-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNew Approaches to Preventing, Diagnosing, and Treating Neonatal Sepsis
article has not abstract
Published in the journal: . PLoS Med 7(3): e32767. doi:10.1371/journal.pmed.1000213
Category: Research in Translation
doi: https://doi.org/10.1371/journal.pmed.1000213Summary
article has not abstract
Five Key Papers on Preventing, Diagnosing, and Treating Neonatal Sepsis
-
Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, et al (2008) Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 359 : 1555–1564. Recent large-scale randomised trial which conclusively demonstrated that maternal immunisation can reduce serious respiratory infections in young infants and is a feasible strategy for improving health outcomes in mothers and young infants.
-
Mullany LC, Darmstadt GL, Khatry SK, Katz J, LeClerq SC, et al (2006) Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet 367 : 910–918. The first trial to show that commonly used antiseptics could have major impacts in reducing neonatal infections and mortality in rural field-based settings in low-income countries.
-
Boyer KM, Gotoff SP (1986) Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 314 : 1665–1669. The first trial to demonstrate conclusively that risk-based intrapartum antibiotic prophylaxis can prevent early-onset neonatal group B streptococcal disease in high-income settings.
-
Stevens DY, Petri CR, Osborn JL, Spicar-Mihalic P, McKenzie KG, et al (2008) Enabling a microfluidic immunoassay for the developing world by integration of on-card dry reagent storage. Lab Chip 8 : 2038–2045. One of a series of studies that has demonstrated that microfluidic microtechnologies can be used to create robust point-of-care diagnostic systems for low-income countries and that results can be fast, accurate, and reproducible.
-
Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD (1999) Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India Lancet 354 : 1955–1961. The first study that demonstrated that home visiting from village health workers in the first days of life and the provision of parenteral antibiotics at home could substantially reduce neonatal sepsis and neonatal mortality in low-income countries.
Neonatal sepsis or septicaemia is a clinical syndrome characterized by systemic signs of circulatory compromise (e.g., poor peripheral perfusion, pallor, hypotonia, poor responsiveness) caused by invasion of the bloodstream by bacteria in the first month of life. In the pre-antibiotic era neonatal sepsis was usually fatal. Case fatality rates in antibiotic treated infants now range between 5% and 60% with the highest rates reported from the lowest-income countries [1]. The World Health Organization (WHO) estimates that 1 million deaths per year (10% of all under-five mortality) are due to neonatal sepsis and that 42% of these deaths occur in the first week of life [2]. There are wide disparities in neonatal care between high - and low-income countries. In high-income countries the major concern is the increasing numbers of extremely premature infants with high nosocomial infection rates due to multiresistant organisms in intensive care units. Health facility infections are also a major problem in low-income countries, but the more pressing issues are the high proportion of home deliveries in unclean environments predisposing to sepsis and ensuring that all neonates have access to effective interventions from health care providers in the first days of life2. Indeed, new strategies that can prevent, diagnose, and treat neonates with sepsis are needed in both low - and high-income settings.
Pathogenesis of Neonatal Infections
Distal risk factors for neonatal sepsis include poverty and poor environmental conditions. Proximate factors include prolonged rupture of membranes, preterm labour, maternal pyrexia, unhygienic intrapartum and postnatal care, low birth weight, and prelacteal feeding of contaminated foods and fluids [3]–[5].
The bacteria that cause neonatal sepsis are acquired shortly before, during, and after delivery (Figure 1). They can be obtained directly from mother's blood, skin, or vaginal tract before or during delivery or from the environment during and after delivery. Streptococcus agalactiae (Group B streptococcus, GBS) is the most common cause of neonatal sepsis in many countries, though low rates are reported from many low-income countries, especially those in south Asia.[6]–[8]; gram-negative bacilli (Escherichia coli, Klebsiella spp., Pseudomonas spp., Acinetobacter spp.) and gram-positive cocci (such as Staphylococcus aureus and Staphylococcus epidermidis) are other important causes [6]–[8]. However, there are many difficulties in interpreting aetiological neonatal sepsis data, because many studies report selected populations of high-risk infants. Specimens from infants in the first 24 hours of life are also seriously under-represented, especially those from low birth-weight babies and babies born outside health facilities [6],[9]–[11]. Intrapartum antibiotic prophylaxis against S. agalactiae has also led to a substantial change in the bacteria responsible for early onset neonatal sepsis; gram-negative bacilli and Staphylococcus spp. predominate in countries implementing these programs [12].
Fig. 1. Pathogenesis of congenital and neonatal infections. 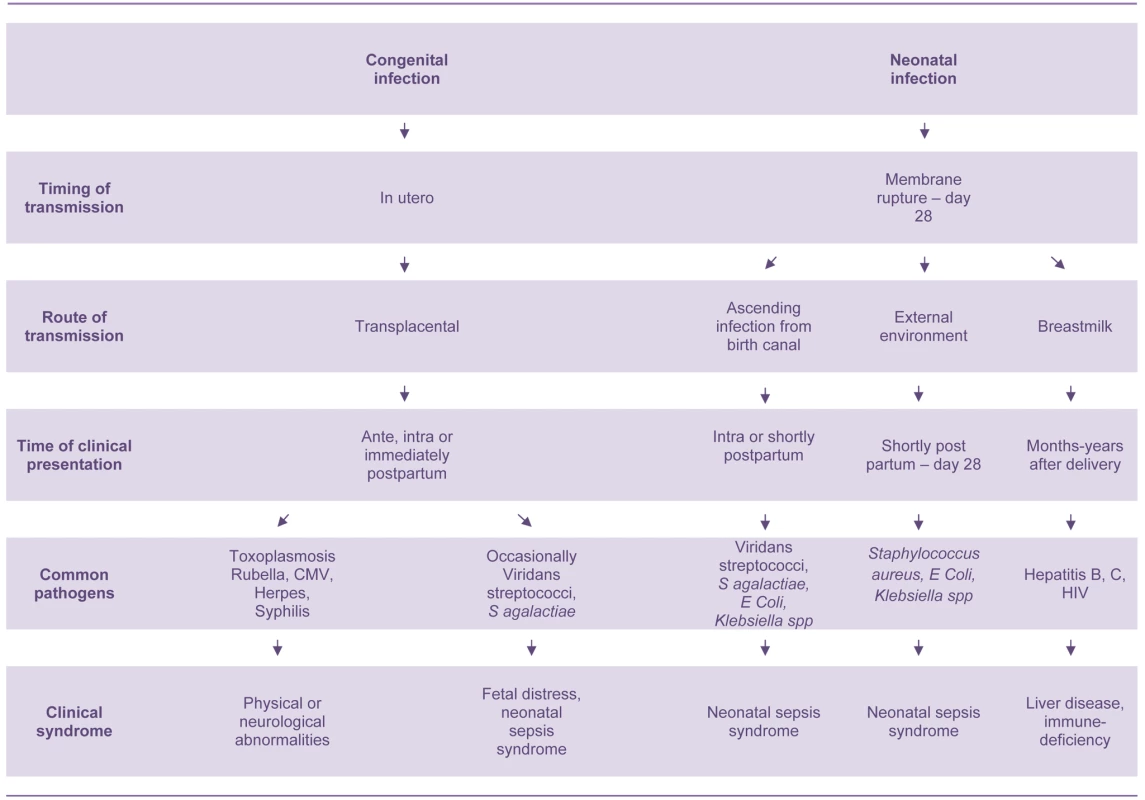
There are also many other important neonatal infectious disease pathogens that are not associated with the sepsis syndrome including: Treponema pallidum, rubella virus, herpes simplex virus, cytomegalovirus, toxoplasmosis, Clostridium tetani, HIV, hepatitis B virus, and Bordetella pertussis (Figure 1) [1],[7],[13]. These infectious pathogens cause serious morbidities in young infants and multifaceted disease syndromes including congenital anomalies, developmental disabilities, chronic liver disease, neonatal tetanus, and apnoea. They are also important causes of morbidity and mortality in older age groups. However, only pathogens that cause neonatal sepsis are discussed in this paper.
Neonatal Immunity
Neonates have a functionally immature immune system. They have extremely low immunoglobulin (Ig) levels except for IgG to specific maternal antigens transferred passively across the placenta during the last trimester of pregnancy [14],[15]. T cell function is relatively unimpaired but complement activity is half that of healthy adults. Neonates have a low neutrophil storage pool, and their existing neutrophils have impaired capacity to migrate from the blood to sites of infection [16].
The basal expression of Toll-like receptors (TLRs, receptors that detect the presence of microbes) is similar in the neonate and adult [17]. However, innate immune responses of neonatal mononuclear cells are characterised by markedly reduced release of the proinflammatory Th1-polarizing cytokines tumour necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) with relative preservation of anti-inflammatory Th2-polarizing cytokines such as interleukin 6 (IL6) [18]. These findings may reflect in utero requirements, including the avoidance of harmful inflammatory immune reactions [19].
These immunological problems are reflected in the clinical presentation of neonatal sepsis. Neonates have a rapid and fulminant progression of septicaemic disease, nonspecific clinical signs of infection, and difficult-to-interpret laboratory results including haematological and immunological biomarkers of infection and inflammation. Low birth-weight (preterm and small for gestational age) infants have even poorer functional immunity, and are especially at risk of sepsis [19].
However, neonates do have well-functioning cationic membrane-active antimicrobial proteins and peptides (APPs) which have microbicidal properties [15],[19]. These APPs can be found in the vernix caseosa covering the skin at birth, and in the neonatal gastrointestinal and respiratory tracts.
Advances in Prevention
Before Delivery
Many older studies have demonstrated that improving maternal health and nutrition before delivery is directly associated with improved neonatal health outcomes [3]. Randomised controlled trials (RCTs) of maternal protein-calorie and multiple micronutrient and supplementation have demonstrated significant improvements in rates of prematurity and birth weight and variable impact on mortality; but no studies have examined their impact on rates of neonatal sepsis [20],[21].
Maternal immunisation is an important method of providing neonates with appropriate antibodies as soon as they are born [22]. This approach is less sensitive to obstacles in accessing the health care system than are other approaches, and examples of successful interventions include maternal tetanus toxoid and influenza immunisations [23],[24]. Studies of maternal immunisation with S. agalactiae type III conjugate vaccine have demonstrated excellent placental transfer and persistence of protective levels in 2-month-old infants [22]. Phase I and II trials of other serotypes in nonpregnant women have also demonstrated safety and immunogenicity. A recent modelling study estimated that vaccination with S. agalactiae vaccine would prevent 4% of US preterm births and 60%–70% of neonatal S. agalactiae infections [25]. Encouraging results are also emerging from studies of maternal immunisation with pneumococcal polysaccharide and conjugate vaccines [22],[26]. The vaccines all have excellent safety profiles. However, barriers to maternal immunisation include: liability issues for vaccine manufacturers in developed countries; education of the public and health care providers regarding the benefits of maternal immunisation; and poor ascertainment of data from low-income countries [22].
During Labour and Delivery
There is strong evidence that clean delivery practices and handwashing during delivery reduces rates of neonatal sepsis in both home and health facility settings [27]–[29]. Interventions to improve handwashing rates have been remarkably successful in research settings [30],[31]. The reasons for lack of successful scale-up of handwashing interventions into policy, programs, and behaviour change are less clear [32].
New studies from Malawi and Nepal indicate that maternal antisepsis interventions such as vaginal chlorhexidine during labour may have a significant impact on rates of neonatal mortality and sepsis in developing countries [33]. However, other studies from high-income countries have demonstrated little effect on rates of HIV or neonatal infections [34].
Intrapartum antibiotic prophylaxis has been highly effective in reducing both early-onset neonatal bacterial and maternal sepsis in developed countries [35]. Chemoprophylaxis in the US has halved the incidence of early-onset neonatal bacterial sepsis caused by S. agalactiae from 1.7 per 1,000 live births in 1993 to 0.6 per 1,000 in 1998 [36]. Clear protocols are in place in high-income countries for the management of women with risk factors for neonatal sepsis [37]. Risk factors for early-onset neonatal bacterial sepsis in low-income settings are probably similar to resource-rich settings, but have not been evaluated in the context of high rates of maternal undernutrition, anaemia, HIV, and malaria.
After Delivery
There is also strong evidence that handwashing by health care providers after delivery can reduce neonatal sepsis and infection rates, especially in hospitals [27],[28]. There is less evidence for the importance of rigorous handwashing and use of antiseptics in mothers of their own infants.
In high-income settings, studies have not shown an advantage of antibiotics or antiseptics over simply keeping the umbilical cord clean [2]. However, umbilical stump chlorhexidine cleansing has recently been shown to substantially reduce neonatal deaths in Nepal [38]. Other studies investigating the effects of chlorhexidine on prevention of omphalitis are currently underway in several countries [39].
There is emerging evidence that neonatal skin antisepsis preparations such as sunflower seed oil provides cheap, safe, and effective protection against nosocomial infections in hospitalized preterm neonates and infants in studies in south Asia. Application of chlorhexidine to neonatal skin has also been shown to be effective in reducing neonatal sepsis in studies from south Asia [39],[40].
Neonatal immunisation has long been considered an important method of reducing neonatal infections. However, response varies according to the antigen [15]. BCG, polio, and hepatitis B vaccines are highly immunogenic when given at birth [41]. However, maternal antibodies interfere with a neonate's response to measles vaccine when administered under six months. Protein antigen vaccines (e.g., pertussis and tetanus toxoid) given at birth have been shown to produce poor responses compared to the same antigen given at two months of age and are associated with later tolerance [41]. Studies also indicate that S. agalactiae and Streptococcus pneumoniae vaccines are both likely to be ineffective when given in the neonatal period [15].
Breastmilk contains secretory IgA, lysozymes, white blood cells, and lactoferrin and has been shown to encourage the growth of healthy lactobacilli and reduce the growth of E. coli and other gram-negative pathogenic bacteria [15]. RCTs that focused on increasing early initiation and exclusive breastfeeding rates demonstrated significant reductions in diarrhoea and acute respiratory infections in neonates and older infants in India [42]. Other observational studies have demonstrated impact on infection specific mortality rates and all-cause mortality during the neonatal period [43]–[45].
Neonatal micronutrient supplementation trials have focused on vitamin A supplementation. Older studies have shown significant reductions in respiratory disease in low birth-weight infants after the administration of parenteral vitamin A [46]. More recently, trials of newborn vitamin A supplementation have shown encouraging reductions in neonatal mortality, and more trials are underway [47].
In high-income countries, clinical trials of immune stimulants such as granulocyte/monocyte colony stimulating factor (GM-CSF) to enhance the quantity and quality of neonatal neutrophils and monocytes appear promising but have not yet shown a significant clinical benefit [15]. The evaluation of recombinant APPs as adjunctive therapy for neonatal infection are still under evaluation. The impact of TLR agonists to improve defences against microorganisms are also being evaluated [15].
Advances in Diagnosis
Neonatal clinical sepsis syndrome identification is difficult as the clinical signs of neonatal septicaemia can be very similar to those of other life-threatening diseases such as necrotising enterocolitis, hyaline membrane disease, and perinatal asphyxia [48],[49]. However, recent studies in middle - and low-income countries have provided seven danger signs which can be used to identify infants with very severe disease including neonatal sepsis (Table 1) [49]. These signs provide high sensitivity and moderate specificity for detecting serious illness in newborns in low-resource settings and have now been incorporated into the new neonatal WHO Integrated Management of Childhood Illness (n-IMCI) guidelines.
Tab. 1. Clinical symptoms and signs of severe neonatal illness including sepsis. 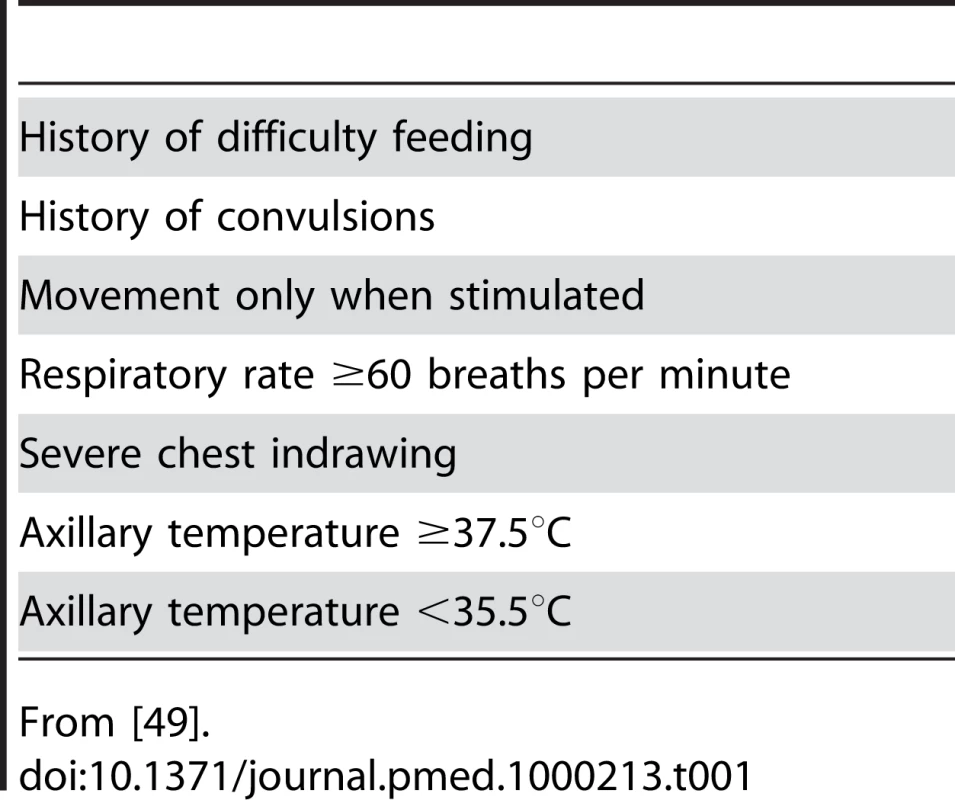
From [49]. Identification of neonatal sepsis before delivery also remains challenging. A combination of maternal risk factors and clinical signs and symptoms is currently used [50]. However, peripartum proteomic analysis of the amniotic fluid is now offering the opportunity for early and accurate diagnosis of early-onset neonatal sepsis in the select population of women undergoing amniocentesis in high-risk pregnancies [51],[52].
Confirmation of pathogenic organisms allows targeted antibiotic therapy. However, identification of pathogenic organisms in neonates with sepsis syndrome is fraught with difficulties. Bacterial load may be low due to mothers receiving antepartum or intrapartum antibiotics and because only small amounts of blood can often be taken from newborns [53]. Contamination rates may also be very high due to the technical difficulties of sterile venipuncture in small babies. There may also be misinterpretation of the role of coagulase-negative staphylococci (e.g., S. epidermidis), as these organisms are both normal skin flora and pathogenic organisms in preterms and infants with indwelling blood vessel catheters [54].
Automated blood culture systems have long been considered the gold standard for microbiological diagnosis. However, despite improvements in growth media and instrumentation, results of blood culture can be delayed by up to 48 hours [53],[55]. The condition of a neonate with true sepsis can deteriorate quickly, thus the most common approach is to initiate empiric broad-spectrum antibiotic therapy in all young infants with suspected bacterial infection [49]. A negative blood culture after 48 hours may allow cessation of antibiotic therapy in a well infant. While appropriately cautious, this practice leads to antibiotic exposure in a large number of newborns for whom antibiotic treatment may be unnecessary since blood cultures are positive in only 5%–10% of suspected sepsis cases, even at highly resourced facilities [56].
Antigen detection techniques allow rapid detection and identification of microorganisms without culturing. The most commonly used commercially available test is the latex agglutination assay, which is based on specific agglutination by bacterial cell wall antigens of antibody-coated latex particles. However, these tests can only detect specific organisms such as S. agalactiae and are associated with high false positive and negative rates [57]. New urinary antigen tests for pneumococcus are more encouraging but are also associated with false positives from pneumococcal carriage [58].
The polymerase chain reaction (PCR) has been widely used in biomedical research laboratories for pathogen identification in neonatal sepsis and in some clinical hospital laboratories. The high sensitivity of PCR allows detection of bacterial DNA even when concentrations are low [57]. Conventional assays are being replaced by a newer “real-time” system, which is faster and associated with lower contamination rates because amplification and detection occur simultaneously in a closed system [59]. The real-time PCR is based on the measurement of a fluorescent signal generated during each amplification cycle. It produces quantitative results within 30 minutes and calculates bacterial load. Broad-range real-time PCR uses a single primer to detect the universal bacterial genome (16S RNA or 23S RNA) which is a conserved ribosomal genome sequence across all bacterial genera [60]. Broad-range real-time PCR can be used to distinguish bacterial septicaemic disease from other causes of neonatal illness such as asphyxia or complications of prematurity. However, it has been used with varying success in the analysis of whole blood for neonatal sepsis; specificity is generally high but sensitivity can be as low as 40% [60],[61]. In contrast, multiplex PCR involves the parallel amplification of different targets but is focused only on specific pathogens, and false negatives can occur if the aetiologic agent of interest is not included in the database [62]. Real-time PCR is now often used to screen for microbial load, followed by sequence-based targeting and identification of PCR amplicons (pyrosequencing) [62]. This process can detect very small copy numbers of specific nucleic acid sequences. There is also a new commercially available multiplex pyrosequencing PCR assay which can identify up to 40 different bacterial and fungal pathogens directly from whole blood [63]. Real-time PCR and pyrosequencing of the universal 23S rRNA gene has also recently been used successfully in neonatal blood culture samples [64]. Further tests on neonatal whole blood have been planned by a number of different research groups.
The biggest problem with real time PCR testing is that the specimen must be collected with a sterile venipuncture, which may be difficult in young neonates. Neonatal capillary heel prick specimens are easier to collect but highly contaminated by skin flora. There is also high potential for contamination of enrichment media, reagents, or the sample during collection and processing [61] Other problems include low sensitivity due to competition from human DNA in whole blood, especially if white cell counts are high. Also, bacterial organisms require lysis before their DNA can be available for analysis, and gram-positive organisms are difficult to lyse because of their resilient cell wall [61]. Real-time PCR technologies are also expensive and currently can be used only by highly trained staff.
Important haematological tests include microscopic examination of the blood for white cells (total leucocyte count, differential, neutrophil count, and immature neutrophil to total neutrophil ratio). Advantages are that these specimens do not require sterility and a heel prick specimen can be used. However many of these indices are falsely low in a septic neonate.
Biological biomarkers are human blood components that increase in response to infection. The most commonly used acute phase reactant is the C-reactive protein (CRP). However, the CRP takes 12–24 hours to increase to measurable levels; its half life is very long and it takes 5–7 days to normalize after eradication of the infectious agent. Cytokines such as IL6, IL8, TNF-α, and procalcitonin have also been extensively studied [65],[66]. Cytokines rise quickly after infection even in neonates, and are more sensitive to low concentrations of pathogens than CRP [66]. However, cord and postnatal blood cytokine concentrations can be depressed in the presence of pregnancy-induced hypertension and can rise after induced vaginal or urgent cesarean delivery, delivery room intubation, muscular damage, and inflammation from other causes [57]. Simultaneous measurement of multiple biomarkers may improve both sensitivity and specificity [66],[67]. However, biomarker assays are likely to be less acceptable to physicians who often place higher value on tests that confirm biological agents and allow targeting of antibiotic therapy [57].
Microtechnologies, especially microfluidics, have provided the greatest recent contribution to the diagnosis of neonatal sepsis. Microfluidics is the study of the behaviour, precise control, and manipulation of fluids geometrically constrained to submillimetre (nanolitre or picolitre) channels [68]. Microfluidic technology uses the unique proprieties of continuous flow micro-volume channels: viscosity, surface tension, energy dissipation, and fluidic resistance, and also includes micro pneumatic pump and valve systems. One specific application of microfluidics is bacterial DNA protein microarray hybridization [69]. In this test, DNA probes specific to selected targets are spotted on a glass or silicon slide in a known order. Target DNA fragments are labelled with a reporter molecule, combined into a single hybrid, and measured using fluorescent signals [62],[68]. This technique has been used in the identification of the specific sepsis pathogen in bacterial meningitis, acute viral respiratory tract infections, and neonatal sepsis, and also in the detection of their antimicrobial resistance and virulence genes in research settings [63].
Microfluidic technology has also allowed sample preparation and a number of different assays to be combined in small, disposable, single-use diagnostic cartridges or cards that have been called a “lab on-a-chip” or LOC (Figure 2) [68]. Some LOCs have combined sample preparation, biomarkers, real-time PCR, and DNA microarrays to provide information about indices of inflammation, pathogen identification, and antimicrobial susceptibility patterns at the point of care [68],[70]. LOCs have been reported to perform assays at sensitivity, specificity, and reproducibility levels similar to those of central laboratory analysers, but yet require little user input other than the insertion of the sample. Single drops of blood, faeces, and saliva have all been tested with encouraging results. LOCs are currently being evaluated for use in sepsis, endocarditis, HIV, tuberculosis, severe acute respiratory syndrome (SARS), and pneumonia [68]. However, they are not yet in clinical use nor licensed by regulatory authorities.
Fig. 2. Example of “lab on a chip” point-of-care device. 
Advances in Treatment
As neonatal sepsis can be rapidly fatal if left untreated, highly effective antibiotic therapy must be used and delays in the provision of care must be minimised. Treatment must be effective against the causative pathogen, safe for the newborn, and feasible to deliver reliably in the hospital or community setting.
Parenteral (intravenous or intramuscular) regimens for neonatal sepsis currently recommended by national paediatric associations are a combination of penicillin/ampicillin and gentamicin, or third-generation cephalosporins (e.g., ceftriaxone or cefotaxime) for 10–14 days. These antibiotics are safe and retain efficacy when administered at extended intervals (e.g., twice daily or daily dosing) [56]. These regimens are very effective against Streptococcus spp., but Staphylococcus spp. can be highly resistant [71]. Gram-negative antimicrobial susceptibility to ampicillin and gentamicin can also be poor, especially for Klebsiella spp. [8],[71]. Emerging E. coli resistance to ampicillin, gentamicin, and third-generation cephalosporins in hospital nurseries in both developed and developing countries is also causing increasing concern [8]. The potential for significant life-threatening toxicity among neonates associated with chloramphenicol makes it the least preferred empiric parenteral therapy [56].
Oral antibiotic therapy must be considered in settings where referral is not possible and there are no health care providers trained to give parenteral antibiotics [72]. The incremental benefit of injectable over oral antibiotics is not known, and oral antibiotic therapy is better than no antibiotic therapy at all. A series of trials are currently evaluating the impact of home and clinic-based short course (7 days) intramuscular and oral antibiotic therapy for neonatal sepsis in low-income countries [72]. Most data are available on the effect of oral cotrimoxazole in community-based treatment of serious neonatal bacterial infections from Nepal and India. However, there are concerns about high resistance rates, and side effects such as neonatal jaundice have been reported [71]. Oral amoxicillin is highly efficacious against Streptococcus spp. and some gram-negative bacilli and has an excellent safety record. However, it has no anti-Staphylococcus coverage and resistance is emerging in gram-negative bacilli such as E. coli. New, better-absorbed oral antibiotics are also being considered. The new second-generation cephalosporins (e.g., cefadroxil and cefuroxime) have an excellent safety profile, a spectrum of activity similar to cotrimoxazole, and may be more effective given the high resistance of neonatal pathogens to cotrimoxazole. Ciprofloxacin also is increasingly accepted as safe in neonates and warrants further investigation for treatment of infections in newborns. However, the current cost of these agents and potential for exacerbating antimicrobial resistance may limit widespread use in developing countries [72].
Poor maternal-neonatal health systems, low levels of care-seeking, and lack of access to sick newborns during the first day of life, when mortality risks are highest, are also important concerns [73]. Recent studies have shown that community health workers can deliver antibiotic treatment to neonates with very severe infections at home safely and acceptably when hospitalization is not feasible [74]. Trials are currently evaluating the effectiveness, quality of care, and coverage of these community health worker programmes in Asia and Africa [73]. Barriers to large-scale implementation include high cost, poor staff training and retention, and difficulties with referral (e.g., lack of ambulances and poor institutional links).
Summary and Next Steps
Newborn sepsis is a major cause of child mortality across the world. Industrialized countries have made remarkable progress in reducing newborn sepsis and sepsis-related mortality by providing access to hygienic skilled delivery for all women, risk-based intrapartum antibiotic prophylaxis, and high-quality intensive care for newborns that need it. Although resource constraints preclude whole-scale adoption of these strategies in developing countries, there are a number of low-cost proven interventions and promising approaches that have the potential to significantly reduce the burden of neonatal sepsis worldwide (Table 2).
Tab. 2. Effective current measures and new approaches to prevent, diagnose, and treat neonatal sepsis. 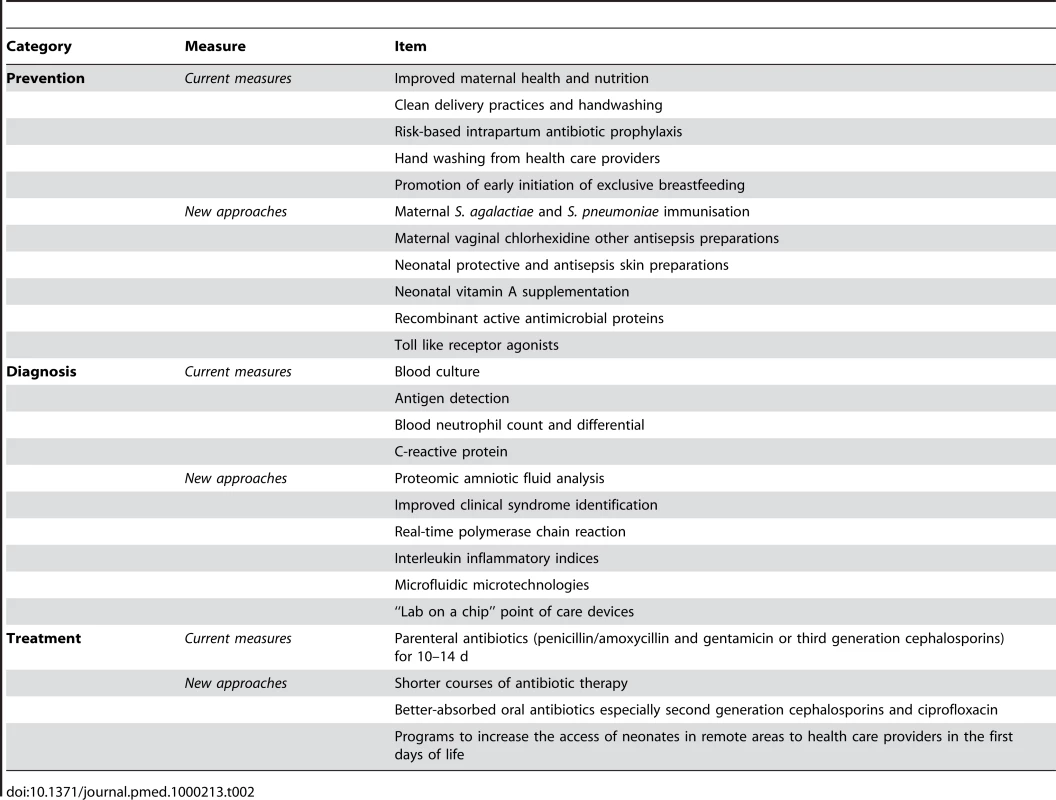
However, practicability of implementing these new advances must be considered. Skilled attendance at delivery is increasing in low - and middle-income countries. Thus, intrapartum approaches such as risk-based antibiotic prophylaxis and improved hand washing during delivery are likely to be both cost-effective and feasible in these countries. More challenges face the implementation of diagnostic technologies. It may take many years for technologies such as the “lab-on-a-chip” to be sufficiently robust and affordable for scale-up to low-income countries. Home-based antibiotic treatment of neonatal sepsis also faces major obstacles to large-scale implementation. Concerns such as “one law for the rich and another for the poor” have already been raised. A careful assessment of the risks and benefits of new technologies and interventions is clearly needed. In low-income settings there are also difficulties with care-seeking for neonatal illnesses, and home visiting programs are needed to identify sick newborns early in life. Neonatal sepsis is also one of the most rapidly fulminating clinical diseases, and many practitioners, including experienced neonatologists, administer parenteral antibiotics rather than wait for the results of any diagnostic tests. These practitioners rightly consider that the individual patient's health is more important than the potential risks of emerging antibiotic resistance.
Thus, front-line health workers and families must be partners in all research and evaluation planning. Detailed assessment of end-user attitudes and preferences using formative and qualitative research methods must be included in the development of programs to reduce morbidity and mortality from neonatal sepsis. Finally, advocacy for equitable resource allocation across and within countries must be a priority and modelling techniques to assess public health impact of neonatal sepsis interventions must be developed and used more widely.
Zdroje
1. ThaverD
ZaidiAK
2009 Burden of neonatal infections in developing countries: a review of evidence from community-based studies. Pediatr Infect Dis J 28 S3 9
2. LawnJE
CousensS
ZupanJ
2005 4 million neonatal deaths: when? Where? Why? Lancet 365 891 900
3. DarmstadtGL
BhuttaZA
CousensS
AdamT
WalkerN
2005 Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet 365 977 988
4. BahlR
MartinesJ
AliN
BhanMK
CarloW
2009 Research priorities to reduce global mortality from newborn infections by 2015. Pediatr Infect Dis J 28 S43 48
5. SchuchatA
ZywickiSS
DinsmoorMJ
MercerB
RomagueraJ
2000 Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics 105 21 26
6. ZaidiAK
ThaverD
AliSA
KhanTA
2009 Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatr Infect Dis J 28 S10 18
7. StollBJ
1997 The global impact of neonatal infection. Clin Perinatol 24 1 21
8. ZaidiAK
HuskinsWC
ThaverD
BhuttaZA
AbbasZ
2005 Hospital-acquired neonatal infections in developing countries. Lancet 365 1175 1188
9. StollBJ
HansenN
FanaroffAA
WrightLL
CarloWA
2002 Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med 347 240 247
10. SealeAC
MwanikiM
NewtonCR
BerkleyJA
2009 Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis 9 428 438
11. TiskumaraR
FakhareeSH
LiuCQ
NuntnarumitP
LuiKM
2009 Neonatal infections in Asia. Arch Dis Child Fetal Neonatal Ed 94 F144 148
12. SchragSJ
HadlerJL
ArnoldKE
Martell-ClearyP
ReingoldA
2006 Risk factors for invasive, early-onset Escherichia coli infections in the era of widespread intrapartum antibiotic use. Pediatrics 118 570 576
13. MenezesEV
YakoobMY
SoomroT
HawsRA
DarmstadtGL
2009 Reducing stillbirths: prevention and management of medical disorders and infections during pregnancy. BMC Pregnancy Childbirth 9 Suppl 1 S4
14. StiehmER
FudenbergHH
1966 Serum levels of immune globulins in health and disease: a survey. Pediatrics 37 715 727
15. LevyO
2007 Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 7 379 390
16. LevyO
MartinS
EichenwaldE
GanzT
ValoreE
1999 Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 104 1327 1333
17. LevyO
2005 Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res 11 113 116
18. AngeloneDF
WesselsMR
CoughlinM
SuterEE
ValentiniP
2006 Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res 60 205 209
19. MarodiL
2006 Neonatal innate immunity to infectious agents. Infect Immun 74 1999 2006
20. KramerMS
2000 Balanced protein/energy supplementation in pregnancy. Cochrane Database Syst Rev CD000032
21. HaiderBA
BhuttaZA
2006 Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev CD004905
22. HealyCM
BakerCJ
2007 Maternal immunization. Pediatr Infect Dis J 26 945 948
23. DemicheliV
BaraleA
RivettiA
2005 Vaccines for women to prevent neonatal tetanus. Cochrane Database Syst Rev CD002959
24. ZamanK
RoyE
ArifeenSE
RahmanM
RaqibR
2008 Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 359 1555 1564
25. SinhaA
LieuTA
PaolettiLC
WeinsteinMC
PlattR
2005 The projected health benefits of maternal group B streptococcal vaccination in the era of chemoprophylaxis. Vaccine 23 3187 3195
26. QuiambaoBP
NohynekH
KayhtyH
OllgrenJ
GozumL
2003 Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine 21 3451 3454
27. BhuttaZA
DarmstadtGL
HasanBS
HawsRA
2005 Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics 115 519 617
28. RheeV
MullanyLC
KhatrySK
KatzJ
LeClerqSC
2008 Maternal and birth attendant hand washing and neonatal mortality in southern Nepal. Arch Pediatr Adolesc Med 162 603 608
29. MeeganME
ConroyRM
LengenySO
RenhaultK
NyangoleJ
2001 Effect on neonatal tetanus mortality after a culturally-based health promotion programme. Lancet 358 640 641
30. BiranA
SchmidtWP
WrightR
JonesT
SeshadriM
2009 The effect of a soap promotion and hygiene education campaign on handwashing behaviour in rural India: a cluster randomised trial. Trop Med Int Health 14 1303 1314
31. LubySP
AgboatwallaM
PainterJ
AltafA
BillhimerWL
2004 Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA 291 2547 2554
32. CurtisVA
DanquahLO
AungerRV
2009 Planned, motivated and habitual hygiene behaviour: an eleven country review. Health Educ Res 24 655 673
33. McClureEM
GoldenbergRL
BrandesN
DarmstadtGL
WrightLL
2007 The use of chlorhexidine to reduce maternal and neonatal mortality and morbidity in low-resource settings. Int J Gynaecol Obstet 97 89 94
34. LumbiganonP
ThinkhamropJ
ThinkhamropB
TolosaJE
2004 Vaginal chlorhexidine during labour for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV). Cochrane Database Syst Rev CD004070
35. OhlssonA
ShahVS
2009 Intrapartum antibiotics for known maternal Group B streptococcal colonization. Cochrane Database Syst Rev CD007467
36. 2005 Early-onset and late-onset neonatal group B streptococcal disease–United States, 1996-2004. MMWR Morb Mortal Wkly Rep 54 1205 1208
37. www.aap.org accessed 3rd September 2009
38. MullanyLC
DarmstadtGL
KhatrySK
KatzJ
LeClerqSC
2006 Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet 367 910 918
39. MullanyLC
DarmstadtGL
TielschJM
2006 Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J 25 665 675
40. DarmstadtGL
SahaSK
AhmedAS
ChowdhuryMA
LawPA
2005 Effect of topical treatment with skin barrier-enhancing emollients on nosocomial infections in preterm infants in Bangladesh: a randomised controlled trial. Lancet 365 1039 1045
41. SiegristCA
2003 Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21 3406 3412
42. BhandariN
BahlR
MazumdarS
MartinesJ
BlackRE
2003 Effect of community-based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet 361 1418 1423
43. EdmondKM
ZandohC
QuigleyMA
Amenga-EtegoS
Owusu-AgyeiS
2006 Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics 117 e380 386
44. EdmondKM
KirkwoodBR
Amenga-EtegoS
Owusu-AgyeiS
HurtLS
2007 Effect of early infant feeding practices on infection-specific neonatal mortality: an investigation of the causal links with observational data from rural Ghana. Am J Clin Nutr 86 1126 1131
45. MullanyLC
KatzJ
LiYM
KhatrySK
LeClerqSC
2008 Breast-feeding patterns, time to initiation, and mortality risk among newborns in southern Nepal. J Nutr 138 599 603
46. DarlowBA
GrahamPJ
2007 Vitamin A supplementation to prevent mortality and short and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev CD000501
47. GogiaS
SachdevHS
2009 Neonatal vitamin A supplementation for prevention of mortality and morbidity in infancy: systematic review of randomised controlled trials. BMJ 338 b919
48. EnglishM
NgamaM
MwalekwaL
PeshuN
2004 Signs of illness in Kenyan infants aged less than 60 days. Bull World Health Organ 82 323 329
49. 2008 Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet 371 135 142
50. CromwellD
JoffeT
HughesR
MurphyD
DhillonC
2008 The local adaptation of national recommendations for preventing early-onset neonatal Group B Streptococcal disease in UK maternity units. J Health Serv Res Policy 13 Suppl 2 52 57
51. BuhimschiCS
BhandariV
HamarBD
BahtiyarMO
ZhaoG
2007 Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med 4 e18 doi:10.1371/journal.pmed.0040018
52. BuhimschiCS
DulayAT
Abdel-RazeqS
ZhaoG
LeeS
2009 Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG 116 257 267
53. NealPR
KleimanMB
ReynoldsJK
AllenSD
LemonsJA
1986 Volume of blood submitted for culture from neonates. J Clin Microbiol 24 353 356
54. BenjaminDKJr
MillerW
GargesH
BenjaminDK
McKinneyREJr
2001 Bacteremia, central catheters, and neonates: when to pull the line. Pediatrics 107 1272 1276
55. KurlatI
StollBJ
McGowanJEJr
1989 Time to positivity for detection of bacteremia in neonates. J Clin Microbiol 27 1068 1071
56. DarmstadtGL
BatraM
ZaidiAK
2009 Parenteral antibiotics for the treatment of serious neonatal bacterial infections in developing country settings. Pediatr Infect Dis J 28 S37 42
57. PetersRP
van AgtmaelMA
DannerSA
SavelkoulPH
Vandenbroucke-GraulsCM
2004 New developments in the diagnosis of bloodstream infections. Lancet Infect Dis 4 751 760
58. MoisiJC
SahaSK
FaladeAG
Njanpop-LafourcadeBM
OundoJ
2009 Enhanced diagnosis of pneumococcal meningitis with use of the Binax NOW immunochromatographic test of Streptococcus pneumoniae antigen: a multisite study. Clin Infect Dis 48 Suppl 2 S49 56
59. JordanJA
DursoMB
2000 Comparison of 16S rRNA gene PCR and BACTEC 9240 for detection of neonatal bacteremia. J Clin Microbiol 38 2574 2578
60. JordanJA
DursoMB
2005 Real-time polymerase chain reaction for detecting bacterial DNA directly from blood of neonates being evaluated for sepsis. J Mol Diagn 7 575 581
61. JordanJA
DursoMB
ButchkoAR
JonesJG
BrozanskiBS
2006 Evaluating the near-term infant for early onset sepsis: progress and challenges to consider with 16S rDNA polymerase chain reaction testing. J Mol Diagn 8 357 363
62. WeileJ
KnabbeC
2009 Current applications and future trends of molecular diagnostics in clinical bacteriology. Anal Bioanal Chem 394 731 742
63. AndradeSS
BispoPJ
GalesAC
2008 Advances in the microbiological diagnosis of sepsis. Shock 30 Suppl 1 41 46
64. JordanJA
Jones-LaughnerJ
DursoMB
2009 Utility of pyrosequencing in identifying bacteria directly from positive blood culture bottles. J Clin Microbiol 47 368 372
65. Verboon-MaciolekMA
ThijsenSF
HemelsMA
MensesM
van LoonAM
2006 Inflammatory mediators for the diagnosis and treatment of sepsis in early infancy. Pediatr Res 59 457 461
66. FranzAR
SteinbachG
KronM
PohlandtF
1999 Reduction of unnecessary antibiotic therapy in newborn infants using interleukin-8 and C-reactive protein as markers of bacterial infections. Pediatrics 104 447 453
67. EdgarJD
WilsonDC
McMillanSA
CrockardAD
HallidayMI
1994 Predictive value of soluble immunological mediators in neonatal infection. Clin Sci (Lond) 87 165 171
68. YagerP
EdwardsT
FuE
HeltonK
NelsonK
2006 Microfluidic diagnostic technologies for global public health. Nature 442 412 418
69. ZhangY
OzdemirP
2009 Microfluidic DNA amplification–a review. Anal Chim Acta 638 115 125
70. StevensDY
PetriCR
OsbornJL
Spicar-MihalicP
McKenzieKG
2008 Enabling a microfluidic immunoassay for the developing world by integration of on-card dry reagent storage. Lab Chip 8 2038 2045
71. ThaverD
AliSA
ZaidiAK
2009 Antimicrobial resistance among neonatal pathogens in developing countries. Pediatr Infect Dis J 28 S19 21
72. DarmstadtGL
BatraM
ZaidiAK
2009 Oral antibiotics in the management of serious neonatal bacterial infections in developing country communities. Pediatr Infect Dis J 28 S31 36
73. BhuttaZA
ZaidiAK
ThaverD
HumayunQ
AliS
2009 Management of newborn infections in primary care settings: a review of the evidence and implications for policy? Pediatr Infect Dis J 28 S22 30
74. BangAT
BangRA
BaituleSB
ReddyMH
DeshmukhMD
1999 Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet 354 1955 1961
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials
- BMI and Risk of Serious Upper Body Injury Following Motor Vehicle Crashes: Concordance of Real-World and Computer-Simulated Observations
- Preventing Road Deaths—Time for Data
- Essential Surgery at the District Hospital: A Retrospective Descriptive Analysis in Three African Countries
- Drivers of Inequality in Millennium Development Goal Progress: A Statistical Analysis
- Where Will the Next Generation of Stroke Treatments Come From?
- Unravelling the Genetics of Ischaemic Stroke
- Chronic Obstructive Pulmonary Disease: Effects beyond the Lungs
- The Promise of Prevention: The Effects of Four Preventable Risk Factors on National Life Expectancy and Life Expectancy Disparities by Race and County in the United States
- Can Animal Models of Disease Reliably Inform Human Studies?
- New Approaches to Preventing, Diagnosing, and Treating Neonatal Sepsis
- Protecting Vulnerable Road Users from Injury
- Providing Alcohol-Related Screening and Brief Interventions to Adolescents through Health Care Systems: Obstacles and Solutions
- Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- Accelerating Policy Decisions to Adopt Type b Vaccine: A Global, Multivariable Analysis
- Human Resource and Funding Constraints for Essential Surgery in District Hospitals in Africa: A Retrospective Cross-Sectional Survey
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- BMI and Risk of Serious Upper Body Injury Following Motor Vehicle Crashes: Concordance of Real-World and Computer-Simulated Observations
- Unravelling the Genetics of Ischaemic Stroke
- Providing Alcohol-Related Screening and Brief Interventions to Adolescents through Health Care Systems: Obstacles and Solutions
- Human Resource and Funding Constraints for Essential Surgery in District Hospitals in Africa: A Retrospective Cross-Sectional Survey
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



