-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Use of Nonhuman Primate Models in HIV Vaccine Development
article has not abstract
Published in the journal: . PLoS Med 5(8): e173. doi:10.1371/journal.pmed.0050173
Category: Guidelines and Guidance
doi: https://doi.org/10.1371/journal.pmed.0050173Summary
article has not abstract
In April 2006, the National Institute of Allergy and Infectious Disease (NIAID)-funded HIV Vaccine Trials Network and the NIAID Division of AIDS sponsored a workshop at which nonhuman primate (NHP) researchers and clinical trial scientists with HIV vaccine research expertise discussed how to more effectively use NHPs for evaluating HIV-1 vaccine candidates. This workshop precipitated a broad discussion on what types of NHP studies should be targeted in the critical preclinical pathway for HIV-1 vaccine candidates, especially those designed to elicit HIV-1-specific T cell responses. This paper describes the two-stage NHP screening strategy for T cell–based HIV-1 vaccines that emerged from discussions among the authors during the past year and a half. While conceived prior to the recent release of results for the phase IIB trial (STEP Study) of the Merck replication-incompetent adenovirus serotype 5 (Ad5)-HIV gag/pol/nef vaccine, we think the approach outlined below will be particularly useful for preclinical evaluation of vaccine candidates in the current vaccine pipeline for two reasons. First, the proposed strategy will eliminate suboptimal vaccine candidates early in the testing process (i.e., before initiation of phase I clinical trials). Second, the strategy would provide comparative immune response data in NHPs and humans for each promising HIV-1 vaccine product, information that could help the design of future vaccine candidates.
The most rigorous approach to judging the potential efficacy of HIV-1 vaccine candidates in NHP models is to determine if prototype vaccine candidates can protect the animals from the uncontrolled replication of a virulent challenge virus that recapitulates the pathogenic effects of HIV-1 in humans. At present, the most extensively studied HIV-1 vaccines are intended primarily to induce T cell immunity. While binding antibodies are reliably detected, if neutralizing antibodies are detected they are usually of low titer and narrow in their breadth. The primary expected outcome for T cell vaccines is the control of viral replication after experimental challenge with simian immunodeficiency virus (SIV) in NHPs or natural infection with HIV-1 in humans. This control should result in reduced viral load in plasma or tissue and preservation of CD4+ T cell counts, thereby preventing or delaying progression to AIDS. Progress toward developing an AIDS vaccine has been hampered by the lack of clarity about what host immune responses are required to prevent HIV-1 transmission or protect against disease progression. An immune correlate of HIV-1 vaccine-mediated protection can only be identified after analysis of the results from one or more efficacy trials of effective vaccine(s). There are clear examples of vaccines that are capable of providing modest control of viral replication after SIV challenge of macaques. No single immune effector function, however, has been consistently associated with these vaccine-mediated effects. Thus, at present, protection against disease progression in NHP studies is the best surrogate measure of vaccine efficacy that can be used in preclinical assessments of vaccine approaches.
An assumption that underlies the current approach to HIV-1 vaccine testing has been that vaccine immunogenicity is an essential surrogate measure of vaccine efficacy but, as noted above, the validity of this assumption is unknown. Some HIV-1 vaccine candidates have moved into phase I clinical testing with positive immune responses in NHPs, but without challenge data (Figure 1). Others have been tested only in rodents, whose immune responses have not reliably predicted either the consistency or level of immune responses in people. Overall, many HIV-1 vaccine candidates that are immunogenic in animals have elicited only weak and/or transient immune responses in humans. Critically, only a very few vaccine candidates have demonstrated any efficacy in NHP challenge models using pathogenic SIV isolates, the model that best recapitulates the key features of HIV-1 infection and pathogenesis according to the following criteria: (1) persistent, progressive systemic infection after experimental inoculation; (2) use of the CCR5 coreceptor; (3) acute depletion of memory CD4+ T cells, especially from mucosal sites; and (4) establishment of an initially disease-free plateau phase in the majority of animals, followed by (5) progression to AIDS over a period of several months to years. The key requirements, relevance, and limitations of specific NHP models for evaluating HIV-1 vaccine candidates have recently been reviewed in detail [1–10].
Fig. 1. Current Preclinical Testing Strategy for Candidate HIV-1 Vaccines 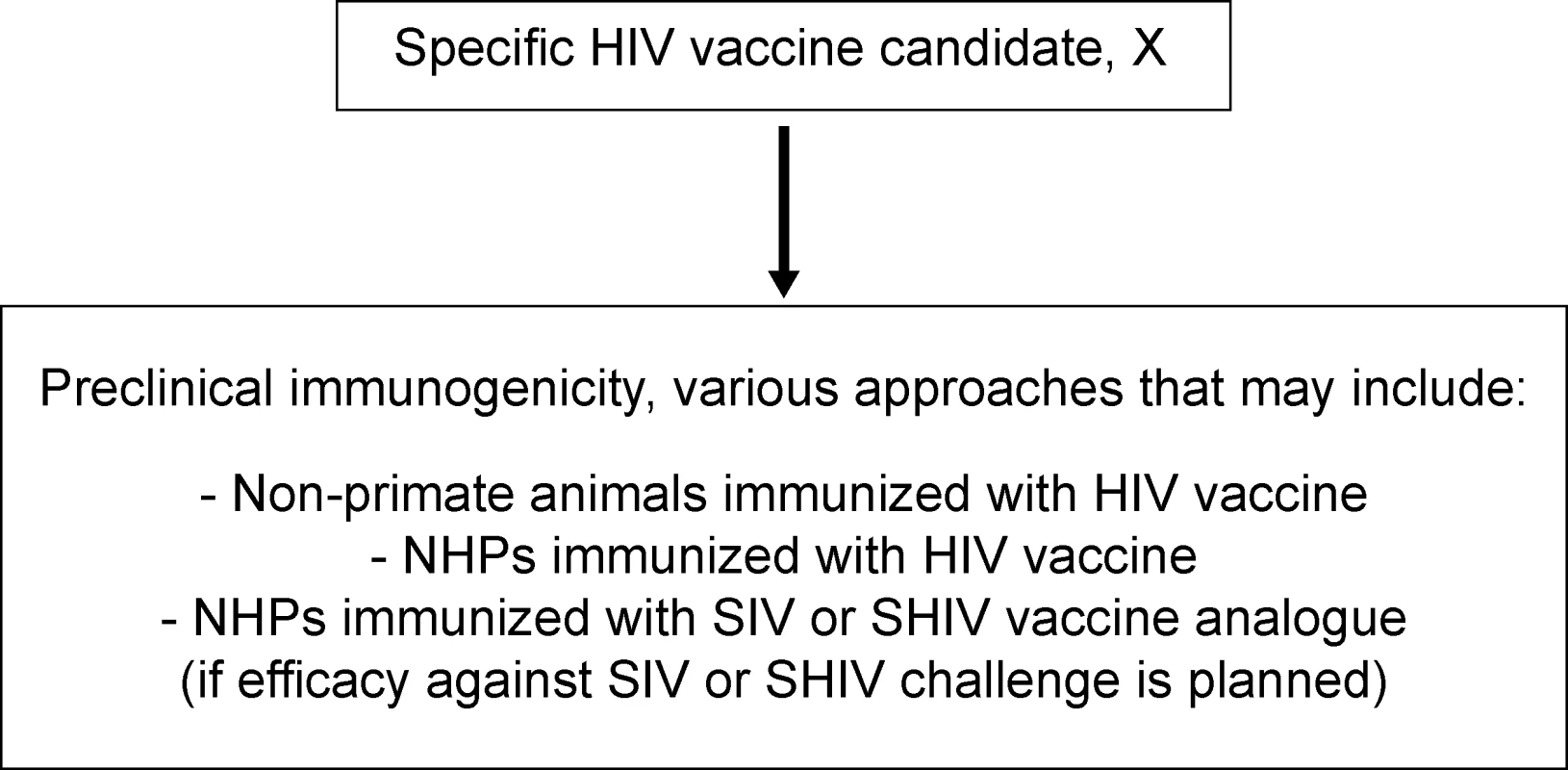
A variety of approaches are used to evaluate the immunogenicity of a specific candidate HIV-1 vaccine, X. These approaches may or may not include testing immunogenicity of the HIV-1 vaccine itself in non-primates (e.g., rodents, rabbits, guinea pigs, etc.) or NHPs. In addition, the immunogenicity of an SIV or SHIV analogue of the HIV-1 vaccine may be tested in NHPs; this is done before NHP efficacy trials, which require challenge of NHPs with SIV or SHIV. Currently, neither immunogenicity nor challenge studies in NHPs are required preclinical assessments for candidate HIV-1 vaccines. It is important to recognize that, in the past, there has been a reluctance to give NHP models any “gatekeeper” status in the HIV-1 vaccine testing pathway, out of concern that potentially effective HIV-1 vaccine candidates might thereby be missed. Specific concerns include the imperfect (albeit close) recapitulation by the SIV-infected macaque model of HIV-1 infection of humans, and the possibility that some experimental challenges used in NHP models might be more stringent than is relevant for typical transmissions of HIV-1 between humans. The last few years, however, have seen the development of vaccines that are more consistently immunogenic in NHPs. Hence, identifying immunogens with increased immunogenicity and screening out those vaccine candidates likely to have low efficacy has now become a much higher priority element of the preclinical HIV-1 vaccine evaluation process. The cost and the regulatory requirements associated with the manufacture of vaccines for human clinical trials are additional factors; specifically, the number of HIV-1 vaccine candidates now available for phase I testing has increased, resulting in the need to develop cost-effective strategies to filter the vaccine pipeline. Thus, the role of NHP models in the process of selecting HIV-1 vaccines for clinical trials is now being reconsidered.
Shared Vision and Goals for Expediting HIV-1 Vaccine Development
There is a clear consensus that we must expedite the process of moving HIV-1 vaccine candidates from preclinical to clinical studies, using the most scientifically rigorous, efficient, and cost-effective strategies available. Furthermore, stringent criteria are needed to identify the HIV-1 vaccine candidates most likely to lead to a safe and effective vaccine product. To accomplish these shared goals, we strongly recommend identifying and implementing a strategy that places standardized, coordinated NHP studies in a central role in the preclinical evaluation of HIV-1 vaccines. Moreover, we recommend that HIV-1 vaccine candidates now advancing in the clinical pipeline also be evaluated by this NHP strategy to provide essential comparative data; determining which NHP studies should be conducted for each product currently in clinical trials should be done on a case-by-case basis. Our consensus proposal is a two-pronged strategy for evaluation of candidate vaccines in NHPs, using biologically based, clinically relevant endpoints to define go/no-go decision points prior to entry into phase I clinical trials (Figure 2).
Fig. 2. Proposed Strategy for the Effective Use of NHPs in Preclinical Testing of Candidate HIV-1 T Cell–Based Vaccines 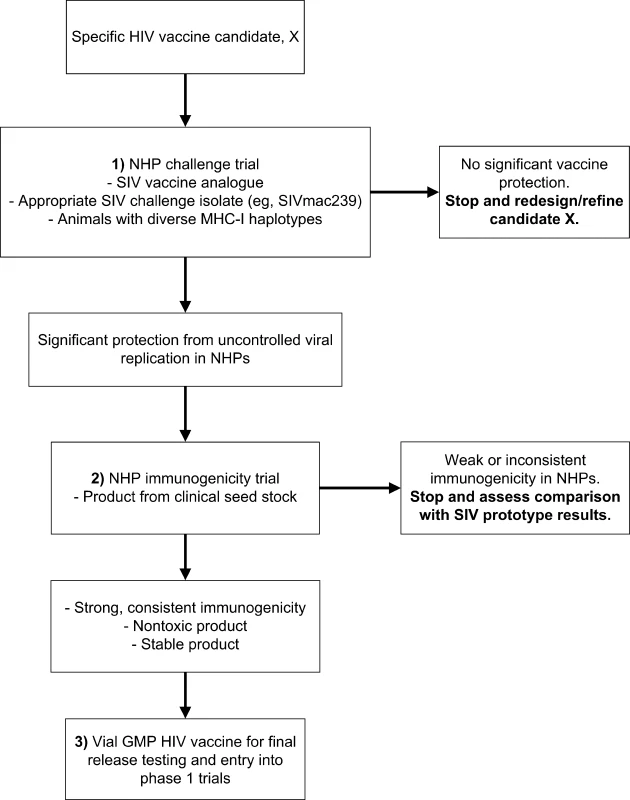
This strategy would be required for advancing an HIV-1 vaccine candidate to human testing. The first phase of the proposed evaluation strategy for cytotoxic T lymphocyte–based vaccines would use the effectiveness of viral replication control following virulent SIV challenge of NHPs vaccinated with a prototype SIV vaccine as the primary criterion (Step 1 in Figure 2) for continuing the development of the vaccine candidate. Further testing would proceed only if the prototype vaccine showed consistent and statistically significant reduction of viremia compared with control animals. Given the diversity in HIV-1 sequences, there will almost certainly be greater divergence between vaccine and challenge virus sequences in the clinical setting than occurs in SIV challenge studies, so NHP studies may overestimate vaccine effectiveness. Conversely, the failure to demonstrate a degree of effectiveness in SIV challenge studies using closely matched vaccine and challenge sequences would argue strongly against the clinical efficacy of the vaccine approach in humans. The ability of the candidate vaccine to contain viral replication should be followed for six to eight months; longer periods of containment would constitute an additional important, but not definitive, evaluation criterion. This approach proposes that a specific HIV-1 vaccine candidate whose SIV prototype vaccine did not provide protection against SIV challenge should not proceed further in the developmental pathway.
In retrospect, the proposed approach outlined in Figure 2 predicts the lack of efficacy observed for the Merck Ad5 gag/pol/nef HIV-1 vaccine in a human clinical trial. Studies published in 2002 showed that an SIV gag version of the Merck Ad5 HIV-1 gag vaccine was strongly immunogenic in macaques and protected the animals from uncontrolled replication of simian/human immunodeficiency virus (SHIV) 89.6P [11]. SHIV 89.6P, however, is a virus that uses CXCR4 as its dominant coreceptor, not the CCR5 coreceptor used by incident HIV-1 isolates, and the disease course that SHIV 89.6P induces differs markedly from what occurs during most human infections with HIV-1 [2]. Thus, challenge of rhesus macaques with SHIV 89.6P, considered by some vaccinologists to be a predictive NHP challenge model five years ago, has been shown by the STEP Study results to be an inadequate predictor of subsequent human efficacy trials.
Studies published after the human trials of the HIV-1 vaccine were underway demonstrated that the SIV version of the Merck Ad5 gag-only vaccine was not effective in reducing postinfection viremia of vaccinated rhesus macaques after SIVmac239 challenge; furthermore, an SIV gag DNA prime plus Ad5 SIV gag vaccine was only effective at reducing postinfection viral load in macaques with a specific major histocompatibility complex (MHC) class I allele, Mamu A*01, which is associated with an SIV cytotoxic T lymphocyte epitope that exhibits strong control of SIV replication in both vaccinated and control macaques [12]. Very recently, a DNA prime plus Ad5 SIV gag/tat/rev/nef vaccine was shown to be effective at reducing postinfection viral load in Mamu A*01+ macaques challenged with SIVmac239 [13], but this vaccine has not yet been evaluated in macaques that do not have the Mamu A*01 allele. The above NHP vaccine studies used a homologous challenge virus; it is possible that less robust protection would be observed using a challenge virus less well matched to the vaccine construct, a scenario that would more closely mimic human exposures to HIV-1. For example, in macaques immunized with live-attenuated SIV, reduced control of viremia is observed after challenge with heterologous (SIVsmE660) compared to homologous (SIVmac239) virus [14].
Taken together, these recent NHP results suggest that candidate HIV-1 vaccines should be tested in NHPs with a range of MHC-1 haplotypes, excluding (or not solely relying on) animals with MHC alleles associated with an increased capacity to control viral replication, even in the absence of immunization, such as Mamu A*01, Mamu B*17, and Mamu B*08. The results also suggest that reduction of viral load in challenge models employing pathogenic, CXCR4-using SHIVs, which represent a less stringent challenge than pathogenic R5 SIV isolates [2,8], appears not to be an effective model for predicting the clinical efficacy of T cell–based HIV-1 vaccine candidates. Overall, the outcomes from NHP studies of the SIV versions of the Merck Ad5-based HIV-1 vaccine provide support for modifying the way in which NHPs are used in preclinical HIV-1 vaccine research, as outlined above and summarized in Figure 2. For any promising HIV-1 vaccine candidate that demonstrates efficacy in NHPs against challenge with an SIV isolate containing envelope (or other antigens) homologous to the vaccine, challenge with an SIV isolate containing heterologous envelope could then be performed. Whether heterologous NHP challenge models can accurately predict HIV-1 vaccine efficacy will require further clinical testing.
It is important that the initial phase in the screening strategy uses a measure for efficacy that is consistent with the desired clinical endpoint. Using the ability to control viremia after SIV challenge as the endpoint for the initial screening step of candidate HIV-1 vaccines intended to induce T cell immunity is cost-effective and resource-efficient for three reasons. First, the immune responses required for any vaccine against HIV-1 or SIV to be effective are currently unknown. Second, the demonstration that a vaccine is effective against viral replication is a more direct and relevant criterion than its immunogenicity when selecting an HIV-1 vaccine for clinical evaluation, because the immune responses associated with vaccine protection are not known. There are more SIV vaccines that elicit moderate to robust SIV-specific immune responses, as measured by interferon-gamma enzyme-linked immunosorbent spot or cytokine flow cytometry assays, but that do not protect NHPs against SIV challenge, than there are SIV vaccines that do protect against challenge. Third, it is most cost-effective to eliminate candidate vaccines before they undergo good manufacturing practices (GMP) manufacture and evaluation in human clinical trials.
One area of HIV-1 vaccine research that needs consideration is developing NHP models to define whether a vaccine candidate might increase HIV-1 acquisition. A provocative aspect of the STEP Study results is the increased rate of acquisition seen among men with neutralizing antibodies against Ad5 prior to vaccination or men who were uncircumcised when they entered the trial [15]. Availability of a NHP model to define the potential mechanisms for such findings could be a valuable tool in preclinical testing of candidate HIV-1 vaccines.
To facilitate the comparative evaluation of efficacy of various vaccine candidates, the design of the challenge studies should be standardized as much as possible, at least when similar classes of vaccine candidate are being evaluated (e.g., DNA vaccines, recombinant adenovirus vector-based vaccines). Relevant factors that should be standardized include the SIV vaccine insert isolate, SIV challenge virus, challenge route, challenge dose, endpoint measurements, and the macaque species. Specimens from NHP challenge studies should be evaluated for immunogenicity, both to evaluate potential immune correlates of control of viral replication and to link the results to subsequent immunogenicity studies conducted in macaques using corresponding vaccine products with HIV-1 inserts, including products manufactured under GMP conditions (see below).
The second phase in the proposed NHP-based HIV-1 vaccine screening strategy would be to determine if an HIV-1 vaccine product from the clinical grade seed stock is sufficiently immunogenic in NHPs to warrant advancement into human trials (Step 2 in Figure 2). The rationale for this step is that the HIV-1 vaccine should be tested for immunogenicity in NHPs to avoid the costs of manufacturing and testing defective or low immunogenicity products in humans. To avoid delays in clinical development, NHP immunogenicity trials could be conducted either with a vaccine product derived from the seed stock to be used for human clinical trials, or from the GMP lot itself. The immunization regimen tested in the NHPs should represent the one to be evaluated in the proposed clinical trial. Similarly, when immunogenicity is evaluated in NHP models, validated T cell assays similar to those used in human trials should be used. The availability of central testing laboratories would facilitate the standardized testing of defined T cell responses in NHP vaccination studies, and thereby help obtain robust data for comparing immune responses in NHPs and humans.
As outlined in Figure 2, a phase I clinical trial would be undertaken only if the product proved to be safe, immunogenic, and effective when tested in recommended, standardized NHP models. The immune responses elicited in NHPs by the HIV-1 vaccine should not be grossly different than those elicited by the SIV prototype. Further development of promising HIV-1 vaccine candidates that fail the immunogenicity-testing step might still be warranted if the manufacturing process had created an inactive product for a reason that was remediable. An added value of evaluating an HIV-1 vaccine product in NHPs and humans is the ability to compare immune responses more directly in these species, an ability that will be critical in the iterative process of improving future HIV-1 vaccine products. However, allowances will need to be made for certain vaccine types for which the human vaccines are not well suited for immunogenicity evaluation in NHPs; examples include peptide vaccines that are designed using epitopes recognized by human MHC alleles and vaccine vectors that do not replicate in NHPs.
Finally, there is consensus that conducting immunogenicity trials of multiple HIV-1 T cell vaccine candidates in NHPs, solely to establish a “rank order” for selecting candidates for evaluation in phase I clinical trials, may not be the best use of NHP resources; as noted above, it is not yet known what immune responses correlate with vaccine efficacy. Whether the use of comparative immunogenicity studies in NHPs that are intended to rank and reject various iterations of a specific candidate vaccine prior to entry into phase I trials is an effective strategy is at present unclear.
The most effective use of NHPs for predicting the clinical effectiveness of neutralizing antibody-based HIV-1 vaccines is unclear. Although vaccine-induced HIV-1-neutralizing antibodies of a sufficient titer can provide potent protection of NHPs against a single homologous challenge virus, neutralizing antibodies will need to be broadly cross-reactive to protect against the multiple HIV-1 genetic variants in the human population. When HIV-1 immunogens that elicit broadly neutralizing antibodies in primates become available, standardized in vitro assessments of vaccine-elicited neutralizing antibody responses, as measured against a wide spectrum of HIV-1 variants, may provide important information that complements the outcome of NHP challenge studies.
In summary, we believe that it is time for the HIV vaccine field to consider a more standardized and rigorous approach to the preclinical testing of candidate HIV-1 vaccines. It is our opinion that the strategy proposed here is the best approach among currently available options.
Zdroje
1. DesrosiersRC
2004
Prospects for an AIDS vaccine.
Nat Med
10
221
223
2. FeinbergMBMooreJP
2002
AIDS vaccine models: Challenging challenge viruses.
Nat Med
8
207
210
3. HaigwoodNL
2004
Predictive value of primate models for AIDS.
AIDS Rev
6
187
198
4. HuSL
2005
Non-human primate models for AIDS vaccine research.
Curr Drug Targets Infect Disord
5
193
201
5. JohnsonRP
2006
HIV pathogenesis and vaccine development.
Top HIV Med
14
8
15
6. KoffWCJohnsonPRWatkinsDIBurtonDRLifsonJD
2006
HIV vaccine design: Insights from live attenuated SIV vaccines.
Nat Immunol
7
19
23
7. LetvinNL
2006
Progress and obstacles in the development of an AIDS vaccine.
Nat Rev Immunol
6
930
939
8. LifsonJDMartinMA
2002
One step forwards, one step back.
Nature
415
272
273
9. MalkevitchNVRobert-GuroffM
2004
A call for replicating vector prime-protein boost strategies in HIV vaccine design.
Expert Rev Vaccines
3
S105
S117
10. RobinsonHLAmaraRR
2005
T cell vaccines for microbial infections.
Nat Med
11
S25
S32
11. ShiverJWFuTMChenLCasimiroDRDaviesME
2002
Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity.
Nature
415
331
335
12. CasimiroDRWangFSchleifWALiangXZhangZQ
2005
Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag.
J Virol
79
15547
15555
13. WilsonNAReedJNapoeGSPiaskowskiSSzymanskiA
2006
Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239.
J Virol
80
5875
5885
14. WyandMSMansonKMontefioriDCLifsonJDJohnsonRP
1999
Protection by live, attenuated simian immunodeficiency virus against heterologous challenge.
J Virol
73
8356
8363
15. RobertsonMMehrotraDFitzgeraldDDuerrACasimiroD
2008
Efficacy results from the Step Study (Merck V520 Protocol 023/HVTN 502)—A phase II test-of-concept trial of the MRKAd5 HIV-1 Gag/Pol/Nef Trivalent vaccine [abstract 88LB].
15th Conference on Retroviruses and Opportunistic Infections; 3–6 February 2008; Boston, Massachusetts, United States of America. Available: http://www.hvtn.org/science/step_buch.html. Accessed 10 July 2008
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 8- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
- Greater Response to Placebo in Children Than in Adults: A Systematic Review and Meta-Analysis in Drug-Resistant Partial Epilepsy
- Can a Topical Microbicide Prevent Rectal HIV Transmission?
- Developing a Prognostic Model for Traumatic Brain Injury—A Missed Opportunity?
- Assessing Antimalarial Efficacy in a Time of Change to Artemisinin-Based Combination Therapies: The Role of Médecins Sans Frontières
- Children Are Not Just Small Adults: The Urgent Need for High-Quality Trial Evidence in Children
- The Use of Nonhuman Primate Models in HIV Vaccine Development
- More Evidence Against a Causal Association between C-Reactive Protein and Diabetes
- Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries
- Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
- An Acute Evolving Flaccid Quadriparesis in an Elderly Woman
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
- Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
- The Use of Nonhuman Primate Models in HIV Vaccine Development
- Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



