-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaVariable Pathogenicity Determines Individual Lifespan in
A common property of aging in all animals is that chronologically and genetically identical individuals age at different rates. To unveil mechanisms that influence aging variability, we identified markers of remaining lifespan for Caenorhabditis elegans. In transgenic lines, we expressed fluorescent reporter constructs from promoters of C. elegans genes whose expression change with age. The expression levels of aging markers in individual worms from a young synchronous population correlated with their remaining lifespan. We identified eight aging markers, with the superoxide dismutase gene sod-3 expression being the best single predictor of remaining lifespan. Correlation with remaining lifespan became stronger if expression from two aging markers was monitored simultaneously, accounting for up to 49% of the variation in individual lifespan. Visualizing the physiological age of chronologically-identical individuals allowed us to show that a major source of lifespan variability is different pathogenicity from individual to individual and that the mechanism involves variable activation of the insulin-signaling pathway.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1002047
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002047Summary
A common property of aging in all animals is that chronologically and genetically identical individuals age at different rates. To unveil mechanisms that influence aging variability, we identified markers of remaining lifespan for Caenorhabditis elegans. In transgenic lines, we expressed fluorescent reporter constructs from promoters of C. elegans genes whose expression change with age. The expression levels of aging markers in individual worms from a young synchronous population correlated with their remaining lifespan. We identified eight aging markers, with the superoxide dismutase gene sod-3 expression being the best single predictor of remaining lifespan. Correlation with remaining lifespan became stronger if expression from two aging markers was monitored simultaneously, accounting for up to 49% of the variation in individual lifespan. Visualizing the physiological age of chronologically-identical individuals allowed us to show that a major source of lifespan variability is different pathogenicity from individual to individual and that the mechanism involves variable activation of the insulin-signaling pathway.
Introduction
A fundamental property of aging in all animals is stochasticity, which refers to the large and unpredictable variability in the lifespan of individuals in a population [1]. For example, human lifespan expectancy at birth in modern societies is about 78 years, but there is a large variability in the age of death of individuals as 29% die after age 85 and 27% die before age 65 (coefficient of variation is ∼0.2) [2]. Understanding the reasons that make some individuals die earlier than others would provide key insights about why some succumb to major killers such as infection, cardiovascular disease, cancer and stroke, whereas others do not. The causes of the variability in the aging process are poorly understood.
Aging stochasticity can best be studied in model organisms that are genetically identical and can be grown under controlled environmental conditions, such as the nematode C. elegans, a model organism with a normal lifespan of about two weeks [3]. For instance, an isogenic population of worms of the same age grown under identical conditions shows a great deal of individual variability in lifespan, with a coefficient of variation of ∼0.24 [4]. Individual animals appear to age at different rates, such that animals that are the same chronological age may have aged to different extents (younger or older) and have different physiological ages [5].
Several studies have characterized behavioral, morphological and molecular changes in old worms, These age-related changes include decline in locomotion [5], [6], decrease in the rate of pumping of the pharynx [7], and increase in an age-related pigment called lipofuscin [6], [8], [9]. Herndon et al. used electron microscopy to identify ultra-structural changes in different tissues and observed that the nervous system appeared to undergo much less dramatic changes during aging than the muscular system [5]. They also found a stochastic component for age-related decline in individuals. Muscle sarcomeres become damaged in old worms, and this damage can be visualized using a muscle myosin protein tagged with GFP (MYO-3::GFP) [5]. DNA microarray analysis has identified genes that change expression with age [10], [11], [12].
In some cases, the age-related changes have been shown to be markers of physiological, rather than just chronological age. Individual worms can age at different rates, such that some individuals may be physiologically older or younger than others even though they are the same chronological age. One test of whether an age-related change is a marker of physiological age is to see if it predicts remaining lifespan of individuals of the same chronological age. For instance, high lipofucsin levels in moderately aged worms correlates with short lifespans. Another example is expression of hsp-16.2 [13]. Heat shock protects cells and extends lifespan by inducing a number of heat shock response genes, including hsp-16.2. Following heat shock, levels of expression of hsp-16.2 correlate with remaining lifespan of individual worms [13]. However, it is not clear whether differences between individuals occur naturally or are due to the heat shock treatment.
We wanted to understand the mechanisms underlying aging stochasticity. To pursue this goal we first identified C. elegans genes whose expression in individual worms was predictive of their remaining lifespan. We used sod-3, one of the genes whose expression was predictive of remaining lifespan, to investigate mechanisms underlying variable aging in individuals. Our results indicate that pathogenicity from Escherichia coli used as food is a major source of lifespan variability due to variable activation of the insulin-signaling pathway.
Results
Identification of molecular markers predictive of remaining lifespan
We wanted to understand the underlying factors causing aging variability in C. elegans. To achieve this goal, our strategy was to find markers that not only change with age, but that are predictive of remaining lifespan of individuals in a synchronous population. A marker whose expression is connected to an age-related process that ultimately limits lifespan should reveal which individuals will die early or live long. We tested eight fluorescent reporters corresponding to genes that change expression with age (Figure 1A, Figure S1, Table 1). For each fluorescent reporter we measured fluorescence of individual worms at the point when they had lived ∼50% of their mean lifespan. We then recorded the remaining lifespan of each individual, and compared the level of fluorescence expression with their lifespan. For a sod-3::mCherry reporter (fluorescent mCherry protein driven by the superoxide dismutase-3 promoter), we found that mCherry abundance showed a Pearson correlation of 0.57 with lifespan, thus explaining 32% of the variation in lifespan between individuals (Figure 1B). We separated the worms into two groups according to the abundance of sod-3 mCherry, and found that worms with more sod-3 expression lived on average 22% longer than their siblings with less expression (Figure 1C). Besides the sod-3::mCherry reporter, we were able to correlate fluorescence abundance and remaining lifespan using two other sod-3 reporters: a transcriptional GFP reporter containing multiple integrated copies [14] and a transcriptional reporter expressing histone H2B fused to GFP on an extrachomosomal array [10]. The remaining seven reporters showed a Pearson correlation between fluorescent intensity and remaining lifespan, ranging from 0.35 to 0.51 (Table 1). In summary, we found a correlation between gene expression and lifespan for eight genes whose expression changes with age. For the remainder of this paper, we focus our attention on sod-3, which is the best marker of physiological age of those tested.
Fig. 1. sod-3 expression correlates with remaining lifespan. 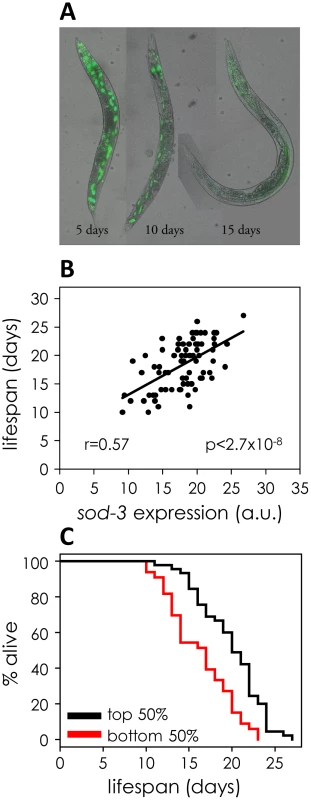
(A) sod-3::GFP expression decreases in old worms. Shown are images of three worms at 5, 10 and 15 days of adulthood expressing extrachromosomal sod-3::GFP. (B) Correlation of sod-3 expression at day 9 with remaining lifespan (n = 78). x-axis shows mCherry fluorescence in arbitrary units. y-axis shows lifespan in days. The correlation between expression and lifespan is r = 0.57, with a p-value<2.7×10−8. (C) Worms with sod-3 expression in the top 50% at day 9 live 22% longer than those with expression in the bottom 50% (p = 5.76×10−5 (log rank)). y-axis shows percentage of worms alive. x-axis shows lifespan in days. Data are from the same worms as in (B). Tab. 1. Correlation of fluorescent markers with remaining lifespan. 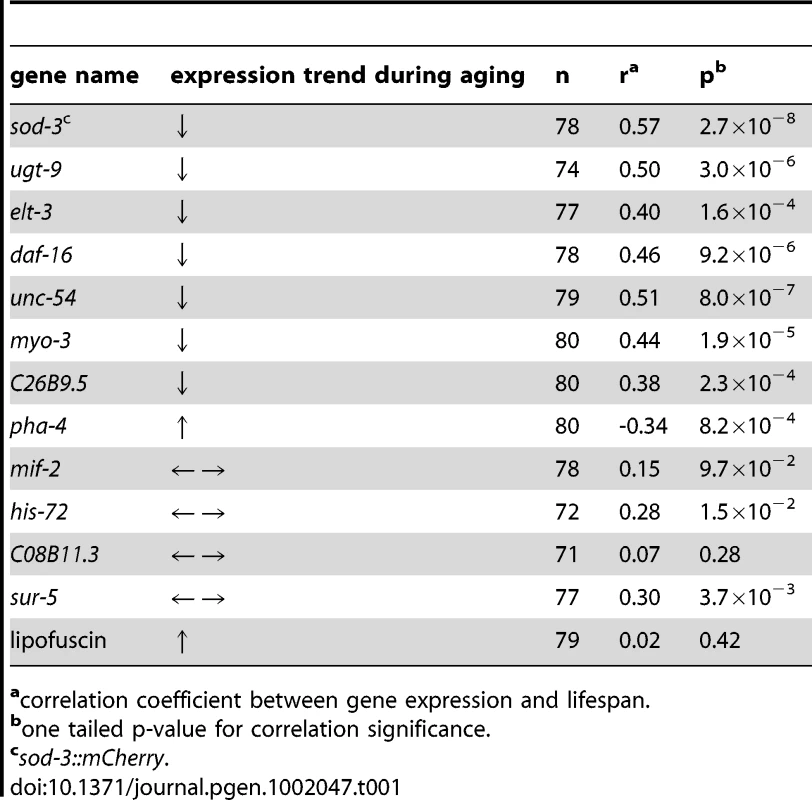
correlation coefficient between gene expression and lifespan. We examined sod-3 expression at different ages to find the optimal time for using sod-3 expression as an aging marker. At ages earlier than day 9, we found that sod-3 expression can still correlate with remaining lifespan in individual worms, although not as well as in 9 day old worms (∼50% of mean lifespan) (Table S1). At ages later than day 9, sod-3 expression is not as useful as a molecular marker because worms begin to show overt signs of aging such as decreased locomotion, decreased pharyngeal pumping and vacuolar appearance. Next, we analyzed sod-3::mCherry expression in the same worm at different ages in a longitudinal assay (Figure S2). This experiment revealed the age-related downward slope in expression of sod-3 for each individual worm, in addition to levels of sod-3 expression at day 9. However, using linear regression, we found that the age-related slope of sod-3 expression did not improve the prediction of remaining lifespan beyond the prediction made using sod-3 expression at 9 days of age alone (p<0.21). In summary, we measured sod-3 expression in a variety of ways and found that expression at day 9 was most informative for predicting remaining lifespan in individual worms.
sod-3 activity might directly extend longevity by itself, or it might be a marker for other processes in the worm that affect lifespan. sod-3 null mutants are known to have wild-type lifespans [15], [16], [17]. We created transgenic lines containing many copies of wild-type sod-3, and found no differences in lifespan compared to controls (Figure S3). These results indicate that sod-3 activity does not extend lifespan per se, but rather that sod-3 expression is a reporter of remaining lifespan.
As a control, we selected four genes at random from the genome (mif-2, his-72, C08B11.3, and sur-5), measured their expression level in individual worms when they had lived ∼50% of their mean lifespan, and found that none correlated with remaining lifespan as well as the eight age-regulated genes (Table 1). Thus, not every gene can serve as a marker for aging. Therefore, the correlation between expression of age-related genes and remaining lifespan is not due to a general decrease in gene expression during aging.
Finally, we tested whether amounts of the age-related pigment lipofuscin correlated with remaining lifespan in middle-aged worms. Lipofuscin is composed of a set of fluorescent breakdown products that accumulate in the gut with age (Figure S1) [3], [8]. Previous studies have shown that lipofuscin levels correlate with remaining lifespan; specifically, in a chronologically old population, there is a small amount of worms that can barely move (Class C), and these worms have high levels of lipofucsin and short remaining lifetimes [9]. We tested whether lipofucsin autofluorescence correlates with remaining lifespan in middle-aged worms (day 9), before overt signs of aging appear. We found no correlation between lipofucsin levels and remaining lifespan (Table 1).
Molecular and environmental sources of lifespan variability
There are many potential sources that could lead to variable abundance of sod-3 between worms, including sources that are intrinsic (variable activity of transcription factors at the sod-3 promoter) or extrinsic (variable effects from the environment). To begin to characterize the types of mechanisms that could be responsible for the correlation between variable lifespan and variable sod-3 expression, we examined whether expression of two different sod-3 reporters fluctuated either together or independently in individual worms from a synchronous population. We created a transgenic line expressing sod-3::GFP and sod-3::mCherry, and compared the fluorescence intensity of GFP and mCherry in individual worms. There was a high degree of correlation (r = 0.87) between sod-3 expression from the GFP and mCherry reporters (Figure 2). This indicates that variability in sod-3 expression is caused by a mechanism that is variable from worm to worm, but has similar effects on both reporter genes within an individual worm.
Fig. 2. Correlation of sod-3::GFP and sod-3::mCherry reporter expression. 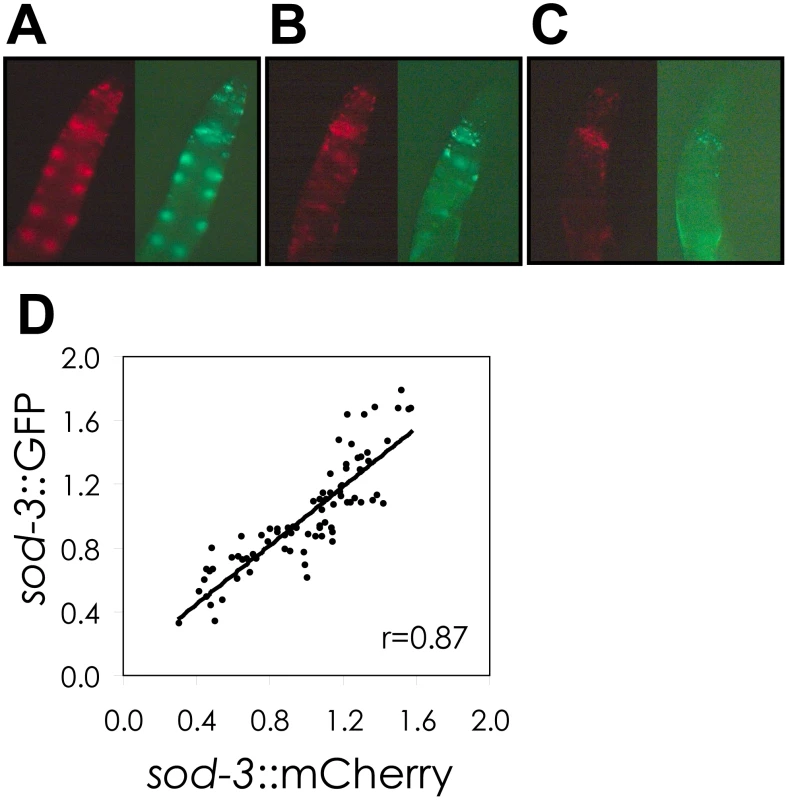
(A–C), Shown are GFP and mCherry images of the anterior portion of adult hermaphrodites at day 8 of adulthood. The individual worms show high (A), medium (B), and low (C) abundance of GFP and mCherry. (D) Scatterplot comparing sod-3 GFP and mCherry expression for 8 day old worms. Each dot represents expression from an individual worm. Data are from 80 sod-3::mCherry/extrachromosomal sod-3::GFP individuals. Correlation between relative levels of GFP and mCherry expression is r = 0.87. We tested several environmental factors that could vary between worms and be responsible for fluctuation in expression of sod-3 and stochasticity in lifespan.
One possibility is that variability in individual lifespan might result from differences in feeding on bacteria provided as food on the culture plate, which could lead to heterogeneity in caloric restriction. However, sod-3::GFP fluorescence from worms grown on plates with an even lawn of E. coli correlated with remaining lifespan as well or better than that of worms grown on plates on which the supply of E. coli was restricted to one small spot (Figure 3A and 3C), indicating that heterogeneity in caloric restriction does not contribute to the link between variability in sod-3 expression and lifespan.
Fig. 3. Variable pathogenicity as a source of variability in sod-3 expression and lifespan. 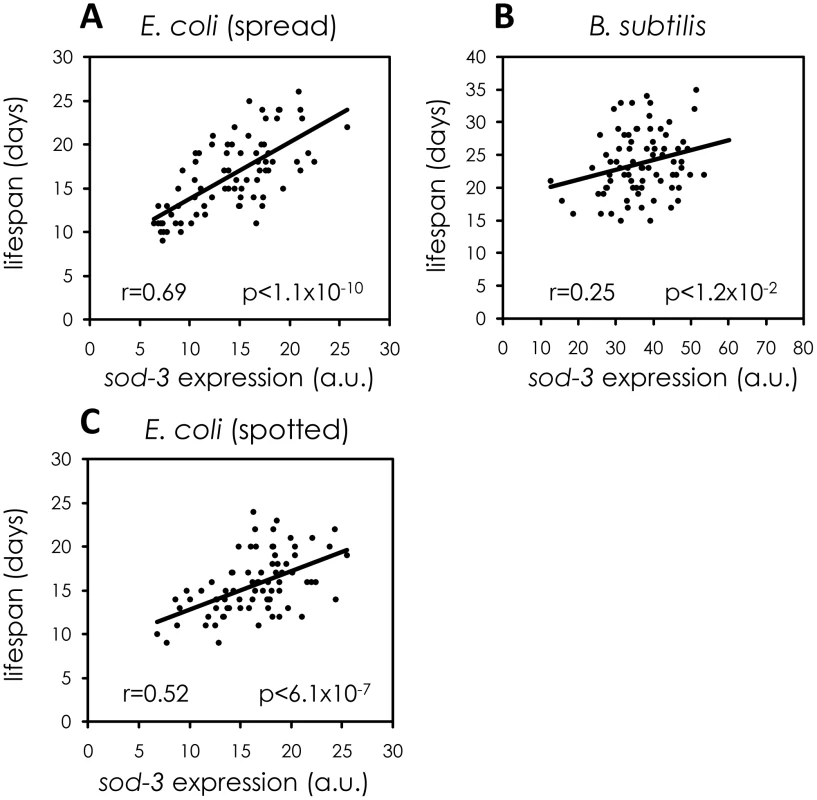
Shown are scatterplots correlating sod-3::GFP expression to their remaining lifespan. x-axis shows expression levels in arbitrary units. y-axis shows lifespan in days. The Pearson correlation between expression and remaining lifespan and p-value are shown in each plot. (A) sod-3::GFP expression in worms (n = 78) grown on plates with evenly spread E. coli. sod-3::GFP expression was measured at day 8 (49% of their mean lifespan). (B) sod-3::GFP expression in worms (n = 79) maintained on B. subtilis. sod-3::GFP expression was measured at day 12 (50% of their mean lifespan). (C) sod-3::GFP expression from worms (n = 79) grown on plates with a spot of E. coli in the middle. sod-3::GFP expression was measured at day 8 of adulthood (50% of their mean lifespan). Another possibility is that variable pathogenicity from bacterial food could lead to variable sod-3 expression and lifespan. E. coli, the common diet for worms, is mildly pathogenic whereas Bacillus subtilis is not pathogenic [18]. Accordingly, worms fed B. subtilis live longer than worms grown on E. coli [19], [20] (Figure S4). We tested whether variable pathogenicity could lead to individual differences in sod-3 expression and lifespan in two ways. First, we examined whether growing worms on B. subtilis rather than E. coli reduced variability in sod-3 expression. We cultured worms on E. coli or on B. subtilis for 8, 12, and 14 days and calculated variability in sod-3 abundance (defined as Standard Deviation/mean) at each age. We found that the variability in sod-3 expression was lower in worms fed B. subtilis than it was in worms fed E. coli at all ages (Figure 4 and Table S2). Second, we showed that the correlation between abundance of sod-3::GFP and remaining lifespan was considerably lower for worms maintained on B. subtilis than for worms grown on E. coli when worms had lived 50% of their mean lifespan (Figure 3A and 3B). Besides B. subtilis, we obtained similar results using two other non-pathogenic diets, UV-killed E. coli and Caulobacter crescentus. Specifically, worms fed these diets lived longer than E. coli fed worms (Figure S4), and the correlation between sod-3 abundance and remaining lifespan was lower for worms fed these non-pathogenic diets than for E. coli fed worms when worms had lived 50% of their mean lifespan (Figure S5). Thus, these results suggest that variable pathogenicity from individual to individual may cause variation in abundance of sod-3 and lifespan variability.
Fig. 4. sod-3 expression variability is lower for worms fed B. subtilis compared to worms fed E. coli. 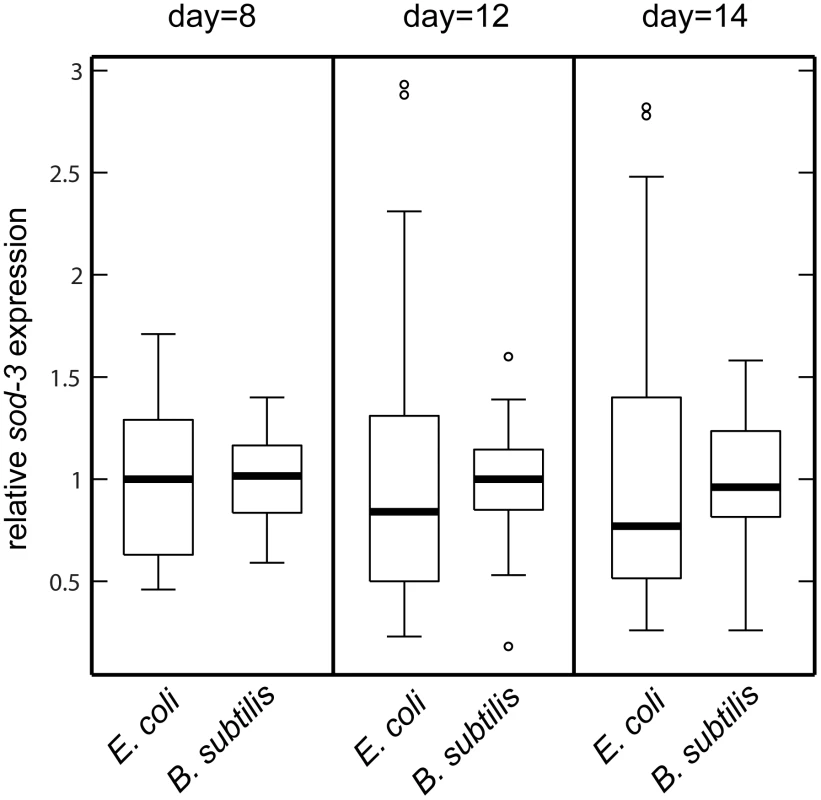
Shown are boxplot distributions for sod-3::GFP expression in worms maintained on E. coli or B. subtilis at 20°C at different ages. The boxes define the interquartile range and the thick line is the median. Bars represent the expression range. n = 60 in each group. The finding that the amount of sod-3 expression present in a middle-aged worm is correlated with its remaining lifespan indicates that events have occurred that affect its future aging trajectory. If so, feeding a worm either E. coli or B. subtilis should have greatest effect when it is young rather than when it is old. To test this, we fed worms one type of bacteria (E. coli or B. subtilis) when they were young and then shifted them to the other type of bacteria at day 8 of adulthood. Young worms fed E. coli had short lifespans, no matter what they ate when they were old. Conversely, young worms fed B. subtilis had long lifespans no matter what they ate when they were old (Figure 5 and Table S3). This result indicates that pathogenicity or some other factor associated with E. coli initiates changes in young worms that affect their time of death later on.
Fig. 5. The effect of E. coli pathogenicity persists after worms are shifted to B. subtilis. 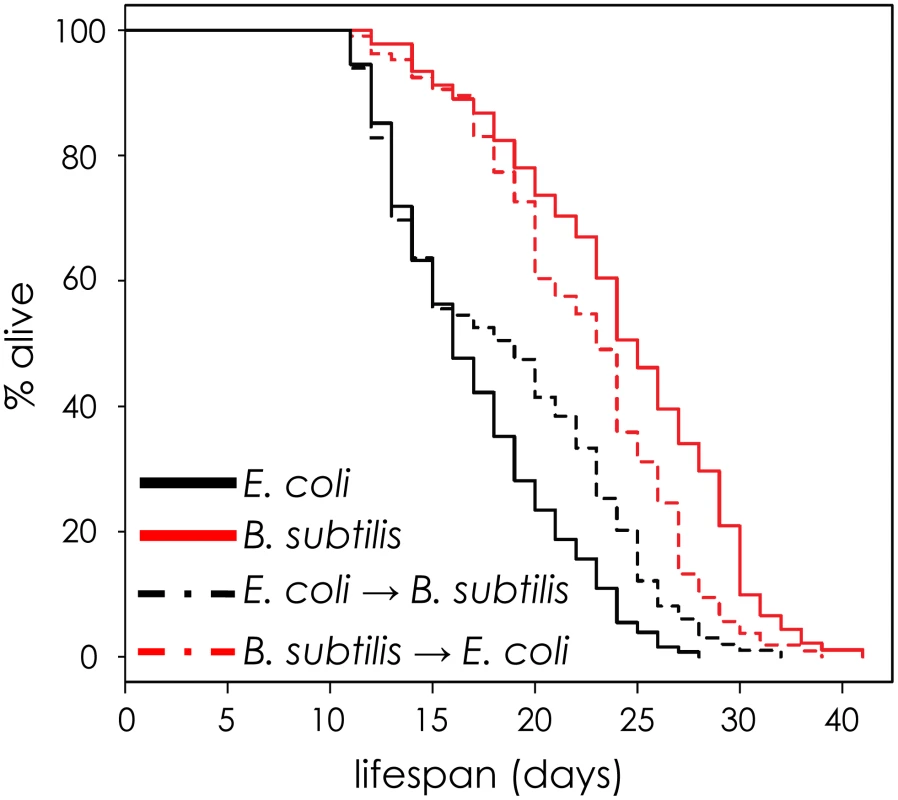
Lifespan curves for worms maintained on one type of bacteria (E. coli or B. subtilis) and then shifted to the other bacteria at day 8. All lifespans were done at 20°C. y-axis indicates % of worms that are alive. x-axis indicates day of adulthood. Pathogenicity might affect sod-3 abundance through regulation of the insulin-like signaling pathway because this pathway mediates response to pathogen infection [19], [21], [22]. Furthermore, the terminal step in the insulin-like signaling pathway is daf-16, which encodes a FOXO transcription factor that directly regulates sod-3 expression [23]. Fluctuation in the activity of daf-16 FOXO could be a source of lifespan stochasticity in individual worms. We tested this possibility using four approaches. First, we showed that a daf-16 null mutation eliminated the correlation between sod-3 expression and lifespan (Figure 6C and Figure S6). The daf-16 null mutation also prevents sod-3 from acting as an aging marker at younger ages (Figure 6D). The lack of correlation between sod-3 expression and lifespan in daf-16 null mutants is not simply because sod-3 is less abundant. sod-3::GFP was also less abundant in mutant worms for elt-3 GATA (Figure S7), another transcription factor that regulates sod-3 expression [10], but sod-3 abundance in elt-3 GATA mutants still correlated with remaining lifespan (Figure 6B).
Fig. 6. Variable daf-16 activity as a source of sod-3 expression and lifespan variability. 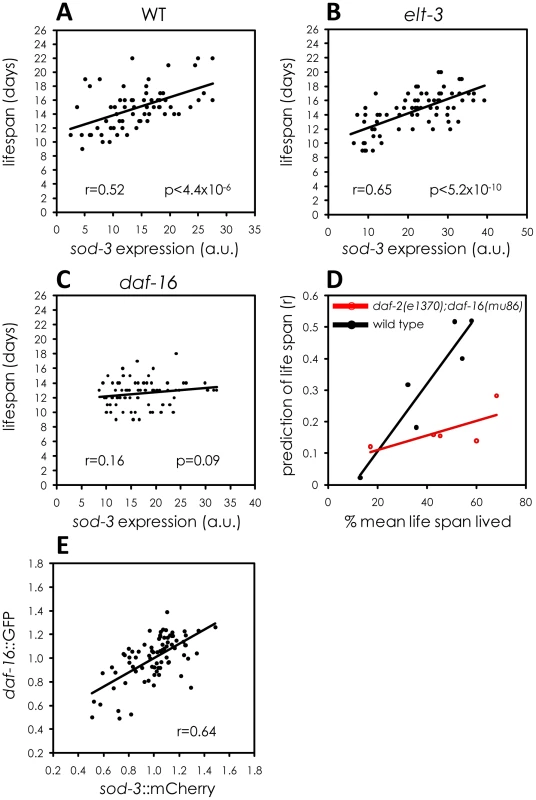
(A–C) Shown are scatterplots comparing sod-3::GFP expression with their remaining lifespan. x-axis shows expression levels in arbitrary units. y-axis shows lifespan in days. The Pearson correlation between expression and remaining lifespan and p-value are shown in each plot. sod-3::GFP expression and lifespan are shown for (A) daf-16(+);elt-3(+) 9 day old worms (n = 68). (B) elt-3(vp1) 8 day old worms (n = 70). (C) daf-16(mu86) 8 day old worms (n = 76). (D) Shown is a scatterplot that summarizes data from several assays. Each point represents an experiment comparing sod-3::GFP expression with remaining lifespan. The y-axis shows the correlation coefficient and the x-axis shows the age of the worms when sod-3 expression was measured. Black indicates worms that were daf-2(+);daf-16(+) and red indicates worms that were daf-2(e1370);daf-16(mu86) mutants. (E) Correlation between daf-16::GFP and sod-3::mCherry relative abundance in individual worms at day 9 (n = 78). Second, we tested whether the specific amount of DAF-16 in an individual worm led to corresponding changes in sod-3 expression. The insulin-like signaling pathway controls activity of DAF-16 FOXO primarily by affecting protein phosphorylation, which controls nuclear versus cytoplasmic localization [24], [25]. However, it is possible that insulin-signaling may also affect the levels of total DAF-16 protein in the cell, which can be measured using a daf-16::GFP translational reporter. We constructed a strain that expressed a fusion of GFP with DAF-16 [24] as well as mCherry under the control of the sod-3 promoter. We measured expression of GFP and mCherry in individual worms. Accumulation of these reporter proteins in individual worms was highly correlated (Figure 6E). This result is consistent with the model that DAF-16 is a direct regulator of sod-3 expression.
Third, since daf-16 is a direct regulator of sod-3 expression [14] and expression of these genes is highly correlated with one another, one might expect that independently measuring expression of these two genes in the same worm would be either partially or wholly redundant. To test this, we evaluated a regression model that included expression of both genes to find out if expression of both genes together was more informative about remaining lifespan than expression of sod-3 alone. We found that measuring expression of both genes together or sod-3 alone showed little difference in predicting remaining lifespan (p = 0.26) (Table 2). This shows redundancy in lifespan information from daf-16 and sod-3 expression.
Tab. 2. Correlation of two fluorescent markers with remaining lifespan. 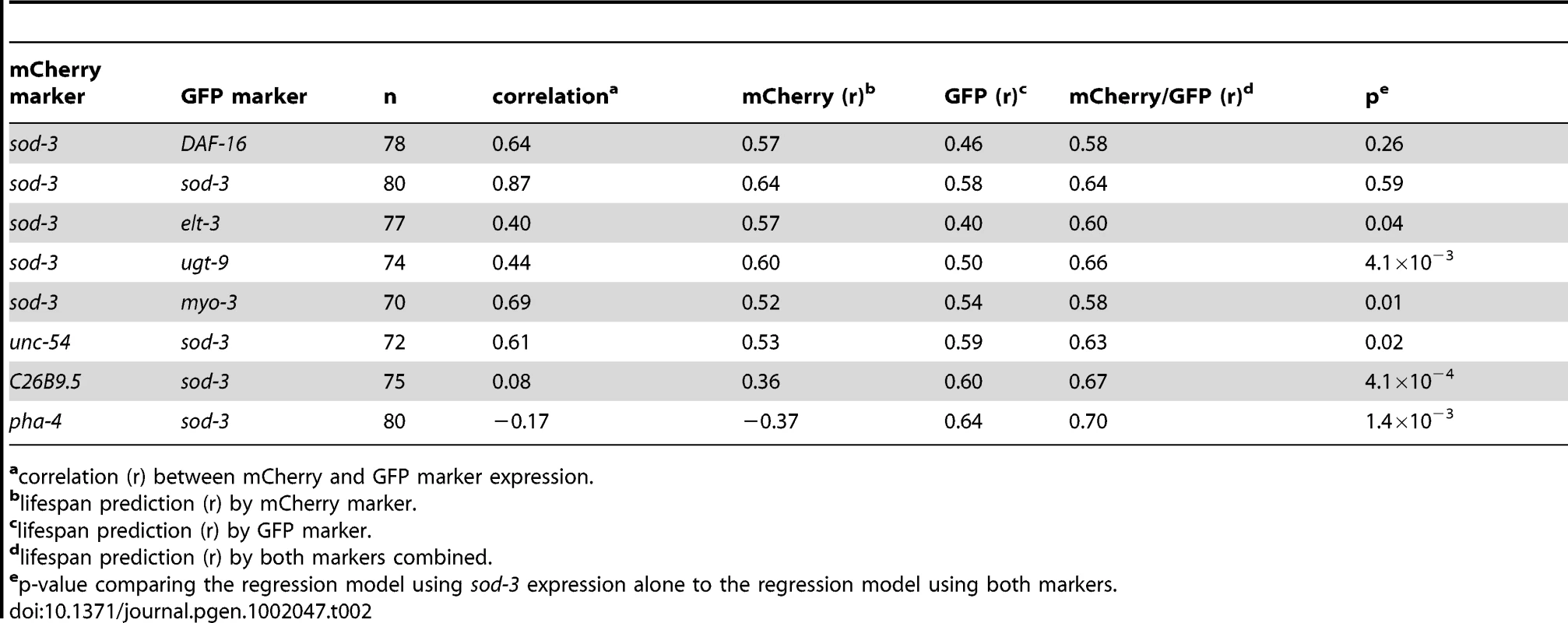
correlation (r) between mCherry and GFP marker expression. Fourth, we tested whether pathogenicity from E. coli used as food induces expression of daf-16. We fed worms either E. coli or non-pathogenic B. subtilis and measured the level of expression of DAF-16 during aging. We found that expression of DAF-16::GFP was significantly higher for worms fed E. coli than for worms fed B. subtilis at days two, five, and eight of adulthood (Figure S8). In summary, results from all four experiments indicate that fluctuations in DAF-16 FOXO activity account for much of the individual variability in sod-3 expression and lifespan in individual worms.
The intestine is a major tissue determining variable individual lifespan
sod-3 is expressed throughout the worm, including many cells in the head as well as the intestine. The intestine is a primary site for response to pathogenic infection [8] and also for transcriptional regulation of daf-16 FOXO [14]. Three results indicate that the intestine is a major tissue responsive to variable pathogenicity, which in turn determines individual lifespan. First, we measured mCherry fluorescence produced from the sod-3 promoter separately in the head and the anterior intestine. Expression in the intestine showed a higher correlation with lifespan than did expression in the head, demonstrating that intestinal sod-3 expression is the main contributor to the lifespan correlation (Figure 7A and 7B). Second, E. coli and B. subtilis had different effects on sod-3 expression in the intestine, but similar effects on expression in the head throughout lifespan (Figure S9B and S9C). This result is consistent with the idea that differences in lifespan due to growth on E. coli or B. subtilis are due to effects in the intestine. Third, we observed that there were variable levels of sod-3 expression in the head and intestine in individual worms of the same age (Figure S9A). However, sod-3 expression in the head showed little correlation to expression in the intestine in individual worms (Figure 7C).
Fig. 7. The intestine is a major tissue determining variable individual lifespan. 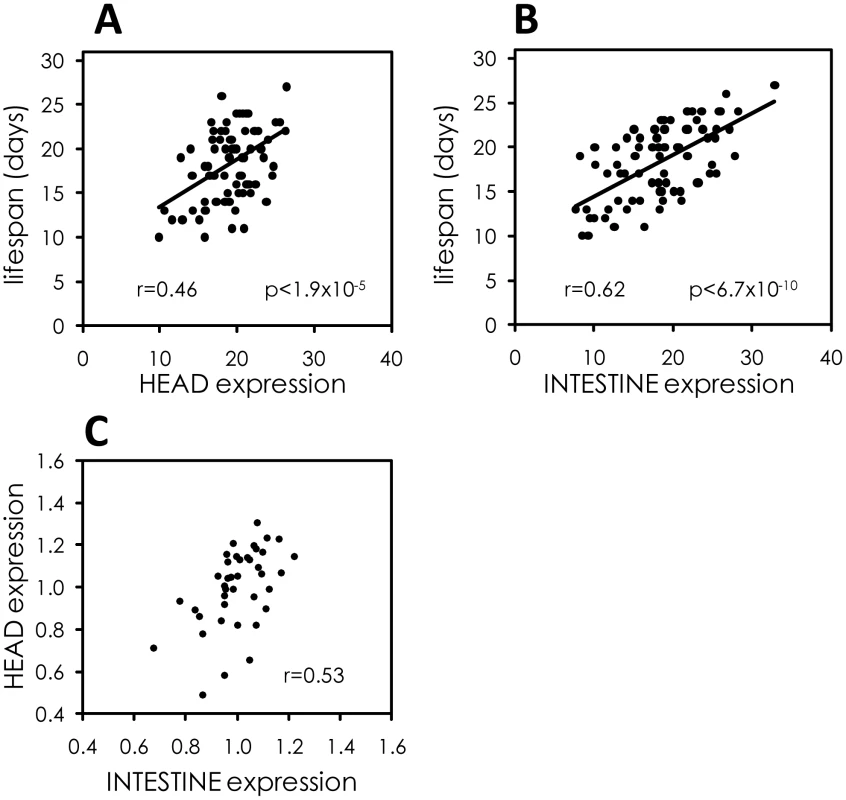
(A) Scatterplot comparing expression of sod-3::mCherry in the head at day 9 of adulthood to remaining lifespan. Data are from 78 sod-3::mCherry expressing worms. Pearson correlation and p-value are shown. Expression levels are in arbitrary units. (B) Scatterplot comparing expression of sod-3::mCherry in the intestine at day 9 of adulthood to remaining lifespan. Data are from 78 sod-3::mCherry expressing worms. Expression levels are in arbitrary units. (C) Correlation between relative sod-3 expression in the head and the intestine within individual sod-3::mCherry expressing worms at day 9 of adulthood (n = 40, r = 0.53). Improved prediction of remaining lifespan from combinations of molecular markers
Expression from two markers could provide more information about remaining lifespan than one marker alone if, for example, expression of the markers were to vary independently from each other in individuals. We calculated the correlation between marker expression and remaining lifespan for sod-3, each of the seven other marker genes, and for each of the seven double combinations with sod-3 (Table 2). For six genes (ugt-9, unc-54, myo-3, pha-4, C26B9.5 and elt-3), we found a higher correlation with remaining lifespan using these markers in combination with sod-3 expression than using one marker alone (p<0.05 linear regression). The best combinations were sod-3 expression combined with expression from ugt-9, C26B9.5 or pha-4, which had correlations with lifespan between 0.66 and 0.70, accounting between 43% and 49% of the variability in individual lifespan. As discussed above, the seventh gene is daf-16, which acts in the same pathway as sod-3 and thus daf-16 expression provides redundant information with sod-3 expression about remaining lifespan in individual worms. As a control, we looked at the correlation between expression of sod-3::GFP and sod-3::mCherry in individual worms. As expected, we found that the combined GFP and mCherry expression levels did not improve the correlation with remaining lifespan compared to either sod-3 marker alone (Table 2). These results show that a combination of aging markers can significantly improve the correlation with remaining lifespan in individual worms.
Discussion
This paper shows that a common cause of death for worms grown under normal lab conditions is pathogenicity from ingested food. We propose a model in which there is a variable effect of pathogenicity between individuals in an isogenic and chronologically identical population, resulting in differences in lifespan (Figure 8). E. coli is mildly pathogenic [18], [19], [20], and individuals may be affected to different extents when eating E. coli as food. The pathogenicity from the ingested bacteria primarily affects the intestine. Ingestion of E. coli activates daf-16 FOXO activity via the insulin-like signaling pathway, which induces a beneficial stress response that is protective and extends lifespan. sod-3 is a downstream target of DAF-16, and expression of sod-3 indicates levels of DAF-16 activation. High levels of sod-3 expression correspond to high levels of DAF-16 activation and longer lifespan, and vice versa for low levels of sod-3 expression. Mutations in sod-3 do not affect lifespan, indicating that sod-3 activity is not in itself functionally important for lifespan but that sod-3 expression is a marker for physiological age because it reports the level of activity of daf-16 FOXO. This paper provides novel insights about a common cause of death for worms grown under normal laboratory conditions, and highlights the complex and interconnected roles of aging and disease in specifying lifespan.
Fig. 8. Model for lifespan variability in C. elegans. 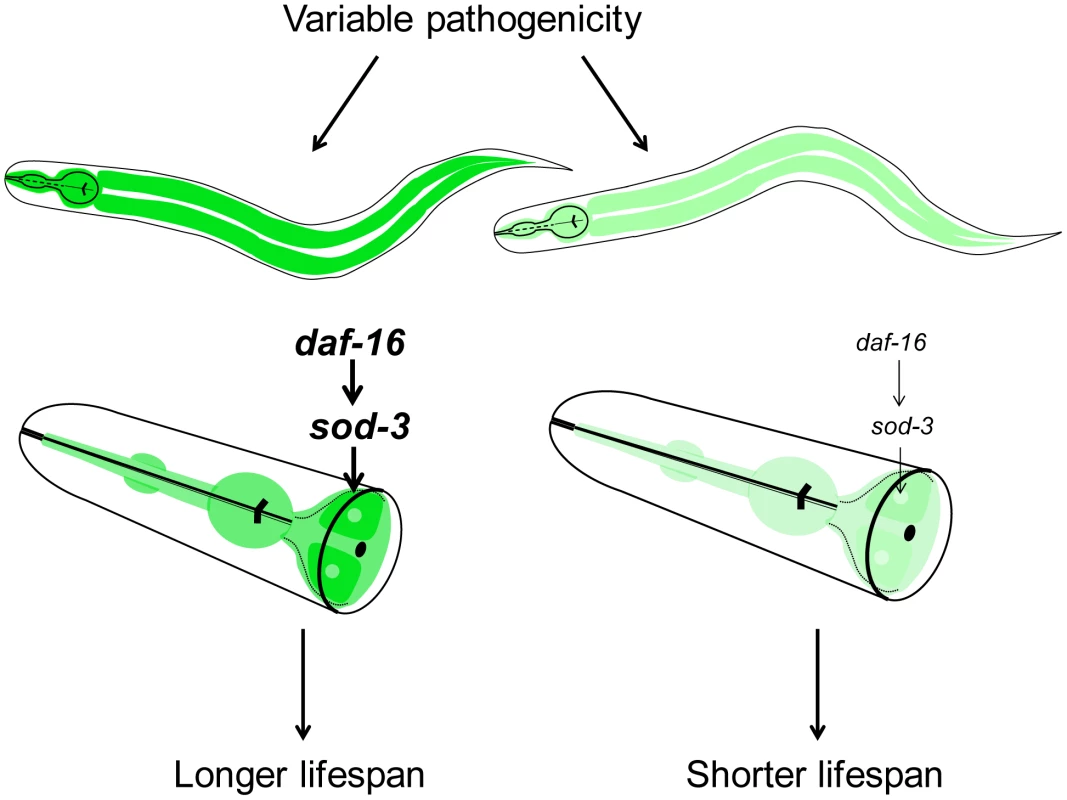
Variable pathogenicity in young adulthood leads to older or younger physiological age of individuals. Variable pathogenicity affects daf-16 activity, which then regulates sod-3 expression. Darker green and lighter green represent higher and lower sod-3 expression in the intestine, respectively. Higher sod-3 expression from worm to worm is correlative with longer individual lifespan. Where does the variability in bacterial pathogenicity between individuals arise? One possibility is that the variability arises from extrinsic environmental differences. For instance, there might be variability in pathogenicity of the E. coli itself in different regions of the plate, which might affect worms to different extents depending on which region they occupy. However, this appears unlikely because worms can traverse all regions of a plate in a day, and because we see a correlation between sod-3 expression and remaining lifespan even when the plates are specially prepared to have even lawns of E. coli. A more likely possibility is that the variability might be intrinsic to the worm itself. Different worms might express different levels of DAF-16 stemming from intrinsic differences such as noise in the expression machinery. When confronted by mildly pathogenic E. coli, some worms could mount a stronger protective response than others, giving rise to correlated differences in sod-3 expression and variability in lifespan.
Evidence that bacterial pathogenicity in the intestine is a major source of stochasticity in physiological aging in individual worms comes from comparing the effects caused by feeding worms live E. coli versus three other food sources (B. subtilis, UV-killed E. coli, and C. crescentus). The most obvious differences between these foods is that live E. coli is mildly pathogenic whereas the others are either non-pathogenic or have less pathogenicity. It seems unlikely that the effects on lifespan seen with UV-killed E. coli, B. subtilis and C. crescentus are due to caloric restriction stemming from difficulty in ingesting these foods. Worms fed these three bacteria appear normal in size rather than thin, develop normally and produce a normal brood size [19], indicating that these worms are not dietary restricted.
What happens when worms are switched from E. coli as a food source (mildly pathogenic) to non-pathogenic bacteria such as B. subtilis? According to the model, worms fed E. coli experience variable levels of pathogenicity whereas this variability is reduced or absent for worms fed B. subtilis, resulting in four measurable differences. First, worms have a longer lifespan on non-pathogenic bacteria (Figure S4). Second, since sod-3 expression is induced by pathogenicity, expression is lower on non-pathogenic bacteria compared to E. coli (Figure S9B and S9C). Third, the variable pathogenicity arising from growth on E. coli generates more fluctuation in sod-3 expression compared to growth on B. subtilis (Figure 4). Fourth, variable pathogenicity from growth on E. coli results in a correlation between expression of sod-3 and remaining lifespan of individual worms (Figure 3). Finally, when worms are grown on B. subtilis, they die at a later time and the cause of death is currently unknown. The cause of death for B. subtilis-fed worms could have more, less or similar variability between individuals compared to death from E. coli-derived pathogenicity. Hence, lifespan of worms fed B. subtilis could show more, less or the same amount of variability as worms fed E. coli, depending on the cause of death. In fact, we found that the variability of lifespan of worms grown on B. subtilis is 20% less than that of E. coli-fed worms (A.S.B., unpublished observations).
Our results identify a number of fluorescent reporters that can be used as markers of physiological age. Previously, the most common way to determine the rates of aging in C. elegans was to measure the lifespan of a population of worms. The aging markers presented in this paper will be useful tools to study aging because one can measure the age of individual worms and because expression analysis of fluorescent markers in individual worms is less time-consuming than lifespan analysis of a population of worms. hsp-16::GFP has been previously used as a marker for physiological age [13], but this marker requires heat shock treatment in order to predict remaining lifespan and this treatment prolongs lifespan in itself. Additionally, drosomycin, hsp22 and hsp70 are partially predictive of remaining lifespan in Drosophila [26], [27].
In addition to sod-3, we identified seven other aging biomarkers that could provide information about mechanisms responsible for variability in aging. For six of the biomarkers (ugt-9, pha-4, myo-3, unc-54, C26B9.5, and elt-3), combinations of sod-3 and a second marker provide better prediction of remaining lifespan than just one of the markers alone. This result suggests that the different markers may be responsive to aging pathways that are distinct from those controlling sod-3 expression. For example, age-related decrease in expression of the GATA transcription factor ELT-3 is caused by drift of the upstream regulatory network that controls ELT-3 expression [10]. Thus, simultaneous measurement of sod-3 and elt-3 expression in a worm would show the status of two aging pathways (pathogenic induction and developmental drift) in that individual, and therefore provide better information about its physiological age and remaining lifespan. For the remaining five aging biomarkers, DNA microarray experiments indicate that they are not regulated by daf-16 FOXO [10], [28]. Future studies may reveal the source of variation governing the other aging markers described in this paper, which will illuminate other mechanisms that limit lifespan of worms grown under normal lab conditions.
Materials and Methods
Bacterial growth
UV-killed E. coli was prepared as described [8]. C. crescentus was grown as described [29]. Since C. crescentus does not grow on NGM plates, plates were seeded with 300 µl of resuspended C. crescentus, allowed to dry, and then subjected to UV irradiation as described [8].
C. elegans fluorescent reporters
We used three sod-3 fluorescent reporters. The first is a multi-copy insertion reporter that expresses cytoplasmic GFP from the sod-3 promoter (referred to as sod-3::GFP) [14]. The second is a low-copy insertion reporter that expresses histone H2B fused to mCherry from the sod-3 promoter (referred to as sod-3::mCherry) [30]. The third is a multi-copy extrachromosomal array reporter that expresses histone H2B fused to GFP from the sod-3 promoter (referred to as extrachromosomal sod-3::GFP) [10].
daf-16::GFP is a multi-copy integrated translational reporter that expresses GFP at the C terminus of the DAF-16 protein [24]. myo-3::GFP is an multi-copy integrated reporter that expresses nuclear-targeted GFP–LacZ from the myo-3 promoter [31]. unc-54::mCherry and pha-4::mCherry are low-copy integrated reporters that express histone H1 fused to mCherry from the unc-54 or the pha-4 promoter [30]. ugt-9::GFP and elt-3::GFP are multi-copy extrachromosomal array reporters that expresses histone H2B fused to GFP from the ugt-9 or the elt-3 promoter [10].
Promoter::H1::mCherry constructs for C26B9.5 and mif-2 were made using promoters from the Vidal lab promoterome [32] in combination with a modified Gateway cloning system [33] vector (pD4H1cherry [30]). Transgenic lines with integrated copies of the reporter were made by microparticle bombardment [34].
Lifespan prediction assay
Approximately 80 age-synchronized worms were transferred to 1 mM aldicarb-NGM plates for 2–3 hours to induce paralysis [35]. Worms were then individually transferred to FUDR plates. Each worm was then photographed using 20× lens. Images were analyzed using ImageJ. For any given comparison, all pictures were taken on the same day with the same microscope settings. Each plate containing a single worm was labeled with the corresponding picture number and plates were scored for dead worms daily.
Lifespan analyses were conducted at 20°C as previously described [36]. Age refers to days following adulthood, and p values were calculated using the log-rank (Mantel-Cox) method. Individuals were excluded from the analysis when their gonad was extruded, or when they desiccated by crawling onto the edge of the housing plate.
Lipofuscin autofluorescence quantification
Worm gut autofluorescence was imaged using a 525 nm bandpass filter. A Zeiss Axioplan microscope equipped with Zeiss AxioVision 4.6 software was used for quantitative fluorescence microscopy. Images were captured with 10× lens and analyzed using ImageJ. Gut autofluorescence time courses were done using at least 15 worms per age. All pictures were taken on the same day with the same microscope settings.
Statistical analyses
To test remaining lifespan prediction of the age-related slope in the longitudinal sod-3::mCherry expression experiment, linear regression analysis was used with the following model:Yi is the lifespan of worm i, day 9i is the fluorescence of worm i at day 9, slopei is the slope of fluorescence by age for worm i and εi is a random error term. The coefficients were estimated by least squares from the data. Expression at day 9 ( term) was statistically significant (p<3.0×10−7). Age-related slope of sod-3 expression ( term) was not statistically significant (p>0.21).
To test the combined effect of multiple measurements within single worms, multiple regression analysis was used with the following model: is the lifespan of worm i, sod-3 marker and 2nd marker are the sod-3 and additional marker expression levels respectively, is a random error term, and and are regression coefficients. Likelihood ratio F-tests were used to determine the significance of and . For example, to test whether addition of a daf-16::GFP second marker adds significant information about lifespan prediction compared to using sod-3::mCherry alone, we test the hypothesis that . This is done by comparing the following two predictions of lifespan:
-
Model (1):
-
Model (2): .
Y is the original matrix of measured lifespans, is the matrix of predicted lifespans from the full model (1), and is the matrix of predicted lifespans from the partial model (2) that leaves out the daf-16::GFP term. With n independent worms, the likelihood ratio test compares the residual sum of squares of the two models and rejects the hypothesis that if , where is the cumulative distribution of an F-distribution with 1 and n-2 degrees of freedom.
Using this test, we determined that addition of the daf-16::GFP expression term did not significantly improve the model compared to using sod-3::mCherry alone (p-value>0.2, and so we cannot reject the hypothesis that ). A similar approach showed that adding sod-3::mCherry expression significantly improved a model using daf-16::GFP expression alone (p-value<0.001, so we can reject the hypothesis that ).
Worm transgenic lines
The sod-3 gene and regulatory regions (2.57 kbp fragment) were amplified by PCR from wild type N2 worm genomic DNA (forward primer: 5′ ATT CGC AGA AAA AAG TCG TTG C 3′, reverse primer: 5′ TTT CAG TGT ACC GAG TGA AGT TC 3′). The sod-3 PCR fragment was cloned into the TOPO pCR 2.1 vector (Invitrogen). TOPO pCR 2.1 plasmid with the coding sod-3 region was coinjected at different concentrations (5 ng/µl and 40 ng/µl) with pha-1 encoding plasmid into pha-1 mutant worms to generate extrachromosomal array sod-3 overexpressing lines. A control line was generated by injecting pha-1 encoding plasmid alone into pha-1 mutant worms.
Supporting Information
Zdroje
1. KirkwoodTBFederMFinchCEFranceschiCGlobersonA 2005 What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mech Ageing Dev 126 439 443
2. EdwardsRDTuljapurkarS 2005 Inequality in Life Spans and a New Perspective on Mortality Convergence Across Industrialized Countries. Population Devel Rev 31 4 645 674
3. KlassMR 1977 Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev 6 413 429
4. KirkwoodTBFinchCE 2002 Ageing: the old worm turns more slowly. Nature 419 794 795
5. HerndonLASchmeissnerPJDudaronekJMBrownPAListnerKM 2002 Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419 808 814
6. HosonoRSatoYAizawaSIMitsuiY 1980 Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegans. Exp Gerontol 15 285 289
7. HuangCXiongCKornfeldK 2004 Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A 101 8084 8089
8. GariganDHsuALFraserAGKamathRSAhringerJ 2002 Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics 161 1101 1112
9. GerstbreinBStamatasGKolliasNDriscollM 2005 In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell 4 127 137
10. BudovskayaYVWuKSouthworthLKJiangMTedescoP 2008 An elt-3/elt-5/elt-6 GATA transcription circuit guides aging in C. elegans. Cell 134 291 303
11. GoldenTRHubbardADandoCHerrenMAMelovS 2008 Age-related behaviors have distinct transcriptional profiles in Caenorhabditis elegans. Aging Cell 7 850 865
12. LundJTedescoPDukeKWangJKimSK 2002 Transcriptional profile of aging in C. elegans. Curr Biol 12 1566 1573
13. ReaSLWuDCypserJRVaupelJWJohnsonTE 2005 A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet 37 894 898
14. LibinaNBermanJRKenyonC 2003 Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115 489 502
15. DoonanRMcElweeJJMatthijssensFWalkerGAHouthoofdK 2008 Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 22 3236 3241
16. Van RaamsdonkJMHekimiS 2009 Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet 5 e1000361 doi:10.1371/journal.pgen.1000361
17. YenKPatelHBLublinALMobbsCV 2009 SOD isoforms play no role in lifespan in ad lib or dietary restricted conditions, but mutational inactivation of SOD-1 reduces life extension by cold. Mech Ageing Dev 130 173 178
18. PartridgeLGemsD 2007 Benchmarks for ageing studies. Nature 450 165 167
19. GarsinDAVillanuevaJMBegunJKimDHSifriCD 2003 Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300 1921
20. HahmJHKimSPaikYK 2010 GPA-9 is a novel regulator of innate immunity against Escherichia coli foods in adult Caenorhabditis elegans. Aging Cell
21. ChavezVMohri-ShiomiAMaadaniAVegaLAGarsinDA 2007 Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics 176 1567 1577
22. KurzCLTanMW 2004 Regulation of aging and innate immunity in C. elegans. Aging Cell 3 185 193
23. OhSWMukhopadhyayADixitBLRahaTGreenMR 2006 Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet 38 251 257
24. HendersonSTJohnsonTE 2001 daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol 11 1975 1980
25. KenyonC 2005 The plasticity of aging: insights from long-lived mutants. Cell 120 449 460
26. LandisGNAbduevaDSkvortsovDYangJRabinBE 2004 Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A 101 7663 7668
27. YangJTowerJ 2009 Expression of hsp22 and hsp70 transgenes is partially predictive of drosophila survival under normal and stress conditions. J Gerontol A Biol Sci Med Sci 64 828 838
28. MurphyCTMcCarrollSABargmannCIFraserAKamathRS 2003 Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 277 283
29. IniestaAAShapiroL 2008 A bacterial control circuit integrates polar localization and proteolysis of key regulatory proteins with a phospho-signaling cascade. Proc Natl Acad Sci U S A 105 16602 16607
30. LiuXLongFPengHAerniSJiangM 2009 Analysis of gene regulation and cell fate from single-cell gene expression profiles in C. elegans. Cell 139 623 633
31. FireAXuSMontgomeryMKKostasSADriverSE 1998 Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 806 811
32. DupuyDLiQRDeplanckeBBoxemMHaoT 2004 A first version of the Caenorhabditis elegans Promoterome. Genome Res 14 2169 2175
33. CheoDLTitusSAByrdDRHartleyJLTempleGF 2004 Concerted assembly and cloning of multiple DNA segments using in vitro site-specific recombination: functional analysis of multi-segment expression clones. Genome Res 14 2111 2120
34. PraitisVCaseyECollarDAustinJ 2001 Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157 1217 1226
35. MahoneyTRLuoSNonetML 2006 Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat Protoc 1 1772 1777
36. ApfeldJKenyonC 1999 Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402 804 809
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



