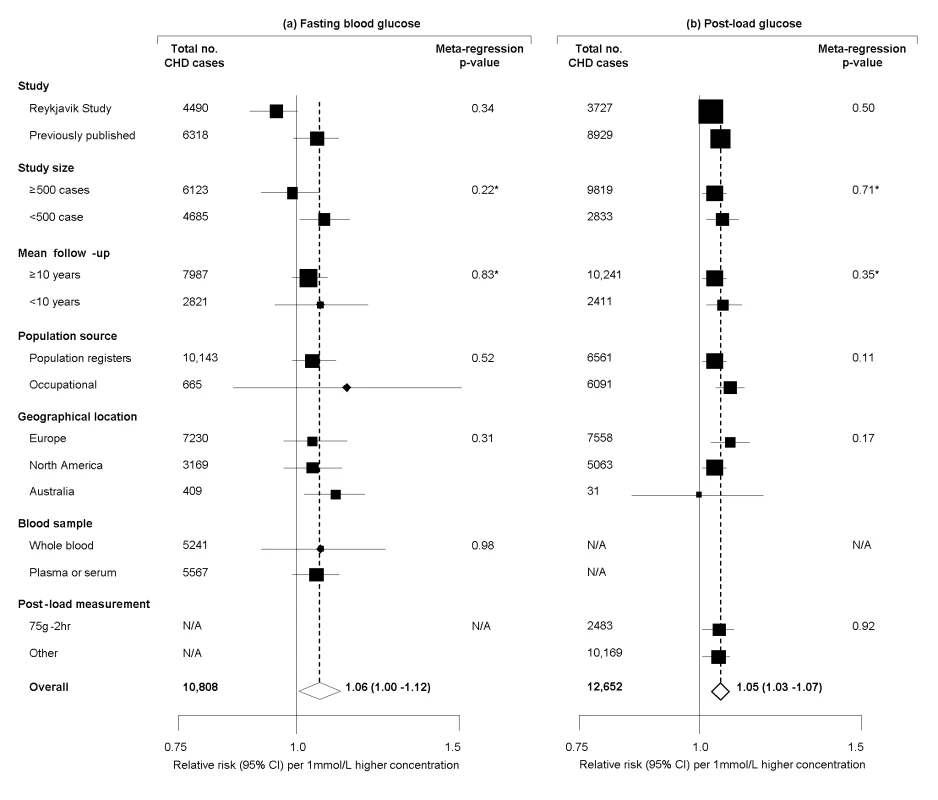
Meta-regression analyses separately considered each characteristic presented. *Assessed as continuous factors. Discussion
The current data indicate that fasting and post-load glucose and HbA1c each have reasonably high degrees of long-term within-person reproducibility (i.e., broadly comparable to such reproducibility values for total cholesterol and systolic blood pressure). The current meta-analysis of data from population-based Western prospective studies involving a total of >300,000 people (≈17,000 incident CHD cases) indicate that fasting glucose concentration is modestly associated with CHD risk in people without diabetes, i.e., RR for CHD was about 1.06 per 1 mmol/l higher fasting glucose. Furthermore, in the Reykjavik prospective study, RRs were generally modest and nonsignificant across glucose values below the diabetes definition (i.e., fasting glucose <7 mmol/l). We observed similar results in relation to post-load glucose. The current findings contrast with those from some smaller previous studies, which have suggested that glucose values are log-linearly and more strongly associated with CHD risk (including at glucose values lower than those defining diabetes). Because previous epidemiological estimates have influenced scientific guideline statements [40], clinical risk assessment strategies [41], burden of disease estimates [42], and public health policy recommendations [43], it may be helpful to review such efforts in the light of the current updated epidemiological evidence. Careful consideration may also need to be given to the design and interpretation of trials of CHD prevention using glucose-lowering agents in people without diabetes, as the current findings suggest that trial sample sizes required may be larger than previously anticipated [44]–[47].
In contrast with findings for glucose concentration, the current meta-analysis has indicated a RR for CHD of 1.20 per 1% higher HbA1c in people without diabetes. Although RRs for CHD appear stronger with HbA1c than those with glucose concentration, this possibility requires careful interpretation because: (1) the comparison is an indirect one (i.e., fasting glucose and HbA1c measurements were typically not made in the same participants) and (2) fewer than one-fifth as many incident CHD cases have been reported with HbA1c as with glucose concentration (so associations with HbA1c cannot be quantified as reliably as those with glucose levels). Nevertheless, because the current data suggest that fasting glucose and HbA1c have similar levels of within-person variability over several years, such variability seems unlikely to account for differences seen in RRs with different measures of dysglycaemia. It remains uncertain whether HbA1c is a more informative measure of dysglycaemia than are fasting or post-load glucose levels, more accurately reflects processes relevant to vascular damage in response to glycation, or some combination of these possibilities [2],[48].
The strengths and potential limitations of this study merit consideration. For fasting glucose, the Reykjavik study involves more incident CHD cases than in any previous prospective study. It identified participants in population registers, achieved high response and follow-up rates, and entailed robust ascertainment of incident MI and fatal CHD. We have demonstrated the validity of the glucose measurements in capillary whole blood samples by showing: the expected strong associations of fasting glucose levels with CHD in people with values ≥7.0 mmol/l; long-term within-person consistency of glucose concentration comparable to that for systolic blood pressure and total cholesterol concentration; and similar findings as in previous studies that used plasma or serum. For post-load glucose, RRs in the Reykjavik study (involving assessment 1 h after ingestion of a 50 g glucose load) were very similar to RRs in studies involving assessment 2 h after ingestion of a 75 g glucose load.
As findings in the Reykjavik study were reinforced by a meta-analysis of tabular data from 26 other long-term prospective studies located in ten Western countries, it increases the likelihood that these results can be extrapolated, at least to other Western populations. Although our review focused only on Western cohorts to reduce heterogeneity, it may be relevant to note that the largest available prospective study in East Asia (≈3,100 incident MI outcomes) has reported similar findings to those described here, concluding that fasting glucose concentration has no clear association with MI risk below the diabetes definition [49]. Our meta-analysis included >85% of the relevant data identified by the systematic review (and a literature-based sensitivity analyses of noncontributing studies yielded broadly similar findings). Although we noted heterogeneity, it was not explained by the characteristics recorded here. Because some previous reviews did not consistently exclude people with diabetes at baseline [3],[4] or involved only fatal CHD [5], it is difficult to compare their RRs directly with the RRs observed here. A more detailed consideration of available prospective studies, perhaps on the basis of combination of individual participant data, will enable more reliable analyses under a broader range of circumstances and a more detailed investigation of potential sources of diversity.
Conclusions
In people without diabetes, fasting and post-load glucose levels were modestly associated with CHD risk. Associations of HbA1c with CHD risk in such people appeared somewhat stronger. Scientific guidelines, policies, and trial designs premised on the existence of strong, log-linear associations of fasting and post-load glucose concentration with CHD risk may benefit from review in light of these epidemiological findings.
Note Added in Proof
Investigators of an additional study of fasting glucose concentration and CHD [50], involving a further 6,447 participants and 862 incident CHD cases, provided tabular data while this article was in proof. After addition of these data to the meta-analysis, the combined adjusted relative risk for CHD was 1.05 (1.00–1.10) per 1 mmol/l higher fasting glucose concentration (24 cohorts, 11,670 cases, 261,618 participants).
Supporting Information
Zdroje
1. GoldsteinDE
LittleRR
LorenzRA
MaloneJI
NathanDM
2003 Tests of glycemia in diabetes. Diabetes Care 26 Suppl 1 S106 8
2. BeckmanJA
CreagerMA
LibbyP
2002 Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287 2570 81
3. CoutinhoM
GersteinHC
WangY
YusufS
1999 The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22 233 40
4. LevitanEB
SongY
FordES
LiuS
2004 Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med 164 2147 55
5. DECODE Study Group, the European Diabetes Epidemiology Group 2001 Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 161 397 405
6. HelfandM
BuckleyDI
FreemanM
FuR
RogersK
2009 Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med 151 496 507
7. UlmerH
KelleherC
DiemG
ConcinH
2004 Why Eve is not Adam: prospective follow-up in 149650 women and men of cholesterol and other risk factors related to cardiovascular and all-cause mortality. J Womens Health (Larchmt) 13 41 53
8. EberlyLE
PrineasR
CohenJD
VazquezG
ZhiX
2006 Metabolic syndrome: risk factor distribution and 18-year mortality in the multiple risk factor intervention trial. Diabetes Care 29 123 30
9. SmithNL
BarzilayJI
ShafferD
SavagePJ
HeckbertSR
2002 Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the Cardiovascular Health Study. Arch Intern Med 162 209 16
10. SaydahSH
MiretM
SungJ
VarasC
GauseD
2001 Postchallenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care 24 1397 402
11. Cruz-VidalM
Garcia-PalmieriMR
CostasRJr
SorliePD
HavlikRJ
1983 Abnormal blood glucose and coronary heart disease: the Puerto Rico Heart Health Program. Diabetes Care 6 556 61
12. FerrieJE
Singh-ManouxA
KivimäkiM
MindellJ
BreezeE
2009 Cardiorespiratory risk factors as predictors of 40-year mortality in women and men. Heart 95 1250 7
13. WelbornTA
WearneK
1979 Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Diabetes Care 2 154 60
14. Scheidt-NaveC
Barrett-ConnorE
WingardDL
CohnBA
EdelsteinSL
1991 Sex differences in fasting glycemia as a risk factor for ischemic heart disease death. Am J Epidemiol 133 565 76
15. OnatA
HergençG
CanG
2007 Prospective validation in identical Turkish cohort of two metabolic syndrome definitions for predicting cardiometabolic risk and selection of most appropriate definition. Anadolu Kardiyol Derg 7 29 34
16. BalkauB
ShipleyM
JarrettRJ
PyoralaK
PyoralaM
1998 High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 21 360 7
17. YarnellJW
PickeringJE
ElwoodPC
BakerIA
BaintonD
1994 Does non-diabetic hyperglycemia predict future IHD? Evidence from the Caerphilly and Speedwell studies. J Clin Epidemiol 47 383 8
18. WangJ
RuotsalainenS
MoilanenL
LepistöP
LaaksoM
2007 The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J 28 857 64
19. SternMP
FatehiP
WilliamsK
HaffnerSM
2002 Predicting future cardiovascular disease: do we need the oral glucose tolerance test? Diabetes Care 25 1851 6
20. SelvinE
CoreshJ
GoldenSH
BrancatiFL
FolsomAR
2005 Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med 165 1910 6
21. BjornholtJV
ErikssenG
AaserE
SandvikL
Nitter-HaugeS
1999 Fasting blood glucose: an underestimated risk factor for cardiovascular death. Results from a 22-year follow-up of healthy nondiabetic men. Diabetes Care 22 45 9
22. RodriguezBL
LauN
BurchfielCM
AbbottRD
SharpDS
1999 Glucose intolerance and 23-year risk of coronary heart disease and total mortality: the Honolulu Heart Program. Diabetes Care 22 1262 5
23. PyöräläM
MiettinenH
HalonenP
LaaksoM
PyöräläK
2000 Insulin resistance syndrome predicts the risk of coronary heart disease and stroke in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Arterioscler Thromb Vasc Biol 20 38 44
24. MarinA
MedranoMJ
GonzalezJ
PintadoH
CompairedV
2006 Risk of ischaemic heart disease and acute myocardial infarction in a Spanish population: observational prospective study in a primary-care setting. BMC Public Health 6 38
25. MeigsJB
NathanDM
D'AgostinoRBSr
Wilson PW; Framingham Offspring Study. 2002 Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 25 1845 50
26. LawlorDA
SmithGD
EbrahimS
2006 Does the new International Diabetes Federation definition of the metabolic syndrome predict CHD any more strongly than older definitions? Findings from the British Women's Heart and Health Study. Diabetologia 49 41 8
27. AdamsRJ
AppletonSL
HillCL
WilsonDH
TaylorAW
2009 Independent association of HbA(1c) and incident cardiovascular disease in people without diabetes. Obesity (Silver Spring) 17 559 63
28. BarrEL
ZimmetPZ
WelbornTA
JolleyD
MaglianoDJ
2007 Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 116 151 7
29. BrunnerEJ
ShipleyMJ
WitteDR
FullerJH
Marmot MG. 2006 Relation between blood glucose and coronary mortality over 33 years in the Whitehall Study. Diabetes Care 29 26 31
30. OrenciaAJ
DaviglusML
DyerAR
WalshM
GreenlandP
1997 One-hour postload plasma glucose and risks of fatal coronary heart disease and stroke among nondiabetic men and women: the Chicago Heart Association Detection Project in Industry (CHA) Study. J Clin Epidemiol 50 1369 76
31. KhawKT
WarehamN
BinghamS
LubenR
WelchA
2004 Association of haemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 141 413 20
32. JonsdottirLS
SigfussonN
GudnasonV
SigvaldasonH
ThorgeirssonG
2002 Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. J Cardiovasc Risk 9 67 76
33. HoffmanWS
1973 Rapid photoelectric method for the determination of glucose in blood and urine. J Biol Chem 120 51 4
34. EastonDF
PetoJ
BabikerAG
1991 Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med 10 1025 35
35. ClarkeR
ShipleyM
LewingtonS
YoungmanL
CollinsR
1999 Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 150 341 353
36. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus 2003 Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26 3160 7
37. HigginsJP
ThompsonSG
DeeksJJ
AltmanDG
2003 Measuring inconsistency in meta-analyses. BMJ 327 557 60
38. EggerM
Davey SmithG
SchneiderM
Minder C 1997 Bias in meta-analysis detected by a simple, graphical test. BMJ 315 629 34
39. WHO consultation report. Definition, diagnosis and classification of diabetes mellitus and its complications. WHO/NCD/NCS/ 91.2
40. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD). 2007 Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J 28 88 136
41. KahnR
BuseJ
FerranniniE
SternM
2005 The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28 2289 304
42. DanaeiG
LawesCM
Vander HoornS
MurrayCJ
Ezzati M 2006 Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet 368 1651 9
43. AvendanoM
MackenbachJP
2006 Blood glucose levels: facing a global crisis. Lancet 368 1631 2
44. GersteinH
YusufS
RiddleMC
RydenL
Origin Trial Investigators 2008 Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 155 26 32
45. HolmanRR
2007 A new era in the secondary prevention of CVD in prediabetes - the Acarbose Cardiovascular Evaluation (ACE) trial. Diab Vasc Dis Res 4Suppl 1 S40
46. DagenaisGR
GersteinHC
HolmanR
BudajA
DREAM Trial Investigators 2008 Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care 31 1007 1014
47. The NAVIGATOR Study Group, Holman RR, Haffner SM, McMurray JJ, et al. 2010 Effect of Nateglinide on the Incidence of Diabetes and Cardiovascular Events. N Engl J Med 362 1463 1476
48. KhawKT
WarehamN
2006 Glycated haemoglobin as a marker of cardiovascular risk. Curr Opin Lipidol 17 637 43
49. SungJ
SongYM
EbrahimS
LawlorDA
2009 Fasting blood glucose and the risk of stroke and myocardial infarction. Circulation 119 812 9
50. PreissD
WelshP
MurrayHM
ShepherdJ
PackardC
2010 Fasting plasma glucose in non-diabetic participants and the risk for incident cardiovascular events, diabetes, and mortality: results from WOSCOPS 15-year follow-up. Eur Heart J. Epub ahead of print doi:10.1093/eurheartj/ehq095
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 5- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Suicide Prevention for Older Adults in Residential Communities: Implications for Policy and Practice
- Non-Communicable Diseases in Sub-Saharan Africa: The Case for Cohort Studies
- Early Pandemic Influenza (2009 H1N1) in Ho Chi Minh City, Vietnam: A Clinical Virological and Epidemiological Analysis
- Anatomy of the Epidemiological Literature on the 2003 SARS Outbreaks in Hong Kong and Toronto: A Time-Stratified Review
- Prognostic Significance of Subtype Classification for Short- and Long-Term Survival in Breast Cancer: Survival Time Holds the Key
- Negotiating Equitable Access to Influenza Vaccines: Global Health Diplomacy and the Controversies Surrounding Avian Influenza H5N1 and Pandemic Influenza H1N1
- Which New Approaches to Tackling Neglected Tropical Diseases Show Promise?
- Self-Injurious Behavior in Adolescents
- Markers of Dysglycaemia and Risk of Coronary Heart Disease in People without Diabetes: Reykjavik Prospective Study and Systematic Review
- Can Foreign Policy Make a Difference to Health?
- A Population-Based Evaluation of a Publicly Funded, School-Based HPV Vaccine Program in British Columbia, Canada: Parental Factors Associated with HPV Vaccine Receipt
- Genetic Markers of Adult Obesity Risk Are Associated with Greater Early Infancy Weight Gain and Growth
- New Complexities and Approaches to Global Health Diplomacy: View from the U.S. Department of State
- The Impact of Phenotypic and Genotypic G6PD Deficiency on Risk of Infection: A Case-Control Study amongst Afghan Refugees in Pakistan
- The Malawi Developmental Assessment Tool (MDAT): The Creation, Validation, and Reliability of a Tool to Assess Child Development in Rural African Settings
- Journals, Academics, and Pandemics
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Self-Injurious Behavior in Adolescents
- Non-Communicable Diseases in Sub-Saharan Africa: The Case for Cohort Studies
- The Malawi Developmental Assessment Tool (MDAT): The Creation, Validation, and Reliability of a Tool to Assess Child Development in Rural African Settings
- Journals, Academics, and Pandemics
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



