-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Phylogenetic Origin of Coincided with the
Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the
Holometabola
The establishment of the germline is a critical, yet surprisingly evolutionarily
labile, event in the development of sexually reproducing animals. In the fly
Drosophila, germ cells acquire their fate early during
development through the inheritance of the germ plasm, a specialized maternal
cytoplasm localized at the posterior pole of the oocyte. The gene
oskar (osk) is both necessary and
sufficient for assembling this substance. Both maternal germ plasm and
oskar are evolutionary novelties within the insects, as the
germline is specified by zygotic induction in basally branching insects, and
osk has until now only been detected in dipterans. In order
to understand the origin of these evolutionary novelties, we used comparative
genomics, parental RNAi, and gene expression analyses in multiple insect
species. We have found that the origin of osk and its role in
specifying the germline coincided with the innovation of maternal germ plasm and
pole cells at the base of the holometabolous insects and that losses of
osk are correlated with changes in germline determination
strategies within the Holometabola. Our results indicate that the invention of
the novel gene osk was a key innovation that allowed the
transition from the ancestral late zygotic mode of germline induction to a
maternally controlled establishment of the germline found in many holometabolous
insect species. We propose that the ancestral role of osk was
to connect an upstream network ancestrally involved in mRNA localization and
translational control to a downstream regulatory network ancestrally involved in
executing the germ cell program.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1002029
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002029Summary
The establishment of the germline is a critical, yet surprisingly evolutionarily
labile, event in the development of sexually reproducing animals. In the fly
Drosophila, germ cells acquire their fate early during
development through the inheritance of the germ plasm, a specialized maternal
cytoplasm localized at the posterior pole of the oocyte. The gene
oskar (osk) is both necessary and
sufficient for assembling this substance. Both maternal germ plasm and
oskar are evolutionary novelties within the insects, as the
germline is specified by zygotic induction in basally branching insects, and
osk has until now only been detected in dipterans. In order
to understand the origin of these evolutionary novelties, we used comparative
genomics, parental RNAi, and gene expression analyses in multiple insect
species. We have found that the origin of osk and its role in
specifying the germline coincided with the innovation of maternal germ plasm and
pole cells at the base of the holometabolous insects and that losses of
osk are correlated with changes in germline determination
strategies within the Holometabola. Our results indicate that the invention of
the novel gene osk was a key innovation that allowed the
transition from the ancestral late zygotic mode of germline induction to a
maternally controlled establishment of the germline found in many holometabolous
insect species. We propose that the ancestral role of osk was
to connect an upstream network ancestrally involved in mRNA localization and
translational control to a downstream regulatory network ancestrally involved in
executing the germ cell program.Introduction
Germ cells are essential for the transfer of heritable information and, therefore, the determination of their fate is a critical event in the development and evolution of sexually reproducing organisms. Two general strategies for generating the germline have evolved in animals: cytoplasmic inheritance or zygotic induction. Inheritance requires that determinants of the germ cell fate (mRNAs and proteins that form the pole plasm) are maternally generated and provisioned to the oocyte. In contrast, induction involves the acquisition de novo of the germ cell fate in a subset of cells later during embryonic development [1], [2].
Some of the first experiments that proved the existence of a maternally generated substance capable of inducing the germline fate were conducted in insects. It had been observed that in many insect species, a distinct region of cytoplasm (called pole plasm, or oosome) is localized to the posterior pole of the oocyte during oogenesis. This pole plasm remains at the posterior during early embryogenesis, until cleavage nuclei reach the embryo cortex. Those nuclei that reach the posterior pole of the embryo interact with the pole plasm, bud from the posterior pole, and become cellularized precociously in comparison to the other blastodermal nuclei [3]. These cells are termed pole cells, and will give rise to the germline [4], [5]. Classical embryonic manipulations showed that the pole plasm is both necessary [6], and sufficient [7] to produce the primordial germ cells.
Genetic analyses have identified numerous molecular factors that are required for the proper production of the pole plasm and pole cells in Drosophila. Only one of these, oskar (osk), is both necessary and sufficient to induce the production of polar granules and pole cells [8]. Due to the sufficiency of Osk to induce germ plasm, it must be tightly regulated to prevent ectopic induction of germline fate. To this end, genes upstream of osk are generally required to regulate translation of osk mRNA and to mediate its transport between the time it is transcribed in the nurse cells and the time it is properly posteriorly localized in the oocyte [9]. Genes downstream of osk are generally required to assemble the polar granules or to mediate proper behavior of the pole cells [9], and have highly conserved functions in the germline throughout the Metazoa [10]–[12].
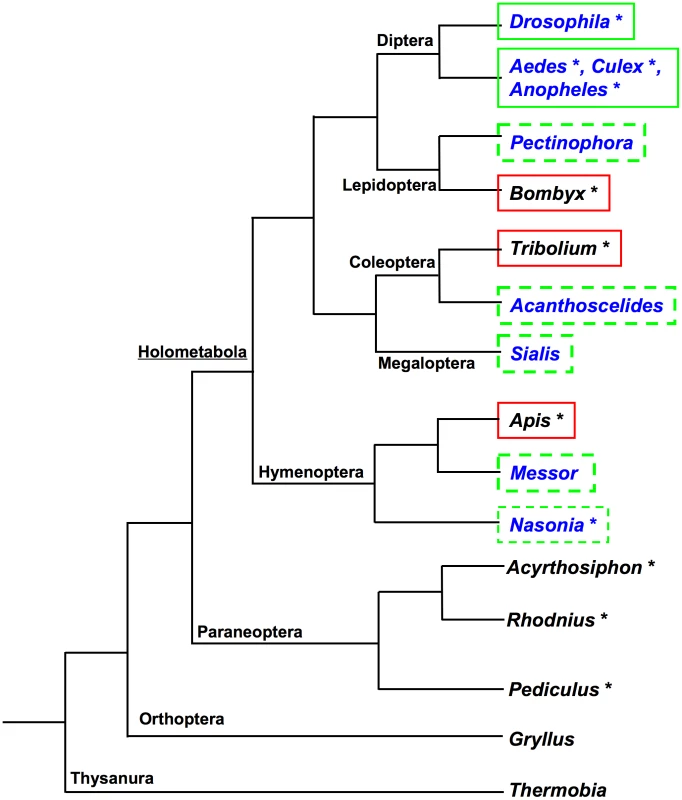
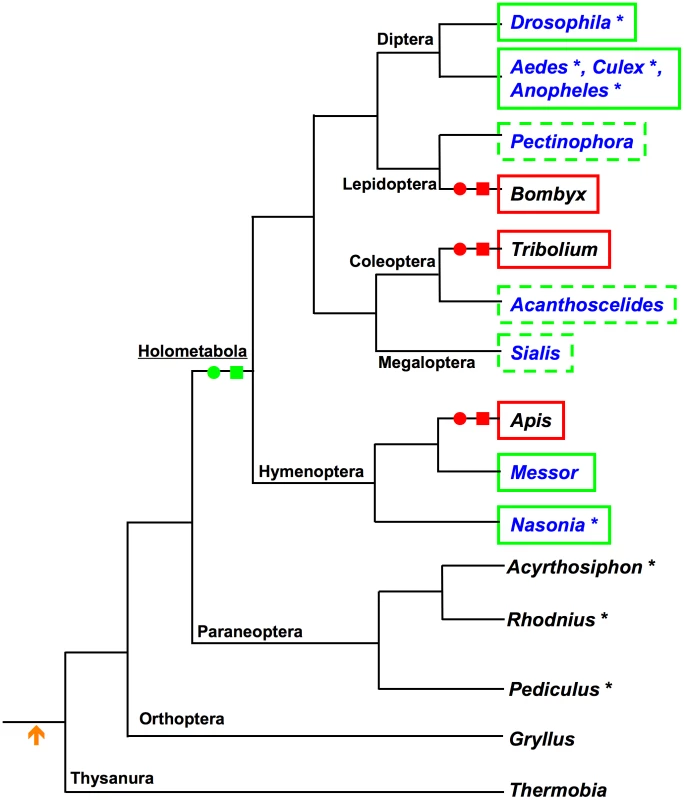
Current data suggest that the mode of germline determination found in Drosophila is not the ancestral mode among the insects. So far neither unequivocal maternal germ plasm nor pole cells have been detected in representatives of basally branching hemimetabolous insect orders. Rather, species from these orders instead appear to rely on zygotic induction mechanisms to specify their germline [13]–[17] (Figure 1). Consistent with absence of cytoplasmic inheritance of germline determinants and the production of pole cells, the processes for which osk is required, orthologs of osk have not been detected in any of the sequenced genomes of the hemimetabolous insects Acyrthosiphon pisum [18], Rhodnius prolixis (http://genome.wustl.edu/genomes/view/rhodnius_prolixus/), and Pediculus humanus http://phumanus.vectorbase.org/SequenceData/Genome/ (Figure 1, Table S1).
Among the Holometabola, osk orthologs are also apparently absent from the sequenced genomes of the silk moth Bombyx mori (Lepidoptera) [19], the beetle Tribolium castaneum (Coleoptera) [20], and the honeybee Apis mellifera (Hymenoptera) [21] (Figure 1, Table S1). Consistent with this absence osk, Bombyx, Tribolium, and Apis all also lack maternal germ plasm, do not produce pole cells, and appear to rather use zygotic inductive strategies to generate the germline [22]–[25] (Figure 1).
These observations led to the idea that osk may have been a novelty that originated within the dipteran lineage [26], [27]. However, Drosophila-like modes of germline determination through posteriorly localized maternal germ plasm and pole cells are also found throughout the Holometabola, including most major lineages of the Hymenoptera (e.g., Nasonia vitripennis [28] sawflies [29] and multiple ant species [30], [31]), the Coleoptera (e.g., Acanthoscelides obtectus [32], Dermestes frischi [33]), Megaloptera (Sialis misuhashii [34]) and Lepidoptera (Pectinophora gossypiella [35]) (Figure 1). Despite the similarity of the strategies for germline determination in the above species to that employed in Drosophila, osk orthologs have only been identified in the genomes of the dipterans Anopheles gambiae, Aedes aegypti, and Culex pipiens [36], [37] (Figure 1).
These observations raised the question of evolutionary origin of osk in the insects and whether or not this gene is associated with the evolution of the inheritance mode of germline specification. To answer these fundamental questions, we examined the molecular basis of maternal germ plasm production in the wasp Nasonia vitripennis. We chose Nasonia because its genome was recently sequenced [38], it is amenable to functional manipulation by pRNAi [39], and its key phylogenetic position within the most basally branching holometabolous order, the Hymenoptera [40], [41]. We show that the regulatory network underlying the production of maternal germ plasm and pole cells is largely conserved between Nasonia and Drosophila, and argue that these features had a common phylogenetic origin at the base of the Holometabola. In addition, we provide evidence that the possession of an oskar ortholog is a general feature of insects that produce pole cells, and that oskar has likely been lost independently multiple times within the Holometabola in correlation with shifts in strategies for establishing the germline.
Results
Cloning and sequence analysis of Nv-Osk
Attempts to detect a Nasonia ortholog by BLAST [42] searches using the Drosophila Osk sequence as the query failed to return significant hits. However, using Oskar sequences identified in the mosquitoes Culex and Aedes, we identified a Nasonia genomic region that showed significant similarity to the mosquito sequences. Using the predicted peptide sequence in this region, reciprocal BLAST against the mosquito and Drosophila genome databases returned results with significant E-values that corresponded to osk genes in each of these species (Table S1). We thus hypothesized that the region in the wasp genome detected by mosquito Osk BLASTs corresponded to Nasonia osk, and cloned a 1500 base pair fragment representing the full length complementary DNA of Nasonia osk using RACE PCR. This sequence contains an open reading frame that is predicted to generate a protein of 375 amino acids.
The overall Nv-Osk sequence is similar to that of Drosophila Osk (16% identity, 33% similarity, 44% gaps), and many of the residues critical for fly Osk function are conserved in the Nasonia sequence (Figure 2). However, we could identify two regions that appear to be unique to the fly sequence. One is the region that is specific to the Drosophila long-Osk isoform [43] (Figure 2, red text). No similarity to this region appears to be encoded in the Nv-osk mRNA, nor is it present in mosquito Osk sequences. The other region that is absent in Nv-Osk includes amino acids 290 to 396 in Dm-Osk (Figure 2, blue text), which corresponds to the domain interacting with LASP to regulate Osk anchoring to the actin cytoskeleton [44]. Interestingly, this region is also absent from the mosquito Osk sequences, which appear to be more similar to Nv-Osk in sequence and general structure (Culex/Nasonia: 24% identity, 42% similarity, 22% gaps).
Fig. 2. Sequence features of Nv-Osk protein. 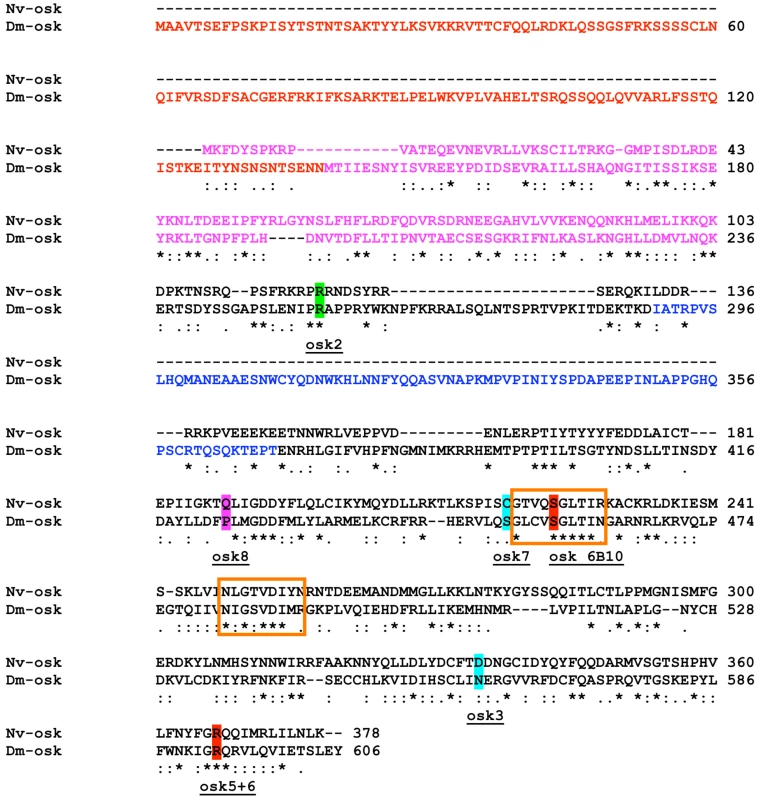
A search in the Conserved Domain Database indicates that the central portion of the Nv-Osk protein shares similarity with a GDSL/SGNH-hydrolase or lipase-like domain (Figure 2, orange boxes), consistent with similar observations made for C. pipiens and A. aegypti Osk orthologs [36]. This domain is weakly detected in Drosophila Osk and it is not clear whether it is necessary for Osk function in pole plasm assembly.
In addition, the N-terminal region of Nv-Osk shows strong similarity to a domain also present at the N-termini of highly conserved tudor-domain containing proteins. This domain has been independently identified in silico as either the Lotus domain [45], or Tejas domain [46]. This domain is present at the N-terminus of orthologs of tudor-domain-containing-7 and -5 (tdrd7, tdrd5), and related tudor domain containing genes [47], and is detected only weakly in fly Osk. tdrd7 and tdrd5 orthologs are found throughout the Metazoa, including all sequenced insect genomes (JAL, personal observation), and are characterized by the presence of Tudor domains toward the C-terminus of the protein, which are absent in Osk proteins. The N-terminal 100 amino acids of Nv-Osk show strong homology to Tdrd7 orthologs throughout the Metazoa, ranging from 39% identical (BLAST E-value 8e-09) to the Apis ortholog, 31% identical (BLAST E-value 1e-05) to the Hydra ortholog, and 29% identical (BLAST E-value 7e-05) for the Danio (zebrafish) ortholog. In comparison, the Apis and Danio Tdrd7 C-termini are 49% identical (BLAST E-value 2e-13), and Apis and Hydra proteins are 30% identical (BLAST E-value 3e-09) in the N-terminal region.
In zebrafish, tdrd7 has a role in controlling germ granule morphology and number during embryogenesis [48]. Furthermore, the Drosophila tdrd5 ortholog, tejas, has a critical role in germline development, and the N-terminal region of this protein (including the Tejas domain, which is similar to the N-terminus of Nv-Osk) has been shown to physically interact with Vas [46]. Finally, a bioinformatic analysis of proteins containing domains similar to those found in Osk and Tdrd7/5 N-termini (termed by the authors OST-HTH) indicated that these domains may bind double-stranded RNA [49]. These results indicate that Oskar is at least partially related to genes that had ancestral germline and/or RNA binding functions.
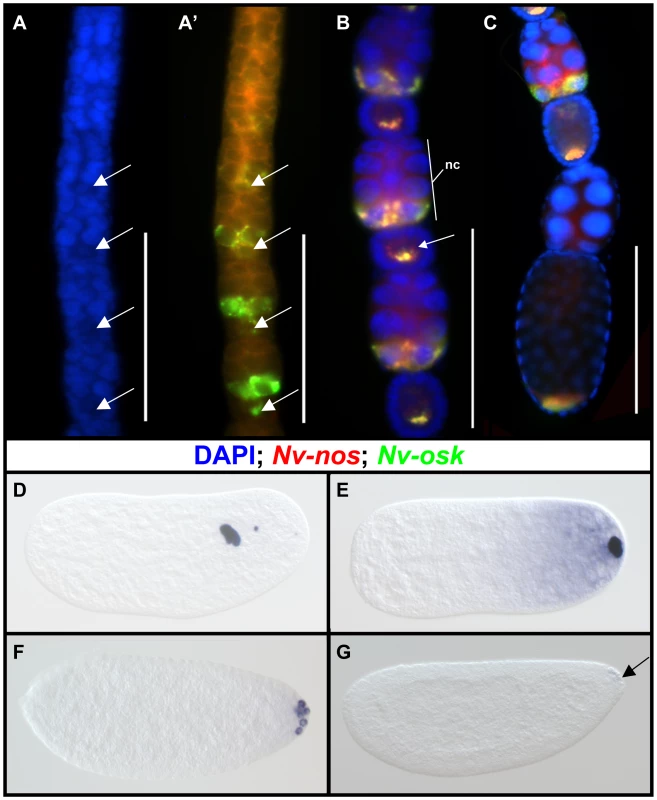
Nv-osk is expressed in the germline and is localized to the posterior of the oocyte and early embryos
Nasonia oogenesis occurs in ovarioles of the polytrophic-meroistic type, where each oocyte is associated with its own population of nurse cells, and has been described in detail previously [50]. Nv-osk mRNA is detected quite early in oogenesis, just after the time that the nurse cells become distinguishable from the oocyte (Figure 3A, 3A′). As the egg chambers mature (Figure 3B), Nv-osk is expressed at very high levels in only the posterior nurse cells nearest to the oocyte. Within these cells, Nv-osk mRNA is incorporated into particles (Figure 3B), a pattern similar to that of Nv-otd1 [51]. From the very early stages of oogenesis, Nv-osk is transported from the nurse cells to the oocyte, where it is localized to the posterior pole in a pattern similar to that of Nv-nos (Figure 3A′, 3B, 3C). During late oogenesis, Nv-osk mRNA levels go from high to barely detectable in the nurse cells of adjacent egg chambers (Figure 3C). This likely indicates the onset of nurse cell dumping, as from this point on the nurse cells will become progressively smaller and eventually disappear. This pattern of rapid transfer of mRNA is similar to what is seen for Nv-otd1 during late oogenesis, except that Nv-otd1 mRNA accumulates at the anterior pole of the oocyte at this stage [51].
In the early embryo, Nv-osk mRNA remains localized to the posterior pole, and most of the mRNA is associated with the oosome, a large, discreet structure associated with the posterior pole. The oosome migrates within the embryo during the early cleavages (Figure 3D), before returning to the posterior pole just before the formation of pole cells (Figure 3E, see [51] for details). At this stage, a population of Nv-osk mRNA not contained within the oosome is observed in a gradient at the posterior pole, a pattern which is typical for oosome associated mRNAs (e.g., otd1and nanos in Nasonia [52]). Nv-osk mRNA still associated with the oosome is then incorporated into the pole cells (Figure 3F), while the cytoplasmic population remains in the embryo proper (not shown, but see [51] for expression of Nv-nos mRNA, which shows identical behavior at these stages). Both populations of mRNA are finally degraded as the cellular blastoderm begins to form (Figure 3G).
Nv-osk is required for oosome assembly and pole cell formation
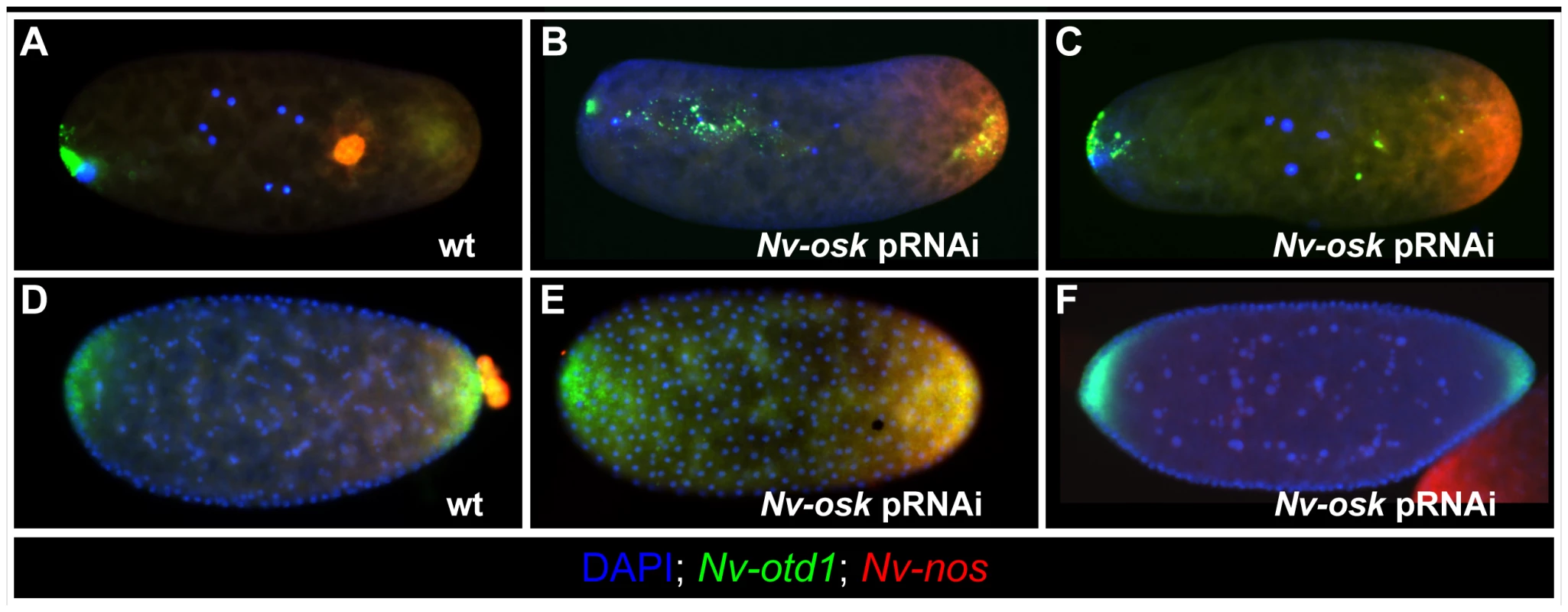
We used parental RNA interference (pRNAi) to analyze the function of Nv-osk during Nasonia development. We obtained specific phenotypes that vary in terms of intensity allowing us to infer a number of potential functions for Nv-osk during oogenesis and early embryogenesis.
In ovarioles showing the strongest Nv-osk pRNAi effect, only a few egg chambers are produced (Figure 4B, compare to 4A) indicating that Nv-osk has an early role in promoting oogenesis. This may be related to a similar phenotype produced by mRNA null mutations in fly osk [53].
Fig. 4. Effects of <i>Nv-osk</i> pRNAi during oogenesis. 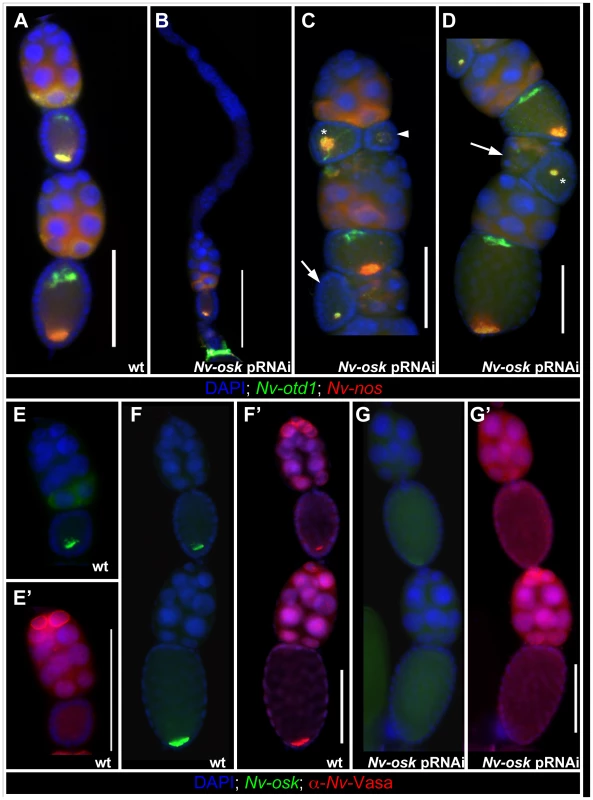
The Nasonia ovariole normally consists of a linear array of egg chambers, with the oocytes always lying directly posterior to their sister nurse cells and directly anterior to the next older egg chamber (Figure 4A). In the milder phenotypes of Nv-osk pRNAi, this linear arrangement is disrupted, and egg chambers arranged perpendicularly to the long axis of the ovariole (arrows in Figure 4C and 4D), or with reversed polarity (arrowhead Figure 4C) are observed. Egg chamber polarity defects are also observed after pRNAi against Nv-vas (not shown) and Nv-tud (see below), indicating that there is a novel role for germ plasm components in establishing polarity of egg chambers within the ovarioles of Nasonia. Due to the variability in the final morphology of ovarioles after pRNAi for Nv-vas, -osk, and -tud, it is not clear whether these phenotypes are all the result of the disruption of a single developmental process.
Within the oocytes, Nv-nos and otd1 mRNAs are sometimes localized more loosely than normal (asterisk and arrowhead Figure 4C) or mislocalized in relation to the AP axis of the oocyte (asterisk Figure 4D) after Nv-osk pRNAi. These phenotypes may represent a disruption of the internal polarity of the oocytes and/or proper anchoring of localized mRNAs. A more detailed understanding of oocyte cytoskeletal polarity and mRNA anchoring mechanisms in Nasonia will be required to resolve this uncertainty. In any case, these results indicate that Nv-osk is required for germline development, for establishing the polarity of the egg chambers, and for the proper localization of the pole plasm to the posterior pole.
In Drosophila, the recruitment of Vas protein to the posterior pole of the oocyte by Osk is a critical step in polar granule assembly. To test whether Nv-Osk functions in a similar way, we examined the distribution of Nv-Vas using a Nasonia specific Vasa antiserum in wild type and Nv-osk pRNAi ovaries. During early oogenesis, Nv-Vas protein is detected primarily on the surface of the nuclei of the most anterior nurse cells (Figure 4E′). This is consistent with the strong transcription of Nv-vas detected in these cells (Figure S1A). Localized Nv-Vas protein is not seen in early oocytes (Figure 4E′), even though Nv-osk is already localized at high levels at the posterior (Figure 4E). Localized Nv-Vas becomes visible in the oocyte relatively late in oogenesis, when the oocyte is of the same size as the nurse cell cluster (Figure 4F, 4F′). This accumulation of Nv-Vas at the posterior pole is abolished after Nv-osk pRNAi (Figure 4G), while Nv-Vas production in anterior nurse cells appears unaffected (Figure 4G′). Thus, the role of Osk in recruiting germ plasm components to the posterior pole is conserved between Drosophila and Nasonia.
Posteriorly localized mRNAs (e.g., Nv-nos, Nv-otd1 and Nv-osk) are incorporated into the oosome in early Nasonia embryos (Figure 5A). After Nv-osk pRNAi, these mRNAs remain in a homogenous cap at the posterior pole of the embryo, and the oosome is not formed (100% penetrance, N = 60) (Figure 5B, 5C). In addition, the anterior localization of Nv-otd1 mRNA is disrupted. Rather than being tightly localized at the anterior pole, Nv-otd1 mRNA is often seen in particles distributed throughout the anterior half of the embryo (Figure 5B). This part of the phenotype may be related to the polarity defects observed in Nv-osk pRNAi oocytes.
pRNAi against Nv-osk also results in the completely penetrant (N = 57) loss of pole cells (Compare wild type in Figure 5D to 5E). In the absence of the protective environment of the pole cells, all Nv-nos mRNA is lost from the embryo by the late blastoderm stage (Figure 5F). A similar phenomenon is seen after Nv-vas pRNAi [51]. Nv-osk pRNAi also causes embryonic patterning phenotypes that result in larval lethality (42%, N = 75). Only a portion (13%) showed phenotypes similar to Nv-nos pRNAi [51], while the remainder of affected cuticles showed defects in head patterning, or more severe patterning disruptions of unclear origin. This range of phenotype was also seen for Nv-vasa [51], and these observations indicate that the roles of Nasonia germ plasm assembly factors in embryonic patterning are much more complicated than they are in the fly, where nos mRNA translation is the main embryonic patterning output of germ plasm assembly [54].
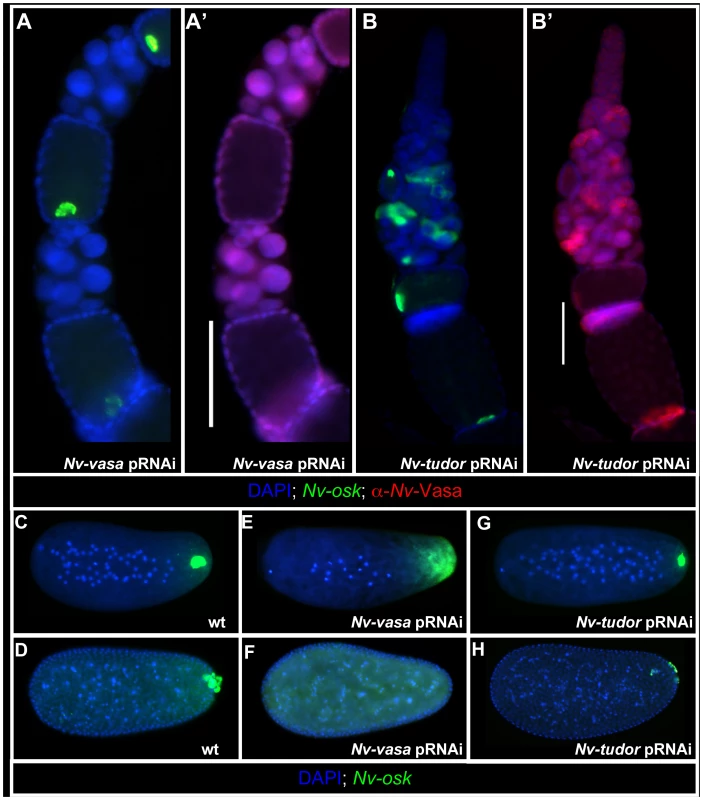
Nv-osk function is upstream of Nv-vas and Nv-tud
In Drosophila, Oskar acts through two main downstream proteins to produce polar granules: Vas and Tud [9]. As shown above, Nv-Osk functions upstream of Nv-Vas recruitment to the posterior during oogenesis (Figure 4G′). However, the functional relationship between Nv-Osk and Nv-Vas in the ovary may not be strictly hierarchical, as Nv-Vas knockdown (Figure 6A′) leads to defects in the proper anchoring and tight localization of Nv-osk mRNA to the posterior pole of the oocyte (Figure 6A). In the embryo, Nv-vasa pRNAi results in the completely penetrant loss of the oosome (Figure 6E) and pole cells (Figure 6F), similar to the effects of Nv-osk pRNAi.
In contrast to Nv-osk and Nv-vas pRNAi, knockdown of Nv-tud, which is expressed weakly and ubiquitously in the nurse cells and oocyte (Figure S1B), has only a minor effect on posterior accumulation of Nv-Vas protein in the oocyte, even when strong polarity defects within the ovariole are observed (Figure 6B, 6B′). In the embryo, the oosome is still formed, but is significantly reduced in size (Compare Figure 6G to 6C). In line with these apparently weaker effects, Nv-tud pRNAi leads to a reduction in the number of pole cells, and those that do form are smaller, less spherical, and less segregated from the somatic nuclei at the posterior pole which may indicate that they are not completely differentiated as primordial germ cells (Compare Figure 6H to 6D). These results indicate that, similar to fly tud [8], [55], Nv-tud function is downstream of Nv-vas and Nv-osk in the production of the germ plasm. However, due to the incompleteness and variability of pRNAi efficiency, we cannot exclude the possibility that the weaker defects are the result of general weaker knockdown of Nv-tud with pRNAi.
Regulation of Nv-osk function
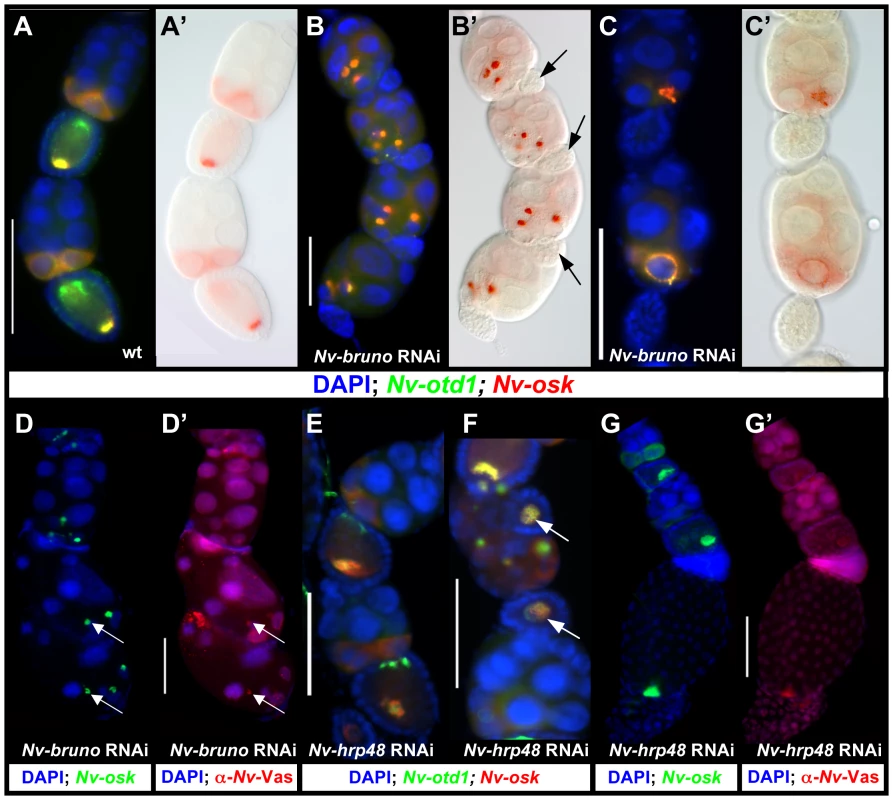
In Drosophila, the localization and regulation of osk translation is tightly regulated in order to prevent ectopic pole plasm and disruptions in segmental patterning. A critical factor in ensuring proper control of osk translation is the RNA binding protein Bruno, which binds the UTRs of osk mRNA and represses its translation. This repression is relieved under normal circumstances only upon localization of osk mRNA to the posterior pole of the oocyte [56]. We analyzed the function of Nasonia bruno to test whether a similar mechanism of translational repression operates in Nasonia to prevent the ectopic assembly of the oosome.
In wild-type egg chambers, Nv-osk and otd1 mRNAs are co-expressed in the posterior nurse cells and localized at the posterior pole of the oocyte, while Nv-otd1 is additionally localized to the anterior pole (Figure 7A, 7A′). The distribution of these mRNAs is dramatically altered after Nv-bruno RNAi: both Nv-osk and Nv-otd1 (and Nv-nos, data not shown) mRNAs are concentrated in large, dense, spheroid particles in the posterior-most nurse cells (Figure 7B, 7B′). These large particles seem to originate at the nuclear envelope, and smaller particles are observed on the surface of the nurse cell nuclear membranes in some egg-chambers (Figure 7C, 7C′). The morphology (density, large size, spheroidal shape) and molecular composition of the ectopic particles seen after Nv-bruno RNAi are similar to the corresponding features of the oosome, indicating that this structure is being ectopically produced in the nurse cells.
If the role of Nv-bruno is similar to that of its Drosophila ortholog, the production of these oosome-like structures in the nurse cells could be due to the ectopic translation of Nv-osk in the nurse cells in the absence of Nv-bruno. In support of this conclusion, the large particles are only produced in the most posterior nurse cells nearest to the oocyte, to which Nv-osk is restricted (Figure 3), while Nv-bruno is expressed in nurse cells located more anteriorly (Figure S1C). However, we cannot exclude that the restriction of large oosome-like particles to the posterior nurse cells is a result of higher levels of Nv-Bruno protein in these cells. In addition, in late Nv-bruno pRNAi egg chambers, Nv-Vas protein is associated with the dense accumulation of Nv-osk mRNA (Figure 7D), further indicating that oosome formation is being completed ectopically within the nurse cells. Conclusive evidence for a direct role of Nv-Bruno in repressing Nv-osk translation will come only with the availability of an antibody against Nv-Osk protein.
Another Drosophila RNA binding protein, Hrp48, is critical for both silencing of unlocalized osk mRNA translation, and for the proper initiation of its translation once the mRNA is localized to the posterior [57], [58]. Nv-hrp48 is expressed strongly throughout the nurse cells in the wasp ovary (Figure S1D), and when its function is knocked down, ectopic oosome-like structures are not seen in the nurse cells (Figure 7E, 7F), in contrast to what is seen after Nv-bruno pRNAi. In most egg chambers, both Nv-osk and Nv-otd1 mRNAs are expressed normally in the nurse cells, and are transported to the oocyte (Figure 7E). Once in the oocyte, however, these mRNAs do not become localized normally. The extent of mislocalization varies from oocytes that show a looser localization of posterior mRNAs (Figure 7E) to those where Nv-osk and Nv-otd1 mRNAs fail to localize to a distinct cortical location, and are diffusely expressed throughout the smaller than usual oocytes (Figure 7F, arrow). In more weakly affected egg chambers, which have established normal polarity, the pattern of Nv-Vas accumulation appears to be only weakly affected, with the protein appearing at slightly lower levels, and loosely organized, likely reflecting a mild disruption in the proper assembly of the oosome during late oogenesis (Figure 7G, 7G′).
Thus, Nv-hrp48 appears to have a conserved role in the assembly of the germ plasm in Nasonia, and by extension may have a conserved function in regulating the translation of Nv-osk. Our results indicate that the primary role of this factor is to promote oosome assembly (and thus, by analogy to Drosophila, Nv-osk function). However, we cannot completely exclude a second role, such as that seen in Drosophila, for Nv-hrp48 in Nasonia in repressing the translation of unlocalized Nv-osk in the oocyte [57], [58].
osk is present in a close relative of Apis, and likely in a close relative of Tribolium
Our results show that a regulatory network of protein interaction centered on Nv-Osk is required for the maternal production of germ plasm, and that this network is highly similar to that found in Drosophila. This suggests that, given the basally branching phylogenetic position of the Hymenoptera among the Holometabola, this regulatory network arose in a common ancestor of all Holometabola, and that transitions to the zygotic induction mode of germ cell specification are associated with secondary disruptions of this network. To test this hypothesis, we sought to determine if osk, as the central component of this network, is conserved in other species that produce maternal germ plasm and pole cells.
Multiple ant species have been shown to specify their pole cells through the assembly of a posterior pole plasm that is incorporated into pole cells during early embryogenesis [30], [31]. Consistent with our hypothesis, we successfully cloned an osk ortholog in the ant Messor pergandei, whose protein sequence shows 46.4% similarity to that of Nv-Osk. Moreover, Messor osk (Mp-osk) mRNA is localized to the posterior pole of the oocyte during oogenesis (Figure 8A), and embryogenesis (Figure 8B). This pattern of Mp-osk mRNA accumulation is similar to that of insects that specify germ cell through cytoplasmic inheritance (e.g., Nasonia and Drosophila), and suggests that its function in germ cell specification is conserved in ants. In addition, the localization of Mp-osk corresponds well to the previously observed localization of Vasa protein and nanos mRNA in the oocyte and embryo at equivalent stages in Messor and other closely related ant species [30], [31]. Messor is a much closer relative of Apis than is Nasonia [59], and the discovery of osk in this ant species strongly indicates that the absence of osk in the bee genome is a derived state.
Fig. 8. Oskar and oosomes in other Holometabolan species. 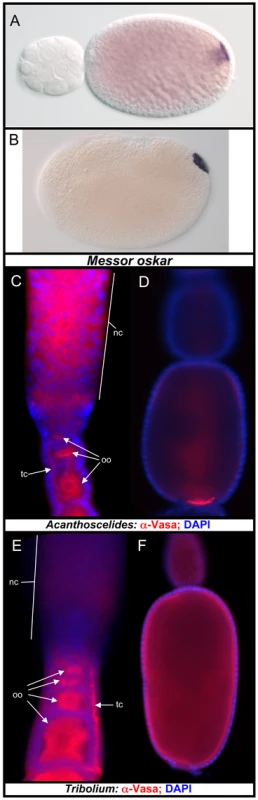
We also analyzed the molecular basis of maternal germ plasm formation in the beetle Acanthoscelides obtectus, which, like Nasonia, but unlike Tribolium, produces an oosome and pole cells [32]. Like Tribolium and many other beetle species, Acanthoscelides possesses teletrophic ovarioles. In this type of oogenesis, a common pool of nurse cells is located at the anterior of the ovariole, which is connected with progressively maturing oocytes toward the posterior by actin and microtubule-rich structures called trophic cords [60]. In early oogenesis, Vas protein is highly enriched around the surface of the oocyte nucleues (Figure 8C). The presence of Vas protein is also detected in the nurse cells and trophic cords. In more mature oocytes, Vas protein is strongly enriched at the posterior pole, where the oosome will be formed (Figure 8D). This indicates that, despite employing a mode of oogenesis quite divergent from that seen in Nasonia and Drosophila, this beetle possesses similar capabilities for directing the localization and assembly of the germ plasm components to the posterior pole.
This is in contrast to Tribolium, where Vas protein is never found in a localized pattern in later oocytes (Figure 8F) despite its presence in the cytoplasm of early oocytes and in the trophic cords (Figure 8E), correlating well with the absence of pole cells and maternal germ plasm in this species. Based on the similarity of the pattern of Vasa protein accumulation in Acanthoscelides to the osk dependent Vas localization patterns in Nasonia and Drosophila, we predict that an osk ortholog is present in the genome of Acanthoscelides, and that it functions in recruiting Vas protein to the posterior pole of the oocyte and in assembling the oosome similar to its orthologs in Nasonia and Drosophila. Attempts to clone osk from the beetle by degenerate PCR have so far failed, and transcriptome or genome sequencing may be required to resolve this question.
Discussion
The origin of germ plasm and pole cells in holometabolous insects
Taken together, our results reveal a new picture for the origin and evolution of oskar, maternally provisioned germ plasm, and pole cells. We propose that the origins of these features represent evolutionary novelties of the Holometabola in relation to the rest of the insects, and that the appearance of the latter two features is strongly correlated with the presence of osk (Figure 9). Our conclusions are based on: (1) the presence of osk orthologs in the genomes of Nasonia and Messor, two distantly related hymenopteran species that also both have maternal germ plasm and pole cells; (2) the molecular and developmental similarity of the germ plasm of Acanthoscelides to that of Drosophila and Nasonia, which is consistent with the presence of an osk ortholog in this beetle; (3) the conserved interactions of Nv-Osk with upstream regulators (such as Nv-Bruno and Nv-Hrp48) and downstream partners (such as Nv-Vas and Nv-Tud), which indicate that a protein interaction network centered on Osk for generating maternal germ plasm and pole cells was present at the latest in the most recent common ancestor of the Hymenoptera and Diptera (which, based on current phylogenies would also be the common ancestor of all Holometabola) (Figure 9); and finally (4) the absence of maternal germ plasm, pole cells and osk in hemimetabolous insects, suggesting that the absence of these features is ancestral for the insects (Figure 9), and that these features likely arose after the divergence of the Holometabola from its sister group the Paraneoptera (true bugs, lice, and thrips).
The mapping of our findings on the insect phylogeny also indicates that Apis, Tribolium, and Bombyx may have lost these characters through independent evolutionary events (Figure 9). In addition, the correlation of the loss of maternal germ plasm and pole cells with the absence of oskar in these species (Figure 9), indicate that osk is a key factor in the evolution of germline determination mechanisms in the Holometabola.
Since production of the germline is a critical event in development and evolution, it is surprising that dramatic changes in how this cell fate is established have occurred several times in insect evolution. Such transitions could have been facilitated if redundant mechanisms for generating the germline existed in the ancestors of lineages that eventually lost the ability to maternally specify the germline.
In Drosophila there appears to be no remaining inductive capability: if pole cells are not produced, or are destroyed before reaching the gonad, the resulting fly is sterile. However, this is not the case in all insects. Destruction or removal of the oosome from the embryo of the wasp Pimpla turionellae resulted in the complete absence of pole cells, consistent with the role of the oosome in generating these cells. In spite of this, when embryos subject to these manipulations were examined later, a majority appeared to have germ cells populating the late embryonic gonads [61]. As Pimpla is a close relative of ants and bees, it is possible that both maternally provisioned germ plasm and the ability to zygotically induce germline fate coexisted in an ancestor of Apis, and the loss of the former capability thus may not have had dire consequences for the fecundity of species within the lineage leading to Apis. Once the presence of pole cells and maternal germ plasm was no longer selected for, it may have been relatively easy to lose osk, as long as another strategy for either localizing posterior nanos, or another mechanism for patterning the posterior is present.
The question of why an insect would lose the capacity to produce pole cells is also difficult to address directly. The likelihood that maternal provisioning of germline determinants evolved independently multiple times among animals [1], [2] implies that this strategy for germline determination has, at least under certain circumstances, selective benefits. Reciprocally, the multiple independent losses of this strategy indicate that, in other circumstances, zygotic induction may be favored. Broader sampling of germline specification strategies among the animals could shed light on the possible ecological or embryological traits correlated with the retention of or transition away from maternal synthesis of germline determinants and early segregation of the germ cell fate.
The origin of oskar
Our finding that Oskar was a critical innovation for the transition to the maternal inheritance mode of germline determination in insects leads to the question of how such a novel protein could have been invented.
The strong similarity of the N-terminus of Nv-Osk to the N-terminus of Tdrd-7 orthologs found throughout the Metazoa, indicates that the origin of osk involved the duplication and divergence of this locus in an ancestor of the Holometabola. However, unlike tdrd-7 genes, osk orthologs lack Tudor domains toward the C-terminus, and rather have a domain with structural similarity to SGNH/GDSL class hydrolases. Since such a domain is not found in Tdrd-7 orthologs, it may be that osk arose by a fusion of a tdrd7 paralog, and a gene possessing a hydrolase domain.
While proteins of the SGNH/GDSL hydrolase family are found in all insect species, Osk orthologs show no significant homology to these sequences in BLAST analyses (E-value cutoff = 10). Rather, the highest scoring non-Oskar BLAST hits for the C-terminal portion (i.e., excluding the first 100 amino acids) of Osk proteins are often SGNH/GDSL hydrolases of Bacteria (e.g., Mp-Osk finds ZP_05979902.1 from Subdoligranulum variabile at an E-value of 0.17, and Cp-Osk finds YP_001491067.1 from Arcobacter butzleri at an E-value of 0.006). These observations raise the possibility that osk could have arisen by the combination of horizontal gene transfer from bacteria and gene fusion events. The fact that horizontal gene transfer from endosymbiotic bacteria occurs in insects is now well established [38], [62], and a source for a potential horizontal transfer could be the endosymbionts that are tightly associated with the early germ cells and gonads of many insect species (e.g., [63], [64]).
While the most parsimonious explanation for the observed distribution of osk orthologs among the Holometabola is that there was a single origin for this gene in a common ancestor of the holometbolan clade, we cannot formally exclude the possibility that the similarity in structure and function between the hymenopteran and dipteran Osk sequences was the result of two lineage specific events of convergent evolution responding to independent instances of selective pressure to establish cytoplasmic inheritance of germline components. However, it seems highly unlikely that the molecular events required to invent a novel gene such as osk would occur in almost identical ways twice in evolution before a different solution is found, let alone the unlikelihood of such a gene being fixed in a population, and then subsequently integrated into a novel regulatory network.
However, the invention of a novel factor required for cytoplasmic inheritance of germ plasm components may not be an occurrence unique to the Holometabola. In zebrafish, the bucky ball gene has an osk-like function in generating maternal germ plasm, but is molecularly unrelated to osk, and is only found in vertebrate genomes [11], [65]. This indicates that there is nothing intrinsic in the primary structure of Osk protein that is required for maternal assembly of germ plasm, and that there are many possible solutions to the problem of generating this substance. Further sampling of metazoan germline establishment strategies will give insight into how common the generation of novel genes is in the process of evolving maternally generated germ plasm.
The origin of a protein regulatory network for restricting germ plasm production to the posterior pole
The process of maternal germ plasm assembly must be precisely controlled, and abnormalities in this process result in deep and sometimes spectacular consequences for the embryonic anterior-posterior axis [8]. Based on our results with Nv-bruno and Nv-hrp48, a common mechanism to spatially regulate osk localization and translation was likely already present at the origin of the Holometabola. This, along with the fact that factors such as Vas and Tud have conserved roles downstream of Osk in Nasonia, indicates that a complex protein interaction network for localized production of germ plasm during oogenenesis existed in a common ancestor of the Holometabola. This raises the question as to when during evolution has this network been assembled, and through which molecular mechanisms.
Proteins downstream of Osk, such as Tud, Vas, and Nos, have conserved roles in the specification and function of germ cells throughout the Metazoa, including those without maternal specification of the germline [11], and therefore are able to function without Osk to generate germ cell characteristics. Similarly, the proteins upstream of Osk, such as Bruno, Hrp48, and Staufen, are also highly conserved throughout the metazoa, and have conserved functions in mRNA localization and translational control in a variety of cellular contexts outside of the germline. Thus, Osk seems to have been intercalated between two ancient pre-existing regulatory networks. The position of Osk as the nexus between these two networks allows its specific and precisely controlled function in specifying the germline fate.
The fact that both the up - and downstream networks were already well established before the evolution of osk indicates that relatively few evolutionary steps may have been required to integrate Osk between them. In addition, since Osk is at least partially derived from a tdrd7/5-like gene, orthologs of which have well described functions in the germline in vertebrates and invertebrates, the ancestral Osk may have been predisposed to interact with other germ plasm components.
The localization of osk likely also had an evolutionary antecedent, as the presence of posteriorly localized patterning factors has been detected in some hemimetabolous species, e.g., [64], [66]. Since germ cells arise at the posterior pole just after gastrulation in some hemimetabolous species [16], [67], [68], it is possible that factors that predispose posterior nuclei to take germline fate are also localized at the posterior pole in these species. The molecular nature of any such factor, and whether its role is direct or indirect, remains to be determined. Testing the function and regulation of orthologs of genes both up - and downstream of osk in hemimetabolous, and other holometabolous, insect species should give insights into the functioning of the ancestral germline regulatory network, and could provide further clues as to how osk could have been integrated into it.
Materials and Methods
A BLAST based strategy was used to identify potential osk orthologs in sequenced insect genomes (see ). The following databases were searched: for Bombyx mori , Silkworm Genome Assembly at silkdb.org [69]; for Tribolium castaneum, BeetleBase3_NCBI_DB at beetlebase.org [70]; for Nasonia vitripennis, Nasonia Scaffolds Assmebly Nvit_1.0 at hymenopteragenome.org/nasonia/ [71]; For Apis melifera, Scaffolds Assembly 2 at hymenopteragenome.org/beebase/; for Acyrthosiphon pisum, genome (reference only) at http://www.ncbi.nlm.nih.gov/projects/genome/seq/BlastGen/BlastGen.cgi?taxid=7029; for Rhodnius prolixus, Harpegnathos saltator and Camponotus floridanus, species-specific Whole-genome shotgun reads (wgs) databases were selected at blast.ncbi.nlm.nih.gov; for Culex pipiens Assembly CpipJ1 - Johannesburg Strain, Supercontigs at http://cquinquefasciatus.vectorbase.org/Tools/BLAST/, and for Pediculus humanus, Assembly PhumU1, Supercontigs - USDA Strain at http://phumanus.vectorbase.org/Tools/BLAST/. These databases were queried using tblastn with default parameters (except the E-value cut off was raised to 10 where necessary) with the following Oskar protein sequences: NP_731295.1 (Drosophila), XP_001848641.1 (Culex), ADK94458.1 (Nasonia), and HM992570 (Messor). To identify osk orthologs among EST sequences, the same query sequences and tblastn parameters were used at blast.ncbi.nlm.nih.gov to search the (est others) database.
Templates for probe and dsRNA production were generated as in [72]. dsRNA was produced using T7 Megascript kit (Ambion) following manufacturers instructions. Fragments used to generate dsRNA and probes were as follows: Nv-osk—bases 161–843 of Genbank accession HM535628.1, Nv-bruno—bases 534–1394 of Genbank accession XM_001605096.1, Nv-vasa—bases 827–1613 of Genbank accession XM_001603906.1, Nv-hrp48—bases 434–1727 of Genbank accession XM_001600216.1, Nv-tudor—bases 6053–6811 of Gnomon model hmm120984.
RNAi experiments were performed as described in [39]. dsRNAs were used at the following concentrations: Nv-osk - 3.5 mg/mL, Nv-vasa - 3 mg/mL, Nv-bruno-2.5 mg/mL, Nv-hrp48 - 1.0 mg/mL, Nv-tudor - 2.5 mg/mL. Knockdown was confirmed by comparing expression levels of the gene of interest in ovaries of wild-type wasps to those from pRNAi treated wasps. All genes showed clearly reduced levels of expression after their corresponding dsRNA injections, but the degree of knockdown was variable from egg chamber to egg chamber.
RACE PCR for Nv-osk was performed using the SMART-RACE kit (Takara) according to manufacturer's instructions.
The Nasonia Vasa antibody was generated using the custom peptide antibody service of Sigma-Genosys with the peptide CVLRHDTMKPPGERQ as the antigen. It was used at 1∶500, and detected using anti-rabbit Alexa 555 (Invitrogen) at 1∶750
The cross reactive Drosophila Vasa antiserum used in Tribolium and Acanthoscelides was a generous gift from Akira Nakamura [73]. It was used at 1∶1000 and detected as above.
in situ hybridization and immunohistochemistry were performed as described in [51].
The ant osk sequence was found in the course of a genome sequencing project (unpublished) and cloned from the ant Messor pergandei using the following primers: AntOsk forward ATGGAWGAAACAGTGGCATTRRTMAAAT and AntOsk reverse GGAACCARTCGTAWTCYGTRRTRTACGTT. The cloned 1057 base pair fragment was validated by sequencing, submitted to Genbank with accession # HM992570 and used to generate an antisense Digoxigenin labeled probe for in situ hybridization. Embryos and ovaries of Nasonia and the beetles were collected and fixed as in [72]. Ant embryos and ovaries were prepared and stained for osk mRNA as described in [74].
Supporting Information
Zdroje
1. ExtavourCGAkamM
2003
Mechanisms of germ cell specification across the metazoans:
epigenesis and preformation.
Development
130
5869
5884
2. ExtavourCGM
2007
Evolution of the bilaterian germ line: lineage origin and
modulation of specification mechanisms.
Integrative and Comparative Biology
47
770
785
3. SchwalmF
1988
Insect Morphogenesis;
SauerHW
Basel
Karger
4. HegnerRW
1914
Studies on germ cells. I. The history of the germ cells in
insects with special reference to the Keimbahn-determinants. II. The origin
and significance of the Keimbahn-determinants in animals.
Journal of Morphology
25
375
509
5. MahowaldAP
2001
Assembly of the Drosophila germ plasm.
International Review of Cytology - a Survey of Cell Biology
Vol 203 203
187
213
6. HegnerRW
1911
Experiments with Chrysomelid Beetles. III. The Effects of Killing
Parts of the Eggs of Leptinotarsa decemlineata.
Biological Bulletin
20
237
251
7. IllmenseeKMahowaldAP
1974
Transplantation of Posterior Polar Plasm in Drosophila. Induction
of Germ Cells at the Anterior Pole of the Egg.
Proceedings of the National Academy of Sciences of the United States of
America
71
1016
1020
8. EphrussiALehmannR
1992
Induction of Germ-Cell Formation by Oskar.
Nature
358
387
392
9. RongoCLehmannR
1996
Regulated synthesis, transport and assembly of the Drosophila
germ plasm.
Trends in Genetics
12
102
109
10. ThomsonTLaskoP
2005
Tudor and its domains: germ cell formation from a Tudor
perspective.
Cell Research
15
281
291
11. Ewen-CampenBSchwagerEEExtavourCGM
2010
The Molecular Machinery of Germ Line
Specification.
Molecular Reproduction and Development
77
3
18
12. RazE
2000
The function and regulation of vasa-like genes in germ-cell
development.
Genome Biol
1
REVIEWS1017
13. KlagJ
1977
Differentiation of primordial germ cells in the embryonic
development of Thermobia domestica, Pack. (Thysanura): an ultrastructural
study.
Journal of Embryology and Experimental Morphology
38
14. MitoTNakamuraTSarashinaIChangCCOgawaS
2008
Dynamic expression patterns of vasa during embryogenesis in the
cricket Gryllus bimaculatus.
Development Genes and Evolution
218
381
387
15. ChangCCDeardenPAkamM
2002
Germ line development in the grasshopper Schistocerca gregaria:
vasa as a marker.
Developmental Biology
252
100
118
16. MellanbyH
1935
Memoirs: The Early Embryonic Development of Rhodnius prolixus
(Hemiptera, Heteroptera).
Quarterly Journal of Microscopical Science
s2
71
90
17. MiuraTBraendleCShingletonASiskGKambhampatiS
2003
A comparison of parthenogenetic and sexual embryogenesis of the
pea aphid Acyrthosiphon pisum (Hemiptera : Aphidoidea).
Journal of Experimental Zoology Part B-Molecular and Developmental
Evolution
295B
59
81
18. The International Aphid Genomics Consortium
2010
Genome Sequence of the Pea Aphid Acyrthosiphon
pisum.
PLoS Biol
8
e1000313
doi:10.1371/journal.pbio.1000313
19. XiaQYZhouZYLuCChengDJDaiFY
2004
A draft sequence for the genome of the domesticated silkworm
(Bombyx mori).
Science
306
1937
1940
20. RichardsSGibbsRAWeinstockGMBrownSJDenellR
2008
The genome of the model beetle and pest Tribolium
castaneum.
Nature
452
949
955
21. WeinstockGMRobinsonGEGibbsRAWorleyKCEvansJD
2006
Insights into social insects from the genome of the honeybee Apis
mellifera.
Nature
443
931
949
22. NagyLRiddifordLKiguchiK
1994
Morphogenesis in the Early Embryo of the Lepidopteran
Bombyx-Mori.
Developmental Biology
165
137
151
23. SchroderR
2006
vasa mRNA accumulates at the posterior pole during blastoderm
formation in the flour beetle Tribolium castaneum.
Development Genes and Evolution
216
277
283
24. NelsonJA
1915
The Embryology of the Honey Bee
Princeton
Princeton University Press
282
25. DeardenPK
2006
Germ cell development in the Honeybee (Apis mellifera); Vasa and
Nanos expression.
Bmc Developmental Biology
6
-
26. DeardenPKWilsonMJSablanLOsbornePWHavlerM
2006
Patterns of conservation and change in honey bee developmental
genes.
Genome Research
16
1376
1384
27. ShigenobuSBickelRDBrissonJAButtsTChangCC
2010
Comprehensive survey of developmental genes in the pea aphid,
Acyrthosiphon pisum: frequent lineage-specific duplications and losses of
developmental genes.
Insect Molecular Biology
19
47
62
28. BullAL
1982
Stages of Living Embryos in the Jewel Wasp
Mormoniella-(Nasonia)-Vitripennis-(Walker)(Hymenoptera,
Pteromalidae).
International Journal of Insect Morphology & Embryology
11
1
23
29. NakaoHHatakeyamaMLeeJMShimodaMKandaT
2006
Expression pattern of Bombyx vasa-like (BmVLG) protein and its
implications in germ cell development.
Development Genes and Evolution
216
94
99
30. KhilaAAbouheifE
2008
Reproductive constraint is a developmental mechanism that
maintains social harmony in advanced ant societies.
Proceedings of the National Academy of Sciences of the United States of
America
105
17884
17889
31. KhilaAAbouheifE
2010
Evaluating the role of reproductive constraints in ant social
evolution.
Philosophical Transactions of the Royal Society B-Biological
Sciences
365
617
630
32. JungE
1966
Untersuchungen am Ei des Speisebohnenkäfers
Bruchidius obtectus Say (Coleoptera).
Development Genes and Evolution
157
320
392
33. KuetheH-W
1966
Das Differenzierungszentrum als selbstregulierendes
Faktorensystem für den Aufbau der Keimanlage im Ei von Dermestes
frischi (Coleoptera) Roux' Archiv für
Entwicklungsmechanik.
157
121
302
34. SuzukiNShimizuSAndoH
1981
Early Embryology of the Alderfly, Sialis-Mitsuhashii Okamoto
(Megaloptera, Sialidae).
International Journal of Insect Morphology & Embryology
10
409
418
35. BergGJGassnerG
1978
Fine structure of the blastoderm embryo of the pink bollworm,
Pectinophora Gossypiella (Saunders) (lepidoptera:
Gelechiidae).
International Journal of Insect Morphology and Embryology
7
81
105
36. JuhnJMarinottiOCalvoEJamesAA
2008
Gene structure and expression of nanos (nos) and oskar (osk)
orthologues of the vector mosquito, Culex quinquefasciatus.
Insect Molecular Biology
17
545
552
37. JuhnJJamesAA
2006
oskar gene expression in the vector mosquitoes, Anopheles gambiae
and Aedes aegypti.
Insect Molecular Biology
15
363
372
38. WerrenJHRichardsSDesjardinsCANiehuisOGadauJ
2010
Functional and Evolutionary Insights from the Genomes of Three
Parasitoid Nasonia Species.
Science
327
343
348
39. LynchJADesplanC
2006
A method for parental RNA interference in the wasp Nasonia
vitripennis.
Nature Protocols
1
486
494
40. SavardJTautzDRichardsSWeinstockGMGibbsRA
2006
Phylogenomic analysis reveals bees and wasps (Hymenoptera) at the
base of the radiation of Holometabolous insects.
Genome Research
16
1334
1338
41. WiegmannBMTrautweinMDKimJWCasselBKBertoneMA
2009
Single-copy nuclear genes resolve the phylogeny of the
holometabolous insects.
Bmc Biology
7
-
42. AltschulSFGishWMillerWMyersEWLipmanDJ
1990
Basic local alignment search tool.
J Mol Biol
215
403
410
43. MarkussenFHMichonAMBreitwieserWEphrussiA
1995
Translational Control of Oskar Generates Short Osk, the Isoform
That Induces Pole Plasm Assembly.
Development
121
3723
3732
44. SuyamaRJennyACuradoSBerkelWPVEphrussiA
2009
The actin-binding protein Lasp promotes Oskar accumulation at the
posterior pole of the Drosophila embryo.
Development
136
95
105
45. CallebautIMornonJP
2010
LOTUS, a new domain associated with small RNA pathways in the
germline.
Bioinformatics
26
1140
1144
46. PatilVSKaiT
2010
Repression of Retroelements in Drosophila Germline via piRNA
Pathway by the Tudor Domain Protein Tejas.
Current Biology
20
724
730
47. ArkovALRamosA
Building RNA-protein granules: insight from the
germline.
Trends Cell Biol
20
482
490
48. StrasserMJMackenzieNCDumstreiKNakkrasaeLISteblerJ
2008
Control over the morphology and segregation of Zebrafish germ
cell granules during embryonic development.
Bmc Developmental Biology
8
-
49. AnantharamanVZhangDPAravindL
2010
OST-HTH: a novel predicted RNA-binding domain.
Biology Direct
5
-
50. OlesnickyECDesplanC
2007
Distinct mechanisms for mRNA localization during embryonic axis
specification in the wasp Nasonia.
Developmental Biology
306
134
142
51. LynchJADesplanC
2010
Novel modes of localization and function of nanos in the wasp
Nasonia.
Development
137
3813
3821
52. LynchJABrentAELeafDSPultzMADesplanC
2006
Localized maternal orthodenticle patterns anterior and posterior
in the long germ wasp Nasonia.
Nature
439
728
732
53. JennyAHachetOZavorszkyPCyrklaffAWestonMDJ
2006
A translation-independent role of oskar RNA in early Drosophila
oogenesis.
Development
133
2827
2833
54. GavisERLehmannR
1994
Translational regulation of nanos by RNA
localization.
Nature
369
315
318
55. HayBJanLYJanYN
1990
Localization of Vasa, a Component of Drosophila Polar Granules,
in Maternal-Effect Mutants That Alter Embryonic Anteroposterior
Polarity.
Development
109
425
433
56. Kim-HaJKerrKMacdonaldPM
1995
Translational Regulation of Oskar Messenger-Rna by Bruno, an
Ovarian Rna-Binding Protein, Is Essential.
Cell
81
403
412
57. HuynhJRMunroTPSmith-LitiereKLepesantJAJohnstonDS
2004
The Drosophila hnRNPA/B homolog, Hrp48, is specifically required
for a distinct step in osk mRNA localization.
Developmental Cell
6
625
635
58. YanoTLopez de QuintoSMatsuiYShevchenkoAShevchenkoA
2004
Hrp48, a Drosophila hnRNPA/B homolog, binds and regulates
translation of oskar mRNA.
Developmental Cell
6
637
648
59. DowtonMAustinAD
1994
Molecular Phylogeny of the Insect Order Hymenoptera - Apocritan
Relationships.
Proceedings of the National Academy of Sciences of the United States of
America
91
9911
9915
60. BüningJ
1994
The insect ovary
London
Chapman & Hall
61. AchteligMKrauseG
1971
Experiments on Uncleared Egg of Pimpla-Turionellae L.
(Hymenoptera) for Functional Analysis of Oosome Region.
Wilhelm Roux Archiv Fur Entwicklungsmechanik Der Organismen
167
164
&
62. HotoppJCDClarkMEOliveiraDCSGFosterJMFischerP
2007
Widespread lateral gene transfer from intracellular bacteria to
multicellular eukaryotes.
Science
317
1753
1756
63. KochA
1931
Die Symbiose von Oryzaephilus surinamensis L.
(Cucujidae, Coleoptera).
Zoomorphology
23
389
424
64. SanderK
1969
Specification of the basic body pattern in insect embryogenesis.
Advances in Insect Physiology,
TreherneJEBerridgeMJWigglesworthVB
12
Academic Press
125
235
65. BontemsFSteinAMarlowFLyauteyJGuptaT
2009
Bucky Ball Organizes Germ Plasm Assembly in
Zebrafish.
Current Biology
19
414
422
66. LallSLudwigMZPatelNH
2003
Nanos plays a conserved role in axial patterning outside of the
Diptera.
Curr Biol
13
224
229
67. HemingBS
1979
Origin and Fate of Germ-Cells in Male and Female Embryos of
Haplothrips-Verbasci (Osborn) (Insecta, Thysanoptera,
Phlaeothripidae).
Journal of Morphology
160
323
&
68. JohannsenOAButtFH
1941
Embryology of Insects and Myriapods
New York
McGraw-Hill
69. DuanJLiRQChengDJFanWZhaXF
2010
SilkDB v2.0: a platform for silkworm (Bombyx mori) genome
biology.
Nucleic Acids Research
38
D453
D456
70. KimHSMurphyTXiaJCarageaDParkY
2010
BeetleBase in 2010: revisions to provide comprehensive genomic
information for Tribolium castaneum.
Nucleic Acids Research
38
D437
D442
71. Munoz-TorresMReeseJCPCAKBJPS
2010
Hymenoptera Genome Database: integrated community resources for
insect species of the order Hymenoptera.
Nucleic Acids Research
72. LynchJAPeelADDrechslerAAverofMRothS
2010
EGF Signaling and the Origin of Axial Polarity among the
Insects.
Current Biology
20
1042
1047
73. Hanyu-NakamuraKKobayashiSNakamuraA
2004
Germ cell-autonomous Wunen2 is required for germline development
in Drosophila embryos.
Development
131
4545
4553
74. KhilaAAbouheifE
2009
In situ hybridization on ant ovaries and embryos.
Cold Spring Harb Protoc
2009
pdb prot5250
75. WheelerWCWhitingMWheelerQDCarpenterJM
2001
The phylogeny of the extant hexapod orders.
Cladistics
17
113
169
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání