-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCell Polarity and Patterning by PIN Trafficking through Early Endosomal Compartments in
PIN-FORMED (PIN) proteins localize asymmetrically at the plasma membrane and mediate intercellular polar transport of the plant hormone auxin that is crucial for a multitude of developmental processes in plants. PIN localization is under extensive control by environmental or developmental cues, but mechanisms regulating PIN localization are not fully understood. Here we show that early endosomal components ARF GEF BEN1 and newly identified Sec1/Munc18 family protein BEN2 are involved in distinct steps of early endosomal trafficking. BEN1 and BEN2 are collectively required for polar PIN localization, for their dynamic repolarization, and consequently for auxin activity gradient formation and auxin-related developmental processes including embryonic patterning, organogenesis, and vasculature venation patterning. These results show that early endosomal trafficking is crucial for cell polarity and auxin-dependent regulation of plant architecture.
Published in the journal: . PLoS Genet 9(5): e32767. doi:10.1371/journal.pgen.1003540
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003540Summary
PIN-FORMED (PIN) proteins localize asymmetrically at the plasma membrane and mediate intercellular polar transport of the plant hormone auxin that is crucial for a multitude of developmental processes in plants. PIN localization is under extensive control by environmental or developmental cues, but mechanisms regulating PIN localization are not fully understood. Here we show that early endosomal components ARF GEF BEN1 and newly identified Sec1/Munc18 family protein BEN2 are involved in distinct steps of early endosomal trafficking. BEN1 and BEN2 are collectively required for polar PIN localization, for their dynamic repolarization, and consequently for auxin activity gradient formation and auxin-related developmental processes including embryonic patterning, organogenesis, and vasculature venation patterning. These results show that early endosomal trafficking is crucial for cell polarity and auxin-dependent regulation of plant architecture.
Introduction
Plant hormone auxin locally accumulates in plant tissues and regulates multiple processes of plant growth and development [1], [2]. Directional intercellular transport of auxin underlies most of known auxin-dependent control of development, including embryogenesis, root and shoot organogenesis, vascular tissue formation and asymmetric phototropic and gravitropic growths [3]. This polar auxin transport is achieved by collective actions of auxin efflux and influx transporters [4]–[6]. PIN-FORMED (PIN) family proteins asymmetrically localize at the plasma membrane (PM) in different plant tissues [7] and exhibit auxin efflux activities [8]. The polar localization of PIN proteins, together with their molecular role as auxin efflux facilitators, correlates well with known direction of polar auxin transport in different plant tissues. Furthermore, manipulation of polar PIN localization causes changes in auxin distribution and altered developmental and/or growth responses [7], [9]. Supported by these lines of evidence, it is widely accepted that polar localization of PIN proteins is essential in regulating auxin distribution in plant tissues.
Detailed observations of PIN family proteins have revealed that their polar localization changes dynamically during plant development [10]–[12] including responses to environmental cues [13]–[15]. PIN proteins are rapidly and constitutively shuttling between the PM and endosomes, providing a potential mechanism for their dynamic relocation [16], [17]. Fungal toxin brefeldin A (BFA) is known to inhibit vesicle transport that involves GDP-GTP exchange factors for small G proteins of ARF class (ARF GEFs). In Arabidopsis thaliana root, recycling of PIN1 protein preferentially to the basal side of cells requires a GBF-type ARF GEF, GNOM, which is highly sensitive to BFA [18]. As such, treatment with BFA of Arabidopsis roots results in intracellular accumulation of PIN1 proteins in agglomerated endomembrane compartments called ‘BFA compartments’[16].
By using BFA as a tool to visualize early endocytic trafficking defects, we have identified bfa-visualized endocytic trafficking defective (ben) mutants, which accumulate less PIN1-GFP proteins in BFA compartments [19]. BEN1 encodes a putative ARF GEF, which belongs to BIG class of ARF GEF subfamily and localizes to early endosomes [19]. However, information on the molecular components involved in endocytic trafficking remains scarce. It has also been elusive to what extent the early endosomal trafficking events are important for polar localization of proteins and thus to polarized development.
To gain better understanding of early endosomal trafficking in plants, we identified additional regulators of this process, manipulated it by genetic and pharmacological means and revealed its impact on cell polarity and development.
Results
BEN1 and BEN2 are involved in different steps of early endosomal trafficking
To dissect the early endosomal trafficking pathway in Arabidopsis root epidermal cells, we examined effects of a chemical inhibitor Endosidin1 (ES1), which affect actin dynamics and interfere with trafficking of endocytic cargoes at the trans-Golgi-network/early endosome (TGN/EE) [20], [21]. As shown previously [20], ES1 causes accumulation of PIN2 in agglomerated intracellular compartment (ES1 body) in wild type root epidermal cells (Figure 1A, 1B). Remarkably, accumulation of PIN2 protein in ES1 body was more pronounced in ben1 mutants than in wild type, indicating that ben1 mutation and ES1 treatment synergistically inhibited trafficking at the TGN/EE (Figure 1B, 1C).
Fig. 1. BEN1 and BEN2 are involved in distinct steps of early endosomal trafficking. 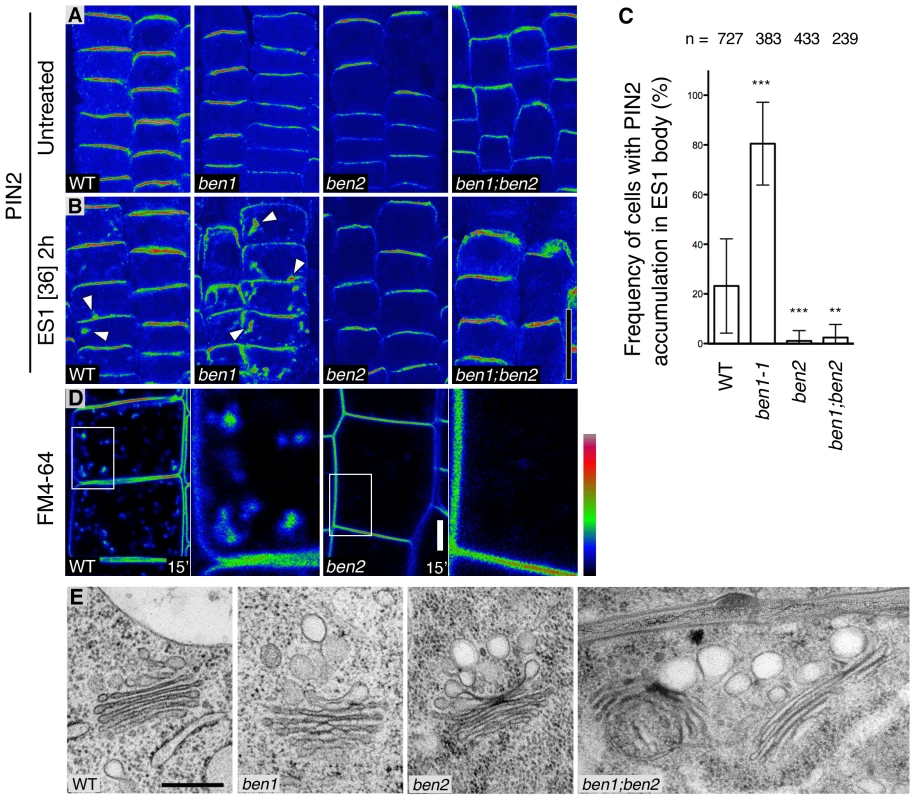
(A,B) PIN2 immunofluorescence signals in root epidermal cells without drug treatment (A) and after ES1 treatment (36 µM, 2 h) (B). Arrowheads indicate intracellular agglomerations of PIN2 signals. (C) Quantitative analyses of intracellular accumulation of PIN2 in ES1 treated root epidermal cells. Asterisks indicate significant difference from wild type control (**: P<0.01; ***: P<0.0001 by t-test). Error bars indicate standard deviation among individual roots. N: number of cells examined. (D) Uptake of endocytic tracer FM4-64 in wild type (left) and ben2 (right) root epidermal cells, 15 minutes after the onset of FM4-64 labeling. Magnified views of the boxed regions are indicated. Signal intensity is represented by the color code as indicated. (E) Ultrastructure of membranes associated with the Golgi apparatus in root epidermal cells of wild type and mutants. Scale bars: 20 µm in (B) for (A,B); 5 µm for (D); 300 nm for (E). Similar examination of ben2 mutant, which exhibits reduced agglomeration of PM proteins upon BFA treatment [19] (Figure S1A), revealed a less pronounced intracellular accumulation of PIN2 upon ES1 treatment (Figure 1B, 1C). The distinct responses to ES1 prompted us to determine the genetic relationship between ben1 and ben2. After incubation with ES1 under the same condition, ben1; ben2 double mutant cells did not show strong intracellular accumulation of PIN2 as compared with ben1 mutant (Figure 1B, 1C), indicating that ben2 mutation is epistatic in terms of responses to ES1.
Next, we tested if ben2 mutation affects endocytic trafficking by using a lipophilic styryl dye FM4-64, which is commonly used as endocytic tracer. As shown in Figure 1D, accumulation of endocytosed FM4-64 in endosomal compartments was also decreased in ben2. At later time point, however, FM4-64 stained vacuolar membrane in wild type as well as ben2 mutant (Figure S1B), implying that endocytic transport is operational to some extent in ben2 mutant. Therefore, it seems that BEN2 promotes transport of endocytosed cargo proteins to ES1-sensitive compartment (i.e. TGN/EE), whereas BEN1 functions in an ES1-insensitive pathway promoting transport of cargo-containing vesicles from the TGN/EE. As these observations indicated that BEN1 and BEN2 are functionally required for trafficking at the TGN/EE, we examined morphology of this compartment. Ultrastructural analysis revealed that enlarged vesicle-like structures often associated with the Golgi apparatus in ben1 and ben2 mutant cells (Figure 1E, Figure S1C). Additive features of both mutants were observed in the double mutant cells (Figure 1E, Figure S1C). Consistently, ben1 and ben2 mutations had a synergistic effect on seedling growth (Figure S1D, S1E). Taken together, these observations suggest the distinct roles of BEN1 and BEN2 in intracellular trafficking processes at the TGN/EE.
BEN2 encodes a Sec1/Munc18 family protein AtVPS45
To clone the BEN2 gene, we narrowed down the chromosomal region carrying ben2 mutation to a 140-kb region by fine mapping (Figure S2A). Subsequently, we took a candidate approach and found a mutation in At1g77140 gene, encoding a Sec1/Munc18 (SM) family protein VACUOLAR PROTEIN SORTING 45 (AtVPS45) [22]. The mutation in ben2 was predicted to cause amino-acid substitution of aspartic acid at position 129, which is well conserved in VPS45 of eukaryotic organisms, to asparagine (Figure 2A, Figure S2B). In ben2 mutant transformed with VPS45-GFP transgene, BFA responses as well as growth defects were restored (Figure 2B, Figure S2C), confirming that the mutation in VPS45 is responsible for ben2 phenotypes.
Fig. 2. BEN2 encodes a SM protein AtVPS45. 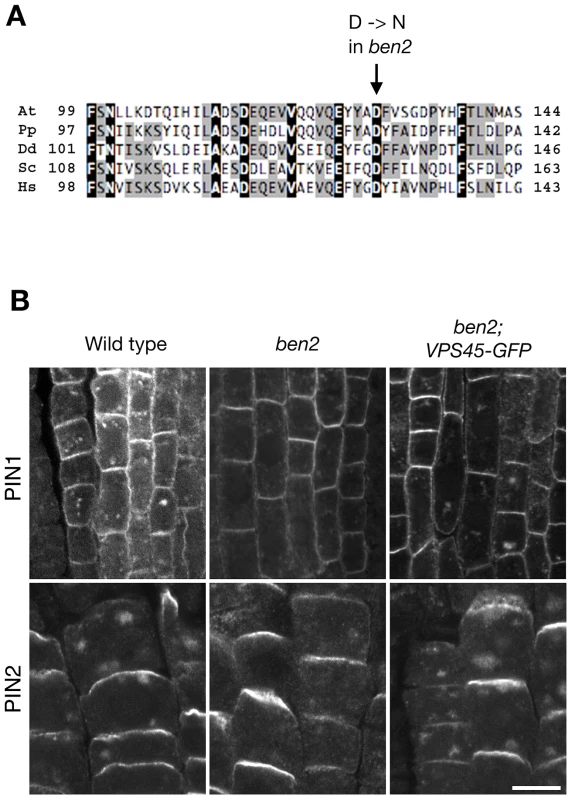
(A) Alignment of VPS45 protein sequences from various organisms. At: Arabidopsis thaliana, (At1g77140); Pp: Physcomitrella patens (XP_001758633); Dd: Dictyostelium discoideum (XP_635835); Sc: Saccharomyces cerevisiae (NP_011420); Hs: Homo sapiens (NP_009190). (B) Complementation by VPS45-GFP of ben2 mutant phenotypes. After BFA treatment (25 µM for 2 h), PIN1 accumulated in intracellular compartments in wild type root vascular tissue. Whereas intracellular accumulation of PIN1 was less pronounced in ben2 mutant, clear accumulation of PIN1 was detected in ben2 mutant harboring VPS45-GFP. Similarly, PIN2 accumulation in root epidermal cells upon BFA treatment was recovered in ben2; VPS45-GFP. Scale bar: 10 µm. BEN2/VPS45 resides in early endocytic route
VPS45 is a member of SM family proteins, which are universal components for membrane fusion in eukaryotic cells. Distinct combinations of SM and SNARE (soluble N-ethylmaleimide-sensitive factor attachment receptor) proteins are thought to ensure specificity of membrane fusions [23]. In yeast, Vps45p binds to SNARE proteins Pep12p and Tlg2p and functions in trafficking to the vacuole [24]. Analogously, AtVPS45 forms a complex with TGN-localized SNARE proteins, which collectively play critical roles in vacuolar trafficking [22], [25]–[27]. In addition, recent studies have demonstrated crucial roles of VPS45 homologs in endocytic trafficking in yeast and animals [28]–[30]. As ben2 mutant phenotypes suggest that AtVPS45 is involved in endocytic trafficking, we tested if AtVPS45 resides in endocytic route. Indeed, our colocalization study revealed that endocytosed FM4-64 rapidly colocalized with VPS45-GFP in root epidermal cells (Figure 3A). Consistently, VPS45-GFP or VPS45-RFP signals colocalized well with a TGN/EE marker VHA-a1-GFP and BEN1, but only marginally with a marker for Golgi apparatus SYP32-YFP (Figure 3B, Figure S3A). When we tested if PIN2-GFP proteins are detectable in BEN1 or BEN2-positive endosomes, we detected only a few colocalization under drug-untreated condition, probably because intracellular trafficking through the TGN/EE is a transient event (Figure S3B, S3C). However, upon treatment with BFA, both BEN1 and PIN2-GFP accumulated in BFA compartments (Figure S3B). On the other hand, VPS45-RFP was mainly detected at the periphery of agglomerated PIN2-GFP signals (Figure S3C).
Fig. 3. Double labeling experiments reveal early endosomal localization of VPS45 in root epidermal cells. 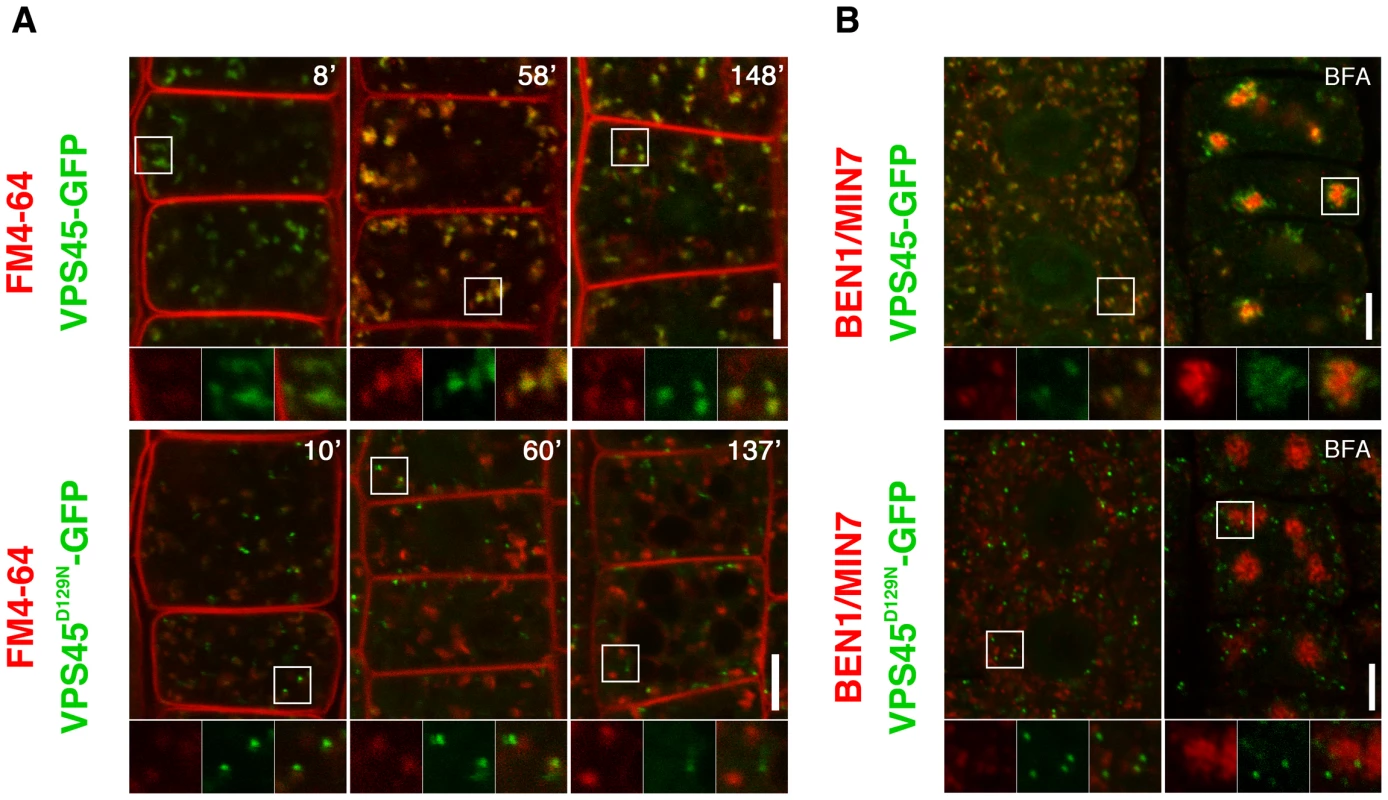
(A) Wild type VPS45-GFP (green) colocalized with FM4-64 (red) within 10 minutes after the onset of staining. Additional endocytic compartments were labeled by prolonged incubation (upper panels). Mutated VPS45-GFP carrying ben2 mutant sequence (VPS45D129N-GFP, green) did not colocalize with FM4-64 under the same conditions (lower panels). (B) Immunostaining of BEN1 (red) and VPS45-GFP (green). Whereas wild type version of VPS45-GFP partially colocalized with BEN1 (upper left panel), ben2 mutation abolished the colocalization (lower left panel). BEN1 and VPS45-GFP responded differently to BFA (upper right panel). Whereas BEN1 accumulates to the center of the BFA compartment in BFA-treated cells, majority of VPS45-GFP localized to the periphery of the BFA compartment. ben2 mutation caused mislocalization of the VPS45-GFP protein (green), although it did not affect the agglomeration of BEN1 signal (lower right panel). Magnified views of the regions indicated by white squares are shown in the bottom panels. The right panels show merged images. Scale bars: 5 µm. Considering the proposed role of VPS45 in membrane fusion at the TGN [22] and the current model of early endocytic trafficking in Arabidopsis [31], it is reasonable to think that VPS45 resides in early endocytic route. The early endocytic trafficking defect observed in ben2 mutant (Figure 1) is also consistent with the early endosomal localization of VPS45.
ben2 mutation influences BEN2/VPS45 localization
In order to know how the amino acid substitution by the ben2 mutation affects the gene product, we generated a VPS45-GFP construct with the ben2 mutant sequence (VPS45D129N-GFP) under the native promoter. Although transgenic plants expressing VPS45D129N-GFP fusion proteins exhibited punctate GFP signals in root epidermal cells, the GFP signals did not overlap with FM4-64 at early time point (Figure 3A, lower left panel), VPS45-RFP (Figure S3D), and BEN1 (Figure 3B, lower panel). In the presence of BFA, whereas VPS45-GFP partially colocalized with BEN1 mainly at the periphery of the BFA compartment, VPS45D129N-GFP did not show clear colocalization with BEN1 (Figure 3B, right panels). Thus, it seems that the ben2 mutation compromised VPS45 function by affecting its association with TGN/EE. To examine the effect of ben2 mutation on the subcellular localization of VPS45 protein in more detail, we tested prolonged incubation with the endocytic tracer FM4-64, which is known to stain late endocytic compartments and vacuolar membrane in time-dependent manner [32]. As shown in Figure 3A, after prolonged incubation, whereas most of the VPS45-GFP positive endosomes were labeled with FM4-64, some other intracellular FM4-64 signals were not colocalized with VPS45-GFP in root epidermal cells, suggesting that late endocytic compartments were also labeled at these time points (Figure 3A). In contrast, in VPS45D129N-GFP expressing seedling roots, most of the GFP-positive endosomes were not clearly labeled with FM4-64 over two hours. We also used an acidotropic probe LysoTracker red to visualize endosomal compartments. Whereas signals from VPS45-GFP and LysoTracker partially overlapped, VPS45D129N-GFP did not show major overlap (Figure S3D). Immunostaining with anti-SEC21 (a Golgi marker) and anti-BiP (an ER marker) also revealed no colocalization with VPS45D129N-GFP. Together, these results suggest that the ben2 mutation caused mislocalization of VPS45D129N-GFP protein to a cryptic compartment other than ER, Golgi apparatus, TGN/EE, and the late compartments.
To gain more insight into the mechanistic impact of the ben2 (D129N) mutation on the function of VPS45 protein, we examined whether the interaction between VPS45-GFP and TGN-localized Qa-SNARE SYP4 is affected by the D129N mutation. For this purpose, we immunoprecipitated VPS45-GFP and VPS45D129N-GFP with an anti-GFP antibody. As shown in Figure S3E, SYP4 was co-immunoprecipitated with VPS45-GFP, consistent with previous reports [22], [25]. However, SYP4 protein did not co-immunoprecipitate with VPS45D129N-GFP even though SYP4 protein was detectable in total protein extract, suggesting that the ben2 mutation has compromised the physical association between VPS45 and SYP4.
BEN1 - and BEN2-dependent trafficking is involved in polar localization of PIN proteins
In order to test the biological relevance of BEN1 - and BEN2-dependent trafficking through the TGN/EE, we examined the subcellular localization of PIN proteins in wild type and mutant roots. Our immunofluorescent study and quantitative analysis revealed that ben1 and ben2 mutation compromised the polar localization of PIN1 in the root tip (Figure 4A, Figure S4A). Whereas polar localization of PIN2 protein was not significantly affected in ben1 and in ben2 single mutants, the polar localization was significantly impaired in ben1; ben2 double mutant. And in severe cases, PIN2 signals were almost non-polar in the root epidermal cells (Figure 4B, Figure S4B).
Fig. 4. BEN1 and BEN2 genes are required for polar localization of PIN proteins. 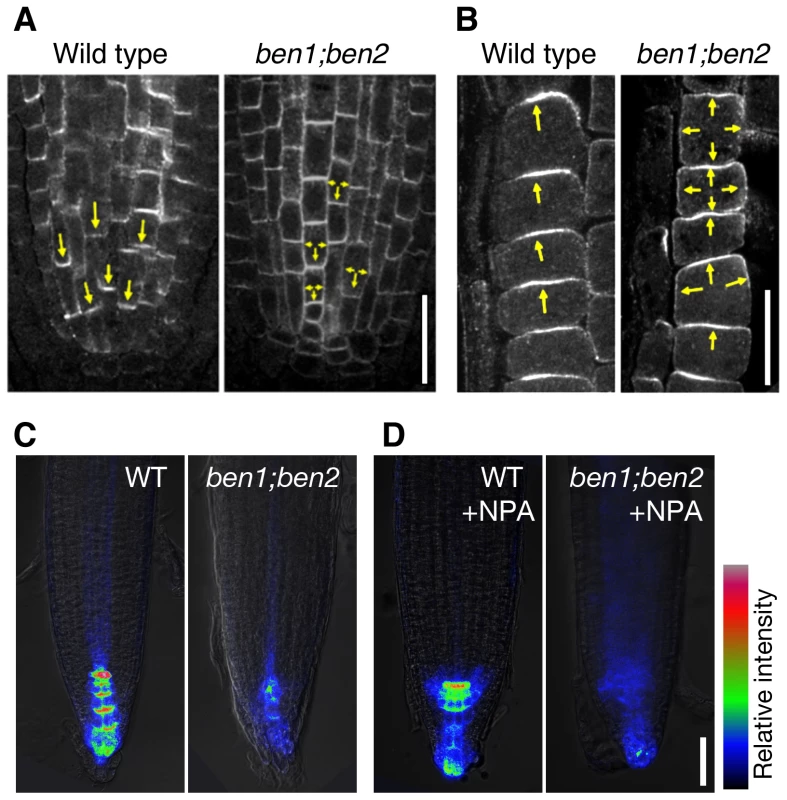
(A,B) Antibody staining of endogenous PIN1 protein in vascular tissue (A) and PIN2 on epidermal cells (B) of wild type and ben1; ben2 double mutant roots. Arrows indicate polar localization of PIN proteins. (C,D) ben mutations and chemical inhibitor of auxin transport collectively diminish auxin response maxima. Auxin response maxima were visualized by DR5rev::GFP with color coding of signal intensities. Seedlings of indicated genotypes (3d) were transferred to mock (C) or NPA-containing media (50 µM) and grown for 2 days before imaging. Scale bars: 20 µm for (A,B); 50 µm in (D) for (C,D). For polarization of PIN proteins, a tissue-dependent feedback regulation by auxin has been demonstrated [33]. A well-characterized example is root endodermal cells, where auxin induces PIN1 localization to the inner lateral side [33]. As previously described, treatment with naphthalene-1-acetic acid (NAA) induced significant enrichment of PIN1 to the inner side in wild type roots (Figure S4C, S4D). However, in the ben1 and ben2 mutants as well as ben1; ben2 double mutant, PIN1 polarity is less pronounced without exogenously applied auxin and treatment with auxin did not dramatically induce relocation of PIN1 proteins (Figure S4C, S4D). As TGN/EE could be a sorting platform for trafficking pathways including secretion and vacuolar targeting [31], [34], we speculated that defects in BEN1 and BEN2 might affect multiple trafficking pathways. We expected that if secretion is severely impaired in ben1 or ben2 mutant, we might be able to see defect in recovery of PM proteins by a fluorescence recovery after photobleaching (FRAP) analysis. To test this possibility, we performed a FRAP experiment using PIN2-GFP. As shown in Figure S5A, when PIN2-GFP in a root epidermal cell was photobleached, the PM signal was recovered over 4 hours to approximately 13% of the initial signal intensity in PIN2-GFP; eir1 line as control. Similarly, ben1 and ben2 mutants also exhibited recovery of the PM signals, approximately to 15 to 16% on average, revealing no discernible defect in the recovery rate (Figure S5B). Next, we evaluated if vacuolar targeting of PIN2 is affected. For this purpose, we incubated seedlings in the darkness before observation, to allow accumulation of GFP in the lytic vacuole [35], [36]. As reported previously, this allowed detection of vacuolar localized GFP signals in PIN2-GFP expressing root epidermal cells [36] (Figure S5C, S5D). Interestingly, ben1 and ben2 mutants as well as ben1; ben2 double mutants tended to have weaker vacuolar GFP signals than control line did (Figure S5C, S5D), implying possible involvement of BEN1 and BEN2 also in vacuolar transport.
Taken together, these results support a scenario in which BEN1 and BEN2 are involved in intracellular trafficking including early endosomal trafficking through the TGN/EE that is required for polar localization of PIN1 and PIN2 proteins.
BEN1 - and BEN2-dependent trafficking is required for root architecture
Next, we addressed if BEN1 - and BEN2-dependent trafficking is required for auxin-dependent developmental processes. As described above, whereas ben1 and ben2 single mutants exhibited only mild defects in terms of root growth, ben1; ben2 double mutant showed a stronger defect (Figure S1D, S1E). Thus, our finding is consistent with previously suggested role of VPS45 in supporting plant growth [25], although ben2 single mutant exhibited only minor phenotypes probably due to the hypomorphic nature of the ben2 mutant allele. To gain more insights into the developmental roles of BEN1/MIN7 and BEN2/VPS45, we characterized the phenotypes in terms of localization of PIN proteins and auxin response gradient, mainly focusing on ben1; ben2 double mutant.
Consistent with the PIN polarity defects as well as root growth defects, auxin response maxima as visualized by DR5rev::GFP [11] was reduced in ben1; ben2 double mutant roots (Figure 4C) and it was further decreased in the presence of a polar auxin transport inhibitor naphtylphthalamic acid (NPA) (Figure 4D). Whereas the growth of primary roots was significantly slower, the formation of lateral roots was not inhibited in ben1; ben2 plantlets, causing a short and bushy appearance of root system architecture (Figure S6A).
To investigate the roles of BEN genes in lateral root (LR) development, we induced LR formation by auxin and followed the development of primordia. When grown in the presence of NAA, formation of lateral root primordia (LRP) is induced in wild type seedlings within 3 days with gradual relocation of PIN1-GFP from anticlinal sides to tipward of newly formed LRP [10] (Figure 5A). In ben1; ben2 double mutant, PIN1-GFP did not efficiently relocate (Figure 5B), LRPs were deformed and auxin response maxima less confined to root tips (Figure 5C). Later on, wild type LRPs continued growing, forming dense array of lateral roots with strong DR5 response maximum at each root tip (Figure 5D). At the same time point, however, additional primordia with DR5 maxima were formed on growing LRPs in ben1; ben2 double mutant (Figure 5D). In such LRPs, PIN1-GFP was ectopically expressed in the epidermis of the mutant LRPs and polarized as if it might have created ectopic auxin maxima (Figure 5E, Figure S6B), although PIN1 in provascular tissues drives auxin flow toward the tips of developing root primordia in normal course as well as auxin-induced LRP initiation [10] (Figure 5E).
Fig. 5. ben mutations alter the pattern of organ initiation and primordia morphology. 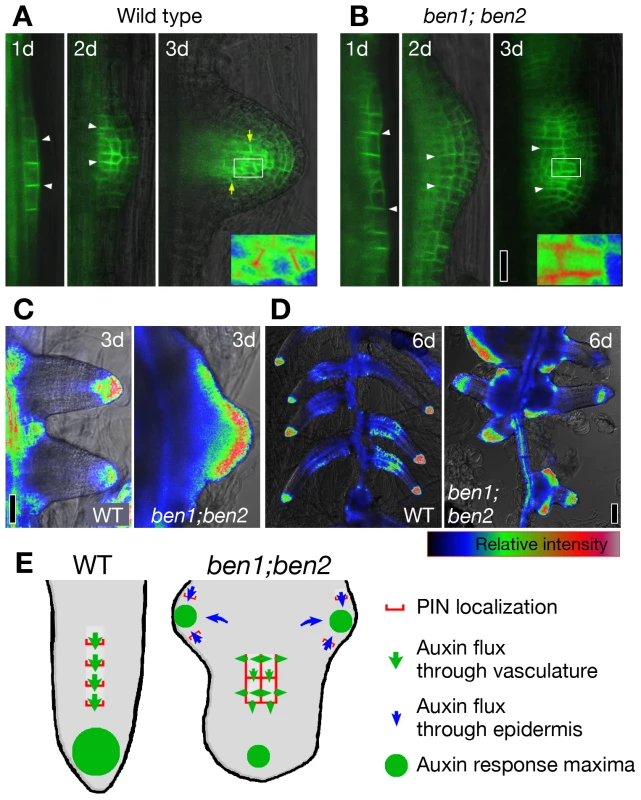
(A,B) Localization of PIN1-GFP in developing LRPs. PIN1-GFP localizes to the anticlinal sides of young LRPs (white arrowheads) and gradually shifts its localization toward the tip of developing LRP within three days (A; yellow arrows). Relocation was less clear in the ben1; ben2 double mutant (B). (C,D) Auxin response maxima as visualized by DR5rev::GFP reporter. Whereas sharp peaks were formed at the tips of LRPs in wild type, DR5 expression was broader in malformed LRPs of ben1; ben2 three days after the onset of induction (C). Sharp peaks of auxin response maxima were maintained over time in wild type. In contrast, new auxin response maxima were generated in the base of LRPs in ben1; ben2 (D). (E) A model to explain altered root architecture. PIN1 relocates towards the tip of LRP and facilitates directional auxin flow in provascular tissue (green arrows), resulting in generation of auxin maxima at the tip of LRP (green circle). PIN relocation is compromised in ben1; ben2 double mutant. PIN1 is ectopically expressed in the epidermis by unknown mechanism, which in turn generates atypical auxin flow (blue arrows). Scale bars: 20 µm in (B) for (A,B); 50 µm in (C); 100 µm in (D). BEN1 and BEN2 are required for multiple auxin-dependent developmental processes
Because dynamic relocation of PIN proteins is involved in multiple developmental processes including root and shoot organogenesis, embryonic patterning, and vasculature venation patterns [10], [11], [37], we next examined if BEN-dependent trafficking is commonly required for these processes. Whereas ben1 and ben2 single mutants showed only moderate shoot growth defect, ben1; ben2 mutant exhibited severely impaired shoot growth (Figure S6C). We also noticed that positions of siliques were often irregular in the ben1; ben2 double mutant plants (Figure S6D). During embryogenesis, whereas ben1 and ben2 single mutants exhibited only moderate patterning defect (Figure S6F), patterning in developing embryos were more often disrupted in the ben1; ben2 mutant (Figure S6E, S6F). In such abnormal embryos, polar localization of PIN1-GFP and auxin response maxima were less pronounced (Figure S6G, S6H). Similarly, whereas ben1 single mutant exhibited a moderate defects in cotyledon venation pattern as reported previously [19] and ben2 single mutation did not show discernible defect, ben1; ben2 double mutant exhibited a severe venation pattern defects (Figure S7A–S7C). During the course of leaf primordial development, PIN1 expression precedes the development of vasculature and PIN1 polarity typically points toward preexisting veins when the PIN1 expression domain becomes connected [37] (Figure S7D). However, PIN1-GFP expression often remained disconnected in ben1; ben2 leaf primordia with less pronounced polar localization of PIN1-GFP at the PM (Figure S7E).
Discussion
The data presented here demonstrated that BEN1 and BEN2 genes have clear biological relevance in polar PIN localization, local auxin response, patterning and plant architecture. PIN-dependent regulation of local auxin distribution is a plant-specific mechanism, which is repeatedly utilized in different aspects of growth and development [2]. Polar localization of PIN proteins requires clearance from the PM by endocytosis [38], [39], retargeting to and retention at the polar domain [18], [40]. This work specifically reveals that the trafficking at the level of early endosomes is a critical mechanism for polar distribution of proteins exemplified by PIN auxin transporters.
In the current study, we showed that BEN2 is identical to AtVPS45, which has been known to function in the vacuolar targeting pathway. Reportedly, when the VPS45 function is inactivated by RNAi, vacuolar cargo proteins containing the C-terminal vacuolar sorting determinants are mistargeted and instead transported to the apoplast [25]. Our finding may represent a parallel situation, as the ben2 mutation compromised vacuolar localization of PIN2-GFP and together with ben1 mutation caused a relatively strong PIN2 polarity defect at the PM. It is also possible that reduced activities of BEN1/MIN7 and BEN2/VPS45 might have caused dysfunction of TGN/EE to compromise multiple trafficking pathways that include vacuolar targeting and endocytic recycling. Results of our ultrastructural analysis fit with the later hypothesis, because ben2 mutation, which might interfere with membrane fusion at the TGN/EE, resulted in accumulation of enlarged vesicle-like structures especially when combined with ben1 mutation, as if membrane budding might be inhibited and/or identity of the accumulating vesicles might be altered. Although we still do not know the detailed mechanisms regulating PIN trafficking at the TGN/EE, our results suggest that BEN1/MIN7 and BEN2/VPS45 are important components.
In addition to the steady-state polar localization of PIN proteins in established tissues, dynamic changes of PIN localization are involved in plant development. It has also been demonstrated that the polar localization and amount of PIN proteins at the PM are controlled by different environmental stimuli [13], [14] as well as by endogenous cues [10], [11], [37]. Plant hormones auxin and cytokinin have strong impact in regulating PIN level and/or localization. Interestingly, each of these plant hormones regulates PINs by different modes (i.e. transcriptional and posttranslational control). For example, whereas auxin inhibits endocytosis via regulation of an auxin receptor ABP1 [41]–[43], it also stimulates relocation of PIN1 by a manner that requires transcriptional regulation, probably through another class of auxin receptor TIR1/AFBs [33]. On the other hand, cytokinin not only modifies transcription of PIN genes in root meristem [44]–[47] but also promotes degradation of PIN1 protein in LRP [48]. A possible link between cytokinin-regulated PIN degradation and BEN genes has also recently been provided [48], although the underlying mechanisms remain elusive. Here, we showed that defects in early endosomal components had strong impact on auxin-mediated PIN relocation as well as developmental processes that involve dynamic changes of PIN polarity. We speculate that efficient trafficking through TGN/EE is involved in rapid changes of PIN polarity and/or levels in response to the stimuli. The mechanism by which multiple stimuli regulate PIN localization via BEN-dependent trafficking is an important issue to be addressed in future.
Although our results highlight the roles of early endosomal trafficking in PIN-dependent regulation of plant development, many other proteins might be transported via BEN1 - and BEN2-dependent pathways. In this respect, it is noteworthy that roles of trafficking at the level of TGN/EE on defense responses and vacuolar targeting are recently emerging [25], [27], [48], [49].
Materials and Methods
Plant materials and phenotypic analysis
Following Arabidopsis thaliana mutants and transgenic lines have been described: PIN1-GFP [10], ben1-1, ben1-2, ben2-1 [19], DR5rev::GFP [11], VHA-a1-GFP [50], Wave 22Y [51]. PIN2-GFP [52] was introduced into ben1-1, ben2 and ben1-1; ben2 background by genetic crossing. Measurements of root length and clearing of seedlings and embryos were performed as described previously [19].
Chemical treatments
Treatment with BFA (Invitrogen B7450) and staining with FM4-64 (Sigma S6689) were performed as described [19]. ES1 [20] (3.6 mM stock in DMSO) was diluted with liquid media. For LRP induction, young seedlings (3d) were transferred to solid plate containing 10 µM of NAA and vertically grown for indicated period. For evaluating auxin-induced PIN1 lateralization, seedlings were treated with 10 µM of NAA for 4 hours as described [33].
Immunodetection and microscopy
Whole-mount immunolocalization on Arabidopsis roots were performed as described [19]. Antibodies were diluted as follows: rabbit anti-PIN1 (1∶1000) [41], rabbit anti-PIN2 (1∶1000) [53], rabbit anti-MIN7/BEN1 (1∶1000) [49], rabbit anti-SEC21 (1∶1000; Agrisera AS08 327), rabbit anti-BiP (1∶1000; Agrisera AS09 481), Cy3-conjugated secondary anti-rabbit (1∶600; Sigma C2306) antibodies. For staining with LysoTracker red, 1 mM stock in DMSO (Invitrogen L7528) was diluted with liquid media for Arabidopsis to make 2 µM solution and incubated for one hour before observation. Fluorescence imaging was done either by Carl Zeiss LSM5 exciter or LSM710 confocal microscopes. For calculating polarity index of PIN1 and PIN2, medial sections of root tips or paradermal sections through root epidermis were imaged at least from 10 roots in each line. Signal intensities of the polar domains and lateral PM were measured by ImageJ using the line function and statistic analyses were performed using PRISM software (version 5.0a, GraphPad Software, Inc.). Photobleaching was performed essentially as described [54] using at least 6 seedlings from each genotype. To visualize vacuolar localization of GFP in PIN2-GFP expressing lines, seedlings on vertical plates were kept in the darkness for 5 hours before observation. Vacuolar signals were evaluated by using color-coded images of paradermal confocal sections, typically containing 15 to 20 epidermal cells per root. Transmission electron microscopy was performed as described [19].
Molecular cloning of BEN2 gene and DNA construction
Polymorphic F2 seedlings obtained from crossing ben2 mutant with a Landsberg erecta plant were treated with BFA and screened for ben2 mutant phenotype by epifluorescense microscopy. DNA was isolated from each of 220 homozygous F2 plants and subjected to fine mapping. The ben2 mutation was mapped between two markers F22K20-Eco72I (28.98 Mb) and T5M16-IPI (29.12 Mb) on chromosome 1. In this 140-kb region, we found a mutation in AtVPS45 gene (At1g77140). For genotyping, the ben2-1 mutation was detected by PCR with primers BEN2-863D(m): 5′-GGTTTTTTATATTGCAGGAGTATTCTGC-3′ and BEN2-970R: 5′-CGACAACTGCGGGGATCA-3′ followed by digestion with restriction enzyme PstI. To generate VPS45-GFP and VPS45-RFP fusion constructs under control of CaMV 35S promoter, VPS45 sequence was amplified from Arabidopsis cDNA (ecotype Col-0) with primers VPS45-GFP B1 : 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTATGGTTTTGGTTACGTCTGTGCGT-3′ and VPS45-GFP B2 : 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCCACCATATGGCTACCTGATC-3′. The PCR-amplified VPS45 cDNA was cloned behind cauliflower mosaic virus 35S promoter (P35S) of Gateway-compatible binary vectors pH7FWG2 [55] and pK7RWG2 (https://gateway.psb.ugent.be/; RFP was kindly provided by Dr. Tsien, HHMI, UCSD). The resultant constructs pH7FWG2-p35S::VPS45-GFP and pK7RWG2-p35S::VPS45-RFP were transformed by agrobacterium-mediated floral-dip transformation procedure into ben2-1 mutant and wild type Col-0, respectively. For generating genomic VPS45 fused to GFP under control of native promoter, genomic fragment was amplified from wild type and ben2 mutant with following primers: pVPS45-B1: GGGGACAAGTTTGTACAAAAAAGCAGGCTCGCAAAACGGTGCGTATTAGGAAAAT; VPS45-GFP-B2: GGGGACCACTTTGTACAAGAAAGCTGGGT T CACCATATGGCTACCTGATC. Amplified fragments were cloned into a binary vector pH7FWG,0 (https://gateway.psb.ugent.be/) to generate binary constructs pH7FWG-pVPS45::VPS45-GFP and pH7FWG-pVPS45::VPS45D129N-GFP, respectively. These constructs were transformed into Col-0 wild type plants. Transgenic plants were selected on solid media containing Kanamycin (25 mg/L) or Hygromycin (15 mg/L).
Immunoprecipitation and immunoblot experiments
Immunoprecipitation from detergent extracts was carried out using the micro-MACS GFP-tagged protein isolation kit (Miltenyi Biotec), according to the manufacturer's instructions using 12 day-old A. thaliana plantlets (0.5 g) as the starting material. Immunoblot analysis was performed as described [27] with antibody against SYP4 (Uemura, T., unpublished) and anti-GFP antibody (Nacalai Tesque).
Supporting Information
Zdroje
1. MockaitisK, EstelleM (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24 : 55–80.
2. VannesteS, FrimlJ (2009) Auxin: a trigger for change in plant development. Cell 136 : 1005–1016.
3. GrunewaldW, FrimlJ (2010) The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J 29 : 2700–2714.
4. KerrID, BennettMJ (2007) New insight into the biochemical mechanisms regulating auxin transport in plants. Biochem J 401 : 613–622.
5. PetrášekJ, FrimlJ (2009) Auxin transport routes in plant development. Development 136 : 2675–2688.
6. GeislerM, MurphyAS (2006) The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett 580 : 1094–1102.
7. WiśniewskaJ, XuJ, SeifertovaD, BrewerPB, RůžičkaK, et al. (2006) Polar PIN localization directs auxin flow in plants. Science 312 : 883.
8. PetrášekJ, MravecJ, BouchardR, BlakesleeJJ, AbasM, et al. (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312 : 914–918.
9. ZhangJ, NodzynskiT, PencikA, RolcikJ, FrimlJ (2010) PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc Natl Acad Sci U S A 107 : 918–922.
10. BenkováE, MichniewiczM, SauerM, TeichmannT, SeifertováD, et al. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 : 591–602.
11. FrimlJ, VietenA, SauerM, WeijersD, SchwarzH, et al. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426 : 147–153.
12. SorefanK, GirinT, LiljegrenSJ, LjungK, RoblesP, et al. (2009) A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 459 : 583–586.
13. FrimlJ, WiśniewskaJ, BenkováE, MendgenK, PalmeK (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415 : 806–809.
14. DingZ, Galvan-AmpudiaCS, DemarsyE, LangowskiL, Kleine-VehnJ, et al. (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13 : 447–452.
15. RakusováH, Gallego-BartolomeJ, VanstraelenM, RobertHS, AlabadiD, et al. (2011) Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J 67 : 817–826.
16. GeldnerN, FrimlJ, StierhofYD, JürgensG, PalmeK (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413 : 425–428.
17. DhonuksheP, AnientoF, HwangI, RobinsonDG, MravecJ, et al. (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17 : 520–527.
18. Kleine-VehnJ, DhonuksheP, SauerM, BrewerPB, WiśniewskaJ, et al. (2008) ARF GEF-Dependent Transcytosis and Polar Delivery of PIN Auxin Carriers in Arabidopsis. Curr Biol 18 : 526–531.
19. TanakaH, KitakuraS, De RyckeR, De GroodtR, FrimlJ (2009) Fluorescence imaging-based screen identifies ARF GEF component of early endosomal trafficking. Curr Biol 19 : 391–397.
20. RobertS, CharySN, DrakakakiG, LiS, YangZ, et al. (2008) Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc Natl Acad Sci U S A 105 : 8464–8469.
21. TothR, Gerding-ReimersC, DeeksMJ, MenningerS, GallegosRM, et al. (2012) Prieurianin/endosidin 1 is an actin-stabilizing small molecule identified from a chemical genetic screen for circadian clock effectors in Arabidopsis thaliana. Plant J 71 : 338–352.
22. BasshamDC, SanderfootAA, KovalevaV, ZhengH, RaikhelNV (2000) AtVPS45 complex formation at the trans-Golgi network. Mol Biol Cell 11 : 2251–2265.
23. SüdhofTC, RothmanJE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323 : 474–477.
24. ToonenRF, VerhageM (2003) Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol 13 : 177–186.
25. ZouharJ, RojoE, BasshamDC (2009) AtVPS45 Is a Positive Regulator of the SYP41/SYP61/VTI12 SNARE Complex Involved in Trafficking of Vacuolar Cargo. Plant Physiol 149 : 1668–1678.
26. SurpinM, ZhengH, MoritaMT, SaitoC, AvilaE, et al. (2003) The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15 : 2885–2899.
27. UemuraT, KimH, SaitoC, EbineK, UedaT, et al. (2012) Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc Natl Acad Sci U S A 109 : 1784–1789.
28. LewisMJ, NicholsBJ, Prescianotto-BaschongC, RiezmanH, PelhamHR (2000) Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell 11 : 23–38.
29. Gengyo-AndoK, KuroyanagiH, KobayashiT, MurateM, FujimotoK, et al. (2007) The SM protein VPS-45 is required for RAB-5-dependent endocytic transport in Caenorhabditis elegans. EMBO Rep 8 : 152–157.
30. MorrisonHA, DionneH, RustenTE, BrechA, FisherWW, et al. (2008) Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell 19 : 4167–4176.
31. ViottiC, BubeckJ, StierhofYD, KrebsM, LanghansM, et al. (2010) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22 : 1344–1357.
32. UedaT, YamaguchiM, UchimiyaH, NakanoA (2001) Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J 20 : 4730–4741.
33. SauerM, BallaJ, LuschnigC, WiśniewskaJ, ReinohlV, et al. (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20 : 2902–2911.
34. ScheuringD, ViottiC, KrugerF, KunzlF, SturmS, et al. (2011) Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell 23 : 3463–3481.
35. TamuraK, ShimadaT, OnoE, TanakaY, NagataniA, et al. (2003) Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J 35 : 545–555.
36. Kleine-VehnJ, LeitnerJ, ZwiewkaM, SauerM, AbasL, et al. (2008) Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci U S A 105 : 17812–17817.
37. ScarpellaE, MarcosD, FrimlJ, BerlethT (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20 : 1015–1027.
38. DhonuksheP, TanakaH, GohT, EbineK, MähönenAP, et al. (2008) Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456 : 962–966.
39. KitakuraS, VannesteS, RobertS, LöfkeC, TeichmannT, et al. (2011) Clathrin Mediates Endocytosis and Polar Distribution of PIN Auxin Transporters in Arabidopsis. Plant Cell 23 : 1920–1931.
40. Kleine-VehnJ, WabnikK, MartinièreA, ŁangowskiL, WilligK, et al. (2011) Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol Syst Biol 7 : 540.
41. PaciorekT, ZažímalováE, RuthardtN, PetrášekJ, StierhofYD, et al. (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435 : 1251–1256.
42. RobertS, Kleine-VehnJ, BarbezE, SauerM, PaciorekT, et al. (2010) ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143 : 111–121.
43. NagawaS, XuT, LinD, DhonuksheP, ZhangX, et al. (2012) ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol 10: e1001299 doi:10.1371/journal.pbio.1001299.
44. Dello IoioR, NakamuraK, MoubayidinL, PerilliS, TaniguchiM, et al. (2008) A genetic framework for the control of cell division and differentiation in the root meristem. Science 322 : 1380–1384.
45. RůžičkaK, ŠimáškováM, DuclercqJ, PetrášekJ, ZažímalováE, et al. (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci U S A 106 : 4284–4289.
46. KiefferM, NeveJ, KepinskiS (2010) Defining auxin response contexts in plant development. Curr Opin Plant Biol 13 : 12–20.
47. BishoppA, BenkováE, HelariuttaY (2011) Sending mixed messages: auxin-cytokinin crosstalk in roots. Curr Opin Plant Biol 14 : 10–16.
48. MarhavýP, BielachA, AbasL, AbuzeinehA, DuclercqJ, et al. (2011) Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell 21 : 796–804.
49. NomuraK, DebroyS, LeeYH, PumplinN, JonesJ, et al. (2006) A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science 313 : 220–223.
50. DettmerJ, Hong-HermesdorfA, StierhofYD, SchumacherK (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18 : 715–730.
51. GeldnerN, Denervaud-TendonV, HymanDL, MayerU, StierhofYD, et al. (2009) Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J 59 : 169–178.
52. XuJ, ScheresB (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17 : 525–536.
53. AbasL, BenjaminsR, MalenicaN, PaciorekT, WiśniewskaJ, et al. (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8 : 249–256.
54. GrebeM, XuJ, MobiusW, UedaT, NakanoA, et al. (2003) Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr Biol 13 : 1378–1387.
55. KarimiM, InzéD, DepickerA (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 : 193–195.
Štítky
Genetika Reprodukční medicína
Článek Attachment Site Selection and Identity in Bxb1 Serine Integrase-Mediated Site-Specific RecombinationČlánek Bck2 Acts through the MADS Box Protein Mcm1 to Activate Cell-Cycle-Regulated Genes in Budding YeastČlánek High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple IsolatesČlánek Neuropeptides Function in a Homeostatic Manner to Modulate Excitation-Inhibition Imbalance inČlánek Implicates Tyrosine-Sulfated Peptide Signaling in Susceptibility and Resistance to Root Infection
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 5
-
Všechny články tohoto čísla
- Functional Elements Are Embedded in Structurally Constrained Sequences
- RNA–Mediated Epigenetic Heredity Requires the Cytosine Methyltransferase Dnmt2
- Loss of Expression and Promoter Methylation of SLIT2 Are Associated with Sessile Serrated Adenoma Formation
- Attachment Site Selection and Identity in Bxb1 Serine Integrase-Mediated Site-Specific Recombination
- Human Genetics in Rheumatoid Arthritis Guides a High-Throughput Drug Screen of the CD40 Signaling Pathway
- Genome-Wide Analysis in German Shepherd Dogs Reveals Association of a Locus on CFA 27 with Atopic Dermatitis
- Liver X Receptors Protect from Development of Prostatic Intra-Epithelial Neoplasia in Mice
- Chromosomal Organization and Segregation in
- A Statistical Framework for Joint eQTL Analysis in Multiple Tissues
- Cell Polarity and Patterning by PIN Trafficking through Early Endosomal Compartments in
- Bck2 Acts through the MADS Box Protein Mcm1 to Activate Cell-Cycle-Regulated Genes in Budding Yeast
- High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple Isolates
- Neuropeptides Function in a Homeostatic Manner to Modulate Excitation-Inhibition Imbalance in
- A Compendium of Nucleosome and Transcript Profiles Reveals Determinants of Chromatin Architecture and Transcription
- Wnt Signaling Regulates the Lineage Differentiation Potential of Mouse Embryonic Stem Cells through Tcf3 Down-Regulation
- Filamin and Phospholipase C-ε Are Required for Calcium Signaling in the Spermatheca
- The Specificity and Flexibility of L1 Reverse Transcription Priming at Imperfect T-Tracts
- Imputation-Based Meta-Analysis of Severe Malaria in Three African Populations
- Implicates Tyrosine-Sulfated Peptide Signaling in Susceptibility and Resistance to Root Infection
- Clathrin and AP2 Are Required for Phagocytic Receptor-Mediated Apoptotic Cell Clearance in
- Encodes CDF Transporters That Excrete Zinc from Intestinal Cells of and Act in a Parallel Negative Feedback Circuit That Promotes Homeostasis
- Global Properties and Functional Complexity of Human Gene Regulatory Variation
- DNA Binding of the Cell Cycle Transcriptional Regulator GcrA Depends on N6-Adenosine Methylation in and Other
- Side Effects: Substantial Non-Neutral Evolution Flanking Regulatory Sites
- From Paramutation to Paradigm
- From Mouse to Human: Evolutionary Genomics Analysis of Human Orthologs of Essential Genes
- Distinct Translational Control in CD4 T Cell Subsets
- Female Bias in and Regulation by the Histone Demethylase KDM6A
- ATM–Dependent MiR-335 Targets CtIP and Modulates the DNA Damage Response
- HDAC7 Is a Repressor of Myeloid Genes Whose Downregulation Is Required for Transdifferentiation of Pre-B Cells into Macrophages
- The Majority of Primate-Specific Regulatory Sequences Are Derived from Transposable Elements
- Identification of Meiotic Cyclins Reveals Functional Diversification among Plant Cyclin Genes
- EGL-13/SoxD Specifies Distinct O and CO Sensory Neuron Fates in
- Congruence of Additive and Non-Additive Effects on Gene Expression Estimated from Pedigree and SNP Data
- Using Extended Genealogy to Estimate Components of Heritability for 23 Quantitative and Dichotomous Traits
- Ikbkap/Elp1 Deficiency Causes Male Infertility by Disrupting Meiotic Progression
- Analysis of the Genetic Basis of Disease in the Context of Worldwide Human Relationships and Migration
- Duplication and Retention Biases of Essential and Non-Essential Genes Revealed by Systematic Knockdown Analyses
- Strong Purifying Selection at Synonymous Sites in
- , a Susceptibility Gene for Type 1 and Type 2 Diabetes, Modulates Pancreatic Beta Cell Apoptosis via Regulation of a Splice Variant of the BH3-Only Protein
- Chromosome Movements Promoted by the Mitochondrial Protein SPD-3 Are Required for Homology Search during Meiosis
- The Secretory Pathway Calcium ATPase PMR-1/SPCA1 Has Essential Roles in Cell Migration during Embryonic Development
- The Genomic Signature of Crop-Wild Introgression in Maize
- CDK4 T172 Phosphorylation Is Central in a CDK7-Dependent Bidirectional CDK4/CDK2 Interplay Mediated by p21 Phosphorylation at the Restriction Point
- Genome-Wide Identification of Regulatory RNAs in the Human Pathogen
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Using Extended Genealogy to Estimate Components of Heritability for 23 Quantitative and Dichotomous Traits
- HDAC7 Is a Repressor of Myeloid Genes Whose Downregulation Is Required for Transdifferentiation of Pre-B Cells into Macrophages
- Female Bias in and Regulation by the Histone Demethylase KDM6A
- ATM–Dependent MiR-335 Targets CtIP and Modulates the DNA Damage Response
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



