-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCalibrating the Human Mutation Rate via Ancestral Recombination Density in Diploid Genomes
The rate at which new heritable mutations occur in the human genome is a fundamental parameter in population and evolutionary genetics. However, recent direct family-based estimates of the mutation rate have consistently been much lower than previous results from comparisons with other great ape species. Because split times of species and populations estimated from genetic data are often inversely proportional to the mutation rate, resolving the disagreement would have important implications for understanding human evolution. In our work, we apply a new technique that uses mutations that have accumulated over many generations on either copy of a chromosome in an individual’s genome. Instead of an external reference point, we rely on fine-scale knowledge of the human recombination rate to calibrate the long-term mutation rate. Our procedure accounts for possible errors found in real data, and we also show that it is robust to a range of model violations. Using eight diploid genomes from non-African individuals, we infer a rate of 1.61 ± 0.13 × 10−8 single-nucleotide changes per base per generation, which is intermediate between most phylogenetic and pedigree-based estimates. Thus, our estimate implies reasonable, intermediate-age population split times across a range of time scales.
Published in the journal: . PLoS Genet 11(11): e32767. doi:10.1371/journal.pgen.1005550
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005550Summary
The rate at which new heritable mutations occur in the human genome is a fundamental parameter in population and evolutionary genetics. However, recent direct family-based estimates of the mutation rate have consistently been much lower than previous results from comparisons with other great ape species. Because split times of species and populations estimated from genetic data are often inversely proportional to the mutation rate, resolving the disagreement would have important implications for understanding human evolution. In our work, we apply a new technique that uses mutations that have accumulated over many generations on either copy of a chromosome in an individual’s genome. Instead of an external reference point, we rely on fine-scale knowledge of the human recombination rate to calibrate the long-term mutation rate. Our procedure accounts for possible errors found in real data, and we also show that it is robust to a range of model violations. Using eight diploid genomes from non-African individuals, we infer a rate of 1.61 ± 0.13 × 10−8 single-nucleotide changes per base per generation, which is intermediate between most phylogenetic and pedigree-based estimates. Thus, our estimate implies reasonable, intermediate-age population split times across a range of time scales.
Introduction
All genetic variation—the substrate for evolution—is ultimately due to spontaneous heritable mutations in the genomes of individual germline cells. The most commonly studied mutations are point mutations, which consist of single-nucleotide changes from one base to another. The rate at which these changes occur, in combination with other forces, determines the frequency with which homologous nucleotides differ from one individual’s genome to another.
A number of different approaches have previously been used to estimate the human mutation rate [1–3], of which we mention four categories here. The first method is to count the number of fixed genetic changes between humans and another species, such as chimpanzees [4]. Population genetic theory implies that if the mutation rate remains constant, then neutral mutations (those that do not affect an organism’s fitness) should accumulate between two genomes at a constant rate (the well-known “molecular clock” [5]). Thus, the mutation rate can be estimated based on the divergence time of the genomes, if this can be confidently inferred from fossil evidence. However, even if the age of fossil remains can be accurately determined, assigning their proper phylogenetic positions is often difficult. Moreover, because of shared ancestral polymorphism, the time to the most recent common ancestor is always older—and sometimes far older—than the time of species divergence, meaning that split-time calibrations cannot always be directly applied to genetic divergences.
A second common approach, which has only become possible within the last few years, is to count newly occurring mutations in deep sequencing data from family pedigrees, especially parent-child trios [6–10]. This approach provides a direct estimate but can be technically challenging, as it is sensitive to genotype accuracy and data processing from high-throughput sequencing. In particular, sporadic sequencing and alignment errors can be difficult to distinguish from true de novo mutations. Surprisingly, these sequencing-based estimates have consistently been much lower than those based on the first approach: in the neighborhood of 1–1.2 × 10−8 per base per generation, as opposed to 2–2.5 × 10−8 for those from long-term divergence [1–3].
A third method, and another that is only now becoming possible, is to make direct comparisons between present-day samples and precisely-dated ancient genomes. This method is similar to the first one, but by using two time-separated samples from the same species, it avoids the difficulty of needing an externally inferred split time. A recent study of a high-coverage genome sequence from a 45,000-year-old Upper Paleolithic modern human produced two estimates of this type [11]. Direct measurement of decreased mutational accumulation in this sample led to rate estimates of 0.44–0.63 × 10−9 per base per year (range of 14 estimates), or 1.3–1.8 × 10−8 per base per generation (assuming 29 years per generation [12]). An alternative technique, leveraging time shifts in historical population sizes, yielded an estimate of 0.38–0.49 × 10−9 per base per year (95% confidence interval), or 1.1–1.4 × 10−8 per base per generation, although a re-analysis of different mutational classes led to a total estimate of 0.44–0.59 × 10−9 per base per year (1.3–1.7 × 10−8), in better agreement with the first approach [11].
Finally, a fourth technique is to calibrate the rate of accumulation of mutations using a separate evolutionary rate that is better measured. In one such study, the authors used a model coupling single-nucleotide mutations to mutations in nearby microsatellite alleles to infer a single-nucleotide rate of 1.4–2.3 × 10−8 per base per generation (90% confidence interval) [13]. In principle, this general technique is appealing because it only involves intrinsic information, without any reference points, and yet can leverage the signal of mutations that have occurred over many generations.
In this study, we present a new approach that falls into this fourth category: we calibrate the mutation rate against the rate of meiotic recombination events, which has been measured with high precision in humans [14–17]. Intuitively, our method makes use of the following relationship between the mutation and recombination rates. At every site i in a diploid genome, the two copies of the base have some time to most recent common ancestor (TMRCA) Ti, measured in generations. The genome can be divided into blocks of sequence that have been inherited together from the same common ancestor, with different blocks separated by ancestral recombinations. If a given block has a TMRCA of T and a length of L bases, and if μ is the per-generation mutation rate per base, then the expected number of mutations that have accumulated in either copy of that block since the TMRCA is 2TLμ. This is the expected number of heterozygous sites that we observe in the block today (disregarding the possibility of repeat mutations). We also know that if the per-generation recombination rate is r per base, then the expected length of the block is (2Tr)−1. Thus, the expected number of heterozygous sites per block (regardless of age) is μ/r.
This relationship allows us to estimate μ given a good prior knowledge of r. Our full method is more complex but is based on the same principle. We show below how we can capture the signal of heterozygosity per recombination to infer the historical per-generation mutation rate for non-African populations over approximately the last 50–100 thousand years (ky). A broadly similar idea is also applied in an independent study [18], but over a more recent time scale (up to ∼ 3 ky, via mutations present in inferred identical-by-descent segments), and the two final estimates are in very good agreement.
Results
Overview of methods
One difficulty of the simple method outlined above is that in practice we cannot accurately reconstruct the breakpoints between adjacent non-recombined blocks. Instead, we use an indirect statistic that captures information about the presence of breakpoints but can be computed in a simple way (without directly inferring blocks) and averaged over many loci (Fig 1).
Fig. 1. Explanation of the statistic HS(d). 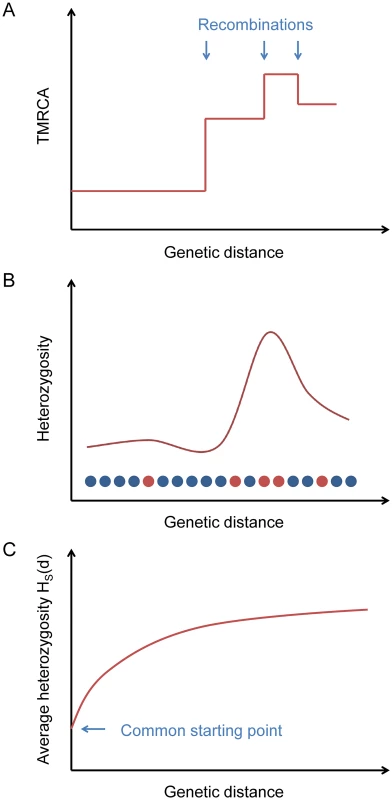
(A) Ancestral recombinations separate chromosomes into blocks of piecewise-constant TMRCA (and hence expected heterozygosity). (B) From the data, we measure local heterozygosity as a function of genetic distance; red and blue circles represent heterozygous and homozygous sites, respectively, along a diploid genome. (C) Our statistic HS(d) is an average heterozygosity as a function of genetic distance over many starting points with similar local heterozygosities, yielding a smooth relaxation toward the genome-wide average. Starting from a certain position in the genome, the TMRCA of the two haploid chromosomes as a function of distance in either direction is a step function, with changes at ancestral recombination points (Fig 1A). Heterozygosity, being proportional to TMRCA in expectation (and directly observable), follows the same pattern on average (Fig 1B).
If we consider a collection of starting positions having similar local heterozygosities, then as a function of the genetic distance d away from them, the average heterozygosity displays a smooth relaxation from the common starting value toward the global mean heterozygosity H ‾ as the probability increases of having encountered recombination points (Fig 1C). We define a statistic HS(d) that equals this average heterozygosity, where S is a set of starting points indexed by the local number of heterozygous sites per 100 kb (we also use S at times to refer to the heterozygosity range itself). The TMRCAs of these points determine the time scale over which our inferred value of μ is measured. Our default choice is to use starting points with a local total of 5–10 heterozygous sites per 100 kb (see Methods).
To estimate μ, we use the fact that the probability of having encountered a recombination as one moves away from a starting point is a function of both d and the starting heterozygosity HS(0), since smaller values of HS(0) correspond to smaller TMRCAs, with less time for recombination to have occurred, and hence longer unbroken blocks. This relationship allows us to calibrate μ against the recombination rate r via the relaxation rate of HS(d). Our inference procedure involves using coalescent simulations to create matching “calibration data” with known values of μ and then solving for the best-fit mutation rate for the test data (see Methods and Fig 2). We note that when comparing HS(d) for real data to the calibration curves, a larger value of μ will correspond to a lower curve. This is because HS(0) is fixed, which means that the TMRCAs at the starting points are proportionally lower for larger values of μ. Thus, recombinations are less frequent as a function of d, leading to a slower relaxation.
Fig. 2. Illustration of the steps of our inference procedure. 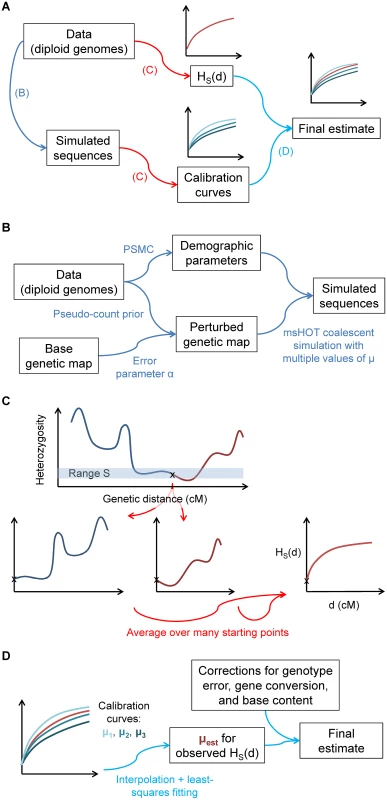
(A) Overview: from the data, we compute both the statistic HS(d) and other parameters necessary to create matching calibration curves with known values of μ. (B) Details of capturing aspects of the real data for the calibration data. (C) Computation of HS(d): the statistic captures the average heterozygosity as a function of genetic distance d from a starting point with heterozygosity in a defined range S, averaged over many such points. (D) For the final inferred value of μ, we compare matched HS(d) curves for the real data and calibration data (with known values of μ). In order for our inferences to be accurate, the calibration curves must recapitulate as closely as possible all aspects of the real data that could affect HS(d) (see Methods, S1 Text, and Fig 2). First, because coalescent probabilities depend on ancestral population sizes, we use PSMC [19] to learn the demographic history of our samples. Next, we adapt a previously developed technique [20] to infer the fine-scale uncertainty of our genetic map. Finally, we correct our raw inferred values of μ for three additional factors in order to isolate the desired mutational signal: (1) we multiply by a correction for genotype errors; (2) we subtract the contribution of non-crossover gene conversion, using a result from [21] adjusted for local recombination rate; and (3) we scale the final value to correspond to genome-wide base content and mutability (see Methods and S1 Text). We also test additional potential model violations through simulations (see S1 Text and S1 Fig). We account for statistical uncertainty using a block jackknife and incorporate confidence intervals for model parameters; all results are given as mean ± standard error.
Simulations
First, for seven different scenarios, including a range of possible model violations, we generated 20 simulated diploid genomes with a known true mutation rate (μ = 2.5 × 10−8 per generation except where otherwise specified) and ran our procedure as we would for real data, with perturbed genetic maps for both the test data and calibration data (variance parameter α = 3000 M−1; see Methods). To measure the uncertainty in our estimates, we performed 25 independent trials of each simulation, and we also compared the standard deviations of the estimates across trials with jackknife-based standard errors (as we would measure uncertainty for real data). Full details of the simulation procedures can be found in Methods and S1 Text.
In all cases, the H5–10(d) curves matched quite well between the test data and the calibration data, and our final results were within two standard errors of the true rate (Fig 3). Furthermore, our jackknife estimates of the standard error were comparable to the realized standard deviations and on average conservative, especially for the most complex simulation (g), despite not incorporating PSMC uncertainty (see Methods): 0.08 × 10−8, 0.04 × 10−8, 0.04 × 10−8, 0.06 × 10−8, 0.09 × 10−8, 0.05 × 10−8, and 0.11 × 10−8, respectively, for the seven scenarios (see Fig 3 for empirical standard deviations). The fact that all of the inferred rates are close to the true values leads us to conclude that none of the aspects of the basic procedure or the tested model violations create a substantial bias.
Fig. 3. Results for simulated data. 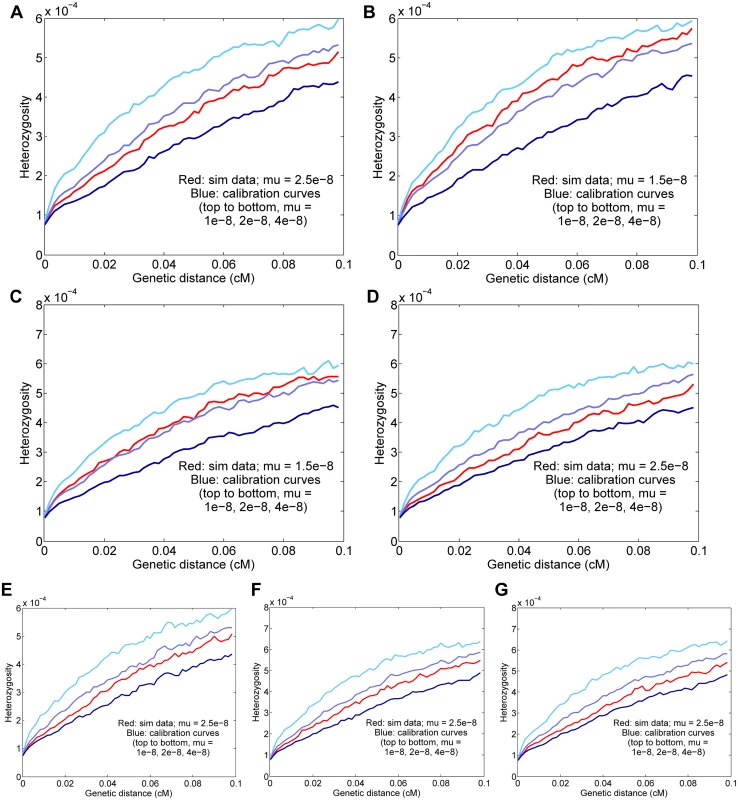
Means and standard deviations of 25 independent trials are given, and the curves displayed are for representative runs matching the 25-trial means. The true simulated rate is μ = 2.5 × 10−8 unless otherwise specified. (A) Baseline simulated data; the inferred rate is μ = 2.47 ± 0.05 × 10−8. (B) Basic simulated data with a true rate of 1.5 × 10−8; the inferred rate is μ = 1.57 ± 0.04 × 10−8. (C) Data with a true rate of 1.5 × 10−8 plus gene conversion; the inferred rate is μ = 1.49 ± 0.05 × 10−8 (corrected from a raw value of 1.70 × 10−8 with gene conversion included). (D) Data with simulated genotype errors; the inferred rate is μ = 2.39 ± 0.06 × 10−8 (corrected from a raw value of 2.71 × 10−8 with genotype errors included). (E) Data simulated with variable mutation rate; the inferred rate is μ = 2.61 ± 0.08 × 10−8. (F) Data from a simulated admixed population; the inferred rate is μ = 2.57 ± 0.07 × 10−8. (G) Simulated data with all three complications as in (D)–(F); the inferred rate is μ = 2.53 ± 0.06 × 10−8 (corrected from a raw value of 2.77 × 10−8). Error parameters
Before obtaining mutation rate estimates from real data, we quantified two important error parameters: the rate of false heterozygous genotype calls and the degree of inaccuracy in our genetic map.
We estimated the genotype error rate by taking advantage of the fact that methylated cytosines at CpG dinucleotides are roughly an order of magnitude more mutable than other bases [3, 7, 8, 10] (see Methods). Thus, such mutations are strongly over-represented among true heterozygous sites as compared to falsely called heterozygous sites. By counting the proportion of CpG mutations out of all heterozygous sites around our ascertained starting points, we inferred an error rate of approximately 1 per 100 kb (1.08 ± 0.28 × 10−5 per base; see Methods and S1 Text), consistent with previous results [22].
It was also necessary for us to estimate the accuracy of our genetic map. We used the “shared” version of the African-American (AA) map from [17] as our base map and a modified version of the error model of [20]: Z ∼ Gamma(αγ(g + πp), α), where Z is the true genetic length of a map interval, g is the observed genetic length, p is the physical length, α is the parameter measuring the accuracy of the map, and γ and π are constants (see Methods). Based on pedigree crossover data from [23], we estimated α = 2802 ± 14 M−1 for the full AA map and α = 3414 ± 13 M−1 for the “shared” map, which should serve as lower and upper bounds (see Methods). For our analyses, we took α = 3100 M−1 (with a standard error of 300 M−1 to account for our uncertainty in the precise value). This means that 1/α ≈ 0.03 cM can be thought of as the length scale for the accuracy of genetic distances according to the base map (see Methods for details). In order to translate the uncertainty in α into its effect on the inferred μ, we repeated our primary analysis with a range of alternative values of α (S2 Fig).
We note that the values of α reported in [20] are substantially lower than ours, which we suspect is because our validation data have much finer resolution than those used previously. (When using the same validation data, the “shared” and HapMap LD [15] maps appear to be relatively similar in accuracy.) If we substitute our new α values for the original application of inferring the date of Neanderthal gene flow into modern humans, we obtain a less distant time in the past, 28–65 ky (most likely 35–49 ky), versus 37–86 ky (most likely 47–65 ky) reported in [20]. While relatively recent, this date range is not in conflict with archaeological evidence or with an estimate of 49–60 ky (95% confidence interval) based on an Upper Paleolithic genome [11].
Estimates for Europeans and East Asians
Our primary results (Fig 4) were obtained from eight diploid genomes of European and East Asian individuals (two each French, Sardinian, Han, and Dai) using our standard parameter settings (see above and Methods). For all real-data applications, to minimize noise from the randomized elements of the procedure (namely, coalescent simulation and generation of the perturbed calibration map), we averaged 25 independent calibrations of the data to obtain our final point estimate. With all eight individuals combined, we estimated a mutation rate of μ = 1.61 ± 0.13 × 10−8 per generation (Fig 4A). Using this value of μ, our starting heterozygosity HS(0) ≈ 7.4 × 10−5 corresponds to a TMRCA of approximately 1550–3100 generations, or 45–90 ky, assuming an average generation time of 29 years [12].
Fig. 4. Results for Europeans and East Asians. 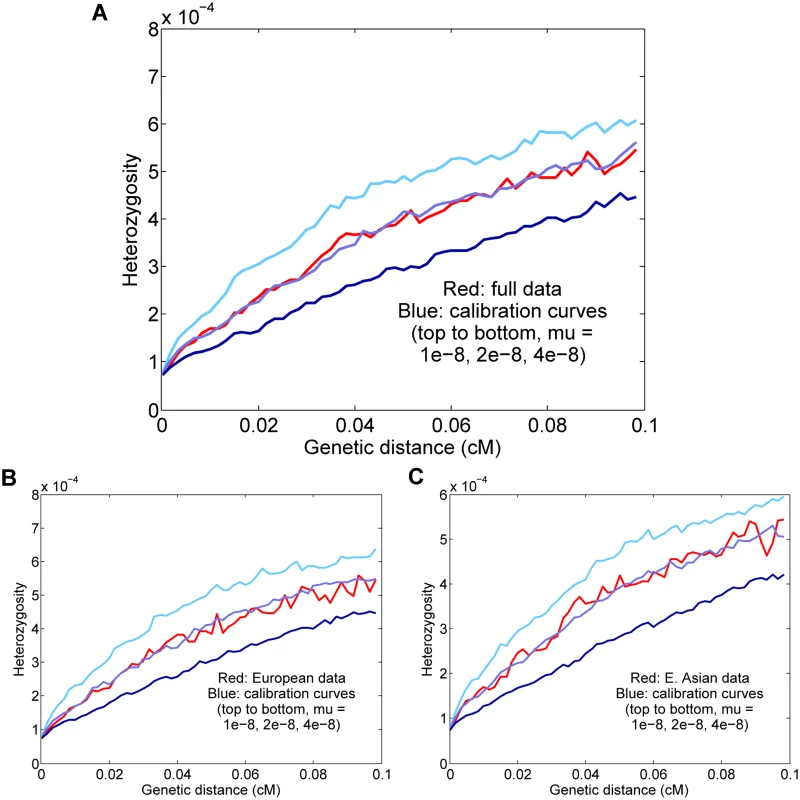
(A) All eight individuals together; the inferred rate is μ = 1.61 ± 0.13 × 10−8 per generation. (B) Results for the four Europeans; the inferred rate is μ = 1.72 ± 0.14 × 10−8. (C) Results for the four East Asians; the inferred rate is μ = 1.55 ± 0.14 × 10−8. For all real-data results, the curves displayed are for representative calibrations matching the overall means. The reported values are also corrected for gene conversion, genotype error, and base content, which explains the apparent discrepancy between the final estimates and the curves (for example, the estimate (A) is corrected from a raw value of 2.00 × 10−8). It is possible that our full estimate could be slightly inaccurate due to population-level differences in either the fine-scale genetic map or demographic history (see S1 Text). However, we expect Europeans and East Asians to be compatible in our procedure both because they are not too distantly related and because they have similar population size histories [19, 24]. To test empirically the effects of combining the populations, we estimated rates for the four Europeans and four East Asians separately (Fig 4B and 4C). Using the same genotype error corrections, we found that the H5–10(d) curves as well as the final inferred values were similar to those for the full data: μ = 1.72 ± 0.14 × 10−8 for Europeans and μ = 1.55 ± 0.14 × 10−8 for East Asians. Thus, in conjunction with our simulation results, it appears that the full eight-genome estimate is robust to the effects of population heterogeneity.
Additionally, to investigate the influence of different mutational types, we estimated rates separately for CpG transitions and all other mutations (see Methods). We inferred values of μ = 0.50 ± 0.06 × 10−8 for CpGs and μ = 1.36 ± 0.13 × 10−8 for non-CpGs (S3 Fig), with a sum (1.87 ± 0.14 × 10−8) that is somewhat higher than our full-data estimate. Since CpG transitions are known to comprise approximately 17–18% of all mutations [8], our full-data and non-CpG estimates appear to be in very good agreement, whereas the CpG-only estimate is likely inflated, perhaps because our method performs poorly with the low density of heterozygous sites (only 1 per 100 kb window for our CpG-only starting points). As a result, we believe that our value of μ = 1.61 × 10−8 is accurate, or at most slightly underestimated, as a total mutation rate for all sites.
Estimates for other populations
We also ran our procedure for three other non-African populations: aboriginal Australians, Karitiana (an indigenous group from Brazil), and Papua New Guineans. Using two genomes per population and computing curves for starting regions with 1–15 heterozygous sites per 100 kb (to increase the number of test regions, with a potential trade-off in accuracy), we inferred rates of μ = 1.86 ± 0.19 × 10−8, μ = 1.37 ± 0.19 × 10−8, and μ = 1.62 ± 0.17 × 10−8 for Australian, Karitiana, and Papuan, respectively (Fig 5). We note that the relatively high (but not statistically significantly different) per-generation value for Australians is consistent with the high average ages of fathers in many aboriginal Australian societies [12, 25]. Overall, given the expected small differences for historical, cultural, or biological reasons (including, as mentioned above, our use of the same “shared” genetic map for all groups), we do not see evidence of substantial errors or biases in our procedure when applied to diverse populations.
Fig. 5. Results for other populations. 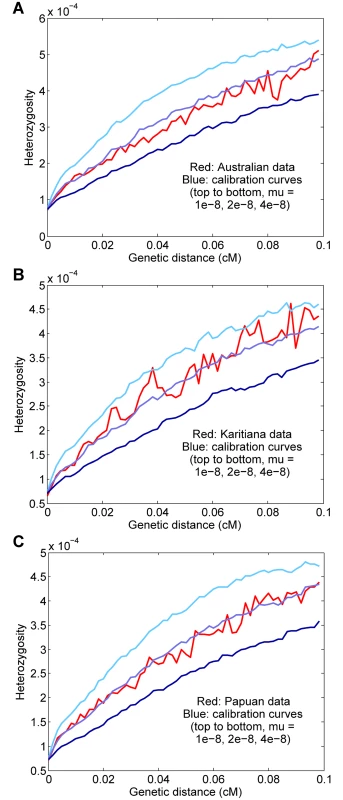
(A) Australian, μ = 1.86 ± 0.19 × 10−8. (B) Karitiana, μ = 1.37 ± 0.19 × 10−8. (C) Papuan, μ = 1.62 ± 0.17 × 10−8. Discussion
Using a new method for estimating the human mutation rate, we have obtained a genome-wide estimate of μ = 1.61 ± 0.13 × 10−8 single-nucleotide mutations per generation. Our approach counts mutations that have arisen over many generations (a few thousand, i.e., several tens of thousands of years) and relies on our excellent knowledge of the human recombination rate to calibrate the length of the relevant time period.
We have shown that our estimate is robust to many possible confounding factors (S1 Fig). In addition to statistical noise in the data, our method directly accounts for ancestral gene conversion and for errors in genotype calls and in the genetic map. We have also demonstrated, based on simulations, that heterogeneity in demographic and genetic parameters, including the mutation rate itself, does not cause an appreciable bias. However, we acknowledge that our estimate requires a large number of modeling assumptions, and while we have attempted to justify each step of our procedure and to incorporate uncertainty at each stage into our final standard error, it is possible that we have not precisely captured the influence of every confounder. Similarly, while we consider a broad range of possible sources of error, we cannot guarantee that there might not be others that we have neglected.
The meaning of an average rate
It is important to note that the mutation rate is not constant at all sites in the genome [26]. As we have discussed, we believe that this variability does not cause a substantial bias in our inferences, but to the extent that some bases mutate faster than others, a rate is only meaningful when associated with the set of sites for which it is estimated. For example, methylated cytosines at CpG positions accumulate point mutations roughly an order of magnitude faster than other bases because of spontaneous deamination [3, 7, 8, 10]. Such effects can lead to larger-scale patterns, such as the higher mutability of exons as compared to the genome as a whole [27].
In our work, we filter the data substantially, removing more than a third of the sites in the genome. The filters tend to reduce the heterozygosity of the remaining portions [24, 28], which is to be expected if they have the effect of preferentially removing false heterozygous sites. We also make a small adjustment to our final value of μ to account for differences in base composition between our ascertained starting points and the (filtered) genome as a whole (see Methods). For reference, in S1 Table, we give heterozygosity levels and human–chimpanzee divergence statistics for sites passing our filters, i.e., the subset of the genome for which our inferred rates are applicable.
Evolutionary implications and comparison to previous estimates
A key application of the mutation rate is for determining the divergence times of human populations from each other and of humans from other species [1]. Published mutation rate estimates are highly discrepant, however, by as much as a factor of 2 between those based on sequence divergence among great apes (2–2.5 × 10−8 per base per generation) versus de novo mutations in present-day families (generally 1–1.2 × 10−8) [1–3]. This uncertainty causes estimated population split times to be highly dependent on whether a high or low rate is assumed. We also note that while the de novo mutation rate and the long-term substitution rate are equal under a neutral model (and assuming a constant value of μ over time), this may not hold in reality. In this case, one might expect rates measured over longer time scales to be somewhat lower, due to purifying selection, with our estimate affected more than de novo values but less than inter-species comparisons.
Here, we infer an intermediate rate of 1.61 ± 0.13 × 10−8 per base per generation, in good agreement with a previous estimate based on linked microsatellite mutations (1.4–2.3 × 10−8) [13] and a concurrent study using mutations present in inferred identical-by-descent segments (1.66 ± 0.04 × 10−8) [18]. Assuming an average generation time of 29 years [12] for the last ∼ 50–100 ky (the time period over which our rate is measured), this value equates to 0.55 ± 0.05 × 10−9 per base per year (which overlaps the high end of the range of 0.4–0.6 × 10−9 inferred from comparisons between modern samples and a Paleolithic modern human genome [11]). Here we make the approximation that because most mutations are paternal in origin and accumulate roughly linearly with the age of the father [8, 10, 29], the per-year mutation rate is more robust to changes in the generation interval than is the per-generation rate. We stress that this conversion is approximate, both because the generation interval is uncertain and because this model is overly simplistic [3], but it allows us a reasonable means to compute long-term population split times even if the generation interval has changed in the past.
We propose that split times derived from our inferred per-year rate are in good agreement with the available paleontological evidence. Importantly, great apes exhibit a large amount of incomplete lineage sorting, which is indicative of large population sizes and high rates of polymorphism in their common ancestors [30, 31]. This results in substantial differences between genetic divergence times and the final split times of species pairs. For example, according to a recent estimate [31], assuming a “fast” rate of 1.0 × 10−9 mutations per base per year (2.5 × 10−8 per base per generation with a generation interval of 25 years) results in an estimated population split time of ∼ 3.8 million years (My) for humans and chimpanzees—with a genetic divergence time approximately 50% older—which seems too recent in light of the fossil record. By contrast, our rate of 0.55 × 10−9 leads to a more reasonable split time of 6.8 ± 0.6 My (or 7.3 ± 0.6 My using our filtered subset of the genome, with 1.23% sequence divergence; see above and S1 Table). Another constraint is the human–orangutan split, which is believed to be no older than about 16 My [1, 32]. In this case, our rate implies a split time of 20.1 ± 1.7 My (with a genetic divergence time substantially older, approximately 29 My) [31]. Although this appears to predate the fossil-inferred split (with some uncertainty), it is reasonable to expect that some changes in the biology (and, specifically, the mutation rate) of ancestral apes have occurred over this time scale (and likewise for older splits [1]). By comparison, however, a rate of ∼ 0.4 × 10−9 per year from de novo studies implies a much more discrepant split time of ∼ 28 My.
Our results can also be assessed in terms of their implications for split times among modern human populations. It has been argued on the basis of results from demographic models that a slower rate fits better with our knowledge of human history [1, 33]. For example, a recent method for estimating population split times from coalescent rates placed the median split of African from non-African populations at 60–80 ky and the split of Native Americans from East Asians at ∼ 20 ky, both assuming a per-generation mutation rate of 1.25 × 10−8 and an average generation interval of 30 years [33]. While both the model and the histories of the populations involved are somewhat complicated, it does seem unlikely that these dates could be half as old (30–40 ky and 10 ky), as would be required for a rate of 2.5 × 10−8. Using our inferred rate also makes the dates more recent, but only modestly so: ∼ 47–62 ky and 15 ky, with some associated uncertainty both from the model and from our estimated rate. Emphasizing the degree to which genetic variation is shared among modern humans, the genome-wide average heterozygosities of our samples (after filtering) range from 5.4–7.5 × 10−4, which corresponds to average TMRCAs of 480–670 ky between two haploid chromosomes (S1 Table).
A possible explanation for the discrepancy between our results and those of trio sequencing studies is that because it is very difficult to separate true de novo mutations from genotype errors in single-generation data, some mutations have been missed in previous work. For example, three recent exome-sequencing studies [34–36], which estimated effective genome-wide mutation rates of approximately 1.5 × 10−8, 1.2 × 10−8, and 1.35 × 10−8 per base per generation, found in follow-up validation that, in addition to virtually all sites from their filtered data sets, many putative sites that did not pass all filters (roughly 70%, 20%, and 90% of tested sites, respectively) were confirmed as true de novo mutations. These results suggest that there may be a subset of de novo mutations having low quality metrics that are missed in trio-based counts as a result of the filtering that is necessary to remove genotype errors. It would be of interest to carry out larger-scale follow-up validation to test if this is the case. In theory, filtered sites can be accounted for by adjusting the denominator in the final rate calculation [10], but it seems possible that site-level filters preferentially remove de novo mutations or that the resulting denominators have not been fully corrected, or both.
Another possibility is that the mutation rate could have changed over time. It has been suggested, for example, that phylogenetic and pedigree-based estimates could be reconciled if the rate has recently slowed in extant great apes [1]. While there is evidence of recent increases in the frequency of certain mutation types in Europeans [37], it seems unlikely that such changes have caused substantial differences in the total mutation rate over the last 50–100 ky. On the other hand, at least part of the discrepancy between our results and previous estimates could plausibly relate to environmental or cultural effects. For example, present-day hunter-gatherers, who may serve as the best available population proxy for our long-term rate estimate, have high paternal ages on average (about 32.3 years) [12]. Using an estimate from a recent de novo study [10] that each additional year of paternal age results in an average of 3.9 × 10−10 more mutations per base per generation, the sex-averaged mutation rate would be expected to be about 0.08 × 10−8 higher for a population with an average paternal age of 32.3 years than for the individuals in that study (average paternal age of 28.4 and inferred mutation rate of 1.27 ± 0.06 × 10−8 [10]), accounting for more than 20% of the discrepancy with our inferred value of 1.61 ± 0.13 × 10−8. One could also speculate that changes in lifestyle, diet, nutrition, or other environmental factors could potentially have contributed to a reduction in the mutation rate in the very recent past. In the future, we expect that new data, including from more diverse populations, combined with new technologies and analytical techniques, will continue to add to our knowledge of the human mutation rate, both in the precision of estimates of its long-term average and in its variability over time, by age and sex, and in different human groups.
Methods
Definition of the statistic HS(d)
Our inferences are based on a statistic that allows us to compare the mutation rate to the (much better measured) recombination rate. Intuitively, we compare local levels of heterozygosity in diploid genomes to the distance scales over which these levels change; the former are proportional to the mutation rate and the latter to the recombination rate, with the same constant of proportionality.
We define a statistic HS(d) equal to the average heterozygosity (local proportion of heterozygous sites) as a function of genetic distance d (measured in cM) from a set S of suitably ascertained starting points within a collection of diploid genomes (see below for more details). This statistic takes the form of a relaxation curve, with the rate of relaxation informative of the average TMRCA at the starting points (via the recombination rate), and the starting heterozygosity in turn informative of the mutation rate. In practice, we compute HS(d) in 60 distance bins from 0 to 0.1 cM.
Locating starting points
In order to maximize signal quality, we would like to measure HS(d) using many starting points in the genome, but within a relatively narrow range of local heterozygosity at those points. For our primary analyses, we use a starting heterozygosity value HS(0) ≈ 7.5 × 10−5, corresponding to points with TMRCA roughly one-tenth of the genome-wide average for non-African individuals. This choice of S has two main advantages (see more detailed discussion below). First, there are relatively many such points in genomes of non-African individuals because it corresponds approximately to the age of the out-of-Africa bottleneck. Second, a smaller HS(0) corresponds to a slower and higher-amplitude (from a lower starting value to the same asymptote) relaxation of HS(d), making the curve easier to fit and less susceptible to genetic map error, an important consideration for our method (see below).
To determine local heterozygosity levels, we tile the genome with 100-kb windows (defined by physical position) and count the proportion of heterozygous sites within each (after filtering, such that that each window will have fewer than 100,000 un-masked sites). The starting points used to compute HS(d) are then the midpoints of the 100-kb regions having a heterozygosity at the desired level, for example 5–10 × 10−5 for out-of-Africa-age blocks with HS(0) ≈ 7.5 × 10−5. We use the notation (for example) H5–10(d) to denote an HS(d) curve computed for starting points with 5–10 heterozygous sites per 100 kb. This scheme may result in choosing starting points with unwanted true heterozygosity if there are recombinations within the 100-kb region, but 100 kb is long enough that most regions within a narrow range of heterozygosity on that scale should be similarly behaved. Additionally, any deviations from the desired heterozygosity range at the midpoints should, on average, be the same for real and simulated data (assuming that the simulator accurately models the genealogical process with recombination; see below), and hence would only cause noise rather than bias in the estimated mutation rate. Similarly, while randomness in the number of accumulated mutations causes the relationship between observed heterozygosity and TMRCA to deviate from strict linearity, this effect is the same in real and simulated data. As an attempt to avoid certain kinds of undesirable behavior (for example, a very low heterozygosity over most of the region and a recombination near one end followed by high heterozygosity), we also require at least one heterozygous site in each half of the window (except for the CpG-only results; see below). Descriptive statistics for regions ascertained with S = 5–10 can be found in S2 Table.
Inference strategy
As described above, HS(d) exhibits a relaxation as a function of d as a result of ancestral recombination events. Recombination can be modeled as a Poisson process (in units of genetic distance), but HS(d) does not have an exponential functional form, because the TMRCAs T1 and T2 at two loci separated by a recombination event are not independent [19, 38, 39]. First, both T1 and T2 must be older than the time at which the recombination occurred, which imposes different constraints on T2 for different values of T1. This dependence becomes especially complicated when the population from which the chromosomes are drawn has changed in size over time. Second, the coalescence at time T2 can involve additional lineages in the ancestral recombination graph, making the expected time different than would be true for two lineages in isolation. For example, with some probability, the two lineages split by the recombination can coalesce together more recently than this combined lineage coalesces with the second chromosome, in which case T1 = T2.
These complicating factors mean that HS(d) is difficult to describe as a closed-form function of d. However, we know that HS(d) relaxes from HS(0) toward the average heterozygosity H ‾, and the rate of relaxation is governed by the relationship between μ and r. Thus, our strategy is to infer the true value of μ by simulating sequence data matching our real data in all respects (see below for more detail) and with a range of different values of μ (typically μ = 1, 2, 4 × 10−8). Then, we can compare the observed HS(d) curve to the same statistic calculated on each simulated data set and infer μ by finding which value gives the best match. Computationally, we interpolate the observed HS(d) curve between the simulated ones (from d = 0 to 0.1 cM), parametrized by the simulated μ values (we use the MATLAB “spline” interpolation; for our main results, other interpolation methods yield results differing by less than 1%). Finally, we perform variance-weighted least-squares to find the single best-fit value of μ.
Population size history
We estimate the historical population sizes for the sampled chromosomes with PSMC [19]. PSMC returns parameters in coalescent units: the scaled mutation rate θ = 4Nμ, the scaled recombination rate ρ = 4Nr, and population sizes going back in (discretized) time, with both the sizes and time intervals in terms of the scaling factor N (the baseline total population size). We do not know N, but the inferred θ together with the population size history are exactly what we need in order to simulate matching data for the calibration curves. We do not use the inferred value of ρ but rather set ρ = θr/μ, where r is the true per-base recombination rate and μ is the fixed mutation rate for a given calibration curve. This maintains the proper ratio between r and μ for that curve, as well as the proper diversity parameter θ. While we only use short regions of the genome in computing HS(d), we run PSMC on the full genome sequences. The exception is that when testing the method in simulations, we run PSMC on the simulated segments, as these are all that are available to us (see below). Further technical details can be found in S1 Text.
Genetic map error
The statistic HS(d) is computed as a function of genetic distance, which we obtain from a previously-estimated genetic map. However, while map distances (i.e., local recombination rates) are known much more precisely than mutation rates, there is still some error in even the best maps, which we must account for in our inferences.
Our approach is not to make a direct correction for map error but rather to include it in a matching fashion in the calibration data. We first select a baseline genetic map from the literature, and we plot HS(d) as a function of d using this base map as the independent variable. To make the calibration curves match the real data, whose intrinsic, true map does not match the base map exactly, we simulate the calibration data using a perturbed version of the base map, with the aim of capturing an equal amount of deviation as in the real data.
The base map we use is the “shared” version of an African-American (AA) genetic map published in [17]. The AA map was derived by tabulating switch points between local African and European ancestry in the genomes of African-Americans, which reflect recombination events since the time of admixture. The “shared” component of the map was estimated as the component of this recombination landscape that is active in non-Africans, particularly Europeans. We also considered using either the 2010 deCODE map [16], which was estimated by observing crossovers in a large Icelandic pedigree cohort, or an LD map estimated from variation in linkage disequilibrium levels in unrelated individuals [14, 15]. However, we were concerned that an LD-based map would cause confounding in our method because it is based on a correlated ancestral recombination signal, while we found that at short distance scales, the “shared” map was less susceptible than the deCODE map to the issue of zero-length intervals (see prior distribution below).
While using as accurate a map as possible is helpful, the key to our approach is in the ability to quantify the amount of error in the base map. In what follows, we describe in detail our methods to measure the degree of genetic map error.
Basic model and previous estimates
Our basic error model is that of [20]. Consider a chromosomal interval whose true genetic length is Z. Given the measured genetic length g of the interval in our base map, we assume that Z ∼ Gamma(αg, α), so E[Z∣g] = g and var(Z∣g) = g/α. The gamma distribution has several desirable properties, in particular that it is scale-invariant. The parameter α measures the per-distance variance of the map (and hence of our perturbation), with a larger value of α corresponding to a smaller variance. It can be interpreted directly as the inverse of the length scale at which the coefficient of variation of the map equals 1: on average, intervals of length 1/α have an equal mean and standard deviation, while longer intervals are relatively more accurately measured and shorter intervals are less accurate.
Briefly, this model can be used to estimate α by comparing genetic distances in the base map to counts of recombination events observed in an independent validation data set: the more accurate the map, the more closely it will predict the observations. From the distribution above, one can specify a full probability model for the validation data in terms of the error in the base map as well as other parameters and infer α via a Bayesian (Gibbs sampling) approach. Full details can be found in [20]. In that study, using a validation data set of recombination events observed in a Hutterite pedigree [40], the authors obtained estimates (in per-Morgan units) of α = 1400 ± 100 M−1 for the deCODE map [16] and α = 1220 ± 80 M−1 for the HapMap LD map [15].
Here, we apply the same method to estimate α, but using a much larger set of validation data, consisting of 2.2 million crossovers from 71,000 meioses in Icelandic individuals [23] (versus 24,000 crossovers from 728 meioses in [40]). A potential complication is that the procedure to build the “shared” map [17] used information from the 2010 deCODE map, which is not independent from our validation set. However, we reasoned that we could constrain the true α by applying the estimation method first to the “shared” map as an upper bound (since the value will be inflated by the non-independence) and then to the full AA map as a lower bound (since the AA map includes African-specific hotspots not active in Europeans and does not use information from the deCODE map).
As noted above, since the “shared” map is estimated from directly-observed crossover events rather than population-level statistics (as for LD-based maps), it should be free from confounding between heterozygosity and recombination (as desired for our rate estimation method). There could potentially be a subtle effect of population heterozygosity on the SNP grid on which the map is defined, which does not have perfectly uniform coverage of the genome, but both the base map data and validation data should be independent. Moreover, the error model described above takes into account differential SNP spacing [20]. Also, we note that most of the power of our procedure comes from regions away from recombination hotspots, so that any potential issues pertaining to hotspots, including differential SNP density, should be minimized.
Modified prior distribution on Z
We also add one modification to the basic model of map error described above. For intervals in the base map with estimated (genetic) length 0, the original model states that the true length of these intervals must be 0 (since Z has mean g = 0 and Z ≥ 0). In fact, though, the data used to build the map might simply have included no crossovers there by chance. Overall, very short intervals in the base map are likely underestimated, while very long intervals are likely overestimated.
To account for this effect, we modify the prior distribution on the true length Z by adding a pseudo-count adjustment π, i.e., a small uniform prior on the true map length. In order for the model still to be additive, it is reasonable for the prior to be in units of genetic distance per physical distance.
Empirically, we observe that without the adjusted prior, the relaxation of HS(d) in the calibration curves is too slow at the smallest values of d and too fast at larger d (S4 Fig). This would be expected if very short intervals in the base genetic map are underestimated, so that the calibration data have too few recombinations in that range compared to real data. By matching the curve shape of real data to the calibration data for different values of the pseudo-count, we find that a value of π = 0.09 cM/Mb properly corrects for the underestimation of very short intervals in the “shared” map.
Perturbed genetic maps for calibration data
For our purposes, once we obtain a value for α, we use the gamma-distribution model to generate the randomized perturbed maps that we input to msHOT to generate the calibration data (see below). Our complete error model is as follows: for an interval of physical length p, we take Z ∼ Gamma(αg′, α), where g′ = γ(g + πp) for a pseudo-count π and the corresponding constant factor γ < 1 that preserves the total map length. Thus, for each interval in the map (between two adjacent SNPs on which the map is defined), if the physical length is p and the genetic length in the base map is g, we create a new genetic length Z for that interval in the perturbed map by drawing from this distribution. When computing HS(d), we then measure all genetic distances in both the real and simulated data by linearly interpolating individual sites within the SNP grid.
Uncertainty in α
The importance of accurately estimating α lies in the fact that the smaller the value of α used to generate the calibration data (i.e., the less accurate the genetic map is taken to be), the smaller the final inferred value of μ will be. This is because the genetic lengths used for simulation will be more discrepant from the base map, and as a result, the final value of HS(d) (computed on the calibration data) for a given d will reflect an average of the heterozygosity over a wider range of perturbed distances around d. Since HS(d) is a concave function of d, this smoothing will cause HS(d) to decrease, or in other words, to make the relaxation appear slower. Thus, in order to match the real data, a calibration curve with a smaller α would have to have a higher scaled recombination rate, and hence, with a fixed θ, a smaller μ.
As described in Results, we assume a standard error of 300 around our estimate of α = 3100 M−1. To determine how much of an effect this uncertainty has on our estimates of μ, we run our procedure with our primary data set using a range of different values of α (2500, 3000, 3500, 4000, and 4500; see S2 Fig). The slope of a linear regression of the inferred μ as a function of α is 1.66 × 10−4 (in units of 10−8 per base per generation per M−1), so that the standard error of 300 for α translates into a standard error of roughly 0.05 × 10−8 per base per generation for μ.
We also note that even if our map error model is properly specified and estimated, there could be a small bias in our final inferred μ if the exact form of the true map is such that the real-data HS(d) curve relaxes slightly faster or slower than the calibration curves built with a perturbed map. Since this uncertainty is analogous to variability in the inferred μ depending on the exact instantiation of the perturbed map, we can compensate for it by using for our final point estimate the average of calibration results for different versions of the perturbed map. In practice, we obtain our final point estimates by averaging 25 independent calibrations of the data, which should remove most randomization noise arising from both the perturbed map and the simulations, and we assume that the resulting reduction in uncertainty (as compared to our single-run jackknife standard error) negates the uncertainty from the exact form of the true map.
Simulation of calibration data with msHOT
As discussed above, the relaxation of HS(d) reflects the decorrelation of heterozygosity (caused by recombination) as a function of genetic distance. However, in the sequence of TMRCAs for the recombination-separated blocks along a chromosome, successive values are not independent, and in fact the sequence is not Markovian, since even lineages that are widely separated along the chromosome can interact within the ancestral recombination graph [38, 39]. It is important, then, that our simulated data be generated according to an algorithm that captures all of the coalescent details that could impact the history of a real-data sample. For this reason, we use msHOT [41] (an extension of ms [42] that allows variable recombination rates) rather than a Markovian simulator, which would have had the advantage of greater speed.
As a result of using a non-Markovian simulator, it is computationally infeasible to generate entire simulated chromosomes. Thus, in practice we define wider “super-regions” around the 100-kb windows and simulate the super-regions independently of each other, matching the physical and genetic coordinates to the human genome. Since we compute HS(d) from d = 0 to 0.1 cM, we define the super-regions to include at least 0.1 cM on both sides of their internal starting point, which typically leads to a total length of several hundred kb per super-region.
Finally, in addition to matching the demographic and genetic map parameters of the calibration data to the test data, we also apply an adjustment to the calibration curves themselves to correct for residual unequal heterozygosity (not precisely captured by PSMC), which would cause the asymptotes of the curves to be mis-aligned. In particular, we multiply the relaxation portion of the calibration curves (i.e., HS(d) − HS(0)) by the ratio of the heterozygosity of the real data (over all of the super-regions) to that of the matching simulated data. In our experience, this correction ranges from 0–10%. We note that in theory, the intercept values HS(0) might also not be recapitulated exactly in the calibration data, but in fact the intercepts match extremely closely in all cases other than the CpG-only estimate (see below). This indicates good reconstruction of the demographic history around the TMRCAs of our test regions.
Genotype error
When we compare matching HS(d) curves for real and simulated data, the real-data curve will be influenced by genotype errors (almost all of which are sites that are in fact homozygous but are mistakenly called as heterozygous). Since the relaxation rates of the curves as a function of d are equal, the starting points will have the same average TMRCAs. The real and simulated regions also have the same average starting heterozygosity HS(0), but since the real data contain both true heterozygous sites and genotype errors, the calibration data must have a higher mutation rate per generation. Thus, false-positive heterozygous sites will artificially inflate our estimates of μ. The upward bias in the estimates will be larger for smaller values of HS(0), since the local ratio of false to true heterozygous sites will be larger; for the same reason, for a fixed error rate, our method will be less sensitive to genotype errors than de novo mutation counts.
We have two main approaches for dealing with errors in genotype calls. First, we have taken a number of steps to filter the data, discussed below, such that the sites we analyze have high-quality calls and are as free from errors as possible. However, this is not sufficient to eliminate all false positives, and thus we also use local abundances of CpG transitions to quantify the proportion of true homozygous sites that are called as heterozygous (for full details, see S1 Text). For our final values of μ, we directly correct for these inferred levels of genotype error, incorporating our uncertainty in the error rate into our final reported standard error. Specifically, for a given starting heterozygosity value HS(0) and genotype error rate ϵ (inferred for the same set of windows S), we multiply the initial estimate of μ by a factor of (HS(0)−ϵ)/HS(0). This correction is based on the idea stated above that while the calibration data are created to mimic the full observed data (including genotype errors), matched curves with the same HS(0) and slope of relaxation (and thus TMRCA) will still differ: the real data will have errors contributing to the local heterozygosity, so that the inferred mutation rate from the calibration will be too high. (The same logic applies for the two sections that follow; see also S1 Text). To ensure that this correction is valid, we perform simulations in which we randomly add false heterozygous sites to the simulated sequence data (see below).
Non-crossover gene conversion
Gene conversion is similar to recombination (as we have used the term; more precisely, crossing-over) in that it results from double-stranded breaks during meiosis and leads to the merging of genetic material between homologous chromosomes. However, whereas crossing-over creates large-scale blocks inherited from the recombining chromosomes, non-crossover gene conversion occurs in very small tracts, on the order of 100 bases in humans [43]. For our purposes, gene conversion is significant primarily because it can introduce heterozygous sites in our test regions if one of the haplotypes has experienced a gene conversion event since the TMRCA. (New mutations that occur in our regions can also be lost via gene conversion, which we take into account, although this rate is much lower because the local heterozygosity is small.)
We choose to account for the effect of gene conversion by applying a correction to our inferred mutation rates, reasoning that a subset of the observed heterozygous sites will be caused by gene conversion events rather than mutations. Since our method relies on the ratio between the recombination and mutation rates at the selected starting points, the key quantity is the number of heterozygous sites near those points that are due to gene conversion, which we subtract from the raw estimate of μ after correcting for genotype error. The gene conversion rate is a combination of two factors: the probability that each base is involved in a gene conversion event and the conditional probability that a polymorphism is introduced. For the former, we use a recent estimate that non-crossover gene conversion affects approximately 5.9 × 10−6 bases per generation (95% confidence interval 4.6–7.4 × 10−6) [21] and adjust for local recombination rate, and for the latter, we use differences between the heterozygosity in the test regions and in the genomes as a whole (see S1 Text). The final correction ranges from 0.13–0.17 × 10−8 per base per generation (with a standard error of 0.02–0.03 × 10−8), approximately 7–10% of the total apparent mutational signal after accounting for genotype error. To confirm that this procedure is accurate, we also apply it to simulated data with gene conversion (see below and S1 Text).
We note that gene conversion events are well known to carry a GC bias, so that when they affect heterozygous sites with one strong (C or G) and one weak (A or T) allele, the strong base is preferentially transmitted (recently estimated as 68 ± 5% of the time [21]). In theory this bias could have an impact on our gene conversion correction, but (a) genome-wide, weak-to-strong and strong-to-weak SNPs are close to equally frequent (the latter about 10% more abundant), and (b) while new mutations are enriched in strong-to-weak substitutions, gene conversion of newly occurring mutations is rare in our test regions because of their recent TMRCAs. Thus, for simplicity, other than a small adjustment when calculating the gene conversion corrections for the CpG-only and non-CpG-only estimates, we do not include GC bias in the gene conversion correction (we note that this is conservative, in the sense that GC bias would cause the gene conversion effect to be slightly weaker than we are assuming).
Base content mutability adjustment
Because not all bases in the genome are equally mutable, and our test regions may deviate from the full genome in their base composition, we apply an adjustment to our estimated rates to convert them to a genome-wide equivalent. The two primary parameters we use are the fraction of CpG sites (which are highly mutable) and total GC content (because G and C are more mutable than A and T, beyond CpG effects). We also include an interaction term between these two quantities. Our final adjustment for the primary eight-genome estimate is a multiplicative factor of 1.027 ± 0.003 (an increase of about 0.04 × 10−8 over the uncorrected value). The uncertainty in the adjustment incorporates confidence intervals on the parameters in our model and a jackknife for the counts of CpG and GC sites. Full details can be found in S1 Text.
Choice of the starting heterozygosity range S
Our use of S = 5–10 per 100 kb for our primary results was guided by several considerations. First, our method works best with relatively recently coalesced starting points (at most perhaps 1/3 of the genome-wide average) because they yield HS(d) curves that relax slowly and have a low intercept HS(0). At a finer scale, we also wish to to balance the impact of different sources of error: while genetic map error is more significant for higher starting heterozygosity, genotype error is relatively more significant for lower starting heterozygosity. Another consideration is that the TMRCAs corresponding to S = 5–10 lie approximately at the deepest portion of the out-of-Africa bottleneck [19, 33], so that more starting points are available at that range (for non-African populations). Moreover, demographic inference with PSMC is less accurate both at very recent times and at the edges of bottlenecks [19]. The combination of these factors motivates our use of S = 5–10.
Noise and uncertainty
Several of the steps in our procedure have some associated statistical uncertainty, while others rely on randomization. These include the computation of HS(d) from a finite number of loci, the population size inference with PSMC, the random genetic map perturbation, and the simulation of calibration data. In order to capture this uncertainty, we use jackknife resampling to obtain standard errors for our estimates of μ, treating each autosome as a separate observation and leaving out one chromosome in each replicate. Our rationale for this scheme is that nearby regions of a chromosome are non-independent, and different individuals can also have correlated coalescent histories for a given locus, but a chromosomal unit encompasses most or all of the dependencies among the data. For real data, we also average the results of 25 independent calibrations to obtain our point estimate of μ, which eliminates most of the noise associated with randomization. We note that we have found that the transformation from PSMC-inferred demographic parameters to calibration data via msHOT is discontinuous and is not properly captured by the jackknife. Thus, we use the same population size history (from the full data) for each replicate (see next section and Results).
The other form of uncertainty in our method is our inexact knowledge of a number of model parameters, including the genetic map variance α and several components of the three final adjustments (genotype error, gene conversion, and base content; see S1 Fig). For these sources of error, we translate our uncertainty in the parameters into uncertainty in μ and combine them with our jackknife results to form a final standard error, using the assumption that the errors are independent and normally distributed (for implicit conversion of standard errors into confidence intervals).
Simulations
To test the accuracy of our procedure in a controlled setting, we first apply it to simulated data generated using msHOT [41]. When not otherwise specified, we create 20 sample genomes with μ = 2.5 × 10−8; an ancestral population size of 10,000 (or 16,666 when using μ = 1.5 × 10−8 so as to maintain the same diversity parameter θ) outside of a 10 × bottleneck from 1000–2000 generations ago (similar to the age of the out-of-Africa bottleneck); and a perturbed version of the “shared” AA genetic map (α = 3000 M−1 and π = 0.09 cM/Mb). We run the full inference procedure as we would with real data, except with a default total of 30 genomes’ worth of data per calibration curve versus 40 for real data. Also, for computational efficiency, when running PSMC on simulated test data, we only include a single copy of each chromosome (chosen at random from among the samples in the simulated data set).
As discussed in more detail in S1 Text, in addition to this basic setup (a), we also run a number of additional simulations. First, we run the procedure (b) with a true rate of μ = 1.5 × 10−8, (c) with a true rate of μ = 1.5 × 10−8 plus gene conversion, and (d) with simulated genotype errors. Then, to test the effects of possible model violations, we simulate (e) samples from an admixed population, (f) mutation rate heterogeneity based on polymorphism levels in present-day African individuals, and (g) all three complications (d)–(f) simultaneously. For simulations (d) and (g), we add false heterozygous sites to the simulated diploid genomes (at a rate of 1 per 100 kb for (d) and 1 per 150 kb for (g)) and apply our standard correction, with one modification: because msHOT does not create individual nucleotides, we directly count the numbers of errors in ascertained regions instead of using our CpG-based estimate.
Data and filtering
As mentioned previously, we generate our estimates using genome sequences from non-African individuals, since the presence of a large number of relatively recently coalesced blocks arising from the out-of-Africa bottleneck gives us more data to work with at starting points with low heterozygosity. We use high-coverage sequences published in [24] and [28].
In order to remove as many genotype errors as possible, we use a filtering scheme based on the one applied to estimate heterozygosity in [28]. This consists of a tandem repeat filter, mapping quality threshold (MQ = 30), genome alignability filter (all possible 35-mers overlapping a given base match uniquely to that position in the genome, with up to one mismatch), and coverage thresholds (central 95% of the depth distribution) [28]. We additionally apply a strict genotype quality threshold in order to preserve the highest-quality calls for analysis. From the GATK output, we compare the PL likelihood score of the heterozygous state to the minimum of the two PL scores of the homozygous states, imposing a quality threshold of 60 along with a prior of 31 (to reflect the genome-wide average heterozygosity). That is, if the heterozygote PL is at least 60 + 31 = 91 lower than either homozygote PL, we call the site heterozygous; if it is at least 60 − 31 = 29 higher, we call the site homozygous; and if it is in between, we mask the site as low-quality. Finally, we also remove all sites 1 or 2 bases away from any masked base under the five filters described.
While filtering is not necessary for the simulated calibration data, we still apply the same filters to the calibration data as to the real sequence data for consistency, on a genome-matching basis (e.g., for a sample of eight real genomes and our default real-data setting of 40 genomes’ worth of calibration data, the base positions that are masked for each real sequence are also masked in five of the simulated sequences). In addition to filtering out individual sites, we impose a missing-data threshold for regions, ignoring any with more than 50% of sites masked (either of the super-region or the 100-kb central window).
Estimates for separate mutation classes
For our estimates of the mutation rate for CpG and non-CpG sites separately, we make several small modifications to our default procedure. First, we divide heterozygous sites into two classes: C-to-T transitions at CpG sites (defined based on the human reference sequence) and all others. For the two estimates, we consider as homozygous all sites in the genome not in the corresponding class of heterozygous sites. This places the CpG, non-CpG, and full-data values on an equal denominator. For the CpG estimate, we select starting points with S = 0.1–2, which, because of our filtering, is equivalent to requiring exactly one heterozygous site in a 100 kb window. We then create calibration curves with μ = 0.2, 0.4, 0.8 × 10−8 and 10 simulated genomes per real-data sequence (two times the usual). We find that, unlike the curves for all of our other results, the CpG-only HS(d) has a noticeably lower y-intercept than the calibration curves, which we believe may be due to relatively poor reconstruction of the demographic history with thin data. In order to make the interpolation more sensible, we translate the calibration curves downward to match the real-data value of HS(0), which results in a decrease in the final inferred μ of approximately 0.04 × 10−8 as compared to the uncorrected fitting. For the non-CpG estimate, we use S = 4.375–8.75 and calibrate as for the full data.
When correcting for genotype error, we use our baseline value of 1.08 × 10−5 per base, multiplied by the fraction of CpG or non-CpG sites. Likewise, we partition the full-data gene conversion estimate by the fraction of heterozygous sites in each class and adjust for GC bias (final correction approximately 0.09 and 0.91 times the full gene conversion rate for CpG and non-CpG, respectively, versus about 0.12 and 0.88 for fractions of heterozygous sites). Finally, we re-compute base content corrections of approximately 1.17 for CpGs and 1.005 for non-CpGs (where the CpG estimate is more strongly affected because of the relatively low proportion of CpG sites in the ascertained regions).
Software
MATLAB code is available at https://github.com/DReichLab/MutationRateCode.
Supporting Information
Zdroje
1. Scally A, Durbin R (2012) Revising the human mutation rate: implications for understanding human evolution. Nat Rev Genet 13 : 745–753. doi: 10.1038/nrg3295 22965354
2. Campbell CD, Eichler EE (2013) Properties and rates of germline mutations in humans. Trends Genet 29 : 575–584. doi: 10.1016/j.tig.2013.04.005 23684843
3. Ségurel L, Wyman MJ, Przeworski M (2014) Determinants of mutation rate variation in the human germline. Annu Rev Genomics Hum Genet 15 : 47–70. doi: 10.1146/annurev-genom-031714-125740 25000986
4. Li WH, Tanimura M (1987) The molecular clock runs more slowly in man than in apes and monkeys. Nature 326 : 93–96. doi: 10.1038/326093a0 3102974
5. Kimura M (1968) Evolutionary rate at the molecular level. Nature 217 : 624–626. doi: 10.1038/217624a0 5637732
6. Roach JC, Glusman G, Smit AF, Huff CD, Hubley R, et al. (2010) Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 328 : 636–639. doi: 10.1126/science.1186802 20220176
7. Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, et al. (2011) Variation in genome-wide mutation rates within and between human families. Nat Genet 43 : 712–714. doi: 10.1038/ng.862 21666693
8. Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, et al. (2012) Rate of de novo mutations and the importance of father’s age to disease risk. Nature 488 : 471–475. doi: 10.1038/nature11396 22914163
9. Campbell CD, Chong JX, Malig M, Ko A, Dumont BL, et al. (2012) Estimating the human mutation rate using autozygosity in a founder population. Nat Genet 44 : 1277–1281. doi: 10.1038/ng.2418 23001126
10. Besenbacher S, Liu S, Izarzugaza JM, Grove J, Belling K, et al. (2015) Novel variation and de novo mutation rates in population-wide de novo assembled Danish trios. Nat Comm 6 : 5969. doi: 10.1038/ncomms6969
11. Fu Q, Li H, Moorjani P, Jay F, Slepchenko SM, et al. (2014) Genome sequence of a 45,000-year-old modern human from western Siberia. Nature 514 : 445–449. doi: 10.1038/nature13810 25341783
12. Fenner J (2005) Cross-cultural estimation of the human generation interval for use in genetics-based population divergence studies. Am J Phys Anthropol 128 : 415–423. doi: 10.1002/ajpa.20188 15795887
13. Sun JX, Helgason A, Masson G, Ebenesersdóttir SS, Li H, et al. (2012) A direct characterization of human mutation based on microsatellites. Nat Genet 44 : 1161–1165. doi: 10.1038/ng.2398 22922873
14. Myers S, Bottolo L, Freeman C, McVean G, Donnelly P (2005) A fine-scale map of recombination rates and hotspots across the human genome. Science 310 : 321–324. doi: 10.1126/science.1117196 16224025
15. The International HapMap Consortium (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449 : 851–861. doi: 10.1038/nature06258 17943122
16. Kong A, Thorleifsson G, Gudbjartsson DF, Masson G, Sigurdsson A, et al. (2010) Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467 : 1099–1103. doi: 10.1038/nature09525 20981099
17. Hinch AG, Tandon A, Patterson N, Song Y, Rohland N, et al. (2011) The landscape of recombination in African Americans. Nature 476 : 170–175. doi: 10.1038/nature10336 21775986
18. Palamara PF, Francioli L, Genovese G, Wilton P, Gusev A, et al. (2015) Leveraging distant relatedness to quantify human mutation and gene conversion rates. Am J Hum Genet. 97. doi: 10.1016/j.ajhg.2015.10.006
19. Li H, Durbin R (2011) Inference of human population history from individual whole-genome sequences. Nature 475 : 493–496. doi: 10.1038/nature10231 21753753
20. Sankararaman S, Patterson N, Li H, Pääbo S, Reich D (2012) The date of interbreeding between Neandertals and modern humans. PLoS Genet 8: e1002947. doi: 10.1371/journal.pgen.1002947 23055938
21. Williams A, Geneovese G, Dyer T, Truax K, Jun G, et al. (2015) Non-crossover gene conversions show strong GC bias and unexpected clustering in humans. eLife 4: e04637. doi: 10.7554/eLife.04637
22. Li H (2014) Towards better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics 30 : 2843–2851. doi: 10.1093/bioinformatics/btu356 24974202
23. Kong A, Thorleifsson G, Frigge ML, Masson G, Gudbjartsson DF, et al. (2014) Common and low-frequency variants associated with genome-wide recombination rate. Nat Genet 46 : 11–16. doi: 10.1038/ng.2833 24270358
24. Meyer M, Kircher M, Gansauge MT, Li H, Racimo F, et al. (2012) A high-coverage genome sequence from an archaic Denisovan individual. Science 338 : 222–226. doi: 10.1126/science.1224344 22936568
25. Cochran G, Harpending H (2013) Paternal age and genetic load. Hum Biol 85 : 515–528. doi: 10.3378/027.085.0401 25019186
26. Francioli LC, Polak PP, Koren A, Menelaou A, Chun S, et al. (2015) Genome-wide patterns and properties of de novo mutations in humans. Nat Genet 47 : 822–826. doi: 10.1038/ng.3292 25985141
27. Neale BM, Kou Y, Liu L, Maayan A, Samocha KE, et al. (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485 : 242–245. doi: 10.1038/nature11011 22495311
28. Prüfer K, Racimo F, Patterson N, Jay F, Sankararaman S, et al. (2014) The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505 : 43–49. doi: 10.1038/nature12886 24352235
29. Genome of the Netherlands Consortium (2014) Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat Genet 46 : 818–825. doi: 10.1038/ng.3021 24974849
30. Mailund T, Halager AE, Westergaard M, Dutheil JY, Munch K, et al. (2012) A new isolation with migration model along complete genomes infers very different divergence processes among closely related great ape species. PLoS Genet 8: e1003125. doi: 10.1371/journal.pgen.1003125 23284294
31. Prado-Martinez J, Sudmant PH, Kidd JM, Li H, Kelley JL, et al. (2013) Great ape genetic diversity and population history. Nature 499 : 471–475. doi: 10.1038/nature12228 23823723
32. Scally A, Dutheil JY, Hillier LW, Jordan GE, Goodhead I, et al. (2012) Insights into hominid evolution from the gorilla genome sequence. Nature 483 : 169–175. doi: 10.1038/nature10842 22398555
33. Schiffels S, Durbin R (2014) Inferring human population size and separation history from multiple genome sequences. Nat Genet 46 : 919–925. doi: 10.1038/ng.3015 24952747
34. Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, et al. (2012) De novo gene disruptions in children on the autistic spectrum. Neuron 74 : 285–299. doi: 10.1016/j.neuron.2012.04.009 22542183
35. Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, et al. (2014) De novo mutations in schizophrenia implicate synaptic networks. Nature 506 : 179–184. doi: 10.1038/nature12929 24463507
36. Iossifov I, ORoak BJ, Sanders SJ, Ronemus M, Krumm N, et al. (2014) The contribution of de novo coding mutations to autism spectrum disorder. Nature 515 : 216–221. doi: 10.1038/nature13908 25363768
37. Harris K (2015) Evidence for recent, population-specific evolution of the human mutation rate. Proc Natl Acad Sci U S A 112 : 3439–3444. doi: 10.1073/pnas.1418652112 25733855
38. McVean GA, Cardin NJ (2005) Approximating the coalescent with recombination. Philos Trans R Soc Lond B Biol Sci 360 : 1387–1393. doi: 10.1098/rstb.2005.1673 16048782
39. Marjoram P, Wall JD (2006) Fast “coalescent” simulation. BMC Genet 7 : 16. doi: 10.1186/1471-2156-7-16 16539698
40. Coop G, Wen X, Ober C, Pritchard JK, Przeworski M (2008) High-resolution mapping of crossovers reveals extensive variation in fine-scale recombination patterns among humans. Science 319 : 1395–1398. doi: 10.1126/science.1151851 18239090
41. Hellenthal G, Stephens M (2007) mshot: modifying hudson’s ms simulator to incorporate crossover and gene conversion hotspots. Bioinformatics 23 : 520–521. doi: 10.1093/bioinformatics/btl622 17150995
42. Hudson RR (2002) Generating samples under a Wright–Fisher neutral model of genetic variation. Bioinformatics 18 : 337–338. doi: 10.1093/bioinformatics/18.2.337 11847089
43. Jeffreys AJ, May CA (2004) Intense and highly localized gene conversion activity in human meiotic crossover hot spots. Nat Genet 36 : 151–156. doi: 10.1038/ng0404-427a 14704667
Štítky
Genetika Reprodukční medicína
Článek A Hereditary Enteropathy Caused by Mutations in the Gene, Encoding a Prostaglandin TransporterČlánek Exocyst-Dependent Membrane Addition Is Required for Anaphase Cell Elongation and Cytokinesis inČlánek Spindle-F Is the Central Mediator of Ik2 Kinase-Dependent Dendrite Pruning in Sensory Neurons
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 11
-
Všechny články tohoto čísla
- Agricultural Genomics: Commercial Applications Bring Increased Basic Research Power
- Ernst Rüdin’s Unpublished 1922-1925 Study “Inheritance of Manic-Depressive Insanity”: Genetic Research Findings Subordinated to Eugenic Ideology
- Convergent Evolution During Local Adaptation to Patchy Landscapes
- The Locus Controls Age at Maturity in Wild and Domesticated Atlantic Salmon ( L.) Males
- A Hereditary Enteropathy Caused by Mutations in the Gene, Encoding a Prostaglandin Transporter
- Absence of Maternal Methylation in Biparental Hydatidiform Moles from Women with Maternal-Effect Mutations Reveals Widespread Placenta-Specific Imprinting
- Calibrating the Human Mutation Rate via Ancestral Recombination Density in Diploid Genomes
- Anaplastic Lymphoma Kinase Acts in the Mushroom Body to Negatively Regulate Sleep
- Connecting Replication and Repair: YoaA, a Helicase-Related Protein, Promotes Azidothymidine Tolerance through Association with Chi, an Accessory Clamp Loader Protein
- UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development
- Mosaic and Intronic Mutations in Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing
- Members of the Epistasis Group Contribute to Mitochondrial Homologous Recombination and Double-Strand Break Repair in
- QTL Mapping of Sex Determination Loci Supports an Ancient Pathway in Ants and Honey Bees
- Genetic Interactions Implicating Postreplicative Repair in Okazaki Fragment Processing
- Genomics of Cancer and a New Era for Cancer Prevention
- Adaptation to High Ethanol Reveals Complex Evolutionary Pathways
- Dynamics of Transcription Factor Binding Site Evolution
- Exocyst-Dependent Membrane Addition Is Required for Anaphase Cell Elongation and Cytokinesis in
- Enhancer Runaway and the Evolution of Diploid Gene Expression
- Cattle Sex-Specific Recombination and Genetic Control from a Large Pedigree Analysis
- Drosophila Mutants Model Cornelia de Lange Syndrome in Growth and Behavior
- Pleiotropic Effects of Immune Responses Explain Variation in the Prevalence of Fibroproliferative Diseases
- Leaderless Transcripts and Small Proteins Are Common Features of the Mycobacterial Translational Landscape
- Tissue-Specific Effects of Reduced β-catenin Expression on Mutation-Instigated Tumorigenesis in Mouse Colon and Ovarian Epithelium
- Genus-Wide Comparative Genomics of Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin
- Mapping of Craniofacial Traits in Outbred Mice Identifies Major Developmental Genes Involved in Shape Determination
- Conserved Genetic Interactions between Ciliopathy Complexes Cooperatively Support Ciliogenesis and Ciliary Signaling
- Metabolomic Quantitative Trait Loci (mQTL) Mapping Implicates the Ubiquitin Proteasome System in Cardiovascular Disease Pathogenesis
- DNA Repair Cofactors ATMIN and NBS1 Are Required to Suppress T Cell Activation
- Spindle-F Is the Central Mediator of Ik2 Kinase-Dependent Dendrite Pruning in Sensory Neurons
- Ernst Rüdin and the State of Science
- ABCs of Insect Resistance to Bt
- Epigenetic Control of O-Antigen Chain Length: A Tradeoff between Virulence and Bacteriophage Resistance
- Encodes Dual Oxidase, Which Acts with Heme Peroxidase Curly Su to Shape the Adult Wing
- The Fanconi Anemia Pathway Protects Genome Integrity from R-loops
- Controls Quantitative Variation in Maize Kernel Row Number
- Genome-Wide Association Study of Golden Retrievers Identifies Germ-Line Risk Factors Predisposing to Mast Cell Tumours
- Insect Resistance to Toxin Cry2Ab Is Conferred by Mutations in an ABC Transporter Subfamily A Protein
- A Cytosine Methytransferase Modulates the Cell Envelope Stress Response in the Cholera Pathogen
- Conserved piRNA Expression from a Distinct Set of piRNA Cluster Loci in Eutherian Mammals
- The Multi-allelic Genetic Architecture of a Variance-Heterogeneity Locus for Molybdenum Concentration in Leaves Acts as a Source of Unexplained Additive Genetic Variance
- The lncRNA Controls Cryptococcal Morphological Transition
- Sae2 Function at DNA Double-Strand Breaks Is Bypassed by Dampening Tel1 or Rad53 Activity
- A Tandem Duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia,
- Ectodysplasin/NF-κB Promotes Mammary Cell Fate via Wnt/β-catenin Pathway
- The QTL within the Complex Involved in the Control of Tuberculosis Infection in Mice Is the Classical Class II Gene
- Identifying Loci Contributing to Natural Variation in Xenobiotic Resistance in
- Variation in Rural African Gut Microbiota Is Strongly Correlated with Colonization by and Subsistence
- A Flexible, Efficient Binomial Mixed Model for Identifying Differential DNA Methylation in Bisulfite Sequencing Data
- Competition between Heterochromatic Loci Allows the Abundance of the Silencing Protein, Sir4, to Regulate Assembly of Heterochromatin
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development
- Metabolomic Quantitative Trait Loci (mQTL) Mapping Implicates the Ubiquitin Proteasome System in Cardiovascular Disease Pathogenesis
- Genus-Wide Comparative Genomics of Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin
- Encodes Dual Oxidase, Which Acts with Heme Peroxidase Curly Su to Shape the Adult Wing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



