-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvidence-Based Tuberculosis Diagnosis
article has not abstract
Published in the journal: . PLoS Med 5(7): e156. doi:10.1371/journal.pmed.0050156
Category: Research in Translation
doi: https://doi.org/10.1371/journal.pmed.0050156Summary
article has not abstract
There is great excitement in the tuberculosis (TB) scientific community over the introduction of new tools into TB control activities. The development of new tools is an important component of the Global Plan to Stop TB and the World Health Organization's new global Stop TB Strategy [1,2]. Anticipating the introduction of new tools, the Stop TB Partnership has established a Retooling Task Force to develop a framework for engaging policy makers to foster accelerated adoption and implementation of new tools into TB control programs [3].
While new tools offer great promise in clinical medicine and in public health, limited resources and the movement toward evidence-based guidelines and policies require careful validation of new tools prior to their introduction for routine use. The world spends an estimated US$1 billion per year on diagnostics for TB [4]. It is important to ensure that such expenditure is backed by strong evidence.
Ideally, clinical and policy decisions must be guided by the totality of evidence on a given topic. This is particularly relevant for TB, where concerns have been raised about the lack of emphasis on evidence of effectiveness in some of the existing TB guidelines and policies [5]. These concerns are being taken seriously [6,7], and the outcome should be evident in upcoming TB guidelines and policies. In fact, the World Health Organization (WHO) recently announced its approach for developing new policies on TB in a document entitled “Moving Research Findings into New WHO Policies” [7]. According to this document, in order to consider a global policy change, WHO must have solid evidence, including clinical trials or field evaluations in high TB prevalence settings. The steps involved in the policy process include a comprehensive review of the evidence, as well as expert opinion and judgment (Box 1).
Box 1. WHO Policy Process for Tuberculosis
1. Identifying the Need for a Policy Change
The need to formulate new or revised policies may arise from WHO's ongoing monitoring of technical developments or from interested parties submitting requests with supporting documentation for policy or guideline development. WHO receives information about a new technology or approach via many channels, with the most direct lines coming from national TB programs and researchers themselves. To consider a global policy change, WHO must have solid evidence, including clinical trials or field evaluations in high TB prevalence settings.
2. Reviewing the Evidence
WHO may carry out or commission a review of the documentation of the technology's clinical or programmatic performance, including newly published and “grey” research or reviews, “proof of principle” reports, large-scale field trials, and demonstration projects in different resource settings. Standardized evaluation criteria have been and are being developed by the New Diagnostics, New Drugs, and New Vaccines Working Groups of the Stop TB Partnership.
3. Convening an Expert Panel
If the evidence base is compelling, WHO will convene an external panel of experts, excluding all original principal investigators from the studies. The panel will review the evidence and make a recommendation or propose draft policies or guidelines to WHO's Strategic and Technical Advisory Group for Tuberculosis (STAG-TB).
4. Assessing Draft Policies and Guidelines
STAG-TB provides objective, ongoing technical and strategic advice to WHO on TB care and control. STAG-TB's objectives are to provide the Director-General, through the Stop TB Department, with an independent evaluation of the strategic, scientific, and technical aspects of WHO's TB activities; review progress and challenges in WHO's TB-related core functions; review and make recommendations on committees and working groups; and make recommendations on WHO's TB activity priorities. STAG-TB reviews the policy drafts and supporting documentation during its annual meeting. STAG-TB may endorse the policy recommendation with or without revisions, request additional information and re-review the evidence in subsequent years, or reject the recommendation.
5. Formulating and Disseminating Policy
New WHO policies and guidelines will be disseminated through different channels to Member States, including through the World Health Assembly, WHO Web site, listservs, and journal publications. WHO also disseminates its recommendations to other agencies and donors engaged in TB control activities.
Source: World Health Organization [7]
High-quality evidence on TB diagnostics is critical for the development of evidence-based policies on TB diagnosis, and, ultimately, for effective control of the global TB epidemic. While primary diagnostic trials are needed to generate data on test accuracy and operational performance, systematic reviews provide the best synthesis of current evidence on any given diagnostic test [8]. Although a large number of trials on TB diagnostics have been published, surprisingly, no systematic reviews were published until recently. In the past few years, at least 30 systematic reviews and meta-analyses have been published on various TB tests [9–38]. These reviews have synthesized the results of more than 1,000 primary studies, providing valuable insights into the diagnostic accuracy of various tests (Table 1, Box 2).
Tab. 1. Findings from Systematic Reviews on TB Diagnostic Tests 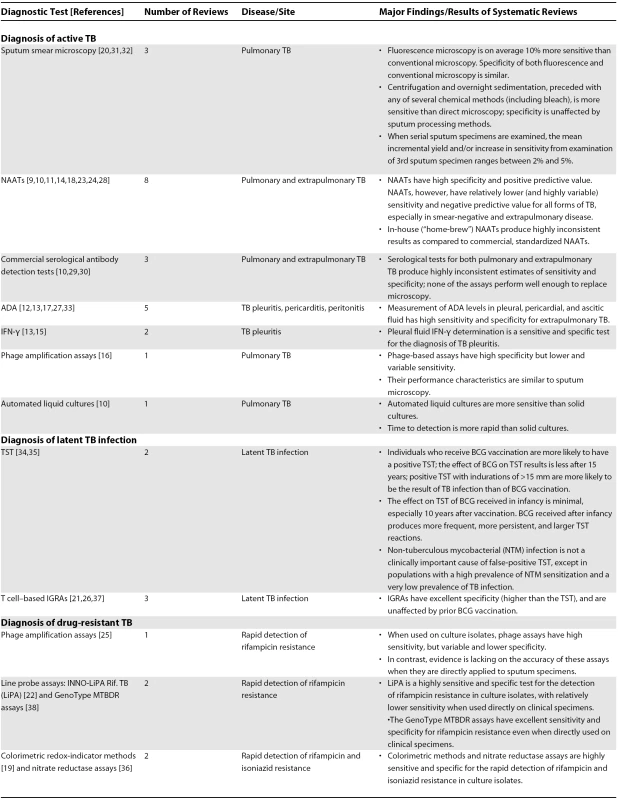
Box 2. Five Key Papers in the Field
Dinnes et al., 2007 [10]. The most comprehensive systematic review of several rapid diagnostic tests for the detection of TB, sponsored by the UK Health Technology Assessment Programme.
Mase et al., 2007 [20]. This review on incremental yield of serial smears showed that the average incremental yield and/or the increase in sensitivity of examining a third sputum specimen ranged between 2% and 5%. This evidence partly informed the new WHO policy on smear microscopy.
Menzies et al., 2007 [21]. This meta-analysis showed that IGRAs for TB infection have excellent specificity (higher than the conventional TST), and are unaffected by prior BCG vaccination. This review also highlighted the key unresolved questions regarding the use of these assays in clinical practice. An update to this meta-analysis was published recently (Pai et al., 2008 [37]).
Steingart et al., 2007 [30]. This meta-analysis showed that serological tests for TB produce highly inconsistent estimates of sensitivity and specificity, and none of the currently available commercial assays perform well enough to replace microscopy. Several initiatives are now ongoing to develop improved point-of-care immune-based rapid tests for TB.
Steingart et al., 2006 [31]. This systematic review reported strong evidence that fluorescence microscopy is more sensitive than conventional microscopy. Several initiatives are now ongoing to develop simple, low-cost fluorescence microscopy systems to optimize smear microscopy.
Implications for Clinical and Laboratory Practice
For clinicians, systematic reviews provide several useful insights for diagnosis of latent TB infection, active TB disease, and drug resistance.
For diagnosis of latent TB, clinicians have used the tuberculin skin test (TST) for decades. Recently, interferon-gamma release assays (IGRAs) have emerged as attractive alternatives. While the TST is known to have poor specificity in populations vaccinated with bacille Calmette-Guérin (BCG) [34], meta-analyses have shown that IGRAs have much higher specificity for TB infection than the TST, and IGRA specificity is unaffected by BCG vaccination [21,26,37]. However, another meta-analysis showed that BCG vaccination received in infancy has a minimal effect on the TST, whereas BCG received after infancy produces more frequent, more persistent, and larger TST reactions [35]. Thus, the TST might retain high specificity in some populations, whereas it may perform poorly in others. IGRAs are particularly attractive in the latter setting. However, meta-analyses on IGRAs have highlighted the lack of evidence on the predictive ability of these assays in identifying those individuals with TB infection who are at highest risk for progressing to active disease. Several cohort studies are ongoing (reviewed elsewhere [39]), and these should provide useful evidence on this unresolved issue.
For active TB, serological tests have been attempted for decades. Two meta-analyses have convincingly shown that existing commercial antibody-based tests have poor accuracy and limited clinical utility [29,30]. Despite this evidence, dozens of commercial serological tests continue to be marketed, mostly in private sectors of countries that lack diagnostic regulatory bodies [4].
Nucleic acid amplification tests (NAATs) were considered to be a major breakthrough in TB diagnosis when they were first introduced. A series of meta-analyses have shown that NAATs have high specificity and positive predictive value, but modest and highly variable sensitivity, especially in smear-negative and extrapulmonary TB [9,11,14,18,23,24,28].
Conventional tests such as smears and cultures perform poorly in extrapulmonary TB. A series of reviews have shown that biomarkers such as adenosine deaminase (ADA) and interferon-gamma (IFN-γ) have excellent accuracy for tuberculous pleural effusion [12,13,15,17]. These biomarkers, especially ADA, are easy to measure and inexpensive. Despite this evidence, these tests appear to be underutilized [40].
For the diagnosis of multidrug-resistant TB (MDR-TB), available data suggest that phage-based assays do not perform well when directly applied to clinical specimens [25]. Line probe assays show great promise for rapid detection of rifampicin resistance in settings with high MDR-TB prevalence [22,38]. Simple tests such as colorimetric redox methods and nitrate reductase assays appear to perform very well, but require culture isolation [19,36]. More evidence is needed on rapid tests for drug resistance, especially since the Global XDR-TB Response Plan calls for wide-scale implementation of rapid methods to screen patients at risk of XDR-TB (extensively drug-resistant TB) and MDR-TB [41].
For laboratory practice, systematic reviews provide strong evidence that fluorescence microscopy is more sensitive than conventional light microscopy (with no significant loss in specificity) [31], that sputum processing methods (e.g., bleach or centrifugation) can be effective in increasing the yield of smear microscopy [32], and that liquid cultures are more rapid and sensitive than solid cultures [10].
Implications for Policies and Guidelines
In addition to informing evidence-based TB diagnosis, systematic reviews have been helpful in informing policy decisions. For example, a series of recent reviews has shown that smear microscopy can be optimized using at least three different approaches: chemical and physical processing for concentration of sputum, use of fluorescence microscopy instead of conventional light microscopy, and the examination of two (as compared to three) sputum specimens [20,31,32]. The findings of these reviews were incorporated into the International Standards for TB Care [42], and have informed policy guidance on the diagnosis of smear-negative TB in people living with HIV/AIDS [43].
The review on incremental yield of serial smears showed that the average incremental yield and/or increase in sensitivity of examining a third sputum specimen ranged between 2% and 5% [20]. This suggested that reducing the recommended number of specimens examined from three to two could potentially benefit TB control programs, and potentially increase case detection for several reasons [20]. Partly based on this evidence and expert opinion, WHO recently revised its policies on smear microscopy [44]. It now recommends that the number of specimens to be examined for screening of TB cases be reduced from three to two, in places where a well-functioning external quality assurance system exists, where the workload is very high, and where human resources are limited [44]. The revised WHO definition of a new sputum smear-positive pulmonary TB case is based on the presence of at least one acid fast bacillus in at least one sputum sample in countries with a well-functioning external quality assurance system [45].
These new policies have major implications for resource-poor settings with high TB prevalence where sputum microscopy is the main or only diagnostic test available, and particularly where laboratory services are being overwhelmed with demand for smear microscopy. Omitting the third smear could potentially reduce costs and alleviate the workload of laboratories, particularly in countries with human resource crises. In these settings, laboratories performing smear microscopy often have to deal with anemia, malaria, and other diseases. Thus, the time saved from the inefficient examination of a third smear may be applied toward improving laboratory testing for other diseases [20]. The adoption of the revised case definition and a two-smear approach may create the opportunity to examine both smears during a patient's first presentation to a health facility, and thereby reduce the large numbers of patients known to drop out during the diagnostic process [46]. While these are reasonable assumptions, it is worth emphasizing that there is no hard evidence that the two-smear policy actually improves TB control in the real world. Such data will have to come from programmatic research at the country level and from data collected in routine public health program settings.
There is strong evidence that liquid cultures are more sensitive and rapid than solid media cultures [10]. Based on a review of available evidence and an expert consultation, WHO recently issued policy guidance on the use of liquid TB culture and drug susceptibility testing in low-resource settings [47]. The WHO policy recommends phased implementation of liquid culture systems as a part of a country-specific comprehensive plan for laboratory capacity strengthening that addresses issues such as biosafety, training, maintenance of infrastructure, and reporting of results [47]. These policies are expected to have an important impact in settings with high HIV prevalence [43] and in countries where MDR-TB is an increasing problem [41], helping to inform the needed global scale-up of culture and drug susceptibility testing capacity.
However, implementation of culture testing requires a well-functioning health care system, adequate laboratory infrastructure, and trained personnel. Therefore, emphasis must be placed on capacity building and health system and laboratory strengthening [43,48]. Recognizing this, the Stop TB Partnership, WHO, and partners have launched a Global Laboratory Initiative to facilitate laboratory policy guidance, technical assistance, quality management, resource mobilization, and advocacy. Again, as in the case of the two-smear strategy, it must be emphasized that there is no strong evidence that the WHO policy on liquid cultures actually improves TB control at the routine programmatic level. Field studies and cost-effectiveness data are needed to better understand the real world implications of this policy.
In June 2008, WHO announced a new policy statement, endorsing the use of line probe assays for rapid screening of patients at risk of MDR-TB (http://www.who.int/tb/en/). This policy statement was based in part on evidence summarized in systematic reviews [22,38], expert opinion, and results of field demonstration projects. The recommended use of line probe assays is currently limited to culture isolates and direct testing of smear-positive sputum specimens. Line probe assays are not recommended as a complete replacement for conventional culture and drug susceptibility testing. Culture is still required for smear-negative specimens, and conventional drug susceptibility testing is still necessary to confirm XDR-TB.
Following this new policy, WHO, UNITAID, the Stop TB Partnership, and the Foundation for Innovative New Diagnostics (FIND) announced a new initiative to improve the diagnosis and treatment of MDR-TB in resource-limited settings (http://www.who.int/tb/features_archive/mdrtb_rapid_tests/en/index.html). As part of this initiative, over the next few years, 16 countries will begin using rapid tests to diagnose MDR-TB, including line probe assays. The countries will receive specially priced tests through the Stop TB Partnership's Global Drug Facility, which provides countries with both drugs and diagnostic reagents.
Implications for Research and Development
Systematic reviews have been helpful in identifying key knowledge gaps and defining research agendas. For example, based on the smear microscopy reviews [20,31,32] and expert opinion, the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) recently launched a major research program aimed at the optimization of smear microscopy [49]. Large-scale field studies are ongoing in more than ten countries on issues such as optimum timing and composition of sputum specimen sets; use of lower-cost light-emitting diode (LED) fluorescence microscopy systems (Figure 1); sputum processing methods involving bleach digestion; and potential for reducing time to diagnosis and number of patient visits required by examining two specimens on the day that the patient first presents. The latter can be expected to reduce the considerable patient drop-out rates during diagnosis that are seen in many settings [46].
Fig. 1. Low-Cost LED-Based Fluorescence Microscopy Being Evaluated at a TDR/WHO Trial Site in Abuja, Nigeria 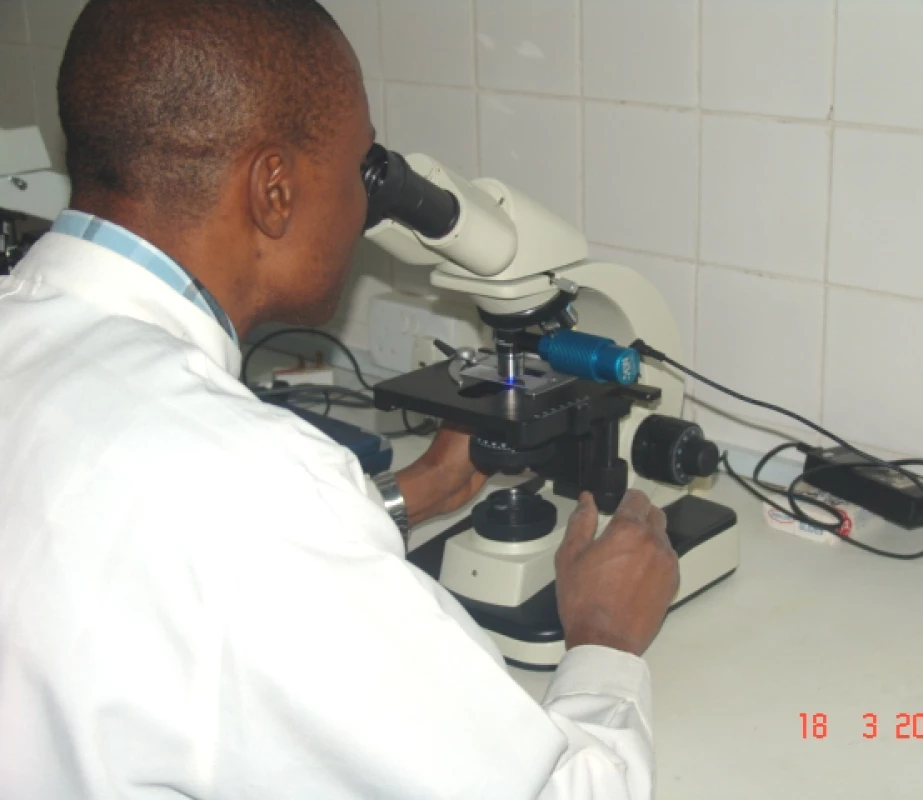
Photographer: Andrew Ramsay (Courtesy of TDR, Geneva) In parallel, FIND recently forged a partnership with Carl Zeiss MicroImaging (http://www.zeiss.com/micro/) to develop an inexpensive, robust LED-based microscope that will be extensively evaluated for routine use in high-burden countries [50].
Systematic reviews on existing commercial serological tests and NAATs have shown that these assays have not performed as well as expected [14,18,29,30]. A recent evaluation of 19 rapid commercial serological tests for TB using specimens from the TDR TB Specimen Bank confirmed the poor accuracy of existing serological tests for TB [51]. Such evidence has informed several initiatives to improve serological assays and NAATs. For example, FIND is supporting the development and evaluation of newer, improved NAATs (Figure 2) [52]. Several groups are working on methods to optimize serological assays, including the use of novel TB-specific antigens, the use of antigen combinations, and the development of point-of-care tests [52].
Fig. 2. A Simplified NAAT Being Evaluated at a FIND Trial Site in India 
Photographer: Ralf Linke (Courtesy of FIND, Geneva) Systematic reviews on IGRAs have informed the development of guidelines and positions statements in many countries [53,54,55]. They have also facilitated the development of a comprehensive research agenda with a specific focus on the use of these assays in resource-limited settings [56].
Systematic reviews on TB diagnostics have revealed deficiencies in the quality of TB diagnostic trials. A recent analysis of systematic reviews showed that trials of TB diagnostics lack methodological rigor, and studies are often poorly reported [57]. Lack of methodological rigor in trials is a cause for concern, as it may prove to be a major hurdle for effective application of diagnostics in TB care and control. Biased results from poorly designed trials can lead to premature adoption of diagnostics that may have little or no benefit. The situation is exacerbated by the fact that most developing countries have poor regulatory mechanisms for licensing and post-marketing surveillance of diagnostics. For example, dozens of commercial serological tests are marketed in developing countries, despite lack of evidence on their utility [29,30,51].
It is clear that efforts are needed to improve both methodological quality and reporting of TB diagnostic trials [57,58]. TDR has developed guidelines for researchers on assessing the performance and operational characteristics of diagnostics for infectious diseases [59], and the STARD (Standards for Reporting Diagnostic Accuracy) initiative was launched to improve the quality of reporting of diagnostic studies [60].
Conclusions
With the publication of several systematic reviews, there is now a strong evidence base to support global policy on TB diagnostics. A key challenge is to maintain the momentum gained in the past few years, and expand the scope and role of evidence synthesis to outcomes that go beyond conventional diagnostic accuracy. These outcomes include: accuracy of diagnostic algorithms (rather than single tests) and their relative contributions to the health care system; incremental or added value of new tests; impact of new tests on clinical decision-making and therapeutic choices; cost-effectiveness in routine programmatic settings; impact on patient-centered outcomes; and societal impact of new tools. Indeed, the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to grading the quality of evidence and strength of recommendations for diagnostic tests recognizes that diagnostic accuracy results are surrogates for patient-centered outcomes, and emphasizes that diagnostic tests are of value only if they result in improved outcomes for patients [61].
In addition to expanding the scope of evidence synthesis, it is also important to ensure that systematic reviews stay current by including new literature. Periodic updates are needed to ensure that systematic reviews provide the most current evidence available for clinical and policy decisions. For example, the literature on IGRAs has exploded in the past few years, and this necessitated an updated meta-analysis on this topic [37].
Recognizing the growing importance of evidence-based TB diagnosis and policy making, the Stop TB Partnership's New Diagnostics Working Group has recently created a new subgroup on Evidence Synthesis for TB Diagnostics [62]. This subgroup will support the development of new systematic reviews, facilitate the development and dissemination of evidence summaries on new diagnostics, and actively promote their use in guideline and policy development processes, along the lines of the GRADE approach.
Zdroje
1. Stop TB Partnership, World Health Organization
2006
The global plan to stop TB 2006–2015.
Available: http://www.stoptb.org/globalplan/. Accessed 16 June 2008
2. RaviglioneMCUplekarMW
2006
WHO's new Stop TB Strategy.
Lancet
367
952
955
3. World Health Organization
2007
New technologies for tuberculosis control: A framework for their adoption, introduction and implementation.
Available: http://whqlibdoc.who.int/publications/2007/9789241595520_eng.pdf. Accessed 16 June 2008
4. World Health Organization, Special Programme for Research and Training in Tropical Diseases
2006
Diagnostics for tuberculosis. Global demand and market potential.
Available: http://www.who.int/tdr/publications/publications/tbdi.htm. Accessed 16 June 2008
5. OxmanADLavisJNFretheimA
2007
Use of evidence in WHO recommendations.
Lancet
369
1883
1889
6. HillSPangT
2007
Leading by example: A culture change at WHO.
Lancet
369
1842
1844
7. World Health Organization
2008
Moving research findings into new WHO policies.
Available: http://www.who.int/tb/dots/laboratory/policy/en/index4.html. Accessed 16 June 2008
8. PaiMMcCullochMEnanoriaWColfordJMJr
2004
Systematic reviews of diagnostic test evaluations: What's behind the scenes.
ACP J Club
141
A11
A13
9. DaleyPThomasSPaiM
2007
Nucleic acid amplification tests for the diagnosis of tuberculous lymphadenitis: A systematic review.
Int J Tuberc Lung Dis
11
1166
1176
10. DinnesJDeeksJKunstHGibsonACumminsE
2007
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
Health Technol Assess
11
1
196
11. FloresLLPaiMColfordJMJrRileyLW
2005
In-house nucleic acid amplification tests for the detection of Mycobacterium tuberculosis in sputum specimens: meta-analysis and meta-regression.
BMC Microbiol
5
55
12. GotoMNoguchiYKoyamaHHiraKShimboT
2003
Diagnostic value of adenosine deaminase in tuberculous pleural effusion: A meta-analysis.
Ann Clin Biochem
40
374
381
13. GrecoSGirardiEMasciangeloRCapoccettaGBSaltiniC
2003
Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: A meta-analysis.
Int J Tuberc Lung Dis
7
777
786
14. GrecoSGirardiENavarraSSaltiniC
2006
The current evidence on diagnostic accuracy of commercial based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis.
Thorax
61
783
790
15. JiangJShiHZLiangQL
2007
Diagnostic value of interferon-g in tuberculous pleurisy: A meta-analysis.
Chest
131
1133
1141
16. KalantriSPaiMPascopellaLRileyLReingoldA
2005
Bacteriophage-based tests for the detection of Mycobacterium tuberculosis in clinical specimens: A systematic review and meta - analysis.
BMC Infect Dis
5
59
17. LiangQLShiHZWangKQinSMQinXJ
2008
Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: A meta-analysis.
Respir Med
102
744
754
18. LingDIFloresLLRileyLWPaiM
2008
Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: Meta-analysis and meta-regression.
PLoS ONE
3
e1536
doi:10.1371/journal.pone.0001536
19. MartinAPortaelsFPalominoJC
2007
Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: A systematic review and meta-analysis.
J Antimicrob Chemother
59
175
183
20. MaseSRRamsayANgVHenryMHopewellPC
2007
Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: A systematic review.
Int J Tuberc Lung Dis
11
485
495
21. MenziesDPaiMComstockG
2007
Meta-analysis: New tests for the diagnosis of latent tuberculosis infection: Areas of uncertainty and recommendations for research.
Ann Intern Med
146
340
354
22. MorganMKalantriSFloresLPaiM
2005
A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: A systematic review and meta-analysis.
BMC Infect Dis
5
62
23. PaiMFloresLLHubbardARileyLWColfordJMJr
2004
Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: A systematic review and meta-analysis.
BMC Infect Dis
4
6
24. PaiMFloresLLPaiNHubbardARileyLW
2003
Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: A systematic review and meta-analysis.
Lancet Infect Dis
3
633
643
25. PaiMKalantriSPascopellaLRileyLWReingoldAL
2005
Bacteriophage-based assays for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: A meta-analysis.
J Infect
51
175
187
26. PaiMRileyLWColfordJMJr
2004
Interferon-gamma assays in the immunodiagnosis of tuberculosis: A systematic review.
Lancet Infect Dis
4
761
776
27. RiquelmeACalvoMSalechFValderramaSPattilloA
2006
Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: A meta-analysis.
J Clin Gastroenterol
40
705
710
28. SarmientoOLWeigleKAAlexanderJWeberDJMillerWC
2003
Assessment by meta-analysis of PCR for diagnosis of smear-negative pulmonary tuberculosis.
J Clin Microbiol
41
3233
3240
29. SteingartKRHenryMLaalSHopewellPCRamsayA
2007
A systematic review of commercial serological antibody detection tests for the diagnosis of extra-pulmonary tuberculosis.
Thorax
62
911
918
30. SteingartKRHenryMLaalSHopewellPCRamsayA
2007
Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: A systematic review.
PLoS Med
4
e202
doi:10.1371/journal.pmed.0040202
31. SteingartKRHenryMNgVHopewellPCRamsayA
2006
Fluorescence versus conventional sputum smear microscopy for tuberculosis: A systematic review.
Lancet Infect Dis
6
570
581
32. SteingartKRNgVHenryMHopewellPCRamsayA
2006
Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A systematic review.
Lancet Infect Dis
6
664
674
33. TuonFFLitvocMNLopesMI
2006
Adenosine deaminase and tuberculous pericarditis—A systematic review with meta-analysis.
Acta Trop
99
67
74
34. WangLTurnerMOElwoodRKSchulzerMFitzGeraldJM
2002
A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements.
Thorax
57
804
809
35. FarhatMGreenawayCPaiMMenziesD
2006
False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria.
Int J Tuberc Lung Dis
10
1192
1204
36. MartinAPanaiotovSPortaelsFHoffnerSPalominoJC
2008
The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: A systematic review and meta-analysis.
J Antimicrob Chemother
62
56
64
37. PaiMZwerlingAMenziesD
2008
Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection—An update.
Ann Intern Med
149
177
184
38. LingDIZwerlingAPaiM
2008
GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: A meta-analysis.
Eur Respir J
E-pub 9 July 2008. doi:10.1183/09031936.00061808
39. AndersenPDohertyTMPaiMWeldinghK
2007
The prognosis of latent tuberculosis: Can disease be predicted.
Trends Mol Med
13
175
182
40. TrajmanAPaiMDhedaKvan zyl SmitRZwerlingA
2008
Novel tests for diagnosing tuberculous pleural effusion: What works and what does not.
Eur Respir J
31
1098
1106
41. World Health Organization
2007
The global MDR-TB and XDR-TB response plan 2007–2008.
Available: http://whqlibdoc.who.int/hq/2007/WHO_HTM_TB_2007.387_eng.pdf. Accessed 16 June 2008
42. HopewellPCPaiMMaherDUplekarMRaviglioneMC
2006
International standards for tuberculosis care.
Lancet Infect Dis
6
710
725
43. GetahunHHarringtonMO'BrienRNunnP
2007
Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: Informing urgent policy changes.
Lancet
369
2042
2049
44. World Health Organization
2007
Reduction of number of smears for the diagnosis of pulmonary TB.
Available: http://www.who.int/tb/dots/laboratory/policy/en/index2.html. Accessed 16 June 2008
45. World Health Organization
2007
Definition of a new sputum smear-positive TB case.
Available: http://www.who.int/tb/dots/laboratory/policy/en/index1.html. Accessed 16 June 2008
46. SquireSBBelayeAKKashotiASalaniponiFMMundyCJ
2005
‘Lost’ smear-positive pulmonary tuberculosis cases: Where are they and why did we lose them.
Int J Tuberc Lung Dis
9
25
31
47. World Health Organization
2007
The use of liquid medium for culture and DST.
Available: http://www.who.int/tb/dots/laboratory/policy/en/index3.html. Accessed 16 June 2008
48. RidderhofJCvan DeunAKamKMNarayananPRAzizMA
2007
Roles of laboratories and laboratory systems in effective tuberculosis programmes.
Bull World Health Organ
85
354
359
49. World Health Organization, Special Programme for Research and Training in Tropical Diseases
2006
Request for applications: Diagnostic trial sites: Improving the diagnosis of tuberculosis through optimization of sputum smear microscopy.
Available: http://www.who.int/tdr/grants/grants/trial_sites.htm. Accessed 16 June 2008
50. Foundation for Innovative New Diagnostics
2008
Market launch of improved fluorescence microscope scheduled for later this year.
Available: http://www.finddiagnostics.org/news/press/zeiss_mar08.pdf. Accessed 16 June 2008
51. CunninghamJ
2005
Rapid serological-based TB test evaluation: Prelim analysis. Stop TB Working Group on New Diagnostics.
Available: http://www.stoptb.org/wg/new_diagnostics/assets/documents/jane_cunningham.pdf. Accessed 16 June 2008
52. PerkinsMDCunninghamJ
2007
Facing the crisis: Improving the diagnosis of tuberculosis in the HIV era.
J Infect Dis
196
Suppl 1
S15
S27
53. Canadian Tuberculosis Committee
2007
Interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS).
Can Commun Dis Rep
33
1
18
54. HPA Tuberculosis Programme Board
2007
Health Protection Agency Position Statement on the use of Interferon Gamma Release Assay (IGRA) tests for tuberculosis (TB): Draft for consultation.
Available: http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1204186168242. Accessed 16 June 2008
55. National Tuberculosis Advisory Committee Australia
2007
Position statement on interferon-gamma release immunoassays in the detection of latent tuberculosis infection, October 2007.
Commun Dis Intell
31
404
405
56. PaiMDhedaKCunninghamJScanoFO'BrienR
2007
T-cell assays for the diagnosis of latent tuberculosis infection: Moving the research agenda forward.
Lancet Infect Dis
7
428
438
57. PaiMO'BrienR
2006
Tuberculosis diagnostics trials: Do they lack methodological rigor.
Expert Rev Mol Diagn
6
509
514
58. SmallPMPerkinsMD
2000
More rigour needed in trials of new diagnostic agents for tuberculosis.
Lancet
356
1048
1049
59. BanooSBellDBossuytPHerringAMabeyD
2006
Evaluation of diagnostic tests for infectious diseases: General principles.
Nat Rev Microbiol
4
S21
S31
60. BossuytPMReitsmaJBBrunsDEGatsonisCAGlasziouPP
2003
Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative.
Ann Intern Med
138
40
44
61. SchünemannHJOxmanADBrozekJGlasziouPJaeschkeR
2008
Grading quality of evidence and strength of recommendations for diagnostic tests and strategies.
BMJ
336
1106
1110
62. Stop TB Partnership Working Group on New TB Diagnostics
2007
Revised strategic plan of the Stop TB Partnership's Working Group on New Diagnostics.
Available: http://www.stoptb.org/wg/new_diagnostics/assets/documents/Draft%20NDWG%20Strategic%20Plan%20for%20Cape%20Town%20Meeting.pdf. Accessed 16 June 2008
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Léčba bolesti u seniorů
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- International Funding for Malaria Control in Relation to Populations at Risk of Stable Transmission
- Immunological Outcomes of New Tuberculosis Vaccine Trials: WHO Panel Recommendations
- A 58-Year-Old Woman with Abdominal Symptoms and Elevated C-Reactive Protein
- Organophosphate Poisoning–Induced Intermediate Syndrome: Can Electrophysiological Changes Help Predict Outcome?
- Evidence-Based Tuberculosis Diagnosis
- Divergent Goals and Commitments in Global Malaria Intervention
- Next Stop, Don't Block the Doors: Opening Up Access to Clinical Trials Results
- The Effects of International Monetary Fund Loans on Health Outcomes
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evidence-Based Tuberculosis Diagnosis
- Organophosphate Poisoning–Induced Intermediate Syndrome: Can Electrophysiological Changes Help Predict Outcome?
- The Effects of International Monetary Fund Loans on Health Outcomes
- Next Stop, Don't Block the Doors: Opening Up Access to Clinical Trials Results
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



