-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNeuropeptides Function in a Homeostatic Manner to Modulate Excitation-Inhibition Imbalance in
Neuropeptides play crucial roles in modulating neuronal networks, including changing intrinsic properties of neurons and synaptic efficacy. We previously reported a Caenorhabditis elegans mutant, acr-2(gf), that displays spontaneous convulsions as the result of a gain-of-function mutation in a neuronal nicotinic acetylcholine receptor subunit. The ACR-2 channel is expressed in the cholinergic motor neurons, and acr-2(gf) causes cholinergic overexcitation accompanied by reduced GABAergic inhibition in the locomotor circuit. Here we show that neuropeptides play a homeostatic role that compensates for this excitation-inhibition imbalance in the locomotor circuit. Loss of function in genes required for neuropeptide processing or release of dense core vesicles specifically modulate the convulsion frequency of acr-2(gf). The proprotein convertase EGL-3 is required in the cholinergic motor neurons to restrain convulsions. Electrophysiological recordings of neuromuscular junctions show that loss of egl-3 in acr-2(gf) causes a further reduction of GABAergic inhibition. We identify two neuropeptide encoding genes, flp-1 and flp-18, that together counteract the excitation-inhibition imbalance in acr-2(gf) mutants. We further find that acr-2(gf) causes an increased expression of flp-18 in the ventral cord cholinergic motor neurons and that overexpression of flp-18 reduces the convulsion of acr-2(gf) mutants. The effects of these peptides are in part mediated by two G-protein coupled receptors, NPR-1 and NPR-5. Our data suggest that the chronic overexcitation of the cholinergic motor neurons imposed by acr-2(gf) leads to an increased production of FMRFamide neuropeptides, which act to decrease the activity level of the locomotor circuit, thereby homeostatically modulating the excitation and inhibition imbalance.
Published in the journal: . PLoS Genet 9(5): e32767. doi:10.1371/journal.pgen.1003472
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003472Summary
Neuropeptides play crucial roles in modulating neuronal networks, including changing intrinsic properties of neurons and synaptic efficacy. We previously reported a Caenorhabditis elegans mutant, acr-2(gf), that displays spontaneous convulsions as the result of a gain-of-function mutation in a neuronal nicotinic acetylcholine receptor subunit. The ACR-2 channel is expressed in the cholinergic motor neurons, and acr-2(gf) causes cholinergic overexcitation accompanied by reduced GABAergic inhibition in the locomotor circuit. Here we show that neuropeptides play a homeostatic role that compensates for this excitation-inhibition imbalance in the locomotor circuit. Loss of function in genes required for neuropeptide processing or release of dense core vesicles specifically modulate the convulsion frequency of acr-2(gf). The proprotein convertase EGL-3 is required in the cholinergic motor neurons to restrain convulsions. Electrophysiological recordings of neuromuscular junctions show that loss of egl-3 in acr-2(gf) causes a further reduction of GABAergic inhibition. We identify two neuropeptide encoding genes, flp-1 and flp-18, that together counteract the excitation-inhibition imbalance in acr-2(gf) mutants. We further find that acr-2(gf) causes an increased expression of flp-18 in the ventral cord cholinergic motor neurons and that overexpression of flp-18 reduces the convulsion of acr-2(gf) mutants. The effects of these peptides are in part mediated by two G-protein coupled receptors, NPR-1 and NPR-5. Our data suggest that the chronic overexcitation of the cholinergic motor neurons imposed by acr-2(gf) leads to an increased production of FMRFamide neuropeptides, which act to decrease the activity level of the locomotor circuit, thereby homeostatically modulating the excitation and inhibition imbalance.
Introduction
Neuropeptides are widespread and diverse modulators of neuronal circuit function, and have long been known to play regulatory roles in complex behaviors, such as learning, feeding, temperature regulation, and pain sensation [1], [2]. Additionally, neuropeptide modulation is implicated in a number of neurological diseases including epilepsy and autism [3]–[8]. In recent years great strides have been made in the recognition of the diverse means by which neuropeptides regulate neuronal circuits [9]–[16]. In particular, numerous studies from C. elegans have revealed important insights on the precise mechanisms underlying endogenous neuropeptide function in animal behaviors [15], [17]–[19].
The C. elegans genome contains over 100 peptide-encoding genes, which are generally classified as flp for FMRFamide-like peptides, ins for insulin-like genes, and nlp for neuropeptide-like proteins [20]. Recent proteomic studies have detected expression of over 150 distinct mature peptides [20]–[23]. As in higher vertebrates and other organisms, neuropeptide precursors are packaged into large dense core vesicles, and are further processed into functionally mature neuropeptides through a series of conserved enzymatic reactions [24], [25]. The release of dense core vesicles occurs in response to Ca2+ influx, and relies on several unique proteins in addition to those that are also involved in fast neurotransmitter release [26].
The two best characterized enzymes for neuropeptide processing in C. elegans are the proprotein convertase (PC2), EGL-3, and the carboxypeptidase E (CPE), EGL-21 [20], [27], [28]. EGL-3/PC2 cleaves the propeptide after the basic amino acid residues located at the C-terminus of the individual peptides [20]. EGL-21/CPE then removes the basic amino acids of the newly cleaved peptides [20]. Both genes are expressed primarily in the nervous system [20], [27], [28]. An early report using an antibody that recognizes fully processed FMRFamide-related peptides showed loss of most staining in egl-21 mutants, and a great reduction of staining in egl-3 mutants [27]. Recent peptidomic analyses fail to detect any processed neuropeptides in egl-3 null mutants [21]. While egl-21 mutants show incomplete processing of the majority of FLP and NLP peptides, they also express a number of fully processed peptides [22]. Thus, these two enzymes are important for the processing and production of most, but not all, mature neuropeptides. egl-3 and egl-21 mutants share similar phenotypes including retention of eggs, sluggish movement, and a reduction in sensitivity to the acetylcholinesterase inhibitor aldicarb [27], [28]. However, egl-3; egl-21 double mutants show increased resistance to aldicarb, compared to either single mutant [27], [28], suggesting that they may not act in a completely linear pathway.
Neuropeptide release in C. elegans is well known to influence neural circuit activity and behavior [15], [29], [30]. The UNC-31 CAPS (Calcium-dependent Activator Protein for Secretion) protein is essential for peptide-containing dense core vesicle release, and unc-31 mutants exhibit many sensory deficits and impaired locomotion [31]–[35]. Examples of specific neuropeptides regulating the locomotor circuit activity include the neuropeptide NLP-12, which is released by the stretch sensitive neuron DVA and can influence cholinergic motor neuron neurotransmitter release [14]. The levels of the FLP-1 FMRFamide peptides can also alter locomotor behavior such that flp-1(lf) mutants are hyperactive while overexpression of flp-1 causes reduced mobility [36].
C. elegans sinusoidal locomotion is the result of coordinated muscle contraction due to innervation by the excitatory cholinergic motor neurons and inhibitory GABAergic motor neurons in the ventral cord [37]. Neuropeptide signaling has been implicated in modulating the activity of both types of motor neurons as well as the muscles [12], [20]. We have previously reported that the ACR-2 nicotinic acetylcholine receptor is expressed in the cholinergic motor neurons and plays a key role in balancing excitatory and inhibitory neurotransmission in the locomotor circuit [38]. Specifically, a gain of function mutation (Val309Met), designated as acr-2(gf), in the pore-lining transmembrane domain of the ACR-2 subunit causes an increase in cholinergic excitation, accompanied with a decrease in GABAergic inhibition. This imbalance in excitation and inhibition results in stochastic convulsive behavior due to spontaneous contractions of body muscles. Thus, the frequency of convulsions of the acr-2(gf) mutant can be used as an indicator for the imbalanced activity of the locomotor circuit.
In this study we examined the roles of neuropeptides in modulating excitation and inhibition imbalance in the locomotor circuit. We show that neuropeptides processed by EGL-3 and released from the cholinergic motor neurons inhibit the convulsions caused by acr-2(gf). We find that two neuropeptide-encoding genes, flp-1 and flp-18, act together to reduce excitation and inhibition imbalance in the locomotor circuit. acr-2(gf) causes a specific up-regulation of flp-18 expression in the cholinergic motor neurons. Electrophysiological recordings of the neuromuscular junctions indicate that egl-3 and flp genes primarily influence GABAergic synaptic transmission. We also identify two neuropeptide receptors, NPR-1 and NPR-5 that are likely involved in the regulation of convulsions by the FLP-18 neuropeptides. These data suggest that neuropeptide production is regulated by activity, and that in turn neuropeptides function in a homeostatic manner to modulate output of the locomotor circuit. Our findings have implications for our understanding of excitation-inhibition imbalance in disease conditions, and support a general notion that neuropeptide modulation can provide effective strategies in disease management.
Results
Loss of function in the proprotein convertase EGL-3 increases the convulsion frequency of acr-2(gf)
To specifically test the roles of neuropeptides on acr-2(gf) induced convulsions, we first examined a set of mutants that are known to disrupt peptide processing. We found that multiple alleles of egl-3 caused a significant increase in the convulsion frequency of acr-2(gf) (Figure 1A). A null mutation in sbt-1, a molecular chaperone necessary for EGL-3 function [23], showed a similar enhancement. egl-3(lf) sbt-1(lf); acr-2(gf) triple mutants showed a similar level of increased convulsions as egl-3(lf); acr-2(gf) and sbt-1(lf); acr-2(gf) double mutants, consistent with SBT-1 and EGL-3 acting in the same pathway. The overall locomotion pattern and speed of sbt-1; acr-2(gf) was indistinguishable from that of acr-2(gf) (Videos S1, S2), supporting the specific effects of SBT-1 and EGL-3 on convulsion frequency.
Fig. 1. Neuropeptide processing and release pathway regulate acr-2(gf) convulsions. 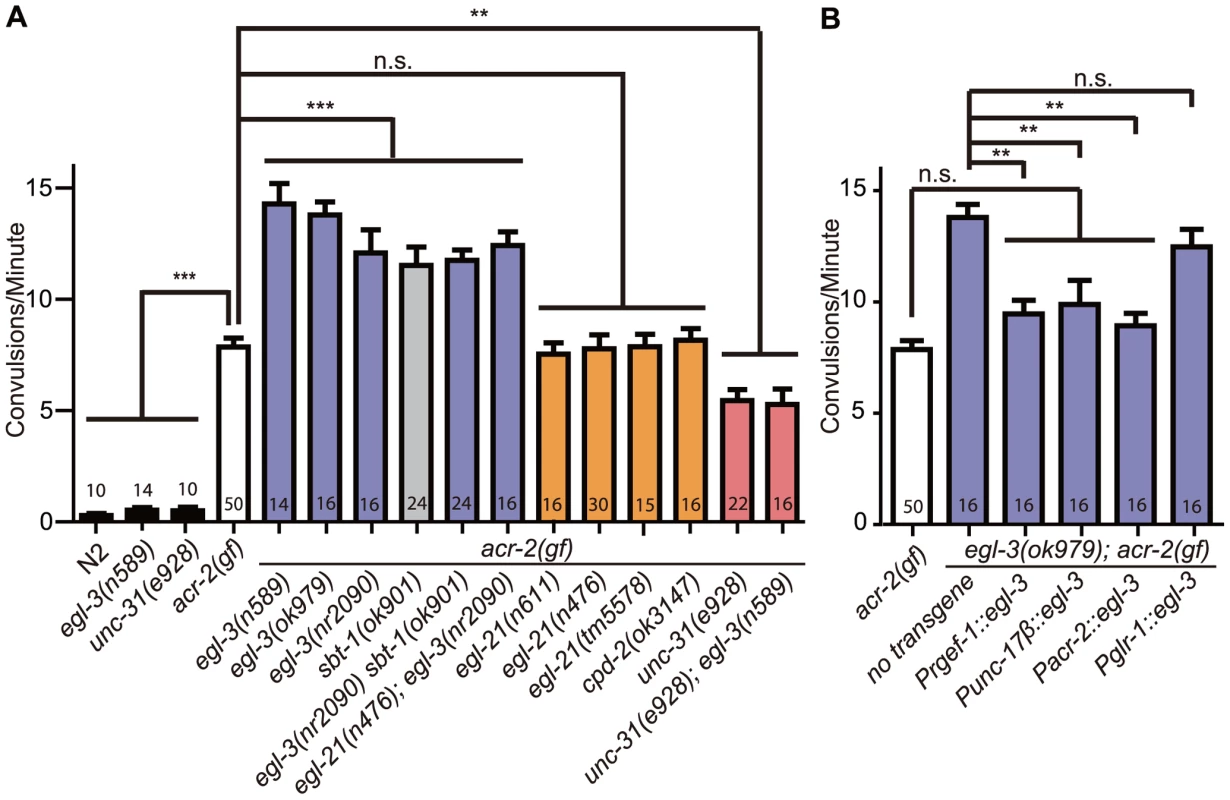
All mutations are loss of function alleles, except for acr-2(gf), which designates acr-2(n2420). Mean convulsion frequencies are shown. Error bars indicate SEM. Numbers in the graph indicate sample sizes. Statistics: ***: p<0.001, **: p<0.01, *: p<0.05 by ANOVA and Bonferroni post hoc test. (A) Loss of function in egl-3 and sbt-1 significantly enhances acr-2(gf) convulsions; and the increased convulsion caused by egl-3(lf) is dependent on unc-31. (B) egl-3 functions in the cholinergic motor neurons to suppress acr-2(gf) convulsions. The number of independent transgenic lines tested are the following: Prgef-1::egl-3; 4 lines, Punc-17β::egl-3; 3 lines, Pglr-1::egl-3; 3 lines, Pacr-2; 2 lines. Quantification data is shown for one representative line. The carboxypeptidase E EGL-21 generally functions together with EGL-3 in producing mature neuropeptides [20]. However, we tested three mutations in egl-21, including a large deletion tm5578, which removes most of the exons 2 and 3 and causes premature stop after 85 amino acids (Table S1), and did not observe any effects on acr-2(gf) convulsions (Figure 1A). A null mutation in cpd-2, another carboxypeptidase, also showed no effects. Moreover, egl-21(lf); egl-3(lf); acr-2(gf) triple mutants behaved similarly to egl-3(lf); acr-2(gf). These observations suggest that egl-21 may not be required, or has a partial role, for processing the specific neuropeptides involved in acr-2(gf) convulsive behavior. As addressed later, we found the latter interpretation to be true. Overall, these observations indicate that EGL-3-dependent neuropeptides modulate the convulsive behavior of acr-2(gf) animals.
The function of neuropeptides is dependent on dense core vesicle release that requires the CAPS protein UNC-31 [26]. To test further the role of neuropeptides in modulating acr-2(gf) convulsions, we introduced a null mutation of unc-31 into the acr-2(gf) background. In contrast to egl-3(lf); acr-2(gf), unc-31(lf); acr-2(gf) double mutants showed a significant reduction in the convulsion frequency as compared to the acr-2(gf) mutants alone (Figure 1A, Videos S1, S3). Importantly, unc-31(lf) blocked the enhancement of egl-3(lf), as egl-3(lf); unc-31(lf); acr-2(gf) triple mutants convulsed to the same degree as unc-31(lf); acr-2(gf) (Figure 1A). Dense core vesicles contain complex components that include neuropeptides, whose processing most likely depends on EGL-3, as well as INS-like peptides, whose processing generally does not depend on EGL-3. Upon release, peptides can act in a combinatorial manner to modulate specific pathways. The fact that the enhanced convulsion in egl-3(lf); acr-2(gf) is dependent on unc-31 led us to propose that the effective mature neuropeptides processed by EGL-3 are a specific subset of dense core vesicle components released via UNC-31.
We further addressed in which cells neuropeptide processing by EGL-3 is required to modulate acr-2(gf). We found that expression of egl-3(+), either pan-neuronally using the rgef-1 promoter [39], or in the cholinergic motor neurons using the unc-17β or the acr-2 promoter [32], fully rescued the enhanced convulsions in egl-3(lf); acr-2(gf), whereas expression of egl-3(+) in pre-motor command neurons, driven by the glr-1 promoter [40], did not show any effect (Figure 1B, Tables S1, S2). Together, these data reveal that neuropeptides processed in the cholinergic motor neurons modulate the convulsive behavior of acr-2(gf), and suggest that the neuropeptide products act to restore the balance of excitation and inhibition in the locomotor circuit.
FMRFamide-like peptides encoded by flp-1 and flp-18 act synergistically to decrease locomotor circuit activity in acr-2(gf) mutants
We next sought to determine the specific neuropeptides responsible for the inhibition of acr-2(gf) convulsions. We tested a set of candidate neuropeptide genes that had either been shown to be expressed in the locomotor circuit, or were known to affect locomotion [20]. Of 23 neuropeptide mutants tested, none showed significant enhancement of the acr-2(gf) convulsion phenotype (Figure 2A–2C, Table S1). We reasoned that the observed inhibitory effects of egl-3 could be due to a group of neuropeptides produced by more than one gene. To test this idea, we made selected double mutants among flp and nlp genes chosen based on similarity in expression patterns or phenotypes. In doing so we found that eliminating both flp-1 and flp-18 resulted in a significant enhancement of acr-2(gf) convulsions (Figure 2D, Figure 3A). Two independent flp-18(lf) mutants, flp-18(tm2179) and flp-18(db99), gave similar effects (Figure 3A). None of the other seven neuropeptide gene double mutants affected acr-2(gf) convulsion frequency (Figure 2D). We note that while flp-1; flp-18 acr-2(gf) mutants display a significant enhancement of the convulsion frequency, the extent of the convulsion is often less obvious than that seen in egl-3(lf); acr-2(gf) animals (Videos S4, S5), suggesting that other as yet unidentified neuropeptides may also be influencing acr-2(gf).
Fig. 2. Loss of both flp-1 and flp-18 enhances acr-2(gf) convulsions. 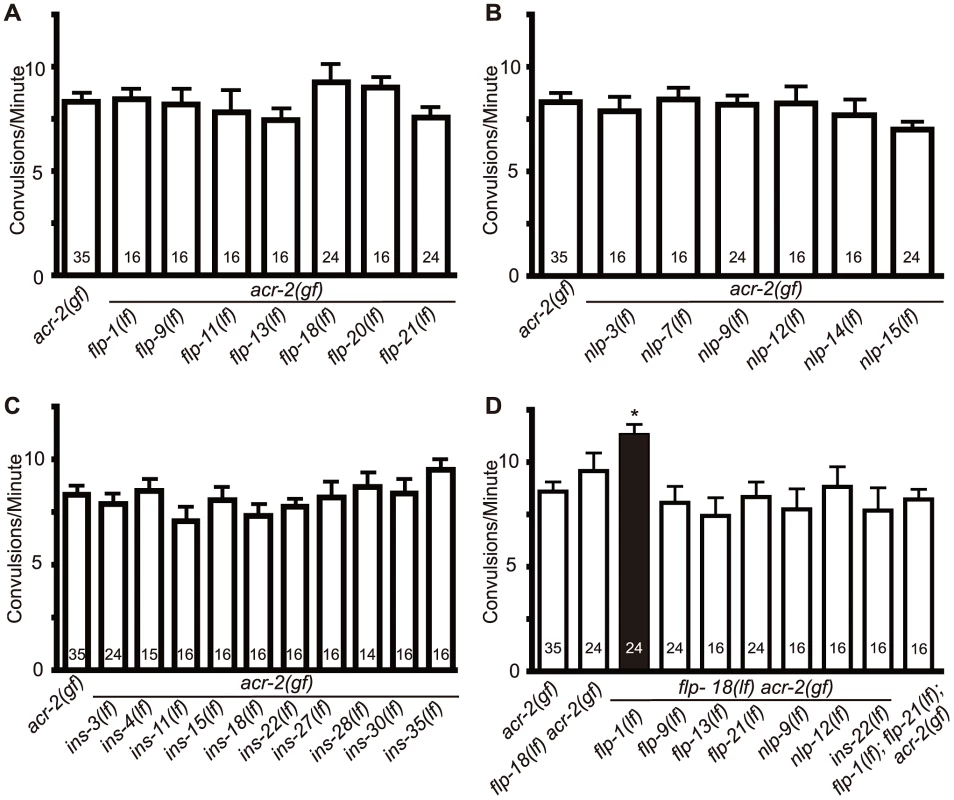
Null mutants of candidate neuropeptide genes were tested for effects on acr-2(gf) convulsions. flp-18(lf) indicates flp-18(tm2179); the allele number for other genes are listed in materials and methods. No significant effects were observed for selected FMRF-amide (flp) (A), neuropeptide like proteins (nls) (B), or insulins (ins) (C). (D) Double mutants of candidate peptide genes with flp-18. Loss of both flp-1 and flp-18 leads to a significant enhancement of acr-2(gf) convulsions. Numbers in the graph indicate sample sizes. Mean convulsion frequencies are shown. Error bars indicate SEM. Statistics: *: p<0.05 by ANOVA and Dunnett's post hoc test. Fig. 3. flp-1 and flp-18 act as inhibitory neuropeptides in the acr-2(gf) background. 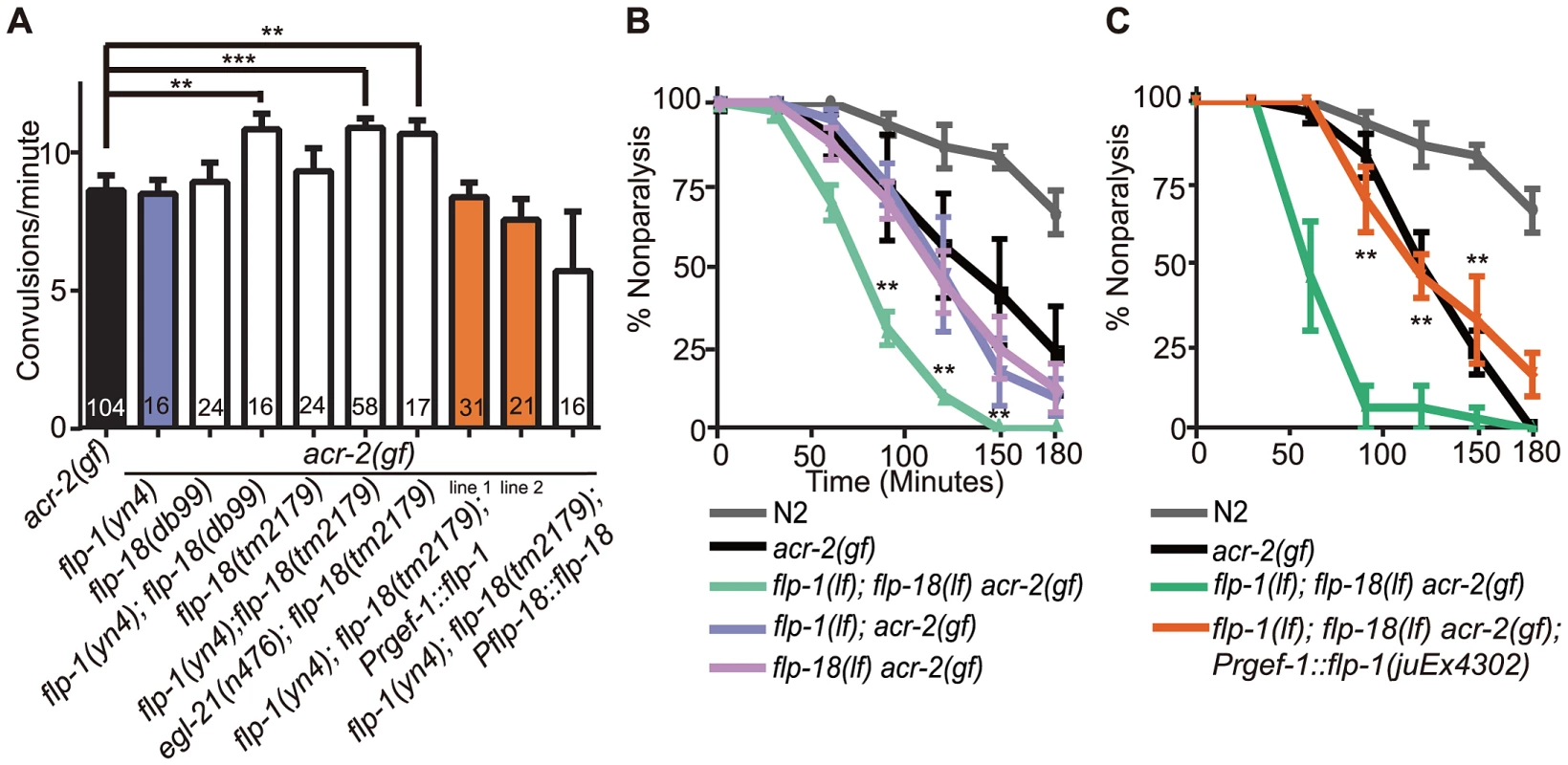
(A) Convulsion frequency of acr-2(gf) in combination with loss of function (lf) mutations in flp-1(yn4), flp-18(tm2179), or flp-18(db99). The enhanced convulsion frequency of flp-1(lf); flp-18(lf) acr-2(gf) animals is rescued with transgenic expression of flp-1 under the pan-neuronal promoter Prgef-1. Two independent transgenic lines were tested as indicated by line 1 and 2. Mean convulsion frequencies are shown. Error bars indicate SEM. Numbers in the graph indicate sample sizes. Statistics: ***: p<0.001, **: p<0.01 by ANOVA and Dunnett's post-hoc test. (B, C) Rate of paralysis on 150 µM aldicarb plates in acr-2(gf) background. flp-1(lf); flp-18(lf) mutants in the acr-2(gf) background showed enhanced aldicarb sensitivity compared to acr-2(gf) (B). Pan-neuronal expression of flp-1 rescues the increased aldicarb sensitivity of the flp-1(lf); flp-18(lf) acr-2(gf) mutants (C). n = 10 for one group per trial; and results of three to five independent trials are shown. Mean rate of paralysis are shown for each time point. Error bars indicate SEM. Two independent transgenic lines were tested, only one is shown in the graph. Statistics in B, C: **: p<0.01, *: p<0.05 by two-way ANOVA and Bonferroni post-hoc test. In the recent peptidomic studies of egl-21(lf) animals, fully processed FLP-1 peptides are reported to be largely undetectable; however, four of the six fully processed mature peptides from FLP-18 are produced [22]. The presence of functional FLP-18-derived peptides would explain why egl-21(lf) single mutants did not show detectable effects on acr-2(gf) (Figure 1A). To test this idea, we constructed egl-21(lf); flp-18(lf) acr-2(gf) triple mutants, and observed that the convulsion frequency in these animals was comparable to that of flp-1(lf); flp-18(lf) acr-2(gf) (Figure 3A). Thus, these observations support a role of EGL-21 in the processing of FLP-1 neuropeptides, and imply other unidentified carboxypeptidases in the processing of FLP-18 neuropeptides.
As an independent assay for the effects of flp-1 and flp-18 neuropeptides on the locomotor circuit activity associated with acr-2(gf) convulsions, we tested the sensitivity of animals to the acetylcholinesterase inhibitor aldicarb [41]. acr-2(gf) animals show hypersensitivity to aldicarb, consistent with increased cholinergic transmission and decreased GABAergic transmission [38] (Figure 3B, 3C). flp-1(lf) mutants showed mild resistance to aldicarb (Figure S1A), consistent with a previous report [29]. flp-18(lf) showed sensitivity to aldicarb similar to wild type and suppressed the resistance of flp-1(lf) (Figure S1A). The hypersensitivity of acr-2(gf) to aldicarb was slightly, but not significantly enhanced by loss of function mutations in either flp-1 or flp-18 alone (Figure 3B). Notably, triple mutants of flp-1(lf); flp-18(lf) acr-2(gf) showed significantly increased sensitivity to aldicarb, compared to acr-2(gf) alone (Figure 3B). Both the increased convulsion frequency and the increased aldicarb sensitivity of the flp-1(lf); flp-18(lf) acr-2(gf) triple mutants were rescued by transgenic expression of flp-18, as well as pan-neuronal expression of flp-1(+) (Figure 3A, 3C), indicating FLP genes act in the nervous system to modulate the excitation-inhibition imbalance caused by acr-2(gf).
EGL-3 and FLP neuropeptides primarily regulate GABAergic inhibition
To address more precisely how neuropeptides influence locomotor circuit activity in the acr-2(gf) background, we performed electrophysiological recordings at the neuromuscular junction. As reported previously [38], [42], when recordings were performed with 2 mM Ca2+ in the bath solution, acr-2(gf) showed slightly increased frequencies of endogenous acetylcholine release (EPSC), but a striking reduction of endogenous GABAergic activity (IPSC) (Figure 4A). Loss of egl-3 function in acr-2(gf) caused a further reduction in endogenous IPSC frequency (Figure 4A). We observed a similar, but milder, effect on IPSC rate in flp-1(lf); flp-18(lf) acr-2(gf) triple mutants, consistent with the milder enhancement in convulsions in these animals (Videos S4, S5). egl-3(lf); acr-2(gf) and flp-1(lf); flp-18(lf) acr-2(gf) both showed slightly reduced endogenous EPSC rates compared to acr-2(gf) single mutant, although the average rate did not significantly differ among the strains (Figure 4A). The amplitudes of endogenous EPSCs and IPSCs were similar in all four genotypes tested (Figure 4B), suggesting that the muscle ACh and GABA receptors are largely unaltered. Thus, the electrophysiology analysis indicates that neuropeptides processed by EGL-3 compensate for the excitation-inhibition imbalance caused by acr-2(gf) primarily by influencing GABAergic transmission, and that FLP-1 and FLP-18 peptides account for most, but not all, of the neuromodulatory effects of EGL-3.
Fig. 4. Neuropeptide modulation primarily affects GABAergic neuromuscular transmission. 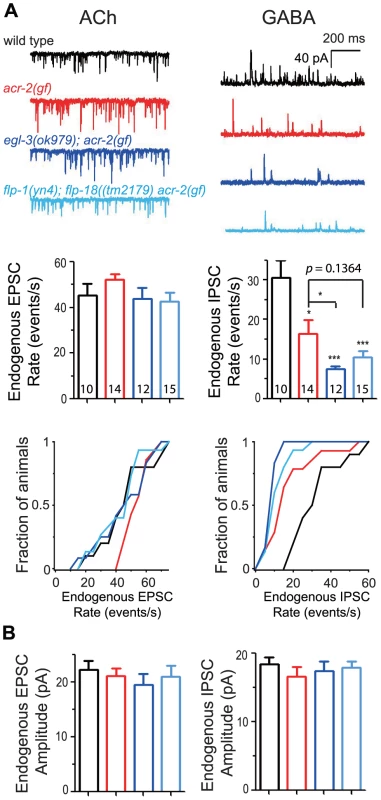
(A) Shown are the electrophysiology recording data on the neuromuscular junctions. Top panels are representative traces of genotypes indicated. Middle panels are mean rates of endogenous EPSCs and IPSCs; and bottom panels are cumulative fractions of the animal number with endogenous EPSC rate or IPSC rate less than indicated values in X-axis of genotype indicated. (B) Mean amplitudes of endogenous EPSCs and IPSCs from genotypes shown in A. The number of animals analyzed is indicated for each genotype. Error bars indicate SEM. Statistics, two-tailed t-test, ***, p<0.001; *, p<0.05. The acr-2(gf) mutation increases FLP-18 expression in the cholinergic motor neurons
The specific effect of flp-1 and flp-18 on acr-2(gf) could be caused by either increased expression or release of these neuropeptides in this mutant background. To address the possibility of increased neuropeptide release, we examined two fluorescent reporters for dense core vesicle release from the cholinergic motor neurons: Punc-129::NLP-21::venus and Punc-129::INS-22::venus [29], [43]. Neither reporter showed significant changes in fluorescence intensity or pattern (Figure S2), suggesting that the general release machinery is largely normal in acr-2(gf).
We next tested for increased expression of neuropeptides using a bicistronic flp-18 reporter that contains the entire genomic locus of flp-18, including the 3.6 kb upstream promoter, followed by a trans-spliced SL2::GFP (designated as Pflp-18::flp-18::SL2::gfp) [44]. In the wild type background this reporter was strongly expressed in several head neurons and was detectable at low levels in the ventral nerve cord. In the acr-2(gf) background, we found that Pflp-18::flp-18::SL2::gfp expression in the ventral cord neurons was strongly enhanced (Figure 5A–5C), while its expression in the head neurons was not changed (Figure S3). GFP expression pattern in the cholinergic motor neurons under the unc-17β promoter was also not affected by acr-2(gf) mutation (Figure S4). We quantified the number of ventral cord neuron cell bodies that showed expression of Pflp-18::flp-18::SL2::gfp, and found that more cell bodies were observed with elevated expression in acr-2(gf) animals than in wild type (Figure 5D). We were not able to examine flp-1 expression due to variable expression patterns of different transgenic flp-1 reporter lines (our unpublished data).
Fig. 5. FLP-18 expression is selectively increased in the cholinergic motor neurons in the acr-2(gf) background. 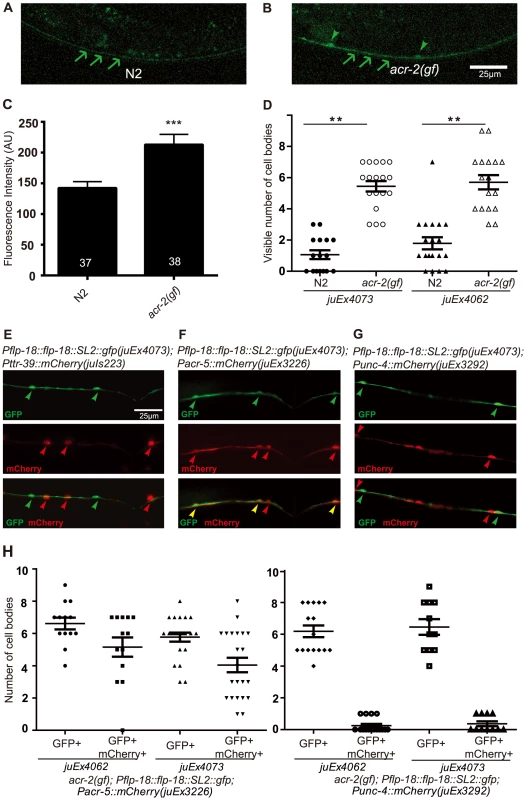
(A–B) Ventral nerve cord expression of Pflp-18::flp-18::SL2::gfp in wild type (N2) and acr-2(gf) background, respectively. Increased fluorescence intensity and cell body expression is seen in the ventral cord in the acr-2(gf) background. Arrows point to the ventral nerve cord posterior to the vulva, and arrowheads point to cell bodies. Scale bar = 25 µm. Two Pflp-18::flp-18::SL2::gfp transgenic lines, juEx4062 and juEx4073, were tested. Images from juEx4073 are shown. (C) Quantification of average fluorescence intensity in the ventral nerve cord posterior to the vulva. Mean fluorescence intensities are shown. N = 37 (wild type), = 38 (acr-2(gf)). ***: p<0.001 by student's t-test. Error bars indicate SEM. (D) Quantification of the number of cell bodies in the ventral cord with visible GFP expression. ***: p<0.001, **: p<0.01 by Student's t-test. Each dot indicates quantification from one animal. Means are indicated by lines. Error bars indicate SEM. Two transgenic lines were tested. (E–H) Identification of the cells showing up-regulation of Pflp-18::flp-18::SL2::gfp in the acr-2(gf) background. (E) Co-expressing mCherry in GABAergic (Pttr-39) motor neurons did not show overlap with Pflp-18::flp-18::SL2::gfp. (F) Expression of mCherry in B-type (Pacr-5) cholinergic motor neurons overlapped extensively with Pflp-18::flp-18:SL2::gfp expression. (G) mCherry expression in A-type (Punc-4) cholinergic motor neurons mostly did not overlap with Pflp-18::flp-18::SL2::gfp expression. (H) Quantification of the number of cell bodies that showed overlapping expression of Pflp-18::flp-18::SL2::gfp and Pacr-5::mCherry or Punc-4::mCherry in F–G. Each dot indicates quantification from one animal. Means are indicated by lines. Error bars indicate SEM. The cells that showed up-regulation of Pflp-18::flp-18::SL2::gfp in acr-2(gf) were evenly spaced along the ventral nerve cord (Figure 5B). To determine in which class of motor neurons Pflp-18::flp-18::SL2::gfp expression was affected, we crossed acr-2(gf); Pflp-18::flp-18::SL2::gfp with a set of mCherry reporter lines driven by specific motor neuron promoters. We observed consistent co-expression of GFP and mCherry in B-type cholinergic motor neurons, labeled by Pacr-5, and occasional expression in A-type cholinergic motor neurons, labeled by Punc-4, but no overlapping expression in GABAergic D-type motor neurons, labeled by Pttr-39 (Figure 5E–5H). These data indicate that acr-2(gf) primarily up-regulates flp-18 expression in the cholinergic B-type motor neurons.
Elevated flp-18 expression correlates with the onset of convulsions and is likely induced by neuronal activity
To further correlate the acr-2(gf)-dependent up-regulation of flp-18 expression, we examined the developmental onset of Pflp-18::flp-18::SL2::gfp expression with respect to the onset of convulsions. We have shown earlier that the onset of convulsions in acr-2(gf) mutants occurs in mid-larval stage [38]. We found that in acr-2(gf) mutants the expression of the flp-18 reporter also increased sharply in mid-larval stages (Figure 6A). The close temporal correlation between the onset of acr-2(gf) convulsions and that of flp-18 up-regulation in cholinergic motor neurons is consistent with flp-18 up-regulation being caused by increased cholinergic activity. Supporting this idea, we observed increased expression of Pflp-18::flp-18::SL2::gfp in wild type animals acutely treated with aldicarb (Figure S5, Protocol S1). In contrast, the expression of Pflp-18::flp-18::SL2::gfp in acr-2(gf) animals was decreased when the animals were grown on plates with the acetylcholine receptor antagonist mecamylamine (Figure S5), which suppresses the convulsion behavior as previously reported [38].
Fig. 6. Induced expression of FLP-18 in acr-2(gf) correlates with the onset of convulsions, and high levels of FLP-18 or FLP-1 suppress convulsions. 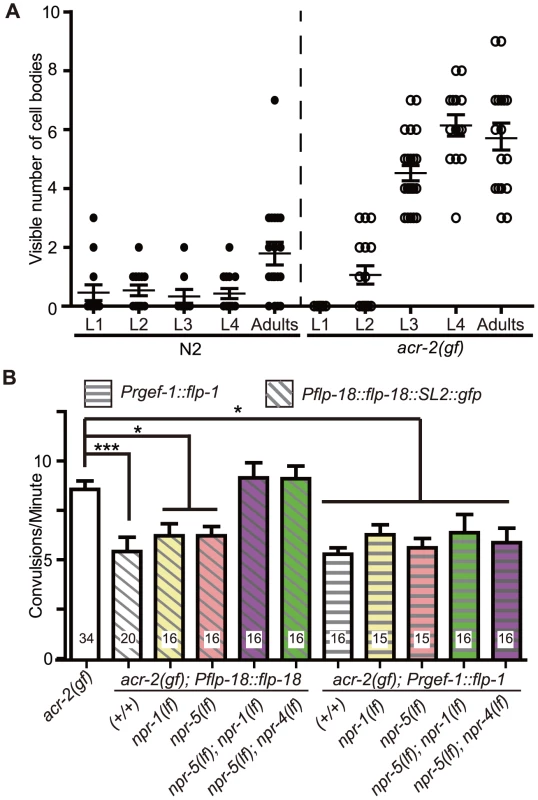
(A) Quantification of the number of cell bodies in the ventral cord that showed Pflp-18::flp-18::SL2::gfp expression in larval and adult stages. Each dot indicates quantification from one animal. Means are indicated by lines. Error bars indicate SEM. Two independent lines juEx4062 and juEx4073 were tested. Result from juEx4073 is shown. (B) Convulsion of acr-2(gf) was suppressed by expression of Pflp-18::flp-18::SL2::gfp or pan-neuronal expression of flp-1. The suppression by flp-18 overexpression was blocked by loss of both npr-1 and npr-5, or npr-4 and npr-5. The same set of npr mutations did not affect the suppression effect of flp-1 overexpression. Mean convulsion frequencies are shown. Error bars indicate SEM. Statistics: ***: p<0.001, *: p<0.05 by ANOVA and Dunnett's post-hoc test. (+/+) indicates strains with no mutations in any of the neuropeptide receptor genes. As FLP-18 functions together with FLP-1 to reduce acr-2(gf) convulsions (Figure 2D, Figure 3A), we hypothesized that the induced expression of flp-18 could be a homeostatic response to the elevated cholinergic neuronal activity in acr-2(gf). If so, overexpression of flp-18(+) or flp-1(+) should ameliorate the extent of convulsions. Indeed, overexpressing flp-18 under the control of its endogenous promoter caused a significant suppression of convulsions (Figure 6B). Overexpression of flp-1, driven by a pan-neuronal promoter, also resulted in a similar suppression of convulsions (Figure 6B). Together, these observations support the conclusion that in the acr-2(gf) background where excitation and inhibition balance is impaired, increased expression of flp-18, and possibly of flp-1, acts as a homeostatic response to dampen imbalanced circuit activity.
npr-1 and npr-5 appear to be the major receptors mediating the suppression of convulsions by FLP-1 and FLP-18
Neuropeptides generally act through G-protein coupled receptors (GPCRs). Next we sought to identify which GPCRs are involved in the regulation of convulsions by the flp neuropeptides. The CKR-2 receptor can be activated by FLP-1 at high concentration, and is also shown to act as a high-affinity receptor for NLP-12 [14], [45]. We found that ckr-2(lf) or ckr-2(lf); flp-18(lf) had no effects on acr-2(gf) (Figure S6), consistent with the observation that nlp-12(lf) did not affect acr-2(gf) either alone or in combination with flp-18 (Figure 2D). Three receptors NPR-1, NPR-4 and NPR-5 can be activated by all six FLP-18 neuropeptides when expressed in Xenopus oocytes [44], [46]–[48]. NPR-1 is expressed in the ventral cord GABAergic motor neurons and in multiple head sensory neurons [46]. NPR-4 is expressed in the AVA, RIV, BDU and PQR neurons as well as in coelomocytes and the intestine [44]. NPR-5 expression is found in the amphid and phasmid neurons, interneurons AIA and AUA, as well as in the muscles [44]. We found that loss of function mutations in individual npr genes neither suppressed nor enhanced acr-2(gf) (Figure 7A). We then made selected double mutant combinations among npr-1, npr-4, and npr-5, in the presence or absence of flp-1(lf). Eliminating both npr-1 and npr-5 in acr-2(gf) resulted in increased convulsions, while npr-4(lf) showed detectable effects only when both npr-5 and flp-1 were eliminated (Figure 7A, 7B). To further test the roles of these npr genes, we examined the suppression effects of acr-2(gf) by the overexpression of flp-18. We found that the suppression of convulsion by overexpression of flp-18 were reduced by either npr-1(lf); npr-5(lf) or npr-4(lf); npr-5(lf) double mutations, but not by npr-1(lf) or npr-5(lf) single mutation (Figure 6B). Based on these observations, we conclude that NPR-1 and NPR-5 likely play a major role in mediating the modulatory action of FLP-18 in acr-2(gf), while NPR-4 has a minor role. Similar npr receptor combinations had no effects on the suppression of convulsion by overexpression of flp-1 driven by a pan-neuronal promoter (Figure 6B). It is possible that this reflects non-physiological effects caused by overexpression of flp-1. Alternatively, FLP-1 and FLP-18 may act through distinct signaling pathways. Supporting the latter idea, we observed a slight but significant difference in aldicarb sensitivity between npr-5(lf); npr-1(lf) acr-2(gf) and flp-1(lf); npr-5(lf); npr-1(lf) acr-2(gf) (Figure S7). Nonetheless, this difference did not result in significant changes in the convulsion frequency of flp-1(lf); npr-5(lf); npr-1(lf) acr-2(gf) from that of npr-5(lf); npr-1(lf) acr-2(gf) (Figure 7A, 7B), which could reflect the limitation of our visual detection methodology.
Fig. 7. NPR-1, NPR-4, and NPR-5 act together to mediate the effects of neuropeptides on convulsions. 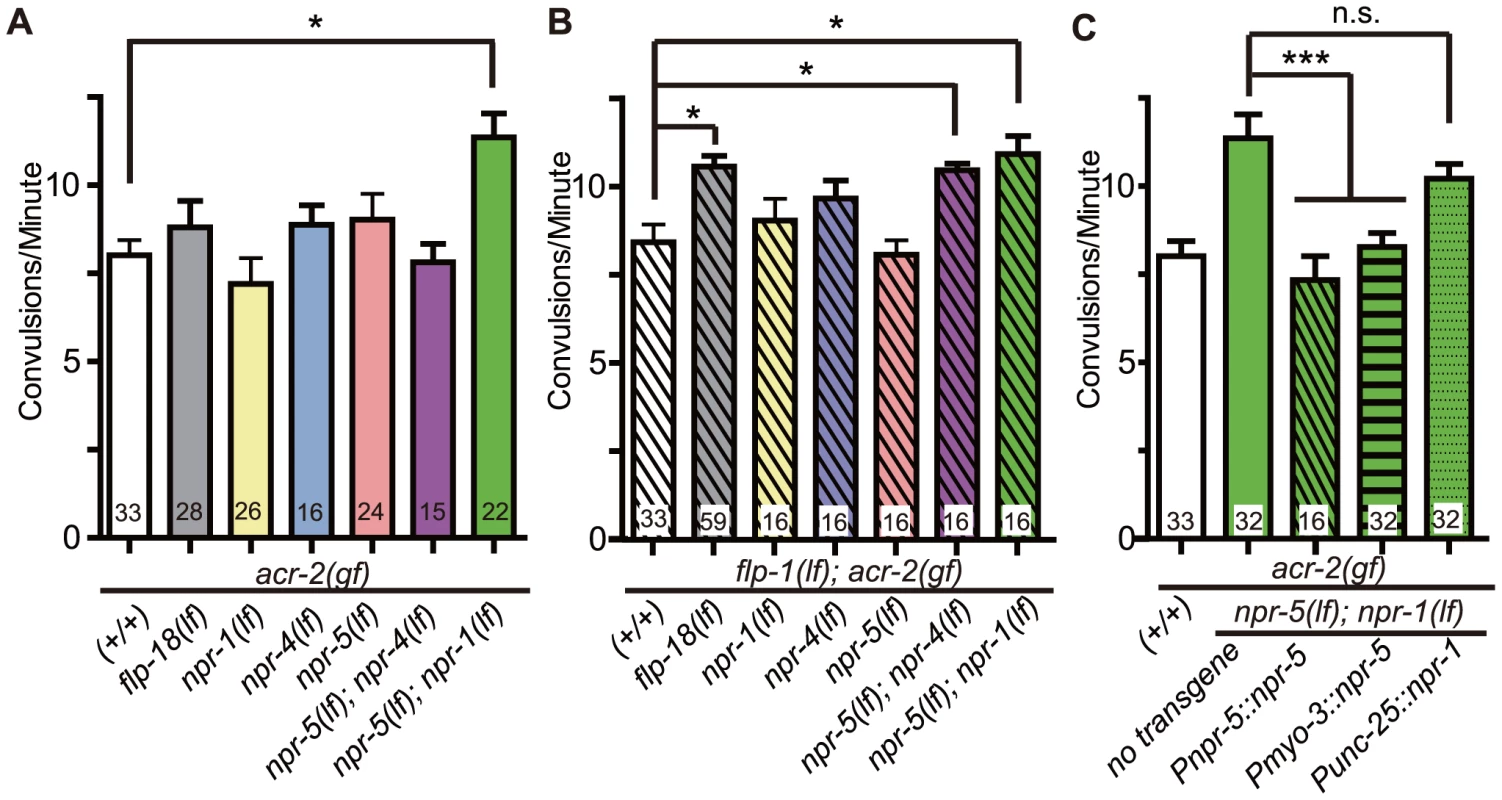
(A–B). Convulsion frequencies of acr-2(gf) combined with loss of function mutations in npr-1(ok1447), npr-4(tm1782), npr-5(ok1583) (A) and with flp-1(yn4) (B). (C) Convulsion frequency of animals with cell type-specific expression of npr-1 and npr-5. npr-5 expression in the muscle rescued the increased convulsion frequency of npr-5(lf); npr-1(lf) acr-2(gf) triple mutant; two independent lines were tested. npr-1 expression in GABAergic motor neurons did not significantly rescue the increased convulsion frequency; three lines were tested. All strains contain acr-2(gf). Mean convulsion frequencies are shown. Error bars indicate SEM. Statistics: ***: p<0.001, *: p<0.05 by ANOVA and Bonferroni post hoc test. (+/+) indicates strain with no mutations in any of the neuropeptide receptor genes. We next addressed the cell types in which npr-1 or npr-5 may act. We found that expression of npr-5 in muscles by the Pmyo-3 promoter reduced the convulsion frequency of npr-5(lf); npr-1(lf) acr-2(gf) animals to a similar degree as did npr-5 expression under its endogenous promoter (Figure 7C). We also expressed npr-1 in the GABAergic motor neurons by the Punc-25 promoter, and did not detect any significant effect on the convulsion frequency of npr-5(lf); npr-1(lf) acr-2(gf). Overall, our analysis supports a conclusion that NPR-5 acts in the muscle, while NPR-1 expressed in other neurons such as sensory head neurons may be contributing to the locomotor circuit activity in an indirect manner.
Discussion
In this study we have identified two neuropeptide-encoding genes, flp-1 and flp-18 that act in a homeostatic manner to dampen the effects of the excitation-inhibition balance of the locomotor circuit caused by the acr-2(gf) mutation. The role of flp-1 and flp-18 in suppressing overexcitation of the locomotor circuit is dependent on neuropeptide processing by egl-3 in the cholinergic motor neurons. We provide electrophysiological evidence that this neuropeptide modulation primarily acts on the GABAergic neural transmission at the neuromuscular junctions. Our previous studies have shown that acr-2(gf) elevates the activity of the cholinergic motor neurons [38]. Here, we find that acr-2(gf) causes up-regulation of flp-18 expression in the cholinergic motor neurons, and that over-expression of flp-18 or flp-1 is able to suppress acr-2(gf). Our analyses of known flp neuropeptide receptors suggest that npr-1 and npr-5 play a major role in mediating the peptide's function. We show that npr-5 primarily acts in the muscles. Yet, the combinatorial effects of these receptors on the excitation-inhibition imbalance caused by acr-2(gf) likely involve multiple cell types.
The production of mature neuropeptides generally requires sequential enzymatic reactions starting with proprotein convertases, followed by carboxypeptidases [20]. Peptide mass spectrometry studies indicate that the majority of mature neuropeptides in C. elegans require EGL-3/PC2, its chaperone SBT-1, and EGL-21/CPE [20]. Previous studies have shown that egl-3 and egl-21 generally exhibit similar behavioral defects, although they differ in severity [27], [28]. Here, we find that loss of function in egl-3 and sbt-1, but not in egl-21, enhances the convulsion frequency of acr-2(gf) animals. Proteomic analyses show that several mature peptides of FLP-18 are present in egl-21 mutants [22]. We find that egl-21(lf); flp-18(lf) acr-2(gf) triple mutants show an increased convulsion frequency similar to flp-1(lf); flp-18(lf) acr-2(gf). These data provide an explanation for the lack of effect on the acr-2(gf) convulsion frequency by the egl-21 mutations, and also imply the involvement of other carboxypeptidases besides EGL-21 in mature neuropeptide production. Once mature peptides are processed in the dense core vesicles, the release of peptides requires UNC-31/CAPS. Intriguingly, we observed a suppression of acr-2(gf) convulsions by unc-31(lf), an opposite effect from that of egl-3(lf). egl-3 is necessary for processing primarily NLP and FLP neuropeptides [21], [27], while unc-31 is required for release of all neuropeptides including INS-like peptides and may also affect fast neurotransmitter release. We find that loss of both flp-1 and flp-18 largely mimicked the effects of egl-3(lf). Importantly, the effects of egl-3(lf) are dependent on unc-31(lf). These data support a conclusion that flp-1 and flp-18 peptides, processed by EGL-3, are released via dense core vesicles and in turn act to modulate the locomotion circuit in an inhibitory manner. We infer that the suppression of convulsions of acr-2(gf) by unc-31(lf) is likely due to the involvement of other pathways or unidentified neuropeptides that play excitatory roles in locomotion.
Previous studies on flp-18 have focused on the functions of FLP-18 released from the head interneurons [44]. flp-18(lf) mutants show defects in fat accumulation and foraging behavior, and the defects can be rescued by flp-18 expression in the AIY or RIG neurons [44]. The role of FLP-18 neuropeptides in the locomotor circuit is unknown. In wild type animals, flp-18 expression, visualized using a reporter that expresses FLP-18 and GFP under the endogenous flp-18 promoter, is generally low in the cholinergic motor neurons [44] (Figure 5A). We find that the transcriptional expression of flp-18 is specifically up-regulated in the cholinergic motor neurons at the onset of convulsions induced by acr-2(gf) and upon upregulation of cholinergic activity by acute aldicarb treatment. This result suggests that up-regulation of flp-18 is likely a homeostatic response to the overexcitation caused by acr-2(gf). We were not able to determine whether flp-1 expression might be similarly regulated, due to inconsistent expression pattern of different transgenic reporter lines (our unpublished data, and C. Li, personal communication). Strong up-regulation of flp-18 is consistently observed in the B type motor neurons, which drive forward locomotion [49], [50]. We have recently found that the AVB neurons, which provide major synaptic input to the B type neurons, are necessary for the onset of convulsions in acr-2(gf) [51]. Together, these observations support that the AVB-B neuron pathway plays a major role in the excitation-inhibition balance of the locomotor network.
FLP-18 can activate NPR-1, NPR-4 and NPR-5 receptors [44], [48]. Our analysis suggests that these receptors act in a combinatorial manner to modulate the convulsion in acr-2(gf) animals, with NPR-1 and NPR-5 having a major, and NPR-4 a minor role. These receptors are expressed in multiple cell types. Our data show that muscle-specific expression of NPR-5 can rescue the increased convulsions, suggesting that the FLP-18 neuropeptides can act directly on muscles to inhibit contraction, or to promote relaxation. NPR-1 and NPR-5 may also be activated by neuropeptides other than FLP-18, since npr-5(lf); npr-1(lf) acr-2(gf) triple mutants show a more severe phenotype than flp-18(lf) acr-2(gf) double mutants. The effect of flp-1 and flp-18 double loss of function is milder than that of egl-3(lf) (Figure 4), implying the involvement of other neuropeptides.
We previously showed that GABAergic transmission at the neuromuscular junctions is reduced in acr-2(gf) animals [38]. Our neuromuscular physiology analysis here shows that the neuropeptide modulation by egl-3 and flp-1 and flp-18 primarily acts on GABAergic transmission. npr-1 is expressed in the GABAergic motor neurons [44], [48]. However, our data suggests that this expression is unlikely to be directly responsible the effect of neuropeptides on GABA neurons. flp-1 is reported to be expressed primarily in the head neurons including AIA, AIY, AVA, AVE, AVK, RIG, RMG, M5 [36]. The effects of flp-1(lf) on convulsions appear to be independent of CKR-2, presently the only known receptor for FLP-1 (Figure S6). npr-1(lf); npr-5(lf) double mutants cause enhanced convulsions of acr-2(gf), similar to flp-1(lf); flp-18(lf) double mutants. Yet, double loss of function in npr-1 and npr-5 does not significantly affect the suppression of convulsions by flp-1(+) overexpression under a pan-neuronal promoter. It is possible that flp-1(+) overexpression activates other inhibitory pathways that do not require npr-1 and npr-5. We observed an enhanced aldicarb sensitivity of the flp-1(lf); npr-5(lf); npr-1(lf) acr-2(gf) animals comparing to npr-5(lf); npr-1(lf) acr-2(gf) (Figure S7). However, this difference did not result in detectable differences in convulsion, which may likely be due to the limitation in our methodology of visual observation of convulsion. The modest effects of these receptors make it difficult to determine the precise contribution of their signaling in the context of convulsive behavior of acr-2(gf). Identification of additional GPCRs that respond to FLP-1 will be necessary for fully understanding the peptidergic transmission pathway that modulates acr-2(gf) convulsions. Overall, our results are consistent with a model in which these neuropeptides act on multiple cell types, one of which is body wall muscle, to coordinate the activity state of the locomotion circuit.
The molecular nature and the physiological basis of C. elegans acr-2(gf) mutants share similarities with mutations causing epileptic seizures including an imbalance between excitation and inhibition of the nervous system. Examples of neuropeptides acting to inhibit altered neuronal circuit activity, such as in seizures, have also been observed in vertebrates. For example, the neuropeptide galanin has been shown to play a key role in epilepsy [5], [52]. Galanin agonists inhibit seizures [5], and expression of galanin is increased in the mouse brain upon the induction of seizures [53]. A model for the role of galanin in epilepsy has been proposed in that increased excitation increases galanin levels in an attempt to normalize the excitation and inhibition balance by reducing glutamatergic transmission [6]. Likewise, our studies have revealed that activity-dependent expression of neuropeptides provides a homeostatic mechanism to modulate neuronal network balance. Together, these findings provide support for manipulations of slow neuropeptide signaling in controlling neuronal circuit activity disruption underlying neurological disorders.
Materials and Methods
Genetics and alleles
All C. elegans strains were grown on NGM plates at room temperature (20–22°C) following standard methods. Deletion mutant strains were backcrossed two times against N2 before being used for strain construction. All double mutants were constructed using standard procedures, and genotypes were confirmed by PCR verification of the deletions. Table S1 lists the information on the alleles and strains. Specific alleles used in the figures are: acr-2(gf) indicates acr-2(n2420), unc-31(e928), egl-3(n589), egl-3(ok979), egl-3(nr2090), sbt-1(ok901), egl-21(n611), egl-21(n476), egl-21(tm5578), cpd-2(ok3147), flp-1(yn4), flp-9(ok2730), flp-11(tm2706), flp-13(tm2427), flp-18(tm2179), flp-20(ok2964), flp-21(ok889) ,nlp-3(tm3023), nlp-7(tm2984), nlp-9(tm3572), nlp-12(ok335), nlp-14(tm1880), nlp-15(ok1512), ins-3(ok2488), ins-4(ok3534), ins-11(tm1053), ins-18(ok3444), ins-22(ok3616), ins-27(ok2474), ins-28(ok2722), ins-30(ok2343), ins-35(ok3297), npr-1(ok1447), npr-4(tm1782), npr-5(ok1583), ckr-2(tm3082), acr-2(ok1887).
Molecular biology and transgenes
Molecular biology was performed according to standard methods [54]. Expression constructs were generated using Gateway recombination technology (Invitrogen, CA), and Table S2 lists the details of the DNA clones generated in this study. An unc-31 cDNA pDONR construct was provided by Dr. Kaveh Ashrafi [33], Punc-17β::unc-31 was provided by Dr. Ken Miller [32], Pglr-1::egl-3 , Pacr-2::egl-3 and Punc-25::npr-1 were provided by Dr. Josh Kaplan ([28] and personal communication), and Pflp-18::flp-18::SL2::gfp and Pnpr-5::npr-5 were provided by Dr. Merav Cohen and Dr. Mario de Bono [44].
Quantification of convulsion behavior
Ten to twenty L4 larvae were placed on freshly seeded NGM plates. The following day, young adults were transferred to fresh plates and recorded by video for 90 seconds, five frames per second. Eight animals were recorded for each genotype per trial and at least two trials were performed per genotype. Videos were scored by an observer blind to genotype. A “convulsion” was defined as a visible shortening in the animal's body length.
Pharmacology analysis
L4 animals were picked the day before an experiment. The day of the experiment ten young adults per genotype were placed on plates containing 150 µM aldicarb, and the effects on animal movement were observed at 30 minute intervals. Animals were scored as paralyzed when no body movements were observed, even in response to touch.
FLP-18 imaging
Confocal images were taken on a Zeiss LSM 510 with 1 µm per section, and processed using ImageJ. Maximum projection images were created from confocal stacks and the average intensity was measured of the ventral cord posterior to the vulva. For cell body counting, L4 animals were picked the day before an experiment, and young adults were observed using a Zeiss Axioplan 2 fluorescence microscope the following day. The number of cell bodies of the ventral nerve cord with visible GFP fluorescence was counted. For identification of the cells expressing Pflp-18::flp-18::SL2::gfp cell bodies with GFP and mCherry fluorescence were observed and counted. For the observation in different stages of animals, animals were synchronized at L1 stage and observed under Zeiss Axioplan 2 Fluorescence microscope at each developmental stage.
Electrophysiology
NMJ dissection methods were adapted from previous studies [42]. In brief, adult worms were immobilized on Sylgard-coated cover slips with cyanoacrylate glue. A dorsolateral incision was made with a sharp glass pipette and the cuticle flap was folded back and glued down to expose the ventral medial body wall muscles. The preparation was then treated by collagenase type IV (Sigma-Aldrich) for ∼30 s at a concentration of 0.4 mg/ml. The bath solution contained (in mM): 127 NaCl, 5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 4 MgCl2, 10 glucose, and sucrose to 340 mOsm, bubbled with 5% CO2, 95% O2 at 20°C. The pipette solution containing (in mM): 120 CH3O3SCs, 4 CsCl, 15 CsF, 4 MgCl2, 5 EGTA, 0.25 CaCl2, 10 HEPES and 4 Na2ATP, adjusted to pH 7.2 with CsOH. Conventional whole-cell recordings from muscle cells were performed at 20°C with 2–3 MΩ pipettes. An EPC-10 patch-clamp amplifier was used together with the Patchmaster software package (HEKA Electronics, Lambrecht, Germany). Endogenous acetylcholine postsynaptic currents were recorded at −60 mV and GABA postsynaptic currents were recorded at 0 mV. The current traces were imported to IGOR Pro (WaveMetrics, Lake Oswego, OR) for further analysis.
Ethics statement
This work does not use human subjects or animals. The research was performed following the ethical conduct rules of University of California San Diego.
Supporting Information
Zdroje
1. KriegerDT (1983) Brain peptides: what, where, and why? Science 222 : 975–985.
2. KowLM, PfaffDW (1988) Neuromodulatory actions of peptides. Annu Rev Pharmacol Toxicol 28 : 163–188.
3. BlakeAD, BadwayAC, StrowskiMZ (2004) Delineating somatostatin's neuronal actions. Curr Drug Targets CNS Neurol Disord 3 : 153–160.
4. FetissovSO, JacobyAS, BrumovskyPR, ShineJ, IismaaTP, et al. (2003) Altered hippocampal expression of neuropeptides in seizure-prone GALR1 knockout mice. Epilepsia 44 : 1022–1033.
5. LernerJT, SankarR, MazaratiAM (2008) Galanin and epilepsy. Cell Mol Life Sci 65 : 1864–1871.
6. MitsukawaK, LuX, BartfaiT (2008) Galanin, galanin receptors and drug targets. Cell Mol Life Sci 65 : 1796–1805.
7. SchwarzerC (2009) 30 years of dynorphins–new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 123 : 353–370.
8. WuG, FederA, WegenerG, BaileyC, SaxenaS, et al. (2011) Central functions of neuropeptide Y in mood and anxiety disorders. Expert Opin Ther Targets 15 : 1317–1331.
9. NasselDR (2002) Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol 68 : 1–84.
10. DavisRE, StrettonAO (1996) The motornervous system of Ascaris: electrophysiology and anatomy of the neurons and their control by neuromodulators. Parasitology 113 (Suppl) S97–117.
11. Harris-WarrickRM, MarderE (1991) Modulation of neural networks for behavior. Annu Rev Neurosci 14 : 39–57.
12. ChalasaniSH, KatoS, AlbrechtDR, NakagawaT, AbbottLF, et al. (2010) Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci 13 : 615–621.
13. RennSC, ParkJH, RosbashM, HallJC, TaghertPH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99 : 791–802.
14. HuZ, PymEC, BabuK, Vashlishan MurrayAB, KaplanJM (2011) A neuropeptide-mediated stretch response links muscle contraction to changes in neurotransmitter release. Neuron 71 : 92–102.
15. BargmannCI (2012) Beyond the connectome: How neuromodulators shape neural circuits. Bioessays 34 (6) 458–65.
16. MarderE (2012) Neuromodulation of neuronal circuits: back to the future. Neuron 76 : 1–11.
17. LeinwandSG, ChalasaniSH (2011) Olfactory networks: from sensation to perception. Curr Opin Genet Dev 21 : 806–811.
18. LuedtkeS, O'ConnorV, Holden-DyeL, WalkerRJ (2010) The regulation of feeding and metabolism in response to food deprivation in Caenorhabditis elegans. Invert Neurosci 10 : 63–76.
19. TaghertPH, NitabachMN (2012) Peptide neuromodulation in invertebrate model systems. Neuron 76 : 82–97.
20. LiC, KimK (2008) Neuropeptides. WormBook 1–36.
21. HussonSJ, ClynenE, BaggermanG, JanssenT, SchoofsL (2006) Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J Neurochem 98 : 1999–2012.
22. HussonSJ, JanssenT, BaggermanG, BogertB, Kahn-KirbyAH, et al. (2007) Impaired processing of FLP and NLP peptides in carboxypeptidase E (EGL-21)-deficient Caenorhabditis elegans as analyzed by mass spectrometry. J Neurochem 102 : 246–260.
23. HussonSJ, SchoofsL (2007) Altered neuropeptide profile of Caenorhabditis elegans lacking the chaperone protein 7B2 as analyzed by mass spectrometry. FEBS Lett 581 : 4288–4292.
24. FullerRS, SterneRE, ThornerJ (1988) Enzymes required for yeast prohormone processing. Annu Rev Physiol 50 : 345–362.
25. JungLJ, SchellerRH (1991) Peptide processing and targeting in the neuronal secretory pathway. Science 251 : 1330–1335.
26. SuudhofTC (2008) Neurotransmitter release. Handb Exp Pharmacol 1–21.
27. JacobTC, KaplanJM (2003) The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci 23 : 2122–2130.
28. KassJ, JacobTC, KimP, KaplanJM (2001) The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci 21 : 9265–9272.
29. SieburthD, Ch'ngQ, DybbsM, TavazoieM, KennedyS, et al. (2005) Systematic analysis of genes required for synapse structure and function. Nature 436 : 510–517.
30. LiC (2005) The ever-expanding neuropeptide gene families in the nematode Caenorhabditis elegans. Parasitology 131 (Suppl) S109–127.
31. AveryL, BargmannCI, HorvitzHR (1993) The Caenorhabditis elegans unc-31 gene affects multiple nervous system-controlled functions. Genetics 134 : 455–464.
32. CharlieNK, SchadeMA, ThomureAM, MillerKG (2006) Presynaptic UNC-31 (CAPS) is required to activate the G alpha(s) pathway of the Caenorhabditis elegans synaptic signaling network. Genetics 172 : 943–961.
33. LeeBH, AshrafiK (2008) A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet 4: e1000213 doi:10.1371/journal.pgen.1000213.
34. LiuT, KimK, LiC, BarrMM (2007) FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. J Neurosci 27 : 7174–7182.
35. ZhouKM, DongYM, GeQ, ZhuD, ZhouW, et al. (2007) PKA activation bypasses the requirement for UNC-31 in the docking of dense core vesicles from C. elegans neurons. Neuron 56 : 657–669.
36. NelsonLS, RosoffML, LiC (1998) Disruption of a neuropeptide gene, flp-1, causes multiple behavioral defects in Caenorhabditis elegans. Science 281 : 1686–1690.
37. WhiteJG, SouthgateE, ThomsonJN, BrennerS (1976) The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 275 : 327–348.
38. JospinM, QiYB, StawickiTM, BoulinT, SchuskeKR, et al. (2009) A neuronal acetylcholine receptor regulates the balance of muscle excitation and inhibition in Caenorhabditis elegans. PLoS Biol 7: e1000265 doi:10.1371/journal.pbio.1000265.
39. Altun-GultekinZ, AndachiY, TsalikEL, PilgrimD, KoharaY, et al. (2001) A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128 : 1951–1969.
40. BrockiePJ, MadsenDM, ZhengY, MellemJ, MaricqAV (2001) Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci 21 : 1510–1522.
41. RandJB (2007) Acetylcholine. WormBook 1–21.
42. StawickiTM, ZhouK, YochemJ, ChenL, JinY (2011) TRPM channels modulate epileptic-like convulsions via systemic ion homeostasis. Curr Biol 21 : 883–888.
43. SieburthD, MadisonJM, KaplanJM (2007) PKC-1 regulates secretion of neuropeptides. Nat Neurosci 10 : 49–57.
44. CohenM, RealeV, OlofssonB, KnightsA, EvansP, et al. (2009) Coordinated regulation of foraging and metabolism in C. elegans by RFamide neuropeptide signaling. Cell Metab 9 : 375–385.
45. JanssenT, MeelkopE, LindemansM, VerstraelenK, HussonSJ, et al. (2008) Discovery of a cholecystokinin-gastrin-like signaling system in nematodes. Endocrinology 149 : 2826–2839.
46. CoatesJC, de BonoM (2002) Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature 419 : 925–929.
47. de BonoM, BargmannCI (1998) Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 94 : 679–689.
48. RogersC, RealeV, KimK, ChatwinH, LiC, et al. (2003) Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat Neurosci 6 : 1178–1185.
49. ChalfieM, SulstonJE, WhiteJG, SouthgateE, ThomsonJN, et al. (1985) The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci 5 : 956–964.
50. WicksSR, RoehrigCJ, RankinCH (1996) A dynamic network simulation of the nematode tap withdrawal circuit: predictions concerning synaptic function using behavioral criteria. J Neurosci 16 : 4017–4031.
51. QiYB, GarrenEJ, ShuX, TsienRY, JinY (2012) Photo-inducible cell ablation in Caenorhabditis elegans using the genetically encoded singlet oxygen generating protein miniSOG. Proc Natl Acad Sci U S A 109 : 7499–7504.
52. LundstromL, ElmquistA, BartfaiT, LangelU (2005) Galanin and its receptors in neurological disorders. Neuromolecular Med 7 : 157–180.
53. ChristiansenSH, WoldbyeDP (2010) Regulation of the galanin system by repeated electroconvulsive seizures in mice. J Neurosci Res 88 : 3635–3643.
54. Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
Štítky
Genetika Reprodukční medicína
Článek Attachment Site Selection and Identity in Bxb1 Serine Integrase-Mediated Site-Specific RecombinationČlánek Bck2 Acts through the MADS Box Protein Mcm1 to Activate Cell-Cycle-Regulated Genes in Budding YeastČlánek High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple IsolatesČlánek Implicates Tyrosine-Sulfated Peptide Signaling in Susceptibility and Resistance to Root Infection
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 5
-
Všechny články tohoto čísla
- Functional Elements Are Embedded in Structurally Constrained Sequences
- RNA–Mediated Epigenetic Heredity Requires the Cytosine Methyltransferase Dnmt2
- Loss of Expression and Promoter Methylation of SLIT2 Are Associated with Sessile Serrated Adenoma Formation
- Attachment Site Selection and Identity in Bxb1 Serine Integrase-Mediated Site-Specific Recombination
- Human Genetics in Rheumatoid Arthritis Guides a High-Throughput Drug Screen of the CD40 Signaling Pathway
- Genome-Wide Analysis in German Shepherd Dogs Reveals Association of a Locus on CFA 27 with Atopic Dermatitis
- Liver X Receptors Protect from Development of Prostatic Intra-Epithelial Neoplasia in Mice
- Chromosomal Organization and Segregation in
- A Statistical Framework for Joint eQTL Analysis in Multiple Tissues
- Cell Polarity and Patterning by PIN Trafficking through Early Endosomal Compartments in
- Bck2 Acts through the MADS Box Protein Mcm1 to Activate Cell-Cycle-Regulated Genes in Budding Yeast
- High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple Isolates
- Neuropeptides Function in a Homeostatic Manner to Modulate Excitation-Inhibition Imbalance in
- A Compendium of Nucleosome and Transcript Profiles Reveals Determinants of Chromatin Architecture and Transcription
- Wnt Signaling Regulates the Lineage Differentiation Potential of Mouse Embryonic Stem Cells through Tcf3 Down-Regulation
- Filamin and Phospholipase C-ε Are Required for Calcium Signaling in the Spermatheca
- The Specificity and Flexibility of L1 Reverse Transcription Priming at Imperfect T-Tracts
- Imputation-Based Meta-Analysis of Severe Malaria in Three African Populations
- Implicates Tyrosine-Sulfated Peptide Signaling in Susceptibility and Resistance to Root Infection
- Clathrin and AP2 Are Required for Phagocytic Receptor-Mediated Apoptotic Cell Clearance in
- Encodes CDF Transporters That Excrete Zinc from Intestinal Cells of and Act in a Parallel Negative Feedback Circuit That Promotes Homeostasis
- Global Properties and Functional Complexity of Human Gene Regulatory Variation
- DNA Binding of the Cell Cycle Transcriptional Regulator GcrA Depends on N6-Adenosine Methylation in and Other
- Side Effects: Substantial Non-Neutral Evolution Flanking Regulatory Sites
- From Paramutation to Paradigm
- From Mouse to Human: Evolutionary Genomics Analysis of Human Orthologs of Essential Genes
- Distinct Translational Control in CD4 T Cell Subsets
- Female Bias in and Regulation by the Histone Demethylase KDM6A
- ATM–Dependent MiR-335 Targets CtIP and Modulates the DNA Damage Response
- HDAC7 Is a Repressor of Myeloid Genes Whose Downregulation Is Required for Transdifferentiation of Pre-B Cells into Macrophages
- The Majority of Primate-Specific Regulatory Sequences Are Derived from Transposable Elements
- Identification of Meiotic Cyclins Reveals Functional Diversification among Plant Cyclin Genes
- EGL-13/SoxD Specifies Distinct O and CO Sensory Neuron Fates in
- Congruence of Additive and Non-Additive Effects on Gene Expression Estimated from Pedigree and SNP Data
- Using Extended Genealogy to Estimate Components of Heritability for 23 Quantitative and Dichotomous Traits
- Ikbkap/Elp1 Deficiency Causes Male Infertility by Disrupting Meiotic Progression
- Analysis of the Genetic Basis of Disease in the Context of Worldwide Human Relationships and Migration
- Duplication and Retention Biases of Essential and Non-Essential Genes Revealed by Systematic Knockdown Analyses
- Strong Purifying Selection at Synonymous Sites in
- , a Susceptibility Gene for Type 1 and Type 2 Diabetes, Modulates Pancreatic Beta Cell Apoptosis via Regulation of a Splice Variant of the BH3-Only Protein
- Chromosome Movements Promoted by the Mitochondrial Protein SPD-3 Are Required for Homology Search during Meiosis
- The Secretory Pathway Calcium ATPase PMR-1/SPCA1 Has Essential Roles in Cell Migration during Embryonic Development
- The Genomic Signature of Crop-Wild Introgression in Maize
- CDK4 T172 Phosphorylation Is Central in a CDK7-Dependent Bidirectional CDK4/CDK2 Interplay Mediated by p21 Phosphorylation at the Restriction Point
- Genome-Wide Identification of Regulatory RNAs in the Human Pathogen
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Using Extended Genealogy to Estimate Components of Heritability for 23 Quantitative and Dichotomous Traits
- HDAC7 Is a Repressor of Myeloid Genes Whose Downregulation Is Required for Transdifferentiation of Pre-B Cells into Macrophages
- Female Bias in and Regulation by the Histone Demethylase KDM6A
- ATM–Dependent MiR-335 Targets CtIP and Modulates the DNA Damage Response
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



