-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenetic Markers of Adult Obesity Risk Are Associated with Greater Early Infancy Weight Gain and Growth
Background:
Genome-wide studies have identified several common genetic variants that are robustly associated with adult obesity risk. Exploration of these genotype associations in children may provide insights into the timing of weight changes leading to adult obesity.Methods and Findings:
Children from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort were genotyped for ten genetic variants previously associated with adult BMI. Eight variants that showed individual associations with childhood BMI (in/near: FTO, MC4R, TMEM18, GNPDA2, KCTD15, NEGR1, BDNF, and ETV5) were used to derive an “obesity-risk-allele score” comprising the total number of risk alleles (range: 2–15 alleles) in each child with complete genotype data (n = 7,146). Repeated measurements of weight, length/height, and body mass index from birth to age 11 years were expressed as standard deviation scores (SDS). Early infancy was defined as birth to age 6 weeks, and early infancy failure to thrive was defined as weight gain between below the 5th centile, adjusted for birth weight. The obesity-risk-allele score showed little association with birth weight (regression coefficient: 0.01 SDS per allele; 95% CI 0.00–0.02), but had an apparently much larger positive effect on early infancy weight gain (0.119 SDS/allele/year; 0.023–0.216) than on subsequent childhood weight gain (0.004 SDS/allele/year; 0.004–0.005). The obesity-risk-allele score was also positively associated with early infancy length gain (0.158 SDS/allele/year; 0.032–0.284) and with reduced risk of early infancy failure to thrive (odds ratio = 0.92 per allele; 0.86–0.98; p = 0.009).Conclusions:
The use of robust genetic markers identified greater early infancy gains in weight and length as being on the pathway to adult obesity risk in a contemporary birth cohort.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(5): e32767. doi:10.1371/journal.pmed.1000284
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000284Summary
Background:
Genome-wide studies have identified several common genetic variants that are robustly associated with adult obesity risk. Exploration of these genotype associations in children may provide insights into the timing of weight changes leading to adult obesity.Methods and Findings:
Children from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort were genotyped for ten genetic variants previously associated with adult BMI. Eight variants that showed individual associations with childhood BMI (in/near: FTO, MC4R, TMEM18, GNPDA2, KCTD15, NEGR1, BDNF, and ETV5) were used to derive an “obesity-risk-allele score” comprising the total number of risk alleles (range: 2–15 alleles) in each child with complete genotype data (n = 7,146). Repeated measurements of weight, length/height, and body mass index from birth to age 11 years were expressed as standard deviation scores (SDS). Early infancy was defined as birth to age 6 weeks, and early infancy failure to thrive was defined as weight gain between below the 5th centile, adjusted for birth weight. The obesity-risk-allele score showed little association with birth weight (regression coefficient: 0.01 SDS per allele; 95% CI 0.00–0.02), but had an apparently much larger positive effect on early infancy weight gain (0.119 SDS/allele/year; 0.023–0.216) than on subsequent childhood weight gain (0.004 SDS/allele/year; 0.004–0.005). The obesity-risk-allele score was also positively associated with early infancy length gain (0.158 SDS/allele/year; 0.032–0.284) and with reduced risk of early infancy failure to thrive (odds ratio = 0.92 per allele; 0.86–0.98; p = 0.009).Conclusions:
The use of robust genetic markers identified greater early infancy gains in weight and length as being on the pathway to adult obesity risk in a contemporary birth cohort.
: Please see later in the article for the Editors' SummaryIntroduction
The increasing prevalence of overweight and obesity even in young preschool children [1] highlights the need to understand the very early determinants and potential targets for prevention of obesity. It has been proposed that there are certain critical periods in childhood for the development of obesity, including gestation and early infancy, the period of adiposity rebound between ages 5 and 7 years, and adolescence [2]. However, the relevance of overweight and obesity in infants and very young children to adult obesity and its comorbidities is unclear [3]. Common genetic variation associated with adult obesity may provide an opportunity to identify the timing of childhood weight changes that are associated with later obesity risk.
Recent technological advances and the massive increases in scale and statistical rigour of genome-wide association (GWA) studies has allowed the identification of common genetic variants associated with adult BMI and obesity risk that are consistently replicable in other populations. The first such common genetic variation shown to be associated with adult body mass index (BMI) was in the FTO gene region, published in 2007 by Frayling et al. [4]. This was closely followed by variation downstream of MC4R in 2008 [5]. Several further loci, in or near TMEM18, GNPDA2, KCTD15, NEGR1, BDNF, ETV5, MTCH2, and SH2B1, were recently reported to be adult obesity risk variants by studies from the GIANT international consortium [6] and the deCODE Genetics group [7].
To date all the published GWA-BMI studies primarily focussed on the association with adult BMI. The initial reports of common variants in/near to FTO and MC4R also included a demonstration of their relevance to childhood BMI and childhood obesity [4],[5], and these have since been confirmed in other childhood studies [8],[9]. A further four of the six new variants for adult BMI identified by the GIANT consortium also showed association with childhood BMI and/or childhood obesity (TMEM18, GNPDA2, KCTD15, and NEGR1) [6]. However, the joint publication by deCODE Genetics did not include any childhood populations [7].
Although cross-sectional associations with childhood BMI have been reported [4]–[6], the effects for most of these obesity risk variants on the rate of growth and weight gain during infancy and childhood have not yet been established. Data from the population-based Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort and other childhood obesity studies have shown the relevance of individual variants in FTO [4], MC4R [5], TMEM18, GNPDA2, KCTD15, and NEGR1 [6] for childhood BMI and body fat mass. By further analysing these adult obesity susceptibility loci in the ALSPAC birth cohort, with the addition of variants in BDNF and ETV5 [7], we aimed to identify the specific timing of childhood weight gain and growth associated with adult obesity risk. As the effects of individual variants are small (in the original studies of adult BMI the replication populations numbered in the tens of thousands [4]–[7]), we used a composite score of obesity risk alleles to maximise statistical power and reduce multiple testing.
Methods
Study Population
ALSPAC is a prospective study that has been described in detail elsewhere [10] (http://www.alspac.bris.ac.uk). Briefly, 14,541 pregnant women living in one of three Bristol-based health districts in the former County of Avon with an expected delivery date between April 1991 and December 1992 were enrolled in the study. Detailed information has been collected using self-administered questionnaires, data extraction from medical notes, and linkage to routine information systems and at research clinics. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and Local Research Ethics Committees.
Growth Measurements
Infancy/early childhood
Birth weight as recorded in the delivery room was obtained from medical records. Birth length was measured by the study team. Infant and early childhood measures of weight and length (up to age 2 y) or height (from 2 y) at around ages 6 wk, 9 mo, and 1.5 and 3.5 y were available from routinely collected measurements performed by health visitors as part of the infant health surveillance programme and were extracted from the local child health database.
Later childhood
Childhood weight and height was measured annually between ages 7 and 11 y at dedicated ALSPAC Focus clinics by a trained research team. Height was measured to the nearest 0.1 cm using a Leicester Height Measure (Holtain Crosswell, Dyfed) and weight while wearing underwear was measured to the nearest 0.1 kg using Tanita electronic scales. Fat mass and fat-free mass was assessed at the 9-year-old research clinic visit by whole body dual energy X-ray absorptiometry (DXA) (Prodigy scanner, Lunar Radiation Corp, Madison, Wisconsin, US).
Genotypes
Genotype information was available for six GWA-obesity variants previously reported to show association with BMI or obesity in children [4]–[6]; these variants were: rs9939609 (in/near to FTO); rs17782313 (MC4R), rs6548238 (TMEM18), rs10938397 (GNPDA2), rs368794 (KCTD15), rs2568958 (NEGR1). New genotype information was generated for two further variants reported to be associated with BMI in adults: rs925946 (BDNF) and rs7647305 (ETV5) [7]. Genotyping was performed by KBiosciences Ltd (Hoddesdon, UK) using their own novel system of fluorescence-based competitive allele-specific PCR (KASPar). Details of assay design are available from the KBiosciences Web site (http://www.kbioscience.co.uk). Call rates were 93.3% for rs925946 (BDNF) and 92.3% for rs7647305 (ETV5). All genotype frequencies met Hardy-Weinberg Equilibrium criteria (p>0.1).
Calculations
BMI was calculated as weight (kg)/height2 (m2). Individual weight, length/height, and BMI values were converted to standard deviation scores (SDS) by comparison to the British 1990 growth reference, adjusted for sex and precise age at measurement [11],[12]. Values at birth were adjusted for gestational age using the growth reference. Children were classified as overweight or obese based on the International Obesity Task Force (IOTF) criteria, which uses population centile-based curves that pass through the 25 kg/m2 and 30 kg/m2 thresholds at age 18 y [13]. Fat and fat-free mass indices were calculated for each child from DXA measurements at age 9 y by dividing fat mass and fat-free mass (kg) by height squared (m2) [14].
Weight gain from birth to 6 wk, conditional on birth weight, was calculated using the following formula: [15]. SDSbirth represents weight SDS at birth, SDS6week weight SDS at the 6 wk measurement and r represents the population correlation between weight SDS at birth and 6 wk. The resulting SDSgain represents a measure of weight gain from birth that is conditional on birth weight. Early infancy “failure to thrive” was defined as infants with the slowest 5% conditional weight gain from birth within the ALSPAC population [16].
Statistical Analysis
Analyses were restricted to singleton white Europeans plus one randomly selected child from each mother for whom more than one child had entered the study. Linear regression was used to analyse whether common genetic variants in BDNF (rs925946) and ETV5 (rs7647305) showed cross-sectional associations with BMI, weight, height, and body composition at 9 y.
An “obesity-risk-allele score” was created by counting the total number of obesity risk alleles across the eight variants that showed association with childhood weight or BMI (in/near to: FTO, MC4R, TMEM18, GNPDA2, NEGR1, KCTD15, BDNF, and ETV5). Only one variant at each locus was chosen and only individuals with complete genotype data at all eight variants were included in the obesity-risk-allele score analyses (n = 7,146 children). A “weighted obesity-risk-allele-score” (where contributions of each variant were weighted according to their apparent effect size on adult BMI) showed essentially the same associations as the currently reported unweighted score (unpublished data). For comparison, a second obesity-risk-allele score was created based on all ten variants (i.e., with the addition of the two variants in/near SH2B1 and MTCH2 with no evidence for association with childhood BMI or obesity [6]).
Linear regression was used to analyse the association between the obesity-risk-allele score and weight, height, and BMI SDS, and with fat mass index and fat-free mass index at age 9 y with adjustment for sex, precise age at measurement, and height. Logistic regression was used to analyse the association between the obesity-risk-allele score and the risks of infancy failure to thrive, and overweight or obesity at age 9 y.
Longitudinal analyses of associations between the obesity-risk-allele score and rates of weight gain were performed using the xtmixed command in STATA 10.1 to fit random intercepts models [17]. By assigning a unique identifier for each individual, this command performs a multi-level mixed effects linear regression analysis, allowing for clustering within individuals. A risk-score × age interaction term was calculated and added to the xtmixed models. This interaction term denotes by how much the effect of the obesity-risk-allele score gets stronger per year, and can be interpreted as the effect of the obesity-risk-allele score on linear change in weight SDS with age (in years). In further models, additional polynomial interaction terms (risk-score × age2 and risk-score × age3) were not significant (unpublished data). All analyses were conducted using STATA version 10.1 [17].
Results
The ALSPAC Population
Characteristics of the ALSPAC sample of children (n = 7,146) with complete genotype data are described in Table 1. Compared to other white European ALSPAC children without genotype data (n = 6,090), participants in this sample were slightly heavier at birth and showed modest differences in childhood BMI (<0.1 SDS difference at any age); their mothers were slightly older and more educated, but there was no difference in maternal BMI (see Table S1). The prevalence of obesity at age 9 y by IOTF criteria was 3.9% and 4.0% in boys and girls, respectively. At the same age, 20.0% of boys and 20.2% of girls met the IOTF criteria for overweight or obese (Table 1).
Tab. 1. Summary of growth measurements by age and sex. 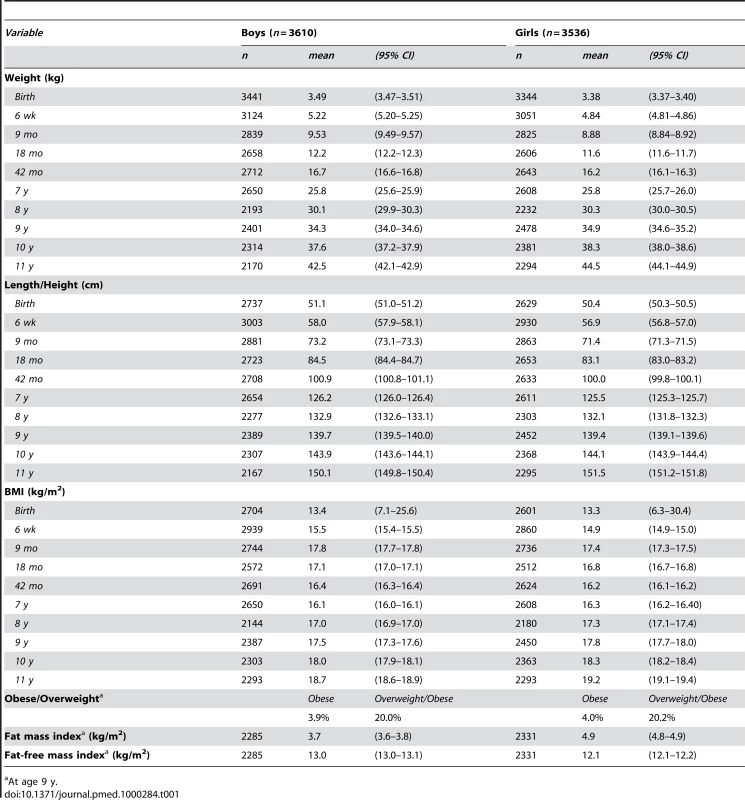
At age 9 y. Association of BDNF and ETV5 with Childhood BMI
Variants in rs925946 (BDNF) and rs7647305 (ETV5), which have not been previously studied in children, were associated with childhood BMI SDS, weight SDS, and height SDS (see Table S2). For example, at age 9 y each obesity-risk-allele at rs925946 was associated with +0.07 SDS (95% CI 0.03–0.12) greater childhood BMI, and +0.06 SDS (0.01–0.12) for rs7647305.
The Obesity-Risk-Allele Score
The obesity-risk-allele score based on genotypes at eight SNPs associated with childhood BMI (in/near to: FTO, MC4R, TMEM18, GNPDA2, NEGR, KCTD15, BDNF, and ETV5) ranged from 2 to 15 alleles. The score approximated a normal distribution and showed a linear association with BMI SDS at age 9 y (Figure 1). On average, each additional obesity risk allele conferred an estimated 0.07 SDS greater weight (95% CI 0.05–0.08; p = 1.2×10−17), 0.08 SDS greater BMI (0.06–0.10; p = 1.4×10−19), and 0.03 SDS greater height (0.01–0.04; p = 0.0003) at age 9 y. The differences in body size at age 9 y between the two extreme groups displayed in Figure 1 (≤4 risk alleles versus ≥13 risk-alleles) equated to 3.5 kg in body weight, 1.4 kg/m2 in BMI, and 2.0 cm in height at age 9 y. The obesity-risk-allele score explained 1.7% of the variance in BMI SDS at age 9 y (see Table S3). Furthermore, the obesity-risk-allele score was associated with increased risk of childhood overweight (odds ratio [OR] per allele = 1.14; 95% CI 1.10–1.19; p = 6.3×10−11) and childhood obesity (OR = 1.17; 1.07–1.26; p = 0.0002) at age 9 years, and was more strongly associated with childhood fat mass index (0.13 kg/m2 per allele; 0.10–0.16, adjusted for sex, age, and height) than with fat-free mass index (0.03 kg/m2 per allele; 0.01–0.04) (Table 2).
Fig. 1. Distribution of the obesity-risk-allele score in ALSPAC children. 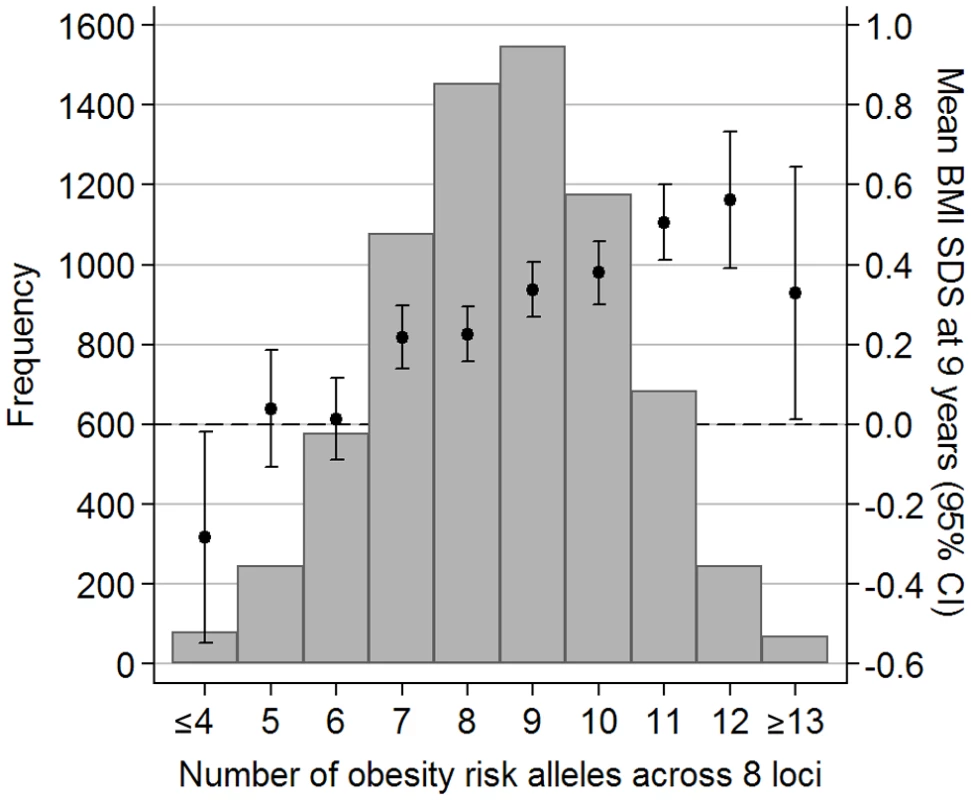
The risk-allele score comprises genotypes at eight loci (FTO, MC4R, TMEM18, GNPDA2, KCTD15, NEGR1, BDNF, and ETV5). Error bars (and 2nd y-axis) display the mean (±95% CI) BMI SDS at age 9 y for each risk score value. Tab. 2. Association of the obesity-risk-allele score with measures of growth and adiposity at age 9 y. 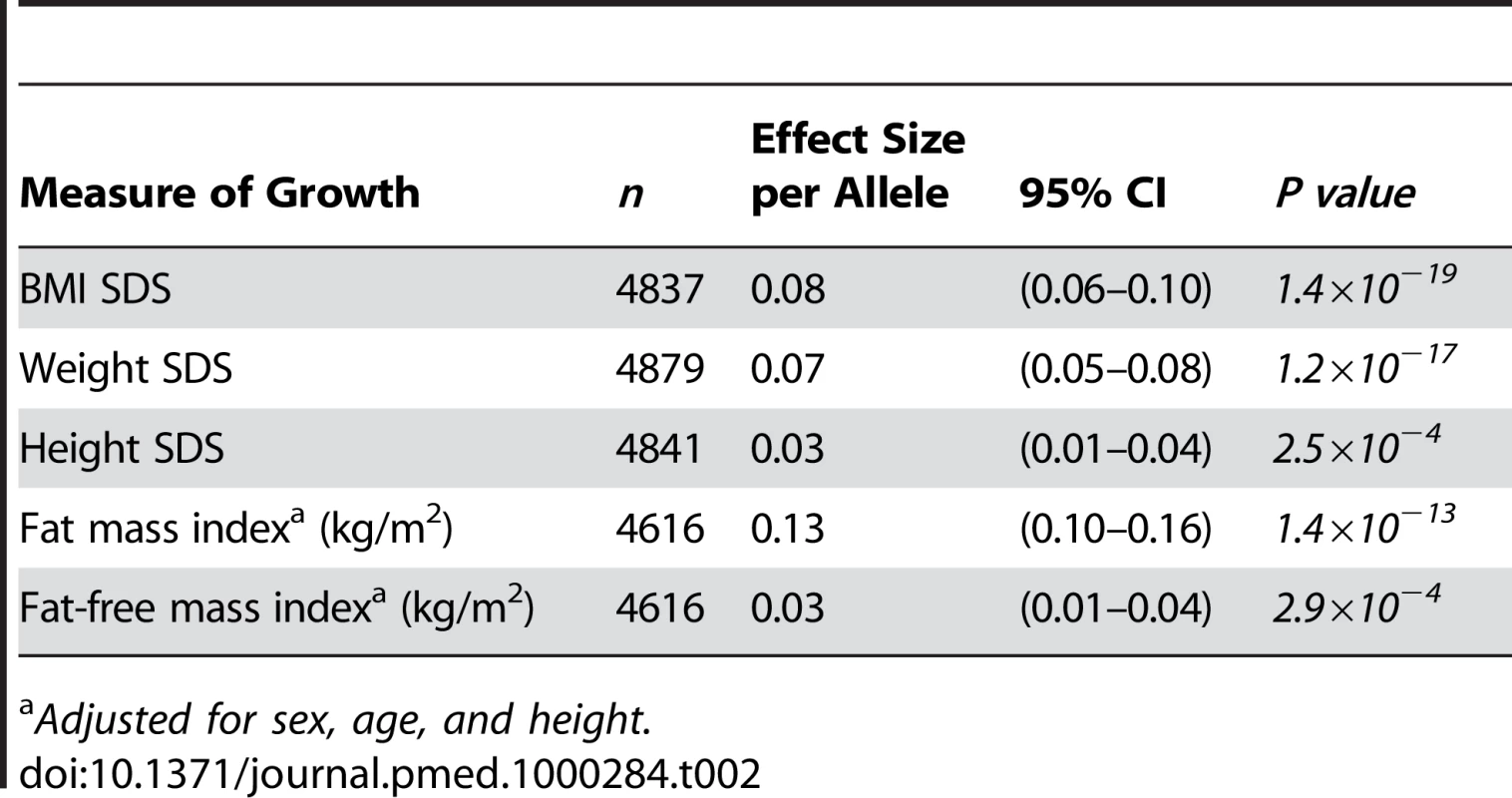
Adjusted for sex, age, and height. Rate of Infancy and Childhood Weight Gain
The obesity-risk-allele score showed little association with birth weight SDS (effect size 0.01 SDS per allele; 95% CI 0.00–0.02; p = 0.2), but showed increasing positive associations with weight SDS from early infancy to childhood (Figure 2A). Even as early as age 6 wk, each additional risk allele was associated with a 0.03 SDS increase in weight (95% CI 0.01–0.04; p = 0.001).
Fig. 2. Longitudinal associations between the obesity-risk-allele score and (A) weight SDS, (B) length/height SDS, and (C) BMI SDS. 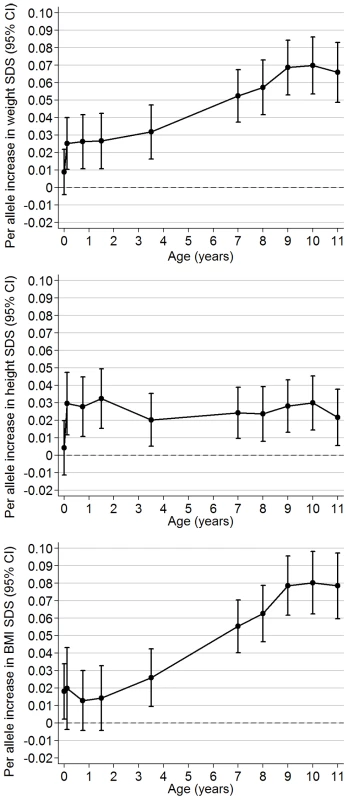
Regression coefficients ±95% CI are shown from linear regression models (adjusted for sex and precise age at measurement) according to age at measurement between birth and 11 y. Longitudinal analyses estimated that the obesity-risk-allele score was positively associated with rate of weight gain between birth to age 11 y (0.005 SDS/allele/y; 95% CI 0.004–0.006). The obesity-risk-allele score had an apparent much larger effect on the rate of early infancy weight gain (birth to 6 wk: 0.119 SDS/allele/y; 0.023–0.216) than on subsequent weight gain (6 wk to 11 y: 0.004 SDS/allele/y; 0.004–0.005).
The obesity-risk-allele score was positively associated with conditional weight gain between birth and age 6 wk (0.03 SDS gain per allele, 0.01–0.04; p = 0.001) and, conversely, was associated with reduced risk of early infancy “failure to thrive” between birth and age 6 wk (OR = 0.92 per allele, 0.86–0.98, p = 0.009).
Childhood Length/Height and BMI
Similar to weight SDS, an association between the obesity-risk-allele score and length/height appeared soon after birth (association with length at 6 wk: 0.03 SDS/allele; 95% CI 0.01–0.05, p = 0.001). However, in contrast to weight, this association did not appear to increase in size subsequently during childhood (Figure 2B). Longitudinal analyses confirmed that the obesity-risk-allele score was positively associated with rate of gain in length during early infancy (birth to 6 wk: 0.158 SDS/allele/y; 0.032–0.284), but not subsequently (6 wk to 11 y: 0.000 SDS/allele/y; −0.001 to 0.001).
The obesity-risk-allele score was positively associated with BMI at birth (0.02 SDS/allele; 95% CI 0.00–0.03, p = 0.03), and longitudinal analyses showed a positive association with rate of gain in BMI between birth to age 11 y (0.006 SDS/allele/y; 0.005–0.007). However, the obesity-risk-allele score showed only weak association with BMI SDS during infancy until age 3.5 y onwards, from which time the association increased rapidly (Figure 2C).
Comparison with the Ten-Variants Risk-Allele Score
Compared to the obesity-risk-allele score based on eight genetic variants, an extended obesity-risk-allele score based on ten variants (i.e., including genotypes at SH2B1 and MTCH2, which were individually unrelated to childhood BMI) showed very similar, but slightly attenuated, associations with childhood body size and body composition (Table S4). The extended obesity-risk-allele score was also significantly associated with infant body weight at age 6 wk, and with conditional weight gain during the first 6 wk of life.
Discussion
This study shows that recently established genetic variants for adult BMI have a combined association with childhood weight gain that is apparent even within the first weeks from birth. The combined association between these variants and childhood BMI, of around 0.5 SDS between the lowest and highest allele risk score groups, was similar in size to that seen with adult BMI (1.5 kg/m2) in terms of proportion of a standard deviation [6]. Therefore, while these risk variants may well influence rate of weight gain in adults [4], we postulate that that their relative influence on the rate of weight gain may be greater during childhood.
Their association with weight gain was already apparent from birth, within the first 6 weeks of life, and these adult obesity risk alleles were, in combination, protective against poor weight gain during the first weeks of life after birth. These findings are striking considering that these variants were originally discovered by association with adult BMI or obesity in populations with mean ages ranging from 40 to 60+ years [6],[7]. Other obesity susceptibility variants in/near MAF, NPC1, PRL, and PTER [18] have been identified in other GWA studies of early-onset and severe obesity, and putative associations with early life weight gain may be more expected with those variants. In addition, we observed that the adult obesity risk alleles were also associated with faster gains in length/height during infancy, but not during childhood. These findings are consistent with the Karlberg model of the endocrine regulation of childhood growth, whereby early infancy growth in length/height is largely controlled by nutritional factors, while the relatively stable trajectory of childhood growth reflects the setting of the growth hormone–insulin-like growth factor–I axis [19]. This potential weight-regulated drive in length gain likely explains the concurrent gains in both length and weight during infancy. Consequently, the obesity-risk-allele score showed a weaker association with BMI than with weight until age 3.5 years.
Rapid infancy weight gain and larger infant body weight have been consistently related to increased risk of obesity in subsequent childhood or adult life [20],[21]. However, life-course disease studies in historical cohort studies have provided conflicting evidence. For example, rapid infant weight gain has been associated with increased risk of obesity and the metabolic syndrome, but with reduced risk of type 2 diabetes [22]. One difficulty is that studies in historical cohorts or in societies that are undergoing nutritional transition may identify life-course associations that are specific to those particular settings [23]. Even in contemporary western studies, the very long-term follow-up needed to record adult disease outcomes may mean that current findings will not be applicable to future infant settings. We propose that the application in contemporary birth cohorts of genetic markers that are robustly associated with adult disease risks may provide a novel approach to life-course epidemiology, by identifying early exposures that are directly relevant to current settings.
Infancy weight gain and growth are markedly influenced by nutritional factors, such as type of milk feeding [24]. Our current findings show that genetic factors also contribute to early weight gain and growth. While the mechanisms of action for these obesity variants have yet to be established, many show high levels of expression, or have known actions, in the central nervous system and could therefore regulate feeding behaviour [6]. However, there is likely to be heterogeneity in their biological actions and in their specific effects during childhood and infancy. Future identification of the biological actions related to individual variants will shed further light on the specific early life mechanisms that lead to obesity. However, in view of their modest effect sizes, much larger studies or collaborations to pool longitudinal data from multiple birth cohorts will be needed to distinguish the specific childhood manifestations of individual variants.
Failure to thrive in infancy, variably defined as underweight or poor weight gain, is a multifactorial condition [25]. After exclusion of a wide range of medical conditions, nonorganic failure to thrive was traditionally considered to be a risk marker for maternal rejection and neglect [26]. However, the majority of infants with failure to thrive likely represent the lower-normal distribution of infancy weight gain. Health professionals have increasingly recognised the important contribution of innate differences in infant food intake rather than simply food provision by the caregiver [26], but until now no such infant factors have been demonstrated. We postulate that genetic factors in the infant that increase appetite and predispose to later obesity are protective against infant failure to thrive.
In conclusion, greater early infancy gains in weight and length represent the start of pathway to adult obesity risk in contemporary settings. Our findings demonstrate the utility of using robust genetic markers of disease risk to identify life-course disease associations with current relevance.
Supporting Information
Zdroje
1. BundredP
KitchinerP
BuchanI
2001 Prevalence of overweight and obese children between 1989 and 1998: population based series of cross sectional studies. BMJ 322 326 328
2. DietzWH
1994 Critical periods in childhood for the development of obesity. American Journal of Clinical Nutrition 59 955 959
3. WhitakerRC
WrightJA
PepeMS
SeidelKD
DietzWH
1997 Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337 869 873
4. FraylingTM
TimpsonNJ
WeedonMN
ZegginiE
FreathyRM
2007 A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 889 894
5. LoosRJ
LindgrenCM
LiS
WheelerE
ZhaoJH
2008 Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40 768 775
6. WillerCJ
SpeliotesEK
LoosRJ
LiS
LindgrenCM
2009 Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41 25 34
7. ThorleifssonG
WaltersGB
GudbjartssonDF
SteinthorsdottirV
SulemP
2009 Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41 18 24
8. CecilJE
TavendaleR
WattP
HetheringtonMM
PalmerCN
2008 An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 359 2558 2566
9. GrantSF
BradfieldJP
ZhangH
WangK
KimCE
2009 Investigation of the Locus Near MC4R With Childhood Obesity in Americans of European and African Ancestry. Obesity (Silver Spring)
10. GoldingJ
PembreyM
JonesR
2001 ALSPAC–the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 15 74 87
11. ColeTJ
FreemanJV
PreeceMA
1995 Body mass index reference curves for the UK, 1990. Arch Dis Child 73 25 29
12. FreemanJV
ColeTJ
ChinnS
JonesPR
WhiteEM
1995 Cross sectional stature and weight reference curves for the UK, 1990. Archives of Disease in Childhood 73 17 24
13. ColeTJ
BellizziMC
FlegalKM
DietzWH
2000 Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj 320 1240 1243
14. WellsJC
ColeTJ
2002 Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord 26 947 952
15. ColeTJ
1995 Conditional reference charts to assess weight gain in British infants. Arch Dis Child 73 8 16
16. DrewettR
BlairP
EmmettP
EmondA
2004 Failure to thrive in the term and preterm infants of mothers depressed in the postnatal period: a population-based birth cohort study. J Child Psychol Psychiatry 45 359 366
17. Stata. Corp 2007 Stata Statistical Software: Release 10. College Station, TX StataCorp LP
18. MeyreD
DelplanqueJ
ChevreJC
LecoeurC
LobbensS
2009 Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 41 157 159
19. KarlbergJ
1987 On the modelling of human growth. Stat Med 6 185 192
20. OngKK
LoosRJ
2006 Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 95 904 908
21. BairdJ
FisherD
LucasP
KleijnenJ
RobertsH
2005 Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 331 929
22. FallCH
SachdevHS
OsmondC
LakshmyR
BiswasSD
2008 Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: Data from the New Delhi Birth Cohort. Diabetes Care 31 2349 2356
23. StettlerN
2007 Commentary: Growing up optimally in societies undergoing the nutritional transition, public health and research challenges. Int J Epidemiol 36 558 559
24. OngKK
PreeceMA
EmmettPM
AhmedML
DungerDB
2002 Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatric Research 52 863 867
25. JolleyCD
2003 Failure to thrive. Curr Probl Pediatr Adolesc Health Care 33 183 206
26. SkuseDH
1985 Non-organic failure to thrive: a reappraisal. Arch Dis Child 60 173 178
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 5- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Suicide Prevention for Older Adults in Residential Communities: Implications for Policy and Practice
- Non-Communicable Diseases in Sub-Saharan Africa: The Case for Cohort Studies
- Early Pandemic Influenza (2009 H1N1) in Ho Chi Minh City, Vietnam: A Clinical Virological and Epidemiological Analysis
- Anatomy of the Epidemiological Literature on the 2003 SARS Outbreaks in Hong Kong and Toronto: A Time-Stratified Review
- Prognostic Significance of Subtype Classification for Short- and Long-Term Survival in Breast Cancer: Survival Time Holds the Key
- Negotiating Equitable Access to Influenza Vaccines: Global Health Diplomacy and the Controversies Surrounding Avian Influenza H5N1 and Pandemic Influenza H1N1
- Which New Approaches to Tackling Neglected Tropical Diseases Show Promise?
- Self-Injurious Behavior in Adolescents
- Markers of Dysglycaemia and Risk of Coronary Heart Disease in People without Diabetes: Reykjavik Prospective Study and Systematic Review
- Can Foreign Policy Make a Difference to Health?
- A Population-Based Evaluation of a Publicly Funded, School-Based HPV Vaccine Program in British Columbia, Canada: Parental Factors Associated with HPV Vaccine Receipt
- Genetic Markers of Adult Obesity Risk Are Associated with Greater Early Infancy Weight Gain and Growth
- New Complexities and Approaches to Global Health Diplomacy: View from the U.S. Department of State
- The Impact of Phenotypic and Genotypic G6PD Deficiency on Risk of Infection: A Case-Control Study amongst Afghan Refugees in Pakistan
- The Malawi Developmental Assessment Tool (MDAT): The Creation, Validation, and Reliability of a Tool to Assess Child Development in Rural African Settings
- Journals, Academics, and Pandemics
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Self-Injurious Behavior in Adolescents
- Non-Communicable Diseases in Sub-Saharan Africa: The Case for Cohort Studies
- The Malawi Developmental Assessment Tool (MDAT): The Creation, Validation, and Reliability of a Tool to Assess Child Development in Rural African Settings
- Journals, Academics, and Pandemics
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



