-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide Patterns of Genetic Variation within and among Alternative Selective Regimes
Evolutionary biologists seek to understand the factors affecting genetic variation. While it is intuitive that environmental heterogeneity should increase levels of variation, theoretical models showed that spatial and temporal heterogeneity differ in how likely they are to maintain polymorphisms affecting fitness. We evolved experimental populations of fruit flies in constant environments or in temporally or spatially varying environments, then examined levels of sequence variation across the genome. For sites associated with ecological selection, polymorphism patterns matched the theoretical expectations with variation greatest in populations evolving in spatially heterogeneous environments, less variation in populations evolving in temporally heterogeneous environments, and least variation in populations evolving in constant environments. However, a different pattern was observed at sites not associated with differential ecological selection (i.e., most of the genome). For these sites, levels of variation were highest at spatially heterogeneous populations but lowest for temporally heterogeneous populations. Populations evolving under temporal heterogeneity also showed the greatest differentiation from one another, suggesting that this selection regime caused more genetic drift than other selection regimes. These results illustrate that environmental heterogeneity affects levels of variation not only at sites subject to differential ecological selection but also genome-wide, though spatial and temporal heterogeneity affect diversity differently.
Published in the journal: . PLoS Genet 10(8): e32767. doi:10.1371/journal.pgen.1004527
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004527Summary
Evolutionary biologists seek to understand the factors affecting genetic variation. While it is intuitive that environmental heterogeneity should increase levels of variation, theoretical models showed that spatial and temporal heterogeneity differ in how likely they are to maintain polymorphisms affecting fitness. We evolved experimental populations of fruit flies in constant environments or in temporally or spatially varying environments, then examined levels of sequence variation across the genome. For sites associated with ecological selection, polymorphism patterns matched the theoretical expectations with variation greatest in populations evolving in spatially heterogeneous environments, less variation in populations evolving in temporally heterogeneous environments, and least variation in populations evolving in constant environments. However, a different pattern was observed at sites not associated with differential ecological selection (i.e., most of the genome). For these sites, levels of variation were highest at spatially heterogeneous populations but lowest for temporally heterogeneous populations. Populations evolving under temporal heterogeneity also showed the greatest differentiation from one another, suggesting that this selection regime caused more genetic drift than other selection regimes. These results illustrate that environmental heterogeneity affects levels of variation not only at sites subject to differential ecological selection but also genome-wide, though spatial and temporal heterogeneity affect diversity differently.
Introduction
Patterns of genetic variation have several major consequences for evolution. First, the amount and type of genetic variation in fitness mediate a population's ability to adapt to selection pressures. Second, the nature of genetic variation determines the fitness consequences of different types of reproduction (e.g., sexual versus asexual reproduction, inbreeding versus outbreeding, random mating versus mate choice), and can therefore be a major cause of selection on reproductive traits. Third, the amount of neutral variation determines the rate at which populations diverge by drift. But what factors shape variation within populations and create differences in levels of variation among them? This is one of the central questions in evolutionary biology [1], [2]. Here we examine how different selective regimes alter patterns of variation across the genome.
When considering genetic variation in fitness, selection must be a major determinant, but the relative importance of different types of selection is unknown. One possibility is that most genetic variation in fitness is due to the constant input of new deleterious mutations balanced by the removal of such variants by negative selection. While mutation-selection balance undoubtedly contributes to variation in fitness, several authors have argued that this model is unable to fully account for empirical observations [3], [4]. The alternative is that some form of balancing selection (e.g., negative frequency-dependent selection, antagonistic pleiotropy, environmental heterogeneity) actively maintains multiple alleles at selected sites.
In theoretical models, the form of selection is imposed by assumption, but, in nature, selection is a consequence of the environment. A common feature of natural environments is that they change over time and space. Here we use experimental evolution to ask how this key feature affects genome-wide patterns of selected and neutral variation. Below we review several simplistic predictions for selected sites but emphasize that these are based on alternative assumptions regarding how environmental heterogeneity changes selection and ignore the effects of linked loci.
Classic theory [5], [6] predicts that genetic variation at selected sites can be maintained by environmental heterogeneity if alternative alleles are favoured in different environments (environmentally antagonistic selection, EAS). However, the type of heterogeneity is important; the conditions for a protected polymorphism through balancing selection are more restrictive with temporal heterogeneity than spatial heterogeneity [6], [7]. From this, we expect to find the most genetic variation for fitness in spatially heterogeneous populations, somewhat less in temporally heterogeneous populations, and the least variation in populations evolving in a constant environment (i.e., VSpatial>VTemp>VConst).
Rather than being environmentally antagonistic, allelic variation could be conditionally neutral (CN) whereby the two alleles at a site are selectively neutral in Environment A but with differential fitness effects in Environment B. The disfavored allele will be purged in Environment B but the polymorphism could persist for much longer in Environment A. For populations that experience both environments, conditionally neutral alleles experience half as much directional selection. Therefore, the heterogeneous regimes are expected to harbor levels of genetic variation for this locus intermediate to that in populations maintained in A or B alone (i.e., VConst_A>VSpatial>VTemp>VConst_B). However, if we consider multiple loci and assume that some experience selection only in A and others only in B, then the average amount of variation across these loci might be lowest in the heterogeneous populations where variation should be purged eventually from both types of loci (i.e., VConst_A, VConst_B>VSpatial>VTemp).
Rather than favoring the phenotypes specialized for either A or B, a third possibility is that heterogeneous environments select for a distinct phenotype (e.g., a robust generalist rather than the maintenance of multiple specialists). In this case, populations from heterogeneous environments would not be genetically intermediate between populations from alternative constant environments; rather, they might be as genetically distinct from the homogenously selected populations as the homogenously selected populations are from one another. Under this scenario, there is no reason to expect levels of variation to differ among selective regimes.
While some sites experience different selection between different environments or between homogeneous versus heterogeneous regimes, most sites will be uniformly selected across environments or neutral in both environments. However, variation at such sites will be influenced by changes in selection at other sites in the genome due to genetic hitchhiking (linkage effect). The extent of this influence will depend on the number of differentially selected sites, the strength, form and timescale of selection on these other sites, as well as their distribution across the genome and the recombination rates between them [8]–[10].
A number of important experimental studies have examined the effect of environmental heterogeneity on genetic variance (quantitative genetic variation: [11]–[17]; allozyme heterozygosity: [18]–[20]). The findings from these studies are quite variable and often there are few significant differences between treatments. Logistical constraints have limited past studies to either quantitative genetic analysis of a small number of traits or diversity measures from a handful molecular markers. It is impossible to know whether unmeasured traits or loci were similarly affected. An additional challenge in interpreting past results is that it is often unclear whether the traits or loci being studied are under differential selection between environments or even closely related to fitness.
High-throughput re-sequencing offers new opportunities to re-examine this issue. This technique has been applied to study the genetic basis of selection response in experimental populations (e.g., [21]–[24]). We applied this technology to replicated experimental populations of Drosophila melanogaster that evolved under different selective regimes. Twenty replicate populations were established from a cross between an ancestral lab population adapted to a salt-enriched larval environment (“Ancestral Salt,” AS) and an ancestral lab population adapted to a cadmium-enriched larval environment (“Ancestral Cad,” AC). These 20 replicates (N = 448 per population) were divided equally among four treatments: two constant treatments in either salt - (Salt) or cadmium-enriched (Cad) larval environments, a temporally heterogeneous treatment (Temp) in which populations were switched between each generation to the alternate environment, and a spatially heterogeneous treatment (Spatial) in which the population was split between the two environments but surviving adults were mixed with equal number from the two environments before egg-laying for next generation. After ∼20 generations, Long et al [25] found striking differences across treatments in inbreeding depression, suggesting important shifts in patterns of variation within populations among treatments.
At generation 42, we performed pooled re-sequencing of each of the 20 populations, the two environmentally specialized ancestral populations (AS and AC), as well as the lab source population (“Grand Ancestor”, GA) from which AS and AC were derived (see Figure 1 for relationship among populations). We first examine genetic differentiation among treatments and identify candidate sites under differential selection. We then compare levels of within-population variation among treatments. There are major differences in levels of variation among treatments and these patterns change between neutral and putatively selected sites.
Fig. 1. Selection history of the experimental populations. 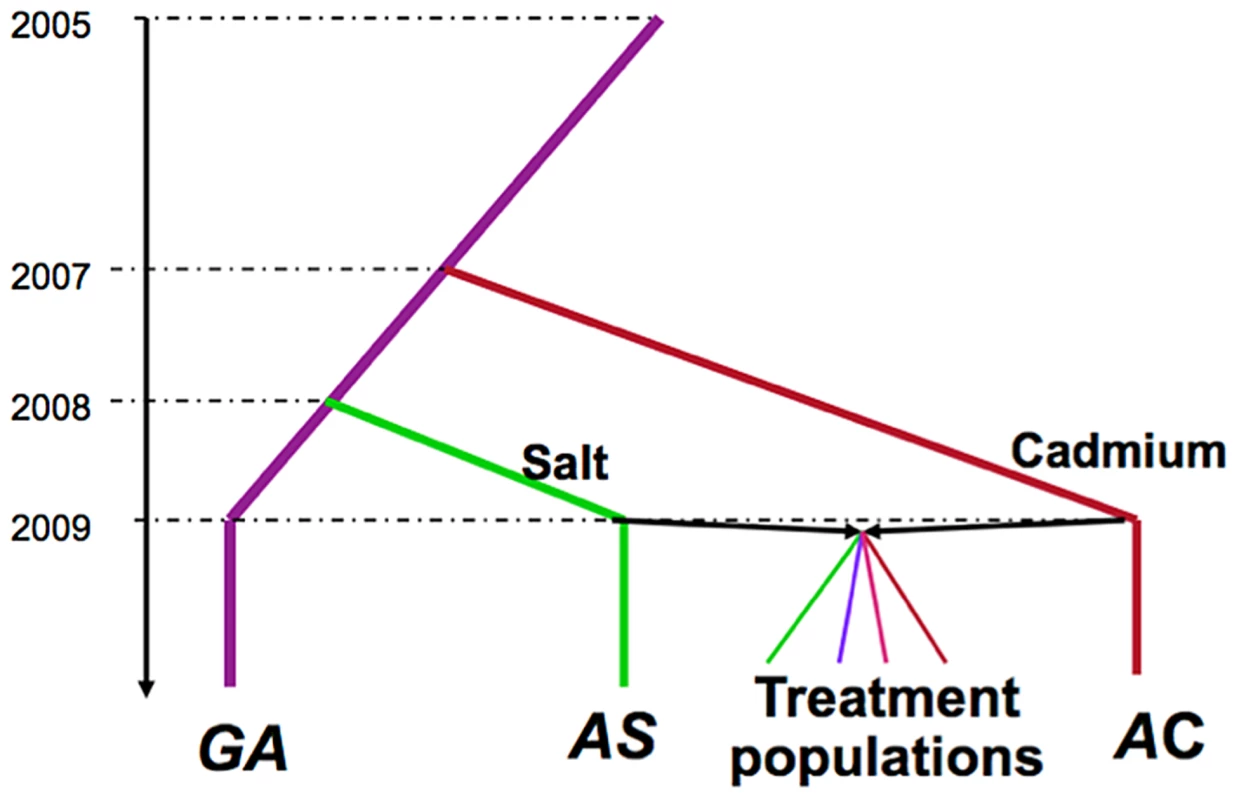
The Grand Ancestor population (GA) was maintained in benign laboratory conditions, and was used to initiate populations maintained on salt-enriched media (AS) or cadmium-enriched media (AC). The treatment populations were produced by crossing two ancestral population AS and AC. There are 5 replicate populations of each of the four treatments (not illustrated). Results
Differentiation for environment-specific survival
We tested the survival of flies from all replicate populations in both environments. Contrasting the two constant treatments, we found the expected patterns of local adaptation, i.e., Cad populations had higher survival than Salt populations when tested in cadmium but the reverse was true in salt (Figure 2). In the salt (cadmium) environment, the survival rates of flies from heterogeneous treatments were considerably higher than flies from Cad (Salt) treatment, indicating populations perform poorly when tested in an environment to which they lack selective exposure. These results are qualitatively similar to those reported by Long et al [25] from an earlier time point (∼generation 20).
Fig. 2. Mean survival in cadmium (A) or salt (B) environment. 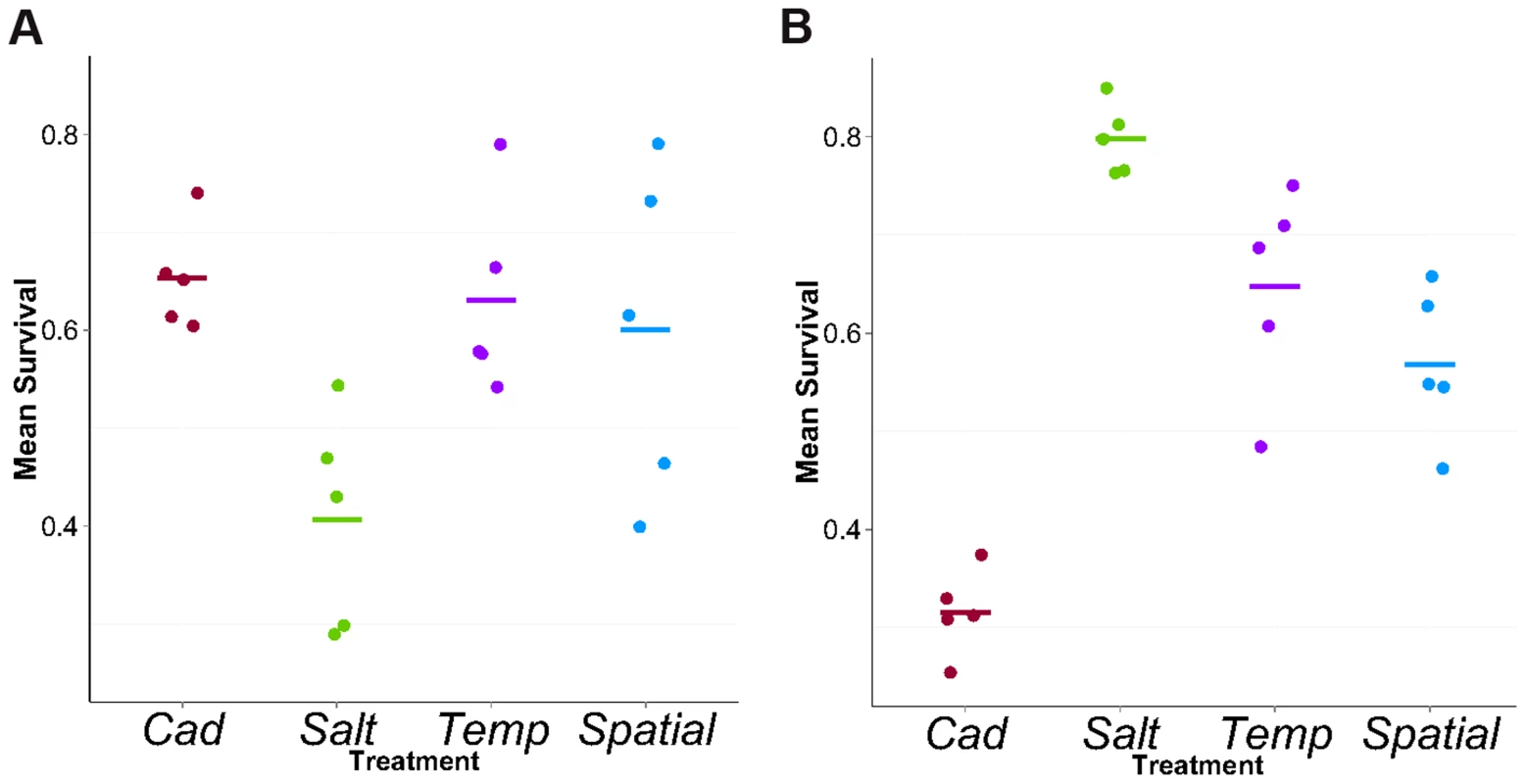
Each point represents the mean survival of ∼140 full-sib families for each population. Each bar represents the mean across five replicate populations for each treatment. Allele frequency differentiation between salt and cadmium environments
In addition to measuring the differentiation in survival, we measured differentiation in the frequencies of single nucleotide polymorphisms (SNPs) between the Salt treatment and Cad treatment. After read mapping and filtering for map and base quality, we had a mean coverage among populations of ∼17.5 fold for euchromatic regions.
To maximize our power in examining differentiation that occurs between populations evolving in the two extreme environments, we compared allele frequencies in the Ancestral Salt (AS) and the five Salt populations with allele frequencies in the Ancestral Cadmium (AC) and the five Cad populations. To identify SNPs, we screened for sites in euchromatic regions that are ≥5-fold coverage in all of these populations. We kept only those sites where the initial diversity (πini) was not too low, specifically πini = 2pini *(1−pini)>5%, where pini is the estimated frequency of the minor allele pooling across the Ancestral Salt (AS) and Ancestral Cad (AC) populations. After applying these screens, there were ∼2*106 sites for testing differentiation; we refer to these sites as the “α-sites”. To maximize our statistical power, we considered allele frequency estimates from the five replicate Salt populations and the Ancestral Salt (AS) population as replicates for a “salt” treatment and the estimates from the five replicate Cad populations and the Ancestral Cad (AC) as replicates for a “cadmium” treatment and then used a screen for allele frequency differentiation between treatments based on the Cochran-Mantel-Haenszel test (CMH) [26]. A total of 123,291 sites passed our screen for significant differentiation with false discovery rate = 0.001%; we refer to these as the “β-sites”. Because differentiation likely occurs from common variants, we expect the variation in the Grand Ancestor population to be greater at β-sites than other α-sites. Indeed, diversity is ∼20% greater (i.e., πGA, β-sites = 0.290; πGA, other α-sites = 0.246).
To evaluate the potential for false positives due to sampling error, allele frequency estimation error or genetic drift, we randomly assigned half of our cadmium populations as well as half of our salt populations to pseudo-treatment “A” and the remaining cadmium and salt populations to pseudo-treatment “B”. We then performed the same analysis looking for differentiation between “A” and “B”. We repeated this process for 15 randomly chosen combinations. Among these combinations, we found the mean number of sites that passed the same criteria above was 93, with the highest number 349. Thus, we expect the majority of the ∼123000 significant differentiated sites are not false positives in a statistical sense.
Nonetheless, it is highly improbable that ∼123000 β-sites are direct targets of differential selection. Much of the differentiation is likely the result of linkage effects. In the most extreme situation, there could be just one or two selected sites per chromosome and all the other sites could differentiate because of linkage. However, there are several observations that would not be expected if linkage effects were massive and there were only a few true targets of selection: (1.1) Rather than observing a single large block of differentiation, we observe numerous distinct peaks of differentiation across each chromosome (plotted as non-overlapping sliding windows Figure 3A–B, Supplementary Information S1A and Figure S1 for whole genome); (1.2) Among the most strongly significantly differentiated sites, there is an enrichment of (i) intragenic to intergenic sites, and (ii) coding to non-coding intragenic sites (Figure 3C–D). (There is no enrichment of nonsynonymous sites relative to 4-fold degenerate sites suggesting strong linkage effects at this small scale; more details in Supplementary Information S1B and Figure S2); (1.3) Differentiation is not random with respect to gene function, rather certain classes of genes (Gene Ontology) show enrichment, including those involved with protein metabolic process and peptide activity (Supplementary Information S1C and Table S1). Moreover, similar functional categories are identified when gene enrichment tests are performed separately for the two major autosomes (Table S1). In addition, some of our differentiated genes had been identified in previous functional studies of cadmium and salt resistance (e.g., MtnB, MTF-1 for cadmium resistance [27], [28] and CG6484, sug, Sik3, CrebA, Crtc for salt resistance [29], [30]).
Fig. 3. Differentiation between salt and cadmium populations. 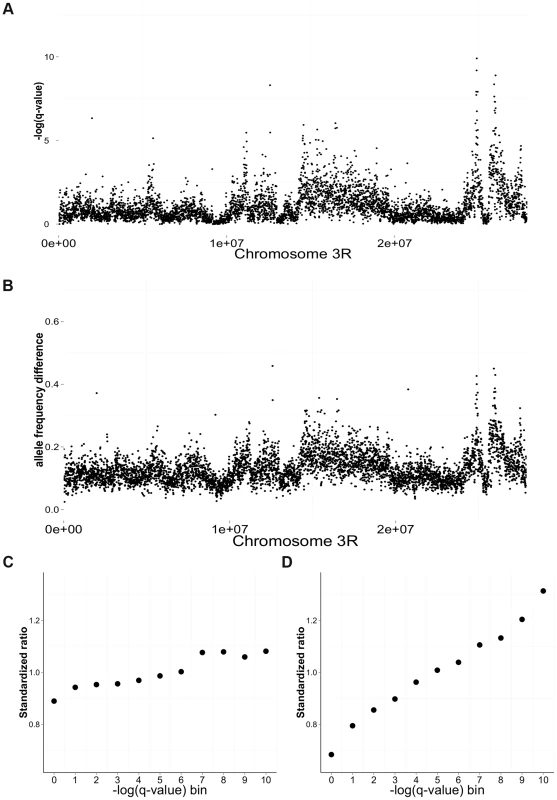
Sliding-window (5 kb non-overlapping) plots of differentiation along chromosome 3R: the average -log(q-value) from the CMH test (A) and the average difference in mean allele frequency (B) between the salt and cadmium populations. For each environment, the data are calculated based on the environment-specific ancestor and the five replicate populations. The ratio of the number of genic to intergenic sites (C) and the ratio of the number of coding sites to intronic sites (D) in different -log(q-value) bins using data from the whole genome. To compare the magnitude of the ratios for different functional catergories, the ratios are standardized around 1 by dividing the mean ratio among the 11 bins. Strong significance corresponds to larger values of -log(q-value). Other patterns suggest that linkage effects are contributing to differentiation but on a scale much smaller than the whole chromosomes: (2.1) The β-sites are strongly (and significantly) clustered relative to the distribution of α sites across genome. However, the level of clustering declines rapidly within 5000 bp, though a lower level of clustering extends much further (Supplementary Information S2A and Figure S3). The pattern of clustering remains similar after controlling for initial diversity in the GA population, which could affect the likelihood of differentiation and the power to detect it (not shown); (2.2) FST between salt and cadmium populations is very high near focal differentiated sites but declines rapidly within the first 500–1000 base pairs (Supplementary Information S2B and Figure S4); (2.3) The linkage disequilibrium (LD) within the paired-end reads (2*100 bp) declines relatively rapidly within the first 100 bp from 0.7 to about 0.4 (Supplementary Information S2C and Figure S5). These patterns remain similar when we only consider the regions that contain β-sites.
To assay the potential impact of inversions on genetic differentiation and as a source of linkage disequilibria, we used previously identified inversion-specific SNP markers [31] to estimate the inversion frequency in our populations (Supplementary Information S2D). The frequency of all potential inversions across the six populations in constant cadmium and six populations in constant salt environment are lower than 5%. Given the average allele frequency differentiation of β-sites is ∼0.4 (Tables S2, S3 and Supplementary Information S3), inversions are unlikely to play a major role. Further, for the two major autosomes, on average, the relative abundance of the β-sites and level of LD do not seem to be elevated in regions of commonly segregating inversions (Tables S4, S5 and Supplementary Information S5). As the linked selection is expected to be stronger in low recombination regions, we calculated the number and proportion of β-sites and α-sites in each of these regions (Table S6). Consistent with the prediction of greater effects of linked selection, there is a higher proportion of significant sites to α-sites in low recombination regions than high recombination regions (7.6% vs 5.4%, the difference is caused by autosome 2).
Recent papers [32]–[34] suggest that “evolve & re-sequence” studies have limited power to identify true targets of selection and our observation of clustering of β-sites and LD is consistent with those concerns. However, for our purposes, it is not essential to identify the true targets, rather our purpose is to demonstrate that the two alternative habitats cause genetic differentiation. Nonetheless, such differentiation is more meaningful if it is not due to selection on only one or two sites. Taken together, the results above suggest that the β-sites are enriched for true targets of selection but that many of the β-sites are differentiated because of linked selection. Linkage effects are likely strong but the patterns reported above (e.g., enrichment of significant sites in coding regions as well as gene ontology enrichment) do not seem consistent with selection on only a few sites. However, we emphasize that we cannot identify true targets of selection or reliably estimate whether the true number of targets is in the tens, hundreds, or more. We conclude only that the salt and cadmium environments cause populations to diverge genetically and that, though much of the differentiation may be due to linkage effects, there are likely multiple targets of selection.
Pairwise genetic differentiation between selective regimes
After establishing the high degree of differentiation across the genome between the two constant environments, we examined the level of differentiation between all other pairs of treatments. In the preceding section we compared the salt and cadmium environments and included the ancestral populations to maximize power for finding β-sites. In this section, we use only the five replicate populations within each experimental selection regime (the ancestral populations are not considered) so that we have similar power to examine differentiation between all pairs of treatments (e.g., Salt vs. Cad; Temp vs. Spatial). The number of significantly differentiated sites between each pair of treatments is shown in Table 1. The number of differentiated sites between the two homogenous treatments (Cad and Salt) is much greater than the numbers between any pair of homogeneous and heterogeneous treatments (e.g., Cad and Spatial). The two heterogeneous treatments (Spatial and Temp), where populations experience both selective agents, have many fewer differentiated sites than any other pair of treatments. The numbers of genes with significantly differentiated sites shows the same relationship among treatment pairs (Table S7).
Tab. 1. The number of highly differentiated sites between each pair of treatments. 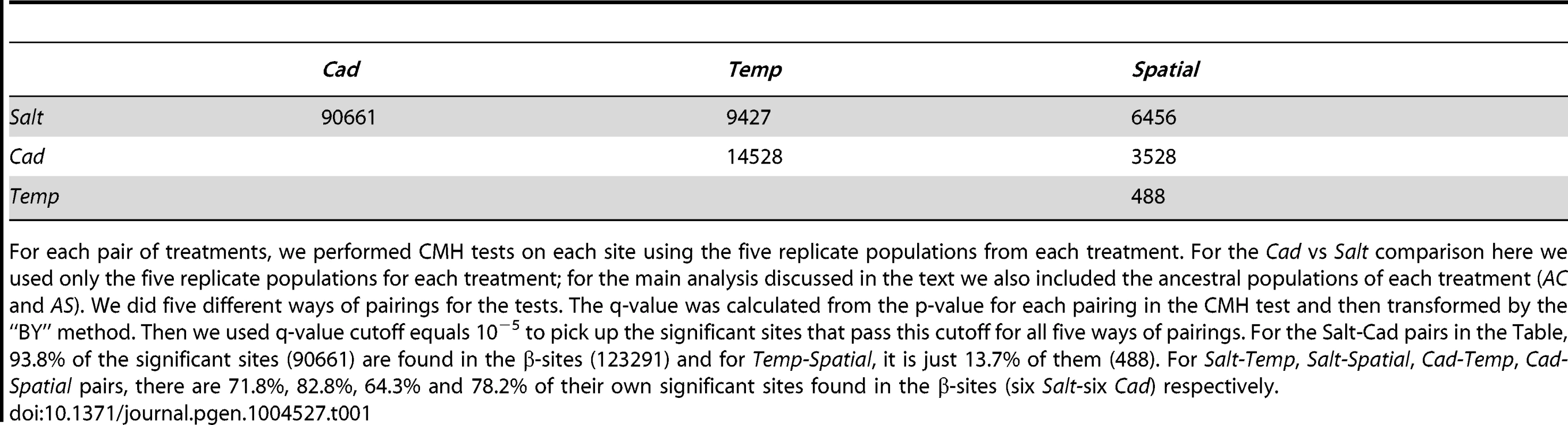
For each pair of treatments, we performed CMH tests on each site using the five replicate populations from each treatment. For the Cad vs Salt comparison here we used only the five replicate populations for each treatment; for the main analysis discussed in the text we also included the ancestral populations of each treatment (AC and AS). We did five different ways of pairings for the tests. The q-value was calculated from the p-value for each pairing in the CMH test and then transformed by the “BY” method. Then we used q-value cutoff equals 10−5 to pick up the significant sites that pass this cutoff for all five ways of pairings. For the Salt-Cad pairs in the Table, 93.8% of the significant sites (90661) are found in the β-sites (123291) and for Temp-Spatial, it is just 13.7% of them (488). For Salt-Temp, Salt-Spatial, Cad-Temp, Cad-Spatial pairs, there are 71.8%, 82.8%, 64.3% and 78.2% of their own significant sites found in the β-sites (six Salt-six Cad) respectively. As an alternative approach to comparing treatments, we measured the correlation of allele frequencies between different pairs of treatments using the sites that are likely under differential selection. To avoid bias in comparing treatments, we identified sites that were significantly differentiated in allele frequencies between AS and AC, the direct ancestors of all treatments (using Fisher's exact test with q-value<0.001). Because a correlation in allele frequency will arise simply from variance among sites in initial allele frequency, we calculated a ‘standardized’ correlation of allele frequency between each treatment pairs by using the correlation for differentially selected sites minus the correlation for non-differentiated control sites (details in Supplementary Information S3 and Table S8). We found the standardized correlation between the Salt and Cad treatments is significantly negative, and more negative than the correlation between any other pair of treatments. The negative correlation possibly suggests that the most strongly favored alleles in one environment are the most strongly disfavored in the other environment, indicating the operation of environmentally antagonistic selection (EAS). On the other hand, Temp and Spatial treatments have the most positive correlation, which is consistent with the differentially selected sites experiencing similar selection pressures between the two heterogeneous treatments (Supplementary Information S3 and Table S8).
Molecular diversity in alternative selective regimes
Our major goal is to compare the molecular variation among different treatments. First, we compared the genome-wide average diversity (π) among treatments. We used non-overlapping sliding windows to calculate Tajima's π over ∼95% of the euchromatic region of the genome for which we have sufficient coverage in all populations [35], [36]. The mean π across the genome for each population is shown in Figure 4. There is significant variation in π among treatments (F3,16 = 9.68, P = 0.0007); the genome-wide level of diversity is highest for the Spatial treatment but lowest for the Temp treatment. Though the census size of all populations is equal, given most of the sites across the genome are neutral, the observed differences in genome-wide average π suggest that treatments differ in effective population size. However, the use of genome-wide averages belies more interesting patterns that emerge at a finer scale.
Fig. 4. Average diversity (π) within experimental populations across the genome. 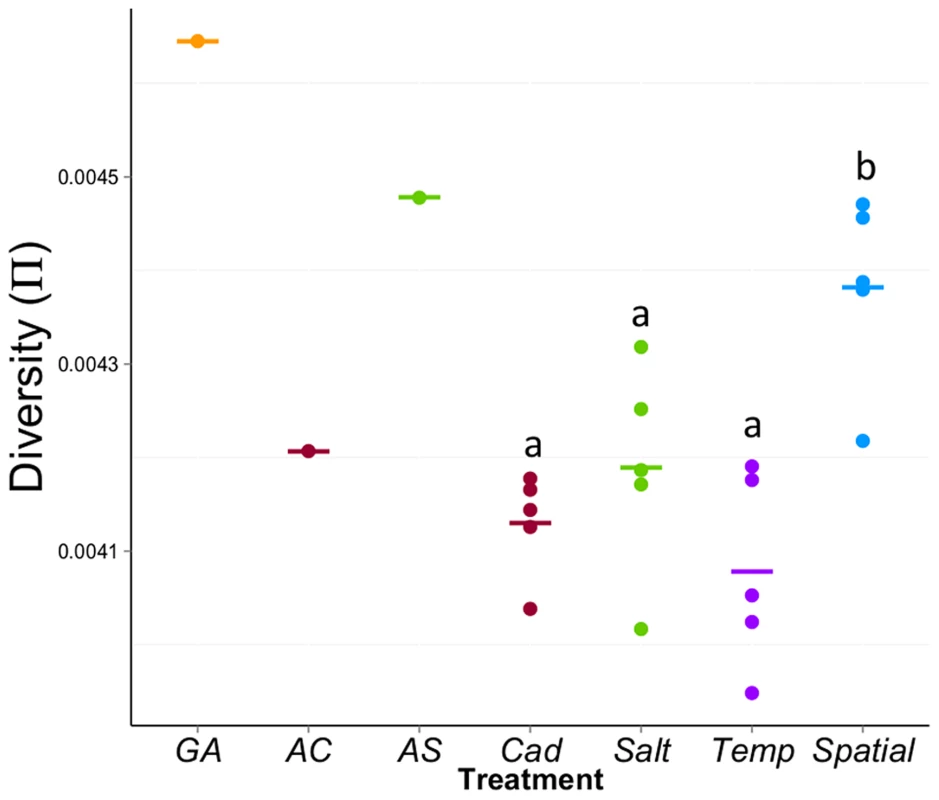
Each point represents the mean π across the common sliding windows (including non-variable sites) for each population. Though ancestral populations are shown for reference, we are primarily interested in the mean diversity (π) among the four treatments that were created by crossing AC and AS. There is significant variation among the four treatments (F3,16 = 9.68, P = 0.0007). The “b” group is significantly higher than the “a” group based on ANOVA Tukey HSD test. Spatial has significantly higher diversity than the Temp (Padj = 0.0006, Tukey HSD), the Salt (Padj = 0.026) and the Cad (Padj = 0.0036). The prediction of VSpatial>VTemp>VConst is for antagonistically selected sites, but many of the variable sites are likely neutral (or effectively neutral). To better test the prediction, we attempted to identify the sites that are likely under differential selection between the salt and cadmium environments. For this section, we want to identify potential targets of differential ecological selection using only the data from the two ancestral populations (AC and AS). This is necessary to avoid biasing tests comparing among selective treatments. To do so, we applied a different screen than used in the earlier sections. First, to increase the accuracy of the estimation of allele frequency, we screened sites that have ≥15-fold coverage in both ancestral populations and have πini>5% (estimated from the average allele frequency in the AC and AS). Also, we screened for the sites that have ≥10-fold coverage for all of the 20 treatment populations. In total, we had 769,924 SNPs for this analysis; we refer to these as the “χ-sites”.
We calculated the degree of differentiation in allele frequency between the two ancestral source populations AS and AC for all of the χ-sites, i.e., d = |pAS−pAC|. Sites under environmentally antagonistic selection will tend to have high d values. Because all treatments were created equally from AS and AC, there should be no initial differences in π among treatments for any given level of d. However, differences in π among treatments should develop due to treatment-specific selection, and the nature of these differences is expected to vary with d (i.e., selection is likely similar across treatments for low d sites whereas high d sites likely experience very different selection in different treatments).
To illustrate how the variance changes as we move from neutral sites to sites putatively under differential selection, we calculated π across the complete range of differentiation (d) values (Figure 5A). For all treatments, π is lower for weakly differentiated sites than strongly differentiated sites. This pattern is expected simply because the experimental populations were founded by crossing AC and AS; thus, sites that are strongly differentiated between AC and AS will start off as highly polymorphic within each experimental population. More interestingly, the rank order of the treatments with respect to diversity changes from weakly differentiated sites (πSpatial>πSalt≈πCad>πTemp for d<0.3) to highly differentiated ones (πSpatial>πTemp>πSalt≈πCad for d>0.7).
Fig. 5. Mean π within treatments as function of differentiation (d) between the ancestral source populations (AC and AS). 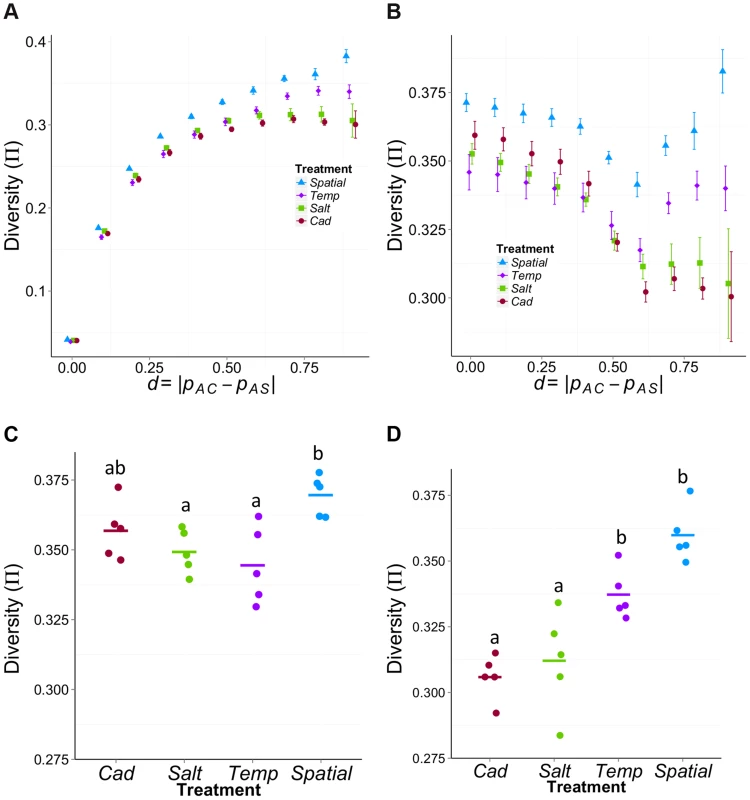
(A) and (B) The average π across different levels of ancestral differentiation for (A) all χ-site SNPs or (B) only those χ-site SNPs that have high initial diversity (πini>0.4). The x-axis is the allele frequency difference between ancestral populations, d = |pAC−pAS|. Error bars represent the standard error among the five replicates for each treatment. (C) and (D) Comparison among treatments in average π for sites that have high initial diversity (πini>0.4) using sites with (C) weak ancestral differentiation (d<0.3) or (D) strong ancestral differentiation (d>0.7). For the weakly differentiated sites (C), Spatial has significantly higher diversity than the Temp (Padj = 0.006, Tukey HSD) and the Salt (Padj = 0.028). For the highly differentiated sites (D), both Spatial and Temp treatments have significantly higher diversity than the two constant treatments (Padj<0.03 between any a & b pairs). To more clearly see the effect of selection, it is helpful to compare π between weakly and strongly differentiated sites that have similar levels of initial diversity. To do so, we re-examined the data using only those sites that have high initial diversity (πini>0.4, where πini = 2pini(1−pini) and pini = ½(pAS+pAC)). Using these sites, the relationship of π to d is very different for heterogeneous treatments than for homogeneous treatments (Figure 5B). In the constant selection environments π declines with d, as expected under directional selection. In contrast, π is relatively high at both low and high values of d for heterogeneous treatments.
When we compare levels of diversity among treatments using the χ-sites with high initial diversity (πini>0.4), we find different patterns for sites that are weakly or strongly differentiated between the ancestral founding populations. For the putatively ‘neutral’ sites (arbitrarily designated as d<0.3), we find significant variation in π among treatments (F3,16 = 5.8, P = 0.007; Figure 5C) with treatments ranked as πSpatial>πCad>πSalt>πTemp. There is also significant variation in π among treatments for the sites likely to be under differential selection (d>0.7, F3,16 = 19.5, P = 1.36E−5; Figure 5D) but the order is πSpatial>πTemp>πSalt>πCad. These differences in diversity are not driven by the potential effect of inversions (which seem to be very rare in our population, Table S2). After excluding the χ-sites located within inversion regions, the diversity patterns are qualitatively similar (Supplementary Information S4 and Figure S6)
Divergence among replicates within treatments
The reduced level of diversity at ‘neutral’ sites within the Temp treatment suggests that populations in this treatment have the lowest Ne. Drift should not only reduce diversity within populations but also cause divergence among populations. To test the latter, we examined FST among the five replicate populations within each treatment. For this analysis, we used ‘neutral’ sites passing a minimum coverage and initial diversity thresholds (i.e., the χ-sites above with πini>0.4 and d<0.3). As expected, FST was higher for X chromosome sites than autosomes in all four treatments, (X FST = 0.156±007; autosome FST = 0.139±008; t = −3.87, df = 3, P = 0.026). We then compared FST among treatments using five regions of the genome that are approximately recombinationally independent: the X chromosome, and the left and right ends of each of the two autosomes (Figure 6A). There was significant variation among treatments in FST values (F3,16 = 6.72, P = 0.004), with post-hoc tests showing this was primarily due to elevated FST in the Temp treatment, consistent with a lower Ne for this treatment. It is worth noting that the treatments do not differ in expected total heterozygosity (HT) for these sites (F3,16 = 1.02, P = 0.41, Figure 6C), which can be a misleading cause of differences in FST [37].
Fig. 6. FST values for each treatment in different regions. 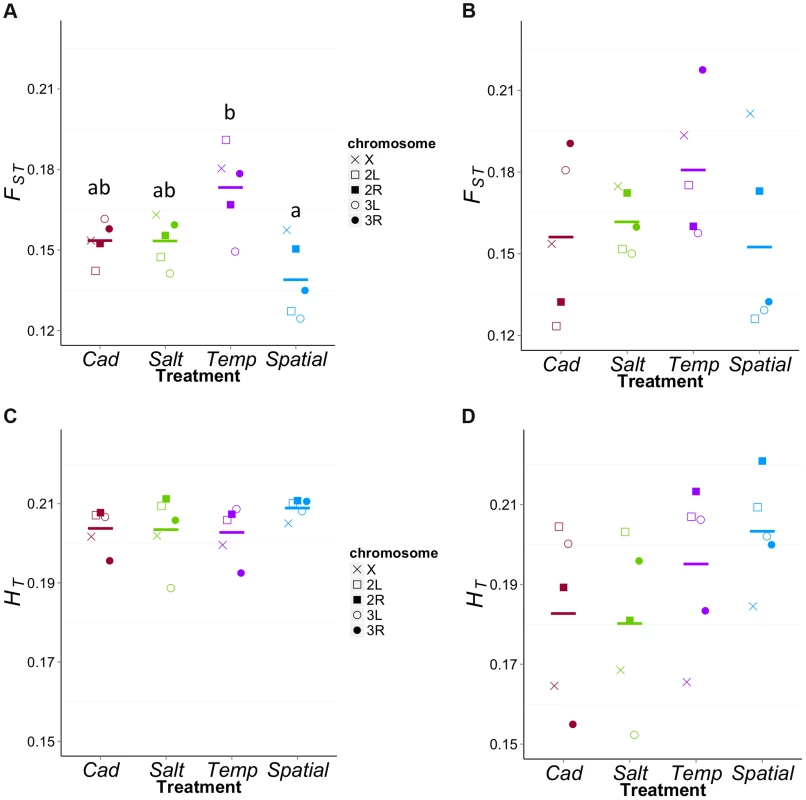
We screened for the χ-sites above with πini>0.4 and divided them into five recombinationally independent regions of the genome. For weakly differentiated sites (d<0.3, Figure 6A), There is significant variation among treatments in FST values with Temp being highest among them (F3,16 = 6.72, P = 0.0038). The Temp has significantly higher FST than Spatial (Padj = 0.002). The difference between Temp and the two constant treatments are marginally non-significant (Temp vs. Salt: Padj = 0.084; Temp vs. Cad: Padj = 0.087). For highly differentiated sites (d>0.7, Figure 6B), the FST values do not significantly differ among treatments (F3,16 = 1.16, P = 0.35). For both weakly and highly differentiated sites, the treatments do not differ in expected total heterozygosity (HT) (F3,16 = 1.02, P = 0.41, Figure 6C; F3,16 = 1.59, P = 0.23, Figure 6D). The equivalent comparison of within-treatment FST for sites likely to be under differential ecological selection (d>0.7) does not show significant differences among treatments (F3,16 = 1.16, P = 0.35, Figure 6B) but the patterns are qualitatively similar to the ‘neutral’ sites. HT does not vary significantly among treatments (F3,16 = 1.59, P = 0.23; Figure 6D), though point estimates of average HT are higher in the heterogeneous treatments than the homogeneous ones. It is worth noting that while both heterogeneous treatments have elevated HT values, the Temp treatment has the highest FST value whereas the Spatial has the lowest. These patterns suggest the differences in Ne between Spatial and Temp affect differentiation among replicates even at sites under selection (or those linked to them). Based on the low within-population diversity of the constant environments (Figure 5B), we might have expected elevated FST for these treatments. However this is not the case and may be attributable to the ‘selected’ sites experiencing stronger net selection in these treatments.
Discussion
In nature, many populations experience environments that are heterogeneous in space or time. Though our experimental populations differ from natural populations in a number of ways (population size, time scale of adaptation, etc.), our study provides insight into how alternative selective regimes affect genome-wide patterns of molecular diversity in an experimentally simplified setting. In our study, we used two environments that cause populations to diverge with respect to environment-specific survival (Figure 2) as well as allele frequency at numerous SNPs (Figure 3). Though we are unable to determine the number or identity of true genetic targets of differential ecological selection, our analyses suggest that there are likely to be more than one or two per chromosome.
For variants that are likely under differential selection between cadmium and salt environments (or statistically linked to such sites), the diversity patterns are consistent with the classic theoretical prediction: VSpatial>VTemp>VConst (Figure 5D). In contrast, the pattern of diversity of other sites is different: VSpatial>VConst>VTemp (Figures 4 and 5C). In other words, the rank order of treatments with respect to genetic diversity differs between ecologically selected sites and other sites. Our classification of ‘selected’ sites likely includes many neutral sites that are statistically linked to true targets of selection, due to physical linkage or, perhaps more importantly, through admixture or stochastic association from the founding of these populations [32]–[34]. However, for our purposes of studying diversity, such sites behave similarly to sites under differential ecological selection because of their statistical association with true targets.
The difference in the rank order of treatments between ‘selected’ and other sites may help to reconcile inconsistencies among classic studies [11]–[20] attempting to assess how alternative selective regimes affect genetic variance in phenotypes or genetic markers. In most of those studies, phenotypes or genetic markers had unknown relationships to experimentally imposed ecological differences in selection. Even in cases where measured phenotypes or markers are unlikely to have any true ecological effect, they might have been statistically linked to genes that did, making it difficult to interpret such results. By categorizing sites based on information from the ancestral populations, we found very different patterns of variation among our experimental treatments for different categories of sites within the context of a single study.
The difference in the rank order of treatments between ‘selected’ and other sites seems to occur primarily because of how selection affects diversity in homogeneous environments. Focusing on a set of sites that should have similar initial levels of diversity (Figures 5B–D), we found that diversity levels in the two heterogeneous treatments were similar between ‘selected’ and ‘neutral’ sites, but with diversity in Temp being consistently ∼6% lower than in the Spatial treatment. In contrast, diversity in homogeneous treatments is ∼14% lower than the Spatial treatment for ‘selected’ sites but only ∼4% lower for neutral sites.
In simplistic simulations with environmentally antagonistic alleles (Supplementary Information S5 and Figure S7), we were able to qualitatively reproduce these patterns by considering physical linkage to selected sites. In constant environments, neutral diversity was reduced in simulations where the neutral site was closer to selected targets compared to simulations where the recombination distance was greater. For simulated populations evolving in spatially heterogeneous environments, physical linkage to selected sites had only weak effect on neutral diversity, unless selection was strong. With temporal heterogeneity there was no noticeable effect of physical linkage on neutral diversity.
Spatial heterogeneity can result in balancing selection. Previous theoretical studies have shown that balancing selection is expected to increase neutral diversity, but only at sites closely linked to the targets of balancing selection [38]. In contrast, Barton [39] showed that temporally fluctuating selection is expected to reduce neutral variation across the genome (see also [40], [41]). When linkage is loose relative to selection, the drift experienced by neutral sites will be increased by an amount proportional to the enhanced amount of additive genetic variation maintained by fluctuating selection. For more closely linked sites, the loss of diversity can be much more extreme if allele frequencies at the selected loci are oscillating between extreme values. Fluctuating selection is only expected to increase diversity for sites with extremely tight linkage and only when time scales are very long [39]. The latter effect results from the build up of mutations during the longer coalescent times of sites linked to targets of balancing selection relative to those sites that are unlinked. However, if fluctuating selection is applied to standing variation for a short period, as in our simulations and experiment, there appears to be little relationship between physical linkage to selected sites and diversity.
The patterns in the simulations are qualitatively similar to those in our data if we assume our strongly differentiated sites (d>0.7; Figure 5D) are more tightly linked to true selected targets than are our ‘neutral’ sites (d<0.3). Another approach to examining the importance of linkage is to compare diversity in regions of high versus low recombination. If we assume that neutral sites will tend to be more tightly linked to a true target in the regions of low versus high recombination, then there should be differences in the diversity between low and high recombination regions but these differences should vary among treatments. Controlling for initial diversity of sites among regions, there is significantly less variation in low recombination regions than in high recombination regions in the Cad (Supplementary Information S6, Figures S8 and S9, Table S9), as expected. However, diversity does not differ between high and low recombination regions in the other treatments. This lack of a difference is reasonably consistent with the predictions of our simulations (Figure S7) and our analysis based on d (Figure 5B) for the two heterogeneous treatments but not for Salt. The contrast between high and low recombination regions used here is a crude comparison because (i) the distribution of true selected targets across these regions is unknown and (ii) even the “low” recombination regions likely include many neutral sites that have low degree of linkage from selected sites.
Differential selection between environments could take two qualitatively different, but not mutually exclusive, forms: environmentally antagonistic selection (EAS) and conditional neutrality (CN). This experiment was not designed to distinguish between them but we can consider the observed patterns in light of these alternatives. For sites strongly differentiated between the ancestral populations (AS and AC), the diversity pattern in the experimental populations is consistent with the prediction from the antagonistic selection model. After controlling for initial diversity, diversity is reduced for putatively selected sites compared to other sites in the constant selection treatments but not in the heterogeneous treatments (Figure 5B). Under CN we would expect the heterogeneous environments to show a decline in diversity for selected sites, albeit a less severe decline than in the constant environments. In contrast, the diversity increases for sites expected to be more strongly selected (i.e., π increases with d for d>0.6, Figure 5B), suggestive of EAS. An additional observation suggestive of antagonistic selection is the negative correlation in allele frequency between Salt and Cad treatments (Supplementary Information S3 and Table S8).
Despite these observations consistent with EAS, we suspect that some sites are conditionally neutral. A curious pattern is the clear dip in diversity seen in Figure 5B for both heterogeneous treatments for sites with intermediate levels of divergence between the two ancestral populations (0.4<d<0.6). One possible explanation emerges from considering the type of sites likely to be found at different levels of d (where d = |pAC−pAS|). Low values of d will be enriched for sites that are neutral or uniformly selected across environments whereas high values of d will be enriched for environmentally antagonistically selected sites. Compared to EAS sites, conditionally neutral sites will tend to show less differentiation between the two ancestral populations (i.e., intermediate values of d) because they are selected in only one ancestor and are neutral in the other. In the heterogeneous habitats, variation from such sites is expected to be eliminated because one allele is deleterious some of the time and never advantageous. This would explain the reduced diversity for sites with intermediate values of d compared to neutral sites (low d sites).
Conditionally neutral alleles should show evidence of being selected in one environment but not the other. We cannot rigorously test for this but, as a first approximation, we can consider the fraction of sites that are differentiated between environments but also show little change in allele frequency from the ancestral state in one of the two habitats. Of sites significantly differentiated between salt and cadmium (β-sites), ∼5% (6620/123291) could be classified as possibly neutral in cadmium based on showing low differentiation between the Ancestral Cadmium population and the Grand Ancestor (|pAC−pGA|<0.1) as well as low differentiation between the five replicate Cad populations and the initial allele frequency (|pCad−pini|<0.1, where pini = (pAC+pAS)/2). Similarly, ∼6% (7498/123291) could be classified as possibly neutral in salt. By this ad hoc categorization, only ∼11% of significantly differentiated sites show a pattern consistent with CN.
The discussion above has focused on scenarios (EAS and CN) where selection in heterogeneous environments is intermediate to that of the two homogeneous environments. However, environmental heterogeneity may select for properties not favoured in either constant environment. We see some evidence suggestive of unique sites being favoured by heterogeneity. Among the several thousands of significantly differentiated sites between any pair of heterogeneous and homogeneous treatments (Table 1), about 20% to 35% of such sites are different from the sites differentiated between Cad and Salt treatments. This means that the divergence of a heterogeneous treatment from a homogeneous treatment is not a simple subset of the divergence between alternative homogenous treatments, suggesting that some sites respond specifically to heterogeneity. Heterogeneity may favor a generalist or plastic genotype (rather than a mix of specialists) and this is thought to be more likely with temporal than spatial heterogeneity [42]. Relative to the Spatial treatment, the Temp treatment has more significantly differentiated sites from the constant treatments (23745 vs. 9920) and these sites are less likely to be differentiated between the two constant treatments (9147/23745 = 39% of sites differentiated between Temp and the constant treatments are not differentiated between the two constant treatments; 2278/9920 = 23% for Spatial; χ2 = 754.7179, df = 1, p-value<2.2e-16).
Understanding the mechanisms maintaining genetic variation is a classic pursuit of evolutionary biology. Environmental heterogeneity has long been postulated to sustain elevated levels of variation through antagonistic pleiotropy between environments, which can result in balancing selection especially if heterogeneity is spatial rather than temporal. However, rather than antagonism, different loci may be selected in each environment (i.e., conditional neutrality). Further, heterogeneity may select for alleles different from those favored in either single habitat (e.g., generalist genotype rather than a mixture of specialists). These three distinct, but not mutually exclusive, possibilities create uncertainty in how environmental heterogeneity will affect genetic variation. Although there is indirect evidence of each of these genetic possibilities in our data, the major patterns are most consistent with environmental antagonism. However, even consideration of EAS alone does not lead to a single prediction because the effect of environmental heterogeneity on neutral sites depends on their linkage to selected sites.
Although the patterns of diversity among treatments are most consistent with the prediction from EAS, it is likely that some populations are not at equilibrium and these patterns could change over time. For example, we expect CN sites specific to each environment would lose variation in heterogeneous treatments but the rate of loss would be slower than in the appropriate homogenous environment where they experience constant selection. Thus, the relative contributions of CN and EAS sites to diversity will change over time and at different rates across treatments. Given that the current patterns appear to be dominated by EAS and that the contribution of CN is expected to decline over time, it seems unlikely that the patterns would change dramatically through this effect. Nonetheless, it would be ideal to re-sequence these populations at several time points in the future. From the changes in allele frequencies during a time series, we could gain a better sense the effects of environmental heterogeneity on the genetic variation and how these change over time.
This experiment serves as a case study of the effects of environmental heterogeneity on genome-wide variation in a simplified setting, demonstrating distinct differences between environmental heterogeneity in space versus time and between sites likely to be closely linked or not to sites under differential ecological selection (Figure 5). Yet this experiment is only a single test of environmental heterogeneity at one time point; different environments, different spatial or temporal scales of heterogeneity, or different organisms could yield different patterns because the results will depend on the genetic basis of adaptation and the nature of selection [43]. A recent study in yeast suggests that antagonistic effects may be common [44]. But other field studies in plants found that patterns of conditional neutrality are more common [45], [46]. A recent study in Brassicaceae quantified the proportion of conditional neutral and EAS QTLs across genome and found that conditional neutrality is more common than EAS (8% vs. 3% of the genome, [47]). The ultimate challenge remains to determine how environmental variation affects patterns of diversity and quantitative genetic variation in nature in different organisms (e.g., [48]). Because the constraints of real systems make it difficult to cleanly test these effects in nature, experimental evolution provides a helpful step towards testing the key principles [49].
Methods
History of selection populations
The selective histories of all populations we used are illustrated in Figure 1. A population of Drosophila melanogaster was collected in the Similkameen Valley, British Columbia in 2005 and maintained in regular benign conditions at large size (∼2000–4000 adults, by S. Yeaman); we refer to this population as the “Grand Ancestor” (GA). In July 2007, a subset of flies from the GA population was used to initiate 12 replicate populations (each has ∼1000 adults) maintained in a cadmium-enriched environment (by C.C Spencer); In April 2008, the 12 replicate populations were pooled to generate a single populations maintained in cadmium environment (with a population size of ∼2000 adults), we refer to this as the “Ancestral Cadmium” (AC) population. In August 2008, a subset of flies from the GA population was used to initiate a population (with a population size of ∼2000 adults) maintained in a salt-enriched environment (by A. Wang); we call this the “Ancestral Salt” (AS) population. During the adaptive history, the amount of cadmium and salt in the environments were progressively increased, reaching 75 µg/ml and 29 mg/ml, respectively at the time to start the experimental evolution project. In October 2009, we collected 448 males and 448 virgin females from both the AC and AS populations and crossed flies from different populations via mass mating. Flies were collected from the next generation and randomly divided them into four selection regimes (by T.A.F. Long): constant salt-enriched environment (Salt), constant cadmium enriched-environment (Cad), alternatively in a salt environment or cadmium environment generation by generation (Temp), and spatially in either salt or cadmium environment for each generation (Spatial). For the Spatial treatment, we ensured that the same number of adult flies produced by the two environments contributed to the next generation (i.e., this can be considered a “soft” selection regime sensu [50]. Each selection regime had five replicate populations with a population size of 448. Further details of the maintenance of these populations are described in Long et al [25].
Phenotypic measurements
To confirm that the populations under constant selection adapted to their own environment and to test the fitness of populations under fluctuating selection, we measured the egg to adult viability of flies from these populations in both salt and cadmium testing environments (75 µg/ml cadmium or 22 mg/ml salt). Before the assay, flies were reared in regular benign condition for one generation to control the maternal environment. We mated each virgin female with one male for each population and then let mated females lay eggs in vials with salt food or cadmium food for about 18 hours and 11.5 to 12 days later scored the number of adult flies from each vial. There were ∼140 vials per population per environment for measuring survival.
Population resequencing
At generation 42 we sampled 70 adult female flies from each of the 23 populations (20 treatment populations plus the three source populations, AS, AC, and GA). For each population, we pooled the females to extract genomic DNA for next-gen sequencing. We obtained 100 bp paired-end short reads from Illumina HiSeq 2000. Paired-end reads were aligned to the D. melanogaster r. 5.42 genome sequences using bwa v. 0.5.9 [51] with default settings and the “-I” option. The alignments were filtered using a mapping quality and base quality (PHRED quality score) of 20 as cutoff by samtools v. 0.1.16 [52] and popoolation2 [53]. After the filtering, the range of coverage among 23 populations for euchromatic regions is 12.2∼26.7.
Allele frequency differentiation between treatments
To look for differentiation between salt and cadmium environments, we first screened for sites in euchromatic regions with at least 5-fold coverage in both ancestral populations (AS and AC) as well as in the five Salt and five Cad populations. We kept only those sites where the initial diversity was not too low, specifically πini = 2pini *(1-pini)>5%, where pini is the frequency of the minor allele pooling across the two ancestral populations (AS and AC). After applying these screens, there were ∼2*106 sites for testing differentiation; we refer to these sites as the “α-sites”. We considered allele frequency data from the five replicate Salt populations and the Ancestral Salt population as six replicates for a “salt” treatment and the data from the five replicate Cad populations and the Ancestral Cadmium population as six replicates for a “cadmium” treatment. The significant differentiated sites between “cadmium” treatment and “salt” treatment were identified by Cochran-Mantel-Haenszel (CMH) test [26] in R (version 2.15.1 R-Development-Core-Team 2012). The CMH test is a paired test and it was sensible to pair AC with AS but the pairings of the replicate Cad and Salt populations are arbitrary. Consequently, we repeated the analysis for each SNP five times, each time pairing AC with AS but using different (and unique) pairings for the replicate Cad and Salt populations (1st pairing: Cad1 vs Salt1, Cad2 vs Salt2, Cad3 vs Salt3, Cad4 vs Salt4, Cad5 vs Salt5; 2nd pairing: Cad1 vs Salt2, Cad2 vs Salt3, Cad3 vs Salt4, Cad4 vs Salt5, Cad5 vs Salt1; …). For the pairwise genetic differentiation between treatment pairs, we did similar tests for different and unique five pairings between five replicate populations from one treatment and those from the another treatment, without the AC and the AS.
The q-value for each site was calculated from the geometric mean p-value of the five different tests and converted to q-value via the “BY” method in the p.adjust package in R [54]. To show the differentiation across genome, we used a 5 kb sliding-window with a step size of 5 kb to calculate the average -log(q-value) and allele frequency differentiation (based on the environment-specific ancestor and the five replicate populations) for sites within windows (Figure 3A, 3B, Supplementary Information S1A and Figure S1). All the figures except Figure 1 were generated using the R library gglot2 [55]. A SNP was only considered differentiated (i.e., a β-site) if it passed a significance cutoff in all five tests (false discovery rate = 0.001% for q-value from each test). To evaluate the potential for false positives including sampling error, allele frequency estimation error or genetic drift, we randomly assigned half of our cadmium populations as well as half of our salt populations to pseudo-treatment “A” and the remaining cadmium and salt populations to pseudo-treatment “B”. We then performed the same analysis looking for differentiation between “A” and “B”. We repeated this process for 15 randomly chosen combinations.
We examined the enrichment of genic SNPs relative to intergenic SNPs for different -log (q-value) bins [56], [57]. Using all the “α-sites”, we calculated the ratio of number of sites located in genic and intergenic region for each -log(q-value) bin. In order to compare the enrichment across different functional categories, we standardized the ratios relative to the mean ratio across the 11 bins. We performed similar enrichment analyses for other functional categories: coding sequences/intron sites, 0-fold sites/4-fold sites in coding region.
Genes overlapping with at least one significant SNP (β-sites) and their Gene Ontology annotations were identified using the FlyBase annotation (release 5.43) [58]. Gene Ontology enrichment tests were performed using Gowinda [59] with β-sites as significant sites and α-sites as background sites and these parameters: simulations: 100000; min-significance: 1; gene-definition: gene; threads: 8. We repeated the enrichment tests for each of the two major autosomes chromosomes separately. The levels of linkage disequilibrium (LD) within two pair-ends (∼250 bp) were estimated by the program LDx [60].
The frequency of each known inversion was estimated from the average frequency of all inversion-specific SNP markers [31], weighted by the coverage on each marker. The high and low recombination rate regions were divided based on the estimations in [61]. The high regions were defined as having a recombination rate greater than 2 cM/Mb.
Molecular diversity
Genome-wide diversity (measured by Tajima's π) was calculated via the program Popoolation [35], [36]. After trimming and mapping the reads (quality threshold 20, min-length 60), we used 10 kb non-overlapping sliding windows across genome for each population. To be included in the analysis, we required that at least 60% of sites in a window have at least 4-fold coverage (parameter: window-size 10000 –step-size 10000 –min-count 2 –min-coverage 4 –max-coverage 400 –min-qual 20 –pool-size 140). There were 11265 common windows that passed the cutoff for all 23 populations, covering about 95% of the euchromatic region of the genome.
To calculate allele frequency differentiation and diversity, we screened sites that had at least 15-fold coverage in both ancestral populations (AC and AS) and had πini>5% (estimated from the allele frequency in the AC and AS), based on the synchronized file generated by Popoolation2 [53]. Also, we screened for the sites that had at least 10-fold coverage in all 20 treatment populations. Further, we calculated the local median coverage among the two ancestral population and 20 treatment populations for all sites in euchromatic region and then the global median among these local medians. We excluded the sites whose local median coverage is higher than two fold of the global median coverage. In total, we had 769,924 SNPs (χ-sites) for the diversity analysis. The differentiation between AC and AS population (d) for each χ-site was calculated via the allele frequencies in AC and AS populations: |pAC−pAS|. The π for each χ-site in was calculated as: π = 1−((A*A)+(G*G)+(C*C)+(T*T)+(D*D)), where A, G, C, T, D are the frequencies of different bases at that site, where D represents a deletion. To control for initial diversity, we screened for high initial diversity (πini>0.4) from the χ-sites and had 86,629 sites from which to recalculate the π in each d category for each treatment.
FST for replicates within each treatment
To assess the divergence among replicates within each treatment, we measured FST among the five replicate populations within each treatment. From the χ-sites, we screened for those with high initial diversity (πini>0.4). The average expected heterozygosity (HS) and total heterozygosity (HT) for each site within each treatment was calculated as follows:
Following Nei 1977 from [62], the mean FST over all sites as First, we calculated the E[FST] for sites located in the two major autosomes and X chromosome for each of the four treatments using putatively neutral sites (i.e., only sites that were weakly differentiated between the ancestral populations (d = |pAC−pAS|<0.3)). We performed a paired t-test to test the FST difference between autosomes and X, pairing the data from the same treatment.To compare the FST among treatments statistically, we selected five regions of the genome that approximately recombinationally independent. The sequence location for these regions are: 2L from 1 to 7,307,159, 2R from 10,368,692 to the end, 3L from 1 to 7,753,553, 3R from 17,055,561 to the end and X chromosome. The two regions on the second (third) chromosome are separated from each other by 50.1 (47.7) cM. Because differences in coverage among treatments could result in artificial differences in FST [63], we used an equivalent level of coverage to measure FST for each treatment. To do so, we performed the following procedure for each site. We first ranked by coverage the five replicate populations within treatments. For each rank i (i∈[1], [5]), we found the minimum coverage across treatments, ni. We sampled without replacement ni alleles from the ith ranked population for each treatment. Based on these resampled allele frequencies, we calculated the average FST based on the equation above for each treatment. We repeated the whole resampling and calculation 10 times and used the mean FST among the 10 results for statistical analysis. We used ANOVA and TukeyHSD test to compare the difference in FST among treatments.
Supporting Information
Zdroje
1. Mitchell-OldsT, WillisJH, GoldsteinDB (2007) Which evolutionary processes influence natural genetic variation for phenotypic traits? Nat Rev Genet 8 : 845–856.
2. LefflerEM, BullaugheyK, MatuteDR, MeyerWK, SégurelL, et al. (2012) Revisiting an old riddle: what determines genetic diversity levels within species? PLoS Biol 10: e1001388 doi:10.1371/journal.pbio.1001388
3. Charlesworth B, Hughes KA (2000) The maintenance of genetic variation in life history traits. Pp. 369–391 in R. S. Singh and C. B. Krimbas, eds. Evolutionary Genetics from Molecules to Morphology. Cambridge University Press, Cambridge, UK.
4. JohnsonT, BartonN (2005) Theoretical models of selection and mutation on quantitative traits. Philosophical Transactions of the Royal Society B: Biological Sciences 360 : 1411–1425.
5. LeveneH (1953) Genetic equilibrium when more than one niche is available. Am Nat 87 : 331–333.
6. FelsensteinJ (1976) The theoretical population genetics of variable selection and migration. Annu Rev Genet 10 : 253–280.
7. DempsterER (1955) Maintenance of genetic heterogeneity. Cold Spring Harbor Symp. Quant Biol 20 : 25–32.
8. HillWG, RobertsonA (1966) The effect of linkage on limits to artificial selection. Genetical Research 8 : 269–294.
9. CharlesworthD, CharlesworthB, MorganMT (1995) The pattern of neutral molecular variation under the background selection model. Genetics 141 : 1619–1632.
10. CharlesworthD (2006) Balancing selection and its effects on sequences in nearby genome regions. PLoS Genet 2: e64 doi:10.1371/journal.pgen.0020064
11. BeardmoreJA (1961) Diurnal temperature fluctuation and genetic variance in Drosophila populations. Nature 189 : 162–163.
12. LongT (1970) Genetic effects of fluctuating temperature in populations of Drosophila melanogaster. Genetics 66 : 401–416.
13. MackayTFC (1981) Genetic variation in varying environments. Genetical Research 37 : 79–93.
14. RiddleRA, DawsonPS, ZirkleDF (1986) An experimental test of the relationship between genetic variation and environmental variation in tribolium flour beetles. Genetics 113 : 391–404.
15. YeamanS, ChenY, WhitlockMC (2010) No effect of environmental heterogeneity on the maintenance of genetic variation in wing shape in drosophila melanogaster. Evolution 64 : 3398–3408.
16. VenailPA, KaltzO, Olivierii, PommierT, MouquetN (2011) Diversification in temporally heterogeneous environments: effect of the grain in experimental bacterial populations. Journal of Evolutionary Biology 24 : 2485–2495.
17. HallssonLR, BjörklundM (2012) Selection in a fluctuating environment leads to decreased genetic variation and facilitates the evolution of phenotypic plasticity. Journal of Evolutionary Biology 25 : 1275–1290.
18. PowellJR (1971) Genetic polymorphisms in varied environments. Science 174 : 1035–1036.
19. McDonaldJF, AyalaFJ (1974) Genetic response to environmental heterogeneity. Nature 250 : 572–574.
20. HaleyCS, BirleyAJ (1983) The genetical response to natural selection by varied environments. II. Observations on replicate populations in spatially varied laboratory environments. Heredity 51 : 581–606.
21. BurkeMK, DunhamJP, ShahrestaniP, ThorntonKR, RoseMR, et al. (2010) Genome-wide analysis of a long-term evolution experiment with Drosophila. Nature 467 : 587–590.
22. TurnerTL, StewartAD, FieldsAT, RiceWR, TaroneAM (2011) Population-Based Resequencing of Experimentally Evolved Populations Reveals the Genetic Basis of Body Size Variation in Drosophila melanogaster. PLoS Genet 7: e1001336 doi:10.1371/journal.pgen.1001336
23. Orozco-terWengelP, KapunM, NolteV, KoflerR, FlattT, et al. (2012) Adaptation of Drosophila to a novel laboratory environment reveals temporally heterogeneous trajectories of selected alleles. Molecular Ecology 21 : 4931–4941.
24. RemolinaSC, ChangPL, LeipsJ, NuzhdinSV, HughesKA (2012) Genomic basis of aging and life-history evolution in Drosophila melanogaster. Evolution 66 : 3390–3403.
25. LongTAF, RoweL, AgrawalAF (2013) The Effects of Selective History and Environmental Heterogeneity on Inbreeding Depression in Experimental Populations of Drosophila melanogaster. The American Naturalist 181 : 532–544.
26. McDonald JH (2009) Handbook of Biological Statistics (2nd ed.). Sparky House Publishing, Baltimore, Maryland 88–94.
27. EgliD, DomènechJ, SelvarajA, BalamuruganK, HuaH, et al. (2006) The four members of the Drosophila metallothionein family exhibit distinct yet overlapping roles in heavy metal homeostasis and detoxification. Genes to Cells 11 : 647–658.
28. YepiskoposyanH, EgliD, FergestadT, SelvarajA, TreiberC, et al. (2006) Transcriptome response to heavy metal stress in Drosophila reveals a new zinc transporter that confers resistance to zinc. Nucleic Acids Research 34 : 4866–4877.
29. StergiopoulosK, CabreroP, DaviesSA, DowJAT (2009) Salty dog, an SLC5 symporter, modulates Drosophila response to salt stress. Physiological Genomics 37 : 1–11.
30. ChoiS, KimW, ChungJ (2011) Drosophila Salt-inducible Kinase and Element-binding Protein. J Biol Chem 286 : 2658–2664.
31. KapunM, SchalkwykHV, McallisterB, FlattT, SchlottererC (2014) Inference of chromosomal inversion dynamics from Pool-Seq data in natural and laboratory populations of Drosophila melanogaster. Molecular Ecology 23 : 1813–1827.
32. Baldwin-BrownJG, LongAD, ThorntonKR (2014) The Power to Detect Quantitative Trait Loci Using Resequenced, Experimentally Evolved Populations of Diploid, Sexual Organisms. Mol Biol Evol 31 : 1040–1055.
33. ToblerR, et al. (2014) Massive habitat-specific genomic response in D. melanogaster populations during experimental evolution in hot and cold environments. Mol Biol Evol 31 : 364–375.
34. KoflerR, SchlöttererC (2013) A guide for the design of evolve and resequencing studies. Mol Biol Evol 31 : 474–83 doi: 10.1093/molbev/mst221
35. KoflerR, Orozco-terWENGELP, De MaioN, PandeyRV, NolteV, et al. (2011) PoPoolation: A toolbox for population genetic analysis of next generation sequencing data from pooled individuals. PLoS ONE 6: e15925 doi:10.1371/journal.pone.0015925
36. FutschikA, SchlöttererC (2010) The next generation of molecular markers from massively parallel sequencing of pooled DNA samples. Genetics 186 : 207–218.
37. JakobssonM, EdgeMD, RosenbergNA (2013) The relationship between FST and the frequency of the most frequent allele. Genetics 193 : 515–528.
38. NordborgM (1997) Structured coalescent processes on different time scales. Genetics 146 : 1501–1514.
39. BartonNH (2000) Genetic hitchhiking. Philos Trans R Soc Lond B 355 : 1553–1562.
40. GillespieJH (1997) Junk ain't what junk does: neutral alleles in a selected context. Gene 205 : 291–299.
41. TaylorJE (2013) The effect of fluctuating selection on the genealogy at a linked site. Theor Pop Bio 87 : 34–50.
42. KassenR (2002) The experimental evolution of specialists, generalists, and the maintenance of diversity. Journal of Evolutionary Biology 15 : 173–190.
43. TurelliM, BartonNH (2004) Polygenic variation maintained by balancing selection: pleiotropy, sex-dependent allelic effects and G×Polygenic varia. Genetics 166 : 1053–1079.
44. QianW, DiMa, XiaoC, WangZ, ZhangJ (2012) The genomic landscape and evolutionary resolution of antagonistic pleiotropy in Yeast. Cell Reports 2 : 1399–1410.
45. AndersonJT, WillisJH, Mitchell-OldsT (2011) Evolutionary genetics of plant adaptation. Trends in Genetics 27 : 258–266.
46. LeinonenPH, RemingtonDL, LeppäläJ, SavolainenO (2012) Genetic basis of local adaptation and flowering time variation in Arabidopsis lyrata. Molecular Ecology 22 : 709–723.
47. AndersonJT, LeeC-R, RushworthCA, ColauttiRI, Mitchell-OldsT (2012) Genetic trade-offs and conditional neutrality contribute to local adaptation. Molecular Ecology 22 : 699–708.
48. SchmidtPS, CondeDR (2006) Environmental heterogeneity and the maintenance of genetic variation for reproductive diapause in Drosophila melanogaster. Evolution 60 : 1602–1611.
49. KaweckiTJ, LenskiRE, EbertD, HollisB, OlivieriI, et al. (2012) Experimental evolution. Trends in Ecology & Evolution 27 : 547–560.
50. WallaceB (1975) Hard and soft selection revisited. Evolution 29 : 465–473.
51. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
52. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
53. KoflerR, PandeyRV, SchlöttererC (2011) PoPoolation2: identifying differentiation between populations using sequencing of pooled DNA samples (Pool-Seq). Bioinformatics 27 : 3435–3436.
54. BenjaminiY, YekutieliD (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29 : 1165–1188.
55. Wickham H (2009) ggplot2: elegant graphics for data analysis. New York: Springer
56. CoopG, PickrellJK, NovembreJ, KudaravalliS, LiJ, et al. (2009) The role of geography in human adaptation. PLoS Genet 5: e1000500 doi:10.1371/journal.pgen.1000500
57. FumagalliM, SironiM, PozzoliU, Ferrer-AdmettlaA, PattiniL, et al. (2011) Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet 7: e1002355 doi:10.1371/journal.pgen.1002355.t003
58. McQuiltonP, St PierreSE, ThurmondJ (2011) the FlyBase Consortium (2011) FlyBase 101 - the basics of navigating FlyBase. Nucleic Acids Research 40: D706–D714.
59. KoflerR, SchlöttererC (2012) Gowinda: unbiased analysis of gene set enrichment for genome-wide association studies. Bioinformatics 28 : 2084–2085.
60. FederAF, PetrovDA, BerglandAO (2012) LDx: Estimation of linkage disequilibrium from high-throughput pooled resequencing data. PLoS ONE 7: e48588 doi:10.1371/journal.pone.0048588.t002
61. Fiston-LavierA-S, SinghND, LipatovM, PetrovDA (2010) Drosophila melanogaster recombination rate calculator. Gene 463 : 18–20.
62. Hedrick PW (2009) Genetics of populations. Pp.491. Jones & Bartlett Learning Press.
63. Kalinowski (2005) Do polymorphic loci require large sample sizes to estimate genetic distances? Heredity 94 : 33–36.
64. JoeH (2006) Generating Random Correlation Matrices Based on Partial Correlations. Journal of Multivariate Analysis 97 : 2177–2189.
65. Christiansen FB (2000) Population genetics of multiple loci. Pp. 32–40. Wiley series in mathematical and computational biology. John Wiley & Sons Inc. Hoboken, NJ.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 8
-
Všechny články tohoto čísla
- DNA Double Strand Break Repair in Mitosis Is Suppressed by Phosphorylation of XRCC4
- Inference of Transposable Element Ancestry
- The Population Genetics of Evolutionary Rescue
- Retinoic Acid Activates Two Pathways Required for Meiosis in Mice
- Pooled Segregant Sequencing Reveals Genetic Determinants of Yeast Pseudohyphal Growth
- Comprehensive Identification of Single Nucleotide Polymorphisms Associated with Beta-lactam Resistance within Pneumococcal Mosaic Genes
- SMA-Causing Missense Mutations in Display a Wide Range of Phenotypes When Modeled in
- Branch Migration Prevents DNA Loss during Double-Strand Break Repair
- Transcriptome Sequencing from Diverse Human Populations Reveals Differentiated Regulatory Architecture
- Genetic Deletion of SEPT7 Reveals a Cell Type-Specific Role of Septins in Microtubule Destabilization for the Completion of Cytokinesis
- Tethering Sister Centromeres to Each Other Suggests the Spindle Checkpoint Detects Stretch within the Kinetochore
- Global Genetic Variations Predict Brain Response to Faces
- Demography and the Age of Rare Variants
- The Response to High CO Levels Requires the Neuropeptide Secretion Component HID-1 to Promote Pumping Inhibition
- Sp6 and Sp8 Transcription Factors Control AER Formation and Dorsal-Ventral Patterning in Limb Development
- The Groucho Co-repressor Is Primarily Recruited to Local Target Sites in Active Chromatin to Attenuate Transcription
- A Transposable Element Insertion Confers Xenobiotic Resistance in Drosophila
- The Genomic Architecture of Population Divergence between Subspecies of the European Rabbit
- Human Social Genomics
- Gene Expansion Shapes Genome Architecture in the Human Pathogen : An Evolutionary Genomics Analysis in the Ancient Terrestrial Mucorales (Mucoromycotina)
- Canonical Non-Homologous End Joining in Mitosis Induces Genome Instability and Is Suppressed by M-phase-Specific Phosphorylation of XRCC4
- Roles of Type 1A Topoisomerases in Genome Maintenance in
- The TRIM-NHL Protein LIN-41 Controls the Onset of Developmental Plasticity in
- Wnt-Mediated Repression via Bipartite DNA Recognition by TCF in the Hematopoietic System
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- Integration of UPR and Oxidative Stress Signaling in the Control of Intestinal Stem Cell Proliferation
- miR171-Targeted Scarecrow-Like Proteins Bind to GT -Elements and Mediate Gibberellin-Regulated Chlorophyll Biosynthesis under Light Conditions
- Syndecan-1 Is Required to Maintain Intradermal Fat and Prevent Cold Stress
- LIN-3/EGF Promotes the Programmed Cell Death of Specific Cells in by Transcriptional Activation of the Pro-apoptotic Gene
- A System for Genome-Wide Histone Variant Dynamics In ES Cells Reveals Dynamic MacroH2A2 Replacement at Promoters
- Multiple Regulation of Rad51-Mediated Homologous Recombination by Fission Yeast Fbh1
- A Genome-Wide Association Study of the Maize Hypersensitive Defense Response Identifies Genes That Cluster in Related Pathways
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- Asymmetric Division and Differential Gene Expression during a Bacterial Developmental Program Requires DivIVA
- A Model-Based Approach for Identifying Signatures of Ancient Balancing Selection in Genetic Data
- Chromatin Insulator Factors Involved in Long-Range DNA Interactions and Their Role in the Folding of the Drosophila Genome
- Conditional Inactivation of Upstream Binding Factor Reveals Its Epigenetic Functions and the Existence of a Somatic Nucleolar Precursor Body
- Evidence for Divisome Localization Mechanisms Independent of the Min System and SlmA in
- Patterns of Admixture and Population Structure in Native Populations of Northwest North America
- Response Regulator Heterodimer Formation Controls a Key Stage in S Development
- A Genetic Strategy to Measure Circulating Insulin Reveals Genes Regulating Insulin Production and Secretion
- EVA-1 Functions as an UNC-40 Co-receptor to Enhance Attraction to the MADD-4 Guidance Cue in
- Dysfunction of the CNS-Heart Axis in Mouse Models of Huntington's Disease
- An Otx/Nodal Regulatory Signature for Posterior Neural Development in Ascidians
- Phosphorylation of a Central Clock Transcription Factor Is Required for Thermal but Not Photic Entrainment
- Genome-Wide Patterns of Genetic Variation within and among Alternative Selective Regimes
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
- Ku-Mediated Coupling of DNA Cleavage and Repair during Programmed Genome Rearrangements in the Ciliate
- Functional Specialization Among Members Of Knickkopf Family Of Proteins In Insect Cuticle Organization
- Playing RNase P Evolution: Swapping the RNA Catalyst for a Protein Reveals Functional Uniformity of Highly Divergent Enzyme Forms
- The Translational Regulators GCN-1 and ABCF-3 Act Together to Promote Apoptosis in
- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- A -Regulatory Mutation of Causes Silky-Feather in Chickens
- VIB1, a Link between Glucose Signaling and Carbon Catabolite Repression, Is Essential for Plant Cell Wall Degradation by
- A Population Genetic Signal of Polygenic Adaptation
- A Conserved Dopamine-Cholecystokinin Signaling Pathway Shapes Context–Dependent Behavior
- The MAP Kinase p38 Is Part of Circadian Clock
- The Cohesin Subunit Rad21 Is Required for Synaptonemal Complex Maintenance, but Not Sister Chromatid Cohesion, during Drosophila Female Meiosis
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání






